Abstract
Somatic expansion of the CAG repeat tract that causes Huntington's disease (HD) is thought to contribute to the rate of disease pathogenesis. Therefore, factors influencing repeat expansion are potential therapeutic targets. Genes in the DNA mismatch repair pathway are critical drivers of somatic expansion in HD mouse models. Here, we have tested, using genetic and pharmacological approaches, the role of the endonuclease domain of the mismatch repair protein MLH3 in somatic CAG expansion in HD mice and patient cells. A point mutation in the MLH3 endonuclease domain completely eliminated CAG expansion in the brain and peripheral tissues of a HD knock-in mouse model (HttQ111). To test whether the MLH3 endonuclease could be manipulated pharmacologically, we delivered splice switching oligonucleotides in mice to redirect Mlh3 splicing to exclude the endonuclease domain. Splice redirection to an isoform lacking the endonuclease domain was associated with reduced CAG expansion. Finally, CAG expansion in HD patient-derived primary fibroblasts was also significantly reduced by redirecting MLH3 splicing to the endogenous endonuclease domain-lacking isoform. These data indicate the potential of targeting the MLH3 endonuclease domain to slow somatic CAG repeat expansion in HD, a therapeutic strategy that may be applicable across multiple repeat expansion disorders.
INTRODUCTION
Huntington's disease (HD) is a dominantly inherited neurodegenerative disorder caused by an expanded CAG trinucleotide repeat in the huntingtin gene (HTT), encoding an extended polyglutamine tract in the huntingtin protein (1). The expanded CAG mutation ultimately results in neuronal dysfunction and death (2), via mechanisms that are as yet unclear (3). The mutant CAG repeat undergoes further expansion in somatic cells in a CAG length-dependent and tissue- or cell-type-specific manner (4–8). Somatic expansion is recapitulated in HD mouse models that also show progressive repeat tract lengthening over time (9–12), with the striatum and liver showing high levels of expansion, particularly the medium spiny neurons and hepatocytes, respectively (9,10). Studies of postmortem HD brains revealed that longer somatic CAG expansions are associated with an earlier age of disease onset (7), with more recent studies showing correlations between age of onset or disease progression and the degree of somatic expansion in HD patient blood (13). Significantly, recent genome-wide association studies (GWAS) of over 9000 HD patients have demonstrated that the rate of disease onset is determined by the length of the pure CAG repeat, rather than the length of the glutamine tract in huntingtin (14). This is supported by additional studies (13,15) and provides compelling support for a model in which the rate of somatic CAG expansion drives the rate of disease onset. Thus, therapies targeting somatic repeat expansions have the potential to delay disease onset and progression.
Genetic knockout studies in HD mouse models have shown that Msh2, Msh3, Mlh1 and Mlh3 genes in the DNA mismatch repair (MMR) pathway are required for somatic expansion (16–19). Human GWAS have identified several genes in this pathway (MSH3, MLH1, PMS1, PMS2) to be associated with age at motor onset (14,20), with MSH3 also showing association with a measure of HD progression (21). The mouse ortholog of an additional HD onset-modifier gene, FAN1 (14,20), with no known function in the MMR pathway, suppressed somatic expansion in HD knock-in mice in a manner that was dependent on Mlh1 (8). Genetic variation in some human onset or progression modifier genes (MSH3, MLH1, MLH3, FAN1) also modified CAG expansion in HD patient blood or blood-derived cells (13,14,22). Together, these data indicate that HD pathogenesis is modulated by naturally occurring variation in genes that act by altering the rate of somatic CAG expansion, and that genes driving this process are potential therapeutic targets for HD. MMR pathway genes also modify somatic expansion in mouse models and in mouse or human cell-based models of a number of other DNA repeat expansion disorders, including myotonic dystrophy type I (DM1) (23–25), Friedreich ataxia (FRDA) (26–29), and the fragile X-related disorders (FXD) (30–34). In addition, polymorphisms in two DNA repair genes (FAN1, PMS2) associated with HD onset were also associated with disease onset in the spinocerebellar ataxias (35). Thus, therapeutic strategies targeting MMR genes may be applicable to multiple repeat expansion diseases.
Our previous genetic knockout studies in HttQ111 knock-in mice demonstrated that MLH3 is an essential component of the CAG repeat expansion process (17). MLH3 can form a heterodimer with MLH1 (MutLγ) and is known to play an important role in meiosis, but a relatively minor role in canonical MMR (36–38). MLH3 contains an evolutionary conserved endonuclease domain (39) that we postulated may be important in its CAG expansion-promoting role in HD (17), with inactivation or elimination of this domain hypothesized to eliminate or reduce somatic expansions. Interestingly, human MLH3 exists primarily as two protein isoforms: isoform 1 (UniProt Q9UHC1-1) is specified by splice variant 1 (CCDS32123) containing exon 7, which encodes the endonuclease domain, while isoform 2 (UniProt Q9UHC1-2) is specified by splice variant 2 (CCDS9837), lacking exon 7, and therefore has no endonuclease domain (28,40). MLH3 splice redirection from predominantly splice variant 1 to predominantly splice variant 2 can be achieved with splice switching oligonucleotides (SSOs) that mask the acceptor and donor sites that surround exon 7 of the MLH3 pre-mRNA (28). In a previous FRDA study, splice redirection of MLH3 to exclude the endonuclease domain resulted in reduced rates of GAA expansion in a human cell-based model and in patient cells (28). This indicated that the MLH3 endonuclease domain was important for GAA repeat expansion and that splice redirection could be used to reduce its contribution to the expansion process. More recently, a point mutation in the MLH3 endonuclease domain was found to abrogate expansion of the CGG repeat tract in a mouse embryonic cell-based FXD model (30).
Here, we have tested the role of the MLH3 endonuclease domain in somatic HTT CAG expansion, using both genetic studies and a pharmacological approach based on splice redirection. We demonstrate that: i) a single point mutation in the MLH3 endonuclease domain, predicted to abrogate endonuclease activity, eliminates somatic CAG expansion in HttQ111 mice; ii) systemic delivery of SSOs to redirect Mlh3 splicing to exclude the endonuclease domain suppresses somatic CAG expansion in HttQ111 mice, and iii) MLH3 splice redirection with SSOs also effectively reduces CAG expansion in HD patient-derived primary fibroblast cells, in the absence of replication. These data highlight the therapeutic potential of targeting the MLH3 endonuclease domain to slow HTT CAG repeat expansion, a strategy that may be applicable across multiple repeat expansion disorders.
MATERIALS AND METHODS
Mice
Htt Q111 HD knock-in mice (formerly HdhQ111) (41) were maintained on a C57BL/6J background (9) by breeding heterozygous males to C57BL/6J wild-type females from The Jackson Laboratory (Bar Harbor, ME). Mlh3DN mice (42) were also maintained on C57BL/6J background. HttQ111/+ and Mlh3WT/DN mice were crossed together to generate HttQ111/+Mlh3WT/DN and Htt+/+Mlh3WT/DN mice, which were subsequently intercrossed to generate heterozygous HttQ111/+ mice (hereafter referred to as HttQ111, for simplicity) that were either wild type (Mlh3WT/WT), heterozygous (Mlh3WT/DN), or homozygous (Mlh3DN/DN) for the Mlh3DN point mutation. Genotyping of the HttQ111 and Mlh3DN alleles in genomic DNA extracted from tail or ear biopsies at weaning, or in adult tissues for genotype confirmation, was carried out as previously described (16,42). All animal procedures were carried out to minimize pain and discomfort, under approved IACUC protocols of the Massachusetts General Hospital. Animal husbandry was performed under controlled temperature and light/dark cycles.
HTT CAG repeat instability analysis
Genomic DNA was isolated from mouse tissues and patient fibroblast cells as previously described (17,28). Analyses of HTT CAG repeat size in both HttQ111 mice and in patient fibroblasts was performed by PCR using human-specific HTT primers and Taq PCR Core Kit with Q solution (Qiagen), as previously described (17,43). The resulting FAM-labelled PCR products, encompassing the HTT CAG repeat, were resolved on the ABI3730xl automated DNA analyzer (Applied Biosystems), with either GeneScan 500-LIZ or 1200-LIZ internal size standards, and analyzed with GeneMapper v5 (Applied Biosystems). When necessary, to increase the PCR signal strength, up to three PCR replicates were combined and concentrated with MinElute PCR Purification Kit (Qiagen). CAG expansion indices were quantified in HttQ111 mice from GeneMapper peak height data, taking into account only expansion peaks (i.e. larger than main allele) and using a 5% peak height threshold, as described previously (44). In the HD patient fibroblasts, CAG expansion was reflected in a shift of the entire CAG length distribution to greater repeat lengths over time, without any discernible impact on the shape of the distribution. To quantify repeat expansion, we determined the average gain of CAG units by calculating the difference in average CAG length before (t = 0, n = 4) and after the 6-week culture period (t = 6 weeks, n = 10). To determine average CAG length in an individual sample, we first applied a 20% peak height threshold to the distribution (i.e. only peaks with height ≥ 20% of tallest peak were used), then multiplied the frequency of each peak (peak height divided by the sum of all peak heights) by the corresponding CAG size, and finally summed these values.
Treatment of HttQ111 mice with Mlh3 SSOs
Mouse-specific SSOs were designed to target the pre-mRNA of Mlh3 exon 6 (Chr12:85,250,302–85,250,373; GRCm38/mm10): mMLH3ac5 (acceptor site) 5′-TCCACCTACAAAATAATCCAGGATT-3′ and mMLH3dr7 (donor site) 5′-AACTACAGACAGATACTTACCAGTA-3′. Oligonucleotides were obtained as ‘Vivo-Morpholinos’ from Gene Tools, LLC (45). HttQ111 mice (CAG 104–109; two males and two females per group) were treated with either 25 mg/kg SSOs (mMLH3ac5 and mMLH3dr7; 1:1 ratio), or PBS, by single tail vein injection of 200 μl. Treatment was initiated at 4 weeks of age and injections were performed three times per week (every 48–72 h). Weight measurements were taken at the beginning of each week to determine weekly weight-adjusted doses. Treatment was stopped at 12 weeks of age, with animal euthanasia and immediate dissection performed 24 h after the final injection. Tissues were rapidly frozen using liquid nitrogen to preserve RNA integrity.
MLH3 splicing analysis
Frozen mouse tissues were mechanically homogenized prior to RNA extraction. Total RNA was extracted from HttQ111 mouse tissues and HD patient cultured primary fibroblasts with TRIzol (Thermo Fisher Scientific) following the manufacturer's protocol. Total RNA (250 ng) was used to generate cDNA with the High Capacity cDNA Reverse Transcription Kit following the manufacturer's protocol (Thermo Fisher Scientific). Human MLH3 splice variants were characterized by Reverse Transcription PCR (RT-PCR) as previously described (28). Mouse Mlh3 splice variants were characterized by RT-PCR with primers mMLH3 × 3-3328F (5′-CCAGTGTTTGCTCGATACCC-3′) and mMLH3 × 11-4028R (5′-GGACAAGGACAGAGCTTCGA-3), at 59°C annealing temperature, for 33 cycles. PCR products were resolved by electrophoresis on a 1.4% agarose gel containing 1 μg/ml ethidium bromide. Real-time RT-PCR (RT-qPCR) characterization of Mlh3 splicing in mouse tissues was performed with TaqMan Gene Expression Assays (Applied Biosystems) following the manufacturer's protocol on the CFX96 Real-Time PCR Detection System (Bio-Rad). The assay used for the Mlh3 splice variant 1 detects the splice junction between exon 6 and exon 7 (Mm00520819_m1, FAM-labeled probe). The assay for Mlh3 splice variant 2, binds at the splice junction between exon 5 and exon 7 (custom design, FAM-labeled probe) and only binds when exon 6 is spliced out. Relative Mlh3 splice variant levels were normalized to the endogenous mouse β-actin (Actb) reference gene (Mm00607939_s1, primer-limited, VIC-labeled probe) in duplex reactions. Each sample was run in triplicate.
Treatment of HD patient primary fibroblasts with MLH3 SSOs
Primary fibroblasts from a juvenile HD patient (GM09197; CAG ∼180/18) (46) were obtained from the Coriell Institute for Medical Research. Cells were maintained in Dulbecco's modified Eagle's medium (DMEM) high glucose (Thermo Fisher Scientific) with 10% fetal bovine serum (FBS; Sigma) at 37°C in an atmosphere containing 5% CO2. To determine the ability of the SSOs to induce MLH3 splice redirection in HD primary fibroblasts, confluent cell cultures of unaltered primary GM09197 cells were treated with a single dose of either 500 nM of MLH3 SSOs (MLH3acr6 and MLH3dnr8, 1:1 ratio, 250 nM each) (28) or 500 nM of scrambled control oligonucleotide (5′-CCTCTTACCTCAGTTACAATTTATA-3′), alongside cells that were left untreated. A total of three independent wells were harvested for each condition. Oligonucleotides were obtained as ‘Vivo-Morpholinos’ from Gene Tools, LLC (45). Cells were treated for 6 h in reduced-serum Opti-MEM media (Thermo Fisher Scientific). The media was then replaced with 20% FBS high glucose DMEM and cells were harvested for RNA isolation after 48 h to analyze changes to MLH3 mRNA splice variants. To test the long-term effect of MLH3 splice redirection on HTT CAG expansion, GM09197 primary HD fibroblasts were first transduced with MSH3- and EGFP-expressing lentiviral particles (PNL-MSH3-IRES2EGFP construct), as previously described (27,28), to induce CAG expansion. Cells were then grown to confluent cultures and treated twice weekly with 500 nM of MLH3 SSOs, as descried above, or left untreated, for a total of six weeks. Cells were then harvested for DNA for HTT CAG instability analysis. A total of four wells were harvested immediately prior to treatment (t = 0) and a total of ten independent wells were harvested at the end of treatment (t = 6 weeks).
Statistical analysis
Statistical analyses were performed using GraphPad Prism v.8. Comparison of HTT somatic CAG expansion indices between HttQ111 mice with different Mlh3DN genotypes and across different tissues was performed using two-way ANOVA, with Tukey multiple comparisons post hoc correction. Comparison of HTT somatic CAG expansion indices in HttQ111 mice treated with Mlh3 SSOs was performed using two-tailed unpaired t tests comparing Mlh3 SSO-treated and PBS-treated mice for each tissue. Comparison of average HTT CAG gain in HD patient fibroblasts treated with MLH3 SSOs was performed using one-way ANOVA (‘t = 0’ versus ‘MLH3 SSOs, t = 6 weeks’ versus ‘untreated, t = 6 weeks’), with Tukey multiple comparisons post hoc correction. Not significant, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
RESULTS
Mutation in the MLH3 endonuclease domain prevents HD CAG expansion in HttQ111 mice
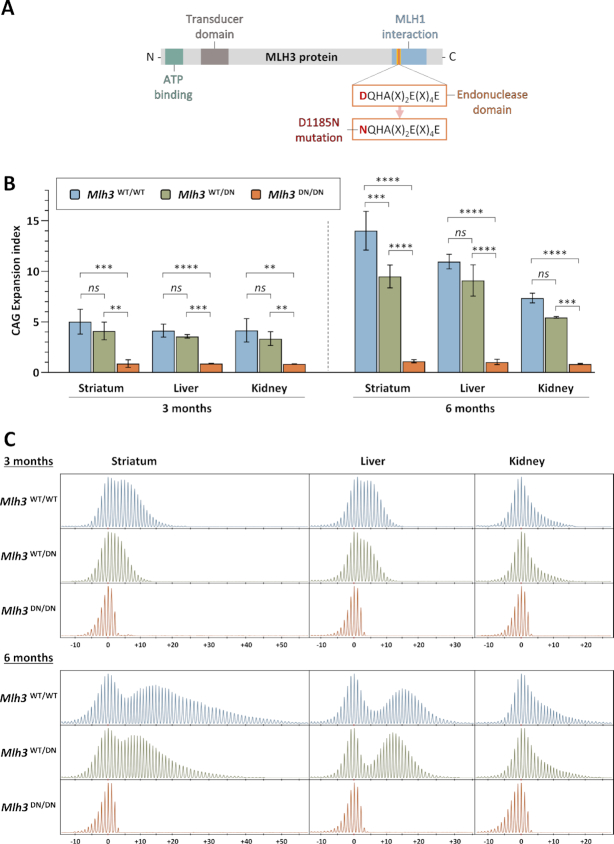
We previously demonstrated that MLH3 is required for somatic CAG repeat expansion in HttQ111 knock-in mice (17). To test the hypothesis that MLH3 endonuclease activity is involved in repeat expansion, we used an Mlh3 mutant mouse line (Mlh3D1185N, abbreviated to Mlh3DN) harboring a point mutation in the endonuclease domain (42). In these mice, GAC is changed to AAC in the genomic sequence to replace the aspartic acid ‘D’ at amino acid 1185 with an asparagine ‘N’ within the conserved DQHA(X)2E(X)4E motif (DQHAAHERIRLE in mouse MLH3) (Figure 1A). An analogous mutation in Saccharomyces cerevisiae or human MLH3 eliminates its endonuclease activity (47–50). Importantly, this D to N substitution in purified mouse, human, and S. cerevisiae proteins did not alter the stability of MLH3 or its interaction with MLH1 (42,50,51). In addition, in the mouse there was no impact on the number or localization of MLH3 or MLH1 foci in spermatocytes or on the recruitment of factors required to initiate crossovers in meiosis (42). Molecular modeling of mouse MLH3 was consistent with the lack of impact of the D1185N substitution on the structure or stability of MLH3 (30). Therefore, the Mlh3DN mutation in the mouse is predicted to disrupt MLH3 endonuclease function in the absence of significant effects on protein expression, stability, or interactions.
Figure 1.
MLH3 endonuclease domain mutation eliminates HTT CAG expansion in HttQ111 mice. (A) Schematic representation of MLH3 protein and its functional domains, as well as mutation of the endonucleolytic motif in Mlh3DN mice (adapted from Toledo et al. (42)). (B) Quantification of HTT CAG expansions in striatum, liver, and kidney at 3 and 6 months (M) of age in HttQ111 knock-in mice that are either wild type (Mlh3WT/WT, 3M CAG 115–122, 6M CAG 114–121), heterozygous (Mlh3WT/DN, 3M CAG 115–121, 6M CAG 116–121), or homozygous (Mlh3DN/DN, 3M CAG 116–121, 6M CAG 116–121) for the D1185N mutation shows that an intact MLH3 endonuclease domain is required for somatic CAG expansion. Striatum, 3M n = 5–6, 6M n = 3; liver, 3M n = 5, 6M n = 3; kidney, 3M n = 4–5, 6M n = 2–3; error bars represent standard deviation of the mean; two-way ANOVA (with genotype and tissue as variables) with Tukey multiple comparisons post hoc correction. Genotype effect: ns, not significant; **P < 0.01; ***P < 0.001; ****P < 0.0001. (C) Representative GeneMapper traces across different tissues at 3 and 6 months of age in HttQ111 knock-in mice (CAG 121) with different Mlh3 genotypes. 3M: Mlh3WT/WT, CAG 119, Mlh3WT/DN and Mlh3DN/DN, CAG 118. 6M: Mlh3WT/WT, Mlh3WT/DN and Mlh3DN/DN, CAG 121. CAG change from modal allele is represented on the x-axis.
To determine the effect of Mlh3DN allele on somatic HTT CAG expansion, we crossed Mlh3DN mice (42) with HttQ111 HD knock-in mice (41) to generate heterozygous HttQ111 mice that were either wild type (Mlh3WT/WT), heterozygous (Mlh3WT/DN) or homozygous (Mlh3DN/DN) for the Mlh3 D1185N point mutation. Analysis of CAG expansions in striatum, liver, and kidney – tissues previously shown to exhibit high levels of instability in HttQ111 mice (9,17) – revealed the absence of somatic expansions in Mlh3DN/DN mice, both at three and six months of age (Figure 1B, C). Notably, the effect of the homozygous Mlh3DN/DN mutation on CAG expansion was indistinguishable from that of a homozygous Mlh3 null mutation (17). Quantification of repeat expansions (44) showed statistically significant reductions in the CAG expansion index in all tissues analyzed (striatum, liver, and kidney) of Mlh3DN/DN mice compared to Mlh3WT/WT mice (Figure 1B). There was a slight reduction in expansion index in the Mlh3WT/DN mice, consistent at both ages and in all tissues analyzed, though only reaching statistical significance in 6-month striata (Figure 1B). The dramatic effect of this mutation, phenocopying the Mlh3 null (17), indicates that an intact endonuclease domain is required for somatic HTT CAG repeat expansion.
In vivo Mlh3 splice redirection suppresses CAG expansion in HttQ111 mice
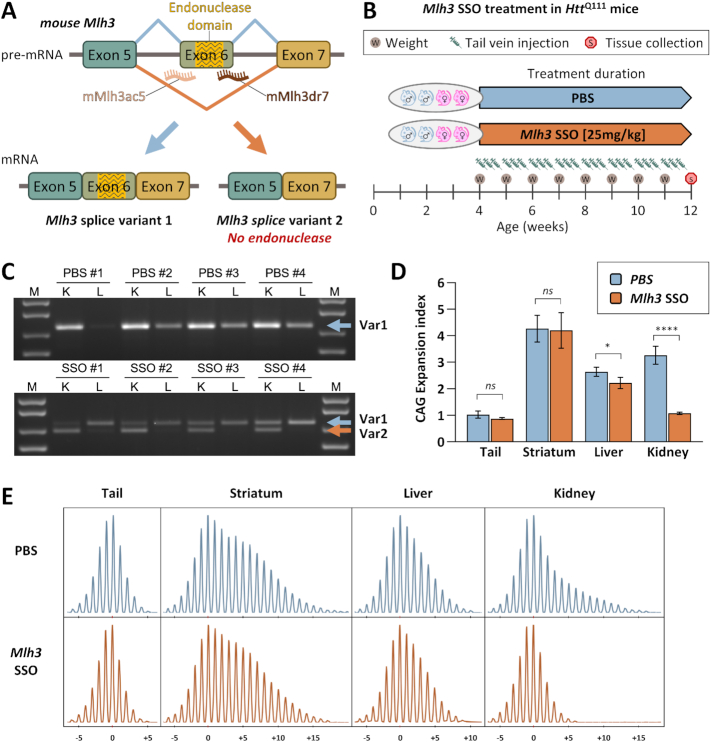
Having demonstrated that the MLH3 endonuclease domain was critical for somatic HTT CAG expansion in HttQ111 mice, we were interested in testing the potential of an Mlh3 splice redirection approach, previously used in FRDA cell-based models and patient cells (28), to slow CAG expansion in the same HD mice. Mice express predominantly one endogenous Mlh3 mRNA splice variant in which exon 6, the homologous exon to human MLH3 exon 7, encodes the endonuclease domain (Figure 2A and Supplementary Figure S1C). For this study, mouse-specific vivo-morpholino-based SSOs were designed to bind both the acceptor and donor splice sites of Mlh3 exon 6 (Figure 2A and Supplementary Figure S1B), as previously reported for human MLH3 (28). Starting at 4 weeks of age, heterozygous HttQ111 mice (n = 4) were treated with 25 mg/kg of Mlh3 SSOs (or PBS) by tail vein injection every 48–72 h until they reached 12 weeks of age (Figure 2B). Note that these in vivo experiments did not incorporate a scrambled ASO control, as preliminary in vitro testing indicated no impact of a scrambled ASO (Figure 3B). HttQ111 tissues were harvested 24 h after the final injection and Mlh3 splice variants were characterized by RT-PCR analysis in liver and kidney (Figure 2C), two peripheral tissues in which CAG expansion was shown to be dependent on an intact MLH3 endonuclease domain (Figure 1B, C) and which can be effectively targeted with systemic delivery of vivo-morpholinos (45,52–54). We analyzed Mlh3 splice variants using RT-PCR, generating a 720 bp product for the exon 6-containing splice variant 1 (Var1) and a 648 bp product for Mlh3 splice variant 2 (Var2), which lacks exon 6 (Supplementary Figure S1C) and is the mouse analog of human MLH3 splice variant 2. As expected, all four PBS-treated mice showed Var1 only in both kidney and liver, while all four Mlh3 SSO-treated mice showed successful splice redirection favoring Var2 in the kidney, with much lower, yet still detectable, Var2 levels in the livers of two treated mice (Figure 2C). We also established a TaqMan-based RT-qPCR assay for quantitative analysis of Mlh3 splice variants and increased sensitivity (Supplementary Figure S2). Using this more sensitive assay, only Var1 was detected in PBS-treated mice, whereas variable expression of Var2 was detected in all SSO-treated samples, with the global pattern of Var1 and Var2 expression in the two tissues consistent with levels detected in the gel-based RT-PCR assay (Supplementary Figure S2). In kidney, the efficiency of splice redirection (% Var2 relative to total Var1 + Var2) was 60–90%, whereas efficiency in liver was only 3–12%. No sex-specific differences were observed. It is important to note, however, that this RT-qPCR quantification reflects Var1 and Var2 mRNA levels at a single timepoint 24 h after the final injection. This does not necessarily reflect levels of these variants throughout the 8 weeks of treatment of 24 individual injections per mouse. The higher levels of splicing redirection in the kidney relative to the liver are consistent with observations in several prior studies (52–54) and may reflect better cellular SSO uptake in the kidney than in the liver, or may be the result of SSOs being actively removed from circulation by the kidney for excretion, limiting SSO availability to other peripheral tissues. However, the relatively low levels of splicing redirection in liver in our study may also indicate specific SSO-dependent differential splicing effects. In summary, we found that Mlh3 SSO-mediated splice redirection to exclude the exon encoding the endonuclease domain of MLH3 can be partially achieved in vivo in a mouse model of HD.
Figure 2.
Successful in vivo Mlh3 splice redirection and suppression of somatic HTT CAG expansion in HttQ111 mice by systemic Mlh3 SSO treatment. (A) Schematic representation of mouse Mlh3 splice redirection with splice switching oligonucleotides (SSOs). SSOs were designed to bind the intron–exon junctions in mouse Mlh3 pre-mRNA at either the splice acceptor (mMlh3ac5) or donor (mMlh3dr7) regions of the endonuclease-coding exon 6, which is analogous to the human MLH3 exon 7, inducing exon skipping and preferential production of Mlh3 splice variant 2, which lacks the endonuclease domain. (B) Summary of Mlh3 SSOs systemic treatment in HD mice. HttQ111 mice (two males and two females per group) were treated with either 25 mg/kg Mlh3 SSOs (mMLH3ac5 and mMLH3dr7, 1:1 ratio; CAG 106–109), or PBS (CAG 104–108), by tail vein injection. Treatment was initiated at 4 weeks of age and injections were performed three times per week (every 48–72 h). Weight measurements were taken at the beginning of each week to determine weekly weight-adjusted doses. Treatment was stopped at 12 weeks of age, with tissue collection performed 24 hours after the final injection. (C) RT-PCR analyses of Mlh3 mRNA splice variants in 12-week-old HttQ111 mice, following treatment with Mlh3 SSOs, reveals successful splice redirection from splice variant 1 (Var1) to variant 2 (Var2) in the kidney (K), and to a lesser extent in the liver (L). (D) Quantification of CAG expansion indices reveals statistically significant reductions in somatic HTTCAG expansion in kidney and, to a lesser extent, in liver of mice that received Mlh3 SSO treatment when compared to PBS. n = 4; error bars represent standard deviation of the mean; two-tailed unpaired t test: ns, not significant; *P < 0.05; ****P < 0.0001. (E) Representative GeneMapper traces of somatic HTTCAG repeat size distributions across different tissues at 12 weeks of age in HttQ111 knock-in mice following PBS (mouse PBS #4, CAG 108) or Mlh3 SSO treatment (mouse SSO #3, CAG 108). CAG change from modal allele is represented on the x-axis.
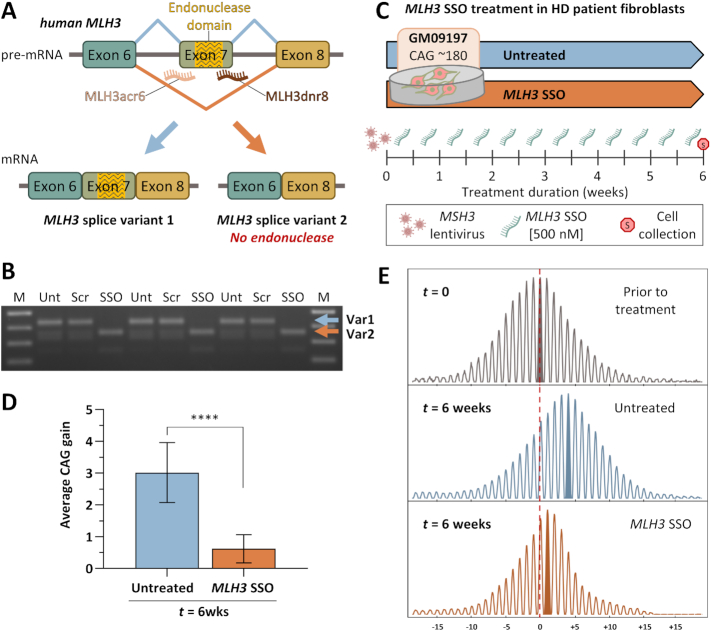
Figure 3.
Successful MLH3 splice redirection and suppression of HTT CAG expansion in HD patient-derived fibroblasts following treatment with MLH3 SSOs. (A) Schematic representation of human MLH3 splice redirection with splice switching oligonucleotides (SSOs) (adapted from Halabi et al. 2018 (28)). SSOs were designed to bind the intron–exon junctions in human MLH3 pre-mRNA at either the splice acceptor (MLH3ac6) or donor (MLH3dnr8) regions of the endonuclease-coding exon 7, inducing exon skipping and preferential production of MLH3 splice variant 2, which lacks the endonuclease domain. (B) RT-PCR analyses of MLH3 mRNA splice variants in HD patient-derived primary fibroblasts (GM09197, CAG ∼180/18) following treatment with 500 nM of either scrambled control vivo-morpholino oligonucleotide (Scr) or MLH3 SSOs (SSO) for 48 hours, alongside untreated controls (Unt) (n = 3 per condition). Splice redirection from predominantly splice variant 1 (Var1) to predominantly splice variant 2 (Var2), which lacks exon 7 and the endonuclease domain, was efficiently achieved with MLH3 SSOs treatment. No splice redirection was observed in scrambled oligo-treated cells, which were identical to untreated cells. (C) In order to induce CAG expansion in HD patient fibroblasts (GM09197, CAG ∼180/18), cells were initially treated with lentiviral particles for stable ectopic expression of MSH3. Cells were then cultured to confluency, to inhibit replication, and treated twice weekly with 500 nM of MLH3 SSOs (MLH3acr6 and MLH3dnr8, 1:1 ratio, 250 nM each), or left untreated, for a total of six weeks when cells were harvested for HTT CAG instability analysis. (D) Quantification of average HTT CAG gain during the 6-week-long treatment (relative to average CAG prior to treatment, ‘t = 0’, n = 4) reveals a potent inhibition of CAG expansion by MLH3 SSOs (average gain of 0.6 CAGs, n = 10), when compared to untreated cells (average gain of 3.0 CAGs, n = 10). Error bars represent standard deviation of the mean; one-way ANOVA with Tukey multiple comparisons post hoc correction; ****P < 0.0001. (E) Representative GeneMapper traces of HTT CAG repeat size distributions from GM09197 HD patient fibroblasts before (t = 0) and after (t = 6 weeks) treatment with MLH3 SSOs (CAG gain = 0.7), or no treatment (CAG gain = 3.6). CAG change from modal allele at t = 0 is represented on the x-axis.
To test whether such Mlh3 splice redirection impacted somatic CAG expansion, we performed qualitative and quantitative analyses of CAG repeat length profiles from the same HttQ111 mice (n = 4) treated with either Mlh3 SSOs or PBS (Figure 2D, E). When compared to the PBS-treated mice, the Mlh3 SSO-treated mice displayed significantly less somatic CAG expansion in the kidney (P < 0.0001), and to a lower extent in the liver (P < 0.05). These effects are consistent with the different degrees of splice redirection achieved in the two tissues. As expected, Mlh3 SSO treatment had no effect on CAG expansion in the striatum, consistent with the inability of these SSOs to efficiently cross the blood brain barrier (45,52,54). As Mlh3 splice redirection from Var1 to Var2 increases the proportion of mRNAs encoding an endonuclease-lacking MLH3 protein, these data support our prior results in Mlh3DN mice showing that an intact endonuclease domain is required for somatic HTT CAG expansion. The Mlh3 SSOs reduced the expansion index in 3-month kidney to a level comparable to that in tail (Figure 2D), which is a relatively stable tissue. In comparison, the expansion index in the kidney of SSO-treated mice was only very slightly higher than that observed in 3-month-old mice homozygous for the Mlh3DN mutation (Figure 1B), which is present from conception. These data indicate that a pharmacological splice redirection strategy, administered in 1-month-old mice once CAG expansions have already accrued, can be used in vivo to impact a function of MLH3 that is critical for somatic CAG expansion.
MLH3 splice redirection suppresses CAG expansion in HD patient-derived fibroblasts
Alternative splicing of human MLH3 pre-mRNA results in endogenous expression of two predominant splice variants: variant 1 (Var1), containing the exon 7-encoded endonuclease domain, and variant 2 (Var2), lacking this domain (Figure 3A, Supplementary Figure S1C and Supplementary Figure S3). We previously showed that MLH3 splice redirection from Var1 to Var2 can be achieved in FRDA patient primary fibroblasts using a combination of SSOs targeting the exon 7 splice acceptor (MLH3acr6) and donor (MLH3dnr8) sites (28). Prolonged treatment with these MLH3 SSOs resulted in suppression of GAA repeat expansion in FRDA cell-based models and patient cells (28). With the goal of testing this strategy in HD, we used primary fibroblasts derived from a juvenile HD patient (GM09197; CAG ∼180/18) (46) with a long repeat tract, predicted to allow repeat expansion in cell culture within a reasonable time frame (27,55). We first tested our ability to induce MLH3 splice redirection following 48 hour treatment with 500 nM of MLH3 SSOs (MLH3acr6 and MLH3dnr8, 1:1 ratio, 250 nM each) compared to treatment with 500 nM of a control vivo-morpholino oligonucleotide with a scrambled sequence or no treatment (Figure 3B). RT-PCR analysis of the MLH3 splice variants generates a 434 bp product for the full-length Var1 and a 362 bp product for Var2. RT-PCR analysis detects MLH3 Var1 as the primary isoform expressed in GM09197 fibroblasts, while treatment with MLH3 SSOs resulted in full redirection of splicing towards Var2 (Figure 3B). No impact on MLH3 splicing was detected following treatment with scrambled control oligonucleotides, where splice variants were identical to untreated cells. We then determined whether splice redirection of MLH3 was capable of slowing CAG expansion in these cells over time. In our studies on FRDA primary fibroblast cells, we found that the GAA repeat tract was stable due to low levels of expression of MSH3, one of the key factors that contribute to both GAA and CAG repeat expansion (27,56). Therefore, we induced CAG expansion in the HD fibroblasts via ectopic expression of MSH3 using lentiviral vector delivery as previously described for FRDA fibroblasts (27,28). These MSH3-expressing fibroblasts were then grown to confluent cultures to prevent replication, before initiating SSO treatment. Prior to SSO treatment, genomic DNA was isolated from untreated cells (t = 0; n = 4) to determine the starting average CAG repeat size. Cells were then treated with 500 nM MLH3 SSOs twice-weekly, or left untreated, for a total of six weeks (t = 6 weeks) (Figure 3C). Quantitative GeneMapper analysis revealed an overt shift of the entire population of CAG alleles towards larger lengths in untreated HD fibroblasts (t = 6wks), relative to the starting population (t = 0), reflected by an increase in average CAG length of 3.0 repeats (P < 0.0001) (Figure 3D, E and Supplementary Figure S4). Treatment with MLH3 SSOs for six weeks significantly suppressed CAG expansion: after six weeks, the MLH3 SSO-treated cells were statistically indistinguishable from the starting population (P = 0.3120, average gain of 0.6 CAG repeats) and significantly different from the untreated cells (P ≤ 0.0001) (Figure 3D, E and Supplementary Figure S4). These data further support our prior results in Mlh3DN and Mlh3 SSO-treated HttQ111 mice, suggesting that the MLH3 endonuclease domain is essential for somatic HTT CAG expansion in HD patient cells, and that the use of MLH3 SSOs constitutes a viable and effective strategy to pharmacologically target this pathway in HD patients.
DISCUSSION
Somatic expansion of the HTT CAG repeat is thought to drive the rate of HD pathogenesis. Repeat expansion is dependent on proteins in the MMR pathway and, therefore, these present potential therapeutic targets. Insight into specific functions or activities of MMR proteins that modify repeat expansion will provide possible opportunities for therapeutic intervention. Here, we provide evidence, using two distinct, yet complementary approaches, that the endonuclease domain of MLH3 is required for somatic HTT CAG expansion. First, a point mutation in a conserved endonuclease domain motif abolishes CAG expansion in HttQ111 mouse tissues, having an effect indistinguishable from a Mlh3 null mutation. Second, CAG expansion both in HttQ111 mice and in HD patient-derived cells is reduced by modulating Mlh3/MLH3 splicing in a manner that excludes the exon encoding the endonuclease motif.
The endonuclease domain of MLH3 is encompassed in the C-terminal domain of the protein, which interacts with MLH1 to form the MutLγ complex (MLH1–MLH3 heterodimer) (57). As described above, whereas the D>N substitution in the first amino acid of the DQHA(X)2E(X4)E motif eliminates MLH3 endonuclease activity (47–50), there is no evidence that this mutation alters the stability of MLH3 or its interaction with MLH1 (30,42,50,51). We have been unable to directly determine MLH3 protein levels in our study due to the lack of availability of suitable antibodies to detect MLH3, which is present at very low levels in most tissues (28,38). More specifically, we could not detect MLH3 in mouse tissues using the same antibody used to detect MLH3 in mouse spermatocytes of Mlh3DN mice, where this protein is relatively highly expressed (42). Importantly, we find that heterozygous or homozygous Mlh3DN alleles have impacts on CAG expansion comparable to heterozygous or homozygous Mlh3 null alleles, respectively (17). This indicates that the Mlh3DN mutation would need to dramatically reduce MLH3 protein levels in order to have an impact on CAG expansion that phenocopies the null allele. Given existing data in the literature (30,42,50,51), this scenario appears highly improbable. Further, the comparable effects of homozygous Mlh3DN and Mlh3 null alleles argue against a dominant negative effect of the Mlh3DN mutation, and rather support a loss of function model. It is therefore likely that the absence of HTT CAG expansion in HttQ111 mice harboring the Mlh3 D1185N mutation is due to loss of MLH3-dependent endonuclease activity.
The impact of Mlh3/MLH3 splice redirection that excludes the DQHA(X)2E(X4)E motif on HTT CAG expansion is also consistent with a requirement for MLH3-dependent endonuclease activity, although we cannot rule out the possibility that deletion of the endonuclease domain-encoding exon in MLH3 due to splice redirection has additional effects on MLH3 stability or function. Published data in this regard provide evidence that the human MLH3Δ7 isoform can interact with MLH1 in yeast 2-hybrid (57) and GST pull down experiments (58), and is recruited to repair foci in response to DNA damage in the same way as the full length MLH3 (59), implying normal protein interactions. However, one study found that MLH3Δ7 was unable to interact with MLH1 in a mammalian 2-hybrid assay (60). Overall, our data from orthogonal genetic and pharmacological-based approaches together provide strong support for a critical role of the MLH3 endonuclease in driving somatic expansion of the HTT CAG repeat.
Of note is the very modest impact of the heterozygous Mlh3DN mutation (Figure 1B) in comparison to the effect of the Mlh3 SSO, which significantly reduced CAG expansion in kidney and in which splice redirection efficiencies of 60–90% were measured. However, direct comparisons between levels of CAG expansion and Mlh3 expression in these experiments are difficult to make for a number of reasons: i) We are unable to correlate Mlh3DN heterozygosity with the extent of MLH3 functional loss. ii) The degree of splice switching, measured in bulk tissue, may not be representative of every cell in the tissue being analyzed. We cannot exclude, therefore, that some cells may have very high levels of splicing redirection whereas other cells have much lower levels. As repeat instability is cell type-dependent (9,10), a strong impact on instability could be observed if the cell types exhibiting high instability incurred the highest levels of splice switching. iii) The degree of splicing redirection only represents a single snapshot 24 h after the final injection. We therefore have no insight into levels of splicing redirection achieved or sustained in a tissue/cell type of interest over the 8-week course of 24 SSO injections. iv) There may be a threshold for functional MLH3, below which, CAG expansion is suppressed, suggested by the similar expansion suppression achieved with a range of splice redirection that varies from 60 to 90% (Supplementary Figure S2B). A threshold for MLH1 function was also indicated in a previous study (17). Thus, further studies examining Mlh3 splice redirection efficiency and CAG expansion over time in single cells would be important to delineate this relationship.
Our findings support and extend previous observations that exclusion of MLH3 exon 7 slowed GAA repeat expansion in FRDA cell models and patient-derived fibroblasts (28), and the Mlh3 D1185N point mutation eliminated expansion of the CGG repeat in a FXD mouse embryonic stem cell model (30). Together, data indicate that the MLH3 endonuclease domain is critical for somatic expansion of different trinucleotide repeat sequences, with implications for cross-disease applicability of therapeutic approaches that target this activity. Recent biochemical studies in cell-free systems have suggested a plausible mechanism by which MLH3 endonuclease activity might lead to repeat expansion (50). These studies provided support that MutLγ cleaves a closed circular plasmid harboring a CAG- or CTG-containing loop on the loop-lacking strand, with repair leading to loop incorporation, effectively corresponding to an expansion event. This observation distinguished the MutLγ endonuclease from the MutLα endonuclease, where MutLα was able to cleave on both the loop-containing and loop-lacking strands, potentially leading to both deletion and expansion events (61). This specific strand-directed activity of MutLγ may, in part, explain the necessity for MLH3 in the somatic expansion of several disease-associated repeats (17,28,31). This is in contrast to PMS2, whose knockout can fully (32) or partially (62) suppress expansion, or can promote expansion (26,28), depending on the repeat sequence and/or biological system. Understanding the role played by PMS2 endonuclease in somatic repeat instability in vivo would also be of significant interest.
The fact that MLH3 splice variants that both include or exclude the endonuclease domain-containing exon 7 occur naturally in humans indicates that the relative levels of these variants might have biological relevance. As exon 7 exclusion resulted in reduced CAG expansion, this prompted us to examine whether the levels of MLH3 Var2 might correlate with tissue-specific expansion propensities of the HTT CAG repeat (43). Examination of MLH3 Var1 and Var2 mRNA expression in the GTEx database (Supplementary Figure S3) did not reveal any obvious correlation with levels of CAG expansion in tissues; where forebrain regions such as the cortex and basal ganglia tend to have high instability, cerebellum exhibits low instability, and in the periphery, liver exhibits high instability (43). While more refined cell type-based analyses would be needed to better understand relationships between CAG instability and trans-modifying factors, these initial observations do not indicate any obvious relationship between naturally occurring MLH3 Var2 and tissue-specific CAG expansion.
How is the MutLγ endonuclease activated to promote repeat expansion? MutLγ endonuclease is also required in canonical MMR in S. cerevisiae and in meiosis (42,51), however the mechanism(s) of endonuclease activation are not yet well understood. In reconstituted systems, MutLγ can bind directly to DNA oligonucleotides, including those containing loop or branched structures, but does not cleave these structures (47–49,63). This, and the further observation that larger DNA molecules are better substrates for the MutLγ endonuclease suggested that MutLγ binds cooperatively to DNA and that higher order polymers are required for endonuclease activation (63). Interestingly, the nicking activity of a 50:50 mixture of wild-type Mlh1-Mlh3 and Mlh1-Mlh3D523N dimers (S. cerevisiae D523N is orthologous to the D1185N mouse mutation) was the same as that elicited by 100% wild-type Mlh1-Mlh3, consistent with the idea that a critical level of DNA binding is needed for endonuclease activity (63). It is unknown whether MutLγ polymers are required in vivo, but this observation suggests one possible explanation for the relatively minor impact on CAG instability in heterozygous Mlh3WT/DN mice.
In reconstituted systems, both human and S. cerevisiae MutLγ are stimulated by MutSβ (human MSH2-MSH3 or S. cerevisiae Msh2-Msh3 respectively), potentially via direct interaction of these two complexes (47,50,64), but human MutLγ was not stimulated by MSH2-MSH6 (MutSα) (64). As MSH3, but not MSH6, is required for trinucleotide repeat expansion (16), MutSβ-stimulation of MutLγ endonuclease may be integral to repeat expansion in vivo. Other protein-protein interactions have been implicated in MutLγ endonuclease activation (64–66) and, interestingly, S. cerevisiae Mlh3 separation of function mutations provided evidence that distinct protein interactions may be required for MutLγ endonuclease activity in MMR and in meiosis (64,65). Activity appears to be variably stimulated by proliferating cell nuclear antigen (PCNA) and replication factor C (RFC), depending on the assay system (47,50,64,66). Studies in S. cerevisiae have also indicated that Mlh3 endonuclease creates DNA breaks in the presence of RNA/DNA hybrids (R-loops) that can lead to increased instability, suggesting a link with transcription (67), though the relevance to repeat expansion in vivo is unclear. Future biochemical and genetic studies will be needed to dissect the mechanism of MutLγ endonuclease activation in its repeat expansion-promoting function.
Is MLH3 a good therapeutic target for HD? In contrast to other MMR pathway genes (MSH3, MLH1 PMS2, PMS1), MLH3 is not currently validated as a disease modifier in human GWAS (14). This may relate to the effect size of a putative modifier SNP(s) and the power to detect a significant effect on a clinical endpoint with the current patient sample size. Nevertheless, the following provide support for MLH3 as a viable therapeutic target in HD: i) MLH3 is part of a pathway involved in disease modification that likely acts via somatic repeat expansion, ii) MLH3 is essential for somatic CAG repeat expansion in HD mice (17), iii) functional MLH3 promotes HTT CAG expansion in HD patient cells (this study), and iv) MLH3 genetic variation is associated with somatic HTT CAG expansion measured in HD patient blood DNA (13). From a safety standpoint, consistent with a minor role of MLH3 in MMR, only a handful of studies have reported associations of MLH3 mutations with cancer, however the pathogenicity of these mutations and their clinical significance are still unclear (68–71). MLH3 is primarily known for playing a critical role in the promotion of meiotic crossover products during homologous recombination repair (HRR). In fact, Mlh3DN/DN males have no spermatozoa and are infertile (42). Interestingly, a recent study has demonstrated that MLH3 endonuclease also promotes HRR of double-strand breaks in non-meiotic cells (72). However, it remains unclear how much this may contribute to tumor suppression in humans. Further, the genome Aggregation Database (gnomAD) reports an extremely low probability for intolerance to loss of function mutations for MLH3 (pLI = 0.00) (73). Therefore, MLH3 appears to be a promising candidate as a safe and effective therapeutic target.
Here, we demonstrate applicability to HD of one possible therapeutic approach targeting the critical endonuclease domain of MLH3 using SSOs to redirect MLH3 splicing. Future studies will be needed to optimize SSO delivery to the brain in order to test the efficacy of MLH3 splice redirection in disease-relevant tissues. Splice redirection allows specific targeting of the MLH3 endonuclease, whilst leaving intact the PMS2 endonuclease that is critical to the major MMR activity in cells. A pharmacological strategy targeting MLH3 endonuclease activity in the protein may also be feasible, but specificity would likely be harder to achieve. Thus, further insights into distinguishing features of these endonucleases may provide additional opportunities for specific inactivation of the MLH3 endonuclease. An alternative oligonucleotide-based approach of simply reducing MLH3 expression is also indicated based on genetic data in Mlh3 knockout mice (17). This strategy would reduce or eliminate all MLH3 function(s), in contrast to splice redirection that is predicted based on genetic data in Mlh3DN/DN mice to retain nuclease-independent activities (42). Therefore, additional understanding of the cellular roles of the MLH3 endonuclease, and of the endonuclease-lacking variant, will help to inform on optimal therapeutic approaches applicable to both HD and other repeat expansion diseases.
DATA AVAILABILITY
This study includes no data deposited in external repositories.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Tammy Gillis and Jacqi Siciliano of the MGH Mission Driven Service Core for DNA Fragment Analysis.
Notes
Present address: Jennie C. L. Roy, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Contributor Information
Jennie C L Roy, Department of Genetics, Louisiana State University Health Sciences Center, New Orleans, LA 70112, USA.
Antonia Vitalo, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA; Department of Neurology, Harvard Medical School, Boston, MA 02115, USA.
Marissa A Andrew, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Eduarda Mota-Silva, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Marina Kovalenko, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Zoe Burch, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Anh M Nhu, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA.
Paula E Cohen, Department of Biomedical Sciences, Cornell University, Ithaca, NY 14853, USA; Center for Reproductive Genomics, Cornell University, Ithaca, NY 14853, USA.
Ed Grabczyk, Department of Genetics, Louisiana State University Health Sciences Center, New Orleans, LA 70112, USA.
Vanessa C Wheeler, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA; Department of Neurology, Harvard Medical School, Boston, MA 02115, USA.
Ricardo Mouro Pinto, Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA 02114, USA; Department of Neurology, Harvard Medical School, Boston, MA 02115, USA; Program in Medical and Population Genetics, Broad Institute of MIT and Harvard, Cambridge, MA 02142, USA.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Huntington's Disease Society of America Berman/Topper Family HD Career Development Fellowship and HD Human Biology Project (to R.M.P.); National Institutes of Health (NS049206 to V.C.W.); Dake Family Foundation (to R.M.P.); Friedreich Ataxia Research Alliance (to E.G.); Harrington Discovery Institute (to E.G.). Funding for open access charge: Huntington's Disease Society of America and National Institutes of Health.
Conflict of interest statement. V.C.W. is a scientific advisory board member of Triplet Therapeutics, a company developing new therapeutic approaches to address triplet repeat disorders such as Huntington's disease and Myotonic Dystrophy, and of LoQus23 Therapeutics, and has provided paid consulting services to Alnylam and Acadia Pharmaceuticals. Her financial interests in Triplet Therapeutics were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. R.M.P. and V.C.W have received sponsored research funding from Pfizer Inc. unrelated to the content of this manuscript. E.G. is a named inventor on a patent related to this work (US10669542B2).
REFERENCES
- 1. Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993; 72:971–983. [DOI] [PubMed] [Google Scholar]
- 2. Vonsattel J.-P., Myers R.H., Stevens T.J., Ferrante R.J., Bird E.D., Richardson E.P. Jr. Neuropathological classification of Huntington's disease. J. Neuropathol. Exp. Neurol. 1985; 44:559–577. [DOI] [PubMed] [Google Scholar]
- 3. McColgan P., Tabrizi S.J.. Huntington's disease: a clinical review. Eur. J. Neurol. 2018; 25:24–34. [DOI] [PubMed] [Google Scholar]
- 4. Kennedy L., Evans E., Chen C.-M.M., Craven L., Detloff P.J., Ennis M., Shelbourne P.F.. Dramatic tissue-specific mutation length increases are an early molecular event in Huntington disease pathogenesis. Hum. Mol. Genet. 2003; 12:3359–3367. [DOI] [PubMed] [Google Scholar]
- 5. Shelbourne P.F., Keller-McGandy C., Bi W.L., Yoon S.R., Dubeau L., Veitch N.J., Vonsattel J.P., Wexler N.S., Norman A., Augood S.J.. Triplet repeat mutation length gains correlate with cell-type specific vulnerability in Huntington disease brain. Hum. Mol. Genet. 2007; 16:1133–1142. [DOI] [PubMed] [Google Scholar]
- 6. Gonitel R., Moffitt H., Sathasivam K., Woodman B., Detloff P.J., Faull R.L.M., Bates G.P.. DNA instability in postmitotic neurons. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:3467–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swami M., Hendricks A.E., Gillis T., Massood T., Mysore J., Myers R.H., Wheeler V.C.. Somatic expansion of the Huntington's disease CAG repeat in the brain is associated with an earlier age of disease onset. Hum. Mol. Genet. 2009; 18:3039–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loupe J.M., Mouro Pinto R., Kim K.-H., Gillis T., Mysore J.S., Andrew M.A., Kovalenko M., Murtha R., Seong I., Gusella J.F.et al.. Promotion of somatic CAG repeat expansion by Fan1 knock-out in Huntington's disease knock-in mice is blocked by Mlh1 knock-out. Hum. Mol. Genet. 2020; 29:3044–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J.M., Mouro Pinto R., Gillis T., St. Claire J.C., Wheeler V.C.. Quantification of age-dependent somatic CAG repeat instability in Hdh CAG knock-in mice reveals different expansion dynamics in striatum and liver. PLoS One. 2011; 6:e23647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovalenko M., Dragileva E., St. Claire J., Gillis T., Guide J.R., New J., Dong H., Kucherlapati R., Kucherlapati M.H., Ehrlich M.E.et al.. Msh2 acts in medium-spiny striatal neurons as an enhancer of CAG instability and mutant Huntingtin phenotypes in Huntington's disease knock-in mice. PLoS One. 2012; 7:e44273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larson E., Fyfe I., Morton A.J., Monckton D.G.. Age-, tissue- and length-dependent bidirectional somatic CAG•CTG repeat instability in an allelic series of R6/2 Huntington disease mice. Neurobiol. Dis. 2015; 76:98–111. [DOI] [PubMed] [Google Scholar]
- 12. Ament S.A., Pearl J.R., Grindeland A., Claire J.S., Earls J.C., Kovalenko M., Gillis T., Mysore J., Gusella J.F., Lee J.M.et al.. High resolution time-course mapping of early transcriptomic, molecular and cellular phenotypes in Huntington's disease CAG knock-in mice across multiple genetic backgrounds. Hum. Mol. Genet. 2017; 26:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciosi M., Maxwell A., Cumming S.A., Hensman Moss D.J., Alshammari A.M., Flower M.D., Durr A., Leavitt B.R., Roos R.A.C., Holmans P.et al.. A genetic association study of glutamine-encoding DNA sequence structures, somatic CAG expansion, and DNA repair gene variants, with Huntington disease clinical outcomes. EBioMedicine. 2019; 48:568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GeM-HD CAG repeat not polyglutamine length determines timing of Huntington's disease onset. Cell. 2019; 178:887–900.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wright G.E.B., Collins J.A., Kay C., McDonald C., Dolzhenko E., Xia Q., Bečanović K., Drögemöller B.I., Semaka A., Nguyen C.M.et al.. Length of uninterrupted CAG, independent of polyglutamine size, results in increased somatic instability, hastening onset of Huntington disease. Am. J. Hum. Genet. 2019; 104:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dragileva E., Hendricks A., Teed A., Gillis T., Lopez E.T., Friedberg E.C., Kucherlapati R., Edelmann W., Lunetta K.L., MacDonald M.E.et al.. Intergenerational and striatal CAG repeat instability in Huntington's disease knock-in mice involve different DNA repair genes. Neurobiol. Dis. 2009; 33:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mouro Pinto R., Dragileva E., Kirby A., Lloret A., Lopez E., St. Claire J., Panigrahi G.B., Hou C., Holloway K., Gillis T.et al.. Mismatch repair genes Mlh1 and Mlh3 modify CAG instability in Huntington's disease mice: genome-wide and candidate approaches. PLos Genet. 2013; 9:e1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheeler V.C., Lebel L.-A., Vrbanac V., Teed A., te Riele H., MacDonald M.E.. Mismatch repair gene Msh2 modifies the timing of early disease in HdhQ111 striatum. Hum. Mol. Genet. 2003; 12:273–281. [DOI] [PubMed] [Google Scholar]
- 19. Owen B.A.L., Yang Z., Lai M., Gajek M., Badger J.D., Hayes J.J., Edelmann W., Kucherlapati R., Wilson T.M., McMurray C.T.. CAG)n-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat. Struct. Mol. Biol. 2005; 12:663–670. [DOI] [PubMed] [Google Scholar]
- 20. GeM-HD Identification of genetic factors that modify clinical onset of Huntington's disease. Cell. 2015; 162:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moss D.J.H., Tabrizi S.J., Mead S., Lo K., Pardiñas A.F., Holmans P., Jones L., Langbehn D., Coleman A., Santos R.D.et al.. Identification of genetic variants associated with Huntington's disease progression: a genome-wide association study. Lancet Neurol. 2017; 16:701–711. [DOI] [PubMed] [Google Scholar]
- 22. Flower M., Lomeikaite V., Ciosi M., Cumming S., Morales F., Lo K., Hensman Moss D., Jones L., Holmans P., Investigators the, T.-H.et al.. MSH3 modifies somatic instability and disease severity in Huntington's and myotonic dystrophy type 1. Brain. 2019; 142:1876–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van den Broek W.J.A.A., Nelen M.R., Wansink D.G., Coerwinkel M.M., te Riele H., Groenen P.J.T.A., Wieringa B.. Somatic expansion behaviour of the (CTG)n repeat in myotonic dystrophy knock-in mice is differentially affected by Msh3 and Msh6 mismatch–repair proteins. Hum. Mol. Genet. 2002; 11:191–198. [DOI] [PubMed] [Google Scholar]
- 24. Savouret C., Brisson E., Essers J., Kanaar R., Pastink A., Te Riele H., Junien C., Gourdon G.. CTG repeat instability and size variation timing in DNA repair-deficient mice. EMBO J. 2003; 22:2264–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foiry L., Dong L., Savouret C., Hubert L., te Riele H., Junien C., Gourdon G.. Msh3 is a limiting factor in the formation of intergenerational CTG expansions in DM1 transgenic mice. Hum. Genet. 2006; 119:520–526. [DOI] [PubMed] [Google Scholar]
- 26. Bourn R.L., de Biase I., Mouro Pinto R., Sandi C., Al-Mahdawi S., Pook M.A., Bidichandani S.I.. Pms2 suppresses large expansions of the (GAA·TTC)n sequence in neuronal tissues. PLoS One. 2012; 7:e47085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halabi A., Ditch S., Wang J., Grabczyk E.. DNA mismatch repair complex MutSb promotes GAA-TTC repeat expansion in human cells. J. Biol. Chem. 2012; 287:29958–29967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halabi A., Fuselier K.T.B., Grabczyk E.. GAA•TTC repeat expansion in human cells is mediated by mismatch repair complex MutLγ and depends upon the endonuclease domain in MLH3 isoform one. Nucleic Acids Res. 2018; 46:4022–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ezzatizadeh V., Sandi C., Sandi M., Anjomani-Virmouni S., Al-Mahdawi S., Pook M.A.. MutLα heterodimers modify the molecular phenotype of Friedreich ataxia. PLoS One. 2014; 9:e100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayward B.E., Steinbach P.J., Usdin K.. A point mutation in the nuclease domain of MLH3 eliminates repeat expansions in a mouse stem cell model of the Fragile X-related disorders. Nucleic Acids Res. 2020; 48:7856–7863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao X., Zhang Y., Wilkins K., Edelmann W., Usdin K.. MutLγ promotes repeat expansion in a Fragile X mouse model while EXO1 is protective. PLos Genet. 2018; 14:e1007719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller C.J., Kim G.-Y., Zhao X., Usdin K.. All three mammalian MutL complexes are required for repeat expansion in a mouse cell model of the Fragile X-related disorders. PLos Genet. 2020; 16:e1008902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lokanga R.A., Zhao X.-N., Usdin K.. The mismatch repair protein MSH2 is rate limiting for repeat expansion in a fragile X premutation mouse model. Hum. Mutat. 2014; 35:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao X.-N., Lokanga R., Allette K., Gazy I., Wu D., Usdin K.. A MutSβ-dependent contribution of MutSα to repeat expansions in fragile X premutation mice. PLos Genet. 2016; 12:e1006190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bettencourt C., Hensman-Moss D., Flower M., Wiethoff S., Brice A., Goizet C., Stevanin G., Koutsis G., Karadima G., Panas M.et al.. DNA repair pathways underlie a common genetic mechanism modulating onset in polyglutamine diseases. Ann. Neurol. 2016; 79:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipkin S.M., Moens P.B., Wang V., Lenzi M., Shanmugarajah D., Gilgeous A., Thomas J., Cheng J., Touchman J.W., Green E.D.et al.. Meiotic arrest and aneuploidy in MLH3-deficient mice. Nat. Genet. 2002; 31:385–390. [DOI] [PubMed] [Google Scholar]
- 37. Cannavo E., Marra G., Sabates-Bellver J., Menigatti M., Lipkin S.M., Fischer F., Cejka P., Jiricny J.. Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res. 2005; 65:10759–10766. [DOI] [PubMed] [Google Scholar]
- 38. Charbonneau N., Amunugama R., Schmutte C., Yoder K., Fishel R.. Evidence that hMLH3 functions primarily in meiosis and in hMSH2-hMSH3 mismatch repair. Cancer Biol. Ther. 2009; 8:1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kadyrov F.A., Dzantiev L., Constantin N., Modrich P.. Endonucleolytic function of MutLα in human mismatch repair. Cell. 2006; 126:297–308. [DOI] [PubMed] [Google Scholar]
- 40. Yates A.D., Achuthan P., Akanni W., Allen J., Allen J., Alvarez-Jarreta J., Amode M.R., Armean I.M., Azov A.G., Bennett R.et al.. Ensembl 2020. Nucleic Acids Res. 2020; 48:D682–D688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wheeler V.C., Auerbach W., White J.K., Srinidhi J., Auerbach A., Ryan A., Duyao M.P., Vrbanac V., Weaver M., Gusella J.F.et al.. Length-dependent gametic CAG repeat instability in the Huntington's disease knock-in mouse. Hum. Mol. Genet. 1999; 8:115–122. [DOI] [PubMed] [Google Scholar]
- 42. Toledo M., Sun X., Brieño-Enríquez M.A., Raghavan V., Gray S., Pea J., Milano C.R., Venkatesh A., Patel L., Borst P.L.et al.. A mutation in the endonuclease domain of mouse MLH3 reveals novel roles for MutLγ during crossover formation in meiotic prophase I. PLos Genet. 2019; 15:e1008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mouro Pinto R., Arning L., Giordano J.V, Razghandi P., Andrew M.A., Gillis T., Correia K., Mysore J.S., Grote Urtubey D.M., Parwez C.R.et al.. Patterns of CAG repeat instability in the central nervous system and periphery in Huntington's disease and in spinocerebellar ataxia type 1. Hum. Mol. Genet. 2020; 29:2551–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee J.-M., Zhang J., Su A.I., Walker J.R., Wiltshire T., Kang K., Dragileva E., Gillis T., Lopez E.T., Boily M.-J.et al.. A novel approach to investigate tissue-specific trinucleotide repeat instability. BMC Syst. Biol. 2010; 4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Morcos P.A., Li Y., Jiang S.. Vivo-Morpholinos: a non-peptide transporter delivers Morpholinos into a wide array of mouse tissues. BioTechniques. 2008; 45:613–623. [DOI] [PubMed] [Google Scholar]
- 46. Sathasivam K., Amaechi I., Mangiarini L., Bates G.. Identification of an HD patient with a (CAG)180 repeat expansion and the propagation of highly expanded CAG repeats in lambda phage. Hum. Genet. 1997; 99:692–695. [DOI] [PubMed] [Google Scholar]
- 47. Rogacheva M.V., Manhart C.M., Chen G., Guarne A., Surtees J., Alani E.. Mlh1-Mlh3, a meiotic crossover and DNA mismatch repair factor, is a Msh2-Msh3-stimulated endonuclease. J. Biol. Chem. 2014; 289:5664–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ranjha L., Anand R., Cejka P.. The Saccharomyces cerevisiae Mlh1-Mlh3 heterodimer is an endonuclease that preferentially binds to holliday junctions. J. Biol. Chem. 2014; 289:5674–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Claeys Bouuaert C., Keeney S.. Distinct DNA-binding surfaces in the ATPase and linker domains of MutLγ determine its substrate specificities and exert separable functions in meiotic recombination and mismatch repair. PLoS Genet. 2017; 13:e1006722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kadyrova L.Y., Gujar V., Burdett V., Modrich P.L., Kadyrov F.A.. Human MutLγ, the MLH1–MLH3 heterodimer, is an endonuclease that promotes DNA expansion. Proc. Natl. Acad. Sci. U.S.A. 2020; 117:3535–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nishant K.T., Plys A.J., Alani E.. A mutation in the putative MLH3 endonuclease domain confers a defect in both mismatch repair and meiosis in Saccharomyces cerevisiae. Genetics. 2008; 179:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou H., Janghra N., Mitrpant C., Dickinson R.L., Anthony K., Price L., Eperon I.C., Wilton S.D., Morgan J., Muntoni F.. A novel morpholino oligomer targeting ISS-N1 improves rescue of severe spinal muscular atrophy transgenic mice. Hum. Gene Ther. 2013; 24:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gallego-Villar L., Viecelli H.M., Pérez B., Harding C.O., Ugarte M., Thöny B., Desviat L.R.. A sensitive assay system to test antisense oligonucleotides for splice suppression therapy in the mouse liver. Mol. Ther. - Nucleic Acids. 2014; 3:e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Salewsky B., Hildebrand G., Rothe S., Parplys A.C., Radszewski J., Kieslich M., Wessendorf P., Krenzlin H., Borgmann K., Nussenzweig A.et al.. Directed alternative splicing in Nijmegen breakage syndrome: proof of principle concerning its therapeutical application. Mol. Ther. 2016; 24:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goold R., Flower M., Moss D.H., Medway C., Wood-Kaczmar A., Andre R., Farshim P., Bates G.P., Holmans P., Jones L.et al.. FAN1 modifies Huntington's disease progression by stabilizing the expanded HTT CAG repeat. Hum. Mol. Genet. 2019; 28:650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakatani R., Nakamori M., Fujimura H., Mochizuki H., Takahashi M.P.. Large expansion of CTG·CAG repeats is exacerbated by MutSβ in human cells. Sci. Rep. 2015; 5:11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kondo E., Horii A., Fukushige S.. The interacting domains of three MutL heterodimers in man: hMLH1 interacts with 36 homologous amino acid residues within hMLH3, hPMS1 and hPMS2. Nucleic Acids Res. 2001; 29:1695–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Santucci-Darmanin S., Neyton S., Lespinasse F., Saunières A., Gaudray P., Paquis-Flucklinger V.. The DNA mismatch-repair MLH3 protein interacts with MSH4 in meiotic cells, supporting a role for this MutL homolog in mammalian meiotic recombination. Hum. Mol. Genet. 2002; 11:1697–1706. [DOI] [PubMed] [Google Scholar]
- 59. Roesner L.M., Mielke C., Fähnrich S., Merkhoffer Y., Dittmar K.E.J.J., Drexler H.G., Dirks W.G.. Stable expression of MutLgamma in human cells reveals no specific response to mismatched DNA, but distinct recruitment to damage sites. J. Cell. Biochem. 2013; 114:2405–2414. [DOI] [PubMed] [Google Scholar]
- 60. Lipkin S.M., Wang V., Jacoby R., Banerjee-Basu S., Baxevanis A.D., Lynch H.T., Elliott R.M., Collins F.S.. MLH3: A DNA mismatch repair gene associated with mammalian microsatellite instability. Nat. Genet. 2000; 24:27–35. [DOI] [PubMed] [Google Scholar]
- 61. Pluciennik A., Burdett V., Baitinger C., Iyer R.R., Shi K., Modrich P.. Extrahelical (CAG)/(CTG) triplet repeat elements support proliferating cell nuclear antigen loading and MutLa endonuclease activation. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:12277–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gomes-Pereira M., Fortune M.T., Ingram L., McAbney J.P., Monckton D.G.. Pms2 is a genetic enhancer of trinucleotide CAG-CTG repeat somatic mosaicism: Implications for the mechanism of triplet repeat expansion. Hum. Mol. Genet. 2004; 13:1815–1825. [DOI] [PubMed] [Google Scholar]
- 63. Manhart C.M., Ni X., White M.A., Ortega J., Surtees J.A., Alani E.. The mismatch repair and meiotic recombination endonuclease Mlh1-Mlh3 is activated by polymer formation and can cleave DNA substrates in trans. PLoS Biol. 2017; 15:e2001164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cannavo E., Sanchez A., Anand R., Ranjha L., Hugener J., Adam C., Acharya A., Weyland N., Aran-Guiu X., Charbonnier J.B.et al.. Regulation of the MLH1–MLH3 endonuclease in meiosis. Nature. 2020; 586:618–622. [DOI] [PubMed] [Google Scholar]
- 65. Al-Sweel N., Raghavan V., Dutta A., Ajith V.P., Di Vietro L., Khondakar N., Manhart C.M., Surtees J.A., Nishant K.T., Alani E.. mlh3 mutations in baker's yeast alter meiotic recombination outcomes by increasing noncrossover events genome-wide. PLos Genet. 2017; 13:e1006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kulkarni D.S., Owens S.N., Honda M., Ito M., Yang Y., Corrigan M.W., Chen L., Quan A.L., Hunter N.. PCNA activates the MutLγ endonuclease to promote meiotic crossing over. Nature. 2020; 586:623–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Su X.A., Freudenreich C.H.. Cytosine deamination and base excision repair cause R-loop–induced CAG repeat fragility and instability in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 2017; 114:E8392–E8401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hansen M.F., Johansen J., Sylvander A.E., Bjørnevoll I., Talseth-Palmer B.A., Lavik L.A.S., Xavier A., Engebretsen L.F., Scott R.J., Drabløs F.et al.. Use of multigene-panel identifies pathogenic variants in several CRC-predisposing genes in patients previously tested for Lynch Syndrome. Clin. Genet. 2017; 92:405–414. [DOI] [PubMed] [Google Scholar]
- 69. Xavier A., Olsen M.F., Lavik L.A., Johansen J., Singh A.K., Sjursen W., Scott R.J., Talseth-Palmer B.A.. Comprehensive mismatch repair gene panel identifies variants in patients with Lynch-like syndrome. Mol. Genet. Genomic Med. 2019; 7:e850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Olkinuora A., Nieminen T.T., Mårtensson E., Rohlin A., Ristimäki A., Koskenvuo L., Lepistö A., Silander G., Kuchinskaya E., Aravidis C.et al.. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet. Med. 2019; 21:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang M., Zhu L., Xu D., Weng S., Fang X., Dong C., Hu H., Yuan Y.. How should we correctly interpret biallelic germline truncating variant of MLH3 in hereditary colorectal cancer. Genet. Med. 2019; 21:2650–2651. [DOI] [PubMed] [Google Scholar]
- 72. Rahman M.M., Mohiuddin M., Shamima Keka I., Yamada K., Tsuda M., Sasanuma H., Andreani J., Guerois R., Borde V., Charbonnier J.-B.et al.. Genetic evidence for the involvement of mismatch repair proteins, PMS2 and MLH3, in a late step of homologous recombination. J. Biol. Chem. 2020; 295:17460–17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P.et al.. The mutational constraint spectrum quantified from variation in 141, 456 humans. Nature. 2020; 581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study includes no data deposited in external repositories.