Abstract
This study aimed to determine the standard amount of antioxidant content and compounds of the propolis for the standardization of propolis. For this purpose, the total flavonoids, total phenolic, CUPRAC antioxidant capacity content and the diversity of phenolic and flavonoid components of these propolis samples were found by HPLC determined at the 23 propolis samples which were collected different regions of Turkey. Beside that, the similarities and differences of these 23 provinces to each other according to their antioxidant capacities were investigated by multidimensional scaling analysis. The total flavonoid content in the propolis samples were determined between 21.28 and 152.56 mg CE/g. The total phenolic content in the propolis samples was found between 34.53 mg and 259.4 mg GAE/g. CUPRAC antioxidant capacity of the propolis samples and antioxidant range was found from 95.35 to 710.43 mg TE/g. Also, 4 flavonoid [Quercetin (min.1.12–max.4.14 mg/g), Galangin (min.0.72–max.40.79 mg/g), Apigenin (min.1.07–max.17.35 mg/g), Pinocembrin (min.1.32–max.39.92 mg/g] and 6 phenolic acid [Caffeic acid (min.1.20–max.7.6 mg/g), p-Coumaric acid (min.1.26–max.4.47 mg/g), trans-Ferulic acid (min.1.28–max.4.92 mg/g), Protocatechuic acid (1.78 mg/g), trans-Cinnamic acid (min.1.05–max.3.83 mg/g), Caffeic Acid Phenethyl Ester (CAPE) (min.1.41–max.30.15 mg/g)] components were detected as mg/g, in different ratios in propolis samples collected from different regions. The feature of this study, so far, is to have the maximum number of samples representing the Turkish propolis, and so is thought to help to national and international propolis standard workings.
Keywords: CUPRAC antioxidant capacity, HPLC, Propolis, Total flavonoid, Total phenolic
Introduction
Propolis or bee glue is a substance containing a mixture of wax and resin collected by honeybees (Apis mellifera L.) from different parts (tree and flower buds, sap flows, mucilage, latex, resin etc.) of plants [1–5]. Honeybees, collect propolis from protective resins of flowers and trees buds with their lower jaws and carry them to the hive in the pollen sacs on their hind legs. They also add substances from their bodies during the resin collection and modeling phase. The collected propolis ensures that the hive is protected from all kinds of diseases and prevents the entrance of insects and animals by closing the small openings in the hive [6, 7].
Propolis, generally consists of 50% balsam, 30% wax, 10% essential oils and 5% pollen. Since the 1950s, scientists have started to isolate important components in propolis with the help of new analytical methods and have shown people that they have many benefits [7]. Propolis and its many compounds show a wide variety of biological and pharmacological activities [8]. It is a supplementary and supportive food that has become popular all over the world in 2000s, thanks to its antimicrobial [9–11], antioxidant [12], anticancer [13], antiulcer [14], antidiabetic [15], anti-inflammatory [16], antigenotoxic [17] and antiviral [18–22] activities. Propolis, which is also used in traditional and complementary medicine, is an important bee product and it is used in “Apitherapy”, which is a treatment method with bee products [23–26]. In particular, it has been determined by many scientists that it is effective on the corona virus in the COVID-19 pandemic in 2020 [18–22]. Therefore, people's interest in propolis has increased more.
The content of propolis varies according to the plant source and when it is collected [27]. In addition, the variety in the content of beeswax affects the chemical composition of the raw propolis [28]. More than 300 different compounds have been detected to date in the propolis [29–31]. The majority of these components are phenolic acids and flavonoids [32].
Plant species in a geographic region determine the amount and type of compounds found in the propolis. In a study in New Zealand, dihydroflavonoids, pinobanksin and pinocembrin accounted for approximately 70% of the flavonoids in the analyzed samples. However, in a similar study conducted in Brazil, Uruguay and China, the dihydroflavonoid in the samples was 10% less than the samples in New Zealand [7]. Moreover, it has been found to vary the amount of flavonoids and phenolic contents in propolis samples collected from different regions of Turkey [33–35]. The most important pharmacological activity elements in propolis are flavones, flavanols and flavanones, which are common names flavonoids, and various phenolics and aromatics [7].
Propolis contains hundreds of different substances with antimicrobial properties, about 80 of which are flavonoids [1, 36–39]. Phenolic compounds in propolis are found in large quantities at about 1 in 3, while flavonoids are only up to 10% (w/w) of the concentrated form of propolis [40, 41]. Among them, pinocembrin and galangin provide antibacterial activity. It also has pinocembrin, fungicidal and local anesthetic properties [36]. Cinnamyl alcohol, cinnamic acid, vanillin, benzyl alcohol, benzoic acid, caffeic acid, coumaric acid and ferulic acid are some phenolics found in propolis [7]. In recent years, studies on propolis have found that pinocembrin, pinobanksin, quercetin, chrysin and galangin flavonoids and caffeic acid and coumaric acid phenolic acids are the most common components in propolis [9, 42–44].
In this study, total flavonoids and total phenolic compounds content and total antioxidant capacity was determined at the 23 propolis samples which were collected different regions of Turkey. In addition, the diversity of phenolic and flavonoid components of these propolis samples was found by HPLC and compared with other studies. Beside that, the similarities and differences of these 23 provinces to each other according to their antioxidant capacities were investigated by multidimensional scaling analysis. On the other hand, there are marketing difficulties for propolis because of the lack of the national or international propolis standard. For this reason, national and international standard studies will progress more easily thanks to studies that reflect the general characteristics of country propolis, such as this study, and this will solve marketing problems.
Material and methods
Collecting of propolis samples
Propolis samples were collected from 23 different cities in Turkey in 2019. Propolis traps placed in the hives in spring season were harvested end of the summer. Traps were kept in the freezer and were removed from the freezer while preparing propolis extracts (Fig. 1).
Fig. 1.
Regions where propolis samples were collected
Preparation of extracts from raw propolis
About 30 mL of 70% ethanol solution is added to the powdered 1 g of raw propolis sample, shaken for 24 h at room temperature in shaker. The upper part is filtered through coarse filter paper and transferred to 100 mL flask. The process is repeated by adding 30 mL of 70% ethanol solution to the remaining solid part. The supernatant is added to the flask in which the first extract is collected, completed to 100 mL with 70% ethanol solution [45].
Total phenolic analysis
Total phenolic content was found by modifying the Meda et al. [46], method. According to this method, the working curve was prepared using varying concentrations (0.25–0.13–0.06–0.03–0.02) of the Gallic acid (GAE) standard (0.5 mg/mL) for calibration. The dilution appropriate for the sample was done with extraction solution and 200 µL of diluted sample was put into the tubes for analysis. For the blank, 200 µL extraction solution was substituted for the sample. For the working curve, 200 µL tubes of varying concentrations of gallic acid were placed in tubes. Then, 1.5 mL of 0.2 N Folin solution was added to the tubes and left for 5 min. The tubes were then vortexed by adding 1.2 mL of NaCO3 (7.5%) solution. It was incubated in the dark at room temperature for 90 min. Finally, the UV-spectrophotometer was read against the curve at a wavelength of 765 nm.
Total flavonoid analysis
Total flovonoid content was found by modifying the Dewanto et al. [47], method. According to this method, the working curve was prepared using varying concentrations (0.1–0.08–0.05–0.02–0.01) of the catechin (CE) standard (1 mg/mL) for calibration. The dilution appropriate for the sample was done with extraction solution and was placed in tubes from 1 mL of diluted sample for analysis. For the blank, 1 mL of extraction solution was substituted for the sample. For the working curve, 1 mL tubes of varying concentrations of catechin were placed in each tube. The timing is started with the stopwatch and 300 µL of 5% NaNO2 (at t = 0 time), 300 µL of 10% AlCl3 (at t = 5 time), 2 mL of 1 M NaOH (at t = 6 time) and finally 2.4 mL distilled water was added and vortexed. Without delay, the UV-spectrophotometer was read against the curve at a wavelength of 510 nm.
CUPRAC antioxidant capacity analysis
CUPRAC antioxidant capacity was detected according to the Apak et al. [48], method. According to this method, the working curve was prepared using varying concentrations (0.5–0.25–0.13–0.06–0.03) of the trolox standard (1 mg/mL) for calibration. Dilution appropriate to the sample was done with extraction solution and 100 µL of diluted sample was put into the tubes for analysis. For the blank, 100 µL extraction solution was substituted for the sample. For the working curve, 100 µL of the varying concentrations of trolox were put into the tubes. Then 1 mL of CuCl2, 1 mL of neocuproin, 1 mL of NH4CH3COO and 1 mL of pure water were added and vortexed, respectively. Incubated for 1 h at room temperature in the dark. Finally, a 450 nm wavelength reading was made on the UV-spectrophotometer.
HPLC component analysis
Modified Aliyazıcıoglu et al. [33], method was used for propolis HPLC component analysis. Powdered 1 g raw propolis sample is weighed into a 50 mL falcon tube. Add 30 mL of 70% ethanol solution, shake for 24 h on a shaker. After centrifugation, the upper phase is transferred to a 100 mL volumetric flask. The shaking process is repeated once more. The upper phase is added to the volumetric flask where the first extract is collected, and complete to 100 mL with 70% ethanol solution. The solution is filtered through a PVDF syringe filter and transferred to the vial and 20 µL is injected to the HPLC device. VWR Hitachi HLC-UV Detector (UV 280 nm) and Supelcosil LC-18 25 cm × 4.6 mm, 5 µm column is used in the HPLC. Mobile phase A: 99% Ultra pure water: 1% Acetic Acid and Mobile phase B: 100% Methanol is used and flow was 0.9 mL/min, a linear gradient was applied by increasing the B mobile phase from 10 to 90%.
Statistical analysis
Multidimensional scaling (MDS) is a way to visualize the level of similarity between binary distances between a series of n objects or units. With multi-dimensional scaling analysis, objects are displayed in a k-dimensional (k > p) space based on the distance determined by p variable between n observations or units [49]. In this study, the similarities between the provinces according to the variables phenolic, flavonoid, and CUPRAC parameters were investigated using the multidimensional scaling analysis with Euclidean distance model. The similarity matrix obtained based on the variables in question was used to show the proximity and distance of the provinces to each other. The differences between provinces in relation to flavonoid was researched with One-Way analysis of variance (ANOVA). The Kruskall Wallis test was used for phenolic and CUPRAC because of normality assumptions are not valid. The differences of group means were detected by Duncan and Bonferroni multiple comparison test, parametric and nonparametric analysis, respectively. The IBM SPSS v25 program was used all statistical analysis.
Results and discussion
Total flavonoid content
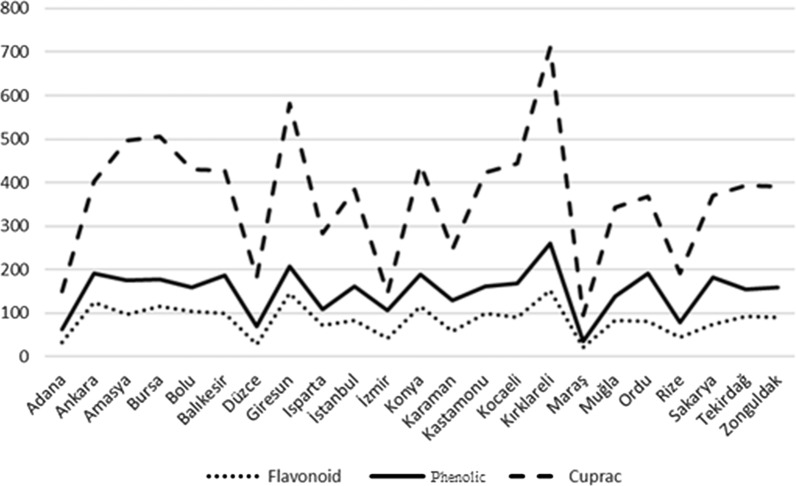
The total flavonoid content in the propolis samples were determined between 21.28 and 152.56 mg CE/g. Kırklareli (152.57), Giresun (146.35), Ankara (125.66) provinces have the highest values, while İzmir (42.1), Düzce (28.35) and Maraş (21.28), provinces have the least values were observed. The average of all provinces were found to be 84.77 mg CE/g value (Table 1, Fig. 2). Many researchers have determined the total flavonoid value in propolis. Some of these researchers and their conclusions are as follows: Zarate et al. [50] in Mexico; 13–379 mg QE/g, Ozdal et al. [51] in Turkey; 522.71 mg QE/g, Narimane et al. [52], in Algeria; 0.57–3.53 mg QE/g, Wang et al. [53] in South Korea; 21–50 mg QE/g and for Brazilia, China and Australia; 33–53 mg QE/g, Socha et al., [54] in Poland; 35.64–62.04 mg QE/g and Bonvehi and Gutierrez [55] in Spain; 72–161 mg QE/g. These results are compatible with our study.
Table 1.
The ANOVA means and standart errors for flavonoid, and Kruskall Wallis mean ranks for phenolic and cuprac according to provinces
| Provinces | N | Flavonoid mg Catechin/g | Phenolic mg Gallic Acid/g | CUPRAC mg TE/g | ||
|---|---|---|---|---|---|---|
| Mean ± sd error | Mean ± std deviation | Mean rank | Mean ± std deviation | Mean rank | ||
| Adana | 3 | 32.20 ± 0.21n | 62.92 ± 0.75 | 8.00a | 150.63 ± 0.65 | 8.00abc |
| Ankara | 3 | 125.66 ± 0.30c | 191.55 ± 0.79 | 62.0abc | 402.65 ± 0.50 | 41.00abc |
| Amasya | 3 | 96.60 ± 2.54gh | 175.10 ± 2.70 | 44.00abc | 495.87 ± 3.33 | 59.00abc |
| Bursa | 3 | 115.27 ± 2.09d | 177.77 ± 3.85 | 57.33abc | 506.27 ± 3.81 | 62.00abc |
| Bolu | 3 | 104.49 ± 0.34e | 158.58 ± 0.95 | 53.00abc | 430.99 ± 1.75 | 50.00abc |
| Balıkesir | 3 | 99.54 ± 0.31fg | 186.78 ± 1.55 | 47.67abc | 427.86 ± 0.85 | 47.00abc |
| Düzce | 3 | 28.35 ± 0.74o | 69.30 ± 3.25 | 5.00abc | 182.70 ± 0.84 | 11.00abc |
| Giresun | 3 | 146.35 ± 0.40b | 208.20 ± 2.64 | 65.00ac | 580.93 ± 3.02 | 65.00ac |
| Isparta | 3 | 71.40 ± 0.86k | 108.13 ± 1.98 | 20.67abc | 282.30 ± 1.93 | 20.00abc |
| İstanbul | 3 | 83.52 ± 0.37j | 161.77 ± 1.86 | 31.00abc | 384.07 ± 2.25 | 32.00abc |
| İzmir | 3 | 42.10 ± 2.14 m | 107.20 ± 0.46 | 12.33 abc | 144.93 ± 2.06 | 5.00a |
| Konya | 3 | 115.50 ± 2.17d | 187.97 ± 2.18 | 57.67abc | 441.53 ± 0.26 | 54.00abc |
| Karaman | 3 | 57.67 ± 0.72l | 129.07 ± 2.80 | 17.00abc | 245.33 ± 3.51 | 17.00abc |
| Kastamonu | 3 | 100.18 ± 0.64f | 160.98 ± 1.56 | 48.00abc | 424.00 ± 2.61 | 44.00abc |
| Kocaeli | 3 | 91.10 ± 0.13i | 167.21 ± 0.40 | 37.00abc | 443.94 ± 0.15 | 55.00abc |
| Kırklareli | 3 | 152.57 ± 1.47a | 259.40 ± 1.73 | 68.00 c | 710.43 ± 2.01 | 68.00c |
| Maraş | 3 | 21.28 ± 1.02p | 34.53 ± 2.10 | 2.00b | 95.35 ± 2.83 | 2.00b |
| Muğla | 3 | 82.95 ± 0.95 j | 139.28 ± 0.67 | 30.00abc | 342.56 ± 0.74 | 23.00abc |
| Ordu | 3 | 80.55 ± 0.48j | 190.92 ± 0.88 | 26.00abc | 368.53 ± 2.50 | 27.00abc |
| Rize | 3 | 44.04 ± 0.96m | 78.06 ± 0.86 | 12.67abc | 191.13 ± 0.40 | 14.00abc |
| Sakarya | 3 | 73.93 ± 1.01k | 182.48 ± 2.69 | 22.33abc | 369.57 ± 0.93 | 28.00abc |
| Tekirdağ | 3 | 93.39 ± 0.53hi | 155.22 ± 1.01 | 42.00abc | 393.62 ± 1.16 | 38.00abc |
| Zonguldak | 3 | 91.20 ± 0.70i | 159.82 ± 0.96 | 36.33abc | 390.42 ± 1.87 | 35.00abc |
| p value | < 0.001 | < 0.001 | < 0.001 | |||
Different letters in the same columns show statistically differences between means (p < 0.05)
Fig. 2.
Comparing the flavonoid, phenolic and CUPRAC results according to the provinces
Total phenolic content
The total phenolic content in the propolis samples was found between 34.53 mg GAE/g and 259.4 mg GAE/g. Kırklareli (259.4), Giresun (208.2), Ankara (191.55), Ordu (190.92) provinces have the highest values, while Kahramanmaraş (34.53), Adana (62.92), Düzce (69.3), Rize (78.06) provinces have the lowest values were determined. The average of all provinces were found to be 150.09 mg GAE/g value and statistically Adana, Kırklareli and Maraş were found statistically different from each other (p < 0.05) (Table 1, Fig. 2). There are many studies on total phenolic compound in propolis. Some of them are as follows: Zarate et al. [50] in Mexico; 68–500 mg GAE/g, Ozdal et al. [51] in Turkey; 314.36 mg GAE/g, Narimane et al. [52] in Algeria; 0.81–8.97 mg GAE/g, Wang et al. [53] in South Korea; 49–239 mg GAE/g and for Brazilia, China and Australia; 127–142 mg GAE/g, Socha et al. [54] in Poland; 150.05–197.14 mg GAE/g, Aliyazıcıoglu et al. [33] in Turkey; 115–210 mg GAE/g and Bonvehi and Gutierrez [55] in Spain; 200–340 mgGAE/g. All results are consistent with our study.
Antioxidant capacity (CUPRAC)
CUPRAC method gives information about reductive capabilities of propolis extracts and based on reduction of Cu+2 to Cu+ by antioxidants [52]. Table 1 and Fig. 2 show the antioxidant capacity of the propolis samples and antioxidant range was found from 95.35 to 710.43 mg TE/g. Kırklareli (710.43), Giresun (580.93), Bursa (506.26) provinces have the highest values, while Maraş (95.35), Adana (150.63), Düzce (182.7), provinces have the lowest values were determined. The average of all provinces was found to be 365.46 mg TE/g value and İzmir, Kırklareli, and Maraş provinces were found statistically different from each other (p < 0.05) (Table 1, Fig. 2). Researchers found the CUPRAC value in propolis in different countries respectively: Bayram et al. [56] in Turkey; 282.8 mg TE/g, Ozdal et al. [51] in Turkey; 1184.94 mg TE/g, Narimane et al. [52] in Algeria 8 µM TE/g, Daraban et al. [57] in Romania; 12404–35721 µM TE/100 mL. These results are similar to our study results.
MDS analysis
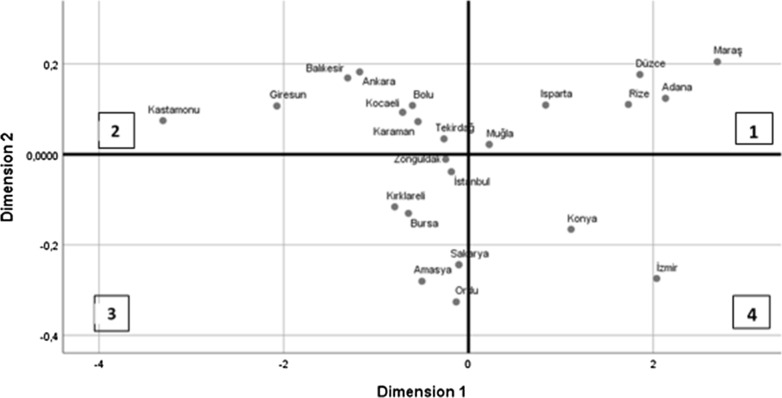
The results of the examination according to the similarities and differences of all provinces according to Flavonoid, Phenolic and CUPRAC antioxidant capacity contents are given in Fig. 3. After several dimensional scaling analysis, two-dimensional (k = 2) scaling was determined the best because of giving the lowest The Kruskal's stress value and higher the coefficient of determination (R2), as 0.004 and 0.99, respectively. Therefore, the results were given, and comments were made on two-dimension scaling.
Fig. 3.
Optimal two dimensional configuration of provinces obtained by MDS
The stimulus coordinates of provinces and configurations of provinces showed that Muğla, Isparta, Düzce, Rize, Adana and Maraş were found similar, Tekirdağ, Karaman, Kocaeli, Bolu, Ankara, Balıkesir, Giresun and Kastamonu were found similar; Zonguldak, İstanbul, Kırklareli, Bursa, Sakarya, Amasya and Ordu were found similar and Konya and İzmir were found similar among each other. Optimal two‐dimensional configuration of provinces based on stimulus coordinates was illustrated in Fig. 3.
HPLC component analysis
Propolis has many biological and pharmacological activities thanks to its large number of phenolic and flavonoid components [33]. For the HPLC method validation of the study, the repeatability, reproducibility, recovery, linearity, limit of detection limit (LOD) and limit of quantitation (LOQ) validation parameters were examined and presented in Table 2.
Table 2.
Validation parameters of HPLC
| Compound | R2 | Recovery (%) | LOD (mg/kg) | LOQ (mg/kg) | 5 mg/L | 20 mg/L | 40 mg/L | |||
|---|---|---|---|---|---|---|---|---|---|---|
| RSRr | RSDR | RSRr | RSDR | RSRr | RSDR | |||||
| Quercetin | 0.9975 | 87.9 | 1.14 | 1.51 | 0.66 | 1.89 | 1.57 | 1.93 | 1.81 | 3.09 |
| Galangin | 0.9936 | 80.0 | 0.93 | 1.58 | 0.72 | 1.51 | 0.78 | 1.67 | 1.09 | 1.81 |
| Apigenin | 0.9987 | 104.9 | 1.13 | 1.90 | 0.75 | 1.01 | 1.05 | 1.26 | 1.16 | 1.93 |
| Pinocembrin | 0.9969 | 110.2 | 0.70 | 1.20 | 0.78 | 1.51 | 1.75 | 2.27 | 1.98 | 2.30 |
| Caffeic acid | 0.9983 | 108.4 | 0.89 | 1.45 | 0.30 | 1.52 | 1.22 | 2.23 | 1.31 | 2.93 |
| p-Coumaric acid | 0.9980 | 106.2 | 0.67 | 0.93 | 0.23 | 1.22 | 1.32 | 1.47 | 1.43 | 1.68 |
| Trans-ferulic acid | 0.9983 | 98.7 | 0.56 | 0.60 | 0.37 | 1.12 | 1.56 | 1.82 | 1.78 | 2.21 |
| Protocatechuic acid | 0.9983 | 108.7 | 0.51 | 0.82 | 0.80 | 1.18 | 0.97 | 1.24 | 1.07 | 1.92 |
| Trans-cinnamic acid | 0.9984 | 88.5 | 0.61 | 0.66 | 0.57 | 1.06 | 0.91 | 1.44 | 1.09 | 1.76 |
|
Caffeic acid Phenethyl ester (CAPE) |
0.9983 | 98.9 | 0.51 | 1.18 | 0.24 | 1.37 | 1.45 | 1.83 | 1.59 | 2.37 |
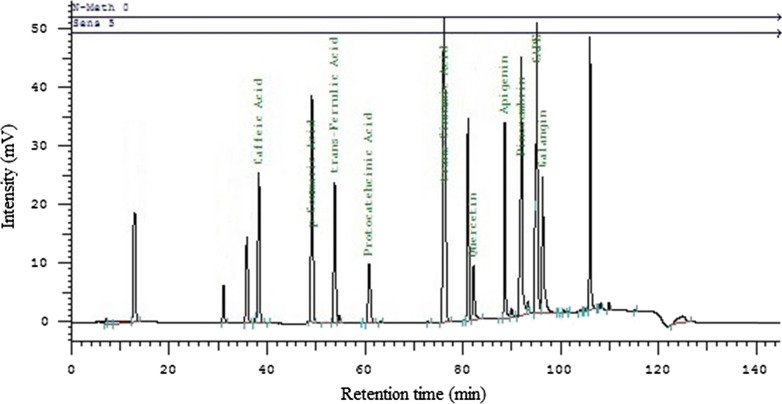
In this study, 4 flavonoid [quercetin (min.1.12–max.4.14 mg/g), galangin (min.0.72–max.40.79 mg/g), apigenin (min.1.07–max.17.35 mg/g), pinocembrin (min.1.32–max.39.92 mg/g] and 6 phenolic acid [caffeic acid (min.1.20–max.7.6 mg/g), p-coumaric acid (min.1.26–max.4.47 mg/g), trans-ferulic acid (min.1.28–max.4.92 mg/g), protocatechuic acid (1.78 mg/g), trans-cinnamic acid (min.1.05–max.3.83 mg/g), caffeic acid phenethyl ester (CAPE) (min.1.41–max.30.15 mg/g)] components were detected as mg/g, in different ratios in propolis samples collected from different regions of Turkey (Table 3, Fig. 4).
Table 3.
Propolis component analysis results by HPLC
| Provinces | Quercetin (mg/g) | Galangin (mg/g) | Apigenin (mg/g) | Pinocembrin (mg/g) | Caffeic acid (mg/g) | p-Coumaric acid (mg/g) | Trans-ferulic acid (mg/g) | Protocatechuic acid (mg/g) | Trans-cinnamic acid (mg/g) | CAPE (mg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Adana | N.D | 1.07 ± 0.39 | 1.96 ± 0.05 | 2.14 ± 0.88 | 4.03 ± 0.2 | 2.33 ± 0.23 | N.D | N.D | 2.26 ± 0.5 | 2.04 ± 0.83 |
| Ankara | N.D | 19.24 ± 0.42 | 1.75 ± 0.04 | 7.93 ± 0.48 | 2.01 ± 0.8 | 1.26 ± 0.03 | N.D | N.D | 1.20 ± 0.07 | 19.26 ± 0.66 |
| Amasya | N.D | 12.86 ± 0.7 | 1.81 ± 0.4 | 6.65 ± 0.45 | 1.55 ± 0.02 | N.D | N.D | N.D | N.D | 19.32 ± 0.19 |
| Bursa | N.D | 10.87 ± 0.09 | N.D | 7.25 ± 0.04 | 4.74 ± 0.07 | N.D | N.D | N.D | 1.33 ± 0.02 | 16.08 ± 0.06 |
| Bolu | 4.14 ± 0.4 | 10.42 ± 0.04 | 1.87 ± 0.3 | 15.39 ± 1.54 | 1.32 ± 0.02 | 2.00 ± 0.05 | 2.26 ± 0.38 | 1.78 ± 0.04 | 1.05 ± 0.02 | 26.99 ± 0.87 |
| Balıkesir | 2.58 ± 0.16 | 9.64 ± 1.92 | 1.20 ± 0.05 | 11.93 ± 3.06 | 2.83 ± 1.1 | N.D | N.D | N.D | 1.31 ± 0.07 | 8.87 ± 0.03 |
| Düzce | 1.58 ± 0.28 | 3.98 ± 0.68 | N.D | 7.39 ± 0.88 | N.D | N.D | 1.28 ± 0.07 | N.D | N.D | 3.65 ± 0.25 |
| Giresun | 2.46 ± 0.04 | 12.52 ± 0.45 | N.D | 5.42 ± 0.05 | 4.82 ± 1.4 | 4.47 ± 0.09 | 4.92 ± 0.05 | N.D | N.D | 1.41 ± 0.04 |
| Isparta | N.D | 1.58 ± 0.04 | 1.19 ± 0.07 | 3.09 ± 0.05 | 1.20 ± 0.03 | N.D | N.D | N.D | N.D | N.D |
| İstanbul | N.D | 7.69 ± 0.4 | 17.35 ± 0.53 | 11.07 ± 0.55 | N.D | N.D | N.D | N.D | 1.80 ± 0.03 | 3.30 ± 0.38 |
| İzmir | N.D | 2.50 ± 0.48 | N.D | 5.97 ± 0.36 | N.D | N.D | N.D | N.D | N.D | 1.58 ± 0.05 |
| Konya | N.D | 6.12 ± 0.44 | N.D | 10.41 ± 0.6 | 2.51 ± 0.6 | 2.59 ± 0.45 | N.D | N.D | 1.12 ± 0.04 | 9.79 ± 0.65 |
| Karaman | N.D | 0.97 ± 0.03 | N.D | 1.32 ± 0.06 | N.D | N.D | N.D | N.D | N.D | 4.03 ± 0.08 |
| Kastamonu | 1.18 ± 0.05 | 2.77 ± 0.26 | 1.07 ± 0.06 | 5.32 ± 0.63 | 7.6 ± 0.06 | 2.69 ± 0.09 | N.D | N.D | 2.13 ± 0.03 | 19.53 ± 0.04 |
| Kocaeli | 1.46 ± 0.28 | 10.89 ± 5.90 | 1.19 ± 0.20 | 29.17 ± 5.08 | 2.07 ± 0.75 | 3.47 ± 0.79 | 2.50 ± 0.43 | N.D | 2.25 ± 0.85 | 25.41 ± 4.41 |
| Kırklareli | 2.10 ± 0.04 | 40.79 ± 0.04 | 4.08 ± 0.06 | 39.92 ± 0.04 | 5.77 ± 0.04 | 3.40 ± 0.26 | N.D | N.D | 3.47 ± 0.04 | 30.15 ± 0.04 |
| Maraş | N.D | 0.72 ± 0.06 | N.D | 1.65 ± 0.06 | N.D | N.D | N.D | N.D | N.D | 3.18 ± 0.18 |
| Muğla | 1.14 ± 0.04 | 6.71 ± 3.73 | 1.46 ± 0.4 | 16.32 ± 0.52 | 1.59 ± 0.07 | N.D | N.D | N.D | 2.16 ± 1.02 | 2.13 ± 0.33 |
| Ordu | N.D | 4.83 ± 0.32 | 2.67 ± 0.23 | 20.22 ± 0.25 | 1.96 ± 0.7 | 1.39 ± 0.05 | N.D | N.D | N.D | 12.54 ± 3.15 |
| Rize | N.D | 3.73 ± 0.05 | 3.77 ± 0.07 | 17.42 ± 0.07 | N.D | N.D | N.D | N.D | 2.01 ± 0.05 | 4.58 ± 0.08 |
| Sakarya | 3.89 ± 0.04 | 16.49 ± 0.03 | 2.98 ± 0.02 | 34.82 ± 0.07 | 2.06 ± 0.04 | 3.25 ± 0.04 | 3.26 ± 0.09 | N.D | 1.15 ± 0.03 | 15.14 ± 0.05 |
| Tekirdağ | 1.12 ± 0.02 | 11.77 ± 1.56 | 1.67 ± 0.42 | 5.88 ± 1.88 | 5.03 ± 0.02 | 1.53 ± 0.05 | 1.32 ± 0.15 | N.D | 3.83 ± 0.03 | 21.79 ± 0.35 |
| Zonguldak | N.D | 6.95 ± 0.04 | N.D | N.D | 2.90 ± 0.02 | N.D | N.D | N.D | 2.55 ± 0.08 | N.D |
Fig. 4.
HPLC chromatogram of the components
Cunha et al. [58] found caffeic acid, p-coumaric acid and ferulic acid in all of the extracts prepared in their study using different solvents to determine the phenolic content of Brazilian propolis. Also, Choi et al., [59] stated that in many propolis samples obtained from Korea contains caffeic acid (min.1.0–8.7 mg/g), p-coumaric acid (min.1.2–7.1 mg/g), ferulic acid (min.0.5–1.9 mg/g), apigenin (min.0.6–2.4 mg/g), pinocembrin (min.1.5–87.8 mg/g) and galangin (min.4.9–max.26.3 mg/g). These results are in line with our results. On the other hand, Lagouri et al., [60] identified that caffeic acid (min.0.64–max.4.17 mg/g), caffeic acid phenyl ester (min.0.36–max.2.04 mg/g), ferulic acid (min.0.53–max.1.41 mg/g), p-coumaric acid (min.0.83–max.3.00 mg/g), apigenin (min.0.48–max.2.74 mg/g) and galangin (min.1.32–max.8.55) components at the lower amounts according to our study at the Greek propolis samples. Also, Keskin and Kolaylı [34] found caffeic acid (min.0.40––max.7.33 mg/g), ferulic acid (min.0.52–max.9.83 mg/g), coumaric acid (min.0.71–max.4.30 mg/g) phenolic compounds amounts similar to our results at the Turkish propolis samples. On the other hand, Aliyazıcıoglu et al. [33] determined similar results for Turkish propolis samples with our results. They found caffeic acid (1446.8–4658.1 µg/g), p-coumaric acid (381.7–4579.8 µg/g) and ferulic acid (223.3–7126.9 µg/g). Ristivojevic et al. [35] also, found phenolic and flavonoid compounds at the another Turkish propolis study. They revealed caffeic acid (min.3.96–max.34.78 mg/mL), ferulic acid (min.1.00–max.19.42 mg/mL), coumaric acid (min.0.19–max.4.91 mg/mL), protocatechuic acid (min.0.45 mg/mL–max.1.69 mg/mL), trans-cinnamic acid (min.3.00–max.5.28 mg/mL), quercetin (min.1.11–max.4.33 mg/mL), galangin (min.0.96–max.2.70 mg/mL), apigenin (min.0.54–max.1.56 mg/mL), pinocembrin (min.0.94–max.2.81 mg/mL]. Their results were similar to our results. Beside that, Pavlovic et al., [61] determined caffeic acid (min.4.21–max.4.37 mg/g), p-coumaric acid (min.1.40–max.6.97 mg/g), ferulic acid (min.1.64–max.7.41 mg/g), pinocembrin (min.17.90–max.19.06 mg/g) at the Italian propolis samples. Also, these results close to our study results. As a result, in this study, we found caffeic acid, caffeic acid phenethyl ester (CAPE), galangin and pinocembrin as major components for the Turkish propolis. Because these components have been detected in almost all provinces and these components can be used for quality determination and standardization of Turkish propolis. As a similar, Sorucu and Oruc [62] determined pinocembrin, CAPE, caffeic acid highest amounts at the propolis samples from the northwest of Turkey.
As a result, the content of raw propolis varies according to the botanical origin of the region where it is obtained. Turkey, where they grow different plant species, is a country with rich botanical resources. Because, there are three phytogeographical regions in Turkey (Euro & Siberian, Mediterranean, Irano & Turanian) and the plant diversity varies from region to region [63]. So, there are about 12,000 plant species in Turkey and 3000 of them are endemic. About 500 plant species are nectar plants and are preferred by honeybees [64]. Since propolis is in very different phytogeographic regions in the 23 cities studied, the botanic origin varies. For this reason, propolis contents also differ greatly and it is very important to making content analysis for propolis standardization.
In this study, total phenolics, total flavonoids and antioxidant capacity amounts were determined and compared statistically at the propolis samples, which were collected from different regions of Turkey. By illuminating the phenolic and flavonoid components contained in propolis samples, components [caffeic acid, caffeic acid phenethyl ester (CAPE), galangin and pinocembrin] that could be markers for Turkish propolis were determined. Thus, for the basic standardization of Turkish propolis, the range in which the total phenolic substance and flavonoid substance amounts should be and the components it should contain were determined. These compounds can be used in the marketing quality control of Turkish propolis.
Acknowledgements
This work was supported by SBS Bilimsel Bio Çözümler Inc. Bee&You Propolis R&D Center.
Authors' contributions
EYÖ, MK, AÖ analyzed data and wrote the manuscript and ÇT made the statistically analyses. AÖ and AETS organized this study and manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by SBS Bilimsel Bio Çözümler Inc. Bee&You Propolis R&D Center.
Availability of data and materials
All data analysed during this study are included in this published article.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bankova V, Popov S, Marekov NL. High performance liquid chromatografic analysis of flavonoids from propolis. J Chromatogr. 1982;242:135–143. doi: 10.1016/S0021-9673(00)87255-6. [DOI] [Google Scholar]
- 2.Wagh VD. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharm Sci. 2013;2013:308249. doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res PTR. 2016;30:894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- 4.Özkök A, Sorkun K, Salih B. The chemical analysis of propolis, which are produced in western of Turkey. Hacettepe J Biol Chem. 2016;44:317–328. [Google Scholar]
- 5.Keskin M, Keskin Ş, Mayda N, Özkök A. Determination of biochemical profile of bilecik propolis. Hacettepe J Biol Chem. 2019;47:403–409. [Google Scholar]
- 6.Ghisalberti EL. Propolis: a review. Bee World. 1979;60:59–84. doi: 10.1080/0005772X.1979.11097738. [DOI] [Google Scholar]
- 7.Krell R (1996) Value-added products from beekeeping, Fao Agricultural Services Bulletin No. 124.
- 8.Schmidt JO, Buchmann SL (1992) Other products of the hive. In the hive and the honeybee. Editorial Graham, J. M. Dadant & Sons, Hamilton, Illinois: USA. pp. 927–988.
- 9.Koru O, Toskay F, Açıkel CH, Tunca YM, Baysallar M, Üsküdar GA, Akça E, Özkök Tüylü A, Sorkun K, Tanyüksel M, Salih B. In vitro antimicrobial activity of propolis samples from different geographical origins against certain oral pathogens. Anaerobe. 2007;13:140–145. doi: 10.1016/j.anaerobe.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Temiz A, Sener A, Özkök Tüylü A, Sorkun K, Salih B. Antibacterial activity of bee propolis samples from different geographical regions of Turkey against two foodborne pathogens, Salmonella enteritidis and Listeria monocytogenes. Turk J Biol. 2011;35:503–511. [Google Scholar]
- 11.Monzote L, Cuesta-Rubio O, Campo Fernandez M, Márquez Hernandez I, Fraga J, Pérez K, Kerstens M, Maes L, Cos P. In vitro antimicrobial assessment of Cuban propolis extracts. Mem Inst Oswaldo Cruz. 2012;107:978–984. doi: 10.1590/S0074-02762012000800003. [DOI] [PubMed] [Google Scholar]
- 12.Russo A, Cardile V, Sanchez F, Troncoso N, Vanella A, Garbarino JA. Chilean propolis: antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004;76:545–558. doi: 10.1016/j.lfs.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Chan GC, Cheung KW, Sze DM. The immunomodulatory and anticancer properties of propolis. Clin Rev Allergy Immunol. 2013;44:262–273. doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- 14.De Barros MP, Lemos M, Maistro EL, Leite MF, Sousa JPB, Bastos JK, de Andrade SF. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J Ethnopharmacol. 2008;120:372–377. doi: 10.1016/j.jep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 15.El-Sayed ES, Abo-Salem OM, Aly HA, Mansour AM. Potential antidiabetic and hypolipidemic effects of propolis extract in streptozotocin-induced diabetic rats. Pak J Pharm Sci. 2009;22:168–174. [PubMed] [Google Scholar]
- 16.Shi H, Yang H, Zhang X, Yu L. Identification and quantification of phytochemical composition and anti-inflammatory and radical scavenging properties of methanolic extracts of Chinese propolis. J Agric Food Chem. 2012;60:12403–12410. doi: 10.1021/jf3042775. [DOI] [PubMed] [Google Scholar]
- 17.Bayram NE, Karadayı M, Güllüce M, Bayram S, Sorkun K, Öz GC, Aydoğan MN, Koç TY, Alaylar B, Salih B. Genotoxic and antigenotoxic evaluation of propolis by using in vitro bacterial assay systems. Mellifera. 2015;15:29–36. [Google Scholar]
- 18.Bachevski D, Damevska K, Simeonovski V, Dimova M. Back to the basics: propolis and COVID-19. Dermatol Ther. 2020;33:e13780. doi: 10.1111/dth.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berretta AA, Silveira MAD, Capcha JMC, De Jong D (2020) Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed Pharmacother 110622 [DOI] [PMC free article] [PubMed]
- 20.Miryan M, Soleimani D, Dehghani L, Sohrabi K, Khorvash F, Bagherniya M, Sayedi SM, Askari G. The effect of propolis supplementation on clinical symptoms in patients with coronavirus (COVID-19): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:1–2. doi: 10.1186/s13063-020-04934-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahlan M, Irdiani R, Flamandita D, Aditama R, Alfarraj S, Ansari MJ, Khayrani AC, Pratami DK, Lischer K. Molecular interaction analysis of Sulawesi propolis compounds with SARS-CoV-2 main protease as preliminary study for COVID-19 drug discovery. J King Saud Univ Sci. 2020;33:101234. doi: 10.1016/j.jksus.2020.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorza CA, Gonçalves VC, Scorza FA, Fiorini AC, de Almeida ACG, Fonseca MC, Finsterer J. Molecular interaction analysis of Sulawesi propolis compounds with SARS-CoV-2 main protease as preliminary study for COVID-19 drug discovery. Complement Ther Clin Pract. 2020;41:101227. doi: 10.1016/j.ctcp.2020.101227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassileth BR (2011) Chapter 36: Apitherapy. The complete guide to complementary therapies in cancer care: essential information for patients, survivors and health professionals. World Scientific. pp. 221–224. ISBN 978-981-4335-66-9
- 24.Barlak Y, Değer O, Colak M, Karataylı SC, Bozdayı AM, Yücesan F. Effect of Turkish propolis extracts on proteome of prostate cancer cell line. Proteome Sci. 2011;9:74. doi: 10.1186/1477-5956-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turan I, Demir S, Misir S, Kilinc K, Mentese A, Aliyazıcıoğlu Y, Değer O. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop J Pharm Res. 2015;14:777–782. doi: 10.4314/tjpr.v14i5.5. [DOI] [Google Scholar]
- 26.Yasar M, Savranlar Y, Karaman H, Sağit M, Silici S, Ozcan I. Effects of propolis in an experimental rat model of allergic rhinitis. Am J Otolaryngol. 2016;37:287–293. doi: 10.1016/j.amjoto.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Salatino A, Fernandes-Silva CC, Righi AA, Salatino MLF. Propolis research and the chemistry of plant products. Nat Prod Rep. 2011;28:925–936. doi: 10.1039/c0np00072h. [DOI] [PubMed] [Google Scholar]
- 28.Crane E. Bees and beekeeping. Science, practice and world resources. New York: Cornell University Press; 1990. p. 593. [Google Scholar]
- 29.Hernández M, Cuesta-Rubio O, Fernández MC, Pérez AR, Oca Porto RM, Piccinelli AL, Rastrelli L. Studies on the constituents of yellow Cuban Propolis: GC-MS determination of triterpenoids and flavonoids. J Agric Food Chem. 2010;58:4725–4730. doi: 10.1021/jf904527n. [DOI] [PubMed] [Google Scholar]
- 30.Farooqui T, Farooqui AA. Beneficial effects of propolis on human health and neurological diseases. Front Biosci. 2012;4:779–793. doi: 10.2741/e418. [DOI] [PubMed] [Google Scholar]
- 31.Huang S, Zhang CP, Wang K, Li GQ, Hu EL. Recent advances in the chemical composition of propolis. Molecules. 2014;19:19610–19632. doi: 10.3390/molecules191219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bankova V, Marcucci MC, Simova S, Nikolova N, Kujumgiev A, Popov S. Antibacterial diterpenic acids from Brazilian propolis. Z Naturforsch C. 1996;51:277–280. doi: 10.1515/znc-1996-5-602. [DOI] [PubMed] [Google Scholar]
- 33.Aliyazıcıoglu R, Sahin H, Erturk O, Ulusoy E, Kolayli S. Properties of phenolic composition and biological activity of propolis from Turkey. Int J Food Prop. 2013;16:277–287. doi: 10.1080/10942912.2010.551312. [DOI] [Google Scholar]
- 34.Keskin M, Kolaylı S. Standardization of Propolis, is it possible? Uludag Bee J. 2018;18:101–110. [Google Scholar]
- 35.Ristivojevic P, Dimkic I, Guzelmeric E, Trifkovic J, Knezevic M, Beric T, Yesilada E, Milojokvic-Opsenica D, Stankovic S. Profiling of Turkish propolis subtypes: comparative evaluation of their phytochemical compositions, antioxidant and antimicrobial activities. LWT-Food Sci Technol. 2018;95:367–379. doi: 10.1016/j.lwt.2018.04.063. [DOI] [Google Scholar]
- 36.Bankova V, Popov S, Marekov NL. A study on flavonoids of propolis. J Nat Prod. 1983;46:471–474. doi: 10.1021/np50028a007. [DOI] [Google Scholar]
- 37.Greenaway W, Scaysbrook T, Whatley FR. The composition and plant origins of propolis: a report of work at Oxford. Bee World. 1990;71:107–118. doi: 10.1080/0005772X.1990.11099047. [DOI] [Google Scholar]
- 38.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. doi: 10.1051/apido:19950202. [DOI] [Google Scholar]
- 39.Park YK, Koo MH, Ikegaki M, Contado JL. Comparison of the flavonoid aglycone contents of Apis mellifera propolis from various regions of Brazil. Contado Arquivos Biologia Tecnologia (Curitiba) 1997;40:97–106. [Google Scholar]
- 40.Bonvehi SJ, Coll VF, Jorda ER. The composition, active components and bacteriostatic activity of propolis in dietetics. J Am Oil Chem Soc. 1994;71:529–532. doi: 10.1007/BF02540666. [DOI] [Google Scholar]
- 41.Campos M, Sabatier S, Amiot MJ, Aubert S. Characterisation of flavonoids in three hive products: bee pollen, propolis, and honey. Planta Med. 1990;56:580–581. doi: 10.1055/s-2006-961183. [DOI] [Google Scholar]
- 42.Falcao SI, Vilas-Boas M, Estevinho LM, Barros C, Domingues MRM, Cardoso SM. Phenolic characterization of Northeast Portuguese propolis: usual and unusual compounds. Anal Bioanal Chem. 2010;396:887–897. doi: 10.1007/s00216-009-3232-8. [DOI] [PubMed] [Google Scholar]
- 43.Sun LP, Chen AL, Hung HC, Chien YH, Huang JS, Huang CY, Chen YW, Chen CN. Chrysin: a histone deacetylase 8 inhibitor with anticancer activity and suitable candidate for the standardization of Chinese propolis. J Agric Food Chem. 2012;60:11748–11758. doi: 10.1021/jf303261r. [DOI] [PubMed] [Google Scholar]
- 44.Kasote D, Suleman T, Chen W, Sandasi M, Viljoen A, Vuuren S. Chemical profiling and chemometric analysis of South African propolis. Biochem Syst Ecol. 2014;55:156–163. doi: 10.1016/j.bse.2014.03.012. [DOI] [Google Scholar]
- 45.Bankova V, Bertelli D, Borba R, Conti BJ, Cunha IBS, Danert C, Eberlin MN, Falcão SI, Isla MI, Moreno MIN, Papotti G, Popova M, Santiago KB, Salas A, Sawaya ACHF, Schwab NV, Sforcin JM, Simone-Finstrom M, Spivak M, Trusheva B, Vilas-Boas M, Wilson M, Zampini C. Standard methods for Apis mellifera propolis research. J Apic Res. 2016;58:1–49. doi: 10.1080/00218839.2016.1222661. [DOI] [Google Scholar]
- 46.Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 47.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 48.Apak R, Güçlü K, Ozyürek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- 49.Davidson ML. Multidimensional scaling. New York: Wiley; 1983. [Google Scholar]
- 50.Zarate MSH, Juarez MRA, Garcia AC, Lopez CO, Chavez AJG, Garfias JJNS, Ramos FA. Flavonoids, phenolic content, and antioxidant activity of propolis various areas of Guanajuato, Mexico. Food Sci Technol. 2018;38:210–215. doi: 10.1590/fst.29916. [DOI] [Google Scholar]
- 51.Ozdal T, Sari-Kaplan G, Mutlu-Altundag E, Boyacioglu D, Capanoglu E. Evaluation of Turkish propolis for its chemical composition, antioxidant capacity, anti-proliferative effect on several human breast cancer cell lines and proliferative effect on fibroblasts and mouse mesenchymal stem cell line. J Apic Res. 2018;57:627–638. doi: 10.1080/00218839.2018.1494888. [DOI] [Google Scholar]
- 52.Narimane S, Demircan E, Salah A, Özçelik B, Salah R. Correlation between antioxidant activity and phenolic acids profile and content of Algerian propolis: influence of solvent. Pak J Pharm Sci. 2017;30:1417–1423. [PubMed] [Google Scholar]
- 53.Wang X, Sankarapandian K, Cheng Y, Woo SO, Kwon HW, Perumalsamy H, Ahn YJ. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement Altern Med. 2016;16:65. doi: 10.1186/s12906-016-1043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Socha R, Gałkowska D, Bugaj M, Juszczak L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat Prod Res. 2015;29:416–422. doi: 10.1080/14786419.2014.949705. [DOI] [PubMed] [Google Scholar]
- 55.Bonvehi S, Gutierrez AL. Antioxidant activity and total phenolics of propolis from the Basque Country (Northeastern Spain) J Am Oil Chem Soc. 2011;88:1387–1395. doi: 10.1007/s11746-011-1792-1. [DOI] [Google Scholar]
- 56.Bayram NE, Gerçek YC, Cevahir Öz G. Screening for antioxidant capacity, pollen types and phytochemical profile by GC/MS and UHPLC from propolis. Progr Nutr. 2020;22:e2020011. [Google Scholar]
- 57.Daraban A, Olah NK, Burtescu RF, Furtuna FP, Hanganu D, Simon I, Bojita M, Heghes CS, Filip L, Pripon F. The evaluation of antioxidant capacity of propolis originating from Western Romania. Farmacia. 2019;67:111–116. doi: 10.31925/farmacia.2019.1.15. [DOI] [Google Scholar]
- 58.Cunha BS, Sawaya ACHF, Caetano FM, Shimizu MT, Marcucci MC, Drezza FT, Povia GS, Carvalho P. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15:964–970. doi: 10.1590/S0103-50532004000600026. [DOI] [Google Scholar]
- 59.Choi SJ, Shimomura K, Kumazawa S, Ahn MR. Antioxidant properties and phenolic composition of propolis from diverse geographic regions in Korea. Food Sci Technol Res. 2013;19:211–222. doi: 10.3136/fstr.19.211. [DOI] [Google Scholar]
- 60.Lagouri V, Prasianaki D, Krysta F. Antioxidant properties and phenolic composition of Greek propolis extracts. Int J Food Prop. 2014;17:511–522. doi: 10.1080/10942912.2012.654561. [DOI] [Google Scholar]
- 61.Pavlovic R, Borgonovo G, Leoni V, Giupponi L, Ceciliani G, Sala S, Bassoli A, Giorgi A. Effectiveness of different analytical methods for the characterization of propolis: a case of study in Northern Italy. Molecules. 2020;25:504. doi: 10.3390/molecules25030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorucu A, Oruc HH. Determination of biologically active phenolic compounds in propolis by LC-MS/MS according to seasons and altitudes. J Food Meas Charact. 2019;13:2461–2469. doi: 10.1007/s11694-019-00166-9. [DOI] [Google Scholar]
- 63.Avcı M. The floristic regions of Turkey and a geographical approach for anatolian diagonal. Türk Coğ Der. 1993;28:225–248. [Google Scholar]
- 64.Sorkun K. Turkey’s nectarious plants, pollen, honey. Ankara: Palme Press; 2008. p. 352. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analysed during this study are included in this published article.