Abstract
Exercise has been recommended as an important strategy to improve glucose metabolism in obesity. Adipose tissue fibrosis is associated with inflammation and is implicated in glucose metabolism disturbance and insulin resistance in obesity. However, the effect of exercise on the progression of adipose tissue fibrosis is still unknown. The aim of the present study was to investigate whether exercise retarded the progression of adipose tissue fibrosis and ameliorated glucose homeostasis in diet-induced obese mice. To do so, obesity and adipose tissue fibrosis in mice were induced by high-fat diet feeding for 12 weeks and the mice subsequently received high-fat diet and exercise intervention for another 12 weeks. Exercise alleviated high-fat diet-induced glucose intolerance and insulin resistance. Continued high-fat diet feeding exacerbated collagen deposition and further increased fibrosis-related gene expression in adipose tissue. Exercise attenuated or reversed these changes. Additionally, PPARγ, which has been shown to inhibit adipose tissue fibrosis, was observed to be increased following exercise. Moreover, exercise decreased the expression of HIF-1α in adipose fibrosis, and adipose tissue inflammation was inhibited. In conclusion, our data indicate that exercise attenuates and even reverses the progression of adipose tissue fibrosis, providing a plausible mechanism for its beneficial effects on glucose metabolism in obesity.
Keywords: exercise, adipose tissue fibrosis, glucose metabolism, PPARγ, inflammation
Introduction
Type 2 diabetes mellitus (T2DM) has become a pandemic and a major global health problem. Obesity, which has a rapidly increasing prevalence, is considered to be a primary cause of T2DM (1, 2). Approximately 60%-90% of patients with T2DM suffer from obesity (3, 4). Overloaded energy intake and physical inactivity result in obesity characterized by rapid expansion of adipose tissue. As obesity progresses, adipose expansion outpaces the growth of vasculature, leading to hypoxia-induced HIF-1α activation (5, 6). In adipose tissue, the transcription factor HIF-1α promotes the mRNA expression of pro-fibrotic genes such as lysyl oxidase (LOX), collagens I (Col1), and collagens III (Col3), consequently leading to adipose tissue fibrosis (7, 8).
Adipocytes are surrounded by a network of extracellular matrix (ECM) mainly composed of collagens and fibronectin. Adipose fibrosis is defined as an excessive accumulation of ECM components (9). It has been demonstrated that adipose tissue fibrosis is strongly associated with inflammation. Adipose tissue inflammation causes insulin resistance, which in turn promotes inflammation (10). Adipose tissue mRNA levels of pro-fibrotic genes were positively correlated with fasting insulin levels and HOMA-IR in Chinese subjects (11). Induction of adipose tissue fibrosis by overexpression of HIF1α led to exacerbated adipose inflammation and aggravated glucose intolerance in mice fed with a high-fat diet (HFD) (7). On the other hand, suppression of fibrosis via HIF1α-inhibitor or adipose-selective expression of GTF2IRD1, a transcription factor that inhibited pro-fibrotic gene expression, alleviated adipose tissue inflammation and improved glucose metabolism in HFD-fed mice (8, 12).
Peroxisome proliferator-activated receptor gamma (PPARγ) is the focus of substantial research as its agonists have emerged as potent insulin sensitizers used in the treatment of type 2 diabetes. PPARγ activation has been proven to downregulate HIF-1α expression and reduce adipose tissue collagen levels (13, 14).
Regular exercise has long been considered as a cornerstone for the prevention and treatment of T2DM. Population studies reveal that moderate exercise each week lowers the risk of T2DM (15, 16). We have previously demonstrated that 12 weeks of moderate-intensity aerobic exercise prevented HFD-induced glucose intolerance and insulin resistance (17). In addition to this, results from numerous studies document the effectiveness of exercise for T2DM treatment. Exercise improves glycemic control, reducing insulin resistance in patients with T2DM (18). Adipose tissue fibrosis, which is implicated as a contributor to insulin resistance, has been demonstrated to be prevented by exercise in a study conducted by Kawanishi et al. (19); however, it is still unknown whether exercise mitigates existing adipose tissue fibrosis.
In this study, mice were fed a HFD for 12 weeks to induce adipose tissue fibrosis followed by 12 weeks of exercise intervention. We observed that exercise elevated PPARγ expression and effectively retarded the HFD-induced ongoing adipose tissue fibrosis. Inflammation in adipose tissue was significantly attenuated by exercise. The exercise-induced regression of adipose tissue fibrosis was accompanied with improved glucose homeostasis. Hence, exercise may have an anti-fibrotic effect on adipose tissue and may have benefits on glucose metabolism in obesity.
Materials and methods
Animal studies
All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Guangzhou Sport University. Male C57BL/6J mice aged 6 weeks were purchased from the Experimental Animal Center of Guangdong Province (Guangzhou, China). After a 2-week adaptation, mice were randomly divided into two groups: control group with normal diet (ND group; AIN-93G, D10012G, Research Diets, New Brunswick, NJ, USA) (n = 14) and high-fat diet-induced obese group fed for 12 weeks with 60% HFD (D12492, Research Diets) (n = 22). After 12 weeks of dietary intervention, six mice from the ND group and six mice from the high-fat diet-induced obese group were sacrificed, and epididymal adipose tissue was collected for adipose fibrosis assessment. The other eight mice in the ND group were continuously fed with the normal diet for another 12 weeks. The remaining 16 mice in the high-fat diet-induced obese group were re-randomized into two groups: high-fat diet group (HFD group) (n = 8) and high-fat diet plus exercise group (HFD+EXE group) (n = 8). The HFD mice and the HFD+EXE mice were fed with the high-fat diet for another 12 weeks. The HFD+EXE mice were subjected to exercise for 12 weeks over the same period. All animals were kept under 12 h light:12 h darkness cycle at 22°C–24°C, with free access to food and water. After the experimental period, all mice were kept sedentary for 2 days and were fasted overnight before being sacrificed for blood and epididymal and subcutaneous adipose tissue collection.
Exercise protocol
Mice were trained on a treadmill at 0% grade 5 days per week. The warm up was performed at 6 m/min for 5 min followed by 20 min of main exercise at 10 m/min, and 5 min of cool down at 6 m/min during the first week for adaptation. A routine consisting of 5 min of warm up at 9 m/min, 50 min of main exercise at 12 m/min (75% maximum oxygen consumption) (20), and 5 min of cool down at 9 m/min were performed from the 2nd week to the last week of the exercise intervention period.
Blood analysis
Mice were fasted overnight to measure the fasting blood glucose and insulin levels. Accu-Chek Performa glucometer (Roche) was used to detect blood glucose. Serum insulin levels was determined by commercial ELISA kit (Alpco Diagnostics, Salem, NH, USA).
Oral glucose tolerance test (OGTT) and insulin tolerance tests (ITT)
OGTT and ITT was conducted on the 10th week and on the 11th week of the exercise intervention period, respectively, as described before (17).
Histological and immunohistochemical analysis
Epididymal adipose samples were fixed in 4% paraformaldehyde solution for 24 h, embedded in paraffin, and sliced into 4-μm sections. The sections of adipose tissue were stained with hematoxylin–eosin (H&E) and Masson’s trichrome dye. The Masson-positive area was measured by ImageJ Software. The F4/80 staining was performed using the F4/80 antibody (Santa Cruz Biotechnology, sc-377009). Six randomly selected fields in each slide were analyzed by two investigators who were blinded to the treatment.
Measurement of hydroxyproline content
Both sides of epididymal adipose tissue were harvested and weighed promptly after sacrifice. Epididymal adipose tissue sample (100 mg) was used for hydroxyproline measurement, which was determined using a Hydroxyproline Assay Kit (Quickzyme Biosciences, Leiden, Netherlands). Hydroxyproline content was normalized to the weight of adipose tissue.
Hepatic triglyceride (TG) and total cholesterol (TC) measurement
Hepatic levels of TG and TC were determined using commercial kits (Applygen Technologies Inc., Beijing, China).
Western blot analysis
Total protein levels were determined using a BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of proteins were separated by SDS-PAGE and subsequently transferred to PVDF membrane. After blocking, the membranes were incubated over night at 4°C with primary antibodies to HIF-1α (Abcam, H1alpha67), PPARγ (Santa Cruz Biotechnology, sc-7273), IRS1 (Santa Cruz Biotechnology, sc-8038), phosphorylated IRS1 at Ser307 (Cell Signaling Technology, #2381), or GAPDH (Cell Signaling Technology, #5174). The membranes were then incubated for 1 h with the secondary antibodies: m-IgGκ BP-HRP (Santa Cruz Biotechnology, sc-516102) or mouse anti-rabbit IgG-HRP (Santa Cruz Biotechnology, sc-2357). Proteins were detected by ECL (Thermo Fisher Scientific), and the intensities of bands were quantified using Quantity One software.
Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from epididymal adipose tissue with TRIzol reagent (Thermo Fisher Scientific), followed by cDNA synthesis. The expression levels of mRNA were determined and normalized to the levels of β-actin. Sequences of primers used are shown in Supplementary Table 1 (see section on supplementary materials given at the end of this article).
Statistical analysis
All data were expressed as mean ± s.d. values. For the data that were normally distributed, the statistical significance between two groups was evaluated by two-tailed Student’s t-test, and the statistical significance among three groups by one-way ANOVA with Bonferroni’s multiple comparison test. For the data that were not normally distributed, the statistical significance between two groups was evaluated by Mann–Whitney test, and the statistical significance among three groups by Kruskal–Wallis test with Dunn’s multiple comparison test. All analyses were performed using SPSS 20.0 and GraphPad Prism 7.00. Differences with a P value less than 0.05 were significant.
Results
Exercise improves glucose homeostasis in mice with diet-induced obesity
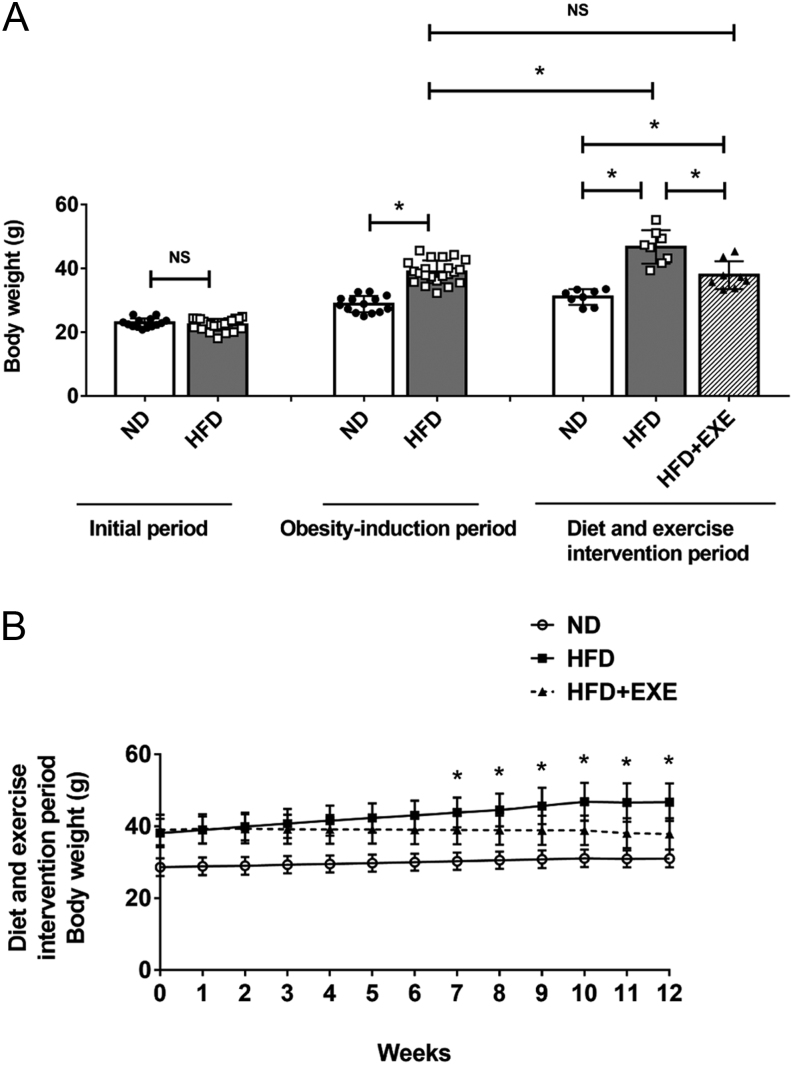
HFD-fed mice displayed significantly increased body weight compared to the ND mice after the 12-week obesity-induction period (Fig. 1A). The HFD-induced obese mice were subsequently subjected to either a HFD feeding or HFD plus exercise intervention for an additional 12 weeks (the diet and exercise intervention period). Continued HFD feeding for another 12 weeks resulted in weight gain in the HFD group mice, but failed to induce increased body weight in the HFD+EXE group mice as shown in Fig. 1. The mice in the HFD+EXE group showed decreased body weight compared to the mice in the HFD group (Fig. 1A and B). Significant decreases in epididymal fat mass and subcutaneous fat mass were observed after 12 weeks of exercise. Hepatic TG and TC levels were also reduced (Table 1).
Figure 1.
Exercise reduces body weight in high-fat diet-induced obese mice. Male C57BL/6J mice (n = 36) were randomly divided into normal diet group (ND group, n = 14) and high-fat diet group (HFD group, n = 22). After 12-week obesity-induction period, the body weight of all mice was measured and six mice from the ND group and six mice from the HFD group were sacrificed. The remaining mice received 12 weeks of diet and exercise intervention (diet and exercise intervention period): the mice in the ND group (n = 8) were continuously fed the normal diet; the high-fat diet-fed mice were divided into the high-fat diet group (HFD group, n = 8) and the high-fat diet plus exercise group (HFD+EXE group, n = 8). (A) Initial body weight, body weight at the end of obesity-induction period and body weight at the end of diet and exercise intervention period. (B) Body weight measured weekly during the diet and exercise intervention period. Data are presented as the mean ± s.d. For panel A, *P < 0.05; for panel B, *P < 0.05 HFD+EXE vs HFD.
Table 1.
Metabolic parameters were detected after the diet and exercise intervention period. C57BL/6J mice received a normal diet (ND), a high-fat diet (HFD) or a high-fat diet plus exercise training (HFD+EXE).
| Metabolic parameters | ND | HFD | HFD+EXE |
|---|---|---|---|
| Epididymal fat weight (g) | 0.41 ± 0.14 | 2.35 ± 0.53a | 0.99 ± 0.53ab |
| Epididymal fat/body weight ratio (%) | 1.32 ± 0.43 | 4.96 ± 0.66a | 2.52 ± 1.14ab |
| Subcutaneous fat weight (g) | 0.29 ± 0.15 | 2.65 ± 0.61a | 1.42 ± 0.56ab |
| Subcutaneous fat/body weight ratio (%) | 0.93 ± 0.44 | 5.61 ± 0.77a | 3.67 ± 1.18ab |
| Hepatic TG (μmol/g protein) | 249.68 ± 77.79 | 942.83 ± 238.77a | 432.26 ± 158.43b |
| Hepatic TC (μmol/g protein) | 23.60 ± 3.74 | 119.37 ± 21.90a | 67.26 ± 26.13ab |
Data are mean ± s,d, n = 8 per group.
aP < 0.05 for difference from ND; bP < 0.05 for difference from HFD.
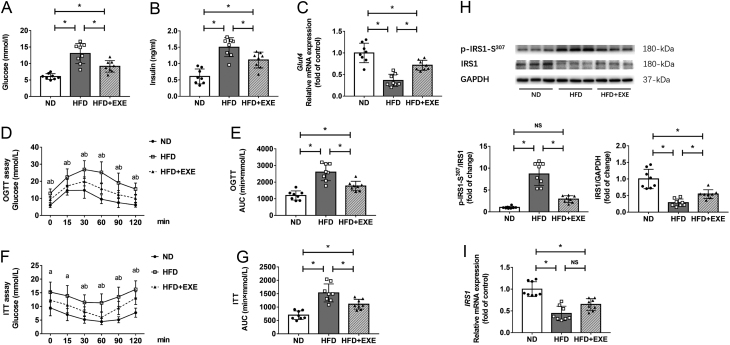
We analyzed parameters related to glucose metabolism after the diet and exercise intervention period. The HFD+EXE mice exhibited reduced levels of fasting blood glucose and decreased levels of serum insulin relative to the HFD mice (Fig. 2A and B). We performed OGTT and ITT to determine the influence of exercise on glucose tolerance and insulin sensitivity. Compared to the HFD mice, the HFD+EXE mice showed improved glucose tolerance and enhanced insulin sensitivity, as indicated by reduced glucose excursion and lower area under the curve (AUC) values during OGTT and ITT (Fig. 2D, E, F and G). Insulin receptor substrate-1 (IRS1) is a key adaptor protein in insulin signaling. Phosphorylation of this protein at Ser-307 has been known to lead to its degradation and subsequent impairment of downstream insulin signaling (21). The HFD feeding increased levels of IRS phosphorylation at Ser-307 and decreased IRS-1 protein levels in adipose tissue. These changes were attenuated by exercise (Fig. 2H). Additionally, exercise caused an insignificant increase in mRNA expression of IRS1 and significantly enhanced glucose transporter 4 (Glut4) mRNA expression (Fig. 2C and I). These data demonstrate that exercise normalizes the disturbed glucose metabolism in diet-induced obese mice.
Figure 2.
Exercise improves glucose metabolism in high-fat diet-induced obese mice. Glucose metabolism-related parameters were measured after the diet and exercise intervention period (n = 8). (A) Fasting blood glucose levels. (B) Serum levels of insulin in fasted state. (C) mRNA levels of Glut4 in adipose tissue. (D) OGTT was performed in mice fasted overnight, with glucose (1 mg/g) administrated intragastrically. (E) AUC analysis during OGTT. (F) For the ITT, mice were fasted for 3 h and insulin (0.75 IU/kg) injected intraperitoneally. (G) AUC analysis during ITT. (H) Western blot analysis of IRS1 and phosphorylation of IRS-1 (p-IRS1-S307). (I) mRNA levels of IRS1 in adipose tissue. Data are presented as the mean ± s.d., *P < 0.05.
Exercise reverses existing collagen deposition in adipose tissue of mice with diet-induced obesity
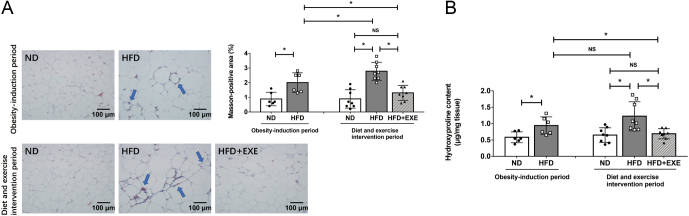
We first examined whether adipose tissue fibrosis was established after the 12-week obesity-induction period. The 12-week HFD feeding triggered excess collagen deposition in adipose tissue compared to ND feeding, as demonstrated by an increased positive Masson staining area and elevated levels of hydroxyproline, an indicator for collagen in adipose tissue (Fig. 3A and B). Then, for an additional 12 weeks, the HFD-induced obese mice received HFD feeding or HFD plus exercise intervention. The 24-week HFD-fed mice showed a greater increase in positive Masson staining area compared to the 12-week HFD-fed mice (Fig. 3A). In addition, we observed that adipose hydroxyproline levels had a tendency to be elevated, although this did not reach statistical significance in mice fed with HFD for 24 weeks (Fig. 3B). These data suggest that the 24-week HFD feeding exacerbates adipose tissue fibrosis to a greater extent compared to the 12-week HFD feeding. We evaluated the effect of exercise on the existing collagen deposition in adipose tissue. Fewer collagen fibers around adipocytes in adipose tissue were observed in the HFD+EXE mice compared to the HFD mice as shown by Masson’s trichrome staining after the 12-week HFD and exercise intervention period (Fig. 3A). Adipose tissue levels of hydroxyproline were also significantly decreased after exercise intervention (Fig. 3B). Positive Masson staining area and hydroxyproline levels in the HFD+EXE mice decreased to levels almost equal to those in the ND mice and were significantly lower than those in the 12-week HFD-induced obese mice. This indicated that exercise retarded and reversed the ongoing adipose tissue fibrosis.
Figure 3.
Exercise reverses existing collagen deposition in high-fat diet-induced obese mice. Masson’s trichrome staining and hydroxyproline content measurement were performed after the obesity-induction period (n = 6) and after the diet and exercise intervention period (n = 8). (A) Representative images (20× objective) of adipose tissue sections stained with Masson’s trichrome dye (collagen appears blue as arrows pointed out). The Masson-positive area was quantified using ImageJ Software. (B) Hydroxyproline content in adipose tissue. Data are presented as the mean ± s.d., *P < 0.05.
Exercise reduces fibrosis-related genes expression in adipose tissue of mice with diet-induced obesity
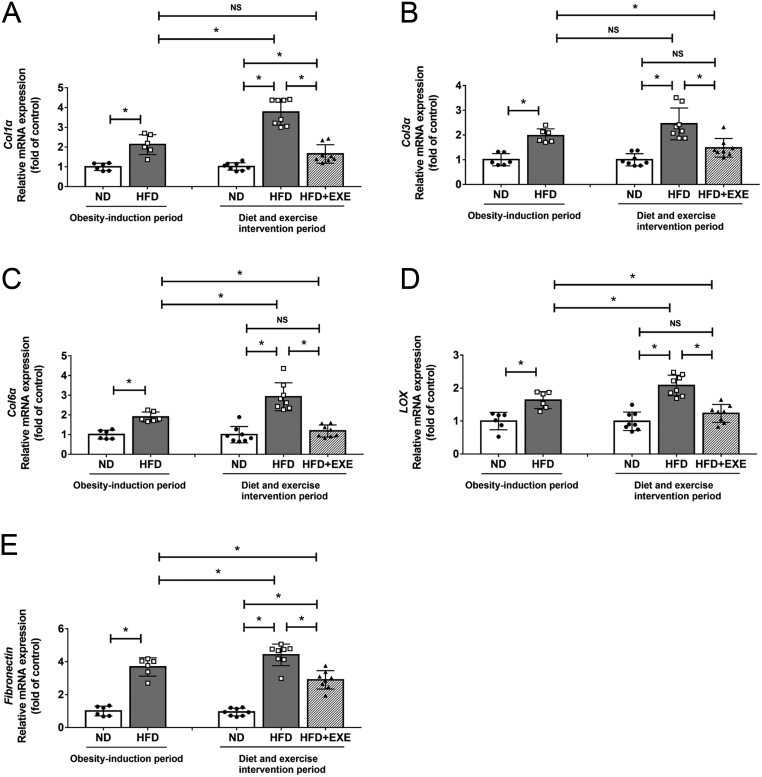
Collagen and fibronectin are the main protein components of adipose ECM (9). LOX is an enzyme responsible for collagen cross-linking. We examined the mRNA levels of Col1α1, Col3α1, Col6α1, three major types of collagen in the ECM, and LOX as well as fibronectin in adipose tissue. All these genes were substantially upregulated in the 12-week HFD feeding as shown in Fig. 4. Col1α1, Col6α1, LOX, and fibronectin levels were further increased after 24 weeks-feeding on HFD than 12 weeks-feeding on HFD. HFD-induced upregulation of fibrosis-related genes expression were effectively inhibited by exercise. The levels of Col3α1, Col6α1, LOX and fibronectin in the HFD+EXE mice were even lower than those in the mice fed HFD for 12 weeks (Fig. 4A, B, C, D and E).
Figure 4.
Exercise reduces fibrosis-related genes expression in adipose tissue. We detected adipose tissue fibrosis-related genes expression after the obesity-induction period (n = 6) and after the diet and exercise intervention period (n = 8). (A) mRNA levels of Col1α1, (B) mRNA levels of Col3α1, (C) mRNA levels of Col6α1, (D) mRNA levels of LOX, (E) mRNA levels of fibronectin. Data are presented as the mean ± s.d., *P < 0.05.
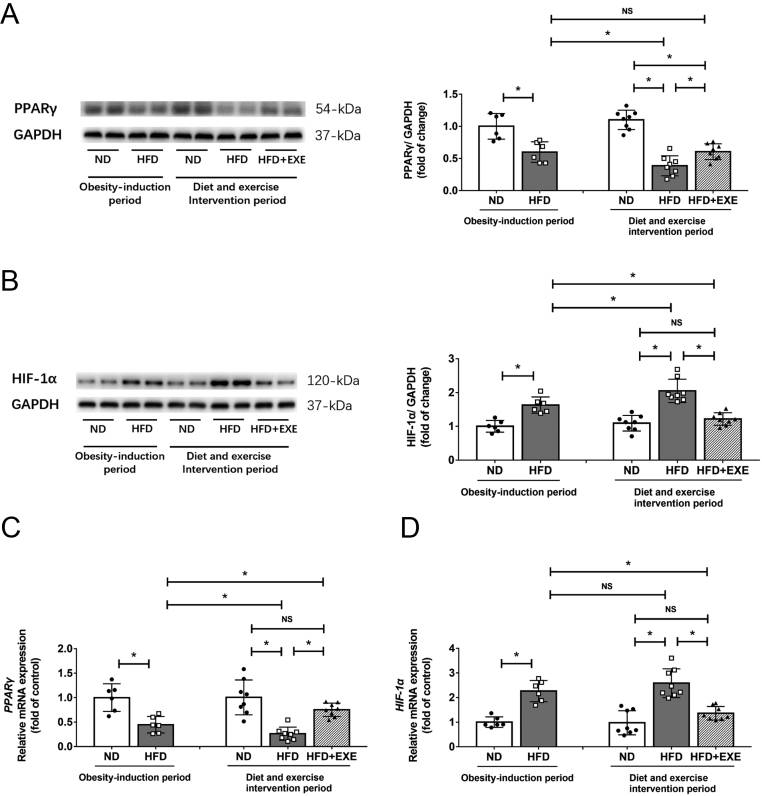
Exercise increased PPARγ expression and decreases HIF-1α levels in adipose tissue of mice with diet-induced obesity
PPARγ activation has been known to decrease HIF-1α expression and ameliorate adipose tissue fibrosis (13, 14). We further investigated the influence of exercise on PPARγ and HIF-1α in adipose tissue. The 12-week HFD feeding caused decreased protein and mRNA expression of PPARγ compared to the normal diet feeding. The levels of PPARγ were reduced further by another 12 weeks of HFD feeding, and these reductions were mitigated by exercise (Fig. 5A and C). Both protein and mRNA expression of HIF-1α were markedly elevated after HFD feeding. The HFD-induced upregulation of HIF-1α was largely suppressed by exercise (Fig. 5B and D).
Figure 5.
Exercise enhances PPARγ levels and down-regulates HIF-1α expression in adipose tissue. PPARγ and HIF-1α levels in adipose tissue were examined after the obesity-induction period (n = 6) and after the diet and exercise intervention period (n = 8). (A) Western blot analysis of PPARγ. (B) Western blot analysis of HIF-1α. (C) mRNA levels of PPARγ, (D) mRNA levels of HIF-1α. Data are presented as the mean ± s.d., *P < 0.05.
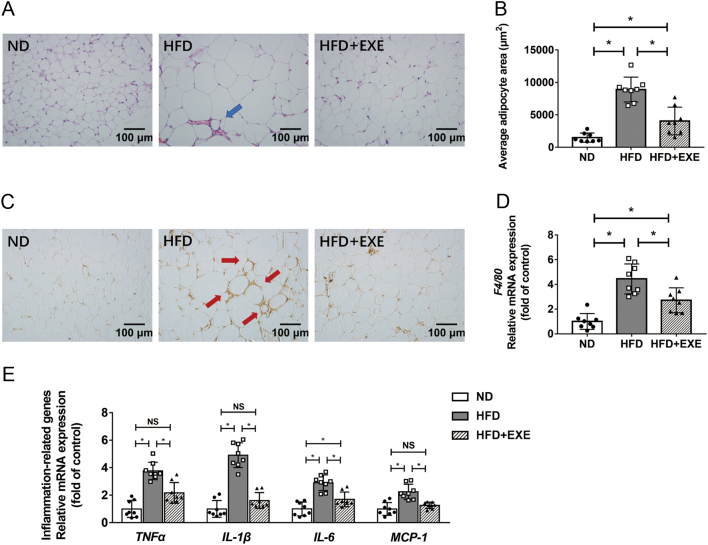
Exercise alleviates adipose tissue inflammation in mice with diet-induced obesity
We explored the effect of exercise on adipose tissue inflammation in HFD-induced obese mice since adipose tissue fibrosis is tightly linked to inflammation, which in return contributes to insulin resistance (10). H&E staining revealed that HFD feeding resulted in adipocyte enlargement, increased infiltration of immune cells in adipose tissue, and formation of crown-like structures (CLS). These effects were significantly, although not completely, attenuated by exercise (Fig. 6A and B). We performed immunohistochemical staining of the macrophage marker F4/80 and examined the mRNA levels of F4/80 to detect the levels of macrophages infiltrated in adipose tissue. Exercise significantly inhibited HFD-induced expression of F4/80 (Fig. 6C and D). Furthermore, exercise reduced the mRNA expression of proinflammatory cytokines TNFα, IL-1β, IL-6 and MCP-1 in adiposes tissue of HFD-induced obese mice (Fig. 6E).
Figure 6.
Exercise attenuates adipose tissue inflammation in high-fat diet-induced obese mice. We detected the effect of exercise on adipose tissue inflammation after the diet and exercise intervention period (n = 8). (A) The H&E staining images of adipose tissue were observed using a 20× objective (the arrows point to the crown-like structures formed by immune cell aggregation). (B) The average adipocyte area was estimated using Leica Application Suite V3. (C) Images of F4/80 stained adipose tissue sections were taken using a 20× objective (the arrows point to the crown-like structures formed by macrophage aggregation). (D) mRNA levels of F4/80 in adipose tissue. (E) mRNA expression of inflammation-related genes in adipose tissue. Data are presented as the mean ± s.d., *P < 0.05.
Discussion
The prevalence of T2DM has greatly increased. In 2013, 382 million adults aged 20–70 suffered from T2DM worldwide. It is estimated that this number will rise to over 592 million by 2035 (22). Sedentary lifestyle has been seen as one of the primary causes of the rapidly rising incidence of T2DM, and exercise is considered integral for T2DM prevention and management. The American Diabetes Association recommendations for T2DM prevention or delay include moderate-intensity physical activity more than 150 min/week (23). In our previous study, mice were fed with HFD and subjected to exercise intervention for 12 weeks over the same period, and the results showed that 12 weeks of HFD feeding resulted in impaired glucose tolerance and insulin resistance. Exercise showed a preventive effect on the HFD-induced disturbance of glucose metabolism (17). In the present study, mice received a 60% HFD for 12 weeks to induce obesity before the 12-week exercise regimen. After 12 weeks of exercise, the HFD+EXE mice had better glucose tolerance and less insulin resistance compared with the HFD mice, demonstrating that exercise was effective in the treatment of HFD-induced impaired systemic glucose metabolism. Liver, adipose tissue and muscle are involved in the maintenance of glucose homeostasis (24). Studies have shown the beneficial effect of exercise against insulin resistance in these organs (17, 25, 26). Our results showed that exercise increased Glut4 and IRS1 levels and decreased phosphorylation of IRS1 at Ser-307 in adipose tissue, suggesting the protective effect of exercise against insulin resistance in adipose tissue.
Obesity is one of the major risk factors for T2DM. Mechanisms linking obesity to deregulated glucose homeostasis are complicated. Since adipose tissue acts as a crucial energy-sensing and storage organ in obesity and is one of the major insulin target tissues, it is natural to speculate that it plays a part in obesity-induced insulin resistance. In fact, it is now generally appreciated that adipose tissue not only serves as a major storage depot for lipids, but also plays an important role in maintaining systemic metabolic homeostasis (27). There are some possible links between adipose tissue function and obesity-induced disturbance of glucose metabolism (28). A growing body of research suggests that adipose tissue fibrosis, a hallmark of adipose tissue dysfunction, promotes inflammation, ultimately driving insulin resistance (10, 12, 29, 30). Inhibition of adipose tissue fibrosis and inflammation emerges as a promising therapeutic approach for obesity-related T2DM (31, 32). Moderate exercise is a novel strategy for T2DM prevention and treatment. However, the underlying mechanisms for the exercise-induced benefits on T2DM are still not fully understood. Here we demonstrated that 12 weeks of exercise suppressed the progression of adipose tissue fibrosis and alleviated inflammation in HFD-induced obese mice, providing a mechanistic explanation for the protective effect of exercise against HFD-induced impairment of glucose tolerance and insulin resistance.
Fibrosis, defined as excess ECM deposition, can occur in all organs and tissues during chronic metabolic diseases, such as fatty liver disease, diabetes and hypertension. In these diseases, pathological fibrosis causes tissue dysfunction and ultimately drives disease progression (33). Although fibrosis was previously considered to be irreversible, growing evidence indicates that fibrosis can be reversed under certain circumstances (34). In the present study, adipose tissue fibrosis was established by the 12-week HFD feeding. The mice with adipose tissue fibrosis were then subjected to the HFD and exercise intervention for another 12 weeks. The continuous HFD feeding exacerbated adipose tissue fibrosis. It is noteworthy that the levels of Masson-positive area, and hydroxyproline contents and fibrosis-associated genes after the HFD plus exercise intervention were significantly lower than those before the HFD plus exercise intervention. These results suggested that the ongoing fibrosis in adipose tissue could be retarded, even reversed by exercise.
The mechanisms underlying adipose fibrosis is complicated. In addition to adipocytes, macrophages and myofibroblasts are also involved in the development of adipose tissue fibrosis (35). During obesity, endogenous and exogenous stimuli promote the shift of anti-inflammatory macrophage M2 polarization to proinflammatory M1 polarization (36). M1 macrophages produce proinflammatory cytokines, which exacerbate adipose tissue fibrosis. On the other hand, a lot of macrophages surround dead or dying adipocytes, forming CLS. Macrophage-inducible C-type lectin in these macrophages activates myofibroblasts and induces fibrosis-related genes expression (35, 37, 38). It has been demonstrated that macrophage depletion mitigates adipose tissue fibrosis (39). In the present study, we observed that exercise reduced the levels of proinflammatory cytokines and CLS formation in adipose tissue. Whether the exercise-mediated anti-inflammatory effect is responsible for its anti-fibrosis effect needs further study.
PPARγ is a predominant transcription factor which regulates glucose metabolism in adipose tissue. PPARγ agonist treatment primes monocytes into anti-inflammatory M2 macrophages and decreases proinflammatory cytokine expression (40). In addition, PPARγ down-regulates HIF-1α and promotes angiogenesis (13, 41). These effects may contribute to anti-fibrosis. Indeed, a previous study has demonstrated that PPARγ activation leads to reduction in fibrosis in adipose tissue (14). In our current study, we found that exercise upregulated PPARγ expression, which may account for exercise-mediated attenuation of adipose tissue fibrosis.
Exercise provides many health benefits due to its weight-loss effect. In the present study, we observed that just 7 weeks of exercise significantly reduced body weight in HFD-induced obese mice. It is generally accepted that exercise-induced weight loss is due to increases in energy expenditure. A recent study conducted by Joshua Cordeira and Daniel Monahan indicated that exercise decreased weight gain by reducing food consumption (42). Weight loss may account for the exercise-induced anti-fibrosis effect. As mentioned earlier, 12 weeks of exercise reduced the levels of fibrosis-related parameters to levels even lower than that in pre-HFD plus exercise intervention, while exercise only decreased body weight to levels approximately equal to that in pre-HFD plus exercise intervention. In other words, the exercise-mediated anti-fibrosis effect seems to be stronger than its weight-loss effect, suggesting that the observed exercise-induced inhibition of fibrosis did not completely depend on the weight-loss effect.
Taken together, the present study demonstrates that exercise retards ongoing adipose tissue fibrosis, and may thereby improve glucose homeostasis and insulin resistance in HFD-induced obese mice.
Supplementary Material
Declaration of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by grants from the Natural Science foundation of Guangdong Province of China (2019A1515012204), the National Natural Science foundation of China (31801006) and the Project of Administration of Traditional Chinese Medicine of Guangdong Province of China (20212112).
References
- 1.DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GIet al. Type 2 diabetes mellitus. Nature Reviews: Disease Primers 2015. 1 15019. ( 10.1038/nrdp.2015.19) [DOI] [PubMed] [Google Scholar]
- 2.Patel P, Abate N.Body fat distribution and insulin resistance. Nutrients 2013. 5 2019–2027. ( 10.3390/nu5062019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selvin E, Parrinello CM, Sacks DB, Coresh J.Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Annals of Internal Medicine 2014. 160 517–525. ( 10.7326/M13-2411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Shen L, Sun X, Liu F, Feng W, Jiang C, Chu X, Ye X, Jiang C, Wang Yet al. Adipose group 1 innate lymphoid cells promote adipose tissue fibrosis and diabetes in obesity. Nature Communications 2019. 10 3254. ( 10.1038/s41467-019-11270-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, Aminuddin A, Senda S, Tsuneyama K, Ikutani Met al. HIF-1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 2016. 65 3649–3659. ( 10.2337/db16-0012) [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Neinast MD, Frank AP, Sun K, Park J, Zehr JA, Vishvanath L, Morselli E, Amelotte M, Palmer BFet al. ERalpha upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Molecular Metabolism 2014. 3 642–651. ( 10.1016/j.molmet.2014.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJet al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and Cellular Biology 2009. 29 4467–4483. ( 10.1128/MCB.00192-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE.Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Molecular and Cellular Biology 2013. 33 904–917. ( 10.1128/MCB.00951-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K, Tordjman J, Clement K, Scherer PE.Fibrosis and adipose tissue dysfunction. Cell Metabolism 2013. 18 470–477. ( 10.1016/j.cmet.2013.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, Kusminski CM, Scherer PE.Adipose tissue remodeling and obesity. Journal of Clinical Investigation 2011. 121 2094–2101. ( 10.1172/JCI45887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alba DL, Farooq JA, Lin MYC, Schafer AL, Shepherd J, Koliwad SK.Subcutaneous fat fibrosis links obesity to insulin resistance in Chinese Americans. Journal of Clinical Endocrinology and Metabolism 2018. 103 3194–3204. ( 10.1210/jc.2017-02301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Ikeda K, Chen Y, Alba DL, Stifler D, Shinoda K, Hosono T, Maretich P, Yang Y, Ishigaki Yet al. Repression of adipose tissue fibrosis through a PRDM16-GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metabolism 2018. 27 180, .e6–194.e6. ( 10.1016/j.cmet.2017.12.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum JI, Bijli KM, Murphy TC, Kleinhenz JM, Hart CM.Time-dependent PPARγ modulation of HIF-1α signaling in hypoxic pulmonary artery smooth muscle cells. American Journal of the Medical Sciences 2016. 352 71–79. ( 10.1016/j.amjms.2016.03.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE.Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and Cellular Biology 2009. 29 1575–1591. ( 10.1128/MCB.01300-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AD, Crippa A, Woodcock J, Brage S.Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies. Diabetologia 2016. 59 2527–2545. ( 10.1007/s00125-016-4079-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayor S.Moderate exercise cuts type 2 diabetes risk but more is better, review finds. BMJ 2016. 355 i5605. ( 10.1136/bmj.i5605) [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Liu L, Wei Y, Fang C, Zhou F, Chen J, Han Q, Huang M, Tan X, Liu Qet al. Exercise ameliorates the FGF21-adiponectin axis impairment in diet-induced obese mice. Endocrine Connections 2019. 8 596–604. ( 10.1530/EC-19-0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amanat S, Ghahri S, Dianatinasab A, Fararouei M, Dianatinasab M.Exercise and Type 2 diabetes. Advances in Experimental Medicine and Biology 2020. 1228 91–105. ( 10.1007/978-981-15-1792-1_6) [DOI] [PubMed] [Google Scholar]
- 19.Kawanishi N, Niihara H, Mizokami T, Yano H, Suzuki K.Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochemical and Biophysical Research Communications 2013. 440 774–779. ( 10.1016/j.bbrc.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 20.Fernando P, Bonen A, Hoffman-Goetz L.Predicting submaximal oxygen consumption during treadmill running in mice. Canadian Journal of Physiology and Pharmacology 1993. 71 854–857. ( 10.1139/y93-128) [DOI] [PubMed] [Google Scholar]
- 21.Gual P, Le Marchand-Brustel Y, Tanti JF.Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 2005. 87 99–109. ( 10.1016/j.biochi.2004.10.019) [DOI] [PubMed] [Google Scholar]
- 22.Ley SH, Hamdy O, Mohan V, Hu FB.Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014. 383 1999–2007. ( 10.1016/S0140-6736(1460613-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association. 5. Prevention or delay of Type 2 diabetes. Diabetes Care 2017. 40 S44–S47. ( 10.2337/dc17-S008) [DOI] [PubMed] [Google Scholar]
- 24.Petersen MC, Shulman GI.Mechanisms of insulin action and insulin resistance. Physiological Reviews 2018. 98 2133–2223. ( 10.1152/physrev.00063.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Wan J, Xu Z, Hua T, Sun Q.Exercise ameliorates insulin resistance via regulating TGFβ-activated kinase 1 (TAK1)-mediated insulin signaling in liver of high-fat diet-induced obese rats. Journal of Cellular Physiology 2019. 234 7467–7474. ( 10.1002/jcp.27508) [DOI] [PubMed] [Google Scholar]
- 26.Reidy PT, Mahmassani ZS, McKenzie AI, Petrocelli JJ, Summers SA, Drummond MJ.Influence of exercise training on skeletal muscle insulin resistance in aging: spotlight on muscle ceramides. International Journal of Molecular Sciences 2020. 21 1514. ( 10.3390/ijms21041514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern JH, Scherer PE.Adipose tissue biology in 2014: advances in our understanding of adipose tissue homeostasis. Nature Reviews: Endocrinology 2015. 11 71–72. ( 10.1038/nrendo.2014.219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpe F, Tan GD.Adipose tissue function in the insulin-resistance syndrome. Biochemical Society Transactions 2005. 33 1045–1048. ( 10.1042/BST0331045) [DOI] [PubMed] [Google Scholar]
- 29.Lawler HM, Underkofler CM, Kern PA, Erickson C, Bredbeck B, Rasouli N.Adipose tissue hypoxia, inflammation, and fibrosis in obese insulin-sensitive and obese insulin-resistant subjects. Journal of Clinical Endocrinology and Metabolism 2016. 101 1422–1428. ( 10.1210/jc.2015-4125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdennour M, Reggio S, Le Naour G, Liu Y, Poitou C, Aron-Wisnewsky J, Charlotte F, Bouillot JL, Torcivia A, Sasso Met al. Association of adipose tissue and liver fibrosis with tissue stiffness in morbid obesity: links with diabetes and BMI loss after gastric bypass. Journal of Clinical Endocrinology and Metabolism 2014. 99 898–907. ( 10.1210/jc.2013-3253) [DOI] [PubMed] [Google Scholar]
- 31.Lee BC, Lee J.Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochimica et Biophysica Acta 2014. 1842 446–462. ( 10.1016/j.bbadis.2013.05.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun K, Park J, Gupta OT, Holland WL, Auerbach P, Zhang N, Goncalves Marangoni R, Nicoloro SM, Czech MP, Varga Jet al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nature Communications 2014. 5 3485. ( 10.1038/ncomms4485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klinkhammer BM, Floege J, Boor P.PDGF in organ fibrosis. Molecular Aspects of Medicine 2018. 62 44–62. ( 10.1016/j.mam.2017.11.008) [DOI] [PubMed] [Google Scholar]
- 34.Jun JI, Lau LF.Resolution of organ fibrosis. Journal of Clinical Investigation 2018. 128 97–107. ( 10.1172/JCI93563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buechler C, Krautbauer S, Eisinger K.Adipose tissue fibrosis. World Journal of Diabetes 2015. 6 548–553. ( 10.4239/wjd.v6.i4.548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lumeng CN, Bodzin JL, Saltiel AR.Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Journal of Clinical Investigation 2007. 117 175–184. ( 10.1172/JCI29881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeBari MK, Abbott RD.Adipose tissue fibrosis: mechanisms, models, and importance. International Journal of Molecular Sciences 2020. 21 6030. ( 10.3390/ijms21176030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, Hamaguchi M, Nishimura S, Manabe I, Matsuda Tet al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nature Communications 2014. 5 4982. ( 10.1038/ncomms5982) [DOI] [PubMed] [Google Scholar]
- 39.Vila IK, Badin PM, Marques MA, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui Pet al. Immune cell toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Reports 2014. 7 1116–1129. ( 10.1016/j.celrep.2014.03.062) [DOI] [PubMed] [Google Scholar]
- 40.Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx Net al. PPARγ activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metabolism 2007. 6 137–143. ( 10.1016/j.cmet.2007.06.010) [DOI] [PubMed] [Google Scholar]
- 41.Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, Hadoke PWF, Walker BR, Iredale JP, Morton NMet al. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11β-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. Journal of Biological Chemistry 2012. 287 4188–4197. ( 10.1074/jbc.M111.259325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cordeira J, Monahan D.Voluntary wheel running reduces weight gain in mice by decreasing high-fat food consumption. Physiology and Behavior 2019. 207 1–6. ( 10.1016/j.physbeh.2019.04.019) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a