Abstract
Patients with cholangiocarcinoma have poor survival since the majority of patients are diagnosed at a stage precluding surgical resection, due to locally irresectable tumors and/or metastases. Optimization of diagnostic strategies, with a principal role for tissue diagnosis, is essential to detect cancers at an earlier stage amenable to curative treatment. Current barriers for a tissue diagnosis include both insufficient tissue sampling and a difficult cyto- or histopathological assessment. During endoscopic retrograde cholangiopancreatography, optimal brush sampling includes obtaining more than one brush within an individual patient to increase its diagnostic value. Currently, no significant increase of the diagnostic accuracy for the new cytology brush devices aiming to enhance the cellularity of brushings versus standard biliary brush devices has been demonstrated. Peroral cholangioscopy with bile duct biopsies appears to be a valuable tool in the diagnostic work-up of indeterminate biliary strictures, and may overcome current technical difficulties of fluoroscopic-guided biopsies. Over the past years, molecular techniques to detect chromosomal instability, mutations and methylation profiling of tumors have revolutionized, and implementation of these techniques on biliary tissue during diagnostic work-up of biliary strictures may be awaited in the near future. Fluorescence in situ hybridization has already been implemented in routine diagnostic evaluation of biliary strictures in several centers. Next-generation sequencing is promising for standard diagnostic care in biliary strictures, and recent studies have shown adequate detection of prevalent genomic alterations in KRAS, TP53, CDKN2A, SMAD4, PIK3CA, and GNAS on biliary brush material. Detection of DNA methylation of tumor suppressor genes and microRNAs may evolve over the coming years to a valuable diagnostic tool for cholangiocarcinoma. This review summarizes optimal strategies for biliary tissue sampling during endoscopic retrograde cholangiopancreatography and focuses on the evolving molecular techniques on biliary tissue to improve the differentiation of benign and malignant biliary strictures.
Keywords: chromosomal imbalance, epigenetics, immunohistochemistry, mutation, next-generation sequencing
Introduction
Biliary strictures are an important clinical condition that may lead to severe symptoms and complications, including jaundice, cholangitis, liver abscesses, and secondary biliary cirrhosis. Transpapillary biliary drainage with stent placement or balloon dilation through endoscopic retrograde cholangiopancreatography (ERCP) is a well-established treatment for these patients and allows for symptom relief of jaundice, pruritus, and pain, and improvement of liver functions.1–4
A biliary stricture may be caused by either benign or malignant etiology. Malignant strictures account for approximately 70% of cases, and most often concern pancreatic cancer, followed by cholangiocarcinoma (CCA), and at a lesser frequency other malignancies (such as gallbladder carcinoma, ampullary carcinoma, metastatic lesions).5 The determination of the etiology of a stricture often requires the combination of several diagnostic modalities, including clinical evaluation, laboratory findings, and endoscopic and radiological imaging.6,7 In addition, tissue sampling for cyto- or histopathological assessment is of paramount importance.8 The level of serum tumor markers, in particular CA-19.9 and CEA, is often evaluated in clinical practice during evaluation of biliary strictures; however, it should be noted that elevated levels of these markers are compatible with both benign and malignant causes of biliary obstruction. Therefore, before ordering tumor markers, consideration of the consequence of elevated levels is warranted, and interpretation of the test results requires combination with other diagnostic modalities, including cross-sectional imaging.9
Although the majority of biliary strictures prove malignant after diagnostic work-up or follow-up, a number of benign conditions, such as primary sclerosing cholangitis (PSC), chronic pancreatitis, and IgG4-related sclerosing cholangitis, frequently mimic malignancy, and differentiation can be challenging.10–14
Despite the combination of the above-mentioned diagnostics, current strategies for the assessment of biliary strictures have a very low diagnostic accuracy. As a result, biliary strictures remain indeterminate in up to 20% of cases.15 Since consequences are devastating when a malignant cause of a biliary stricture is missed or diagnosed at a later stage precluding surgery, improved diagnostic methods with adequate sensitivity are required. In addition to a high sensitivity, these diagnostic methods should also demonstrate a high specificity to avoid unnecessary large surgical procedures (such as pancreaticoduodenectomy). The importance of a high specificity is underlined by the observation that in approximately 15–25% of patients undergoing surgical resection for biliary strictures with a high suspicion of malignancy, pathological examination of the resected specimens reveals benign disease.16,17
A tissue diagnosis remains key for an adequate differentiation of the etiology of biliary strictures. Current barriers for a tissue diagnosis include both insufficient tissue sampling and a difficult cyto- or histopathological assessment mostly in an inflammatory background. Therefore, optimization of diagnostic strategies requires both improved tissue sampling during ERCP in combination with development and implementation of novel molecular diagnostic tools on biliary tissue.
In this review, we summarize current knowledge regarding optimal biliary tissue sampling of biliary strictures during ERCP, and elaborate on promising molecular techniques to differentiate between benign and malignant strictures. It should be noted that these diagnostic strategies will be included in a complex diagnostic algorithm in clinical practice.
Optimizing tissue sampling techniques during ERCP
Cytology
Brush cytology is the most commonly used tissue sampling technique during ERCP. Since the majority of CCAs develop distal (40%) or in the perihilar (50%) region, most strictures are amenable to this technique.18 After transpapillary biliary cannulation, a brush catheter is inserted over a guidewire into the biliary tree, and is advanced to the stricture. After advancing the brush from the catheter, the brush is moved back and forth along the stricture, resulting in attachment of epithelial cells to the brush. Thereafter, the brush is retracted in the catheter and withdrawn from the endoscope. The brush is cut off the catheter and can be smeared onto glass slides or can be stored in CytoLyt fixative solution.19
Due to low diagnostic yield of brush cytology (which is described in detail in the following paragraph on ‘Cytology’), multiple studies have investigated modified techniques and new cytology brush designs to increase its diagnostic yield. These techniques aim at enhancement of exfoliation of epithelial cells and subsequently making material more representative by increasing cellularity. Repeat brushing (>1 brush per patient) increases cancer detection,20 whereas obtaining brush cytology after stricture dilation by a balloon or passage dilating catheter does not result in higher cancer detection rates.20,21 The newest brushes are larger and/or have different types of bristles oriented at an angle greater than the conventional 45 degree angle. However, these more expensive Infinity® brushes (Infinity sampling device, US Endoscopy, Mentor, OH, USA) and the Cytolong® brush (Cook Endoscopy, Winston-Salem, NC, USA) demonstrated an equal diagnostic performance as compared with the conventional brush, and have therefore not widely replaced conventional brushes in clinical practice.22–24 The use of other devices to disrupt the biliary epithelium, such as a scraping brush and stent retriever have been studied in the past years, but study results were inconclusive due to small series. 25–27
In several patients it is a challenge to obtain cyto- or histological specimens with brushing or biopsies due to technical difficulties. Intraductal bile aspiration cytology is an easy and established method and a suitable alternative diagnostic strategy to investigate the morphology of biliary epithelial cells when other methods, in particular brush cytology, fail.28 Biliary aspiration can be performed during either ERCP, via an endoscopic nasobiliary catheter, or a percutaneous transhepatic biliary catheter.29 In general, 10–50 ml bile is aspirated and centrifuged to separate the cells for preparation of a smear, whereafter the slide is stained by standard Papanicolaou technique for cellular examination. These cells are well preserved and can be observed singly, in monolayer, or folded sheets.30 The most important drawbacks of this technique is the low abundance of epithelial cells in bile and the lack of knowledge about the ideal bile volume and the ideal sampling site.31
Finally, another technique that has been described in literature to obtain cytological specimens is by fine needle aspiration (FNA) of the biliary lesion during ERCP. This procedure is performed with a biliary catheter with a retractable needle that can be placed into the target lesion under fluoroscopic guidance.32 The needle is moved back and forth into the lesion, after which the aspirations are processed into smears or are stored in CytoLyt.33 This technique is not commonly applied in routine practice, since its additional value to brush cytology has not been proven.
Histology
Brushes are often combined with fluoroscopy-guided biliary biopsies to increase diagnostic accuracy for malignant biliary strictures. Obtaining ductal biopsies can, however, be technically challenging. Under fluoroscopic guidance, the biopsy forceps are advanced to the level of the stricture, and opened and closed to grasp a specimen from the stricture. It usually provides a sample of bile duct lining epithelium sometimes together with underlying stroma. In patients with an indeterminate biliary stricture it has been shown to improve diagnostic performance as compared with brush cytology alone.34 Endoscopic retrograde transpapillary forceps biopsy demonstrates sensitivities ranging from 40% to 60% and specificities from 97% to 100%.35–40 Because of a limited sampling area for biliary biopsies, obtaining multiple biopsies or complementary biliary brushing is recommended. When biopsy is combined with brush cytology, sensitivity increases from 47% to 86%, while specificity remains close to 100%.41 Furthermore, the combination of bile aspiration cytology, brush cytology, and forceps biopsy results in a sensitivity of 85% and a specificity of 100%.42
Over recent years, many new tools have been developed to optimize the success rate of adequate tissue sampling in the bile duct. One of those methods is the wire-guided biopsy forceps, which can be advanced over a wire through the common bile duct. This technique may overcome the difficulties encountered by freehand insertion of the forceps across the major papilla. In the direct method, the forceps are directly inserted into the bile duct alongside the guidewire. Both direct and wire-grasping methods for obtaining transpapillary forceps biopsies have been explored, resulting in a significantly higher success rate of obtaining adequate specimens using the wire-grasping method (100% versus 50%).43
Cholangioscopy targeted biopsies
Peroral cholangioscopy (POC) allows targeted biopsies by direct endoscopic visualization of the biliary stricture, resulting in a superior diagnostic performance as compared with ERCP-guided cytology brushing and forceps biopsies.44,45 In POC, a slim endoscope is introduced through a working channel of a duodenoscope. The cholangioscope is then introduced into the bile duct, either freehand or over a guidewire. This technique enables direct intraductal visualization of strictures, targeted biopsy sampling of suspected areas and in case of stones, intraductal lithotripsy. POC is considered to be a well-tolerated technique with relatively few complications. The nature and frequency of complications are comparable with those reported for conventional ERCP, including pancreatitis, perforation and bleeding, with a possible exception for the occurrence of cholangitis.46 Retrospective studies have found higher rates of cholangitis among patients undergoing POC as compared with conventional ERCP, although prospective data are lacking.47 In clinical practice, the use of antibiotic prophylaxis could be considered before POC to prevent cholangitis. The success rate with peroral cholangioscopy to achieve a visual diagnosis and a histological diagnosis is reported to be high (up to 90% and 80%, respectively).48–51 The addition of peroral cholangioscopy to biliary brushes or forceps biopsies resulted in a sensitivity of 100% and a specificity of 87%.52 In up to 15% of cases the cholangioscope is not able to pass through the stricture, with PSC as an important risk factor for failed cannulation.44,53 The disposable through the scope Spyglass® single-operator cholangioscopy is a popular tool to obtain visual assessment and (fluoroscopic-guided) targeted biopsies of biliary lesions with a reported sensitivity as well as specificity of 83% for visual diagnosis, and a sensitivity and specificity of 66–86% and 94–100% for representative biopsies.54–59 Further improvement of sensitivity is reached when increasing the number of biopsies for histological assessment.60 However, a critical look at the studies is needed. Many studies have included a small series of samples, a high risk of selection bias exists, and histological assessment is not blinded for clinical characteristics. Furthermore, in the above-mentioned studies, only representative samples were included, meaning that the real sensitivity is lower than the reported sensitivity. Nevertheless, peroral cholangioscopy with bile duct biopsies appears to be a valuable tool in the diagnostic work-up of indeterminate biliary strictures. Although at present the use of cholangioscopy with biopsies is often limited to expert centers, more widespread use can be awaited during coming years.
Confocal laser endomicroscopy
A novel technique for the distinction of benign and malignant biliary strictures is probe-based confocal laser endomicroscopy (pCLE), which provides in vivo evaluation of gastrointestinal mucosal histology.61 A laser illuminates the tissue with a wavelength of 488 nm.62 The light is locally absorbed by fluorophores and the reflected fluorescence is detected by the probe, resulting in multiple two-dimensional images, which can reconstruct a true microscopic three-dimensional structure in real time.63 This technique has shown an excellent sensitivity of 89–98% to detect malignant strictures, but the diagnostic value is hampered by a disappointing interobserver agreement and a low specificity of 67%, mostly due to false positive results in cases with a benign inflammatory stricture.64–66 The Miami and Paris classifications have been developed to improve diagnostic accuracy of the confocal laser endomicroscopy for benign strictures caused by inflammation.67,68 Up to writing, only a small number of studies in a low number of expertise centers have investigated the diagnostic performance of pCLE. Therefore, prior to implementation of this technique in clinical practice, prospective studies need to be awaited to establish the additional diagnostic value of pCLE to existing diagnostic strategies.
Optimizing tissue analysis and new molecular techniques
Cytology
Several processing options exist for biliary brush samples to optimize the assessment of cytology, including smears on glasses, generally stained with May Grünwald–Giemsa (MGG) stain, and fixation in CytoLyt. After fixation in CytoLyt, cellular material can be further processed into sections by the use of ThinPrep® or Cellient™, which are suitable for cytological assessment (Supplementary Figure 1).69–71
Brush cytology is scored by morphological examination and can be classified into the following categories according to the Papanicolaou classification: nondiagnostic, negative for malignancy, atypical, neoplastic (benign or other), suspicious for malignancy, or malignant (Figure 1).72,73 Specimens scored as nondiagnostic cannot be interpreted due to technical or sample issues, including absence of epithelial cells.74 In malignant smears, cells have among others, enlarged nuclei and an irregular nuclear membrane.
Figure 1.
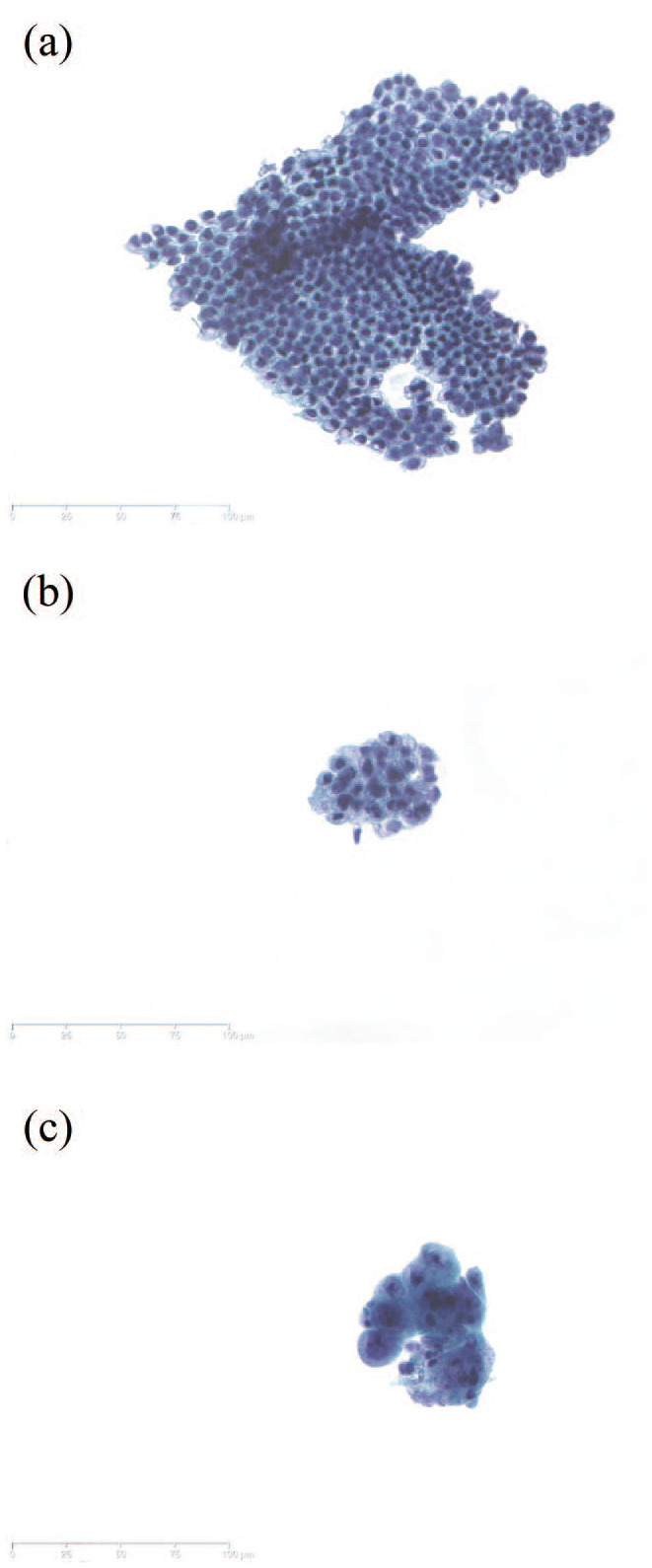
Examples of routine cytology of biliary brush samples. (a) Normal biliary epithelial cells. (b) Atypia; reactive changes with variation in nuclear size, often observed in PSC. (c) Adenocarcinoma, showing variation in nuclear architecture and size. Papanicolaou staining. Original magnification, ×25.
PSC, primary sclerosing cholangitis.
The diagnostic accuracy of brush cytology has been investigated in several studies. Table 1 provides an overview of the accuracy to detect CCA of biliary brush cytology in available studies, including reports on PSC patients.8,26,35,36,40,73,75–128 Specificity has been shown to be close to 100% in all these studies. However, sensitivity rates of brush cytology are rather disappointing, reported within a considerable range from 15% to 70%.104,107,129,130 Main reasons for the high rate of false-negative results are sampling errors and low tissue yield due to technical challenges, including difficult anatomic sites and poor visualization.106 Moreover, the pathological assessment is difficult as illustrated by reports on a considerable interobserver disagreement.102,131 Especially in PSC the distinction between benign and malignant cytology on brush is notoriously difficult due to altered cellular morphology caused by inflammation. Predictors of a positive yield of brush cytology include advanced age, mass size >1 cm, stricture dilation before sampling, stricture length of >1 cm, and increased serum levels of CA-19.9, alanine aminotransferase (ALT), and total bilirubin.114,132
Table 1.
Review of literature. Sensitivity and specificity of brush cytology and FISH analysis in sporadic and PSC-associated CCA.
| Author | No. of brush samples | Brush cytology |
FISH |
|||
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | Analysis | ||
| Bang et al.26 | 114 | 75 | 100 | |||
| Bardales et al.84 | 38 | 75 | 93 | |||
| Baron et al.85 | 110 | 18 | 98 | |||
| Binek et al.86 | 89 | 67 | 92 | |||
| Chaiteerakij et al.87 | 99 | 38 | 100 | 55 | 100 | |
| De Peralta-Venturina et al.88 | 74 | 89 | 96 | |||
| Desa et al.89 | 24 | 40 | NR | |||
| Eiholm et al.93 | 75 | 66 | 100 | |||
| Elek et al.90 | 103 | 53 | 100 | |||
| Farrell et al.94 | 46 | 57 | 80 | |||
| Ferrari et al.95 | 74 | 56 | 100 | |||
| Fritcher et al.91 | 284 | 15 | 100 | 44 | 81 | Polysomy or trisomy 7 |
| Fritcher et al.97 | 498 | 20 | 99 | 43 | 99 | |
| Fritcher et al.92 | 272 | 19 | 100 | 65 | 93 | Probes 1q21, 7p12, 8q24, and 9p21 |
| Foutch et al.96 | 7 | 57 | 100 | |||
| Glasbrenner et al.98 | 86 | 56 | 91 | |||
| Gonda et al.99 | 100 | 32 | 100 | 51 | 100 | FISH + cytology |
| Govil et al.100 | 278 | 68 | 100 | |||
| Hamada et al.101 | 60 | 57 | 100 | |||
| Harewood et al.102 | 113 | 18 | 100 | |||
| Howell et al.103 | 28 | 58 | 100 | |||
| Kipp et al.104 | 131 | 15 | 98 | 34 | 91 | |
| Kitajima et al.105 | 51 | 73 | 100 | |||
| Kocjan and Smith106 | 267 | 44 | 100 | |||
| Kurzawinski et al.107 | 100 | 69 | 100 | |||
| Kushnir et al.108 | 101 | 26 | 98 | 39 | 100 | HGD samples included |
| Layfield et al.109 | 108 | 44 | 98 | |||
| Lee et al.110 | 149 | 37 | 100 | |||
| Liew et al.111 | 30 | 54 | 82 | 31 | 100 | |
| Logrono et al.112 | 183 | 48 | 98 | |||
| Macken et al.113 | 123 | 63 | 96 | |||
| Mahmoudi et al.114 | 199 | 61 | 98 | |||
| Mehmood et al.73 | 142 | 65 | 100 | |||
| Mohammad Alizadeh et al.115 | 105 | 33 | 100 | |||
| Ponchon et al.35 | 210 | 35 | 97 | |||
| Pugliese et al.116 | 22 | 73 | 100 | |||
| Pugliese et al.36 | 42 | 53 | 100 | |||
| Rabinovitz et al.117 | 65 | 40 | 100 | |||
| Rösch et al.118 | 50 | 46 | 100 | |||
| Ryan119 | 69 | 44 | 100 | |||
| Salomao et al.120 | 73 | 40 | 94 | 64 | 94 | |
| Schoefl et al.121 | 65 | 47 | 100 | |||
| Scudera et al.122 | 25 | 50 | 100 | |||
| Singhi et al.123 | 132 | 35 | 98 | |||
| Smoczynski et al.128 | 81 | 35 | 100 | 52 | 89 | |
| Sugiyama et al.124 | 43 | 48 | 100 | |||
| Weber et al.40 | 58 | 41 | 100 | |||
| Zhai125 | 22 | 14 | 100 | 55 | 62 | |
| PSC-associated CCA | ||||||
| Bangarulingam et al.82 | 235 | 72 | 57 | Polysomy or trisomy 3/7 | ||
| Boberg et al.75 | 61 | 73 | 95 | |||
| Charatcharoenwitthaya et al.126 | 230 | 46 | 97 | 88 | 73 | Polysomy or trisomy |
| Furmanczyk et al.76 | 107 | 63 | 100 | |||
| Halme et al.127 | 102 | 58 | 62 | |||
| Lal et al.77 | 21 | 67 | 94 | |||
| Levy et al.8 | 34 | 7 | 100 | 29 | 100 | |
| Lindberg et al.78 | 20 | 71 | 100 | |||
| Moreno Luna et al.79 | 86 | 18 | 100 | 70 | 86 | |
| Ponsioen et al.80 | 47 | 60 | 89 | |||
| Singhi et al.123 | 37 | 8 | 100 | |||
| Siqueira et al.81 | 318 | 46 | 100 | |||
| Von Seth et al.83 | 208 | 80 | 74 | FISH + cytology | ||
CCA, cholangiocarcinoma; FISH, fluorescence in situ hybridization; HGD, high grade dysplasia; NR, not reported; PSC, primary sclerosing cholangitis.
Similar to biliary brush cytology, bile aspiration cytology specimens from neoplastic biliary strictures demonstrate identical cellular alterations as compared with specimens from inflammatory biliary strictures, including hyperplasia or regenerative or degenerative alterations. In order to confirm neoplasia, increased nuclear–cytoplasmic ratio, variation in shape, and clumping of chromatin are essential features in cytological material.30 Further research is required to explore the diagnostic accuracy of cytological examination from bile aspiration, since mainly small series were published with conflicting results and large ranges of sensitivity. Although promising results of bile aspiration were reported with a significantly higher sensitivity as compared with routine brushes (89% versus 37%), no difference in sensitivity between brushing and bile aspiration were observed when only adequate samples were examined and bile aspiration cytology needed to be repeated several times to obtain an acceptable sensitivity.29,133 Moreover, other studies did not confirm these findings and reported markedly lower sensitivities of 41–55%.30,134,135
Immunohistochemistry
Immunohistochemical staining is a widely available method in order to investigate specific tumorigenesis-related protein expression patterns in brush and biopsy samples. In CCA, patterns of multiple antibodies have been evaluated in several studies, including insulin-like growth factor II mRNA-binding protein-3 (IMP3), tumor-associated protein p53, Mac-2 binding protein (Mac-2BP), interferon α-inducible protein 27 (IFI27), urokinase-type plasminogen activator (uPA), and S100P. Sensitivity and specificity of immunohistochemical staining of these proteins are reported in Table 2.101,136–149
Table 2.
Review of literature. Sensitivity and specificity of immunohistochemistry in CCA.
| Author | Protein | No. of samples | Immunohistochemistry |
|
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | |||
| Hart et al.136 | IMP3 | 64 | 64 | 100 |
| Riener et al.137 | IMP3 | 113 | 47 | 100 |
| Diamantis et al.140 | p53 | 39 | 54 | NR |
| Stewart et al.146 | p53 | 66 | 46 | 98 |
| Tascilar et al.147 | p53 | 49 | 40 | 100 |
| Yeo et al.148 | p53 | 139 | 65 | 93 |
| Ordonez et al.145 | Mesothelin | 19 | 37 | NR |
| Yu et al.149 | Mesothelin | 24 | 33 | NR |
| Koopmann et al.142 | Mac-2 | 36 | 94 | NR |
| Chiang et al.139 | IFI27 | 96 | 45 | NR |
| Gibbs et al.141 | uPA | 11 | 73 | NR |
| Hamada et al.101 | S100P | 85 | 83 | 92 |
| S100P + cytology | 90 | 92 | ||
| Ali et al.138 | KOC | 99 | 67 | 99 |
| S100P | 81 | 100 | ||
| Mesothelin | 55 | 98 | ||
| MUC1 | 33 | 86 | ||
| Levy et al.143 | S100P | 72 | 90 | 100 |
| IMP3 | 78 | 94 | ||
| VHL | 93 | 94 | ||
| S100P + IMP3 + VHL | 70 | 97 | ||
| Ligato et al.144 | KOC | 44 | 92 | 95 |
| S100A4 | 79 | 95 | ||
| KOC + S100A4 | 100 | 95 | ||
CCA, cholangiocarcinoma; KOC, K homology domain-containing protein overexpressed in cancer; NR, not reported; uPA, urokinase-type plasminogen activator; VHL, von Hippel-Lindau.
IMP3 is an oncofetal RNA-binding protein expressed in embryologic epithelial cells, myocytes and placental tissue during embryogenesis.137 In several, mainly gastrointestinal malignancies, protein expression can be significantly upregulated.136 It has been demonstrated that 58–78% of bile duct carcinomas show intermediate to strong cytoplasmic staining of IMP3.137,143 IMP3 is also expressed in biliary tissue with low or high-grade dysplasia.137 Sensitivity and specificity of IMP3 immunohistochemistry for the diagnosis of CCA are 64% and 100%, respectively. When staining is combined with routine cytology, sensitivity increases to 72% with a specificity of 100%.136 Positive IMP3 immunohistochemistry is often associated with concurrent p53 overexpression and enhanced tumor proliferation, indicated by a significantly higher ki-67 staining in tumor tissue with a strong IMP3 expression, and is associated with reduced survival rates.
The protein p53 is a product of the TP53 tumor suppressor gene, in which mutations occur in many cancers, including biliary carcinomas.150 Staining biliary brush cytology and biopsies for p53 may be helpful to identify malignant strictures in samples showing indeterminate results on conventional brush cytology, since the majority of CCAs with TP53 mutations demonstrates overexpression of p53 protein.148,151 In 17–60% of CCAs, an increased p53 protein expression is observed, more often in poorly differentiated neoplasia as compared with well differentiated neoplasia.140,146,147,152
Mac-2BP is a cytoplasmic glycoprotein that interacts with galectin-3 on tumor cell surfaces and plays an important role in cell adhesion.153 Approximately 94% of bile duct carcinomas show Mac-2BP expression, suggesting that this protein could be a diagnostic marker for biliary carcinomas.142 Nevertheless, due to a lack of data, Mac-2BP immunohistochemistry is not recommended at present for differentiation between benign and malignant biliary strictures.
IFI27 is an oncogene involving the innate immune system and cell proliferation.139 High expression leads to cell proliferation and invasion. IFI27 knockdown in CCA has been shown to lead to cell cycle arrest in the S-phase, resulting in a lower cell proliferation rate.154 In addition to diagnostic applications, this finding suggests that IFI27 might also be a potential treatment target.
Immunohistochemical analysis of uPA has been performed in a pilot study with 11 patients with known or suspected malignant strictures, demonstrating expression of this protein in 73% of the biliary cytology samples obtained during ERCP.141 uPA plays a role in tumor invasion, growth, and cellular migration leading to the development of metastases.
S100P is a member of the S100 calcium-binding proteins and is involved in multiple cellular processes, including cell cycle progression and differentiation. An increased expression level is frequently observed in CCA, while no expression is seen in healthy bile duct tissue.101 It has also been shown that S100P expression level increases during pancreatic carcinogenesis.155 Moreover, cell proliferation, survival, motility, and invasion are stimulated by S100P expression in an in vitro culture of cancer cells.156
Lastly, biomarker panels have been suggested in the detection of CCAs, including KOC, S100P, and mesothelin.138 At least two of these biomarkers need to be positive to achieve a sensitivity of 100% and a specificity of 99%. K homology domain-containing protein overexpressed in cancer (KOC) is an oncofetal RNA-binding protein, frequently positively expressed in pancreatic carcinomas.157 In addition, one-third of CCAs demonstrates mesothelin overexpression, a differentiation antigen with expression normally limited to mesothelial cells.145,149 Another suggested immunohistochemical biomarker panel includes S100P, von Hippel-Lindau gene product (pVHL), and IMP3. Negative staining of S100P and IMP3 combined with positive staining of pVHL can be found in a significant number (69%) of biliary adenocarcinomas.143 The VHL protein has a tumor suppression function, meaning that carcinogenic development can occur in case of inactivation of both VHL alleles.158
In summary, many immunohistochemical tumor markers have been investigated with varying success rates. However, full appreciation of these ancillary techniques is hindered by the low number of carcinomas evaluated in these studies. Due to this lack of data, none of the available immunohistochemical markers is recommended for routine use in clinical practice.
Chromosomal instability
Biliary tract tumors are genomically unstable, leading to chromosomal copy-number alterations.159 Several techniques can be used to demonstrate DNA quantity and abnormalities, including digital image analysis, microsatellite instability, fluorescence in situ hybridization and next-generation sequencing (NGS).
Digital image analysis (DIA) quantifies DNA content, chromatin distribution, and nuclear morphology, by using a camera interfaced with a microscope. The determination of these characteristics may help to distinguish between benign and malignant biliary strictures. However, the accuracy of DIA is quite disappointing and comparable to routine brush cytology with a described sensitivity of 40% and specificity ranging between 77% and 92%.85,91
Analysis of microsatellite DNA is also widely used for detecting different types of cancer. Microsatellites are short highly polymorphic DNA fragments composed of nucleotide repeats, located on many positions within the genome.160 In genomic unstable tumor cells, microsatellites may be gained or lost, implying allelic imbalance. In addition, in tumor cells, defective in DNA mismatch repair, microsatellites are prone to deletion and insertion mutations during DNA replication. This phenomenon is known as microsatellite instability (MSI) and can be the result of defects in mismatch repair genes, including mutL homolog 1 (MLH1), mutS homolog 2 (MSH2), and mutS homolog 6 (MSH6).161 In addition, tumor clonality can be explored with microsatellite analysis. Microsatellite assays are able to detect identical genetic alterations in body fluids as found in the corresponding primary tumors, illustrating the clonality of the tumors.162
Furthermore, fluorescence in situ hybridization (FISH) is a valuable strategy to add to the diagnostic work-up of biliary strictures. This technique utilizes fluorescently labelled complementary DNA probes targeting specific chromosomes or chromosomal loci of individual cells.163 It allows detecting aneuploidy of chromosomes in biliary brushes or biopsies in order to distinguish between CCA and benign bile duct strictures (Figure 2). Several studies have investigated sensitivity and specificity of FISH on biliary brush samples in CCA and PSC-associated CCA. Table 1 provides an overview of 60 studies on both diagnoses. For CCA (non-PSC) reported sensitivity ranges from 31% to 65% and specificity is consistently reported close to 100%, and for PSC-associated CCA sensitivity ranges from 29% to 88% and specificity from 57% to 100%. All but one studies have included invasive carcinoma as study samples for FISH analysis; only Kushnir et al.108 have included both invasive carcinoma and high-grade dysplasia. The range of reported sensitivities is considerable, which may be caused by various methods and endpoints used in the studies, including different probe sets and polysomy and/or trisomy as endpoints. Mostly, FISH leads to an enhanced accuracy of CCA diagnosis in addition to brush cytology assessment.87,111,120 Polysomy of chromosomes 3, 7, and 17, and loss of chromosome locus 9p21 are well-known genetic alterations in malignancies.99,164 Also in patients with PSC, polysomy is suspicious for the presence of biliary neoplasia, especially in combination with an elevated serum CA-19.9 level.165,166 For the detection of CCA an optimized set of four probes has been suggested, containing DNA probes directed to chromosomes 1q21, 7p12, 8q24, and 9p21.92 CDKN2A is a 9p21 gene of which homozygous deletion and loss of heterozygosity is regularly found in CCA (5% and 20%, respectively).167 In addition, FGFR genetic aberrations are also common in CCA, including FGFR2 fusions and amplification of FGFR19 and FGFR3.168,169 FGFR2 fusions are typically observed in small-duct intrahepatic CCA.170 Amplification of ERBB2 and EGFR are less common in CCA, but both are also described in around 5–8% of patients.150,171 Amplification of these genes leads to overexpression of the corresponding proteins.
Figure 2.
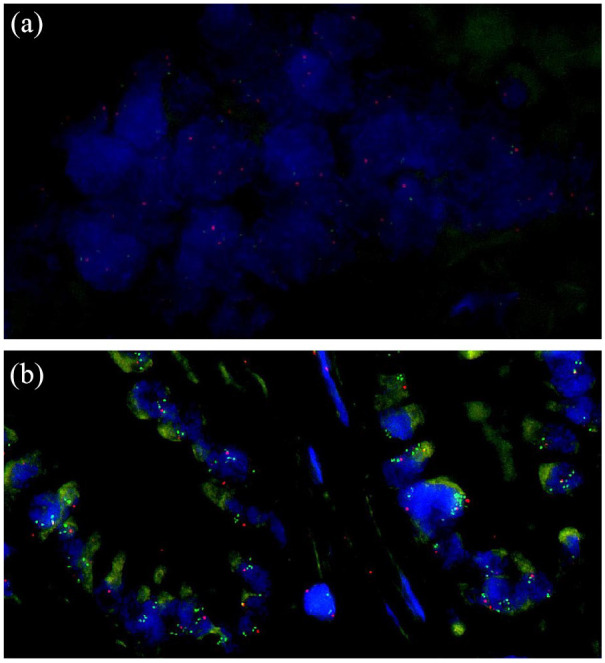
Examples of FISH negative (a) and positive (b) cases for MYC copy-number assessment. (a) Tumor cells with two signals for each of the two probes. (b) Tumor cells with MYC amplification.
MYC (green); centromere-bounded probe (red).
Finally, chromosomal imbalance can also be detected by NGS. Several algorithms have been currently developed to identify copy-number variations based on targeted amplicon sequencing data.172–175 In addition, single-nucleotide polymorphism (SNP) analysis is another suitable NGS strategy to detect chromosomal imbalance.176 During this analysis highly polymorphic SNP amplicons are included in the NGS panel, after which the status of each SNP can be interpreted as homozygous (noninformative), balanced heterozygous, or imbalanced heterozygous (loss or amplification of one allele).
Mutation profiling
Many studies have identified mutations in resection specimens of CCA, including in the genes TP53, KRAS, ARID1A, and IDH1.150,164,177–180 Mutations in TP53 are most common in biliary tumors with a prevalence of 26%.150 Also KRAS mutations are frequently detected in CCA (17%). Some studies have even reported a prevalence of KRAS mutations in resection specimens and biliary brushes up to 30–40%.181–185 In addition to the previous described chromosomal imbalance of FGFR, also mutations have been identified in this gene family, more often in FGFR2 than in FGFR1, FGFR3, and FGFR4.168 In contrast to an aggressive tumor progression in case of TP53 and KRAS mutations, FGFR mutations correlate with a significantly indolent disease course.186 Furthermore, in addition to the previously described amplification of EGFR in a small number of CCA patients, somatic mutations of the EGFR gene have also been described in biliary tract cancer.187 Patients with these mutations might benefit from EGFR inhibitors, such as gefitinib or erlotinib, which are used in EGFR mutant lung cancer.188–190 Mutations in GNAS and PIK3CA are less frequently observed alterations in CCA.183,191
Mutations differ between intrahepatic and extrahepatic CCA. TP53 and KRAS mutations are more common in extrahepatic than in intrahepatic CCA (45% versus 35% and 40% versus 24%, respectively). Furthermore, mutations in IDH1, ARID1A, BAP1, MCL1, NRAS, and PBRM1 are mainly detected in intrahepatic CCA, while ERBB2, SMAD4, FBXW7, BRAF, and CDKN2A alterations are mainly identified in extrahepatic CCA.192–196 A systematic review reported frequencies of IDH1 mutations in intrahepatic CCA ranging from 5% to 56%, while less than 1% of the extrahepatic CCA showed IDH1 mutations.197 Mutations in PSC-associated CCA (PSC-CCA) are largely identical to mutations in sporadic (non-PSC-related) CCA, including alterations in TP53, KRAS, IDH1, and SMAD4.198 In comparison to sporadic CCA, ERBB2 mutations and amplification are more often observed in PSC-CCA.198,199
NGS in both intraductal brushes and biopsies is promising for standard diagnostic care in biliary strictures. Different sequencing technologies are available, including Sanger, targeted sequencing, whole exome sequencing, and whole genome sequencing.200 All methods have unique characteristics, leading to differences in coverage, read length, ideal DNA input, and limit of detection for mutations, and are therefore not all appropriate for mutation profiling in brush cytology. Singhi et al.123 have identified mutations in a considerable number of biliary brushes and biopsies by performing targeted NGS with a large gene panel. With this gene panel a sensitivity of 73% and specificity of 100% was reached for biliary neoplasia. The most prevalent genomic alterations consisted of mutations in KRAS, TP53, CDKN2A, SMAD4, PIK3CA, and GNAS. Overall, when routine cytology was combined with targeted NGS with a large gene panel, sensitivity increased from 36% to 60–80% with a specificity of 99%.123,185 Kushnir et al.108 demonstrated that the combination of brush cytology, FISH analysis, and mutation profiling of KRAS resulted in an increase of the diagnostic performance with a sensitivity of 66% and a specificity of 100%. When a large gene panel of 39 potentially interesting genes was added to brush cytology examination, sensitivity increased to 85%, with a maintained high specificity of 96%.164 The promising data of mutation profiling need further confirmation.
Mutation profiling on liquid biopsies is a promising technique for the diagnosis of CCA, especially when tissue sampling fails, and to monitor tumor response to (targeted) therapy.201–204 Genetic profiles of cell-free (cf)DNA in blood samples have been shown to match the profiles in CCA.205–207 As an alternative, the mutation profiles may be investigated in bile samples. According to a small case series, cfDNA genetic profile analysis in bile has a higher diagnostic value as compared with blood samples for the detection of mutation profiles of CCA.208 In addition, mutations may also be found in cfDNA from the supernatant fluids after biliary flushing during ERCP, similar to observations in samples obtained during bronchoscopy.209 This technique requires further evaluation for biliary lesions. Another promising technique is the detection of circulating free microRNAs. MicroRNAs are small noncoding RNAs (21–23 nucleotides) that play an important role in cancer development by influencing many cellular processes, such as differentiation, proliferation, and apoptosis.210,211 Due to the remarkable stability of cfmicroRNAs in blood, these can be detected by various methods, including northern blotting, in situ hybridization, RT–PCR, microarray, and deep sequencing.212,213 Bernuzzi et al.214 have identified several promising microRNAs as biomarkers for the diagnosis of PSC and CCA, including miR-200c for PSC and miR-194 for CCA. The analysis of microRNAs in serum might differentiate between PSC and CCA. According to a cohort study of 40 PSC, 40 CCA, 20 primary biliary cholangitis patients and 40 controls, the miR-483-5p and miR-222 were significantly more upregulated in CCA as compared with PSC. More data on the diagnostic value of microRNAs for the differentiation of benign and malignant biliary strictures are eagerly awaited.
In addition to diagnostic purposes, identifying mutations might also be important for the treatment of CCA. In several cancer types, suitable molecular targets have been identified for individualized targeted therapy, including EGFR and BRAF mutations in lung cancer and melanoma, respectively.215,216 The development of molecular targeted therapies in CCA is challenging due to the large variety of genetic aberrations.217–219 However, mutations in genes as ERBB2, EGFR, and PIK3CA are detected and agents with these genes as targets have been suggested for targeted therapy in CCA.220 Bankov et al.221 identified 79 mutations in 39 intraductal biopsies of 16 CCA patients with targeted NGS, resulting in potentially therapeutic targets in 6/16 patients: EPHA2, ERBB2, PIK3CA, and PTEN. Unfortunately, several clinical trials have been performed in CCA with inhibitors of various targets in oncogenic pathways, including MEK1/2, EGFR, ERBB2, and BRAF/VEGFR, all of which did not show a pronounced clinical benefit.222–225 Also in PSC-CCA, potentially actionable molecular targets were identified, including ERBB2, EGFR, MET, and MYC.199 However, clinical data of targeted therapy in PSC-CCA is scarce.
Methylation
Aberrant DNA methylation contributes to carcinogenesis in several types of tumors by inactivating tumor suppressor genes.226 Normally, DNA methylation occurs at CpG sites, which are DNA regions where a guanine nucleotide follows a cytosine nucleotide. Hypermethylation is mostly located in the promoter regions, while dense CpG islands are usually devoid. Promoter hypermethylation of the CpG islands leads to transcriptional repression.227 In addition, global hypomethylation is also often found in neoplastic cells.228 However, the precise role of the hypomethylation and the mechanism to silence tumor suppressor genes are unclear. The epigenetic change of aberrant DNA methylation induces silencing of tumor suppressor genes and appears to be an early event in carcinogenesis.229 In many tumor types, including lung cancer, methylation plays a critical role in the initiation and progression of neoplasia.230,231 Tumor suppressor genes MLH1, p14ARF, CDKN2A, CDH1, NPTX2, TFPI-2, APC, TIMP3, RASSF1, and DAPK are commonly methylated in CCA.226,232,233 Approximately 80–85% of the biliary tract tumors shows methylation in at least one known tumor suppressor gene, while around 70% of CCA demonstrates methylation in at least three genes.226,234
In addition, inactivation of tumor suppressor microRNAs by DNA methylation is frequently found in CCA.232 MicroRNAs are involved in several pathways and deregulation of these small molecules can lead to progressive carcinogenesis of different types of cancer.235 For example, tumor suppressor miR-370 can be inactivated by DNA methylation in tumor cells of bile duct carcinomas.236 MiR-370 is downregulated by a high expression of interleukin-6, a pro-inflammatory cytokine. Overexpression of interleukin-6 is often found in CCA and may contribute to tumor cell growth by miRNAs deregulation, including miR-370.237
Detection of DNA methylation of tumor suppressor genes and microRNAs could be a valuable diagnostic tool for CCA. Besides, DNA methylation inhibitors might be promising for the treatment of these tumors. Further investigations of these techniques are required.
Conclusion
CCA is commonly diagnosed at an advanced stage and has a devastating prognosis. A low diagnostic yield of currently available diagnostic methods contributes to the late diagnosis of CCA. Genetic factors and a variety of signaling pathways are involved in the carcinogenesis and are potential diagnostic targets. Current diagnostic work-up consists mainly of routine cytology examination, and in some centers the standard is assisted by FISH analysis or p53 immunohistochemistry. Genetic alterations and aberrant DNA methylation in biliary brushes and/or biopsies are promising diagnostic and prognostic markers for CCA, which are already implemented in the diagnostic work-flow of some laboratories. Amplification of 1q21, 7p12, 8q24, ERBB2, EGFR, and FGFR genes are observed in biliary tumors, as well as CDKN2A loss and FGFR2 fusions. The majority of these potential markers could be identified by FISH or NGS and aid in a timely diagnosis of CCA. Prompted by the described considerations, in case of a high suspicion of malignant stricture, we would recommend obtaining both brush cytology and fluoroscopic-guided biopsies. NGS should be performed on this biliary material when results are inconclusive. A prominent position of molecular analyzes for the diagnosis of CCA may be awaited in the near future.
Supplemental Material
Supplemental material, sj-jpg-1-tag-10.1177_17562848211002023 for Optimal tissue sampling during ERCP and emerging molecular techniques for the differentiation of benign and malignant biliary strictures by Eline J. C. A. Kamp, Winand N. M. Dinjens, Michail Doukas, Marco J. Bruno, Pieter Jan F. de Jonge, Maikel P. Peppelenbosch and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Annemarie C. de Vries  https://orcid.org/0000-0001-5410-9878
https://orcid.org/0000-0001-5410-9878
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Eline J. C. A. Kamp, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, The Netherlands
Winand N. M. Dinjens, Department of Pathology, Erasmus MC Cancer Institute, University Medical Center Rotterdam, The Netherlands
Michail Doukas, Department of Pathology, Erasmus MC Cancer Institute, University Medical Center Rotterdam, The Netherlands.
Marco J. Bruno, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, The Netherlands
Pieter Jan F. de Jonge, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, The Netherlands
Maikel P. Peppelenbosch, Department of Gastroenterology and Hepatology, Erasmus MC, University Medical Center Rotterdam, The Netherlands
Annemarie C. de Vries, Department of Gastroenterology & Hepatology, Erasmus MC, University Medical Center Rotterdam, Doctor Molewaterplein 40, Room Na-609, Rotterdam, 3015 GD, The Netherlands.
References
- 1. Visrodia KH, Tabibian JH, Baron TH. Endoscopic management of benign biliary strictures. World J Gastrointest Endosc 2015; 7: 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graziadei IW, Schwaighofer H, Koch R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl 2006; 12: 718–725. [DOI] [PubMed] [Google Scholar]
- 3. Draganov P, Hoffman B, Marsh W, et al. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc 2002; 55: 680–686. [DOI] [PubMed] [Google Scholar]
- 4. Ponsioen CY, Vrouenraets SM, Prawirodirdjo W, et al. Natural history of primary sclerosing cholangitis and prognostic value of cholangiography in a Dutch population. Gut 2002; 51: 562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tummala P, Munigala S, Eloubeidi MA, et al. Patients with obstructive jaundice and biliary stricture +/− mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol 2013; 47: 532–537. [DOI] [PubMed] [Google Scholar]
- 6. Weber A, Schmid RM, Prinz C. Diagnostic approaches for cholangiocarcinoma. World J Gastroenterol 2008; 14: 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol 2009; 51: 237–267. [DOI] [PubMed] [Google Scholar]
- 8. Levy MJ, Baron TH, Clayton AC, et al. Prospective evaluation of advanced molecular markers and imaging techniques in patients with indeterminate bile duct strictures. Am J Gastroenterol 2008; 103: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsen A, Barbara M, Rosenkranz L. Dilemma of elevated CA 19-9 in biliary pathology. Pancreatology 2018; 18: 862–867. [DOI] [PubMed] [Google Scholar]
- 10. Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017; 67: 1298–1323. [DOI] [PubMed] [Google Scholar]
- 11. Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl 2008; 14: 759–769. [DOI] [PubMed] [Google Scholar]
- 12. Thuluvath PJ, Pfau PR, Kimmey MB, et al. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy 2005; 37: 857–863. [DOI] [PubMed] [Google Scholar]
- 13. Shah BB, Goenka MK. Approach to management of indeterminate biliary stricture. Adv Res Gastroentero Hepatol 2018; 10: 86–90. [Google Scholar]
- 14. Ma MX, Jayasekeran V, Chong AK. Benign biliary strictures: prevalence, impact, and management strategies. Clin Exp Gastroenterol 2019; 12: 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie C, Aloreidi K, Patel B, et al. Indeterminate biliary strictures: a simplified approach. Expert Rev Gastroenterol Hepatol 2018; 12: 189–199. [DOI] [PubMed] [Google Scholar]
- 16. Gerhards MF, Vos P, van Gulik TM, et al. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br J Surg 2001; 88: 48–51. [DOI] [PubMed] [Google Scholar]
- 17. Clayton RA, Clarke DL, Currie EJ, et al. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon 2003; 1: 32–38. [DOI] [PubMed] [Google Scholar]
- 18. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014; 383: 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brugge WR, De Witt J, Klapman JB, et al. Techniques for cytologic sampling of pancreatic and bile duct lesions: the Papanicolaou Society of Cytopathology guidelines. Cytojournal 2014; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Bellis M, Fogel EL, Sherman S, et al. Influence of stricture dilation and repeat brushing on the cancer detection rate of brush cytology in the evaluation of malignant biliary obstruction. Gastrointest Endosc 2003; 58: 176–182. [DOI] [PubMed] [Google Scholar]
- 21. Ornellas LC, Santos Gda C, Nakao FS, et al. Comparison between endoscopic brush cytology performed before and after biliary stricture dilation for cancer detection. Arq Gastroenterol 2006; 43: 20–23. [DOI] [PubMed] [Google Scholar]
- 22. Bank JS, Witt BL, Taylor LJ, et al. Diagnostic yield and accuracy of a new cytology brush design compared to standard brush cytology for evaluation of biliary strictures. Diagn Cytopathol 2018; 46: 234–238. [DOI] [PubMed] [Google Scholar]
- 23. Kylanpaa L, Boyd S, Ristimaki A, et al. A prospective randomised study of dense infinity cytological brush versus regularly used brush in pancreaticobiliary malignancy. Scand J Gastroenterol 2016; 51: 590–593. [DOI] [PubMed] [Google Scholar]
- 24. Fogel EL, deBellis M, McHenry L, et al. Effectiveness of a new long cytology brush in the evaluation of malignant biliary obstruction: a prospective study. Gastrointest Endosc 2006; 63: 71–77. [DOI] [PubMed] [Google Scholar]
- 25. Nakahara K, Michikawa Y, Morita R, et al. Diagnostic ability of endoscopic bile cytology using a newly designed biliary scraper for biliary strictures. Dig Dis Sci 2019; 64: 241–248. [DOI] [PubMed] [Google Scholar]
- 26. Bang KB, Kim HJ, Park JH, et al. Comparison of brush and basket cytology in differential diagnosis of bile duct stricture at endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int 2014; 13: 622–627. [DOI] [PubMed] [Google Scholar]
- 27. Sakuma Y, Kodama Y, Sogabe Y, et al. Diagnostic performance of a new endoscopic scraper for malignant biliary strictures: a multicenter prospective study. Gastrointest Endosc 2017; 85: 371–379. [DOI] [PubMed] [Google Scholar]
- 28. Tsuchiya T, Yokoyama Y, Ebata T, et al. Randomized controlled trial on timing and number of sampling for bile aspiration cytology. J Hepatobiliary Pancreat Sci 2014; 21: 433–438. [DOI] [PubMed] [Google Scholar]
- 29. Abdelghani YA, Arisaka Y, Masuda D, et al. Bile aspiration cytology in diagnosis of bile duct carcinoma: factors associated with positive yields. J Hepatobiliary Pancreat Sci 2012; 19: 370–378. [DOI] [PubMed] [Google Scholar]
- 30. Kurzawinski T, Deery A, Davidson BR. Diagnostic value of cytology for biliary stricture. Br J Surg 1993; 80: 414–421. [DOI] [PubMed] [Google Scholar]
- 31. De Bellis M, Sherman S, Fogel EL, et al. Tissue sampling at ERCP in suspected malignant biliary strictures (part 1). Gastrointest Endosc 2002; 56: 552–561. [DOI] [PubMed] [Google Scholar]
- 32. Howell DA, Beveridge RP, Bosco J, et al. Endoscopic needle aspiration biopsy at ERCP in the diagnosis of biliary strictures. Gastrointest Endosc 1992; 38: 531–535. [DOI] [PubMed] [Google Scholar]
- 33. Selvaggi SM. Biliary brushing cytology. Cytopathology 2004; 15: 74–79. [DOI] [PubMed] [Google Scholar]
- 34. Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther 2014; 39: 282–301. [DOI] [PubMed] [Google Scholar]
- 35. Ponchon T, Gagnon P, Berger F, et al. Value of endobiliary brush cytology and biopsies for the diagnosis of malignant bile duct stenosis: results of a prospective study. Gastrointest Endosc 1995; 42: 565–572. [DOI] [PubMed] [Google Scholar]
- 36. Pugliese V, Conio M, Nicolo G, et al. Endoscopic retrograde forceps biopsy and brush cytology of biliary strictures: a prospective study. Gastrointest Endosc 1995; 42: 520–526. [DOI] [PubMed] [Google Scholar]
- 37. Nanda A, Brown JM, Berger SH, et al. Triple modality testing by endoscopic retrograde cholangiopancreatography for the diagnosis of cholangiocarcinoma. Therap Adv Gastroenterol 2015; 8: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Naitoh I, Nakazawa T, Kato A, et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J Dig Dis 2016; 17: 44–51. [DOI] [PubMed] [Google Scholar]
- 39. Navaneethan U, Njei B, Lourdusamy V, et al. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 2015; 81: 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber A, von Weyhern C, Fend F, et al. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol 2008; 14: 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aabakken L, Karlsen TH, Albert J, et al. Role of endoscopy in primary sclerosing cholangitis: European Society of Gastrointestinal Endoscopy (ESGE) and European Association for the Study of the Liver (EASL) clinical guideline. Endoscopy 2017; 49: 588–608. [DOI] [PubMed] [Google Scholar]
- 42. Lee SJ, Lee YS, Lee MG, et al. Triple-tissue sampling during endoscopic retrograde cholangiopancreatography increases the overall diagnostic sensitivity for cholangiocarcinoma. Gut Liver 2014; 8: 669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamashita Y, Ueda K, Kawaji Y, et al. The wire-grasping method as a new technique for forceps biopsy of biliary strictures: a prospective randomized controlled study of effectiveness. Gut Liver 2016; 10: 642–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawakami H, Kuwatani M, Etoh K, et al. Endoscopic retrograde cholangiography versus peroral cholangioscopy to evaluate intraepithelial tumor spread in biliary cancer. Endoscopy 2009; 41: 959–964. [DOI] [PubMed] [Google Scholar]
- 45. Draganov PV, Chauhan S, Wagh MS, et al. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc 2012; 75: 347–353. [DOI] [PubMed] [Google Scholar]
- 46. Hammerle CW, Haider S, Chung M, et al. Endoscopic retrograde cholangiopancreatography complications in the era of cholangioscopy: is there an increased risk? Dig Liver Dis 2012; 44: 754–758. [DOI] [PubMed] [Google Scholar]
- 47. Moon JH, Choi HJ. The role of direct peroral cholangioscopy using an ultraslim endoscope for biliary lesions: indications, limitations, and complications. Clin Endosc 2013; 46: 537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Korrapati P, Ciolino J, Wani S, et al. The efficacy of peroral cholangioscopy for difficult bile duct stones and indeterminate strictures: a systematic review and meta-analysis. Endosc Int Open 2016; 4: E263–E275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nishikawa T, Tsuyuguchi T, Sakai Y, et al. Comparison of the diagnostic accuracy of peroral video-cholangioscopic visual findings and cholangioscopy-guided forceps biopsy findings for indeterminate biliary lesions: a prospective study. Gastrointest Endosc 2013; 77: 219–226. [DOI] [PubMed] [Google Scholar]
- 50. Navaneethan U, Hasan MK, Kommaraju K, et al. Digital, single-operator cholangiopancreatoscopy in the diagnosis and management of pancreatobiliary disorders: a multicenter clinical experience (with video). Gastrointest Endosc 2016; 84: 649–655. [DOI] [PubMed] [Google Scholar]
- 51. Sioulas AD, El-Masry MA, Groth S, et al. Prospective evaluation of the short access cholangioscopy for stone clearance and evaluation of indeterminate strictures. Hepatobiliary Pancreat Dis Int 2017; 16: 96–103. [DOI] [PubMed] [Google Scholar]
- 52. Fukuda Y, Tsuyuguchi T, Sakai Y, et al. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest Endosc 2005; 62: 374–382. [DOI] [PubMed] [Google Scholar]
- 53. Kalaitzakis E, Webster GJ, Oppong KW, et al. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol 2012; 24: 656–664. [DOI] [PubMed] [Google Scholar]
- 54. Laleman W, Verraes K, Van Steenbergen W, et al. Usefulness of the single-operator cholangioscopy system SpyGlass in biliary disease: a single-center prospective cohort study and aggregated review. Surg Endosc 2017; 31: 2223–2232. [DOI] [PubMed] [Google Scholar]
- 55. Manta R, Frazzoni M, Conigliaro R, et al. SpyGlass single-operator peroral cholangioscopy in the evaluation of indeterminate biliary lesions: a single-center, prospective, cohort study. Surg Endosc 2013; 27: 1569–1572. [DOI] [PubMed] [Google Scholar]
- 56. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015; 82: 608–614.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ramchandani M, Reddy DN, Gupta R, et al. Role of single-operator peroral cholangioscopy in the diagnosis of indeterminate biliary lesions: a single-center, prospective study. Gastrointest Endosc 2011; 74: 511–519. [DOI] [PubMed] [Google Scholar]
- 58. Siddiqui AA, Mehendiratta V, Jackson W, et al. Identification of cholangiocarcinoma by using the Spyglass Spyscope system for peroral cholangioscopy and biopsy collection. Clin Gastroenterol Hepatol 2012; 10: 466–471; quiz e48. [DOI] [PubMed] [Google Scholar]
- 59. Tieu AH, Kumbhari V, Jakhete N, et al. Diagnostic and therapeutic utility of SpyGlass® peroral cholangioscopy in intraductal biliary disease: single-center, retrospective, cohort study. Dig Endosc 2015; 27: 479–485. [DOI] [PubMed] [Google Scholar]
- 60. Hartman DJ, Slivka A, Giusto DA, et al. Tissue yield and diagnostic efficacy of fluoroscopic and cholangioscopic techniques to assess indeterminate biliary strictures. Clin Gastroenterol Hepatol 2012; 10: 1042–1046. [DOI] [PubMed] [Google Scholar]
- 61. Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol 2008; 24: 631–637. [DOI] [PubMed] [Google Scholar]
- 62. Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology 2007; 133: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 63. Shieh FK, Drumm H, Nathanson MH, et al. High-definition confocal endomicroscopy of the common bile duct. J Clin Gastroenterol 2012; 46: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meining A, Chen YK, Pleskow D, et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc 2011; 74: 961–968. [DOI] [PubMed] [Google Scholar]
- 65. Slivka A, Gan I, Jamidar P, et al. Validation of the diagnostic accuracy of probe-based confocal laser endomicroscopy for the characterization of indeterminate biliary strictures: results of a prospective multicenter international study. Gastrointest Endosc 2015; 81: 282–290. [DOI] [PubMed] [Google Scholar]
- 66. Talreja JP, Sethi A, Jamidar PA, et al. Interpretation of probe-based confocal laser endomicroscopy of indeterminate biliary strictures: is there any interobserver agreement? Dig Dis Sci 2012; 57: 3299–3302. [DOI] [PubMed] [Google Scholar]
- 67. Caillol F, Filoche B, Gaidhane M, et al. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris classification. Dig Dis Sci 2013; 58: 1784–1789. [DOI] [PubMed] [Google Scholar]
- 68. Kahaleh M, Giovannini M, Jamidar P, et al. Probe-based confocal laser endomicroscopy for indeterminate biliary strictures: refinement of the image interpretation classification. Gastroenterol Res Pract 2015; 2015: 675210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siddiqui MT, Gokaslan ST, Saboorian MH, et al. Comparison of ThinPrep and conventional smears in detecting carcinoma in bile duct brushings. Cancer 2003; 99: 205–210. [DOI] [PubMed] [Google Scholar]
- 70. Ylagan LR, Liu LH, Maluf HM. Endoscopic bile duct brushing of malignant pancreatic biliary strictures: retrospective study with comparison of conventional smear and ThinPrep techniques. Diagn Cytopathol 2003; 28: 196–204. [DOI] [PubMed] [Google Scholar]
- 71. Prendeville S, Brosnan T, Browne TJ, et al. Automated CellientTM cytoblocks: better, stronger, faster? Cytopathology 2014; 25: 372–380. [DOI] [PubMed] [Google Scholar]
- 72. Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: a review. Cancer Cytopathol 2014; 122: 399–411. [DOI] [PubMed] [Google Scholar]
- 73. Mehmood S, Loya A, Yusuf MA. Biliary brush cytology revisited. Acta Cytol 2016; 60: 167–172. [DOI] [PubMed] [Google Scholar]
- 74. Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology guidelines. Cytojournal 2014; 11(Suppl. 1): 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boberg KM, Jebsen P, Clausen OP, et al. Diagnostic benefit of biliary brush cytology in cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 2006; 45: 568–574. [DOI] [PubMed] [Google Scholar]
- 76. Furmanczyk PS, Grieco VS, Agoff SN. Biliary brush cytology and the detection of cholangiocarcinoma in primary sclerosing cholangitis: evaluation of specific cytomorphologic features and CA19-9 levels. Am J Clin Pathol 2005; 124: 355–360. [DOI] [PubMed] [Google Scholar]
- 77. Lal A, Okonkwo A, Schindler S, et al. Role of biliary brush cytology in primary sclerosing cholangitis. Acta Cytol 2004; 48: 9–12. [DOI] [PubMed] [Google Scholar]
- 78. Lindberg B, Arnelo U, Bergquist A, et al. Diagnosis of biliary strictures in conjunction with endoscopic retrograde cholangiopancreaticography, with special reference to patients with primary sclerosing cholangitis. Endoscopy 2002; 34: 909–916. [DOI] [PubMed] [Google Scholar]
- 79. Moreno Luna LE, Kipp B, Halling KC, et al. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology 2006; 131: 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ponsioen CY, Vrouenraets SM, van Milligen de Wit AW, et al. Value of brush cytology for dominant strictures in primary sclerosing cholangitis. Endoscopy 1999; 31: 305–309. [DOI] [PubMed] [Google Scholar]
- 81. Siqueira E, Schoen RE, Silverman W, et al. Detecting cholangiocarcinoma in patients with primary sclerosing cholangitis. Gastrointest Endosc 2002; 56: 40–47. [DOI] [PubMed] [Google Scholar]
- 82. Bangarulingam SY, Bjornsson E, Enders F, et al. Long-term outcomes of positive fluorescence in situ hybridization tests in primary sclerosing cholangitis. Hepatology 2010; 51: 174–180. [DOI] [PubMed] [Google Scholar]
- 83. von Seth E, Ouchterlony H, Dobra K, et al. Diagnostic performance of a stepwise cytological algorithm for biliary malignancy in primary sclerosing cholangitis. Liver Int 2019; 39: 382–388. [DOI] [PubMed] [Google Scholar]
- 84. Bardales RH, Stanley MW, Simpson DD, et al. Diagnostic value of brush cytology in the diagnosis of duodenal, biliary, and ampullary neoplasms. Am J Clin Pathol 1998; 109: 540–548. [DOI] [PubMed] [Google Scholar]
- 85. Baron TH, Harewood GC, Rumalla A, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol 2004; 2: 214–219. [DOI] [PubMed] [Google Scholar]
- 86. Binek J, Lindenmann N, Meyenberger CM, et al. Malignant biliary stenosis: conventional cytology versus DNA image cytometry. Surg Endosc 2011; 25: 1808–1813. [DOI] [PubMed] [Google Scholar]
- 87. Chaiteerakij R, Barr Fritcher EG, Angsuwatcharakon P, et al. Fluorescence in situ hybridization compared with conventional cytology for the diagnosis of malignant biliary tract strictures in Asian patients. Gastrointest Endosc 2016; 83: 1228–1235. [DOI] [PubMed] [Google Scholar]
- 88. de Peralta-Venturina MN, Wong DK, Purslow MJ, et al. Biliary tract cytology in specimens obtained by direct cholangiographic procedures: a study of 74 cases. Diagn Cytopathol 1996; 14: 334–348. [DOI] [PubMed] [Google Scholar]
- 89. Desa LA, Akosa AB, Lazzara S, et al. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut 1991; 32: 1188–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Elek G, Gyokeres T, Schafer E, et al. Early diagnosis of pancreatobiliary duct malignancies by brush cytology and biopsy. Pathol Oncol Res 2005; 11: 145–155. [DOI] [PubMed] [Google Scholar]
- 91. Barr Fritcher EG, Kipp BR, Slezak JM, et al. Correlating routine cytology, quantitative nuclear morphometry by digital image analysis, and genetic alterations by fluorescence in situ hybridization to assess the sensitivity of cytology for detecting pancreatobiliary tract malignancy. Am J Clin Pathol 2007; 128: 272–279. [DOI] [PubMed] [Google Scholar]
- 92. Barr Fritcher EG, Voss JS, Brankley SM, et al. An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology 2015; 149: 1813–1824. [DOI] [PubMed] [Google Scholar]
- 93. Eiholm S, Thielsen P, Kromann-Andersen H. Endoscopic brush cytology from the biliary duct system is still valuable. Dan Med J 2013; 60: A4656. [PubMed] [Google Scholar]
- 94. Farrell RJ, Jain AK, Brandwein SL, et al. The combination of stricture dilation, endoscopic needle aspiration, and biliary brushings significantly improves diagnostic yield from malignant bile duct strictures. Gastrointest Endosc 2001; 54: 587–594. [DOI] [PubMed] [Google Scholar]
- 95. Ferrari Júnior AP, Lichtenstein DR, Slivka A, et al. Brush cytology during ERCP for the diagnosis of biliary and pancreatic malignancies. Gastrointest Endosc 1994; 40: 140–145. [DOI] [PubMed] [Google Scholar]
- 96. Foutch PG, Harlan JR, Kerr D, et al. Wire-guided brush cytology: a new endoscopic method for diagnosis of bile duct cancer. Gastrointest Endosc 1989; 35: 243–247. [DOI] [PubMed] [Google Scholar]
- 97. Fritcher EG, Kipp BR, Halling KC, et al. A multivariable model using advanced cytologic methods for the evaluation of indeterminate pancreatobiliary strictures. Gastroenterology 2009; 136: 2180–2186. [DOI] [PubMed] [Google Scholar]
- 98. Glasbrenner B, Ardan M, Boeck W, et al. Prospective evaluation of brush cytology of biliary strictures during endoscopic retrograde cholangiopancreatography. Endoscopy 1999; 31: 712–717. [DOI] [PubMed] [Google Scholar]
- 99. Gonda TA, Viterbo D, Gausman V, et al. Mutation profile and fluorescence in situ hybridization analyses increase detection of malignancies in biliary strictures. Clin Gastroenterol Hepatol 2017; 15: 913–919. [DOI] [PubMed] [Google Scholar]
- 100. Govil H, Reddy V, Kluskens L, et al. Brush cytology of the biliary tract: retrospective study of 278 cases with histopathologic correlation. Diagn Cytopathol 2002; 26: 273–277. [DOI] [PubMed] [Google Scholar]
- 101. Hamada S, Satoh K, Hirota M, et al. Calcium-binding protein S100P is a novel diagnostic marker of cholangiocarcinoma. Cancer Sci 2011; 102: 150–156. [DOI] [PubMed] [Google Scholar]
- 102. Harewood GC, Baron TH, Stadheim LM, et al. Prospective, blinded assessment of factors influencing the accuracy of biliary cytology interpretation. Am J Gastroenterol 2004; 99: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 103. Howell DA, Parsons WG, Jones MA, et al. Complete tissue sampling of biliary strictures at ERCP using a new device. Gastrointest Endosc 1996; 43: 498–502. [DOI] [PubMed] [Google Scholar]
- 104. Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol 2004; 99: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 105. Kitajima Y, Ohara H, Nakazawa T, et al. Usefulness of transpapillary bile duct brushing cytology and forceps biopsy for improved diagnosis in patients with biliary strictures. J Gastroenterol Hepatol 2007; 22: 1615–1620. [DOI] [PubMed] [Google Scholar]
- 106. Kocjan G, Smith AN. Bile duct brushings cytology: potential pitfalls in diagnosis. Diagn Cytopathol 1997; 16: 358–363. [DOI] [PubMed] [Google Scholar]
- 107. Kurzawinski TR, Deery A, Dooley JS, et al. A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology 1993; 18: 1399–1403. [PubMed] [Google Scholar]
- 108. Kushnir VM, Mullady DK, Das K, et al. The diagnostic yield of malignancy comparing cytology, FISH, and molecular analysis of cell free cytology brush supernatant in patients with biliary strictures undergoing endoscopic retrograde cholangiography (ERC): a prospective study. J Clin Gastroenterol 2019; 53: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Layfield LJ, Wax TD, Lee JG, et al. Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol 1995; 39: 11–18. [PubMed] [Google Scholar]
- 110. Lee JG, Leung JW, Baillie J, et al. Benign, dysplastic, or malignant–making sense of endoscopic bile duct brush cytology: results in 149 consecutive patients. Am J Gastroenterol 1995; 90: 722–726. [PubMed] [Google Scholar]
- 111. Liew ZH, Loh TJ, Lim TKH, et al. Role of fluorescence in situ hybridization in diagnosing cholangiocarcinoma in indeterminate biliary strictures. J Gastroenterol Hepatol 2018; 33: 315–319. [DOI] [PubMed] [Google Scholar]
- 112. Logrono R, Kurtycz DF, Molina CP, et al. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at 2 university hospitals. Arch Pathol Lab Med 2000; 124: 387–392. [DOI] [PubMed] [Google Scholar]
- 113. Macken E, Drijkoningen M, Van Aken E, et al. Brush cytology of ductal strictures during ERCP. Acta Gastroenterol Belg 2000; 63: 254–259. [PubMed] [Google Scholar]
- 114. Mahmoudi N, Enns R, Amar J, et al. Biliary brush cytology: factors associated with positive yields on biliary brush cytology. World J Gastroenterol 2008; 14: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mohammad Alizadeh AH, Mousavi M, Salehi B, et al. Biliary brush cytology in the assessment of biliary strictures at a tertiary center in Iran. Asian Pac J Cancer Prev 2011; 12: 2793–2796. [PubMed] [Google Scholar]
- 116. Pugliese V, Barone D, Saccomanno S, et al. Tissue sampling from the common bile duct through endoscopic retrograde cholangiopancreatography, endoscopic papillo(sphinctero)tomy and drainage in juxtapapillary malignancies. Surg Endosc 1987; 1: 83–87. [DOI] [PubMed] [Google Scholar]
- 117. Rabinovitz M, Zajko AB, Hassanein T, et al. Diagnostic value of brush cytology in the diagnosis of bile duct carcinoma: a study in 65 patients with bile duct strictures. Hepatology 1990; 12: 747–752. [DOI] [PubMed] [Google Scholar]
- 118. Rosch T, Hofrichter K, Frimberger E, et al. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc 2004; 60: 390–396. [DOI] [PubMed] [Google Scholar]
- 119. Ryan ME. Cytologic brushings of ductal lesions during ERCP. Gastrointest Endosc 1991; 37: 139–142. [DOI] [PubMed] [Google Scholar]
- 120. Salomao M, Gonda TA, Margolskee E, et al. Strategies for improving diagnostic accuracy of biliary strictures. Cancer Cytopathol 2015; 123: 244–252. [DOI] [PubMed] [Google Scholar]
- 121. Schoefl R, Haefner M, Wrba F, et al. Forceps biopsy and brush cytology during endoscopic retrograde cholangiopancreatography for the diagnosis of biliary stenoses. Scand J Gastroenterol 1997; 32: 363–368. [DOI] [PubMed] [Google Scholar]
- 122. Scudera PL, Koizumi J, Jacobson IM. Brush cytology evaluation of lesions encountered during ERCP. Gastrointest Endosc 1990; 36: 281–284. [DOI] [PubMed] [Google Scholar]
- 123. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut. Epub ahead of print 10 April 2019. DOI: 10.1136/gutjnl-2018-317817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sugiyama M, Atomi Y, Wada N, et al. Endoscopic transpapillary bile duct biopsy without sphincterotomy for diagnosing biliary strictures: a prospective comparative study with bile and brush cytology. Am J Gastroenterol 1996; 91: 465–467. [PubMed] [Google Scholar]
- 125. Zhai J. UroVysion multi-target fluorescence in situ hybridization assay for the detection of malignant bile duct brushing specimens: a comparison with routine cytology. Acta Cytol 2018; 62: 295–301. [DOI] [PubMed] [Google Scholar]
- 126. Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008; 48: 1106–1117. [DOI] [PubMed] [Google Scholar]
- 127. Halme L, Arola J, Numminen K, et al. Biliary dysplasia in patients with primary sclerosing cholangitis: additional value of DNA ploidity. Liver Int 2012; 32: 783–789. [DOI] [PubMed] [Google Scholar]
- 128. Smoczynski M, Jablonska A, Matyskiel A, et al. Routine brush cytology and fluorescence in situ hybridization for assessment of pancreatobiliary strictures. Gastrointest Endosc 2012; 75: 65–73. [DOI] [PubMed] [Google Scholar]
- 129. Volmar KE, Vollmer RT, Routbort MJ, et al. Pancreatic and bile duct brushing cytology in 1000 cases: review of findings and comparison of preparation methods. Cancer 2006; 108: 231–238. [DOI] [PubMed] [Google Scholar]
- 130. Stewart CJ, Mills PR, Carter R, et al. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol 2001; 54: 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Adamsen S, Olsen M, Jendresen MB, et al. Endobiliary brush biopsy: intra- and interobserver variation in cytological evaluation of brushings from bile duct strictures. Scand J Gastroenterol 2006; 41: 597–603. [DOI] [PubMed] [Google Scholar]
- 132. Kuzu UB, Odemis B, Turhan N, et al. The diagnostic value of brush cytology alone and in combination with tumor markers in pancreaticobiliary strictures. Gastroenterol Res Pract 2015; 2015: 580254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Curcio G, Traina M, Mocciaro F, et al. Intraductal aspiration: a promising new tissue-sampling technique for the diagnosis of suspected malignant biliary strictures. Gastrointest Endosc 2012; 75: 798–804. [DOI] [PubMed] [Google Scholar]
- 134. Hattori M, Nagino M, Ebata T, et al. Prospective study of biliary cytology in suspected perihilar cholangiocarcinoma. Br J Surg 2011; 98: 704–709. [DOI] [PubMed] [Google Scholar]
- 135. Nieburgs HE, Dreiling DA, Rubio C, et al. The morphology of cells in duodenal-drainage smears: histologic origin and pathologic significance. Am J Dig Dis 1962; 7: 489–505. [DOI] [PubMed] [Google Scholar]
- 136. Hart J, Parab M, Mandich D, et al. IMP3 immunocytochemical staining increases sensitivity in the routine cytologic evaluation of biliary brush specimens. Diagn Cytopathol 2012; 40: 321–326. [DOI] [PubMed] [Google Scholar]
- 137. Riener MO, Fritzsche FR, Clavien PA, et al. IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol 2009; 40: 1377–1383. [DOI] [PubMed] [Google Scholar]
- 138. Ali A, Brown V, Denley S, et al. Expression of KOC, S100P, mesothelin and MUC1 in pancreatico-biliary adenocarcinomas: development and utility of a potential diagnostic immunohistochemistry panel. BMC Clin Pathol 2014; 14: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chiang KC, Huang ST, Wu RC, et al. Interferon alpha-inducible protein 27 is an oncogene and highly expressed in cholangiocarcinoma patients with poor survival. Cancer Manag Res 2019; 11: 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Diamantis I, Karamitopoulou E, Perentes E, et al. p53 protein immunoreactivity in extrahepatic bile duct and gallbladder cancer: correlation with tumor grade and survival. Hepatology 1995; 22: 774–779. [PubMed] [Google Scholar]
- 141. Gibbs JF, Schlieman M, Singh P, et al. A pilot study of urokinase-type Plasminogen Activator (uPA) overexpression in the brush cytology of patients with malignant pancreatic or biliary strictures. HPB Surg 2009; 2009: 805971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Koopmann J, Thuluvath PJ, Zahurak ML, et al. Mac-2-binding protein is a diagnostic marker for biliary tract carcinoma. Cancer 2004; 101: 1609–1615. [DOI] [PubMed] [Google Scholar]
- 143. Levy M, Lin F, Xu H, et al. S100P, von Hippel-Lindau gene product, and IMP3 serve as a useful immunohistochemical panel in the diagnosis of adenocarcinoma on endoscopic bile duct biopsy. Hum Pathol 2010; 41: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 144. Ligato S, Zhao H, Mandich D, et al. KOC (K homology domain-containing protein overexpressed in cancer) and S100A4-protein immunoreactivity improves the diagnostic sensitivity of biliary brushing cytology for diagnosing pancreaticobiliary malignancies. Diagn Cytopathol 2008; 36: 561–567. [DOI] [PubMed] [Google Scholar]
- 145. Ordonez NG. Application of mesothelin immunostaining in tumor diagnosis. Am J Surg Pathol 2003; 27: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 146. Stewart CJ, Burke GM. Value of p53 immunostaining in pancreatico-biliary brush cytology specimens. Diagn Cytopathol 2000; 23: 308–313. [DOI] [PubMed] [Google Scholar]
- 147. Tascilar M, Sturm PD, Caspers E, et al. Diagnostic p53 immunostaining of endobiliary brush cytology: preoperative cytology compared with the surgical specimen. Cancer 1999; 87: 306–311. [DOI] [PubMed] [Google Scholar]
- 148. Yeo MK, Kim KH, Lee YM, et al. The usefulness of adding p53 immunocytochemistry to bile drainage cytology for the diagnosis of malignant biliary strictures. Diagn Cytopathol 2017; 45: 592–597. [DOI] [PubMed] [Google Scholar]
- 149. Yu L, Feng M, Kim H, et al. Mesothelin as a potential therapeutic target in human cholangiocarcinoma. J Cancer 2010; 1: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wardell CP, Fujita M, Yamada T, et al. Genomic characterization of biliary tract cancers identifies driver genes and predisposing mutations. J Hepatol 2018; 68: 959–969. [DOI] [PubMed] [Google Scholar]
- 151. Kamp EJCA, Peppelenbosch MP, Doukas M, et al. THU-014: Primary sclerosing cholangitis-associated biliary neoplasia demonstrates a high inter- and intratumour heterogeneity. J Hepatol 2019; 70: e141–e382. [Google Scholar]
- 152. Itoi T, Takei K, Shinohara Y, et al. K-ras codon 12 and p53 mutations in biopsy specimens and bile from biliary tract cancers. Pathol Int 1999; 49: 30–37. [DOI] [PubMed] [Google Scholar]
- 153. Sasaki T, Brakebusch C, Engel J, et al. Mac-2 binding protein is a cell-adhesive protein of the extracellular matrix which self-assembles into ring-like structures and binds beta1 integrins, collagens and fibronectin. EMBO J 1998; 17: 1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wang H, Qiu X, Lin S, et al. Knockdown of IFI27 inhibits cell proliferation and invasion in oral squamous cell carcinoma. World J Surg Oncol 2018; 16: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dowen SE, Crnogorac-Jurcevic T, Gangeswaran R, et al. Expression of S100P and its novel binding partner S100PBPR in early pancreatic cancer. Am J Pathol 2005; 166: 81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Arumugam T, Simeone DM, Van Golen K, et al. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res 2005; 11: 5356–5364. [DOI] [PubMed] [Google Scholar]
- 157. Yantiss RK, Woda BA, Fanger GR, et al. KOC (K homology domain-containing protein overexpressed in cancer): a novel molecular marker that distinguishes between benign and malignant lesions of the pancreas. Am J Surg Pathol 2005; 29: 188–195. [DOI] [PubMed] [Google Scholar]
- 158. Iliopoulos O, Kibel A, Gray S, et al. Tumour suppression by the human von Hippel-Lindau gene product. Nat Med 1995; 1: 822–826. [DOI] [PubMed] [Google Scholar]
- 159. Shiraishi K, Okita K, Kusano N, et al. A comparison of DNA copy number changes detected by comparative genomic hybridization in malignancies of the liver, biliary tract and pancreas. Oncology 2001; 60: 151–161. [DOI] [PubMed] [Google Scholar]
- 160. Ashley MV, Dow BD. The use of microsatellite analysis in population biology: background, methods and potential applications. EXS 1994; 69: 185–201. [DOI] [PubMed] [Google Scholar]
- 161. Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res 2014; 34: 2061–2068. [PubMed] [Google Scholar]
- 162. Mao L, Lee DJ, Tockman MS, et al. Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci USA 1994; 91: 9871–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Adler DG, Witt B. Cytologic diagnosis of biliary strictures: FISH or cut the sensitivity rate? Dig Dis Sci 2018; 63: 549–550. [DOI] [PubMed] [Google Scholar]
- 164. Dudley JC, Zheng Z, McDonald T, et al. Next-generation sequencing and fluorescence in situ hybridization have comparable performance characteristics in the analysis of pancreaticobiliary brushings for malignancy. J Mol Diagn 2016; 18: 124–130. [DOI] [PubMed] [Google Scholar]
- 165. Barr Fritcher EG, Voss JS, Jenkins SM, et al. Primary sclerosing cholangitis with equivocal cytology: fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol 2013; 121: 708–717. [DOI] [PubMed] [Google Scholar]
- 166. Eaton JE, Gossard AA, Talwalkar JA. Recall processes for biliary cytology in primary sclerosing cholangitis. Curr Opin Gastroenterol 2014; 30: 287–294. [DOI] [PubMed] [Google Scholar]
- 167. Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut 2000; 47: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Jain A, Borad MJ, Kelley RK, et al. Cholangiocarcinoma with FGFR genetic aberrations: a unique clinical phenotype. JCO Precis Oncol 2018; 2: 1–12. [DOI] [PubMed] [Google Scholar]
- 169. Mahipal A, Tella SH, Kommalapati A, et al. FGFR2 genomic aberrations: achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev 2019; 78: 1–7. [DOI] [PubMed] [Google Scholar]
- 170. Kendall T, Verheij J, Gaudio E, et al. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int 2019; 39(Suppl. 1): 7–18. [DOI] [PubMed] [Google Scholar]
- 171. Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 2005; 206: 356–365. [DOI] [PubMed] [Google Scholar]
- 172. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 2013; 31: 1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Boeva V, Popova T, Lienard M, et al. Multi-factor data normalization enables the detection of copy number aberrations in amplicon sequencing data. Bioinformatics 2014; 30: 3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Grasso C, Butler T, Rhodes K, et al. Assessing copy number alterations in targeted, amplicon-based next-generation sequencing data. J Mol Diagn 2015; 17: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Pritchard CC, Salipante SJ, Koehler K, et al. Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn 2014; 16: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Dubbink HJ, Atmodimedjo PN, van Marion R, et al. Diagnostic detection of allelic losses and imbalances by next-generation sequencing: 1p/19q co-deletion analysis of gliomas. J Mol Diagn 2016; 18: 775–786. [DOI] [PubMed] [Google Scholar]
- 177. Yoo KH, Kim NK, Kwon WI, et al. Genomic alterations in biliary tract cancer using targeted sequencing. Transl Oncol 2016; 9: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yoon KA, Woo SM, Kim YH, et al. Somatic mutations from whole exome sequencing analysis of the patients with biliary tract cancer. Genomics Inform 2018; 16: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Braconi C, Roessler S, Kruk B, et al. Molecular perturbations in cholangiocarcinoma: is it time for precision medicine? Liver Int 2019; 39(Suppl. 1): 32–42. [DOI] [PubMed] [Google Scholar]
- 180. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015; 47: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 181. Ahrendt SA, Rashid A, Chow JT, et al. p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. J Hepatobiliary Pancreat Surg 2000; 7: 426–431. [DOI] [PubMed] [Google Scholar]
- 182. Boberg KM, Schrumpf E, Bergquist A, et al. Cholangiocarcinoma in primary sclerosing cholangitis: K-ras mutations and Tp53 dysfunction are implicated in the neoplastic development. J Hepatol 2000; 32: 374–380. [DOI] [PubMed] [Google Scholar]
- 183. Hsu M, Sasaki M, Igarashi S, et al. KRAS and GNAS mutations and p53 overexpression in biliary intraepithelial neoplasia and intrahepatic cholangiocarcinomas. Cancer 2013; 119: 1669–1674. [DOI] [PubMed] [Google Scholar]
- 184. Kipp BR, Fritcher EG, Clayton AC, et al. Comparison of KRAS mutation analysis and FISH for detecting pancreatobiliary tract cancer in cytology specimens collected during endoscopic retrograde cholangiopancreatography. J Mol Diagn 2010; 12: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sturm PD, Rauws EA, Hruban RH, et al. Clinical value of K-ras codon 12 analysis and endobiliary brush cytology for the diagnosis of malignant extrahepatic bile duct stenosis. Clin Cancer Res 1999; 5: 629–635. [PubMed] [Google Scholar]
- 186. Jain A, Kwong LN, Javle M. Genomic profiling of biliary tract cancers and implications for clinical practice. Curr Treat Options Oncol 2016; 17: 58. [DOI] [PubMed] [Google Scholar]
- 187. Leone F, Cavalloni G, Pignochino Y, et al. Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res 2006; 12: 1680–1685. [DOI] [PubMed] [Google Scholar]
- 188. Geurts-Giele WR, Dirkx-van der Velden AW, Bartalits NM, et al. Molecular diagnostics of a single multifocal non-small cell lung cancer case using targeted next-generation sequencing. Virchows Arch 2013; 462: 249–254. [DOI] [PubMed] [Google Scholar]
- 189. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500. [DOI] [PubMed] [Google Scholar]
- 190. Faris JE, Zhu AX. Targeted therapy for biliary tract cancers. J Hepatobiliary Pancreat Sci 2012; 19: 326–336. [DOI] [PubMed] [Google Scholar]
- 191. Moon A, Chin S, Kim HK, et al. EGFR, COX2, p-AKT expression and PIK3CA mutation in distal extrahepatic bile duct carcinoma. Pathology 2016; 48: 35–40. [DOI] [PubMed] [Google Scholar]
- 192. Churi CR, Shroff R, Wang Y, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One 2014; 9: e115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Lee H, Wang K, Johnson A, et al. Comprehensive genomic profiling of extrahepatic cholangiocarcinoma reveals a long tail of therapeutic targets. J Clin Pathol 2016; 69: 403–408. [DOI] [PubMed] [Google Scholar]
- 194. Putra J, de Abreu FB, Peterson JD, et al. Molecular profiling of intrahepatic and extrahepatic cholangiocarcinoma using next-generation sequencing. Exp Mol Pathol 2015; 99: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Weinberg BA, Xiu J, Lindberg MR, et al. Molecular profiling of biliary cancers reveals distinct molecular alterations and potential therapeutic targets. J Gastrointest Oncol 2019; 10: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013; 45: 1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol 2019; 10: 751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Kamp EJCA, Dinjens WNM, Doukas M, et al. THU-015: Mutational signatures during the neoplastic cascade towards cholangiocarcinoma in primary sclerosing cholangitis. J Hepatol 2019; 70: e141–e382. [Google Scholar]
- 199. Goeppert B, Folseraas T, Roessler S, et al. Genomic characterization of cholangiocarcinoma in primary sclerosing cholangitis reveals therapeutic opportunities. Hepatology 2020; 72: 1253–1266. [DOI] [PubMed] [Google Scholar]
- 200. Rehm HL, Bale SJ, Bayrak-Toydemir P, et al. ACMG clinical laboratory standards for next-generation sequencing. Genet Med 2013; 15: 733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Shen N, Zhang D, Yin L, et al. Bile cell-free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol Rep 2019; 42: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Husain H, Melnikova VO, Kosco K, et al. Monitoring daily dynamics of early tumor response to targeted therapy by detecting circulating tumor DNA in urine. Clin Cancer Res 2017; 23: 4716–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rizzo A, Ricci AD, Tavolari S, et al. Circulating tumor DNA in biliary tract cancer: current evidence and future perspectives. Cancer Genomics Proteomics 2020; 17: 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Benesova L, Belsanova B, Suchanek S, et al. Mutation-based detection and monitoring of cell-free tumor DNA in peripheral blood of cancer patients. Anal Biochem 2013; 433: 227–234. [DOI] [PubMed] [Google Scholar]
- 205. Ettrich TJ, Schwerdel D, Dolnik A, et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep 2019; 9: 13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zill OA, Greene C, Sebisanovic D, et al. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov 2015; 5: 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Pishvaian MJ, Joseph Bender R, Matrisian LM, et al. A pilot study evaluating concordance between blood-based and patient-matched tumor molecular testing within pancreatic cancer patients participating in the Know Your Tumor (KYT) initiative. Oncotarget 2016; 8: 83446–83456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Driescher C, Fuchs K, Haeberle L, et al. Bile-based cell-free DNA analysis is a reliable diagnostic tool in pancreatobiliary cancer. Cancers (Basel) 2020; 13: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kawahara A, Fukumitsu C, Taira T, et al. Epidermal growth factor receptor mutation status in cell-free DNA supernatant of bronchial washings and brushings. Cancer Cytopathol 2015; 123: 620–628. [DOI] [PubMed] [Google Scholar]
- 210. Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol 2014; 9: 287–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kong YW, Ferland-McCollough D, Jackson TJ, et al. MicroRNAs in cancer management. Lancet Oncol 2012; 13: e249–e258. [DOI] [PubMed] [Google Scholar]
- 212. Schwarzenbach H. The clinical relevance of circulating, exosomal miRNAs as biomarkers for cancer. Expert Rev Mol Diagn 2015; 15: 1159–1169. [DOI] [PubMed] [Google Scholar]
- 213. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A 2008; 105: 10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Bernuzzi F, Marabita F, Lleo A, et al. Serum microRNAs as novel biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Clin Exp Immunol 2016; 185: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature 2007; 445: 851–857. [DOI] [PubMed] [Google Scholar]
- 216. Janku F, Stewart DJ, Kurzrock R. Targeted therapy in non-small-cell lung cancer–is it becoming a reality? Nat Rev Clin Oncol 2010; 7: 401–414. [DOI] [PubMed] [Google Scholar]
- 217. Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology 2011; 53: 695–704. [DOI] [PubMed] [Google Scholar]
- 218. Lamarca A, Barriuso J, McNamara MG, et al. Molecular targeted therapies: ready for “prime time” in biliary tract cancer. J Hepatol 2020; 73: 170–185. [DOI] [PubMed] [Google Scholar]
- 219. Hezel AF, Deshpande V, Zhu AX. Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol 2010; 28: 3531–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Rahnemai-Azar AA, Abbasi A, Acher AW, et al. Emerging pathways for precision medicine in management of cholangiocarcinoma. Surg Oncol 2020; 35: 47–55. [DOI] [PubMed] [Google Scholar]
- 221. Bankov K, Doring C, Schneider M, et al. Sequencing of intraductal biopsies is feasible and potentially impacts clinical management of patients with indeterminate biliary stricture and cholangiocarcinoma. Clin Transl Gastroenterol 2018; 9: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011; 29: 2357–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006; 24: 3069–3074. [DOI] [PubMed] [Google Scholar]
- 224. Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol 2009; 64: 777–783. [DOI] [PubMed] [Google Scholar]
- 225. Bengala C, Bertolini F, Malavasi N, et al. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer 2010; 102: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lee S, Kim WH, Jung HY, et al. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol 2002; 161: 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Andresen K, Boberg KM, Vedeld HM, et al. Novel target genes and a valid biomarker panel identified for cholangiocarcinoma. Epigenetics 2012; 7: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Baylin SB, Herman JG, Graff JR, et al. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res 1998; 72: 141–196. [PubMed] [Google Scholar]
- 229. Timmer MR, Beuers U, Fockens P, et al. Genetic and epigenetic abnormalities in primary sclerosing cholangitis-associated cholangiocarcinoma. Inflamm Bowel Dis 2013; 19: 1789–1797. [DOI] [PubMed] [Google Scholar]
- 230. Fujiwara K, Fujimoto N, Tabata M, et al. Identification of epigenetic aberrant promoter methylation in serum DNA is useful for early detection of lung cancer. Clin Cancer Res 2005; 11: 1219–1225. [PubMed] [Google Scholar]
- 231. Belinsky SA, Nikula KJ, Palmisano WA, et al. Aberrant methylation of p16INK4a is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA 1998; 95: 11891–11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Nakaoka T, Saito Y, Saito H. Aberrant DNA methylation as a biomarker and a therapeutic target of cholangiocarcinoma. Int J Mol Sci 2017; 18: 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Wong N, Li L, Tsang K, et al. Frequent loss of chromosome 3p and hypermethylation of RASSF1A in cholangiocarcinoma. J Hepatol 2002; 37: 633–639. [DOI] [PubMed] [Google Scholar]
- 234. Yang B, House MG, Guo M, et al. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol 2005; 18: 412–420. [DOI] [PubMed] [Google Scholar]
- 235. Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer 2006; 6: 857–866. [DOI] [PubMed] [Google Scholar]
- 236. Meng F, Wehbe-Janek H, Henson R, et al. Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene 2008; 27: 378–386. [DOI] [PubMed] [Google Scholar]
- 237. Meng F, Yamagiwa Y, Ueno Y, et al. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol 2006; 44: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-jpg-1-tag-10.1177_17562848211002023 for Optimal tissue sampling during ERCP and emerging molecular techniques for the differentiation of benign and malignant biliary strictures by Eline J. C. A. Kamp, Winand N. M. Dinjens, Michail Doukas, Marco J. Bruno, Pieter Jan F. de Jonge, Maikel P. Peppelenbosch and Annemarie C. de Vries in Therapeutic Advances in Gastroenterology


