1. Introduction
Fibromyalgia (FM) is a condition characterized by chronic widespread bodily pain and fatigue that remains difficult for patients and clinicians to manage. Successful management strategies for individuals with FM include non-pharmacological and pharmacological strategies [26,48,67]. Recent clinical practice guidelines for chronic pain suggest that exercise is a first-line treatment and has strong evidence for clinical effectiveness [23,37]. While regular physical activity/exercise is an important and effective non-pharmacological treatment for individuals with chronic pain [7,10–12,27], activity-induced pain is a significant barrier to activity participation [8,52,66]. In people with chronic pain, a single bout of fatiguing exercise enhances pain [18,29,58,63]. To address this paradoxical situation, clinicians must employ evidence-based interventions that have the greatest potential to improve participation in exercise and adherence to an exercise program [26].
Transcutaneous Electrical Nerve Stimulation (TENS) is a safe, effective, economical, and readily available non-pharmacological adjunctive intervention to manage pain [13,34,46]. In our recent randomized controlled clinical trial, women with FM showed reduced pain and fatigue at rest and with movement with addition of active-TENS to standard care [17]. After one month of TENS, 44% (45/103) of the study sample showed at least a 30% reduction in pain, and 45% (46/103) of the study sample showed at least a 20% reduction in fatigue [17], which are considered clinically meaningful reductions [3,22]. Identifying characteristics that predict response to TENS would provide a tool to practicing clinicians supporting a prescription for TENS. Thus, the goal of this study was to identify factors that predicted response to TENS using data from the Fibromyalgia Activity Study with TENS (FAST) trial and validate these models using ROC curves.
To further evaluate clinical utility of TENS, comparison of the risks versus benefits of TENS to other treatments can be done by examining the number needed to treat (NNT) and number needed to harm (NNH). Developed in 1988 [33], NNT represents the number of patients one would need to treat in order to get one additional responder when using the active treatment while NNH is a measure of how many people need to be treated for one person to experience an adverse effect [31]. For medications approved by the Food and Drug Administration (FDA) to treat FM, the NNT range between 6 and 19 people for the following three medications: duloxetine (NNT=7.2), pregabalin (NNT=6.6–12), and milnacipran (NNT=11–19) [4,57,60]. For these same medications, the NNH for individual adverse events range from 3.7 to 29 people [15,19]. We hypothesized that TENS would have similar effectiveness, as measured by NNT, and be safer, as measured by the NNH, to the FDA-approved medications for FM.
2. Methods
The current study is a secondary analysis of data from FAST. This phase II randomized, double-blind, placebo controlled dual-site clinical trial conducted was at The University of Iowa and Vanderbilt Medical Center (NCT01888640). Participants recruited from the University of Iowa and Vanderbilt medical Center and surrounding communities completed 4 study visits over a 9-week period (see Figure 1A for protocol). Visit 1 was for screening and consent. At visit 2 movement-evoked pain was measured with the Numeric Rating Scale (NRS) before and during the final minute of a 6-minute walk test. The same assessments were taken following a 30-minute TENS treatment. Baseline measures were assessed at Visit 2. Between visits 2 and 3, participants used active-TENS placebo-TENS or no-TENS at home for one month based on randomized assignments. Between Visits 3 and 4 all subjects used active-TENS for one month. We refer the reader to the published study protocol and primary outcomes manuscripts for additional study details [17,46].
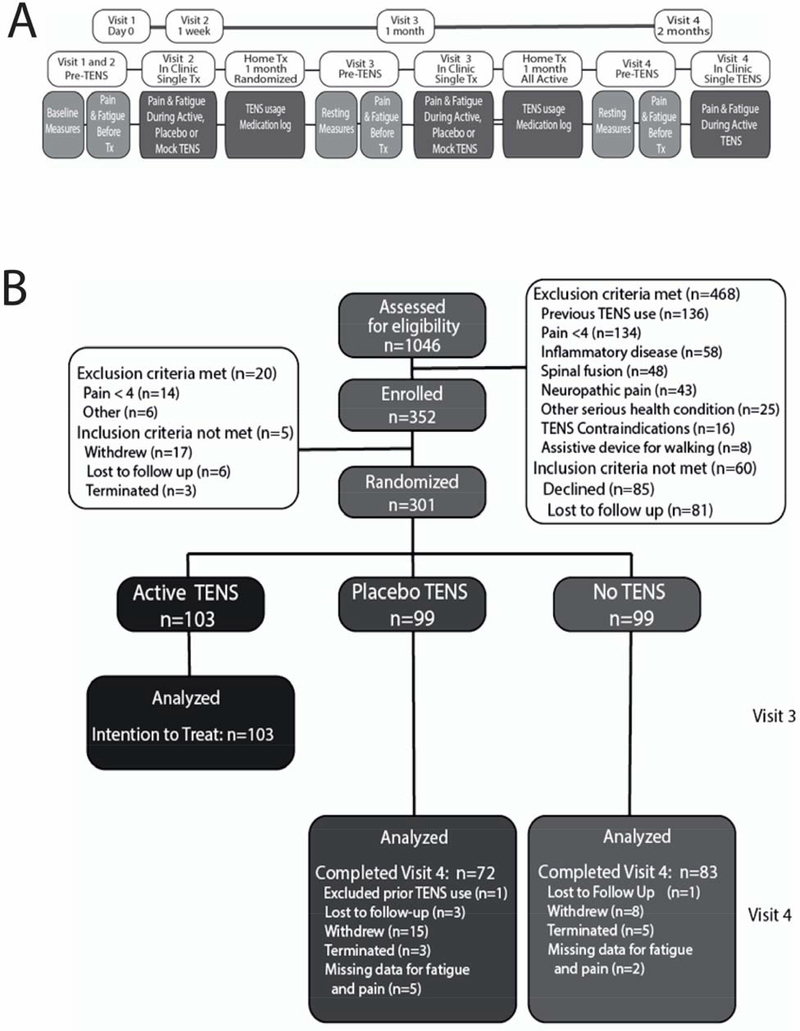
Figure 1.
A. Study Protocol showing the time-course for collections. Reproduced from [17]. B. Consort Diagram for the data used in the current study. The training data set to determine predictors used the active-TENS group with an intention-to-treat analysis with response to TENS data from Visit 3 (n=103). The validation data set used the placebo-TENS and No-TENS groups who completed Visit 4 (n=155). These two groups received active-TENS for 1-month starting at Visit 3 and continuing to Visit 4.
2.1. Participants
Women with FM between 18–70 years were recruited from hospital clinics associated with the study sites as well as from the surrounding communities. Inclusion criteria included meeting the 1990 American College of Rheumatology (ACR) criteria for FM, having a 4-week history of stable medication regimen, female sex, age 18–790 years, and speaking English satisfied inclusion criteria. Participants with self-reported pain ratings of less than 4 on the NRS scale (0–10) on Visit 1 and 2 were excluded to mitigate a “floor effect” for treatment response. Additional exclusion criteria included inability to walk for 6 minutes without assistive device, TENS use in the last 5 years, pacemaker, epilepsy, spinal fusion or metal implants, neuropathic or autoimmune disorder, pregnancy, allergy to nickel, or serious psychiatric or medical condition precluding study participation. Prior to enrollment, participants provided written informed consent.
2.2. Measures
Multiple measures included in the FAST protocol across several domains: pain, fatigue, function, sleep, and psychological characteristics were included in these analyses. The variables examined, along with instrument validity and reliability, are previously published in the study protocol [17,46].
2.2.1. Outcome Measures
Movement-evoked pain and fatigue were measured during (after 5 minutes) the six-minute walk test [59] by asking participants to rate the maximum pain intensity and fatigue level using an 11-point NRS. Movement-evoked pain and fatigue were used to assess responders and non-responders to treatment.
Additional Outcome Measures and Potential Predictors
To further assess pain and characterize the sample, pain intensity and pain interference over the past 24 hours was assessed using the 15 question Brief Pain Inventory (BPI)[61]. Disease impact was assessed with the disease specific Revised Fibromyalgia Impact Questionnaire (FIQR)[6], and quality of life with the 36-item Short-Form Health Survey with physical component score (SF-36 PCS) and mental component score (SF-36 MCS)[39]. American College of Rheumatology (ACR) 2010/2011 classification for FM were assessed and scores were calculated for Widespread Pain Index (WPI) and Symptom Severity scale (SS) and a Fibromyalgia Severity Scale (FS) which represented the WPI and SS added together [68]. Pain catastrophizing, fear of pain with movement, self-efficacy and fatigue were measured with the Pain Catastrophizing Scale (PCS), Tampa Scale of Kinesiophobia (TSK), Pain Self-Efficacy Questionnaire (PSEQ) and the Multidimensional Assessment of Fatigue (MAF)[5,44,49,54,55]. The Patient Reported Outcomes Measurement Information System (PROMIS) short-forms were used to assess anxiety, depression, sleep disturbance and impairment, and satisfaction in social roles and participation [http://www.nihpromis.org/]. The International Physical Activity Questionnaire (IPAQ) assessed self-reported activity [16].
2.3. TENS Intervention Randomization/Allocation/ Blinding
Of the 1046 subjects assessed for eligibility, 352 participants were enrolled with 301 being randomized to one of three treatment groups at Visit 2 to active-TENS [n=103], placebo-TENS [n=99], and No-TENS [n=99]. All active-TENS participants were used to determine responder status (see below) at Visit 3 and to develop the predictor model for pain response and the predictor model for fatigue response (i.e. training data set). After 1 month of randomization, the placebo-TENS and no-TENS groups received active-TENS. Those who completed the final Visit 4 in these two groups, placebo-TENS (n=72) and no-TENS (n=83), were combined (n=155), and used to validate the fitted predictor model identified from the training data set. The CONSORT flow diagram for the study is presented in Figure 1B.
All the assessments were completed by a person (Outcome-Assessor) different to the person allocating and applying the TENS treatment (TENS-Allocator). The Outcome-Assessor was blinded to treatment group while the TENS-Allocator was not blinded to group as they performed the allocation, application, and instructions for home use of TENS. At Visit 2, after randomization, movement-evoked pain and fatigue, and other outcome measures were collected before and during the initial 30-minute TENS treatment. The change from before to during this first TENS treatment provided a measure of the initial response to TENS of movement-evoked pain and fatigue. Similarly, on Visit 3 and Visit 4, movement-evoked pain and fatigue were assessed before and during a single 30-minute TENS treatment.
The TENS intervention protocol has been previously published [17,46]. Briefly, TENS was applied to the upper and lower back with butterfly electrodes using a modulated alternating frequency (2–125 Hz), variable pulse duration, and the highest tolerable intensity. The lumbar and cervical placement were chosen to provide both local and segmental inhibition, and based on our preliminary data testing effects of a single treatment in individuals with TENS [45]. Subjects were instructed to increase the intensity to strong but comfortable. During in Visits 2, 3 and 4, TENS was applied by the TENS allocator in a clinic setting for 30 minutes prior to assessing pain, fatigue and function; the TENS unit remained on for all post-TENS tests. To avoid accommodation to TENS during Visits 2, 3, and 4, the TENS allocator returned to the room every 5 minutes to inquire about patient comfort and to see if they could increase the stimulation intensity. Subjects were instructed to use the TENS unit for 2 hours per day, at least 30 minutes for each session, while doing activities.
The TENS unit captured the number of sessions, total time, and average stimulation intensity of both channels for the 1-month home use by participants. We used this data to determine a per protocol analysis and show similar differences in movement-evoked pain and fatigue to the intention-to-treat analysis; this was reported in the primary manuscript [17].
2.4. Statistical Analysis
A TENS responder was defined as an active-TENS treated participant who experienced at least 30% decrease in movement-evoked pain (pain responder), or a 20% decrease in movement-evoked fatigue (fatigue responder) after 1 month of active TENS use [2, 20].
Candidate variables for inclusion in the responder predictor model for pain and the responder predictor model for fatigue were identified by comparing demographic, and baseline clinical and psychosocial variables between responders and non-responders among those randomized to active-TENS. This was done using two-sample t-test or Wilcoxon rank-sum test for continuous variables, and Pearson Chi-square test for categorical variables. Ordinal variables were compared using Wilcoxon rank-sum exact test. Variables with p-value<0.10 from these tests were then included as predictor variables in the logistic regression analysis that was used to develop two responder predictor models; one for pain and one for fatigue. For model building, a higher p-value (for this study p≤0.10) is used because the more traditional p-value<0.05 can fail in identifying variables known to be important [41]. Even higher p-value<0.25 has been suggested for model building [28] but we decided to use a lower threshold as to not have too many variables in the model for practical clinical application.
The logistic regression analyses involved fitting the models that included various combinations of identified predictor variables [1,28]. In addition, the model fitting process also assessed for linearity with the logit for the continuous independent variables. This was done by creating 4 ordinal categories defined by intervals based on quartiles of the continuous independent variable, which was then used in place of the continuous variable. The logistic regression parameter estimates obtained for this categorized variable were then plotted against the corresponding midpoint of the quartile interval. If this showed a linear trend, then the variable was kept as continuous in the model. Otherwise, the variable was categorized in the model, where adjacent categories with similar estimates (overlapping 95% CI) were combined.
Assessment of the fitted models included, Wald Chi-square tests for the model parameter estimates, Hosmer-Lemeshow test for lack of fit, Akaike information criteria (AIC) for model fit, and cross-validation estimate of the area under the curve of the receiver-operator classification (AUC of the ROC) curve for predictive ability of model. The best models selected from this model development phase were then advanced to the next step for validation. This was done by applying the fitted model to the validation data set to obtain the AUC of the ROC curve.
From the efficacy analysis that compared active-TENS to placebo-TENS and no-TENS groups during the randomized phase, the NTT and NTH was computed from Absolute Risk Difference between the groups. The absolute risk differences is calculated by using the difference in event rates (responder rates for NNT or adverse events for NNH). The NNT and the NNH are the reciprocal the reciprocal of the absolute risk difference.
3.0. Results
During the randomized period of the study, 44% of participants were identified as pain responders and 45% identified as fatigue responders from the training data set for the active-TENS treatment [17]. Demographic and participant characteristics by pain responder status and by fatigue responder status are presented in Table 1. Baseline measures for pain, fatigue, disease severity, quality of life, psychosocial measures, and PROMIS scores of the responder groups are shown in Table 2. A similar proportion of pain responders (46%) and fatigue responders (45%) were identified in the validation data set. Likewise, demographic characteristics and baseline measures of participants in the validation data set were similar to the training data set (Supplemental Tables 1 and 2).
Table 1.
Demographic and Participant Characteristics by Pain and Fatigue Responders at one-month follow-up (Visit 3) using Intention-to-treat for the active-TENS group
| Pain | Fatigue | |||||
|---|---|---|---|---|---|---|
| Responder (≥30%) n=45 |
Non-responder n=58 |
p-value | Responder (≥20%) n=46 |
Non-responder n=57 |
p-value | |
| Age mean (SD) | 41.8 (16.4) | 47.0 (12.2) | 0.078 | 45.9 (14.7) | 43.8 (14.1) | 0.45 |
| Marital status | (n=55) | (n=54) | ||||
| Married/with partner | 16 (36%) | 17 (31%) | 0.62 | 22 (48%) | 11 (20%) | 0.004 |
| Single/widowed/divorced | 29 (64%) | 38 (69%) | 24 (52%) | 43 (80%) | ||
| Education level | ||||||
| High school or less | 6 (13%) | 10 (17%) | 0.80 | 8 (17%) | 8 (14%) | 0.32 |
| Some college/ Vocational/Technical | 21 (47%) | 24 (41%) | 22 (48%) | 23 (40%) | ||
| College degree | 11 (24%) | 14 (24%) | 10 (22%) | 15 (26%) | ||
| Post-graduate degree | 7 (16%) | 10 (17%) | 6 (13%) | 11 (19%) | ||
| Annual income | (n=43) | (n=53) | (n=43) | (n=53) | ||
| < $20K | 14 (33%) | 25 (47%) | 0.20 | 14 (33%) | 25 (47%) | 0.058 |
| $20K-<$60K | 20 (46%) | 16 (30%) | 15 (35%) | 21 (40%) | ||
| ≥$60K | 9 (21%) | 12 (23%) | 14 (32%) | 7 (13%) | ||
| Work status | (n=45) | (n=57) | (n=56) | |||
| Working | 27 (60%) | 29 (51%) | 0.61 | 25 (54%) | 31 (55%) | 0.61 |
| Seeking work/Laid-off | 3 (7%) | 3 (5%) | 4 (9%) | 2 (4%) | ||
| Student/Homemaker/Retired/Other | 15 (33%) | 25 (44%) | 17 (37%) | 23 (41%) | ||
| Body mass index, kg/m2 | 34.4 (8.3) | 35.1 (12.2) | 0.71 | 36.1 (8.2) | 33.7 (9.0) | 0.17 |
| BMI categories | ||||||
| Underweight (<20) | 0 (0%) | 1 (2%) | 0.73 | 0 (0%) | 1 (2%) | 0.54 |
| Normal weight (20-<25) | 8 (18%) | 7 (12%) | 5 (11%) | 10 (18%) | ||
| Overweight (25-<30) | 7 (16%) | 11 (19%) | 7 (15%) | 11 (19%) | ||
| Obese (30–<40) | 18 (40%) | 22 (38%) | 18 (39%) | 22 (39%) | ||
| Morbidly obese (≥40) | 12 (27%) | 17 (29%) | 16 (35%) | 13 (23%) | ||
| ACR 2010 criteria, mean (SD) | (n=44) | (n=45) | ||||
| Widespread pain index | 12.2 (3.7) | 14.1 (3.5) | 0.009 | 12.8 (3.9) | 13.7 (3.5) | 0.21 |
| Somatic symptom score | 8.8 (1.8) | 9.6 (1.9) | 0.030 | 9.1 (1.9) | 9.4 (1.9) | 0.43 |
| Fibromyalgia severity | 21.0 (4.4) | 23.8 (4.7) | 0.003 | 21.9 (4.8) | 23.2 (4.7) | 0.19 |
| Opioid for pain | 9 (20%) | 18 (31%) | 0.21 | 10 (22%) | 17 (30%) | 0.35 |
| TENS dose (minutes/day) | (n=44) 60 (46–92) |
(n=49) 67 (36–87) |
0.92 | 60 (47–93) | (n=47) 61 (39–84) |
0.45 |
| TENS intensity1-lumbar, median(IQR) | (n=44) 38.3 (36.0–40.8) |
(n=50) 37.5 (33.9–41.4) |
0.44 | 38.4 (35.6–40.5) | (n=48) 37.5 (35.6–41.4) |
0.48 |
| TENS intensity2-cervical | 38.0 (36.8–40.7) | 38.3 (37.0–42.0) | 0.76 | 38.3 (37.2–40.5) | 37.3 (36.8–43.5) | 0.96 |
Table 2.
Baseline survey score for pain and fatigue responders and non-responders. Data are presented as mean (SD), median (IQR=25th-75th percentile), or percent of the sample.
| Pain | Fatigue | |||||
|---|---|---|---|---|---|---|
| Responder (≥30%) n=45 |
Non-responder n=58 |
p-value | Responder (≥20%) n=46 |
Non-responder n=57 |
p-value | |
| Pain | ||||||
| Pre-TENS movement pain or fatigue (0–10) | 7 (6–8) | 7 (5–8) | 0.95 | 7.5 (6–8) | 7 (5–8) | 0.16 |
| Initial TENS response to pain or fatigue (% change) | −25 (−33 to −12) | −12 (−22 to 0) | 0.006 | −18 (−29 to −11) | 0 (−20 to 0) | 0.004 |
| BPI Intensity (0–10) | 5.9 (1.4) | 5.8 (1.4) | 0.77 | 5.8 (1.4) | 5.9 (1.4) | 0.71 |
| BPI Interference (0–10) | 6.3 (1.8) | 6.7 (1.9) | 0.23 | 6.2 (1.5) | 6.6 (2.0) | 0.68 |
| Fatigue | ||||||
| MAF GFI (1–50) | 38.3 (32.0–41.8) | 39.8 (34.7–44.1) | 0.16 | 38.9 (32.7–41.8) | 39.6 (33.4–44.1) | 0.25 |
| IPAQ Physical activity level | ||||||
| Low | 18 (40%) | 27 (47%) | 0.70 | 22 (48%) | 23 (40%) | 0.29 |
| Moderate | 15 (33%) | 15 (26%) | 15 (33%) | 15 (26%) | ||
| High | 12 (27%) | 16 (28%) | 9 (20%) | 19 (33%) | ||
| Psychosocial Measures | ||||||
| PCS (0–52) | 24.0 (12.5) | 22.4 (13.5) | 0.55 | 24.5 (13.2) | 21.9 (12.9) | 0.33 |
| PSEQ (0–60) | 31 (21–37) | 29 (14–36) | 0.33 | 27.2 (12.8) | 29.1 (13.7) | 0.48 |
| TSK (17–68) | 35.3 (7.3) | 37.4 (7.9) | 0.17 | 36.8 (7.5) | 36.2 (7.9) | 0.71 |
| Disease State and Quality of Life | ||||||
| FIQR (0–100) | 56.4 (16.6) | 61.4 (16.8) | 0.14 | 57.0 (16.6) | 61.0 (16.9) | 0.23 |
| SF-36 MCS (0–100) | 38.8 (10.4) | 38.6 (9.7) | 0.92 | 40.6 (10.3) | 37.1 (9.5) | 0.076 |
| SF-36 PCS (0–100) | 33.8 (5.9) | 31.7 (6.7) | 0.10 | 31.9 (6.0) | 33.3 (6.7) | 0.25 |
| PROMIS T-score | ||||||
| Anxiety | 58.9 (9.1) | 58.7 (8.5) | 0.90 | 58.7 (8.7) | 58.9 (8.8) | 0.89 |
| Depression | 57.5 (8.7) | 58.5 (7.7) | 0.57 | 58.6 (8.1) | 57.6 (8.1) | 0.57 |
| Satisfaction in Social Participation | 43.4 (7.4) | 41.4 (5.8) | 0.13 | 42.5 (7.3) | 42.2 (6.0 | 0.82 |
| Satisfaction in Social Roles | 38.9 (6.4) | 38.3 (6.7) | 0.65 | 38.9 (7.0) | 38.3 (6.2) | 0.64 |
| Sleep disturbance | 62.0 (9.0) | 61.6 (8.0) | 0.83 | 61.3 (8.1) | 62.2 (8.8) | 0.60 |
| Sleep impairment | 63.0 (7.1) | 64.9 (7.7) | 0.20 | 62.3 (7.3) | 65.5 (7.4) | 0.029 |
From the variables in Table 1 and Table 2, those that were found to differ between pain responders and non-responders were selected for inclusion in the pain responder predictor model. Likewise, the differing variables between fatigue responders and non-responders were selected for inclusion in the fatigue predictor model.
Movement-evoked pain responders had significantly lower ACR 2010/2011 mean scores for WPI (p=0.009), SS (p=0.030), and FS (p=0.003) versus non-responders. Pain responders also had a significant decrease in movement-evoked pain (6MWT; Initial TENS response) during the first in-clinic 30-minute active-TENS treatment (i.e. greater immediate percent change) compared to non-responders (p=0.006) (see Table 2). Age also met the cut-off for possible inclusion as predictor variable with mean age of pain responders 5.2 years younger than non-responders (p=0.08) (Table 1). None of the baseline quality of life or psychosocial measures differed between responders and non-responders.
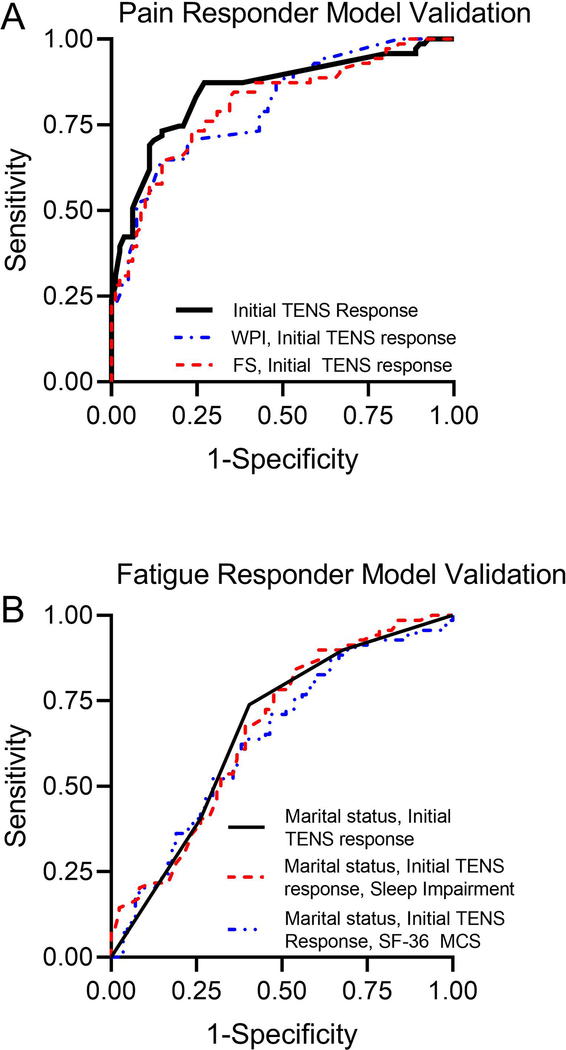
Using these variables (WPI, SS, FS, immediate percentage change in movement-evoked pain, and age), a logistic regression analysis was used to develop a prediction model for TENS pain responders. Since the FS score is the sum of WPI and SS, models with FS and with WPI were separately examined. The parameter estimates and model fit statistics for all the pain responder logistic regression models that were evaluated using the training data are presented in Supplemental Table 3. Of these models, the model with immediate percentage change in movement-evoked pain and WPI (≤13 vs. ≥14; i.e. median split) showed the best fit and highest AUC (0.68; 95%CI: 0.58, 0.79). The model replacing WPI with FS resulted in a slightly smaller AUC (0.67; 95% CI: 0.57, 0.78). The model that included only immediate percentage change in movement-evoked pain (initial TENS response) had ROC of 0.63 (95% CI: 0.52, 0.74).
The parameter estimates and model fit statistics for the selected pain responder predictor models are presented in Table 3. Applying these 3 fitted models to the validation data set resulted in AUC 0.80 (CI: 0.73, 0.87) for model with WPI and initial TENS response for pain, 0.81 (CI: 0.74, 0.88) for model with FM severity score and initial TENS response for pain, and 0.85 (CI: 0.78, 0.91) for model with initial TENS response for pain (Figure 2A, Table 3). Thus, these data show that those with a greater change in movement-evoked pain during the first TENS treatment and those with a lower number of pain areas (WPI score), or lower FS were more likely to respond to TENS with a reduction in pain at one-month follow-up. However, change in movement-evoked pain during the first TENS treatment alone was the best predictor for pain response at one month, and adding WPI or FS to the model did not improve the predictive ability of the model. Based on the model with only initial TENS response for pain as a predictor, obtaining at least a 10% reduction in the initial pain response resulted in 87% sensitivity and 68% specificity for predicting participants achieving at least a 30% decrease in pain at the one month follow up. Positive predictive value was 75% and negative predictive value was 84%, with the cut-off correctly identifying responders and non-responders 77% of the time.
Table 3.
Logistic regression pain responder predictor models and validation model fit statistics
| Model/Parameters | Estimate (SE) | Fit Statistics for Validation Data |
|
|---|---|---|---|
| AIC | AUC (95% CI) | ||
| Model with initial TENS response for pain and WPI score | |||
| Intercept | −1.36 (0.38) | 169.81 | 0.80 (0.73, 0.87) |
| Initial TENS response for pain (% change) | −0.02 (0.010) | ||
| WPI score (≤13 vs. ≥14) | 1.349 (0.442) | ||
| Model with initial TENS response for pain and FM severity (FS) score: | |||
| Intercept | 2.42 (1.126) | 169.04 | 0.81 (0.74, 0.88) |
| Initial TENS response for pain (% change) | −0.02 (0.010) | ||
| Fibromyalgia severity (FS score) | −0.14 (0.049) | ||
| Model with initial TENS response for pain: | |||
| Intercept | −0.63 (0.265) | 168.62 | 0.85 (0.78, 0.91) |
| Initial TENS response for pain (% change) | −0.02 (0.009) | ||
Figure 2.
Receiver operator characteristic (ROC) curves for the predictor models form the validation data set (Visit 4 dataset) for A. Pain. and B. Fatigue. See Table 3 for the summary values for the area under the curve for the ROC.
For fatigue, responders were more likely to be married (p=0.004) and have less sleep impairment (p=0.029) compared to non-responders. Similarly, fatigue responders had greater decreased movement-evoked fatigue (6MWT) during the initial 30-minute TENS treatment (p=0.004). The data also suggested fatigue responders had higher income (p=0.056), and greater SF-36 mental components score (MCS) mean score (p=0.076) (Table 1 and Table 2). Using these variables, logistic regression analysis was used to develop a prediction model for TENS fatigue responder. The parameter estimates and model fit statistics for all the fatigue responder logistic regression models that were evaluated using the training data set are presented in Supplemental Table 4. Of these models, the model with marital status, immediate change in movement-evoked fatigue (initial TENS response to fatigue: decreased vs. no change/increased), and SF-36 MCS score showed the best fit and highest AUC (0.74; 95%CI: 0.64, 0.84). The model replacing MCS score with PROMIS sleep impairment resulted in a similar AUC (0.74; 95% CI: 0.64, 0.84). The model that included only marital status and initial TENS response for fatigue had AUC of 0.65 (95% CI: 0.54, 0.76).
Applying these fitted predictor models for fatigue response to the validation data set resulted in smaller AUC than the training model cross-validation AUC, with AUC of 0.64 (CI: 0.55, 0.73) for model with SF-36 MCS, 0.67 (CI: 0.58, 0.75) for the model with sleep impairment, and 0.66 (CI: 0.58 0.75) for the model with decreased immediate fatigue change, and marital status (Figure 2B, Table 4). These data suggest that those with a decrease in movement-evoked fatigue during the initial TENS treatment, less sleep impairment or higher MCS score, and those who were married were more likely to respond to TENS with a reduction in fatigue. For fatigue, obtaining any degree of reduction in the initial fatigue response resulted in 74% sensitivity and 60% specificity to obtain a 20% reduction in fatigue after 1 month of TENS use. Positive predictive value was 60% and negative predictive value was 74%, which overall correctly identified responders and non-responders 66% of the time.
Table 4.
Logistic regression fatigue responder predictor models and validation model fit statistics
| Model/Parameters | Estimate (SE) | Fit Statistics for Validation Data |
|
|---|---|---|---|
| AIC | AUC (95% CI) | ||
| Model with SF-36 MCS score | |||
| Intercept | −3.12 (1.02) | 230.25 | 0.64 (0.55, 0.73) |
| Married/with partner (vs single/widow/divorce) | 1.34 (0.51) | ||
| Initial TENS response for fatigue (“decreased”) | 1.88 (0.50) | ||
| SF-36 MCS score | 0.04 (0.02) | ||
| Model with PROMIS sleep impairment | |||
| Intercept | 1.24 (2.20) | 221.87 | 0.67 (0.58, 0.75) |
| Married/with partner (vs single/widow/divorce) | 1.32 (0.50) | ||
| Initial TENS response for fatigue (“decreased”) | 1.78 (0.50) | ||
| PROMIS sleep impairment | −0.05 (0.03) | ||
| Model with marital status and initial TENS response for fatigue | |||
| Intercept | −1.76 (0.46) | 218.47 | 0.66 (0.58, 0.75) |
| Married/with partner (vs single/widow/divorce) | 1.39 (0.50) | ||
| Initial TENS response for fatigue (“decreased”) | 1.85 (0.50) | ||
To determine the relative benefits and harms of TENS, we analyzed the NNT and NNH by comparing the results from the active-TENS to the no-TENS group or placebo-TENS group. The NNT was 3.3 for pain compared to no-TENS and 4.5 compared to placebo-TENS (Table 5). The NNT for fatigue was 4.5 when compared to no-TENS and 5.3 compared to placebo-TENS (Table 5). Our previous study showed no serious adverse events [17] and resulted in a NNH for a serious adverse event as non-existent. Fifteen people in the active-TENS group had minor adverse events related to TENS that included skin irritation, itchiness, pain with TENS, nausea, and anxiety. For individual adverse events the NNH ranged between 20 and 100 when compared to either placebo-TENS or no-TENS treatments (Table 5).
Table 5.
Number Needed to Treat (NNT) and Number needed to Harm (NNH) for TENS
| Compared to placebo-TENS | Compared to no-TENS | ||
|---|---|---|---|
| NNT | Pain | 4.5 | 3.3 |
| Fatigue | 5.3 | 4.5 | |
| NNH | Serious adverse events | N/A | N/A |
| Itchiness | 100 | 50 | |
| Anxiety | 50 | 50 | |
| Pain with TENS | 50 | 20 | |
| Nausea | 33 | 50 | |
| Skin Irritation | 25 | 20 | |
| All minor events | 8 | 6 |
4.0. Discussion
The current study for the first time examined predictors of TENS response in women with FM by utilizing a battery of measures, pain characteristics, and patient reported outcomes collected at baseline. We show that the reduction in pain or fatigue during the initial 30-minute TENS treatment predicted who would respond to TENS with a 30% reduction in pain or 20% reduction in fatigue after 1-month of home use. Additionally, the number of pain sites, as measured by the WPI from the 2010/2011 ACR fibromyalgia classification criteria, suggests that those with fewer pain sites are more likely to respond with a reduction in pain. We further show that marital status and sleep predicted a reduction in fatigue with TENS use. The current study showed AUC of ROC curve of 0.85 for pain and 0.66 for fatigue for the initial response to TENS treatment suggesting good discrimination of future responsiveness to TENS. Clinically, examining the response to an initial treatment is simple and thus useful to determine who is most likely to respond to TENS. Our data suggest that clinicians who see a minimum of a 10% reduction in the movement-evoked pain, and any reduction in the movement-evoked fatigue rating during an initial TENS treatment, can predict TENS responders with good sensitivity (87% and 74%, respectively). Similar to TENS, pharmaceutical studies on pain show that early response to treatment predicts later responses to treatment. For duloxetine treatment in FM, the response trajectory for the first 2 weeks, predicted the response to treatment with a 75% positive predictive value and an 85% negative predictive value 3 months later period [65], similar to those observed for pain response to TENS (75% and 84%, respectively). For pain response to opioids in chronic low back pain, early response to a 1-month trial predicted the response 6–12 months later [30]. Thus, these data support that the initial response to a non-pharmacological treatment can predict more long-term response for TENS.
4.1. Patient characteristics may assist in determining if TENS is an effective intervention
ACR 2010/2011 WPI scores predicted TENS responders for decreased pain suggesting that a lower number of pain areas predicted a greater response to TENS. Similarly, in individuals with osteoarthritis, preoperative widespread pain is associated with greater pain after joint replacement [9,70]. Also, those with chronic widespread pain have worse pain and disease activity in inflammatory arthritis conditions [36,56,69] and worse outcomes and greater dysfunction in those with chronic neck and back pain [40,47,64]. Thus, widespread pain is associated with worse outcomes and greater disability.
Greater anxiety, depression and pain catastrophizing are predictors of greater impact of FM symptoms and disability [21,38,43]. A prior study using TENS for postoperative knee pain showed that those with lower anxiety and pain catastrophizing had a greater reduction in movement pain with active-TENS; these effects were not observed for placebo-TENS and a predictor analysis was not performed [51]. In contrast to these findings, the current study did not show that anxiety, depression, fear of movement or pain catastrophizing differed between individuals who responded to treatment and those who did not. The differences between these studies could be related to the pain condition (acute postoperative pain vs. chronic widespread pain), the outcome measure (range of motion pain vs. movement-evoked pain with 6MWT), or the measure of anxiety or depression (Trait Anxiety Scale, Geriatric Depression Scale vs. PROMIS). Similar to the current study, the lack of predictive ability of psychosocial variables was also observed in a trial testing the non-pharmacological interventions of exercise and education [32], and the effect of duloxetine was independent of the effect on mood and anxiety [2]. On the other hand, milnacipran is more effective in those without depression [2]. Thus, widespread pain, but not psychosocial variables, can predict response to TENS as well as poorer outcomes in other diseases.
Interestingly, this study showed that less sleep impairment predicted who will respond to TENS with a reduction in perceived fatigue to a movement task. Home use of TENS also reduced resting perceived fatigue at rest using a numeric rating scale and perceived fatigue using a subjective questionnaire [17]. Fatigue is a significant symptom in individuals with FM, interferes with daily activities and is multifactorial. Sleep alone is a strong predictor of fatigue in those with FM [14,35,62]. For example, a path analysis showed that pain contributed to lower sleep quality which led to greater fatigue [43]. Other predictors of worse fatigue include daily increases in negative events [50] and lower positive affect [71]. Interestingly, one retrospective study in individuals with chronic low back pain showed that those who adhered to TENS treatment total sleep time measured by actigraphy was improved by 29 minutes [24]. The current study showed that those with less sleep impairment predicted who would respond with a reduction in perceived fatigue to a movement task to TENS suggesting that other treatments may be necessary to impact fatigue in individuals with greater sleep impairment.
Surprisingly, marital status was significantly different between responders and non-responders to TENS for movement-evoked fatigue. The reasons for this are unclear. Prior studies show a relationship between marital status and fatigue in in Japanese workers and individuals with multiple sclerosis [20,42], but not in cancer patients [25,53]. Future studies should further investigate this relationship.
4.2. TENS is safe and effective
The current study shows the NNT to reduce pain for active-TENS was 3.3 compared to no-TENS and 4.5 compared to placebo-TENS for pain. In comparison to pharmaceutical drugs approved for medication by the FDA to treat FM, TENS showed similar or better NNT. Duloxetine showed a NNT of 6–7.2, pregabalin NNT ranges from 6.6–12, and milnacipran ranged from NNT between 11–19 [4,57]. For pregabalin, a recent Cochrane review showed that the NNT was 11 [19]. Milnacipran showed the NNT to range between 6 and 11 [15]. Additionally, we show a NNT for reduction in fatigue, of 4.5 and 5.3 when compared to no-TENS or placebo-TENS. Thus, the NNT for TENS was similar or better to that observed for FDA-approved drugs for FM pain. Further, TENS has similar efficacy on movement-evoked fatigue, a symptom with few effective treatments. Thus, TENS should be considered for treatment of individuals with FM as a treatment option for reduction in movement-evoked pain and fatigue.
When compared to TENS, the NNH was significantly lower for FDA-approved pharmaceutical drugs for FM [15,19,57,60]. For duloxetine in individuals with FM, a systematic review reported the NNH for nausea, somnolence, constipation, and reduced appetite was 6.3, 11, 11, and 18 respectively when compared to placebo [60]. For pregabalin in individuals with FM, a Cochrane review showed that the number needed to harm for dizziness, somnolence, weight gain, and peripheral edema, with number needed to harm of 3.7, 7.4, 18, and 19, respectively when compared to placebo [19]. Lastly, for milnacipran for individuals with FM, nausea, constipation, and headache were the most common events with the NNH of 5.7, 13, and 29, respectively [15]. In comparison, the NNH for TENS in individuals with FM for the most common side effects ranged between 25 and 100 and included, nausea, pain with TENS, itchiness, skin irritation and anxiety. The NNH calculations are not representative of responders or non-responders, nor of a particular outcome such pain or fatigue, as the adverse events were collected in response to active TENS and compared to placebo or no TENS were used in the calculation. Thus, these data support that TENS may be safer for pain reduction than commonly used pharmaceutical agents for individuals with FM.
4.3. Conclusion
In summary, the change in movement-evoked pain and fatigue from the first treatment with TENS predicts who will respond one-month later. Other factors that predicted outcomes were the number of pain areas at baseline for pain reduction to TENS, and sleep impairment and marital status for fatigue. Determining if there is a pain or fatigue reduction to a single treatment of TENS is clinically useful and could help to determine who will benefit from TENS. We found that a 10% or greater reduction in movement-evoked pain during 6MWT and any reduction in movement-evoked fatigue were useful clinical thresholds. Movement evoked-pain and fatigue using a NRS before and after 30 minutes of TENS can be quickly calculated by clinicians making this a clinically useful predictor. Conversely, these data suggest that those who do not respond to an initial 30-minute TENS trial should be offered other pain management strategies. The NNT was lower, and the NNH was substantially higher, than many approved FDA pharmaceutical agents, showing that TENS is safe and effective. The fact that TENS is easy to use and inexpensive, safe and effective, and response to an initial treatment predicts outcomes of repeated use, we suggest a one 30-minute treatment trial of TENS in individuals with FM to determine who will benefit.
Supplementary Material
Acknowledgements/Disclosures
Funded by NIH UM1 AR06338, NIH UM1 AR063381-S1, T32 NS045549-12, and K99AR071517. Study data was collected and managed using REDCap electronic data capture tools hosted at University of Iowa (supported by NIH 54TR001013). FAST data collection was completed at the CTSA at University of Iowa (supported by NIH U54TR001356) and Vanderbilt University (supported by NIH UL1TR000445). Active and placebo TENS units and electrodes were provided by DJO, Inc.
Footnotes
Conflicts of Interest/Disclosure statement:
Kathleen A. Sluka, PT, PhD, FAPTA serves as a consultant for Novartis Consumer Healthcare/GSK Consumer Healthcare and Regeneron, Inc. Dr. Sluka receives royalties from IASP Press. The remaining authors of this manuscript have no conflicts of interest that compromise the integrity of this work.
Trial Number: NCT01888640
References
- [1].Allison PD. Logistic Regression Using SAS: Theory and Application, 2nd Ed. Cary, NC: SAS Institute Inc., 2012. [Google Scholar]
- [2].Arnold LM, Clauw DJ, Wohlreich MM, Wang F, Ahl J, Gaynor PJ, Chappell AS. Efficacy of duloxetine in patients with fibromyalgia: pooled analysis of 4 placebo-controlled clinical trials. Prim Care Companion J Clin Psychiatry 2009;11(5):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arnold LM, Williams DA, Hudson JI, Martin SA, Clauw DJ, Crofford LJ, Wang F, Emir B, Lai C, Zablocki R, Mease PJ. Development of responder definitions for fibromyalgia clinical trials. Arthritis and rheumatism 2012;64(3):885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bellato E, Marini E, Castoldi F, Barbasetti N, Mattei L, Bonasia DE, Blonna D. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain research and treatment 2012;2012:426130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Belza BL. Comparison of self-reported fatigue in rheumatoid arthritis and controls. The Journal of rheumatology 1995;22(4):639–643. [PubMed] [Google Scholar]
- [6].Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis research & therapy 2009;11(4):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev 2014;10(1):45–79. [DOI] [PubMed] [Google Scholar]
- [8].Brittain DR, Gyurcsik NC, McElroy M, Hillard SA. General and arthritis-specific barriers to moderate physical activity in women with arthritis. Womens Health Issues 2011;21(1):57–63. [DOI] [PubMed] [Google Scholar]
- [9].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67(5):1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev 2007(4):CD003786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Busch AJ, Webber SC, Brachaniec M, Bidonde J, Bello-Haas VD, Danyliw AD, Overend TJ, Richards RS, Sawant A, Schachter CL. Exercise therapy for fibromyalgia. Curr Pain Headache Rep 2011;15(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Busch AJ, Webber SC, Richards RS, Bidonde J, Schachter CL, Schafer LA, Danyliw A, Sawant A, Dal Bello-Haas V, Rader T, Overend TJ. Resistance exercise training for fibromyalgia. Cochrane Database Syst Rev 2013;12:CD010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carbonario F, Matsutani LA, Yuan SL, Marques AP. Effectiveness of high-frequency transcutaneous electrical nerve stimulation at tender points as adjuvant therapy for patients with fibromyalgia. European journal of physical and rehabilitation medicine 2013;49(2):197–204. [PubMed] [Google Scholar]
- [14].Choy EH. The role of sleep in pain and fibromyalgia. Nature reviews Rheumatology 2015;11(9):513–520. [DOI] [PubMed] [Google Scholar]
- [15].Cording M, Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for pain in fibromyalgia in adults. The Cochrane database of systematic reviews 2015(10):Cd008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise 2003;35(8):1381–1395. [DOI] [PubMed] [Google Scholar]
- [17].Dailey DL, Vance CGT, Rakel BA, Zimmerman MB, Embree J, Merriwether EN, Geasland KM, Chimenti R, Williams JM, Golchha M, Crofford LJ, Sluka KA. Transcutaneous Electrical Nerve Stimulation Reduces Movement-Evoked Pain and Fatigue: A Randomized, Controlled Trial. Arthritis Rheumatol 2020;72(5):824–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Damsgard E, Thrane G, Anke A, Fors T, Roe C. Activity-related pain in patients with chronic musculoskeletal disorders. Disabil Rehabil 2010;32(17):1428–1437. [DOI] [PubMed] [Google Scholar]
- [19].Derry S, Cording M, Wiffen PJ, Law S, Phillips T, Moore RA. Pregabalin for pain in fibromyalgia in adults. The Cochrane database of systematic reviews 2016;9:Cd011790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dochi M, Suwazono Y, Oishi M, Sakata K, Kobayashi E, Nogawa K. The relation between cumulative fatigue and marital status in Japanese workers. Behav Med 2007;33(2):55–65. [DOI] [PubMed] [Google Scholar]
- [21].Edwards RR, Bingham CO 3rd, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis and rheumatism 2006;55(2):325–332. [DOI] [PubMed] [Google Scholar]
- [22].Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- [23].Fitzcharles MA, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choiniere M, Ko G, Moulin DE, Panopalis P, Proulx J, Shir Y. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Res Manag 2013;18(3):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gozani SN, Ferree TC, Moynihan M, Kong X. Impact of transcutaneous electrical nerve stimulation on sleep in chronic low back pain: a real-world retrospective cohort study. Journal of pain research 2019;12:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Haghighat S, Akbari ME, Holakouei K, Rahimi A, Montazeri A. Factors predicting fatigue in breast cancer patients. Support Care Cancer 2003;11(8):533–538. [DOI] [PubMed] [Google Scholar]
- [26].Hauser W, Perrot S, Clauw DJ, Fitzcharles MA. Unravelling Fibromyalgia-Steps Toward Individualized Management. The journal of pain : official journal of the American Pain Society 2018;19(2):125–134. [DOI] [PubMed] [Google Scholar]
- [27].Hoeger Bement MK, Sluka KA. Exercise-induced hypoalgesia: An Evidence-based review. In: Sluka KA, editor. Pain Mechanisms and Management for the Physical Therapist. Philadelphia: Wolters Kluwer, 2016. pp. 177–202. [Google Scholar]
- [28].Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley, 2000. [Google Scholar]
- [29].Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain 2007;11(1):39–47. [DOI] [PubMed] [Google Scholar]
- [30].Kalso E, Simpson KH, Slappendel R, Dejonckheere J, Richarz U. Predicting long-term response to strong opioids in patients with low back pain: findings from a randomized, controlled trial of transdermal fentanyl and morphine. BMC medicine 2007;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Katz N, Paillard FC, Van Inwegen R. A review of the use of the number needed to treat to evaluate the efficacy of analgesics. The journal of pain : official journal of the American Pain Society 2015;16(2):116–123. [DOI] [PubMed] [Google Scholar]
- [32].King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. The Journal of rheumatology 2002;29(12):2620–2627. [PubMed] [Google Scholar]
- [33].Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. The New England journal of medicine 1988;318(26):1728–1733. [DOI] [PubMed] [Google Scholar]
- [34].Lauretti GR, Chubaci EF, Mattos AL. Efficacy of the use of two simultaneously TENS devices for fibromyalgia pain. Rheumatology international 2013;33(8):2117–2122. [DOI] [PubMed] [Google Scholar]
- [35].Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of selective slow wave sleep disruption on musculoskeletal pain and fatigue in middle aged women. The Journal of rheumatology 1999;26(7):1586–1592. [PubMed] [Google Scholar]
- [36].Macfarlane GJ, Barnish MS, Pathan E, Martin KR, Haywood KL, Siebert S, Packham J, Atzeni F, Jones GT. Co-Occurrence and Characteristics of Patients With Axial Spondyloarthritis Who Meet Criteria for Fibromyalgia: Results From a UK National Register. Arthritis & rheumatology (Hoboken, NJ) 2017;69(11):2144–2150. [DOI] [PubMed] [Google Scholar]
- [37].Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, Choy E, Kosek E, Amris K, Branco J, Dincer F, Leino-Arjas P, Longley K, McCarthy GM, Makri S, Perrot S, Sarzi-Puttini P, Taylor A, Jones GT. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 2017;76(2):318–328. [DOI] [PubMed] [Google Scholar]
- [38].Maurel S, Calvo N, Saez-Francas N, Alegre J, Castro-Marrero J. Association between psychological constructs and physical and emotional distress in individuals with fibromyalgia. Clinical and experimental rheumatology 2020. [DOI] [PubMed] [Google Scholar]
- [39].McHorney CA, Ware JE Jr., Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care 1994;32(1):40–66. [DOI] [PubMed] [Google Scholar]
- [40].McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, Bortsov AV, Bair E. Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. Pain 2014;155(2):309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol 1989;129(1):125–137. [DOI] [PubMed] [Google Scholar]
- [42].Mollaoglu M, Ustun E. Fatigue in multiple sclerosis patients. J Clin Nurs 2009;18(9):1231–1238. [DOI] [PubMed] [Google Scholar]
- [43].Nicassio PM, Moxham EG, Schuman CE, Gevirtz RN. The contribution of pain, reported sleep quality, and depressive symptoms to fatigue in fibromyalgia. Pain 2002;100(3):271–279. [DOI] [PubMed] [Google Scholar]
- [44].Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain 2007;11(2):153–163. [DOI] [PubMed] [Google Scholar]
- [45].Noehren B, Dailey DL, Rakel BA, Vance CG, Zimmerman MB, Crofford LJ, Sluka KA. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Phys Ther 2015;95(1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Noehren B, Dailey DL, Rakel BA, Vance CG, Zimmerman MB, Crofford LJ, Sluka KA. Effect of transcutaneous electrical nerve stimulation on pain, function, and quality of life in fibromyalgia: a double-blind randomized clinical trial. Physical therapy 2015;95(1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nordeman L, Gunnarsson R, Mannerkorpi K. Prevalence and characteristics of widespread pain in female primary health care patients with chronic low back pain. Clin J Pain 2012;28(1):65–72. [DOI] [PubMed] [Google Scholar]
- [48].Nuesch E, Hauser W, Bernardy K, Barth J, Juni P. Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: network meta-analysis. Annals of the rheumatic diseases 2013;72(6):955–962. [DOI] [PubMed] [Google Scholar]
- [49].Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. Journal of behavioral medicine 2000;23(4):351–365. [DOI] [PubMed] [Google Scholar]
- [50].Parrish BP, Zautra AJ, Davis MC. The role of positive and negative interpersonal events on daily fatigue in women with fibromyalgia, rheumatoid arthritis, and osteoarthritis. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 2008;27(6):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rakel BA, Zimmerman MB, Geasland K, Embree J, Clark CR, Noiseux NO, Callaghan JJ, Herr K, Walsh D, Sluka KA. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain 2014;155(12):2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rasinaho M, Hirvensalo M, Leinonen R, Lintunen T, Rantanen T. Motives for and barriers to physical activity among older adults with mobility limitations. J Aging Phys Act 2007;15(1):90–102. [DOI] [PubMed] [Google Scholar]
- [53].Respini D, Jacobsen PB, Thors C, Tralongo P, Balducci L. The prevalence and correlates of fatigue in older cancer patients. Crit Rev Oncol Hematol 2003;47(3):273–279. [DOI] [PubMed] [Google Scholar]
- [54].Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain 2004;8(5):495–502. [DOI] [PubMed] [Google Scholar]
- [55].Roelofs J, Sluiter JK, Frings-Dresen MH, Goossens M, Thibault P, Boersma K, Vlaeyen JW. Fear of movement and (re)injury in chronic musculoskeletal pain: Evidence for an invariant two-factor model of the Tampa Scale for Kinesiophobia across pain diagnoses and Dutch, Swedish, and Canadian samples. Pain 2007;131(1–2):181–190. [DOI] [PubMed] [Google Scholar]
- [56].Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8(4):283–291. [DOI] [PubMed] [Google Scholar]
- [57].Serra EA, M. Pharmacotherapy of Fibromyaliga: Focus on Duloxetine. Clinical Medicine Insights:: Therapeutics 2009;1:1617–1627. [Google Scholar]
- [58].Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain 2005;118(1–2):176–184. [DOI] [PubMed] [Google Scholar]
- [59].Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Physical therapy 2002;82(2):128–137. [DOI] [PubMed] [Google Scholar]
- [60].Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC neurology 2008;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. The journal of pain : official journal of the American Pain Society 2004;5(2):133–137. [DOI] [PubMed] [Google Scholar]
- [62].Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. Journal of psychosomatic research 2007;62(2):145–151. [DOI] [PubMed] [Google Scholar]
- [63].Vierck CJ Jr., Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain 2001;2(6):334–344. [DOI] [PubMed] [Google Scholar]
- [64].Viniol A, Jegan N, Leonhardt C, Brugger M, Strauch K, Barth J, Baum E, Becker A. Differences between patients with chronic widespread pain and local chronic low back pain in primary care--a comparative cross-sectional analysis. BMC musculoskeletal disorders 2013;14:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang F, Ruberg SJ, Gaynor PJ, Heinloth AN, Arnold LM. Early improvement in pain predicts pain response at endpoint in patients with fibromyalgia. The journal of pain : official journal of the American Pain Society 2011;12(10):1088–1094. [DOI] [PubMed] [Google Scholar]
- [66].Wilcox S, Der AC, Abbott J, Vrazel J, Ramsey C, Sharpe PA, Brady T. Perceived exercise barriers, enablers, and benefits among exercising and nonexercising adults with arthritis: results from a qualitative study. Arthritis Rheum 2006;55(4):616–627. [DOI] [PubMed] [Google Scholar]
- [67].Winkelmann A, Bork H, Bruckle W, Dexl C, Heldmann P, Henningsen P, Krumbein L, Pullwitt V, Schiltenwolf M, Hauser W. [Physiotherapy, occupational therapy and physical therapy in fibromyalgia syndrome : Updated guidelines 2017 and overview of systematic review articles]. Schmerz (Berlin, Germany) 2017;31(3):255–265. [DOI] [PubMed] [Google Scholar]
- [68].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research 2010;62(5):600–610. [DOI] [PubMed] [Google Scholar]
- [69].Wolfe F, Petri M, Alarcon GS, Goldman J, Chakravarty EF, Katz RS, Karlson EW. Fibromyalgia, systemic lupus erythematosus (SLE), and evaluation of SLE activity. The Journal of rheumatology 2009;36(1):82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152(3):566–572. [DOI] [PubMed] [Google Scholar]
- [71].Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain 2007;128(1–2):128–135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.