Abstract
The amyloid precursor protein is so named, because a proteolytic fragment of it was found associated with a neuropathic disorder now known as Alzheimer’s disease. This fragment, Aβ, along with tau makes up the plaques and tangles that are the hallmark of AD. Iron (and other first-row transition metals) is found associated with these proteinaceous deposits. Much research has focused on the relationship of the plaques and iron to the etiology of the disease. This commentary asks another question, one only more recently addressed namely, what is the physiologic function of the amyloid precursor protein (APP) and of its secretase-generated soluble species? Overall, the data make clear that APP and its products have neurotrophic functions and some data indicate one of these may be to modulate the trafficking of iron in the brain.
Keywords: Amyloid precursor protein, Ferroportin, Iron efflux, Hephaestin, APP-like proteins, Iron metabolism, Cell surface protein, Membrane transport, Metal transport, Metal binding, Alzheimer’s disease
Introduction
What is now known clinically as Alzheimer’s disease (AD) was first described in 1906 by a neuroanatomist, Dr. Alois Alzheimer at a meeting in Tübingen [1, 2]. In his post-mortem of a patient who had presented with the now well-characterized behavioral symptoms of this neurodegenerative disease, he noted distinctive plaques throughout the cerebral cortex and strongly staining fibrils in what remained of intact neurons. In 1986, Selkoe and co-workers demonstrated that the fibrils were composed of microtubule-associated protein (MAP) tau [3]; 1 year later, Masters, Grezschik, Beyreuther, and colleagues mapped the plaque peptide, A4, to a transcript that encoded a 695 residue protein whose locus was found on chromosome 21 connecting this gene product to late-onset Down’s syndrome as well as to AD [4]. Tau was known to be an important contributor to microtubule stability; the physiologic role of the A4 precursor was not known a priori and, thus, has since been known as the amyloid precursor protein, APP. While APP and its processing can be contraindicating when it comes to longevity, selective pressure for protein function dominates in a species’ reproductive years; those selectable physiologic roles for APP—or its processed forms—have been an enigma that has only recently begun to be deciphered [5-11]. One of those physiologic roles appears linked in some fashion to cell iron metabolism [12-19]. This commentary addresses the current state of these latter investigations.
Background: APP and the APP family members
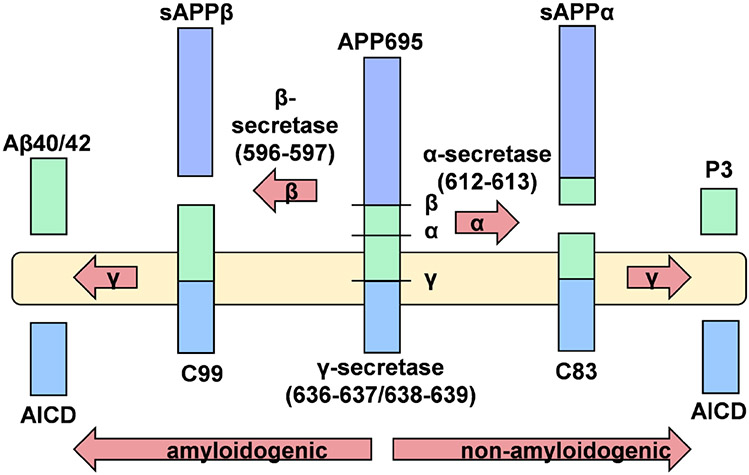
APP and its two mammalian paralogs, APLP1 and APLP2, are Type I membrane proteins which undergo proteolytic cleavage by one of two membrane-resident secretases releasing soluble ectodomains into the extracellular space [20-22]. The most abundant form of APP in the brain, and the only form found in neurons, is a protein of 695 residues. The soluble protein, sAPPα (610 residues), is shed as a result of APP cleavage by ADAM10/ADAM17, while cleavage of APP by BACE1 releases sAPPβ (596 residues). Subsequent action by γ-secretase—which is relatively promiscuous—within the transmembrane domain of the product of BACE1 proteolysis generates two peptides one of which is Aβ42, the initiator of plaque formation in AD. ADAM secretase cleavage is within this Aβ sequence and, thus, initiates what is referred to as the non-amyloidogenic processing pathway. These processing events are illustrated in Fig. 1. Processing of APLP1 and 2 is similar but lacking the Aβ sequence, and these APP family members are constitutively non-amyloidogenic.
Fig. 1.
Secretase processing of native APP. This example uses APP695, the sole splice variant expressed by neurons. Note the promiscuous nature of γ-secretase that leads to two Aβ variants; only Aβ42 is amyloidogenic. AICD refers to the APP carboxyl-terminal intracellular domain
The mature, unprocessed forms of all three family members have been described as having neurotrophic function. Given that all three proteins have epitopes in their ectodomains for a variety of homo- and hetero-oligomeric interactions, they have been described as having essential roles in neurite outgrowth and cell–cell interaction [5, 6, 10, 11]. APP likely plays a role in synapse formation, clustering in the pre-synaptic zone where glutamate is released into the synaptic cleft populated by the glutamatergic calcium channels that characterize the hippocampal neuron [23-25]. These neurons exhibit the long-term potentiation that lead to memory and learning; thus, hippocampal neuronal die-off is central to the progression of AD [26]. Reasonably, APLP1 and 2 support a similar but not totally redundant activity [5, 9]. This general conclusion follows from the pathophysiology reported for mouse knockout lines that fail to express two of the three proteins. While no one single knockout is lethal, the APP/APLP2 and APLP1/APLP2 double KO mice exhibit embryonic lethality or mortality within 14 days of birth suggesting some overlap of function [6, 27].
The physiologic functions of the soluble forms of these three proteins are somewhat more opaque, although, they, too, are broadly considered to support neuronal homeostasis [7, 8, 25]. Neuroprotective sAPPα has been associated with synaptic plasticity, LTP activation, and memory formation, highlighting its role in maintaining cognitive function. In addition, sAPPα has been shown to mediate neuroprotection through modulation of cell signaling processes, primarily through activation of cell survival pathways involving PI3K, NFκB, and ERK, and downregulation of stress-induced pathways including c-JNK [28-32]. Similarly, sAPPβ has been shown to be neuroprotective in so far as it can support axonal outgrowth and differentiation [33]; however, sAPPβ does not have an effect on LTP and any reported neuroprotective effects are greatly reduced compared to those seen with sAPPα [34-37]. APP KO mice have growth and cognitive deficits consistent with impaired LTP and memory; these can be rescued and even further improved with sAPPα but not with sAPPβ [38, 39]. The most compelling data are sAPPα and sAPPβ when used to complement APP function in APP/APLP2 double-knock-out mice. In both paradigms, sAPPα but not sAPPβ restored the long-term potentiation characteristic of hippocampal neurons via the activation of the Ca-permeant NMDA receptor [40, 41]. The molecular mechanism of this effect has not been investigated, but one can envision that it involves a protein–protein interaction at the post-synaptic membrane; this might involve the dimerization of sAPPα mediated by metal binding (Zn2+) to the (Aβ) motif found at this species’ C-terminus; obviously, sAPPβ lacks this potential for protein dimerization. As the reader will see, Zn2+ plays a varied role in APP biology.
An important contributor to the cellular function of the native forms of all three proteins is their robust anterograde and retrograde trafficking, to and from the plasma membrane. A relatively small fraction of any one of these proteins is localized to the plasma membrane on a time-averaged basis; of the three, APLP2 is most abundantly expressed in the plasma membrane [42]. Residence in the plasma membrane is enhanced by Zn2+, likely through the role this divalent metal ion plays in the assembly of APP family member oligomers [43]; with respect to overall function of these proteins, quaternary structure formation restricts secretase-catalyzed shedding of soluble forms putting significant focus on the role of zinc in APP function, both physiologic and pathophysiologic [44, 45].
APP family member metal-binding epitopes
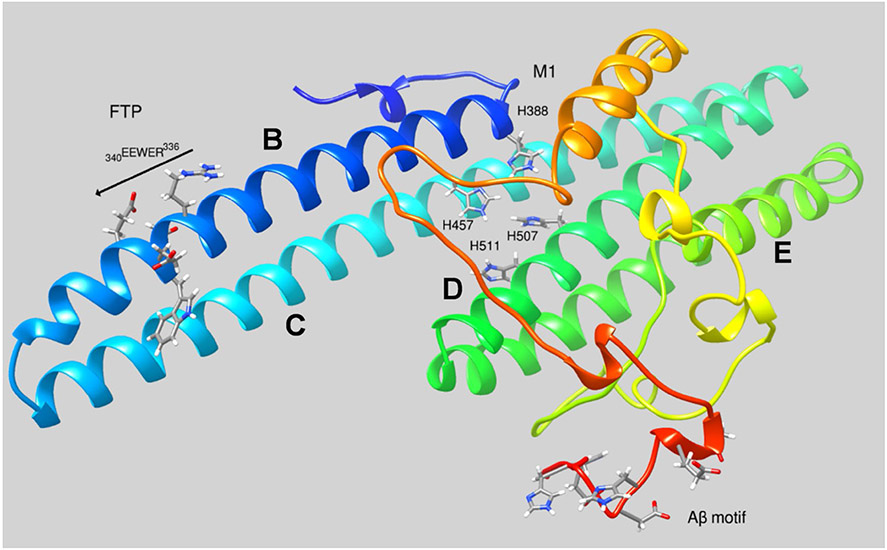
All three APP proteins have motifs that have been demonstrated to bind divalent, first-row transition metal ions, specifically, Zn2+ and Cu2+; one motif found in the N-terminal copper-binding domain appears to be specific for Cu2+, while two others found in the E2 domain have been linked to both Zn2+ and Cu2+ coordination. In addition, there is the motif in the Aβ peptide (a sequence also found at the C-terminus of sAPPα) that appears to bind Zn2+, Cu2+ and Fe2+ [46, 47]. The structure of the sAPPα E2 domain is shown in Fig. 2 highlighting the ligands that contribute to the M1 binding site and the Aβ sequences found at the C-terminus of this ADAM10/17 cleavage product. The role of Zn2+ and Cu2+ in APP family member cell biology is fairly widely understood to be associated with their role in the dimerization of the native, Type 1 membrane proteins. In general, metal binding indirectly promotes either trans-dimerization (the effect of Cu2+ on APP or Zn2+ on APLP1) or cis-dimerization (the effect of Cu2+ on APP) of the membrane bound, native APP family members (indirectly, since metal binding stabilizes a conformation favorable to dimerization without participating in the dimerization interface). Trans-dimerization refers to species in opposing membranes, whereas the latter refers to dimerization of species in the same membrane. In either case, these metal-promoted interactions are thought to be physiologic, not pathophysiologic and linked to mobilization of Zn2+ and Cu1+/2+ from the pre-synaptic neuron upon depolarization. Indeed, this connection between APP and these two transition metals in synaptic function is an underinvestigated area in metallo-neuroscience. Evolution shows us that, together, these three bio-molecules have a selective advantage and we do not really know what that is.
Fig. 2.
Structure of the E2 domain of sAPPα. The three motifs highlighted are: 1—the ferroportin targeting sequence, FTP found in the B helix: REWEE; 2—the M1 Zn2+- and Cu2+-binding site that spans helices B–D; and 3—the C-terminal residues in sAPPα that correspond to those found in Aβ
APP and iron: facts, inferences, and controversy
Similarly, in contrast to the plethora of citations referencing iron, APP, and the pathophysiology associated with AD, there is a limited number that examine roles that APP might play in iron metabolism as one of its evolutionarily selected physiologic functions. The most explicit finding linking iron to APP is the presence of an iron response element in the 5’-UTR of the APP transcript [12, 14, 18], a stem-loop structure to which the iron response-binding protein (the des-Fe, S cluster form of cytosolic aconitase) binds blocking translation of the downstream APP coding sequence. In short, expression of APP is regulated by the relative Fe, S cluster occupancy of this IRE-binding protein (IREBP) [48, 49]. This occupancy is downstream from the assembly of cytoplasmic Fe, S clusters. While the details of this assembly in humans have not been fully characterized, there is no debate that it relies on cell iron status, thus completing the link between cellular iron and APP expression [50-54].
Given this relationship between cell iron and APP protein synthesis, a reasonable premise is that APP itself plays some role in the trafficking of iron that modulates the protein’s expression. This then represents one of the physiologic roles that APP may play when we consider “it’s not just amyloid” [8]. However, the initial findings on the IRE in the APP transcript and its link to iron regulation were interpreted in terms of pathophysiology, interpreting the iron-dependent upregulation of APP synthesis in terms of amyloid production, not iron metabolism [18, 55]. A common perspective is reflected in the following from a recent review: “Therefore, these findings demonstrate that iron accumulation can be a pathological factor triggering the upregulated expression of the APP holo-protein and subsequent Aβ deposition” [56]. This view is counter-intuitive, however. If the presumption is that iron plus amyloid is toxic, then increased iron would be expected to reduce not increase expression of the amyloid precursor, APP. More reasonable is the model that more APP synthesis is induced by cell iron, because APP, either full-length or a soluble, secretase product (sAPP) plays some role in managing that iron, not commandeering it to kill the cell. These competing views represent the principle dichotomy with respect to how investigators perceive the APP–iron connection [57-59].
The other widely recognized link between APP species and iron metabolism is with respect to ferroportin-dependent cell iron efflux. FPN is the sole iron exporter in mammals (transporting ferrous iron, exclusively) whose activity is regulated primarily by its residency in the plasma membrane; the steady-state level of this membrane expression is, in turn, dependent on the relative rates of antero- and retrograde trafficking. Little is known about these processes with the exception of the action of the hormone hepcidin which binds to a motif in the FPN’s ecto-domain stimulating internalization and degradation [60, 61].
Another well-established aspect of FPN function is the linkage between transport of ferrous iron and its oxidation to Fe3+ via the action of one or both of the mammalian multicopper ferroxidases, ceruloplasmin, and hephaestin. Data indicate that not only do these enzymes support the conversion of FPN-transported Fe2+ to extracellular Fe3+, but their presence and/or activity increases FPN plasma membrane presentation presumably by altering FPN forward or reverse flow, or both [62, 63]. In as much that inactive forms of ceruloplasmin fail to stabilize FPN in the membrane indicate that it is the conversion of Fe2+ to Fe3+ that promotes FPN membrane occupancy [64].
How does an APP species fit into this iron efflux framework? A third well-established fact is that the product of the 21q21.3 locus in the human genome—APP—has an REWEE motif in the B helix of the protein’s E2 domain (part of the protein’s ~ 600 residue ecto-domain, see Fig. 2) that binds to FPN and, in so doing, results in an increase in FPN’s occupancy on the surface of a cell in a fashion that functionally is antagonistic to the action of hepcidin [17, 65-67]. There is little controversy about this experimental observation. The questions that remain are “how” and “why.” There are differing views on both aspects of the FPN–APP iron link.
The “how” is first a question of whether an FPN–APP interaction occurs between native, integral membrane APP, and FPN, or a soluble, sAPP species, and FPN or both. The data that bear on this question are somewhat in conflict, although experiments designed to answer this question explicitly are consistent with the model that the physiologically relevant interaction is between FPN and sAPP (likely independent of whether the product of alpha- or beta-secretase action). The three most definitive results supporting this conclusion are pull-down assays using epitope-tagged sAPP species [17, 65]; quantification of surface FPN biotinylated in the presence of endogenous, native APP in comparison to the effect of exogenous, soluble APP species [67, 68]; and FRET analyses of potential interactions between FPN and either hephaestin or APP in the cell membrane [67]. The outcomes of all three approaches are consistent with the conclusion that only exogenous, soluble APP species interact with FPN. In contrast, for example, while no interaction between fluorescently tagged FPN and native APP was detected, FRET was readily evident between FPN–CFP and HEPH–YFP, a finding consistent with the generally accepted model of the cellular relationship between this protein pair essential to iron efflux [63, 67].
In addition, there is evidence that secretase-dependent processing is stimulated by iron, a finding that is consistent with the premise that a processed, soluble APP species has been selected for in response to an increased cellular iron load. However, the iron-dependent regulation of secretase expression and processing remains controversial in the field, and may very well be cell-type dependent. Reports of regulation of both ADAM10 and BACE1 protein expression are frequent, particularly in primary neurons and neuronal cell lines [69, 70]. In contrast, studies in other CNS cell types indicate that iron might alter the processing activity of the secretases and not their expression [14, 71].
Is this upregulation of processing a simple case of increased APP substrate itself pushing the enzymatic reaction towards production of the secretase cleavage products? This is likely true in cases where native APP and its cleavage products are increased in response to iron, without a concurrent change in secretase expression or activity. Not all cases can be so simply explained: often, native APP and secretase protein, as well as secretase activity (determined by fluorescence reporter assays) remain unchanged with iron treatment, yet the levels of sAPP and the C-terminal cleavage fragments are greatly increased. This could potentially be explained as altered localization of the substrate and the enzyme, either increased surface presentation of native APP bringing it into close proximity of the α-secretase, or a switch in the trafficking of β-secretase and native APP leading to co-localization in the endosomal pathway. In both cases, the amount of overall substrate is unchanged, while the localized concentration of substrate in proximity to the active secretases is greatly increased, directly leading to an increase in cleavage. That there might be this upstream iron link to APP localization and processing would be consistent with the premise that shedding of sAPP is a physiologic response to a change in iron status, not a consequence of some aberrant signaling.
As to the question of function, irrespective of the details of the experiment, the consistent finding is that the FPN–APP interaction potentiates iron efflux whether quantified by direct measurement or by inference based on the finding of a decrease in steady-state cell iron load [17, 65, 67]. However, another critical aspect of this stimulation is its ferroxidase requirement; that is, surface presentation of FPN increased by interaction with an REWEE-containing soluble APP species supports iron efflux only in the presence of ferroxidase activity. This fact was strongly demonstrated by a number of reports following the initial suggestion that APP itself was a ferroxidase [65]; it is not [17, 68, 72].
Indeed, there are reports associating APP with either metallo-oxidase or reductase activity involving iron and copper. One such report demonstrated that Cu2+ bound at the tetra-His site in the E2 domain (cf. Fig. 2) turned over O2 in the presence of ascorbate; this was not strictly catalytic in that APP oxidation products were also formed [73]. In such studies, one needs to keep in mind the aqueous redox chemistry of ionic iron and copper given their reduction potentials. That for the Cu1+/2+ couple is in the low millivolt range and for iron at neutral pH in the presence of any ligand that has a preference for ferric versus ferrous iron; its potential will be lowered into this range, as well. These properties underlie the well-recognized pro-oxidant activity of each metal given the ease of electron transfer to dioxygen from their low valent forms and redox cycling in the presence of any common biologic reducing equivalent [74]. In short, reports of ferro-/cupro-oxidase or ferri-/cupri-reductase activity must be validated by appropriate controls. The original report of the ferroxidase activity of APP lacked these controls, ones only reported on too late to retract the claim [68].
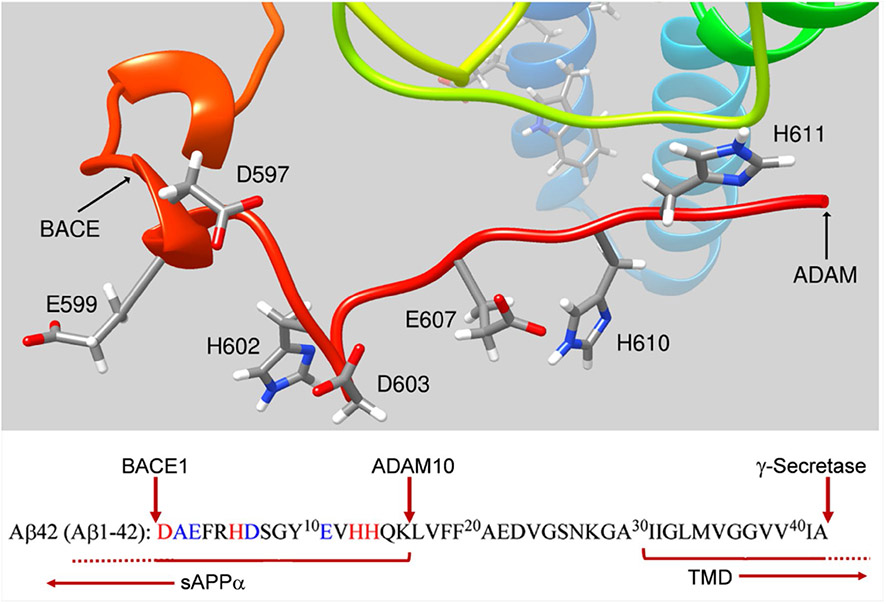
Another under-developed premise concerning a possible physiologic role for sAPPα, specifically, in iron trafficking is its retention of the metal-binding epitopes so well studied in Aβ [45-47]; this is illustrated in Fig. 3. Given the extended, unordered nature of this sAPPα ‘tail’, there is no reason a priori to discount the likelihood that it retains a metal-binding portfolio similar to that established for Aβ. In general, the focus of studies evaluating the metal coordination by Aβ has focused on Cu2+ and Zn2+ in their competition and in the formation of bi-metallic complexes [46, 47, 75]. Whereas Zn2+ has been considered a factor in APP species oligomerization (as noted above), Cu2+ binding to Aβ has been investigated in terms of the apparent ROS generation associated with the resulting complex [47]. Although not pursued further, reactions programed with Fe3+ rather than Cu2+ exhibited 10% of this activity, a reactivity difference between Cu2+ and Fe3+ noted by Buettner in 1986 [76].
Fig. 3.
Retention of metal-binding epitopes in sAPPα. ADAM10/17 processing of APP cleaves the native APP at the carboxyl-terminal end of the well-characterized Aβ motif associated with metal binding (highlighted in red and blue and noted in the structure). The amyloidogenic processing pathway associated with BACE1 processing is indicated, also. In either pathway, membrane-associated secretase function ‘cleans up’ the residual transmembrane domain fragment releasing the C-terminal domain into the cytosol (Fig. 1). Reasonably, sAPPα should exhibit whatever metal binding has been attributed to Aβ. The residue numbering in the sequence reflects that used for Aβ, while the residue numbering in the structure is for sAPPα generated from APP695, the most abundant neuronal isoform
Epilogue
There is no controversy about the fact that a major fraction of AD patients exhibit elevated iron levels in numerous brain regions and that there is a positive correlation with brain iron and Aβ plaque formation. There is, however, no consensus as to the cause-and-effect relationship between these AD-distinctive parameters. A confounding aspect of this connection is the plethora of diseases that can be (and have been) grouped as Neurodegeneration with Brain Iron Accumulation, NBIA [77]. While this designation was created for a group of relatively rare specific gene-linked pathologies, it includes ones like aceruloplasminemia and Friedreich’s ataxia, as well, in as much as these diseases have neuropathic features as well as brain iron accumulation [78, 79]. In short, making a specific link between Alzheimer’s disease and iron certainly obscures our view of the physiologic roles that APP must play—including managing iron—and appears to restrict our asking the question Why? And why is brain iron accumulation typically observed in a patient exhibiting the cognitive and motor deficits common to most neuropathology [80]? These are the two questions: this commentary proposes as significant subjects for more extensive consideration.
Acknowledgements
The work in the Kosman lab is supported by a grant from the National Institute of Neurological Disorders and Stroke, NS102337. This support is gratefully acknowledged.
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dahm R (2006) Curr Biol 16:R906–910 [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer A (1906) Zeit Psych Psych Med 64:146–148 [Google Scholar]
- 3.Kosik KS, Joachim CL, Selkoe DJ (1986) Proc Natl Acad Sci USA 83:4044–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B (1987) Nature 325:733–736 [DOI] [PubMed] [Google Scholar]
- 5.Cousins SL, Dai W, Stephenson FA (2015) J Neurochem 133:879–885 [DOI] [PubMed] [Google Scholar]
- 6.Klevanski M, Saar M, Baumkotter F, Weyer SW, Kins S, Muller UC (2014) Mol Cell Neurosci 61:201–210 [DOI] [PubMed] [Google Scholar]
- 7.Ludewig S, Korte M (2016) Front Mol Neurosci 9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller UC, Deller T, Korte M (2017) Nat Rev Neurosci 18:281–298 [DOI] [PubMed] [Google Scholar]
- 9.Weyer SW, Klevanski M, Delekate A, Voikar V, Aydin D, Hick M, Filippov M, Drost N, Schaller KL, Saar M, Vogt MA, Gass P, Samanta A, Jaschke A, Korte M, Wolfer DP, Caldwell JH, Muller UC (2011) EMBO J 30:2266–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilling S, Mehr A, Ludewig S, Stephan J, Zimmermann M, August A, Strecker P, Korte M, Koo EH, Muller UC, Kins S, Eggert S (2017) J Neurosci 37:5345–5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plummer S, Van den Heuvel C, Thornton E, Corrigan F, Cappai R (2016) Aging Dis 7:163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT (2010) J Biol Chem 285:31217–31232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerreiro C, Silva B, Crespo AC, Marques L, Costa S, Timoteo A, Marcelino E, Maruta C, Vilares A, Matos M, Couto FS, Faustino P, Verdelho A, Guerreiro M, Herrero A, Costa C, de Mendonca A, Martins M, Costa L (2015) Biochim Biophys Acta 1852:2116–2122 [DOI] [PubMed] [Google Scholar]
- 14.Guo LY, Alekseev O, Li Y, Song Y, Dunaief JL (2014) Exp Eye Res 129:31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy RC, Kosman DJ (2015) Front Mol Neurosci 8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy RC, Kosman DJ (2015) Cell Mol Life Sci 72:709–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy RC, Park YH, Kosman DJ (2014) EMBO Rep 15:809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR (2002) J Biol Chem 277:45518–45528 [DOI] [PubMed] [Google Scholar]
- 19.Yanatori I, Richardson DR, Imada K, Kishi F (2016) J Biol Chem 291:17303–17318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow VW, Mattson MP, Wong PC, Gleichmann M (2010) NeuroMol Med 12:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien RJ, Wong PC (2011) Annu Rev Neurosci 34:185–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobsen KT, Iverfeldt K (2009) Cell Mol Life Sci 66:2299–2318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoe HS, Fu Z, Makarova A, Lee JY, Lu C, Feng L, Pajoohesh-Ganji A, Matsuoka Y, Hyman BT, Ehlers MD, Vicini S, Pak DT, Rebeck GW (2009) J Biol Chem 284:8495–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Wang B, Yang L, Guo Q, Aithmitti N, Songyang Z, Zheng H (2009) J Neurosci 29:10788–10801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagna E, Dorostkar MM, Herms J (2017) Front Mol Neurosci 10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu Y, Gage FH (2011) Mol Neurodegener 6:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weyer SW, Zagrebelsky M, Herrmann U, Hick M, Ganss L, Gobbert J, Gruber M, Altmann C, Korte M, Deller T, Muller UC (2014) Acta Neuropathol Commun 2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copanaki E, Chang S, Vlachos A, Tschäpe J-A, Müller UC, Kögel D, Deller T (2010) Mol Cell Neurosci 44:386–393 [DOI] [PubMed] [Google Scholar]
- 29.Kogel D, Schomburg R, Copanaki E, Prehn JH (2005) Cell Death Differ 12:1–9 [DOI] [PubMed] [Google Scholar]
- 30.Cheng G, Yu Z, Zhou D, Mattson MP (2002) Exp Neurol 175:407–414 [DOI] [PubMed] [Google Scholar]
- 31.Greenberg SM, Kosik KS (1995) Neurobiol Aging 16:403–407 [DOI] [PubMed] [Google Scholar]
- 32.Guo Q, Robinson N, Mattson MP (1998) J Biol Chem 273:12341–12351 [DOI] [PubMed] [Google Scholar]
- 33.Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B (2011) PLoS ONE 6:e16301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor CJ, Ireland DR, Ballagh I, Bourne K, Marechal NM, Turner PR, Bilkey DK, Tate WP, Abraham WC (2008) Neurobiol Dis 31:250–260 [DOI] [PubMed] [Google Scholar]
- 35.Mockett BG, Guevremont D, Elder MK, Parfitt KD, Peppercorn K, Morrissey J, Singh A, Hintz TJ, Kochen L, Tom Dieck S, Schuman E, Tate WP, Williams JM, Abraham WC (2019) J Neurosci 39:3188–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP (1996) J Neurochem 67:1882–1896 [DOI] [PubMed] [Google Scholar]
- 37.Tackenberg C, Nitsch RM (2019) Mol Brain 12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corrigan F, Vink R, Blumbergs PC, Masters CL, Cappai R, van den Heuvel C (2012) J Neurochem 122:208–220 [DOI] [PubMed] [Google Scholar]
- 39.Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC (2007) J Neurosci 27:7817–7826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hick M, Herrmann U, Weyer SW, Mallm JP, Tschape JA, Borgers M, Mercken M, Roth FC, Draguhn A, Slomianka L, Wolfer DP, Korte M, Muller UC (2015) Acta Neuropathol 129:21–37 [DOI] [PubMed] [Google Scholar]
- 41.Moreno L, Rose C, Mohanraj A, Allinquant B, Billard JM, Dutar P (2015) J Alzheimers Dis 48:927–935 [DOI] [PubMed] [Google Scholar]
- 42.Kaden D, Voigt P, Munter LM, Bobowski KD, Schaefer M, Multhaup G (2009) J Cell Sci 122:368–377 [DOI] [PubMed] [Google Scholar]
- 43.Mayer MC, Schauenburg L, Thompson-Steckel G, Dunsing V, Kaden D, Voigt P, Schaefer M, Chiantia S, Kennedy TE, Multhaup G (2016) J Neurochem 137:266–276 [DOI] [PubMed] [Google Scholar]
- 44.Wild K, August A, Pietrzik CU, Kins S (2017) Front Mol Neurosci 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerber H, Wu F, Dimitrov M, Garcia Osuna GM, Fraering PC (2017) J Biol Chem 292:3751–3767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bousejra-ElGarah F, Bijani C, Coppel Y, Faller P, Hureau C (2011) Inorg Chem 50:9024–9030 [DOI] [PubMed] [Google Scholar]
- 47.Atrian-Blasco E, Gonzalez P, Santoro A, Alies B, Faller P, Hureau C (2018) Coord Chem Rev 371:38–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rouault TA (2006) Nat Chem Biol 2:406–414 [DOI] [PubMed] [Google Scholar]
- 49.Kuhn LC (2015) Metallomics 7:232–243 [DOI] [PubMed] [Google Scholar]
- 50.Lill R, Muhlenhoff U (2008) Annu Rev Biochem 77:669–700 [DOI] [PubMed] [Google Scholar]
- 51.Braymer JJ, Lill R (2017) J Biol Chem 292:12754–12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ciofi-Baffoni S, Nasta V, Banci L (2018) Metallomics 10:49–72 [DOI] [PubMed] [Google Scholar]
- 53.Pandey AK, Pain J, Dancis A, Pain D (2019) J Biol Chem 294:9489–9502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Condo I, Malisan F, Guccini I, Serio D, Rufini A, Testi R (2010) Hum Mol Genet 19:1221–1229 [DOI] [PubMed] [Google Scholar]
- 55.Rogers JT, Randall JD, Eder PS, Huang X, Bush AI, Tanzi RE, Venti A, Payton SM, Giordano T, Nagano S, Cahill CM, Moir R, Lahiri DK, Greig N, Sarang SS, Gullans SR (2002) J Mol Neurosci 19:77–82 [DOI] [PubMed] [Google Scholar]
- 56.Zhou ZD, Tan EK (2017) Mol Neurodegener 12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters DG, Connor JR, Meadowcroft MD (2015) Neurobiol Dis 81:49–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uranga RM, Salvador GA (2018) Oxid Med Cell Longev 2018:2850341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long JM, Maloney B, Rogers JT, Lahiri DK (2019) Mol Psychiatry 24:345–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drakesmith H, Nemeth E, Ganz T (2015) Cell Metab 22:777–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA, Vieth KR, Jung G, Casu C, Rivella S, Jormakka M, Mackenzie B, Ganz T, Nemeth E (2018) Blood 131:899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonaccorsi di Patti MC, Cutone A, Polticelli F, Rosa L, Lepanto MS, Valenti P, Musci G (2018) Biometals 31:399–414 [DOI] [PubMed] [Google Scholar]
- 63.Ji C, Steimle BL, Bailey DK, Kosman DJ (2018) Cell Mol Neurobiol 38:941–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kono S, Yoshida K, Tomosugi N, Terada T, Hamaya Y, Kanaoka S, Miyajima H (2010) Biochim Biophys Acta 1802:968–975 [DOI] [PubMed] [Google Scholar]
- 65.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez K, Johanssen T, Greenough MA, Cho HH, Galatis D, Moir RD, Masters CL, McLean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI (2010) Cell 142:857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarthy RC, Kosman DJ (2013) J Biol Chem 288:17932–17940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dlouhy AC, Bailey DK, Steimle BL, Parker HV, Kosman DJ (2019) J Biol Chem 294:4202–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong BX, Tsatsanis A, Lim LQ, Adlard PA, Bush AI, Duce JA (2014) PLoS ONE 9:e114174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong K, Cai H, Luo X-G, Struble RG, Clough RW, Yan X-X (2007) Exp Brain Res 181:435–446 [DOI] [PubMed] [Google Scholar]
- 70.Kim CH, Yoo YM (2013) Korean J Physiol Pharmacol 17:189–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Steven Bodovitz MTF, Frail DE, Klein WL (1995) J Neurochem 64:307–315 [DOI] [PubMed] [Google Scholar]
- 72.Honarmand Ebrahimi K, Dienemann C, Hoefgen S, Than ME, Hagedoorn PL, Hagen WR (2013) PLoS ONE 8:e72177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Young TR, Pukala TL, Cappai R, Wedd AG, Xiao Z (2018) Biochemistry 57:4165–4176 [DOI] [PubMed] [Google Scholar]
- 74.Kosman DJ (2013) Coordin Chem Rev 257:210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Atrian-Blasco E, Conte-Daban A, Hureau C (2017) Dalton T 46:12750–12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buettner GR (1986) Free Radic Res Commun 1:349–353 [DOI] [PubMed] [Google Scholar]
- 77.Schneider SA, Dusek P, Hardy J, Westenberger A, Jankovic J, Bhatia KP (2013) Curr Neuropharmacol 11:59–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kruer MC (2013) Int Rev Neurobiol 110:165–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schneider SA (2016) Curr Neurol Neurosci Rep 16:9. [DOI] [PubMed] [Google Scholar]
- 80.Ndayisaba A, Kaindlstorfer C, Wenning GK (2019) Front Neurosci 13:180. [DOI] [PMC free article] [PubMed] [Google Scholar]