Significance
We use distribution data on 48,661 species to show that marine biodiversity has been responding to climate warming at a global scale. We show that marine species richness levels off or declines in latitudinal bands with average annual sea surface temperatures exceeding 20 °C. This results in a dip in species richness around the equator that has become more pronounced as the climate has warmed, especially for pelagic species. Previous studies have either only predicted such effects or have provided data at regional scales or for limited numbers of taxa.
Keywords: species richness, latitudinal gradient, climate change, OBIS, GAM
Abstract
The latitudinal gradient in species richness, with more species in the tropics and richness declining with latitude, is widely known and has been assumed to be stable over recent centuries. We analyzed data on 48,661 marine animal species since 1955, accounting for sampling variation, to assess whether the global latitudinal gradient in species richness is being impacted by climate change. We confirm recent studies that show a slight dip in species richness at the equator. Moreover, richness across latitudinal bands was sensitive to temperature, reaching a plateau or declining above a mean annual sea surface temperature of 20 °C for most taxa. In response, since the 1970s, species richness has declined at the equator relative to an increase at midlatitudes and has shifted north in the northern hemisphere, particularly among pelagic species. This pattern is consistent with the hypothesis that climate change is impacting the latitudinal gradient in marine biodiversity at a global scale. The intensification of the dip in species richness at the equator, especially for pelagic species, suggests that it is already too warm there for some species to survive.
The latitudinal gradient in species richness is a striking biogeographic pattern in both terrestrial and marine realms that is likely to reflect evolutionary history and current environmental conditions (1–4). It is strongly correlated with temperature (5–8) (SI Appendix, Table S1) and may thus serve as a natural laboratory to study the impact of climate change (9). A unimodal latitudinal gradient in species richness peaking at the equator had been assumed to be the general pattern for most taxa (10–15). However, the majority of global studies have been limited to a specific taxonomic group, and multitaxon studies have been regional, making generalizations difficult. Recently, in a review of 27 studies and a dataset of 65,000 species, Chaudhary et al. (10, 16) suggested that the distribution of marine diversity was bimodal, with a dip at the equator, and that all marine taxa followed this pattern, with the possible exception of planktonic radiolarians (17), which are found deeper in tropical waters (the so-called “tropical submergence”) (18). Species distribution models forced by Earth system models predict that the leading (cool) edge of species’ distributions will move away from the equator in the future (19), which could further depress equatorial richness relative to midlatitudes. This begs the question: Is climate change already altering the global latitudinal gradient in species richness? Here, we analyze the latitudinal pattern in species richness for a suite of taxonomic groups based on 48,661 marine species to assess whether there was a consistent dip in species richness at the equator and what role ocean warming might play as a driver of changing latitudinal distribution of marine biodiversity.
Results
Bimodality in Species Richness with Latitude.
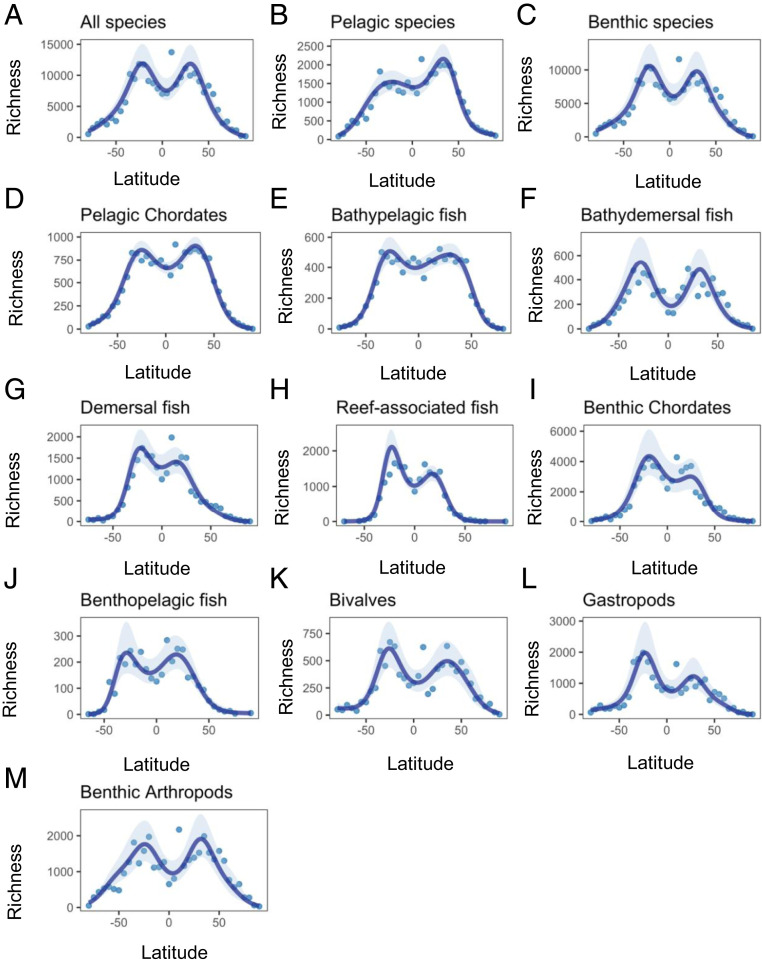
Estimates of asymptotic diversity for entire assemblages of marine organisms derived using Hill numbers (20) confirmed that the latitudinal gradient in species richness was bimodal for all individual taxonomic groups, as well as when these groups were pooled by habitat (pelagic species or benthic species) or all considered together (all species) (Fig. 1). The generalized additive models (GAMs) explained 88–99% of the variation in richness (SI Appendix, Table S2). There was a clear symmetrical bimodal pattern for all species, benthic species, and most of the taxonomic groups, except for pelagic species, reef-associated fish, and gastropods (Fig. 1). All groups showed sharp declines in richness toward the poles.
Fig. 1.
The latitudinal distribution of species richness in marine taxa at the scale of 5° latitudinal bands based on GAMs (the effect of latitude adjusting for shelf area) (SI Appendix, Table S2): (A) all species; (B) pelagic species; (C) benthic species; (D and E) organisms in the pelagic environment; and (F–M) organisms living near, on, or in the seabed. The shaded region in each graph shows the 95% confidence envelope for the fit.
Temperature and Other Potential Predictors of Species Richness.
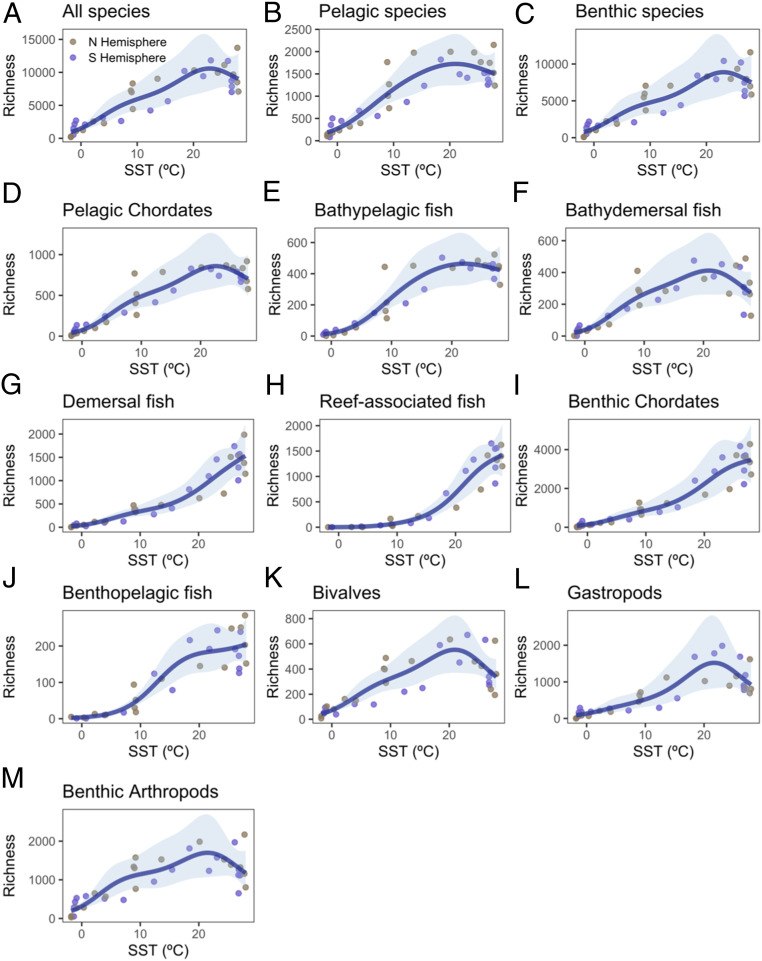
Mean annual sea surface temperature (SST) consistently explained less variation in species richness across all groups than did latitude (61–89%; SI Appendix, Table S3). Species richness declined or plateaued in latitudinal bands with waters >20 °C for all, pelagic, and benthic species (Fig. 2), as well as all individual taxonomic groups except demersal and reef-associated fish (Fig. 2). As expected, taxa that exhibited a greater tendency toward decline in species richness at temperatures >20 °C—such as bathydemersal fish, bivalves, gastropods, and benthic arthropods—generally showed the clearest dip in species richness near the equator (Fig. 1).
Fig. 2.
The relationship between species richness and SST based on GAMs (SI Appendix, Table S3): (A) all species; (B) pelagic species; (C) benthic species; (D and E) organisms in the pelagic environment; and (F–M) seabed-associated organisms. The shaded region in each graph shows the 95% confidence envelope for the fit.
Impact of Ocean Warming.
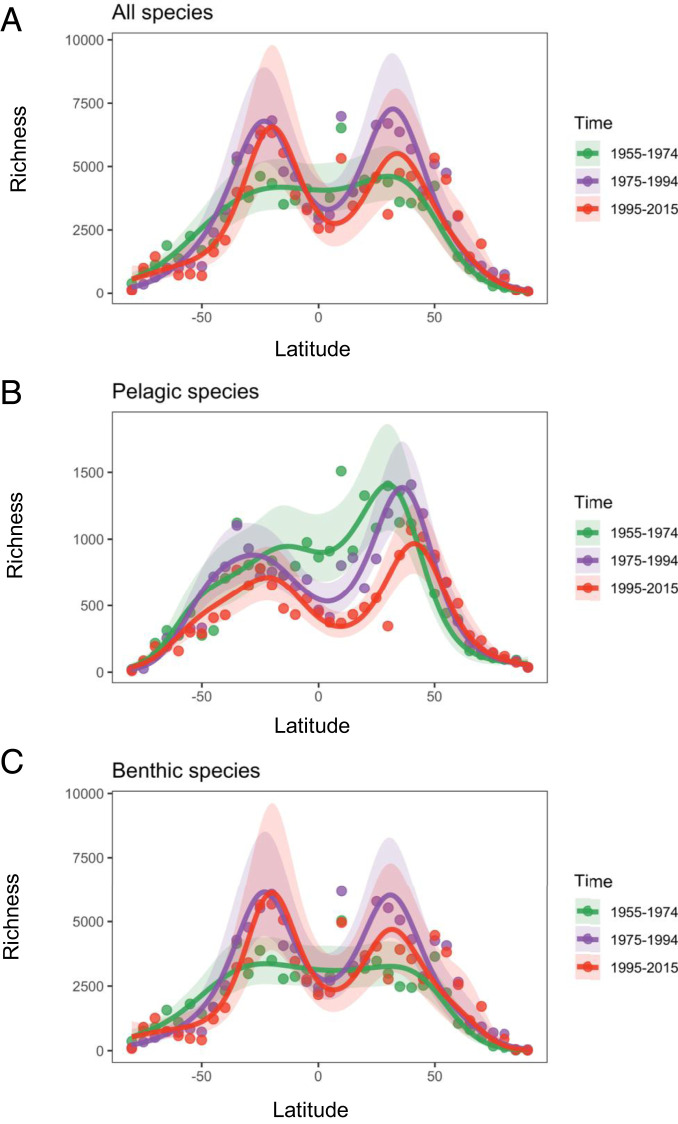
To investigate the potential effect of ocean warming, we examined how the latitudinal gradient of species richness changed over three time periods: 1955 to 1974, 1975 to 1994, and 1995 to 2015 (Fig. 3). There was 0.06 °C warming from 1955 to 1974, 0.10 °C warming from 1975 to 1994, and 0.08 °C from 1995 to 2015 (SI Appendix, Fig. S1). The GAMs explained >72% of the variation in all, pelagic, and benthic species in each time period (SI Appendix, Table S4). From 1955 to 1974, the distribution of pelagic species were weakly bimodal, with a larger peak in the northern hemisphere (Fig. 3B), and benthic species had a broad equatorial peak in biodiversity with no bimodality (Fig. 3C). However, from 1975, the patterns changed markedly. For pelagic species, richness declined at the equator and there was relatively higher richness in the subtropics over successive periods as the temperature warmed, resulting in a more pronounced trough in diversity around the equator. The equatorial dip not only intensified but also widened and shifted northward from 0° to 10°N (Fig. 3B). For benthic species, there was only a hint of a decline in richness at the equator from 1975, but richness clearly increased in the subtropics, again resulting in a more pronounced dip at the equator, consistent with warming (SI Appendix, Fig. S1). These distinct peaks in benthic species in the subtropics did not previously exist (Fig. 3C).
Fig. 3.
Latitudinal distribution in species richness using GAMs (the effect of latitude adjusting for shelf area in each 5° latitudinal band) (SI Appendix, Table S4) in (A) all species, (B) pelagic, and (C) benthic species in three time periods: 1955 to 1974 (green), 1975 to 1994 (purple), and 1995 to 2015 (red). Shaded regions represent 95% confidence envelopes for models fit to each of the periods, with shades (from green to red) reflecting progression of time periods from older to more recent.
Changes in the shape of the latitudinal diversity gradient are most likely explained by the effect of warming causing the geographic range expansion of species and migration into new latitudinal zones. This is especially clear in the northern hemisphere, where there was a rapid poleward shift in the biodiversity peak from 30°N in 1955 to 1974, to 45°N in 1995 to 2015, which is not as clear in the southern hemisphere (SI Appendix, Fig. S2). The progressive poleward expansion of the leading (cool) edges of species’ distributional ranges is evident through time, especially for species found predominantly in the northern hemisphere (SI Appendix, Fig. S2C). For species found in both hemispheres, southern range edges initially located close to the equator moved progressively south through time (SI Appendix, Fig. S2B), although patterns were less clear for northern range edges (SI Appendix, Fig. S2A).
Discussion
The latitudinal gradient in species richness is perhaps the best known global biogeographic pattern and has been presumed to be stable over centuries. Using OBIS (https://obis.org/), the largest available dataset on global biodiversity, we show that the dip in the latitudinal pattern of marine species richness around the equator has deepened with global warming, as predicted (8, 19). Ocean warming is thus causing large-scale changes in the global latitudinal distribution of marine biodiversity. Despite less warming in the ocean than on land, marine species are shifting their distributions as fast or faster in response to warming than those on land (21, 22). It remains an open question whether the latitudinal gradient in species richness is also changing on land.
We also confirm bimodality in the latitudinal distribution pattern of marine species richness. While it has been suggested that the cause of the equatorial dip in species richness was undersampling in the tropics (23), we accounted for this here by using Hill numbers to estimate asymptotic diversity (see Materials and Methods). The application of this approach to the Ocean Biodiversity Information System (OBIS) data seems robust in that the greater sampling in the northern hemisphere did not produce uniformly higher richness peaks (considering confidence envelopes, there were few clear differences in species richness peaks between northern and southern hemispheres, except for pelagic species). A global analysis of fish species ranges that minimized the effect of sampling gaps found a similar bimodal pattern (24), as did other studies on tropical pelagic biodiversity (8), marine fish (24), amphipods (25), polychaetes (26), and a suite of fossil and living taxa (27). We found the equatorial dip in species richness when considering all taxa, including pelagic and benthic chordates, and benthic invertebrates (bivalves, gastropods, and arthropods), despite variations in their ecology, methods used to sample them, ease of identification, and the amount of taxonomic attention each group has received.
The strong nonlinear relationship between species richness and SST—with richness plateauing and sometimes declining in latitudinal bands with water temperatures above 20 °C for most taxa—is a clear explanation for the deepening equatorial dip over time as the ocean has warmed. A similar pattern has been found in other studies on benthic and pelagic species, although these had restricted taxonomic and geographic coverage (7, 8, 24–29).
The greater latitudinal shift in species richness in the northern than southern hemisphere species is likely to be a consequence of the greater warming in that hemisphere (21). Further, in the northern hemisphere the greater latitudinal shift in the richness of pelagic species compared with benthic species post-1975 suggests that pelagic species respond more quickly to climate warming than benthic ones (22, 30). This may be because demersal and benthic species have access to thermal refugia in deeper water, as suggested by studies on demersal fish (31, 32). Alternatively, pelagic species might simply be more responsive to climate change by virtue of their greater motility.
The increasing equatorial dip and movement of the richness peaks toward the subtropics with climate change should not be surprising given that it has been clearly observed in the fossil record in response to previous warming events. For example, during the last Pleistocene interglacial period, reef corals also exhibited an equatorial decline and shift toward the subtropics (33). Similarly, there was a sudden loss in equatorial diversity in the late Quaternary (8) and early Triassic (34) in response to warming. An abrupt loss of diversity at the tropics has also been predicted in the future under different climatic scenarios (34). Our results, together with previous research, show that equatorial biodiversity is threatened by and is responding to climate change now. The decreasing relative richness at the equator since 1975, especially for pelagic species, suggests that the equator is already too hot for some species to survive and indicates that further low-latitude declines of species are likely with continued warming.
Materials and Methods
Species Data.
Data on the distribution of species used in our analyses were downloaded from OBIS (35). These data, collected since 1920, included species that could be defined as benthic or pelagic based on a literature review, and had sufficient occurrences for global analysis based on their distribution. We further cleaned the data and removed low-quality observations with high probability of errors. The final quality-controlled data used for analysis had 6,917,656 observations for 48,661 species, with 43,249 benthic species (4,386,802 records) and 5,412 pelagic species (2,530,854 records).
Taxa with most occurrence records were Chordata, Arthropoda, Cnidaria, Echinodermata, and Mollusca. Each of these five taxonomic groups had observations for >3,000 species and >150,000 occurrences (SI Appendix, Table S5). Species in these groups were defined as benthic if they were exclusively benthic or had any benthic life stage (except 74 species of jellyfish, which were considered pelagic). Species were defined as pelagic if they had no benthic life stage, according to FishBase (36), MolluscaBase (37), and the literature (38). Chordates comprised fish, tunicates, mammals, birds, and reptiles (Sauria, Squamata, and Testudines).
Fish species were classified based on their depth distribution and functional groups in accordance with FishBase (36): demersal (near or on the seabed) and coral reef-associated fish between 0 m and 200 m depth; bathy-demersal fish below 200 m depth; and bathy-pelagic fish between 1,000 m and 4,000 m. There were insufficient records in other depth zones for separate analyses.
Benthic vertebrates included four fish groups: demersal, reef-associated, bathy-demersal, and bentho-pelagic. Benthic chordates included these vertebrates and benthic tunicates. Pelagic chordates included pelagic fish, tunicates, mammals, birds, cephalochordates, and reptiles (Sauria, Squamata, and Testudines) and bathy-pelagic fish. Benthic invertebrates with sufficient geographic data for inclusion separately in the analysis were arthropods, bivalves, and gastropods. Thus, there were five groups of fish, three groups of invertebrates, two groups of chordates (benthic and pelagic), and collectively, thirteen benthic and pelagic taxonomic groups, including all species, pelagic species, and benthic species.
To ensure a sufficient number of data points for robust statistical analysis among the ten taxonomic groups, we aggregated the data into 5° latitudinal bands, starting with a band centered at the equator. To analyze the change in latitudinal pattern in species richness over time, data for benthic and pelagic species were subdivided into three time periods, 1955 to 1974, 1975 to 1994, and 1995 to 2015 (data before 1955 were too scarce to include as a separate period). These periods were selected to ensure that there were sufficient data with global spatial coverage in each period (SI Appendix, Table S6 and Fig. S3) and so that the strong warming signal in ocean temperature was captured over the time range (SI Appendix, Fig. S1). Since the 1980s, global warming has intensified, with the greatest increases in the North Atlantic (39).
Temperature Data.
We used SST as our common index of warming for pelagic and benthic species to remain consistent for all groups analyzed within the study and with the literature and, also, because of the unavailability of observed sea bottom temperatures globally during the earlier part of our data range (to correlate with benthic species richness). There is a high correlation (0.84) between SST and seabed temperature (http://gmed.auckland.ac.nz/layersd.html). In addition to the poor temporal and spatial coverage of seabed temperatures, they seem to correlate less well with demersal fish responses to climate warming (40). SST is also the most common predictor used in studies of climate change ecology (22, 31) (SI Appendix, Table S1). We thus used SST as a reasonable proxy of temperature for all fish and invertebrate species. We used monthly data from the Hadley Centre Global Sea Ice and Sea Surface Temperature (HadISST 1.1) database (41). We computed the (area-weighted) mean decadal SST for the 100-y period 1920 to 2019 (decade starting from 1920) in the R (42) package raster (43).
Geographical Data.
Higher coastal diversity has also been attributed to the presence of more biogenic habitats in the shallow waters of the continental shelves (40), although these are often also species in their own right. The presence of mangroves, seagrass, and coral reefs in tropical coastal regions creates greater habitat heterogeneity and higher productivity. In 7 of 29 studies reviewed here (SI Appendix, Table S1), food availability and productivity have been reported as the most common explanations for the latitudinal gradient in species richness after temperature. Thus, it is possible that latitudes with more continental shelf area would harbor more species than latitudes with less continental shelf area. Thus, we assessed the effect of ocean and shelf area on the latitudinal pattern in species richness. The ocean areas per 5° latitudinal band were calculated in R (42) using a 1:10,000,000 shapefile for global land area from Natural Earth (https://www.naturalearthdata.com/downloads/10m-physical-vectors/) to define the global ocean, together with appropriate shapefiles for the continental shelf and oceanic slope from Blue Habitats (44). In each instance, shapefiles were rasterized (43) to 0.05° resolution before summing areas for ocean, slope, and shelf, respectively, per 5° latitudinal band.
Data Analysis.
Sampling bias.
Sampling bias can be taxonomic, methodological, geographic, or temporal. To minimize taxonomic bias, we analyzed only those records identified to species. Methodological bias can arise because different methods are used to sample species from different habitats and body sizes (45). Analyses across a wide range of taxa and habitats—as undertaken here—is more likely to subsume methodological biases. In terms of geographic biases, coastal areas and surface waters have been sampled more than the deep sea (45), and the frequency of sampling varies over time and location (46). Here, we accounted for such biases using the framework of Hill numbers to obtain asymptotic diversity estimates with which to infer true diversities of entire assemblages (20). To account for the different sampling effort in each latitudinal band and its effect on estimates of species richness, we used the Hill number of order q = 0 for presence data. This is based on the relative probability of species detection in any occurrence record (20, 47). We used the R package iNEXT (48) to extrapolate the expected number of species, using the incidence rates of species (20, 47). We further rounded extrapolations to the nearest whole number so that they were counts.
GAM.
GAM was used to investigate the expected nonlinear relationship of the estimated number of species with environmental and geographical predictors using the package “mgcv” (49) in R. GAMs sum a series of smoothed functions of individual covariates and can thus capture nonlinearity (50). Two models were developed: a model of species richness as a function of latitude and a model of species richness as a function of SST. We fitted the models with a Poisson and negative binomial error structures and based on diagnostic plots of their residuals (improved homogeneity of variance and normality of residuals) and lower AIC, we chose negative binomial (SI Appendix, Table S7). After initial inspection of results, we set the basis dimension of the spline smoother (related to the flexibility and the estimated degrees of freedom of the smooth) to be nine for latitude and five for SST to ensure consistency across the different models for each response variable. However, using generalized cross validation to estimate the degree of smoothness for each model fit gives very similar results (SI Appendix, Figs. S4–S6). To adjust for other predictors that might be important for predicting marine species richness, we included the oceanic area and the shelf area as linear terms in the models with latitude. The slope area was highly correlated with the oceanic area (0.69) and added complexity to the models considering the relatively few degrees of freedom (n = 35); it was thus excluded from the models. Oceanic area was found to be not significant, but shelf area was in most of the models. We thus included shelf area in all models with latitude (SI Appendix, Tables S2 and S4), but have only shown latitude in the main figures.
To compare potential range shifts among species in the three periods, we selected the species that were common among these periods (11,252 species). For each species and year, we calculated the 2.5th, 97.5th percentile and median latitude of available observations (assuming north is positive, so the 97.5th percentile is in the north and the 2.5th percentile is in the south). These were then aggregated to year, using the minimum, maximum, and median values (of the 2.5th, 97.5th, and 50th percentiles, respectively)—i.e., the extremes across years within each species for each period. Based on the number of observations in the northern and southern hemisphere per period, we classified “northern species” (>75% of observations in the north), “southern species” (>75% of observations in the south), or cosmopolitan (the remaining species). We then constructed kernel density plots across species of extreme latitudes of observation. These represent the poleward-advancing “leading” (cool) range edge.
Supplementary Material
Acknowledgments
We thank Ward Appeltans and Pieter Provost for facilitating access to the Ocean Biodiversity Information System and all the data providers of the database. C.C. was part-funded by the European Marine Observation Data Network (EMODnet) Biology project (https://www.emodnet-biology.eu/) funded by the European Commission’s Directorate-General for Maritime Affairs and Fisheries (DG MARE) to M.J.C. The authors acknowledge funding from the University of Auckland International Office to visit the University of Queensland and departmental funding for the preparation of this manuscript. We thank Dr. Irawan Asaad, Dr. Rakshan Roohi, Dr. Dinusha Jayathilake, Julian Uribe Palamino, and Thomas Morris for helpful discussions, and Dr. Qianshuo Zhao for his help in preparation of temperature data.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015094118/-/DCSupplemental.
Data Availability
Data have been deposited in The University of Auckland research repository (https://doi.org/10.17608/k6.auckland.12672884.v1).
References
- 1.Hillebrand H., On the generality of the latitudinal diversity gradient. Am. Nat. 163, 192–211 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Brown J. H., Why are there so many species in the tropics? J. Biogeogr. 41, 8–22 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schemske D. W., Mittelbach G. G., Cornell H. V., Sobel J. M., Roy K., Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 40, 245–269 (2009). [Google Scholar]
- 4.Condamine F. L., Sperling F. A., Wahlberg N., Rasplus J. Y., Kergoat G. J., What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 15, 267–277 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Macpherson E., Large-scale species-richness gradients in the Atlantic ocean. Proc. Biol. Sci. 269, 1715–1720 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rombouts I., et al., Global latitudinal variations in marine copepod diversity and environmental factors. Proc. R. Soc. B 276, 3053–3062 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeedi H., Dennis T. E., Costello M. J., Bimodal latitudinal species richness and high endemicity in razor clams (Mollusca). J. Biogeogr. 44, 592–604 (2017). [Google Scholar]
- 8.Yasuhara M., et al., Past and future decline of tropical pelagic biodiversity. Proc. Natl. Acad. Sci. U.S.A. 117, 12891–12896 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Frenne P., et al., Latitudinal gradients as natural laboratories to infer species’ responses to temperature. J. Ecol. 101, 784–795 (2013). [Google Scholar]
- 10.Chaudhary C., Saeedi H., Costello M. J., Bimodality of latitudinal gradients in marine species richness. Trends Ecol. Evol. 31, 670–676 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Powell M. G., Glazier D. S., Asymmetric geographic range expansion explains the latitudinal diversity gradients of four major taxa of marine plankton. Paleobiology 43, 1–13 (2017). [Google Scholar]
- 12.Hillebrand H., Strength, slope and variability of marine latitudinal gradients. Mar. Ecol. Prog. Ser. 273, 251–267 (2004). [Google Scholar]
- 13.Yasuhara M., Hunt G., Dowsett H. J., Robinson M. M., Stoll D. K., Latitudinal species diversity gradient of marine zooplankton for the last three million years. Ecol. Lett. 15, 1174–1179 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Yasuhara M., Tittensor D. P., Hillebrand H., Worm B., Combining marine macroecology and palaeoecology in understanding biodiversity: Microfossils as a model. Biol. Rev. Camb. Philos. Soc. 92, 199–215 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Yasuhara M., et al., Cenozoic dynamics of shallow‐marine biodiversity in the Western Pacific. J. Biogeogr. 44, 567–578 (2017). [Google Scholar]
- 16.Chaudhary C., Saeedi H., Costello M. J., Marine species richness is bimodal with latitude: A reply to Fernandez and Marques. Trends Ecol. Evol. 32, 234–237 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Boltovskoy D., Correa N., Planktonic equatorial diversity troughs: Fact or artifact? Latitudinal diversity gradients in radiolaria. Ecology 98, 112–124 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Ishitani Y., Takahashi K., Okazaki Y., Tanaka S., Vertical and geographic distribution of selected radiolarian species in the North Pacific. Micropaleontology 54, 27–39 (2008). [Google Scholar]
- 19.Molinos J. G., et al., Climate velocity and the future global redistribution of marine biodiversity. Nat. Clim. Chang. 6, 83–88 (2016). [Google Scholar]
- 20.Chao A., et al., Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 35, 292–314 (2020). [Google Scholar]
- 21.Burrows M. T., et al., The pace of shifting climate in marine and terrestrial ecosystems. Science 334, 652–655 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Poloczanska E. S., et al., Global imprint of climate change on marine life. Nat. Clim. Chang. 3, 919–925 (2013). [Google Scholar]
- 23.Menegotto A., Rangel T. F., Mapping knowledge gaps in marine diversity reveals a latitudinal gradient of missing species richness. Nat. Commun. 9, 4713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H.-Y., Corkrey R., Kaschner K., Garilao C., Costello M. J., Latitudinal diversity gradients for five taxonomic levels of marine fish in depth zones. Ecol. Res. 36, 266–280 (2020). [Google Scholar]
- 25.Arfianti T., Costello M. J., Global biogeography of marine amphipod crustaceans: Latitude, regionalisation, and beta diversity. Mar. Ecol. Prog. Ser. 638, 83–94 (2020). [Google Scholar]
- 26.Pamungkas J., Glasby C. J., Costello M. J., Biogeography of polychaete worms (Annelida) of the world. Mar. Ecol. Prog. Ser. 657, 147–159 (2021). [Google Scholar]
- 27.Powell M. G., Beresford V. P., Colaianne B. A., The latitudinal position of peak marine diversity in living and fossil biotas. J. Biogeogr. 39, 1687–1694 (2012). [Google Scholar]
- 28.Brayard A., Escarguel G., Bucher H., Latitudinal gradient of taxonomic richness: Combined outcome of temperature and geographic mid‐domains effects? J. Zool. Syst. Evol. Res. 43, 178–188 (2005). [Google Scholar]
- 29.Hobday A. J., Ensemble analysis of the future distribution of large pelagic fishes off Australia. Prog. Oceanogr. 86, 291–301 (2010). [Google Scholar]
- 30.Burrows M. T., et al., Thermal affinities and vertical temperature gradients explain recent responses to warming in ocean communities. Nat. Clim. Chang. 9, 959–963 (2019). [Google Scholar]
- 31.Pinsky M. L., Eikeset A. M., McCauley D. J., Payne J. L., Sunday J. M., Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Kiessling W., Simpson C., Beck B., Mewis H., Pandolfi J. M., Equatorial decline of reef corals during the last Pleistocene interglacial. Proc. Natl. Acad. Sci. U.S.A. 109, 21378–21383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song H., et al., Flat latitudinal diversity gradient caused by the Permian–Triassic mass extinction. Proc. Natl. Acad. Sci. U.S.A. 117, 17578–17583 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trisos C. H., Merow C., Pigot A. L., The projected timing of abrupt ecological disruption from climate change. Nature 580, 496–501 (2020). [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary C., Costello M., Occurrence records of marine species. Figshare. 10.17608/k6.auckland.12672884.v1. Deposited 19 July 2020. [DOI]
- 36.Froese R., Pauly D., FishBase. Version 10/2016. https://www.fishbase.in/. Accessed 15 July 2016.
- 37.MolluscaBase Editors , MolluscaBase. http://www.molluscabase.org. Accessed 4 February 2016.
- 38.Brusca R. C., Brusca G. J., Invertebrates (Sinauer Associates, Sunderland, MA, 2003), p. 2. [Google Scholar]
- 39.Solomon S., Qin D., Manning M., Averyt K., Marquis M., Eds., “Observations: Oceanic climate change and sea level” in Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC, (Cambridge University Press, New York, 2007), vol. 4. [Google Scholar]
- 40.Costello M. J., Chaudhary C., Marine biodiversity, biogeography, deep-sea gradients, and conservation. Curr. Biol. 27, R511–R527 (2017). [DOI] [PubMed] [Google Scholar]
- 41.UK Meteorological Office, Hadley Centre, Data from “HadISST 1.1–Global sea-ice coverage and SST (1870-Present).” British Atmospheric Data Centre. https://catalogue.ceda.ac.uk/uuid/facafa2ae494597166217a9121a62d3c. Accessed 7 October 2016.
- 42.R Core Team , R: A Language and Environment for Statistical Computing (Version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria, 2020). https://www.R-project.org/. Accessed 25 July 2015.
- 43.Robert J., Hijmans raster: Geographic Data Analysis and Modeling (Version 3.4-5, R package, 2020). https://cran.r-project.org/package=raster. Accessed 11 December 2020.
- 44.Harris P. T., Macmillan-Lawler M., Rupp J., Baker E. K., Seafloor geomorphic Features Map by geomorphology of the oceans. Mar. Geol. 352, 4–24 (2014). [Google Scholar]
- 45.Costello M. J., et al., Marine biogeographic realms and species endemicity. Nat. Commun. 8, 1057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costello M. J., Vanhoorne B., Appeltans W., Conservation of biodiversity through taxonomy, data publication, and collaborative infrastructures. Conserv. Biol. 29, 1094–1099 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Chao A., et al., Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014). [Google Scholar]
- 48.Hsieh T. C., Ma K. H., Chao A., iNEXT: iNterpolation and EXTrapolation for species diversity (Version 2.0.20, R package, 2020). https://cran.r-project.org/web/packages/iNEXT/index.html. Accessed 4 December 2020.
- 49.Wood S. N., Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73, 3–36 (2011). [Google Scholar]
- 50.Hastie T. J., Tibshirani R. J., Generalized Additive Models 43 (CRC Press, London, 1990). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited in The University of Auckland research repository (https://doi.org/10.17608/k6.auckland.12672884.v1).