Abstract
Limited evidence has suggested that terpenes found in Cannabis sativa are analgesic, and could produce an “entourage effect” whereby they modulate cannabinoids to result in improved outcomes. However this hypothesis is controversial, with limited evidence. We thus investigated Cannabis sativa terpenes alone and with the cannabinoid agonist WIN55,212 using in vitro and in vivo approaches. We found that the terpenes α-humulene, geraniol, linalool, and β-pinene produced cannabinoid tetrad behaviors in mice, suggesting cannabimimetic activity. Some behaviors could be blocked by cannabinoid or adenosine receptor antagonists, suggesting a mixed mechanism of action. These behavioral effects were selectively additive with WIN55,212, suggesting terpenes can boost cannabinoid activity. In vitro experiments showed that all terpenes activated the CB1R, while some activated other targets. Our findings suggest that these Cannabis terpenes are multifunctional cannabimimetic ligands that provide conceptual support for the entourage effect hypothesis and could be used to enhance the therapeutic properties of cannabinoids.
Subject terms: Neuroscience, Pain
Introduction
Cannabis sativa is a dioecious plant belonging to the Cannabaceae family, along with another popular plant, Humulus lupulus (hops)1. The plant itself is a “biopharmacy” containing hundreds of phytochemicals2, many with medicinal indications. Of these, the phytocannabinoids and terpenes have been the most studied in regard to their medicinal and therapeutic properties3,4. Terpenes, which are the basic constituents of essential oils found in many plants, have been used for thousands of years for therapeutic purposes. They also provide flavor and aroma for cannabis and other plants. Studies in animal models and humans have identified analgesic, anti-microbial, anti-inflammatory, and similar therapeutic properties for terpene treatment5–7. Phyto-cannabinoids, most notably Δ9-tetrahydrocannabinol (THC), have been the main focus of research for mechanistic and therapeutic studies4. While cannabis contains both of these families of phytochemicals, the terpenes have been less studied than the phytocannabinoids, and the potential interaction between terpenes and phytocannabinoids when the plant is consumed for recreational and medicinal purposes has barely been studied at all.
The hypothesized interactions between various phytocannabinoids and terpenes to produce unique outcomes from either chemical alone is known as the “entourage effect”3,5,8. The evidence for the entourage effect is comprised of deductive reasoning arguments5,9, some clinical suggestions10,11, and a few pre-clinical investigations8,12–14. There is also skepticism within the literature15, and some evidence against cannabinoid and terpene interactions from preclinical studies7,16. It remains unclear whether terpenes can influence the activity of cannabinoids, and if they do, whether this modulation is a result of direct influence on cannabinoid receptors, as with β-caryophyllene13, or indirect modulation via other mechanisms.
If terpenes can be shown to modulate cannabinoid activity, it could provide a powerful tool to improve cannabinoid therapy. The main phytocannabinoids THC and cannabidiol (CBD) work through cannabinoid and non-cannabinoid mechanisms4,17 to evoke therapeutic benefits, most notably treatment for chronic pain18,19. However efficacy in these studies tends to be modest, and THC induces burdensome psychoactive and somatic side effects19–21. If terpene compounds modulate phytocannabinoids like THC, then it might be possible to identify terpenes that maximize the therapeutic efficacy of cannabinoids while reducing unwanted side effects. Therapeutically, this could take the form of specific chemovar plant strains, or purified and defined terpene/cannabinoid mixtures.
In this study we thus aimed to assess the functional and modulatory actions of various terpenes in vivo and in vitro both alone and in combination with an established cannabinoid agonist. Our results establish direct interaction between cannabinoids and terpenes by demonstrating that selected terpenes have poly-pharmacological effects at both cannabinoid and non-cannabinoid receptors, and selectively modulate canonical cannabinoid agonist activity.
Results
Terpenes induce cannabinoid tetrad behaviors in mice
Our first set of experiments sought to determine whether selected terpenes/terpenoids (α-Humulene, β-Pinene, Linalool [terpenoid], Geraniol [terpenoid], and β-Caryophyllene as a putative cannabinoid receptor type 2 [CB2] agonist control) had activity in the cannabinoid tetrad of behaviors mediated by the CB1 receptor: antinociception, hypolocomotion, catalepsy, and hypothermia20. These terpenes were selected based on their quantities in Cannabis sativa, with α-Humulene, β-Pinene, Linalool, and β-Caryophyllene all being found in higher quantities and Geraniol in lower to underdetermined quantities. Although it is not clear if geraniol is present in higher quantities, we nonetheless chose this ligand due to its reported anti-nociceptive activity (e.g.22). Terpenes were administered at several doses (50–200 mg/kg) i.p. and assessed in the tail flick assay in male and female CD-1 mice. As β-Caryophyllene has been identified as a selective CB2 agonist, and induced CB2-mediated effects at 50 mg/kg13, we administered it at 100 mg/kg as a known CB2 agonist for our behavioral assays. Thus, if selective, it should not exhibit the distinct CB1-mediated tetrad behaviors.
Terpenes induced a range of efficacies in the tail flick assay (Fig. S1A-E). Geraniol and α-Humulene exhibited moderate ~ 40–50% efficacy in a dose-dependent manner (Fig. S1A,D) while β-Pinene showed low efficacy but not in a dose-dependent manner (Fig. S1B), suggesting partial agonist activity at the top of the dose range. Linalool demonstrated dose-dependent low efficacy (Fig. S1C), as did the β-Caryophyllene at the dose tested (Fig. S1E). The positive control WIN55,212-2 demonstrated dose-dependent increases in thermal latency with greater efficacy than any of the tested compounds reaching near-threshold values at a 10 mg/kg dose (Fig. S1F).
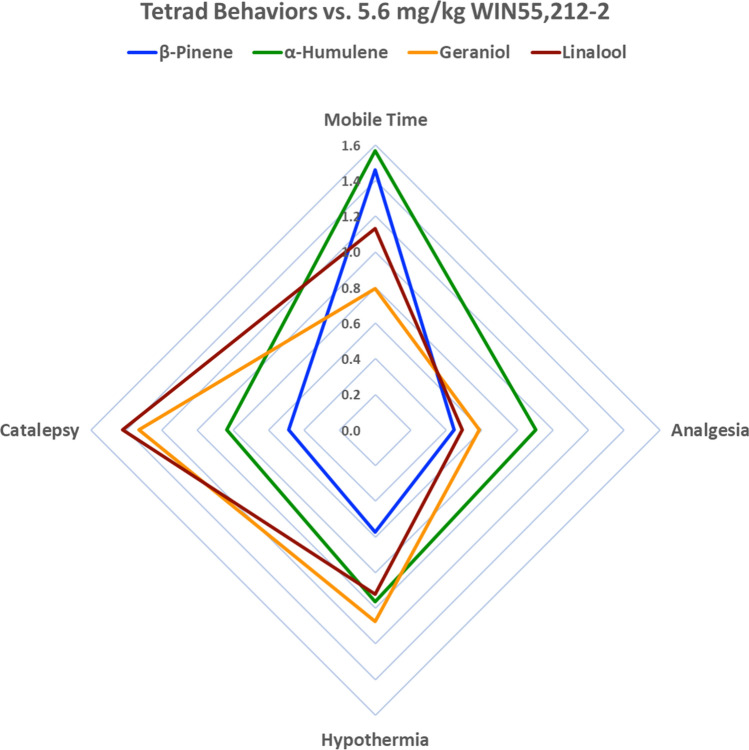
Terpenes were next assessed in the remaining tetrad behaviors during the peak effect window observed in the tail flick assay (i.e. 0–30 min post-injection). α-Humulene, β-Pinene, Geraniol and Linalool, as well as the control WIN55,212-2, induced significant hypothermia (Fig. S2A), significant increases in cataleptic behavior (Fig. S2B), and significant reductions in locomotor activity (Fig. S2C,D) compared to their baseline values. The CB2-selective control, β-Caryophyllene, as well as Vehicle control did not induce hypothermia and catalepsy. Hypolocomotion observations were likely confounded by mouse habituation of the open field boxes between baseline and post-injection measurements, as shown in Vehicle treatment in Fig. S2C,D. Therefore, the experiment was repeated without a baseline recording. In this experiment WIN55,212-2, β-Pinene, Geraniol and Linalool all displayed reductions in distance traveled and mobile time, whereas Vehicle, β-Caryophyllene or α-Humulene treatment did not result in significant reductions (Fig. S3A,B). However, α-Humulene did trend towards significance (p = 0.057). Together these results suggest that our terpenes are cannabimimetic, inducing at least 3 out of 4 of the classic cannabinoid tetrad behaviors each, while Vehicle and β-Caryophyllene controls did not. A radar chart depiction of the impact of the different terpenes on tetrad behavior is shown in Fig. 1.
Figure 1.
Terpenes Induce Cannabimimetic Tetrad Behaviors in Mice. Behavioral analysis data from Figs. S1-S3 represented in a radar chart format. The percent change from baseline for each terpene in each behavior was determined; the peak effect for tail flick was selected (Fig. S1) while the other behaviors only had a single time point. This percent change was then normalized to the % change of 5.6 mg/kg WIN55,212-2 (1.0 on the chart above). The chart demonstrates that while all terpenes induce all 4 tetrad behaviors, they do so with varying efficacy; they also vary whether the effect is stronger or weaker than the positive control WIN55,212-2.
Terpene tail flick antinociception is CB1 mediated and is additive with cannabinoid
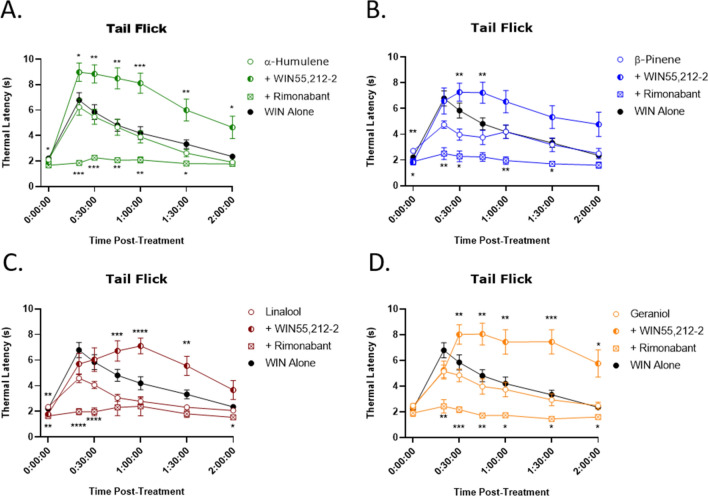
To determine the role of the CB1 receptor in potentially mediating these tetrad behaviors induced by terpenes we used the CB1 selective antagonist/inverse agonist rimonabant. We first showed that rimonabant could fully or partially reverse the tetrad behaviors induced by the positive control cannabinoid WIN55,212-2 (Fig. S4). We then used this drug in terpene tail flick anti-nociception, and showed that rimonabant pretreatment fully blocked terpene response in this assay, suggesting that terpenes induce tail flick anti-nociception via the CB1 (Fig. 2).
Figure 2.
Terpenes Induce CB1 Dependent and Cannabinoid Additive Antinociception in the Tail Flick Assay. Mice were treated with 200 mg/kg terpene alone, combined with 5.6 mg/kg WIN55,212-2, or after pretreatment with 10 mg/kg rimonabant, all injected i.p.. Mice were then assessed in the tail flick test over 2 h. (A) α-Humulene, (B) β-Pinene, (C) Linalool and (D) Geraniol. Data represents the mean ± SEM of tail flick latency (n = 10–14/group). Statistics analyzed via RM two-way ANOVA, Dunnett’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared to terpene alone at same time point. The animal response to 5.6 mg/kg WIN55,212-2 (“WIN Alone”) from Fig. S1 is included in each graph for reference.
In order to test potential terpene/cannabinoid interaction, terpene was combined with WIN55,212-2 in the tail flick assay. When a given terpene was combined with a lower dose of WIN55,212-2, the combined effect was increased compared to terpene or WIN55,212-2 alone (Fig. 2), demonstrating a terpene/cannabinoid interaction in modulating antinociception. Whether this interaction is additive or synergistic in nature is currently under investigation.
However, as an inverse agonist, rimonabant has the potential to reverse tail flick responses through a systems level inverse agonism effect on antinociception. In other words, rimonabant could demonstrate blockade of antinociception through pro-nociceptive activity. To control for this, we assessed the effect of rimonabant pretreatment on morphine-induced antinociception in the tail flick assay (Fig. S5A,B), at an equal-efficacy dose compared to our terpenes, and on baseline thermal latency responses at a reduced water bath temperature (Fig. S5C). Rimonabant had no significant effect on morphine-induced antinociception or baseline thermal latency responses, further suggesting terpenes mediate their antinociceptive actions through a CB1-dependent mechanism.
Terpene hypothermia is additive with cannabinoid but mostly not mediated by CB1
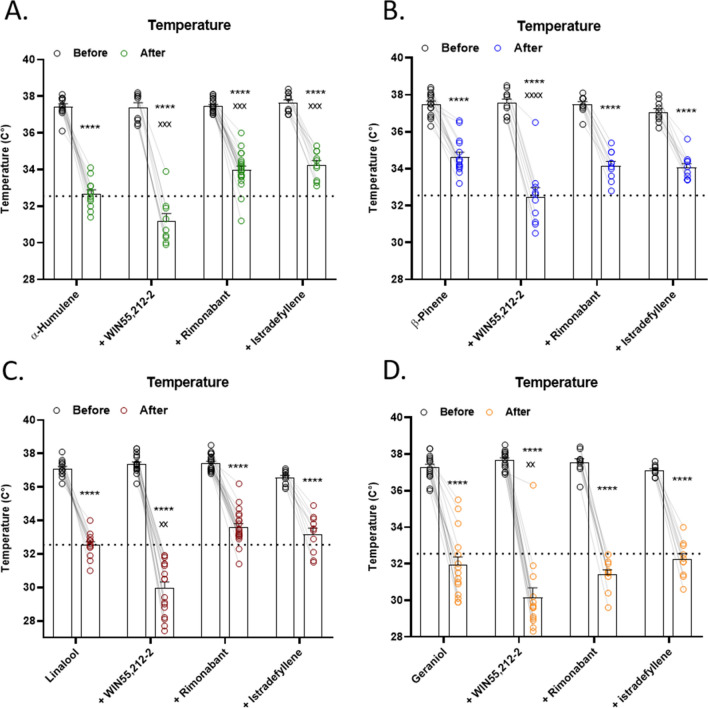
Following the experimental design used for tail flick antinociception above, we next sought to determine the mechanisms of terpene-induced hypothermia. First, when we co-injected both terpene and WIN55,212-2, hypothermia was increased over either treatment alone for all terpenes tested (Fig. 3). This is similar to the finding for tail flick anti-nociception, and further lends support to terpene/cannabinoid interaction.
Figure 3.
Terpenes Induce Hypothermia Through Mostly Non-CB1/A2a Mechanisms and are Additive with Cannabinoid. Mice were baselined for temperature, injected with 200 mg/kg terpene alone, combined with 5.6 mg/kg WIN55,212-2, or after pretreatment with 10 mg/kg rimonabant or 10 mg/kg istradefyllene. After 30 min, temperature was assessed again. (A) α-Humulene, (B) β-Pinene, (C) Linalool and (D) Geraniol. Data represents the mean ± SEM temperature (n = 10–20/group). Statistics analyzed via RM two-way ANOVA, Tukey’s post hoc; ****p < 0.0001 compared to each baseline; xx p < 0.01, xxx p < 0.001, xxxx p < 0.0001 compared to terpene post-treatment. The dashed line represents the hypothermic response to 5.6 mg/kg WIN55,212-2 alone, taken from Fig. S2A.
However, unlike for tail flick, rimonabant treatment was only able to partially reverse α-humulene hypothermia (Fig. 3A), and had no effect on the other terpenes (Fig. 3B-D). While as shown above rimonabant only partially reversed WIN55,212-2 hypothermia (Fig. S4C), this still suggests that CB1 may only mediate α-humulene hypothermia, and has no role for the other terpenes. This further suggests that while the terpenes are cannabimimetic, they may induce these effects through both CB1-dependent and independent mechanisms.
Seeking to test the involvement of other receptor systems, we tested the role of adenosine A2a receptors (A2a) in terpene-induced hypothermia. Activation of A2a can induce hypothermia, hypolocomotion and cataleptic-like behaviors23 and many studies have started to investigate the interactions between the cannabinoid and adenosine systems24–28. In fact, a known cannabis terpene, D-limonene, is an agonist at the A2a receptor29. We thus hypothesized that activation of A2a receptors may contribute to the rimonabant-insensitive behaviors induced by the studied terpenes. However, pretreatment with the A2a antagonist istradefyllene partially reversed α-humulene hypothermia (Fig. 3A) while having no effect on the other terpenes (Fig. 3B-D), suggesting no role for the A2a much like the CB1 for terpene-induced hypothermia. Further complicating the analysis of the A2a in our behaviors, we found that istradefyllene alone had a small but significant hypothermic and hyperlocomotive effect, suggesting these behaviors be carefully interpreted (Fig. S6).
Terpene catalepsy is partially additive with cannabinoid and mostly mediated by A2a
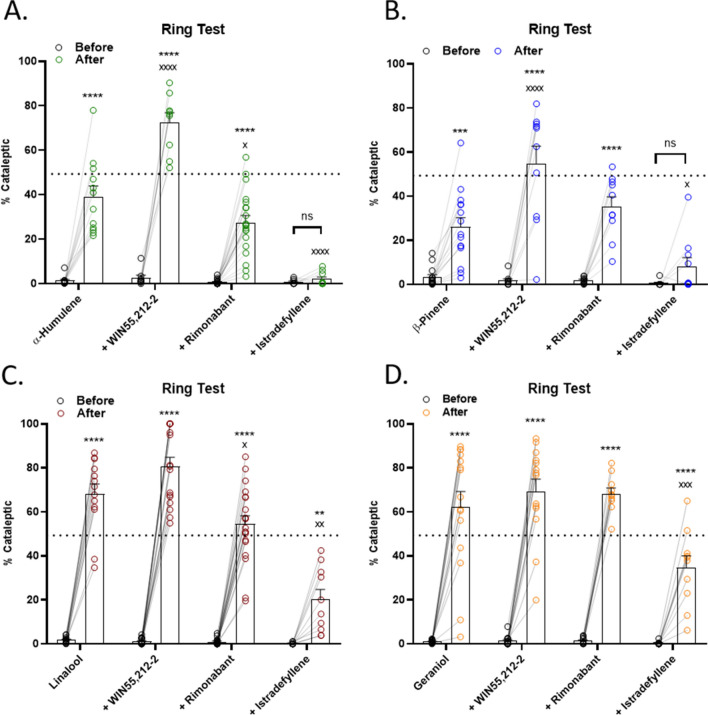
We continued our mechanistic analysis with terpene-induced catalepsy. In contrast to the above findings, combining terpene with WIN55,212-2 did produce additive effects with α-humulene and β-pinene (Fig. 4A-B), but not Linalool and Geraniol (Fig. 4C,D). Here we begin to see differentiation of our terpene/cannabinoid interaction evidence, which suggests that different terpenes could be used to modulate different parts of the cannabinoid response. We also found that rimonabant had no impact on the catalepsy response for Geraniol and β-pinene, and a small but significant reduction for α-humulene and Linalool, suggesting this terpene behavior is mostly CB1-independent (Fig. 4).
Figure 4.
Terpene Induced Catalepsy is Partially Mediated by CB1, Strongly Mediated by A2a, and is Partially Additive with Cannabinoid. Mice were baselined in the ring test for 5 min, injected with 200 mg/kg terpene alone, combined with 5.6 mg/kg WIN55,212-2, or after pretreatment with 10 mg/kg rimonabant or 10 mg/kg istradefyllene. After 15 min, mice were tested in the ring test again for 5 min. (A) α-Humulene, (B) β-Pinene, (C) Linalool and (D) Geraniol. Data represents the mean ± SEM of % catalepsy (n = 10–20/group). Statistics analyzed via RM two-way ANOVA, Tukey’s post hoc; **p < 0.01, ***p < 0.001, ****p < 0.0001, not significant (ns) compared to baseline; x p < 0.05, xx p < 0.01, xxx p < 0.001, xxxx p < 0.0001 compared to terpene post-treatment. The dashed line represents the cataleptic response to 5.6 mg/kg WIN55,212-2 alone, taken from Fig. S2B.
However, we did find a major role for the A2a in this behavior. Istradefyllene pretreatment completely blocked the catalepsy response for α-humulene and β-pinene, suggesting that the A2a is a necessary component of catalepsy for these terpenes (Fig. 4A,B). Istradefyllene also partially blocked catalepsy for Linalool and Geraniol, showing that it is still a significant part of the mechanism for these terpenes (Fig. 4C,D). Our results thus suggest that the A2a has a major role in terpene-induced catalepsy, and that the CB1 has a minor or no role in mediating terpene-induced catalepsy.
Terpene hypolocomotion is partially additive with cannabinoid and partially A2a mediated
Our analysis of mobile time as a measure of hypolocomotion is shown in Fig. 5. We did observe a further decrease in mobile time when terpene was combined with WIN55,212-2 for α-humulene, β-pinene, and Linalool (Fig. 5A-C) but not Geraniol (Fig. 5D). This continues our theme of generally showing terpene/cannabinoid additive effects, albeit with specific behavioral impacts for each terpene. Also of note, rimonabant had no reversal effect on mobile time for any terpene, suggesting this behavior is also CB1-independent. However, we did observe significant reversal with istradefyllene for β-pinene (Fig. 5B) and Geraniol (Fig. 5D), showing that the A2a is mediating this behavior for some terpenes. We also report the distance traveled measurement of hypolocomotion in Fig. S7; this data was in general less robust than the mobile time, although we did observe istradefyllene reversal with β-pinene (Fig. S7B) and Geraniol (Fig. S7D), confirming this finding from Fig. 5.
Figure 5.
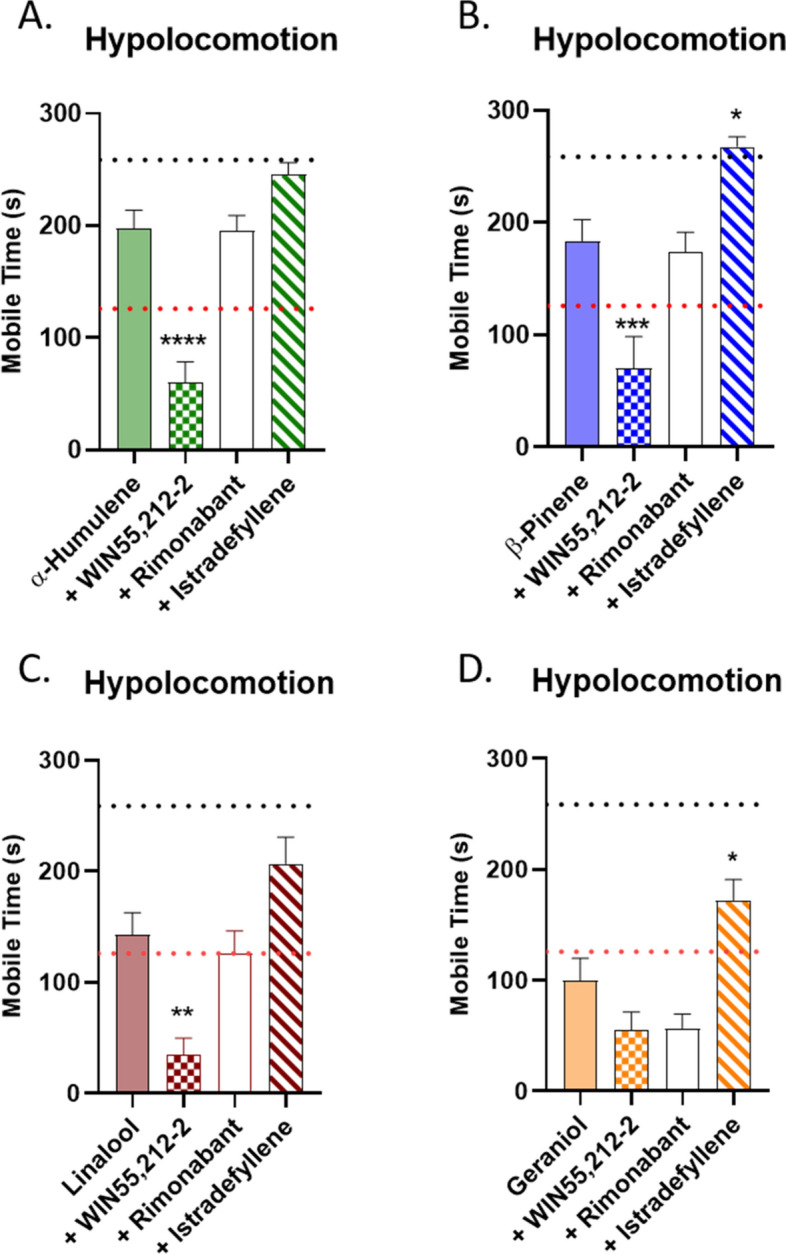
Terpene Induced Hypolocomotion is Partially Mediated by A2a and is Additive with Cannabinoid. Mice were injected with 200 mg/kg terpene alone, combined with 5.6 mg/kg WIN55,212-2, or after pretreatment with 10 mg/kg rimonabant or 10 mg/kg istradefyllene, i.p.. After 10 min mice were then placed back into the open field box for a 5 min test. (A) α-Humulene, (B) β-Pinene, (C) Linalool and (D) Geraniol. Data represents the mean ± SEM of mobile time seconds (n = 10–20/group). Statistics analyzed via one-way ANOVA, Dunnett’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to terpene alone. The black dotted line denotes vehicle levels of mobile time for reference, while the red dotted line represents the effect of 5.6 mg/kg WIN55,212-2 alone, both taken from Fig. S3A.
Overall, our mechanistic studies suggest that the terpenes tested generally although selectively increase the behavioral effects of the cannabinoid WIN55,212-2, supporting the potential modulation of cannabinoids by terpenes. Our findings also suggest a mix of CB1-dependent, A2a-dependent, and independent mechanisms for terpene behavioral effects. These findings overall suggest that Cannabis terpenes can have significant pharmacological effects, and could be used to selectively modulate the impact of Cannabis/cannabinoid therapy.
Linalool has specific sex differences
For all experiments above, both male and female mice were used, and in nearly every case, no differences were observed. However, we did observe specific sex differences for Linalool. First, in the tail flick assay, both males and females had the same response to Linalool alone, and both were fully blocked by rimonabant. However, we observed a sex difference when Linalool was combined with WIN55,212-2; males showed greater additive effects of combining both that occurred earlier in the time course, while females showed a delay in response and no potentiation over Linalool alone (Fig. S8A). For Linalool hypolocomotion, we also observed a mechanistic difference; males showed rimonabant reversal of the behavior while females showed istradefyllene reversal, suggesting this behavior is mediated by CB1 in males and A2a in females (Fig. S8B-C). These observations suggest that other terpene sex differences could be found, although the mechanism for this difference is unknown.
Terpenes activate the CB1 in vitro
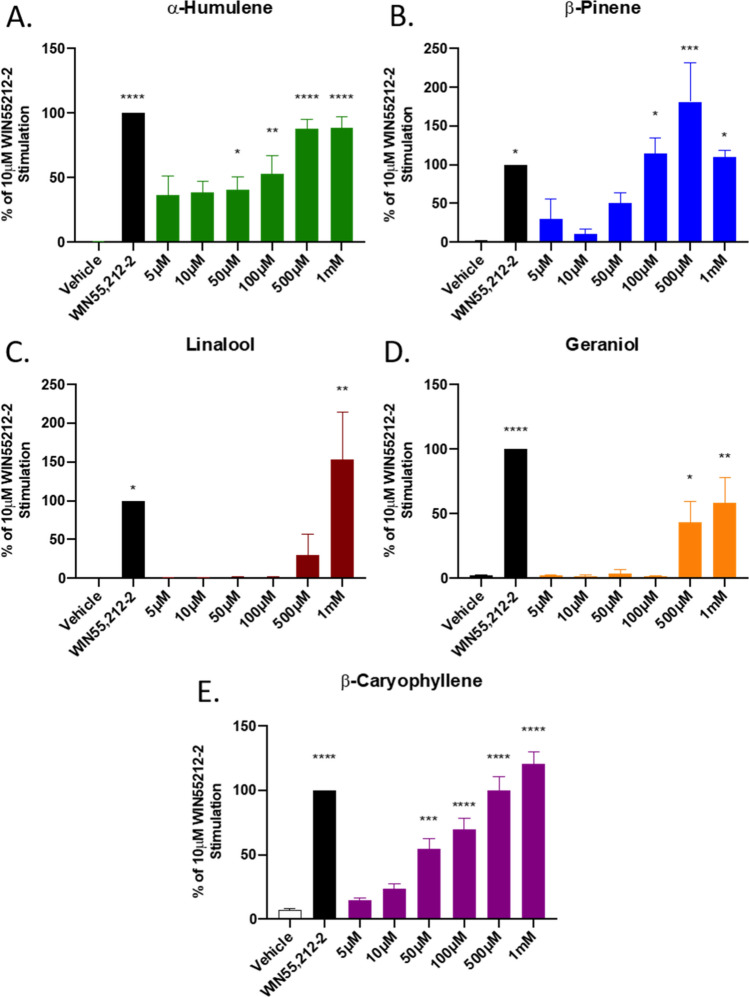
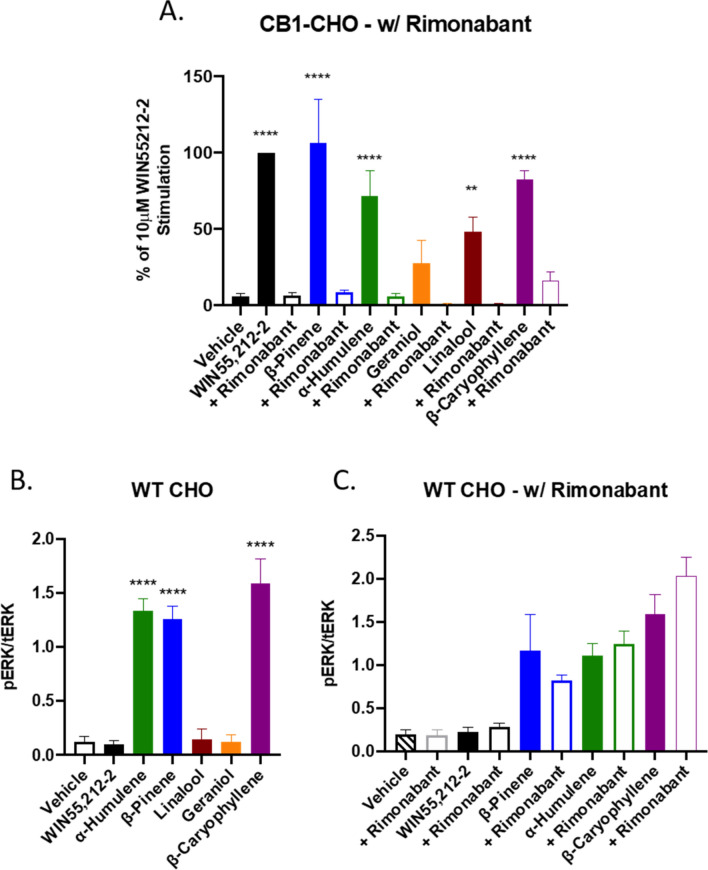
Our behavioral results suggested that the terpenes potentially interact with the CB1 receptor, and likely others. We thus sought to determine whether these selected terpenes acted as CB1 receptor agonists in vitro, first by assessing CB1-dependent ERK activation. Each of the terpenes tested, including the putative CB2 agonist β-Caryophyllene, activated downstream ERK signaling in CB1-CHO cells (Figs. 6, S9). This activation was rimonabant-sensitive (Figs. 7A, S10A,B), suggesting that terpenes act as CB1 agonists in vitro, further supporting our in vivo findings.
Figure 6.
Terpene Treatment Activates the CB1 In Vitro. CB1-CHO cells were serum starved for 1 h then treated with varying concentrations of (A) α-Humulene, (B) β-Pinene, (C) Linalool, (D) Geraniol, (E) β-Caryophyllene, along with 10 μM WIN55,212-2 positive control or matched vehicle control, for 5 min. Lysates were then subjected to Western analysis and blotted for phospho-ERK and total-ERK (see Methods). Graph represents the quantified Western bands (pERK/tERK). Data expressed as a % of WIN55,212-2 stimulation (n = 3 independent experiments). Statistics analyzed via one-way ANOVA, Dunnett’s post hoc; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared to vehicle stimulation.
Figure 7.
Terpenes Induce CB1-Dependent and Independent Signaling In Vitro. (A) CB1-CHO cells were serum starved for 1 h then pretreated with 10 μM rimonabant or vehicle for 5 min. Cells were then treated with 500 μM terpene, 10 μM WIN55,212-2, or matched vehicle, for 5 min. Lysates were then subjected to Western analysis and blotted for phospho-ERK and total-ERK (see Methods). Graph represents the quantitation of ERK phosphorylation induced by terpene and rimonabant combinations (pERK/tERK). Data expressed as a % of WIN55,212-2 stimulation (n = 3 independent experiments for each). (B) WT CHO cells were serum starved for 1 h then treated with 500 μM terpene, 10 μM WIN55,212-2, or matched vehicle, for 5 min, and analyzed as above. (C) WT CHO cells were serum starved for 1 h, pretreated with 10 μM rimonabant or vehicle, then treated with 500 μM terpene, 10 μM WIN55,212-2, or matched vehicle, for 5 min, and analyzed as above. Data analyzed via one-way ANOVA, Dunnett’s post hoc; **p < 0.01, ****p < 0.0001, compared to vehicle stimulation.
However, we found evidence that some terpenes act at other receptors or effectors to activate ERK in vitro when we screened the terpenes in WT-CHO cells presumably lacking any CB receptor (Figs. 7B, S10C). Linalool, Geraniol, and WIN55,212-2 did not cause ERK phosphorylation in WT CHO cells, however, α-Humulene, β-Pinene and β-Caryophyllene activated ERK in a rimonabant-insensitive manner (Figs. 7C, S10D-E). As rimonabant can act as an inverse agonist, potentially confounding the results above, we tested several concentrations of rimonabant against fetal bovine serum (FBS)-induced ERK activation (Fig. S11). Rimonabant did not significantly reduce ERK phosphorylation due to FBS treatment. This evidence suggests that each of the tested terpenes induced phosphorylation of ERK that is dependent on the CB1 receptor while some activated non-CB1 targets in these cells.
Of note, each of the terpenes tested also caused ERK phosphorylation in CB2-expressing cells (Fig. S12), suggesting they may interact with CB2 as already described for β-Caryophyllene13. Together, this evidence supports terpene poly-pharmacology, that can evoke behavioral and cellular changes via CB1-dependent and CB1-independent mechanisms (e.g. Adenosine A2a and CB2 from above).
However, these results could also be explained by non-specific or non-receptor interactions of the terpenes. We thus tested for the ability of the terpenes to activate the mu opioid receptor, in a separate GPCR family from CB1/2. β-pinene, α-humulene, β-caryophyllene, and morphine positive control all produced ERK stimulation in MOR-CHO cells, while geraniol and linalool had no response (Fig. S13A). Notably, these same terpenes produce stimulation in WT-CHO cells above (Fig. 7), suggesting the stimulation may not occur through the opioid receptor. Indeed, the antagonist naloxone was able to block morphine stimulation, but had no impact on terpene stimulation (Fig. S13B). When compared to the CB1-CHO and WT-CHO results above, this experiment suggests that the terpenes cannot activate the mu opioid receptor, providing support that their receptor interaction with CB1/2 is specific and receptor-mediated.
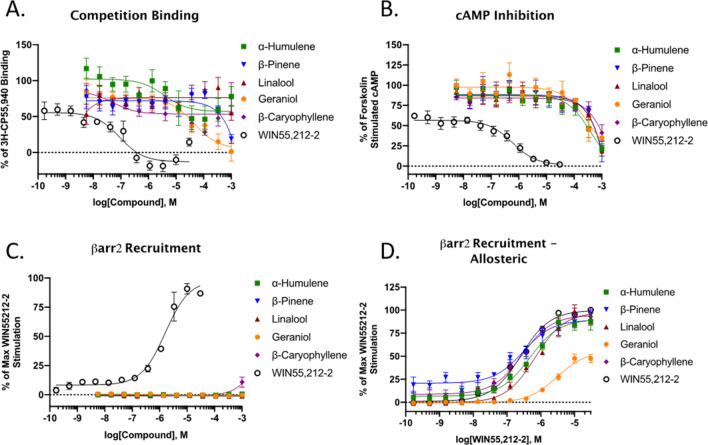
We next followed up with a more comprehensive analysis of the pharmacological properties of each terpene at CB1. We first ran competition binding assays in CB1-CHO membrane preparations to determine whether each would compete for the orthosteric binding site against CP55,940 (Fig. 8A). As shown, WIN55,212-2 induced a typical concentration response-curve, fully competing CP55,940 away at higher concentrations. Of the terpenes assessed, Geraniol was the only one that displayed full competition. α- Humulene and β-Caryophyllene both displayed some competition and semi-biphasic properties. Linalool and β-Pinene displayed little to no competition. These results suggest both orthosteric and potential allosteric binding mechanisms for some of the different terpenes at CB1.
Figure 8.
Binding and Functional Analysis of Terpenes at the CB1. Fitted curve values reported as the mean with the 95% confidence interval. (A) CB1-CHO membranes were subjected to competition binding assay with terpenes and WIN55,212-2 against 3H-CP55,940. Data represents the mean ± SEM of specific binding (n = 5 independent experiments). IC50 values: WIN55,212-2 = 90.4 nM (36–206); Geraniol = 44.2 μM (11.1–193). Other terpenes did not provide competition curves that could be fit. (B) CB1-CHO cells were treated with varying concentrations of terpene or WIN55,212-2 for 30 min. The ability to inhibit forskolin-stimulated cAMP accumulation was measured accordingly (see Methods). Data represents the mean ± SEM of % of forskolin-stimulated cAMP (n = 4 independent experiments). IC50 value: WIN55,212-2 = 591 nM (289–1,180). Terpenes did not provide full inhibition curves. (C) CB1-CHO-DX cells were treated with varying concentrations of compounds and βarrestin2 recruitment was assessed after 1.5 h of treatment (see Methods). Data represents the mean ± SEM of max WIN55,212-2 recruitment (n = 3 independent experiments). EC50 value: WIN55,212-2 = 1,600 nM (1,110–2,310). (D) CB1-CHO-DX cells were pretreated with 500 μM terpene for 5 min, followed by varying concentrations of WIN55,212-2 for 1.5 h (see Methods). Data represents the mean ± SEM of max WIN55,212-2 recruitment (n = 3 independent experiments). EC50 values: WIN55,212-2 alone = 254 nM (194–331); WIN + Geraniol = 3,080 nM (2180–4350); WIN + α-Humulene = 471 nM (295–743); WIN + Linalool = 679 nM (480–956); WIN + β-Pinene = 302 nM (147–621); WIN + β-Caryophyllene = 283 nM (169–470).
Following activation of the CB1 receptor, two downstream signaling pathways can be initiated; inhibition of adenylyl cyclase and β-arrestin2 recruitment. We first looked at the ability of the terpenes to inhibit adenylyl cyclase in CB1-CHO cells. Each of the terpenes tested demonstrated partial inhibition of forskolin-stimulated cAMP but only at high micromolar concentrations, while WIN55,212-2 displayed high potency and full efficacy (Fig. 8B). This effect by WIN55,212-2 and terpenes was partially reversed by rimonabant, except in the case of β-Pinene (Fig. S14A). Rimonabant had no effect on forskolin-stimulated cAMP formation by itself, suggesting no inverse agonism in this assay (Fig. S14B). These results suggest CB1-mediated downstream signaling induced by the terpenes.
When assessed in a βarrestin2 recruitment assay, terpenes displayed no agonism at any concentration tested, while the positive control WIN55,212-2 did (Fig. 8C). We next determined whether the terpenes may prevent or promote arrestin recruitment in an allosteric fashion and screened each terpene in combination with WIN55,212-2 (Fig. 8D). Only Geraniol displayed any presence of potential allosteric interaction and suggested it may be a negative allosteric modulator (NAM) or a biased partial agonist vs. WIN55,212, discussed below. When a full dose-range of Geraniol was tested against WIN55,212-2 it showed dose-dependent reductions in WIN55,212-2 recruitment (Fig. S14C).
Discussion
As reviewed above, terpene compounds have been tested for their therapeutic properties in a number of studies, identifying anti-inflammatory, anti-nociceptive, anti-microbial, and similar beneficial properties3,4. However very few studies have made any attempt to identify molecular targets and mechanisms for these compounds. Similarly, while terpenes and cannabinoids have been hypothesized to interact to produce an “entourage effect”, the few studies performed to date have shown no interaction16,30. This study is thus the first to show that terpenes and cannabinoids can produce an additive effect when combined. This study is also the first to identify the CB1 and A2a receptors as terpene targets, and describe the role of these receptors in producing terpene cannabimimetic effects in vivo.
One question engendered by these findings is why our study found evidence for terpene/cannabinoid interactions while two other studies did not. There are several potential explanations. One study used an AtT20 cell line transfected with human CB1 or CB2 receptors, with a specific outcome of membrane potential change16. This study did not observe CB1/2 effects by terpenes while we did; however, the study was performed in a highly specific cell line with a single signaling output, membrane potential change. It is thus quite possible that the terpenes may not produce membrane potential changes via the CB1/2, just as we observed ERK and cAMP changes but not arrestin recruitment (Fig. 8). The second study used a similar in vitro approach, finding no receptor binding or cAMP signaling of the terpenes tested30. However, this study used mixtures of terpenes, with no single terpene exceeding 50% of the mixture; they also used a maximum concentration of 10 μM, while we observed receptor activation at higher concentrations. These differences explain our observed results, and in addition, we used an in vivo model, which will capture a broader range of potential activity than an in vitro model with very specific outputs.
Another notable aspect of our study was the generally high concentrations of terpene needed to see activation. This was especially apparent in our in vitro studies, where > 10 μM, or up to 500 μM depending on the terpene, was needed to see activation. Although high concentrations were required in vitro, this activation was still fully CB1-dependent, as it could be fully reversed by rimonabant treatment (Fig. 7A). However, the doses needed in vivo were not as extreme, producing full responses in most assays for most terpenes at 200 mg/kg. This is consistent with the hypothesis that low potency multifunctional compounds may have benefits over selective single target compounds by inducing a significant systemic effect via multiple molecular effectors31–34. In this light, compounds, such as these terpenes, that have low potency at multiple receptor targets are likely to generate a substantial systemic effect (depending on the targets thereof). This idea is aligned with our in vitro data showing low potency effects and our in vivo data suggesting significant behavioral effects compared to a full CB1 agonist. Although it is clear that these selected terpenes can interact with the CB1 receptor to produce signaling, much more work is necessary to determine the mechanism of such action, and the collective group of terpene targets. Low potency drugs are also not necessarily less desirable for therapy. A number of low potency drugs are in use in the clinic, including ibuprofen (dosed at 600–2400 mg/day), proglumide (dosed up to 2400 mg/day), and metformin (maximum dose of 2550 mg/day) (dosing information from UpToDate).
These observations have shown an important role for the CB1, among others, in mediating the effects of the test terpenes both in vitro and in vivo. Although appearing to be low potency agonists at CB1, there are alternative hypotheses for the mechanism of action based on these results. Two alternatives to direct CB1 agonism are (1) direct modulation of membrane dynamics, shifting CB1 activation equilibrium to favor the activated receptor; and (2) terpene modulation of endocannabinoid synthesis and/or degradation, which would then result in CB1 activation by these endocannabinoids. It has been suggested that membrane composition and dynamics heavily influence the cannabinoid receptor35. Indeed, this has also been described for other receptors36 whereby membrane composition alters activation equilibrium. Terpenes are highly lipophilic in nature, and thus likely have direct interactions with the membrane environment, potentially also including membrane microdomains such as lipid rafts. If these interactions lead to a thermodynamically favored “active” CB1 receptor, the inverse agonism properties of rimonabant would still block signaling. In regards to the second point, it has been shown that CHO cells can participate in autocrine and paracrine signaling via endocannabinoid synthesis37,38, in these cases 2-arachadonylglycerol (2-AG). In our assays using CHO cells, it is thus a possibility that terpenes modulate the synthesis or degradation of 2-AG to stimulate CB1 signaling, which would be blocked by rimonabant. These alternative hypotheses are further supported by the generally weak competition binding observed at the CB1 by terpenes (Fig. 8A). This is under current investigation in our laboratory. However, these alternate mechanisms must still possess some selectivity, as the terpenes were unable to activate the mu opioid receptor, a GαI-coupled GPCR similar to the CB1/2 (Fig. S13). Nonetheless, non-specific mechanisms of terpene action like colloid formation39 must still be ruled out.
Our studies suggest terpene poly-pharmacology, with multiple receptor targets, including CB1/2 and A2a. Other authors who tested some of these terpenes in the context of essential oils have detected glutamatergic40, serotonergic41, or dopaminergic42 activities. Co-activation of such GPCR systems or ion channels with CB1 and/or CB2 receptors may explain why we observed differences between the terpenes for interaction with cannabinoid and the influence of CB1 and A2a on different tetrad behaviors. Thus, it seems that the observed effects produced by these terpenes are the complex outcomes of the activation or inhibition of multiple receptor systems.
This complex poly-pharmacology may provide a unique means to use terpenes to enhance cannabinoid or other therapies. In our findings, we see that all terpenes synergize with WIN55,212-2 to produce enhanced anti-nociception (Fig. 2) while interacting variably with WIN55,212-2 in the other behaviors (Figs. 3–5). In principle, this suggests that terpenes could be used to enhance the analgesic properties of cannabis/cannabinoid therapy, without worsening the side effects of cannabinoid treatment. However, this must be confirmed using relevant phytocannabinoids like THC instead of the synthetic cannabinoid WIN55,212-2 used in this study. Identifying specific terpene:cannabinoid combinations with a maximized therapeutic index for a particular disease state could provide a new means to improve human therapy with these drugs.
Materials and methods
Materials
WIN55,212-2 (Tocris, #1038), α-Humulene (Sigma Aldrich, #53675), β-Pinene (Alfa Aesar, #A17818), Linalool (Alfa Aesar, #A14424), Geraniol (Alfa Aesar, #13736), and β-Caryophyllene (Cayman, #21572) were all prepared as stock solutions in DMSO. Working solutions were then diluted in 10% DMSO, 10% Tween-80, and 80% USP saline for injections. Rimonabant (Tocris, #0923) was made up in DMSO and then diluted to 20% DMSO, 10% Tween-80, and 70% USP saline for injections. Istradefyllene (Tocris, #5147) was made up in DMSO and then diluted to 20% DMSO, 10% Tween-80 and 70% saline for injections. Morphine sulfate pentahydrate (from the NIDA Drug Supply Program) was dissolved in USP saline. Vehicle injections were matched accordingly as a control in each experiment. All solutions were made immediately before use without long-term storage. For in vitro experiments, 100 mM stocks of terpenes, and 10 mM stocks of all other compounds, were made up in DMSO. DMSO concentrations in assays were maintained at 1% or lower. Vehicle controls were matched accordingly.
Animals
All experiments were performed on male and female CD-1 (a.k.a. ICR) mice obtained from Charles River in age-matched cohorts of 5–6 weeks of age. All mice were recovered for at least 5 days after shipping prior to experimentation, and housed no more than 5 per cage. The animals were maintained in an AAALAC-accredited vivarium at the University of Arizona in temperature and humidity-controlled rooms on a 12-h light/dark cycle. Standard chow and water were available ad libitum. For all behavioral experiments, mice were brought to the testing room in their home cages for at least 30 min prior to the experiment for acclimation. Mice were randomized to treatment group by randomization of mice to cages upon arrival, and block random assignment of cages to treatment group. The experimenter was blinded to treatment group by the delivery of coded drug vials. The groups were not decoded until all data had been acquired. All experiments were approved by the University of Arizona IACUC, and were carried out in accord with the standards of the NIH Guide for the Care and Use of Laboratory Animals. We also adhered to the guidelines of ARRIVE; no adverse events were noted for any of the animals.
Tail flick
Antinociception was tested using the tail flick thermal latency test. Mice were baselined by gently restraining the animal and lowering the distal portion of the tail into a water bath set to 52 °C or 47 °C where stated, and the latency to flick the tail recorded with a stopwatch, with a 10 s cutoff. Mice were then injected with compounds intraperitoneally (i.p.). Thermal latency was then assessed over a time course of 2 h, or at a single time-point as noted. For assays using rimonabant, rimonabant was injected 10 min prior to terpene or morphine injection. For assays using terpene/WIN55,212-2 co-treatment, WIN55,212-2 was co-administered with terpene in the same solution.
Catalepsy
Potential induction of catalepsy was assessed using the ring test as described43. Mice were baselined, injected with compound, and then assessed with the ring test at 15 min post-injection. Data was represented as the percentage (%) of time in a “cataleptic” immobile state over the 5 min observation period.
Open field test
The open field test was used to determine potential hypolocomotive properties induced by terpene and cannabinoid treatment. The open field box was a white box with an open top and black floor. The tracking area was 30 cm × 28 cm. Mice were baselined by placing the mouse in the center of the box and recording from a video camera ~ 1.5 m above for a period of 5 min. Mice were then injected with compound and placed back in the open field box 10–15 min post-injection. In follow-up experiments, mice were not baselined, injected with compound, and placed in the open field box from 10 to 15 min. The total distance traveled and mobile time of mice were analyzed using AnyMaze software.
Hypothermia
Changes in core temperature were assessed using a lubricated rectal thermometer placed 1.0 cm into the mouse rectum. Temperature was assessed before treatment and 30 min post-treatment.
Cell culture
WT-CHO cells were obtained from ATCC (#CCL-61). CHO cells stably expressing the human CB1 receptor were obtained from PerkinElmer (#ES-110-C) and utilized for western blots (CB1-CHO-C2) or binding assays (CB1-CHO-C3). HitHunter (CB1-CHO-cAMP) and PathHunter (CB-CHO-βarr2) assays were performed on respective CB1-CHO lines, and were a kind gift from Dr. Robert Laprairie44. CHO cells stably expressing cloned CB2 (CB2-CHO) were produced by electroporation with the human CB2 N-3xHA tag cDNA (GeneCopoeia). A stable expressing population was selected for with 500 μg/mL G418. CHO cells stably expressing the human mu opioid receptor were obtained from PerkinElmer (#ES-542-C). All cells were grown on 10 cm dishes in DMEM/F-12 50/50 mix w/ L-glutamine and 15 mM HEPES (Corning) containing 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, 100 units/mL streptomycin, and 400 μg/mL G418 (CB1-CHO-C2, CB1-CHO-C3, CB2-CHO, MOR-CHO); 800 μg/mL G418 (CB1-CHO-cAMP); 800 μg/mL G418 and 300 μg/mL hygromycin B (CB1-CHO-βarr2). WT CHO were grown as stated above without selection antibiotic. Cells were incubated in a humidified incubator at 37 °C with 5% CO2 and were sub-cultured every 2–3 days.
Cell treatments for ERK
Cells were plated in 6-well plates 24 h prior to the start of the experiments. Cells were serum starved for 1 h then treated for 5 min with compound. In antagonist experiments, cells were pretreated for 5 min before agonist treatment for 5 min. Media was then aspirated, cells were placed on ice, washed with ice-cold PBS once, and lysis buffer (20 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Nonidet P-40, 0.25% deoxycholate, 1 mM sodium orthovanadate, 1 mM PMSF, 1 mM sodium fluoride, and a protease inhibitor cocktail (EMD Millipore)), was added. Cells were scraped and collected. Lysates were vortexed then centrifuged at 13 k rpm at 4 °C for 10 min. Lysates were then processed for Western blots or stored at − 80 °C.
Electrophoresis and western blotting
Cell lysate protein was quantified with a modified BCA protein assay using manufacturers protocol (Bio-Rad). Samples (20–30 μg) were ran on precast gels (10% Bis–Tris, Bolt brand, from ThermoFisher). Gels were wet-transferred to nitrocellulose membranes at 30 V for at least 60 min at 4 °C. Blots were blocked with 5% non-fat dry milk in TBS for 30 min, washed 3 × for 5 min with TBS + 0.1% Tween-20 (TBST), and incubated with primary antibody overnight at 4 °C. Primary antibody: pERK and tERK (Cell Signaling) at 1:1000 dilution in 5% BSA in TBST. Blots were then washed 3 × with TBST then incubated with secondary antibodies for 90 min at room temperature. Secondary antibodies: Goat anti-Rabbit 800 W and Goat anti-Mouse 680 diluted at 1:10,000 and 1:20,000 (Licor), respectively, in 5% non-fat milk in TBST. Blots were washed 3X with TBST and 1 × with TBS, then imaged on a Licor Odyssey Fc or Azure Sapphire. Bands were analyzed using Image-J and reported as pERK/tERK normalized to the standard, or simply as pERK/tERK.
Radioligand binding
Radioligand binding was performed as previously reported45. Briefly, CB1-CHO-C3 cells were homogenized in 50 mM Tris–HCl containing 1 mM PMSF, then centrifuged at 30,000g for 30 min at 4 °C. The resultant pellet was resuspended in the same buffer. 30–40 μg of membrane protein was incubated with varying concentrations of compound and 0.3 nM [3H]-CP55,940 (PerkinElmer) for 90 min at room temperature. Data reported as the % of specific [3H]-CP55,940 binding.
PathHunter assay
CB1-CHO-βarr2 cells were plated in 384-well plates (5000 cells/well) in Opti-MEM with 1% FBS. The following day cells were treated with varying concentrations of ligand for 1.5 h. In allosteric assays, cells were pre-treated with the first compound for 5 min. Manufacturers protocol was then followed. Luminescence was read on a CLARIOstar plate reader. Data reported as the % of maximum recruitment by WIN55,212-2 reference standard.
HitHunter assay
CB1-CHO-cAMP cells were plated in 384-well plates (5000 cells/well) in Opti-MEM with 1% FBS. The following day, the media was removed and replaced with PBS. Cells were then treated with varying concentrations of compounds in solution containing 20 μM forskolin (Enzo) for 30 min. In antagonist experiments cells were pre-treated for 5 min. Following agonist treatment, the manufacturers protocol was followed. Data reported as the % of forskolin stimulated cAMP.
Data analysis and statistics
All data was analyzed using GraphPad Prism 8. For behavioral studies graphs show combined male and female data. When sex differences were observed (p < 0.05 by 2 Way ANOVA), males and females were separated for analysis. For dose–response curves, non-linear fit curves were generated using Prism. The behavioral data was all reported in raw values, without normalization. Statistical analysis details are noted in the Fig. Legends. For RM 2 Way ANOVA, the Geisser-Greenhouse correction was used to account for a potential lack of sphericity of the data, permitting valid RM ANOVA. ANOVA post hoc tests were only performed when ANOVA F values indicated a significant difference, and there was homogeneity of variance (permitting parametric analysis). In all cases, significance was defined as p < 0.05. A sample size of N = 10 was targeted for all animal experiments based on our previous work using similar behavioral assays (e.g.46). In some cases, the sample size range was large (e.g. N = 10–20/group). This was due to the fact that some experiments were performed separately, such as rimonabant vs. istradefyllene pre-treatment, however, each separate experiment would have matched controls. Those controls would be combined at the end to result in a larger range of sample sizes. All sample sizes given represent biological replicates; either individual mice in the in vivo experiments or fully independent experiments for in vitro studies. No outliers were removed from the experiments and all data was included (no exclusions).
Supplementary Information
Acknowledgments
The authors would like to acknowledge Drs. Tally Largent-Milnes and Todd Vanderah from the University of Arizona for sharing their behavioral equipment and expertise. This study was funded by institutional funds from the University of Arizona. JMS has an equity stake in Botanical Results, LLC, a local cannabidiol company; no company products or interests were tested in this study.
Abbreviations
- 2-AG
2-Arachidonoylglycerol
- A2a
Adenosine A2a receptor
- CBD
Cannabidiol
- CB1/2
Cannabinoid receptor type ½
- ERK
Extracellular signal-regulated kinase
- FBS
Fetal bovine serum
- NAM
Negative allosteric modulator
- THC
Δ9-tetrahydrocannabinol
- TBST
Tris-buffered saline with tween
Author contributions
J.E.L. designed research, performed research, analyzed data, wrote paper. R.H. performed research, analyzed data, edited paper. A.K. performed research, analyzed data, edited paper. J.M.S. analyzed data, edited paper.
Data availability
All data generated or analyzed during this study are included in the manuscript and supporting files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-87740-8.
References
- 1.Russo EB. The case for the entourage effect and conventional breeding of clinical cannabis: No "strain," No Gain. Front Plant Sci. 2018;9:1969. doi: 10.3389/fpls.2018.01969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartsel, J.A., J. Eades, B. Hickory, A. Makriyannis, and R.C. Gupta, Chapter 53 - Cannabis sativa and Hemp. In Nutraceuticals 735–754 (Academic Press, Boston, 2016)
- 3.Russo EB, Marcu J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2017;80:67–134. doi: 10.1016/bs.apha.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 5.Russo EB. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163(7):1344–64. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Cássia da Silveira, E.S.R., Lima, T.C., da Nóbrega, F.R., de Brito, A.E.M., & de Sousa, D.P.: Analgesic-like activity of essential oil constituents: An update.Int. J. Mol. Sci.18(12), 2392 (2017). [DOI] [PMC free article] [PubMed]
- 7.Harris HM, Rousseau MA, Wanas AS, Radwan MM, Caldwell S, Sufka KJ, ElSohly MA. Role of cannabinoids and terpenes in cannabis-mediated analgesia in rats. Cannabis Cannabinoid Res. 2019;4(3):177–182. doi: 10.1089/can.2018.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998;353(1):23–31. doi: 10.1016/S0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 9.Ferber SG, Namdar D, Hen-Shoval D, Eger G, Koltai H, Shoval G, Shbiro L, Weller A. The "entourage effect": Terpenes coupled with cannabinoids for the treatment of mood disorders and anxiety disorders. Curr Neuropharmacol. 2020;18(2):87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J. Pain Symptom Manag. 2010;39(2):167–179. doi: 10.1016/j.jpainsymman.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Pamplona FA, da Silva LR, Coan AC. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: Observational data meta-analysis. Front Neurol. 2018;9:759. doi: 10.3389/fneur.2018.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blasco-Benito S, Seijo-Vila M, Caro-Villalobos M, Tundidor I, Andradas C, Garcia-Taboada E, Wade J, Smith S, Guzman M, Perez-Gomez E, et al. Appraising the "entourage effect": Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018;157:285–293. doi: 10.1016/j.bcp.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. U. S. A. 2008;105(26):9099–104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namdar, D., Voet, H., Ajjampura, V., Nadarajan, S., Mayzlish-Gati, E., Mazuz, M., Shalev, N., & Koltai, H. Terpenoids and phytocannabinoids co-produced in cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules24(17), 3031 (2019). [DOI] [PMC free article] [PubMed]
- 15.Cogan, P.S. The 'entourage effect' or 'hodge-podge hashish': The questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Rev. Clin. Pharmacol. 1–11 (2020). [DOI] [PubMed]
- 16.Santiago M, Sachdev S, Arnold JC, McGregor IS, Connor M. Absence of entourage: Terpenoids commonly found in cannabis sativa do not modulate the functional activity of Δ(9)-THC at human CB(1) and CB(2) receptors. Cannabis Cannabinoid Res. 2019;4(3):165–176. doi: 10.1089/can.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172(20):4790–805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King KM, Myers AM, Soroka-Monzo AJ, Tuma RF, Tallarida RJ, Walker EA, Ward SJ. Single and combined effects of Delta(9)-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 2017;174(17):2832–2841. doi: 10.1111/bph.13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uberall MA. A review of scientific evidence for THC:CBD oromucosal spray (nabiximols) in the management of chronic pain. J Pain. Res. 2020;13:399–410. doi: 10.2147/JPR.S240011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metna-Laurent M, Mondésir M, Grel A, Vallée M, Piazza P. Cannabinoid-Induced Tetrad in Mice. Current Protocols in Neuroscience. 2018;80(1):9.59.1–9.59.10. doi: 10.1002/cpns.31. [DOI] [PubMed] [Google Scholar]
- 21.Boyaji S, Merkow J, Elman RNM, Kaye AD, Yong RJ, Urman RD. The role of cannabidiol (CBD) in chronic pain management: An assessment of current evidence. Curr. Pain Headache Rep. 2020;24(2):4. doi: 10.1007/s11916-020-0835-4. [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Zhang L, Li N, Mai N, Zhang Y, Pan S. Geraniol promotes functional recovery and attenuates neuropathic pain in rats with spinal cord injury. Can. J. Physiol. Pharmacol. 2017;95(12):1389–1395. doi: 10.1139/cjpp-2016-0528. [DOI] [PubMed] [Google Scholar]
- 23.Carlin JL, Jain S, Duroux R, Suresh RR, Xiao C, Auchampach JA, Jacobson KA, Gavrilova O, Reitman ML. Activation of adenosine A2A or A2B receptors causes hypothermia in mice. Neuropharmacology. 2018;139:268–278. doi: 10.1016/j.neuropharm.2018.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno E, Chiarlone A, Medrano M, Puigdellívol M, Bibic L, Howell LA, Resel E, Puente N, Casarejos MJ, Perucho J, et al. singular location and signaling profile of adenosine A(2A)-cannabinoid CB(1) receptor heteromers in the dorsal striatum. Neuropsychopharmacology. 2018;43(5):964–977. doi: 10.1038/npp.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiodi V, Ferrante A, Ferraro L, Potenza RL, Armida M, Beggiato S, Pèzzola A, Bader M, Fuxe K, Popoli P, et al. Striatal adenosine-cannabinoid receptor interactions in rats over-expressing adenosine A2A receptors. J. Neurochem. 2016;136(5):907–917. doi: 10.1111/jnc.13421. [DOI] [PubMed] [Google Scholar]
- 26.Köfalvi A, Moreno E, Cordomí A, Cai NS, Fernández-Dueñas V, Ferreira SG, Guixà-González R, Sánchez-Soto M, Yano H, Casadó-Anguera V, et al. Control of glutamate release by complexes of adenosine and cannabinoid receptors. BMC Biol. 2020;18(1):9. doi: 10.1186/s12915-020-0739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aso E, Fernández-Dueñas V, López-Cano M, Taura J, Watanabe M, Ferrer I, Luján R, Ciruela F. Adenosine A(2A)-cannabinoid CB(1) receptor heteromers in the hippocampus: Cannabidiol Blunts Δ(9)-tetrahydrocannabinol-induced cognitive impairment. Mol Neurobiol. 2019;56(8):5382–5391. doi: 10.1007/s12035-018-1456-3. [DOI] [PubMed] [Google Scholar]
- 28.Mouro FM, Köfalvi A, André LA, Baqi Y, Müller CE, Ribeiro JA, Sebastião AM. Memory deficits induced by chronic cannabinoid exposure are prevented by adenosine A(2A)R receptor antagonism. Neuropharmacology. 2019;155:10–21. doi: 10.1016/j.neuropharm.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Park HM, Lee JH, Yaoyao J, Jun HJ, Lee SJ. Limonene, a natural cyclic terpene, is an agonistic ligand for adenosine A(2A) receptors. Biochem. Biophys. Res. Commun. 2011;404(1):345–348. doi: 10.1016/j.bbrc.2010.11.121. [DOI] [PubMed] [Google Scholar]
- 30.Finlay DB, Sircombe KJ, Nimick M, Jones C, Glass M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020;11:359. doi: 10.3389/fphar.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho TT, Tran QT, Chai CL. The polypharmacology of natural products. Future Med. Chem. 2018;10(11):1361–1368. doi: 10.4155/fmc-2017-0294. [DOI] [PubMed] [Google Scholar]
- 32.Medina-Franco JL, Giulianotti MA, Welmaker GS, Houghten RA. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today. 2013;18(9–10):495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anighoro A, Bajorath J, Rastelli G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014;57(19):7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 34.Pang MH, Kim Y, Jung KW, Cho S, Lee DH. A series of case studies: Practical methodology for identifying antinociceptive multi-target drugs. Drug Discov. Today. 2012;17(9–10):425–434. doi: 10.1016/j.drudis.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Maccarrone M, Bernardi G, Agrò AF, Centonze D. Cannabinoid receptor signalling in neurodegenerative diseases: A potential role for membrane fluidity disturbance. Br. J. Pharmacol. 2011;163(7):1379–90. doi: 10.1111/j.1476-5381.2011.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida K, Nagatoishi S, Kuroda D, Suzuki N, Murata T, Tsumoto K. Phospholipid membrane fluidity alters ligand binding activity of a G protein-coupled receptor by shifting the conformational equilibrium. Biochemistry. 2019;58(6):504–508. doi: 10.1021/acs.biochem.8b01194. [DOI] [PubMed] [Google Scholar]
- 37.Turu G, Simon A, Gyombolai P, Szidonya L, Bagdy G, Lenkei Z, Hunyady L. The role of diacylglycerol lipase in constitutive and angiotensin AT1 receptor-stimulated cannabinoid CB1 receptor activity. J. Biol. Chem. 2007;282(11):7753–7757. doi: 10.1074/jbc.C600318200. [DOI] [PubMed] [Google Scholar]
- 38.Turu G, Várnai P, Gyombolai P, Szidonya L, Offertaler L, Bagdy G, Kunos G, Hunyady L. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J. Biol. Chem. 2009;284(25):16914–21. doi: 10.1074/jbc.M109.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson KM, Bisson J, Singh G, Graham JG, Chen SN, Friesen JB, Dahlin JL, Niemitz M, Walters MA, Pauli GF. The essential medicinal chemistry of cannabidiol (CBD) J. Med. Chem. 2020;63(21):12137–12155. doi: 10.1021/acs.jmedchem.0c00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Batista PA, Werner MF, Oliveira EC, Burgos L, Pereira P, Brum LF, Santos AR. Evidence for the involvement of ionotropic glutamatergic receptors on the antinociceptive effect of (−)-linalool in mice. Neurosci. Lett. 2008;440(3):299–303. doi: 10.1016/j.neulet.2008.05.092. [DOI] [PubMed] [Google Scholar]
- 41.Martinez AL, Gonzalez-Trujano ME, Pellicer F, Lopez-Munoz FJ, Navarrete A. Antinociceptive effect and GC/MS analysis of Rosmarinus officinalis L. essential oil from its aerial parts. Planta Med. 2009;75(5):508–511. doi: 10.1055/s-0029-1185319. [DOI] [PubMed] [Google Scholar]
- 42.Peana AT, De Montis MG, Nieddu E, Spano MT, D'Aquila PS, Pippia P. Profile of spinal and supra-spinal antinociception of (−)-linalool. Eur. J. Pharmacol. 2004;485(1–3):165–174. doi: 10.1016/j.ejphar.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 43.Pertwee RG. The ring test: A quantitative method for assessing the 'cataleptic' effect of cannabis in mice. Br. J. Pharmacol. 1972;46(4):753–63. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laprairie RB, Mohamed KA, Zagzoog A, Kelly MEM, Stevenson LA, Pertwee R, Denovan-Wright EM, Thakur GA. Indomethacin enhances type 1 cannabinoid receptor signaling. Front Mol. Neurosci. 2019;12:257. doi: 10.3389/fnmol.2019.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olson, K.M., Duron, D.I., Womer, D., Fell, R., & Streicher, J.M. Comprehensive molecular pharmacology screening reveals potential new receptor interactions for clinically relevant opioids.PLoS One14(6), 0217371 (2019). PMC6553708 This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials. The authors have no other relevant competing interests to declare. [DOI] [PMC free article] [PubMed]
- 46.Duron, D.I., Lei, W., Barker, N.K., Stine, C., Mishra, S., Blagg, B.S.J., Langlais, P.R., & Streicher, J.M. Inhibition of Hsp90 in the spinal cord enhances the antinociceptive effects of morphine by activating an ERK-RSK pathway. Sci. Signal, 13(630), eaaz1854 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the manuscript and supporting files.