Abstract
The Southwestern United States has a legacy of industrial mining due to the presence of rich mineral ore deposits. The relationship between environmental inhaled particulate matter (PM) exposures and neurological outcomes within an autoimmune context is understudied. The aim of this study was to compared two regionally-relevant dusts from high-priority abandoned mine-sites, Claim 28 PM, from Blue Gap Tachee, AZ and St. Anthony mine PM, from the Pueblo of Laguna, NM and exposed autoimmune-prone mice (NZBWF1/J). Mice were randomly assigned to one of three groups (n=8/group): DM (dispersion media, control), Claim 28 PM, or St. Anthony PM, subjected to oropharyngeal aspiration of (100 μg/50 μl), once per week for a total of 4 consecutive doses. A battery of immunological and neurological endpoints was assessed at 24 weeks of age including: bronchoalveolar lavage cell counts, lung gene expression, brain immunohistochemistry, behavioral tasks and serum autoimmune biomarkers. Bronchoalveolar lavage results demonstrated a significant increase in number of polymorphonuclear neutrophils following Claim 28 and St. Anthony mine PM aspiration. Lung mRNA expression showed significant upregulation in CCL-2 and IL-1ß following St. Anthony mine PM aspiration. In addition, neuroinflammation was present in both Claim 28 and St. Anthony mine-site derived PM exposure groups. Behavioral tasks resulted in significant deficits as determined by Y-maze new arm frequency following Claim 28 aspiration. Neutrophil elastase was significantly upregulated in the St. Anthony mine exposure group. Interestingly, there were no significant changes in serum autoantigens suggesting systemic inflammatory effects may be mediated through other molecular mechanisms following low-dose PM exposures.
Keywords: autoimmune, pulmonary, particulate matter, neurobehavioral, cognitive, autoantibodies
Introduction
There are over 500 abandoned uranium mines in the Southwestern United States, many with a history of poor reclamation, or insufficient restoration of these sites to their natural state following the cessation of mining activities (Lewis et al 2017; Hoover et al. 2017). Poor or incomplete reclamation of mine-sites results in residential exposure to metal-based waste including vanadium (V), arsenic(As), and uranium (U) via inhalation and other routes such as ingestion (Lewis et al 2017; Dashner-Titus et al. 2018; Harmon et al. 2017; 2018). Recent evidence suggests that residential proximity to abandoned mine-sites leads to in increased serum cumulative inflammatory potential independent of metals-intake via drinking water (Harmon et al. 2017). In addition, findings from our lab demonstrated that pulmonary exposure to metal-based particulate matter (PM), results in vascular and neurological dysfunction (Aragon et al. 2017; Tyler et al. 2016), mediated by the blood brain barrier (BBB). Blood-brain barrier breakdown following inhaled exposures involves inflammatory molecules and complement proteins with both remodeling and destabilization of endothelial tight and adherens junctions (Aragon et al. 2016). While canonical cytokines are clearly ediating lung inflammation, several investigators indicated that canonical cytokines are not necessarily the primary culprit, but rather circulating, serum-borne peptidomic and/or exosomal fragments may be responsible for observed toxicity (Mostovenko et al. 2019; Sanchez et al. 2020). Furthermore, inhaled air pollutant exposures have been linked to both autoimmune disease (Zhao et al. 2019; Gawda et al. 2017; Sigaux et al. 2019) and circulating inflammatory potential (Aragon et al. 2017; Harmon et al. 2017; Sanchez et al. 2020).
Exposures to these mine-dust metals are potential instigators of autoimmune responses and several investigators examined the relationship between heavy metal exposure and development of systemic lupus erythematosus, rheumatoid arthritis and Alzheimer’s disease (Barbhaiya and Costenbader 2016; Parks et al. 2017; Afridi et al. 2014; Murphy et al. 2016). In occupational settings McCormic et al (2010) and Yahya et al (2014) noted that respirable exposure to silica dust in the form of quartz particulates is a risk factor for systemic autoimmune diseases such as scleroderma and rheumatoid arthritis. In addition, Erdei et al (2019) found elevated autoimmunity biomarkers in residents living near abandoned metal-rich U mine sites on the Navajo Nation. In this study, anti-native DNA/-chromatin autoantibodies were linked with U ingestion via drinking water. Life-long metals exposure was associated with anti-denatured DNA antibodies predominantly in men. However, As consumption through water and urinary As levels were negative predictors of autoantibody presence. In another smaller cohort study, pooled and stratified urinary metals data in Navajo and Nicaraguan men revealed that Nicaraguan men exhibited higher urinary metals than Navajo or NHANES (Scammell et al. 2020). Antinuclear autoantibody positivity was observed in individuals with significant urinary barium, cesium, lead, strontium and tungsten.
Silica exposure, in particular, has been linked with both instigation and progression of autoimmune diseases (Scammell et al. 2020; Pollard 2016; Mayeux et al. 2018), yet few studies examined head-to-head comparisons of silica-based dusts, in combination with other metals, that may be driving autoimmune development. Furthermore, few investigators determined the role in toxicological exposures driving neurological outcomes in autoimmune models, despite the fact that neurobehavioral sequelae are evident in autoimmune disorders (Lepri et al. 2019; Appenzeller 2016; Lynall 2018). Sakic et al (1993) showed that rapid progression of humoral autoimmunity impacts emotionality in MRL-lpr mice and that neurocognitive learning and memory abnormalities in children from mothers with SLE are significant, regardless of gender, race and socio-economic status (Urowitz et al. 2008). However, the contributions of inhaled exposures to these outcomes are largely unknown.
The aim of this study was to compare molecular biological effects of two regional, metal-rich dusts, Claim 28, from an abandoned U-V mine in Blue Gap Tachee, AZ and St. Anthony mine, a privately owned, inactive, U mine, via oropharyngeal aspiration in NZBWF1/J (autoimmune-prone) mice. Furthermore, pulmonary, serum-borne, circulating biomarkers and neurological outcomes were determined. It was postuated that exposure to U-Fe-Si dust (St. Anthony mine) might result in a more severe autoimmune phenotype than a K-V-Si (Claim 28) dust following repeated oropharyngeal aspiration. To our knowledge, this is the first assessment of 1) biological impacts resulting from two regional mine-related dusts and 2) examination of neurological endpoints following dust exposure in an NZBWF1/J mouse model.
Materials and Methods
Solid samples
Solid samples were collected from the Blue Gap Tachee (Claim 28), AZ in June 2014. The mineralized deposits from the Claim 28 site correspond to the Cretaceous Mesa Verde Group (Blake et al. 2015). A more detailed description of the geology of this site may be found in a previous study from our group (Blake et al. 2015). Other solid samples were collected in June 2017 from the St. Anthony mine in NM. The mineralized deposits form the St. Anthony mine correspond to the Jackpile Sandstone Member of the Morrison Formation. A more detailed description of the geology of this region may be found in other studies. (Blake et al. 2017; Hansley and Spirakis 1992; Leventhal et al 1986; Leventhal 1980)
Solid analyses
Thermal analyses were conducted on samples to estimate the organic matter content. Solid phase analyses were conducted by X-Ray Fluorescence (XRF), electron probe microanalysis (EPMA) to determine the bulk composition of the samples and spatial association of co-occurring elements in the samples.
The volume median diameter (VMD) of the PM samples was determined by laser diffraction using the cuvette disperser (Helos/KF-OM, Sympatec GmbH, Germany). Briefly, PM samples were vortex-mixed and suspended in water. Approximately 100 μl aliquot of PM suspensions were added to a 6 ml glass cuvette containing 5 ml water. Measurements were taken using the R3 lens in triplicate.
Thermal analyses for solids
The natural organic matter (NOM) content in the mineralized deposit solid samples from the St. Anthony mine was estimated by loss on ignition (LOI) and thermogravimetric analyses (TGA). Samples were dried at 105°C for 12 hr in ceramic crucibles to remove the moisture then heated in a muffle furnace at 550°C for 5 he. The weight difference between 105°C and 550°C is attributed to the NOM content (Cawley et al. 2017; Velasco et al. 2019). The samples from Claim 28 were predominantly inorganic and contained limited carbon content, thus thermal analyses were not pursued (Avasarala 2018).
X-Ray fluorescence
Bulk analysis was performed on solid samples using X-Ray Fluorescence (XRF) with a Rigaku ZSX Primus II with Rhodium X-Ray tube that quantitatively determines major and minor atomic elements, from B to U. The software is ZSX Primus II that performs both qualitative and quantitative analysis.
Aqueous Chemical Extraction.
To assess the acid extractable elements from the mineralized surface deposits from the Jackpile Mine samples, acid digestions were conducted on triplicates by adding 2 ml nitric acid (HNO3) and 6 ml hydrochloric acid (HCl) and 3 ml concentrated hydrofluoric acid (HF) into 50 ml Teflon digestion tubes containing 2 ± 0.002 g of homogenized sample. All reagents used were ultra-high purity. The digestion tubes were then heated in a Digi prep MS SCP Science block digester at 95° C for 2 hr. Following heating, acid extracts were diluted to 50 ml with mega ohm waster and filtered through a 0.45 μm filter to remove suspended solids. Metal concentrations in solution were measured using ICP-OES and ICP-MS.
Electron probe microanalysis (EMPA) x-ray mapping
Solid samples were embedded in epoxy. The surface was then ground flat and polished using successive grits of silicon carbide, diamond, and alumina to achieve a surface smoothness of 0.05μm and coated with 150 nm of carbon to provide electrical conductivity under the electron beam. The samples were examined in a JEOL JXA8200 electron microprobe equipped with 5 wavelength X-ray spectrometers (WDS) and an energy dispersive X-ray spectrometer (EDS). Operating conditions were 15 kV accelerating voltage and 30 nA beam current. Backscattered electron (BSE) imaging was conducted on the surface of the epoxy-embedded mine waste fragments to locate targets for X-ray mapping. Maps were acquired over an area of 1 × 1 mm at a pixel resolution of 4 μm with 80 msec dwell per pixel, taking approximately 90 min for each. Maps for O, P, K and U were acquired by WDS simultaneously with C, Si, Ca and Fe acquired by EDS.
Particulate matter exposure
Briefly, 6–7 week old NZBWF1/J, female mice were purchased from Taconic Labs (Rensselaer, New York) and acclimated in standard animal housing for one week according to the University of New Mexico IACUC protocol. This mouse strain and gender was selected based upon its development of autoimmune disease and autoantibody production that mimics human systemic lupus erythematous (SLE). Other similarities to human SLE include its deposition of pathogenic kidney autoantibodies and development of glomerulonephritis (Nakajima et al. 1997).
Mice were randomly assigned to one of three groups (n=8/treatment) and subjected to either 4 weekly doses (100 μg/ 50μl) of Claim 28 PM, St. Anthony mine PM or dispersion media control (DM) starting at 8 weeks old via oropharyngeal aspiration, as described in Figure 1, based upon previous studies (Bates et al. 2015; 2018). Dispersion media consisted of 600 μl albumin stock (1mg albumin/1ml PBS), 399 μL USP Grade PBS, 1 μL dipalmitoylphosphatidylcholine (DPPC) stock (1mg DPPC/10μl 10% ethanol). Mice were then aged several weeks until a lupus-like phenotype developed and were euthanized at 24 weeks, as NZBWF1 mice develop glomerulonephritis around this timeframe. Urinary proteinuria suggestive of renal injury and glomerulonephritis was confirmed at > 300mg/dl to confirm disease development (Chang et al. 2011; Wang et al. 2009).
Figure 1. Study design.
Mice were subjected to PM exposures via oropharyngeal aspiration (100μg/50μl). Three groups of mice (n=8) were exposed to either dispersion media (DM), Claim 28 dust, or St. Anthony mine dust, repeatedly, once per week (weeks. 8–11). Behavioral assays (open field, novel object, Y-maze, and O-maze) were performed 4 days (one assay each day) prior to week. 24 euthanasia.
Bronchoalveolar lavage
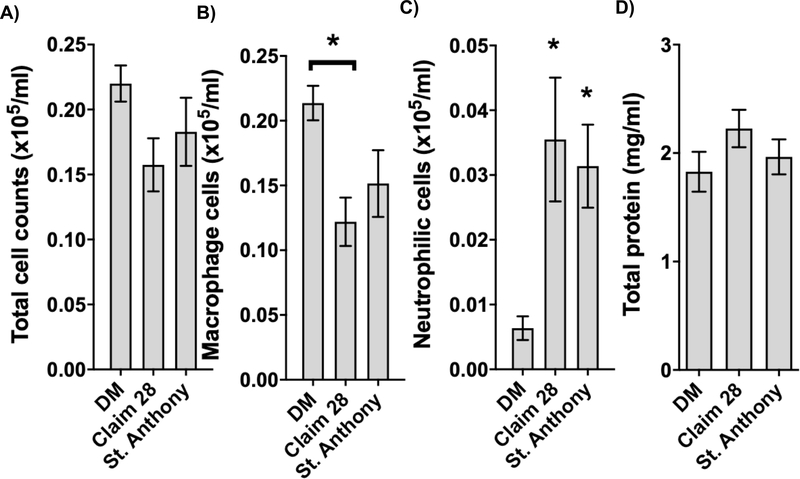
Bronchoalveolar lavage fluid (BALF) was collected by inserting a cannula through a small incision in the trachea. Approximately 1 ml phosphate-buffered saline (PBS) was injected and withdrawn using a sterile syringe for collection. Total cell, neutrophil, and macrophage counts were assessed from 6–8 subjects per group using a cytospin (ThermoFisher Scientific, Waltham, MA). Sample total protein was analyzed using Bradford’s assay (Harlow and Lane 2006). Results were expressed as cells/volume as illustrated in Figure 3.
Figure 3. Bronchoalveolar lavage (BAL) fluid parameters.
Following euthanasia of each mouse, BAL was collected via tracheal puncture and assessed for number of inflammatory cells for A) total cell counts (cells x105) B) macrophage cells (cells x105) C) neutrophilic cells (cells x105) and D) total protein Statistics were executed using a one-way ANOVA followed by a Kruskal-Wallis post hoc test and considered significant at p<0.05.
Lung transcriptional responses to PM
Following euthanasia, lungs were isolated from mice and immediately frozen in liquid nitrogen and subsequently stored at −80°C. Quantitative polymerase chain reaction (qPCR) was then used to assess transcriptional alterations (Cung et al. 2015). Lung samples were homogenized using a QIAGEN RNA Fibrous mini-kit and RNA was extracted following the manufacturer’s protocol (QIAGEN, Valencia, CA). Reverse transcription of RNA was executed using a high-capacity cDNA reverse transcriptase, immediately prior to qPCR. Genes probed using TaqMan primers included Mmp-2, Tgf-, Ccl-2, IL-1B, IL-6 and Cxcl-1. These targets were selected based upon previous published PM studies (Tyler et al. 2016; Zychowski et al. 2018; Begay et al. 2020), and amplified using TaqMan Universal Master Mix. TATA boxbinding protein (TBP) was implemented as the housekeeping gene. The 2ΔΔCT method (Livak and Schmittgen 2001) was used to analyze relative gene expression quantification and the relative amount of mRNA for each sample and normalized to TBP (TATA binding protein) (Garcia et al. 2020; Zychowski et al. 2018; 2019).
Neuroinflammatory immunohistochemistry
Brains were dissected from the skull and halved. The right hemisphere was embedded in optimal cutting temperature (OCT) media, frozen and sectioned at 5–10 μm. The sections were mounted on positively charged slides, fixed in a 50/50 mixture of formalin/ethanol and then transferred to reaction buffer on the autostainer, the Ventana Discovery platform (Roche, Basel, Switzerland). GFAP (glial fibrillary acidic protein) rabbit polyclonal antibody (Abcam, Cambridge, MA) was diluted at 1:5000 and incubated for 20 min at 36°C. In a second set of slides, Iba-1 (ionized calcium binding adaptor molecule 1) rabbit polyclonal antibody (Abcam, Cambridge, MA) was diluted 1:100 in Discovery PSS and incubated for 32 min at 36°C. The enzyme conjugate (anti-HQ HRP, horse radish peroxidase) was applied for 12 min followed by DAB (3,3’-diaminobenzidine) detection and hematoxylin counterstain with bluing solution. Slides were then removed from the autostainer and coverslipped.
Behavioral tasks
Behavioral outcomes and tasks related to stress, anxiety or learning and spatial memory were conducted one week prior to euthanasia. A battery of 4 assays were executed to assess these outcomes including the Open Field test, a test of stress and anxiety, Novel Object test, a test of learning and memory, Y-maze, a test of learning and memory, and O-maze, a test of stress and anxiety. These tasks were executed at the University of New Mexico’s Center for Brain Recovery and Repair Preclinical Core. Each mouse was placed in an open field arena with motion tracking capabilities with a novel object for a 5-min trial. The total duration of interactions was assessed using Noldus Ethovision XT software to quantify overall response to learning and memory deficits in addition to stress and anxiety.
Serum autoimmune biomarkers
Serum neutrophil elastase was assessed via ELISAs (enzyme-linked immunoassay) from a commercial vendor (Signosis, Santa Clara, CA). Using a proteomic microarray containing autoantigens (UT Southwestern), serum IgG and IgM autoantibodies were also determined in a high-throughput platform from exposed and unexposed treatment groups. Key autoantigens included in the panel were selected based upon recent relevant autoimmune literature including, but not limited to Sjøgren’s syndrome, scleroderma, polymyositis, systemic lupus erythematosus, mixed connective tissue disease, polymyositis and rheumatoid arthritis. Selected autoantigens were adjusted to final concentration of 1mg/ml with PBS and immobilized onto nitrocellulose membrane-coated glass slides using a non-contact micro-dispenser Nonoplotter NP2.1 (GeSim, Gemany). A 1:50 dilution of mouse serum was incubated with the array and anti-mouse IgG and anti-mouse IgM secondary antibodies tagged with two differing fluorophores (cy3 for IgG and cy5 for IgM) were used to determine autoantibody levels in each sample (Rajasinghe et al. 2020).
Statistical analysis
Most assays were assessed via ANOVA with Bonferroni’s multiple comparisons test, post-hoc. Data presented in all graphs are mean+/− SEM. P values <0.05 were considered statistically significant. The heatmap was constructed using open source software (Babicki et al. 2016) using the mean linkage clustering method according to each row (autoantibody).
Results
Particulate matter (PM) characterization
The Blue Gap Tachee (Claim 28) sample used in this investigation is the same sample from previously-published studies (Zychowski et al. 2018; Blake et al. 2015; Avasarala et al. 2017). The XRF analysis indicated that this sample contained 0.66% U by weight (Blake et al. 2015), and ICP analyses for the acid digestions detected 0.19% U by weight. Respirable particles from this sample are composed of carnotite, which is a U-V mineral (Zychowski et al. 2018). Electron probe microanalysis demonstrated the association of K-U-V which is consistent with the stoichiometry of the mineral carnotite [K2(UO2)2 ]. The association of Ca-U-V was also detected and is consistent with the stoichiometry of the mineral tyuyamunite [Ca(UO2)2V2O8·(5–8)H2O] (Figure 2A). These findings are consistent with previous investigations from our research group related to the minerology of Claim 28 site (Blake et al. 2015; Avasarala et al. 2017).
Figure 2. Electron microprobe mapping.
Particulate matter PM collected from (A) Claim 28 and (B) Saint Anthony Mine showing a BSE image of the map area in the upper left, and maps for U, V, K, O, Si, Ca, P, Fe, and C. Yellow, red, pink and white colors represent higher concentration of elements. (C) % elemental composition analysis between St. Anthony and Claim 28 using XRF and acid extractable techniques. *Blake, J. M.; Avasarala, S.; Artyushkova, K.; Ali, A.-M. S.; Brearley, A. J.; Shuey, C.; Robinson, W. P.; Nez, C.; Bill, S.; Lewis, J.; Hirani, C.; Pacheco, J. S. L.; Cerrato, J. M., Elevated Concentrations of U and Co-occurring Metals in Abandoned Mine Wastes in a Northeastern Arizona Native American Community. Environ. Sci. Technol. 2015, 49, (14), 8506–8514. **Avasarala, S., Lichtner, P., Ali, A.S., González-Pinzón, R., Blake, J.M., and Cerrato, J.M., (2017) Reactive transport of U and V from abandoned mine wastes. Environ Sci Technol, 51, 12385–12393.
The XRF analysis conducted on the Saint Anthony mine samples detected 2.32 % U by weight, and ICP analyses for the acid digestions detected 1.13% U by weight. Electron probe microanalysis shows the association of U-Ca-P which is consistent with the stoichiometry of autunite [Ca(UO₂)₂(PO₄)₂·10–12H₂O]. The U-P phases found in this sample was observed at other sites of the Jackpile Sandstone Member of the Morrison formation.(Avasarala et al. 2019; Blake et al. 2017) In addition, these analyses also indicate areas with significant content of Si. Other investigators detected the mineral coffinite [USiO4] in samples from this formation.(Avasarala et al. 2017; Blake et al. 2017; Deditius et al 2008) Our findings showed 19.69±1.04% mass loss by LOI and 19.85±0.79 % by TGA due to volatilization of NOM. Previous investigations conducted by our research group found similar results in samples from the Jackpile Sandstone Member of the Morrison Formation (Velasco et al. 2019; Ruiz et al 2016). The mineralogy of these samples is complicated given the high content of organic matter and co-occurrence of various U-bearing phases.
The average PM size distribution for both samples was approximately 2.43 μm volume mean diameter (VMD) for Claim 28 and approximately 1.76 μm volume mean diameter for St. Anthony mine using the laser diffraction technique (Supplemental Figure 1). Further, any PM aggregation was not observed for either of the samples based upon the unimodal particle size distribution.
Bronchoalveolar lavage cell counts
Total cell counts did not significantly vary between DM, Claim 28, or St. Anthony exposures (Figure 3A). However, macrophage cells were significantly decreased following repeated Claim 28 PM exposure, compared to DM (Figure 3B). Neutrophilic cells were significantly upregulated when comparing DM vs. Claim 28 and DM vs. St. Anthony dust exposure (Figure 3C). Total protein (mg/ml) did not vary markedly between treatment groups (Figure 3D).
Lung gene expression
Lung gene expression of CCL-2 and IL-1ß was significantly upregulated in the St. Anthony exposure group, but not the Claim 28, relative to DM (Figure 4). Other genes assessed, including MMP-2, TGF- ß, IL-6 and CXCL-1, did not vary markedly between each of the treatment groups.
Figure 4. Lung gene expression.
Inflammatory gene expression including MMP-2, TGF-ß, IL-1 ß, IL-6, CCL-2 and CXCL-1. Statistics were executed using a one-way ANOVA followed by a Kruskal-Wallis post hoc test and considered significant at p<0.05.
Brain immunohistochemistry
Quantification of two key markers, Iba-1 (Ionized calcium binding adaptor molecule-1, a marker of activated microglia) and GFAP (glial fibrillary acidic protein, a marker of astroglial injury) revealed significantly increased protein expression in both Claim 28 and St. Anthony exposure groups of both in a cross-section of the right brain, including cortex and hippocampus (Figure 5). Quantification was based upon the number of cells per field and was determined using Image J software (Rueden et al 2017).
Figure 5. Brain immunohistochemistry.
Quantification of A) GFAP and B) IBA-1 in a cross-section of the brain. C) representative images of brains from DM, Claim 28 and St. Anthony mine dust exposure groups. Statistics were executed using a one-way ANOVA followed by a Kruskal-Wallis post hoc test and values considered significant at p<0.05.
Learning and memory behavioral tasks
Y-maze new arm frequency was significantly downregulated following Claim 28 exposure (Figure 6). Novel object cumulative duration, novel object frequency and Y-maze new arm cumulative duration trended downward in both the Claim 28 and St. Anthony exposure groups, although these did not demonstrate significant differences compared to controls. These data suggest reduced exploratory behavior, and possible memory impairment in the Claim 28 exposure group compared to controls.
Figure 6. Learning and memory tasks following aspirated dust exposure.
Novel object and Y-maze new arm cumulative duration and frequency, measures of spatial memory, using the NZBWF1/J mouse model. Statistics were executed using a one-way ANOVA followed by a Kruskal-Wallis post hoc test and values were considered significant at p<0.05.
Serum neutrophil elastase and serum autoantibody array
Serum neutrophil elastase, a biomarker elevated in a diseases such as atherosclerosis, hypertension, obstructive pulmonary disease and autoimmune disorders was significantly upregulated in the St. Anthony mine exposure group (Figure 7A) (El-Eshmawy et al. 2011; Bates et al. 2015). It is noteworthy that autoantibodies were consistently and mostly downregulated in the Claim 28 exposure group compared to DM and St. Anthony. Autoantibodies were quantitatively upregulated in the St. Anthony exposure group, (Figure 7B); however, these values across treatment groups were not significantly different from each other. IgM autoantibody values also did not markedly vary across treatment groups (Supplemental Figure 2).
Figure 7. Serum autoimmune biomarkers.
A) neutrophil elastase B) autoantigen array Biomarkers in mouse serum (DM, Claim 28 and St. Anthony) was evaluated using ELISA and an autoantibody array. Statistics were executed using a one-way ANOVA followed by a Kruskal-Wallis post hoc test and values were considered significant at p<0.05.
Discussion
The relevant aspect of these findings is key to understanding differential PM-mediated adverse effects. Due to improper reclamation of abandoned mine-sites in the Southwestern United States, tribal communities are chronically exposed to metal-mixtures including arsenic (As), vanadium (V), uranium (U) and other toxic metals (Dashner-Titus et al. 2018; Lewis et al 2017). Pulmonary inhalation of these toxic dusts is currently an active area of study (Zychowski et al. 2018; Sanchez et al. 2020). Findings from this study are also potentially translational to workers with occupational dust exposure, such as active and former ore miners and communities residing in close proximity to the poorly-remediated mine-sites (Rusibamayila et al 2018; Costello et al. 2018), such as the community living in Paguate, NM, near St. Anthony mine.
Our data suggest that 1) K-V-Si PM (Claim 28) and 2) Fe-U-Si PM (St. Anthony mine) exhibit differing, yet complementary toxicities when comparing lung, serum biomarkers and neurological outcomes. Based upon our evidence, these metals may act additively or synergistically with silica (Si), thereby potentiating their adverse effects. The U-Fe component to St. Anthony PM might serve as the driver of upregulation in lung gene expression, as Claim 28 contained relatively minimal U and Fe in the respirable dust (Zychowski et al. 2018; Assad et al. 2018). Silica has been cited as one key driver of autoimmune lung diseases including silicosis, pulmonary fibrosis (Fubini and Hubbard 2003) and sarcoidosis (Vihlborg et al. 2017). Repeated oropharyngeal aspiration of these dusts over 8–11 weeks resulted in significantly increased lung mRNA expression of IL-1ß, indicative of lung injury, and CCL-2, a monocyte chemoattractant protein, even after a hiatus from dust exposure for 13 weeks. (Deshmane et al. 2009). Both of these factors contribute to inflammatory diseases such as chronic obstructive pulmonary disease (COPD) and asthma (Jiang and Zhu 2016). In addition, neutrophils have been implicated in autoimmune disease development, in particular, NETosis and extracellular trap formation (Gupta et al. 2016, Cheng and Palaniyar 2013). Persistence of NETs in autoimmune disease is partially responsible for autoantibody development (Papayannopoulous and Zychlinsky 2009). NETs expand readily to pulmonary alveoli and are directly able to induce epithelial and endothelial cell death (Porto and Stein 2016). Neutrophil infiltration into the lung and NET formation were implicated in pulmonary disease progression including interstitial lung diseases, COPD and cystic fibrosis. Our data exemplify the long-term inflammatory nature of synergistic, U-Fe-Si-exposure, compared to another Si-based dust. Steenland and Goldsmith (1995) reported that exposure to Si as an environmental agent led to autoimmune diseases including systemic sclerosis, rheumatoid arthritis, lupus and chronic renal disease. Factory workers with exposure to occupational metals and Si exhibited symptoms of systemic illness comparable to systemic sclerosis, Sjogren’s syndrome, and systemic lupus erythematosus (Sanchez-Roman et al. 1993). Inhaled PM damages pulmonary endothelial cells resulting in macrophage recruitment, thereby contributing to the fibrotic milieu following toxicological insult.
Previous studies identified the blood brain barrier (BBB) as a susceptible endothelial target following aspirated and inhaled PM. The BBB prevents systemic soluble molecules and regulates uptake and efflux of these serum-borne factors into the parenchyma. Pro-inflammatory mechanisms responsible for disrupting the BBB include activation of MMP-9 and CD-36 (Aragon et al. 2016; 2017). Cerebrovascular endothelial cells may undergo apoptosis in the BBB, which leads to activation of astrocytes and microglia in adjacent brain tissue. Blood-brain barrier disruption through cytokine induction and peptidomic interaction with scavenger receptors in endothelial cells (ECs) results in increased leakage of peptides and molecules across the BBB, thereby inappropriately accessing the brain parenchyma (Mumaw et al. 2016, Aragon et al. 2017, Mostovenko et al. 2019). Nanoscale particulate matter from urban traffic was found to induce upregulation of Iba-1 and GFAP (Cheng et al. 2016). Particulate matter exposure was also associated with triggering a depressive-like response in wild-type mice following exposure (Liu et al. 2018). Bhatt et al (2015) noted that PM exposure was correlated with a rise in biomarkers of inflammation, including IL-1α, TNF-α and other molecules downstream of NF-kB.
Neuropsychiatric manifestations of autoimmunity are understudied and the contributions of environmental exposures to these neurological sequelae remain relatively unknown. For example, neuropsychiatric lupus (NPSLE) occurs more frequently in children than adults and is characterized by minor difficulties such as decline in cognitive function and difficulties thinking, to more severe health outcomes such as strokes and seizures. Neuropsychiatric lupus is a disease manifestation of systemic lupus erythematosous and occurs in approximately 20–40% of lupus patients (Kivity et al. 2015; Shaban and Leira 2019). Patients with NPSLE display symptoms including cognitive decline, acute confusion, psychosis and depression (Hanly 2014), and the pathophysiology driving these manifestations are poorly understood. Several investigators suggested that BBB permeability is altered in patients with SLE (Chi et al. 2019; Gulati et al. 2017). Magnetic resonance imaging (MRI) data revealed that patients with SLE exhibit significantly enhanced BBB permeability in the hippocampus compared to other regions of the brain, which may be one particular culprit of learning and memory deficits present in SLE patients (Shapira-Lichter et al. 2013). Much of the behavioral sequelae may be attributed to BBB dysfunction and neuroinflammation, as seen in human clinical cohorts and in animal models (Duarte-Delgado et al 2019; Hammer et al. 2014; Liu et al. 2020). Our data demonstrated significant elevation in brain inflammatory markers including IBA-1 and GFAP in both Claim 28 and St. Anthony exposure groups compared to DM. In addition, using a battery of behavioral tasks, a significantly diminished Y-maze new arm frequency following Claim 28 exposure was found accompanied by decreased novel object cumulative duration, frequency and Y-maze cumulative duration in both St. Anthony mine and Claim 28 exposure groups, although these impacts were not statistically significant. Y-maze entrance frequency, which utilizes the inherent motivation of an animal to explore an unknown environment, demonstrated impairment of working memory and cognition following the Claim 28 PM exposure (Cleal et al. 2020). Further delineation of basic biological mechanisms is warranted.
One striking finding from this preliminary experiment is that autoantibodies did not significantly vary between groups, although there was a trending reduction in the Claim 28 autoantigen production compared to controls (Figure 7). This may suggest that inflammation related to Claim 28 exposure night not be mediated by autoantibody production, but rather other serum-borne factors, within the time course of the experimental timeframe. Increased autonuclear antibody (ANA), anti-neutrophil cytoplasmic autoantibodies, and anti-citrullinated protein antibodies were noted in population-based, case-controlled studies following Si and metals exposure (Hogan et al. 2001; Zaghi et al. 2010). Similarly, the St. Anthony exposure group exhibited the most significant presence of both neutrophil elastase and numerical increase in autoantibodies, although this was not statistically significant compared to DM controls. Future studies may focus on mechanisms mediating this effect, especially in relation to U and Fe-bearing, metal-based PM.
Our study has several strengths and is the first to subject mice to repeated lower-dose metal-based PM (100 μg/aspiration), in an autoimmune model. Previously Bates et al (2015) instilled high levels of SiO2 (0.25 and 1 mg each week for 4 weeks.) to examine various physiological effects. It is noteworthy that our findings demonstrated that even lower-dose aspirations exhibit inflammatory exacerbations in older, lupus-prone NZBWF1/J mice. It is important to note that the one limitation of this study involved the usage of oropharyngeal aspiration, rather than inhalation for dosing. Although inhalation exposure is more physiologically and environmentally relevant than oropharyngeal aspiration, prior studies hnoted that both techniques result in similar pulmonary inflammation, airway gene expression and biological pathway alterations (Kinaret et al. 2017). Furthermore, this study is unique due to the head-to-head comparison of regionally-relevant dusts. These data may not only have relevance on nearby, at-risk communities, but also exert an impact to occupationally-exposed individuals who are subject to high levels of dust exposure such as ore miners, millers, stonemasons and others.
Conclusions
Particulate matter exposure, specifically Si-based PM, in conjunction with other metals such as U and Fe, may negatively affect pulmonary and serum-borne biomarkers of autoimmunity and systemic inflammation that may impact the brain, more so than other Si-based dusts. This is the first study to examine the impact of neuroinflammatory impacts of aspirated dust exposure in the NZBWF1/J mouse model. In addition, diminished learning and memory, as determined by the Y-maze behavioral task, was also noted. Further research is necessary to decipher the mechanism driving pulmonary and neuropsychiatric effects following environmental exposures to metal and Si-based dusts.
Supplementary Material
Supplemental Figure 2. IgM autoantibody array. Red suggests upregulation of autoantigens and green indicates decreased expression.
Supplemental Figure 1. Size distribution of aspirated particulates. Claim 28 (green), St. Anthony mine (purple)
Acknowledgements
The authors would like to thank Cathy Martinez and the UNM-Human Tissue Repository for their assistance with protocol development for IHC staining. The research in this paper was supported by the National Institutes of Health (NIH) under the following grant numbers: NIH/NCI 2P30CA118100, NIH/NIEHS K99 and R00 ES029104, R01 ES026673 and Superfund P42 ES025589.
Footnotes
Competing interests and disclosure statement
The authors declare no competing interests. The authors do not have any direct financial interests from this research.
Supplemental Information
Aqueous Chemical Extraction. To assess the acid extractable elements from the mineralized surface deposits from the Jackpile Mine samples, acid digestions were conducted on triplicates by adding 2 ml HNO3 and 6 mL HCl and 3 ml concentrated HF into 50 ml Teflon digestion tubes containing 2 ± 0.002 g of homogenized sample. All reagents used were ultra-high purity. The digestion tubes were then heated in a Digi prep MS SCP Science block digester at 95° C for 2 hr. Following heating, acid extracts were diluted to 50 ml with mega ohm waster and filtered through a 0.45 μm filter to remove suspended solids. Metal concentrations in solution were measured by ICP-OES and ICP-MS.
Data availability statement
All original data will be made available upon reasonable request.
Ethical Approval and Consent to participate
Studies were conducted with full approval by the Institutional Animal Care and Use Committees (IACUC) of the University of New Mexico.
References
- Afridi H, Kazi TG, Talpur FN, Naher S, and Brabazon D. 2014. Relationship between toxic metals exposure via cigarette smoking and rheumatoid arthritis. Clin Lab 60:1735–1745. [DOI] [PubMed] [Google Scholar]
- Appenzeller S 2016. Neuropsychiatric manifestations in autoimmune diseases. Rev Bras Reumatol Engl 56:189–190. [DOI] [PubMed] [Google Scholar]
- Aragon M, Erdely A, Bishop L, Salmen R, Weaver J, Liu J, Hall P, Eye T, Kodali V, Zeidler-Erdely P, Stafflinger JE, Ottens AK, and Campen MJ. 2016. MMP-9-dependent serum-borne bioactivity caused by multiwalled carbon nanotube exposure induces vascular dysfunction via the CD36 scavenger receptor. Toxicol Sci 150:488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragon MJ, Topper L, Tyler CR, Sanchez B, Zychowski K, Young T, Herbert G, Hall P, Erdely A, Eye T, Bishop L, Saunders SA, Muldoon PP, Ottens AK, and Campen MJ. 2017. Serum-borne bioactivity caused by pulmonary multiwalled carbon nanotubes induces neuroinflammation via blood-brain barrier impairment. Proc Natl Acad Sci USA 114: E1968–E1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad N, Sood A, Campen MJ, and Zychowski KE. 2018. Metal-Induced pulmonary fibrosis. Curr Environ Health Rep 5: 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasarala S. 2018. Physical and Chemical Interactions Affecting U and V Transport from Mine Wastes. Dissertation, Civil Eng., University of New Mexico. [Google Scholar]
- Avasarala S, Lichtner PC, Ali AM, González-Pinzón R, Blake JM, and Cerrato JM. 2017. Reactive transport of U and V from abandoned uranium mine wastes. Environ Sci Technol 51: 12385–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasarala S, Torres C, Ali AM, Thomson BM, Spilde MN, Peterson EJ, Artyushkova K, Dobrica E, Lezama-Pacheco JS, and Cerrato JM. 2019. Effect of bicarbonate and oxidizing conditions on U(IV) and U(VI) reactivity in mineralized deposits of New Mexico. Chem Geol 524: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, and Wishart DS. 2016. Heatmapper: Web-enabled heat mapping for all. Nucl Acids Res 44: W147–W153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya M, and Costenbader KH. 2016. Environmental exposures and the development of systemic lupus erythematosus. Curr Opin Rheumatol 28: 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MA, Akbari P, Gilley KN, Wagner JG, Li N, Kopec AK, Wierenga KA, Jackson-Humbles D, Brandenberger C, Holian A and Benninghoff AD, 2018. Dietary docosahexaenoic acid prevents silica-induced development of pulmonary ectopic germinal centers and glomerulonephritis in the lupus-prone NZBWF1 mouse. Front Immunol 9: 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates MA, Brandenberger C, Langohr I, Kumagai K, Harkema JR, Holian A, and Pestka JJ. 2015. Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-orone NZBWF1 mouse. PLoS One 10: e0125481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begay J, Sanchez B, Wheeler A, Baldwin F Jr., Lucas S, Herbert G, Ordonez Suarez Y, Shuey C, Klave Z, Harkema JR, and Wagner JG, 2021. Assessment of particulate matter toxicity and physicochemistry at the Claim 28 uranium mine site in Blue Gap, AZ. J Toxicol Environ Health, A 84: 31–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt DP, Puig KL, Gorr MW, Wold LE, and Combs CK, 2015. A pilot study to assess effects of long-term inhalation of airborne particulate matter on early Alzheimer-like changes in the mouse brain. PLoS One, 10: e0127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake JM, Avasarala S, Artyushkova K, Ali AM, Brearley AJ, Shuey C, Robinson WP, Nez C, Bill S, Lewis J. 2015. Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a Northeastern Arizona Native American community. Environ. Sci. Technol 49: 8506–8514. [DOI] [PubMed] [Google Scholar]
- Blake JM, De Vore CL, Avasarala S, Ali AM, Roldan C, Bowers F, Spilde MN, Artyushkova K, Kirk MF, Peterson E et al. 2017. Uranium mobility and accumulation along the Rio Paguate, Jackpile Mine in Laguna Pueblo, NM. Environ. Sci-Proc. Imp 19: 605–621. [DOI] [PubMed] [Google Scholar]
- Cawley KM, Hohner AK, Podgorski DC, Cooper WT, Korak JA, and Rosario-Ortiz FL. 2017. Molecular and spectroscopic characterization of water extractable organic matter from thermally altered soils reveal insight into disinfection byproduct precursors. Environ. Sci. Technol 51:771–779. [DOI] [PubMed] [Google Scholar]
- Chang JM, Lee YR, Hung LM, Liu SY, Kuo MT, Wen WC, and Chen P. 2011. An extract of Antrodia camphorata mycelia attenuates the progression of nephritis in systemic lupus erythematosus-prone NZB/W F1 mice. Evid Based Complement Altern Med 2011:465894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Saffari A, Sioutas C, Forman HJ, Morgan TE and Finch CE. 2016. Nanoscale particulate matter from urban traffic rapidly induces oxidative stress and inflammation in olfactory epithelium with concomitant effects on brain. Environ Health Perspect 124:1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng OZ, & Palaniyar N, 2013. NET balancing: A problem in inflammatory lung diseases. Front Immunol 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi JM, Mackay M, Hoang A, Cheng K, Aranow C, Ivanidze J, Volpe B, Diamond B, and Sanelli PC. 2019. Alterations in blood-brain barrier permeability in patients with systemic lupus erythematosus. Am J Neuroradiol 40: 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Attfield MD, Lubin JH, Neophytou AM, Blair A, Brown DM, Stewart PA, Vermeulen R, Eisen EA, and Silverman DT. 2018. Ischemic heart disease mortality and diesel exhaust and respirable dust exposure in the diesel exhaust in miners study. Am J Epidemiol 187: 2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal M, Fontana BD, Ranson DC, McBride SD, Swinny JD, Redhead ES and Parker MO, 2020. The free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behavior Res Meth : 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cung H, Aragon MJ, Zychowski KE, Anderson JR, Nawarskas J, Roldan C, Sood A, Qualls C, and Campen MJ. 2015. Characterization of a novel endothelial biosensor assay reveals increased cumulative serum inflammatory potential in stabilized coronary artery disease patients. J Transl Med 13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashner-Titus EJ, Hoover J, Li L, Lee JH, Du R, Liu KJ, Traber MG, Ho E, Lewis J, and Hudson LG. 2018. Metal exposure and oxidative stress markers in pregnant Navajo Birth Cohort Study participants. Free Radic Biol Med 124:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deditius AP, Utsunomiya S, Ewing RC 2008. The chemical stability of coffinite, USiO4 center dot nH(2)O; 0 b n b 2, associated with organic matter: a case study from Grants uranium region, New Mexico, USA. Chem. Geol 1–4: 33–49. [Google Scholar]
- Deshmane SL, Kremlev S, Amini S and Sawaya BE, 2009. Monocyte chemoattractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Delgado NP, Vásquez G, and Ortiz-Reyes BL. 2019. Blood-brain barrier disruption and neuroinflammation as pathophysiological mechanisms of the diffuse manifestations of neuropsychiatric systemic lupus erythematosus. Autoimmun Rev 18: 426–432. [DOI] [PubMed] [Google Scholar]
- El-Eshmawy MM, El-Adawy EH, Mousa AA, Zeidan AE, El-Baiomy AA, Abdel-Samie ER, and Saleh OM. 2011. Elevated serum neutrophil elastase is related to prehypertension and airflow limitation in obese women. BMC Womens Health 11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei E, Shuey C, Pacheco B, Cajero M, Lewis J, and Rubin RL. 2019. Elevated autoimmunity in residents living near abandoned uranium mine sites on the Navajo Nation. J Autoimmun 99: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B, and Hubbard A. 2003. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34:1507–1516. [DOI] [PubMed] [Google Scholar]
- Gawda A, Majka G, Nowak B, and Marcinkiewicz J. 2017. Air pollution, oxidative stress, and exacerbation of autoimmune diseases. Cent Eur J Immunol 42: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Salazar R, Wilson T, Lucas S, Herbert G, Young T, Begay J, Denson JL, Zychowski K, Ashley R and Byrum S, 2020. Early gestational exposure to inhaled ozone impairs maternal uterine artery and cardiac function. Toxicol Sci In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati G, Jones JT, Lee G, Altaye M, Beebe DW, Meyers-Eaton J, Wiley K, Brunner HI, and DiFrancesco MW. 2017. Altered blood-brain barrier permeability in patients with systemic lupus erythematosus: A novel imaging approach. Arthritis Care Res 69: 299–305. [DOI] [PubMed] [Google Scholar]
- Gupta S and Kaplan MJ. 2016. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 12: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Sirén AL, Pardo LA, Sperling S, Mohd Jofrry S, Gurvich A, Jensen N, Ostmeier K, Lühder F, Probst C, Martens H, Gillis M, Saher G, Assogna F, Spalletta G, Stöcker W, Schulz TF, Nave KA, and Ehrenreich H. 2014. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiat 19:1143–1149. [DOI] [PubMed] [Google Scholar]
- Hanly JG 2014. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol 10: 338–347. [DOI] [PubMed] [Google Scholar]
- Hansley P, and Spirakis C. 1992. Organic matter diagenesis as the key to a unifying theory for the genesis of tabular uranium-vanadium deposits in the Morrison Formation, Colorado Plateau. Econ. Geol 87: 352–365. [Google Scholar]
- Harlow E, and Lane D. 2006. Bradford assay. CSH Protoc 2006. [DOI] [PubMed] [Google Scholar]
- Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Pacheco B, Erdei E, Ramone S, Nez T, Campen MJ, and Gonzales M. 2018. Arsenic association with circulating oxidized low-density lipoprotein in a Native American community. J Toxicol Environ Health A 81: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon ME, Lewis J, Miller C, Hoover J, Ali AS, Shuey C, Cajero M, Lucas S, Zychowski K, Pacheco B, Erdei E, Ramone S, Nez T, Gonzales M, and Campen MJ. 2017. Residential proximity to abandoned uranium mines and serum inflammatory potential in chronically exposed Navajo communities. J Expo Sci Environ Epidemiol 27: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan SL, Satterly KK, Dooley MA, Nachman PH, Jennette JC, and Falk RJ. 2001. Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol 12:134–142. [DOI] [PubMed] [Google Scholar]
- Hoover J, Gonzales M, Shuey C, Barney Y, and Lewis J. 2017. Elevated arsenic and uranium concentrations in unregulated water sources on the Navajo Nation, USA. Expo Health 9: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z and Zhu L, 2016. Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulmon Pharmacol Therap 37: 1–8. [DOI] [PubMed] [Google Scholar]
- Kinaret P, Ilves M, Fortino V, Rydman E, Karisola P, Lahde A, Koivisto J, Jokiniemi J, Wolff H, Savolainen K and Greco D, 2017. Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano, 11: 291–303. [DOI] [PubMed] [Google Scholar]
- Kivity S, Agmon-Levin N, Zandman-Goddard G, Chapman J, and Shoenfeld Y. 2015. Neuropsychiatric lupus: A mosaic of clinical presentations. BMC Med 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepri G, Rigante D, Bellando Randone S, Meini A, Ferrari A, Tarantino G, Cunningham MW, and Falcini F. 2019. Clinical-serological characterization and treatment outcome of a large cohort of Italian children with pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection and pediatric acute neuropsychiatric syndrome. J Child Adolesc Psychopharmacol 29: 608–614. [DOI] [PubMed] [Google Scholar]
- Leventhal JS. 1980. Organic geochemistry and uranium in grants mineral belt. New Mexico Bureau Mines Mineral Resources Memoir 38:10. [Google Scholar]
- Leventhal JS, Daws TA, and James S. Frye. 1986. Organic geochemical analysis of sedimentary organic matter associated with uranium. Appl. Geochem. 1: 241–247. [Google Scholar]
- Lewis J, Hoover J, and MacKenzie D. 2017. Mining and environmental health disparities in Native American Communities. Curr Environ Health Rep 4:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Qian X, Xing J, Wang J, Sun Y, Wang QG, and Li H (2018). Particulate matter triggers depressive-like response associated with modulation of inflammatory cytokine homeostasis and brain-derived neurotrophic factor signaling pathway in mice. Toxicol Sci 164: 278–288. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ma Y, Du B, Wang Y, Yang GY, and Bi X. 2020. Mesenchymal stem cells attenuated blood-brain barrier disruption via downregulation of aquaporin-4 expression in EAE mice. Mol Neurobiol 57: 3891–3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, and Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lynall M 2018. Neuropsychiatric symptoms in lupus. Lupus 27 (1_Suppl): 18–20. [DOI] [PubMed] [Google Scholar]
- Mayeux JM, Escalante GM, Christy JM, Pawar RD, Kono DH, and Pollard KM. 2018. Silicosis and silica-induced autoimmunity in the diversity outbred mouse. Front Immunol 9: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormic ZD, Khuder SS, Aryal BK, Ames AL, and Khuder SA. 2010. Occupational silica exposure as a risk factor for scleroderma: a meta-analysis. Int Arch Occup Environ Health 83: 763–769. [DOI] [PubMed] [Google Scholar]
- Mostovenko E, Young T, Muldoon PP, Bishop L, Canal CG, Vucetic A, Zeidler-Erdely PC, Erdely A, Campen MJ, and Ottens AK. 2019. Nanoparticle exposure driven circulating bioactive peptidome causes systemic inflammation and vascular dysfunction. Part Fibre Toxicol 16: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumaw CL, Levesque S, McGraw C, Robertson S, Lucas S, E Stafflinger J, and Block ML. 2016. Microglial priming through the lung—brain axis: The role of air pollution-induced circulating factors. FASEB J 30: 1880–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D, Mathew A, James B, and Hutchinson D. 2016. Could the inhalation of cadmium and other metals in addition to textile dust inhalation account for the observed increased risk of rheumatoid arthritis in textile workers? Ann Rheum Dis 75: e30. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Hirose S, Yagita H, and Okumura K (1997). Roles of IL-4 and IL-12 in the development of lupus in NZB/W F1 mice. J Immunol 158: 1466–1472. [PubMed] [Google Scholar]
- Papayannopoulos V, and Zychlinsky A 2009. NETs: A new strategy for using old weapons. Trends Immunol 30: 513–521. [DOI] [PubMed] [Google Scholar]
- Parks CG, de Souza Espindola Santos A, Barbhaiya M, and Costenbader KH. 2017. Understanding the role of environmental factors in the development of systemic lupus erythematosus. Best Pract Res Clin Rheumatol 31: 306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard KM 2016. Silica, silicosis, and autoimmunity. Front Immunol 7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto BN, and Stein RT 2016. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Front Immunol 7: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasinghe LD, Li QZ, Zhu C, Yan M, Chauhan PS, Wierenga KA, Bates MA, Harkema JR, Benninghoff AD, and Pestka JJ. 2020. Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice. Autoimmunity: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, & Eliceiri KW, (2017). ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz OF, Thomson BM and Cerrato JM 2016. Investigation of in situ leach (ISL) mining of uranium in New Mexico and post-mining reclamation. Paper read at New Mexico Geology. [Google Scholar]
- Rusibamayila M, Meshi E, and Mamuya S. 2018. Respiratory impairment and personal respirable dust exposure among the underground and open cast gold miners in Tanzania. Ann Glob Health 84:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šakić B, Szechtman H, Denburg S, Carbotte R, and Denburg JA (1993). Spatial learning during the course of autoimmune disease in MRL mice. Behav Brain Res 54: 57–66. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Zhou X, Gardiner AS, Herbert G, Lucas S, Morishita M, Wagner JG, Lewandowski R, Harkema JR, Shuey C et al. 2020. Serum-borne factors alter cerebrovascular endothelial microRNA expression following particulate matter exposure near an abandoned uranium mine on the Navajo Nation. Part Fibre Toxicol 17:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roman J, Wichmann I, Salaberri J, Varela JM, and Nuñez-Roldan A. 1993. Multiple clinical and biological autoimmune manifestations in 50 workers after occupational exposure to silica. Ann Rheum Dis 52:534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell MK, Sennett C, Laws RL, Rubin RL, Brooks DR, Amador JJ, López-Pilarte D, Ramirez-Rubio O, Friedman DJ, McClean MD 2020. Urinary metals concentrations and biomarkers of autoimmunity among Navajo and Nicaraguan men. Int J Environ Res Public Health 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban A, and Leira EC. 2019. Neurological complications in patients with systemic lupus erythematosus. Curr Neurol Neurosci Rep 19: 97. [DOI] [PubMed] [Google Scholar]
- Shapira-Lichter I, Vakil E, Litinsky I, Oren N, Glikmann-Johnston Y, Caspi D, Hendler T, and Paran D. 2013. Learning and memory-related brain activity dynamics are altered in systemic lupus erythematosus: A functional magnetic resonance imaging study. Lupus 22: 562–573. [DOI] [PubMed] [Google Scholar]
- Sigaux J, Biton J, André E, Semerano L, and Boissier MC. 2019. Air pollution as a determinant of rheumatoid arthritis. Joint Bone Spine 86: 37–42. [DOI] [PubMed] [Google Scholar]
- Steenland K, and Goldsmith DF. 1995. Silica exposure and autoimmune diseases. Am J Ind Med 28: 603–608. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Zychowski KE, Sanchez BN, Rivero V, Lucas S, Herbert G, Liu J, Irshad H, McDonald JD, Bleske BE et al. 2016. Surface area-dependence of gas-particle interactions influences pulmonary and neuroinflammatory outcomes. Part Fibre Toxicol 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urowitz MB, Gladman DD, Tom BD, Ibanez D, & Farewell VT, (2008). Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 35: 2152–2158. [DOI] [PubMed] [Google Scholar]
- Velasco CA,., Artyushkova K, Ali AS, Osburn CL, Gonzalez-Estrella J, Lezama-Pacheco JS, Cabaniss SE, and Cerrato JM. 2019. Organic functional group chemistry in mineralized deposits containing U(IV) and U(VI) from the Jackpile Mine in New Mexico. Environ Sci Technol 53: 5758–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihlborg P, Bryngelsson IL, Andersson L, and Graff P. 2017. Risk of sarcoidosis and seropositive rheumatoid arthritis from occupational silica exposure in Swedish iron foundries: a retrospective cohort study. BMJ Open 7: e016839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XC, Feng J, Huang F, Fan YS, Wang YY, Cao LY, and Wen CP. 2009. Effects of shikonin isolated from zicao on lupus nephritis in NZB/W F1 mice. Biol Pharm Bull 32: 1565–70. [DOI] [PubMed] [Google Scholar]
- Yahya A, Bengtsson C, Larsson P, Too CL, Mustafa AN, Abdullah NA, Muhamad NA, Klareskog L, Murad S, and Alfredsson L. 2014. Silica exposure is associated with an increased risk of developing ACPA-positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case-control study. Mod Rheumatol 24:271–274. [DOI] [PubMed] [Google Scholar]
- Zaghi G, Koga F, Nisihara RM, Skare TL, Handar A, Rosa Utiyama SR, and Silva MB. 2010. Autoantibodies in silicosis patients and in silica-exposed individuals. Rheumatol Int 30: 1071–1075. [DOI] [PubMed] [Google Scholar]
- Zhao CN, Xu Z, Wu GC, Mao YM, Liu LN, Qian Wu, Dan YL, Tao SS, Zhang Q, Sam NB et al. 2019. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev 18: 607–614. [DOI] [PubMed] [Google Scholar]
- Zychowski KE, Tyler CRS, Sanchez B, Harmon M, Liu J, Irshad H, McDonald JD, Bleske BE, and Campen MJ, 2019. Vehicular particulate matter (PM) characteristics impact vascular outcomes following inhalation. Cardiovasc Toxicol : 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychowski KE, Kodali V, Harmon M, Tyler CR, Sanchez B, Ordonez Suarez Y, Herbert G, Wheeler A, Avasarala S, Cerrato JM et al. 2018. Respirable uranyl-vanadate-containing particulate matter derived from a legacy uranium mine site exhibits potentiated cardiopulmonary toxicity. Toxicol Sci 164:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2. IgM autoantibody array. Red suggests upregulation of autoantigens and green indicates decreased expression.
Supplemental Figure 1. Size distribution of aspirated particulates. Claim 28 (green), St. Anthony mine (purple)