Abstract
Adrenocortical carcinoma (ACC) is a rare malignancy that arises from the adrenal cortex and can be classified as either non-functioning or functioning. A patient with non-functioning ACC may present no specific symptoms. Imaging analysis can provide some information to a clinician who suspects ACC, such as tumor size, density, washout, necrosis, hemorrhage, and calcification. Histopathology is used to confirm and determine the origin of the malignancy and can provide relevant prognostic information. Microscopic findings can be used to obtain information such as the Weiss score, resection surface features, Ki-67 proliferative index, and the degree of capsular and vascular invasion. Surgery can be curative for localized tumors, and adjuvant therapy using mitotane and cytotoxic chemotherapy is often employed for advanced-stage tumors. We describe a case report of a 32-year-old man with a non-functioning ACC that highlights the importance of radiological and pathological features in the diagnosis of ACC and their use as prognostic factors.
Keywords: Adrenal tumor, Adrenocortical carcinoma, Rare neoplasm
Introduction
Adrenocortical carcinoma (ACC) is a rare and aggressive endocrine malignancy that originates from the adrenal cortex and is associated with a dismal prognosis [1]. The estimated incidence of ACC is 0.5-2 cases per million population each year [2]. ACC can be classified as either functioning (which results in the production of a higher than normal hormone concentration level) or non-functioning (which results in the production of manufacturing a lower than normal hormone concentration level). Functioning tumors are more common than non-functioning tumors [3]. ACC can present with symptoms consistent with excess hormone secretion or as an abdominal mass, or ACC can be discovered incidentally during the course of investigating other clinical issues [4]. Imaging plays important roles in the identification and characterization of ACC, the determination of a malignancy risk, staging evaluation, and follow-up [5]. Histological and immunohistochemical features can be used to confirm the identity of an adrenocortical tumor, provide advanced knowledge that can be used to determine prognosis, and guide the application of appropriate therapeutic options for these infrequent neoplasms [6].
Case report
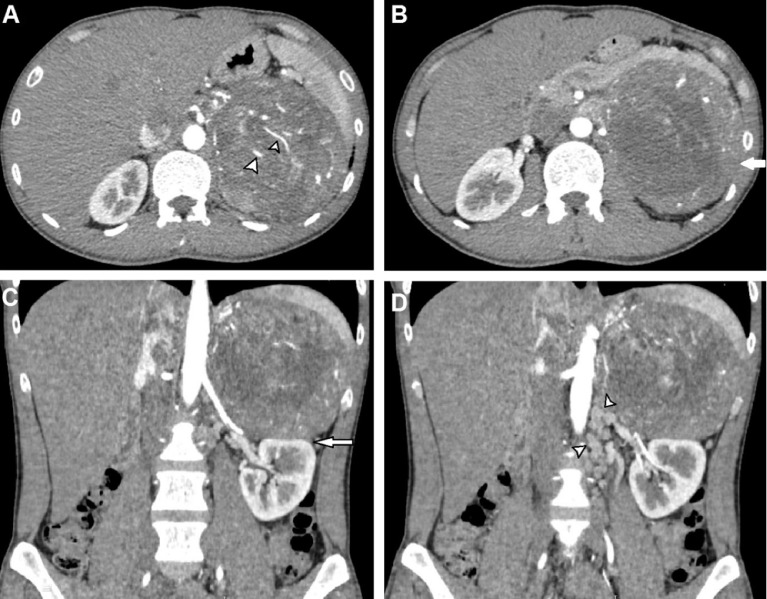
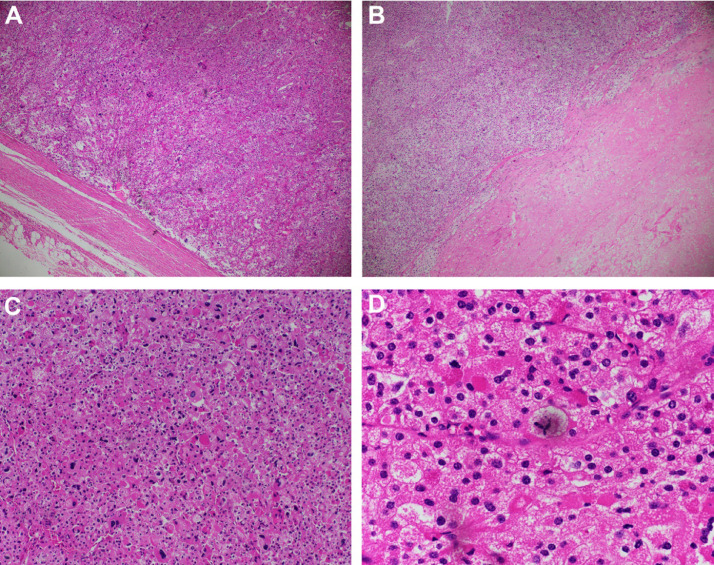
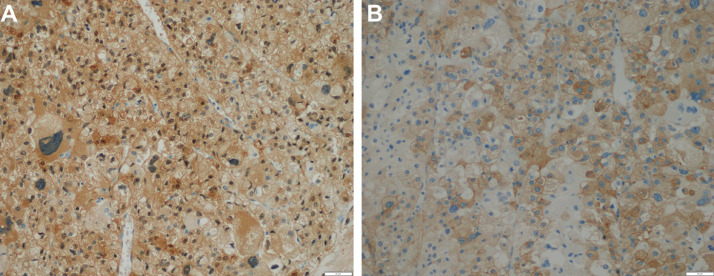
A 32-year-old male with a normal medical history presented with left, upper-quadrant, dull pain for 1 month. An abdominal examination revealed a large mass located in the left upper quadrant. An abdominal computed tomography (CT) scan was performed. On the pre-contrast phase CT, a large mass was identified, situated in the left adrenal gland, measuring 12 × 12 × 11 cm3. The mass had a heterogeneous density, with an average density of 35 Hounsfield (HU). The lowest density was recorded at −18 HU, whereas the highest density was 60 HU (Fig. 1). On the arterial phase, the mass appeared hyper vascular (Fig. 2A), with heterogeneous enhancement (Fig. 2B). The mass compressed the left kidney (Fig. 2C) and the left renal vein, resulting in the dilatation of the left gonadal vein (Fig. 2D). The mass showed a slightly elevated level of enhancement in the venous phase (Fig. 3). No abnormal lymph nodes were noticed. This mass was suspected to be a malignant adrenal mass. The patient's serum cortisol level was 350 nmol/L, which is within the normal range [7]. The levels of electrolytes in the blood and the blood pressure were normal, and the patient did not present with the symptoms of Cushing syndrome. The patient underwent total gross tumor resection. Macroscopically, the mass was large and had a thin capsule (Fig. 4). The cut surface showed a heterogeneous mass, with a white to red appearance. An area of hemorrhage was identified, and the adipose tissue presented with a yellow appearance. Microscopically, the tumor had a fibrous capsule (Fig. 5A) and a necrotic region (Fig. 5B). The typical patterns associated with adrenal cortical adenomas were replaced by a broad trabecular appearance and a diffuse architecture, characterized as nuclear grades III and IV, with > 20 mitotic figures/50 high power fields (HPFs, Fig. 5C). No signs of capsular or vascular invasion were observed (Fig. 5A). Atypical mitotic figures were observed (Fig. 5D). The tumor margins were free. Immunohistochemical analysis showed that the tumor cells stained positively for calretinin (Fig. 6A) and synaptophysin (Fig. 6B) and negatively for chromogranin (Fig. 7A), Melan A (Fig. 7B), S100 (Fig 7C); the Ki67 index was > 5% (Fig. 7D). This tumor was determined to be of adrenal cortical origin, with a Weiss score of five. Thus, the final diagnosis for this patient was ACC. The patient was treated with adjuvant chemotherapy, consisting of ondansetron, cisplatin, and etoposide.
Fig. 1.
Axial computed tomography (CT) scan, pre-contrast. The left adrenal mass was heterogeneous, with a low-density of −18 HU and a high density of 60 HU (arrows).
Fig. 2.
Axial (A and B) and coronal reconstruction (C and D) CT scans in the arterial phase. The mass was hypervascular (A, arrowheads), with heterogeneous enhancement (B, arrow). The mass compressed the left kidney (C, arrow) and the left renal vein, resulting in the dilatation of the left gonadal vein (D, arrowheads).
Fig. 3.
Axial CT scan in the venous phase. The mass was heterogeneous, with an elevated level of enhancement in the venous phase (arrows).
Fig. 4.
The cut surface revealed a heterogeneous mass with a white to red appearance. Areas of hemorrhage and adipose tissue were identified.
Fig. 5.
Hematoxylin and eosin (HE) staining. The tumor had a fibrous capsule, without evidence of capsular or vascular invasion (A, × 40), and a necrotic region (B, × 40). The patterns that are typical of adrenal cortical adenomas were replaced by a diffuse trabecular pattern, characterized as nuclear grades III and IV. Greater than 20 mitotic figures/50 HPFs were observed (C, × 100). Atypical mitotic figures were identified (D, × 400).
Fig. 6.
Positive immunohistochemistry results (× 200) for calretinin (A) and synaptophysin (B).
Fig. 7.
Negative immunohistochemistry results for (× 200) (A) chromogranin, (B) Melan A, and (C) S100. (D) The Ki67 index was > 5%.
Discussion
Adrenocortical carcinoma is an uncommon disease that can develop at any age. The underlying causes of most ACCs are unknown, but ACC can be associated with hereditary tumor syndromes, such as Li-Fraumeni syndrome, Lynch syndrome, multiple endocrine neoplasia type one, and familial adenomatous polyposis [8]. Most adrenal tumors are unilateral, although bilateral ACC can also occur [9]. A functioning adrenocortical tumor overproduces the following hormones: cortisol, aldosterone, testosterone, and estrogen. Therefore, functioning ACCs can present with the symptoms associated with several different endocrine syndromes, including Cushing's syndrome and virilization [10]. In contrast, patients with non-functioning ACC may present with a variety of nonspecific symptoms, such as abdominal pain, fatigue, and symptoms related to mass effects. However, the discovery of asymptomatic adrenal masses has become more common due to the development of innovative imaging modalities [11]. The preoperative assessment of basal levels of cortisol, adrenocorticotropic hormone, dehydroepiandrostenedione sulfate, 17-hydroxyprogesterone, testosterone, androstenedione, and estradiol, the performance of a dexamethasone suppression test, and the assessment of urinary free cortisol levels are recommended [3]. These tests may assist in the establishment of the adrenocortical origins of the tumor and provide information regarding the malignant potential of the tumor. They are also necessary to evaluate the risks of postoperative adrenal insufficiency, and these hormones can serve as tumor markers during postoperative follow-up [12]. Imaging plays a vital role in the diagnosis of ACC. Adrenal tumors with a high risk of malignancy are often characterized by large tumor size (> 4 cm), high density (>10 HU), heterogeneity, absolute washout values below 60%, relative washout values below 40%, necrosis, hemorrhage, and ossification [3]. On a CT scan, ACC may appear large, with irregular margins, heterogeneity, an average density > 10 HU, and evidence of calcification, necrosis, and hemorrhage. Approximately 30% of ACC tumors display calcification [13]. On CT scans performed with contrast enhancement, tumors often display heterogeneous enhancement, typically characterized by the increased enhancement of the peripheral regions compared with the central region due to the presence of necrosis [12]. The identification of invasion, venous thrombus, and lymphadenopathy are clearly suggestive of malignancy. On magnetic resonance imaging (MRI), normal adrenal tissue shows low to intermediate signal intensity on T1- and T2-weighted images [3]. MRI can be useful for determining the lipid contents of a tumor through the use of fat-suppression techniques. MRI can, therefore, be used to support the differentiation between adrenal adenoma and ACC. On T1-weighted images, ACCs often appear isointense to hypointense relative to the normal liver parenchyma, and high signal intensity on T1-weighted images can often be observed due to the presence of hemorrhage [5]. ACCs are hyperintense relative to the normal liver parenchyma on T2-weighted images, with heterogeneous enhancement [5]. MRI can also be used to evaluate the presence of invasion and metastasis indicators. Histopathology remains necessary to reach a final and conclusive diagnosis of ACC. Macroscopically, ACCs are typically large and heterogeneous, and the cut surface may appear brown or yellow, depending on the presence of hemorrhage, necrosis, and lipid contents [14]. Microscopically, the patterns associated with ACC, including solid, broad, nested trabecula, replace the patterns that are typical of adrenal cortical adenoma [15]. Thick fibrosis capsules are also associated with carcinoma. Fibrosis brands, tumor necrosis, and hemorrhage are common features. Tumor invasion of both the capsule and vessels and evidence of mitosis are strong predictors of ACC [16]. ACCs express specific markers for steroid-producing cells, such as steroidogenic factor-one, inhibin alpha, Melan A, and calretinin, which can be detected by immunohistochemical staining. ACCs are frequently positive for synaptophysin, chromogranin A [17]. Some diagnostic algorithms have been used to differentiate benign from malignant adrenocortical tumors, such as the Hough and Weiss systems; however, the Weiss system is the most commonly used system [14]. Tumors with a Weiss score ≥ 3 are considered malignant, whereas scores of 0-2 define benign tumors [18]. The Weiss system examines nine different items, including nuclear atypia, atypical mitoses, the mitotic rate, cytoplasmic characteristics, tumor cell architecture, necrosis, invasion of the venous structures, invasion of the sinusoidal structure, and invasion of the tumor capsule [18]. The proliferation index, determined according to the Ki67 immunomarker or mitotic count, can help to define the diagnosis and prognosis of ACC. ACCs characterized with a Ki67 index value ≥ 5% and a mitotic count > 20 mitoses/50 HPF are associated with poor prognosis [19,20]. Surgery is the first-line therapeutic option, with the potential to cure ACC, and adjuvant mitotane is recommended for patients at high risk for recurrence [3]. Moreover, adjuvant chemotherapy, radiation, and targeted therapies have also been used to treat ACC [12]. The 5-year survival of ACC patients may reach as high as 77% [4].
In this case report, the patient was diagnosed with a non-functioning ACC, presenting with nonspecific symptoms and a serum cortisol level within the normal range. At the time of diagnosis, imaging revealed a very large tumor, with a heterogeneous density, including some fat density areas, and an average density greater than 10 HU. These features all suggested a malignant tumor. Histopathology analyses revealed a Ki67 index value >5% and a mitotic count >20 mitoses/50 HPF. Together, these imaging and histopathological features support the diagnosis and treatment planning of a malignant ACC.
Conclusion
Adrenocortical carcinoma is an unusual neoplasm that originates from the adrenal cortex. Patients with non-functioning ACC are often diagnosed late, with large masses. CT scans can be useful for characterizing indeterminate adrenal lesions or lesions suspected of malignancy. Histopathological diagnoses associated with the Weiss score and the Ki67 index can support both treatment and prognosis.
Patient consent
Appropriate written informed consent was obtained for the publication of this case report and accompanying images.
Authors’ contributions
Bui-Van L and Nguyen MD contributed to this article as co-first authors. All authors have read the manuscript and agree to the contents.
Footnotes
Acknowledgements: Self-financed.
Competing interests: The authors do not report any conflicts of interest.
References
- 1.Kostiainen I., Hakaste L., Kejo P., Parviainen H., Laine T., Löyttyniemi E. Adrenocortical carcinoma: presentation and outcome of a contemporary patient series. Endocrine. 2019;65(1):166–174. doi: 10.1007/s12020-019-01918-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerkhofs T.M.A., Verhoeven R.H.A., Van der Zwan J.M., Dieleman J., Kerstens M.N., Links T.P. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49(11):2579–2586. doi: 10.1016/j.ejca.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Stigliano A., Chiodini I., Giordano R., Faggiano A., Canu L., Della Casa S. Management of adrenocortical carcinoma: a consensus statement of the Italian Society of Endocrinology (SIE) J Endocrinol Invest. 2016;39(1):103–121. doi: 10.1007/s40618-015-0349-9. [DOI] [PubMed] [Google Scholar]
- 4.Lucon A.M., Pereira M.A., Mendonça B.B., Zerbini M.C., Saldanha L.B., Arap S. Adrenocortical tumors: results of treatment and study of Weiss's score as a prognostic factor. Rev Hosp Clínicas. 2002;57(6):251–256. doi: 10.1590/s0041-87812002000600002. [DOI] [PubMed] [Google Scholar]
- 5.Bharwani N., Rockall A.G., Sahdev A., Gueorguiev M., Drake W., Grossman A.B. Adrenocortical carcinoma: the range of appearances on CT and MRI. Am J Roentgenol. 2011;196(6):W706–W714. doi: 10.2214/AJR.10.5540. [DOI] [PubMed] [Google Scholar]
- 6.Erickson L.A. Challenges in surgical pathology of adrenocortical tumours. Histopathology. 2018;72(1):82–96. doi: 10.1111/his.13255. [DOI] [PubMed] [Google Scholar]
- 7.El-Farhan N., Rees D.A., Evans C. Measuring cortisol in serum, urine and saliva – are our assays good enough? Ann Clin Biochem. 2017;54(3):308–322. doi: 10.1177/0004563216687335. [DOI] [PubMed] [Google Scholar]
- 8.Fassnacht M., Libé R., Kroiss M., Allolio B. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol. 2011;7(6):323–335. doi: 10.1038/nrendo.2010.235. [DOI] [PubMed] [Google Scholar]
- 9.Ozimek A., Diebold J., Linke R., Heyn J., Hallfeldt K., Mussack T. Bilateral primary adrenal non-Hodgkin's lymphoma and primary adrenocortical carcinoma - review of the literature preoperative differentiation of adrenal tumors. Endocr J. 2008;55(4):625–638. doi: 10.1507/endocrj.k08e-035. [DOI] [PubMed] [Google Scholar]
- 10.Puglisi S., Perotti P., Pia A., Reimondo G., Terzolo M. Adrenocortical carcinoma with hypercortisolism. Endocrinol Metab Clin North Am. 2018;47(2):395–407. doi: 10.1016/j.ecl.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M., Arlt W., Bancos I., Dralle H., Newell-Price J., Sahdev A. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016;175(2):G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 12.Else T., Kim A.C., Sabolch A., Raymond V.M., Kandathil A., Caoili E.M. Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. doi: 10.1210/er.2013-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H.M., Perrier N.D., Grubbs E.G., Sircar K., Ye Z.X., Lee J.E. CT features and quantification of the characteristics of adrenocortical carcinomas on unenhanced and contrast-enhanced studies. Clin Radiol. 2012;67(1):38–46. doi: 10.1016/j.crad.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Libé R. Adrenocortical carcinoma (ACC): diagnosis, prognosis, and treatment. Front Cell Dev Biol. 2015;3 doi: 10.3389/fcell.2015.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labelle P., Kyles A.E., Farver T.B., de Cock H.E.V. Indicators of malignancy of canine adrenocortical tumors: histopathology and proliferation Index. Vet Pathol. 2004;41(5):490–497. doi: 10.1354/vp.41-5-490. [DOI] [PubMed] [Google Scholar]
- 16.Hoang M.P., Ayala A.G., Albores-Saavedra J. Oncocytic Adrenocortical carcinoma: a morphologic, immunohistochemical and ultrastructural study of four cases. Mod Pathol. 2002;15(9):973–978. doi: 10.1038/modpathol.3880638. [DOI] [PubMed] [Google Scholar]
- 17.Weissferdt A., Phan A., Suster S., Moran C.A. Adrenocortical carcinoma: a comprehensive immunohistochemical study of 40 cases. Appl Immunohistochem Mol Morphol. 2014;22(1):24–30. doi: 10.1097/PAI.0b013e31828a96cf. [DOI] [PubMed] [Google Scholar]
- 18.Weiss L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am J Surg Pathol. 1984;8(3):163–170. doi: 10.1097/00000478-198403000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Beuschlein F., Weigel J., Saeger W., Kroiss M., Wild V., Daffara F. Major prognostic role of ki67 in localized adrenocortical carcinoma after complete resection. J Clin Endocrinol Metab. 2015;100(3):841–849. doi: 10.1210/jc.2014-3182. [DOI] [PubMed] [Google Scholar]
- 20.Miller B.S., Gauger P.G., Hammer G.D., Giordano T.J., Doherty G.M. Proposal for modification of the ENSAT staging system for adrenocortical carcinoma using tumor grade. Langenbecks Arch Surg. 2010;395(7):955–961. doi: 10.1007/s00423-010-0698-y. [DOI] [PubMed] [Google Scholar]