Abstract
Despite considerable global investment, only 60% of people who live with HIV currently receive antiretroviral therapy. The sustainability of current programmes remains unknown and key incidence rates are declining only modestly. Given the complexities and expenses associated with lifelong medication, developing an effective curative intervention is now a global priority. Here we review why and where a cure is needed, and how it might be achieved. We argue for expanding these efforts from resource-rich regions to sub-Saharan Africa and elsewhere: for any intervention to have an effect, region-specific biological, therapeutic and implementation issues must be addressed.
Although much effort has been devoted to providing suppressive antiretroviral therapy (ART) to all of those in need, current trajectories suggest that this goal may not be attainable. A safe, effective and durable intervention that completely eliminates the HIV infection (eradication) or that suppresses viraemia in the absence of antiretroviral therapy (remission) (here we refer to both as a ‘cure’) could serve as an important adjunct in the control of the HIV epidemic (Fig. 1). Although this seems a daunting goal, the scientific motivation is clear: long-term remission if not eradication has been observed in at least two people following transplantation of bone-marrow progenitor cells that lack the viral co-receptor CCR51,2; and durable remission occurs in approximately 1% of individuals who are infected with HIV (elite controllers) and in 5–10% of those who are treated early in infection and then stop treatment (post-treatment controllers)3,4. Recent advances in animal models suggest that an HIV cure might be induced by some interventions, alone or in combination, such as through the provision of broadly neutralizing antibodies, the generation of an effective antiviral CD8+ T cell response or the knockout of CCR5.
Fig. 1 |. Pathways towards a cure.
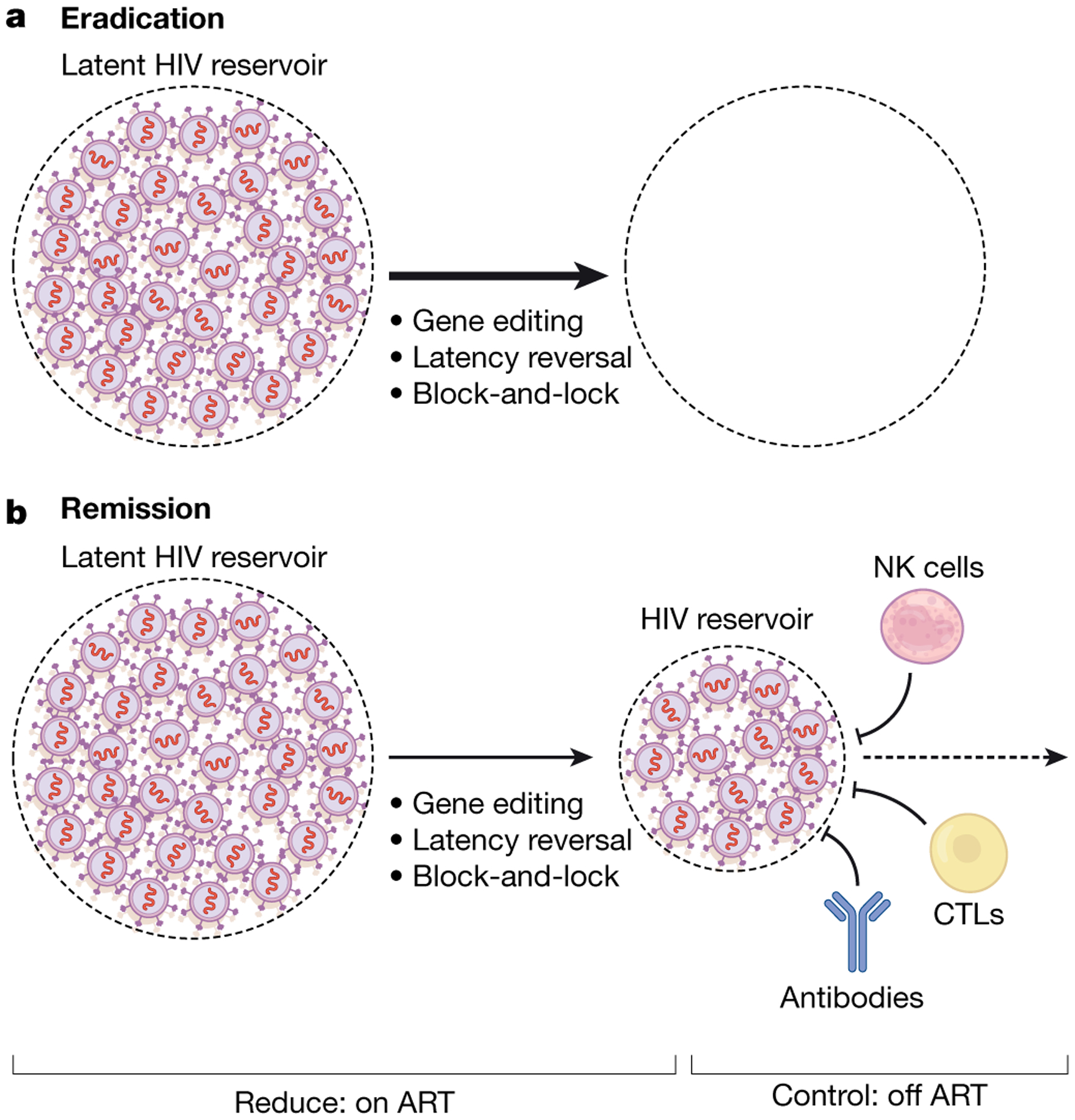
There are two broadly defined pathways for a treatment-free period of virus control. a, Eradication. The ideal outcome for a curative intervention would be the complete eradication of all replication-competent virus; gene-editing, latency reversal and block-and-lock approaches are all aimed at reducing the reservoir size and, if fully effective, could lead to complete eradication of the virus within an individual.
b, Remission. Given observations made in elite and post-treatment controllers, a more plausible strategy may be to reduce the reservoir to more manageable levels while also enhancing immune control. Multiple combination approaches are now being pursued. CTLs, cytotoxic T lymphocytes.
Despite some progress, a number of fundamental issues remain (Box 1). There is, for example, no consensus on why a cure is needed. Previous studies have focused on the needs of the individual, but as ART has become safer, more effective and more affordable, it is unclear whether a cure will ever compete with current therapies. In this Review, we argue that the public-health implications of a cure might prove to be at least as important as the benefits for the individual. There is also no consensus on what a cure needs to do. Will a partially effective intervention that provides some with the ability to remain healthy in the absence of therapy for a few years be sufficient, or should we focus our efforts on complete eradication of the virus? Similarly, will an expensive cure that requires specialized laboratories be useful, or should we seek to develop an approach that is scalable and that could address the main unmet needs globally? Achieving a consensus on these fundamental questions is needed as these decisions will enable the prioritization of competing strategies going forward5.
Box 1. Unresolved questions.
The road to an HIV cure will be long and unpredictable. There are, however, several important issues that can be acknowledged and addressed.
The nature of a curative intervention.
Complete eradication of the virus from an individual is the ideal outcome of a cure intervention; however, short of aggressive interventions (for example, haematopoietic stem-cell transplantation), this may not be possible, at least with current technologies. Short of such a cure, the induction of a durable immune response (leading to sustained ART-free remission) is appealing. Such a response must be primed, expanded and topologically disposed to prevent viral spread as soon as it begins. Ideally, it would prevent rebound of the virus from endogenous sources and block viraemia upon re-exposure. It is likely that combination approaches will be required and, given the distribution of the epidemic around the world, curative interventions of this type must be safe, effective and scalable to be truly effective. It is hoped that future advances in gene targeting and editing in vivo may eventually lead to a more definitive and scalable cure.
The clinical evaluation of candidate cures.
At present, treatment interruption is the only way to discern the effect of most interventions. Although strategies have been developed to address associated risks to the treated individual, such interruptions also pose substantial risks to sexual partners, and there is no consensus about the mitigation of these risks. A high priority now is to identify circulating non-viral biomarkers that can predict the rebound of infectious virus. Ideally, such biomarkers would ultimately form the substrate of diagnostic tests that could be used by individuals to detect the failure of a candidate cure—that is, viral rebound—should it occur.
Why and where a cure is needed
There are two main considerations driving the desire to cure HIV: to improve long-term health for individuals who are infected with HIV and to reduce on a community level the transmission of the virus to other individuals. The interests of these two motivating factors are generally well-aligned: for a cure to have an impact on either level, it will need to be safe, effective, durable, scalable and cost-effective. Ideally, it should also protect against reinfection.
The individual perspective
Many individuals living with HIV are able to obtain and adhere to an effective and typically well-tolerated treatment regimen of a single tablet once daily. Assuming such treatment options remain accessible, these individuals have few unmet needs and may not need a cure. Still, not everyone with access to ART does well. Although current regimens are generally safe, they are not benign. Increased risk of cardiovascular, kidney and bone disease has been associated with more-commonly used drugs. Even the recently developed integrase inhibitor class may have long-term health consequences, including weight gain and obesity6,7. Many individuals developed drug-resistant HIV during the early years of the treatment era; complex multidrug regimens are often needed to maintain virus control. As people age and need other medications, polypharmacy has emerged as a concern for nearly all ageing people who have HIV.
Stigma remains a problem for many people living with HIV and is now recognized as a major factor that affects health and well-being8. In many communities worldwide, prevalent social constructs make it highly stigmatizing to take ART and/or to attend HIV clinics9. Knowledge of HIV infection also affects perceptions of social worth and peer relationships10. It is not yet clear whether an HIV cure can address these challenges, but this would certainly be the goal.
The public-health perspective
Effective control of HIV with ART eliminates the risk of transmission (‘undetectable equals untransmissible’)11,12, and the ability to test and treat with the aim of reducing transmission is now a universal goal. Currently, approximately half of the infected global population is believed to be on effective ART13 and—should this treatment scenario not improve in the future—it is highly unlikely that the epidemic will be contained14 (Fig. 2). Although nearly all countries have experienced progress in the roll-out of ART, the outcomes have been uneven15,16 and the widespread availability of ART has led to only a modest reduction in HIV incidence in some communities with a high prevalence17.
Fig. 2 |. The cascade of treatment and control.
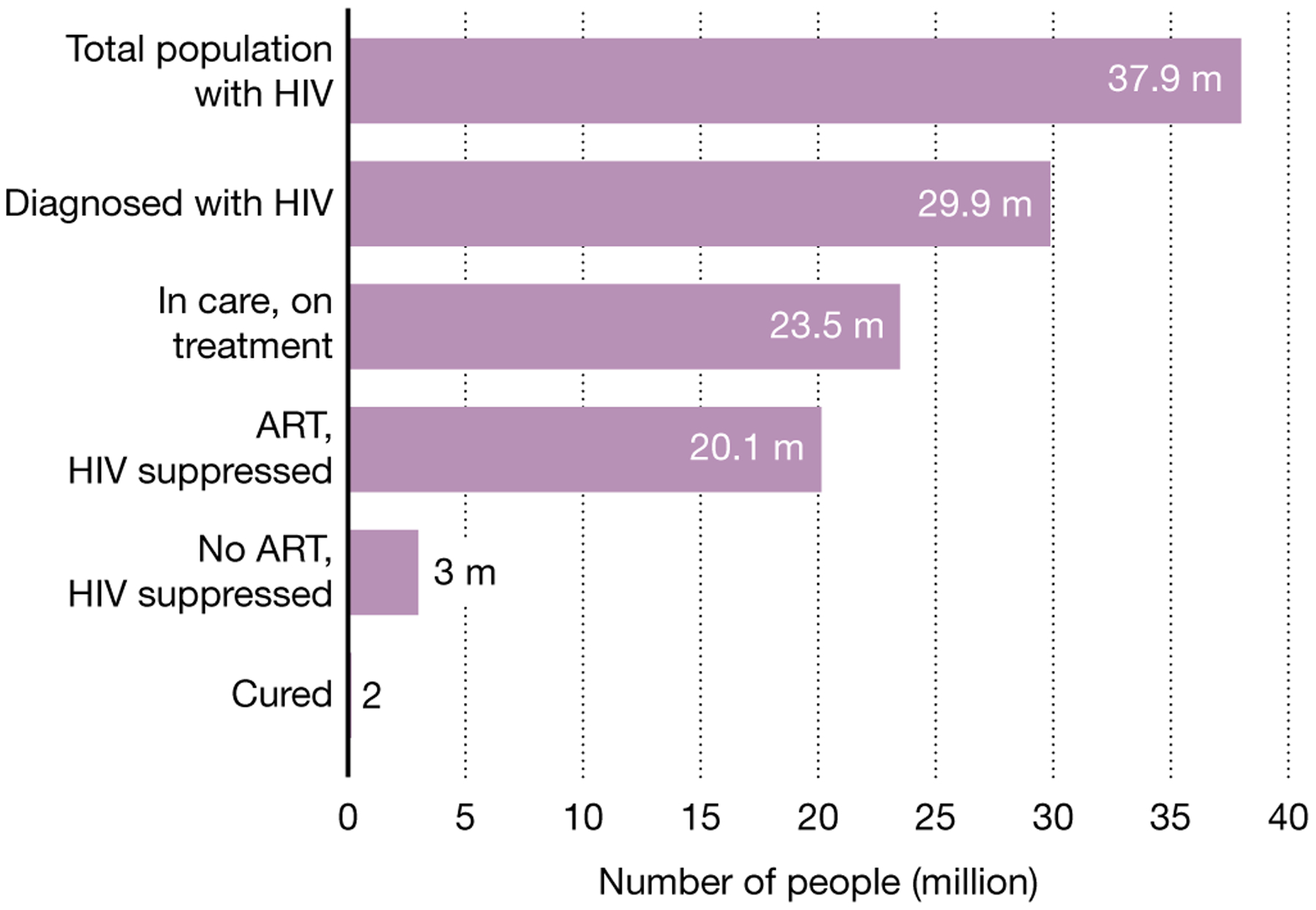
According to the most recent UNAIDS estimates, of the 37.9 million people living with HIV, only about 79% have been diagnosed and only about 53% are on effective therapy. It is estimated that about 1% are doing well in the absence of therapy (‘elite’ control and rarely ‘post-treatment control’). Only two individuals are believed to have been cured.
Antiretroviral treatment programmes in many countries are heavily reliant on international donor partners, raising concerns about the sustainability of these initiatives should the political and economic climate change. Indeed, UNAIDS estimates that US$26.2 billion in funding (nearly a 30% increase compared to current funding levels) will be required by the year 2020 to achieve their ‘90–90–90’ treatment goals (that is, 90% of people living with HIV should know their status, 90% of those people should be on ART and 90% of those people should have an undetectable viral load)18. The global commitment to funding is stagnating: whereas the compound annual growth rate for global funding in low- and middle-income countries between 2000 and 2010 was 13%, this number has declined to 1.2% in the last five years.
Theoretically, a short-term, affordable and effective intervention that results in sustained periods of virus control in the absence of any therapy will reduce the overall strain on public-health systems, freeing up resources for other healthcare imperatives including other aspects of HIV prevention and care. Ideally, such a regimen will need to be as safe and effective as ART—a bar that might not be possible to achieve, particularly in adherent populations in which treatment response rates now approach 100%. This raises questions of how such a regimen will obtain regulatory approval and how it will be implemented. Discussions among key stakeholders will be needed to define in which situations a regimen that is less effective than ART will be effective and how such an approach will achieve regulatory approval.
On a global level, approximately 20% of individuals with HIV do not know their status13 (Fig. 2). Multiple barriers to getting tested exist, including stigma and concerns about being diagnosed with an incurable disease. The availability of an effective cure might boost HIV control programmes by encouraging disenfranchised individuals with HIV to proactively seek testing and treatment for HIV, as has been documented for syphilis19.
Why ART is not curative
Many of the curative interventions that are currently being explored address virological and immunological factors that limit the ability of ART itself to cure HIV infection, as described below.
Latency
As a retrovirus, the HIV genome fully integrates into the host genome and persists for the lifetime of the infected cell20–22. The vast majority of these integrated genomes appear to be transcriptionally silent (that is, latent)23. This state of latency—in which viral proteins are not produced—enables infected cells to escape immune recognition and clearance. Latency is maintained by multiple mechanisms, including expression of unique and complex transcriptional pathways that may prevent reactivation24, the upregulation of anti-apoptotic genes25 and blocks to various post-initiation transcriptional pathways (such as elongation, polyadenylation and multiple splicing)26.
Reservoir dynamics
Approximately 0.01–1% of circulating CD4+ T cells contain an integrated genome, only a small proportion (<5–10%) of which are fully intact27, and only a small proportion of intact genomes may be readily inducible and able to support virus replication post-ART27,28.
There is intense interest in further defining the cell populations that are more likely to be infected. CD32 was reported to be a putative biomarker29, but subsequent studies have failed to confirm this finding. PD-1 has been associated with the reservoir in several studies30–32. Other cell populations enriched for HIV include those that express other checkpoint receptors31,33,34, markers of activation or proliferation35, members of the tumour necrosis factor family25,36 and markers of cell adhesion and migration37,38. The virus may also be enriched in the more differentiated effector memory cell population35,37, such as the T helper 1 and 17 subsets39,40. As these associations are modest and highly variable, none will prove useful as biomarkers or as targets for host-directed immunotherapies. These findings, however, provide insights into how HIV establishes latency.
The fate of latently infected cells is largely dictated by the physiological pathways of T cell homeostasis24,30,41–43; infected cells undergo clonal expansion and are then maintained by those same factors that control the size and diversity of the memory T cell pool41. During long-term ART, individual clonal populations wax and wane as the entire reservoir becomes increasingly clonal in nature44. Some integration events can disrupt the regulation of cell growth, leading to massive expansions42,43. The local chromatin environment in which the virus integrates is also important: genomes integrated in largely silenced regions are less likely to be inducible45,46; indeed, some may be permanently silenced. These silenced genomes may be selected for and enriched during long-term ART.
Emerging data suggest that the intact genome may decay more rapidly than the defective genomes47,48, although some studies have argued the opposite49. It has also been suggested that the inducibility of intact genomes declines over time as the reservoir increasingly enters a relative state of deep latency45,47; if confirmed, this would suggest that virus populations during long-term ART will be less likely to initiate rounds of virus replication after the cessation of therapy.
HIV replication
HIV may also persist in the face of suppressive ART owing to low but detectable levels of virus replication in lymphoid microenvironments50, although the fact that the virus does not evolve over time argues that ART can be fully suppressive51. Even if this is the case, ART can only prevent cells from becoming newly infected; cells that are already infected must be eliminated by other means.
Host clearance mechanisms
The capacity of the immune system to clear infected cells is also likely to be an important factor that contributes to the persistence of HIV52. During acute infection, the virus evolves rapidly to escape the immune system and these mutants are retained in the reservoir53. The numbers of HIV-specific CD8+ T cells during ART are low (as expected given low antigen levels), often dysfunctional (as defined by expression of PD-1 and other markers) and often target HIV variants that have already escaped immune recognition54–57. The administration of ART during acute HIV infection may prevent many of these abnormalities from emerging, but cells are often not sufficiently primed to be effective58–60. Many of the immunotherapies that are under development for an HIV cure seek to reverse these defects.
How to define a cure
The ideal outcome for any curative intervention would be the complete eradication of all replication-competent HIV particles—that is, a sterilizing cure (Fig. 1). As it will not be possible to prove that all infected cells that have replication-competent HIV have been eliminated61, a potentially cured individual will never really know whether any virus particles persist that can restart a systemic infection. Indeed, in several cases of very early ART treatment or allogeneic bone marrow transplants, the virus was no longer detectable yet rebounded within weeks to months after ART interruption62–65. As an infected cell can initiate acute viraemia at any given time, new point-of-care or preferably at-home tests that can reliably determine whether the virus has rebounded may prove to be necessary for any strategy that is less than 100% effective66. This inability to predict whether and when a rare infected cell will initiate an episode of acute viraemia argues for interventions that confer durable host-mediated control of a residual reservoir.
Most strategies that are currently being pursued seek to durably induce a state in which the virus is maintained at such low levels that it can no longer cause disease or be transmitted to others (Fig. 1). Operationally, such a functional cure or state of durable virus remission will probably be defined as the sustained maintenance of a viral load that is less than the level of quantification with standard assays (for example, 40–50 copies of RNA per ml of plasma for some yet-to-be-defined period). Of note, remission strategies that achieve a low but persistently detectable level of viraemia may provide important benefits for individuals who are unable to adhere to standard regimens, including those with multidrug resistance or disenfranchised individuals who are unable to access or adhere to therapy.
How to measure a cure
A major barrier to the development of curative interventions is the lack of a well-validated surrogate biomarker for the total reservoir of replication-competent virus in the body. This is especially crippling for ‘go/no-go’ decisions at the proof-of-concept stage and a key impediment to the initiation of investment in this new therapeutic area by biotechnological or pharmaceutical companies.
Although HIV DNA can be readily measured in the CD4+ T cell compartment of ART-suppressed individuals, most of it (>95%) is defective and unable to contribute to new rounds of infection within the host or be transmitted to others27. Assays that directly quantify or estimate the circulating reservoir of replication-competent virus are under development67, but the degree to which these approaches quantify viruses that can initiate new rounds of infection (the rebound-competent viral reservoir) has yet to be determined. Additionally, it is not clear whether and to what extent any measurement using blood will be able to quantify the level and disposition of viral reservoirs that are contained within tissues, where the vast preponderance of the replication-competent pool resides68. Novel approaches that quantify the virus within tissues directly (for example, using biopsies or imaging using labelled anti-HIV antibodies) or indirectly (for example, by assessing the host response to the virus by quantifying antibody titres) are in development69,70.
Short of an unexpected breakthrough across the above barriers, the only way to determine the effectiveness of a putative curative intervention is to interrupt treatment with ART and then measure either the time-to-rebound (test of cure) or the level of set-point viraemia after rebound. The former approach is relatively safe if the viral load is measured frequently (usually weekly) and ART is resumed immediately upon the detection of rebound62,63,71. Defining the post-interruption set point is more informative but far more complicated. People who are destined to control their virus in the absence of therapy may first experience a rapid but transient burst in viraemia3,72, which can cause harm to the immune system of the individual and poses risks to sexual partners73. Strategies that aim to reduce the risk to participants and their sexual partners have been developed74.
What a cure needs to achieve
Cure research remains focused in academic medical centres located in low HIV burden, resource-rich countries. As such, there has been limited public discussion on the practicalities of product development, particularly as they relate to sub-Saharan Africa. Failure early on to define a target product profile (TPP) risks developing a strategy that fails to be effective. In principle, a TPP should provide a broad outline of the minimal attributes of an effective therapy, guiding drug development while engaging all stakeholders, including the community, the regulators, the funders, the implementers and industry (Box 2).
Box 2. TPP of a potential cure.
Efforts are underway to identify those characteristics of a curative intervention that will be necessary for the intervention to be effective. It is expected that the first generation of cures will be expensive, require access to advanced technologies and have limited scalability. For a cure to truly alter the course of the global epidemic, a number of factors will need to be considered, all of which contribute to the target product profile (TPP). The minimal and optimal characteristics of a cure TPP should be discussed among all stakeholders as early in drug development as possible. Here we discuss the factors and associated characteristics that should be considered for an intervention to be effective.
Clinical effect. The desired outcome (such as remission or the elimination of viral reservoir) and efficacy threshold should be well-defined.
Indication. The target populations to be included (for example, adults and children with HIV viral load < 100,000).
Re-exposure and reuse. The number and frequency of doses required for the cure to be effective and the requirements for pretreatment.
Dosing and administration. In addition to the number and frequency of doses required for the cure to be effective and the requirements for pretreatment, the treatment route (such as infusion or oral administration) should be considered.
Storage and handling. The supply chain (for example, cold storage), formulation (such as the need for compounding) and shelf life need to be assessed.
Follow up. Clinical monitoring, biomarker testing (such as viral load) and confirmatory testing (test of latent reservoir) need to be carried out.
Contraindications. Comorbidities (such as chronic kidney disease), concurrent therapies, social factors (that is, adherence to the treatment regime) and other conditions (such as pregnancy) need to be taken into account.
Safety and toxicity. Side effects (such as nausea, diarrhoea and mood disturbances) and toxicity (to the renal or hepatic system, for example) need to be assessed.
Cost. The goal price in target markets needs to be such that this intervention will reach those people with the highest need.
An unresolved question is whether a cure will need to compete with and potentially replace ART. If this is the case, it will have to be as safe and effective as standard ART regimens, including emerging long-acting formulations—a high bar that is unlikely to be reached by currently available interventions (for example, CCR5Δ32 bone-marrow transplantation).
As modern ART regimens are remarkably safe and well-tolerated, any intervention associated with serious toxicities will probably be unacceptable to most stakeholders. With regard to efficacy, short-term failures in which a cure is administered and then quickly found to have failed could easily be managed by continuing or resuming ART. Long-term failures in which the virus rebounds after an unpredictable period of time would potentially be disastrous. In this setting, a burst of viraemia may go unnoticed for months, leaving the person and his or her sexual partner(s) at risk. Modern cure studies generally have indefinite periods of close monitoring to avoid this scenario63. Inexpensive at-home diagnostics that are able to rapidly detect rebound might be needed when a curative strategy is implemented.
Other factors are probably also important. If a cure leaves an individual susceptible to reinfection, then at least one model suggests it will rarely ever be effective from a public-health perspective, given that those who acquire HIV once will presumably have sustained exposures and risks for acquiring the infection a second time14. The ‘Berlin patient’, for example (one of the two known cases in whom HIV is potentially cured), has disclosed publicly that he is now taking pre-exposure prophylaxis (PrEP); in other words, he has switched from a three-drug to a two-drug regimen and is not living in a state of ART-free viral suppression.
A critical consideration for resource-limited settings will be ease of administration. Healthcare systems in most low- and middle-income countries are already strained by the existing disease burden and will not be able to cope with cure strategies that require the administration of multiple products, inpatient evaluations, a specialized infrastructure or extensive cold chain management.
Finally, a curative intervention will only have a global influence if it is cost-effective. In the context of the epidemic in Zimbabwe, for instance, an accessible intervention would only make sense financially as a replacement for ART if it costs less than US$1,400 and enables ART interruption in 95% of treated individuals with a rate of viral rebound of approximately 5% per year75. For South African people with access to effective therapy, only a safe and highly effective curative intervention would be cost-effective76.
How to achieve a cure
There are five broadly defined approaches to achieving an eradicative cure or remission: early ART, genetic modifications, ‘shock-and-kill’, ‘block-and-lock’ and immunotherapy.
Early ART
Starting therapy immediately after the diagnosis of infection with HIV preserves immune function, limits virus diversification, minimizes HIV-related complications in the treated individual and prevents transmission to others by lowering viraemia59,77. Although early ART may also prove to be curative (as suggested in a study of non-human primates)78, experience to date suggests that this will not often be observed in adults who are infected with HIV. Thus, in a carefully performed study of ten adults in Thailand who started ART in the first few weeks after infection, the virus rebounded almost immediately after ART was interrupted62. Similarly, an individual who started pre-exposure prophylaxis immediately (probably within a day or two) after a recent infection nevertheless experienced viral rebound once ART was discontinued63.
Although immediate ART will probably never cure an adult, it might under some circumstances provide benefits to infants. In the well-known case of the ‘Mississippi baby’, for instance, an infant was infected in utero, born with a viral load of approximately 20,000 copies of RNA per ml, started on ART within 30 h of her birth, stopped therapy 18 months later and then experienced 30 months of virus-free remission before the occurrence of rebound65. This case raises the possibility that treatment in infants might prove curative if started earlier or maintained longer.
Even if not curative, early ART appears to reshape the association between the virus and the immune system, allowing some people to eventually control their virus in the absence of treatment. Approximately 5–10% of those treated early during the course of disease develop a state of ART-free viral remission3,4. These so-called post-treatment controllers generally start ART in the first few weeks to months of their infection, remain on ART for several years and then stop therapy for various reasons4. Less commonly, post-treatment control can occur in perinatally infected children who begin ART treatment soon after birth79,80.
Genetic modifications and cell therapy
There are two known cases in whom HIV is potentially cured: the Berlin patient1 and the ‘London patient’2. In each case, an HIV-seropositive patient with cancer received fully ablative chemotherapy and an allogeneic stem-cell transplant from donors who naturally lacked CCR5 (CCR5Δ32/Δ32), a co-receptor used by many circulating virus strains to enter CD4+ T cells. Once 100% chimaerism was achieved, all available CD4+ T cells were resistant to new infections and the virus was unable to spread to uninfected cells. Although associated with multiple toxicities and high cost, these cases have provided proof-of-concept evidence that genetic and/or cellular modifications of the affected host might lead to a state of durable viral remission.
Given these cases, the question arises of whether there are safer and more scalable ways to achieve the same result. The two transplant cases and limited studies in animal models81,82 argue that, if CCR5 can be safely disrupted in T cells (including key progenitor cells), a cure may be achievable. As there are a number of approaches to genetically edit or suppress the function of CCR583, a major hurdle is the manner of delivery, which ideally would be done in vivo and not require ex vivo manipulation and transplantation. Given the strong interest in developing in vivo gene-editing strategies in other areas of medicine84, it seems likely that a safe and scalable delivery approach will eventually be able to target gene-modifying systems to appropriate, long-lived cell populations in vivo, precisely and safely disrupting or inhibiting the function of CCR5 and leading to an effective cure. CCR5 may be an ideal candidate for such a technology as its disruption would probably prove to be safe and effective. There may be a threshold below which the frequency of susceptible cells is insufficient to support systemic infection85, arguing that even incomplete editing will be curative.
Although editing CCR5 is attractive, the limitations of this approach are not trivial. Many individuals have viruses that utilize CXCR4 for cell entry. Indeed, one recipient of an allogeneic stem-cell transplant from a donor with homozygous deletion of CCR5 stopped therapy and exhibited rapid rebound of a pre-existing CXCR4-utilizing variant86. Furthermore, although individuals who are homozygous for the CCR5Δ32 allele generally do well, it remains unknown whether disruption of this pathway by gene modification will be safe.
Gene-editing approaches that target and excise the integrated provirus would also be potentially curative87. Nucleases that are specific to conserved areas of the provirus have been developed and validated in vitro, although selection for virus populations that are resistant to editing can occur. Combination approaches that target multiple sites in the virus may be needed, just as a combination of antiretroviral drugs is necessary to prevent HIV resistance88. Delivering a universally effective combination of gene-editing enzymes to all cells in the body that are infected with replication-competent viruses will be challenging but necessary for this strategy to work.
Alternatively, novel genetic modifications that lead to an eradicative cure or remission might be delivered. Theoretically, if a cell can be re-engineered to produce an effective antiviral response indefinitely, then a state of durable control will be achieved. In a proof-of-concept study, SHIV-infected rhesus monkeys were infected with adeno-associated virus vectors that encoded three potent neutralizing antibodies against HIV; in one animal, high levels of the antibodies were durably produced, resulting in sustained control of the virus89. Efforts to repeat this in humans have been slowed by the development of anti-drug antibodies to the vector90.
Studies of individuals who naturally control HIV in the absence of therapy (elite controllers) have demonstrated that functional HIV-specific CD8+ T cells that target vulnerable regions of the virus can control the virus for years, suggesting that this mechanism may be harnessed for a functional cure. Although early attempts to re-engineer CD8+ T cells to express a chimeric antigen receptor (CAR) that targets HIV failed91, more mature versions of this technology are now being used for the management of B cell malignancies92 and actively repurposed for studies researching an HIV cure93–95. Barriers that will need to be overcome include limited sensitivity (the density of virus proteins on the surface may be too low to be readily recognized), limited persistence of the CAR-T cells in vivo, escape mutations and safety.
Latency reversal
With the hope that the induction of viral proteins could lead to immune-mediated recognition and destruction of infected cells, attempts have been made to reverse latency (called shock-and-kill or ‘kick-and-kill’). Multiple first-generation latency-reversing agents have been studied in the clinic, and several have been shown to stimulate the transcription of HIV mRNA96–98 and perhaps even the production of viral particles99,100. A second generation of approaches has focused instead on non-specific inducers of T cell activation, for example, Toll-like receptor (TLR) agonists101,102. Given the complexity and heterogeneity of the reservoir46, it seems likely that combinations of approaches may be needed103–105.
A fundamental problem with latency reversal is that, in the absence of the concomitant generation of other factors (for example, an immune response that can suppress viraemia), it may be necessary to eliminate most if not all of the reservoir. Although one model predicts that a 10,000-fold reduction in the reservoir will be needed to prevent virus rebound106, recent clinical data suggest that even with exceedingly small and undetectable reservoirs the virus will rebound within months63. As complete or near-complete elimination may prove to be impossible with latency-reversing agents alone, these approaches are now being proposed as a way to reduce the size of the reservoir to a more manageable level, possibly enhancing the effectiveness of other (for example, immune-based) strategies (Figs. 1, 3). This approach is analogous to tumour debulking in cancer therapeutics.
Fig. 3 |. HIV remission pathway.
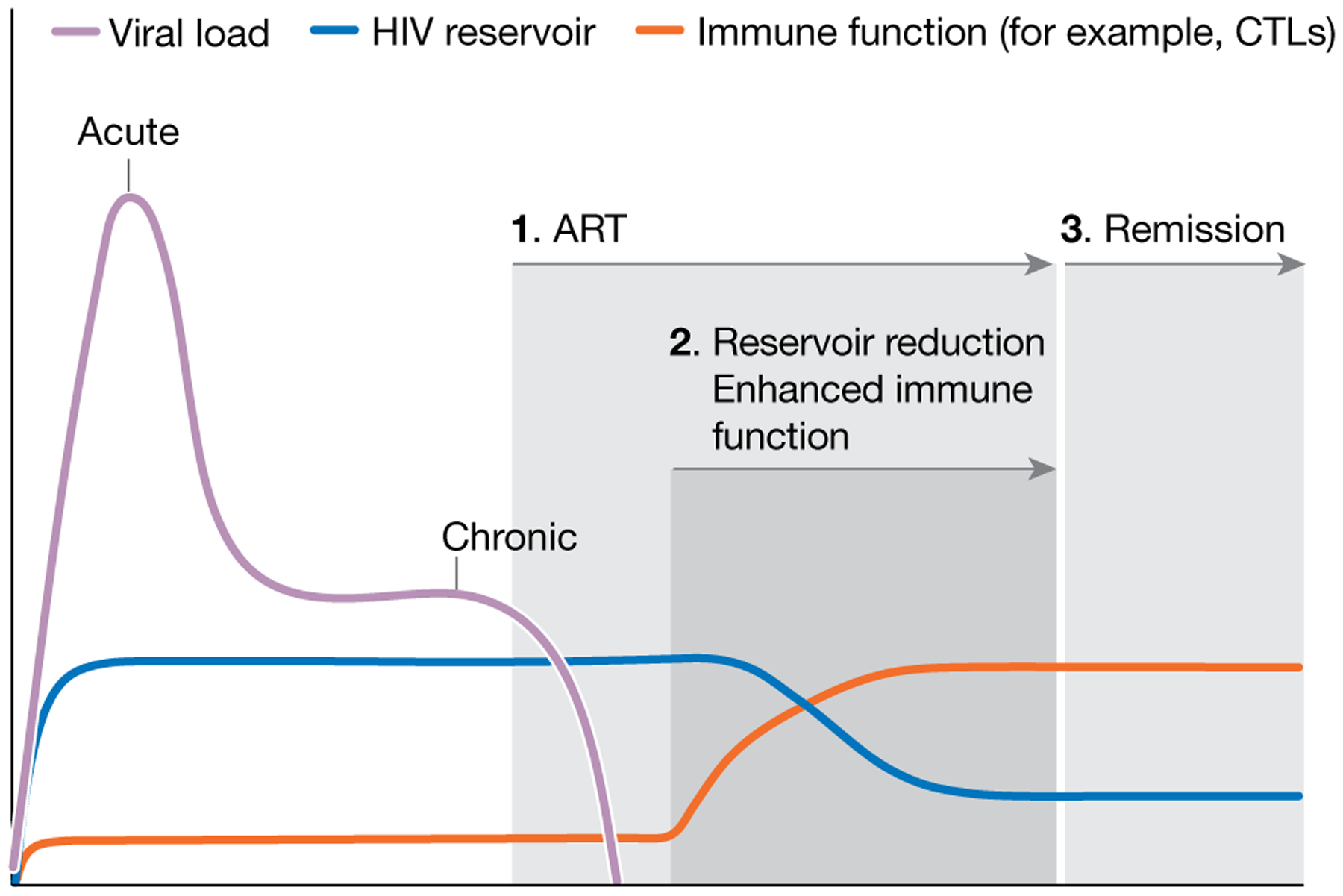
Many strategies that are currently in development are aimed at achieving durable control, rather than complete eradication. All interventions require individuals to first control HIV with ART. Interventions that are aimed at reducing the reservoir size (shock-and-kill, block-and-lock) are then initiated, followed by combination approaches to enhance immune control. ART is eventually interrupted, followed by an expected transient period of transient viraemia and eventually control of HIV. Post-ART monitoring of viral load may prove to be the most challenging and expensive component of this strategy.
Deep latency
In studies carried out ex vivo, it has been found to be difficult to induce some proviruses out of latency27,28, suggesting that inducing a state of deep latency might contribute to a cure. During long-term ART, intact genomes may become enriched in non-genic chromosomal regions that are less likely to support efficient transcription of the viral genome45. Viral genomes that are therapeutically placed into such a state of deep latency would be effectively eliminated as a source of virus recrudescence. For example, didehydro-cortistatin A107 (a Tat inhibitor) caused a modest delay in virus rebound post-ART in a humanized mouse model108. As with the latency reversing approach, it seems likely that this approach (generally referred to as block-and-lock) might work best to reduce the effective reservoir size, thus enhancing the efficacy of other approaches.
Immunotherapy
Propelled by revolutionary advances in the field of oncology, multiple ongoing or planned clinical trials are testing various immunotherapies in people with HIV109. Most of these studies have targeted CD8+ T cells and multiple factors probably account for the fact that all have failed. The size of the reservoir in some people may be too large110. Many of the initial CD8+ T cell responses target highly variable regions of the virus, resulting in rapid selection of escape mutants53. Sustained exposure to the antigen drives T cells towards a dysfunctional state marked by upregulation of PD-1 and other regulatory pathways. These immuno-dominant and dysfunctional responses that target escaped epitopes are typically the first to be expanded therapeutically with vaccines and other approaches111. HIV infection induces a potent inflammatory response that is reduced but not eliminated by ART; this inflammatory state induces counter-regulatory responses that prevent T cells and other effectors from working optimally. Finally, some of the reservoir resides in the B cell follicle32, an anatomical space from which CD8+ T cells, natural killer (NK) cells, and other effector responses are typically excluded. For an immunotherapeutic regimen to work, it may be necessary to breach these barriers (Figs. 1, 3).
There are, however, examples of success, particularly in the non-human primate model. A re-engineered replication-deficient CMV vector with simian immunodeficiency virus (SIV) inserts induced an unusual (MHC class II- or MHC-E-restricted) T cell response of unprecedented breadth112. Although the vaccine failed to prevent SIV acquisition, it did lead to control and eventually the elimination of the infection in approximately 50% of exposed animals113. The administration of broadly neutralizing antibodies to infant macaques either cleared or prevented the establishment of a latent reservoir114. A therapeutic vaccine and a vaccine adjuvant (a TLR7 agonist) induced post-ART remission mediated by CD8+ T cells in a subset of SIV-infected monkeys115. In ART-treated macaques, a TLR7 agonist alone was shown to induce the expression of SIV RNA, a reduction of viral DNA, the activation of innate and adaptive immune cells and viral control for more than two years101. A broadly neutralizing antibody that targets the viral envelope combined with the same TLR7 agonist either eliminated the reservoir or generated a post-ART state of remission116. Finally, therapies using broadly neutralizing antibodies during acute SHIV infection induced subsequent remission117, possibly though a ‘vaccinal effect’, as described below. These approaches are now being translated into clinical studies.
Arguably, broadly neutralizing antibodies have been the linchpin in most of the successful immunotherapeutic studies. When used in combination, broadly neutralizing antibodies have a potent antiviral effect that is comparable to that of ART118. In contrast to ART, however, broadly neutralizing antibodies can theoretically clear reservoir cells by stimulating antibody-dependent cellular toxicity and other Fc-receptor-dependent cytotoxic effects. Antibody–antigen immune complexes can also activate Fc receptors on antigen-presenting cells and induce a sustained T cell effect (a vaccinal effect). A combination of two antibodies may have induced long-term remission in two individuals through such an effect118. A newer generation of antibodies that bind to two or three sites on the viral envelope (bi-specific and tri-specific antibodies) has been shown to be effective in a macaque model119 and the use of such antibodies is moving towards clinical applications. Dual-specific antibodies that bind to HIV and the T cell surface receptor CD3 have the potential to stimulate T cells to target and eliminate HIV-infected target cells120.
Therapeutic vaccines—fuelled by innovations in adjuvants, immunogen design and delivery modalities such as novel DNA and RNA systems, viral vectors and ex vivo loading of dendritic cells—are showing promise in the treatment of cancer and chronic infections, including HIV121. Most of these vaccines are designed to target conserved or vulnerable regions of the HIV virus122.
Borrowing heavily from the oncology field, ongoing studies are testing agents that directly stimulate or redirect immune responses (for example, the cytokine IL-15 and TLR agonists), or that reverse the dysfunction of T cells that is induced by chronic exposure to HIV (for example, inhibitors of PD-1 and CTLA-4). These agents are inherently activating and hence might have the added benefit of reversing latency. PD-1 inhibitors, for example, induce virus production ex vivo123 and may enhance HIV-specific T cell responses in vivo124, thus resulting in a combined shock-and-kill effect.
Non-specific therapies that reverse the chronic inflammatory state of treated HIV might induce a more-effective immune response. Inhibitors of IL-10, IFNα, TGFβ, mTOR, IL-1β, JAK–STAT, and other pathways are being tested in animals and/or people. Although most of these approaches are being pursued as potential strategies to reduce the burden of inflammation-associated co-morbidities in treated HIV disease, some might plausibly enhance the ability of the adaptive immune system to reduce or control the reservoir.
To facilitate the entry of CD8+ T cells and NK cells into the B cell follicle, agents that deplete B cells (rituximab) or that enhance the migration of CD8+ T cells (IL-15) are being tested as adjuncts for immunotherapies in animal studies125. By slowly reducing the inflammatory state that maintains inflamed follicles and the production or replication of the virus32,126, long-term ART might prove to be the most effective way to deal with this potential barrier.
Combination approaches
Most studies of curative interventions that are now in the clinic are designed to test safety and to provide proof-of-concept that a relevant pathway is effectively targeted, and it is not likely that any of these approaches will effect an eradicative cure or remission when used alone. There is accordingly an evolving perspective that the field should more rapidly advance combination therapies that are designed to achieve a complete cure or remission127,128. A popular combination approach is to reduce the reservoir to levels that a therapeutically enhanced immune system can control (‘reduce and control’; Figs. 1, 3). Clinical trials using analytical treatment interruptions are increasingly being used to study these combination approaches74.
What is needed to achieve a scalable cure
Research on a cure in those areas of the world where the need is greatest is essentially non-existent. From a biological perspective, there are many virological, immunological and socioeconomic factors that are unique to Africa and elsewhere that might conceivably affect the impact of a cure intervention129,130 (Box 3). The HIV-infected populations in resource-rich areas are older and predominantly male, whereas in Africa they are younger and predominantly female. Recent evidence suggests that ART will have differential toxicities in these populations6,7 and that sex influences reservoir activity and the immune response131. From an implementation perspective, failure to develop an intervention that is effective, affordable and easy to administer risks limiting the ultimate impact of the strategy, and failure to engage communities early on in the research process could result in delays in implementation that cost lives when a product becomes available.
Box 3. Unresolved questions regarding cure studies in sub-Saharan Africa.
Arguably, an effective strategy for a HIV cure is most urgently needed in sub-Saharan Africa, where the prevalence and incidence of HIV are highest. However, most studies that have tried to find a HIV cure have been conducted in low-burden, resource-rich countries. Unless this research imbalance is corrected, there is a potential to develop cure strategies that may not work in all populations, or that may prove difficult to scale and implement in those communities in which the need is greatest. Some of the critical questions that need to be addressed are:
What do those individuals living with HIV in Africa want from a cure?
Who are the target populations that might best benefit from a cure?
What are the characteristics of an intervention that lead to an eradicative cure or remission that would be best suited to these target populations?
What will a cure need to cost to be accessible and affordable?
What kind of interventions can be practically given in non-urban healthcare clinics?
Will the HIV subtype have an effect on the effectiveness of current cure interventions?
Will therapies developed for subtype B (for example, vaccines and antibodies) need to be redesigned for other subtypes?
Will persistent inflammation be a concern? Will common prevalent co-infections (for example, with tuberculosis, malaria and helminthic worms) affect or preclude the use of immunotherapy?
Are the replication-competent reservoirs that are found among African people qualitatively and quantitatively similar to those studied elsewhere (for example, in the United States and Europe)? In particular, might differential aspects of age, gender, race and concomitant co-infections have an effect?
Will there be a practical, scalable, affordable and acceptable method available for detecting failure (that is, rebound viraemia), should that occur?
The lack of adequate clinical and laboratory infrastructure is a major barrier to expanding the cure agenda to Africa. Currently, most HIV clinical research sites in Africa perform observational cohort studies or late-stage clinical trials of ART or vaccines, with little involvement in early-stage clinical research. If resource-poor, high-burden areas such as Africa are to play a more prominent part in this endeavour, the laboratory and clinical infrastructure needed to support this kind of research will require substantial improvement. Considering the previous experience with antiretroviral drug research in Africa, these are not insurmountable problems.
Outlook
Although HIV treatment with ART has improved public health worldwide and resulted in a decrease in the incidence of HIV in most countries, lifelong treatment poses financial, logistical, health and psychosocial challenges. A safe, effective, scalable and cost-effective intervention that is fully curative or that allows for a sustained period of virus control in the absence of any therapy would provide a powerful adjunct for the eventual control of the epidemic. Although research towards developing such an intervention remains in its infancy, recent observations provide insight, suggest progress and prompt continued interest in experimental medicine studies and small early-phase clinical trials of promising strategies. Ultimately, for an intervention that leads to an eradicative cure or remission of HIV disease to reshape the course of the epidemic, these studies will need to be conducted in communities that are most likely to benefit from such research.
Acknowledgements
T.N. is supported in part by grants from the Bill & Melinda Gates Foundation, Gilead Sciences (grant no. 00406), the International AIDS Vaccine Initiative (IAVI) (UKZNRSA1001), the NIAID (R37AI067073) and the South African Department of Science and Technology through the National Research Foundation. T.N. is also partially supported through the sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative (grant no. DEL-15-006). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and is supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (grant no. 107752/Z/15/Z) and the UK government. J.M.M. is an employee of the Bill & Melinda Gates Foundation. S.G.D. is supported by the amfAR Institute for HIV Cure Research (amfAR 109301), the Delaney AIDS Research Enterprise (DARE; A127966), and the NIAID (K24 AI069994). We thank W. Greene for assistance with Fig. 1. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, the UK government, the Bill & Melinda Gates Foundation or amfAR.
Footnotes
Competing interests S.G.D. receives grant support from Gilead, Merck and ViiV. He is a member of the scientific advisory boards for BryoLogyx and Enochian Biosciences and has consulted for AbbVie, Biotron and Eli Lilly. T.N. receives grant support from Gilead.
References
- 1.Hütter G et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N. Engl. J. Med 360, 692–698 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Gupta RK et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature 568, 244–248 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; Two HIV-infected adults1,2 with haematological malignancies were apparently cured of HIV after an effective stem-cell transplant from an allogeneic donor whose T cells lacked CCR5, a key co-receptor for virus entry.
- 3.Namazi G et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J. Infect. Dis 218, 1954–1963 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sáez-Cirión A et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9, e1003211 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; A subset of HIV-infected adults who start ART early and remain on therapy for a sustained period are able to effectively control HIV replication after treatment interruption; although the mechanism(s) at play remain unclear, these ‘post-treatment controllers’ provide strong evidence that the host–virus association can in some settings be slanted towards ART-free viral remission.
- 5.Deeks SG et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med 22, 839–850 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venter WDF et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N. Engl. J. Med 381, 803–815 (2019). [DOI] [PubMed] [Google Scholar]
- 7.The NAMSAL ANRS 12313 Study Group. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N. Engl. J. Med 381, 816–826 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Rueda S et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open 6, e011453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz IT et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J. Int. AIDS Soc 16, 18640 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CE et al. Exploring the social meaning of curing HIV: a qualitative study of people who inject drugs in Guangzhou, China. AIDS Res. Hum. Retroviruses 31, 78–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisinger RW, Dieffenbach CW & Fauci AS HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. J. Am. Med. Assoc 321, 451–452 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Rodger AJ et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 393, 2428–2438 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. Global HIV & AIDS Statistics — 2019 Fact Sheet https://www.unaids.org/en/resources/fact-sheet (2019)
- 14.Beacroft L & Hallett TB The potential impact of a “curative intervention” for HIV: a modelling study. Glob. Health Res. Policy 4, 18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuadros DF et al. Towards UNAIDS Fast-Track goals: targeting priority geographic areas for HIV prevention and care in Zimbabwe. AIDS 33, 305–314 (2019). [DOI] [PubMed] [Google Scholar]
- 16.GBD 2017 HIV collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 6, e831–e859 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moyo S et al. Cross-sectional estimates revealed high HIV incidence in Botswana rural communities in the era of successful ART scale-up in 2013–2015. PLoS ONE 13, e0204840 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.UNAIDS. Fast-Track Update On Investments Needed In The AIDS Response https://www.unaids.org/sites/default/files/media_asset/UNAIDS_Reference_FastTrack_Update_on_investments_en.pdf (2016).
- 19.Gelpi A & Tucker JD A cure at last? Penicillin’s unintended consequences on syphilis control, 1944–1964. Sex. Transm. Infect 91, 70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finzi D et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Wong JK et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Chun TW et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl Acad. Sci. USA 94, 13193–13197 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiegand A et al. Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc. Natl Acad. Sci. USA 114, E3659–E3668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohn LB et al. Clonal CD4+ T cells in the HIV-1 latent reservoir display a distinct gene profile upon reactivation. Nat. Med 24, 604–609 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Using single-cell analyses, the transcriptional profile of HIV-infected CD4+ T cells was characterized, revealing the expression of pathways that suppress HIV transcription.
- 25.Kuo H-H et al. Anti-apoptotic protein BIRC5 maintains survival of HIV-1-infected CD4+ T cells. Immunity 48, 1183–1194 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yukl SA et al. HIV latency in isolated patient CD4+ T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med 10, eaap9927 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; Multiple blocks to HIV transcription exist in infected CD4+ T cells; it may be necessary to overcome each using diverse approaches before sufficient amounts of HIV protein are made for the cell to be recognized and eliminated.
- 27.Ho YC et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; Most (>90%) integrated HIV genomes are defective and unable to support HIV replication, complicating the measurement of the inducible replication-competent viral reservoir.
- 28.Hosmane NN et al. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J. Exp. Med 214, 959–972 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Descours B et al. CD32a is a marker of a CD4 T-cell HIV reservoir harbouring replication-competent proviruses. Nature 543, 564–567 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Chomont N et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med 15, 893–900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fromentin R et al. CD4+ T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 12, e1005761 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Banga R et al. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat. Med 22, 754–761 (2016). [DOI] [PubMed] [Google Scholar]; During effective antiretroviral therapy, transcriptionally active HIV is enriched in PD-1-expressing T follicular helper cells that reside in lymph nodes.
- 33.McGary CS et al. CTLA-4+PD-1− memory CD4+ T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity 47, 776–788 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chew GM et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 12, e1005349 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hiener B et al. Identification of genetically intact HIV-1 proviruses in specific CD4+ T cells from effectively treated participants. Cell Rep. 21, 813–822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogan LE et al. Increased HIV-1 transcriptional activity and infectious burden in peripheral blood and gut-associated CD4+ T cells expressing CD30. PLoS Pathog. 14, e1006856 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pardons M et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 15, e1007619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoury G et al. Persistence of integrated HIV DNA in CXCR3+ CCR6+ memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS 30, 1511–1520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee GQ et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4+ T cells. J. Clin. Invest 127, 2689–2696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wacleche VS et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology 13, 59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z et al. Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc. Natl Acad. Sci. USA 115, E2575–E2584 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner TA et al. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maldarelli F et al. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; As demonstrated in these two studies42,43, proliferation of memory T cells is the main mechanism by which the reservoir is maintained indefinitely; during long-term therapy, the population of infected cells becomes increasingly clonal with integration of defective HIV genomes in genes associated with cell growth (including oncogenes) and/or within intergenic regions or silent genes.
- 44.Cohn LB et al. HIV-1 integration landscape during latent and active infection. Cell 160, 420–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einkauf KB et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J. Clin. Invest 129, 988–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Battivelli E et al. Distinct chromatin functional states correlate with HIV latency reactivation in infected primary CD4+ T cells. eLife 7, e34655 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinzone MR et al. Longitudinal HIV sequencing reveals reservoir expression leading to decay which is obscured by clonal expansion. Nat. Commun 10, 728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee GQ et al. HIV-1 DNA sequence diversity and evolution during acute subtype C infection. Nat. Commun 10, 2737 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang SH et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J. Clin. Invest 128, 876–889 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fletcher CV et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA 111, 2307–2312 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearney MF et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 10, e1004010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shan L et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36, 491–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng K et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Migueles SA et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J. Virol 83, 11876–11889 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peretz Y et al. CD160 and PD-1 co-expression on HIV-specific CD8 T cells defines a subset with advanced dysfunction. PLoS Pathog. 8, e1002840 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hersperger AR et al. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood 117, 3799–3808 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tauriainen J et al. Perturbed CD8+ T cell TIGIT/CD226/PVR axis despite early initiation of antiretroviral treatment in HIV infected individuals. Sci. Rep 7, 40354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takata H et al. Delayed differentiation of potent effector CD8+ T cells reducing viremia and reservoir seeding in acute HIV infection. Sci. Transl. Med 9, eaag1809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ndhlovu ZM et al. Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci. Transl. Med 11, eaau0528 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ndhlovu ZM et al. Magnitude and kinetics of CD8+ T cell activation during hyperacute HIV infection impact viral set point. Immunity 43, 591–604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yukl SA et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9, e1003347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Colby DJ et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat. Med 24, 923–926 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henrich TJ et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med. 14, e1002417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; Studies62,63 have demonstrated that even with highly effective interventions that result in multi-log reductions in the reservoir, a small undetectable reservoir of replication-competent HIV can persist and rebound many months after ART is interrupted.
- 64.Henrich TJ et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann. Intern. Med 161, 319–327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Persaud D et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med 369, 1828–1835 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fidler S et al. A pilot evaluation of whole blood finger-prick sampling for point-of-care HIV viral load measurement: the UNICORN study. Sci. Rep 7, 13658 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bruner KM et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estes JD et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat. Med 23, 1271–1276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santangelo PJ et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat. Methods 12, 427–432 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keating SM et al. HIV antibody level as a marker of HIV persistence and low-level viral replication. J. Infect. Dis 216, 72–81 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothenberger MK et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc. Natl Acad. Sci. USA 112, E1126–E1134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sneller MC et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci. Transl. Med 9, eaan8848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lelièvre J-D & Hocqueloux L Unintended HIV-1 transmission to a sex partner in a study of a therapeutic vaccine candidate. J. Infect. Dis 220, S5–S6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Julg B et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV 6, e259–e268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Phillips AN et al. Identifying key drivers of the impact of an HIV cure intervention in sub-Saharan Africa. J. Infect. Dis 214, 73–79 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paltiel AD et al. Setting performance standards for a cost-effective human immunodeficiency virus cure strategy in South Africa. Open Forum Infect. Dis 4, ofx081 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dong KL et al. Detection and treatment of Fiebig stage I HIV-1 infection in young at-risk women in South Africa: a prospective cohort study. Lancet HIV 5, e35–e44 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okoye AA et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med 24, 1430–1440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Violari A et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat. Commun 10, 412 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frange P et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 3, e49–e54 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Holt N et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol 28, 839–847 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peterson CW et al. Differential impact of transplantation on peripheral and tissue-associated viral reservoirs: implications for HIV gene therapy. PLoS Pathog. 14, e1006956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haworth KG, Peterson CW & Kiem HP CCR5-edited gene therapies for HIV cure: closing the door to viral entry. Cytotherapy 19, 1325–1338 (2017). [DOI] [PubMed] [Google Scholar]
- 84.Yin H, Kauffman KJ & Anderson DG Delivery technologies for genome editing. Nat. Rev. Drug Discov 16, 387–399 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Davenport MP et al. Functional cure of HIV: the scale of the challenge. Nat. Rev. Immunol 19, 45–54 (2019). [DOI] [PubMed] [Google Scholar]
- 86.Kordelas L et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N. Engl. J. Med 371, 880–882 (2014). [DOI] [PubMed] [Google Scholar]
- 87.Dash PK et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat. Commun 10, 2753 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang G, Zhao N, Berkhout B & Das AT A combinatorial CRISPR–Cas9 attack on HIV-1 DNA extinguishes all infectious provirus in infected T cell cultures. Cell Rep. 17, 2819–2826 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Martinez-Navio JM et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50, 567–575 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Priddy FH et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6, e230–e239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deeks SG et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther 5, 788–797 (2002). [DOI] [PubMed] [Google Scholar]
- 92.June CH, O’Connor RS, Kawalekar OU, Ghassemi S & Milone MC CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Zhen A et al. Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 13, e1006753 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leibman RS et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLoS Pathog. 13, e1006613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anthony-Gonda K et al. Multispecific anti-HIV duoCAR-T cells display broad in vitro antiviral activity and potent in vivo elimination of HIV-infected cells in a humanized mouse model. Sci. Transl. Med 11, eaav5685 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Archin NM et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elliott JH et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 10, e1004473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elliott JH et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2, e520–e529 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rasmussen TA et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1, e13–e21 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Søgaard OS et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 11, e1005142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lim S-Y et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci. Transl. Med 10, eaao4521 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vibholm L et al. Short-course Toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis 64, 1686–1695 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laird GM et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J. Clin. Invest 125, 1901–1912 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macedo AB et al. Dual TLR2 and TLR7 agonists as HIV latency-reversing agents. JCI Insight 3, e122673 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rochat MA, Schlaepfer E & Speck RF Promising role of Toll-like receptor 8 agonist in concert with prostratin for activation of silent HIV. J. Virol 91, e02084–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hill AL, Rosenbloom DI, Fu F, Nowak MA & Siliciano RF Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc. Natl Acad. Sci. USA 111, 13475–13480 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mousseau G et al. The Tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. mBio 6, e00465–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kessing CF et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep. 21, 600–611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barr EL & Jefferys R A landscape analysis of HIV cure-related clinical trials and observational studies in 2018. J. Virus Eradication http://viruseradication.com/journal-details/A_landscape_analysis_of_HIV_cure-related_clinical_trials_and_observational_studies_in_2018/ (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Conway JM & Perelson AS Post-treatment control of HIV infection. Proc. Natl Acad. Sci. USA 112, 5467–5472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Casazza JP et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J. Infect. Dis 207, 1829–1840 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hansen SG et al. Broadly targeted CD8+ T cell responses restricted by major histocompatibility complex E. Science 351, 714–720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hansen SG et al. Immune clearance of highly pathogenic SIV infection. Nature 502, 100–104 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hessell AJ et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med 22, 362–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Borducchi EN et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540, 284–287 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borducchi EN et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; In these two related non-human primate studies115,116, a combination of immune therapies resulted in enhanced immune control and, in some cases, possible elimination of SIV/SHIV; these strategies are now being tested in people.
- 117.Nishimura Y et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 543, 559–563 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mendoza P et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; These two related studies117,118 demonstrate that broadly neutralizing antibodies administered during a period of acute SHIV infection of non-human primates or immediately after interruption of ART in HIV-infected humans potentially induce long-term immune-mediated control (the ‘vaccinal effect’); in the case of SHIV-infected non-human primates, such suppression was shown to be mediated by CD8+ T cells.
- 119.Xu L et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science 358, 85–90 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sung JA et al. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Invest 125, 4077–4090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leal L et al. New challenges in therapeutic vaccines against HIV infection. Expert Rev. Vaccines 16, 587–600 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Gaiha GD et al. Structural topology defines protective CD8+ T cell epitopes in the HIV proteome. Science 364, 480–484 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fromentin R et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat. Commun 10, 814 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gay CL et al. Clinical trial of the anti-PD-L1 antibody BMS-936559 in HIV-1 infected participants on suppressive antiretroviral therapy. J. Infect. Dis 215, 1725–1733 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Webb GM et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv. 2, 76–84 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petrovas C et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci. Transl. Med 9, eaag2285 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ananworanich J & Barré-Sinoussi F Is it time to abandon single intervention cure trials? Lancet HIV 2, e410–e411 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Leth S et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 3, e463–e472 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Rossouw T, Tucker JD, van Zyl GU, Sikwesi K & Godfrey C Barriers to HIV remission research in low- and middle-income countries. J. Int. AIDS Soc 20, 21521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kityo C et al. Lymphoid tissue fibrosis is associated with impaired vaccine responses. J. Clin. Invest 128, 2763–2773 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scully EP et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J. Infect. Dis 219, 1084–1094 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


