Abstract
Background:
The role and timing of whole or stereotaxic brain radiotherapy (BR) in patients with advanced non-small cell lung cancer (aNSCLC) and asymptomatic brain metastases (aBMs) are not well established. This study investigates whether deferring BR until cerebral progression was superior to upfront BR for patients with aNSCLC and aBM.
Methods:
This open-label, multicenter, phase III trial, randomized (1:1) aNSCLC patients with aBMs to receive upfront BR and chemotherapy: platin–pemetrexed and bevacizumab in eligible patients, followed by maintenance pemetrexed with or without bevacizumab, BR arm, or the same chemotherapy with BR only at cerebral progression, chemotherapy (ChT) arm. Primary endpoint was progression-free survival (PFS), secondary endpoints were overall survival (OS), global, extra-cerebral and cerebral objective response rate (ORR), toxicity, and quality of life [ClinicalTrials.gov identifier: NCT02162537].
Results:
The trial was stopped early because of slow recruitment. Among 95 included patients, 91 were randomized in 24 centers: 45 to BR and 46 to ChT arms (age: 60 ± 8.1, men: 79%, PS 0/1: 51.7%/48.3%; adenocarcinomas: 92.2%, extra-cerebral metastases: 57.8%, without differences between arms.) Significantly more patients in the BR-arm received BR compare with those in the ChT arm (87% versus 20%; p < 0.001); there were no significant differences between BR and ChT arms for median PFS: 4.7, 95% confidence interval (CI):3.4–7.5 versus 4.8, 95% CI: 2.4–6.5 months, for median OS: 8.5, 95% CI:.6–11.1 versus 8.3, 95% CI:4.5–11.5 months, cerebral and extra-cerebral ORR (27% versus 13%, p = 0.064, and 30% versus 41%, p = 0.245, respectively). The ChT arm had more grade 3/4 neutropenia than the BR arm (13% versus 6%, p = 0.045); others toxicities were comparable.
Conclusion:
The significant BR rate difference between the two arms suggests that upfront BR is not mandatory in aNSCLC with aBM but this trial failed to show that deferring BR for aBM is superior in terms of PFS from upfront BR.
Keywords: bevacizumab, cerebral metastasis, management, non-small–cell lung cancer, pemetrexed, radiotherapy
Introduction
Among patients with non-small cell lung cancers (NSCLCs), 10–20% have brain metastases (BMs),1 usually multiple, at diagnosis. Moreover, the incidence of BMs at diagnosis seems to be increasing,2 partly because of the more widespread use of brain magnetic resonance imaging (MRI). Most of these patients are not included in clinical trials that lead to the marketing of new therapeutics, chemotherapies, targeted therapies targeting anti-programmed cell death protein-1 (PD-1) or its ligand-1 (PD-L1), for first- or second-line or more treatments. Furthermore, very few trials have been dedicated exclusively to patients with BMs. The quality of life (QOL) and prognosis for these patients are poorer than for those without BMs at diagnosis.3 The prognosis is associated with age, Eastern Cooperative Oncology Group performance status (ECOG PS), extra-cerebral metastases, number of BMs, and the presence of functional neurological symptoms.4
Management of these patients has not been clearly codified and BM management varies widely in Europe5; it is often complex to avoid functional and cognitive deterioration and to control extra-cerebral disease. Treatment has long relied on whole brain radiation therapy (WBRT), 30 Gy in 10 fractions,6 but the indications are limited by its toxicity and have been even more restricted since the QUARTZ trial.7 WBRT is increasingly being replaced by stereotaxic radiotherapy when the number and size of the cerebral lesions permit it.8 In addition, although BMs have long been considered less sensitive to chemotherapy because of the blood–brain barrier,9 the results of several studies have shown that use of modern molecules, such as pemetrexed and bevacizumab, achieved response and control rates of the cerebral disease close to those obtained for extra-cerebral disease in patients with asymptomatic BMs (aBM).10,11 Results of a phase II trial demonstrated the efficacy of the cisplatin-pemetrexed combination in this setting, with 38% and 43% overall response rate (ORR) and cerebral response rates (CRR), respectively, for non-squamous NSCLCs.11 More recently, BRAIN trial findings showed the efficacy and good tolerance of first- or second-line platin-based chemotherapy combined with bevacizumab for non-squamous NSCLCs with aBMs.12
In this context, for NSCLC patients with aBMs and in good general condition, eligible for platin-based chemotherapy, the role and the timing of brain radiotherapy (BR), WBRT, or stereotaxic radiotherapy, have not yet been established clearly. The objective of this phase III trial was to compare two management modalities for non-squamous NSCLC with aBMs at diagnosis: initial BR followed by cisplatin-pemetrexed chemotherapy combined with bevacizumab for eligible patients (BR arm) or this same protocol of first-line chemotherapy with BR only at cerebral disease progression (ChT arm).
Methods
Study design and participants
This multicenter, open, randomized (1:1) phase III trial stratified patients according to ECOG PS and bevacizumab eligibility. The main inclusion criteria were: histologically proven non-squamous NSCLC, with no or unknown epidermal growth factor receptor (EGFR) mutation or ALK translocation, and aBMs at diagnosis or after treatment with corticosteroids and/or anticonvulsants, at least one measurable cerebral lesion on contrast-enhanced MRI according to Response Evaluation Criteria in Solid Tumours 1.1 (RECIST), ECOG PS <2, adequate bone marrow reserve and organs functions.
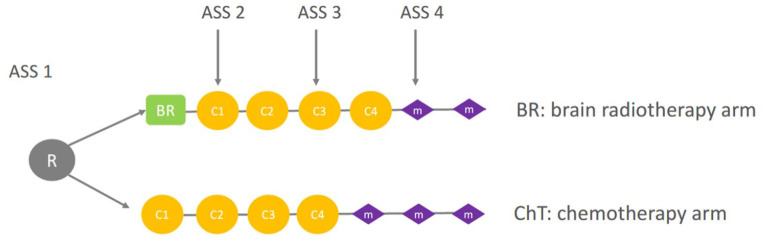
The randomization and treatment plan are shown in Figure 1.
Figure 1.
Randomization and treatment schedule. M, C, ASS (BR) arm: initial BR followed by cisplatin-pemetrexed chemotherapy combined with bevacizumab for eligible patients; ChT arm: cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients with BR only at cerebral disease progression.
ASS, assessment; BR, brain radiotherapy; C, cycle of chemotherapy; ChT, chemotherapy; M, chemotherapy maintenance; R, randomization.
Eligible patients were assigned randomly to receive either first-line BR followed by chemotherapy (BR arm) or first-line of the same chemotherapy and BR only if clinical or radiological cerebral progression (ChT arm).
Chemotherapy consisted of cisplatin [75 mg/m2 intravenously (IV)] and pemetrexed (500 mg/m2 IV) with or without bevacizumab (7.5 mg/kg on day 1 every 3 weeks, starting with the second chemotherapy cycle). In the case of cisplatin contraindication, carboplatin [area under the curve (AUC) 5] could be used. Patients received standard oral dexamethasone (or equivalent) prophylaxis of (4 mg, twice daily) the day before, the day of, and the day after each day-1 treatment. All patients took oral folic acid (400 µg) daily and received a vitamin B12 injection (1000 µg) every 9 weeks, beginning at least 1 week before the first dose and continuing until 3 weeks after the last dose of study treatment.
After four induction cycles in both treatment arms, patients without progression or residual toxicity could receive maintenance chemotherapy with pemetrexed (500 mg/m2 IV) combined, if eligible, with bevacizumab (7.5 mg/kg, IV, every 3 weeks) until progression or toxicity.
Patients requiring one pemetrexed- or cisplatin-dose reduction received the reduced dose for the remainder of the study. For patients needing two dose reductions and who experienced toxicity requiring a third dose reduction, the study drugs were discontinued. Cycle delays of up to 42 days were permitted to recover from adverse events (AEs). Concomitant supportive therapies (e.g., erythropoietic agents or granulocyte colony-stimulating factors) were allowed, in accordance with the American Society of Clinical Oncology guidelines. Second-line therapy was left to the investigator’s discretion.
Radiotherapy, initially WBRT, consisted of lateral opposed fields to the whole brain; patients received a total dose of 30 Gy in 10 daily fractions of 3 Gy over 2 weeks.
Because of changes in practices and recruitment difficulties, a protocol amendment was adopted (19 November 2015) allowing stereotaxic radiotherapy (18–35 Gy in 1–5 fractions as a function of lesion size) to be delivered if the patient had fewer than five BMs.
Radiological assessments were repeated every two cycles, including chest–abdomen computed-tomography (CT) scans and mandatory MRI to assess BMs. Therapeutic responses were evaluated according to RECIST 1.1 criteria. Each patient’s tumor response was reviewed centrally by four medical oncologist blinded to the study arm of the subject.
Endpoints
The principal objective was progression-free survival (PFS). The main secondary outcomes were global, cerebral and extra cerebral ORR, overall survival (OS), the number of patients requiring BR, treatment-related adverse events (CTCAE v4.0) and QOL (EUROQOL 5D and MOCA).
Statistical analyses
This trial was designed as a superiority study, to assess PFS in patients of the ChT (experimental) compared with those of the BR (reference) arm. The initial hypothesis was that median PFS would be 4.5 months for the ChT arm and 3 months in the BR arm, i.e., a hazard ratio (HR) of 0.67. With recruitment lasting 2 years and minimum follow up of 6 months for the last-included patient, a two-sided α of 5%, and β of 0.20, screen failure of 10%, and loss to follow up of 5%, it was calculated that 190 events and 210 patients should be included (to achieve a total of 105 patients in each randomization group).
The one-sided Kaplan–Meier method was used to estimate PFS (from randomization to progression or death) and OS (from randomization to death) for each arm. The efficacy analysis was done on an intention-to-treat basis. Safety analyses included all enrolled patients who received at least one dose of the study drug or one course of radiotherapy. The statistical analyses were computed with SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
This trial was closed early due to slow recruitment. Between 30 December 2013 and 30 December 2017, 24 centers included 95 patients, among whom 91 (4 were not eligible) were randomized: 45 in the BR arm, 46 in the ChT arm.
Patient median age was 60 ± 8.1 years, with majorities of men (79.2%) and smokers or ex-smokers (92.2%); 51.7% had an ECOG PS = 0; and 56% had no weight loss. Kirsten rat-sarcoma viral oncogene (KRAS) mutation was found in 21 (23.1%) patients; 57.8% had extra-cerebral metastasis; 85 (93.4%) of the patients had less than five metastasis and the median number of BMs was 3 ± 2. All these characteristics were comparable for the two arms (Table 1). A total of 38 patients were randomized after the amendment adoption allowing delivery of stereotaxic radiotherapy according to local practices (19 patients in each arm).
Table 1.
Patient characteristics according to arms. BR arm: initial BR followed by cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients. ChT arm: cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients with BR only at cerebral disease progression.
| Characteristic | Total (n = 91) | BR arm (n = 45) | ChT arm (n = 46) |
|---|---|---|---|
| Age (median ± SD) | 60 ± 8.1 | 59 ± 8.9 | 60.5 ± 7.3 |
| Male sex | 70 (76.9) | 34 (73.5) | 36 (78.3) |
| Tobacco use | |||
| Ex-smoker | 44 (48.3) | 22 (47.7) | 22 (47.9) |
| Smoker | 40 (44) | 21 (46.5) | 19 (41.3) |
| Non-smoker | 7 (7.7) | 2 (5.8) | 5 (10.8) |
| Histology | |||
| Adenocarcinoma | 83 (92.2) | 41 (93.2) | 42 (91.3) |
| Undifferentiated carcinoma | 8 (7.8) | 4 (6.8) | 4 (8.7) |
| Extracerebral metastases | 52 (57.8) | 25 (56.8) | 27 (58.7) |
| Brain metastasis <5 | 85 (93.4) | 42 (93.3) | 43 (93.5) |
| ECOG PS | |||
| 0 | 47 (51.7) | 23 (51) | 24 (52) |
| 1 | 44 (48.3) | 22 (49) | 22 (48) |
| Weight loss >5% | 40 (44) | 19 (42.2) | 21 (45.7) |
| KRAS mutation | 21 (23.1) | 12 (26.7%) | 9 (19.6%) |
| Bevacizumab treated | 38 (41.7%) | 19 (42.2) | 19 (41.3) |
| Brain radiotherapy | 40 (87%)a | 10 (20%)a,b | |
| Platin-doublet cycles, median n | 4 | 4 | 4 |
| Maintenance | |||
| Pemetrexed | 19 (42.2) | 28 (60.1) | |
| Bevacizumab + pemetrexed | 7 (15.6) | 16 (36)c | |
| Reason for stopping | |||
| Progression | 37 (84) | 36 (79) | |
| Death | 3 (6) | 4 (8) | |
| Toxicity | 3 (6) | 4 (8) | |
| Others | 2 (4) | 2 (5) | |
Values are expressed as n (%) unless stated otherwise.
Six received stereotaxic radiotherapy.
p < 0.001.
p < 0.03.
BR, brain radiotherapy; ChT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; KRAS, Kirsten rat-sarcoma viral oncogene; SD, standard deviation.
All patients received at least one cycle of the planned platin–pemetrexed chemotherapy, combined for more than a third with bevacizumab (41.7%), with no significant between-arm differences. The two arms were comparable for the median duration of chemotherapy administration. Comparing BR and ChT arms, fewer patients received pemetrexed maintenance therapy (42.2% versus 60.1%, respectively) but the difference was not significant; significantly fewer also received bevacizumab + pemetrexed maintenance therapy (15.6% versus 36%, respectively; p < 0.03). The main reason for stopping chemotherapy was progression (Table 1) with no difference between arms. For the BR and ChT arms, 42.2% and 43.5% of patients received second-line systemic treatment/chemotherapy, predominantly docetaxel monotherapy for 53% and 60% of cases, respectively.
Patients in the ChT arm received significantly less BR compared with those in the BR arm (20% versus 87%, p < 0.001 (in the BR arm, six patients did not receive BR because of early progression, n = 1 or death, n = 5).
Efficacy
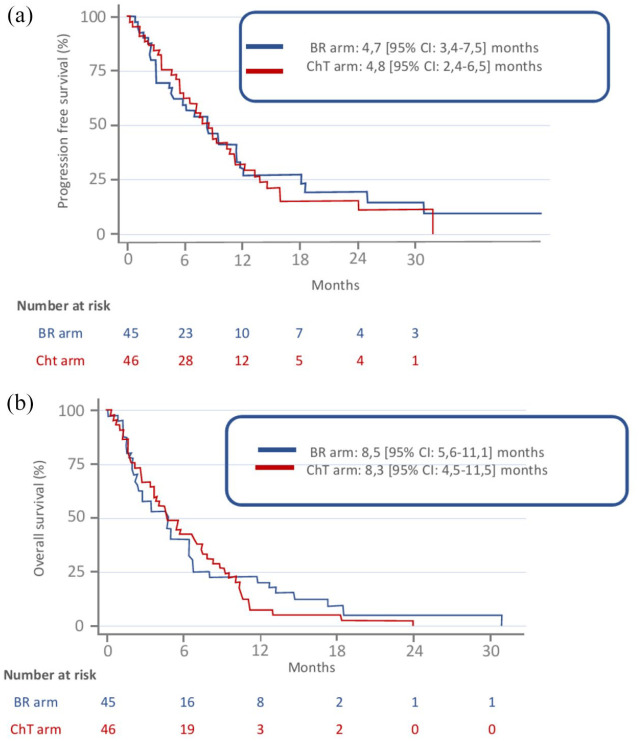
According to intention-to-treat analysis, at the time of data cut-off (28 May 2018), after a median follow up of 28 (95% CI: 12.4–34.2) months, no PFS (primary end point) difference was observed between BR and ChT arms, respectively: 4.7, 95% CI: 3.4–7.5 and 4.8, 95% CI: 2.4–6.5 months (Figure 2a). Also, their respective OS durations did not differ significantly (8.5, 95% CI: 5.6–11.1 and 8.3, 95% CI: 4.5–11.5 months) (Figure 2b), and there was no significant difference for cerebral, extracerebral, and global ORRs between the two arms (Table 2).
Figure 2.
Probability of PFS (a) or OS (b), according to treatment arms. BR arm: initial BR followed by cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients. ChT arm: cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients with BR only at cerebral disease progression.
BR, brain radiotherapy; ChT, chemotherapy; OS, overall survival; PFS, progression-free survival.
Table 2.
Patients’ responses to first-line according to arms. BR arm: initial BR followed by cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients. ChT arm: cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients with BR only at cerebral disease progression.
| Response | Total (n = 91) | BR arm (n = 45) | ChT arm (n = 46) |
|---|---|---|---|
| Cerebral | |||
| Stable | 37 (41) | 13 (30) | 24 (52) |
| Partial | 18 (20) | 12 (27) | 6 (13) |
| Progression | 35 (39) | 19 (43) | 16 (35) |
| Extracerebral | |||
| Stable | 32 (36) | 13 (30) | 19 (41) |
| Partial | 19 (21) | 8 (18) | 11 (24) |
| Progression | 39 (43) | 23 (52) | 16 (35) |
| Global | |||
| Stable | 28 (31) | 12 (27) | 16 (35) |
| Partial | 21 (23) | 9 (21) | 12 (26) |
| Progression | 41 (46) | 23 (52) | 18 (39) |
BR, brain radiotherapy; ChT, chemotherapy.
Safety
Overall, 21/45 (46.7%) and 28/46 (62.2%) (p = 0.09) patients in the BR and ChT arms, respectively, developed a grade 3/4 AE or serious AE (Table 3). No treatment-attributable death occurred. No QOL differences were observed, but the small number of patients included and the high numbers of missing data (more than half of the patients filled out less than three QOL questionnaires) precluded drawing any definitive conclusions.
Table 3.
AEs according to according to arms. BR arm: initial BR followed by cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients. ChT arm: cisplatin-pemetrexed ChT combined with bevacizumab for eligible patients with BR only at cerebral disease progression.
| BR arm n = 45 (%) | ChT arm n = 46 (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Gr 1 | Gr 2 | Gr 3 | Gr 4 | Gr 1 | Gr 2 | Gr 3 | Gr 4 | |
| Anemia | 5 (22.) | 13 (29) | 3 (7) | − | 7 (15) | 8 (17) | 5 (11) | 1 (2) |
| Anorexia | − | 2 (4) | 2 (4) | − | 5 (11) | 1 (2) | − | − |
| Neutropenia | 3 (7) | 11 (24) | 2 (4) | 4 (9) | 3 (7) | 6 (13) | 10 (22) | 3 (6) |
| Asthenia | 9 (20) | 17 (38) | 4 (9) | − | 19 (41) | 8 (17) | 3 (7) | 1 (2) |
| Headache | 8 (178) | 3 (7) | − | − | 4 (9) | 2 (4) | − | − |
| Renal failure | 5 (22.) | 4 (9) | − | 1 (2) | 3 (7) | 4 (8) | 1 (2) | − |
| Diarrhea | 6 (13.) | 2 (4) | − | − | 1 (2) | 1 (2) | 1 (2) | − |
| Dysgeusia | 3 (7) | − | − | − | 3 (7) | − | − | − |
| Epistaxis | 3 (7) | 3 (7) | − | − | 2 (4) | 1 (2) | − | − |
| Arterial hypertension | − | 2 (4) | 1 (2) | − | 1 (2) | 1 (2) | − | − |
| Mucositis | 2 (4.) | − | − | 1 (2) | − | 2 (4) | − | |
| Nausea | 12 (27) | 5 (22) | 1 (2) | − | 14 (29) | 7 (15) | − | − |
| Vomiting | 5 (22) | 3 (7) | 2 (4) | 1 (2) | 3 (7) | 5 (10) | 2 (4) | − |
AE, adverse event; BR, brain radiotherapy; ChT, chemotherapy.
Discussion
Early discontinuation of the trial because of insufficient inclusions precluded drawing conclusions to the relevance to delay BR in aNSCLC patients with aBM at diagnosis. Analysis of the 91 randomized patients did not show a significant difference for the primary judgement criterion – PFS – or the secondary end points, especially OS. In contrast, only 20% of ChT arm patients underwent BR, which favors the current concept according to which BR can be delayed or withheld for neurologically asymptomatic NSCLC patients.13 Lung cancer BMs have long been considered a final event and were treated with either WBRT or palliative care. The most commonly used WBRT schedules, i.e., classical 30-Gy dose in 10 fractions or a short 20-Gy course of five fractions, obtained overlapping efficacy.14
Two randomized clinical trials compared best supportive care (BSC) and steroids, with or without WBRT. The former dates back to the 1970s and demonstrated a slight advantage of radiotherapy for remission duration and OS based on a heterogeneous population of 48 patients, 60% of whom had lung cancer, but the percentage with small-cell histology was not specified.15 The QUARTZ trial included a larger population of NSCLC patients, and was published in 2016 when we started recruiting for our trial. The QUARTZ study showed the non-inferiority of BSC alone arm in terms of quality-adjusted life years (QALYs).7 But, in that study, most subjects had an ECOG PS of 3 or worse, which might explain their findings.
WBRT exposes the patient to acute and late toxicity, which can impact QOL and negatively affect later systemic treatments.16,17 Stereotaxic radiotherapy without WBRT is an evolving paradigm for the management of patients with limited intracranial disease (1–4 metastases).18
However, since the arrival of new systemic and targeted therapies, the need for early WBRT for NSCLC patients with asymptomatic BMs has been controversial. Few studies have analyzed the relevance of a systematic BR in this context.10,19,20 One study randomized 171 NSCLC patients with no surgical BMs to receive cisplatin–vinorelbine chemotherapy with either WBRT at cerebral progression or systematic.10 The respective between-arm ORRs did not differ significantly (21% versus 20%), nor did the cerebral response rate (27% versus 33%) or OS (6 versus 5.3 months). OS for both arms was shorter than that obtained for the two arms in the present study, which can be explained by the inclusion of patients with PS 2, that brain MRI was not mandatory, and the chemotherapy administered was less effective against BMs.
Another trial on 48 randomized NSCLC patients with aBMs treated with first-line gemcitabine–vinorelbine chemotherapy with either WBRT at cerebral progression or systematic did not find any difference in between-arm ORR (28.0% versus 39.1%) or OS (9.1 versus 9.9 months).19
More recently, 106 Asians with NSCLCs with asymptomatic oligoBMs were randomized to receive stereotaxic brain radiosurgery plus chemotherapy or chemotherapy alone.20 Several chemotherapy doublets were given: cisplatin–gemcitabine or pemetrexed or docetaxel or paclitaxel, with carboplatin replacing cisplatin in the case of contraindications. Again, no between-arm differences were noted for OS (14.6 and 15.3 months); the prolonged OS in this latter study were attributed to the 30% of the patients having EGFR-mutated tumors with a high percentage previously treated by an EGFR-tyrosine kinase inhibitor (TKI). Two retrospective studies also suggested that systematic BR or BR at cerebral progression have equivalent efficacy.21,22 For patients with EGFR-mutated NSCLC and asymptomatic BMs, a recent retrospective study also indicated that the outcomes of administering first-line EGFR-TKI or brain radiotherapy followed by EGFR-TKI were comparable.23 For patients managed with immunotherapy or immunochemotherapy, the results of a phase II study showed that, for patients whose tumor cells expressed >1% PD-L1, pembrolizumab achieved a 29.7% cerebral response rate.24,25
One of the difficulties encountered in our trial was the rapid evolution of treatments, with stereotaxic radiotherapy indications becoming broader and increasingly accepted for cerebral efficacy of systemic treatments.18 The results of a number of prospective trials on NSCLC patients demonstrated the activity of first-line cisplatin-based chemotherapy combinations for NSCLC with aBMs, with cerebral response rates of 23–50%. The efficacy of doublets containing pemetrexed was confirmed in several studies.11,26–28 In a phase II study including 43 chemotherapy-naïve NSCLC patients with aBMs ineligible for (radio)surgery, ECOG PS 0–2, eligible to cisplatin and pemetrexed doublets, intent-to-treat analysis yielded cerebral, extracerebral, and ORRs of 41.9%, 34.9%, and 34.9%, respectively. Median OS and PFS lasted 7.4 and 4.0 months, respectively, with an acceptable safety profile.11 In addition, for eligible patients, bevacizumab was also shown to be of interest for this indication. Suspicions about safety of antiangiogenic therapy in BM have been resolved, with the results of one prospective trial in which bevacizumab did not add any risk of cerebral bleeding in patients with untreated BM.29 Moreover, the pooled analysis of 131 patients with treated BMs given bevacizumab in either the PASSPORT or ATLAS trial showed no symptomatic grade >2 brain hemorrhage during the main treatment phases of studies.30 The most suggestive observation came from the BRAIN study, in which treatment-naïve patients given bevacizumab + chemotherapy achieved a considerable 62.7% ORR12; intra-cerebral and extra-cerebral ORR in that non-comparative phase II trial were quite similar. In our study, almost half of the patients were eligible and received bevacizumab, with no between-arm differences. In the BR arm, almost a third of the patients were also eligible for bevacizumab maintenance therapy, twice the number in the ChT group.
To conclude, even though the trial had to be stopped prematurely for lack of recruitment, the results suggest that systematic BR is not mandatory in aNSCLC patients with aBMs, but this trial failed to show that deferring BR for aBM is superior in term of PFS from upfront BR. Prospective studies are now needed to determine the best timing for stereotaxic brain radiotherapy, when this is possible.
Acknowledgments
We thank the Department of Clinical Research at the Centre Hospitaller de Creteil (Jung Camille) for promoting this clinical trial.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributors: IM, AV, GR, and CC wrote the protocol, participated in the implementation of the study, were responsible for data collection, and wrote the manuscript. LG, MG, HL, and HB participated in the search for funding and contributed to the writing of the protocol. AV, J-BA, and RG participated in data management and pharmacovigilance. HJ, JC, RL, JL, HLC, ED, AM, P-AR, GLG, LF, and RS contributed to the writing of successive versions of the manuscript. PS performed the statistical analysis. All authors approved the final version of the manuscript. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Data availability: An English synopsis of the study protocol is available in the appendix. Individual participant data that underlie the reported results will not be made available.
Ethical statement: The study was conducted in accordance with the Declaration of Helsinki and was approved by a local independent Ethics Committee (“Comité de Protection des Personnes Ouest-2” n°13908). Written informed consent was obtained from each patient. This trial is registered [ClinicalTrials.gov identifier: NCT02162537].
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by an academic grant from Roche and Lilly. The study funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
ORCID iDs: Jean Bernard. Auliac  https://orcid.org/0000-0002-6400-7506
https://orcid.org/0000-0002-6400-7506
Christos Chouaïd  https://orcid.org/0000-0002-4290-5524
https://orcid.org/0000-0002-4290-5524
Contributor Information
Isabelle Monnet, Service de Pneumologie, CHI Créteil, Créteil, France.
Alain Vergnenègre, Service de Pneumologie, CHU Limoges, Limoges, France.
Gilles Robinet, Service de Pneumologie, CHU Brest, Brest, France.
Henri Berard, Service de Pneumologie, Hôpital d’instruction des armées Sainte-Anne, Toulon, France.
Regine Lamy, Service de Pneumologie, CH Bretagne Sud, Lorient, France.
Lionel Falchero, Service de Pneumologie, Centre Hospitalier de Villefranche de Rouergue, Villefranche, France.
Sabine Vieillot, Service d’oncologie, Centre Saint Pierre, Perpignan, France.
Roland Schott, Service d’Oncologie, Centre Paul Strauss, Strasbourg, France.
Charles Ricordel, Service de Pneumologie, CHU Rennes, Rennes, France.
Stephane Chouabe, Service de Pneumologie, CH Charleville Mézière, Charleville Mézière, France.
Pascal Thomas, Service de Pneumologie, CH de gap, Gap, France.
Radj Gervais, Service d’Oncologie, Centre François Baclesse, Caen, France.
Anne Madroszyk, Service d’Oncologie, Institut Paoli-Calmettes, Marseille, France.
Samir Abdiche, Service d’Oncologie, CH Libourne, Libourne.
Anne Marie Chiappa, Service de Pneumologie, CH de Quimper, Quimper, France.
Laurent Greillier, Department of Multidisciplinary Oncology and Therapeutic Innovations, APHM, Hôpital Nord, Marseille, France.
Chantal Decroisette, Service de Pneumologie, CH d’Annecy, Annecy, France.
Jean Bernard. Auliac, Service de Pneumologie, CHI Créteil, Créteil, France Service de Pneumologie, CH de Mantes la Jolie, Mantes la Jolie, France.
Christos Chouaïd, Service de Pneumologie, CHI Créteil, 40 avenue de Verdun, Créteil, 94010, France; Institut Mondor de Recherche Biomédicale, U955 Inserm—Université Paris Est Créteil, Créteil, France.
References
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004; 22: 2865–2872. [DOI] [PubMed] [Google Scholar]
- 2. Davis FG, Dolecek TA, McCarthy BJ, et al. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol 2012; 14: 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016; 45: 139–162. [DOI] [PubMed] [Google Scholar]
- 4. Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (lung-molGPA). JAMA Oncol 2017; 3: 827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy A, Faivre-Finn C, Hasan B, et al. Diversity of brain metastases screening and management in non-small cell lung cancer in Europe: results of the European Organisation for research and treatment of Cancer Lung Cancer Group survey. Eur J Cancer 2018; 93: 37–46. [DOI] [PubMed] [Google Scholar]
- 6. Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 2012; 1: CD003869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016; 388: 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015; 91: 710–717. [DOI] [PubMed] [Google Scholar]
- 9. Fortin D. The blood–brain barrier: its influence in the treatment of brain metastases. Curr Cancer Drug Targets 2012; 12: 247–259. [DOI] [PubMed] [Google Scholar]
- 10. Robinet G, Thomas P, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Français de Pneumo Cancérologie (GFPC) Protocol 95-1. Ann Oncol 2001; 12: 59–67. [DOI] [PubMed] [Google Scholar]
- 11. Barlesi F, Gervais R, Léna H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases (BM): a multicenter phase II trial (GFPC 07-01). Ann Oncol 2011; 22: 2466–2470. [DOI] [PubMed] [Google Scholar]
- 12. Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with non-squamous non-small-cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a non-randomised, phase II study. Clin Cancer Res 2015; 21: 1896–1903. [DOI] [PubMed] [Google Scholar]
- 13. Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev 2014; 40: 716–722. [DOI] [PubMed] [Google Scholar]
- 14. Rades D, Schild SE, Lohynska R, et al. Two radiation regimens and prognostic factors for brain metastases in nonsmall cell lung cancer patients. Cancer 2007; 110: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 15. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med 1971; 111: 334–336. [DOI] [PubMed] [Google Scholar]
- 16. Greene-Schloesser D, Robbins ME, Peiffer AM, et al. Radiation-induced brain injury: a review. Front Oncol 2012; 2: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009; 10: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 18. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 19. Lee DH, Han J-Y, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: result of a randomized pilot study. Cancer 2008; 113: 143–149. [DOI] [PubMed] [Google Scholar]
- 20. Lim SH, Lee JY, Lee MY, et al. A randomized phase III trial of stereotactic radiosurgery (SRS) versus observation for patients with asymptomatic cerebral oligo-metastases in non-small-cell lung cancer. Ann Oncol 2015; 26: 762–768. [DOI] [PubMed] [Google Scholar]
- 21. Moscetti L, Nelli F, Felici A, et al. Up-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: survey by Outcome Research Network for evaluation of treatment results in Oncology. Cancer 2007; 109: 274–281. [DOI] [PubMed] [Google Scholar]
- 22. Kim KH, Lee J, Lee JI, et al. Can upfront systemic chemotherapy replace stereotactic radiosurgery or whole brain radiotherapy in the treatment of non-small cell lung cancer patients with asymptomatic brain metastases? Lung Cancer 2010; 68: 258–263. [DOI] [PubMed] [Google Scholar]
- 23. Hyun D, Choi C, Lee D. Outcomes according to initial and subsequent therapies following intracranial progression in patients with EGFR-mutant lung cancer and brain metastasis. PLoS One 2020; 15: e0231546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020; 21: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 26. Bailon O, Chouahnia K, Augier A, et al. Upfront association of carboplatin plus pemetrexed in patients with brain metastases of lung adenocarcinoma. Neuro Oncol 2012; 14: 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bearz A, Garassino I, Tiseo M, et al. Activity of pemetrexed on brain metastases from non-small cell lung cancer. Lung Cancer 2010; 68: 264–268. [DOI] [PubMed] [Google Scholar]
- 28. Dinglin X-X, Huang Y, Liu H, et al. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol 2013; 112: 461–466. [DOI] [PubMed] [Google Scholar]
- 29. Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol 2009; 27: 5255–5261. [DOI] [PubMed] [Google Scholar]
- 30. Besse B, Lasserre SF, Compton P, et al. Bevacizumab safety in patients with central nervous system metastases. Clin Cancer Res 2010; 16: 269–278. [DOI] [PubMed] [Google Scholar]