Abstract
A new pandemic is ongoing in several parts of the world. The agent responsible is the newly emerged severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The symptoms associated with this virus are known as the coronavirus disease-2019 (COVID-19). In this review, we summarize the published data on virus specific antibodies in hospitalized patients with COVID-19 disease, patients recovered from the disease and the individuals who are asymptomatic with SARS-CoV-2 infections. The review highlights the following: i) an adjunct role of antibody tests in the diagnosis of COVID-19 in combination with RT-PCR; ii) status of antibodies from COVID-19 convalescent patients to select donors for plasma therapy; iii) the potential confounding effects of other coronaviruses, measles, mumps and rubella in antibody testing due to homology of certain viral genes; and iv) the role of antibody testing for conducting surveillance in populations, incidence estimation, contact tracing and epidemiologic studies.
Keywords: Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), Diagnosis of COVID-19, Antibody tests, RT-PCR
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2); Diagnosis of COVID-19; Antibody tests; RT-PCR.
1. Introduction
The emergence of a new virus, initially known as 2019-novel coronavirus (2019-nCoV), was reported in Wuhan, China in December 2019 in patients with atypical pneumonia. This outbreak spread to other cities in China as well as other countries. The virus was renamed as severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2) and the disease caused by this virus is currently known as coronavirus disease-2019 (COVID-19) (Li et al., 2020c; Zhu et al., 2020). The virus infections in individuals lead to a range of clinical manifestations including asymptomatic, fever, nasal congestion, runny nose, cough, expectoration, chest tightness, abdominal distension, acute respiratory distress symptoms, diarrhea, pneumonia and fatality in a fraction of cases (Chen et al., 2020a; Guan et al., 2020; Holshue et al., 2020; Huang et al., 2020c; Wang et al., 2020a). The virus was first isolated from the bronchoalveolar lavage fluid (Zhu et al., 2020). SARS-CoV-2 is the latest addition to the coronavirus family known to infect humans. Of this group, four members (229E, NL63, OC43 and HKU1) cause only mild symptoms in the infected individuals. SARS-CoV was the cause of an outbreak in 2002–2003 (Drosten et al., 2003; Zhong et al., 2003) involving a total of 8096 confirmed cases and 774 deaths spanning 32 countries with a mortality rate of approximately 10% (Drosten et al., 2003). Middle east respiratory syndrome coronavirus (MERS-CoV) was associated with an outbreak in Saudi Arabia in 2012. The cases and deaths due to this virus were 2494 and 858, respectively with a high fatality rate of approximately 33% (Zaki et al., 2012).
SARS-CoV-2 has led to significant infections involving more than 200 countries around the world. This is a highly contagious pathogen and the reproductive number or R nought (RO) supports this view (Zhao et al., 2020a). This has led to the declaration of a pandemic by WHO on March 11, 2020. As of April, 15, 2021, there are 140,322,903 cases and 3,003,794 deaths, respectively at the Global level. The therapeutic options were non-existent or limited during the early stage of epidemic for reducing deaths and duration of hospitalizations. This situation has been improved as we learnt more about the disease and the symptoms. Specifically, studies showed that remdesivir, steroids, plasma and monoclonal antibody therapies provide relief to patients with advanced COVID-19.
The lack of availability of vaccines during the early days of pandemic and limited drug options scenario turned healthcare officials’ attention to rely more on measures such as wearing a mask to limit the spread of the virus. This has been shown to prevent the transmission of virus through droplets and aerosol from the infected individuals. In addition, testing for virus in COVID-19 patients and suspected individuals, contact tracing, quarantine, social distancing and personal protective equipment have resulted in a reduced number of infections. The characteristics of SARS-CoV-2 infection include a median incubation period of around 5–10 days for the development of disease related symptoms from the time of exposure to the virus. Hence, it is important to develop tests that have the ability to detect the virus during the acute phase of infection. For this reason, several molecular tests based on viral nucleic acid detection have been developed. These include real time RT-PCR and others (Azzi et al., 2020; Broughton et al., 2020; Huang et al., 2020c; Li et al., 2020c; Lu et al., 2020; Moran et al., 2020; Smithgall et al., 2020b; Xiao et al., 2020). Nucleic acid tests (NATs) are considered the gold standard for the diagnosis of COVID-19. The molecular tests (such as Cobas and others) show sufficient specificity and sensitivity to detect SARS-CoV-2 in infected individuals using specimens including throat swabs, nasopharyngeal swabs, bronchoalveolar lavage, sputum and saliva. These tests require a laboratory infrastructure or depend on mobile laboratory to perform the tests. Recently approved tests such as Xpert Xpress SARS-CoV-2 (Cepheid) and ID Now (Abbott) can be carried out within 45 min and 13 min, respectively in comparison to 3.5 h for Cobas test (Basu et al., 2020; Collier et al., 2020; Smithgall et al., 2020b). In addition to nucleic acid tests, viral antigen assays have also been developed for detecting the virus (La Marca et al., 2020; Mak et al., 2020; Smithgall et al., 2020a). These tests have the potential to be carried out both in clinical laboratories and also in point-of-care settings. While these assays are easy to perform (immunochromatography tests), the sensitivity depends on monoclonal/polyclonal antibody combinations employed in different tests. The antibodies, induced in response to SARS-CoV-2 infection and present in the sera/plasma/saliva, serve as analytes for the serological tests. The serological tests share the flexibilities as viral antigen assays. The antibodies have been reported to appear in the sera/plasma within the first week or second week following the onset of symptoms in patients and persist for up to 3–6 months (Grzelak et al., 2020; Long et al., 2020b; Prevost et al., 2020; Zhou et al., 2020). Hence, the tests will identify ongoing and past infections with SARS-CoV-2. In addition, serological tests can serve as an adjunct test for RT-PCR as there are reports that PCR negative cases were detected by serological tests (Guo et al., 2020; Mlcochova et al., 2020; To et al., 2020; Xu et al., 2020; Zhang et al., 2020a; Zhao et al., 2020b).
In this review, our aim is to summarize the virus specific antibody status in response to SARS-CoV-2 infections in hospitalized patients with COVID-19, patients recovered from disease and infected individuals who are asymptomatic. The presence of antiviral IgM, IgA and IgG antibodies has been demonstrated by a number of technologies including ELISA, immunochromatography, ELISpot, Cell-ELISA, Chemiluminescence and luciferase immunoprecipitation methods. The functional aspects of antibodies were also evaluated by virus neutralization assays. The information on the kinetics of antibody responses and their persistence over time in the patient groups provide opportunities in the diagnostic and therapeutic arenas. Further, antibody assays have been and will continue to be useful for identifying convalescing patients as donors for plasma therapy and for conducting surveillance of SARS-CoV-2 infections in the population.
2. SARS-CoV-2: virus and genome organization
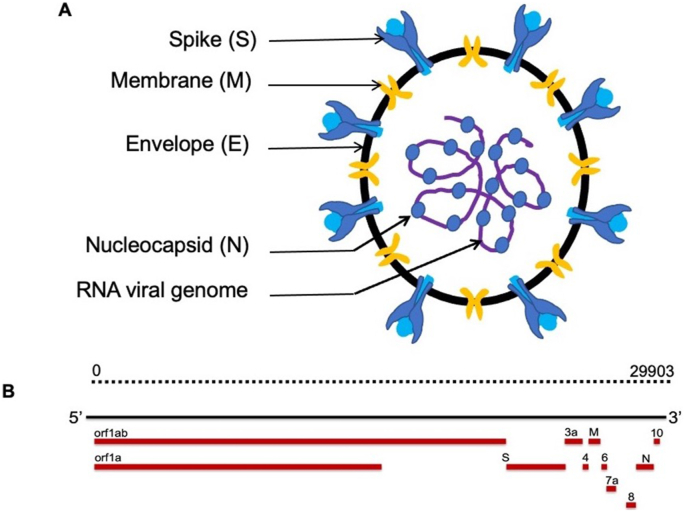
The schematic representation of SARS-CoV-2 virus is shown in Figure 1A. The virus particle has spherical or oval structure and the proteins present on the surface of virion confer unique appearance as noted with other coronaviruses (Jin et al., 2020). There are four proteins contributing to the structure of the particle. These include spike glycoprotein (S), Envelope (E), Membrane protein (M) and Nucleocapsid (N) protein. The viral RNA genome is complexed with N protein which is present in the core surrounded by membrane in which the glycoproteins (S, E and M) are anchored. The size of virus particles, in images through transmission electron microscopy, ranges from 60 to 140 nm in diameter with protrusions of S protein on the surface in the range of 8–12 nm (Jin et al., 2020; Zhu et al., 2020). There is no information available regarding the stoichiometry of the structural proteins present in the virus particles.
Figure 1.
A, Cross-sectional view of SARS-CoV-2 virus particle; B, Schematic representation of SARS-CoV-2 viral genome.
SARS-CoV-2 viral RNA genome is similar to that of other coronaviruses as shown in Figure 1B and the genome size is approximately 30 Kb (Ciotti et al., 2019). The complete sequence of the viral genome, generated through next generation sequencing (NGS), was released on January 10, 2020 (Jin et al., 2020; Zheng et al., 2020; Zhong et al., 2003). Since then thousands of sequences of viral genomes have been acquired and published (Chen and Li, 2020; Forster et al., 2020; Lu et al., 2020; Saha et al., 2020; Stefanelli et al., 2020; Yadav et al., 2020; Zhou and Zhao, 2020). The investigators from University College London reported on the analysis of 7500 SARS-CoV-2 genomes and noted 198 mutations that appear to have independently occurred more than once (van Dorp et al., 2020). Mercatelli and Giorgi (2020) carried out an analysis of 48,635 SARS-CoV-2 complete genomes keeping Wuhan genome (NC-045512.2) as a reference (Mercatelli and Giorgi, 2020). The mutations noted predominantly are of single nucleotide polymorphisms (SNPs) and short insertion/deletion events (indels). The latter includes frameshift and in-frame deletions. Recently, Korber and colleagues from Los Alamos National Laboratory showed a specific mutation in Spike protein changing from D614 to G (Korber et al., 2020a). The virus containing spike D614G variant was found to be more infectious in several cell types in comparison to ancestral form (Korber et al., 2020b; Li et al., 2020b; Shi et al., 2020; Yurkovetskiy et al., 2020). The structural analysis of this variant showed that the conformation is shifted toward ACE2 binding competent state (Yurkovetskiy et al., 2020). The variant virus results in high virus titers in the upper respiratory tracts of COVID-19 patients. There is also a report associating the variant to a higher fatality rate (Becerra-Flores and Cardozo, 2020). However, this needs to be verified in other cohorts of patients. Several variants have also been recently reported which include the U.K. B.1.1.7, Brazil P.1 and South African B.1.351 (Burki, 2021). Further, sequence analysis also showed that SARS-CoV-2 genome exhibits 82% similarity to SARS-CoV and 56% to MERS-CoV (Chan et al., 2020; Lu et al., 2020). In addition to D614G, notable mutations affecting change in amino acids are in non-structural protein12 (NSP12), N, ORF3a, ORF8, NSP2, NSP6 and NSP13 proteins (Mercatelli and Giorgi, 2020).
3. Diagnostic platforms used for the detection of SARS-CoV-2 infection
3.1. Nucleic acid tests
The nucleic acid test is dependent on the amplification methods utilizing nasopharyngeal or oropharyngeal swabs collected from individuals in addition to other specimens including sputum and saliva. As the genome of SARS-CoV-2 is a positive-sense single-stranded RNA, the method utilizes reverse transcriptase enzyme to transcribe viral RNA to DNA followed by PCR amplification of the DNA. A positive test result in this method indicates the presence of viral RNA due to viral infection and replication in the individuals. The primers used for amplification of viral target genes include ORF 1a/b, E, RdRp and N. There are several versions of the tests available (Azzi et al., 2020; Huang et al., 2020c; Lee et al., 2020; Liu et al., 2020c; Smithgall et al., 2020b) (Moran et al., 2020; Xiao et al., 2020). The time required for the completion of assays range from 3.5 h to 5–10 min (Basu et al., 2020; Collier et al., 2020; Smithgall et al., 2020b). Further, assays using alternate methods such as loop-mediated and helicase dependent amplification are also available in the market. The availability of tests based on near patient platforms is likely to accelerate speed and volume of testing capacity.
3.2. Viral antigen tests
The available ELISA and lateral flow assay kits are designed to detect N, S and RBD of S proteins (La Marca et al., 2020; Mak et al., 2020). The specimens used for this test include nasal and throat swabs and saliva. A positive result by this test indicates the presence of viral protein due to virus infection and replication in the individual. These tests utilize monoclonal antibodies or a combination of monoclonal and polyclonal antibodies with reactivities against viral proteins. Specifically, the assays capture S and N proteins present in the virus particles. While ELISA may require 2–3 h for completion, lateral flow assays can be completed within 15 min.
3.3. Serologic tests
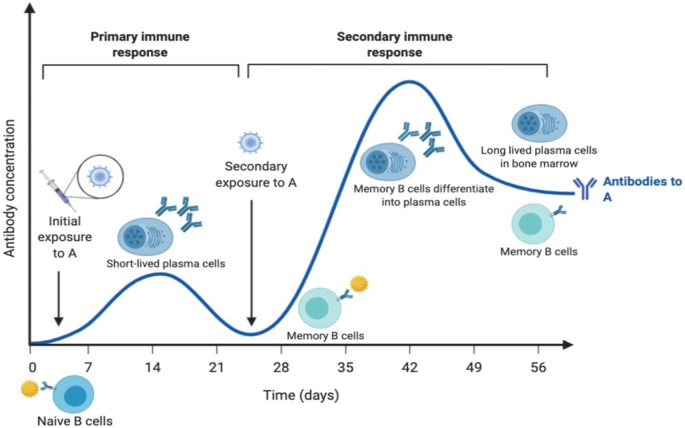
As noted with other infectious agents, SARS-CoV-2 infection leads to eliciting innate and adaptive immune responses in the host. Regarding the latter, antigen presenting cells (APC) present antigen to the CD4 T helper cells (Th1) and also secrete IL-12 for further stimulation of Th1 cells (Rabi et al., 2020). This results in priming of CD8+ T cells that can target the cells harboring foreign antigens for elimination and also the stimulation of B cells. Antigen-antibody interactions at the appropriate B cell surfaces drive virus-specific cell proliferation, antibody isotype switching (through class switch recombination), cell maturation and cell residence in systemic and mucosal sites (Hurwitz, 2020). Upon engagement with the pathogen/vaccine antigens, naïve B cells will be primed. Activated B cells internalize the antigens for processing and present MHC Class II restricted peptides to follicular T helper cells (Tfh cells). Tfh cells provide stimulatory signals to the primed B cells in the form of ligands or soluble cytokines, resulting in proliferation of the B cells in the dark zone of germinal centers (Crotty, 2014). Proliferating B cells also undergo somatic hypermutation and class switching to enable more potent binding of their BCRs to the antigen, as those B cells with lower affinity to the antigens will not survive. Interaction of the Germinal Center (GC) B cells with Tfh cells can also promote differentiation of the GC B cells into short-lived plasma cells and long-lived memory B cells (Akkaya et al., 2020). Short-lived plasma cells reside in lymphoid organs and are responsible for secretion of antibodies into the systemic circulation (Khodadadi et al., 2019). However, as the name implies, short-lived plasma cells have relatively short lifespan, and the death of this population ultimately results in waning antibody titers, a process known as the primary antibody response (Figure 2). Memory B cells, however, have long lifespan and primarily reside in periphery of the body where there is a high likelihood of encountering pathogens, such as the palatine tonsils and Peyer's patches (Jones et al., 2016). Upon re-engagement with the antigens (frequently the pathogens), they can undergo rapid proliferation and differentiation into antibody secreting plasma cells in a process known as the secondary antibody response or memory B cell response. Memory B cell responses also result in the formation of long-lived plasma cells, which frequently reside in the bone marrow and constantly secrete antibodies to maintain systemic antibody titers against the specific pathogens (Brynjolfsson et al., 2018). This has been shown in the case of SARS-CoV where innate and adaptive immune arms came into play upon infection (Azkur et al., 2020; Zhong et al., 2003). The analysis of survivors and non-survivors revealed that survivors mounted adaptive immune response in the form of antibodies in comparison to non-survivors (Ahmed et al., 2020a). The studies published on SARS-CoV-2 also indicate a similar scenario (Azkur et al., 2020).
Figure 2.
Overview of the primary and secondary immune responses.
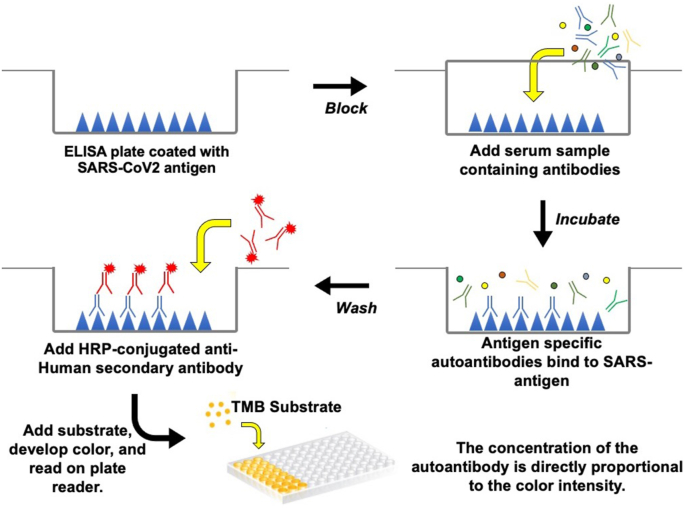
The antibody testing assays are designed to evaluate the humoral immune responses elicited in patients in response to SARS-CoV-2 infections. Specifically, the presence of antibodies in the sera/plasma/saliva of patients is determined using ELISA (Figure 3) and lateral flow immunochromatography assays (Figure 4). Chemiluminescence immunoassays (CLIA) are also being used to detect antibodies in both ELISA and rapid assay formats. The use of luminescent chemical as a substrate enhances the sensitivity in comparison to the chromogen based ELISA. In addition, methods such as ELISPOT and cell-ELISA are used to quantify antibody-secreting cells from peripheral blood mononuclear cells (Boonyaratanakornkit and Taylor, 2019; Zarletti et al., 2020). A positive result in the assay indicates that the individual was exposed to SARS-CoV-2. The antibodies detected belong to three different classes including IgG, IgM and IgA. The antibodies serve to provide defense against the virus through mechanisms including neutralization of virus and ADCC in mucosal surfaces, tissues and blood. While the antibody assays may not be useful in the diagnosis of SARS-CoV-2 infections, they can be used in combination with RT-PCR assays in specific cases. The advantages with these assays are quick turnaround and visual interpretation of the results in case of lateral flow assays. Further they are useful for estimating the infection rates in the population.
Figure 3.
SARS-CoV-2 antibody detection by ELISA.
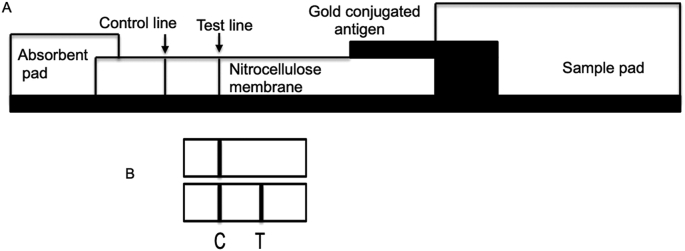
Figure 4.
(A) Schematic representation of the test strip for ICGA. (B). A typical test result of an ICGA. Top panel, control human serum; Bottom panel, human serum positive for antibodies for the virus. C, control; T, test line.
4. SARS-CoV-2 viral proteins
4.1. Spike (S) protein
S codes for a protein of 1273 amino acids (aa) with respect to Wuhan-Hu1 strain. The features of the protein are the following: Signal peptide, 1–12 aa; Ectodomain, 13–1213 aa; Transmembrane domain, 1214–1234 aa; Cytoplasmic domain, 1235–1273 aa. S is a type I membrane protein and contains S1 (13–685 aa) and S2 (686–1273 aa) subunits which carry out binding to ACE2 receptor and fusion of viral and cell membrane, respectively (Hoffmann et al., 2020; Ou et al., 2020; Walls et al., 2020; Wang et al., 2020a). These characteristics make S protein as a target for host immune response and also a candidate for vaccine and therapeutic interventions. S protein is present on the surface of virus particles and forms homomeric trimers (Walls et al., 2020). The homology of SARS-CoV-2 S protein to SARS-CoV is 76% and is much less (35%) to MERS-CoV (Ahmed et al., 2020a; Grifoni et al., 2020). Zheng and Song showed through bioinformatic analysis that the non-conserved regions of SARS-CoV-2 has higher antibody epitope score (Zheng and Song, 2020). There are several forms of S protein generated as substrates to measure the antibodies present using the whole blood, finger-stick blood and sera/plasma/saliva from COVID-19 patients. These include full length S protein (1–1213 aa) without the transmembrane and cytoplasmic tail, S1 subunit of S, N-terminal of S (1–294 aa) and RBD (329–538 aa). S protein and truncated variants are generally expressed using insect and mammalian cells through eukaryotic expression vectors. Considering the trimeric feature of spike protein on the virus particles, Krammer and colleagues generated ectodomain of S and S1 subunit with the addition of a trimerization domain at the c-terminus (Stadlbauer et al., 2020). The predominant S variant containing D614G mutation has also been included in the antibody evaluation (Yurkovetskiy et al., 2020).
4.2. N protein
N codes for a protein of 419 amino acids and is abundantly expressed in virus infected cells. The RNA binding domain (41–186 aa) and dimerization domain (258–361 aa) are key features. N is phosphorylated at residue 176 and present in the virus particles in association with viral RNA through specific interaction with M protein. This is a conserved protein among coronaviruses as it shows 90% and 48% homology to SARS-CoV and MERS-CoV, respectively (Ahmed et al., 2020a; Grifoni et al., 2020; Jaimes et al., 2020; Zhang and Holmes, 2020). The assays screening for antibodies against this protein utilize N expressed and purified using bacterial expression system (Azkur et al., 2020; La Marca et al., 2020).
4.3. E protein
E codes for a protein of 75 amino acids, a membrane protein and is involved in virus morphogenesis and assembly. The features of this protein include a surface domain (1–16 residues), transmembrane domain (17–37 aa) and an intravirion domain (38–75 aa). This is a conserved protein among coronaviruses and exhibits a homology of 94% and 36% to SARS-CoV and MERS-CoV E proteins, respectively. In our survey, we noted that one study utilized E protein in the evaluation of immune responses in patients (Zhang and Holmes, 2020).
4.4. M protein
M is an abundant membrane protein present in the virus particles. This protein has been shown to have a pleiotropic role in functions including virus assembly and morophogenesis, regulation of replication and packages viral RNA into the virions. M ORF codes for a protein of 222 amino acids with molecular weight in the range of 25 kDa. M contains three transmembrane domains and the surface exposed residues correspond to 2–19 aa and 72–79 aa. The intravirion residues correspond to 101–222 aa (Korber et al., 2020b; Zhang et al., 2020a).
5. Synthetic peptides as substrates for evaluation of antibody responses
Peptides representing ORF1a/b, N and S proteins have been used as substrates in a chemiluminescent immunoassay (Cai et al., 2020; Long et al., 2020a; Yu et al., 2020). Peptide library covering the entire S protein was utilized for the screening and resulted in the identification of two highly reactive peptides (TESNKKFLPFQQFGRDIA and PSKPSKRSFIEDLLFNKV) overlapping RBD and fusion regions, respectively (Poh et al., 2020).
6. Serological assay platforms
6.1. ELISA
ELISA is the work horse of the diagnostic laboratories. This method has been utilized by several investigators for detecting antibodies against SARS-CoV-2 (Amanat et al., 2020b). In addition to in-house developed tests, assays from commercial vendors were also used in a substantial number of studies. An advantage with this assay is that it enables the quantification of antibodies. The assays fall into two versions. In one version, antigen bound antibodies were detected by anti-human antibodies conjugated to HRP. In another version, antigen coated microtiter wells were incubated with sera and the bound antibodies were quantified by antigen conjugated with HRP. The latter version improves the specificity of the assay. The key steps involved in the assay are presented in Figure 3. Given the variation in terms of samples used for analysis (sera from patients and healthy controls) and cut-off values set in each case, it is difficult to compare the data between studies. The other assays include chemiluminescence immunoassays (CLIA) and fluorescence immunoassays (FIA) (Kontou et al., 2020). CLIA utilizes chemical probe (luminescent molecule) as a substrate for quantification of antigen-antibody complexes.
6.2. Lateral flow immunochromatography
The rapid test is based on the principles of immunochromatography (ICGA) to detect virus specific antibodies using gold conjugated antigen or antibodies as described (Koo et al., 2005). The test strip consists of four distinct regions designated as sample pad, conjugate pad, nitrocellulose membrane coated with the test antigen as a line and absorbent pad (Figure 4). All the pads will be overlapped to enable migration of samples along the test strip. For detecting the antibodies against the viral proteins, recombinant protein or anti-human antibody will be conjugated to colloidal gold (40 nm) for use as a detector reagent in the conjugate pad of the device. Both the detection reagent (gold particles) and sample migrate by capillary action. In addition, the recombinant protein will also be used to coat the formatted test line. In a typical ICGA, anti-SARS-CoV-2 antibody in the sample serum can be captured on an antigen-colloidal gold complex using N or S proteins. The complex moves through a sample pad and is then trapped at a test line band containing recombinant SARS-CoV-2 protein.
6.3. Luciferase immunoprecipitation system assay
This assay is based on an enzymatic reaction and has been used to detect antibodies in the human sera against pathogens and self-antigens (Burbelo et al., 2005; Haljasmagi et al., 2020). The assay utilizes a chimeric protein, in which the viral antigen is fused to luciferase enzyme coding sequences, as a substrate for capturing specific antibodies present in the sera. The luciferase activity in this assay is proportional to the amount of antibodies bound to the chimeric protein upon incubation with the sera. The advantage with this assay is that it does not require purified recombinant protein for detecting antibodies as other assays require. The cell lysate, collected after transfection of cells with the plasmid DNA encoding chimeric protein, is used directly for the assay. Body fluids from patients/individuals suspected of infection used for evaluation of antibodies against SARS-CoV-2 include serum, plasma, whole blood, finger-stick blood and saliva.
6.4. SARS-CoV-2 neutralization assays
The correlates of protective immunity for SARS-CoV-2 are not known. Studies published on SARS-CoV and MERS-CoV have implicated that humoral and cellular responses may contribute to this process (Ahmed et al., 2020b; Azkur et al., 2020). Hence, the evaluation of neutralizing antibodies is useful for reasons including plasma therapy. The serological tests are not designed to detect neutralizing antibodies. However, the reactivity of antibodies to spike protein correlate with the neutralizing titer (Suhandynata et al., 2020b). While plaque reduction neutralization test is the gold standard for evaluating neutralizing antibodies (Smithgall et al., 2020a), pseudotype virus assay is commonly used for this purpose. The SARS-CoV-2 pseudovirus is produced by co-transfection of HEK293T cells with 1:1 ratio of DNA plasmid encoding SARS-CoV-2 Spike protein and backbone plasmid pNL4-3.Luc.R−E-. The supernatant containing pseudovirus is harvested 48 h post-transfection and the pseudovirus is titrated using the stable commercially available CHO-ACE2 cell line. The neutralization assay is carried out in 96-well plates with 10,000 CHO-ACE2 cells. The samples (sera/plasma), upon serial dilution, are incubated with SARS-CoV-2 pseudovirus at room temperature for 90 min, before the mixture is added to the already plated CHO-ACE2 cells. The cells are incubated for 72 h, and subsequently harvested and lysed with BriteLite reagent (PerkinElmer, USA). Luminescence from the plates are recorded with a BioTek plate reader and used to compute percentage neutralization of the samples at each dilution.
7. Evaluation of antibodies against SARS-CoV-2 in the sera/plasma of COVID-19 patients
There are several reports on the serological studies of COVID-19 patients (Amanat et al., 2020a; Bloch et al., 2020; Chen et al., 2020a; Guan et al., 2020; Long et al., 2020a; Ni et al., 2020; Stringhini et al., 2020; Walls et al., 2020; Weiss and Murdoch, 2020; Wolfel et al., 2020). Globally, there are hundreds of companies that offer tests for virus specific antibody detection. However, these tests vary in their sensitivity and specificity for the detection of virus specific antibodies. The results from studies using these multitude of tests are compounded by differences between them. These include: i) the methods used for the assays were different; ii) studies have utilized both in-house developed and commercial ELISAs; iii) lateral flow chromatography method was used for rapid tests; iv) luciferase immunoprecipitation method was utilized; v) Western blot method was utilized; vi) utilized pseudotype virus assays for evaluating neutralizing antibodies; vii) the number of patient samples used between the studies varies widely; viii) recombinant proteins derived from prokaryotic and eukaryotic expression systems have been used as substrates for detecting antibodies; ix) full length N protein and peptides from N were used; and x) full length S, S1 subunit of S, S2 subunit of S, RBD, N-terminal domain of S, peptides from S and E proteins were used for the assays.
A representative summary of the published studies on antibodies against SARS-CoV-2 in patients is listed in Table 1. These studies are highlighted as they show differences in the substrate (recombinant viral protein/peptides) and technologies used for evaluation of antibodies. A large number of the studies focused on addressing the time of appearance of antibodies in COVID-19 patients. It is clear that it is difficult to pinpoint the exact timing of infection in patients. In individual reports, based on the information available from hospital records, the incubation period from the time of infection to developing disease related symptoms ranges from 5 to 20 days. Hence, the investigators in the field opted to report their findings with respect to the detection of IgM, IgA and IgG antibodies in the sera in terms of days after the onset of symptoms in patients.
Table 1.
Detection of antibodies by different platform assays using recombinant viral antigens as substrates.
| Viral antigen used | Assay format | Source | Antibodies tested | Reference |
|---|---|---|---|---|
| N, S | Lateral Flow assay | Sera | IgM | (Shen et al., 2020) |
| N | ELISA | Plasma | IgM, IgG | (Zhang and Holmes, 2020) |
| N, RBD | Chemiluminescent assay | Sera | IgM, IgA, IgG | (Ma et al., 2020) |
| U | Lateral Flow assay | Sera | IgM, IgG | (Wu et al., 2020a; Yong et al., 2020) |
| N, RBD | Lateral Flow assay, ELISA | Sera, Saliva | IgM, IgG | (To et al., 2020) |
| N, RBD | ELISA | Sera | IgM, IgG | (Ni et al., 2020) |
| RBD, S1, S2 | ELISA | Plasma | IgG | (Wu et al., 2020b) |
| RBD | ELISA | Sera | IgG | (Duan et al., 2020) |
| RBD | ELISA | Sera | IgM, IgG | (Shen et al., 2020) |
| U | Chemiluminescent assay | Sera | IgM, IgG | (Yeh et al., 2004) |
| RBD | ELISA | MAb | IgG | (Cao et al., 2020) |
| S peptides | ELISA | Sera | IgG | (Poh et al., 2020) |
| RBD | ELISA | Plasma | Total antibodies, IgM, IgG | (Zhao et al., 2020a) |
| U | Lateral Flow assay | Sera | IgM, IgG | (Pan et al., 2020) |
| S peptide | Chemiluminescence assay | Sera | IgA, IgM, IgG | (Yu et al., 2020) |
| S1, N | ELISA | Sera | IgG | (Zhao et al., 2020b) |
| E, N | ELISA | Sera | IgM, IgG | (Zhang and Holmes, 2020) |
| N | ELISA | Sera | IgM, IgG | (Tan et al., 2020) |
| N | ELISA | Sera | IgM, IgG | (Xiang et al., 2020) |
| N | ELISA | Sera | IgM, IgA, IgG | (Guo et al., 2020) |
| N, S | Chemiluminescence assay | Sera | IgM, IgG | (Xiao et al., 2020) |
| N, S | Chemiluminescence assay | Sera | IgM, IgG | (Hou et al., 2020) |
| N, S1 | ELISA | Sera | IgM, IgG | (Sun et al., 2020) |
| S1 subunit | ELISA | Sera | IgA, IgG | (Jaaskelainen et al., 2020) |
| N, S | Chemiluminescence assay | Sera | IgM, IgG | (Ou et al., 2020) |
| N | Lateral flow assay | Sera | IgM, IgG | (Lee et al., 2020) |
| S, N | Chemiluminescent assay, ELISA | Sera | IgA, IgM, IgG | (Padoan et al., 2020a) |
| N, S peptide | Chemiluminescence assay | Sera | IgG | (Long et al., 2020a) |
| RBD | ELISA | Sera | IgM, IgG | (Pereira et al., 2020) |
| N, S | ELISA | Sera | IgM, IgG | (Liu et al., 2020c) |
| S peptide | Chemiluminescence assay | Sera | IgM, IgG | (Cao et al., 2020) |
| S ectodomain, S1, S1 (1–294), RBD | ELISA | Sera | IgA, IgM, IgG | (Okba et al., 2020) |
| S1 | ELISA | Sera | IgA, IgG | (Beavis et al., 2020) |
| RBD | ELISA | Sera | Total antibodies, IgM, IgG | (Lou et al., 2020) |
| N | Lateral flow assay | Sera | IgM, IgG | (Lou et al., 2020) |
| S, S1, RBD, N | Single molecule array assay | Plasma | IgM, IgA, IgG | (Norman et al., 2020) |
| RBD | ELISA | Sera | IgM, IgG | (Premkumar et al., 2020) |
| N, RBD | Luminex | Saliva, Sera | IgM, IgA, IgG | (Randad et al., 2020) |
| N, S | Chemiluminescence | Sera | IgM, IgG | (Nuccetelli et al., 2020) |
| S | LFA | Sera | IgM, IgG | (Mlcochova et al., 2020; Pickering et al., 2020) |
| S, N | LFA | Sera | IgM, IgG | (Mlcochova et al., 2020) |
| S1 | cell-ELISA | PBMCs | IgG | (Zarletti et al., 2020) |
U, substrate not mentioned.
Zhao et al. investigated the antibody status using N and S1 subunit of spike proteins expressed in bacteria and mammalian cells, respectively as the antibody capturing antigens (Zhao et al., 2020b). Antibodies were detected in patients after the onset of symptoms and antibody (Piccoli et al., 2020) levels were shown to increase. The antibody dynamics and responses were the subject of investigations by Xiang and co-workers (Xiang et al., 2020). The results showed detection of IgM and IgG antibodies against N protein on the fourth day after the onset of symptoms. Generally, the appearance of IgM antibodies was with a median of 5 days though studies show 1–7 days (Zhao et al., 2020b) and IgG specific antibodies were seen towards the end of first week (day 7) or in the second week after the onset of symptoms (Rokni et al., 2020; Tan et al., 2020). Similarly, Gao et al. used ELISA based on N protein to monitor IgM, IgA and IgG specific antiviral antibodies in plasma samples (Gao et al., 2020). IgM and IgA antibodies were detected 5 days after disease onset and IgG was detected 14 days after the onset of disease (Lee et al., 2020). In contrast, microbeads assay using N and S proteins detected IgM and IgG with a median of 5 and 4 days, respectively after symptoms onset (Suhandynata et al., 2020a).
Studies involving a chemiluminescence immunoassay format also showed that sera were positive for both IgM and IgG in week 3 after symptoms. While IgM showed a declining trend, IgG are observed to continuously increase (Lee et al., 2020; Xiao et al., 2020). The evaluation of total antibodies in patients by ELISA showed an increase in the antibodies against RBD containing S protein. The analysis of sera collected on day 1–3, 4–7, 8–14 and 15–39 days from symptoms onset exhibited a positive rate of 28.6%, 53.6%, 98.2% and 100%, respectively (Zhao et al., 2020b). In another study involving total antibodies, IgM and IgG, total antibodies were detected first followed by IgM and IgG. This reflected in the sensitivity of 64.1% for total antibodies during early stage (0–7 days post onset) in contrast to 33.3% for IgM and IgG (Lou et al., 2020). Similar reactivities were also evident in the immunochromatography assays carried out by Imai et al. (2020). Analysis of sera from 112 patients showed IgM seropositive rate of 22.8%, 48.0% and 95.8% within 1 week, 1–2 weeks and >2 weeks, respectively. The seropositive rate with IgG registered 3.3%, 8,0% and 62.5% for the same sample set. The analysis of sera from 16 patients collected at 14 days or longer after symptoms onset showed rates of seropositivity for N protein (94% and 88% for IgG and IgM, respectively) and RBD (100% and 94% for IgG and IgM, respectively). The analysis of sera or plasma revealed an increase in seropositive rate with time interval showing 81.8–100% in samples taken>20 days after symptoms onset (Lassaunière et al., 2020) (Tan et al., 2020) (Wolfel et al., 2020) (Long et al., 2020a).
A longitudinal analysis of sera from 338 patients, with different severity of illnesses and positive for SARS-CoV-2 confirmed by RT-PCR, revealed that IgM was detected in the first week after symptoms onset, peaked in second week and started to decline to background level. On the other hand, IgG was maintained for a long time. Interestingly, IgM level was found to decline rapidly in recovered patients whereas in deceased patients, IgM remained high or both IgM and IgG were undetected during disease course (Hou et al., 2020). This trend was also noted by Sun et al. (2020). Both N and S specific IgM reached peak in second week while IgG increased in third week. It was noted that a combined detection of N and S specific IgM and IgG could identify up to 75% of patients in the first week after the onset of symptoms. However, Qu et al. observed antibody responses between 17 and 23 days after onset of illness (Qu et al., 2020). Stronger antibody responses were observed in patients under critical conditions. The appearance of IgA and IgG antibodies against S1 showed a trend noted with IgM and IgG (Jaaskelainen et al., 2020). Padoan et al. reported that IgA response peaked at week 3 and was stronger and persistent than IgM response (Padoan et al., 2020b). A similar pattern in terms of appearance of IgM and IgG was noted when E and N antigens were used as substrates (Zhang and Holmes, 2020). Further, based on the analysis of the serial samples collected from patients, an increase of both IgM and IgG was noted 6–7 days after the onset of symptoms (Padoan et al., 2020a, 2020b). This is also echoed in the studies by Long et al. where 100% of patients were tested positive for IgG within days after the onset of symptoms (Long et al., 2020a).
The dynamics of antibody responses was analyzed by Pereira et al. (2020) using RBD expressed in insect cells in an ELISA. While none of the four sera collected within first 4 days of illness showed reactivities, sera collected from 5-9, 11–18, 19–28 and 29–42 days after onset registered IgM and IgG antibodies (Pereira et al., 2020). Montesinos et al., used a panel of 128 PCR confirmed cases and 72 controls to compare the results generated by ELISA and lateral flow assays (Montesinos et al., 2020). Both assays revealed similar values with the sera collected 14 days after onset of symptoms. Kruttgen et al. compared ELISA assays from four companies (Euroimmun, Epitope Diagnostics, Mikogen and Viramed) using a panel of 75 sera from virus positive patients (Kruttgen et al., 2020). The IgG antibodies showed a sensitivity of 86.4%, 100%, 86.4 and 77.37%, respectively. The ultrasensitive high-resolution profiling of antibodies by Single Molecule Array assay showed superior sensitivity in detecting antibodies in the first week after symptoms onset (Norman et al., 2020). Analysis of plasma from COVID-19 patients indicated 86% sensitivity in detecting IgM, IgA and IgG in comparison to other assays.
The immunochromatography assays detecting IgM and IgG antibody was recently used to test a panel of 97 PCR positive cases. This assay showed positive result for 71.1% of the samples. The assay also detected positive cases among 53 PCR negative samples which may also be a reflection of the sensitivity of the PCR assay. The IgM and IgG antibodies were evaluated for their diagnostic feasibility by ELISA assay using N and S antigens. These studies showed that the positive rate was low in early stage sera (Liu et al., 2020b). Okba et al. utilized ELISA with a truncated S protein as a substrate for evaluating IgA, IgM and IgG antibodies in COVID-19 patients (Okba et al., 2020). IgA showed a strong correlation with COVID infection. However, IgM was more sensitive but less specific than IgG assay. Burbelo et al. analyzed the antibodies against N and S (1–538 and 1–513 aa) of SARS-CoV-2 using LIPS assay (Burbelo et al., 2005). In accordance with the results noted with ELISA, antibodies were observed 8–10 days after the onset of symptoms. The comparison of antibody pattern in asymptomatic group of SARS-CoV-2 positive individuals with the symptomatic group showed that IgG antibody positive rates were 81.1% and 83.8% for asymptomatic and symptomatic groups, respectively in 3–4 weeks after exposure. IgM values were 62.2% and 78.4% for the same groups (Long et al., 2020a). The levels of IgG antibodies were higher in symptomatic than asymptomatic groups during the acute phase of infection. Similar data were also reported by other groups (Shirin et al., 2020; Long et al., 2020b). The investigation into the early convalescent phase (8 weeks after discharge from the hospital) showed a decline in IgG level and also a decrease in neutralizing antibodies within 2–3 months. It was reported that IgG titers showed a decline with a half-life of 49 days (Piccoli et al., 2020). Unlike adults, it has been reported that SARS-CoV-2 infected children exhibit less severe symptoms. The analysis of S protein specific antibodies showed that the pediatric patients had more active B cells and neutralizing antibodies (Bahar et al., 2020; Lei et al., 2020; Lynch et al., 2020; Mairesse et al., 2020; Wang et al., 2020b). A difference in the antibodies has also been reported in female versus male patients (Zeng et al., 2020). Analysis of sera from mild, general, severe and recovering patients, a high level of IgG was noted in females than males in severe disease status. In a recent review, Nikolich-Zugich et al. noted that the onset of immune response is slower in older adults (Nikolich-Zugich et al., 2020). Based on the studies with SARS-CoV and SARS-CoV-2, failure to switch from innate to adaptive immune responses impacts on antibody development. It has been noted that sicker COVID-19 individuals showed a high titer S specific antibodies in comparison to asymptomatic and also patients recovered from the disease.
In addition to the studies listed above, there are several reports recently published on the kinetics of antibody responses in COVID-19 (Bailey et al., 2020; Guthmiller et al., 2020; Huang et al., 2020b; Li et al., 2020b; Mlcochova et al., 2020; Piccoli et al., 2020). Overall, a typical scenario regarding the kinetics is the following: i) the incubation period in the individuals lasts anywhere from 5-10 days after infection to show the symptoms; ii) IgM and IgA antibodies appeared in the first week and IgG antibodies showed up in the second week though IgG antibodies have also been noted within the first week after the onset of symptoms (Mlcochova et al., 2020); iii) IgA and IgG antibodies persist for an extended period than IgM; iv) patients with severe illness showed high titer of antibodies in contrast to individuals who are asymptomatic; v) IgG antibodies against SARS-CoV-2 proteins present in the sera/plasma of individuals for periods ranging from 3-6 months in comparison to IgM/IgA antibodies; vi) it has been suggested that detection of antibodies in individuals gives an indication about the ongoing and past infections with SARS-CoV-2; vii) the timing of appearance of antibodies after the onset of symptoms precludes the antibody tests in the realm of COVID-19 diagnosis; viii) SARS-CoV-2 viral proteins show varying antigenicity profiles.
8. Characteristics of antibodies against SARS-CoV-2 in convalescing COVID-19 patients
As pointed out earlier, currently there are three options available for treating COVID-19 disease. These include remdesivir, steroids and plasma therapy. Regarding the latter, there are patients available who have recovered from the disease (Bloch et al., 2020; Chen et al., 2020b; Kumar et al., 2020; Rojas et al., 2020). Such a strategy to use plasma derived from patients who have recovered from infections goes back more than hundred years and has been successfully used in SARS-CoV and MERS-CoV outbreaks and influenza (H1N1) cases (Cheng et al., 2005; Zhou et al., 2007; Hung et al., 2011; Ko et al., 2018). There are several reports regarding the use of plasma from recovered COVID patients to treat severely ill COVID-19 patients (Cao et al., 2020; Duan et al., 2020; Ni et al., 2020; Shen et al., 2020; Wang et al., 2020a; Zhang and Holmes, 2020). The plasma samples, collected from recovered patients 13–27 days after their discharge, were weakly positive for IgM while high IgG titers were noted in ELISA using N protein as the substrate (Zhang and Holmes, 2020) in addition to the demonstration of high IgG titers against RBD of Spike protein as substrate (Ni et al., 2020; Shen et al., 2020; Xu et al., 2020). Virus neutralizing activity was also shown using a pseudotype virus entry assay and/or infectious virus in the plasma of these patients (Shen et al., 2020). Poh et al. screened the sera with peptides covering the entire spike protein and identified two peptides (TESNKKFLPFQQFGRDIA and PSKPSKRSFIEDLLFNKV) overlapping receptor binding region and fusion peptide region with high reactivities in the sera of convalescent patients (Poh et al., 2020). As a large differences noted in the antibody response to viral proteins in patients, it is likely that data regarding antibodies and neutralization may be useful in the selection of plasma donors for plasma therapy. It is likely that data regarding antibodies and neutralization may be useful in the selection of plasma donors for therapy. It is important to note that neutralizing antibodies were detected in all samples positive for SARS-CoV-2 by PCR assay. Hospitalized patients demonstrated high titers of antibodies to viral protein and this correlated with high neutralizing antibody titer (Suhandynata et al., 2020b). It is likely that commercial assays have the potential to predict the presence of neutralizing antibodies. The investigation into the early convalescent phase (8 weeks after discharge from hospital) showed a decline in IgG level indicating an important role for antibody tests in plasma therapy and also a decrease in antibodies within 2–3 months (Long et al., 2020b).
9. Role of serologic tests in diagnosis and seroprevalence in the population
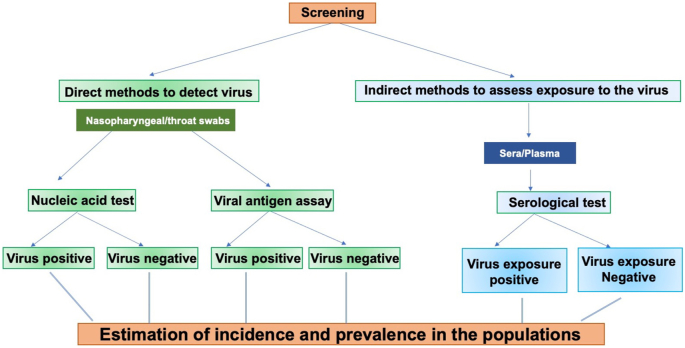
Serologic testing is not currently used for diagnosis of COVID-19. There are several reasons for this: i) Acute SARS-CoV-2 infections quickly lead to the onset of symptoms in the patients. The duration from infection to symptoms takes 5–10 days in a majority of cases; ii) The symptoms precede the generation of antibody response in the host; iii) Molecular tests demonstrate the presence of virus through amplification of viral RNA (Theel et al., 2020), a direct measure of the presence of the virus. Based on the low sensitivity noted with immunoassays (ELISA and immunochromatography) during the early stage of infection, these assays may not be stand-alone assays for the diagnosis of COVID-19 disease. The retrospective analysis of the samples, registered negative for SARS-CoV-2 by RT-PCR, showed the presence of antiviral antibodies (Gao et al., 2020) (To et al., 2020) (Li et al., 2020c; Xu et al., 2020; Zhao et al., 2020a). These data suggest that combining serological tests should improve early diagnosis. Serological assays have been used to evaluate the individuals’ exposure to SARS-CoV-2 who may or may not be symptomatic. Healthcare workers (HCW), who were in close proximity to patients with COVID-19 disease, were the subject of several studies evaluating antibodies against SARS-CoV-2. Chen et al. examined 105 HCW using ELISA with RBD and N proteins and detected a positive rate of 17.14% (18/105) (Chen and Li, 2020). Similarly, a seropositive rate of 9.3% (54/578) was reported (Garcia-Basteiro et al., 2020). However, Korth et al. reported a rate of 1.6% (5/316), a rate likely due to the safe practices adhered to by HCW in that study (Korth et al., 2020). Seroprevalence studies involving a total of 17,368 individuals in Wuhan and other regions noted seropositive rate between 3.2-3.8% (Xu et al., 2020). Stringhini et al. conducted a seroprevalence study in Geneva, Switzerland involving 2,766 participants (Stringhini et al., 2020). Screening of individuals for IgG antibodies against S1 domain of S protein showed a prevalence of 4.8%, 8.65%, 10.9%, 6.6% and 10.8% for week 1–5, respectively. Recently, a large study undertaken in Spain, which suffered heavily due to SARS-CoV-2, involved sera from 70,000 individuals and a, positive rate of around 5% was seen. A strikingly different picture emerges from the analysis of sera from children attending the hospital for other diseases in Seattle area where ~1% positive was observed (Dingens et al., 2020). Sera from individuals attending hospital in San Francisco showed a positive rate of 0.26% in a total of 387 patients (Ng et al., 2020). These results indicate that the extent of positive rate of antibodies depends on several factors including location being close to the endemic areas for SARS-CoV-2. The results from the seroprevalence studies should be interpreted with caution. This is partly due to the fact that studies have been carried out with different assays with varying sensitivity and specificity. The serological assays can also be utilized to Identify donors for convalescent plasma therapy and provide information about the correlates of protection (Amanat et al., 2020a). The screening of general and defined populations for estimation of incidence and seroprevalence against SARS-CoV-2 is outlined in Figure 5. The disappearance of antibodies in the sera of individuals infected with SARS-CoV-2 over time is an important issue. The approach of verifying plasma from convalescing COVID-19 patients for the treatment of severely ill patients may be impacted due to this. The use of vaccines based on Spike protein and inactivated viruses further add a layer of complexity to the seroprevalence studies. Further studies are needed in this area.
Figure 5.
Screening of defined and general populations for estimation of incidence and seroprevalence for SARS-CoV-2.
10. Antibody cross reactivities with coronavirus N, S and E structural proteins: impact on the effectiveness of SARS-CoV-2 tests?
Of the structural proteins S, N, E and M, the homologies between SARS-CoV-2 and SARS-CoV are 76.0%, 90.6%, 94.7% and 90.1%, respectively (Ahmed et al., 2020a) (Grifoni et al., 2020). The extent of similarities points out that there is potential for cross reactivite antibodies between viruses. Hence, a positive result in an antibody test using the N protein is likely the result of reactivities against N of SARS-CoV or SARS-CoV-2. However, the homology is much less in specific regions of the S protein of these viruses. As SARS-CoV has not been in circulation since the earlier outbreak and the limitation regarding the persistence of antibodies, it is likely that a combination of two viral proteins may confirm the seroconversion due to SARS-CoV-2. The common cold coronaviruses (strains 229E, NL63. OC43 and HKU1) also exhibit homology to structural proteins of SARS-CoV-2. This prompted us to start thinking about the preexisting immunity against these viruses in the population and the influence it may have on antibody testing against the virus causing COVID-19. There was around 70% seropositivity against S protein from 229E, NL63, OC43 and HKU1 in the general population (Zhou et al., 2013) and also seropositivity ranging from 90.6 to 91.8% in adults for 229E, NL63 and OC43 and 59.2% against HKU1 using N protein ELISA (Severance et al., 2008). This is similar to the data reported by other investigators (Hruskova et al., 1990; Mourez et al., 2007). Recently it was reported that a total of 45 B cell epitopes are identical in S and N proteins between SARS-CoV and SARS-CoV-2. These results raise concerns regarding the potential to have false positive cases of COVID-19. As noted in Table 1, several antibody assay kits are based on N only and/or S proteins and hence may likely lead to the over estimation of the prevalence of SARS-CoV-2. The answer to this false positive test results is in developing test kits targeting the unique non-conserved immunogenic regions of SARS-CoV-2 S protein such as RBD as shown and also pointed out by the bioinformatic analysis (Premkumar et al., 2020).
Recently it was revealed that there is a limited homology between SARS-CoV-2 and rubella, mumps and measles (Franklin et al., 2020). Specifically, Macro domains present in the non-structural protein 3 (NSP3) of SARS-CoV-2 and p150 of rubella share 29% amino acid sequence identity. Similarly, homology was also noted between SARS-CoV-2 S2 domain of S protein with the fusogenic proteins (F) of mumps and measles viruses in post-fusion conformation. The investigators have hypothesized that these homologous regions may contribute to the less severe effect observed in children with COVID-19 disease. It has been reported that rubella IgG titer was high in COVID-19 patients with severe disease in comparison to patients with moderate disease. These results warrant follow up studies to understand their significance. The emergence of SARS-CoV-2 has raised questions about the effectiveness of currently approved vaccines. Specifically, antibodies elicited by Spike protein based vaccines showed reduced effect in in vitro neutralization assays against U.K and South African variant viruses resulting from an altered interaction between RBD and ACE2. As antibody tests depend on a number of B cell epitopes present in the Spike protein, specific mutations in RBD region may not alter antigen-antibody interactions in other parts of Spike protein.
11. Use of antibody tests for vaccine development and clinical trials
The serological tests, that detect and quantify antibodies against SARS-CoV-2 S and N proteins in COVID-19 patients, provide relevant information for the design of candidate vaccines and their clinical evaluation. The correlates of protective immunity in the case of SARS-CoV-2 are not clear. It is likely that a combination of cellular and humoral responses against the viral proteins contribute to the protection of host as noted with other pathogens (Florindo et al., 2020; Huang et al., 2020a; Poland et al., 2020; Sariol and Perlman, 2020). This may be applicable to SARS-CoV-2. The benefit of antibodies present in convalescent COVID-19 patients has been demonstrated in the form of plasma therapy to severely ill patients (Liu et al., 2020a; Schwartz et al., 2020). The presence of distinct subtypes of antibodies including IgG and IgA with neutralizing activity towards SARS-CoV-2 lend to their role in providing relief to the recipients of plasma therapy (Casadevall and Pirofski, 2020; Li et al., 2020a; Liu et al., 2020a; Schwartz et al., 2020). It was recently reported that individuals, negative for SARS-CoV-2 infection by RT-PCR, have been shown to harbor antibodies which reacted with S2 domain of S protein of SARS-CoV-2. It was suggested that prior infection with related common cold coronaviruses may be the source of such antibodies. The preexisting antibodies exhibited neutralizing activity against SARS-CoV-2 live virus and S protein pseudotyped virus in cell cultures. These studies highlighted that a candidate vaccine representing S protein would be an ideal candidate as shown recently with mRNA, DNA and vectored vaccine platforms (Krammer, 2020; Poland et al., 2020). Interestingly, the preexisting antibodies present in healthy individuals are of IgG subtype. On the other hand, antibody pattern in COVID-19 patients consists of IgM, IgA and IgG subtypes. These observations serve critical roles: i) facilitate the selection of plasma donors for therapy among convalescing COVID-19 patients; ii) help to distinguish between preexisting vs. de novo synthesis of antibodies during infection and vaccine trials.
12. Conclusions
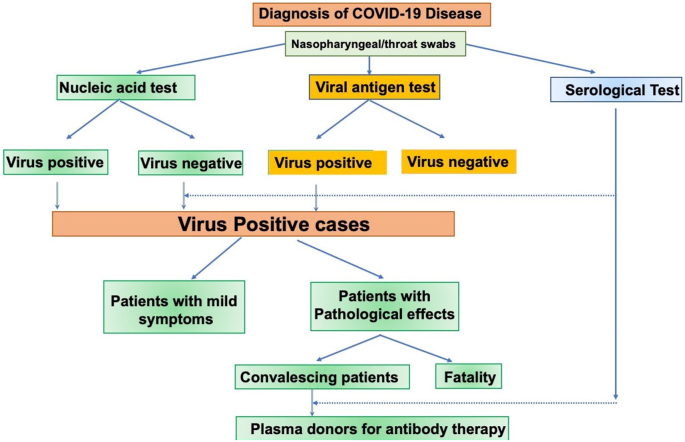
Here we have discussed the data on antibodies observed in patients from the perspective of breadth, kinetics, cross reactivity with related coronaviruses and other viral agents and surveillance of populations for the rate of virus infection. The anti viral IgM, IgA and IgG antibodies in individuals with COVID-19 disease were detected in the first week and second week, respectively after symptoms onset. By weeks 2–3, tests for IgG specific antibodies are seen in 100% of the infected individuals. A combined use of IgM and IgG tests enhanced the sensitivity of detection of antibodies close to symptoms onset. The current paradigm used for the diagnosis of COVID-19 is presented in Figure 6. As shown here, nucleic acid test detects viral RNA and plays a major role in the diagnosis. Another method for this purpose is the antigen assay which detects viral proteins.
Figure 6.
Diagnosis and follow up of patients with COVID-19. Acute infection with SARS-CoV-2 is detected by nucleic acid and antigen assays.
Due to the sensitivity issues with RT-PCR tests, the antibody tests may complement with RT-PCR for diagnosis of COVID-19 disease in specific cases. This is supported by the reports that PCR negative cases were detected by serological (Basu et al., 2020; Chen and Li, 2020; Collier et al., 2020; Huang et al., 2020c; Li et al., 2020b; Mlcochova et al., 2020; Pickering et al., 2020; Smithgall et al., 2020a). The antibody tests are useful for the analysis of antibody status in patients recovered from COVID-19 disease. The analysis of plasma showed predominantly of IgG type and a low level of IgA and IgM. Recently, U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the use of convalescent plasma for the treatment of hospitalized patients with COVID-19 disease (https://www.fda.gov/media/141477/download). The plasma therapy has been in use in several countries. In this regard, it should also be mentioned that monoclonal antibodies have also been approved for use in humans with COVID-19 disease (Focosi and Farrugia, 2020; Nagoba et al., 2020). Further, results of the seroprevalence studies using IgG and IgM antibody tests can be used as evidence of past exposure to the virus at the population level. Specifically, antibody tests in areas with large number of infections such as Spain and Wuhan, showed positive cases of around 5% and 3.8%, respectively. However, the prevalence rate is much lower in locations away from infectious clusters. The serological tests will also be useful in epidemiologic studies, incidence estimation and contact tracing. Interestingly, pediatric patients showed less severe disease and antibody development. Similarly, women with COVID-19 had strong antibody response and also less severe illness. Future studies should expand on these results with a large cohorts of patients. Another area of interest is the time course of antibody development in different populations such as asymptomatic, mild, severe and convalescent stages of virus infected individuals. A related question is that whether antibody levels predict immunity to re-infection by SARS-CoV-2. Barouch and colleagues reported that rhesus macaques, infected with SARS-CoV-2, showed resistance to reinfection with the same virus later due to immune protection (Mercado et al., 2020). Though there are reports in support of reinfection of individuals previously infected with SARS-CoV-2 (Mulder et al., 2020; Tillett et al., 2020; Zhang et al., 2020b), this area needs further investigation. Overall, the availability of tests for determining the status and functionality of antibodies in patients and general population will be useful for controlling SARS-CoV-2 epidemic in the future.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Figures were made in part using BioRender.
References
- Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12 doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkaya M., Kwak K., Pierce S.K. B cell memory: building two walls of protection against pathogens. Nat. Rev. Immunol. 2020;20:229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Arunkumar G.A., Jurczyszak D., Polanco J. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020;26:1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M., Jiang K., Asthagiri Arunkumar G., Jurczyszak D., Polanco J. medRxiv; 2020. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkur A.K., Akdis M., Azkur D., Sokolowska M., van de Veen W., Bruggen M.C., O'Mahony L., Gao Y., Nadeau K., Akdis C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi L., Carcano G., Gianfagna F., Grossi P., Gasperina D.D., Genoni A., Fasano M., Sessa F., Tettamanti L., Carinci F. Saliva is a reliable tool to detect SARS-CoV-2. J. Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar B., Jacquot C., Mo Y.D., DeBiasi R.L., Campos J., Delaney M. Kinetics of viral clearance and antibody production across age groups in children with severe acute respiratory syndrome coronavirus 2 infection. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C.S., Rasoulinejad P., Taylor D., Sequeira K., Miller T., Watson J., Rosedale R., Bailey S.I., Gurr K.R., Siddiqi F. Surgery versus conservative care for persistent sciatica lasting 4 to 12 months. N. Engl. J. Med. 2020;382:1093–1102. doi: 10.1056/NEJMoa1912658. [DOI] [PubMed] [Google Scholar]
- Basu A., Zinger T., Inglima K., Woo K.M., Atie O., Yurasits L., See B., Aguero-Rosenfeld M.E. Performance of abbott ID Now COVID-19 rapid nucleic acid amplification test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York city academic institution. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.01136-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis K.G., Matushek S.M., Abeleda A.P.F., Bethel C., Hunt C., Gillen S., Moran A., Tesic V. Evaluation of the EUROIMMUN anti-SARS-CoV-2 ELISA assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020;129:104468. doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Flores M., Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int. J. Clin. Pract. 2020 doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L., van Buskirk C., Grossman B.J., Joyner M., Henderson J.P. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J. Clin. Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit J., Taylor J.J. Techniques to study antigen-specific B cell responses. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynjolfsson S.F., Persson Berg L., Olsen Ekerhult T., Rimkute I., Wick M.J., Martensson I.L., Grimsholm O. Long-lived plasma cells in mice and men. Front. Immunol. 2018;9:2673. doi: 10.3389/fimmu.2018.02673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo P.D., Goldman R., Mattson T.L. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Understanding variants of SARS-CoV-2. Lancet. 2021;397:462. doi: 10.1016/S0140-6736(21)00298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z., Zhang Y., Zhang H., Jia R., Liu P. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Su B., Guo X., Sun W., Deng Y., Bao L., Zhu Q., Zhang X., Zheng Y., Geng C. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell. 2020;182:73–84. doi: 10.1016/j.cell.2020.05.025. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F., Kok K.H., Zhu Z., Chu H., To K.K., Yuan S., Yuen K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect. Dis. 2020;20:515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Yang D., Yang C., Zheng L., Huang K. Response to Comment on Chen et al. Clinical Characteristics and Outcomes of Patients With Diabetes and COVID-19 in Association With Glucose-Lowering Medication. Diabetes Care. 2020;43:e165–e166. doi: 10.2337/dc20-0660. 1399-1407. [DOI] [PubMed] [Google Scholar]
- Cheng Y. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciotti M., Angeletti S., Minieri M., Giovannetti M., Benvenuto D., Pascarella S., Sagnelli C., Bianchi M., Bernardini S., Ciccozzi M. COVID-19 outbreak: an overview. Chemotherapy. 2019;64:215–223. doi: 10.1159/000507423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier E.K., Hsiao J.L., Shi V.Y. Conducting clinical trials during the COVID-19 pandemic. J. Dermatol. Treat. 2020;31:330–332. doi: 10.1080/09546634.2020.1759770. [DOI] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingens A.S., Crawford K.H., Adler A., Steele S.L., Lacombe K., Eguia R., Amanat F., Walls A.C., Wolf C.R., Murphy M. medRxiv; 2020. Seroprevalence of SARS-CoV-2 Among Children Visiting a Hospital during the Initial Seattle Outbreak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florindo H.F., Kleiner R., Vaskovich-Koubi D., Acurcio R.C., Carreira B., Yeini E., Tiram G., Liubomirski Y., Satchi-Fainaro R. Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 2020;15:630–645. doi: 10.1038/s41565-020-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D., Farrugia A. The art of the possible in approaching efficacy trials for COVID19 convalescent plasma. Int. J. Infect. Dis. 2020;102:244–246. doi: 10.1016/j.ijid.2020.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster P., Forster L., Renfrew C., Forster M. Reply to Sanchez-Pacheco et al., Chookajorn, and Mavian et al.: explaining phylogenetic network analysis of SARS-CoV-2 genomes. Proc. Natl. Acad. Sci. U. S. A. 2020;117:12524–12525. doi: 10.1073/pnas.2007433117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R., Young A., Neumann B., Fernandez R., Joannides A., Reyahi A., Modis Y. medRxiv; 2020. Homologous Protein Domains in SARS-CoV-2 and Measles, Mumps and Rubella Viruses: Preliminary Evidence that MMR Vaccine Might Provide protection against COVID-19. [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Basteiro A.L., Chaccour C., Guinovart C., Llupia A., Brew J., Trilla A., Plasencia A. Monitoring the COVID-19 epidemic in the context of widespread local transmission. Lancet Respir. Med. 2020;8:440–442. doi: 10.1016/S2213-2600(20)30162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680. doi: 10.1016/j.chom.2020.03.002. e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzelak L., Temmam S., Planchais C., Demeret C., Huon C., Guivel-Benhassine F., Staropoli I., Chazal M., Dufloo J., Planas D. medRxiv; 2020. SARS-CoV-2 Serological Analysis of COVID-19 Hospitalized Patients, Pauci-Symptomatic Individuals and Blood Donors. [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Ren L., Yang S., Xiao M., Chang Yang F., Dela Cruz C.S., Wang Y., Wu C., Xiao Y. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19) Clin. Infect. Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller J.J., Stovicek O., Wang J., Changrob S., Li L., Halfmann P., Zheng N.Y., Utset H., Stamper C.T., Dugan H.L. bioRxiv; 2020. SARS-CoV-2 Infection Severity Is Linked to superior Humoral Immunity against the Spike. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haljasmagi L., Remm A., Rumm A.P., Krassohhina E., Sein H., Tamm A., Kisand K., Peterson P. LIPS method for the detection of SARS-CoV-2 antibodies to spike and nucleocapsid proteins. Eur. J. Immunol. 2020 doi: 10.1002/eji.202048715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W., Zhang W., Jin R., Liang L., Xu B., Hu Z. Risk factors for disease progression in hospitalized patients with COVID-19: a retrospective cohort study. Inf. Disp. 2020;52:498–505. doi: 10.1080/23744235.2020.1759817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruskova J., Heinz F., Svandova E., Pennigerova S. Antibodies to human coronaviruses 229E and OC43 in the population of C.R. Acta Virol. 1990;34:346–352. [PubMed] [Google Scholar]
- Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Tu M., Wang S., Chen S., Zhou W., Chen D., Zhou L., Wang M., Zhao Y., Zeng W. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Trav. Med. Infect. Dis. 2020:101606. doi: 10.1016/j.tmaid.2020.101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz J.L. B cells, viruses, and the SARS-CoV-2/COVID-19 pandemic of 2020. Viral Immunol. 2020;33:251–252. doi: 10.1089/vim.2020.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K., Tabata S., Ikeda M., Noguchi S., Kitagawa Y., Matuoka M., Miyoshi K., Tarumoto N., Sakai J., Ito T. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J. Clin. Virol. 2020;128:104393. doi: 10.1016/j.jcv.2020.104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen A.J., Kekalainen E., Kallio-Kokko H., Mannonen L., Kortela E., Vapalahti O., Kurkela S., Lappalainen M. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.18.2000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes J.A., Andre N.M., Chappie J.S., Millet J.K., Whittaker G.R. Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop. J. Mol. Biol. 2020;432:3309–3325. doi: 10.1016/j.jmb.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., Duan G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12 doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G.W., Hill D.G., Jones S.A. Understanding immune cells in tertiary lymphoid organ development: it is all starting to come together. Front. Immunol. 2016;7:401. doi: 10.3389/fimmu.2016.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadadi L., Cheng Q., Radbruch A., Hiepe F. The maintenance of memory plasma cells. Front. Immunol. 2019;10:721. doi: 10.3389/fimmu.2019.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir. Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- Kontou P.I., Braliou G.G., Dimou N.L., Nikolopoulos G., Bagos P.G. Antibody tests in detecting SARS-CoV-2 infection: a meta-analysis. Diagnostics. 2020;10 doi: 10.3390/diagnostics10050319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo O.M., Rubinstein I., Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Korber B., Fischer W., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Foley B., Giorgi E., Bhattacharya T., Parker M. bioRxiv; 2020. Spike Mutation Pipeline Reveals the Emergence of a More Transmissible Form of SARS-CoV-2. [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827 e819. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth J., Wilde B., Dolff S., Anastasiou O.E., Krawczyk A., Jahn M., Cordes S., Ross B., Esser S., Lindemann M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Kruttgen A., Cornelissen C.G., Dreher M., Hornef M., Imohl M., Kleines M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020;128:104394. doi: 10.1016/j.jcv.2020.104394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Verma H., Singhvi N., Sood U., Gupta V., Singh M., Kumari R., Hira P., Nagar S., Talwar C. Comparative genomic analysis of rapidly evolving SARS-CoV-2 reveals mosaic pattern of phylogeographical distribution. mSystems. 2020;5 doi: 10.1128/mSystems.00505-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A., Capuzzo M., Paglia T., Roli L., Trenti T., Nelson S.M. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online. 2020;41:483–499. doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C., Fomsgaard A., Krogfelt K.A., Jørgensen C.S. medRxiv; 2020. Evaluation of Nine Commercial SARS-CoV-2 Immunoassays. [Google Scholar]
- Lee C.Y., Lin R.T.P., Renia L., Ng L.F.P. Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front. Immunol. 2020;11:879. doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. J. Am. Med. Assoc. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020 doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F., Rodriguez D., Tandon P., Bassily-Marcus A., Bander J. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat. Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J., Hu J.L., Xu W., Zhang Y., Lv F.J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- Lou B., Li T., Zheng S., Su Y., Li Z., Liu W., Yu F., Ge S., Zou Q., Yuan Q. medRxiv; 2020. Serology Characteristics of SARS-CoV-2 Infection since the Exposure and post Symptoms Onset. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch K.L., Whitman J.D., Lacanienta N.P., Beckerdite E.W., Kastner S.A., Shy B.R., Goldgof G.M., Levine A.G., Bapat S.P., Stramer S.L. Magnitude and kinetics of anti-SARS-CoV-2 antibody responses and their relationship to disease severity. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Zeng W., He H., Zhao D., Jiang D., Zhou P., Cheng L., Li Y., Ma X., Jin T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020;17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairesse A., Favresse J., Eucher C., Elsen M., Tre-Hardy M., Haventith C., Gruson D., Dogne J.M., Douxfils J., Gobbels P. High clinical performance and quantitative assessment of antibody kinetics using a dual recognition assay for the detection of SARS-CoV-2 IgM and IgG antibodies. Clin. Biochem. 2020 doi: 10.1016/j.clinbiochem.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C.S., Lam E.T., Chan R.C., Tsang D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129:104500. doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020 doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercatelli D., Giorgi F.M. Geographic and genomic distribution of SARS-CoV-2 mutations. Front. Microbiol. 2020;11:1800. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P., Collier D., Ritchie A., Assennato S.M., Hosmillo M., Goel N., Meng B., Chatterjee K., Mendoza V., Temperton N. Combined point-of-care nucleic acid and antibody testing for SARS-CoV-2 following emergence of D614G spike variant. Cell Rep. Med. 2020;1:100099. doi: 10.1016/j.xcrm.2020.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos I., Gruson D., Kabamba B., Dahma H., Van den Wijngaert S., Reza S., Carbone V., Vandenberg O., Gulbis B., Wolff F. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020;128:104413. doi: 10.1016/j.jcv.2020.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A., Beavis K.G., Matushek S.M., Ciaglia C., Francois N., Tesic V., Love N. Detection of SARS-CoV-2 by use of the cepheid Xpert xpress SARS-CoV-2 and roche cobas SARS-CoV-2 assays. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00772-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourez T., Vabret A., Han Y., Dina J., Legrand L., Corbet S., Freymuth F. Baculovirus expression of HCoV-OC43 nucleocapsid protein and development of a Western blot assay for detection of human antibodies against HCoV-OC43. J. Virol. Methods. 2007;139:175–180. doi: 10.1016/j.jviromet.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder M., van der Vegt D.S.J.M., Oude Munnink B.B., GeurtsvanKessel C.H., van de Bovenkamp J., Sikkema R.S., Jacobs E.M.G., Koopmans M.P.G., Wegdam-Blans M.C.A. Reinfection of severe acute respiratory syndrome coronavirus 2 in an immunocompromised patient: a case report. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoba B., Gavkare A., Jamadar N., Mumbre S., Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID-19. J. Infect. Publ. Health. 2020 doi: 10.1016/j.jiph.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K., Poon B.H., Kiat Puar T.H., Shan Quah J.L., Loh W.J., Wong Y.J., Tan T.Y., Raghuram J. COVID-19 and the risk to health care workers: a case report. Ann. Intern. Med. 2020;172:766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L., Ye F., Cheng M.L., Feng Y., Deng Y.Q., Zhao H., Wei P., Ge J., Gou M., Li X. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971–977. doi: 10.1016/j.immuni.2020.04.023. e973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M., Gilboa T., Ogata A.F., Maley A.M., Cohen L., Cai Y., Zhang J., Feldman J.E., Hauser B.M., Caradonna T.M. medRxiv; 2020. Ultra-Sensitive High-Resolution Profiling of Anti-SARS-CoV-2 Antibodies for Detecting Early Seroconversion in COVID-19 Patients. [Google Scholar]
- Nuccetelli M., Pieri M., Grelli S., Ciotti M., Miano R., Andreoni M., Bernardini S. SARS-CoV-2 infection serology: a useful tool to overcome lockdown? Cell Death Dis. 2020;6:38. doi: 10.1038/s41420-020-0275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., Lamers M.M., Sikkema R.S., de Bruin E., Chandler F.D. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg. Infect. Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padoan A., Cosma C., Sciacovelli L., Faggian D., Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin. Chem. Lab. Med. 2020;58:1081–1088. doi: 10.1515/cclm-2020-0443. [DOI] [PubMed] [Google Scholar]
- Padoan A., Sciacovelli L., Basso D., Negrini D., Zuin S., Cosma C., Faggian D., Matricardi P., Plebani M. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: a longitudinal study. Clin. Chim. Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Zhou P., Fan T., Wu Y., Zhang J., Shi X., Shang W., Fang L., Jiang X., Shi J. Immunoglobulin fragment F(ab')2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antivir. Res. 2020:104868. doi: 10.1016/j.antiviral.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M.R., Mohan S., Cohen D.J., Husain S.A., Dube G.K., Ratner L.E., Arcasoy S., Aversa M.M., Benvenuto L.J., Dadhania D.M. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am. J. Transplant. 2020;20:1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L., Park Y.J., Tortorici M.A., Czudnochowski N., Walls A.C., Beltramello M., Silacci-Fregni C., Pinto D., Rosen L.E., Bowen J.E. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020 doi: 10.1016/j.cell.2020.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering S., Betancor G., Galao R.P., Merrick B., Signell A.W., Wilson H.D., Kia Ik M.T., Seow J., Graham C., Acors S. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh C.M., Carissimo G., Wang B., Amrun S.N., Lee C.Y., Chee R.S., Fong S.W., Yeo N.K., Lee W.H., Torres-Ruesta A. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 2020;11:2806. doi: 10.1038/s41467-020-16638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland G.A., Ovsyannikova I.G., Kennedy R.B. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A., Cornaby C., Bartelt L., Weiss S., Park Y. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost J., Gasser R., Beaudoin-Bussieres G., Richard J., Duerr R., Laumaea A., Anand S.P., Goyette G., Benlarbi M., Ding S. Cross-sectional evaluation of humoral responses against SARS-CoV-2 spike. Cell Rep. Med. 2020;1:100126. doi: 10.1016/j.xcrm.2020.100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu R., Ling Y., Zhang Y.H., Wei L.Y., Chen X., Li X.M., Liu X.Y., Liu H.M., Guo Z., Ren H. Platelet-to-lymphocyte ratio is associated with prognosis in patients with coronavirus disease-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabi F.A., Al Zoubi M.S., Kasasbeh G.A., Salameh D.M., Al-Nasser A.D. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9 doi: 10.3390/pathogens9030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randad P.R., Pisanic N., Kruczynski K., Manabe Y.C., Thomas D., Pekosz A., Klein S., Betenbaugh M.J., Clarke W.A., Laeyendecker O. medRxiv; 2020. COVID-19 Serology at Population Scale: SARS-CoV-2-specific Antibody Responses in Saliva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M., Rodriguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramirez-Santana C., Diaz-Coronado J.C., Manrique R. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokni M., Ghasemi V., Tavakoli Z. Immune responses and pathogenesis of SARS-CoV-2 during an outbreak in Iran: comparison with SARS and MERS. Rev. Med. Virol. 2020;30 doi: 10.1002/rmv.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Malaker R., Sajib M.S.I., Hasanuzzaman M., Rahman H., Ahmed Z.B., Islam M.S., Islam M., Hooda Y., Ahyong V. Complete genome sequence of a novel coronavirus (SARS-CoV-2) isolate from Bangladesh. Microbiol. Resour. Announc. 2020;9 doi: 10.1128/MRA.00568-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53:248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S.P., Thompson P., Smith M., Lercher D.M., Rimland C.A., Bartelt L., Park Y.A., Weiss S., Markmann A.J., Raut R. Convalescent plasma therapy in four critically ill pediatric patients with coronavirus disease 2019: a case series. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance E.G., Bossis I., Dickerson F.B., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H., Viscidi R.P. Development of a nucleocapsid-based human coronavirus immunoassay and estimates of individuals exposed to coronavirus in a U.S. metropolitan population. Clin. Vaccine Immunol. 2008;15:1805–1810. doi: 10.1128/CVI.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Wang N., Zou Q.M. [Progress and challenge of vaccine development against 2019-novel coronavirus (2019-nCoV)] Zhonghua Yufang Yixue Zazhi. 2020;54:614–619. doi: 10.3760/cma.j.cn112150-20200317-00366. [DOI] [PubMed] [Google Scholar]
- Shirin T., Bhuiyan T.R., Charles R.C., Amin S., Bhuiyan I., Kawser Z., Rahat A., Alam A.N., Sultana S., Aleem M.A. Antibody responses after COVID-19 infection in patients who are mildly symptomatic or asymptomatic in Bangladesh. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.09.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall M.C., Dowlatshahi M., Spitalnik S.L., Hod E.A., Rai A.J. Types of assays for SARS-CoV-2 testing: a review. Lab. Med. 2020;51:e59–e65. doi: 10.1093/labmed/lmaa039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall M.C., Scherberkova I., Whittier S., Green D.A. Comparison of cepheid Xpert xpress and abbott ID Now to roche cobas for the rapid detection of SARS-CoV-2. J. Clin. Virol. 2020;128:104428. doi: 10.1016/j.jcv.2020.104428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protoc. Microbiol. 2020;57 doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli P., Faggioni G., Lo Presti A., Fiore S., Marchi A., Benedetti E., Fabiani C., Anselmo A., Ciammaruconi A., Fortunato A. Whole genome and phylogenetic analysis of two SARS-CoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.13.2000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S., Wisniak A., Piumatti G., Azman A.S., Lauer S.A., Baysson H., De Ridder D., Petrovic D., Schrempft S., Marcus K. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396:313–319. doi: 10.1016/S0140-6736(20)31304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J. Appl. Lab. Med. 2020 doi: 10.1093/jalm/jfaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J. Appl. Lab. Med. 2020;5:908–920. doi: 10.1093/jalm/jfaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J. Med. Virol. 2020;92:612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Wang Q., Zhang D., Ding J., Huang Q., Tang Y.Q., Wang Q., Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct. Target Ther. 2020;5:33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theel E.S., Slev P., Wheeler S., Couturier M.R., Wong S.J., Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillett R.L., Sevinsky J.R., Hartley P.D., Kerwin H., Crawford N., Gorzalski A., Laverdure C., Verma S.C., Rossetto C.C., Jackson D. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C., Cai J.P., Chan J.M., Chik T.S. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L., Owen C.J., Pang J., Tan C.C.S., Boshier F.A.T. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect. Genet. Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292 e286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Du Q., Guo B., Mu D., Lu X., Ma Q., Guo Y., Fang L., Zhang B., Zhang G. A method to prevent SARS-CoV-2 IgM false positives in gold immunochromatography and enzyme-linked immunosorbent assays. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.00375-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wu J.L., Tseng W.P., Lin C.H., Lee T.F., Chung M.Y., Huang C.H., Chen S.Y., Hsueh P.R., Chen S.C. Four point-of-care lateral flow immunoassays for diagnosis of COVID-19 and for assessing dynamics of antibody responses to SARS-CoV-2. J. Infect. 2020;81:435–442. doi: 10.1016/j.jinf.2020.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in hubei province, China. JAMA Ophthalmol. 2020 doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang F., Wang X., He X., Peng Z., Yang B., Zhang J., Zhou Q., Ye H., Ma Y., Li H. Antibody detection and dynamic characteristics in patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao A.T., Tong Y.X., Gao C., Zhu L., Zhang Y.J., Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J. Clin. Virol. 2020;127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P.D., Potdar V.A., Choudhary M.L., Nyayanit D.A., Agrawal M., Jadhav S.M., Majumdar T.D., Shete-Aich A., Basu A., Abraham P. Full-genome sequences of the first two SARS-CoV-2 viruses from India. Indian J. Med. Res. 2020;151:200–209. doi: 10.4103/ijmr.IJMR_663_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E.A., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113:e73–76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- Yong S.K., Su P.C., Yang Y.S. Molecular targets for the testing of COVID-19. Biotechnol. J. 2020;15 doi: 10.1002/biot.202000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.Q., Sun B.Q., Fang Z.F., Zhao J.C., Liu X.Y., Li Y.M., Sun X.Z., Liang H.F., Zhong B., Huang Z.F. Distinct features of SARS-CoV-2-specific IgA response in COVID-19 patients. Eur. Respir. J. 2020 doi: 10.1183/13993003.01526-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurkovetskiy L., Wang X., Pascal K.E., Tomkins-Tinch C., Nyalile T., Wang Y., Baum A., Diehl W.E., Dauphin A., Carbone C. bioRxiv; 2020. SARS-CoV-2 Spike Protein Variant D614G Increases Infectivity and Retains Sensitivity to Antibodies that Target the Receptor Binding Domain. [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D., Fouchier R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zarletti G., Tiberi M., De Molfetta V., Bossù M., Toppi E., Bossù P., Scapigliati G. A cell-based ELISA to improve the serological analysis of anti-SARS-CoV-2 IgG. Viruses. 2020;12:1274. doi: 10.3390/v12111274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in wuhan, China. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Litvinova M., Liang Y., Wang Y., Wang W., Zhao S., Wu Q., Merler S., Viboud C., Vespignani A. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368:1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Lau J.Y.-N., Yang L., Ma Z.-G. SARS-CoV-2 reinfection in two patients who have recovered from COVID-19. Precision Clin. Med. 2020 doi: 10.1093/pcmedi/pbaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Li M., Song H., Chen J., Ren W., Feng Y., Gao G.F., Song J., Peng Y., Su B. Early detection of SARS-CoV-2 antibodies in COVID-19 patients as a serologic marker of infection. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.Y., Zhang M., Yang C.X., Zhang N., Wang X.C., Yang X.P., Dong X.Q., Zheng Y.T. Elevated exhaustion levels and reduced functional diversity of T cells in peripheral blood may predict severe progression in COVID-19 patients. Cell. Mol. Immunol. 2020;17:541–543. doi: 10.1038/s41423-020-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Song L. Novel antibody epitopes dominate the antigenicity of spike glycoprotein in SARS-CoV-2 compared to SARS-CoV. Cell. Mol. Immunol. 2020;17:536–538. doi: 10.1038/s41423-020-0385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J., Li Y.M., Poon Xie Z.H., Chan K.H., Li P.H., Tan S.Y., Chang Q., Xie J.P. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. Treatment with convalescent plasma for influenza A (H5N1) infection. New Engl. J. Med. 2007;357(14):1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G., Zhao Q. Perspectives on therapeutic neutralizing antibodies against the Novel Coronavirus SARS-CoV-2. Int. J. Biol. Sci. 2020;16:1718–1723. doi: 10.7150/ijbs.45123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Wang W., Wang H., Lu R., Tan W. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect. Dis. 2013;13:433. doi: 10.1186/1471-2334-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.