INTRODUCTION
Metal-catalyzed cross-couplings represent one of the most important reaction platforms in modern synthetic chemistry (Figure 1A).1 Although the field enjoys a rich history, there remains fervent interest in expanding the frontiers of cross-coupling chemistry. Some areas of current exploration include the development of new modes of reactivity (dual catalysis,2 photoredox catalysis,3 cross-electrophile couplings,4 chemoenzymatic transformations,5 etc.), stereoselective couplings,6 and the utilization of new classes of electrophiles. With respect to the latter, non-precious-metal catalysis has been particularly enabling.
Figure 1.
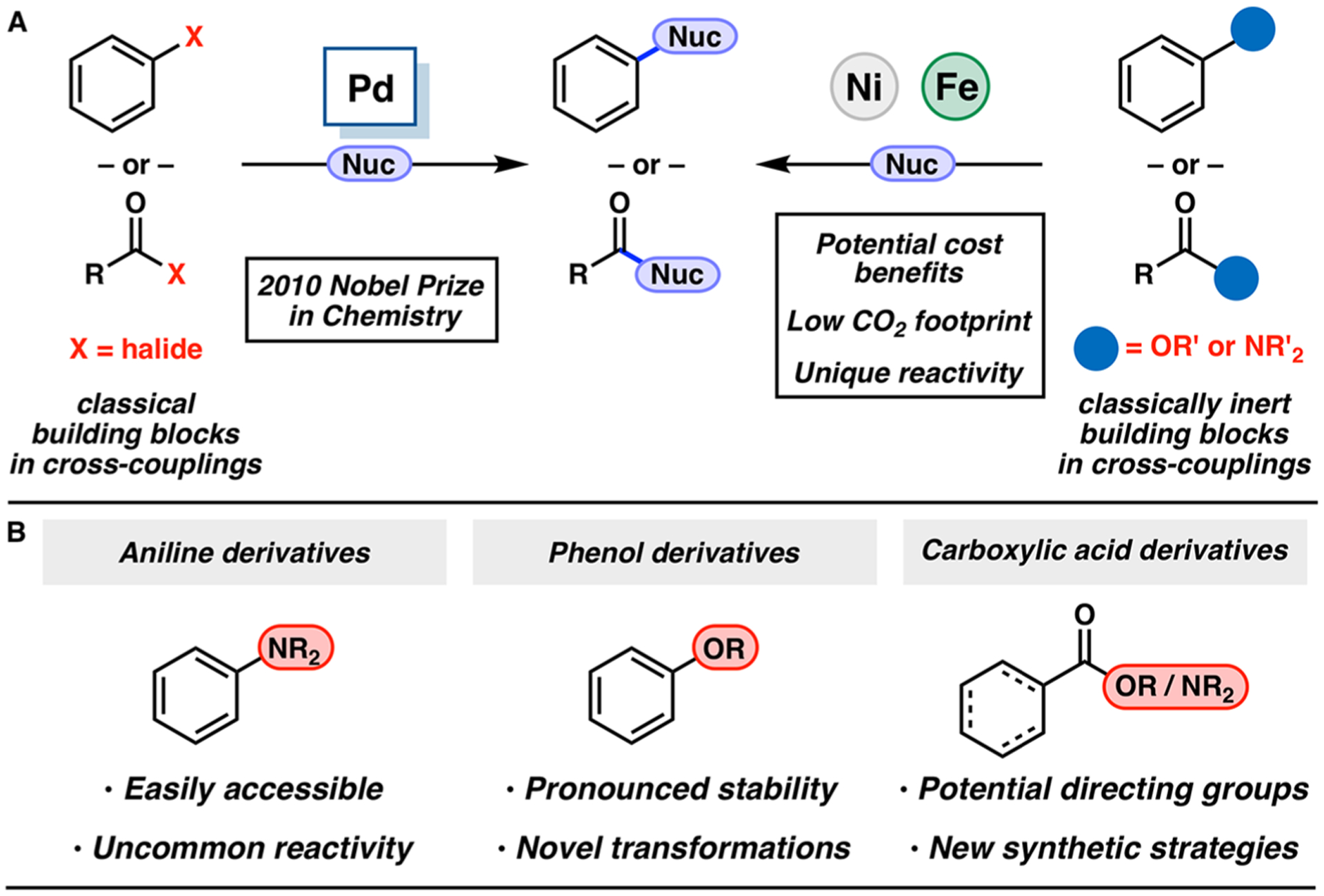
(A) Classical building blocks and those historically considered inert under traditional cross-coupling conditions. (B) Overview of recently explored electrophiles in cross-coupling reactions.
In addition to potential cost, toxicity, and environmental benefits relative to precious-metal alternatives, non-precious-metal catalysts can effect unique and challenging transformations (Figure 1A).7 In particular, iron catalysis7a,f,j and nickel catalysis7f–j have become focal points in this arena. At the time our laboratory began in 2007, nickel-catalyzed cross-couplings were known but significantly underexplored, compared to palladium-catalyzed variants. Since 2007, more than 1200 manuscripts involving nickel-catalyzed cross-couplings have been published.8 Moreover, several reviews covering advances in nickel catalysis have been published over the past decade, highlighting the rapid growth of the field.7 This expansion has largely been driven by recognition of nickel’s ability to participate in single-electron processes9 and to activate strong bonds10 historically considered inert in cross-coupling reactions.7e,11 Notably, the use of nontraditional building blocks (e.g., aniline, phenol, and carboxylic acid derivatives) in cross-couplings offers several advantages, because of their abundance, bench stability, utility as directing groups, and orthogonal reactivity to more common aryl and acyl halide electrophiles (Figure 1B). As a result, these methodologies can enable novel disconnections and improvements in synthetic strategy.
In the context of strong bond activation,12 our laboratory has been particularly interested in the activation and cross-coupling of phenol, amide, and ester electrophiles, using nickel or iron catalysis. Herein, we highlight our laboratory’s contributions in this area, which are summarized in Figure 2. First, we consider cross-couplings of phenol-derived pivalates, carbonates, carbamates, and sulfamates to form C–N and C–C bonds (Figure 2A). In addition, we discuss our efforts to develop nickel-catalyzed activations of esters and amides (Figures 2B and 2C, respectively). Where illustrative, we also outline some of the many contributions from others in the field. Lastly, we provide insight into potential future directions in these areas.
Figure 2.

Scope of this review, which focuses on the activation of phenol, amide, and ester electrophiles.
Activation of Aryl C–O Bonds.
The use of phenol derivatives in cross-couplings is particularly attractive, because of their broad availability, stability, and utility as directing groups in aromatic ring functionalization (Figure 3).13 Although cross-couplings of aryl sulfonates are common,14 methodologies that employ inexpensive phenol derivatives that are unreactive under Pd catalysis and can serve as directing groups offer practical and conceptual advantages.15–18 For example, phenolic electrophiles could be leveraged in conjunction with orthogonal cross-coupling handles to allow for the facile construction of privileged polyfunctionalized aromatics.19 Although couplings of aryl and vinyl methyl ethers had been described by Wenkert and Chatani, respectively,20–24 when our laboratory opened in 2007, cross-couplings of simple O-acylated phenols were unknown.
Figure 3.

Overview of our laboratory’s studies on using phenol derivatives as cross-coupling electrophiles in base-metal-catalyzed C–C and C–N bond-forming reactions.
Our initial efforts in this area focused on nickel-catalyzed C–C and C–N bond-forming reactions of pivalates, carbonates, and carbamates (Figure 4).25 We first developed the nickel-catalyzed Suzuki–Miyaura coupling of aryl pivalates. Of note, this reaction avoids competitive activation of the acyl C–O bond, which has been observed in related cross-couplings of aryl ethers.26,27 An example of the pivalate cross-coupling methodology is shown in Figure 4 involving the synthesis of disubstituted naphthalene derivative 4. First, regioselective bromination of naphthyl-1-pivalate at C4 gave naphthyl bromide 1, which underwent Suzuki–Miyaura coupling with indolylboronic ester 2 to deliver indole 3. Subsequently, nickel-catalyzed cross-coupling of aryl pivalate 3 with phenylboronic acid gave 4 in 88% yield.28 This concise route to 4 underscores the value of O-acylated phenols as orthogonal cross-coupling electrophiles in the synthesis of polyaromatic molecules.19 In a subsequent study, we found that aryl tert-butylcarbonates could serve as cross-coupling electrophiles, as illustrated by the nickel-catalyzed coupling of carbonate 5 to forge biaryl 6 in 65% yield.29
Figure 4.

Select examples of Ni- and Fe-catalyzed cross-couplings of pivalates, carbonates, and carbamates reported by our laboratory.
Aryl carbamates also proved to be versatile electrophiles in C–C and C–N bond-forming cross-coupling reactions, as demonstrated by the examples shown in Figure 4.29 Importantly, this substrate class can be used to functionalize the ortho position on aromatic rings via directed lithiation.30 Using NiCl2(PCy3)2, naphthyl carbamate 7 smoothly underwent nickel-catalyzed cross-coupling to furnish biaryl product 8 in 86% yield.29 The corresponding amination of aryl carbamates was enabled by the use of N-heterocyclic carbene ligand, SIPr, and could be used to generate naphthylmorpholine (11) in excellent yield.31 Developing methodologies to construct sp2–sp3 C–C bonds represents another frontier in cross-couplings.32 Toward this end, we turned to iron catalysis to achieve the Kumada coupling of aryl carbamates,33,34 which allowed for the formation of sterically hindered sp2–sp3 C–C bonds, such as that found in 12.35 Finally, a catalytic reduction of aryl carbamates (7 → 14; see Figure 4) was achieved using inexpensive 1,1,3,3-tetramethyldisiloxane (TMDSO) as the reductant,36 thereby providing a means to achieve the deoxygenation of aromatic rings. In addition, one can perform net cine substitution processes using directed ortho-metalation/functionalization, followed by reductive removal of the carbamate. Since our initial studies in this area, there have been many other contributions from other groups toward expanding the scope and mechanistic understanding of cross-couplings of O-acylated phenol derivatives.37
The cross-coupling of sulfamate electrophiles presented another attractive opportunity, as they are also common directing groups in ortho-metalation reactions as established by Snieckus.30 Nickel-catalyzed Kumada couplings of aryl sulfamates had been reported,18 but milder C–C and C–N bond-forming cross-couplings of these substrates were unknown. With regard to the former challenge, sulfamates proved to be viable substrates in nickel-catalyzed Suzuki–Miyaura cross-couplings. In fact, subsequent experimental and computational studies performed in collaboration with the Houk group revealed aryl sulfamates to be more reactive electrophiles, in comparison to aryl carbamates.38 Importantly, this transformation avoids the use of highly basic and nucleophilic organometallic reagents, allowing for coupling of vinyl sulfamate 15 to give substituted cyclohexenone 17 in 75% yield (Figure 5).29 Sulfamates were also competent electrophiles in iron-catalyzed Kumada couplings, as shown by the formation of tricyclic product 19 in 82% yield.35 In addition, the use of aryl sulfamates in catalytic amination reactions highlights their versatility as cross-coupling handles and allowed for the rapid synthesis of linezolid (22) from fluorosulfamate 20. Following sulfamate-directed ortho-functionalization,29 aryl sulfamate 20 underwent a nickel-catalyzed cross-coupling with morpholine (10) to deliver arylated amine 21 in 84% yield.39 As previously mentioned, subsequent computational and experimental efforts from others in the field have greatly contributed to the rapid growth in understanding and scope of base-metal-catalyzed cross-couplings of nontraditional phenol-derived electrophiles.37k,40
Figure 5.

Select examples of nickel- and iron-catalyzed cross-couplings of sulfamates reported by our laboratory.
A common limitation of nickel-catalyzed methodologies, including those developed by our own laboratory, is the air and moisture sensitivity of the precatalysts and/or ligands employed. To avoid the need for glovebox manipulations in the nickel-catalyzed amination of carbamates and sulfamates, we investigated the use of a variety of air-stable Ni(II) complexes.41 NiCl2(DME) (23) and PhB(pin) were identified as a suitable precatalyst and mild reductant, respectively, to achieve a range of nickel-catalyzed aminations on the benchtop. For example, the methodology tolerated electron-deficient and heterocyclic substrates as well as a variety of amine nucleophiles, giving rise to 21 and 24–26 in yields of 50%–98% (see Figure 6).42 Recognizing that the use of industrially friendly solvents could further enhance the practicality of this methodology, through a collaboration with the ACS Green Chemistry Institute’s Pharmaceutical Roundtable, we evaluated the coupling of (hetero)aryl sulfamates with amine nucleophiles in 2-methyl-THF using nickel catalysis.43 The robustness of this method was evidenced by the gram-scale coupling of trifluoromethyl aryl sulfamate 27 with morpholine (10) to give arylated amine 28 in 97% yield, using only 3 mol % NiCl2(DME).
Figure 6.

Benchtop aminations of aryl sulfamates and carbamates and gram-scale coupling of 27 in a green solvent.
Activation of Aryl C–N Bonds.
Although not a focus of our laboratory’s research, base-metal-catalyzed cross-couplings of aniline derivatives also represent an active field of inquiry (see Figure 7). Historically, aniline-derived diazonium salts have been extensively employed in palladium-catalyzed cross-couplings.44,45 However, the use of safer and more robust aniline derivatives in coupling reactions has recently garnered significant attention.46 MacMillan and co-workers reported a nickel-catalyzed Suzuki–Miyaura coupling of aryltrimethylammonium triflates in 2003,47 building upon the foundational report by Wenkert on Kumada couplings of these species.48 More recently, Shi described a directing group-free nickel-catalyzed Suzuki–Miyaura coupling of N,N-dimethylaryl amines, overcoming a key limitation in comparable Ru-catalyzed reactions.49,50 Moreover, a nickel-catalyzed Suzuki–Miyaura coupling of azoles was published by Robins,51 which allowed for the synthesis of important arylated purine nucleoside analogues 29 and 30 in good yields.52 Finally, the Nakao group and others have investigated transition-metal-catalyzed cross-couplings of nitroarenes and this strategy has been applied toward the syntheses of polycyclic aromatic hydrocarbons.53 We are optimistic that base-metal-catalyzed aryl C–N bond activation will continue to see use in complex molecule synthesis.
Figure 7.

Overview of aniline derivatives employed in metal-catalyzed cross-coupling reactions.
Activation of Acyl C–O Bonds.
Interest in the metal-catalyzed cleavage of the acyl C–O bonds of esters dates back to Yamamoto’s seminal 1976 report utilizing stoichiometric nickel complexes.54 Although cross-couplings of esters, including decarboxylative variants pioneered by Itami55 and Gooßen,56 have since established the feasibility of metal-catalyzed acyl C–O bond activation for subsequent functional group interconversion, these reports were limited to the coupling of structurally or electronically activated substrates. Specifically, aryl esters employed in these couplings featured metal-chelating57 or electron-withdrawing58 O-substituents to facilitate oxidative addition (see Figure 8).59 These substrates are often synthesized from carboxylic acid precursors and are generally more reactive than alkyl esters. In contrast, simple methyl esters are naturally abundant, commercially available, and unreactive under Pd catalysis. As a result, chemists could consider sequential cross-coupling strategies that take advantage of this orthogonal reactivity.
Figure 8.

Historical approaches to metal-catalyzed ester acyl C–O bond activation and advantages of alkyl esters as substrates.
At the time our laboratory entered this field,60 cross-couplings of methyl esters were unknown. Notably, these transformations feature high kinetic barriers to oxidative additions, because of resonance stabilization of the acyl C–O bond.61 However, we hypothesized that the unique reactivity of nickel(0) in the activation of strong bonds7e,f,10,11 may allow for catalytic cross-couplings of methyl esters. Noting the importance of amide bond formations in industry,62 we first pursued a nickel-catalyzed amidation of methyl esters.60 Ultimately, the combined use of a Ni/NHC catalyst and Al(Ot-Bu)3 additive was found to effect the desired transformation. Computations performed by the Houk group60 indicated that the Al(Ot-Bu)3 additive facilitates the rate-limiting oxidative addition step and drives product formation through the generation of a favorable Lewis acid–base complex with the amide product. Although this methodology was limited to the activation of naphthyl-derived substrates, its utility was illustrated in the synthesis of complex anilide 33 (see Figure 9). Treatment of 31, which was prepared via sequential Buchwald–Hartwig and DCC couplings of a suitable methyl naphthoate precursor, with optimized amidation conditions, generated anilide 33 in 60% yield without observable epimerization. This sequence highlights the mildness of the methodology and illustrates the utility of late-stage methyl ester activations in complex molecule synthesis.
Figure 9.

Recent advances in the nickel-catalyzed amidation of methyl esters.
Further studies have improved the efficiency and scope of nickel-catalyzed amidations of methyl esters. Newman and co-workers have described an additive-free variant of this methodology utilizing elevated temperatures.63 In doing so, they were able to achieve amidations of enantioenriched aliphatic methyl esters to access products such as furanylamide 34 in high yields (see Figure 9). A range of aromatic substrates could also be coupled utilizing these conditions, allowing for the improved synthesis of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR) positive modulator 35. In a subsequent survey of NHC ligands in this transformation,64 the same group reported the selective amidation of an alkyl methyl ester in the presence of an aryl methyl ester to form 36 in 65% yield. A reductive cross-coupling of nitroarenes and methyl esters has also been described.65 Notably, nitroarenes are widely used in industry as inexpensive precursors to aryl amines. In an impressive application of this methodology, Hu and co-workers treated known ester 37 with their optimized Ni/Zn conditions to generate (−)-rhazinilam 38 in 59% yield.65,66 As a result, the synthesis of the natural product was completed with improved step economy and comparable efficiency.
Additional advances have broadened the scope of bond formations possible using nickel-catalyzed activations of methyl esters (Figure 10). Similar to Heck-type reactions of amides developed in our laboratory (vida infra),67 Newman recently reported domino Heck reactions of methyl esters that form a C–C bond intramolecularly as well as a terminating C–C or C–H bond in a single catalytic transformation.68 The generation of indanones 40 and 41 in 51% and 79% yields, respectively, from methyl ester substrate 39 highlights the potential utility of this methodology in divergent synthesis. Furthermore, nickel-catalyzed activations of methyl esters that proceed with decarbonylation have been reported. Specifically, the Rueping laboratory has used methyl esters to generate a variety of aryl stannanes, such as substituted pyridine 44.69 A decarbonylative methylation of aryl methyl esters was reported by Yamaguchi and co-workers, and was utilized to access 2-methylindole 45 in 48% yield.70 We anticipate that the exploration of new bond formations and modes of reactivities will continue to be an important driver of innovation in this field.
Figure 10.

Nickel-catalyzed domino Heck and decarbonylative cross-couplings of methyl esters.
Activation of Acyl C–N Bonds.
Although amide C–N bond cleavage is common in Nature, synthetic methods for activation of the amide C–N bond remain challenging, because of well-understood resonance effects (Figure 11).71 However, the pronounced stability of amides renders them ideal functional handles to be carried through multistep synthetic sequences. Moreover, as they are also common directing groups in C–H activations,72 strategies to merge amide-directed C–H functionalization with subsequent late-stage C–N bond activation could allow for the rapid assembly of molecular architectures.
Figure 11.

Resonance stabilization of amides and potential synthetic utility of amide C–N bond activations.
Recognizing the potential utility of amides as useful synthetic handles, many strategies for the functional group interconversion of amides have been reported.73 For example, the use of amides featuring chelating N-substituents such as N-methoxy-N-methylamides or “Weinreb amides”,71a,74 has enabled the single addition of organometallic nucleophiles to amides to access ketone products. In addition, single-electron reduction of imides can generate reactive radical anion intermediates for subsequent functionalizations.75 Notably, electrophilic activation of amides to generate versatile imidate intermediates has a rich history, with many elegant examples of this strategy being showcased by the Maulide group in recent years.76 Another strategy for amide activation involves hydrosilylation using Vaska’s complex (IrCl(CO)(PPH3)2), which has been leveraged by the Dixon group77 and others78 for a variety of reductive functionalizations of tertiary amides. Finally, our group and others have been interested in amide activation through direct oxidative addition by a transition-metal catalyst into the amide C–N bond for subsequent cross-coupling reactions. Although unknown at the time our laboratory entered the field in 2015, we envisioned that this latter approach could provide an alternative synthetic tool for the activation of amides that avoids the use of either highly nucleophilic or electrophilic reagents.7g,79
Beginning with our first disclosure in 2015,80 our laboratory has reported several nickel-catalyzed transformations that proceed with amide C–N bond activation. Our early investigations focused on the use of amide substrates derived from aromatic carboxylic acids (which are often referred to as “aryl amides” and are distinct from N-aryl amides mentioned below), with select examples shown in Figure 12. We identified the conversion of amides to esters, a historically challenging transformation that often proceeds under harshly acidic or basic conditions,71a as an exciting starting point for our studies.80
Figure 12.

Recent advances in the nickel-catalyzed activation of aryl amide C–N bonds by our laboratory.
In collaboration with the Houk laboratory, we first computationally and experimentally investigated the effect of amide N-substituents in the nickel-catalyzed conversion of benzamides to methyl benzoate. These studies revealed two salient features of this transformation. First, N-substituents had a profound influence on the change in Gibbs free-energy values for the overall reactions (ΔG). Although esterifications of N,N-dialkyl benzamides were calculated to be largely thermodynamically unfavorable or thermoneutral, esterifications of N-aryl amides were found to be thermodynamically favorable, with a calculated ΔG value of −6.8 kcal/mol for the conversion of N-phenyl-N-methyl benzamide to methyl benzoate. The effect of amide N-substituents on the oxidative addition barrier using nickel catalysis with commercially available NHC ligand SIPr81 was also evaluated computationally. In comparison to the calculated barriers for N-dialkyl amides, those for N-aryl amides were estimated to be more reasonable, with the oxidative addition barrier for N-phenyl-N-methyl benzamide calculated to be 26 kcal/mol. Overall, the computational predictions were supported by experiments as we observed quantitative yield in the nickel-catalyzed conversion of N-phenyl-N-methyl benzamide to methyl benzoate. These initial collaborative studies with the Houk laboratory greatly informed our understanding of the impact of N-substituents on the performance of amides in various nickel-catalyzed transformations. Moreover, the Szostak group has bolstered the field by providing valuable insight into the physical properties and reactivities of nonplanar, or “twisted”, amides (in particular, glutaramides).79a,82
Using N-alkyl-N-phenyl benzamides, we found that the use of the nickel precatalyst/ligand combination of Ni(cod)2/SIPr allowed for the mild coupling with a range of alcohol nucleophiles to furnish ester products, such as menthyl ester 46, in high yields (see Figure 12).80 We then explored additional C–heteroatom bond-forming reactions of N-alkyl-N-phenyl benzamides using nickel catalysis. Although water could not be directly employed as a nucleophile to access carboxylic acids from amides,83 we designed a one-pot, two-step net-hydrolysis reaction. Specifically, in situ generation of a silyl ester, followed by subsequent deprotection provided carboxylic acids, such as 47, in good yields using mild reaction conditions.84 We were also interested in overcoming the longstanding challenge of secondary amide transamidation utilizing our nickel catalysis platform.85 Toward this end, a two-step, Boc-activation/nickel catalysis approach enabled the catalytic transamidation of secondary amides, notably providing ready access to amino-acid-derived amide 48 on gram-scale.86 Reasoning that these reactions could proceed through a Ni(0)/Ni(II) catalytic cycle, a hypothesis supported by density functional theory (DFT) calculations performed by the Houk group, we became interested in leveraging well-established M(0)/M(II) cross-coupling platforms to form C–C bonds.80 Indeed, we found that aryl amides could smoothly undergo nickel-catalyzed Suzuki–Miyaura (N-alkyl-N-Boc amides) and Negishi (N-Me-N-tosyl and N-benzyl-N-Boc amides) couplings to form biaryl and aryl–alkyl ketones, respectively.87,88 Notably, both of these methodologies proved to be scalable, providing gram-scale access to ketone 49, an antiproliferative tubulin-binding agent,89 and 50, a key intermediate in Pfizer’s synthesis of a glucagon receptor modulator,90 respectively.
Nickel-catalyzed cross-couplings of amides that build stereocomplexity represent an important frontier of the field. Through the development of intramolecular Mizoroki–Heck cyclizations, we achieved the synthesis of indanones bearing α-quaternary stereocenters from N-benzyl-N-Boc amides (see Figure 13).67 Notably, this methodology provided diastereoselective access to indanone 52, establishing vicinal stereocenters, one of which is quaternary, in a single transformation. Stanley and co-workers have extended this strategy to include domino-Heck reactions incorporating boron nucleophiles.91 We were also interested in pursuing methodologies that generate stereocenters at the originating amide carbonyl carbon through the net addition of two nucleophiles. Ultimately, a one-pot chemoenzymatic synthesis of enantioenriched alcohols was achieved through the combined use of nickel and biocatalysis in collaboration with Codexis.92 For example, enantioenriched diarylmethanol 54 was accessed in 72% yield and 97% enantiomeric excess (ee) utilizing the methodology, which combined a Suzuki–Miyaura cross-coupling and ketoreductase (KRED)-mediated reduction in a one-pot, sequential process. These studies validated the utility of amide C–N bond activation in stereocomplexity-generating transformations. We view the development of asymmetric methods as a fruitful area for further exploration in this field.
Figure 13.

Asymmetric reactions of aryl amides developed by our laboratory.
Notably, the aforementioned methodologies were limited to the activation of aryl amide substrates. The activation of amides derived from aliphatic carboxylic acids (often referred to as “aliphatic amides”) presents an even greater challenge as a result of increased steric requirements and a presumed lack of catalyst–substrate precomplexation.93 In considering esterifications of aliphatic amides, the use of terpyridine as the ligand proved effective in a collaborative study with Boehringer–Ingelheim (see Figure 14).94 This methodology provided efficient access to steroidal ester 55, and it could be used in a macrocyclic ring-opening to form ester 56. Subsequently, we evaluated C–C and C–N bond-forming reactions of alkyl amides through the use of the electron-rich NHC ligand precursor, Benz-ICy·HCl.81,95–97 Specifically, we developed a Suzuki–Miyaura coupling of aliphatic amides, which provided access to ketone 57 from an enantioenriched amide with minimal racemization. Importantly, this methodology affords enolizable ketone products, which can then be further elaborated. For example, a Suzuki–Miyaura cross-coupling of a tetrahydropyranyl amide and concatenate Fischer indolization provided spiroindolenine 58 in rapid fashion. Transamidation of aliphatic secondary amides was also possible using this catalytic system, following C–N bond activation via N-functionalization. Of note, the stereoretentive transamidation of a prolinamide to provide secondary amide 59 in 60% yield and >99% ee was possible utilizing the methodology.95 With the development of these protocols, our laboratory established the viability of aliphatic amides in C–O, C–C, and C–N bond-forming reactions using nickel catalysis.
Figure 14.

Recent advances in the nickel-catalyzed activation of aliphatic amide C–N bonds by our laboratory.
As previously noted, our laboratory has an interest in improving the practicality of nickel-catalyzed methods. Toward this end, we sought to optimize the catalytic efficiency of the esterification of amides reported by our laboratory.98 A highlight of this collaborative effort with AbbVie, which combined experimentation and kinetic modeling,99 was the realization of a 5-g scale coupling of aryl amide 60 and menthol (61) at a reduced temperature (45 °C vs 80 °C), using <1 mol % Ni(cod)2 to give ester 46 in 97% yield (see Figure 15). Another key challenge in the area of nickel catalysis lies in the development of glovebox-free cross-couplings.100 Encapsulating air- and moisture-sensitive reagents in paraffin wax has been an effective means to perform transition-metal-catalyzed reactions on the benchtop,101,102 including in undergraduate instructional laboratories.103 In this regard, we successfully employed paraffin–Ni(cod)2/Benzy-ICy·HCl capsules in the benchtop Suzuki–Miyaura coupling of piperidinyl amide 62 to give polyheterocyclic ketone 64 in 73% yield on the gram-scale. We are hopeful that future advances in these areas will promote greater use of base-metal catalysis in academia and industry.
Figure 15.
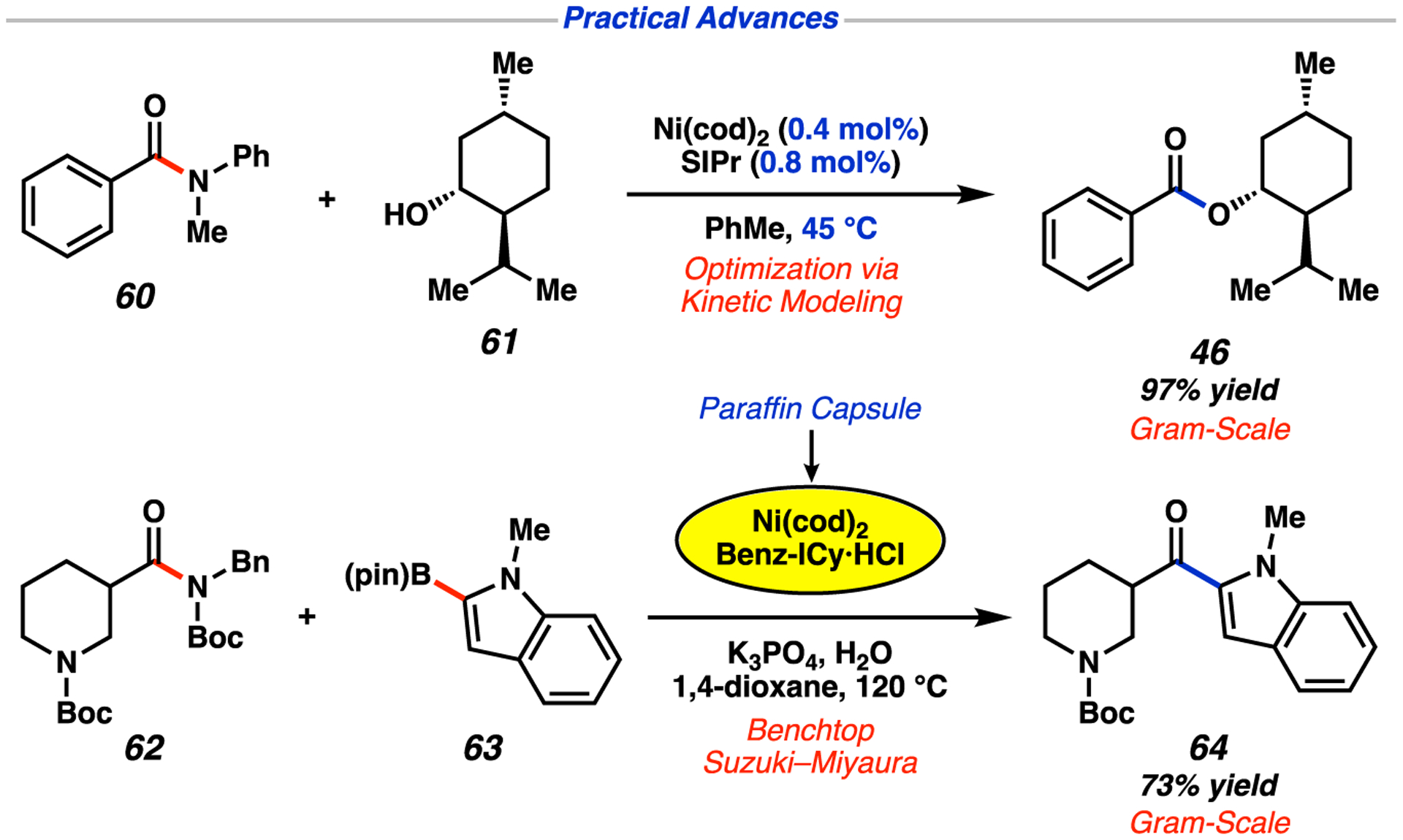
Practical advances in nickel-catalyzed activations of amides disclosed by our laboratory.
The field of amide C–N bond activation using transition-metal catalysis, including breakthroughs with palladium catalysis led by Szostak,104 has flourished in recent years, particularly when considering the introduction of alternative amide electrophiles and novel modes of reactivity. More than 85 studies involving transition-metal-catalyzed amide C–N bond activation have been reported since our initial study in 2015.105 Select examples of amide derivatives that have been employed successfully by other groups in nickel-catalyzed cross-couplings are shown in Figure 16.106 Amides have also seen use as acyl synthons in emerging cross-coupling manifolds, testifying to the rapid growth of this field. For instance, Hu and co-workers demonstrated a nickel-catalyzed reductive transamidation of N-benzyl-N-Boc benzamides using nitroarenes.66,107 Notably, this methodology was tolerant of a range of functional groups, including aryl bromides, allowing for the formation of aryl amide 67 in 69% yield. In addition, Molander and co-workers reported a Ni/Ir dual-catalytic photoredox approach to access alkyl–alkyl ketones such as 1,4-dicarbonyl 70.108 Finally, several nickel-catalyzed decarbonylative reactions of amides have been reported, expanding the scope of functional groups directly accessible from amides, as illustrated by the synthesis of compounds 71–73.109–111 The continued exploration of new modes of reactivity and bond-forming reactions is critical to advancing amides as useful building blocks in complex molecule synthesis.
Figure 16.

N-substituent variation, alternative reaction modes, and decarbonylative reactions of amides utilizing nickel catalysis.
OUTLOOK AND FUTURE DIRECTIONS
Although the field of base-metal-catalyzed activation of strong bonds has grown rapidly in recent years, there remains tremendous opportunity for future discovery. For example, expanding the scope of substrates that can undergo activation to include (sp3)C–O/N electrophiles112,113 and developing catalytic systems to activate amides, phenols, and anilines without the need for electron-withdrawing or chelating substituents114 could allow chemists to directly manipulate common moieties in commodity chemicals and natural products. Another frontier for this field lies in the application of cross-couplings involving the activation of strong bonds to the synthesis of complex molecules. In this regard, the development of both chemoselective and stereoselective reactions is expected to be highly enabling. In addition, collaborations between academia and industry, as well as among academic research groups, would help achieve the future directions outlined above and expedite the adoption of novel methodologies in industrial settings.115 Finally, computational investigations into reaction mechanisms and selectivities will undoubtedly lead to improvements in existing methodologies, inspire the development of others, and lead to the disclosure of tools to predict selectivities and outcomes in these transformations. Of note, although nickel catalysis has seen widespread use in the activation of strong bonds, there remains significant interest in exploring the utility of alternative non-precious-metal catalysts in this arena and more broadly in cases where it could lower the cost of drug manufacturing.115,116 We also envision that the optimization of reaction conditions and catalyst loadings, the discovery of new inexpensive ligand frameworks,117 and the disclosure of well-defined, air-stable precatalysts118 will promote the use of base-metal catalysis in academia and industry. We hope and anticipate that strong bond activation using base-metal catalysts will continue to thrive as a field and be viewed as an increasingly valuable strategy in organic synthesis.
ACKNOWLEDGMENTS
The authors thank NIH-NIGMS (No. R01-GM117016, to N.K.G.), the Trueblood Family (N.K.G.), the Foote family (A.S.B. and J.E.D.), the California Tobacco-Related Disease Research Program (No. 28DT-0006, to T.B.B.), the NSF (No. DGE-1144087, to J.E.D.) and the University of California, Los Angeles, for financial support. The Houk laboratory is gratefully acknowledged for their insight and collaborations throughout the studies described.
Footnotes
The authors declare no competing financial interest.
Complete contact information is available at: https://pubs.acs.org/10.1021/acscatal.0c03334
Contributor Information
Timothy B. Boit, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States.
Ana S. Bulger, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States.
Jacob E. Dander, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States.
Neil K. Garg, Department of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, United States.
REFERENCES
- (1).(a) Suzuki A Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct C–C Bonds. Angew. Chem., Int. Ed 2011, 50, 6722–6737. [DOI] [PubMed] [Google Scholar]; (b) Negishi E Magical Power of Transition Metals: Past, Present, and Future. Angew. Chem., Int. Ed 2011, 50, 6738–6764. [DOI] [PubMed] [Google Scholar]; (c) Johansson Seechurn CCC; Kitching MO; Colacot TJ; Snieckus V Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed 2012, 51, 5062–5085. [DOI] [PubMed] [Google Scholar]
- (2).(a) For representative reviews, see:Duschek A; Kirsch SF Combining the Concepts: Dual Catalysis with Carbophilic Lewis Acids. Angew. Chem., Int. Ed 2008, 47, 5703–5705. [DOI] [PubMed] [Google Scholar]; (b) Hirner JJ; Shi Y; Blum SA Organogold Reactivity with Palladium, Nickel, and Rhodium: Transmetalation, Cross-Coupling and Dual Catalysis. Acc. Chem. Res 2011, 44, 603–613. [DOI] [PubMed] [Google Scholar]; (c) Hanna LE; Jarvo ER Selective Cross-Electrophile Coupling by Dual Catalysis. Angew. Chem., Int. Ed 2015, 54, 15618–15620. [DOI] [PubMed] [Google Scholar]; (d) Afewerki S; Córdova A Combinations of Aminocatalysts and Metal Catalysts: A Powerful Cooperative Approach in Selective Organic Synthesis. Chem. Rev 2016, 116, 13512–13570. [DOI] [PubMed] [Google Scholar]; (e) Krautwald S; Carreira EM Stereodivergence in Asymmetric Catalysis. J. Am. Chem. Soc 2017, 139, 5627–5639. [DOI] [PubMed] [Google Scholar]; (f) Romiti F; del Pozo J; Paioti PHS; Gonsales SA; Li X; Hartrampf FWW; Hoveyda AH Different Strategies for Designing Dual-Catalytic Enantioselective Processes: From Fully Cooperative to Non-Cooperative Systems. J. Am. Chem. Soc 2019, 141, 17952–17961. [DOI] [PubMed] [Google Scholar]
- (3).(a) For representative reviews, see:Zuo Z; Ahneman DT; Chu L; Terrett JA; Doyle AG; MacMillan DWC Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem 2017, 1, 0052. [Google Scholar]; (c) Hopkinson MN; Tlahuext-Aca A; Glorius F Merging Visible Light Photoredox and Gold Catalysis. Acc. Chem. Res 2016, 49, 2261–2272. [DOI] [PubMed] [Google Scholar]; (d) Douglas JJ; Sevrin MJ; Stephenson CRJ Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents. Org. Process Res. Dev 2016, 20, 1134–1147. [Google Scholar]
- (4).(a) For representative reviews, see:Lucas EL; Jarvo ER Stereospecific and Stereoconvergent Cross-Couplings Between Alkyl Electrophiles. Nat. Rev. Chem 2017, 1, 65. [Google Scholar]; (b) Weix DJ Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Acc. Chem. Res 2015, 48, 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hanna LE; Jarvo ER Selective Cross-Electrophile Coupling by Dual Catalysis. Angew. Chem., Int. Ed 2015, 54, 15618–15620. [DOI] [PubMed] [Google Scholar]; (d) Everson DA; Weix DJ Cross-Electrophile Coupling: Principles of Reactivity and Selectivity. J. Org. Chem 2014, 79, 4793–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) For representative publications on combining chemocatalysis and biocatalysis, see:Rudroff F; Mihovilovic MD; Gröger H; Snajdrova R; Iding H; Bornscheuer WT Opportunities and Challenges for Combining Chemo- and Biocatalysis. Nat. Catal 2018, 1, 12–22. [Google Scholar]; (b) Denard CA; Hartwig JF; Zhao H Multistep One-Pot Reactions Combining Biocatalysis and Chemical Catalysts for Asymmetric Synthesis. ACS Catal. 2013, 3, 2856–2864. [Google Scholar]; (c) Gröger H; Hummel W Combining the ‘Two Worlds’ of Chemocatalysis and Biocatalysis Towards Multi-Step One-Pot Processes in Aqueous Media. Curr. Opin. Chem. Biol 2014, 19, 171–179. [DOI] [PubMed] [Google Scholar]; (d) Ríos-Lombardía N; García-Álvarez J; González-Sabín J One-Pot Combination of Metal-and Biocatalysis in Water for the Synthesis of Chiral Molecules. Catalysts 2018, 8, 75. [Google Scholar]; (e) Sirasani G; Tong L; Balskus EP A Biocompatible Alkene Hydrogenation Merges Organic Synthesis with Microbial Metabolism. Angew. Chem., Int. Ed 2014, 53, 7785–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wallace S; Balskus EP Interfacing Microbial Styrene Production with a Biocompatible Cyclopropanation Reaction. Angew. Chem., Int. Ed 2015, 54, 7106–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) For representative publications, see:Lucas EL; Jarvo ER Keeping Track of the Electrons. Acc. Chem. Res 2018, 51, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brozek LA; Ardolino MJ; Morken JP Diasterocontrol in Asymmetric Allyl–Allyl Cross-Coupling: Stereocontrolled Reaction of Prochiral Allylboronates with Prochiral Allyl Chlorides. J. Am. Chem. Soc 2011, 133, 16778–16781. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kainz QM; Matier CD; Bartoszewicz A; Zultanski SL; Peters JC; Fu GC Asymmetric Copper-Catalyzed C–N Cross-Couplings Induced by Visible Light. Science 2016, 351, 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Wang Z; Yin H; Fu GC Catalytic Enantioconvergent Coupling of Secondary and Tertiary Electrophiles with Olefins. Nature 2018, 563, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Huo H; Gorsline BJ; Fu GC Catalyst-Controlled Doubly Enantioconvergent Coupling of Racemic Alkyl Nucleophiles and Electrophiles. Science 2020, 367, 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Bolm C; Legros J; Le Paih J; Zani L Iron-Catalyzed Reactions in Organic Synthesis. Chem. Rev 2004, 104, 6217–6254. [DOI] [PubMed] [Google Scholar]; (b) Enthaler S; Junge K; Beller M Sustainable Metal Catalysis with Iron: From Rust to Rising Star? Angew. Chem., Int. Ed 2008, 47, 3317–3321. [DOI] [PubMed] [Google Scholar]; (c) Bauer EB Recent Advances in Iron Catalysis in Organic Synthesis. Curr. Org. Chem 2008, 12, 1341–1369. [Google Scholar]; (d) Fürstner A Iron Catalysis in Organic Synthesis: A Critical Assessment of What it Takes to Make This Base Metal a Multitasking Champion. ACS Cent. Sci 2016, 2, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Fürstner A; Leitner A; Méndez M; Krause H Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc 2002, 124, 13856–13863. [DOI] [PubMed] [Google Scholar]; (f) Mesganaw T; Garg NK Ni- and Fe-Catalyzed Cross-Coupling Reactions of Phenol Derivatives. Org. Process Res. Dev 2013, 17, 29–39. [Google Scholar]; (g) Tasker SZ; Standley EA; Jamison TF Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ananikov VP Nickel: the “Spirited Horse” of Transition Metal Catalysis. ACS Catal. 2015, 5, 1964–1971. [Google Scholar]; (i) Dander JE; Garg NK Breaking Amides Using Nickel Catalysis. ACS Catal. 2017, 7, 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Su B; Cao Z-C; Shi Z-J Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations. Acc. Chem. Res 2015, 48, 886–896. [DOI] [PubMed] [Google Scholar]
- (8).Based on a SciFinder search for the research topic “nickel cross-coupling” and narrowing the search to only journal articles (i.e., excluding review articles) published between 2008 and 2020 (accessed July 30, 2020).
- (9).(a) Nickel readily participates in radical pathways, because of the relative stability of open-shell nickel complexes. For theoretical and experimental studies, see:Poli R; Cacelli I Orbital Splitting and Pairing Energy in Open-Shell Organometallics: A Study of Two Families of 16-Electron Complexes [Cp2M] (M = Cr, Mo, W) and [CpM(PH3)] (M = Co, Rh, Ir). Eur. J. Inorg. Chem 2005, 2005, 2324–2331. [Google Scholar]; (b) Tsou TT; Kochi J Mechanism of Oxidative Addition. Reaction of Nickel(0) Complexes with Aromatic Halides. J. Am. Chem. Soc 1979, 101, 6319–6332. [Google Scholar]; (c) Schley ND; Fu GC Nickel-Catalyzed Negishi Arylations of Propargylic Bromides: A Mechanistic Investigation. J. Am. Chem. Soc 2014, 136, 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) In comparison to palladium, nickel has a smaller atomic radius, is less electronegative, and is more easily oxidized. In turn, oxidative addition is more facile to Ni(0), relative to Pd(0). For theoretical and experimental studies, see:Mann JB; Meek TL; Knight ET; Capitani JF; Allen LC Configuration Energies of the d-Block Elements. J. Am. Chem. Soc 2000, 122, 5132–5317. [Google Scholar]; (b) Lide DR CRC Handbook of Chemistry and Physics, 87th Edition; CRC Press: Boca Raton, FL, 2008. Chapter 4, p 1. [Google Scholar]; (c) Batsanov SS Van der Waals Radii of Elements. Inorg. Mater 2001, 37, 871–885. [Google Scholar]; (d) Hartwig JF Organotransition Metal Chemistry: From Bonding to Catalysis; Murdzek J, Ed.; University Science Books: Mill Valley, CA, 2010; p 8. [Google Scholar]
- (11).(a) Diccianni J; Lin W; Diao T Mechanisms of Nickel-Catalyzed Coupling Reactions and Applications in Alkene Functionalization. Acc. Chem. Res 2020, 53, 906–919. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Diccianni JB; Diao T Mechanisms of Nickel-Catalyzed Cross-Coupling Reactions. Trends. Chem 2019, 1, 830–844. [Google Scholar]
- (12).In the context of this Viewpoint, we consider a “strong bond” to be one that has been traditionally inert to oxidative addition using palladium catalysis.
- (13).(a) Rosen BM; Quasdorf KW; Wilson DA; Zhang N; Resmerita A-M; Garg NK; Percec V Nickel-Catalyzed Cross-Couplings Involving Carbon–Oxygen Bonds. Chem. Rev 2011, 111, 1346–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu D-G; Li B-J; Shi Z-J Exploration of New C–O Electrophiles in Cross-Coupling Reactions. Acc. Chem. Res 2010, 43, 1486–1495. [DOI] [PubMed] [Google Scholar]; (c) Li B-J; Yu D-G; Sun C-L; Shi Z-J Activation of “Inert” Alkenyl/Aryl C–O Bond and Its Application in Cross-Coupling Reactions. Chem. - Eur. J 2011, 17, 1728–1759. [DOI] [PubMed] [Google Scholar]; (d) Gooßen LJ; Gooßen K; Stanciu C C(Aryl)–O Activation of Aryl Carboxylates in Nickel-Catalyzed Biaryl Syntheses. Angew. Chem., Int. Ed 2009, 48, 3569–3571. [DOI] [PubMed] [Google Scholar]
- (14).(a) For reviews, see:Miyaura N; Suzuki A Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev 1995, 95, 2457–2483. [Google Scholar]; (b) Suzuki AJ Recent Advances in the Cross-Coupling Reactions of Organoboron Derivatives with Organic Electrophiles. J. Organomet. Chem 1999, 576, 147–168. [Google Scholar]; (c) Miyaura N In Advances in Metal–Organic Chemistry, Vol. 6; Liebeskind LS, Ed.; JAI: London, 1998; pp 187–243. [Google Scholar]; (d) Suzuki A In Metal-Catalyzed Cross-Coupling Reactions; Diederich F, Stang PJ, Eds.; Wiley–VCH: New York, 1998; pp 761–813. [Google Scholar]; (e) Stanforth SP Catalytic Cross-Coupling Reactions in Biaryl Synthesis. Tetrahedron 1998, 54, 263–303. [Google Scholar]
- (15).Approximate reagent costs by Aldrich Chemical Co., Inc. are as follows: (a) triflic anhydride, $280 per mol; (b) methanesulfonyl chloride, $12 per mol; (c) trimethylacetyl chloride (pivaloyl chloride), $15 per mol; and (d) iodomethane, $68 per mol.
- (16).(a) Although aryl sulfonates have been established as viable cross-coupling partners, their utility in ortho-lithiation reactions is limited. For examples of alkyl benzenesulfonates as directing groups in ortho-metalation reactions, see:Bonfiglio JN Directed Ortho-Lithiation of Alkyl Arene Sulfonates. J. Org. Chem 1986, 51, 2833–2835. [Google Scholar]; (b) Spangler LA A Novel Method for the Preparation of 2,6-Disubstituted Benzenesulfonates and Benzenesulfonyl Chlorides Utilizing the Powerful Alkyl Sulfonate Ortho Directing Group. Tetrahedron Lett. 1996, 37, 3639–3642. [Google Scholar]
- (17).(a) For examples of aryl mesylates and tosylates in cross-coupling reactions, see:Zim D; Lando VR; Dupont J; Monteiro AL NiCl2(PCy3)2: A Simple and Efficient Catalyst Precursor for the Suzuki Cross-Coupling of Aryl Tosylates and Arylboronic Acids. Org. Lett 2001, 3, 3049–3051. [DOI] [PubMed] [Google Scholar]; (b) Tang Z-Y; Hu Q-S Room-Temperature Ni(0)-Catalyzed Cross-Coupling Reactions of Aryl Arenesulfonates with Arylboronic Acids. J. Am. Chem. Soc 2004, 126, 3058–3059. [DOI] [PubMed] [Google Scholar]; (c) Percec V; Golding GM; Smidrkal J; Weichold O NiCl2(dppe)-Catalyzed Cross-Coupling of Aryl Mesylates, Arenesulfonates, and Halides with Arylboronic Acids. J. Org. Chem 2004, 69, 3447–3452. [DOI] [PubMed] [Google Scholar]; (d) Zhang L; Meng T; Wu J Palladium-Catalyzed Suzuki–Miyaura Cross-Couplings of Aryl Tosylates with Potassium Aryltrifluoroborates. J. Org. Chem 2007, 72, 9346–9349. [DOI] [PubMed] [Google Scholar]; (e) Munday RH; Martinelli JR; Buchwald SL Palladium-Catalyzed Carbonylation of Aryl Tosylates and Mesylates. J. Am. Chem. Soc 2008, 130, 2754–2755. [DOI] [PubMed] [Google Scholar]
- (18).Macklin TK; Snieckus V Directed Ortho Metalation Methodology. The N,N-Dialkyl Aryl O-Sulfamate as a New Directed Metalation Group and Cross-Coupling Partner for Grignard Reagents. Org. Lett 2005, 7, 2519–2522. [DOI] [PubMed] [Google Scholar]
- (19).(a) Polyfunctionalized aromatics are important scaffolds in medicines, ligands for catalysis, and materials chemistry. For examples, see:Kotha S; Lahiri K; Kashinath D Recent Applications of the Suzuki–Miyaura Cross-Coupling Reaction in Organic Synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar]; (b) Kertesz M; Choi CH; Yang S Conjugated Polymers and Aromaticity. Chem. Rev 2005, 105, 3448–3481. [DOI] [PubMed] [Google Scholar]; (c) Martin R; Buchwald SL Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res 2008, 41, 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Surry DS; Buchwald SL Biaryl Phosphane Ligands in Palladium-Catalyzed Amination. Angew. Chem., Int. Ed 2008, 47, 6338–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Wenkert E; Michelotti EL; Swindell CS Nickel-Induced Conversion of Carbon-Oxygen into Carbon-Carbon Bonds. One-Step Transformations of Enol Ethers into Olefins and Aryl Ethers into Biaryls. J. Am. Chem. Soc 1979, 101, 2246–2247. [Google Scholar]; (b) Wenkert E; Michelotti EL; Swindell CS; Tingoli M Transformation of Carbon-Oxygen into Carbon-Carbon Bonds Mediated by Low-Valent Nickel Species. J. Org. Chem 1984, 49, 4894–4899. [Google Scholar]
- (21).Dankwardt JW Nickel-Catalyzed Cross-Coupling of Aryl Grignard Reagents with Aromatic Alkyl Ethers: An Efficient Synthesis of Unsymmetrical Biaryls. Angew. Chem., Int. Ed 2004, 43, 2428–2432. [DOI] [PubMed] [Google Scholar]
- (22).Guan B-T; Xiang S-K; Wu T; Sun Z-P; Wang B-Q; Zhao K-Q; Shi Z-J Methylation of Arenes via Ni-catalyzed Aryl C–O/F Activation. Chem. Commun 2008, 1437–1439. [DOI] [PubMed] [Google Scholar]
- (23).Tobisu M; Shimasaki T; Chatani N Nickel-Catalyzed Cross-Coupling of Aryl Methyl Ethers with Aryl Boronic Esters. Angew. Chem., Int. Ed 2008, 47, 4866–4869. [DOI] [PubMed] [Google Scholar]
- (24).More recently, Sergeev and Hartwig disclosed the nickel-catalyzed hydrogenolysis of diaryl ethers. See: Sergeev AG; Hartwig JF Selective, Nickel-Catalyzed Hydrogenolysis of Aryl Ethers. Science 2011, 332, 439–443. [DOI] [PubMed] [Google Scholar]
- (25).The Suzuki–Miyaura coupling of aryl pivalates was reported simultaneously by our laboratory and the laboratory of Shi: Guan B-T; Wang Y; Li B-J; Yu D-G; Shi Z-J Biaryl Construction via Ni-catalyzed C–O Activation of Phenolic Carboxylates. J. Am. Chem. Soc 2008, 130, 14468–14470. [DOI] [PubMed] [Google Scholar]
- (26).(a) Ishizu J; Yamamoto T; Yamamoto A Selective Cleavage of C–O Bonds in Esters Through Oxidative Addition to Ni(0) Complexes. Chem. Lett 1976, 5, 1091–1094. [Google Scholar]; (b) Yamamoto T; Ishizu J; Kohara T; Komiya S; Yamamoto A Oxidative Addition of Aryl Carboxylates to Nickel(0) Complexes Involving Cleavage of the Acyl-Oxygen Bond. J. Am. Chem. Soc 1980, 102, 3758–3764. [Google Scholar]
- (27).Generally, while evaluating the feasibility of new methodologies, we often employ naphthyl substrates, because oxidative addition to these substrates has a tendency to be more facile.
- (28).Quasdorf KW; Tian X; Garg NK Cross-Coupling Reactions of Aryl Pivalates with Boronic Acids. J. Am. Chem. Soc 2008, 130, 14422–14423. [DOI] [PubMed] [Google Scholar]
- (29).Quasdorf KW; Riener M; Petrova KV; Garg NK Suzuki–Miyaura Coupling of Aryl Carbamates, Carbonates, and Sulfamates. J. Am. Chem. Soc 2009, 131, 17748–17749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Snieckus V Directed Ortho Metalation. Tertiary Amide and O-Carbamate Directors in Synthetic Strategies for Polysubstituted Aromatics. Chem. Rev 1990, 90, 879–933. [Google Scholar]
- (31).Mesganaw T; Silberstein AL; Ramgren SD; Fine Nathel NF; Hong X; Liu P; Garg NK Nickel-Catalyzed Amination of Aryl Carbamates and Sequential Site-Selective Cross-Couplings. Chem. Sci 2011, 2, 1766–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).(a) For reviews in the area of sp2–sp3 C–C cross-couplings, see:Tellis JC; Kelly CB; Primer DN; Jouffroy M; Patel NR; Molander GA Single-Electron Transmetalation via Photoredox/Nickel Dual Catalysis: Unlocking a New Paradigm for sp3–sp2 Cross-Coupling. Acc. Chem. Res 2016, 49, 1429–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Iwasaki T; Kambe N Ni-Catalyzed C–C Couplings Using Alkyl Electrophiles. Top. Curr. Chem 2016, 374, 66. [DOI] [PubMed] [Google Scholar]; (c) Miyaura N; Suzuki A Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev 1995, 95, 2457–2483. [Google Scholar]
- (33).(a) For examples of iron-catalyzed alkylations of aryl halides, triflates, and sulfonates, see:Fürstner A; Leitner A Iron-Catalyzed Cross-Coupling Reactions of Alkyl-Grignard Reagents with Aryl Chlorides, Tosylates, and Triflates. Angew. Chem., Int. Ed 2002, 41, 609–612. [Google Scholar]; (b) Fürstner A; Leitner A; Méndez M; Krause H Iron-Catalyzed Cross-Coupling Reactions. J. Am. Chem. Soc 2002, 124, 13856–13863. [DOI] [PubMed] [Google Scholar]; (c) Fürstner A; Martin R; Krause H; Seidel G; Goddard R; Lehmann CW Preparation, Structure, and Reactivity of Nonstabilized Organoiron Compounds. Implications for Iron-Catalyzed Cross Coupling Reactions. J. Am. Chem. Soc 2008, 130, 8773–8787. [DOI] [PubMed] [Google Scholar]
- (34).(a) For the iron-catalyzed alkylation of heteroaromatic sulfonates and phosphates, see:Gøgsig TM; Lindhardt AT; Skrydstrup T Heteroaromatic Sulfonates and Phosphates and Electrophiles in Iron-Catalyzed Cross-Couplings. Org. Lett 2009, 11, 4886–4888. [DOI] [PubMed] [Google Scholar]; (b) Klei-mark J; Larsson P-F; Emamy P; Hedström A; Norrby P-O Low Temperature Studies of Iron-Catalyzed Cross-Coupling of Alkyl Grignard Reagents with Aryl Electrophiles. Adv. Synth. Catal 2012, 354, 448–456. [Google Scholar]
- (35).Silberstein AL; Ramgren SD; Garg NK Iron-Catalyzed Alkylations of Aryl Sulfamates and Carbamates. Org. Lett 2012, 14, 3796–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Mesganaw T; Fine Nathel NF; Garg NK Cine Substitution of Arenes Using the Aryl Carbamate as a Removable Directing Group. Org. Lett 2012, 14, 2918–2921. [DOI] [PubMed] [Google Scholar]
- (37).(a) Yoshikai N; Matsuda H; Nakamura E Hydroxyphosphine Ligand for Nickel-Catalyzed Cross-Coupling Through Nickel/Magnesium Bimetallic Cooperation. J. Am. Chem. Soc 2009, 131, 9590–9599. [DOI] [PubMed] [Google Scholar]; (b) Li B-J; Xu L; Wu Z-H; Guan B-T; Sun C-L; Wang B-Q; Shi Z-J Cross-Coupling of Alkenyl/Aryl Carboxylates with Grignard Reagent via Fe-Catalyzed C–O Bond Activation. J. Am. Chem. Soc 2009, 131, 14656–14657. [DOI] [PubMed] [Google Scholar]; (c) Xu L; Li B-J; Wu Z-H; Lu X-Y; Guan B-T; Wang B-Q; Zhao K-Q; Shi Z-J Nickel Catalyzed Efficient and Practical Suzuki–Miyaura Coupling of Alkenyl and Aryl Carbamates with Aryl Boroxines. Org. Lett 2010, 12, 884–887. [DOI] [PubMed] [Google Scholar]; (d) Shimasaki T; Tobisu M; Chatani N Nickel-Catalyzed Amination of Aryl Pivalates by the Cleavage of Aryl C–O Bonds. Angew. Chem., Int. Ed 2010, 49, 2929–2932. [DOI] [PubMed] [Google Scholar]; (e) Tobisu M; Yamakawa K; Shimasaki T; Chatani N Nickel-Catalyzed Reductive Cleavage of Aryl–Oxygen Bonds in Alkoxy- and Pivaloxyarenes Using Hydrosilanes as a Mild Reducing Agent. Chem. Commun 2011, 47, 2946–2948. [DOI] [PubMed] [Google Scholar]; (f) Zhao F; Yu D-G; Zhu R-Y; Xi Z; Shi Z-J Cross-Coupling of Aryl/Alkenyl Silyl Ethers with Grignard Reagents Through Nickel-Catalyzed C–O Bond Activation. Chem. Lett 2011, 40, 1001–1003. [Google Scholar]; (g) Yu DG; Shi Z-J Mutual Activation: Suzuki–Miyaura Coupling Through Direct Cleavage of the sp2 C–O Bond of Naphtholate. Angew. Chem., Int. Ed 2011, 50, 7097–7100. [DOI] [PubMed] [Google Scholar]; (h) Huang K; Yu D-G; Zheng S-F; Wu Z-H; Shi Z-J Borylation of Aryl and Alkenyl Carbamates Through Ni-Catalyzed C–O Activation. Chem. - Eur. J 2011, 17, 786–791. [DOI] [PubMed] [Google Scholar]; (i) Zhao F; Zhang Y-F; Wen J; Yu D-G; Wei J-B; Xi Z; Shi Z-J Programmed Selective sp2 C–O Bond Activation Toward Multiarylated Benzenes. Org. Lett 2013, 15, 3230–3233. [DOI] [PubMed] [Google Scholar]; (j) Hong X; Liang Y; Houk KN Mechanisms and Origins of Switchable Chemoselectivity of Ni-Catalyzed C(Aryl)–O and C(Acyl)–O Activation of Aryl Esters with Phosphine Ligands. J. Am. Chem. Soc 2014, 136, 2017–2025. [DOI] [PubMed] [Google Scholar]; (k) Yue H; Guo L; Liu X; Rueping M Nickel-Catalyzed Synthesis of Primary Aryl and Heteroaryl Amines via C–O Bond Cleavage. Org. Lett 2017, 19, 1788–1791. [DOI] [PubMed] [Google Scholar]
- (38).Quasdorf KW; Antoft-Finch A; Liu P; Silberstein AL; Komaromi A; Blackburn T; Ramgren SD; Houk KN; Snieckus V; Garg NK Suzuki–Miyaura Cross-Coupling of Aryl Carbamates and Sulfamates: Experimental and Computational Studies. J. Am. Chem. Soc 2011, 133, 6352–6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Ramgren SD; Silberstein AL; Yang Y; Garg NK Nickel-Catalyzed Amination of Aryl Sulfamates. Angew. Chem., Int. Ed 2011, 50, 2171–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).(a) Jezorek RL; Zhang N; Leowanawat P; Bunner MH; Gutsche N; Pesti AKR; Olsen JT; Percec V Air-Stable Nickel Precatalysts for Fast and Quantitative Cross-Coupling of Aryl Sulfamates with Aryl Neopentylglycolboronates at Room Temperature. Org. Lett 2014, 16, 6326–6239. [DOI] [PubMed] [Google Scholar]; (b) Beromi MM; Nova A; Balcells D; Brasacchio AM; Brudvig GW; Guard LM; Hazari N; Vinyard DJ Mechanistic Study of an Improved Ni Precatalyst for Suzuki–Miyaura Reactions of Aryl Sulfamates: Understanding the Role of Ni(I) Species. J. Am. Chem. Soc 2017, 139, 922–936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang N; Hoffman DJ; Gutsche N; Gupta J; Percec V Comparison of Arylboron-Based Nucleophiles in Ni-Catalyzed Suzuki–Miyaura Cross-Coupling with Aryl Mesylates and Sulfamates. J. Org. Chem 2012, 77, 5956–5964. [DOI] [PubMed] [Google Scholar]; (d) Muto K; Yamaguchi J; Lei A; Itami K Isolation, Structure, and Reactivity of an Arylnickel(II) Pivalate Complex in Catalytic C–H/C–O Biaryl Coupling. J. Am. Chem. Soc 2013, 135, 16384–16387. [DOI] [PubMed] [Google Scholar]; (e) Leowanawat P; Zhang N; Safi M; Hoffman DJ; Fryberger MC; George A; Percec V Trans-Chloro(1-naphthyl)bis(triphenylphosphine)nickel(II)/PCy3 Catalyzed Cross-Coupling of Aryl and Heteroaryl Neopentylglycolboronates with Aryl and Heteroaryl Mesylates and Sulfamates at Room Temperature. J. Org. Chem 2012, 77, 2885–2892. [DOI] [PubMed] [Google Scholar]; (f) Leowanawat P; Zhang N; Percec V Nickel Catalyzed Cross-Coupling of Aryl C–O Based Electrophiles with Aryl Neopentylglycolboronates. J. Org. Chem 2012, 77, 1018–1025. [DOI] [PubMed] [Google Scholar]; (g) Leowanawat P; Zhang N; Resmerita A-M; Rosen BM; Percec V Ni(COD)2/PCy3 Catalyzed Cross-Coupling of Aryl and Heteroaryl Neopentylglycolboronates with Aryl and Heteroaryl Mesylates and Sulfamates in THF at Room Temperature. J. Org. Chem 2011, 76, 9946–9955. [DOI] [PubMed] [Google Scholar]
- (41).(a) For representative publications on the development of air-stable Ni(II) precatalysts, see:Standley EA; Jamison TF Simplifying Nickel(0) Catalysis: an Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes. J. Am. Chem. Soc 2013, 135, 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Strieth-Kalthoff F; Longstreet AR; Weber JM; Jamison TF Bench-Stable N-Heterocyclic Carbene Nickel Precatalysts for C–C and C–N Bond-Forming Reactions. Chem-CatChem 2018, 10, 2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Weber JM; Longstreet AR; Jamison TF Bench-Stable Nickel Precatalysts with Heck-Type Activation. Organometallics 2018, 37, 2716–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shields JD; Gray EE; Doyle AG A Modular, Air-Stable Nickel Precatalyst. Org. Lett 2015, 17, 2166–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Hie L; Ramgren SD; Mesganaw T; Garg NK Nickel-Catalyzed Amination of Aryl Sulfamates and Carbamates Using an Air-Stable Precatalyst. Org. Lett 2012, 14, 4182–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Fine Nathel NF; Kim J; Hie L; Jiang X; Garg NK Nickel-Catalyzed Amination of Aryl Chlorides and Sulfamates in 2-Methyl THF. ACS Catal. 2014, 4, 3289–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Roglans A; Pla-Quintana A; Moreno-Mañas M Diazonium Salts as Substrates in Palladium-Catalyzed Cross-Coupling Reactions. Chem. Rev 2006, 106, 4622–4643. [DOI] [PubMed] [Google Scholar]
- (45).Although Cu(I)-catalyzed reactions of aryl diazononium salts are well-established, these transformations typically proceed via single-electron transfer and aryl radical reactivity, rather than through traditional M(0)/M(II) cross-coupling catalytic cycles.
- (46).For a comprehensive review, see: Ouyang K; Hao W; Zhang W-X; Xi Z Transition-Metal-Catalyzed Cleavage of C–N Single Bonds. Chem. Rev 2015, 115, 12045–12090. [DOI] [PubMed] [Google Scholar]
- (47).Blakey SB; MacMillan DWC The First Suzuki Cross-Couplings of Aryltrimethylammonium Salts. J. Am. Chem. Soc 2003, 125, 6046–6047. [DOI] [PubMed] [Google Scholar]
- (48).Wenkert E; Han A-L; Jenny C-J Nickel-Induced Conversion of Carbon–Nitrogen into Carbon–Carbon Bonds. One-Step Transformations of Aryl, Quaternary Ammonium Salts into Alkylarenes and Biaryls. J. Chem. Soc., Chem. Commun 1988, 975–976. [Google Scholar]
- (49).Cao Z-C; Xie S-J; Fang H; Shi Z-J Ni-Catalyzed Cross-Coupling of Dimethyl Aryl Amines with Arylboronic Esters Under Reductive Conditions. J. Am. Chem. Soc 2018, 140, 13575–13579. [DOI] [PubMed] [Google Scholar]
- (50).(a) For examples, see:Ueno S; Chatani N; Kakiuchi F Ruthenium-Catalyzed Carbon–Carbon Bond Formation via the Cleavage of an Unreactive Aryl Carbon–Nitrogen Bond in Aniline Derivatives with Organoboronates. J. Am. Chem. Soc 2007, 129, 6098–6099. [DOI] [PubMed] [Google Scholar]; (b) Koreeda T; Kochi T; Kakiuchi F Cleavage of C–N Bonds in Aniline Derivatives on a Ruthenium Center and its Relevance to Catalytic C–C Bond Formation. J. Am. Chem. Soc 2009, 131, 7238–7239. [DOI] [PubMed] [Google Scholar]; (c) Koreeda T; Kochi T; Kakiuchi F Substituent Effects on Stoichiometric and Catalytic Cleavage of Carbon–Nitrogen Bonds in Aniline Derivatives by Ruthenium–Phosphine Complexes. Organometallics 2013, 32, 682–690. [Google Scholar]; (d) Koreeda T; Kochi T; Kakiuchi F Ruthenium-Catalyzed Reductive Deamination and Tandem Alkylation of Aniline Derivatives. J. Organomet. Chem 2013, 741–742, 148–152. [Google Scholar]; (e) Zhao Y; Snieckus V Beyond Directed Ortho Metalation: Ruthenium-Catalyzed Amide-Directed CAr–N Activation/C–C Coupling Reaction of Anthranilamides with Organoboronates. Org. Lett 2014, 16, 3200–3203. [DOI] [PubMed] [Google Scholar]
- (51).Liu J; Robins MJ Azoles as Suzuki Cross-Coupling Leaving Groups: Syntheses of 6-Arylpurine 2′-Deoxynucleosides and Nucleosides from 6-(Imidazol-1-yl)- and 6-(1,2,4-Triazol-4-yl)purine Derivatives. Org. Lett 2004, 6, 3421–3423. [DOI] [PubMed] [Google Scholar]
- (52).(a) For reports on the application of nucleoside analogues as potential therapeutics or chemical probes, see:Hocek M; Holy A; Votruba I; Dvořáková H Synthesis and Cytostatic Activity of Substituted 6-Phenylpurine Bases and Nucleosides: Application of the Suzuki–Miyaura Cross-Coupling Reactions of 6-Chloropurine Derivatives with Phenylboronic Acids. J. Med. Chem 2000, 43, 1817–1825. [DOI] [PubMed] [Google Scholar]; (b) Perez OD; Chang Y-T; Rosania G; Sutherlin D; Schultz PG Inhibition and Reversal of Myogenic Differentiation by Purine-Based Microtubule Assembly Inhibitors. Chem. Biol 2002, 9, 475–483. [DOI] [PubMed] [Google Scholar]
- (53).(a) Palladium-catalyzed cross-couplings of nitroarenes have been reported by Nakao and co-workers. See:Yadav MR; Nagaoka M; Kashihara M; Zhong R-L; Miyazaki T; Sakaki S; Nakao Y The Suzuki–Miyaura Coupling of Nitroarenes. J. Am. Chem. Soc 2017, 139, 9423–9426. [DOI] [PubMed] [Google Scholar]; (b) Inoue F; Kashihara M; Yadav MR; Nakao Y Buchwald–Hartwig Amination of Nitroarenes. Angew. Chem., Int. Ed 2017, 56, 13307–13309. [DOI] [PubMed] [Google Scholar]; (c) Rocard L; Hatych D; Chartier T; Cauchy T; Hudhomme P Original Suzuki–Miyaura Coupling Using Nitro Derivatives for the Synthesis of Perylenediimide-Based Multimers. Eur. J. Org. Chem 2019, 2019, 7635–7643. [Google Scholar]; (d) Zhou F; Zhou F; Su R; Yang Y; You J Build-Up of Double Carbohelicenes Using Nitroarenes: Dual Role of the Nitro Functionality as an Activating and Leaving Group. Chem. Sci 2020, 11, 7424–7428. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Muto K; Okita T; Yamaguchi J Transition-Metal-Catalyzed Denitrative Coupling of Nitroarenes. ACS Catal. 2020, 10, 9856–9871. [Google Scholar]
- (54).Yamamoto T; Ishizu J; Kohara T; Komiya S; Yamamoto A Oxidative Addition of Aryl Carboxylates to Nickel (0) Complexes Involving Cleavage of the Acyl–Oxygen Bond. J. Am. Chem. Soc 1980, 102, 3758–3764. [Google Scholar]
- (55).(a) Amaike K; Muto K; Yamaguchi J; Itami K Decarbonylative C–H Coupling of Azoles and Aryl Esters: Unprecedented Nickel Catalysis and Applications to the Synthesis of Muscoride A. J. Am. Chem. Soc 2012, 134, 13573–13576. [DOI] [PubMed] [Google Scholar]; (b) Meng L; Kamada Y; Muto K; Yamaguchi J; Itami K C–H Alkenylation of Azoles with Enols and Esters by Nickel Catalysis. Angew. Chem., Int. Ed 2013, 52, 10048–10051. [DOI] [PubMed] [Google Scholar]; (c) Muto K; Yamaguchi J; Musaev DG; Itami K Decarbonylative Organoboron Cross-Coupling of Esters by Nickel Catalysis. Nat. Commun 2015, 6, 7508. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Muto K; Hatakeyama R; Itami K; Yamaguchi J Palladium-Catalyzed Decarbonylative Cross-Coupling of Azinecarboxylates with Arylboronic Acids. Org. Lett 2016, 18, 5106–5109. [DOI] [PubMed] [Google Scholar]
- (56).(a) Gooßen LJ; Paetzold J Pd-Catalyzed Decarbonylative Olefination of Aryl Esters: Towards a Waste-Free Heck Reaction. Angew. Chem., Int. Ed 2002, 41, 1237–1241. [DOI] [PubMed] [Google Scholar]; (b) Gooßen LJ; Paetzold J Decarbonylative Heck Olefination of Enol Esters: Salt-Free and Environmentally Friendly Access to Vinyl Arenes. Angew. Chem., Int. Ed 2004, 43, 1095–1098. [DOI] [PubMed] [Google Scholar]
- (57).(a) For the chelation-assisted transition-metal-catalyzed activation of esters, see:Chatani N; Tatamidani H; Ie Y; Kakiuchi F; Murai S The Ruthenium-Catalyzed Reductive Decarboxylation of Esters: Catalytic Reactions Involving the Cleavage of Acyl–Oxygen Bonds of Esters. J. Am. Chem. Soc 2001, 123, 4849–4850. [DOI] [PubMed] [Google Scholar]; (b) Tatamidani H; Yokota K; Kakiuchi F; Chatani N Catalytic Cross-Coupling Reaction of Esters with Organoboron Compounds and Decarbonylative Reduction of Esters with HCOONH4: A New Route to Acyl Transition Metal Complexes Through the Cleavage of Acyl–Oxygen Bonds in Esters. J. Org. Chem 2004, 69, 5615–5621. [DOI] [PubMed] [Google Scholar]; (c) Tatamidani H; Kakiuchi F; Chatani N A New Ketone Synthesis by Palladium-Catalyzed Cross-Coupling Reactions of Esters with Organoboron Compounds. Org. Lett 2004, 6, 3597–3599. [DOI] [PubMed] [Google Scholar]
- (58).For the use of electronically activated esters in transitionmetal-catalyzed couplings, see refs 55 and 56.; (b) LaBerge NA; Love JA Nickel-Catalyzed Decarbonylative Coupling of Aryl Esters and Arylboronic Acids. Eur. J. Org. Chem 2015, 2015, 5546–5553. [Google Scholar]; (c) Ben Halima T; Vandavasi JK; Shkoor M; Newman SG A Cross-Coupling Approach to Amide Bond Formation from Esters. ACS Catal. 2017, 7, 2176–2180. [Google Scholar]; (d) Kruckenberg A; Wadepohl H; Gade LH Bis(diisospropylphosphinomethyl)amine Nickel(II) and Nickel(0) Complexes: Coordination Chemistry, Reactivity, and Catalytic Decarbonylative C–H Arylation of Benzoxazole. Organometallics 2013, 32, 5153–5170. [Google Scholar]; (e) Yue H; Guo L; Liao H-H; Cai Y; Zhu C; Rueping M Catalytic Ester and Amide to Amine Interconversion: Nickel-Catalyzed Decarbonylative Amination of Esters and Amides by C–O and C–C Bond Activation. Angew. Chem., Int. Ed 2017, 56, 4282–4285. [DOI] [PubMed] [Google Scholar]; (f) Yu H; Guo L; Lee S-C; Liu X; Rueping M Selective Reductive Removal of Ester and Amide Groups from Arenes and Heteroarenes Through Nickel-Catalyzed C–O and C–N Bond Activation. Angew. Chem., Int. Ed 2017, 56, 3972–3976. [DOI] [PubMed] [Google Scholar]; (g) Ben Halima T; Zhang W; Yalaoui I; Hong X; Yang Y; Houk KN; Newman SG Palladium-Catalyzed Suzuki–Miyaura Coupling of Aryl Esters. J. Am. Chem. Soc 2017, 139, 1311–1318. [DOI] [PubMed] [Google Scholar]
- (59).(a) For transition-metal-catalyzed cross-couplings of acid anhydrides, see:Wang D; Zhang Z Palladium-Catalyzed Cross-Coupling Reactions of Carboxylic Anhydrides with Organozinc Reagents. Org. Lett 2003, 5, 4645–4648. [DOI] [PubMed] [Google Scholar]; (b) Cook MJ; Rovis T Enantioselective Rhodium-Catalyzed Alkylative Desymmetrization of 3,5-Dimethylglutaric Anhydride. Synthesis 2009, 2009, 335–338. [Google Scholar]; (c) Bercot EA; Rovis T A Mild and Efficient Catalytic Alkylative Monofunctionalization of Cyclic Anhydrides. J. Am. Chem. Soc 2002, 124, 174–175. [DOI] [PubMed] [Google Scholar]; (d) Bercot EA; Rovis T Highly Efficient Nickel-Catalyzed Cross-Coupling of Succinic and Glutaric Anhydrides with Organozinc Reagents. J. Am. Chem. Soc 2005, 127, 247–254. [DOI] [PubMed] [Google Scholar]; (e) Johnson JB; Cook MJ; Rovis T Ligand Differentiated Complementary Rh-Catalyst Systems for the Enantioselective Desymmetrization of Meso-Cyclic Anhydrides. Tetrahedron 2009, 65, 3202–3210. [Google Scholar]; (f) Rogers RL; Moore JL; Rovis T Alkene-Directed Regioselective Nickel-Catalyzed Cross-Coupling of Cyclic Anhydrides with Diorganozinc Reagents. Angew. Chem., Int. Ed 2007, 46, 9301–9304. [DOI] [PubMed] [Google Scholar]; (g) Bercot EA; Rovis T A Palladium-Catalyzed Enantioselective Alkylative Desymmetrization of Meso-Succinic Anhydrides. J. Am. Chem. Soc 2004, 126, 10248–10249. [DOI] [PubMed] [Google Scholar]; (h) Johnson JB; Yu RT; Fink P; Bercot EA; Rovis T Selective Substituent Transfer from Mixed Zinc Reagents in Ni-Catalyzed Anhydride Alkylation. Org. Lett 2006, 8, 4307–4310. [DOI] [PubMed] [Google Scholar]; (i) Johnson JB; Bercot EA; Rowley JM; Coates GW; Rovis T Ligand-Dependent Catalytic Cycle and Role of Styrene in Nickel-Catalyzed Anhydride Cross-Coupling: Evidence for Turnover-Limiting Reductive Elimination. J. Am. Chem. Soc 2007, 129, 2718–2725. [DOI] [PubMed] [Google Scholar]; (j) Cook MJ; Rovis T Rhodium-Catalyzed Enantioselective Desymmetrization of Meso-3,5-Dimethyl Glutaric Anhydride: A General Strategy to syn-Deoxypolypropionate Synthons. J. Am. Chem. Soc 2007, 129, 9302–9303. [DOI] [PubMed] [Google Scholar]; (k) Stephan MS; Teunissen AJJM; Verzijl GKM; de Vries JG Heck Reactions Without Salt Formation: Aromatic Carboxylic Anhydrides as Arylating Agents. Angew. Chem., Int. Ed 1998, 37, 662–664. [DOI] [PubMed] [Google Scholar]; (l) Gooßen LJ; Ghosh K Palladium-Catalyzed Synthesis of Aryl Ketones from Boronic Acids and Carboxylic Acids or Anhydrides. Angew. Chem., Int. Ed 2001, 40, 3458–3460. [DOI] [PubMed] [Google Scholar]; (m) Kajita Y; Kurahashi T; Matsubara S Nickel-Catalyzed Decarbonylative Addition of Anhydrides to Alkynes. J. Am. Chem. Soc 2008, 130, 17226–17227. [DOI] [PubMed] [Google Scholar]
- (60).Hie L; Fine Nathel NF; Hong X; Yang Y-F; Houk KN; Garg NK Nickel-Catalyzed Activation of Acyl C–O Bonds of Methyl Esters. Angew. Chem., Int. Ed 2016, 55, 2810–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Carey FA; Sundberg RJ Advanced Organic Chemistry Part A: Structure and Mechanisms; Springer: New York, 2000; pp 9–11. [Google Scholar]
- (62).Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59, 4443–4458. [DOI] [PubMed] [Google Scholar]
- (63).Ben Halima T; Masson-Makdissi J; Newman SG Nickel-Catalyzed Amide Bond Formation from Methyl Esters. Angew. Chem., Int. Ed 2018, 57, 12925–12929. [DOI] [PubMed] [Google Scholar]
- (64).Zheng Y-L; Newman SG Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide Bonds. ACS Catal. 2019, 9, 4426–4433. [Google Scholar]
- (65).Cheung CW; Ploeger ML; Hu X Direct Amination of Esters with Nitroarenes. Nat. Commun 2017, 8, 14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Hu and coworkers note that, rather than proceeding via nickel-catalyzed oxidative addition to the acyl C–O bond, this transformation likely proceeds through a nickel nitrene intermediate, which could undergo insertion into the acyl C–O bond to generate a Ni(II) amido species. See ref 65 for further discussion of the mechanism.
- (67).Medina JM; Moreno J; Racine S; Du S; Garg NK Mizoroki–Heck Cyclizations of Amide Derivatives for the Introduction of Quaternary Centers. Angew. Chem., Int. Ed 2017, 56, 6567–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Zheng Y-L; Newman SG Nickel-Catalyzed Domino Heck-Type Reactions Using Methyl Esters as Cross-Coupling Electrophiles. Angew. Chem., Int. Ed 2019, 58, 18159–18164. [DOI] [PubMed] [Google Scholar]
- (69).Yue H; Zhu C; Rueping M Catalytic Ester to Stannane Functional Group Interconversion via Decarbonylative Cross-Coupling of Methyl Esters. Org. Lett 2018, 20, 385–388. [DOI] [PubMed] [Google Scholar]
- (70).Okita T; Muto K; Yamaguchi J Decarbonylative Methylation of Aromatic Esters by a Nickel Catalyst. Org. Lett 2018, 20, 3132–3135. [DOI] [PubMed] [Google Scholar]
- (71).(a) The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Greenberg A, Breneman CM, Liebman JF, Eds.; Wiley: Hoboken, NJ, 2003; pp 33–43. [Google Scholar]; (b) Pauling L; Corey RB; Branson HR The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. U. S. A 1951, 37, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).For select examples of amide-directed C–H functionalization, see: Rouquet G; Chatani N Catalytic Functionalization of C(sp2)–H and C(sp3)–H Bonds by Using Bidentate Directing Groups. Angew. Chem., Int. Ed 2013, 52, 11726–11743 and references therein. [DOI] [PubMed] [Google Scholar]
- (73).Kaiser D; Bauer A; Lemmerer M; Maulide N Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev 2018, 47, 7899–7925. [DOI] [PubMed] [Google Scholar]
- (74).Nahm S; Weinreb SM N-Methoxy-N-Methylamides as Effective Acylating Agents. Tetrahedron Lett 1981, 22, 3815–3818. [Google Scholar]
- (75).(a) Jensen CM; Lindsay KB; Taaning RH; Karaffa J; Hansen AM; Skrydstrup T Can Decarbonylation of Acyl Radicals Be Overcome in Radical Addition Reactions? En Route to a Solution Employing N-Acyl Oxazolidinones and SmI2/H2O. J. Am. Chem. Soc 2005, 127, 6544–6545. [DOI] [PubMed] [Google Scholar]; (b) Shi S; Lalancette R; Szostak R; Szostak M Highly Chemoselective Synthesis of Indolizidine Lactams by SmI2-Induced Umpolung of the Amide Bond via Aminoketyl Radicals: Efficient Entry to Alkaloid Scaffolds. Chem. - Eur. J 2016, 22, 11949–11953. [DOI] [PubMed] [Google Scholar]; (c) Huang H-M; Procter DJ Radical-Radical Cyclization Cascades of Barbiturates Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc 2016, 138, 7770–7775. [DOI] [PubMed] [Google Scholar]; (d) Huang H-M; Procter DJ Dearomatizing Radical Cyclizations and Cyclization Cascades Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. J. Am. Chem. Soc 2017, 139, 1661–1667. [DOI] [PubMed] [Google Scholar]; (e) Huang H-M; Bonilla P; Procter D-J. Selective Construction of Quaternary Stereocentres in Radical Cyclisation Cascades Triggered by Electron-Transfer Reduction of Amide-Type Carbonyls. Org. Biomol. Chem 2017, 15, 4159–4164. [DOI] [PubMed] [Google Scholar]
- (76).(a) For recent reviews, see:Madelaine C; Valerio V; Maulide N Unexpected Electrophilic Rearrangements of Amides: A Stereoselective Entry to Challenging Substituted Lactones. Angew. Chem., Int. Ed 2010, 49, 1583–1586. [DOI] [PubMed] [Google Scholar]; (b) Peng B; Geerdink D; Maulide N Electrophilic Rearrangements of Chiral Amides: A Traceless Asymmetric α-Allylation. J. Am. Chem. Soc 2013, 135, 14968–14971. [DOI] [PubMed] [Google Scholar]; (c) Tona V; Maryasin B; de la Torre A; Sprachmann J; González L; Maulide N Direct Regioselective Synthesis of Tetrazolium Salts by Activation of Secondary Amides Under Mild Conditions. Org. Lett 2017, 19, 2662–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).(a) Gregory AW; Chambers A; Hawkins A; Jakubec P; Dixon DJ Iridium-Catalyzed Reductive Nitro-Mannich Cyclization. Chem. - Eur. J 2015, 21, 111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fuentes de Arriba ÁL; Lenci E; Sonawane M; Formery O; Dixon DJ Iridium-Catalyzed Reductive Strecker Reaction for Late-Stage Amide and Lactam Cyanation. Angew. Chem., Int. Ed 2017, 56, 3655–3659. [DOI] [PubMed] [Google Scholar]; (c) Xie L-G; Dixon DJ Tertiary Amine Synthesis via Reductive Coupling of Amides with Grignard Reagents. Chem. Sci 2017, 8, 7492–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Gabriel P; Xie L-G; Dixon DJ Iridium-Catalyzed Reductive Coupling of Grignard Reagents and Tertiary Amides. Org. Synth 2019, 96, 511–527. [Google Scholar]; (e) Xie LG; Dixon DJ Iridium-Catalyzed Reductive Ugi-Type Reactions of Tertiary Amides. Nat. Commun 2018, 9, 2841. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Gabriel P; Gregory AW; Dixon DJ Iridium-Catalyzed Aza-Spirocyclization of Indole-Tethered Amides: An Interrupted Pictet–Spengler Reaction. Org. Lett 2019, 21, 6658–6662. For a review, see: [DOI] [PubMed] [Google Scholar]; (g) Matheau-Raven D; Gabriel P; Leitch JA; Almehmadi YA; Yamazaki K; Dixon DJ Catalytic Reductive Functionalization of Tertiary Amides Using Vaska’s Complex: Synthesis of Complex Tertiary Amine Building Blocks and Natural Products. ACS Catal. 2020, 10, 8880–8897. [Google Scholar]
- (78).(a) Nakajima M; Sato T; Chida N Iridium-Catalyzed Chemoselective Reductive Nucleophilic Addition to N-Methoxyamides. Org. Lett 2015, 17, 1696–1699. [DOI] [PubMed] [Google Scholar]; (b) Huang P-Q; Ou W; Han F Chemoselective Reductive Alkynylation of Tertiary Amides by Ir and Cu(I) Bis-Metal Sequential Catalysis. Chem. Commun 2016, 52, 11967–11970. [DOI] [PubMed] [Google Scholar]; (c) Hu X-N; Shen T-L; Cai D-C; Zheng J-F; Huang P-Q The Iridium-Catalysed Reductive Coupling Reaction of Tertiary Lactams/Amides with Isocyanoacetates. Org. Chem. Front 2018, 5, 2051–2056. [Google Scholar]; (d) Takahashi Y; Sato T; Chida N Iridium-Catalyzed Reductive Nucleophilic Addition to Tertiary Amides. Chem. Lett 2019, 48, 1138–1141. [DOI] [PubMed] [Google Scholar]
- (79).(a) Meng G; Shi S; Szostak M Cross-Coupling of Amides by N–C Bond Activation. Synlett 2016, 27, 2530–2540. [Google Scholar]; (b) Takise R; Muto K; Yamaguchi J Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev 2017, 46, 5864–5888. [DOI] [PubMed] [Google Scholar]; (c) Kaiser D; Bauer A; Lemmerer M; Maulide N Amide Activation: An Emerging Tool for Chemoselective Synthesis. Chem. Soc. Rev 2018, 47, 7899–7925. [DOI] [PubMed] [Google Scholar]
- (80).Hie L; Fine Nathel NF; Shah TK; Baker EL; Hong X; Yang Y; Liu P; Houk KN; Garg NK Conversion of Amides to Esters by Nickel-Catalysed Activation of Amide C–N Bonds. Nature 2015, 524, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).(a) Huynh HV Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev 2018, 118, 9457–9492. [DOI] [PubMed] [Google Scholar]; (b) Dröge T; Glorius F The Measure of All Rings–N-Heterocyclic Carbenes. Angew. Chem., Int. Ed 2010, 49, 6940–6952. [DOI] [PubMed] [Google Scholar]; (c) Kelly RA III; Clavier H; Giudice S; Scott NM; Stevens ED; Bordner J; Samardjiev I; Hoff CD; Cavallo L; Nolan SP Determination of N-Heterocyclic Carbene (NHC) Steric and Electronic Parameters Using the [(NHC)Ir(CO)2Cl] System. Organometallics 2008, 27, 202–210. [Google Scholar]; (d) Gómez-Suárez A; Nelson DJ; Nolan SP Quantifying and Understanding the Steric Properties of N-Heterocyclic Carbenes. Chem. Commun 2017, 53, 2650–2660. [DOI] [PubMed] [Google Scholar]
- (82).Liu C; Szostak M Twisted Amides: From Obscurity to Broadly Useful Transition-Metal-Catalyzed Reactions by N–C Amide Bond Activation. Chem. - Eur. J 2017, 23, 7157–7173. [DOI] [PubMed] [Google Scholar]
- (83).Using our previously established conditions for the nickel-catalyzed esterification of amides, the use of H2O as a nucleophile delivered no product. Moreover, in the presence of benzoic acid, the nickel-catalyzed esterification of N-phenyl-N-methyl benzamide failed to deliver product, indicating catalyst poisoning.
- (84).Knapp RR; Bulger AS; Garg NK Nickel-Catalyzed Conversion of Amides to Carboxylic Acids. Org. Lett 2020, 22, 2833–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).(a) Transamidation of secondary amides has been historically difficult, because of the resonance stability of amides and the often thermoneutral nature of this conversion. For further reading on this topic, see:Lanigan RM; Sheppard TD Recent Developments in Amide Synthesis: Direct Amidation of Carboxylic Acids and Transamidation Reactions. Eur. J. Org. Chem 2013, 2013, 7453–7465. [Google Scholar]; (b) Mucsi Z; Chass GA; Csizmadia IG Amidicity Change as a Significant Driving Force and Thermodynamic Selection Rule of Transamidation Reactions. A Synergy Between Experiment and Theory. J. Phys. Chem. B 2008, 112, 7885–7893. [DOI] [PubMed] [Google Scholar]
- (86).Baker EL; Yamano MM; Zhou Y; Anthony SM; Garg NKA A Two-Step Approach to Achieve Secondary Amide Transamidation Enabled by Nickel Catalysis. Nat. Commun 2016, 7, 11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Weires NA; Baker EL; Garg NK Nickel-Catalyzed Suzuki–Miyaura Coupling of Amides. Nat. Chem 2016, 8, 75–79. [DOI] [PubMed] [Google Scholar]
- (88).Simmons BJ; Weires NA; Dander JE; Garg NK Nickel-Catalyzed Alkylation of Amide Derivatives. ACS Catal. 2016, 6, 3176–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Hu L; Jiang J; Qu J; Li Y; Jin J; Li Z; Boykin DW Novel Potent Antimitotic Heterocyclic Ketones: Synthesis, Antiproliferative Activity, and Structure–Activity Relationships. Bioorg. Med. Chem. Lett 2007, 17, 3613–3617. [DOI] [PubMed] [Google Scholar]
- (90).Apnes GE; Didluk MT; Filipski KJ; Guzman-Perez A; Lee ECY; Pfefferkorn JA; Stevens BD; Tu MM Glucagon Receptor Modulators. U.S. Patent No. 20120202834, August. 9, 2012.
- (91).Walker JA Jr.; Vickerman KL; Humke JN; Stanley LM Ni-Catalyzed Alkene Carboacylation via Amide C–N Bond Activation. J. Am. Chem. Soc 2017, 139, 10228–10231. [DOI] [PubMed] [Google Scholar]
- (92).Dander JE; Giroud M; Racine S; Darzi ER; Alvizo O; Entwistle D; Garg NK Chemoenzymatic Conversion of Amides to Enantioenriched Alcohols in Aqueous Medium. Communications Chemistry 2019, 2, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Computations suggest that the active Ni–NHC catalyst coordinates to aryl substrates prior to amide C–N bond activation, thereby lowering the entropic cost associated with the barrier to oxidative addition (see ref 80).
- (94).Hie L; Baker EL; Anthony SM; Desrosiers J; Senanayake C; Garg NK Nickel-Catalyzed Esterification of Aliphatic Amides. Angew. Chem., Int. Ed 2016, 55, 15129–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Dander JE; Baker EL; Garg NK Nickel-Catalyzed Transamidation of Aliphatic Amide Derivatives. Chem. Sci 2017, 8, 6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Boit TB; Weires NA; Kim J; Garg NK Nickel-Catalyzed Suzuki–Miyaura Coupling of Aliphatic Amides. ACS Catal. 2018, 8, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).The NHC ligand, Benz-ICy, is formed from the precursor salt, Benz-ICy·HCl, via deprotonation in situ. See: Arduengo AJ III; Harlow RL; Kline M A Stable Crystalline Carbene. J. Am. Chem. Soc 1991, 113, 361–363. [Google Scholar]
- (98).Weires NA; Caspi DD; Garg NK Kinetic Modeling of the Nickel-Catalyzed Esterification of Amides. ACS Catal. 2017, 7, 4381–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Our collaborator, Dr. Daniel Caspi, employed DynoChem software to model the kinetics of the transformation and perform in silico simulations to calculate the ideal reaction conditions.
- (100).(a) For air-stable Ni(0)-olefin precatalysts recently reported by the Cornella and Engle laboratories, see:Tran VT; Li Z-Q; Apolinar O; Derosa J; Joannou MV; Wisniewski SR; Eastgate MD; Engle KM Ni(COD)(DQ): An Air-Stable 18-Electron Ni(0)-Olefin Precatalyst. Angew. Chem., Int. Ed 2020, 59, 7409–7413. [DOI] [PubMed] [Google Scholar]; (b) Nattmann L; Saeb R; Nöthling N; Cornella J An Air-Stable Binary Ni(0)-Olefin Catalyst. Nat. Catal 2020, 3, 6–13. [Google Scholar]
- (101).(a) Sather AC; Lee HG; Colombe JR; Zhang A; Buchwald SL Dosage Delivery of Sensitive Reagents Enables Glovebox Free Synthesis. Nature 2015, 524, 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fang Y; Liu Y; Ke Y; Guo C; Zhu N; Mi X; Ma Z; Hu Y A New Chromium-Based Catalyst Coated with Paraffin for Ethylene Oligomerization and the Effect of Chromium State on Oligomerization Selectivity. Appl. Catal., A 2002, 235, 33–38. [Google Scholar]; (c) Taber DF; Frankowski KJ Grubbs’ Catalyst in Paraffin: An Air-Stable Preparation for Alkene Metathesis. J. Org. Chem 2003, 68, 6047–6048. [DOI] [PubMed] [Google Scholar]
- (102).Mehta MM; Boit TB; Dander JE; Garg NK Ni-Catalyzed Suzuki–Miyaura Cross-Coupling of Aliphatic Amides on the Benchtop. Org. Lett 2020, 22, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).(a) For additional examples of using a paraffin-encapsulation strategy from our laboratory, see:Dander JE; Weires NA; Garg NK Benchtop Delivery of Ni(cod)2 Using Paraffin Capsules. Org. Lett 2016, 18, 3934–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dander JE; Morrill LA; Nguyen MM; Chen S; Garg NK Breaking Amide C–N Bonds in an Undergraduate Organic Chemistry Laboratory. J. Chem. Educ 2019, 96, 776–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).(a) For Pd-catalyzed amide C–N bond activations, see:Li X; Zou G Acylative Suzuki Coupling of Amides: Acyl-Nitrogen Activation via Synergy of Independently Modifiable Activating Groups. Chem. Commun 2015, 51, 5089–5092. [DOI] [PubMed] [Google Scholar]; (b) Yada A; Okajima S; Murakami M Palladium-Catalyzed Intramolecular Insertion of Alkenes into the Carbon–Nitrogen Bond of β-Lactams. J. Am. Chem. Soc 2015, 137, 8708–8711. [DOI] [PubMed] [Google Scholar]; (c) Meng G; Szostak M Palladium-Catalyzed Suzuki–Miyaura Coupling of Amides by Carbon–Nitrogen Cleavage: General Strategy for Amide N–C Bond Activation. Org. Biomol. Chem 2016, 14, 5690–5705. [DOI] [PubMed] [Google Scholar]; (d) Meng G; Szostak M General Olefin Synthesis by the Palladium-Catalyzed Heck Reaction of Amides: Sterically-Controlled Chemoselective N–C Activation. Angew. Chem., Int. Ed 2015, 54, 14518–14522. [DOI] [PubMed] [Google Scholar]; (e) Meng G; Szostak M Sterically Controlled Pd-Catalyzed Chemoselective Ketone Synthesis via N–C Cleavage in Twisted Amides. Org. Lett 2015, 17, 4364–4367. [DOI] [PubMed] [Google Scholar]; (f) Liu C; Meng G; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M N-Acylsaccharins: Stable Electrophilic Amide-Based Acyl Transfer Reagents in Pd-Catalyzed Suzuki–Miyaura Coupling via N–C Cleavage. Org. Lett 2016, 18, 4194–4197. [DOI] [PubMed] [Google Scholar]; (g) Lei P; Meng G; Szostak M General Method for the Suzuki–Miyaura Cross-Coupling of Amides Using Commercially Available, Air-and Moisture-Stable Palladium/ NHC (NHC = N-Heterocyclic Carbene) Complexes. ACS Catal. 2017, 7, 1960–1965. [Google Scholar]; (h) Liu C; Liu Y; Liu R; Lalancette R; Szostak R; Szostak M Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling of N-Mesylamides by N–C Cleavage: Electronic Effect of the Mesyl Group. Org. Lett 2017, 19, 1434–1437. [DOI] [PubMed] [Google Scholar]; (i) Liu C; Meng G; Szostak M N-Acylsaccharins as Amide-Based Arylating Reagents via Chemoselective N–C Cleavage: Pd-Catalyzed Decarbonylative Heck Reaction. J. Org. Chem 2016, 81, 12023–12030. [DOI] [PubMed] [Google Scholar]; (j) Meng G; Shi S; Szostak M Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling of Amides via Site-Selective N–C Bond Cleavage by Cooperative Catalysis. ACS Catal. 2016, 6, 7335–7339. [Google Scholar]; (k) Cui M; Wu H; Jian J; Wang H; Liu C; Stelck D; Zeng Z Palladium-Catalyzed Sonogashira Coupling of Amides: Access to Ynones via C–N Bond Cleavage. Chem. Commun 2016, 52, 12076–12079. [DOI] [PubMed] [Google Scholar]; (l) Wu H; Li Y; Cui M; Jian J; Zeng Z Suzuki Coupling of Amides via Palladium-Catalyzed C–N Cleavage of N-Acylsaccharins. Adv. Synth. Catal 2016, 358, 3876–3880. [Google Scholar]; (m) Shi S; Szostak M Decarbonylative Cyanation of Amides by Palladium Catalysis. Org. Lett 2017, 19, 3095–3098. [DOI] [PubMed] [Google Scholar]; (n) Lei P; Meng G; Ling Y; An J; Szostak M Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki–Miyaura Cross-Coupling Reactions of Amides. J. Org. Chem 2017, 82, 6638–6646. [DOI] [PubMed] [Google Scholar]; (o) Meng G; Szostak R; Szostak M Suzuki–Miyaura Cross-Coupling of N-Acylpyrroles and Pyrazoles: Planar, Electronically Activated Amides in Catalytic N–C Cleavage. Org. Lett 2017, 19, 3596–3599. [DOI] [PubMed] [Google Scholar]; (p) Meng G; Lalancette R; Szostak R; Szostak M N-Methylamino Pyrimidyl Amides (MAPA): Highly Reactive, Electronically-Activated Amides in Catalytic N–C(O) Cleavage. Org. Lett 2017, 19, 4656–4659. [DOI] [PubMed] [Google Scholar]; (q) Osumi Y; Liu C; Szostak M N-Acylsuccinimides: Twist-Controlled, Acyl Transfer Reagents in Suzuki–Miyaura Cross-Coupling by N–C Amide Bond Activation. Org. Biomol. Chem 2017, 15, 8867–8871. [DOI] [PubMed] [Google Scholar]; (r) Lei P; Meng G; Ling Y; An J; Nolan SP; Szostak M General Method for the Suzuki–Miyaura Cross-Coupling of Primary Amide-Derived Electrophiles Enabled by [Pd(NHC)(cin)–Cl] at Room Temperature. Org. Lett 2017, 19, 6510–6513. [DOI] [PubMed] [Google Scholar]; (s) Li X; Zou G Palladium-Catalyzed Acylative Cross-Coupling of Amides with Diarylboronic Acids and Sodium Tetraarylborates. J. Organomet. Chem 2015, 794, 136–145. [Google Scholar]; (t) Liu C; Li G; Shi S; Meng G; Lalancette R; Szostak R; Szostak M Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon–Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar]; (u) Meng G; Szostak M Palladium/NHC (NHC = N-Heterocyclic Carbene)-Catalyzed β-Alkyl Suzuki Cross-Coupling of Amides by Selective N–C Bond Cleavage. Org. Lett 2018, 20, 6789–6793. [DOI] [PubMed] [Google Scholar]; (v) Shi S; Szostak M Decarbonylative Borylation of Amides by Palladium Catalysis. ACS Omega 2019, 4, 4901–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Zhou T; Li G; Nolan SP; Szostak M [Pd(NHC)(acac)Cl]: Well-Defined, Air-Stable, and Readily Available Precatalysts for Suzuki and Buchwald–Hartwig Cross-Coupling (Transamidation) of Amides and Esters by N–C/O–C Activation. Org. Lett 2019, 21, 3304–3309. [DOI] [PubMed] [Google Scholar]; (x) Rahman MM; Buchspies J; Szostak M N-Acylphthalimides: Efficient Acyl Coupling Reagents in Suzuki–Miyaura Cross-Coupling by N–C Cleavage Catalyzed by Pd-PEPPSI Precatalysts. Catalysts 2019, 9, 129. [Google Scholar]; (y) Liu C; Lalancette R; Szostak R; Szostak M Sterically Hindered Ketones via Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling of Amides by N–C(O) Activation. Org. Lett 2019, 21, 7976–7981. [DOI] [PubMed] [Google Scholar]; (z) Li G; Zhou T; Poater A; Cavallo L; Nolan SP; Szostak M Buchwald–Hartwig Cross-Coupling by Air-and Moisture-Stable [Pd(NHC)-(allyl)Cl] Pre-Catalysts: Catalyst Evaluation and Mechanism. Catal. Sci. Technol 2020, 10, 710–716. [Google Scholar]
- (105).Conservatively estimated on the basis of SciFinder search results (accessed July 30, 2020).
- (106).(a) Shi S; Szostak M Nickel-Catalyzed Diaryl Ketone Synthesis by N–C Bond Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site-Selective N,N-di-Boc Activation. Org. Lett 2016, 18, 5872–5875. [DOI] [PubMed] [Google Scholar]; (b) Shi S; Szostak M Efficient Synthesis of Diaryl Ketones by Nickel-Catalyzed Negishi Cross-Coupling of Amides via Carbon–Nitrogen Bond Cleavage at Room Temperature Accelerated by Solvent Effect. Chem. - Eur. J 2016, 22, 10420–10424. [DOI] [PubMed] [Google Scholar]; (c) Dey A; Sasmal S; Seth K; Lahiri GK; Maiti D Nickel-Catalyzed Deamidative Step-Down Reduction of Amides to Aromatic Hydrocarbons. ACS Catal. 2017, 7, 433–437. [Google Scholar]; (d) Ni S; Zhang W; Mei H; Han J; Pan Y Ni-Catalyzed Reductive Cross-Coupling of Amides with Aryl Iodide Electrophiles via C–N Bond Activation. Org. Lett 2017, 19, 2536–2539. [DOI] [PubMed] [Google Scholar]; (e) Shi S; Szostak M Nickel-Catalyzed Negishi Cross-Coupling of N-Acylsuccinimides: Stable, Amide-Based, Twist-Controlled Acyl Transfer Reagents via N–C Activation. Synthesis 2017, 49, 3602–3608. [Google Scholar]; (f) Huang P-Q; Chen H Ni-Catalyzed Cross-Coupling Reactions of N-Acylpyrrole-Type Amides with Organoboron Reagents. Chem. Commun 2017, 53, 12584–12587. [DOI] [PubMed] [Google Scholar]; (g) Deguchi T; Xin H-L; Morimoto H; Ohshima T Direct Catalytic Alcoholysis of Unactivated 8-Aminoquinoline Amides. ACS Catal. 2017, 7, 3157–3161. [Google Scholar]
- (107).Cheung CW; Ploeger ML; Hu X Nickel-Catalyzed Reductive Transamidation of Secondary Amides with Nitroarenes. ACS Catal. 2017, 7, 7092–7096. [Google Scholar]
- (108).(a) Amani J; Alam R; Badir S; Molander GA Synergistic Visible-Light Photoredox/Nickel-Catalyzed Synthesis of Aliphatic Ketones via N–C Cleavage of Imides. Org. Lett 2017, 19, 2426–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) A dual-catalytic photoredox synthesis of ketones from imides using alkyl halides as radical precursors was recently reported by the Amgoune laboratory: Kerackian T; Reina A; Bouyssi D; Monteiro N; Amgoune A Silyl Radical Mediated Cross-Electrophile Coupling of N-Acyl-imides with Alkyl Bromides under Photoredox/Nickel Dual Catalysis. Org. Lett 2020, 22, 2240–2245. [DOI] [PubMed] [Google Scholar]
- (109).Srimontree W; Chatupheeraphat A; Liao H-H; Rueping M Amide to Alkyne Interconversion via a Nickel/Copper-Catalyzed Deamidative Cross-Coupling of Aryl and Alkenyl Amides. Org. Lett 2017, 19, 3091–3094. [DOI] [PubMed] [Google Scholar]
- (110).Hu J; Wang M; Pu X; Shi Z Nickel-Catalysed Retro-Hydroamidocarbonylation of Aliphatic Amides to Olefins. Nat. Commun 2017, 8, 14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Liu C; Szostak M Decarbonylative Phosphorylation of Amides by Palladium and Nickel Catalysis: The Hirao Cross-Coupling of Amide Derivatives. Angew. Chem., Int. Ed 2017, 56, 12718–12722. [DOI] [PubMed] [Google Scholar]
- (112).(a) For cross-couplings of acyclic alkyl C–O electrophiles, see:Robbins WR; Hartwig JF Sterically Controlled Alkylation of Arenes Through Iridium-Catalyzed C–H Borylation. Angew. Chem., Int. Ed 2013, 52, 933–937. [DOI] [PubMed] [Google Scholar]; (b) Lucas EL; Jarvo ER Stereospecific and Stereoconvergent Cross-Couplings Between Alkyl Electrophiles. Nat. Rev. Chem 2017, 1, 0065. [Google Scholar]; (c) Zhang S; Taylor BLH; Ji C; Gao Y; Harris MR; Hanna LE; Jarvo ER; Houk KN; Hong X Mechanism and Origins of Ligand-Controlled Stereoselectivity of Ni-Catalyzed Suzuki–Miyaura Coupling with Benzylic Esters: A Computational Study. J. Am. Chem. Soc 2017, 139, 12994–13005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shugrue CR; Sculimbrene BR; Jarvo ER; Mercado BQ; Miller SJ Outer-Sphere Control for Divergent Multicatalysis with Common Catalytic Moieties. J. Org. Chem 2019, 84, 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sanford AB; Tollefson EJ; Jarvo ER Stereospecific Cross-Coupling Reactions Provide Conformationally-Biased Arylalkanes with Anti-Leukemia Activity. Isr. J. Chem 2020, 60, 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sanford AB; Thane TA; McGinnis TM; Chen P-P; Hong X; Jarvo ER Nickel-Catalyzed Alkyl–Alkyl Cross-Electrophile Coupling Reaction of 1,3-Dimesylates for the Synthesis of Alkylcyclopropanes. J. Am. Chem. Soc 2020, 142, 5017–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Zhou Q; Srinivas HD; Dasgupta S; Watson MP Nickel-Catalyzed Cross-Couplings of Benzylic Pivalates with Arylboroxines: Stereospecific Formation of Diarylalkanes and Triarylmethanes. J. Am. Chem. Soc 2013, 135, 3307–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).(h) For cross-couplings of alkyl C–N electrophiles, see: Calet S; Urso F; Alper H Enantiospecific and Stereospecific Rhodium(I)-Catalyzed Carbonylation and Ring Expansion of Aziridines. Asymmetric Synthesis of β-Lactams and the Kinetic Resolution of Aziridines. J. Am. Chem. Soc 1989, 111, 931–934. [Google Scholar]; (i) Ney JE; Wolfe JP Synthesis and Reactivity of Azapalladacyclobutanes. J. Am. Chem. Soc 2006, 128, 15415–15422. [DOI] [PubMed] [Google Scholar]; (j) Lin BL; Clough CR; Hillhouse GL Interactions of Aziridines with Nickel Complexes: Oxidative-Addition and Reductive-Elimination Reactions That Break and Make C–N Bonds. J. Am. Chem. Soc 2002, 124, 2890–2891. [DOI] [PubMed] [Google Scholar]; (k) Huang C-Y; Doyle AG Nickel-Catalyzed Negishi Alkylations of Styrenyl Aziridines. J. Am. Chem. Soc 2012, 134, 9541–9544. [DOI] [PubMed] [Google Scholar]; (l) Nielsen DK; Huang C-Y; Doyle AG Directed Nickel-Catalyzed Negishi Cross-Coupling of Alkyl Aziridines. J. Am. Chem. Soc 2013, 135, 13605–13609. [DOI] [PubMed] [Google Scholar]; (m) Huang C-Y; Doyle AG Electron-Deficient Olefin Ligands Enable Generation of Quaternary Carbons by Ni-Catalyzed Cross-Coupling. J. Am. Chem. Soc 2015, 137, 5638–5641. [DOI] [PubMed] [Google Scholar]
- (114).(a) Liu C; Li G; Shi S; Meng G; Lalancette R; Szostak R; Szostak M Acyl and Decarbonylative Suzuki Coupling of N-Acetyl Amides: Electronic Tuning of Twisted, Acyclic Amides in Catalytic Carbon–Nitrogen Bond Cleavage. ACS Catal. 2018, 8, 9131–9139. [Google Scholar]; (b) Iranpoor N; Panahi F; Jamedi F Nickel-Catalyzed One-Pot Synthesis of Biaryls from Phenols and Arylboronic Acids via C–O Activation Using TCT Reagent. J. Organomet. Chem 2015, 781, 6–10. [Google Scholar]; (c) Chen L; Lang H; Fang L; Zhu M; Liu J; Yu J; Wang L Nickel-Catalyzed One-Pot Suzuki–Miyaura Cross-Coupling of Phenols and Arylboronic Acids Mediated by N,N-Ditosylaniline. Eur. J. Org. Chem 2014, 2014, 4953–4957. [Google Scholar]; (d) Chen G; Huang J; Gao L; Han F Nickel-Catalyzed Cross-Coupling of Phenols and Arylboronic Acids Through an In Situ Phenol Activation Mediated by PyBroP. Chem. - Eur. J 2011, 17, 4038–4042. [DOI] [PubMed] [Google Scholar]; (e) Oger N; d’Halluin M; Le Grognec E; Felpin F Using Aryl Diazonium Salts in Palladium-Catalyzed Reactions Under Safer Conditions. Org. Process Res. Dev 2014, 18, 1786–1801. [Google Scholar]
- (115).Hardy MA; Wright BA; Bachman JL; Boit TB; Haley HMS; Knapp RR; Lusi RF; Okada T; Tona V; Garg NK; Sarpong R Treating a Global Health Crisis With a Dose of Synthetic Chemistry. ACS Cent. Sci 2020, 6, 1017–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (116).(a) For reviews on cross-couplings catalyzed by base metals, see:Thapa S; Shrestha B; Gurung SK; Giri R Copper-Catalysed Cross-Coupling: An Untapped Potential. Org. Biomol. Chem 2015, 13, 4816–4827. [DOI] [PubMed] [Google Scholar]; (b) Cahiez G; Moyeux A Cobalt-Catalyzed Cross-Coupling Reactions. Chem. Rev 2010, 110, 1435–1462. [DOI] [PubMed] [Google Scholar]; (c) Mako TL; Byers JA Recent Advances in Iron-Catalysed Cross-Coupling Reactions and Their Mechanistic Underpinning. Inorg. Chem. Front 2016, 3, 766–790. [Google Scholar]
- (117).Expensive NHC ligands are often required for nickel-catalyzed activations of strong bonds.
- (118).Hazari N; Melvin PR; Beromi MM Well-Defined Nickel and Palladium Precatalysts for Cross-Coupling. Nat. Rev. Chem 2017, 1, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]


