Abstract
Background and Purpose
Serum insulin-like growth factor-1 (IGF-1) is known to have a neuroprotective effect. This study aimed to determine the effects of serum IGF-1 on the severity and clinical outcome of acute ischemic stroke (AIS).
Methods
This study included 446 patients with AIS who were admitted to Hallym University Sacred Heart Hospital within 7 days of stroke onset from February 2014 to June 2017. Serum IGF-1 levels were measured within 24 hours of admission. Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) score at admission, and the functional outcome at 3 months after symptom onset was assessed using the modified Rankin Scale score. The effects of serum IGF-1 levels on stroke severity and 3-month functional outcomes were analyzed using multivariate logistic regression analysis.
Results
This study evaluated 379 patients with AIS (age 67.2±12.6 years, mean±standard deviation; 59.9% males) after excluding 67 patients who had a history of previous stroke (n=25) or were lost to follow-up at 3 months (n=42). After adjusting for clinically relevant covariates, a higher serum IGF-1 level was associated with a lower NIHSS score at admission (adjusted odds ratio=0.44, 95% confidence interval=0.24–0.80, p=0.01), while there was no significant association at 3 months.
Conclusions
This study showed that a higher serum IGF-1 level is associated with a lower NIHSS score at admission but not at 3 months. Further studies are required to clarify the usefulness of the serum IGF-1 level as a prognostic marker for ischemic stroke.
Keywords: acute ischemic stroke, insulin-like growth factor I, modified Rankin Scale
INTRODUCTION
Ischemic stroke is one of the leading causes of death worldwide, and poststroke functional disabilities may negatively affect the quality of life.1 The prevalence of ischemic stroke in Korea is currently 1.6% and increasing. The mortality rate at 90 days after stroke is reportedly as high as 3–7%.2 Poor functional outcomes, defined as a modified Rankin Scale (mRS) score of >3, occur in around 33% and 30% of cases at 3 months and 1 year after stroke, respectively.2
Serum biomarkers enable earlier diagnoses and are more cost-effective than imaging methods such as magnetic resonance imaging.3 Therefore, the importance of serum biomarkers in predicting the risk and prognosis of acute ischemic stroke (AIS) is being emphasized in clinical practice. Some well-known prognostic predictors of AIS include age, initial neurological severity, infarct volume, and infarct location.4,5,6 Apart from these known prognostic factors for AIS, various serum biomarkers have been suggested for clinical applications such as predicting the prognosis, guiding treatment, and identifying at-risk patients.7 However, their successful application in clinical practice has not yet been reported, which is due to the presence of heterogeneity in the appropriate cutoff values to use and in the causes of stroke. Some limitations are also attributed to the confounding effect of the blood-brain barrier, the serum biomarker concentration may not show a significant correlation with the outcome.7,8
Previous studies found that low serum insulin-like growth factor-1 (IGF-1) levels were associated with ischemic heart disease, acute myocardial infarction, and diabetes mellitus (DM), which are risk factors for ischemic stroke.9,10 Serum IGF-1 was shown to have a neuroprotective effect,11 and be negatively correlated with cerebrovascular events and the prognosis.12,13 Other previous studies have investigated the correlations between initial stroke severity, poststroke functional outcome, and initial serum IGF-1 level.14,15,16,17,18 However, inconsistent results were found for the relationship between serum IGF-1 levels and initial stroke severity, with some studies showing an negative correlation,17,18 while others showed no definite correlation between these two factors.19 Increased poststroke serum IGF-1 levels and the IGF-1/IGF-binding protein 3 ratio were reported to be related to unfavorable functional outcomes at 3 months after stroke.20
The relationship between the serum IGF-1 level and stroke severity therefore needs to be evaluated further, and so the present study evaluated the predictive value of the serum IGF-1 level in stroke severity and functional outcomes at 3 months after stroke onset.
METHODS
Study design and subjects
This single-center retrospective observational study analyzed data from patients with AIS from the Hallym Stroke Registry from February 2014 to June 2017. Patients were enrolled in the study by applying the following inclusion criteria: 1) admission within 7 days of symptom onset, 2) serum IGF-1 sampled within 24 hours of admission, and 3) AIS documented on diffusion-weighted imaging (DWI). We excluded patients whose functional 3-month outcome was not evaluated or who had a prestroke mRS score of >3.
This study was approved by the Hallym University Sacred Heart Hospital Institutional Review Board (IRB 2019-02-012).
Clinical variables
Clinical information and demographics were collected during hospitalization, including age, sex, height, weight, body mass index (BMI), prestroke mRS score, previous stroke history, hypertension, DM, dyslipidemia, atrial fibrillation (AF), current smoking, and the initial National Institutes of Health Stroke Scale (NIHSS) score. Stroke subtype was classified as follows in accordance with the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification:21 large-artery atherosclerosis (LAA), cardioembolism (CE), small-vessel occlusion (SVO), stroke of other determined etiology (ODE), and stroke of undetermined etiology (UDE). Laboratory data were also collected, including serum IGF-1, high-sensitivity C-reactive protein (hsCRP), serum creatinine, fasting glucose, hemoglobin A1c, low-density lipoprotein cholesterol, and DWI.
Measurement of serum IGF-1
The blood samples for measuring serum IGF-1 levels were collected within 24 hours of admission. Blood was sampled between 6:30 a.m. and 8:00 a.m. after an overnight fast, placed in a serum separating tube, and subjected to centrifugal separation. A chemiluminescence immunoassay (LIAISON® IGF-1, DiaSorin, Saluggia, Italy) was then used to quantify the serum levels.
Outcomes
The primary outcome was stroke severity quantified as the NIHSS score at admission. The NIHSS score was dichotomized into two groups based on the median NIHSS score: mild (≤5) and moderate to severe (≥6). The secondary outcome was the functional outcome assessed using the mRS score at 3 months after symptom onset. A poor functional outcome was defined as an mRS score of 3–6. The 3-month mRS score was collected prospectively in outpatient clinics or through telephone interviews by neurologists or well-trained stroke nurses.
Statistical analyses
Baseline characteristics are presented as mean±standard deviation, median (interquartile range), or frequency and percentage values. Variables for which >5% of the total observations were missing were excluded from the analysis. For variables with <5% missing values, we performed imputation using the mean or median values. No data were missing for the categorical variables in this study. In order to compare baseline characteristics for primary and secondary outcomes, we used Student's t-tests and Mann-Whitney U tests for continuous variables, and Pearson's chi-square tests, Fisher's exact tests, or linear-by-linear associations for categorical variables, as appropriate.
An analysis of the linear correlation between serum IGF-1 level and NIHSS score was performed using Spearman's correlation test. Patients were divided into four groups based on the quartile of the serum IGF-1 level, namely Q1, Q2, Q3, and Q4. Multivariate logistic analysis was applied to calculate the adjusted odds ratios (ORs) and their 95% confidence intervals (CIs) for the potential predictors of outcomes. The following predetermined covariates were included in the multivariate model: clinically relevant factors identified in previous studies, age, sex, and variables with a p value of <0.05 in a bivariate analysis. The predetermined subgroup analysis was performed according to the history of DM or chronic kidney disease (CKD), which have previously been associated with serum IGF-1 levels.22,23 CKD was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2 based on the Modification of Diet in Renal Disease (MDRD) study. All analyses were performed using SPSS for Windows (version 24, IBM Corp., Armonk, NY, USA), and a p value of <0.05 was considered statistically significant.
RESULTS
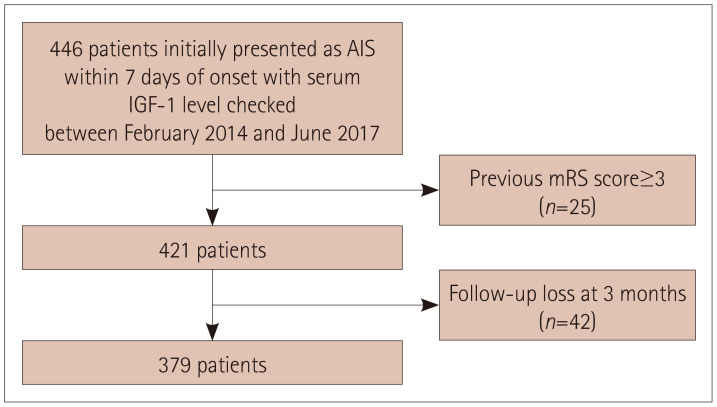
This study analyzed 379 of 446 patients with AIS who were admitted to Hallym University Sacred Heart Hospital during the study period and in whom the serum IGF-1 level was measured (Fig. 1).
Fig. 1. Flowchart of patient enrollment in the study. AIS: acute ischemic stroke, IGF-1: insulin-like growth factor-1, mRS: modified Rankin Scale.
Clinical characteristics
The patients were aged 67.2±12.6 years, and 59.9% of them were male. The median NIHSS score at admission was 5 (interquartile range=9), and 365 of the 379 patients had a prestroke mRS score of 0 or 1. The serum IGF-1 level was 116.0±54.9 ng/mL. Table 1 summarizes the baseline characteristics, risk factors, and initial clinical characteristics. The serum IGF-1 level was negatively correlated with age. The coefficient of determination (r) ranged from 0.09 to 0.30 for the different AIS subtypes, being highest in LAA and lowest in ODE (Supplementary Fig. 1 in the online-only Data Supplement).
Table 1. Baseline characteristics of the study population.
| Total (n=379) | |
|---|---|
| Age (years) | 67.2±12.6 |
| Sex, male | 230 (59.9) |
| Body mass index (kg/m2) | 23.7±3.1 |
| Stroke characteristics | |
| Prestroke mRS score of 0 or 1 | 365 (96.3) |
| NIHSS score at admission | 5.0 [2.0.11.0] |
| Onset to admission time (min) | 482 [95.1188] |
| TOAST classification | |
| Large-artery atherosclerosis | 123 (32.5) |
| Small-vessel occlusion | 83 (21.9) |
| Cardioembolism | 108 (28.5) |
| Stroke of other determined etiology | 48 (12.6) |
| Stroke of undetermined etiology | 17 (4.5) |
| Risk factor | |
| Hypertension | 233 (61.5) |
| Diabetes mellitus | 119 (31.4) |
| Hyperlipidemia | 134 (35.4) |
| Atrial fibrillation | 101 (26.6) |
| Smoking | 131 (34.6) |
| Previous stroke | 74 (19.5) |
| Systolic blood pressure (mm Hg) | 151.6±24.7 |
| Laboratory data | |
| Insulin-like growth factor-1 (ng/mL) | 116.0±54.9 |
| White blood cell count (×103/μL) | 8.39±3.01 |
| Initial glucose (mg/dL) | 152.8±63.1 |
| High-sensitivity C-reactive protein (mg/dL) | 2.74 [1.00.9.11] |
| Hemoglobin A1c (%) | 6.2±1.2 |
| Serum creatinine (mg/dL) | 0.94±0.89 |
| Low-density lipoprotein cholesterol (mg/dL) | 111.8±36.4 |
Data are mean±standard deviation, median [interquartile range], or n (%) values.
mRS: modified Rankin scale, NIHSS: National Institutes of Health Stroke Scale, TOAST: Trial of Org 10172 in Acute Stroke Treatment.
Subjects were divided into the following four groups according to their serum IGF-1 levels: Q1, <77.1 ng/mL (n=96); Q2, 77.1–108.0 ng/mL (n=98); Q3, 108.0–151.0 ng/mL (n=93); and Q4, > 151.0 ng/mL (n=92). Patients in Q4 were younger than those in Q1 (60.6±12.3 years vs. 75.0±10.2 years, p<0.05) and less likely to have history of AF (16.3% vs. 40.6%, p<0.05), a high hsCRP level (2.19 mg/dL vs. 4.85 mg/dL, p<0.05), and severe stroke (4.0 vs. 6.5, p<0.05) (Table 2).
Table 2. Patient characteristics according to quartiles of serum IGF-1 level.
| Serum IGF-1 (ng/mL) | p | ||||
|---|---|---|---|---|---|
| Q1, <77.1 | Q2, 77.1–108.0 | Q3, 108.0–151.0 | Q4, >151.0 | ||
| Age (years) | 75.0±10.2 | 69.9±11.8 | 63.9±10.7 | 60.6±12.3 | <0.001* |
| Sex, male | 35 (36.5) | 55 (56.1) | 65 (69.9) | 72 (78.3) | <0.001† |
| Body mass index (kg/m2) | 23.0±3.3 | 23.2±2.7 | 24.1±3.0 | 24.4±2.9 | <0.001* |
| Stroke characteristics | |||||
| Prestroke mRS score of 0 or 1 | 94 (97.9) | 92 (93.9) | 89 (95.7) | 90 (97.8) | 0.867† |
| NIHSS score at admission | 6.5 [2.25–14.00] | 5.0 [2.00–10.00] | 4.0 [1.00–7.50] | 4.0 [1.00–9.00] | <0.001§ |
| TOAST classification | |||||
| Large-artery atherosclerosis | 26 (27.1) | 26 (26.5) | 32 (34.4) | 39 (42.4) | 0.013‡ |
| Small-vessel occlusion | 19 (19.8) | 22 (22.4) | 27 (29.0) | 15 (16.3) | 0.855‡ |
| Cardioembolism | 42 (43.8) | 28 (28.6) | 22 (23.7) | 16 (17.4) | <0.001‡ |
| Stroke of other determined etiology | 3 (3.1) | 3 (3.1) | 3 (3.2) | 7 (7.6) | 0.145‡ |
| Stroke of undetermined etiology | 6 (6.3) | 19 (19.4) | 9 (9.7) | 15 (16.3) | 0.188‡ |
| Risk factor | |||||
| Hypertension | 59 (60.4) | 65 (66.3) | 58 (62.4) | 52 (56.5) | 0.491‡ |
| Diabetes mellitus | 24 (25.0) | 36 (36.7) | 28 (30.1) | 31 (33.7) | 0.363‡ |
| Hyperlipidemia | 30 (31.3) | 32 (32.7) | 41 (44.1) | 31 (33.7) | 0.386‡ |
| Atrial fibrillation | 39 (40.6) | 30 (30.6) | 17 (18.3) | 15 (16.3) | <0.001‡ |
| Smoking | 18 (18.8) | 28 (28.6) | 40 (43.0) | 45 (48.9) | <0.001‡ |
| Previous stroke | 16 (16.7) | 17 (17.3) | 25 (26.9) | 16 (17.4) | 0.511‡ |
| Systolic blood pressure (mm Hg) | 149.9±22.9 | 155.0±26.8 | 154.0±27.0 | 147.5±20.5 | 0.116* |
| Laboratory data | |||||
| White blood cell count (×103/µL) | 8.31±3.14 | 8.31±3.37 | 8.59±2.87 | 8.35±2.62 | 0.902* |
| Glucose (mg/dL) | 148.46±61.01 | 155.47±62.37 | 150.42±55.68 | 156.72±72.88 | 0.774* |
| High-sensitivity C-reactive protein (mg/dL) | 4.85 [1.94–14.18] | 2.42 [0.88–5.08] | 2.09 [1.06–8.50] | 2.19 [0.52–8.56] | 0.001§ |
| Serum creatinine (mg/dL) | 0.90±0.50 | 0.85±0.30 | 1.02±1.32 | 0.99±1.03 | 0.490* |
| Hemoglobin A1c (%) | 6.23±1.38 | 6.16±1.18 | 6.18±1.15 | 6.25±1.20 | 0.949* |
| Low-density lipoprotein cholesterol (mg/dL) | 102.9±33.8 | 111.5±33.6 | 115.0±39.2 | 118.1±37.6 | 0.026* |
Data are mean±standard deviation, median [interquartile range], or n (%) values.
*ANOVA, †Linear-by-linear association, ‡Chi-square test, §Mann-Whitney U test.
IGF-1: insulin-like growth factor-1, mRS: modified Rankin scale, NIHSS: National Institutes of Health Stroke Scale, TOAST: Trial of Org 10172 in Acute Stroke Treatmen.
Serum IGF-1 level and initial NIHSS score
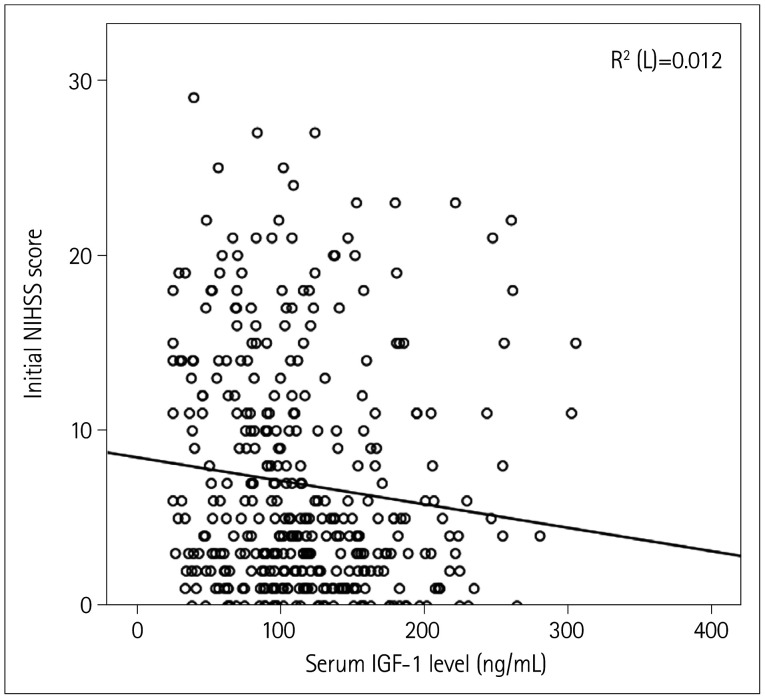
The serum IGF-1 level was negatively correlated with the NIHSS score at admission (r=−0.01, p<0.001) (Fig. 2). We additionally analyzed the association between serum IGF-1 level and initial NIHSS score using multivariate linear regression while adjusting for age, sex, and BMI, which revealed a negative correlation between these variables (r=−0.07, p<0.05). Compared to those in Q1, patients in Q4 had a lower initial NIHSS score (OR=0.45, 95% CI=0.25–0.82). In model 3, with Q1 as the reference, the highest and second-highest IGF-1 levels were independently associated with mild stroke (adjusted OR=0.44 and 0.39, 95% CI=0.24–0.84 and 0.20–0.74, respectively) (Table 3).
Fig. 2. Correlation between serum IGF-1 level and initial NIHSS score. IGF-1: insulin-like growth factor-1, NIHSS: National Institutes of Health Stroke Scale.
Table 3. Association between serum IGF-1 level and initial stroke severity.
| Serum IGF-1 | OR [95% CI] | p |
|---|---|---|
| Model 1 | <0.001 | |
| Q1 | Reference | |
| Q2 | 0.78 [0.44-1.37] | 0.39 |
| Q3 | 0.37 [0.20-0.67] | <0.001 |
| Q4 | 0.45 [0.25-0.82] | 0.01 |
| Model 2 | 0.05 | |
| Q1 | Reference | |
| Q2 | 0.85 [0.48-1.50] | 0.57 |
| Q3 | 0.44 [0.24-0.82] | 0.01 |
| Q4 | 0.57 [0.30-1.09] | 0.09 |
| Model 3 | <0.001 | |
| Q1 | Reference | |
| Q2 | 0.94 [0.50-1.74] | 0.84 |
| Q3 | 0.39 [0.20-0.74] | <0.001 |
| Q4 | 0.44 [0.24-0.84] | <0.001 |
Model 1: crude OR; model 2: model 1 adjusted for age and sex; model 3: model 2 adjusted for body mass index, Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, atrial fibrillation, smoking, white blood cell count, and high-sensitivity C-reactive protein.
CI: confidence interval, IGF-1: insulin-like growth factor-1, OR: odds ratio.
The subgroup analysis of patients without CKD also showed that the highest serum IGF-1 level was significantly associated with mild stroke (adjusted OR=0.37, 95% CI=0.20–0.71) (Supplementary Table 1 in the online-only Data Supplement). Additionally, we conducted subgroup analyses of patients in each subtype of ischemic stroke: LAA, SVO, and CE. There was no significant association between serum IGF-1 level and initial NIHSS score for any of the tested subtypes of ischemic stroke (Supplementary Table 2 in the online-only Data Supplement).
Serum IGF-1 level and 3-month functional outcome
There was a favorable secondary outcome in 233 patients (61.5%). The results from unadjusted and adjusted analyses of the relationship between serum IGF-1 level and 3-month functional outcome are presented in Table 4. Using Q1 as the reference, the unadjusted analysis showed that the OR for patients in Q4 having a good functional outcome was 1.95 (95% CI=1.07–3.57); however, the OR after adjusting for sex and age was 0.81 (95% CI=0.40–1.63). In addition, after adjusting for additional covariates that were statistically significant in the bivariate analyses of the primary outcome, there was no significant association between serum IGF-1 level and 3-month functional outcome (adjusted OR=0.62, 95% CI=0.27–1.43).
Table 4. Association between serum IGF-1 level and 3-month functional outcome.
| Serum IGF-1 | OR [95% CI] | p |
|---|---|---|
| Model 1 | 0.177 | |
| Q1 | Reference | |
| Q2 | 1.23 [0.69-2.17] | 0.481 |
| Q3 | 1.23 [0.69-2.19] | 0.486 |
| Q4 | 1.95 [1.07-3.57] | 0.029 |
| Model 2 | 0.424 | |
| Q1 | Reference | |
| Q2 | 0.90 [0.49-1.65] | 0.722 |
| Q3 | 0.59 [0.30-1.14] | 0.115 |
| Q4 | 0.81 [0.40-1.63] | 0.547 |
| Model 3 | 0.172 | |
| Q1 | Reference | |
| Q2 | 0.71 [0.34, 1.51] | 0.762 |
| Q3 | 0.35 [0.15, 0.80] | 0.773 |
| Q4 | 0.62 [0.27, 1.43] | 0.172 |
Model 1: crude OR; model 2: model 1 adjusted for age and sex; model 3: model 2 adjusted for prestroke modified Rankin Scale score, body mass index, Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification, diabetes mellitus, previous stroke, smoking, initial National Institutes of Health Stroke Scale score, white blood cell count, initial glucose level, and high-sensitivity C-reactive protein.
CI: confidence interval, IGF-1: insulin-like growth factor-1, OR: odds ratio.
Sensitivity analysis
We performed a sensitivity analysis of the 365 patients whose mRS score before admission was 0 or 1. The association of the initial NIHSS score with the highest serum IGF-1 levels remained significant (OR=0.43, 95% CI=0.23–0.81) (Supplementary Table 3 in the online-only Data Supplement), whereas that with the 3-month functional outcome did not (OR=0.60, 95% CI=0.24–1.49) (Supplementary Table 4 in the online-only Data Supplement).
DISCUSSION
This study found that higher serum IGF-1 levels measured within 24 hours of admission were associated with mild stroke as defined by the NIHSS score at admission. However, no significant association was found between serum IGF-1 level and functional outcome at 3 months after stroke.
Our study supports previous suggestions of high serum IGF-1 levels exerting neuroprotective effects on stroke severity. A previous study that evaluated the associations of serum IGF-1 levels with stroke severity and outcomes in 221 patients with AIS found a negative correlation between IGF-1 level and initial stroke severity, and that serum IGF-1 levels were lower in patients with severe AIS.18 However, conflicting results have also been reported for the relationship between serum IGF-1 level and stroke severity. A study that analyzed 255 patients with ischemic stroke found that the mean NIHSS score did not differ significantly between groups with low and high serum IGF-1 levels.15 These conflicting results may have been caused by differences in the baseline characteristics of the enrolled patients; compared to the patients enrolled in our study, the patients in the study of De Smedt et al.15 were older (74±11 years vs. 67±13 years) and higher proportions of them had hypertension (63% vs. 61.5%) and AF (30% vs. 26.6%). These risk factors might influence stroke severity more significantly than does the IGF-1 level, thereby masking the effect of serum IGF-1.
IGF-1, along with growth hormone, is critical in the development of many major organs, including the central nervous system (CNS). IGF-1 plays an essential role in cell proliferation and the survival of many cells in the body.13 In the CNS, IGF-1 aids in regulating neural development, such as in neurogenesis, synaptogenesis, and myelination, while inhibiting apoptosis and cell division.24 Additionally, serum IGF-1 reduces vascular inflammatory responses and atherosclerotic plaque progression, thus acting as a potent neuroprotective compound.25 There have been a few reports of the neuroprotective effect of IGF-1 on initial stroke severity, with serum IGF-1 levels being lower in patients with more-severe stroke.17,18
The present bivariate analysis showed that the 3-month functional outcomes differed significantly between the lowest and highest quartiles of the serum IGF-1 level, but the significance of the association was lost in the multivariate models. The present findings were somewhat inconsistent with those from previous studies. One study found that higher serum IGF-1 levels in patients with AIS are associated with better functional outcomes,15 while another study found these correlations in elderly patients.16 These different results may be attributable to the baseline characteristics differing between the serum IGF-1 levels in different quartiles. Our study included more patients with DM and LAA compared with previous studies, which may have influenced the 3-month functional outcomes.
This study was subject to several limitations. First, the serum IGF-1 level was measured once at admission in all AIS patients within 7 days of symptom onset. In contrast, most previous studies included AIS patients within 24 hours of symptom onset. Mattlage et al.26 suggested that a decrease in IGF-1 during the first week of stroke onset, with the uptake of serum IGF-1 into the brain from the circulation, is related to a better outcome. Because the serum IGF-1 level was measured once within the first week of symptom onset, the level might have already decreased by the time of sampling. However, the serum IGF-1 levels did not differ significantly between groups divided by stroke onset time in our cohort. Second, our study had a single-center, observational design, which might reduce the generalizability of the obtained results. However, our study had the strength of including a larger sample than those in previous studies.
Conclusion
Our findings suggest that the serum IGF-1 level is positively associated with the initial stroke severity. However, unlike some previous studies, we could not find an association between serum IGF-1 levels and functional outcomes after ischemic stroke. Further studies are required to clarify the usefulness of serum IGF-1 levels as a prognostic marker for ischemic stroke.
Acknowledgements
This research was supported by a fund (2020-ER6303-00) by Research of Korea Centers for Disease Control and Prevention.
Footnotes
- Conceptualization: all authors.
- Data curation: all authors.
- Formal analysis: Jeeun Lee, Mi Sun Oh, Byung-Chul Lee.
- Investigation: all authors.
- Methodology: Jeeun Lee, Jae-Sung Lim, Mi Sun Oh, Byung-Chul Lee.
- Supervision: Mi Sun Oh, Byung-Chul Lee.
- Writing—original draft: Jeeun Lee.
- Writing—review & editing: Jeeun Lee, Mi Sun Oh, Byung-Chul Lee.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2021.17.2.206.
Correlation between serum IGF-1 level and age according to stroke subtype: all patients (A), and in patients with large-artery atherosclerosis (B), small-vessel occlusion (C), cardioembolism (D), stroke of other determined etiology (E), and stroke of undetermined etiology (F). IGF-1: insulin-like growth factor-1.
Initial National Institutes of Health Stroke Scale score based on quartiles of serum IGF-1 levels in patients without chronic kidney disease (n=337)
Initial National Institutes of Health Stroke Scale score based on quartiles of serum IGF-1 levels for the different subtypes of ischemic stroke
Association between serum IGF-1 level and initial stroke severity
Association between serum IGF-1 level and 3-month functional outcome
References
- 1.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 2.Hong KS, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the Korean Stroke Society and Clinical Research Center for Stroke. J Stroke. 2013;15:2–20. doi: 10.5853/jos.2013.15.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–188. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC German Stroke Study Collaboration. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 5.Vogt G, Laage R, Shuaib A, Schneider A VISTA Collaboration. Initial lesion volume is an independent predictor of clinical stroke outcome at day 90: an analysis of the Virtual International Stroke Trials Archive (VISTA) database. Stroke. 2012;43:1266–1272. doi: 10.1161/STROKEAHA.111.646570. [DOI] [PubMed] [Google Scholar]
- 6.Frankel MR, Morgenstern LB, Kwiatkowski T, Lu M, Tilley BC, Broderick JP, et al. Predicting prognosis after stroke: a placebo group analysis from the National Institute of Neurological Disorders and Stroke rt-PA Stroke Trial. Neurology. 2000;55:952–959. doi: 10.1212/wnl.55.7.952. [DOI] [PubMed] [Google Scholar]
- 7.Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–360. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maas MB, Furie KL. Molecular biomarkers in stroke diagnosis and prognosis. Biomark Med. 2009;3:363–383. doi: 10.2217/bmm.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jørgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 10.Rajpathak SN, He M, Sun Q, Kaplan RC, Muzumdar R, Rohan TE, et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes. 2012;61:2248–2254. doi: 10.2337/db11-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng WH, Kar S, Doré S, Quirion R. Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic factor acting via the Akt kinase pathway. J Neural Transm Suppl. 2000;(60):261–272. doi: 10.1007/978-3-7091-6301-6_17. [DOI] [PubMed] [Google Scholar]
- 12.Aberg ND, Brywe KG, Isgaard J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci World J. 2006;6:53–80. doi: 10.1100/tsw.2006.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol. 2015;68:76–81. doi: 10.1016/j.exger.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bondanelli M, Ambrosio MR, Onofri A, Bergonzoni A, Lavezzi S, Zatelli MC, et al. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. J Clin Endocrinol Metab. 2006;91:3928–3934. doi: 10.1210/jc.2006-1040. [DOI] [PubMed] [Google Scholar]
- 15.De Smedt A, Brouns R, Uyttenboogaart M, De Raedt S, Moens M, Wilczak N, et al. Insulin-like growth factor I serum levels influence ischemic stroke outcome. Stroke. 2011;42:2180–2185. doi: 10.1161/STROKEAHA.110.600783. [DOI] [PubMed] [Google Scholar]
- 16.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, et al. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117:312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Tang JH, Ma LL, Yu TX, Zheng J, Zhang HJ, Liang H, et al. Insulin-like growth factor-1 as a prognostic marker in patients with acute ischemic stroke. PLoS One. 2014;9:e99186. doi: 10.1371/journal.pone.0099186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Dong X, Chang G, Ji XF, Tao DB, Wang YX. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One. 2014;9:e94845. doi: 10.1371/journal.pone.0094845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Shaheen H, Sobhy S, El Mously S, Niazi M, Gomaa M. Insulin-like growth factor-1 in acute ischemic stroke. Egypt J Neurol Psychiatr Neurosurg. 2018;54:42. doi: 10.1186/s41983-018-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armbrust M, Worthmann H, Dengler R, Schumacher H, Lichtinghagen R, Eschenfelder CC, et al. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 predict three-months outcome after ischemic stroke. Exp Clin Endocrinol Diabetes. 2017;125:485–491. doi: 10.1055/s-0043-103965. [DOI] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson E, Carrero JJ, Heimbürger O, Hellberg O, Lindholm B, Stenvinkel P. A cohort study of insulin-like growth factor 1 and mortality in haemodialysis patients. Clin Kidney J. 2016;9:148–152. doi: 10.1093/ckj/sfv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teppala S, Shankar A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care. 2010;33:2257–2259. doi: 10.2337/dc10-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Åberg D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr Dev. 2010;17:63–76. doi: 10.1159/000262529. [DOI] [PubMed] [Google Scholar]
- 25.Sukhanov S, Higashi Y, Shai SY, Vaughn C, Mohler J, Li Y, et al. IGF-1 reduces inflammatory responses, suppresses oxidative stress, and decreases atherosclerosis progression in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:2684–2690. doi: 10.1161/ATVBAHA.107.156257. [DOI] [PubMed] [Google Scholar]
- 26.Mattlage AE, Rippee MA, Sandt J, Billinger SA. Decrease in insulin-like growth factor-1 and insulin-like growth factor-1 ratio in the first week of stroke is related to positive outcomes. J Stroke Cerebrovasc Dis. 2016;25:1800–1806. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between serum IGF-1 level and age according to stroke subtype: all patients (A), and in patients with large-artery atherosclerosis (B), small-vessel occlusion (C), cardioembolism (D), stroke of other determined etiology (E), and stroke of undetermined etiology (F). IGF-1: insulin-like growth factor-1.
Initial National Institutes of Health Stroke Scale score based on quartiles of serum IGF-1 levels in patients without chronic kidney disease (n=337)
Initial National Institutes of Health Stroke Scale score based on quartiles of serum IGF-1 levels for the different subtypes of ischemic stroke
Association between serum IGF-1 level and initial stroke severity
Association between serum IGF-1 level and 3-month functional outcome