Ultraviolet radiation from the sun is responsible for a variety of familiar photochemical reactions, including photochemical smog, bleaching of paints and decay of plastics. Conjugated bonds in organic molecules such as proteins and DNA absorb the UV radiation, which can damage these molecules. By a fortunate evolutionary event, the oxygen produced by photosynthesis forms a filter in the outer reaches of our atmosphere that absorbs the most energetic and harmful UV radiation, with wavelengths below 240 nm (in the UVC band [wavelength 100-280 nm]). In the process, the oxygen molecules split up and recombine to form ozone (Fig. 1). This rarified ozone layer (spread out between 10 and 50 km in the stratosphere but only 3 mm thick were it compressed at ground level) in turn efficiently absorbs UV radiation of higher wavelenghts (up to about 310 nm). A part of the UV radiation in the UVB band (wavelength 280-315 nm) still reaches ground level and is absorbed in sufficient amounts to have deleterious effects on cells. The less energetic radiation in the UVA band (wavelength 315-400 nm, bordering the visible band [wavelength 400-800 nm]) is not absorbed by ozone and reaches ground level without much attenuation through a clear atmosphere (i.e., no clouds, no air polution). Although not completely innocuous, the UVA radiation in sunlight is much less photochemically active and therefore generally less harmful than UVB radiation.
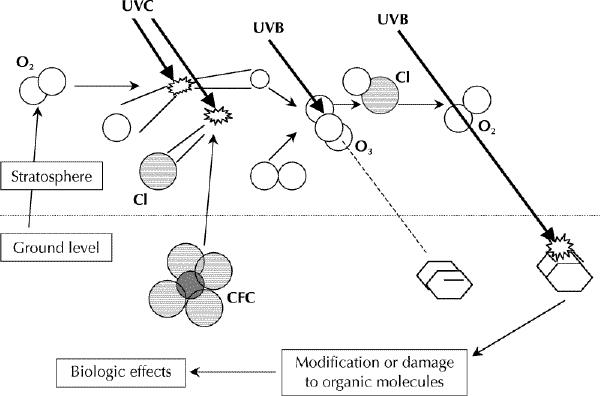
Fig. 1: Simplified scheme of how the stratospheric ozone layer is formed and absorbs ultraviolet B (UVB) radiation and how chlorofluorocarbons (CFCs) degrade the ozone layer and increase UVB-induced biological effects. Molecular oxygen (O2) and CFCs diffuse upward into the stratosphere, where they are decomposed by shortwave UV radiation (UVC). Atomic oxygen (O) recombines with molecular oxygen to form ozone (O3). Ozone absorbs the short wavelengths in the UVB band, blocking photochemical reactions in organic molecules. Reactive chlorine (Cl) released from CFCs catalyzes the breakdown of ozone to molecular oxygen, which does not absorb UVB radiation. More UVB radiation thus passes through the stratosphere, enhancing photochemical reactions in organic molecules and increasing corresponding biological effects.
Life on earth has adapted itself to the UV stress, particularly UVB stress, for example by forming protective UV-absorbing surface layers, by repairing cell damage or by replacing damaged cells entirely. Human skin shows all of these adaptive features. Our eyes are less well adapted, but they are shielded by the brows and by squinting.
Ozone depletion
In 1974 Molina and Rowland,1 who together with Crutzen received the 1995 Nobel Prize in Chemistry for their work in atmospheric chemistry, proposed that stratospheric ozone might be destroyed by industrially produced chlorine- and bromine-containing stable substances, such as chlorofluorocarbons commonly used in spray cans, refrigerators and air conditioners. These inert substances can reach the stratosphere, where they are decomposed by high-energy UV radiation, and reactive chlorine and bromine are released (Fig. 1).
Although complicated by substantial daily, seasonal and annual fluctuations (in the order of 20%) a gradual downward trend in the amount of stratospheric ozone has been measured in temperate and polar climate zones over the last 2 decades (e.g., 3%-6 % per decade at mid-latitudes).2,3 The exceptionally low levels of stratospheric ozone (over 50% depletion) that occur over Antarctica in the early austral spring were unanticipated.4 This ozone "hole" develops because of the generation of reactive chlorine on the surface of ice crystals in high-altitude polar clouds.
The important consequence of a hole in the ozone layer is an increased flux of UV radiation at the earth's surface and in the top layers of the ocean. The productivity of phytoplankton was found to be reduced by 6%-12% directly under the ozone hole.5 Transient effects of increased UV radiation have thus been measured on organisms at the bottom of the marine food chain. What the long-term effects of ozone depletion will be on marine and terrestrial ecosystems is still uncertain.
Health effects
Ozone depletion will not simply increase all UVB-related effects, because adaptive reactions will dampen or even neutralize the impact of an increase in UVB radiation even if sun-exposure behaviour remains the same. For example, the UVB-driven production of vitamin D3 in the skin is self-limiting and the serum level of the ultimately biologically active metabolite (1,25-dihydroxyvitamin D) is controlled by feedback mechanisms that maintain an approximately constant level.6 Therefore, the beneficial effects from vitamin D3 are not expected to be enhanced in a large majority of people: the usual daily sun exposure of head and hands is already sufficient. Among people with regular sun exposure (not sunbathers) rates of sunburn are not likely to increase either, because the human skin can adapt very efficiently to gradual increases in UVB exposure. Photodermatoses may even become less frequent. The loss of adaptation to UV radiation during winter generally increases the incidence and severity of photodermatoses. Because the ozone depletion will be strongest in wintertime, this loss of adaptation will decrease.
The health effects that are believed to be affected by an increased flux in UV radiation include skin cancer, cataract and cellular immunity; the decreased immunity may cause increased severity of infections, less effective vaccinations and perhaps an increase in non-Hodgkin's lymphoma.
Skin cancer
UVB radiation is known to damage DNA at specific sites (neighbouring pyrimidines). Mutations in the p53 tumour suppressor gene in human skin carcinomas were found at precisely these sites.7,8 (The p53 protein plays an important role in the cellular response to DNA damage [cell cycle arrest to allow more time for DNA repair, and apoptosis if the cell is overly damaged]. Its dysfunction can cause genomic instability and thus lead to the dysfunction of other genes.) These point mutations in the p53 gene resembled those found in experiments with UV-irradiated cell cultures (mainly a cytosine substituted by a thymine, and sometimes even 2 neighbouring cytosines replaced by 2 thymines).7,8 The high frequency and apparent selection of the p53 mutations in the tumours appears to constitute the most direct evidence that UVB radiation contributes to the formation of skin cancer. The risk of squamous cell carcinoma appears to increase with lifelong sun exposure, whereas accumulated exposure does not appear to contribute to the risk of basal cell carcinoma.9 The risk of basal cell carcinoma10 and cutaneous malignant melanoma11 appears to be related to childhood exposure and to intermittent overexposures. Skin cancer is by far the most common form of cancer among white people in the United States, and the incidence rates of all 3 types of skin cancer increase with increasing ambient UVB radiation loads, although each type of cancer has a different dose-response curve.12,13 Surprisingly, however, experiments in fish14 and opossums15 have indicated that UVA radiation may be more important than UVB radiation in the induction of melanoma.
The effective carcinogenic UV dose of a certain spectrum of sunlight (which varies with solar angle and ozone thickness) can be ascertained by adding up the effective doses contributed by different wavelengths. The wavelength dependence of squamous cell carcinoma induction in humans can be estimated from data obtained in mouse experiments,16,17 and it crudely parallels the wavelength dependence of sunburn (peaking between 290 and 300 nm). Thus, forecasting UV indices to warn against sunburn should help to minimize carcinogenic UV exposures. In general, people need daily exposure to ordinary levels of UVB radiation in order to maintain their vitamin D levels and their adaptation to sun exposure, but overexposure will contribute to adverse effects such as immunosuppression and skin cancer.
The relation between ambient UVB levels and skin cancer incidence rates at different geographic locations can be used to estimate the impact of ozone depletion on skin cancer rates.18 However, it is not well established whether melanoma induction in humans is dominated by UVB radiation; it may be dominated by UVA radiation, as explained earlier. It is therefore uncertain how melanoma will be affected by ozone depletion, which will change only the UVB component of sunlight. Because melanomas constitute a small proportion (< 10%) of skin cancers, they will not greatly affect skin cancer incidence projections. Melanomas are, however, the most lethal type of skin cancer and, if their incidence is not affected by ozone depletion, the projected increases in skin cancer mortality will be overestimated by 25%-50%.
Based on the international agreements to phase out ozone-depleting substances, as described in the Montreal Protocol of 1987 and its later amendments,19 the thickness of the stratospheric ozone layer is expected to reach a minimum around the turn of the century, after which the incidence of skin cancer is projected to reach a maximum (10% higher than current rates) around 2060 and then to fall.18 However, this projection assumes full compliance of all nations and industries with the international agreements. Unrestrained emissions of chlorofluorocarbons and the use of methylbromide in agriculture would be expected to produce a gradual and persistent degradation of the ozone layer and will likely lead to a dramatic increase in skin cancer incidence, which we have estimated could quadruple by the year 2100.18
Cataract
The effects of ozone depletion and UV radiation on skin cancer are well studied. Other health effects are less certain, mainly because the wavelength and UV dose dependencies are not adequately established. By assuming that the UVB dominance found in cataract formation in animal experiments also holds for cataract development in humans, we have estimated that the incidence of cataract would ultimately rise by 0.5% for every 1% persistent decrease in ozone.20 Dolin21 reviewed epidemiological studies and concluded that there is good evidence for a relation between (cortical) cataracts and sunlight.
Immune effects
Most of the data on immunomodulatory effects of UV radiation stem from mouse experiments, but the suppression of contact hypersensitivity by UV light also occurs in humans, along with many of the cellular effects (disappearance of Langerhans cells, influx of macrophage-like cells and release of certain cytokines such as interleukin-1, tumour necrosis factor α and interleukin-10).22,23 UV radiation can alter organic molecules in the skin and thus form novel antigens ("neo-antigens"), which could potentially evoke immune reactions. The UV-induced immunosuppression could therefore serve to prevent these illicit immune reactions (such as occur in photodermatoses). However, this immunosuppresion can clearly have adverse effects. UV effects on viral, bacterial, parasitic and fungal infections have been established in animal experiments.24,25 Infections that have a phase in the skin, like malaria and leprosy, are likely to be influenced. The herpes simplex "cold sore" triggered by sun exposure is a good example of an infection in humans brought forth by UV-induced immunosuppression.26 Reports by Finsen 100 years ago showed that UV irradiation can influence infections both beneficially (skin tuberculosis or lupus vulgaris) and adversely (smallpox).27
Immunosuppression is a risk factor for both skin cancer and non-Hodgkin's lymphoma. UV-induced immunosuppression might be expected to play a role in the prevalence of these cancers.28 The risk of non-Hodgkin's lymphoma is associated with skin cancer, and the incidence rates of both types of cancer have risen significantly over the past decades. This does not necessarily imply that increased sun exposure is the common causative factor. Unlike skin cancer, non-Hodgkin's lymphoma does not show a clear latitudinal gradient over the United States.29 More recently, however, it has been reported that the incidence of non-Hodgkin's lymphoma in Sweden tends to increase toward the south, but to a considerably lesser extent than that of squamous cell carcinoma.30 Experimental data showed that a combination of a chemical carcinogen and UV exposure induced lymphomas in hairless mice, whereas each agent on its own did not.31
Indirect health effects
There is the possibility that human health would not only be directly affected by the increased levels of ambient UVB radiation, but also be indirectly affected by changes to the environment because of stratospheric ozone depletion. For example, UVB radiation contributes to photochemical smog: it can increase ozone at ground level and thus aggrevate respiratory afflictions. A potentially more dramatic effect could stem from a decrease in food production because of the effects of stratospheric ozone depletion on certain plants and animals. These indirect health effects are, however, even less easily quantified than the direct effects on human health.
Conclusion
Overall, compliance with the international agreements on phasing out ozone-depleting substances has been good, but there is no reason for complacency. As the production of the bulk of such substances in developed countries is curbed, other sources become more important. These other sources may prove to be more difficult to phase out; for example, the production and replacement of ozone-depleting substances can be difficult to control in some developing countries because there is no suitable infrastructure or no proper channelling of international support to overcome the locally prohibitive costs for the required changes.2 Moreover, there is always the danger that certain countries that have or have not ratified the agreements will continue to produce or increase production of ozone-depleting substances. To discourage any subversive production of such substances, consumers in developed countries should remain vigilant and not purchase anything operating or produced with ozone-depleting substances when good alternatives are available.
Despite a long-standing concern about the effects of ozone depletion, our understanding of the problem is still incomplete and limited to only a few effects. Our knowledge of the effects of current levels of UV exposure on ecosytems and human health is scanty, which hampers any extrapolation to effects at higher levels of UV exposure. There is an obvious need to remedy the situation, if only to weigh benefits against costs of the next human activity that may threaten the ozone layer.
Additional reading .
Longstreth J, De Gruijl FR, Kripke ML, Abseck S, Arnold F, Slaper HI, et al. Health risks. J Photochem Photobiol B 1998;46:20-39.
Madronich S, McKenzie RL, Björn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth's surface. J Photochem Photobiol B 1998;46:5-19.
Related Web sites .
European Ozone Research Coordinating Unit: www.ozone-sec.ch.cam.ac.uk
Impacts of a projected ozone depletion: www.gcrio.org/CONSEQUENCES/summer95/impacts.html
Ozone Depletion, US Environmental Protection Agency: www.epa.gov/ozone/index.html
Ozone Secretariat, United Nations Environment Programme: www.unep.org/ozone
Stratospheric Ozone and Human Health Project, Center for International Earth Science Information Network: sedac.ciesin.org/ozone
US Global Change Research Information Office: www.gcrio.org
Footnotes
Articles to date in this series
McCally M. Environment and health: an overview. CMAJ 2000;163(5):533-5.
Speidel JJ. Environment and health: 1. Population, consumption and human health. CMAJ 2000;163(5):551-6.
Haines A, McMichael AJ, Epstein PR. Environment and health: 2. Global climate change and health. CMAJ 2000;163(6):729-34.
This article has been peer reviewed.
Acknowledgements: We thank the Dutch Ministry of Housing, Zonal Planning and Environment for financially supporting our work for the UNEP Panel on the Environmental Effects of an Ozone Depletion. We also thank the European Commission and the Netherlands Cancer Foundation for subsidizing our research.
Reprint requests to: Dr. Frank R. de Gruijl, Department of Dermatology, University Hospital of Utrecht/AZU, PO Box 85500, NL-3508 GA Utrecht, the Netherlands; F.deGruijl@digd.azu.nl
References
- 1.Molina MJ, Rowland FS. Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature 1974;249:810-2.
- 2.Ozone Secretariat, United Nations Environment Programme. Synthesis of the reports of the Scientific, Environmental Effects, and Technology and Economic Assessment Panels of the Montreal Protocol — 1999. Nairobi: The Secretariat; 1999. Available: www.unep.org/ozone and www.unep.ch/ozone (accessed 2000 Aug 31).
- 3.McKenzie R, Connor B, Bodeker G. Increased summertime UV radiation in New Zealand in response to ozone loss. Science 1999;285:1709-11. [DOI] [PubMed]
- 4.Farman JC, Gardiner BG, Shanklin. Large losses of total ozone in Antarctica reveal seasonal ClOx NOx interaction. Nature 1985;315:207-10.
- 5.Smith RC, Prezelin BB, Baker KS, Bidigare RR, Boucher NP, Coley T, et al. Ozone depletion: ultraviolet radiation and phytoplankton biology in Antarctic waters. Science 1992;255:893-1040. [DOI] [PubMed]
- 6.Holick MF, MacLaughlin JAM, Parrish JA, Anderson RR. The photochemistry and photobiology of vitamin D3. In: Regan JD, Parrish JA, editors. The science of photomedicine. New York: Plenum Press; 1982. p. 195-218.
- 7.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A 1991;88:10124-8. [DOI] [PMC free article] [PubMed]
- 8.Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A 1993;90:4216-20. [DOI] [PMC free article] [PubMed]
- 9.Vitasa BC, Taylor HR, Strickland PJ, Rosenthal FS, West S, Abbey H, et al. Association of nonmelanoma skin cancer and actinic keratosis with cumulative solar ultraviolet exposure in Maryland watermen. Cancer 1990;65:2811-7. [DOI] [PubMed]
- 10.Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? A case-control study in Western Australia. Int J Cancer 1995;60:489-94. [DOI] [PubMed]
- 11.Holman CDJ, Armstrong BK. Cutaneous malignant melanoma anf indicators of total accumulated exposure to the sun: an analysis separating histogenic types. J Natl Cancer Inst 1984;73:75-82. [PubMed]
- 12.Scotto J, Fears TR. Incidence of nonmelanoma skin cancer in the United States. Washington: US Department of Health and Human Services; 1981. Cat no NIH 82-2433.
- 13.Scotto J, Fears TR. The assosciation of solar ultraviolet and skin melanoma incidence among Caucasians in the United States. Cancer Invest 1987;5:275-83. [PubMed]
- 14.Setlow RB, Grist E, Thompson K, Woodhead AP. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci U S A 1993;90:6666-70. [DOI] [PMC free article] [PubMed]
- 15.Ley RD. Ultraviolet radiation A-induced precursors to cutaneous melanoma in Monodelphis domestica. Cancer Res 1997;57:3682-4. [PubMed]
- 16.De Gruijl FR, Sterenborg HJCM, Forbes PD, Davies RE, Kelfkens G, Van Weelden H, et al. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res 1993;53:53-60. [PubMed]
- 17.De Gruijl FR, Van der Leun JC. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Phys 1994;67:319-25. [DOI] [PubMed]
- 18.Slaper H, Velders GJM, Daniel JS, De Gruijl FR, Van der Leun JC. Estimates of ozone depletion and skin cancer incidence to examine the Vienna Convention achievements. Nature 1996;384:256-8. [DOI] [PubMed]
- 19.Ozone Secretariat, United Nations Environment Programme. The 1987 Montreal protocol on substances that deplete the ozone layer [as adjusted and amended by the second, fourth, seventh and ninth meetings of the parties]. Nairobi: The Secretariat; 1997. Available: www.unep.ch/ozone/mont_t.htm (accessed 2000 Aug 31).
- 20.Van der Leun JC, De Gruijl FR. Influence of ozone depletion on human health. In: Tevini M, editor. UV-B radiation and ozone depletion. Boca Raton (FL): Lewis Publishers; 1993. p. 95-123.
- 21.Dolin PJ. Ultraviolet radiation and cataract: a review of epidemiological evidence. Br J Ophthalmol 1994;78:178-82. [DOI] [PMC free article] [PubMed]
- 22.Yosikawa T, Rae V, Bruin-Slot W, Van den Berg W, Taylor JR, Streilein JW. Susceptibility to effects of UV-B radiation on the induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol 1990;95:530-6. [DOI] [PubMed]
- 23.Cooper KD, Oberhelman L, Hamilton TA, Baardsgaard O, Terhune M, LeVee G, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a- DR+ epidermal macrophage induction and Langerhans cell deletion. Proc Natl Acad Sci U S A 1992;89:8497-501. [DOI] [PMC free article] [PubMed]
- 24.Jeevan A, Brown E, Kripke ML. UV and infectious diseases. In: Krutmann J, Elmets CA, editors. Photoimmunology. Oxford (UK): Blackwell Science; 1995. p. 153-63.
- 25.Goettsch W, Garssen J, De Gruijl FR, Van Loveren H. Effects of UV-B on the resistance against infectious diseases. Toxicol Lett 1994;72:259-63. [DOI] [PubMed]
- 26.Miura S, Kulka M, Smith CC, Imafuku S, Burnett JW, Aurelian L. Cutaneous ultraviolet radiation inhibits herpes simplex virus-induced lymphoproliferation in latently infected subjects. Clin Immunol Immunopathol 1994;72:62-9. [DOI] [PubMed]
- 27.Finsen NR. Über die bedeutung der chemische strahlen des lichtes für medicin und biologie. Leipzig: Vogel Publishing; 1899.
- 28.Goldberg LH. Basal-cell carcinoma as predictor for other cancers. Lancet 1997; 349:664-5. [DOI] [PubMed]
- 29.Hartge P, Devesa SS, Grauman D, Fears TR, Fraumeni JF Jr. Non-Hodgkin's lymphoma and sunlight. J Natl Cancer Inst 1996;88:298-300. [DOI] [PubMed]
- 30.Adami J, Gridley G, Nyren O, Dosemeci M, Linet M, Glimelius B, et al. Sunlight and non-Hodgkin's lymphoma: a population-based cohort study in Sweden. Int J Cancer 1999;80:641-5. [DOI] [PubMed]
- 31.Husain Z, Pathak MA, Flotte T, Wick MM. Role of ultraviolet radiation in the induction of melanocytic tumors in hairless mice following 7,12-dimethylbenz(a)antracene application and ultraviolet irradiation. Cancer Res 1991;51: 4964-70. [PubMed]


