Abstract
The year 2021 marks the 50th anniversary of the National Cancer Act of 1971 and President Richard Nixon’s declaration of a “war on cancer”. In 1971 cancer was the second leading cause of death in the USA, and today it is still the second leading cause of death with an estimated 606,520 Americans dying of cancer in the year 2020. The half a century campaign to eliminate cancer reveals at least two important public health lessons that must be heeded for the next 50 years of the war against the disease—(1) recognizing the limits of behaviour control and (2) recognizing the significance of rate (of ageing) control. These two lessons result in a somewhat paradoxical conclusion in that we must have both humility and ambition in our attitudes towards future preventative medicine for the world’s ageing populations. Geroscience must become an integral part of public health if serious headway is to be made preventing not only cancer but most of the other chronic conditions of late life.
Keywords: Ageing, Cancer, Geroscience, National Cancer Institute, National Institute on Aging, Preventative medicine
The year 2021 marks the 50th anniversary of the National Cancer Act of 1971 and President Richard Nixon’s declaration of a “war on cancer”. In 1971 cancer was the second leading cause of death in the USA, and President Nixon converted the Army’s former biological warfare facilities at Fort Detrick, Maryland, to house research activities on the causes, prevention and treatment of cancer. Half a century later, approximately 606,520 Americans died of cancer in the year 2020 [1]. Today cancer is still the second leading cause of death. And the associated cost of cancer care is estimated to have risen 27% from 124.57 billion to 157.77 billion 2010 US dollars from 2010 to 2020 [2]. The war on cancer has been fought on two fronts—with cancer prevention and with cancer treatment. From a public health perspective, cancer prevention is among one of the most significant and challenging problems facing the ageing populations of the world today.
There is no prospect of “winning” the war on cancer without more effective preventative medicine. And the half a century campaign to eliminate cancer reveals at least two important public health lessons that must be heeded for the next 50 years of the war against the disease. These two lessons are:
Lesson #1: Some of the modifiable behavioural risk factors for cancer mortality—like smoking and obesity—have proven to be very intractable. Thus lesson #1 is recognizing the limits of behaviour control.
Lesson #2: The most significant risk factor for cancer (and most other chronic conditions) is age. For the future health of ageing populations, it is imperative that biomedical research aspire to make the most important cancer risk factor—biological ageing—a “modifiable” risk factor. Thus lesson #2 is recognizing the significance of rate control.
These two lessons result in a somewhat paradoxical conclusion in that we must have both humility and ambition in our attitudes towards future preventative medicine. Humility teaches us that just because some cancer risk factors are within human control (e.g. obesity, smoking and alcohol consumption), this does not mean that it will be easy (or even possible) to substantively modify them at the population level. And ambition teaches us that something we previously thought was an unmodifiable risk factor just a few decades ago, like biological ageing, may turn out to be something we can in fact modify. This paradoxical conclusion has great significance for how we should envision cancer prevention for the next half a century. Geroscience, the interdisciplinary scientific field of inquiry which strives to understand how ageing enables chronic disease and seeks to develop novel multi-disease preventative and therapeutic approaches [3], must become an integral part of public health if serious headway is to be made preventing not only cancer but most of the other chronic conditions of late life.
Smoking and obesity: intractable modifiable risk factors
Over the past 50 years, significant advancements have been made in identifying and understanding the proximate causation of different types of cancer. Modifiable risk factors, such as smoking and obesity, have been identified and are a critical part of public health campaigns to prevent cancer. But acquiring knowledge about modifiable cancer risk factors and translating such knowledge into compliant behavioural changes are two very different things.
Despite decades of promoting smoking cessation public health campaigns, the CDC estimates that smoking accounts for more than 480,000 deaths every year, with about 15 of every 100 adult men (15.3%) and nearly 13 of every 100 adult women still smoking [4]. And smoking cessation is not a silver bullet to protect past smokers from eventually developing lung cancer, as four of ten lung cancers occur in former smokers with more than 15 years since quitting [5].
Obesity, defined in adults as a body mass index (BMI) ≥ 30, is associated with higher incidence of a number of cancers [6], which has become increasingly more prevalent over the past three decades. The CDC’s NCHS Data Brief “Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018” estimates that the age-adjusted prevalence of obesity in adults is 42.4% [7]. Higher levels of physical activity are associated with lower cancer incidence [8] which is encouraging news, until one acknowledges the reality that approximately 80% of US adults and adolescents are insufficiently active, based on the Physical Activity Guidelines for Americans [9].
Obesity-related diseases are estimated to increase the chances of dying and lessen life years by 0.2 to 11.7 years depending on gender, race, BMI classification and age [10]. This shorter life expectancy from obesity could be because obesity accelerates ageing. In their review of how obesity and ageing are “two sides of the same coin”, Tam, Morais and Santosa [11] contend that both obesity and ageing promote cellular senescence, inflammation, mitochondrial dysfunction, the aggregation of misfolded proteins, the attrition of telomeres, etc. Developing new interventions to modulate obesity and/or ageing would help prevent disease in growing portions of the obese and ageing population.
Ageing and cancer: preventative medicine for ageing populations
Since 1951, the year all state and federal agencies in the USA were required to adopt a standard list of contributing and underlying causes of death; no one in the USA has died from “old age” [12]. But most cancer mortality occurs in late life, as Table 1 illustrates for all cancer morality per 100,000 people in different age groups for the US population. The probability of developing (all sites) cancer within the first 5 decades from birth to age 49 is only 1 in 29, but this rises to 1 in 3 by age 70 [14]. Prioritizing the aspiration to conquer specific diseases like cancer, vs intervening in ageing, has led to the sizeable disparity between the percentage of NIH Congressional Appropriations allocated to the National Cancer Institute (NCI) vs the National Institute on Aging (NIA) over the past half a century (see Graph 1).
Table 1.
Age-specific mortality rates per 100,000 people for all cancers, all races and both sexes (based on 2019 CDC submission data (1999–2017) [13]
| Age group (years) | Age-specific rate |
|---|---|
| <1 | 1.5 |
| 1–4 | 2.0 |
| 5–9 | 2.1 |
| 10–14 | 2.1 |
| 15–19 | 2.7 |
| 20–24 | 3.7 |
| 25–29 | 5.6 |
| 30–34 | 10.6 |
| 35–39 | 19.4 |
| 40–44 | 34.7 |
| 45–49 | 62.7 |
| 50–54 | 122.5 |
| 55–59 | 217.0 |
| 60–64 | 336.8 |
| 65–69 | 485.7 |
| 70–74 | 677.0 |
| 75–79 | 938.7 |
| 80–84 | 1240.9 |
| 85+ | 1599.9 |
Graph 1.
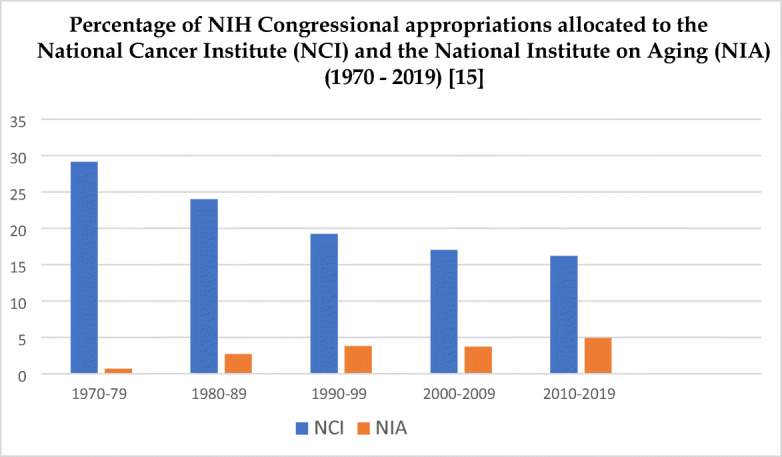
Percentage of NIH Congressional appropriations allocated to the National Cancer Institute (NCI) and the National Institute on Aging (NIA) (1970–2019) [15]
Nearly 200 years ago, the British actuary Benjamin Gompertz made the important observation that there is a law of progression describing the exponential rise in death rates between sexual maturity and old age [16]. The link between cancer and ageing is not accidental; it is a legacy of our Darwinian past which is an evolutionary history that prioritized our species’ survival during the period of the human lifespan when successful reproduction and the continuation of generations could be achieved.
When biomedical research fixates on disease elimination, it adopts a myopic lens because it marginalizes the importance of research on exceptional healthy ageing. Rather than invest so heavily in training biomedical researchers to have expertise sequencing and manipulating the genes of young mice to prevent and treat cancer, a greater emphasis needs to be placed on unravelling the biology of the aged physiology. To focus solely on cancer genomics would be imprudent as the biological puzzles of exceptional longevity might also reveal importance insights for cancer prevention. The naked mole rate, for example, is the longest living (age > 28 years) rodent and displays a low incidence of cancer [17, 18]. Non-traditional models like the naked mole rat have become increasingly important in human disease research, such as cancer, as they often display unusual biological features (e.g. genome stability, protein stability, oxidative metabolism) [19]. In humans cancer in the very elderly is relatively uncommon as a disease and as a cause of death, characterized by a slow growth and a modest life-threatening potential [20]. Understanding how the biology (e.g. genetics) of centenarians (age ≥ 100) and supercentenarians (age ≥ 100) enables these rare individuals to surpass the current human life expectancy by 20+ years could transform preventative medicine beyond the limited focus on behaviour control.
Since research in the 1930s, scientists have known that by feeding rats a calorie-restricted diet, these rodents would stay youthful longer and suffer fewer late-life diseases. Caloric restriction is an “anti-ageing” intervention that induces stress response pathways in organisms, and while it is too burdensome an intervention to be pursed as an ageing intervention for human populations, the prospect of developing a drug that mimics calorie restriction might be a viable way to safely and effectively retard ageing.
For preventative medicine to be most effective, any significant intervention must be (1) cheap, (2) minimally burdensome and (3) accessible to a wide portion of the population. Physical exercise, for example, is cheap and easily accessible to most people, but for many persons, it is too burdensome to be utilized regularly to get its full health benefits. A pill that modulates the inborn ageing process could confer significant health benefits for the 2 billion persons expected to be over age 60 by the middle of this century. And if affordable, consuming a daily pill would likely have a high compliance rate compared to more burdensome behavioural modifications.
Talk of an eventual “anti-ageing” pill often conjures up concerns of a Gattaca-like dystopia where only the affluent can afford to purchase a superior biology while the majority of the population will be left to flounder with their innate biological clocks and the inevitable frailty, disability and multi-morbidity that will occur for most people after age 70. But such a dystopic future need not become a reality, especially if we make an applied gerontological intervention (like we have with COVID-19 vaccines) a public health priority for all.
A fertile source for therapies slowing ageing is FDA-approved drugs whose safety has been investigated [21]. The oral antidiabetic drug metformin, for example, was first used by the French physician Jean Sterne to treat diabetes in 1957 and today is the first-line oral blood glucose-lowering agent to manage type 2 diabetes [22]. Epidemiological studies have established the biological plausibility of a cancer preventive effect of metformin given the multiple ways it can interfere with cancer signalling pathways [23]. Because of its low cost and proven safety over many decades, metformin is among one of the top candidates for a likely first generation of applied gerontological interventions. TAME (Targeting Ageing with Metformin) is a clinical trial to test the drug metformin as a safe and effective intervention against several age-related diseases [24].
Rapamycin is another potential drug that could target ageing. Discovered in the soil on Easter Island more than half a century ago [25], initial studies identified its capacity to inhibit cancer cell proliferation in mouse models, while parallel studies explored the potential of rapamycin as an immunosuppressant for organ transplants [26]. But more recent experiments have found that consuming rapamycin can extend lifespan, including in mammals. In a 2009 study of mice [27] that were already 600 days old (which is roughly equivalent to a 60-year-old human) before being fed rapamycin, Harrison et al. found that this intervention increased the median and maximal lifespan of both male and female mice. The initial study concludes that rapamycin may extend lifespan by postponing death from cancer, by retarding mechanisms of ageing or both. Since this initial report in 2009, there have been fourteen additional studies showing that rapamycin increased the lifespans of male and female mice and these studies on mouse data demonstrate that this molecule is effective in preventing, even reversing, a broad range of age-related conditions and thus warrants being described as an “anti-ageing” intervention [28].
The critical “pivot” for preventative medicine
Reflecting on the realities of the past 50 years of the “war on cancer”, and the reality of the prevalence of comorbidity for populations surviving to the upper limits of the human lifespan, we cannot continue on the same course originally plotted out by the National Cancer Act of 1971. Today cancer is still the second leading cause of death in America. The project of behaviour control has not successfully altered this outcome, in large part because it does not alter the most significant risk factor for cancer—age.
I am not suggesting that public health should concede the battle and abandon the important preventative measures of smoking cessation and a physically active lifestyle. Of course not. But we should have the humility to recognize that doubling down on these efforts for the next half a century is unlikely to yield a significant health dividend for today’s ageing populations. Strategic innovation in preventative medicine will be required. The strategy of behaviour control must be supplemented with the strategy of rate control.
The public health lessons of the 50-year campaign to defeat cancer in the USA ought to inform global public health more generally. The European Commission, for example, has recently identified cancer as a “mission area”, which means it is an integral part of the Horizon Europe framework programme that began in 2021. The EU report titled Conquering Cancer: Mission Possible [29] states that its mission is to save more than 3 million lives, living longer and better by 2030. Like President Nixon’s mission half a century ago, there is no acknowledgement of the reality of comorbidity in late life and the fact that ageing is the major risk factor for cancer mortality. The report recommends focusing on alcohol, food and sugar-sweetened beverages and tobacco consumption, workplace carcinogens, air pollution and behavioural risk factors. Other recommendations include implementing personalized medicine approaches to cancer as well as early diagnostics and minimally invasive treatment. But the major omission from its thirteen recommendations is an action plan to tackle the most significant risk factor for cancer—ageing itself. In order to unify the aspirations to “save people” from cancer mortality while also ensuring they live longer and better lives, the inborn ageing process must also be targeted. To fixate on disease elimination without also aspiring to alter the rate of ageing will prove costly with diminishing health returns because it does not increase the healthspan.
By contrast, in 2021 a House of Lords “Science and Technology Select Committee” UK report [30] was released, the catalyst of which was a 2019 assessment of the feasibility of the Government’s Ageing Society Grand Challenge mission. Chapter 6 of this report is entitled “The Ageing Society Grand Challenge”, and it sets the goal of increasing healthy life expectancy by 5 years by 2035. The report acknowledges (177) there has been a lack of effort since the 2005 report to ensure priority is given to research into ageing vs research into specific age-related diseases. The report notes that this may have contributed to the poor translation of basic research into clinical trials or new medicine. The recommendation is made that UK Research and Innovation commit to funding further research into the biology of ageing as a priority to support studies to improve healthspan.
The World Health Organization dedicated the decade 2021–2030 as the decade of “healthy ageing” [31]. The campaign identifies four main areas of action—age-friendly environments, combating ageism, integrated care and long-term care. These are all morally laudable, but incomplete, prescriptions. Like the EU report on defeating cancer, what is missing from the World Health Organization’s action plan is undertaking the committed action to develop an applied gerontological intervention to increase the human healthspan. Geroscience is an integral element of public health for today’s ageing populations. Redressing the imbalance between the research funding invested in tackling specific chronic diseases vs the most significant risk factor for chronic diseases is critical. The past half a century war on cancer reveals the limitations of continuing on the path of disease elimination for populations that are approaching the upper limits of the human lifespan.
Strategic innovations in preventative medicine are required if we hope to improve the healthspan of today’s ageing populations. To make serious headway in cancer prevention, we must target the most significant risk factor—biological ageing. Despite the limits facing behaviour control, there is good reason for optimism that the development of an applied gerontological intervention could help us achieve the important goal of rate control. Age retardation would ensure we improve the quality of life for older people vs simply preventing death by helping older populations manage multi-morbidity.
When President Nixon declared a “war on cancer” nearly 50 years ago, the success of the war was equated with disease elimination. That is a noble but unrealistic goal. Waging a war against an unrealistic goal is harmful for two reasons. Firstly, it means that large investments of public funds are invested into something that is not attainable (of the 200+ types of cancer, none have been cured or eliminated). Secondly, and more importantly, that investment in disease elimination imposed a hefty opportunity cost. Had those same funds been invested elsewhere, for example, targeting the ageing process itself, it could have yielded the population a much more significant health dividend. The primary challenge for today’s ageing populations is not to eradicate cancer mortality but rather to increase the human healthspan. Rather than continue to prioritize the goal of extending life via disease elimination for populations reaching the upper limits of human lifespan, the more important goal of public health, medicine, biotechnology, and the health sciences should now shift towards delaying and compressing the period of the lifespan when frailty and disability increase substantially [32].
Acknowledgements
I am grateful to two anonymous referees for their helpful comments and suggestions on an earlier version of this paper.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Cancer Institute. Cancer statistics. https://www.cancer.gov/about-cancer/understanding/statistics. Accessed 6 April 2021.
- 2.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Accessed 6 April 2021.
- 5.Tindle HA, Duncan MS, Greevy RA, Vasan RS, Kundu S, Massion PP, Freiberg MS. Lifetime smoking history and risk of lung cancer: results from the Framingham heart study. J Natl Cancer Inst. 2018;110(11):1201–1207. doi: 10.1093/jnci/djy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F. Straif K; International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer--viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Adult obesity facts. https://www.cdc.gov/obesity/data/adult.html. Accessed 6 April 2021.
- 8.Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, Winters-Stone K, Gerber LH, George SM, Fulton JE, Denlinger C, Morris GS, Hue T, Schmitz KH, Matthews CE. American College of Sports Medicine roundtable report on physical activity, sedentary behavior, and cancer Prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–2402. doi: 10.1249/MSS.0000000000002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SH, Pollack LM, Colditz GA. Life years lost associated with obesity-related diseases for U.S. non-smoking adults. PLoS One. 2013;8(6):e66550. doi: 10.1371/journal.pone.0066550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes Rev. 2020;21(4):e12991. doi: 10.1111/obr.12991. [DOI] [PubMed] [Google Scholar]
- 12.Hayflick L. Has anyone ever died of old age? New York: International Longevity Centre; 2003. [Google Scholar]
- 13.U.S. Cancer Statistics Working Group. U.S. Cancer statistics data visualizations tool, based on 2019 submission data (1999-2017): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. www.cdc.gov/cancer/dataviz. Accessed 1 April 2021.
- 14.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. Office of Budget. https://officeofbudget.od.nih.gov/approp_hist.html. Accessed 1 April 2021.
- 16.Gompertz B. On the nature of the function expressive of the law of human mortality and on a new mode of determining life contingencies. Phil Trans R Soc. 1825;115:513–85. doi: 10.1098/rstl.1825.0026. [DOI] [Google Scholar]
- 17.Buffenstein R, Jarvis JU. The naked mole rat--a new record for the oldest living rodent. Sci Aging Knowledge Environ. 2002;29(21):pe7. doi: 10.1126/sageke.2002.21.pe7. [DOI] [PubMed] [Google Scholar]
- 18.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178(4):439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 19.Shepard A, Kissil JL. The use of non-traditional models in the study of cancer resistance-the case of the naked mole rat. Oncogene. 2020;39(28):5083–5097. doi: 10.1038/s41388-020-1355-8. [DOI] [PubMed] [Google Scholar]
- 20.Pavlidis N, Stanta G, Audisio RA. Cancer prevalence and mortality in centenarians: a systematic review. Crit Rev Oncol Hematol. 2012;83(1):145–152. doi: 10.1016/j.critrevonc.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Snell TW, Johnston RK, Srinivasan B, Zhou H, Gao M, Skolnick J. Repurposing FDA-approved drugs for anti-aging therapies. Biogerontology. 2016;17(5-6):907–920. doi: 10.1007/s10522-016-9660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60(9):1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 23.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia. 2017;60(9):1639–1647. doi: 10.1007/s00125-017-4372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a tool to target aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 26.Arriola Apelo SI, Lamming DW. Rapamycin: an inhibiTOR of aging emerges from the soil of Easter island. J Gerontol A Biol Sci Med Sci. 2016;71(7):841–849. doi: 10.1093/gerona/glw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selvarani R, Mohammed S, Richardson A. Effect of rapamycin on aging and age-related diseases-past and future. Geroscience. 2020;Epub ahead of print. 10.1007/s11357-020-00274-1. [DOI] [PMC free article] [PubMed]
- 29.Directorate-General for Research and Innovation (European Commission). Proposed mission, conquering cancer: mission possible. Luxembourg: Publications Office of the European Union; 2020. https://ec.europa.eu/info/sites/info/files/research_and_innovation/funding/documents/ec_rtd_mission-board-report-cancer.pdf. Accessed 6 April, 2021.
- 30.House of Lords, Science and Technology Select Committee 1st Report of Session 2019-2021. Ageing: science, technology and healthy living. https://committees.parliament.uk/publications/4286/documents/43456/default/. Accessed 6 April 2021.
- 31.World Health Organization. UN decade of healthy ageing 2021-2030. https://www.who.int/initiatives/decade-of-healthy-ageing. Accessed 6 April 2021.
- 32.Olshansky SJ. From lifespan to healthspan. JAMA. 2018;320(13):1323–1324. doi: 10.1001/jama.2018.12621. [DOI] [PubMed] [Google Scholar]


