Abstract
Massage is a viable mechanotherapy to improve protein turnover during disuse atrophy and improve muscle regrowth during recovery from disuse atrophy in adult muscle. Therefore, we investigated whether massage can cause beneficial adaptations in skeletal muscle from aged rats during normal weight-bearing (WB) conditions, hindlimb suspension (HS), or reloading (RE) following HS. Aged (30 months) male Fischer 344/Brown Norway rats were divided into two experiments: (1) WB for 7 days (WB, n = 8), WB with massage (WBM, n = 8), HS for 7 days (HS7, n = 8), or HS with massage (HSM, n = 8), and (2) WB for 14 days (WB14, n = 8), HS for 14 days (HS14, n = 8), reloading (RE, n = 10), or reloading with massage (REM, n = 10) for 7 days following HS. Deuterium oxide (D2O) labeling was used to assess dynamic protein and ribosome turnover in each group and anabolic signaling pathways were assessed. Massage did have an anabolic benefit during RE or WB. In contrast, massage during HS enhanced myofibrillar protein turnover in both the massaged limb and contralateral non-massaged limb compared with HS, but this did not prevent muscle loss. Overall, the data demonstrate that massage is not an effective mechanotherapy for prevention of atrophy during muscle disuse or recovery of muscle mass during reloading in aged rats.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00215-y) contains supplementary material, which is available to authorized users.
Keywords: Aging, Disuse atrophy, Protein turnover, Ribosome biogenesis, Mechanotherapy
Introduction
Age-related muscle loss is a primary contributor to loss of independence and all-cause mortality in aged populations (Hirani et al. 2015; Marsh et al. 2011; Rantanen et al. 1999; Szulc et al. 2010). In addition, aged muscle does not adequately recover muscle mass and strength after periods of inactivity or bed rest, further contributing to loss of functional mobility in the aged (English and Paddon-Jones 2010; Magne et al. 2011; Mosoni et al. 1999; White et al. 2015). The failed muscle regrowth following a period of disuse atrophy often leads to disability in previously independent elderly individuals (Covinsky et al. 2003; Kortebein 2008; Kortebein et al. 2008; Suetta 2017). Thus, regaining muscle mass and function during recovery from disuse or intervening during a period of inactivity to attenuate atrophy is of critical importance in older adults.
It is not clear why skeletal muscle in older individuals is resistant to regrowth after a period of atrophy. In various studies, this resistance has been attributed to dampened rates of protein synthesis (Fry et al. 2011; West et al. 2018), reduced activation of Akt/mTOR signaling (Fry et al. 2011; Funai et al. 2006; Parkington et al. 2004; Thomson and Gordon 2006), reduced sensitivity to amino acids (Burd et al. 2013), blunted ribosome biogenesis (Kirby et al. 2015), and reduced insulin sensitivity (Rasmussen et al. 2006) compared with adult muscle. Aging induces changes in anatomical structure and stiffness of the muscle that may impact its mechanosensing ability to mechanical stimuli (Kennedy et al. 2020). Basal metabolic traits, such as mitochondrial capacity, have also been shown to be impaired in skeletal muscle with aging and can certainly impede the ability of aged muscle to adapt to exercise and mechanotherapies (Lagerwaard et al. 2020). However, not all studies agree with these findings as some indicate that there are no issues with anabolic signaling or mechanosensitivity in aged muscle (Hornberger et al. 2005; Magne et al. 2011; Miller et al. 2019; Moro et al. 2018; Paddon-Jones et al. 2004; White et al. 2015). Since it is incompletely understood why aged muscle does not regrow in a similar manner to adult muscle, therapeutic interventions to facilitate regrowth are lacking.
Our work has demonstrated that the massage mimetic, cyclic compressive loading (CCL), may be a useful mechanotherapy to minimize skeletal muscle atrophy during a period of unloading (Lawrence et al. 2020) and aids in regrowth during reloading in adult muscle (Miller et al. 2018). In these studies, we showed that massage elevates rates of myofibrillar protein synthesis in the massaged limb, while also providing beneficial effects on the contralateral non-massaged muscle (Lawrence et al. 2020; Miller et al. 2018). To date, our studies have mainly focused on adult muscle, while the potential therapeutic effect of CCL in old muscle remains largely unknown. In one previous study, we used a single bout of massage on unperturbed muscle indicating that massage was largely ineffective at stimulating anabolic responses, including protein synthesis, in either adult or aged muscle (Van Pelt et al. 2019). However, a single bout of massage did increase the satellite cell abundance in both adult and aged skeletal muscle (Hunt et al. 2019), which may be beneficial for recovery from injury. Whether repeated bouts of massage are useful as a mechanotherapy in aged muscle during disuse, reloading, or unperturbed conditions (i.e., normal weight bearing) is currently unknown.
Muscle size regulation is regulated by the balance of protein synthesis and degradation, and it is important to understand how each contributes to atrophy, regrowth, and response to massage. We previously used deuterium oxide (D2O) labeling to show that compared with normal loading, there is lower protein synthesis and higher protein degradation during disuse atrophy and that massage can offset these changes by stimulating protein synthesis and suppressing degradation (Lawrence et al. 2020). In order to understand the mechanistic underpinnings of changes in protein synthesis, we used D2O to measure ribosomal biogenesis and degradation. In adult muscle during disuse atrophy, we found that the rate of ribosomal degradation was much greater than during normal weight bearing without a change in ribosome biogenesis. We also found that massage was able to attenuate the elevation in ribosome degradation (Lawrence et al. 2020). Ribosome biogenesis is an important factor for muscle hypertrophy (reviewed in Figueiredo and McCarthy (2019)), and by directly measuring changes in turnover, we were able to show that ribosome degradation may be a more important factor that limits translational capacity during atrophic conditions (Lawrence et al. 2020). In this study, we leverage D2O labeling techniques to further understand dynamic protein and ribosomal turnover in aged muscle with atrophy, regrowth, and with massage.
The purpose of the present study was to assess if massage is an effective mechanotherapy in muscle of older rats during unperturbed weight-bearing (WB) conditions, during disuse from hindlimb suspension (HS), and during reloading after disuse (RE). Our study used long-term stable isotope measurements of protein and ribosome turnover to better understand the mechanisms underlying massage as a mechanotherapy in aged muscle. Based on our previous work in adult rats (Lawrence et al. 2020; Miller et al. 2018), we hypothesized that multiple bouts of massage applied during weight-bearing conditions would not affect muscle size or protein turnover, whereas massage applied during disuse and reloading would protect against muscle loss and aid in recovery of muscle size, respectively, by influencing protein and ribosome turnover processes. Finally, we hypothesized that massage would also have positive effects on the contralateral non-massaged limb as we have shown previously in adult rats.
Materials and methods
Study design and animal experimentation
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. The study was conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals. Thirty-month-old male Fischer 344/Brown Norway (F344BN) F1 hybrid rats (National Institute on Aging, Bethesda, MD) were housed in a temperature- and humidity-controlled room and maintained on a 12:12-h light–dark cycle with ad libitum access to food and water within the Division of Laboratory Animal Resources at the University of Kentucky.
Experimental groups and D2O labeling
In order to determine if massage had the same beneficial response in aged muscle as we observed in adult rats, we repeated our previous study designs (Lawrence et al. 2020; Miller et al. 2018). To explore the effect of massage during WB and HS (Fig. 1a) rats were randomly assigned to four groups: normal ambulatory weight bearing (WB; n = 8), weight bearing with massage (WBM; n = 9), hindlimb suspension for 7 days (HS7; n = 8), and hindlimb suspension for 7 days with massage (HSM; n = 9). To determine the effect of massage during RE (Fig. 1b), rats were randomly assigned to an additional four groups: ambulatory weight bearing for 14 days (WB14, n = 8), hindlimb suspension for 14 days (HS14, n = 8), hindlimb suspension for 14 days followed by reloading (RE, n = 10), or reloading with massage (REM, n = 10) for 7 days. For massage, CCL (described below) was applied to the right gastrocnemius muscle of WBM, HSM, or REM rats every other day starting on day 0 for a total of 4 bouts (Lawrence et al. 2020; Miller et al. 2018). Rats were euthanized 24 h after the last bout of massage or at indicated times (Fig. 1).
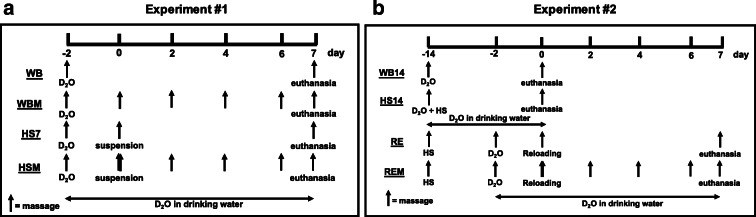
Fig. 1.
Overview of experimental designs. a WB, weight bearing for 7 days; WBM, weight bearing with massage; HS7, hindlimb suspension for 7 days; HSM, hindlimb suspension with massage. b WB14, weight bearing for 14 days; HS14, hindlimb suspension for 14 days; RE, 14 days of hindlimb suspension followed by 7 days of reloading; REM, 14 days of hindlimb suspension followed by 7 days of reloading with massage. Grey arrows indicate when massage was applied through CCL and black arrows with D2O indicate time points of heavy water injections. Rats received D2O in drinking water after the injections until euthanasia
In the first experiment, all groups received an i.p. bolus of (99%) D2O 2 days prior to the start of the experiment followed by drinking water enriched with 8% D2O until sacrifice (Fig. 1a). The experimental period (WB or HS) lasted 7 days with 4 bouts of massage in the appropriate groups (Lawrence et al. 2020). For the second experiment (Fig. 1b), all groups were given an i.p. D2O bolus followed by 8% D2O-enriched drinking water until euthanasia, which totaled 14 days for WB14 and HS14 and 9 days for RE and REM (Miller et al. 2018).
Hindlimb suspension
For hindlimb suspension, a tail device containing a hook was attached with gauze and cyanoacrylate glue while the animals were anesthetized with isoflurane (2% by inhalation) (Lawrence et al. 2020; White et al. 2015). After the animal regained consciousness, the tail device was connected via a thin cable to a pulley sliding on a vertically adjustable stainless steel bar running longitudinally above a high-sided cage. The system was designed in such a way that the rats could not rest their hindlimbs against any side of the cage (Lawrence et al. 2020; White et al. 2015).
CCL, a massage mimetic, of gastrocnemius muscle
CCL, consisted of 30-min bouts of mechanical loading over the gastrocnemius muscle at 4.5 N load and 0.5 Hz as described previously (Lawrence et al. 2020; Miller et al. 2018; Waters-Banker et al. 2014). For CCL application, rats were anesthetized using isoflurane (5% induction, 2% maintenance isoflurane/500 ml oxygen via a nose cone) and placed left lateral recumbent on a heated sling with the right hindlimb secured to a small platform by athletic tape encircling the talocrural joint. The lateral aspect of the gastrocnemius muscle was placed facing superiorly for the application of CCL by a custom-fabricated CCL device (Butterfield et al. 2008; Lawrence et al. 2020; Miller et al. 2018; Van Pelt et al. 2019). A spring-loaded strut mechanism was designed to allow a cylinder to roll longitudinally over the contoured mass of the right gastrocnemius and displace vertically in response to the normal force exerted upwards from the tissue to the roller during an oscillating movement. A force transducer enabled continuous, real-time voltage output, calibrated to known loads, permitting tuning of the normal force applied to the roller. The roller cycled along the length of the gastrocnemius muscle, compressing both the medial and lateral heads. Non-massaged groups were anesthetized and placed lateral recumbent without application of CCL (sham treatment) for 30 min (Butterfield et al. 2008; Lawrence et al. 2020; Miller et al. 2018; Van Pelt et al. 2019).
Blood and tissue collection
Rats were euthanized by i.p. injection of sodium pentobarbital followed by exsanguination through cardiac puncture (Lawrence et al. 2020; Miller et al. 2018). Blood was collected, clotted at room temperature for 30 min, and then centrifuged at 2000g for 10 min at 4 °C. Serum was aliquoted and frozen at − 80 °C until analyses. Gastrocnemius muscles were quickly harvested, trimmed of connective tissue and fat, weighed, and cut midbelly with the distal half mounted for tissue sectioning, while the remainder was flash frozen in liquid nitrogen. For rats in the non-massaged groups (WB, HS7, WB14, HS14, RE), only the right gastrocnemius muscle was analyzed, while for WBM, HSM, and REM, both the non-massaged left (WBM-L, HSM-L, REM-L, respectively) and massaged right muscle were analyzed to be able to determine effects of massage on the contralateral side to the massaged limb.
Total RNA isolation
Total RNA was isolated from a frozen section of muscle (~ 30–75 mg) in 1 ml TRIzol (Thermo Fisher Scientific, Rockford, IL, USA) using a handheld homogenizer (Lawrence et al. 2020; Miller et al. 2019). The homogenate was centrifuged at 12,000g for 10 min at 4 °C. The resulting supernatant was removed and 200 μl of chloroform was added. The mixture was shaken by hand vigorously then centrifuged at 12,000g for 15 min at 4 °C. The upper aqueous layer was isolated, mixed with 500 μl of isopropanol, and then left to incubate at room temperature for 20 min. After incubation, the mixture was centrifuged at 12,000g for 10 min at 4 °C to pellet RNA. The RNA pellet was isolated, rinsed with 1 ml of 75% ethanol, and resuspended in 50 μl of molecular biology grade H2O. RNA integrity was determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and RNA concentration was measured using a NanoDrop (Thermo Fisher Scientific) to then calculate total RNA per milligram muscle (Lawrence et al. 2020; Miller et al. 2019).
Determination of protein and RNA fractional synthesis rate
For the determination of protein synthesis rates, muscle tissue was homogenized 1:10 in isolation buffer (100 mM KCl, 40 mM Tris HCl, 10 mM Tris Base, 5 mM MgCl2, 1 mM EDTA, 1 mM ATP, pH = 7.5) with phosphatase and protease inhibitors (HALT, Thermo Fisher Scientific) using a bead homogenizer (Next Advance Inc., Averill Park, NY, USA). After homogenization, subcellular fractions were isolated via differential centrifugation (Drake et al. 2014; Drake et al. 2015; Drake et al. 2013; Lawrence et al. 2020; Miller et al. 2019). Once protein pellets were isolated and purified, 250 μl 1 M NaOH was added and pellets were incubated for 15 min at 50 °C while slowly mixing. Protein was hydrolyzed by incubation for 24 h at 120 °C in 6 N HCl. The pentafluorobenzyl-N,N-di(pentafluorobenzyl) derivative of alanine was analyzed on an Agilent 7890A GC coupled to an Agilent 5975C MS (Drake et al. 2013, 2014, 2015; Lawrence et al. 2020; Miller et al. 2019).
For ribosomal turnover, we isolated RNA from ~ 15–25 mg of frozen muscle using a TRIzol kit as described above. The isolated RNA was hydrolyzed overnight at 37 °C with nuclease S1 and potato acid phosphatase. Hydrolysates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analyzed on an Agilent 7890A GC coupled to an Agilent 5975C MS. For GC-MS analysis, we used a DB-17 column and negative chemical ionization, with helium as the carrier and methane as the reagent gas. The fractional molar isotope abundances at m/z 212 (M0) and 213 (M1) of the pentafluorobenzyl triacetyl derivative of purine ribose were quantified using the MassHunter software. All analyses were corrected for abundance with an unenriched pentafluorobenzyl triacetyl purine ribose derivative standard (Lawrence et al. 2020; Mathis et al. 2017; Miller et al. 2019; Sieljacks et al. 2019).
To determine body water enrichment, 125 μl of serum was placed into the inner well of o-ring screw cap and inverted on an 80 °C heating block overnight. Two microliters of 10 M NaOH and 20 μl of acetone were added to all samples and to 20 μl 0–20% D2O standards and then capped immediately (Lawrence et al. 2020; Miller et al. 2018, 2019). Samples were vortexed at low speed and left at room temperature overnight. Extraction was performed by the addition of 200 μl hexane. The organic layer was transferred through anhydrous Na2SO4 into GC vials and analyzed via electron ionization (EI) mode (Lawrence et al. 2020; Miller et al. 2018, 2019).
Calculations
The newly synthesized fraction (f) of proteins was calculated from the enrichment of alanine bound in muscle proteins over the entire labeling period, divided by the true precursor enrichment (p), using plasma D2O enrichment with MIDA adjustment (Busch et al. 2006; Lawrence et al. 2020; Miller et al. 2018, 2019). Similarly, RNA synthesis (~ 85% of total RNA exists as ribosomal RNA) was determined by deuterium incorporation into purine ribose of RNA (Lawrence et al. 2020; Mathis et al. 2017; Miller et al. 2019; Sieljacks et al. 2019) with MIDA adjustment of the equilibration of the enrichment of the body water pool with purine ribose.
If there was a significant change in protein or RNA mass, we used our previously published equations that account for non-steady-state conditions (Lawrence et al. 2020; Miller et al. 2015, 2018). The mass of protein or RNA at time t, P(t), obeys the differential equation:
| 1 |
where ksyn is the synthesis rate, with dimensions of mass over time, and kdeg is the degradation constant, with dimensions of inverse time. From this relationship:
| 2 |
Western analysis for determination of protein abundance
A portion (~ 30 mg) of gastrocnemius muscle representing both lateral and medial parts was homogenized and centrifuged, and protein concentration (BCA) was determined of the supernatant. For quantification of protein abundance, samples were separated by SDS-PAGE using 4–15% polyacrylamide gels (Bio-Rad) and then transferred to a PVDF (Bio-Rad) membrane. Transfer efficiency and equal loading among lanes were then determined by staining membranes with Ponceau S (Sigma-Aldrich) and imaging. After blocking membranes (5% non-fat dry milk in TBS-T) for 1 h, proteins of interest (below) were probed with primary antibody overnight at 4 °C. Membranes were then serially washed in TBS-T, incubated with appropriate secondary antibody in blocking buffer for 1 h and again serially washed in TBS-T. For all proteins except FAK and p-FAK, HRP activity was detected by enhanced chemiluminescence substrate (SuperSignal West Dura, Thermo Fisher Scientific). Images were taken for quantification by densitometry using a FluorChem E imager (ProteinSimple, San Jose, CA). Protein content was quantified and corrected for local background using the AlphaView analysis software (ProteinSimple). Membranes were then stripped (Restore Western Blot Stripping Buffer, Thermo Fisher Scientific), checked for adequate stripping, and re-probed (Lawrence et al. 2020). For FAK and p-FAK, we used previous procedures specific to protein quantification on an Odyssey (Licor) infrared imaging system (Lawrence et al. 2020; Miller et al. 2018).
Primary antibodies and dilutions used were as follows: phospho-ERKThr202/Tyr204 (Cell Signaling Technology; CST no. 4370, Beverly, MA, USA), 1:1000; ERK (CST no. 4695), 1:1000; phospho-AktSer473 (CST no. 4058), 1:500; Akt (CST no. 4685), 1:500; phospho-FOXO3ASer235 (CST no. 9466), 1:500; FOXO3A (CST no. 12829), 1:500; phospho-eEF2Thr56 (CST no. 2331), 1:1000; eEF2 (CST no. 2332), 1:1000; phospho-FAKTyr397 (CST no. 3283), 1:1000; FAK (CST no. 3285), 1:1000; phospho-S6K1Thr389 (CST no. 9234), 1:500; S6K1 (CST no. 9202), 1:1000; phospho-rpS6Ser235/236 (CST no. 4858), 1:1000; rpS6 (CST no. 2217), 1:1000; phospho-4EBP1Thr37/46 (CST no. 9459), 1:1000; 4EBP1 (CST no. 9452), 1:1000; MuRF-1 (ECM Biosciences no. MP3401), 1:1000; phospho-UBF-1Ser484 (Abcam, no. ab182583, Cambridge, MA, USA), 1:500; UBF-1 (Thermo Fisher Scientific no. PA5-36153), 1:500; and c-Myc (CST no. 13987), 1:1000. For every protein except FAK and p-FAK goat anti-rabbit, HRP-linked secondary antibody (CST no. 7074) at 1:5000 was used. FAK and p-FAK primary antibodies were reacted with goat anti-rabbit highly cross-absorbed infrared-labeled secondary antibody (Licor, Lincoln, NE, USA) at 1:15,000. The ratio of phosphorylated over total protein is not presented here, because it is mostly determined by changes in the abundance of total protein levels, particularly in HS, thereby artificially inflating the ratios, as described by us in Lawrence et al. (2020) and Miller et al. (2018).
Myosin heavy chain (MyHC) determination
Mean and fiber-type-specific CSA was determined as described (Murach et al. 2017) with modifications for rat muscle (Lawrence et al. 2020; Miller et al. 2018). Gastrocnemius cross-sections were incubated with primary antibodies for MyHC I (1:100, BA.D5, Developmental Studies Hybridoma Bank (DHSB), Iowa City, IA), MyHC IIa (1:2, SC.71, DSHB), MyHC IIb (1:2, BF-F3, DHSB), and laminin (1:50, Sigma) overnight at 4 °C. Sections were then serially washed with PBS prior to application of appropriate secondary antibodies (Lawrence et al. 2020). Sections were washed, post-fixed in absolute methanol, cover slipped, and imaged using an upright fluorescent microscope (Axio Imager MI, Zeiss). A total of 5 regionally representative images totaling at least 600 total muscle fibers of both medial and lateral gastrocnemius muscle were imaged and quantified for fiber-type-specific CSA. Quantification and fiber-type distribution of laminin-outlined MyHC expressing fibers was performed using the MyoVision automated analysis software (Wen et al. 2018); MyHC IIx expressing fibers were inferred from unstained fibers.
Statistical analyses and scientific rigor
For experiment no. 1, we mimicked our study in adult rats (Lawrence et al. 2020) by comparing massage performed during WB and HS separately to independently explore the effects of massage on unperturbed and disuse loading states (i.e., HS versus HSM and WB versus WBM using two-tailed independent sample t tests). For experiment no. 2, we compared all groups using a one-way ANOVA with Holm–Sidak post hoc. For both experiments, when there was a massage treatment, we compared both the massaged limb and the contralateral non-messaged limb to the appropriate non-massaged control with a two-tailed independent sample t tests. Prior to any statistical analyses, the data were tested for normal distribution and equal variances to determine the appropriate statistical test. All statistical analyses and figures were created using GraphPad Prism 8 (San Diego, CA, USA). All values reported are mean ± standard error (SE) and statistical significance was assumed at p < 0.05. For analyses on muscle tissues, the assessors were blinded to the groups to which the animals were assigned. Assignment to groups was performed using block randomization technique to ensure equal sample size (Suresh 2011).
Results
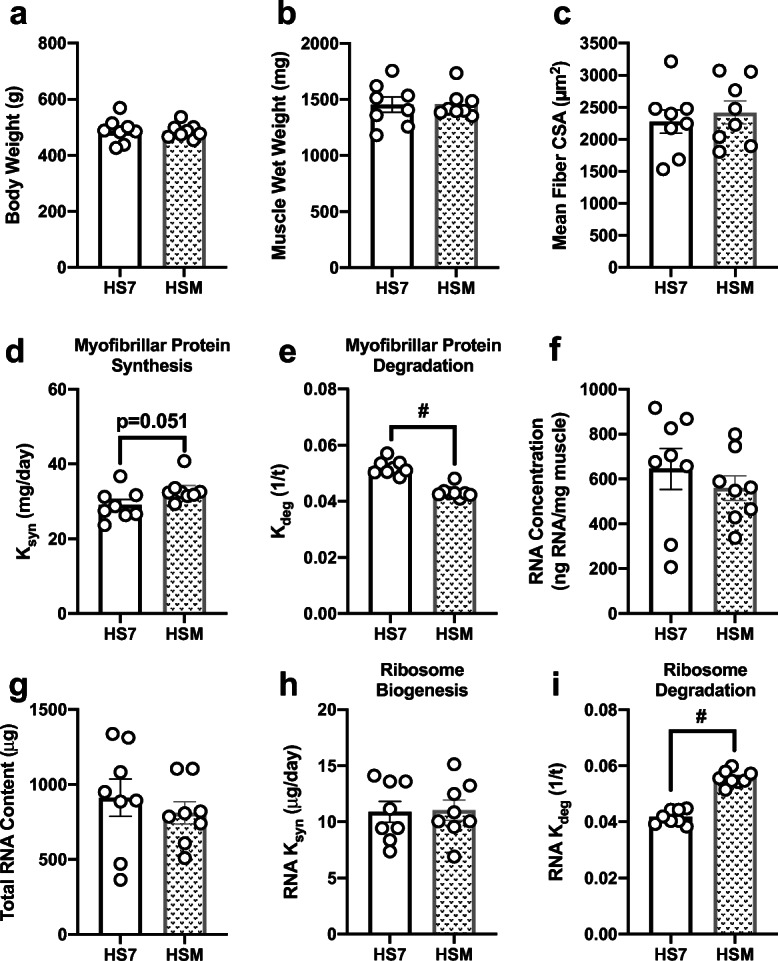
Effects of massage during normal ambulation
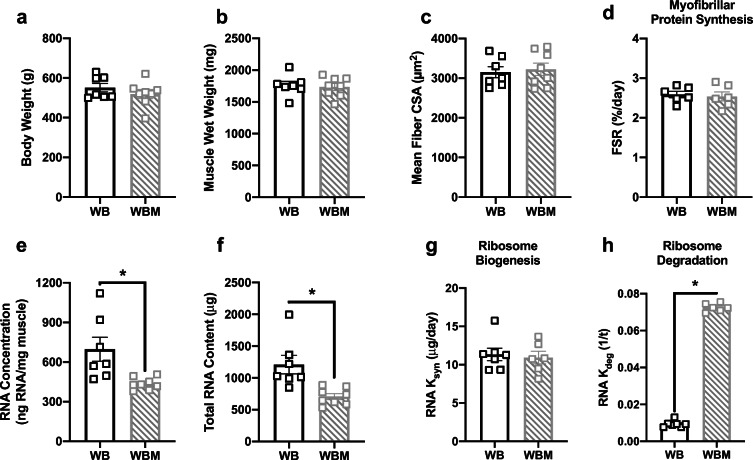
There were no differences in body weight (Fig. 2a), muscle wet weight (Fig. 2b), mean fiber CSA (Fig. 2c), fiber-type-specific CSA (Table 1), fiber-type distribution (Table 2), or myofibrillar protein synthesis (Fig. 2d) when comparing WBM with WB. In contrast, WBM had lower RNA concentration (Fig. 2e) and total RNA content (Fig. 2f), compared with WB. The lower ribosomal content with massage was most likely driven by the higher ribosome degradation in WBM compared with WB (Fig. 2h), since there were no differences in ribosome biogenesis between the groups (Fig. 2g). Levels of intracellular signaling proteins involved in the regulation of anabolic processes such as protein translation, proteasomal degradation, and ribosome biogenesis were not different between WB and WBM (Table 3).
Fig. 2.
Massage during normal ambulatory weight bearing (WB) stimulates ribosome degradation, but has no effect on myofibrillar protein synthesis, ribosome biogenesis, muscle mass, or muscle fiber CSA in the massaged limb. Body weight (a), muscle wet weight (b), mean fiber CSA (c), myofibrillar fractional synthesis rate (FSR) (d), RNA concentration (e), total RNA content (f), and calculated RNA ksyn (g) and RNA kdeg (h) rate from gastrocnemius muscles of WB (n = 7) and WB massaged limb, WBM (n = 8). Values are mean ± SEM. Two-tailed independent sample t tests were used to determine statistical significance between WB and WBM. *p < 0.05, different from WB
Table 1.
Gastrocnemius fiber-type CSA in response to massage
| WB | WBM | HS7 | HSM | WBM-L | HSM-L | |
|---|---|---|---|---|---|---|
| Type I | 2133 ± 191 | 2296 ± 211 | 1786 ± 92 | 1820 ± 71 | 2251 ± 117 | 1777 ± 109 |
| Type IIA | 2095 ± 217 | 2143 ± 193 | 1584 ± 150 | 1766 ± 184 | 2052 ± 143 | 1729 ± 171 |
| Type IIX | 3386 ± 134 | 3379 ± 254 | 2480 ± 96 | 2582 ± 182 | 2840 ± 151* | 2572 ± 204 |
| Type IIB | 4070 ± 291 | 4156 ± 234 | 3327 ± 189 | 3708 ± 177 | 3860 ± 119 | 2870 ± 147 |
| Hybrid | 2136 ± 172 | 1991 ± 113 | 1786 ± 111 | 1878 ± 93 | 1801 ± 103 | 1840 ± 181 |
Cross-sectional area (CSA, μm2) for individual fiber types for WB (n = 7), WB massaged limb WBM (n = 8), HS for 7 days HS7 (n = 8), HS massaged limb HSM (n = 9), WB non-massaged contralateral limb WBM-L (n = 8), and HS non-massaged contralateral limb HSM-L (n = 8). Two-tailed independent sample t tests between the respective control (WB or HS7) and either the massaged limb (WBM or HSM) or contralateral non-massaged limb (WBM-L or HSM-L) were used to determine statistical significance. Values are mean ± SEM. Significance is assumed at p < 0.05
*Significant difference from WB
Table 2.
Gastrocnemius fiber-type distribution in response to massage during weight bearing (WB) and hindlimb suspension (HS)
| WB | WBM | HS7 | HSM | WBM-L | HSM-L | |
|---|---|---|---|---|---|---|
| Type I | 9.3 ± 1.8 | 16.5 ± 2.7 | 13.3 ± 1.7 | 12.6 ± 1.4 | 13.7 ± 2.3 | 13.8 ± 2.0 |
| Type IIA | 14.4 ± 2.4 | 21.4 ± 2.7 | 19.9 ± 4.2 | 19.5 ± 1.7 | 16.9 ± 3.1 | 12.7 ± 1.6 |
| Type IIX | 35.5 ± 6.3 | 27.8 ± 3.5 | 27.6 ± 3.7 | 27.4 ± 2.4 | 27.8 ± 3.3 | 31.5 ± 3.0 |
| Type IIB | 39.6 ± 6.2 | 31.9 ± 3.5 | 37.1 ± 1.3 | 39.3 ± 1.9 | 39.3 ± 3.6 | 40.2 ± 3.5 |
| Hybrid | 1.2 ± 0.3 | 2.4 ± 0.6 | 2.1 ± 0.5 | 1.2 ± 0.3 | 2.4 ± 0.8 | 1.9 ± 0.8 |
Individual fiber-type distribution given as percentages (%) for WB (n = 7), WB massaged limb WBM (n = 8), HS (n = 8), HS massaged limb HSM (n = 8), WB non-massaged contralateral limb WBM-L (n = 8), and HS non-massaged contralateral limb HSM-L (n = 8). Values are mean ± SEM. Two-tailed independent sample t tests between the respective control (WB or HS) and either the massaged limb (WBM or HSM) or contralateral non-massaged limb (WBM-L or HSM-L) were used to determine statistical significance
Table 3.
Western blot analysis of intracellular signaling pathways in massaged limb (WBM or HSM) and contralateral non-massaged limb (WBM-L or HSM-L) in response to massage
| WB | WBM | HS7 | HSM | WBM-L | HSM-L | |
|---|---|---|---|---|---|---|
| Total Akt | 0.94 ± 0.04 | 1.00 ± 0.12 | 1.23 ± 0.12 | 1.28 ± 0.09 | 1.09 ± 0.07 | 1.31 ± 0.05 |
| Phospho-Akt | 0.97 ± 0.14 | 1.31 ± 0.23 | 1.12 ± 0.07 | 1.00 ± 0.15 | 1.01 ± 0.15 | 1.10 ± 0.17 |
| Total FOXO3A | 1.04 ± 0.15 | 0.92 ± 0.09 | 1.05 ± 0.09 | 1.01 ± 0.09 | 0.98 ± 0.08 | 1.19 ± 0.06 |
| Phospho-FOXO3A | 1.08 ± 0.13 | 1.10 ± 0.09 | 1.20 ± 0.08 | 1.30 ± 0.12 | 1.33 ± 0.09 | 1.32 ± 0.08 |
| Total FAK | 4.74 ± 0.61 | 4.38 ± 0.70 | 4.25 ± 0.73 | 4.68 ± 0.59 | 3.89 ± 0.56 | 4.92 ± 0.52 |
| Phospho-FAK | 0.87 ± 0.05 | 1.15 ± 0.15 | 1.00 ± 0.12 | 1.11 ± 0.09 | 0.92 ± 0.12 | 0.94 ± 0.08 |
| Total ERK1/2 | 1.01 ± 0.07 | 1.07 ± 0.03 | 1.19 ± 0.09 | 1.26 ± 0.04 | 1.24 ± 0.10 | 1.11 ± 0.06 |
| Phospho-ERK1/2 | 1.07 ± 0.05 | 1.08 ± 0.05 | 1.08 ± 0.05 | 1.09 ± 0.05 | 1.08 ± 0.05 | 1.13 ± 0.06 |
| Total eEF2 | 1.19 ± 0.10 | 1.18 ± 0.07 | 1.14 ± 0.05 | 1.06 ± 0.07 | 1.24 ± 0.08 | 1.13 ± 0.04 |
| Phospho-eEF2 | 1.05 ± 0.07 | 1.15 ± 0.06 | 0.91 ± 0.09 | 1.03 ± 0.07 | 1.08 ± 0.08 | 1.16 ± 0.12 |
| Total S6K1 | 1.11 ± 0.06 | 1.03 ± 0.09 | 0.95 ± 0.06 | 1.05 ± 0.04 | 1.12 ± 0.02 | 1.04 ± 0.05 |
| Phospho-S6K1 | 1.51 ± 0.21 | 1.47 ± 0.25 | 0.91 ± 0.17 | 0.63 ± 0.07 | 1.76 ± 0.39 | 0.74 ± 0.20 |
| Total 4EBP1 | 0.90 ± 0.02 | 0.91 ± 0.04 | 1.18 ± 0.08 | 1.18 ± 0.07 | 0.87 ± 0.04 | 1.09 ± 0.07 |
| Phospho-4EBP1 | 0.82 ± 0.08 | 0.77 ± 0.07 | 1.19 ± 0.14 | 1.10 ± 0.07 | 0.72 ± 0.07 | 1.22 ± 0.11 |
| Total rpS6 | 1.08 ± 0.15 | 1.10 ± 0.18 | 0.89 ± 0.15 | 0.87 ± 0.10 | 0.84 ± 0.04 | 0.77 ± 0.13 |
| Phospho-rpS6 | 1.33 ± 0.16 | 1.17 ± 0.19 | 0.80 ± 0.16 | 0.72 ± 0.19 | 1.15 ± 0.09 | 0.63 ± 0.17 |
| Total MuRF-1 | 1.04 ± 0.11 | 1.02 ± 0.09 | 1.06 ± 0.09 | 1.10 ± 0.09 | 1.05 ± 0.06 | 1.06 ± 0.06 |
| Total UBF-1 | 0.81 ± 0.13 | 0.91 ± 0.08 | 0.72 ± 0.07 | 0.85 ± 0.08 | 0.79 ± 0.12 | 0.78 ± 0.11 |
| Phospho-UBF-1 | 1.28 ± 0.11 | 1.15 ± 0.08 | 0.97 ± 0.12 | 1.06 ± 0.06 | 1.28 ± 0.13 | 1.01 ± 0.10 |
| Total c-Myc | 1.44 ± 0.13 | 1.16 ± 0.19 | 1.30 ± 0.21 | 1.31 ± 0.21 | 1.11 ± 0.19 | 0.97 ± 0.18 |
Total and phosphorylated analysis of signaling proteins for WB (n = 7), WB massaged limb WBM (n = 8), HS for 7 days HS7 (n = 8), HS massaged limb HSM (n = 8), WB non-massaged contralateral limb WBM-L (n = 8), and HS non-massaged contralateral limb HSM-L (n = 8). Two-tailed independent sample t tests between the respective control (WB or HS) and either the massaged limb (WBM or HSM) or contralateral non-massaged limb (WBM-L or HSM-L) was used to determine statistical significance. All values are in arbitrary density units. Values are mean ± SEM. Representative images for the data within this table are in Supplemental Fig. 1
Effects of massage during hindlimb suspension
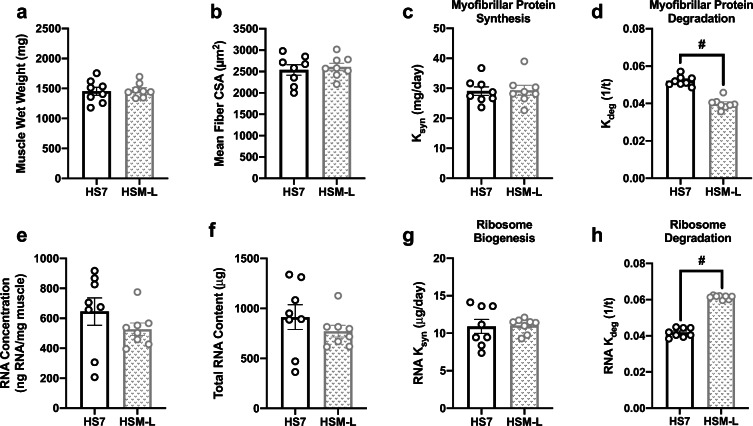
There were no differences in body weight (Fig. 3a), muscle wet weight (Fig. 3b), mean fiber CSA (Fig. 3c), fiber-type-specific CSA (Table 1), or fiber-type distribution (Table 2) comparing HSM with HS7. However, HSM displayed a trend (p = 0.051) for higher myofibrillar protein synthesis rate (Fig. 3d) and had significantly lower myofibrillar protein degradation rate (Fig. 3e) compared with HS7. There were no differences in RNA concentration (Fig. 3f), total RNA content (Fig. 3g), or ribosome biogenesis (Fig. 3h), but there was significantly higher ribosome degradation in HSM compared with HS7 (Fig. 3i). Levels of intracellular signaling markers were not different between HS and HSM (Table 3).
Fig. 3.
Massage during hindlimb suspension (HS) alters myofibrillar protein turnover and ribosomal turnover, without any effect on muscle mass or size. Body weight (a), muscle wet weight (b), mean fiber CSA (c), calculated myofibrillar ksyn (d) and myofibrillar kdeg (e), RNA concentration (f), total RNA content (g), and calculated RNA ksyn (g, h) and RNA kdeg (i) from gastrocnemius muscles following 7 days of HS (HS7, n = 8) and HS massaged limb, HSM (n = 8). Values are mean ± SEM. Independent sample t tests were used to determine statistical significance between HS7 and HSM: #p < 0.05, different from HS7
Effects of massage during reloading after hindlimb suspension
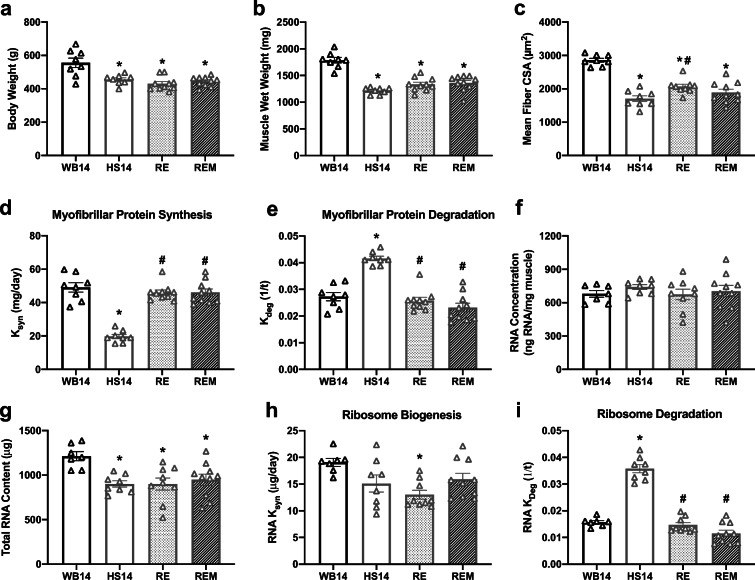
HS14, RE, and REM had significantly lower body weights compared with WB14 (Fig. 4a). Furthermore, HS14 had significant atrophy of the gastrocnemius as measured by muscle wet weight (Fig. 4b) and mean fiber CSA (Fig. 4c), compared with WB14. Moreover, reloading with or without massage resulted in failed regrowth as evidenced by significantly lower muscle wet weights in RE and REM compared with WB14 and no difference with HS14 (Fig. 4b). Also, muscle fiber CSA was not different between REM and HS or RE, despite RE having a higher mean fiber CSA compared with HS14, indicating no effect of massage on muscle fiber size (Fig. 4c). HS14 had lower myofibrillar protein synthesis (Fig. 4d) and higher myofibrillar protein degradation (Fig. 4e) compared with WB14. RE exhibited a reversal of the effects of HS, as RE had significantly higher myofibrillar protein synthesis (Fig. 4d) and lower myofibrillar protein degradation compared with HS14 (Fig. 4e). Massage did not augment protein synthesis (Fig. 4d) or protein degradation (Fig. 4e) during reloading as demonstrated by the lack of difference between these variables when comparing RE with REM. No differences were observed across any group for RNA concentration (Fig. 4f), but disuse (HS14) and reloading without (RE) and with (REM) massage displayed significantly lower total RNA content compared with WB14 (Fig. 3g). HS14 had a similar rate of ribosome biogenesis (Fig. 4h), but significantly higher ribosome degradation (Fig. 4i) compared with WB14. RE had significantly lower ribosome biogenesis rate compared with WB14 (Fig. 4h). In addition, ribosome degradation was higher in HS14 compared with WB14 and this was reversed with reloading with and without massage (Fig. 4i). Also, there were no differences in fiber-type distribution (Table 4).
Fig. 4.
Massage during reloading (RE) following hindlimb suspension (HS) has no effect on myofibrillar protein turnover, ribosome turnover, or muscle mass or size. Body weight (a), muscle wet weight (b), mean fiber CSA (c), calculated myofibrillar ksyn (d) and myofibrillar kdeg (e), RNA concentration (f), total RNA (g), and calculated RNA ksyn (h) and RNA kdeg (i) from gastrocnemius muscles of WB14 (n = 8), weight bearing for 14 days; HS14 (n = 8), HS for 14 days; RE (n = 10), 14 days of HS followed by 7 days of RE; REM (n = 10), 14 days of HS followed by 7 days of reloading with massage through CCL. Values are mean ± SEM. A one-way ANOVA with a Holm–Sidak post hoc test for multiple comparisons were used to determine statistical significance between groups: *p < 0.05, different from WB14; #p < 0.05, different from HS14
Table 4.
Gastrocnemius fiber-type distribution in response to massage during reloading (RE)
| WB14 | HS14 | RE | REM | |
|---|---|---|---|---|
| Type I | 10.8 ± 2.8 | 10.2 ± 1.6 | 10.3 ± 1.7 | 11.7 ± 2.0 |
| Type IIA | 13.2 ± 2.0 | 8.0 ± 1.5 | 11.1 ± 0.8 | 12.7 ± 1.4 |
| Type IIB | 44.3 ± 3.0 | 45.5 ± 4.7 | 48.3 ± 3.5 | 45.1 ± 2.4 |
| Type IIX | 29.9 ± 3.0 | 34.2 ± 2.8 | 26.8 ± 1.9 | 28.3 ± 1.6 |
Individual fiber-type distribution given as percentages (%) for weight bearing WB (n = 8), hindlimb suspension for 14 days HS14 (n = 8), reloading RE (n = 10), and RE massaged limb REM (n = 10). Values are mean ± SEM. A one-way ANOVA with Holm–Sidak post hoc analyses were used to determine statistical significance
Cross-over effects of massage during WB
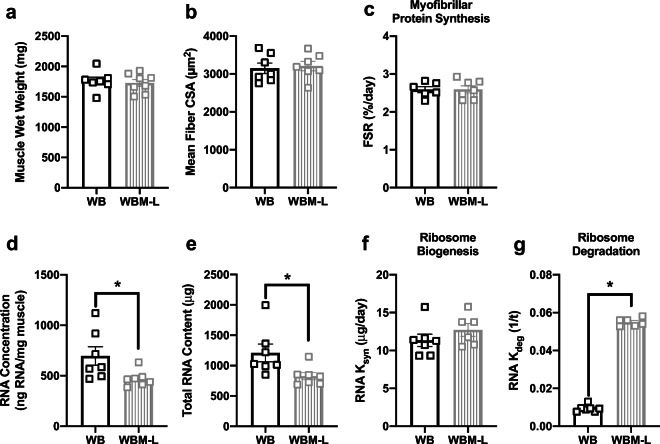
Massage of the right gastrocnemius had no impact on the muscle wet weight (Fig. 5a) or mean fiber CSA (Fig. 5b) in the non-massaged left gastrocnemius (WBM-L) compared with WB right gastrocnemius. WBM-L had significantly smaller type IIX-specific CSA (Table 1) compared with WB, but no other differences were observed in any other fiber-type-specific CSA (Table 1) or fiber-type distribution (Table 2). WBM-L displayed no differences in myofibrillar protein synthesis compared with WB (Fig. 5c). However, WBM-L had less ribosomes compared with WB as measured by RNA concentration (Fig. 5d) and total RNA content (Fig. 5e). The lower ribosome content in WBM-L was associated with no difference in ribosome biogenesis (Fig. 5f), but significantly higher ribosome degradation (Fig. 5g) compared with WB. Levels of intracellular signaling proteins assessed were not different between WB and WBM-L (Table 3).
Fig. 5.
Massage during normal ambulatory weight bearing (WB) stimulates ribosome degradation, but has no effect on myofibrillar protein synthesis, ribosome biogenesis, muscle mass, or muscle size in the contralateral non-massaged limb. Muscle wet weight (a), mean fiber CSA (b), myofibrillar fractional synthesis rate (FSR) (c), RNA concentration (d), total RNA content (e), and calculated RNA ksyn (f) and RNA kdeg (g) from gastrocnemius muscles of WB (n = 7) and WB contralateral non-massaged limb, WBM-L (n = 8). Values are mean ± SEM. Two-tailed independent sample t tests were used to determine statistical significance between WB and WBM-L: *p < 0.05, different from WB
Cross-over effects of massage during HS
The contralateral non-massaged left limb (HSM-L) in the animals that received massage compared with animals who did not receive massage during disuse (HS7) displayed no differences in muscle wet weight (Fig. 6a), mean fiber CSA (Fig. 6b), fiber-type-specific CSA (Table 1), or fiber-type distribution (Table 2). However, HSM-L exhibited a significantly lower myofibrillar protein degradation rate (Fig. 6d) compared with HS7. No differences were observed in myofibrillar protein synthesis rate (Fig. 6c), RNA concentration (Fig. 6e), or total RNA content (Fig. 6f) between HSM-L and HS7. Despite no differences in ribosome content, HSM-L displayed a significantly elevated ribosome degradation rate (Fig. 6h). There were no differences in any intracellular signaling markers assessed between HSM-L and HS7 (Table 3).
Fig. 6.
Massage during hindlimb suspension (HS) alters myofibrillar protein turnover, without an effect on ribosome biogenesis or muscle mass or size in the contralateral non-massaged limb. Muscle wet weight (a), mean fiber CSA (b), calculated myofibrillar ksyn (c) and myofibrillar kdeg (d), RNA concentration (e), total RNA content (f), and calculated RNA ksyn (g) and RNA kdeg (h) from gastrocnemius muscles following 7 days of HS (HS7, n = 8) and HS non-massaged limb, HSM-L (n = 8). Values are mean ± SEM. Independent sample t tests were used to determine statistical significance between HS7 and HSM-L: #p < 0.05, different from HS7
Cross-over effects of massage during RE
There were no differences in muscle wet weight (Fig. 7a), mean fiber CSA (Fig. 7b), myofibrillar protein synthesis rate (Fig. 7c), or myofibrillar protein degradation rate (Fig. 7d) in REM-L versus RE. However, REM-L had higher RNA concentration (Fig. 7e) and total RNA content (Fig. 7f) compared with RE. Furthermore, the increased ribosome content was associated with significantly higher ribosome biogenesis (Fig. 7g) and lower ribosome degradation (Fig. 7h) in REM-L compared with RE.
Fig. 7.
Massage during reloading (RE) following hindlimb suspension (HS) stimulates ribosomal turnover, but has no effect on myofibrillar protein turnover or muscle mass or size. Muscle wet weight (a), mean fiber CSA (b), calculated myofibrillar ksyn (c) and myofibrillar kdeg (d), RNA concentration (e), total RNA content (f), and calculated RNA ksyn (g) and RNA kdeg (h) from gastrocnemius muscles of RE (n = 10) and RE non-massaged limb, REM-L (n = 10). Values are mean ± SEM. Independent sample t tests were used to determine statistical significance between RE and REM-L: ^p < 0.05, different from RE
Discussion
The primary goal of this study was to determine if massage during disuse atrophy and reloading in aged rats has similar anabolic benefits as those previously observed in adult rats (Lawrence et al. 2020; Miller et al. 2018). Herein, we show that the impact of massage on aged muscle was context dependent. In particular, we show that massage does not augment protein turnover, ribosome turnover, or muscle regrowth during reloading in aged rats. However, aged muscle that was massaged during disuse atrophy had greater protein synthesis and less protein degradation compared with muscle that did not receive massage, although this did not translate into measurable preservation of muscle mass or size. A novel finding of this study was that massaged muscle had higher levels of ribosome degradation during weight-bearing conditions and reloading and suggests massage as a mechanotherapy may have an impact on ribosome turnover in aged muscle. Collectively, our data demonstrate that massage has limited utility for promoting anabolism, preserving muscle mass during disuse, or improving muscle regrowth during reloading in aged muscle.
Massage elevates protein synthesis and lowers degradation during disuse
Aged muscle exhibited small, but significant, changes in protein turnover with massage during disuse, although muscle atrophy was not decreased. Massage during disuse was associated with a 14% higher myofibrillar protein synthesis and 17% lower myofibrillar protein degradation rate compared with normal disuse. The magnitude of the difference in myofibrillar protein synthesis in aged muscle in response to massage was much lower compared with the 45% elevation we previously reported for adult rats (Lawrence et al. 2020). In addition, muscle from adult rats did not show a difference in protein degradation with massage, unlike in the aged, which suggest differences in the regulation of protein turnover with aging and disuse. Recent work from Miller et al. (2019) using long-term (14 days) D2O labeling in F344/BN male rats found that the fast-twitch predominant plantaris and tibialis anterior hindlimb muscles displayed elevated myofibrillar protein synthesis rates in aged compared with adult muscle. We did not compare gastrocnemius protein synthesis rates between adult and aged rats, but previous work in rat gastrocnemius using short-term labeling approaches has shown elevated myofibrillar protein synthesis rates in aged compared with adult (Fluckey et al. 1996; Kimball et al. 2004). Thus, it is possible that the elevation in myofibrillar protein synthesis observed with advancing age in muscle may also give rise to a concomitant increase in myofibrillar protein degradation, which may help explain the differences observed between adult and aged muscle in response to massage during disuse. More work using long-term assessments of both aspects of protein turnover is clearly needed to help delineate the effects of massage, disuse, and mechanical loading in muscle with aging.
Aged muscle often exhibits a blunted protein synthetic response to mechanical stimuli, such as resistance exercise and other mechanotherapies (Kumar et al. 2009; Miller et al. 2019; West et al. 2018) and this “anabolic resistance” is often hypothesized to be one of the primary culprits driving sarcopenia (Barclay et al. 2019; Burd et al. 2013). Our current data support an age-related anabolic resistance as evidenced by a blunted elevation in myofibrillar protein synthesis from massage when comparing the magnitude of changes between adult and aged rats. However, neither the adult rats from our previous work (Lawrence et al. 2020) nor the aged rats in this study had an attenuated loss of muscle mass with massage, suggesting that the current massage treatment regimen is insufficient to overcome the influence of chronic disuse on protein turnover and loss of muscle mass in adult or aged muscle. Nevertheless, despite the lack of effect on muscle loss, massage during disuse in both adult and aged rats does improve myofibrillar protein turnover, albeit at differing magnitudes, and may influence myofibrillar protein quality and/or preserve function. Further work is needed to validate whether muscle function is preserved or improved by massage or whether the addition of another anabolic stimulus would enhance the response.
Massage does not improve muscle recovery during reloading in aged muscle
Massage had no impact on protein turnover during reloading and was not associated with higher muscle mass and CSA. The lack of impact of massage on myofibrillar protein synthesis (measured as ksyn) during reloading is similar to what we had previously found in adult muscle (Miller et al. 2018). Furthermore, we found no impact of massage on myofibrillar protein degradation in aged muscle, contrasting the elevation in protein degradation caused by massage in our previous work in adult rats (Miller et al. 2018). Markers of protein degradation, such as Murf1 and Mafbx, are often elevated with mechanical and hypertrophic stimuli in muscle, and inhibition of proper proteasome activity is detrimental to muscle growth during an overload stimulus, indicating that protein turnover is needed to increase muscle size (Baehr et al. 2014; Fernandez-Gonzalo et al. 2013; Louis et al. 2007; Marino et al. 2008; White et al. 2015). Therefore, the inability of massage to alter protein degradation in aged muscle may partially explain why massage was unable to further elevate the recovery in muscle mass with reloading, unlike what we observed in adult rats (Miller et al. 2018).
Ribosome degradation dictates ribosome loss during disuse and is elevated by massage
Disuse atrophy is associated with a decrease in ribosome content (Haddad et al. 2006; Lawrence et al. 2020; Miller et al. 2018) which is typically used as a marker for ribosome biogenesis and translational capacity (Kirby et al. 2015; West et al. 2018; You et al. 2015). Furthermore, aged muscle shows an inability to increase ribosome content with an overload stimulus (Kirby et al. 2015; Stec et al. 2015). Taken together, these data have led many studies to conclude that decreased ribosome biogenesis is a primary culprit dictating the loss of translational capacity and loss of muscle mass with disuse and aging. However, we previously showed in adult rats that lower ribosome content from disuse atrophy was primarily the result of elevated ribosome degradation and is not due to reduced ribosome biogenesis (Lawrence et al. 2020). We now show that this paradigm is similar with aging, as atrophying muscle from aged rats had highly elevated ribosome degradation and no difference in ribosome biogenesis compared with weight-bearing rats. However, the response to massage was distinct in aged rats since it increased ribosome degradation, unlike the reduction in adult muscle (Lawrence et al. 2020). Elevated ribosome degradation and lower ribosome content in weight-bearing conditions with massage demonstrates that the ribosome degradation response to the mechanical stimulus is consistent across multiple conditions in aged muscle. The mechanisms dictating the attenuation of ribosome degradation by massage in adult but not aged muscle remain unknown, but may be the result of differential responses in mechanotransduction pathways (as discussed below). In fact, the role of ribosome biogenesis and degradation with aging, atrophy, or muscle growth in response to mechanical loading is not well-defined. Similar to the patterns of protein synthesis, aged muscle typically has either an equivalent or elevated basal ribosome content and biogenesis compared with younger muscle (Kirby et al. 2015; Miller et al. 2019; Roberts et al. 2010; Stec et al. 2015; West et al. 2018). While not directly compared within our current study, our data agree that aged rats have similar total ribosome content compared with adult rats (Lawrence et al. 2020). We previously showed that aged muscle has elevated RNA oxidation, which is exacerbated by disuse (Hofer et al. 2008). It is possible that elevated RNA oxidation interferes with the ability of massage to attenuate ribosome degradation in aged muscle, but this remains to be investigated. Nevertheless, our current results add further support that changes in ribosome content with disuse and with reloading are highly dictated by ribosome degradation and are not simply driven by ribosome biogenesis.
Massage does not alter intracellular signaling during weight bearing or disuse
The lack of any changes in intracellular signaling with massage during WB or HS in aged muscle matches our previous investigations in adult muscle during disuse and reloading (Lawrence et al. 2020; Miller et al. 2018). We harvested muscles 24 h after the last bout of massage since resistance exercise elevates anabolic signaling for up to 48 h (Bolster et al. 2003; Cuthbertson et al. 2006; MacDougall et al. 1995; Parkington et al. 2004; Phillips et al. 1997). However, the mechanical stimulus from resistance training is likely far more robust than massage and, as such, there may have been transient changes in intracellular signaling that returned to basal levels by the time we harvested the tissue. The intracellular signaling response may also be dampened after multiple bouts of massage as the tissue adapts to the mechanical stimulus. Both adult and aged muscle were previously shown to increase the protein abundance of Murf1 24 h after a single bout of massage (Van Pelt et al. 2019). In the current study, however, Murf1 protein expression was not altered by massage in either weight-bearing or disuse conditions. Murf1 is well established as a critical component in proteasomal degradation and atrophy (Bodine et al. 2001; Gumucio and Mendias 2013), yet Murf1 and protein degradation signaling are also elevated in response to hypertrophic stimuli in both rodents and humans for normal remodeling adaptations (Baehr et al. 2014; Fernandez-Gonzalo et al. 2013; Louis et al. 2007; Marino et al. 2008; White et al. 2015). A single bout of massage may transiently increase protein degradation signaling similar to other hypertrophic stimuli and initiate muscle remodeling, but this response is returned to basal conditions after multiple bouts of massage. The lack of intracellular signaling may also be the result of a more fibrotic and stiffer extracellular matrix (ECM) in aged muscle that dampens mechanosensitivity (Brack et al. 2007; Gao et al. 2008; Wood et al. 2014). Adult muscle has higher expression of integrins compared with aged muscle and this was associated with higher activation of ILK after a single bout of massage (Van Pelt et al. 2019). Additionally, aged muscle was protected from elevated membrane permeability after a single bout of massage when compared with adult muscle, which we believe was the result of increased ECM in the aged (Alnaqeeb et al. 1984; Gosselin et al. 1994; Hunt et al. 2019; Kovanen et al. 1984). It is therefore possible that the more abundant and stiffer ECM in aged muscle diminishes the transmission of mechanical loads to the fibers and dampens mechanosensitivity. It remains to be determined whether an increase in the force applied with massage may overcome the dampening of mechanotransduction caused by the stiffer ECM. Higher doses or different volumes of mechanical stimuli have been shown to overcome the anabolic resistance in aged muscle and cause hypertrophy (Bickel et al. 2011). The massage stimulus we use in this study was originally developed to mimic the force applied during Swedish massage in humans and was calculated using allometric scaling (Butterfield et al. 2008). The 4.5 N load developed from these methods has successfully caused a number of beneficial adaptations in adult skeletal muscle including enhanced recovery from eccentric exercise (Butterfield et al. 2008), immunomodulation (Waters-Banker et al. 2014), and improved regrowth after disuse (Miller et al. 2018), but may not be optimal in aged muscle.
Massage stimulates ribosome turnover in contralateral non-massaged muscle
A clinically important finding from our previous studies on massage as a mechanotherapy is the cross-over effect to the contralateral gastrocnemius and even plantaris muscle that did not directly receive the massage (Lawrence et al. 2020; Miller et al. 2018). It is established that unilateral resistance exercise can cause increased strength gains in the contralateral musculature (Carroll et al. 2006; Munn et al. 2004; Munn et al. 2005). It has also been shown that muscle size can be spared during atrophic conditions via a cross-over effect of resistance exercise (Andrushko et al. 2018a, b). The left non-massaged gastrocnemius muscle in our aged rats undergoing weight-bearing conditions or hindlimb suspension had similar elevations in ribosome degradation in response to massage as the massaged muscle. Interestingly, the cross-over effect during reloading conditions differed from disuse and weight-bearing conditions as the contralateral gastrocnemius had elevated ribosome content as a consequence of elevated ribosome biogenesis and reduced degradation. The exact mechanisms dictating this cross-over effect remain unknown but may be neurally mediated, controlled by endocrine-like factors (i.e., myokines, extracellular vesicles), or a combination of both (Guay and Regazzi 2017; Lee and Jun 2019; Ruddy and Carson 2013; Whitham et al. 2018).
Limitations
There are limitations to our approach for measuring protein and RNA turnover because it is not possible to make sequential measurements of RNA or protein content in the same animal. Therefore, we calculated a change in protein and RNA content from the average value immediately prior to interventions as has been performed in the past by others (Bederman et al. 2015) and us (Lawrence et al. 2020; Miller et al. 2018). Finally, measuring intracellular signaling 24 h after the last bout of massage may have resulted in missing acute, transient signaling changes. Yet, the measurements at 24 h did allow us to directly compare with our previous findings in adult muscle (Lawrence et al. 2020). Future work is needed to determine the acute (minutes to hours) intracellular signaling response to massage in adult and aged muscle.
Conclusions
In summary, massage at the dose provided in this study is not an effective mechanotherapy for preventing muscle loss during disuse or augmenting muscle growth during recovery from disuse in aged muscle. The lack of robust effects of massage in aged muscle warrants additional research to identify whether different massage load, frequency, or duration could mimic its anabolic effects in adult muscle.
Electronic supplementary material
(PDF 5634 kb)
Availability of data and material
Data available upon request.
Authors’ contributions
All experimentation was performed at the University of Kentucky, except for the analysis of protein and RNA synthesis, and western analyses, which were performed at the Oklahoma Medical Research Foundation. M.L. and D.V.P. were responsible for acquisition, analysis, and interpretation of data, and drafting and revising the manuscript. B.M. was responsible for acquisition, analysis, and interpretation of data and for critically revising the manuscript. A.C., E.H., Z.H., J.L., J.R., and F.P. were responsible for acquisition, analysis, and interpretation of data; T.B. and E.D.V. were responsible for conception and design of the experiments, acquisition of data, interpretation of the results, and critically revising the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding information
This work was supported by National Institute of Health grants AT009268 and AG042699 (E.D.V, T.B., B.M.). M.L. was supported by a National Institute of Health, National Institute of Aging Training Grant AG052363.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All animal procedures were conducted in accordance with institutional guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committee of the University of Kentucky. The study was conducted in adherence to the NIH Guide for the Care and Use of Laboratory Animals.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Marcus M. Lawrence and Douglas W. Van Pelt are co-first authors.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat. 1984;139(Pt 4):677–689. [PMC free article] [PubMed] [Google Scholar]
- Andrushko JW, Gould LA, Farthing JP. Contralateral effects of unilateral training: sparing of muscle strength and size after immobilization. Appl Physiol Nutr Metab. 2018;43:1131–1139. doi: 10.1139/apnm-2018-0073. [DOI] [PubMed] [Google Scholar]
- Andrushko JW, Lanovaz JL, Bjorkman KM, Kontulainen SA, Farthing JP. Unilateral strength training leads to muscle-specific sparing effects during opposite homologous limb immobilization. J Appl Physiol. 2018;124:866–876. doi: 10.1152/japplphysiol.00971.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol. 2014;5:69. doi: 10.3389/fphys.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay RD, Burd NA, Tyler C, Tillin NA, Mackenzie RW. The role of the IGF-1 signaling cascade in muscle protein synthesis and anabolic resistance in aging skeletal muscle. Front Nutr. 2019;6:146. doi: 10.3389/fnut.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederman IR, Lai N, Shuster J, Henderson L, Ewart S, Cabrera ME. Chronic hindlimb suspension unloading markedly decreases turnover rates of skeletal and cardiac muscle proteins and adipose tissue triglycerides. J Appl Physiol. 2015;119:16–26. doi: 10.1152/japplphysiol.00004.2014. [DOI] [PubMed] [Google Scholar]
- Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Med Sci Sports Exerc. 2011;43:1177–1187. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara T, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Burd NA, Gorissen SH, van Loon LJ. Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev. 2013;41:169–173. doi: 10.1097/JES.0b013e318292f3d5. [DOI] [PubMed] [Google Scholar]
- Busch R, Kim YK, Neese RA, Schade-Serin V, Collins M, Awada M, Gardner JL, Beysen C, Marino ME, Misell LM, Hellerstein MK. Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim Biophys Acta. 2006;1760:730–744. doi: 10.1016/j.bbagen.2005.12.023. [DOI] [PubMed] [Google Scholar]
- Butterfield TA, Zhao Y, Agarwal S, Haq F, Best TM. Cyclic compressive loading facilitates recovery after eccentric exercise. Med Sci Sports Exerc. 2008;40:1289–1296. doi: 10.1249/MSS.0b013e31816c4e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Herbert RD, Munn J, Lee M, Gandevia SC. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101:1514–1522. doi: 10.1152/japplphysiol.00531.2006. [DOI] [PubMed] [Google Scholar]
- Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, Burant CJ, Landefeld CS. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- Drake JC, Peelor FF, 3rd, Biela LM, Watkins MK, Miller RA, Hamilton KL, Miller BF. Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J Gerontol A Biol Sci Med Sci. 2013;68:1493–1501. doi: 10.1093/gerona/glt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JC, Bruns DR, Peelor FF, 3rd, Biela LM, Miller RA, Hamilton KL, Miller BF. Long-lived crowded-litter mice have an age-dependent increase in protein synthesis to DNA synthesis ratio and mTORC1 substrate phosphorylation. Am J Physiol Endocrinol Metab. 2014;307:E813–E821. doi: 10.1152/ajpendo.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake JC, Bruns DR, Peelor FF, 3rd, Biela LM, Miller RA, Miller BF, Hamilton KL. Long-lived Snell dwarf mice display increased proteostatic mechanisms that are not dependent on decreased mTORC1 activity. Aging Cell. 2015;14:474–482. doi: 10.1111/acel.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English KL, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, Lundberg TR, Tesch PA. Acute molecular responses in untrained and trained muscle subjected to aerobic and resistance exercise training versus resistance training alone. Acta Physiol. 2013;209:283–294. doi: 10.1111/apha.12174. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, McCarthy JJ. Regulation of ribosome biogenesis in skeletal muscle hypertrophy. Physiology. 2019;34:30–42. doi: 10.1152/physiol.00034.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol A Biol Sci Med Sci. 1996;51:B323–B330. doi: 10.1093/gerona/51a.5.b323. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Parkington JD, Carambula S, Fielding RA. Age-associated decrease in contraction-induced activation of downstream targets of Akt/mTor signaling in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1080–R1086. doi: 10.1152/ajpregu.00277.2005. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin LE, Martinez DA, Vailas AC, Sieck GC. Passive length-force properties of senescent diaphragm: relationship with collagen characteristics. J Appl Physiol. 1994;76:2680–2685. doi: 10.1152/jappl.1994.76.6.2680. [DOI] [PubMed] [Google Scholar]
- Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19(Suppl 1):137–146. doi: 10.1111/dom.13027. [DOI] [PubMed] [Google Scholar]
- Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol. 2006;100:433–441. doi: 10.1152/japplphysiol.01203.2005. [DOI] [PubMed] [Google Scholar]
- Hirani V, Blyth F, Naganathan V, le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: the concord health and ageing in men project. J Am Med Dir Assoc. 2015;16:607–613. doi: 10.1016/j.jamda.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol. 2008;43:563–570. doi: 10.1016/j.exger.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger TA, Mateja RD, Chin ER, Andrews JL, Esser KA. Aging does not alter the mechanosensitivity of the p38, p70S6k, and JNK2 signaling pathways in skeletal muscle. J Appl Physiol. 2005;98:1562–1566. doi: 10.1152/japplphysiol.00870.2004. [DOI] [PubMed] [Google Scholar]
- Hunt ER, Confides AL, Abshire SM, Dupont-Versteegden EE, Butterfield TA. Massage increases satellite cell number independent of the age-associated alterations in sarcolemma permeability. Physiol Rep. 2019;7(17):e14200. doi: 10.14814/phy2.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy P, Barnhill E, Gray C, Brown C, van Beek EJR, Roberts N, Greig CA. Magnetic resonance elastography (MRE) shows significant reduction of thigh muscle stiffness in healthy older adults. Geroscience. 2020;42:311–321. doi: 10.1007/s11357-019-00147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball SR, O’Malley JP, Anthony JC, Crozier SJ, Jefferson LS. Assessment of biomarkers of protein anabolism in skeletal muscle during the life span of the rat: sarcopenia despite elevated protein synthesis. Am J Physiol Endocrinol Metab. 2004;287:E772–E780. doi: 10.1152/ajpendo.00535.2003. [DOI] [PubMed] [Google Scholar]
- Kirby TJ, Lee JD, England JH, Chaillou T, Esser KA, McCarthy JJ. Blunted hypertrophic response in aged skeletal muscle is associated with decreased ribosome biogenesis. J Appl Physiol. 2015;119:321–327. doi: 10.1152/japplphysiol.00296.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortebein P. RE: effect of short-term hospitalization on functional capacity in patients not restricted to bed. Am J Phys Med Rehabil. 2008;87:425–425; author reply 426. doi: 10.1097/PHM.0b013e31816dd045. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1076–1081. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- Kovanen V, Suominen H, Heikkinen E. Collagen of slow twitch and fast twitch muscle fibres in different types of rat skeletal muscle. Eur J Appl Physiol. 1984;52:235–242. doi: 10.1007/BF00433399. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerwaard B, Nieuwenhuizen AG, de Boer VCJ, Keijer J. In vivo assessment of mitochondrial capacity using NIRS in locomotor muscles of young and elderly males with similar physical activity levels. Geroscience. 2020;42:299–310. doi: 10.1007/s11357-019-00145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MM, Van Pelt DW, Confides AL, Hunt ER, Hettinger ZR, Laurin JL, Reid JJ, Peelor FF 3rd, Butterfield TA, Dupont-Versteegden EE, Miller BF. Massage as a mechanotherapy promotes skeletal muscle protein and ribosomal turnover but does not mitigate muscle atrophy during disuse in adult rats. Acta Physiol. 2020;e13460. 10.1111/apha.13460. [DOI] [PMC free article] [PubMed]
- Lee JH, Jun HS. Role of myokines in regulating skeletal muscle mass and function. Front Physiol. 2019;10:42. doi: 10.3389/fphys.2019.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol. 2007;103:1744–1751. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- Magne H, Savary-Auzeloux I, Vazeille E, Claustre A, Attaix D, Anne L, Véronique SL, Philippe G, Dardevet D, Combaret L. Lack of muscle recovery after immobilization in old rats does not result from a defect in normalization of the ubiquitin-proteasome and the caspase-dependent apoptotic pathways. J Physiol. 2011;589:511–524. doi: 10.1113/jphysiol.2010.201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino JS, Tausch BJ, Dearth CL, Manacci MV, McLoughlin TJ, Rakyta SJ, Linsenmayer MP, Pizza FX. Beta2-integrins contribute to skeletal muscle hypertrophy in mice. Am J Physiol Cell Physiol. 2008;295:C1026–C1036. doi: 10.1152/ajpcell.212.2008. [DOI] [PubMed] [Google Scholar]
- Marsh AP, Rejeski WJ, Espeland MA, Miller ME, Church TS, Fielding RA, Gill TM, Guralnik JM, Newman AB, Pahor M, for the LIFE Study Investigators (see Appendix 1) Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) J Gerontol A Biol Sci Med Sci. 2011;66:1376–1383. doi: 10.1093/gerona/glr158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis AD, Naylor BC, Carson RH, Evans E, Harwell J, Knecht J, Hexem E, Peelor FF, III, Miller BF, Hamilton KL, Transtrum MK, Bikman BT, Price JC. Mechanisms of in vivo ribosome maintenance change in response to nutrient signals. Mol Cell Proteomics. 2017;16:243–254. doi: 10.1074/mcp.M116.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Wolff CA, Peelor FF, 3rd, Shipman PD, Hamilton KL. Modeling the contribution of individual proteins to mixed skeletal muscle protein synthetic rates over increasing periods of label incorporation. J Appl Physiol. 2015;118:655–661. doi: 10.1152/japplphysiol.00987.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF, 3rd, Butterfield TA, Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol. 2018;596:83–103. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Baehr LM, Musci RV, Reid JJ, Peelor FF, 3rd, Hamilton KL, Bodine SC. Muscle-specific changes in protein synthesis with aging and reloading after disuse atrophy. J Cachexia Sarcopenia Muscle. 2019;10:1195–1209. doi: 10.1002/jcsm.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro T, Brightwell CR, Deer RR, Graber TG, Galvan E, Fry CS, Volpi E, Rasmussen BB. Muscle protein anabolic resistance to essential amino acids does not occur in healthy older adults before or after resistance exercise training. J Nutr. 2018;148:900–909. doi: 10.1093/jn/nxy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoni L, Malmezat T, Valluy MC, Houlier ML, Attaix D, Mirand PP. Lower recovery of muscle protein lost during starvation in old rats despite a stimulation of protein synthesis. Am J Physiol Endocrinol Metab. 1999;277:E608–E616. doi: 10.1152/ajpendo.1999.277.4.E608. [DOI] [PubMed] [Google Scholar]
- Munn J, Herbert RD, Gandevia SC. Contralateral effects of unilateral resistance training: a meta-analysis. J Appl Physiol. 2004;96:1861–1866. doi: 10.1152/japplphysiol.00541.2003. [DOI] [PubMed] [Google Scholar]
- Munn J, Herbert RD, Hancock MJ, Gandevia SC. Training with unilateral resistance exercise increases contralateral strength. J Appl Physiol. 2005;99:1880–1884. doi: 10.1152/japplphysiol.00559.2005. [DOI] [PubMed] [Google Scholar]
- Murach KA, Confides AL, Ho A, Jackson JR, Ghazala LS, Peterson CA, Dupont-Versteegden EE. Depletion of Pax7+ satellite cells does not affect diaphragm adaptations to running in young or aged mice. J Physiol. 2017;595:6299–6311. doi: 10.1113/JP274611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J Appl Physiol. 2004;97:243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Phys. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick EM, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the women’s health and aging study. Arch Phys Med Rehabil. 1999;80:130–135. doi: 10.1016/S0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MD, Kerksick CM, Dalbo VJ, Hassell SE, Tucker PS, Brown R. Molecular attributes of human skeletal muscle at rest and after unaccustomed exercise: an age comparison. J Strength Cond Res. 2010;24:1161–1168. doi: 10.1519/JSC.0b013e3181da786f. [DOI] [PubMed] [Google Scholar]
- Ruddy KL, Carson RG. Neural pathways mediating cross education of motor function. Front Hum Neurosci. 2013;7:397. doi: 10.3389/fnhum.2013.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieljacks P, Wang J, Groennebaek T, Rindom E, Jakonsgaard JE, Herskind J, Gravholt A, Moller AB, Musci RV, de Paoli FV, Hamilton KL, Miller BF, Vissing K. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front Physiol. 2019;10:649. doi: 10.3389/fphys.2019.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol. 2015;119:851–857. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetta C. Plasticity and function of human skeletal muscle in relation to disuse and rehabilitation: influence of ageing and surgery. Dan Med J. 2017;64(8):B5377. [PubMed] [Google Scholar]
- Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4:8–11. doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Szulc P, Munoz F, Marchand F, Chapurlat R, Delmas PD. Rapid loss of appendicular skeletal muscle mass is associated with higher all-cause mortality in older men: the prospective MINOS study. Am J Clin Nutr. 2010;91:1227–1236. doi: 10.3945/ajcn.2009.28256. [DOI] [PubMed] [Google Scholar]
- Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pelt DW, Confides AL, Abshire SM, Hunt ER, Dupont-Versteegden EE, Butterfield TA. Age-related responses to a bout of mechanotherapy in skeletal muscle of rats. J Appl Physiol. 2019;127:1782–1791. doi: 10.1152/japplphysiol.00641.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Banker C, Butterfield TA, Dupont-Versteegden EE. Immunomodulatory effects of massage on nonperturbed skeletal muscle in rats. J Appl Physiol. 2014;116:164–175. doi: 10.1152/japplphysiol.00573.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Murach KA, Vechetti IJ, Jr, Fry CS, Vickery C, Peterson CA, McCarthy JJ, Campbell KS. MyoVision: software for automated high-content analysis of skeletal muscle immunohistochemistry. J Appl Physiol. 2018;124:40–51. doi: 10.1152/japplphysiol.00762.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DWD, Marcotte GR, Chason CM, Juo N, Baehr LM, Bodine SC, Baar K. Normal ribosomal biogenesis but shortened protein synthetic response to acute eccentric resistance exercise in old skeletal muscle. Front Physiol. 2018;9:1915. doi: 10.3389/fphys.2018.01915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JR, Confides AL, Moore-Reed S, Hoch JM, Dupont-Versteegden EE. Regrowth after skeletal muscle atrophy is impaired in aged rats, despite similar responses in signaling pathways. Exp Gerontol. 2015;64:17–32. doi: 10.1016/j.exger.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham M, et al. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. 2018;27:237–251.e4. doi: 10.1016/j.cmet.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Wood LK, Kayupov E, Gumucio JP, Mendias CL, Claflin DR, Brooks SV. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol. 2014;117:363–369. doi: 10.1152/japplphysiol.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You JS, Anderson GB, Dooley MS, Hornberger TA. The role of mTOR signaling in the regulation of protein synthesis and muscle mass during immobilization in mice. Dis Model Mech. 2015;8:1059–1069. doi: 10.1242/dmm.019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 5634 kb)