Abstract
Sleep deprivation is highly prevalent and is associated with increased cardiovascular disease (CVD) morbidity and mortality. Age-related alterations in sleep and chronobiology may exaggerate CVD susceptibility in older individuals. The mechanisms responsible for the association between sleep deprivation and CVD are not fully understood, but endothelial dysfunction may play a central role. Our objective was to conduct a systematic literature review to evaluate the evidence on the effects of sleep deprivation on endothelial function (EF). This review adhered to the PRISMA guidelines and was pre-registered with PROSPERO (#CRD42020192485, 07/24/2020). We searched PubMed, Web of Science, Embase, and Cochrane Library for articles published through May 1, 2020. Eligibility criteria included publication in English and use of well-established EF methodologies in adult humans. Two investigators independently performed the literature search, study selection, data extraction, risk-of-bias assessment, and qualitative data synthesis. Out of 3571 articles identified, 24 articles were included in the systematic review. Main findings include the following: (1) shorter sleep duration is associated with lower macrovascular EF; (2) not sleeping 7–9 h/night is linked with impaired microvascular EF; (3) sleep restriction impairs micro- and macrovascular EF; (4) acute total sleep deprivation impairs micro- and macrovascular EF but data on macrovascular EF are less consistent; and (5) shift work impairs macrovascular EF. In conclusion, sleep deprivation impairs EF, which may explain the link between insufficient sleep and CVD. Future investigations should fully elucidate the underlying mechanisms and develop strategies to combat the adverse endothelial effects of sleep deprivation across the lifespan.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-020-00312-y.
Keywords: Sleep deprivation, Endothelial function, Cardiovascular aging, Chronobiology
Introduction
Sleep deprivation is one of the top modifiable risk factors to human health. The American Heart Association and CDC advocate that adults receive at least 7 h of sleep each night to promote optimal health and reduce the risk for disease [1, 2]. In agreement with this, the National Sleep Foundation recommends that adults obtain 7–9 h of sleep per night [3], yet nearly 50% fail to meet this recommendation [4]. Sleep deprivation is also an important issue in the elderly, over half of whom report sleep complaints including a diminished ability to achieve sufficient sleep duration and quality [5], which is likely secondary to the effects of age-related chronobiological disruption [6–8]. The consequences of insufficient sleep are far from benign. Indeed, there is compelling evidence that reduced sleep duration and poor sleep quality both dramatically increase the morbidity and mortality associated with cardiovascular disease (CVD) [2, 9–12], the leading cause of death in the USA [13]. Importantly, aging, a recognized CVD risk factor [14], may act in concert with insufficient sleep to accelerate cardiovascular aging and increase CVD risk. Promoting successful cardiovascular aging may start with extending the same consideration to sleep as has been given to other traditional CVD risk factors (e.g., hypertension, dyslipidemia, obesity, and physical inactivity) [15].
That sleep is ubiquitous among all organisms implies that it is indispensable [16]. Adequate sleep at the appropriate biological time is also critical for homeostatic maintenance. Endogenous chronobiological rhythms evolved to synchronize physiological processes with environmental and behavioral stimuli (e.g., day and night, feeding and fasting, activity and rest) [17]. The human cardiovascular system is not exempt from chronobiological influence. Circadian clocks are present in cardiac, smooth muscle, and endothelial cells, regulating processes such as heart rate, blood pressure, and endothelial function [18], all of which exhibit diurnal fluctuations. Unfortunately, modern lifestyles encourage living out of sync with our circadian biology [19], which leads to chronobiological disruption and is likely the cause of many preventable diseases including CVD [20].
The burden of chronobiological disruption and sleep deprivation on CVD risk may be exacerbated in the aging population which is expected to grow dramatically in the coming decades [21]. Aging is independently associated with chronobiological disruptions and alterations in sleep duration and quality that ultimately predispose older individuals to sleep deprivation [22]. Even though aging is associated with a reduced amount and quality of sleep, there is no evidence to conclude that the need for sleep decreases with age [22]. As such, the dysfunctional circadian rhythms in older individuals may impair sleep and exacerbate their predisposition to CVD by negatively impacting cardiovascular health. Given that sleep is largely a voluntary behavior, raising awareness about the interaction of sleep and health may support prioritizing healthy sleep behaviors to promote optimal cardiovascular health across the lifespan [2].
Humans experience insufficient sleep in a variety of ways, all of which likely have acute negative physiological effects and when experienced chronically, predispose to disease. Chronic sleep restriction occurs when an individual receives less sleep than their optimal sleep duration for days, weeks, months, or even years, and is likely the most prevalent form of sleep deprivation. Humans also experience insufficient sleep due to shift work which affects more than 15% of the workforce [23]. Shift workers frequently experience sleep restriction concomitant with abnormal sleep schedules that are often out of line with human biological rhythms [24–26]. Finally, extended wakefulness of 24 h or more, termed total sleep deprivation (TSD), can have dramatic physiological effects, although this form of sleep insufficiency is perhaps less prevalent, as the majority of individuals do not routinely stay awake for 24 h or more at a time. Regardless of how sleep deprivation is experienced, the resulting chronodisruption may impair cardiovascular function and increase the risk for CVD.
Identifying the mechanisms underlying the role of insufficient sleep in the pathogenesis of CVD could facilitate the development of therapeutic strategies to attenuate the adverse effects of sleep deprivation on human cardiovascular health. While various cardiometabolic risk factors have been proposed [2], a compelling but understudied mechanism by which sleep deprivation may be associated with CVD is endothelial dysfunction. Endothelial dysfunction is the initiating event in the development of atherosclerosis, detectable before clinical manifestations of CVD [27], and therefore an ideal target for investigating the early effects of sleep deprivation on cardiovascular health. Furthermore, endothelial function measured in coronary and peripheral arteries predicts future CVD events (i.e., myocardial infarction and stroke) and death [28–30]. Endothelial dysfunction is a hallmark of cardiovascular aging, even in the absence of other CVD risk factors [31]. Importantly, age-related impairments in micro- and macrovascular endothelial function contribute to increased susceptibility to CVD in the elderly (reviewed in Ghebre et al. [32]). Therefore, elucidating the role of sleep in preserving endothelial function may be paramount to successful vascular aging.
Sleep deprivation may accelerate the development of age-associated endothelial dysfunction and through this mechanism contribute to the pathogenesis and progression of CVD. Reviewing the evidence on how sleep deprivation impacts endothelial function may offer a lens into how sleep and chronobiological disruptions throughout life may negatively impact the trajectory of cardiovascular aging. This could aid in the development of recommendations for healthy lifestyle behaviors for successful aging. It could also stimulate the design of experimental studies to define the mechanisms by which sleep deprivation affects vascular aging. Therefore, we aimed to conduct a systematic review of the literature to evaluate the observational and experimental evidence on the effects of sleep deprivation on endothelial function. We also sought to discuss the potential underlying mechanisms and construct a conceptual model linking sleep deprivation to CVD.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [33] and adhered to the PRISMA standards and checklist. The protocol for this systematic review was pre-registered online through the PROSPERO International Prospective Register of Systematic Reviews (registration #CRD42020192485) and can be accessed at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=192485.
Eligibility criteria
We sought to provide a comprehensive synthesis of the current state of knowledge; therefore, we did not restrict our systematic review to specific study designs. Articles were included if they (1) were full-length peer-reviewed primary research articles (i.e., no reviews, dissertations, or conference abstracts were considered); (2) focused on adult humans; and (3) assessed micro- or macrovascular endothelial function using well-established methodologies. Articles were excluded if they (1) focused exclusively on animal models due to confounders associated with experimental sleep deprivation (e.g., stress) which may limit their relevance to humans; (2) included individuals younger than 18 years of age due to potential developmental differences in sleep and cardiovascular physiology; and (3) focused on sleep disorders because these conditions are associated with other comorbidities that could confound the association between sleep and endothelial function.
This review evaluated diverse lines of evidence based on observational and experimental studies. Observational studies compared endothelial function in adults who receive short vs. normal sleep duration or assessed the association of sleep duration with endothelial function. Experimental studies manipulated sleep duration and compared endothelial function following normal sleep and sleep deprivation. Sleep deprivation models included (1) TSD, where participants were deprived of sleep for 24 h or more; (2) prolonged sleep restriction, where nightly sleep was limited to a fraction of the participants’ habitual sleep duration for several days to weeks; and (3) shift work, where participants worked an overnight or extended shift of 24 h or more.
Primary outcome
The primary outcome of this systematic review was micro- and macrovascular endothelial function including assessments of blood vessels in the coronary circulation, periphery, or skin using vasodilation or blood flow responses to physiological or pharmacological stimuli.
Information sources
Studies were identified by two investigators (BJH and SSL) from independent computerized literature searches of the following databases: PubMed, Web of Science, Embase, and Cochrane Register of Controlled Trials. Each database was searched using a customized search string to optimize results. Searches were restricted to (1) human studies and (2) studies published in the English language. Additional articles were identified by scanning the references of identified articles. All searches were conducted between the earliest available search date for each database and the last search date of May 1, 2020.
Search strategy
The search strategy used for this systematic review is provided below. The following customized search string and limits were used: (sleep deprivation OR sleep restriction OR insufficient sleep OR sleep curtailment OR short sleep duration OR sleep loss) AND (brachial artery flow-mediated dilation OR brachial artery flow-mediated vasodilation OR brachial artery OR vasodilation OR vascular reactivity OR microvascular function OR vascular function OR endothelial function OR FMD OR vascular health OR microvascular OR vascular tone OR arterial stiffness OR pulse wave velocity OR PWV OR hemodynamics OR wave reflection OR augmentation index OR vascular stiffness OR aortic stiffness). Filters: Humans, English.
Study selection
Search results from each database were downloaded and imported into EndNote X9 to identify and remove duplicates. Two review team members (BJH and SSL) independently screened the titles and abstracts of all records to identify additional duplicates. After removal of duplicate articles, BJH and SSL reviewed the title and abstract of each record to determine its potential for inclusion based on the participants, intervention, comparison, outcomes, and study (PICOS) types. Relevant articles were downloaded as full-text and further screened to determine eligibility based on our specific inclusion and exclusion criteria. Any discrepancies between the two reviewers were reconciled through discussion and consultation with a third review team member (DDC) was sought if a consensus could not be reached. Included and excluded records were tracked within EndNote, and reasons for exclusion were documented in a Microsoft Excel spreadsheet.
Data collection and data items
Data collection and extraction were performed independently by BJH and SSL, while a third review team member (DDC) was available for mediation. Extracted data were inputted into a pre-formatted Microsoft Excel spreadsheet using open-text responses. If data were missing or clarifications were needed, the study’s corresponding author was contacted with a request to obtain unreported data or additional details. Following data extraction, review team members discussed and reconciled any discrepancies. The following variables were extracted from each study: authors, publication year, participant characteristics (average age, # of males/females), study design, sample size, method used for evaluating/manipulating sleep and assessing endothelial function, and key results.
Data synthesis
Data on study selection and inclusion are presented quantitatively in the text and in a flow diagram (Fig. 1) and include number of articles identified at each stage, number of articles included/excluded, and reasons for exclusion. Study characteristics are summarized in text and presented in Tables 1 and 2. Outcome data were organized by study design consisting of observational and experimental studies and within each study design, data were summarized based on sleep deprivation model. Data were then qualitatively synthesized to examine similarities and differences in key findings. When findings conflicted, subject characteristics and other methodological aspects were closely examined as potential explanatory factors. There was no plan to conduct a meta-analysis due to the diverse methodologies used to implement sleep deprivation and assess sleep and endothelial function. The mechanisms responsible for the adverse effects of sleep deprivation on endothelial function were investigated only in some of the studies included in this systematic review. Although not a formal objective of our review, mechanistic data are discussed in the text and incorporated in a conceptual model linking sleep deprivation to CVD.
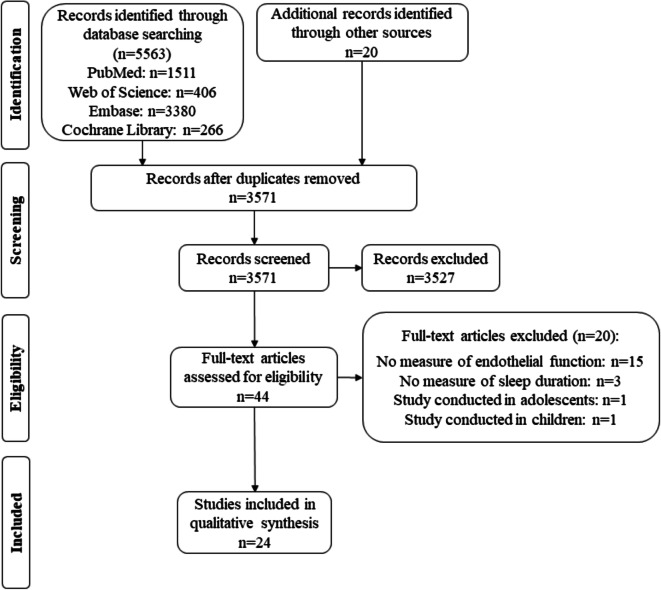
Fig. 1.
Flow chart summarizes the results of the screening process and final article selection
Table 1.
Main characteristics of observational studies included in the systematic review
| Study | Study design | Participants (age in years) | Sample size | Sleep duration assessment | Endothelial function assessment | Results |
|---|---|---|---|---|---|---|
| Bonsen 2015 [34] | Cross-sectional | Healthy adults (42) | 259 (116M/143F) |
Self-reported SSD: < 7 h/night NSD: ≥ 7 h/night |
Capillary recruitment to reactive hyperemia | ↓ Capillary function in women with SSD (β = − 11.17, P = 0.04) but not in men (β = − 0.79, P = 0.81) |
| Bain 2017 [35] | Cross-sectional |
Sedentary men SSD: 55 ± 2 NSD: 58 ± 2 |
30 (15 SSD/15 NSD) |
Self-reported SSD: < 7 h/night NSD: ≥ 7 h/night |
FBF in response to ACh |
↓ Vasodilation to ACh in SSD: FBF: (SSD: 11.7 ± 1 vs. NSD: 14.5 ± 0.5 ml/100 ml tissue/min, P < 0.05) FBFauc: (SSD: 53 ± 6 vs. NSD: 74 ± 6 ml/100 ml tissue/min, P < 0.05) |
| Weil 2013 [36] | Cross-sectional |
Sedentary men SSD: 50 ± 2 NSD: 50 ± 3 |
30 (15 SSD/15 NSD) |
Self-reported SSD: 6.1 ± 0.2 h/night NSD: 7.6 ± 0.2 h/night |
FBF in response to BK | No group diff. in FBF response (SSD: 16.1 ± 0.8 vs. NSD: 14.9 ± 0.9, ml/100 ml tissue/min, P ≥ 0.05) |
| Weil 2010 [37] | Cross-sectional | Sedentary adults (57 ± 1) | 80 (50 NSD [32M/18F]/30 SSD [17M/13F]) |
Self-reported SSD: 6.1 ± 0.1 h/night NSD: 7.6 ± 0.1 h/night |
FBF response to BQ-123 | ~ 3-fold ↑ dilation to BQ-123 infusion in SSD (P < 0.05, means not reported) |
| Hall 2017 [38] | Prospective |
Adults SSD: 61.5 ± 8.4 NSD: 56.8 ± 10.3 |
141 (94 in SSD [39M/55F]/47 in NSD [10M/37F]) |
Sleep monitored using polysomnography SSD: < 7 h/night NSD: ≥ 7 h/night |
Brachial artery FMD |
No group diff. in FMD: (SSD: 6.1 ± 4.7% vs. NSD: 7.3 ± 4.2%, P > 0.05) ↓Sleep duration associated with ↓ FMD (β = 0.171, P = 0.04) |
| Aggarwal 2018 [39] | Cross-sectional | Women (31 ± 9) | 26 | Sleep monitored using actigraphy over 14 days (7.62 ± 0.45 h) | Brachial artery FMD | No association of sleep duration with FMD (r and P values not reported) |
| Behl 2014 [40] | Cross-sectional | Healthy adults (48 ± 11) | 684 (219 M/465F) | Self-reported | Brachial artery FMD | No association of sleep duration with FMD (P = 0.07, r value not provided) |
ACh acetylcholine, BK bradykinin, BQ-123 selective ET-A receptor antagonist, F females, FBF forearm blood flow, FBFauc forearm blood flow area under the curve, FMD flow-mediated dilation, M males, NSD normal sleep duration, SSD short sleep duration
Table 2.
Main characteristics of experimental studies included in the systematic review
| Study | Study design | Participants (age in years) | Sample size | Sleep deprivation model | Endothelial function assessment | Results |
|---|---|---|---|---|---|---|
| Total sleep deprivation | ||||||
| Grassi 2016 [41] | Randomized crossover | Healthy adults (25.3 ± 3.6) | 32 (16M/16F) |
Supervised TSD SD: 24-h TSD NS: FS |
Brachial artery FMD | FMD ↓ after TSD (SD: 5.52 ± 0.53% vs. NS: 6.53 ± 0.63%, P = 0.02) |
| Wehrens 2012 [42] | Non-randomized crossover |
Men Shift workers (35.7 ± 7.2) Non-shift workers (32.5 ± 6.2) |
25 (11 shift/14 non-shift workers) |
Supervised TSD Polysomnography SD: 30.5-h TSD NS: 7.5–8 h |
Brachial artery FMD | No change in FMD after TSD (means and P value not reported) |
| Sauvet 2009 [43] | Non-randomized crossover | Healthy men (29.1 ± 3.3) | 12 |
Supervised TSD Polysomnography SD: 40-h TSD NS: FS |
CVC in response to ACh |
CVCpeak and CVCauc to ACh ↓ after 29 h TSD: CVCpeak (SD: 283 ± 163 vs. NS: 454 ± 158 PU/mmHg, P = 0.03); CVCauc (SD: 8.1 ± 2.2 vs. NS: 16.7 ± 6.5 PU/mmHg, P = 0.02) |
| Sauvet 2017 [44] | Non-randomized crossover | Healthy men (27.3 ± 5.4) | 16 |
Supervised TSD Polysomnography SD: 40-h TSD NS: 8 h |
CVC in response to ACh/insulin/heat |
CVCpeak after TSD in response to ACh/insulin/heat ↓: ACh (SD: 67.2 ± 8.2 vs. NS: 106.5 ± 7.5 PU/mmHg, P = 0.001); insulin (SD: 19.7 ± 3.2 vs. NS: 35.8 ± 5.1 PU/mmHg, P = 0.001); heat (SD: 115.5 ± 11.0 vs. NS: 131.7 ± 9.6 PU/mmHg, P = 0.004) |
| Sauvet 2012 [45] | Randomized crossover | Healthy men (30.6 ± 2.1) | 10 |
Supervised TSD Polysomnography SD: 29-h TSD NS: FS |
CVC in response to CWI |
No change in baseline CVC after TSD (SD: 0.6 ± 0.2 vs. NS: 0.8 ± 0.2, P = 0.9). CVC during CWI lower after TSD: CVC (SD: 30.0 ± 40.2 vs. NS: 143.6 ± 78 PU/mmHg, P = 0.04), CVCmax (SD: 240 ± 133.7 vs. NS: 385.9 ± 169.7 PU/mmHg, P = 0.04), CVCauc (SD: 225.3 ± 123.9 vs. NS: 995.0 ± 181.6 PU/mmHg, P = 0.04) |
| Yang 2012 [46] | Randomized crossover | Healthy adults (22 ± 1) | 28 (14M/14F) |
Supervised TSD SD: 24- h TSD NS: FS |
FBF and FVC in response to reactive hyperemia |
No change in FBF or FVC to reactive hyperemia after TSD: FBF: (Δ67 ± 12% vs. Δ83 ± 13%, P = 0.2); FVC: (Δ47 ± 8% vs. Δ59 ± 11%, P = 0.25) |
| Sleep restriction | ||||||
| Sekine 2010 [47] | Randomized crossover | Healthy men (29 ± 6) | 26 |
Self- reported SR SD: 3.7 ± 0.9 h NS: 7.1 ± 0.2 h |
CFVR to ATP | CFVR to ATP ↓ after SR (SD: 3.3 ± 0.6 vs. NS: 4.2 ± 0.9, P < 0.001) |
| Calvin 2014 [48] | Randomized parallel-group | Healthy adults (SD: 24.1 ± 4.5; NS: 25.1 ± 5.0) |
16: 8 NS (5M/3F) 8 SD (5M/3F) |
Supervised SR Polysomnography SD: 5.1 ± 0.4 h/8 nights NS: 6.8 ± 0.8 h/8 nights |
Brachial artery FMD |
FMD ↓ after SR but not NS: SD: 8.6 ± 4.6% to 5.2 ± 3.4%, P = 0.01 NS: 6.7 ± 2.9% to 5.0 ± 3.0%, P = 0.1 Between group difference: − 4.4%, P = 0.003 |
| Takase 2004 [49] | Non-randomized crossover | Healthy men (21.7 ± 1.1) | 30 |
Self-reported SR SD: < 80% sleep/4 weeks NS: habitual sleep/1 week |
Brachial artery FMD | FMD ↓ after SR (SD: 3.7 ± 2.3% vs. NS: 7.4 ± 3.0%, P < 0.05) |
| Dettoni 2012 [50] | Randomized crossover | Healthy men (31 ± 2) | 13 |
Monitored SR Actigraphy SD: 4.5 ± 0.3 h/5 nights NS: 8 ± 0.5 h/5 nights |
Endothelium-dependent venodilation to ACh | Dilation to ACh ↓ after SR (P < 0.001, means not reported) |
| Sauvet 2015 [51] | Non-randomized crossover | Healthy men (29.3 ± 5.2) | 12 |
Supervised SR and polysomnography SD: 3.68 ± 0.18 h/6 nights NS: 7 ± 0.3 h/1 night |
CVC in response to MCh/cathode current/heat |
CVCpeak to MCh and heat ↓ after SR day 6; (P < 0.05, means not reported) CVC to cathode current did not change after SR (P > 0.05, means not reported) |
| Shift work | ||||||
| Garcia-Fernandez 2002 [52] | Randomized crossover | Healthy medical residents (27–35) | 15 (9M/6F) |
Unmonitored SW SD: 24-h shift NS: regular shift/FS |
Brachial artery FMD | FMD ↓ after SW (SD: 3.4 ± 2.8% vs. NS: 11.3 ± 7.0%, P < 0.001) |
| Zheng 2006 [53] | Randomized crossover | Healthy medical residents (29) | 22 (15M/7F) |
Unmonitored SW Self-reported SD: 30-h shift NS: 6-h shift |
Brachial artery FMD | FMD ↓ after SW (SD: 3.2% vs. NS: 7.9%, P < 0.001) |
| Tarzia 2011 [54] | Randomized crossover | Healthy medical trainees (27.3 ± 1) | 20 (9M/11F) |
Unmonitored SW Self-reported SD: night shift NS: FS |
Brachial artery FMD | FMD ↓after SW (SD: 8.0 ± 1.4% vs. NS: 8.6 ± 1.7%, P = 0.025) |
| Shimada 2011 [55] | Non-randomized crossover | Healthy male medical staff (32 ± 7) | 19 |
Unmonitored SW Self-reported SD: night shift NS: FS |
Brachial artery FMD | FMD ↓ after SW (SD: 10.4 ± 1.8% vs. NS: 12.5 ± 1.7% P < 0.001) |
| Kim 2011 [56] | Non-randomized crossover | Healthy female nurses (30.1 ± 4.1) | 22 |
Unmonitored SW SD: 3 night shifts NS: regular workday/FS |
Brachial artery FMD | FMD ↓ after SW (SD: 7.6 ± 2.4% vs. NS: 13.3 ± 3.5%, P < 0.001) |
| Amir 2004 [57] | Non-randomized crossover | Healthy physicians (35 ± 4) | 30 (23M/7F) |
Unmonitored SW Self-reported SD: 24-h shift NS: regular workday/FS |
Brachial artery FMD | FMD ↓ after SW (SD: 6.7 ± 4.8% vs. NS: 10.5 ± 4.5%, P < 0.0001) |
ACh acetylcholine, ATP adenosine triphosphate, CFVR coronary flow velocity reserve, CVC cutaneous vascular conductance, CVCauc cutaneous vascular conductance area under the curve, CVCmax maximal cutaneous vascular conductance, CVCpeak peak cutaneous vascular conductance, CWI cold-water immersion, FBF forearm blood flow, F female, FMD flow-mediated dilation, FS full night of sleep, FVC forearm vascular conductance, M male, MCh methacholine, NS normal sleep, PU perfusion units, SD sleep deprivation, SR sleep restriction, SW shift work, TSD total sleep deprivation
Risk of bias
Risk of bias was assessed independently by BJH and SSL and any discrepancies between reviewers were resolved through discussion. Risk of bias was conducted for each study at the outcome level for the effect of assignment to intervention. For observational and non-randomized experimental studies, the risk of bias was assessed using the Risk of Bias in Non-Randomized Studies—of Interventions (ROBINS-I) [58] that focuses on 7 domains which include bias arising from (1) confounding; (2) selection of participants; (3) classification of interventions; (4) deviations from intended interventions; (5) missing outcome data; (6) measurement of outcome; and (7) selection of the reported result. For randomized experimental studies, the risk of bias was assessed using the Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB 2) [59] that focuses on 5 domains which include bias arising from (1) randomization process; (2) deviations from intended interventions; (3) missing outcome data; (4) measurement of outcome; and (5) selection of the reported result. Determination of risk of bias was aided using the Cochrane Risk of Bias Tools [58, 59]. Risk of bias for each domain and overall risk of bias across all domains for each study were graphically presented using the Risk-of-bias VISualization (robvis) software [60]. Additionally, risk of bias for each domain were graphically presented across all observational/non-randomized experimental studies and across all randomized experimental studies using the same software.
Results
Study selection
Detailed information regarding study identification, screening, eligibility, exclusion from and inclusion in the review is presented in the flow diagram (Fig. 1). The initial literature search produced 5563 articles which after removal of duplicates yielded 3571 articles. After an initial screening, 3527 articles were excluded based on title and abstract and 44 articles were assessed for eligibility based on full-text using our inclusion and exclusion criteria. Following full-text screening, a total of 20 articles [61–80] were excluded for the following reasons: no measure of endothelial function (n = 15), no measure of sleep duration (n = 3), and inclusion of children or adolescents (n = 2). In total, 24 articles were included in this systematic review. Excluded articles and reasons for exclusion can be found in Supplementary table 1 in the Online Supplementary Material.
Study characteristics
Our search resulted in 7 observational [34–40] (Table 1) and 17 experimental [41–57] (Table 2) studies involving a total of 1598 participants (51.3% female) ranging from 20 to 60 years of age. Eleven studies (45.8%) enrolled both males and females, 11 (45.8%) enrolled only males, and 2 (8.3%) enrolled only females. Study characteristics are presented in Tables 1 and 2.
Observational studies
General characteristics
Seven observational studies were included which enrolled a total of 1250 participants (58% female) ranging from 20 to 60 years of age. Methodologies to assess endothelial function differed widely across studies and included macrovascular function using brachial artery flow-mediated dilation (FMD) [38–40] and microvascular function using forearm blood flow (FBF) response to acetylcholine (ACh), bradykinin (BK), or a selective endothelin 1 (ET-1) receptor A antagonist (BQ-123) [35–37], and capillary recruitment response to reactive hyperemia [34]. Methodologies to assess sleep duration also varied widely with the majority of studies using self-reported methods based on validated sleep questionnaires (Stanford Physical Activity Questionnaire, Pittsburgh Sleep Quality Index, and Epworth Sleepiness Scale) [34–37, 40] while 2 studies used objective sleep tracking (actigraphy or overnight polysomnography) [38, 39]. Observational studies assessed the cross-sectional [39, 40] or prospective [38] association of habitual sleep duration with endothelial function, or compared endothelial function in individuals with short vs. normal habitual sleep duration [34–38].
Sleep duration and endothelial function
Three studies examined the association of sleep duration with macrovascular endothelial function assessed by brachial artery FMD. In a cohort of men and women, shorter sleep duration assessed by polysomnography was prospectively associated with reduced FMD ~ 18 years later [38], though not after adjusting for known CVD risk factors. However, in another study in men and women, there was no significant association between self-reported sleep duration and FMD [40]. Similarly, in a study in women, no association was observed between actigraphy-assessed sleep duration and FMD [39]. No studies examined the association of sleep duration with microvascular endothelial function. Collectively, these studies show that sleep duration is directly associated with macrovascular endothelial function, but this association is not consistently observed and the association with microvascular endothelial function remains unexplored.
Four studies examined differences in microvascular function between adults with short and normal self-reported sleep duration. In adults who did not receive the recommended amount of sleep (≥ 7 h), vasodilation to ACh was reduced by 20% [35]. Additionally, adults who did not receive the recommended 7 h of sleep/night had a ~ 3-fold greater dilator response to infusion of an ET-1 receptor A antagonist [37]. One study also found that women with short sleep duration (< 7 h) had impaired capillary recruitment in response to reactive hyperemia, while the results in men were not significant [34]. Lastly, no differences were found in forearm blood flow responses to BK between adults with short (< 7 h) and normal sleep duration [36]. No studies have compared macrovascular endothelial function between short and normal sleepers. Collectively, these studies consistently demonstrate that adults who do not meet the recommended amount of sleep have reduced microvascular endothelial function, but whether they also have reduced macrovascular endothelial function remains unknown.
Experimental studies
General characteristics
Seventeen experimental studies were included, which enrolled a total of 348 participants (26% female) ranging from 20 to 40 years of age. Endothelial function was assessed using coronary artery flow velocity reserve response to adenosine triphosphate [47], brachial artery FMD [41, 42, 48–50, 52–57], FBF and forearm vascular conductance (FVC) response to reactive hyperemia [46], venodilation to ACh [50], and cutaneous vascular conductance (CVC) to ACh, MCh, insulin, heat, cold, and/or cathode current [43–45, 51]. Methods used to manipulate sleep duration included TSD lasting 24–40 h [41–46], sleep restriction lasting 1–8 nights [47, 48, 50, 51] or 4 weeks [49], and shift work involving overnight [54–56] and extended shifts of 24–30 h [52, 53, 57].
Effects of TSD on endothelial function
Two studies examined the effect of supervised TSD on macrovascular endothelial function assessed by brachial artery FMD. Compared to 7–8 h of sleep, FMD was attenuated following 24 h of TSD in men and women [41] but remained unchanged after 30.5 h of TSD in men [42]. Four studies investigated the effects of TSD on microvascular function, with 3 studies reporting impaired microvascular function after TSD compared to 7–8 h of sleep. CVC to ACh, insulin, and heat was reduced after 40 h of TSD in men [44]. Similarly, CVC to ACh was reduced after 29 h of TSD in men [43]. CVC in response to cold-water immersion was impaired after 29 h of TSD in men, although no impairments were found prior to cold-water immersion [45]. One study found that FBF and FVC in response to reactive hyperemia did not change after 24 h of TSD in men and women [46]. Collectively these studies indicate that macrovascular and skin but not forearm microvascular endothelial function may be impaired in response to acute TSD.
Effects of sleep restriction on endothelial function
Two studies investigated the effects of sleep restriction on macrovascular endothelial function assessed by brachial artery FMD. FMD was reduced after 4 weeks of sleep restriction to < 80% of habitual sleep duration in men [49] and following 8 nights of sleep restricted to ~ 5 h per night in men and women [48]. Interestingly, endothelium-independent dilation measured in response to sublingual nitroglycerin did not change after sleep restriction [48, 49], indicating that vascular smooth muscle responsiveness to nitric oxide (NO) was preserved. Three studies investigated the effects of sleep restriction on microvascular endothelial function. Microvascular function assessed by CVC to MCh and heat was reduced after 6 nights of sleep restricted to < 4 h per night in men, while CVC in response to cathode current did not change [51]. Venous endothelium-dependent dilation to ACh was also attenuated following 5 nights of sleep restricted to 4.5 h per night in men [50]. Coronary flow velocity reserve to ATP was reduced following a single night of sleep restricted to ≤ 4 h in men [47]. Collectively, these studies demonstrate that sleep restriction negatively impacts endothelial function regardless of vascular bed or sleep restriction duration.
Effects of shift work on endothelial function
Six studies investigated the effects of shift work on macrovascular endothelial function using brachial artery FMD. FMD was reduced after working for a 24- [52, 57] and 30-h shift [53] and following a single night shift in medical staff [54, 55]. Similarly, FMD was reduced following 3 sequential night shifts in female nurses compared to a day with no previous night shift [56]. No studies investigated the effect of shift work on microvascular function. Collectively, these studies provide consistent evidence that macrovascular function is negatively impacted by shift work, but the effect on microvascular endothelial function remains unknown.
Risk of bias
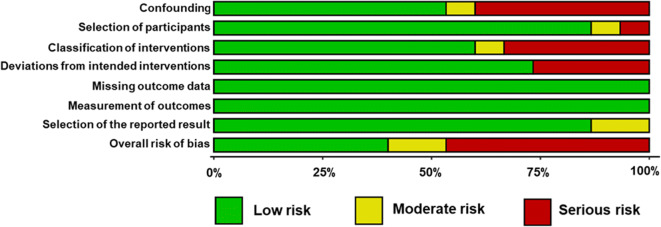
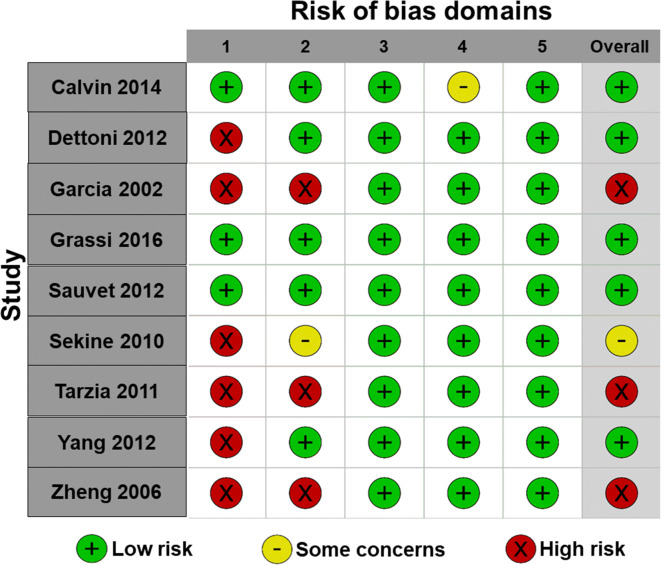
Risk of bias for each observational and non-randomized experimental study is presented in Fig. 2. Additionally, risk of bias for each domain is presented across all observational/non-randomized experimental studies in Fig. 3. Risk of bias arising from confounding was serious in 6, moderate in 1, and low in 8 studies and resulted from inclusion of participants who were shift workers [42] or who had comorbidities [38, 57] and from not controlling important factors known to influence endothelial function (e.g., food and caffeine intake during sleep deprivation, changes in weight during sleep restriction, and standardization of menstrual cycle phase) [50, 51, 57]. Risk of bias due to selection of participants was serious in 1, moderate in 1, and low in 13 studies. Risk of bias arising from classification of interventions was serious in 5, moderate in 1, and low in 9 studies and was mainly due to intervention groups being non-explicitly defined. Risk of bias due to deviations from the intended intervention was serious in 4 and low in 11 studies and this was mainly related to the confounding effects of physical work and mental stress during shift work. However, we recognize that these are not indicative of flaws in the study designs per se, but rather that they introduce a minor bias in interpreting the results on the independent effects of sleep deprivation on endothelial function. Risk of bias due to the selection of the reported results was moderate in 2 and low in 13 studies. For all observational and non-randomized experimental studies, the risk of bias due to missing data and measurement of outcome was low. Overall, none of the observational and non-randomized experimental studies had flaws that prevented them from significantly contributing to this review.
Fig. 2.
Risk of bias for observational and non-randomized experimental studies. Domain 1: bias due to confounding; domain 2: bias due to selection of participants; domain 3: bias in classification of interventions; domain 4: bias due to deviations from intended interventions; domain 5: bias due to missing outcome data; domain 6: bias in measurement of outcomes; domain 7: bias in selection of the reported result
Fig. 3.
Risk of bias domains across all observational/non-randomized experimental studies
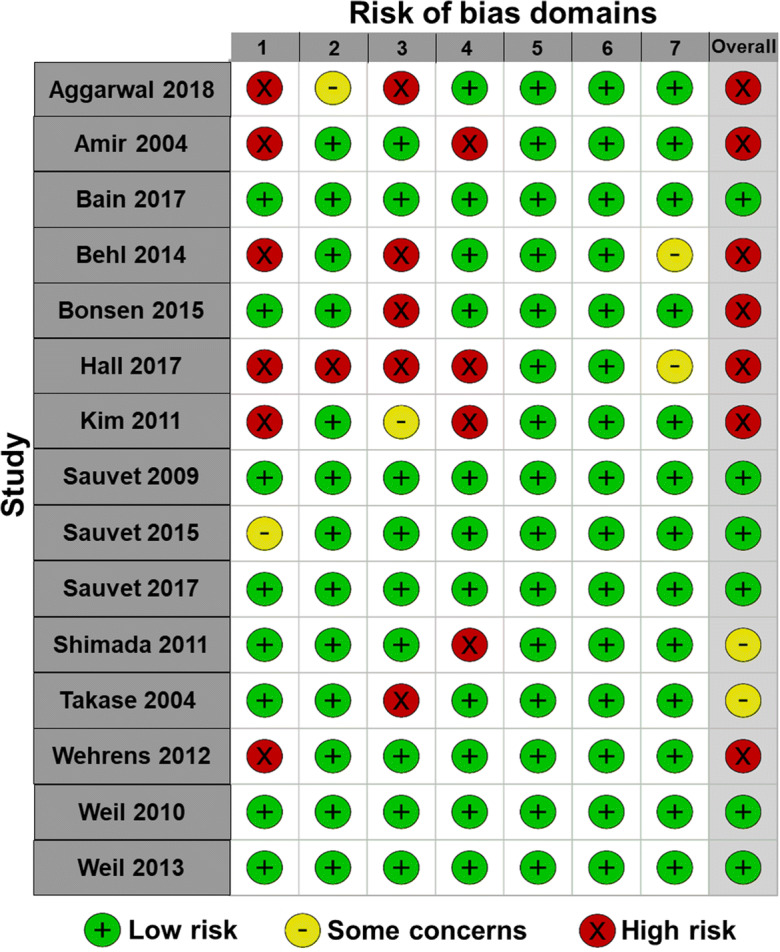
For randomized experimental studies, risk of bias for each study is presented in Fig. 4. Additionally, risk of bias for each domain is presented across all randomized experimental studies in Fig. 5. Risk of bias arising from the randomization process was high in 6 and low in 3 studies and was mainly due to lack of information on the method of sequence generation and allocation concealment. We acknowledge that although this information was not reported it does not mean that the methodology was inappropriate. Risk of bias due to deviations from the intended intervention was assessed in regard to the scope of this review, which was to investigate the independent effects of sleep deprivation on endothelial function. Three studies which focused on shift work included high mental stress or physical work, in addition to sleep deprivation, and were therefore deemed to have high risk of bias for this domain. In addition, 1 study raised some concerns and 5 had low risk of bias. Although deviations regarding this domain could introduce bias into the results of the current review, they were not deviations inherent to the study methods. None of the randomized experimental studies had missing outcome data and thus had low risk for this domain. All randomized experimental studies except 1 were deemed to have low risk of bias due to measurement of the outcome and bias from the awareness of outcome assessors to the intervention. When blinding of the assessor was not reported, studies were considered low risk. When blinding of participants could not occur, studies were also considered low risk because this would not have influenced the endothelial function measure. Risk of bias arising from selection of the reported results was low in all studies. Overall, none of the randomized experimental studies had flaws that prevented them from significantly contributing to this review.
Fig. 4.
Risk of bias for randomized experimental studies. Domain 1: bias arising from the randomization process; domain 2: bias due to deviations from intended intervention; domain 3: bias due to missing outcome data; domain 4: bias in measurement of the outcome; domain 5: bias in selection of the reported result
Fig. 5.
Risk of bias domains across all randomized experimental studies
Discussion
This is the first systematic review to focus on the effects of sleep deprivation on endothelial function. Our main findings include the following: (1) receiving less sleep is associated with reduced macrovascular endothelial function, but data are inconsistent and the association with microvascular endothelial function remains unexplored; (2) not meeting the sleep recommendations for adults (i.e., 7–9 h/night) is linked to impaired microvascular endothelial function, but the effect on macrovascular endothelial function remains unknown; (3) sleep restriction impairs both micro- and macrovascular endothelial function; (4) acute TSD impairs micro- and macrovascular endothelial function, but data on macrovascular endothelial function are less consistent; and (5) extended or overnight shift work impairs macrovascular endothelial function, but the effect on microvascular endothelial function remains unknown.
Observational studies
Correlational data on the association of sleep duration with macrovascular endothelial function are inconsistent and the association with microvascular endothelial function remains unexplored. This is likely due to the inclusion of a limited range of sleep duration in some studies and the challenges associated with quantifying long-term habitual sleep duration. Cross-sectional data consistently demonstrate that short sleepers have reduced microvascular endothelial function. These data complement epidemiological data showing that habitually receiving insufficient sleep is associated with greater risk for future CVD events [81] and highlight endothelial dysfunction as a potential mediator of CVD pathogenesis in short sleepers. Observational evidence provides preliminary support for insufficient sleep as a potential modifiable CVD risk factor, but well-controlled adequately powered studies are needed to provide definitive evidence. Our review supports current recommendations from leading health organizations that adults obtain between 7 and 9 h of sleep each night [82].
Experimental studies
The perils of pulling an all-nighter
The negative effects of TSD on macro- and microvascular function were consistent across most experimental studies, providing strong evidence that endothelial function is directly impacted by an acute lack of sleep. The exception to these findings is two studies which found no effect of TSD on brachial artery FMD [42] or FVC [46]. One of these studies included a group of shift workers who had reduced baseline endothelial function compared with non-shift workers, which likely influenced the impact of TSD on endothelial function [42]. The second study assessed endothelial function following TSD in combination with mental stress and a cold-pressor test; therefore, it is unknown how TSD impacted endothelial function per se [46]. Notwithstanding these two studies, the cumulative evidence supporting that a single day of acute TSD has detrimental endothelial effects is alarming.
While the TSD model may not be as generalizable to real-world settings as sleep restriction or shift work models, TSD is a well-established experimental model to understand the acute effects of insufficient sleep on physiological function in humans and animals. Even though TSD-related endothelial dysfunction is likely reversible in the near-term with adequate recovery sleep, repeated bouts of TSD-induced endothelial dysfunction could lead to the development of CVD. Indeed, endothelial dysfunction is one of the primary initiating events in the development of atherosclerosis [27]. Furthermore, endothelial function governs blood flow to the heart, brain, skeletal muscle, and skin [83], impacting quality of life, activities of daily living, and exercise tolerance. Therefore, our review underscores the importance of receiving an adequate amount of sleep for optimal function and warns of the perils of pulling an all-nighter.
Restricting sleep has vascular consequences
Our review demonstrates that moderately restricting sleep, equivalent to ~ 2–3 h per night, impairs endothelial function throughout the vascular tree. This includes endothelial function impairments in coronary, peripheral, and skin vasculature, which hold strong prognostic value for future CVD and CVD events [84–87]. While only one study assessed coronary endothelial function [47], the finding that a single night of < 4 h of sleep reduces coronary artery function is a cause for concern. A night of insufficient sleep could significantly elevate the risk for acute myocardial ischemia and other cardiovascular events, especially in the morning when this risk is already elevated [88]. Furthermore, peripheral endothelial dysfunction is evident in all experimental studies included in our review independent of the vascular bed or the duration or magnitude of sleep restriction.
While the effects of experimental sleep restriction are acute and likely reversible once a normal and consistent sleep pattern is resumed, findings have relevance to adults who are chronically not meeting the recommended sleep duration. As our review shows, habitually receiving less than the recommended 7 h of sleep/night is associated with micro- and macrovascular endothelial dysfunction. Therefore, experimental sleep restriction data align with observational data and provide a potential mechanism for explaining the association of short habitual sleep duration and CVD.
Working the night shift
Shift work is common among > 15% of the workforce [23], with potentially wide-ranging effects on cardiovascular health. Indeed, our review finds compelling evidence that extended or overnight shift work impairs macrovascular endothelial function assessed by brachial artery FMD, a measure that is highly predictive of future CVD events [28–30, 86]. While multiple mechanisms have been proposed for the increased CVD risk in shift workers including psychosocial stress, circadian misalignment, weight gain, smoking, and increased prevalence of metabolic syndrome [25], our review identifies endothelial dysfunction as a novel potential mechanism by which long-term shift work may increase the risk for CVD. In support of this, a longer duration of shift work is associated with a lower brachial artery FMD [42, 57, 89], suggesting that acute endothelial dysfunction likely progresses along a continuum to persistent endothelial dysfunction, atherosclerosis, and CVD as the duration of shift work increases. These findings are cautionary for individuals involved in professions where extended or night shift work is common. Shift workers do not appear to adapt to chronic sleep deprivation and chronobiological disruption. Indeed, evidence supports that < 3% of night-shift workers ever experience a complete adaptation of endogenous circadian rhythms to this schedule [90]. Adequate physical activity, proper nutrition, and strategic timing of these circadian time-givers [91] may have increased importance in shift workers to mitigate the CVD risk imposed by their work schedule.
Mechanisms
The mechanisms responsible for sleep deprivation–induced impairments in endothelial function have not been fully elucidated. This section will discuss direct mechanistic evidence focusing on inflammation and oxidative stress and autonomic nervous system dysregulation, the main areas investigated in the studies included in this systematic review. Novel potential mechanisms related to chronobiological disruption, a rapidly evolving area of research, will also be discussed.
Inflammation and oxidative stress
Sleep is an important modulator of the immune system and thus, sleep deprivation may cause endothelial dysfunction in part by increasing vascular inflammation and reactive oxygen species (ROS) production [92]. Inflammation due to acute or chronic sleep deprivation could be the result of a chronically elevated sympathetic stress response and increased blood pressure. While blood pressure normally dips 10–20% during nighttime sleep, absence of dipping or elevations in blood pressure during sleep deprivation activate the endothelium, causing the release of inflammatory mediators, vascular adhesion molecules, and factors promoting coagulation that contribute to endothelial dysfunction [93]. This would also explain why chronic sleep deprivation, by preventing resolution of this inflammatory response, is associated with chronic low-grade inflammation and inflammatory diseases [93, 94].
Sleep deprivation has been shown to cause acute changes in inflammatory biomarkers including C-reactive protein (CRP), tumor necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6). In some studies, endothelial dysfunction was accompanied by acute elevations in inflammatory biomarkers. CRP was increased after a 30-h work shift [53] and 10 days of chronic sleep restriction [95]. TNF-α was elevated following 1, 2, and 6 days of sleep restriction [51, 96, 97]. IL-6 was also increased after a 30-h work shift [53], a single night of sleep restriction [96, 97], and 24-h TSD [44]. Interestingly, 7 weeks of aerobic exercise training prior to TSD abolished the inflammatory response and preserved endothelial function after TSD [44], suggesting a causal role of inflammation in endothelial dysfunction and highlighting exercise as a promising intervention that may prevent sleep deprivation–induced endothelial dysfunction.
Acute inflammation causes endothelial dysfunction by promoting oxidative stress within the vasculature and by reducing the bioavailability of NO [98]. ROS cause endothelial damage, apoptosis, and cell proliferation and directly/indirectly reduce NO bioavailability [98]. Superoxide anions (O2−) rapidly react with NO and form peroxynitrite (ONOO−), both of which may cause lipid, protein, and DNA damage [99]. This reaction reduces NO bioavailability while also raising ROS within the endothelium [99]. ROS further reduce NO bioavailability by causing endothelial nitric oxide synthase (eNOS) uncoupling, a process that results in the paradoxical eNOS formation of ROS, rather than NO [99]. Reduced NO bioavailability impairs endothelium-dependent vasodilatory capacity. There is some evidence based on studies included in our review that reduced NO bioavailability, perhaps a result of increased oxidative stress, may be an underlying cause of sleep deprivation–induced endothelial dysfunction. Men who reported sleeping < 7 h per night had a reduced contribution of NO to endothelium-dependent dilation compared to men sleeping > 7 h each night, suggesting that sufficient sleep is necessary for adequate NO availability/action [35]. Additionally, after working 3 consecutive night shifts, female nurses had reduced levels of NO metabolites [56], though whether this was due to altered NO production or increased degradation was not investigated.
Opposing the actions of NO is ET-1, which when bound to endothelial ETA receptors induces vascular smooth muscle constriction. ETA receptor binding also increases ROS production and interferes with NO production and actions [100, 101], thereby implicating ET-1 in the pathogenesis of endothelial dysfunction. One study in our review found that short sleepers had greater ET-1-mediated vasoconstrictor tone [37]. Acute sleep deprivation may also impair NO/ET-1 balance. One study found that acute TSD increased plasma ET-1 without altering levels of plasma nitrites [43], thus favoring vasoconstriction and promoting endothelial dysfunction. Therefore, low-grade inflammation, increased ROS, and their influence on the vasodilator/vasoconstrictor balance likely play a major role in endothelial dysfunction associated with acute and chronic sleep deprivation.
Autonomic nervous system dysregulation
With the exception of REM sleep, parasympathetic nervous system activity is dominant during the majority of sleep duration [102]. Thus, acute sleep deprivation may induce autonomic dysregulation, characterized by a state of sympathetic nervous system (SNS) hyperactivity [102]. SNS stimulation causes augmented vascular smooth muscle tone, alters NO bioavailability and activity, elevates ROS production, and stimulates vasoconstrictor pathways, predisposing to endothelial dysfunction [103]. Indeed, there is an inverse relationship between measures of endothelial function and sympathetic activity, and acutely raising SNS activity reduces endothelial function [103], potentially triggering the negative effects of short- and long-term sleep deprivation.
Several studies included in this systematic review reported evidence of TSD-induced SNS elevation occurring in tandem with endothelial dysfunction. SNS activity measured using heart-rate variability (HRV) was shown to increase after 32 and 40 h of TSD [43, 104], and 5 nights of partial sleep restriction [50], while other studies found no change in HRV after 24 h of TSD [42, 61]. One of these studies observed no change in HRV after TSD, but shift workers in this study had lower baseline HRV indicating higher SNS activity in these individuals [42]. Other measures known to be associated with SNS activity including epinephrine [49], norepinephrine [43, 50, 53], cortisol [47], and blood pressure [41, 43, 46, 47, 49, 52] increase in response to sleep deprivation. However, some studies found no increase in blood pressure [50, 51], catecholamines [45, 51], or muscle sympathetic nerve activity [46] after acute sleep deprivation, or found that endothelial dysfunction preceded sympathetic activation [43], suggesting that endothelial dysfunction can occur in the presence or absence of elevated SNS activity.
Chronobiological disruption
Human experimental data on the effects of acute or chronic sleep deprivation on endogenous circadian clock gene expression, circadian rhythmicity, or chronobiological function are limited. As this is an emerging field of research, we believe it is worthwhile to speculate on the potential ways sleep deprivation may contribute to chronobiological disruption of endothelial function predisposing to CVD development. Acute overnight sleep deprivation has been shown to alter epigenetic and transcriptional circadian clock gene profiles in humans [105]. The presence of circadian clocks in vascular cells and time-of-day variance in vascular tone and endothelial function [106–108] likely function to anticipate external and internal stimuli. The specific time-of-day release of circulating metabolites including NO and ET-1 [109] likely helps to promote optimal vascular function. Sleep deprivation may promote endothelial dysfunction by altering circadian release profiles of NO, ET-1, and endothelial and vascular smooth muscle cell sensitivity to these vasoactive stimuli. Indeed, animal models of circadian disruption show evidence of vascular senescence, endothelial dysfunction, and loss of diurnal variation in endothelial function and blood pressure [110].
Reductions in blood pressure and shear stress during sleep, when cellular turnover is active, suppress pro-hypertrophic stimuli, thereby favoring repair mechanisms (reviewed in Rana et al. [17]). An absence of the nocturnal blood pressure dip and other regenerative functions during the night is likely to impair vascular remodeling in humans, increase vascular and endothelial inflammation and oxidative stress, and promote cell senescence, mechanisms implicated in the pathogenesis of vascular aging [111]. Furthermore, sleep deprivation and shift work require being awake at night when, according to human biology, one should be sleeping, likely causing circadian rhythm desynchrony. Being awake during the night also exposes one to time cues known as zeitgebers (e.g., light, food, and physical activity) that disrupt normal circadian rhythms [112–114]. Shift workers who suffer from chronic insufficient sleep have disrupted circadian clocks, as evidenced by a lack of complete entrainment of their circadian rhythms to the night shift [115], which may promote endothelial dysfunction.
Conceptual model on link of sleep deprivation to CVD
The current systematic review demonstrates that sleep deprivation impairs endothelial function. Chronic sleep deficiency across the lifespan may have cumulative adverse effects on endothelial function, thus accelerating vascular aging and promoting CVD development (Fig. 6). Human experimental evidence supports the role of inflammation, oxidative stress, and autonomic nervous system dysregulation in endothelial dysfunction associated with acute and chronic sleep deprivation. However, other mechanisms may be involved and should be elucidated in future investigations. Human evidence on the role of chronobiological disruption is limited but presents an exciting new direction for future studies. Long-term exposure to sleep deprivation may have devastating chronobiological consequences and provides an attractive target for the development of therapeutic strategies focusing on clock re-entrainment by optimizing timing of cues such as light, food intake, and physical activity to preserve/restore endothelial function.
Fig. 6.
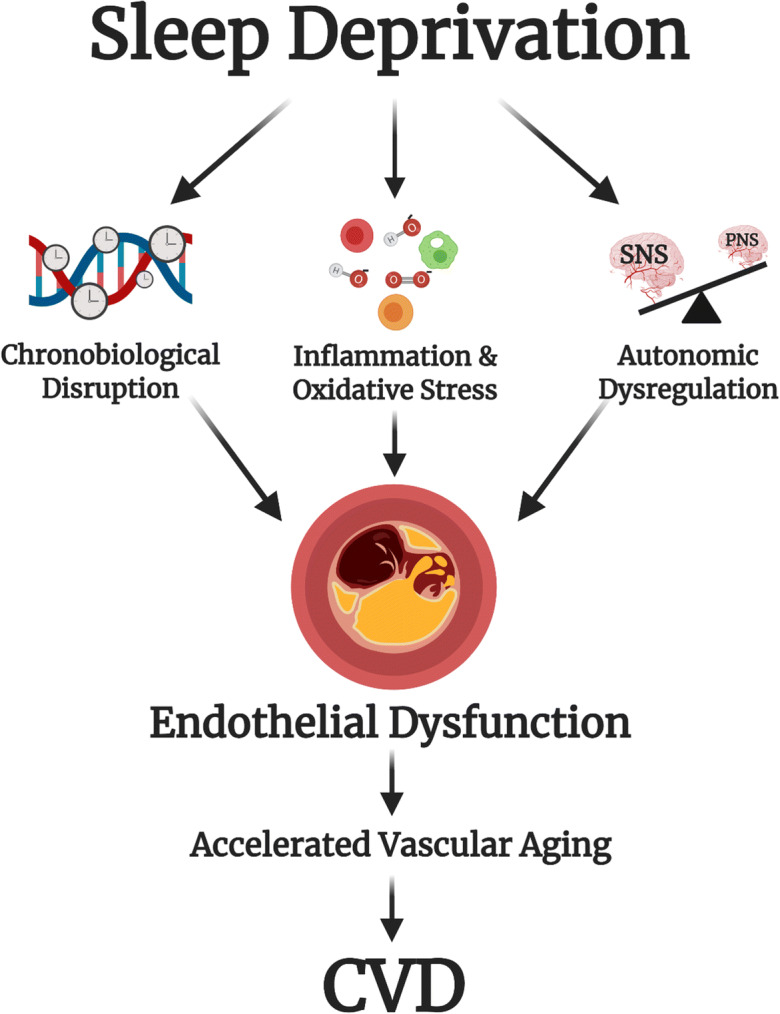
Conceptual model of the link between sleep deprivation and cardiovascular disease. Sleep deprivation leads to inflammation, oxidative stress, autonomic nervous system dysregulation, and chronobiological disruption, which impair endothelial function and may lead to accelerated vascular aging and cardiovascular disease. SNS, sympathetic nervous system; PNS, parasympathetic nervous system; CVD, cardiovascular disease. Created with BioRender.com
Implications for human aging
Adequate sleep should be encouraged in individuals of all ages given that chronic sleep deficiency likely has cumulative adverse cardiovascular effects that may lead to accelerated vascular aging. The evidence summarized in this review clearly demonstrates that achieving adequate sleep should be considered indispensable to promote optimal cardiovascular health. Whether insufficient sleep impairs endothelial function to the same extent in individuals older than 60 years of age remains to be determined because all studies that were included in this review focused on individuals 20 to 60 years of age. The well-documented age-related biological changes in sleep characteristics and chronobiology including reductions in sleep duration and quality [5–8] are concerning considering the evidence from our review that even a moderate amount of sleep restriction has adverse vascular consequences. As individuals age and receive a reduced amount of sleep, this could have compounding effects on vascular aging. Therefore, healthy sleep behaviors should be encouraged in older individuals. Future research in the field of gerontology and aging chronobiology should investigate the influence of sleep deprivation on chronobiological disruption and develop interventions to reduce the negative effects of sleep deprivation on endothelial health in this population.
Limitations
It was not possible to conduct a meta-analysis on the effects of sleep deprivation on endothelial function due to the significant heterogeneity in the methods used to evaluate endothelial function. However, this has resulted in a comprehensive assessment of the effects of sleep deprivation on endothelial function across the vascular tree. We included both observational and experimental studies to maximize the impact of our review but realize the limitations of observational data in informing conclusions about causation. Observational studies that rely on self-reported sleep duration are limited in their ability to make definitive conclusions about the association of sleep duration with endothelial function, especially given that self-reported sleep duration is only moderately correlated with objectively measured sleep duration [112, 113]. Shift work studies also have some limitations. These investigations rarely controlled for work stress, sleep time, and food and beverage consumption. Although this maximizes external validity, it limits direct comparisons with well-controlled sleep deprivation studies. Some studies in this review included women varying in menopausal status [34, 37, 38, 41, 46, 48, 52–54, 56, 57], individuals who smoked [53, 57], or who had hypertension, diabetes, major depressive disorder, and obesity [38], which could confound the association of short sleep duration with endothelial function. Limitations notwithstanding, the inclusion of observational and shift work studies greatly complements evidence of well-controlled experimental studies.
Conclusions
We present the first systematic review of the literature on the effects of sleep deprivation on endothelial function. Taken together, evidence from observational and experimental studies link sleep deprivation to endothelial dysfunction, which may be the link between insufficient sleep and CVD. Current guidelines state that adults should get between 7 and 9 h of sleep each night, and the current review supports this recommendation in the context of reducing CVD risk. Given the pervasiveness of sleep deprivation, interventions to combat its adverse effects on endothelial health are urgently needed. Getting an adequate amount of sleep should become a priority for cardiovascular health across the lifespan.
Supplementary Information
(PDF 96 kb)
Author contributions
BJH and DDC conceived and designed the systematic review. BJH and SSL performed the literature search and screening, data extraction and synthesis, and risk-of-bias assessment. BJH wrote the initial draft of the manuscript. DDC, SSL, and DEJS critically revised the manuscript for intellectual content. All authors reviewed and approved the final manuscript.
Funding
DDC was supported by the National Institute on Aging of the National Institutes of Health [grant AG063143].
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
This systematic review was based on data acquired through previously published articles.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults--United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65(6):137–41. 10.15585/mmwr.mm6506a1. [DOI] [PubMed]
- 2.St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, Bhatt DL, American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–ee86. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 4.NSF. 2010 Bedroom Poll: Summary of Findings. 2012:1–79.
- 5.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 6.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 7.Duffy JF, Zitting KM, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin. 2015;10(4):423–434. doi: 10.1016/j.jsmc.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington JJ, Lee-Chiong T., Jr Sleep and older patients. Clin Chest Med. 2007;28(4):673–684. doi: 10.1016/j.ccm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Covassin N, Singh P. Sleep duration and cardiovascular disease risk: epidemiologic and experimental evidence. Sleep Med Clin. 2016;11(1):81–89. doi: 10.1016/j.jsmc.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Q, Liu X, Wang X, Wu S. Age- and gender-specific associations between sleep duration and incident hypertension in a Chinese population: the Kailuan study. J Hum Hypertens. 2016;30(8):503–507. doi: 10.1038/jhh.2015.118. [DOI] [PubMed] [Google Scholar]
- 11.Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33(6):739–744. doi: 10.1093/sleep/33.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am. 2012;96(1):87–91. doi: 10.1016/j.mcna.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.deGoma EM, Knowles JW, Angeli F, Budoff MJ, Rader DJ. The evolution and refinement of traditional risk factors for cardiovascular disease. Cardiol Rev. 2012;20(3):118–129. doi: 10.1097/CRD.0b013e318239b924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6(8):e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rana S, Prabhu SD, Young ME. Chronobiological influence over cardiovascular function: the good, the bad, and the ugly. Circ Res. 2020;126(2):258–279. doi: 10.1161/CIRCRESAHA.119.313349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato M, Matsuo T, Atmore H, Akashi M. Possible contribution of chronobiology to cardiovascular health. Front Physiol. 2013;4(409):409. doi: 10.3389/fphys.2013.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta NJ. Lifestyle and circadian health: where the challenges lie? Nutr Metab Insights. 2019;12:1178638819869024. doi: 10.1177/1178638819869024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Tang Q, Chen G, Xie M, Yu S, Zhao J, Chen L. New insights into the circadian rhythm and its related diseases. Front Physiol. 2019;10(682):682. doi: 10.3389/fphys.2019.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortman J, Velkoff V, Hogan H. An aging nation: the older population in the United States. US Census Bureau; 2014.
- 22.Miner B, Kryger MH. Sleep in the aging population. Sleep Med Clin. 2017;12(1):31–38. doi: 10.1016/j.jsmc.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright KP, Jr, Bogan RK, Wyatt JK. Shift work and the assessment and management of shift work disorder (SWD) Sleep Med Rev. 2013;17(1):41–54. doi: 10.1016/j.smrv.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Rajaratnam SM, Howard ME, Grunstein RR. Sleep loss and circadian disruption in shift work: health burden and management. Med J Aust. 2013;199(8):S11–S15. doi: 10.5694/mja13.10561. [DOI] [PubMed] [Google Scholar]
- 25.Puttonen S, Harma M, Hublin C. Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health. 2010;36(2):96–108. doi: 10.5271/sjweh.2894. [DOI] [PubMed] [Google Scholar]
- 26.James SM, Honn KA, Gaddameedhi S, Van Dongen HPA. Shift work: disrupted circadian rhythms and sleep-implications for health and well-being. Curr Sleep Med Rep. 2017;3(2):104–112. doi: 10.1007/s40675-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 28.Ras RT, Streppel MT, Draijer R, Zock PL. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int J Cardiol. 2013;168(1):344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 29.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4(11). 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed]
- 31.Seals DR, Alexander LM. Vascular aging. J Appl Physiol (1985). 2018;125(6):1841–2. 10.1152/japplphysiol.00448.2018. [DOI] [PubMed]
- 32.Ghebre YT, Yakubov E, Wong WT, Krishnamurthy P, Sayed N, Sikora AG et al. Vascular aging: implications for cardiovascular disease and therapy. Transl Med (Sunnyvale). 2016;6(4). 10.4172/2161-1025.1000183. [DOI] [PMC free article] [PubMed]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (reprinted from Annals of Internal Medicine) Phys Ther. 2009;89(9):873–880. doi: 10.1093/ptj/89.9.873. [DOI] [PubMed] [Google Scholar]
- 34.Bonsen T, Wijnstok NJ, Hoekstra T, Eringa EC, Serne EH, Smulders YM, et al. Sleep quality and duration are related to microvascular function: the Amsterdam Growth and Longitudinal Study. J Sleep Res. 2015;24(2):140–147. doi: 10.1111/jsr.12256. [DOI] [PubMed] [Google Scholar]
- 35.Bain AR, Weil BR, Diehl KJ, Greiner JJ, Stauffer BL, DeSouza CA. Insufficient sleep is associated with impaired nitric oxide-mediated endothelium-dependent vasodilation. Atherosclerosis. 2017;265:41–46. doi: 10.1016/j.atherosclerosis.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Weil BR, Greiner JJ, Stauffer BL, Desouza CA. Self-reported habitual short sleep duration is associated with endothelial fibrinolytic dysfunction in men: a preliminary report. Sleep. 2013;36(2):183–188. doi: 10.5665/sleep.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, Stauffer BL, et al. Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol. 2010;88(8):777–781. doi: 10.1139/Y10-046. [DOI] [PubMed] [Google Scholar]
- 38.Hall MH, Mulukutla S, Kline CE, Samuelsson LB, Taylor BJ, Thayer JF et al. Objective sleep duration is prospectively associated with endothelial health. Sleep. 2017;40(1). 10.1093/sleep/zsw003. [DOI] [PMC free article] [PubMed]
- 39.Aggarwal B, Makarem N, Shah R, Emin M, Wei Y, St-Onge MP et al. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2018;7(12). 10.1161/JAHA.118.008590. [DOI] [PMC free article] [PubMed]
- 40.Behl M, Bliwise D, Veledar E, Cunningham L, Vazquez J, Brigham K, et al. Vascular endothelial function and self-reported sleep. Am J Med Sci. 2014;347(6):425–428. doi: 10.1097/MAJ.0b013e31829bc950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grassi D, Socci V, Tempesta D, Ferri C, De Gennaro L, Desideri G, et al. Flavanol-rich chocolate acutely improves arterial function and working memory performance counteracting the effects of sleep deprivation in healthy individuals. J Hypertens. 2016;34(7):1298–1308. doi: 10.1097/HJH.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 42.Wehrens SM, Hampton SM, Skene DJ. Heart rate variability and endothelial function after sleep deprivation and recovery sleep among male shift and non-shift workers. Scand J Work Environ Health. 2012;38(2):171–181. doi: 10.5271/sjweh.3197. [DOI] [PubMed] [Google Scholar]
- 43.Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, Van Beers P et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108(1):68–75. 10.1152/japplphysiol.00851.2009. [DOI] [PubMed]
- 44.Sauvet F, Arnal PJ, Tardo-Dino PE, Drogou C, Van Beers P, Bougard C, et al. Protective effects of exercise training on endothelial dysfunction induced by total sleep deprivation in healthy subjects. Int J Cardiol. 2017;232:76–85. doi: 10.1016/j.ijcard.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 45.Sauvet F, Bourrilhon C, Besnard Y, Alonso A, Cottet-Emard JM, Savourey G, Launay JC. Effects of 29-h total sleep deprivation on local cold tolerance in humans. Eur J Appl Physiol. 2012;112(9):3239–3250. doi: 10.1007/s00421-011-2297-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Durocher JJ, Larson RA, Dellavalla JP, Carter JR. Total sleep deprivation alters cardiovascular reactivity to acute stressors in humans. J Appl Physiol (1985). 2012;113(6):903–8. 10.1152/japplphysiol.00561.2012. [DOI] [PMC free article] [PubMed]
- 47.Sekine T, Daimon M, Hasegawa R, Toyoda T, Kawata T, Funabashi N, Komuro I. The impact of sleep deprivation on the coronary circulation. Int J Cardiol. 2010;144(2):266–267. doi: 10.1016/j.ijcard.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Calvin AD, Covassin N, Kremers WK, Adachi T, Macedo P, Albuquerque FN, Bukartyk J, Davison DE, Levine JA, Singh P, Wang S, Somers VK. Experimental sleep restriction causes endothelial dysfunction in healthy humans. J Am Heart Assoc. 2014;3(6):e001143. doi: 10.1161/JAHA.114.001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takase B, Akima T, Uehata A, Ohsuzu F, Kurita A. Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol. 2004;27(4):223–227. doi: 10.1002/clc.4960270411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dettoni JL, Consolim-Colombo FM, Drager LF, Rubira MC, Souza SB, Irigoyen MC et al. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J Appl Physiol (1985). 2012;113(2):232–6. 10.1152/japplphysiol.01604.2011. [DOI] [PubMed]
- 51.Sauvet F, Drogou C, Bougard C, Arnal PJ, Dispersyn G, Bourrilhon C, Rabat A, van Beers P, Gomez-Merino D, Faraut B, Leger D, Chennaoui M. Vascular response to 1 week of sleep restriction in healthy subjects. A metabolic response? Int J Cardiol. 2015;190:246–255. doi: 10.1016/j.ijcard.2015.04.119. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Fernandez R, Garcia Perez-Velasco J, Milian AC, Peix Gonzalez A, Garcia-Barreto D. Endothelial dysfunction in cardiologists after 24 hours on call. Rev Esp Cardiol. 2002;55(11):1202–1204. doi: 10.1016/s0300-8932(02)76784-5. [DOI] [PubMed] [Google Scholar]
- 53.Zheng H, Patel M, Hryniewicz K, Katz SD. Association of extended work shifts, vascular function, and inflammatory markers in internal medicine residents: a randomized crossover trial. JAMA. 2006;296(9):1049–1050. doi: 10.1001/jama.296.9.1049. [DOI] [PubMed] [Google Scholar]
- 54.Tarzia P, Milo M, Di Franco A, Di Monaco A, Cosenza A, Laurito M, et al. Effect of shift work on endothelial function in young cardiology trainees. Eur J Prev Cardiol. 2012;19(5):908–913. doi: 10.1177/1741826711422765. [DOI] [PubMed] [Google Scholar]
- 55.Shimada K, Fukuda S, Maeda K, Kawasaki T, Kono Y, Jissho S, Taguchi H, Yoshiyama M, Yoshikawa J. Aromatherapy alleviates endothelial dysfunction of medical staff after night-shift work: preliminary observations. Hypertens Res. 2011;34(2):264–267. doi: 10.1038/hr.2010.228. [DOI] [PubMed] [Google Scholar]
- 56.Kim W, Park HH, Park CS, Cho EK, Kang WY, Lee ES, Kim W. Impaired endothelial function in medical personnel working sequential night shifts. Int J Cardiol. 2011;151(3):377–378. doi: 10.1016/j.ijcard.2011.06.109. [DOI] [PubMed] [Google Scholar]
- 57.Amir O, Alroy S, Schliamser JE, Asmir I, Shiran A, Flugelman MY, Halon DA, Lewis BS. Brachial artery endothelial function in residents and fellows working night shifts. Am J Cardiol. 2004;93(7):947–949. doi: 10.1016/j.amjcard.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 60.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): an R package and shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2020. 10.1002/jrsm.1411. [DOI] [PubMed]
- 61.Slomko J, Zawadka-Kunikowska M, Kozakiewicz M, Klawe JJ, Tafil-Klawe M, Newton JL, et al. Hemodynamic, autonomic, and vascular function changes after sleep deprivation for 24, 28, and 32 hours in healthy men. Yonsei Med J. 2018;59(9):1138–1142. doi: 10.3349/ymj.2018.59.9.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akdemir I, Altunbas G, Ercan S, Arslan S, Davutoglu V. Impact of acute sleep deprivation on aortic elastic properties in healthy workers. Turk J Med Sci. 2013;43:279–282. doi: 10.3906/shg-1206-77. [DOI] [Google Scholar]
- 63.Anujuo K, Stronks K, Snijder MB, Jean-Louis G, Rutters F, van den Born BJ, Peters RJ, Agyemang C. Relationship between short sleep duration and cardiovascular risk factors in a multi-ethnic cohort - the helius study. Sleep Med. 2015;16(12):1482–1488. doi: 10.1016/j.sleep.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 64.Anujuo K, Stronks K, Snijder MB, Jean-Louis G, van den Born BJ, Peters RJ, Agyemang C. Relationship between sleep duration and arterial stiffness in a multi-ethnic population: the HELIUS study. Chronobiol Int. 2016;33(5):543–552. doi: 10.3109/07420528.2016.1158721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao X, Zhou J, Yuan H, Chen Z. Association between sleep condition and arterial stiffness in Chinese adult with nonalcoholic fatty liver disease. J Thromb Thrombolysis. 2016;42(1):127–134. doi: 10.1007/s11239-016-1356-1. [DOI] [PubMed] [Google Scholar]
- 66.Chen S, Yang Y, Cheng GL, Jia J, Fan FF, Li JP, Huo Y, Zhang Y, Chen DF. Association between short sleep duration and carotid atherosclerosis modified by age in a Chinese community population. J Epidemiol Community Health. 2018;72(6):539–544. doi: 10.1136/jech-2017-209464. [DOI] [PubMed] [Google Scholar]
- 67.Cooper DC, Ziegler MG, Milic MS, Ancoli-Israel S, Mills PJ, Loredo JS, von Känel R, Dimsdale JE. Endothelial function and sleep: associations of flow-mediated dilation with perceived sleep quality and rapid eye movement (REM) sleep. J Sleep Res. 2014;23(1):84–93. doi: 10.1111/jsr.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Culver MN, Langan SP, Carreker J, Flatt AA, Ratchford SM, Grosicki GJ. Self-reported sleep quality is associated with central hemodynamics in healthy individuals. Sleep Breath. 2020;24(3):1083–1088. doi: 10.1007/s11325-020-02082-5. [DOI] [PubMed] [Google Scholar]
- 69.Dominguez F, Fuster V, Fernandez-Alvira JM, Fernandez-Friera L, Lopez-Melgar B, Blanco-Rojo R, et al. Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol. 2019;73(2):134–144. doi: 10.1016/j.jacc.2018.10.060. [DOI] [PubMed] [Google Scholar]
- 70.Hijmans JG, Levy M, Garcia V, Lincenberg GM, Diehl KJ, Greiner JJ, et al. Insufficient sleep is associated with a pro-atherogenic circulating microRNA signature. Exp Physiol. 2019;104(6):975–982. doi: 10.1113/EP087469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim CW, Chang Y, Zhao D, Cainzos-Achirica M, Ryu S, Jung HS, Yun KE, Choi Y, Ahn J, Zhang Y, Rampal S, Baek Y, Lima JA, Shin H, Guallar E, Cho J, Sung E. Sleep duration, sleep quality, and markers of subclinical arterial disease in healthy men and women. Arterioscler Thromb Vasc Biol. 2015;35(10):2238–2245. doi: 10.1161/ATVBAHA.115.306110. [DOI] [PubMed] [Google Scholar]
- 72.Logan JG, Kang H, Lobo JM, Sohn MW, Lin GM, Lima JAC, Punjabi NM, Redline S, Kwon Y. Actigraphy-based sleep characteristics and aortic stiffness: the multi-ethnic study of atherosclerosis. J Am Soc Hypertens. 2018;12(12):841–849. doi: 10.1016/j.jash.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Gomez D, Eisenmann JC, Gomez-Martinez S, Hill EE, Zapatera B, Veiga OL, Marcos A, AFINOS Study Group Sleep duration and emerging cardiometabolic risk markers in adolescents. The AFINOS study Sleep Med. 2011;12(10):997–1002. doi: 10.1016/j.sleep.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, Shide N, Shinkaiya H, Okita K. Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb. 2017;24(8):819–826. doi: 10.5551/jat.36517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nagai M, Hoshide S, Nishikawa M, Shimada K, Kario K. Sleep duration and insomnia in the elderly: associations with blood pressure variability and carotid artery remodeling. Am J Hypertens. 2013;26(8):981–989. doi: 10.1093/ajh/hpt070. [DOI] [PubMed] [Google Scholar]
- 76.Sands MR, Lauderdale DS, Liu K, Knutson KL, Matthews KA, Eaton CB, Linkletter CD, Loucks EB. Short sleep duration is associated with carotid intima-media thickness among men in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Stroke. 2012;43(11):2858–2864. doi: 10.1161/STROKEAHA.112.660332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsai TC, Wu JS, Yang YC, Huang YH, Lu FH, Chang CJ. Long sleep duration associated with a higher risk of increased arterial stiffness in males. Sleep. 2014;37(8):1315–1320. doi: 10.5665/sleep.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weil BR, Maceneaney OJ, Stauffer BL, Desouza CA. Habitual short sleep duration and circulating endothelial progenitor cells. J Cardiovasc Dis Res. 2011;2(2):110–114. doi: 10.4103/0975-3583.83039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoshioka E, Saijo Y, Kita T, Okada E, Satoh H, Kawaharada M, Kishi R. Relation between self-reported sleep duration and arterial stiffness: a cross-sectional study of middle-aged Japanese civil servants. Sleep. 2011;34(12):1681–1686. doi: 10.5665/sleep.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zonoozi S, Ramsay SE, Papacosta O, Lennon L, Ellins EA, Halcox JPJ, Whincup PH, Goya Wannamethee S. Self-reported sleep duration and napping, cardiac risk factors and markers of subclinical vascular disease: cross-sectional study in older men. BMJ Open. 2017;7(6):e016396. doi: 10.1136/bmjopen-2017-016396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aggarwal S, Loomba RS, Arora RR, Molnar J. Associations between sleep duration and prevalence of cardiovascular events. Clin Cardiol. 2013;36(11):671–676. doi: 10.1002/clc.22160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, Kheirandish-Gozal L, Neubauer DN, O’Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 2015;1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Toda N. Age-related changes in endothelial function and blood flow regulation. Pharmacol Ther. 2012;133(2):159–176. doi: 10.1016/j.pharmthera.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 84.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123(2):163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 85.Limberg JK, Casey DP, Trinity JD, Nicholson WT, Wray DW, Tschakovsky ME, Green DJ, Hellsten Y, Fadel PJ, Joyner MJ, Padilla J. Assessment of resistance vessel function in human skeletal muscle: guidelines for experimental design, Doppler ultrasound, and pharmacology. Am J Physiol Heart Circ Physiol. 2020;318(2):H301–HH25. doi: 10.1152/ajpheart.00649.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thijssen DHJ, Bruno RM, van Mil A, Holder SM, Faita F, Greyling A, et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur Heart J. 2019;40(30):2534–2547. doi: 10.1093/eurheartj/ehz350. [DOI] [PubMed] [Google Scholar]
- 87.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27(10):2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest. 2018;128(6):2157–2167. doi: 10.1172/JCI80590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charles LE, Zhao S, Fekedulegn D, Violanti JM, Andrew ME, Burchfiel CM. Shiftwork and decline in endothelial function among police officers. Am J Ind Med. 2016;59(11):1001–1008. doi: 10.1002/ajim.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Folkard S. Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm. Chronobiol Int. 2008;25(2):215–224. doi: 10.1080/07420520802106835. [DOI] [PubMed] [Google Scholar]
- 91.Tahara Y, Shibata S. Entrainment of the mouse circadian clock: effects of stress, exercise, and nutrition. Free Radic Biol Med. 2018;119:129–138. doi: 10.1016/j.freeradbiomed.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 92.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24(5):775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solarz DE, Mullington JM, Meier-Ewert HK. Sleep, inflammation and cardiovascular disease. Front Biosci (Elite Ed). 2012;4:2490–501. 10.2741/e560. [DOI] [PubMed]
- 95.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43(4):678–683. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 96.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166(16):1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 97.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24(1):54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, et al. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64(1):172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 99.Schulz E, Gori T, Munzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 100.Bohm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76(1):8–18. doi: 10.1016/j.cardiores.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Dai DZ, Dai Y. Role of endothelin receptor A and NADPH oxidase in vascular abnormalities. Vasc Health Risk Manag. 2010;6:787–794. doi: 10.2147/vhrm.s6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(Pt B):321–9. 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed]
- 103.Bruno RM, Ghiadoni L, Seravalle G, Dell’oro R, Taddei S, Grassi G. Sympathetic regulation of vascular function in health and disease. Front Physiol. 2012;3:284. doi: 10.3389/fphys.2012.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glos M, Fietze I, Blau A, Baumann G, Penzel T. Cardiac autonomic modulation and sleepiness: physiological consequences of sleep deprivation due to 40 h of prolonged wakefulness. Physiol Behav. 2014;125:45–53. doi: 10.1016/j.physbeh.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Cedernaes J, Osler ME, Voisin S, Broman JE, Vogel H, Dickson SL, Zierath JR, Schiöth HB, Benedict C. Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J Clin Endocrinol Metab. 2015;100(9):E1255–E1261. doi: 10.1210/JC.2015-2284. [DOI] [PubMed] [Google Scholar]
- 106.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106(5):833–841. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thosar SS, Berman AM, Herzig MX, McHill AW, Bowles NP, Swanson CM, et al. Circadian rhythm of vascular function in midlife adults. Arterioscler Thromb Vasc Biol. 2019;39(6):1203–1211. doi: 10.1161/ATVBAHA.119.312682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thosar SS, Berman AM, Herzig MX, Roberts SA, Lasarev MR, Shea SA. Morning impairment in vascular function is unrelated to overnight sleep or the inactivity that accompanies sleep. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R986–RR93. doi: 10.1152/ajpregu.00143.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Elherik K, Khan F, McLaren M, Kennedy G, Belch JJ. Circadian variation in vascular tone and endothelial cell function in normal males. Clin Sci (Lond) 2002;102(5):547–552. doi: 10.1042/cs1020547. [DOI] [PubMed] [Google Scholar]
- 110.Shang X, Pati P, Anea CB, Fulton DJ, Rudic RD. Differential regulation of BMAL1, CLOCK, and endothelial signaling in the aortic arch and ligated common carotid artery. J Vasc Res. 2016;53(5–6):269–278. doi: 10.1159/000452410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a Geroscience perspective: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):931–941. doi: 10.1016/j.jacc.2019.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol. 2017;27(12):1768–1775. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fonken LK, Aubrecht TG, Melendez-Fernandez OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythm. 2013;28(4):262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolff CA, Esser KA. Exercise timing and circadian rhythms. Curr Opin Physiol. 2019;10:64–69. doi: 10.1016/j.cophys.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boivin DB, Tremblay GM, James FO. Working on atypical schedules. Sleep Med. 2007;8(6):578–589. doi: 10.1016/j.sleep.2007.03.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 96 kb)
Data Availability Statement
Not applicable.