Abstract
In humans, there is an endogenous, near 24-hour (i.e., circadian) variation in mood with the best mood occurring during the circadian day and the worst mood occurring during the circadian night. Only positive affect, and not negative affect, has been shown to contribute to this circadian rhythm. We discovered a sharp circadian peak in negative affect during the circadian night coincident with a circadian trough in positive affect. These findings may help explain the association of depression with insomnia, the increased risk of suicide with nocturnal wakefulness and the correlation between circadian misalignment and symptom severity in Major Depressive Disorder.
Keywords: circadian, mood disorders, sleep
1. Introduction
The hypothalamic circadian pacemaker coordinates near-24-hour rhythmicity in a wide range of physiological, behavioral and psychological functions, including mood (Boivin et al. 1997). For example, when evaluated using visual analogue scales that required participants to rate their emotional state along an axis between “happy” and ‘”sad”, the happiest mood occurred during the circadian day while the saddest mood occurred in the circadian night (Boivin et al. 1997). However, when mood was parsed into the independent components (Norris et al. 2010) of positive and negative affect using the Positive and Negative Affect Schedule (PANAS), in which participants rated a variety of pleasant and unpleasant feelings using Likert scales, only the positive feelings were found to have a circadian rhythm (Murray et al. 2009). This is despite the fact that variations in negative affect have been repeatedly observed under naturalistic conditions during waking hours (Porto et al. 2006, Stone et al. 2006, Miller et al. 2015).The clinical relevance of these rhythms is highlighted by the finding that abnormal timing of the circadian clock relative to the timing of biologically important behaviors such as eating and sleeping (termed circadian misalignment, as occurs in night shift work) has adverse psychiatric sequlae (Baron and Reid 2014). Indeed, we, and others, have shown that the degree of circadian misalignment correlates with symptom severity in Major Depressive Disorder (Emens et al. 2009, Hasler et al. 2010). Specifically, the later the pacemaker is set relative to habitual sleep timing, the worse the mood (Emens et al. 2009). The mechanism by which a shift in the timing of the pacemaker worsens mood has not been elucidated. We hypothesized that negative affect would exhibit a circadian rhythm that peaks during the circadian night. If such a pattern exists, this might help explain the association between circadian misalignment and mood disorders (Emens et al. 2009, Baron and Reid 2014).
2. Methods
2.1. Participants
21 healthy adults (52 ± 7 years; 11 females) were studied. All participants provided written informed consent and the protocol was approved by the Institutional Review Board at Oregon Health & Science University. Health status was evaluated via medical history, physical and psychiatric examination (Sheehan et al. 1998), 12-lead electrocardiogram, home sleep apnea screening (WatchPAT, Itamar Medical, Israel), and screening laboratory studies. Exclusion criteria included chronic medical conditions, BMI >40 kg/m2, psychiatric illness or psychotropic medication use in the prior 12 months, use of tobacco or recreational drugs, and any prescription or non-prescription medication use. Participants with a history of travel across >3 time zones in the prior 3 months or night shift work in the prior 6 months were excluded. The current study represents part of a larger study of circadian rhythms and cardiovascular physiology (Thosar et al., 2019).
2.2. Ambulatory Protocol
For 1–2 weeks prior to the start of the in-laboratory protocol, each participant refrained from prescription and over-the-counter medication, alcohol, and caffeine use and maintained a consistent, self-selected sleep-wake schedule with an 8 hour sleep opportunity, which was confirmed by actigraphy (Actigraph wGT3X-BT, Actigraph, Pensacola, FL), sleep diary and twice-daily phone calls to a time-stamped voicemail box.
2.3. Laboratory Protocol
Participants were admitted to the Oregon Clinical and Translational Research Institute at Oregon Health & Science University and lived in an environment free of external time cues for 5 days. Throughout their stay, all activities, including sleep opportunities, were scheduled and light levels remained <3 lux at the angle of gaze during scheduled wakefulness.
2.3.1. Forced Desynchrony Protocol
After a baseline day, participants completed a protocol that evenly distributes all behaviors across the circadian cycle thereby unmasking endogenous rhythms controlled by the circadian pacemaker (Boivin et al. 1997, Czeisler et al. 1999). This protocol was comprised of 10 recurring, 5-hour and 20-minute identical behavioral cycles, consisting of 2 hour and 40 minute sleep and 2 hour and 40 minute wake episodes with an identical isocaloric meal towards the end of each wake period (Thosar et al. 2019).
2.3.2. Assessment of Circadian Phase
Salivary samples were provided every ~30 minutes during the evening of admission and every 40–70 minutes during subsequent scheduled wake periods. Samples were assayed for melatonin via radioimmunoassay (ALPCO Ltd., Windham, NH). The lower limit of sensitivity is 0.2 pg/ml. Circadian phase (the timing of the endogenous biological clock) was determined using the dim light melatonin onset (DLMO), defined as the interpolated time when salivary melatonin crossed a 3 pg/ml threshold (in one participant whose salivary melatonin never dipped below 3 pg/ml, 4 pg/ml (Benloucif et al. 2008) was used as the threshold) (Voultsios et al. 1997, Lewy et al. 1999).
2.3.3. Mood Assessments
Participants completed the PANAS (Watson et al. 1988) and Profile of Mood States-Brief (POMS-B) (McNair et al. 1992) questionnaires ~45 minutes into the start of each wake episode, ten times across the forced desynchrony protocol. Positive affect was reported using the 10-item Positive Affect subscale (PANAS PA); negative affect was reported using the 10-item Negative Affect subscale (PANAS NA). Total mood disturbance (TMD) score, a composite score of the POMS-B, was used as an additional measure of negative affect.
2.4. Statistical Analysis
Each participant’s mood data were normalized (z-scored) and assigned circadian phases relative to their individual DLMO (assigned 0°), with the duration of one circadian cycle equal to 360°. PANAS PA, NA and POMS-B TMD subscale data were assessed for circadian rhythmicity via cosinor analysis (2-harmonic parametrization) (Hu et al. 2011).
3. Results
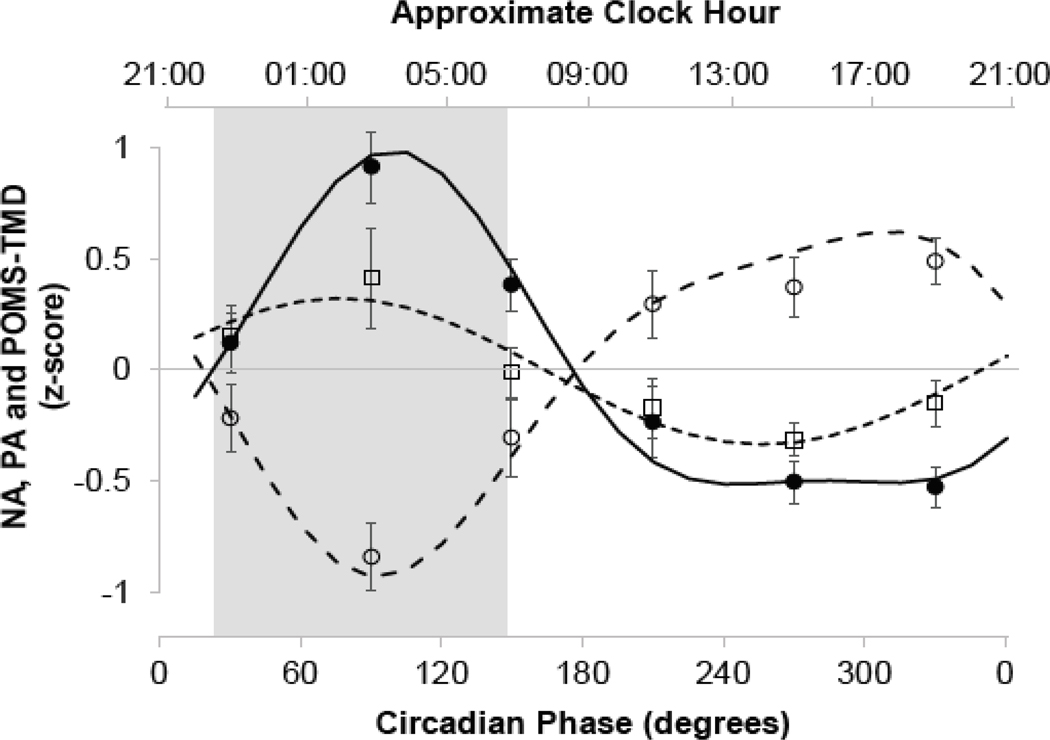
The average bedtimes and waketimes (±SD) prior to entering the lab were 22:27 ± 1:07 and 06:39 ± 1:09, respectively, and the average clock hour of the baseline DLMO occurred at 20:51 ± 1:45. PANAS PA demonstrated a robust circadian rhythm (F[5,202]=16.84, p<0.0001, r2=0.28) with a sharp trough in positive affect during the circadian night (~6 hours after DLMO or ~03:00, Figure 1). We discovered that PANAS NA (F[5,202]=2.83, p = 0.02, r2=0.04) and POMS-B TMD (F[5,202]=17.11, p<0.0001, r2=0.28) also exhibited circadian rhythms that mirrored that of positive affect with both negative affect and total mood disturbance sharply peaking during the circadian night at approximately the same circadian phase as the trough in PANAS PA (Figure 1). The greatest positive affect occurred during the circadian afternoon and evening (without a well-defined peak), and this was mirrored by negative affect and total mood disturbance which were lowest across the circadian afternoon and evening (Figure 1). There were no significant time-into-protocol effects on PANAS NA (p=0.66) but there was a reduction in PANAS PA (p=0.001) and increase in POMS-B TMD (p=0.03) (Amira et al. 2020).
Figure 1:
Endogenous circadian rhythms in mood. Shown are z-scores (mean ± SEM) for the PANAS negative (□) and positive (○) affect scores, and POMS-B total mood disturbance (TMD) score (●). Data are plotted accordingly to circadian time in bin sizes of 60° (~4 hours) with the DLMO defined as 0°. Mixed model cosinor analyses (conducted on non-binned data) are shown for PANAS negative (---) and positive (– –) affect scores, and POMS-B TMD (—). The shaded rectangle corresponds to the participants’ average sleep timing prior to entering the laboratory. For all analyses, p<0.05.
4. Discussion
Our results confirm prior findings of a robust endogenous circadian rhythm of positive affect that peaks during the circadian day. We extended these observations to show that there also exists an endogenous circadian rhythm of negative affect, which peaks during the circadian night.
4.1. Implications for the Development of Mood Disorders
Individuals who are awake across the night, such as those with insomnia or performing night shiftwork, have an increased risk of developing mood disorders (Buysse 2013, Baron and Reid 2014). This association may be at least partially due to wakefulness during the circadian nadir in mood since these individuals would experience negative mood states normally “shielded” by sleep. This is analogous to the increased errors seen in individuals who are awake during the circadian trough in cognitive performance (Goel et al. 2013). Similarly, shifting the timing of the circadian pacemaker without shifting the sleep period, as can occur with exposure to artificial light (Czeisler et al. 1989, Stothard et al. 2017), could cause the endogenous minimum in mood to occur during wakefulness. Indeed, such a mechanism might explain our previous finding that the shifts in the circadian pacemaker to a later time relative to sleep correlate with increased symptom severity in major depressive disorder (Emens et al. 2009). Individuals with an evening diurnal preference have a higher risk of depression (Reid et al. 2012) and this too may be related to shifts in the timing of the pacemaker to a later hour (Emens et al. 2009). It is also conceivable that insomnia would expose individuals to low mood while awake during the night, potentially explaining how insomnia is associated with worse treatment outcomes in depression (Buysse 2013) as well as increased risk of recurrence (Armitage 2007). We speculate that while wakefulness during the circadian night may not cause depression, it may exacerbate or perpetuate depression in predisposed individuals. Moreover, the circadian rhythm in negative affect might also help explain the recently demonstrated increased risk of suicide with nocturnal wakefulness (Perlis et al. 2016).
4.2. Implications for the Management of Mood Disorders
These rhythms underscore the potential of targeting insomnia for both the prevention and treatment of mood disorders (Buysse 2013, McCall et al. 2019). These findings also highlight the potential of using circadian resetting agents such as light (Lam et al. 2016) or melatonin (Lewy et al. 2006) in the treatment of mood disorders and offer a potential mechanism by which such agents may act (i.e., by shifting peaks in negative affect into the sleep episode). Our findings and those of Perlis and colleagues (Perlis et al. 2016), also furthers the notion that the time of presentation and individual circadian timing should be considered in the assessment of suicide risk.
4.3. Limitations and Future Studies
We studied a small number of euthymic and middle-aged participants. A larger data set might allow for an item analysis to determine if certain mood states (e.g., anxious versus depressed) contribute more to the overall circadian rhythm in mood and negative affect in particular (although an exploratory analysis showed that removal of any one of the six subscales on the POMS-TMD did not result in a loss of statistical significance). Additionally, it was beyond the scope of this study to determine whether negative and positive affect represent truly distinct entities or whether they describe moods that exist across a single spectrum (Norris et al. 2010). That said, there is a significant body work demonstrating that positive and negative affect can be independent of each other even if they are not necessarily localized in distinct regions of the brain (Lindquist et al. 2016). The greater amplitude in PANAS PA compared to PANAS NA scores may reflect greater circadian regulation of the reward-related motivational system (associated with positive affect) compared to the threat-related motivational system (associated with negative affect) as discussed by Murray and colleagues (Murray et al. 2009).
It is possible that the rhythmicity in positive and negative affect that we described are driven by circadian variations in the level of arousal, as opposed to affective valence. However, prior literature suggests that this is not the case, because mood and alertness become uncoupled depending on circadian phase and time spent awake (Boivin et al. 1997). We only conducted a single mood assessment per wake episode and therefore we were unable to assess the impact of time-since-awakening on mood (Boivin et al. 1997). Future studies could determine whether the amplitude and timing of these endogenous rhythms differ in men versus women, across the age span, among chronotypes and in individuals with and without mood disorders and whether the magnitude of evoked changes in mood also vary across the circadian cycle.
HIGHLIGHTS.
Mood has a circadian rhythm with the worst mood during the circadian night
Only positive affect has been shown to contribute to this circadian rhythm
It was found that negative affect also has a circadian rhythm that peaks at night
The rhythm in negative affect may have diagnosis and treatment implications for mood disorders
Acknowledgements
We wish to acknowledge the study participants and the OCTRI nursing staff.
Funding
This work was supported by the National Institutes of Health grants R01-HL125893, R01-HL142064, R01- HL140577, F32-HL131308, KL2-TR002370, and UL1-TR000128; NSBRI NCC 9-58 American Sleep Medicine Foundation; Ford Foundation; and the Oregon Institute of Occupational Health Sciences.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amira SA, Bressler BL, Lee JH, Czeisler CA and Duffy JF, 2020. Psychological Screening for Exceptional Environments: Laboratory Circadian Rhythm and Sleep Research. Clocks & Sleep. 2, 153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage R, 2007. Sleep and circadian rhythms in mood disorders. ACTA Psychiatrica Scandinavica. 115 (Suppl. 433), 104–115. [DOI] [PubMed] [Google Scholar]
- Baron KG and Reid KJ, 2014. Circadian misalignment and health. International Review of Psychiatry. 26 (2), 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benloucif S, Burgess HJ, Klerman EB, Lewy AJ, Middleton B, Murphy PJ, Parry BL and Revell VL, 2008. Measuring melatonin in humans. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 4 (1), 66. [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P. and Waterhouse JM, 1997. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Archives of General Psychiatry. 54, 145–152. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, 2013. Insomnia. JAMA. 309 (7), 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN and Ronda JM, 1989. Bright light induction of strong (Type O) resetting of the human circadian pacemaker. Science. 244, 1328–1333. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J and Kronauer RE, 1999. Stability, precision and near-24-hour period of the human circadian pacemaker. Science. 284, 2177–2181. [DOI] [PubMed] [Google Scholar]
- Emens JS, Lewy AJ, Kinzie JM, Arntz D. and Rough J, 2009. Circadian misalignment in major depressive disorder. Psychiatry Research. 168 (3), 259–261. [DOI] [PubMed] [Google Scholar]
- Emens JS, Yuhas K, Rough J, Kochar N, Peters D. and Lewy AJ, 2009. Phase Angle of Entrainment in Morning- and Evening-Types under Naturalistic Conditions. Chronobiology International. 26 (3), 474–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Basner M, Rao H. and Dinges DF, 2013. Circadian Rhythms, Sleep Deprivation, and Human Performance. Progress in Molecular Biology and Translational Science. 119, 155–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Buysse DJ, Kupfer DJ and Germain A, 2010. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: Further evidence for circadian misalignment in non-seasonal depression. Psychiatry Research. 178 (1), 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Laker M, Smales C. and Shea SA, 2011. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation. 123 (9), 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, Morehouse R, Ramasubbu R, Yatham LN and Tam EM, 2016. Efficacy of Bright Light Treatment, Fluoxetine, and the Combination in Patients With Nonseasonal Major Depressive Disorder. A Randomized Clinical Trial. JAMA Psychiatry. 73 (1), 56–63. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL and Sack RL, 1999. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 14 (3), 227–236. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS and Bauer VK, 2006. The circadian basis of winter depression. Proceedings of the National Academy of Science USA. 103 (19), 7414–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist K, Satpute A, Wager T, Weber J. and Feldman Barrett L, 2016. The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cerebral Cortex. 26, 1910–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall WV, Benca RM, Rosenquist PB, Youssef NA, McCloud L, Newman JC, Case D, Rumble ME, Szabo ST, Phillips MEA and Krystal AD, 2019. Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT): A Randomized Clinical Trial. American Journal of Psychiatry. 176, 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M. and Droppleman LF, 1992. Manual: Profile of Mood States, Revised 1992. San Diego, CA, Educational and Industrial Testing Service. [Google Scholar]
- Miller MA, Rothenberger SD, Hasler BP, Donofry SD, Wong PM, Manuck SB, Kamarck TW and Roecklein KA, 2015. Chronotype predicts positive affect rhythms measured by ecological momentary assessment. Chronobiology International. 32 (3), 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB and Trinder J, 2009. Nature’s Clocks and Human Mood: The Circadian System Modulates Reward Motivation. Emotion. 9 (5), 705–716. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Gollan J, Berntson GG and Cacioppo JT, 2010. The current status of research on the structure of evaluative space. Biological Psychiatry. 84, 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Grandner MA, Brown GK, Basner M, Chakravorty S, Morales KH, Gehrman PR, Chaudhary NS, Thase ME and Dinges DF, 2016. Nocturnal Wakefulness as a Previously Unrecognized Risk Factor for Suicide. Journal of Clinical Psychiatry. 77 (6), e726–e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto R, Duarte L. and Menna-Barreto L, 2006. Circadian variation of mood: comparison between different chronotypes. Biological Rhythm Research. 37 (5), 425–431. [Google Scholar]
- Reid KJ, Jaksa AA, Eisengart JB, Baron KG, Lu B, Kane P, Kang J. and Zee PC, 2012. Systematic evaluation of Axis-I DSM diagnoses in delayed sleep phase disorder and evening-type circadian preference. Sleep Medicine. 13 (9), 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R. and Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 59 (Suppl 20), 22–57. [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Schkade D, Schwarz N, Krueger A. and Kahneman D, 2006. A Population Approach to the Study of Emotion: Diurnal Rhythms of a Working Day Examined With the Day Reconstruction Method. Emotion. 6 (1), 139–149. [DOI] [PubMed] [Google Scholar]
- Stothard ER, McHill AW, Depner CM, Birks BR, Moehlman TM, Ritchie HK, Guzzetti JR, Chinoy ED, LeBourgeois MK, Axelsson J. and Wright KP Jr, 2017. Circadian Entrainment to the Natural Light-Dark Cycle across Seasons and the Weekend. Current Biology. 27 (4), 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar S, Berman AM, Herzig M, McHill A, Bowles N, Swanson C, Clemons N, Butler M, Clemons A, Emens J. and Shea S, 2019. Circadian Rhythm of Vascular Function in Midlife Adults. Arteriosclerosis, Thrombosis & Vascular Biology. 39 (6), 1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ and Dawson D, 1997. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. Journal of Biological Rhythms. 12 (5), 457–466. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA and Tellegen A, 1988. Development and Validation of Brief Measures of Positive and Negative Affect: The PANAS Scales. Journal of Personlaity and Social Psychology. 54 (6), 1063–1070. [DOI] [PubMed] [Google Scholar]