Abstract
Aortic stiffening, assessed as pulse-wave velocity (PWV), increases with age and is an important antecedent to, and independent predictor of, cardiovascular diseases (CVD) and other clinical disorders of aging. Aerobic exercise promotes lower levels of aortic stiffness in older adults, but the underlying mechanisms are incompletely understood, largely due to inherent challenges of mechanistic studies of large elastic arteries in humans. Voluntary wheel running (VWR) is distinct among experimental animal exercise paradigms in that it allows investigation of the physiologic effects of aerobic training without potential confounding influences of aversive molecular signaling related to forced exercise. In this study, we investigated whether VWR in mice may be a suitable model for mechanistic studies (i.e., “reverse translation”) of the beneficial effects of exercise on arterial stiffness in humans. We found that 10 weeks of VWR in old mice (~ 28 months) reversed age-related elevations in aortic PWV assessed in vivo (Old VWR: 369 ± 19 vs. old sedentary: 439 ± 20 cm/s, P < 0.05). The de-stiffening effects of VWR were accompanied by normalization of age-related increases in ex vivo mechanical stiffness of aortic segments and aortic accumulation of collagen-I and advanced glycation end products, as well as lower levels of aortic superoxide and nitrotyrosine. Our results suggest that late-life VWR in mice recapitulates the aortic de-stiffening effects of exercise in humans and indicates important mechanistic roles for decreased oxidative stress and extracellular matrix remodeling. Therefore, VWR is a suitable model for further study of the mechanisms underlying beneficial effects of exercise on arterial stiffness.
Keywords: Arterial stiffness, Voluntary aerobic exercise, Aging
Introduction
Age-related stiffening of the large elastic arteries is an important antecedent to, and independent risk factor for, the development of cardiovascular diseases (CVD), which remain the leading cause of death in developed nations (Virani et al. 2020; Timmis et al. 2020; Heidenreich et al. 2011; Lakatta and Levy 2003; Sutton-Tyrrell et al. 2005). Aortic pulse-wave velocity (PWV), most commonly assessed as carotid-femoral PWV, is the gold-standard clinical measure for assessing arterial stiffness in humans and increases with aging independent of other CVD risk factors (Ben-Shlomo et al. 2014; Mitchell et al. 2004; Mitchell et al. 2010; Sutton-Tyrrell et al. 2005). Higher aortic PWV is also associated with many other age-related clinical disorders including renal and cognitive dysfunction (Waldstein et al. 2008; Elias et al. 2009; Scuteri et al. 2005; Mitchell 2008; Safar et al. 2004). Importantly, interventions which decrease or preserve arterial stiffness at lower levels have the potential to lower the risk of CVD and other diseases of aging, as well as associated morbidity and health care costs (Heidenreich et al. 2011).
Age-associated arterial stiffening is mediated largely by structural changes to the arterial wall including excess deposition of collagen and its cross-linking by advanced glycation end-products (AGEs), though age-related changes in functional influences such as increased vasomotor tone and vascular endothelial dysfunction also contribute (Fleenor 2015; Lakatta and Levy 2003; Seals 2014; Soucy et al. 2006; McEniery et al. 2006). A primary underlying molecular mechanism driving these age-related structural changes in arteries is oxidative stress, characterized by excessive superoxide production and associated oxidant damage (Fleenor 2015; Zieman et al. 2005; Lakatta 2003; Seals 2014).
Regular aerobic exercise is associated with preservation of arterial stiffness at lower, more healthy levels with aging (Tanaka et al. 1998; Gando et al. 2010; Laurent et al. 2011; Vaitkevicius et al. 1993; Tanaka et al. 2018), and some data suggest late-life aerobic exercise interventions in healthy older adults may decrease arterial stiffness (Tanaka et al. 2000; Moreau et al. 2003), including when assessed as PWV (Nowak et al. 2018; Yoshizawa et al. 2009; Fujie et al. 2020; Okamoto et al. 2019). An understanding of the cellular mechanisms responsible for the de-stiffening effects of aerobic exercise has important clinical potential to inform novel interventional strategies to prevent or attenuate arterial stiffening, but these mechanisms are presently incompletely characterized. Given the challenges inherent to mechanistic studies of large elastic artery stiffening in older humans, particularly the infeasibility of sampling central arterial tissue, a model system to investigate the de-stiffening effects of exercise could have great utility (Seals 2014).
There are several established rodent aerobic exercise paradigms, including both forced (e.g., swimming, treadmill) and voluntary (primarily wheel running) modalities (Goh and Ladiges 2015; Manzanares et al. 2018). Voluntary wheel running (VWR) has been advanced as a translational model of aerobic endurance training in humans (Goh and Ladiges 2015; Manzanares et al. 2018; Durrant et al. 2009; Fleenor et al. 2010) and is distinct from forced exercise in that it allows for investigation of the physiologic effects of aerobic training without the potential confounding influences of aversive molecular signaling related to the stress of forced exercise (Goh and Ladiges 2015; Manzanares et al. 2018).
We have previously reported that late-life voluntary wheel running in mice is associated with lower (versus a sedentary cage-control group) stiffness of carotid arteries assessed ex vivo (Fleenor et al. 2010), but the effects of late-life voluntary aerobic exercise (wheel running) on aortic PWV, assessed in vivo, have never been established. Therefore, the purpose of this study was to investigate the effects of a late-life voluntary wheel running intervention on arterial stiffening, as assessed by aortic PWV, and to determine whether this exercise paradigm may be a suitable model for mechanistic studies (i.e., “reverse translation”) seeking to elucidate the beneficial effects of exercise on arterial stiffness in humans.
We performed a 10-week late-life voluntary wheel running intervention in old mice (aged ~ 26 months at start of intervention period), with young (6 months) and old (28 months) sedentary cage-control mice serving as reference groups. Our primary outcome was aortic stiffening as assessed in vivo by aortic PWV, but we also assessed intrinsic mechanical stiffness via ex vivo stress-strain testing of aortic segments, aortic abundance of collagen and key cross-linking compound AGEs, and markers of oxidative stress.
Methods
Ethical and institutional approval
This study was approved by the Animal Care and Use Committee at the University of Colorado Boulder and adhered to all standards as set forth the in Guide for Care and Use of Laboratory Animals (National Research Council, 2011).
Mice
Male C57BL/6 mice, a model of age-related arterial stiffening (Donato et al. 2013; LaRocca et al. 2014; Gioscia-Ryan et al. 2018; Fleenor et al. 2012; Sindler et al. 2011), were obtained from Charles River at approximately 4 (young, n = 15) and 24 months (old, n = 25) of age and allowed to acclimate to our facilities for at least 2 weeks. Mice were singly housed in our facility with a 12-h light:dark cycle and consumed normal chow (Harlan 7917) and water ad libitum.
Voluntary wheel running intervention
Old mice (n = 10) were transferred to cages equipped with running wheels at approximately 26 months of age and were permitted to run ad libitum for 10 weeks, a duration we have previously shown to be effective for improving arterial function and decreasing ex vivo stiffness in old mice (Fleenor et al. 2012; Lesniewski et al. 2013; Durrant et al. 2009; Gioscia-Ryan et al. 2016). Running distance was recorded for 72 continuous hours once weekly during the final 8 weeks of the intervention and is reported as average distance covered per 24 h. Three mice whose daily running distance was < 10% of the group mean were excluded from analysis.
Aortic pulse-wave velocity
Aortic pulse-wave velocity was assessed in the Old VWR mice both prior to (Pre, ~ 26 months of age) and following (Post, ~ 28 months of age) the 10-week exercise intervention period, using the Doppler ultrasound procedure previously described by our laboratory (Sindler et al. 2011; Fleenor et al. 2012; LaRocca et al. 2014). Briefly, mice were maintained under light anesthesia with inhaled isoflurane (1.5–2%) and positioned supine on a warmed platform with paws secured to ECG leads. Pulse waves were detected by Doppler probes placed at the transverse aortic arch and abdominal aorta. Three consecutive 2-s recordings were used to determine time delay between the ECG R-wave and the foot of the Doppler signal at each site (timeabdominal and timetransverse). Aortic PWV was calculated as (physical distance between the two probes)/(timeabdominal-timetransverse) and reported in cm/s. Measurements in young and old sedentary reference groups were performed at approximately 6 months of age (Young Sed) and 28 months of age (Old Sed). Heart rate was maintained between ~ 400 and 500 beats per minute for all assessments of PWV to minimize the potential modulatory influence of that factor (Lantelme et al. 2002).
Intrinsic mechanical wall stiffness
At the end of the intervention period, mice were euthanized via cardiac puncture under inhaled isoflurane anesthesia. Aorta were harvested, rinsed in cold physiological saline solution, and cleared of perivascular connective tissue. Two ~ 1-mm segments of the thoracic aorta were used for determination of intrinsic mechanical stiffness by incremental stress-strain testing via wire myography, as described previously by our laboratory (Fleenor et al. 2012; LaRocca et al. 2014; Gioscia-Ryan et al. 2018). Briefly, aortic segments were loaded into a warmed (37 °C) wire myograph chamber (DMT, Arhaus, Denmark) filled with calcium-free phosphate-buffered saline. Following three cycles of pre-stretching, ring diameter was increased to achieve 1 mN force and then incrementally stretched by ~ 10% every 3 min until failure. The force corresponding to each stretching interval was recorded and used to calculate stress and strain, defined as follows:
L = one-dimensional load; H = wall thickness determined by histology; D = vessel length
The slope of the stress-strain curve was used to determine the elastic modulus (collagen-dominant region) as the slope of the linear regression fit to the final four points of the stress-strain curve, as reported previously (Fleenor et al. 2012; Gioscia-Ryan et al. 2018; LaRocca et al. 2014).
Aortic superoxide
One 1-mm segment of thoracic aorta was used for determination of aortic superoxide bioactivity via electron paramagnetic resonance spectroscopy using the spin probe CMH, as reported previously (Fleenor et al. 2012; LaRocca et al. 2014).
Aortic Western blotting
The remaining length of each aorta was snap frozen in liquid nitrogen and stored at − 80 C. A subset (n ~ 8/group) of the aorta preserved in this fashion were used for determination of protein abundance via standard Western blotting techniques. Aorta were lysed in ice-cold RIPA buffer containing protease and phosphatase inhibitors, and protein concentration was determined using the Pierce BCA method (ThermoFisher, Waltham, MA). Approximately 15 μg of protein were loaded into 4–12% gradient gels and separated by electrophoresis. Separated proteins were transferred to nitrocellulose membranes using the TransBlot Turbo system (Bio-Rad) and blocked in 5% nonfat milk in TBS-T overnight at 4 °C. Membranes were incubated overnight at 4 °C with the following primary antibodies: Collagen-I (1:1000, #PA1-26204, ThermoFisher), 3-Nitrotyrosine (1:1000, #ab7048, Abcam, Cambridge, UK), Advanced glycation end-products (AGEs; 1:2000, #GTX20055, Gene Tex, Irvine, CA), and GAPDH (normalizer; 1:2000, #5174, Cell Signaling, Danvers, MA). Membranes were incubated with secondary antibodies (Jackson Laboratory, Bar Harbor, ME) for 1 h at room temperature, developed with Pierce ECL substrate (ThermoFisher), and imaged using the Chemi-DocIt system. Images were analyzed using ImageJ. To control for differences in protein loading, protein abundance in each sample was normalized to expression of GAPDH assessed on the same blot. Data are presented relative to the mean of the young sedentary reference group. For Collagen-I, the band from one sample in the Old Sed group was not resolvable and was therefore not included in the analysis.
Additional experiments were performed using the carotid arteries and aortic lysates from a subset of these same mice, and the results are reported in a previous publication from our laboratory (Gioscia-Ryan et al. 2016). All aortic stiffness and biochemistry data reported herein have not been published previously.
Statistical approach
Data were assessed for outliers using the Grubb’s test (GraphPad online calculator) prior to statistical analysis, with an exclusion threshold of 0.05. Outliers were identified for the following parameters: PWV (1 Old Sed), aortic superoxide (1 Young Sed, 1 Old Sed), aortic collagen-I expression (1 Old Sed, 1 Old VWR), and aortic nitrotyrosine (1 Young Sed, 1 Old VWR). All analyses were performed using SPSS Version 26 (IBM). After confirming normality, data were analyzed using one-way analysis of variance (cross-sectional comparisons) and paired t tests (pre-intervention/post-intervention comparison). When appropriate following one-way analysis of variance, pair-wise post-hoc comparisons between groups were performed using the least-significant-difference method. P < 0.05 was considered statistically significant, and P < 0.10 was considered a trend.
Results
Morphologic characteristics
Select morphological characteristics and daily running distance are presented in Table 1. Body mass did not differ among groups. There were expected age-related differences in heart mass (higher) and skeletal muscle mass (lower), which were not affected by the exercise intervention (no difference between old sedentary and old voluntary wheel running). Fat mass was lower with aging and further decreased by the voluntary wheel running intervention. The average daily running volume for the Old VWR group was consistent with prior studies in old mice (Fleenor et al. 2010; Durrant et al. 2009; Gioscia-Ryan et al. 2016; Lesniewski et al. 2013; Goh and Ladiges 2015).
Table 1.
Select morphological characteristics and voluntary wheel running distance. Data are presented as the mean (standard deviation). aP < 0.05 vs. young sedentary; bP < 0.05 vs. young sedentary and old sedentary
| Young Sed | Old Sed | Old VWR | |
|---|---|---|---|
| Body mass (g) | 30.1 (1.9) | 31.6 (2.0) | 29.8 (2.3) |
| Heart mass (mg) | 155.1 (19.6) | 187.7 (27.4)a | 219.1 (66.3)a |
| Liver mass (g) | 1.63 (0.24) | 2.14 (0.79) | 1.89 (0.38) |
| Visceral adipose mass (mg) | 597.0 (163.2) | 450.0 (137.2)a | 181.1 (85.2)b |
| Subcutaneous adipose mass (mg) | 273.7 (59.6) | 189.1 (51.1)a | 132.9 (55.0)b |
| Quadriceps mass (mg) | 348.2 (42.8) | 284.4 (24.2)a | 252.3 (47.5)a |
| Gastrocnemius mass (mg) | 314.1 (25.1) | 250.5 (22.5)a | 222.4 (51.4)a |
| Soleus mass (mg) | 18.9 (4.8) | 14.5 (3.2)a | 14.3 (7.1)a |
| Average wheel running distance (m/24 h) | n/a | n/a | 2935.9 (1642) |
n/a not applicable
Late-life voluntary wheel running reverses age-related arterial stiffening assessed in vivo as aortic pulse-wave velocity
Aortic pulse-wave velocity was ~ 30% higher in old versus young sedentary mice, consistent with age-associated aortic stiffening. The 10-week late-life voluntary wheel running intervention ameliorated ~ 70% of the mean age-related difference in aortic stiffening, such that aortic PWV was significantly lower post-intervention in Old VWR mice compared to age-matched sedentary controls (Fig. 1a). The exercise mediated de-stiffening effect was consistent, as evidenced by 6 of the 7 individual Old VWR mice demonstrating pronounced decreases in aortic PWV after versus before the 10-week exercise intervention (Fig. 1b).
Fig. 1.
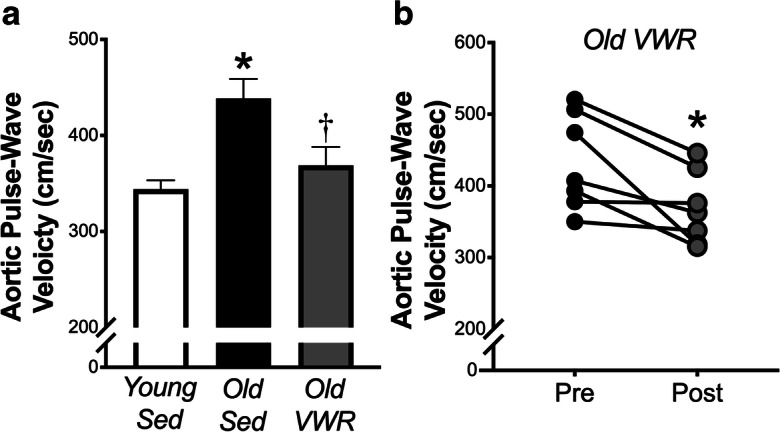
Late-life voluntary wheel running reverses age-related arterial stiffening assessed in vivo as aortic pulse-wave velocity. a Aortic pulse-wave velocity (aPWV) in young (6 months, n = 14) and old (28 months, n = 11) sedentary (Sed) mice and old mice following 10 weeks of voluntary wheel running (Old VWR, n = 7). *P < 0.05 Old Sed vs. Young Sed, †P < 0.05 Old Sed vs. Old VWR. b aPWV before (Pre) and after (Post) 10 weeks of VWR in old mice. *P < 0.05 Pre vs. Post. Data are the mean ± SEM
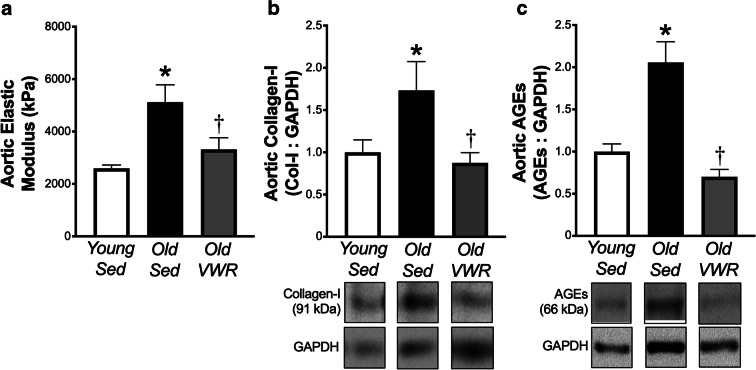
Late-life voluntary wheel running normalizes intrinsic aortic stiffness and aortic abundance of collagen-I and AGEs
Intrinsic mechanical stiffness, assessed via ex vivo stress-strain testing of aortic segments, was ~ 2-fold higher with aging, accompanied by 1.7–2.0-fold increases in aortic abundance of collagen-I and AGEs in old versus young sedentary mice. Late-life voluntary wheel running normalized intrinsic mechanical stiffness and aortic collagen-I and AGEs such that levels in Old VWR mice were similar to those of young sedentary mice (Fig. 2).
Fig. 2.
Late-life voluntary wheel running normalizes age-related increases in intrinsic aortic stiffness and aortic abundance of collagen-I and advanced glycation end-products. a Aortic elastic modulus (intrinsic mechanical stiffness) in young (6 months, n = 13) and old (28 months, n = 11) sedentary (Sed) mice and old mice following 10 weeks of voluntary wheel running (Old VWR, n = 8). b and c Aortic protein abundance of collagen-I and advanced glycation end-products (AGEs) in Young and Old Sed and Old VWR mice, normalized to GAPDH. Representative images of each protein are from the same blot under identical exposure conditions, with images of GAPDH corresponding to the same samples from the same blot. N = 5 Young Sed; 5 Old Sed; 6 Old VWR for collagen-I and 8/group for AGEs. Data are the mean ± SEM. *P < 0.05 Old Sed vs. Young Sed. †P < 0.05 Old Sed vs. Old VWR
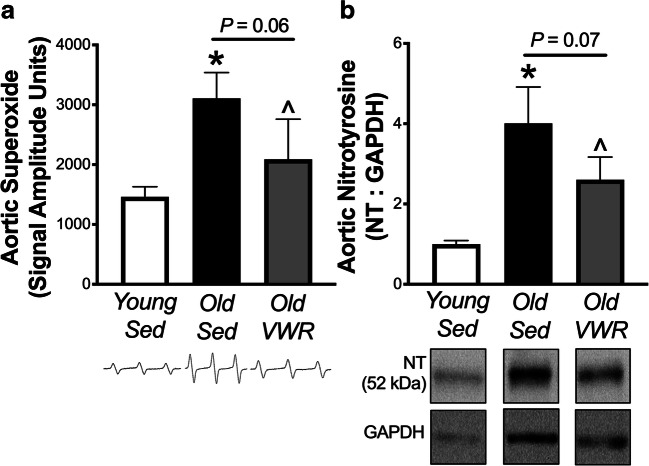
Late-life voluntary wheel running decreases aortic markers of oxidative stress
Aortic superoxide assessed via electron paramagnetic resonance spectroscopy, and protein abundance of 3-nitrotyrosine, a marker of oxidative protein modification, was elevated 2–4-fold in aortic tissue from old compared to young sedentary mice (both P < 0.05). Aortic superoxide and nitrotyrosine levels were ~ 50% lower in the old VWR versus old sedentary mice (P = 0.06 and 0.07, respectively), such that there were no significant differences in these markers of oxidative stress between the old VWR group and the young sedentary reference group (Fig. 3).
Fig. 3.
Late-life voluntary wheel running decreases aortic markers of oxidative stress. a Aortic superoxide in young (6 months, n = 8) and old (28 months) sedentary (Sed, n = 7) mice and old mice following 10 weeks of voluntary wheel running (Old VWR, n = 7). Representative spectra from electron paramagnetic resonance spectroscopy shown below mean data. b Aortic protein abundance of 3-nitrotyrosine in Young (n = 5) and Old Sed (n = 5) and Old VWR (n = 5) mice, normalized to GAPDH. Representative images are from the same blot under identical exposure conditions, with images of GAPDH corresponding to the same samples from the same blot. Data are the mean ± SEM. *P < 0.05 Old Sed vs. Young Sed. ^P < 0.10 Old Sed vs. Old VWR Sed (one-tailed)
Discussion
Overall, the results of this study demonstrate that late-life voluntary wheel running in mice reverses age-related aortic stiffening as assessed in vivo via aortic PWV and that this is mediated at least in part by normalization of the intrinsic stiffness of the aortic wall, accompanied by amelioration of the age-associated increase in aortic collagen-I (e.g., the main structural protein conferring stiffness in the arterial wall) and AGEs. These effects of late-life voluntary aerobic exercise training are further associated with lower aortic superoxide and less oxidative protein modification compared with the sedentary state. Therefore, our results suggest that voluntary wheel running may be a suitable translational model for studying the mechanistic underpinnings of the aortic de-stiffening effects of regular aerobic exercise.
The primary objective of this study was to provide proof of concept that late-life voluntary wheel running in mice is an appropriate model for reverse translation of exercise-mediated de-stiffening of arteries in humans. To our knowledge this is the first study to demonstrate voluntary wheel running-mediated reversal of age-related aortic stiffening, as assessed in vivo by aortic PWV. A prior study from our laboratory showed lower carotid artery incremental stiffness following late-life voluntary wheel running in B6D2F1 mice (Fleenor et al. 2010), and previous investigations employing late-life forced exercise interventions with aging in rodents have primarily reported reductions in ex vivo metrics of large elastic artery stiffness (Nosaka et al. 2003; Matsuda et al. 1993), with one study (Gu et al. 2014) showing lower aortic PWV following forced treadmill exercise. Our results provide important confirmation that voluntary wheel running decreases this integrative, in vivo, measure of arterial stiffness that reflects the clinical gold-standard measurement and independent predictor of CVD risk in humans, further reinforcing the promise of voluntary wheel running as a translational model.
The decrease in aortic PWV following 10 weeks of voluntary wheel running in old mice appears to be at least partially mediated by reversal of age-associated mechanical aortic stiffness as evidenced by normalization of intrinsic stiffness of aortic segments in the old exercising group. Our results further suggest that the de-stiffening effects of VWR may be due to suppression of oxidative stress-mediated aortic collagen deposition and cross-linking by AGEs (e.g., key mechanisms underlying age-associated arterial stiffening in humans). This is consistent with prior studies showing attenuation of age-related vascular oxidative stress (Durrant et al. 2009; Lesniewski et al. 2013; Fleenor et al. 2010; Gu et al. 2014) and age- and disease-associated arterial collagen deposition and/or AGEs cross-linking (Fleenor et al. 2010; Gu et al. 2014; Guers et al. 2019; Hotta et al. 2017; Steppan et al. 2012; Wright et al. 2014) by exercise in rodents. Importantly, our results here indicate that voluntary wheel running is an experimental exercise intervention capable of recapitulating the key physiological changes that occur in arteries in response to aerobic exercise training, underscoring the utility of this model for further mechanistic investigations. Understanding the cellular mechanisms underlying the de-stiffening effects of aerobic exercise could have great potential to inform the development of novel intervention strategies to prevent or attenuate arterial stiffening in humans.
Our data indicate that reversal of age-related aortic stiffness may have been at least partially mediated by attenuation of aortic oxidative stress, but this proof of concept study did not identify the potential source(s) of this oxidative stress. Vascular mitochondria are gaining increasing recognition as an important source of oxidative stress contributing to age-related vascular dysfunction, including arterial stiffening (Zhou et al. 2012), and appear to adapt to exercise training interventions (Keller et al. 2015; Knaub et al. 2013). A previous study in our laboratory showed that aortic levels of mitochondria-specific superoxide were elevated with aging in sedentary mice but normalized (to levels not different from young mice) by 10 weeks of late-life voluntary wheel running, and this was accompanied by favorable changes in aortic protein content of markers of mitochondrial health (Gioscia-Ryan et al. 2016). Consistent with this, a study of late-life forced treadmill exercise in old rats (Gu et al. 2014) demonstrated that decreases in aortic PWV following the exercise intervention were associated with attenuation of oxidative stress and improvements (versus age-matched controls) in several indices of mitochondrial function in aortic tissue. These data suggest the possibility that vascular mitochondrial adaptations, including attenuation of mitochondrial oxidative stress, may underlie the de-stiffening effects of voluntary wheel running, but future studies are needed to more fully elucidate the specific mechanisms and causal relationships involved.
Limitations and future directions
We attempted to assess aortic elastin—a key structural protein whose degradation contributes to age-related arterial stiffening—but were unsuccessful due to technical issues and limited quantity of tissue. However, a previous study in our laboratory showed that elastin content in carotid arteries was unchanged following late-life voluntary wheel running intervention of similar duration to the present study (Fleenor et al. 2010).
Our results suggest that the aortic de-stiffening effects of voluntary wheel running in old mice were at least partially mediated by beneficial changes to arterial structural factors. However, functional influences such as vasomotor tone and vascular endothelial function also affect aortic stiffness in vivo, and changes in aortic stiffness, in turn, have the potential to affect other vascular beds and the downstream organs they supply (Fleenor 2015; Lakatta and Levy 2003; Seals 2014; Soucy et al. 2006; McEniery et al. 2006). Exercise is well established as beneficial in ameliorating many domains of age-related physiological dysfunction, and these effects appear to be transduced via effects on well known (e.g., oxidative stress and inflammation) as well as emerging (e.g., preservation of motor neurons, microvascular IGF-1 signaling, aortic microRNA expression) underlying mechanisms (Gillon et al. 2018; Norling et al. 2020; Kiss et al. 2019). Future work is warranted to better understand the potential interaction among these and other mechanisms in the context of exercise-mediated aortic de-stiffening.
Conclusions
In summary, we show for the first time that late-life voluntary wheel running in mice ameliorates age-related aortic stiffness as assessed in vivo via aortic PWV, reflecting the aortic de-stiffening effects of aerobic exercise in humans. Voluntary wheel running in mice may therefore be a reverse translational exercise intervention model for investigating the mechanisms underlying beneficial effects of exercise on arterial stiffness in humans.
Acknowledgments
The authors would like to thank David Hutton and John Van Hecke for study assistance.
Author contributions
Conception and design (RGR, BSF, MCZ, DRS); data collection (RGR, ZSC, JSE, LCJ, TDE); data analysis and interpretation (RGR, ZSC, BSF, JSE, LCJ, MJR, MCZ, DRS); manuscript writing (RGR, ZSC, DRS); figure development (RGR, ZSC, DRS); critical revision and final approval of manuscript (RGR, ZSC, BSF, JSE, LCJ, MJR, MCZ, TDE, DRS).
Funding information
This work was supported by grants from the National Institutes of Health: AG000279, HL107120, and AG013038.
Data availability
The data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12(5):772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–673. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS. Large elastic artery stiffness with aging: novel translational mechanisms and intervention. Aging Dis. 2015;4(2):76–83. [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR. Arterial stiffening with ageing is associated with transforming growth factor-beta1-related changes in adventitial collagen: reversal by aerobic exercise. J Physiol. 2010;588(20):3971–3982. doi: 10.1113/jphysiol.2010.194753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47:588–594. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujie S, Hasegawa N, Sanada K, Hamaoka T, Maeda S, Padilla J, Martinez-Lemus LA, Iemitsu M. Increased serum salusin-alpha by aerobic exercise training correlates with improvements in arterial stiffness in middle-aged and older adults. Aging. 2020;12(2):1201–1212. doi: 10.18632/aging.102678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando Y, Yamamoto K, Murakami H, Ohmori Y, Kawakami R, Sanada K, Higuchi M, Tabata I, Miyachi M. Longer time spent in light physical activity is associated with reduced arterial stiffness in older adults. Hypertension. 2010;56:540–546. doi: 10.1161/HYPERTENSIONAHA.110.156331. [DOI] [PubMed] [Google Scholar]
- Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. Geroscience. 2018;40(2):177–192. doi: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging. 2016;8(11):2897–2914. doi: 10.18632/aging.101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Eng JS, Murphy MP, Seals DR. Mitochondria-targeted antioxidant therapy with MitoQ ameliorates aortic stiffening in old mice. J Appl Physiol. 2018;124(5):1194–1202. doi: 10.1152/japplphysiol.00670.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol. 2015;5:283–290. doi: 10.1002/9780470942390.mo140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Wang B, Zhang X-F, Ma Y-P, Liu J-D, Wang X-Z. Chronic aerobic exercise training attenuates aortic stiffening and endothelial dysfunction through preserving aortic mitochondrial function in aged rats. Exp Gerontol. 2014;56:37–44. doi: 10.1016/j.exger.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Guers JJ, Farquhar WB, Edwards DG, Lennon SL. Voluntary wheel running attenuates salt-induced vascular stiffness independent of blood pressure. Am J Hypertension. 2019;32(12):1162–1169. doi: 10.1093/ajh/hpz128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ, American Heart Association Advocacy Coordinating Committee. Stroke Council. Council on Cardiovascular Radiology and Intervention. Council on Clinical Cardiology. Council on Epidemiology and Prevention. Council on Arteriosclerosis. Thrombosis and Vascular Biology. Council on Cardiopulmonary. Critical Care. Perioperative and Resuscitation. Council on Cardiovascular Nursing. Council on the Kidney in Cardiovascular Disease. Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD, Muller-Delp JM. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol. 2017;595:3703–3719. doi: 10.1113/JP274172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol. 2015;65(2):137–147. doi: 10.1097/FJC.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Giles CB, Tarantini S, Yabluchanskly A, Balasubramanian P, Gautam T, Cispo T, Nyul-Toth A, Lipecz A, Szabo C, Farkas E, Wren JD, Csiszar A, Ungvari Z. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience. 2019;41(4):419–439. doi: 10.1007/s11357-019-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaub LA, McCune S, Chicco AJ, Miller M, Moore RL, Birdsey N, Lloyd MI, Villareal J, Keller AC, Watson PA, Reusch JE. Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab Vasc Dis Res. 2013;10(3):222–238. doi: 10.1177/1479164112459664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. III Cellular and molecular clues to heart and arterial aging Circulation. 2003;107:490–7. [DOI] [PubMed]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39(6):1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Hearon CM, Jr, Henson GD, Seals DR. Mitochondrial quality control and age-associated arterial stiffening. Exp Gerontol. 2014;58:78–82. doi: 10.1016/j.exger.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent P, Marenco P, Castagna O, Smulyan H, Blacher J, Safar ME. Differences in central systolic blood pressure and aortic stiffness between aerobically trained and sedentary individuals. J Am Soc Hypertens. 2011;5:85–93. doi: 10.1016/j.jash.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Zigler ML, Durrant JR, Nowlan MJ, Folian BJ, Donato AJ, Seals DR. Aging compounds western diet-associated large artery endothelial dysfunction in mice: prevention by voluntary aerobic exercise. Exp Gerontol. 2013;48:1218–1225. doi: 10.1016/j.exger.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares G, Brito-de-Silva G, Gandra PC. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 2018;52:e7830. doi: 10.1590/1414-431X20187830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Nosaka T, Sato M, Ohshima N. Effects of physical exercise on the elasticity and elastic components of the rat aorta. Eur J Appl Physiol Occup Physiol. 1993;66:122–126. doi: 10.1007/BF01427052. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60. [DOI] [PMC free article] [PubMed]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. doi: 10.1016/s0008-6363(02)00777-0. [DOI] [PubMed] [Google Scholar]
- Norling AM, Gerstenecker AT, Buford TW, Oparil S, Lazar RM. The role of exercise in the reversal of IGF-1 deficiencies in microvascular rarefaction and hypertension. Geroscience. 2020;42(1):141–158. doi: 10.1007/s11357-019-00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka H, Tanaka H, Watanabe I, Sato M, Matsuda M. Influence of regular exercise on age-related changes in arterial elasticity: mechanistic insights from wall compositions in rat aorta. Can J Appl Physiol. 2003;28:204–212. doi: 10.1139/h03-016. [DOI] [PubMed] [Google Scholar]
- Nowak KL, Rossman MJ, Chonchol M, Seals DR. Strategies for achieving healthy vascular aging. Hypertension. 2018;71(3):389–402. doi: 10.1161/HYPERTENSIONAHA.117.10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Hashimoto Y, Kobayashi R. Effects of interval walking training compared to normal walking training on cognitive function and arterial function in older adults: a randomized controlled trial. Aging Clin Exp Res. 2019;31(10):1451–1459. doi: 10.1007/s40520-018-1093-8. [DOI] [PubMed] [Google Scholar]
- Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163–168. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211–1216. doi: 10.1097/01.hjh.0000170384.38708.b7. [DOI] [PubMed] [Google Scholar]
- Seals DR. Edward F. Adolph distinguished lecture: the remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol. 2014;117(5):425–439. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell. 2011;10:429–437. doi: 10.1111/j.1474-9726.2011.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- Steppan J, Tran H, Benjo AM, Pellakuru L, Barodka V, Ryoo S, Nyhan SM, Lussman C, Gupta G, White AR, Daher JP, Shoukas AA, Levine BD, Berkowitz DF. Alagebrium in combination with exercise ameliorates age-associated ventricular and vascular stiffness. Exp Gerontol. 2012;47:565–572. doi: 10.1016/j.exger.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–132. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Palta P, Folsom AR, Meyer ML, Matsushita K, Evenson KR, Aguilar D, Heiss G. Habitual physical activity and central artery stiffening in older adults: the atherosclerosis risk in communities study. J Hypertens. 2018;36(9):1889–1894. doi: 10.1097/HJH.0000000000001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Timmis A, Townsend N, Gale CP, Torbica A, Lettino M, Petersen SE, Mossialos EA, Maggioni AP, Kazakiewicz D, May HT, de Smedt D, Flather M, Zuhlke L, Beltrame JF, Huculeci R, Tavazzi L, Hindricks G, Bax J, Casadei B, Achenbach S, Wright L, Vardas P, European Society of Cardiology. Mimoza L, Artan G, Aurel D, Chettibi M, Hammoudi N, Sisakian H, Pepoyan S, Metzler B, Siostrzonek P, Weidinger F, Jahangirov T, Aliyev F, Rustamova Y, Manak N, Mrochak A, Lancellotti P, Pasquet A, Claeys M, Kušljugić Z, Dizdarević Hudić L, Smajić E, Tokmakova MP, Gatzov PM, Milicic D, Bergovec M, Christou C, Moustra HH, Christodoulides T, Linhart A, Taborsky M, Hansen HS, Holmvang L, Kristensen SD, Abdelhamid M, Shokry K, Kampus P, Viigimaa M, Ryödi E, Niemelä M, Rissanen TT, le Heuzey JY, Gilard M, Aladashvili A, Gamkrelidze A, Kereselidze M, Zeiher A, Katus H, Bestehorn K, Tsioufis C, Goudevenos J, Csanádi Z, Becker D, Tóth K, Jóna Hrafnkelsdóttir Þ, Crowley J, Kearney P, Dalton B, Zahger D, Wolak A, Gabrielli D, Indolfi C, Urbinati S, Imantayeva G, Berkinbayev S, Bajraktari G, Ahmeti A, Berisha G, Erkin M, Saamay A, Erglis A, Bajare I, Jegere S, Mohammed M, Sarkis A, Saadeh G, Zvirblyte R, Sakalyte G, Slapikas R, Ellafi K, el Ghamari F, Banu C, Beissel J, Felice T, Buttigieg SC, Xuereb RG, Popovici M, Boskovic A, Rabrenovic M, Ztot S, Abir-Khalil S, van Rossum AC, Mulder BJM, Elsendoorn MW, Srbinovska-Kostovska E, Kostov J, Marjan B, Steigen T, Mjølstad OC, Ponikowski P, Witkowski A, Jankowski P, Gil VM, Mimoso J, Baptista S, Vinereanu D, Chioncel O, Popescu BA, Shlyakhto E, Oganov R, Foscoli M, Zavatta M, Dikic AD, Beleslin B, Radovanovic MR, Hlivák P, Hatala R, Kaliská G, Kenda M, Fras Z, Anguita M, Cequier Á, Muñiz J, James S, Johansson B, Platonov P, Zellweger MJ, Pedrazzini GB, Carballo D, Shebli HE, Kabbani S, Abid L, Addad F, Bozkurt E, Kayıkçıoğlu M, Erol MK, Kovalenko V, Nesukay E, Wragg A, Ludman P, Ray S, Kurbanov R, Boateng D, Daval G, de Benito Rubio V, Sebastiao D, de Courtelary PT, Bardinet I. European Society of Cardiology: cardiovascular disease statistics 2019. Eur Heart J. 2020;41(1):12–85. doi: 10.1093/eurheartj/ehz859. [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner L, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wright JK, Thomas MM, Betik AC, Belke D, Hepple RT. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Exp Gerontol. 2014;50:9–18. doi: 10.1016/j.exger.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M, Maeda S, Miyaki A, Misono M, Saito Y, Tanabe K, Kuno S, Ajisaka R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32–59 years. Br J Sports Med. 2009;43:615–618. doi: 10.1136/bjsm.2008.052126. [DOI] [PubMed] [Google Scholar]
- Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2012;32(3):745–755. doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.