Abstract
Background
To improve child survival, it is necessary to describe and understand the spatial and temporal variation of factors associated with child survival beyond national aggregates, anchored at decentralised health planning units. Therefore, we aimed to provide subnational estimates of factors associated with child survival while elucidating areas of progress, stagnation and decline in Kenya.
Methods
Twenty household surveys and three population censuses conducted since 1989 were assembled and spatially aligned to 47 subnational Kenyan county boundaries. Bayesian spatio-temporal Gaussian process regression models accounting for inadequate sample size and spatio-temporal relatedness were fitted for 43 factors at county level between 1993 and 2014.
Results
Nationally, the coverage and prevalence were highly variable with 38 factors recording an improvement. The absolute percentage change (1993–2014) was heterogeneous ranging between 1% and 898%. At the county level, the estimates varied across space and over time with a majority showing improvements after 2008 which was preceded by a period of deterioration (late-1990 to early-2000). Counties in Northern Kenya were consistently observed to have lower coverage of interventions and remained disadvantaged in 2014 while areas around Central Kenya had and historically have had higher coverage across all intervention domains. Most factors in Western and South-East Kenya recorded moderate intervention coverage although having a high infection prevalence of both HIV and malaria.
Conclusion
The heterogeneous estimates necessitates prioritisation of the marginalised counties to achieve health equity and improve child survival uniformly across the country. Efforts are required to narrow the gap between counties across all the drivers of child survival. The generated estimates will facilitate improved benchmarking and establish a baseline for monitoring child development goals at subnational level.
Keywords: child health, geographic information systems, indices of health and disease and standardisation of rates, epidemiology, health services research
Key questions.
What is already known?
The recent under-five mortality (U5M) decline in Kenya has been uneven across subnational units and over time with widespread inequities. These inequities are likely to be associated with the uneven use of interventions, healthcare utilisation, distribution of resources and disease prevalence. Thus, subnational estimates of factors associated with child survival are required.
What are the new findings?
Overall, the coverage of interventions increased between 1993 and 2014, however, the estimates were heterogeneous with widespread geospatial inequities at county-level over time.
High heterogeneity and varied estimates between the factors ranging from low (less than 35%) for improved sanitation to high (over 65%) for childhood immunisations by 2014.
Across almost all the factors, counties in Northern Kenya were systematically left behind with lower coverage, Central Kenya always had higher coverage while western and south-east counties had moderate coverage and higher HIV and malaria infection prevalence.
What do the new findings imply?
Prioritised targeting in the marginalised Northern Kenya region during allocation of resources, finances, policy formulation and planning to increase coverage, reduce health inequities and improve child survival. Additional efforts to reduce malaria and HIV infection in western and coastal regions of Kenya are needed.
Generated estimates will facilitate benchmarking between counties and form a key baseline for monitoring sustainable development goals and local targets at subnational level.
Need to evaluate the contribution for each factor relative to U5M variation subnationally to aid in granular targeting.
Need for additional data for monitoring coverage during the devolved health planning era in Kenya.
Introduction
The planning and allocation of child health interventions to subnational areas with the greatest need is crucial in improving child survival equitably.1 2 To identify populations that are marginalised from healthcare access and preventive interventions, requires an evaluation of their coverage. This is necessary to achieve universal health coverage on the pathway to equitable improvements of child survival3 and ensure that no child is left behind and that resources do not go to waste on populations with the least need.2 This is enshrined within the sustainable development goals (SDGs) principle of leaving no one behind and reaching the furthest behind, first.4 5
Increased need for improved understanding of gaps in intervention coverage and other factors known to be associated with under-five mortality (U5M) at local health planning units has spurred improvements in the use of geocoded data from household sample surveys6 7 within advanced statistical modelling techniques.8–11 As a result, factors associated with U5M have been mapped at fine-scale spatial and temporal resolution across much of sub-Saharan Africa6 10 12–19 including Kenya.6 10 16–23 Previous approaches in Kenya have not always been comparable over time, nor have these studies harnessed all the available data to make predictions across the country’s subnational units required for decentralised health planning.6 10 16–23 Many of these studies have considered a few individual factors only, however, mapping all factors is fundamental for benchmarking of health systems performance across subnational units.12–15
Here, we leverage data from multiple sources including all available household sample surveys and population census to provide annual estimates of 43 factors known to be associated with changes in U5M at each of the 47 subnational counties used for decentralised health planning in Kenya.24 The generated estimates are used to express spatial and temporal inequities and elucidate areas of marginalisation for the periods between 1993 and 2014.
Methods
Country health context
Kenya’s healthcare system is pluralistic with both public and private healthcare facilities providing services. The service delivery is hierarchical with six tiers spanning between community level and tertiary facilities.25 The government has continually enhanced healthcare utilisation by ensuring healthcare services are affordable and accessible since independence through polices on user fee.3 26–32 Establishment of the Kenya Expanded Programme on Immunization (EPI) in 1980 introduced vaccines for six major killer diseases at the time including tuberculosis, polio, diphtheria, whooping cough, tetanus and measles. Between 2001 and 2014, yellow fever, hepatitis B and haemophilus influenza B type (Hib), pneumococcal conjugate, measles second dose and rotavirus vaccinations were added to the EPI schedule.33
Government-led campaigns such as the school feeding, Malezi Bora (good upbringing), baby-friendly initiatives at the hospital and community levels (Baby-Friendly Community/Hospital Initiative (BFCI and BFHI)) have addressed poor breastfeeding practices and improved nutrition among children.34–36 Initiatives to fight malaria targeted to children and mothers were intensified from 2000 through expanded, free delivery of insecticide-treated nets (ITNs),37 38 replacing failing malaria drugs with efficacious therapeutics,39 40 targeted indoor residual spraying (IRS) and intermittent preventive treatment in pregnancy (IPTp).22 38 Beginning 2000, there was an expansion of HIV prevention interventions including an increase in facilities offering prevention of mother-to-child transmission (PMTCT) interventions and increased uptake of antiretrovirals (ARVs) drugs.41–43
In 1996/1997, the Integrated Management of Childhood Illness was introduced aiming to improve the management of childhood illness such as diarrhoea, pneumonia, malaria, measles and malnutrition. It involves: (i) strengthening health worker skills in managing illnesses, (ii) strengthening health systems (drug availability, supervision, referral and Health Management Information System (HMIS)) and (iii) the improvement of family and community health practices through community involvement and awareness on measures to improve child health.44 After 2003, the millennium development goals created an impetus to improve child survival and led to a proliferation of reproductive, maternal, newborn, and child health policies, programmes and increased funding in Kenya.45
Kenya has made major strides in the promotion of child health through legal frameworks such as the children Act, vision 2030, Big Four agenda and a new constitution.24 46 Conversely, Kenya has been affected by several major disasters including floods, droughts, epidemics and post-election violence47–51 which are antagonistic to child survival. Online supplemental file 1 summarises both the health context and major disasters related to child survival in Kenya since independence.
bmjgh-2020-004544supp001.pdf (209.1KB, pdf)
Following the promulgation of a new constitution in 2010 and the 2013 general elections, Kenya transitioned into a devolved system of government with a central government and 47 semi-autonomous county governments.24 The counties are now used for decentralised health planning52 and were adopted as the unit of analysis (figure 1). Under this system, the central Ministry of Health is mandated with policy-making and regulatory roles while allocation and managing healthcare resources and service provision is under the county governments.53 54
Figure 1.
The map of Kenya showing 8 provinces (coloured) and the 47 subnational units (counties) as dark lines, water bodies and major rivers are shown in blue. Source: author. Coast province: Mombasa (1), Kwale (2), Kilifi (3), Tana River (4), Lamu (5), Taita Taveta (6); north eastern province: Garissa (7), Wajir (8), Mandera (9); eastern province: Marsabit (10), Isiolo (11), Meru (12), Tharaka Nithi (13), Embu (14), Kitui (15), Machakos (16), Makueni (17); central province: Nyandarua (18), Nyeri (19), Kirinyaga (20), Murang'a (21), Kiambu (22); Rift Valley province: Turkana (23), West Pokot (24), Samburu (25), Trans Nzoia (26), Uasin Gishu (27), Elgeyo Marakwet (28), Nandi (29), Baringo (30), Laikipia (31), Nakuru (32), Narok (33), Kajiado (34), Kericho (35), Bomet (36); western province: Kakamega (37), Vihiga (38), Bungoma (39), Busia (40); Nyanza province: Siaya (41), Kisumu (42), Homa Bay (43), Migori (44), Kisii (45), Nyamira (46); Nairobi province: Nairobi (47).
Subnational variations in U5M between 1965 and 2014 in Kenya have previously been described in detail in Macharia et al.1 Briefly, U5M was highly variable in space and time during the period under consideration (1993–2014). In 1993, 15 counties had U5M of ≥100 deaths per 1000 live births and were located in Western Kenya (Homa Bay, Migori, Siaya, Kisumu, Busia, Kakamega, Vihiga, Bungoma and Kisii), parts of coastal Kenya (Kilifi, Tana River, Lamu and Kwale), northern (Turkana, Garissa) and eastern (Kitui) regions. Five counties in central region (Kiambu, Embu, Murang’a, Nyeri and Nyandarua) and three in the neighbouring counties (Nakuru, Kajiado and Laikipia) had the highest probability of child survival, ≤50 deaths per 1000 live births in the same year.
During this period (1993–2014), 39 (83%) counties recorded U5M declines ranging between 1.6% in Kiambu and 58.3% in Mandera county. The counties with huge reductions (western and north eastern) had higher U5M in 1993 compared with counties with lower reductions. No county had U5M rates of ≤25 per 1000 live births, the target for 2030 for SDG 3.2 and by 2014, only three counties in western region (Migori, Homa Bay and Siaya) had U5M of ≥100 deaths per 1000 live births.
Data
We accessed information from multiple household sample surveys and population censuses conducted since 1989 available from online data portals. These included the Integrated Public Use Microdata Series,55 Kenya National Bureau of Statistics,56 Multiple Indicator Cluster Surveys57 and Demographic and Health Survey.58 A survey was included if it contained at least one factor associated with U5M monitored over time, surveyed both in the rural and urban areas and devoid of reported data collection and quality concerns.
The assembled data were spatially misaligned over time due to changes in the number and geographical size of districts (subnational units before counties1) between 1989 and 2010 when county boundaries were formally defined.24 Spatially misaligned historical district boundaries were matched to the current county boundaries (figure 1) as described elsewhere.1 Table 1 summarises the data sources that were included in the analysis.
Table 1.
Household sample surveys and population censuses undertaken since 1989 used in the analysis comprising six DHS, five MICS, three population censuses, two AIS, three MIS, two WMS and two KIHBS
| Survey | Year | Number of counties | Number of households | Clusters/units | Women aged 15–49 years |
| DHS | 1989 | 38 | 8173 | 393 | 7150 |
| 1993 | 40 | 7950 | 520 | 7540 | |
| 1998 | 38 | 8380 | 536 | 7881 | |
| 2003 | 47 | 8561 | 400 | 8195 | |
| 2008/2009 | 47 | 9057 | 400 | 8444 | |
| 2014 | 47 | 36 430 | 1612 | 31 079 | |
| MICS | 2000 | 42 | 9045 | 900 | 10 537 |
| 2007 | 3 | 881 | 62 | 881 | |
| 2008 | 8 | 14 677 | 650 | 13 606 | |
| 2011 | 6 | 6828 | 300 | 5908 | |
| 2013/2014 | 3 | 3744 | 158 | 3348 | |
| Population Census | 1989 | 47 | 224 861 | 36 979 | 238 027 |
| 1999 | 47 | 317 106 | 61 921 | 345 647 | |
| 2009 | 47 | 243 858 | 96 253 | 934 904 | |
| AIS | 2007 | 47 | 9691 | 402 | 5991 |
| 2012 | 44 | 8035 | 371 | 7958 | |
| MIS | 2007 | 43 | 6854 | 200 | 6111 |
| 2010 | 47 | 6538 | 240 | 5749 | |
| 2015 | 47 | 6481 | 245 | 5394 | |
| WMS | 1994 | 47 | 10 860 | 1172 | 13 385 |
| 1997 | 41 | 10 873 | 1107 | 2484 | |
| KIHBS | 2005/2006 | 47 | 13 390 | 1339 | 16 446 |
| 2015/2016 | 47 | 21 773 | 2387 | 23 768 |
Table includes the number of counties covered, clusters and number of women of childbearing (15–49 years).
AIS, AIDS Indicator Survey; DHS, Demographic and Health Surveys; KIHBS, Kenya Integrated Household Budget Survey; MICS, Multiple Indicator Cluster Surveys; MIS, Malaria Indicator Surveys; WMS, Welfare Monitoring Surveys.
Factors associated with U5M
Forty-three factors a priori known to be associated with U5M (table 2 and online supplemental file 2) were identified based on existing frameworks of child survival,59–61 relevance to Kenya’s child health priorities52 and data availability (table 1). The factors were defined following household sample surveys guidelines.56–58 The 43 factors were divided into 8 broad thematic areas including environmental, maternal, child, and household factors, infections, healthcare utilisation, child and maternal health interventions. Table 2 outlines the factors and their thematic groups with detailed definitions and the specific data sources outlined in online supplemental file 2.
Table 2.
The factors associated with child survival and thematic groups as used in the current analysis
| Group | ID | Variable |
| Environmental factors | 1 | Rural residency |
| 2 | Precipitation | |
| 3* | Enhanced vegetation index (EVI) | |
| Maternal factors | 4 | Maternal education |
| 5 | Maternal literacy | |
| 6 | Female headed households | |
| 7 | Short birth spacing | |
| 8 | Use of modern contraceptives | |
| 9 | High parity | |
| Child factors | 10 | Underweight |
| 11 | Wasted | |
| 12 | Stunted | |
| 13 | Breast fed within the first hour of birth | |
| 14 | Exclusive breast feeding | |
| 15 | Continued breast feeding | |
| 16 | Low birth weight (LBW) | |
| Household factors | 17 | Poor household |
| 18 | Improved sanitation | |
| 19 | Access to any form of a toilet | |
| 10 | Improved water | |
| 21 | Access to wells, borehole and piped water | |
| Infections | 22 | HIV infection prevalence |
| 23 | Malaria infection prevalence | |
| Healthcare utilisation | 24 | At least one antenatal care visit (ANC1) |
| 25 | At least four antenatal care visits (ANC4) | |
| 26 | Skilled birth attendance (SBA) | |
| 27 | Health facility deliveries (HFD) | |
| 28 | Diarrhoea treatment-seeking | |
| 29 | Fever/cough treatment-seeking | |
| Child health interventions | 30 | BCG |
| 31 | Three diphtheria–tetanus–pertussis vaccinations (DPT3) | |
| 32 | Three doses of polio (Polio3) | |
| 33 | Measles | |
| 34 | Fully immunised | |
| 35 | Oral rehydration salts (ORS use) | |
| 36* | Vitamin A-children | |
| 37* | Insecticide treated bed nets (ITN) use by children | |
| 38* | Recommended antimalarial use | |
| Maternal health interventions | 39 | Tetanus toxoid injection |
| 40* | Intermittent preventive treatment in pregnancy (IPTp 1) | |
| 41* | IPTp 2 | |
| 42* | Iron supplement | |
| 43* | Vitamin A-mothers |
The definitions and respective data sources of factors are shown in online supplemental file 2.
*2003 is the baseline year because the corresponding factors were either not monitored or had not been rolled out.
bmjgh-2020-004544supp002.pdf (43.5KB, pdf)
The comparativeness of various sources of information considered in the current analysis is non-trivial (table 1) given the variations in period, approaches and tools used during data collection. To ensure comparativeness of the various data sources, we implemented several checks. First, we explored and assessed the responses collected per each factor under consideration across all household sample surveys and population censuses (table 1). We then adopted a definition (online supplemental file 2) that ensured identical meaning across all data sources to maintain temporal comparability and allow for tracking of changes over time. Second, using approaches outlined by Ngandu et al,62 we evaluated the effect of recall bias and missing data on estimates when combining Demographic and Health Surveys (DHS) and Multiple Indicator Cluster Surveys (MICS) data. The results were reasonably comparable using unmatched time lags for 3 years (DHS) and 2 years (MICS) and was adopted for our analyses based on a pragmatic balance between recall data and maintaining a large enough sample for county level estimates. Finally, the use of spatio-temporal methods described in the subnational modelling section allowed for smoothing of data points from the multiple sources.
Subnational modelling
Coverage and/or prevalence for 39 factors were estimated using data defined in table 1 while 4 factors were available either as gridded surfaces or at aggregated geographical units. The four factors included HIV infection prevalence at the provincial level (figure 1) available from Kenya National AIDS Control Council (NACC),63 a temporal gridded malaria risk surface based on parasite prevalence,22 and temporal gridded surfaces of enhanced vegetation index (EVI) and precipitation.64 65 Thirty-five factors were available for the entire analysis period (1993–2014) while eight factors were available from 2003 since they were either rolled out or first monitored from early 2000 (table 2).
Prevalence estimates for each of the 39 factors (table 2) were computed while accounting for sampling design and/or survey weights by survey/census (table 1) at county level. All the household sample surveys (except DHS 2014) were designed to provide precise estimates at national and provincial levels and not powered to provide subnational county-level estimates. To predict across all subnational counties and during non-sampled years and combining raw estimates where more than one survey was conducted in a single year, a Bayesian spatio-temporal Gaussian Process Regression model66 67 with a heteroscedastic error component defined elsewhere1 was used (equation 1). In brief, the modelling framework accounts for large sampling variance and heterogeneity between surveys while exploiting spatio-temporal relatedness to increase predictive power (equation 1).
Spatio-temporal model for smoothing factors associated with U5M:
is the weighted proportion for each factor (table 2) for survey i (table 1), county k (n=47; figure 1) and year t (n=22 or 12 years; table 1, online supplemental file 2); is the intercept, is a spatio-temporal Gaussian process predicted by borrowing strength of information across surveys, counties and years with mean 0 and covariance function . Where is the Kronecker product, RS and RT are the spatial and temporal correlation matrices, respectively. RS is modelled using a conditionally autoregressive (CAR) process while RT is an autoregressive process of the first order. a Gaussian noise modelled with the variance taken to be the product of the log-transformed sample size from a given survey, county and year.1 Further details of the modelling framework are presented in online supplemental file 3 in Macharia et al. 1
bmjgh-2020-004544supp003.pdf (4.1MB, pdf)
The model for each factor was fitted using Markov chain Monte Carlo (MCMC) algorithm based on 10 000 posterior samples by county and year. The algorithm was iterated for 110 000 times and retained every 10th sample after a burn-in of 10 000 samples. Cross-validation was undertaken to assess the predictive performance of the model through a 10% random hold-out of the observed values. The observed and posterior predictions were used to compute the correlation, mean absolute error and root mean square error. The posterior distribution for each factor was summarised by its mean and 95% CIs for each year and county across the study period.
Analyses and data management were conducted in StataCorp 2014 (Stata Statistical Software: Release V.14) and R statistical software (V.3.4.1) while all the cartographies were done in ArcMap V.10.5 (ESRI, Redlands, CA, USA). While estimates were generated for every year between 1993 and 2014, maps were anchored at stable years with substantial data points corresponding to years when majority of the nation-wide sample surveys were conducted (1993, 1998, 2003, 2008 and 2014).
Patient and public involvement
The study used secondary data only (table 1) that are publicly available through links and sources provided within the manuscript.55–58
Results
The data assembled included 20 sample household surveys and 3 population censuses conducted after 1989 covering 870 county-years and 1.7 million women of childbearing age (table 1). The annual predictions spanned either the entire analysis period (1993–2014) for 35 (82%) factors or 12 years (2003–2014) for seven interventions that were introduced or first monitored from the early-2000s.
At the national level, at baseline (1993), child health interventions had high coverage (over 65%). Conversely, rates of healthcare utilisation were moderate (35%–65%) except at least one antenatal care visit (ANC1) which had a high coverage (91%). Both maternal health interventions and household-related factors had either moderate or low coverage (table 3). The coverage of nutritional factors was highly heterogeneous. For example, breastfeeding factors spanned between low (exclusive breast feeding), moderate (breast fed within 1 hour of birth) to high (continued breast feeding) coverage while the prevalence of malnutrition ranged between 7% (acute-malnutrition or wasting) and 40% (chronic-malnutrition or stunting) with underweight (elements of both stunting and wasting) being 19% (table 3). The coverage/prevalence of maternal factors was variable ranging from low (contraceptive use) to high (maternal literacy). The infection prevalence of malaria (24%) and HIV (9%) were high. Table 3 shows the estimate of all factors in 1993 and 2014.
Table 3.
The national coverage and or prevalence of the factors associated with child survival in 1993 and 2014 and change between the two time points
| Thematic group | ID | Factors associated with under-five mortality | Estimate (95% CI) | Change (95% CI) | ||
| 1993 | 2014 | Percentage | Absolute | |||
| Environmental factors | 1 | Rural residency | 84.2 (83.5 to 84.9) | 58.0 (57.7 to 58.8) | −31.3 (−31.7 to −30.9) | −26.4 (−26.8 to −26.0) |
| 2 | Precipitation | 35 455.97 mm | 43 866.85 mm | 19.2 | 8410.9 | |
| 3 | EVI | 0.33 | 0.31 | −5.5 | 0.02 | |
| Maternal factors | 4 | Maternal education less than pry school | 57.7 (56.4 to 59.0) | 36.38 (35.6 to 37.2) | −36.9 (−37.5 to −36.3) | −21.3 (−21.9 to −20.7) |
| 5 | Maternal literacy | 76.7 (75.5 to 77.8) | 84.1 (83.5 to 84.7) | 9.6 (9.1 to 10.2) | 7.4 (6.9 to 7.9) | |
| 6 | Female household head | 33.6 (32.7 to 34.5) | 32.2 (31.7 to 32.7) | −4.2 (−4.6 to −3.8) | −1.4 (−1.8 to −1.0) | |
| 7 | Short birth interval | 25.2 (24.1 to 26.2) | 16.9 (16.4 to 17.5) | −49.1 (−49.4 to −48.8) | −8.3 (−8.6 to −8.0) | |
| 8 | Modern contraceptives use | 20.8 (20.0 to 21.5 | 39.5 (38.7 to 40.3) | 90.8 (90.5 to 91.1) | 18.8 (18.8 to 19.1) | |
| 9 | High parity | 35.9 (35.0 to 36.8) | 25.3 (20.8 to 21.17) | −30.1 (−30.5 to −29.7) | −10.8 (−11.2 to −10.4) | |
| Child factors | 10 | Underweight | 18.9 (18.0 to 19.9) | 10.6 (10.2 to 11.1) | −43.9 (−44.3 to −43.5) | −8.3 (−8.7 to −7.9) |
| 11 | Wasted | 6.8 (6.2 to 7.4) | 4.1 (3.8 to 4.4) | −39.7 (−39.9 to −39.5) | −2.7 (−2.9 to −2.5) | |
| 12 | Stunted | 40.0 (38.8 to 41.2) | 25.8 (25.2 to 26.5) | −35.5 (−36.0 to −35.0) | −14.2 (−14.7 to −13.7) | |
| 13 | Breast fed within first hour of birth | 55.5 (54.1 to 56.8) | 62.8 (61.8 to 64.1) | 13.2 (12.3 to 14.1) | 7.3 (6.4 to 8.2) | |
| 14 | Exclusive breast feeding | 17.6 (14.8 to 20.4) | 60.9 (57.5 to 64.3) | 246.0 (245.1 to 246.9) | 43.3 (42.4 to 44.2) | |
| 15 | Continued breast feeding | 91.6 (90.2 to 92.9) | 87.9 (85.2 to 90.1) | −4.0 (−5.7 to −2.3) | −3.7 (−5.4 to −2.0) | |
| 16 | Low birth weight | 8.7 (7.7 to 9.6) | 7.6 (6.9 to 8.2) | −12.6 (−13.1 to −12.1) | −1.1 (−1.6 to −0.6) | |
| Household factors | 17 | Poor household | 38.4 (37.4 to 39.3) | 34.7 (34.2 to 35.2) | −9.6 (−10.0 to −9.2) | −3.7 (−4.1 to −3.3) |
| 18 | Improved sanitation | 8.4 (8.0 to 8.5) | 17.6 (17.2 to 18.0) | 109.5 (109.2 to 109.8) | 9.2 (8.9 to 9.5) | |
| 19 | Improved and intermediate sanitation | 83.2 (82.5 to 83.9) | 90.1 (89.8 to 90.4) | 8.3 (8.0 to 8.6) | 6.9 (6.6 to 7.2) | |
| 20 | Improved water | 29.9 (28.1 to 30.5) | 43.6 (43.1 to 44.1) | 45.8 (45.4 to 46.2) | 13.7 (13.3 to 14.1) | |
| 21 | Improved and intermediate water | 51.4 (50.5 to 52.4) | 63.4 (62.9 to 63.8) | 23.3 (22.9 to 23.7) | 12.0 (11.6 to 12.4) | |
| Infections | 22 | HIV | 9.16 | 5.01 | −82.8 | −4.2 |
| 23 | Malaria | 23.9 (16.1 to 34.8) | 4.7 (3.6 to 8.4) | −80.3 | −19.2 | |
| Healthcare utilisation | 24 | ANC1 | 91.4 (90.8 to 92.1) | 95.6 (95.3 to 95.9) | 4.6 (4.2 to 5.0) | 4.2 (3.8 to 4.6) |
| 25 | ANC4 | 62.4 (61.1 to 63.6) | 56.3 (55.4 to 57.2) | −9.8 (−10.6 to −9.2) | −6.1 (−6.8 to −5.4) | |
| 26 | Skilled birth attendance | 42.1 (42.0 to 44.3) | 63.5 (62.8 to 64.3) | 50.8 (50.1 to 51.5) | 21.4 (20.7 to 22.1) | |
| 27 | Health facility births | 41.6 (40.3 to 42.9) | 63.0 (62.2 to 63.8) | 51.4 (50.7 to 52.1) | 21.4 (20.7 to 22.1) | |
| 28 | Diarrhoea treatment-seeking | 39.2 (36.3 to 42.0) | 57.8 (56.0 to 59.6) | 47.4 (45.9 to 48.9) | 18.6 (17.1 to 20.1) | |
| 29 | Fever treatment-seeking | 46.0 (44.4 to 47.6) | 72.8 (71.7 to 73.9 | 58.3 (57.3 to 59.3) | 26.8 (25.8 to 27.8) | |
| Child health interventions | 30 | BCG | 96.2 (95.2 to 97.1) | 96.7 (96.1 to 97.2) | 0.5 (0.1 to 1.0) | 0.5 (0.1 to 1.0) |
| 31 | DPT3 | 86.7 (85.0 to 88.4) | 90.1 (89.1 to 91.1) | 3.9 (3.1 to 4.7) | 3.4 (2.6 to 4.2) | |
| 32 | Polio3 | 85.5 (83.7 to 87.2) | 90.9 (90.0 to 91.8) | 6.3 (5.5 to 7.1) | 5.4 (4.6 to 6.2) | |
| 33 | Measles | 83.6 (81.8 to 85.8) | 87.1 (86.0 to 88.1) | 4.2 (3.3 to 5.1) | 3.5 (2.6 to 4.4) | |
| 34 | Fully immunised | 78.1 (76.0 to 80.2) | 78.5 (77.5 to 80.1) | 0.5 (−0.6 to 1.6) | 0.4 (−0.7 to 1.5) | |
| 35 | ORS use | 30.8 (28.0 to 33.5) | 54.7 (52.8 to 56.5) | 77.6 (76.0 to 79.2) | 23.9 (22.3 to 25.5) | |
| 36 | Vitamin A-children* | 34.1 (32.8 to 35.5) | 67.8 (57.1 to 68.5) | 98.8 (98.3 to 99.3) | 33.7 (33.2 to 34.2) | |
| 37 | ITN use by children in malarious areas* | 6.2 (5.4 to 7.0) | 61.9 (61 to 62.8) | 898.4 (897.7 to 899.1) | 55.7 (55.0 to 56.4) | |
| 38 | Antimalarial use in malarious areas* | 13.9 (12.0 to 15.9) | 33.5 (31.9 to 15.2) | 141.0 (139.8 to 142.2) | 19.6 (18.4 to 20.8) | |
| Maternal health interventions | 39 | Tetanus toxoid injection | 51.8 (50.2 to 53.3) | 51.5 (49.3 to 52.0) | −0.6 (−1.5 to 0.3) | −0.3 (−1.2 to 0.6) |
| 40 | IPTp 1 in malarious areas* | 13.3 (11.6 to 15.1) | 41.2 (39.9 to 42.4) | 210.5 (209.7 to 211.3) | 27.9 (27.1 to 28.7) | |
| 41 | IPTp 2 in malarious areas* | 5.6 (4.4 to 6.8) | 25.7 (24.6 to 26.8) | 358.9 (358.4 to 359.4) | 20.1 (19.6 to 20.6) | |
| 42 | Iron supplement* | 46.1 (44.5 to 47.6) | 69.8 (68.7 to 70.9) | 51.4 (50.4 to 52.4) | 23.7 (22.7 to 24.7) | |
| 43 | Vitamin A-mothers* | 14.3 (13.2 to 15.3) | 54.5 (53.3 to 55.6) | 281.1 (280.4 to 281.8) | 40.2 (39.5 to 40.9) | |
*2003 is the baseline year because the corresponding factors were either not monitored or had not been rolled out. The four indicators without CI were externally sourced.
ANC1, one antenatal care visit; ANC4, four antenatal care visits; DPT3, three diphtheria–tetanus–pertussis vaccinations; EVI, enhanced vegetation index; IPTp, intermittent preventive treatment in pregnancy; ITN, insecticide-treated net; ORS, oral rehydration salts.
Nationally, all the factors had either an increase in the coverage of intervention or a decline in the infection prevalence between 1993 and 2014 except four factors (female-headed households, continued breast feeding, at least four antenatal care visits (ANC4) and tetanus toxoid injection) which showed minimal change. The percentage change in each of the four factors was less than 10%. The absolute percentage change was heterogeneous ranging from 1% to 898% across the 43 factors (table 3). Eighteen factors (42%) had an absolute percentage change of less than 25% while seven factors (16%) had a change of over 100%. Table 3 shows the percentage changes highlighting those that stagnated (red), had small (light green), moderate (mild green) or sizeable improvements (dark green).
By 2014, most of the factors had coverage of over 35%. For example, breastfeeding indicators, most maternal factors, household factors (except improved sanitation), maternal health interventions (except antimalarials and IPTp) and healthcare utilisation rates, all had moderate to high coverage (table 3).
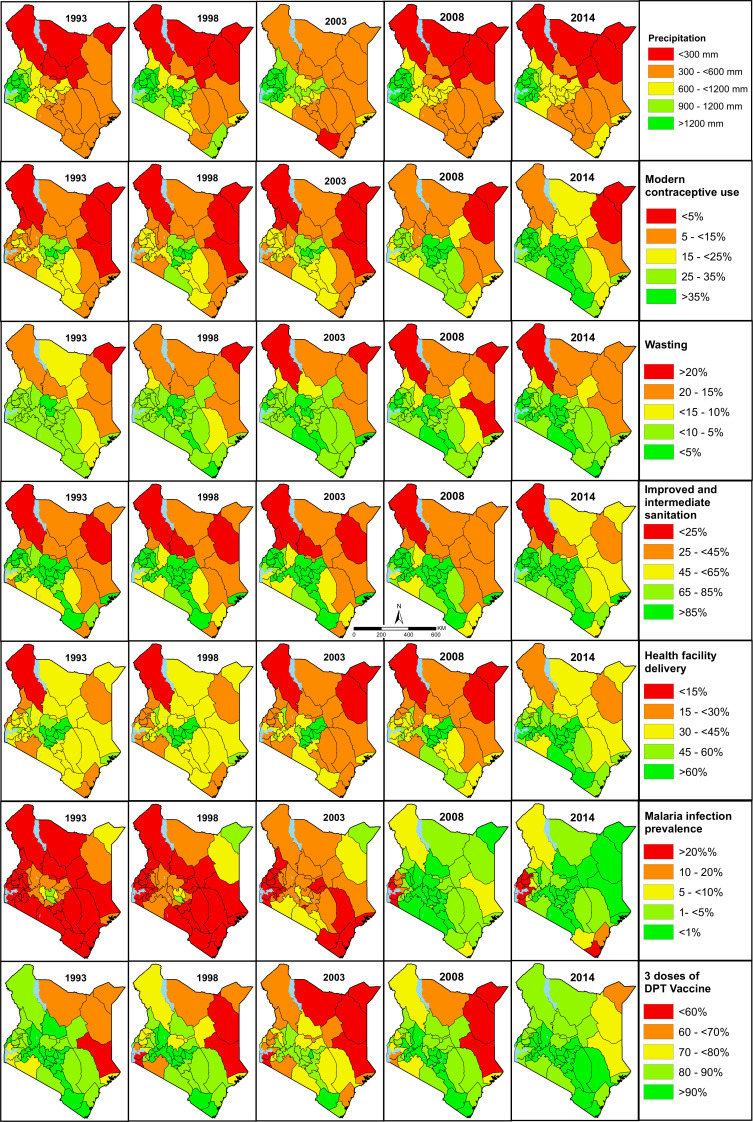
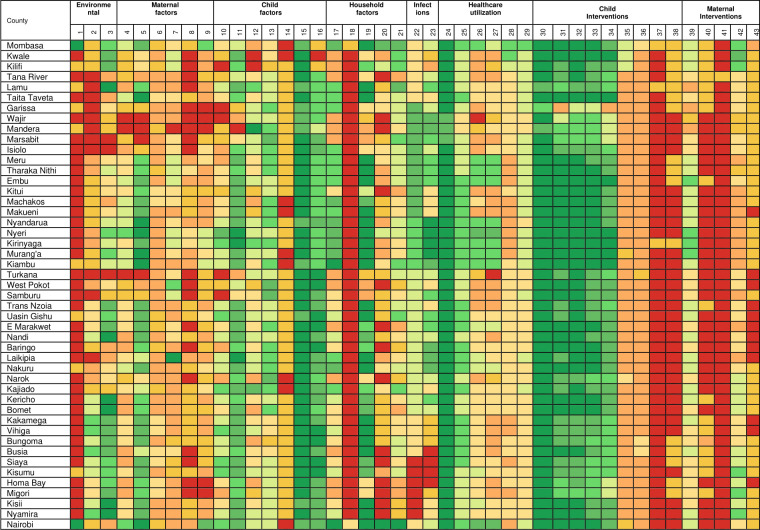
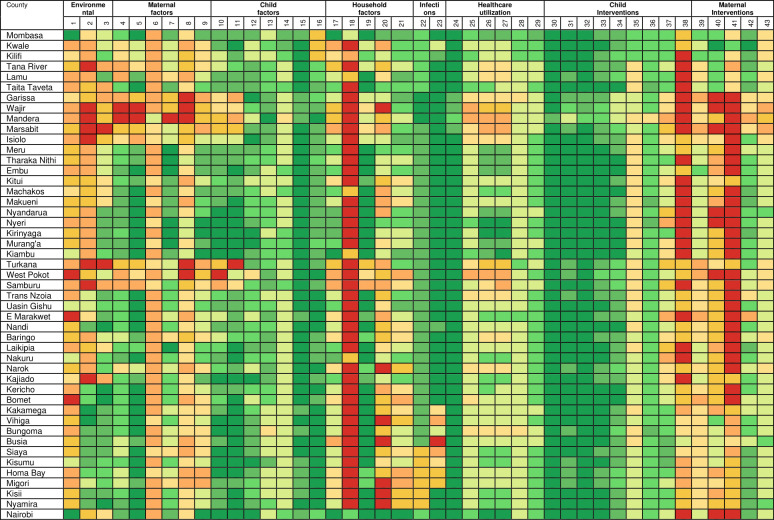
National estimates mask important subnational county differences in the coverage or prevalence of the factors associated with U5M over time. The subnational estimates for all the factors across the study are presented in online supplemental file 3 while a subset of seven factors across seven themes are presented in figure 2. Further, figure 3 (1993) and figure 4 (2014) show scaled heat plots of all 43 factors and the 47 counties across the study period representing the prevalence and coverage estimates. Overall, across the continuum of all factors, intervention coverage was lower and disease infection prevalence higher in 1993 compared with 2014 (figures 2–4 and online supplemental file 3).
Figure 2.
A subset of seven factors associated with child survival including precipitation, use of modern contraceptives, wasting, a combination of intermediate and improved sanitation, health facility delivery, malaria infection prevalence and three doses of diphtheria–tetanus–pertussis (DPT) vaccine. The factors are classified into five classes colour-coded from red (low coverage or high disease prevalence) to green (high coverage or low disease prevalence). The rest of the factors are presented in online supplemental file 3. Source: author.
Figure 3.
Ranking of factors associated with child survival in 1993 from low intervention coverage or high disease prevalence (red) to high intervention coverage or low disease prevalence (green). The second row are the 43 factors (table 2) while online supplemental file 3 has the actual values. Colour codes can be compared within an indicator across the 47 spatial units in 1993 and 2014 but not between factors. Source: author. An octile ranking was used to divide each factor into eight equal classes from <12.5% to ≥87.5% for interventions whose coverage varied from 0% to 100%. For factors whose coverage was not expected to range between 0% and 100% (such as nutrition status), they were first rescaled to 0%–100% and then divided into octiles.
Figure 4.
Ranking of factors associated with child survival in 2014 from low intervention coverage or high disease prevalence (red) to high intervention coverage or low disease prevalence (green). The second row are the 43 factors (table 2) while online supplemental file 3 has the actual values. Colour codes can be compared within a factor across the 47 spatial units in 1993 and 2014 but not between factors. Source: author. An octile ranking was used to divide each factor into eight equal classes from <12.5% to ≥87.5% for interventions whose coverage varied from 0% to 100%. For factors whose coverage was not expected to range between 0% and 100% (such as nutrition status), they were first rescaled to 0%–100% and then divided into octiles.
The reduction in infection prevalence and the increase in intervention coverage was characterised by epochs of improvement, deterioration and reversals in gains made. The improvements were observed mainly after 2008 through to 2014 largely as a result of counties in Western Kenya and those neighbouring central parts catching up with the Central Kenya counties while counties in the northern parts of Kenya showed little improvements. Overall, the improvement phase (2008–2014) was preceded by a period of deterioration and stagnation between the late-1990s to early-2000s while the early-1990s was characterised by moderate coverage of most interventions (figures 2–4 and online supplemental file 3).
Despite the increase in intervention coverage and reduction in disease infection prevalence across factors and counties, there were notable exceptions. Instances, where coverage decreased by more than 5% in several counties included; a reduction in access to safe water, ANC4 coverage, use of recommended antimalarial medicine, tetanus toxoid vaccination coverage and an increase in the proportion of poor households. The counties most affected by these reversals were mainly located in Northern Kenya (Garissa, Wajir, Mandera, Marsabit, Turkana, West Pokot, Samburu) and partly western region (Trans Nzoia and Vihiga) and along the Indian ocean (Lamu) (figures 2–4 and online supplemental file 3).
The coverage of interventions and prevalence between factors was highly variable across the years. For example, there was a huge difference between the coverage of vaccinations such as three diphtheria–tetanus–pertussis vaccinations (DPT3) (mainly over 60% across counties) compared with access to modern contraceptives (mainly below 40% across all counties). Similarly, the usage of recommended antimalarial medicine and improved sanitation were always less than 20% compared with the coverage of ANC1 which was above 75% in a majority of the counties over the entire period (figures 2–4 and online supplemental file 3).
More prominent was the consistent colocation of disadvantaged counties over time for almost all factors. Counties in Northern Kenya had lower coverage for almost all maternal and child health (MCH) interventions, poor health utilisation rates, lower coverage of household factors and higher disease prevalence during the analysis period (online supplemental file 3). However, HIV and malaria infection prevalence were lower in Northern Kenya. Likewise, there was consistent colocation of counties that performed better (Central Kenya) from 1993 to 2014. In western and south-east counties, the coverage of interventions and disease prevalence were moderate except high infection prevalence of HIV and malaria (figures 2–4 and online supplemental file 3). The validation statistics showed a fair agreement between the observed and predicted estimates for a majority of the factors (82%) with a correlation coefficient above 0.6 (online supplemental file 4).
bmjgh-2020-004544supp004.pdf (36.3KB, pdf)
Discussion
The compilation of a large database of household sample surveys, population census and other opportunistic sources allowed for the evaluation of trends, variations and changes in 43 factors associated with child survival at subnational county-level over two decades in Kenya. The geospatial framework applied harmonises previous approaches6 10 16–23 that have not always been comparable. The results depict substantial but heterogeneous gains in the provision and scaling up of MCH interventions between 1993 and 2014. The coverage ranged between suboptimal to moderate levels illustrating widespread disparities and inequities in the continuum of child and maternal healthcare. The trends, variations and changes observed, over time, especially between 2008 and 2014, can be linked to important initiatives and programmes in Kenya as summarised in the country health context and online supplemental file 1.
By 2014, two in every three children received child health interventions except for access to recommended antimalarial medicines (table 3). These include all childhood immunisations, oral rehydration salts, ITNs and vitamin A supplements. Childhood immunisations have been offered free of charge and probably why their coverages have been high over time. However, the proportion of fully immunised children stagnated (0.5% overall change) possibly due to those who do not receive timely immunisation or drop out before completing their immunisation schedule.12 68–70 Demand and supply challenges such as spatial access, health workforce, stockouts, cost of transportation and cold chain could also have hampered vaccination uptake especially in Northern Kenya where coverage was lower.70–75 Child and maternal survival interventions such as supplements have been delivered through the Malezi Bora initiative since 2007, a health facility-based delivery system migrating away from the previous door-to-door approach to reduce implementation cost.35 This might have also encouraged the uptake of immunisations. However, the coverage of maternal interventions was slightly lower than that of childhood interventions.
ITNs were only limited to the private and special project-based distributions until 2000 when they were partially subsidised through to 2004 followed by high subsidies and delivered via MCH clinics. After 2006, ITNs were available free of charge through routine delivery and mass delivery in 2006, 2008, 2011/2012, 2014 and 2015.37 38 Increase in coverage of ITNs among children coincides with these efforts (online supplemental file 3, figures 3 and 4). The regions with high malaria prevalence (counties in western, coastal and partly in the Kenyan highlands) have benefited from targeted ITN distribution and historical clinical trials which might explain their higher coverage relative to other regions.38 The low coverage of recommended antimalarial medicine might be due to frequent changes in first-line treatment of uncomplicated malaria; from chloroquine to the long half-life, single-dose sulfadoxine-pyrimethamine (SP) in 1998 which was later changed to artemisinin-based combination therapy in 2006.38–40
The period of greatest decline in malaria risk occurred prior to the scaling of ITNs and coincided with a period of use of infective drugs. The decline could be linked to the widespread availability of SP and its long half-life providing prophylaxis after single-dose administration.76 On the other hand, increasing HIV infection prevalence in the 1990s (online supplemental file 3) led to the establishment of NACC that put measures that coincided with HIV decline. Since 2000, PMTCT, paediatric HIV programmes, ARVs uptake, testing and behavioural change campaigns have increased steadily.41–43 45 77–81
Increase in healthcare utilisation rates can be linked to policies on user fees. The health voucher programme for maternity services (2006–2016), the abolishment of delivery fees (2007), free maternity services (2013) and suspension of user fee (1990).3 26–32 From 1991, user fees charged for services such as drugs and laboratory services hampered utilisation.82–85 This led to the removal of user fees at dispensaries and health centres (except a for registration fee) in 20043 26 with a health sector services fund introduced in 2010 to compensate for the removal of user fee at dispensaries and health centres.26 Before the suspension of user fees, antenatal care utilisation declined in all counties with Mandera, Wajir, Marsabit, Garissa, Isiolo, Tana River and Turkana counties witnessing larger declines between 1993 and 2003 (online supplemental file 3). Similar trends were observed for other markers of healthcare utilisation such as institutional deliveries. However, an immediate shift in utilisation trends, declining in the 1990s (eg, institutional deliveries) especially in northern counties (Mandera, Isiolo, Garissa, Wajir, Marsabit, Samburu, Laikipia, Baringo and West Pokot) was witnessed from 2006 after the new policies on user fees (online supplemental file 3).
Long physical distances to point of care affect utilisation rates negatively, however, in Kenya, over time, the number of health facilities has increased reducing the travel time needed to seek care.73–75 Therefore, it is plausible that the quality and availability of services offered at points of care might be major factors relative to the physical distance in influencing utilisation rates in some parts of Kenya. The stagnation of ANC4 rates and some of the immunisations could be because they require multiple contacts with the health system, inadequate staff or poor adherence to treatment guidelines.12 21 However, counties in Northern Kenya, have always had poor physical access to healthcare services hence lower utilisation rates. In 2003, only 18% of the total population was within the recommended distance of a health facility (5 km radius) improving marginally to 29% in 2008 compared with national averages of 71% and 89%, respectively.74 For example, mean travel time of up to 120+ minutes was significantly associated with poor immunisation outcomes only in Northern Kenya (Isiolo and Marsabit).71
Low breastfeeding coverage and high malnutrition levels continue to be a public health concern for Kenya with moderate gains witnessed across time.86–89 The small improvements can partly be associated with breastfeeding initiatives (BFCI and BFHI) both at the hospital and community level,36 food fortification, micronutrients, nutritional campaigns, school and community level initiatives (online supplemental file 1). However, the number of children receiving the minimum acceptable diet was low and declined over time.90–93 The piloting and demonstration of BFCI in parts of Siaya (Bondo) and Meru (Igembe North) counties in 2011 showed increased likelihood of participating in ANC, institutional deliveries and initiating breast feeding within an hour of birth94 and appears to be linked to broad increases in coverage (online supplemental file 3). Following the demonstration, BFCI was included in the National Nutrition Action Plan and prioritised as a high impact nutrition intervention.94
The use of modern contraceptive increased by over 90%, however, despite this improvement by 2014, only 40% of the women in need of contraception were covered (table 3). Family planning reduces closely spaced births, ill-timed births and high parity and might explain why there was a moderate reduction in short birth interval and high parity95–97 (online supplemental file 3). There have been several efforts and initiatives to improve the coverage of family planning by addressing drivers of the slow progress.98–102 For, example, The government committed to increase the budget allocated for family planning services in 2012 which may have led to the achievement of 2020 target (58% coverage) and is now focusing on equitable access subnationally.103 104 The marked regional heterogeneities in contraceptive use (online supplemental file 3) have been linked with socioeconomic and cultural environment102 and possibly the regional initiatives addressing areas with lower coverage. For, example, after the launch of AMUA project (a social franchise) to provide family planning among under-served communities led to improved contraceptive use in the focus counties of western and coastal parts of Kenya between 2003 and 2008 (online supplemental file 3).105
The coverage of both improved sanitation and access to clean water at household level has remained low. The poor coverage has been associated with low education attainment, living in rural areas and poverty.106 The government of Kenya committed to focus on the poorest, eliminate open defecation by 2030 and to invest 0.5% of its gross domestic product by 2020 to sanitation.107 However, currently (2019), only a third of the households have access to piped water and 8.2% do not have access to any sanitation facility.108 Minimal improvement in household wealth (online supplemental file 3) compounded the already dire need for access for safe and clean water and improved sanitation.
Historical (1993–2013) county level data on subnational policy and their implications are scarce for periods before 2013 when the devolved government was incorporated; thus, county level discourse is based on exemplar and limited information. At the county level, the coverage of interventions was disproportionately distributed over time; the coverage ranged between high to acutely low in disadvantaged and marginalised areas. Overall, counties in Central and Western Kenya had a moderate to high intervention coverage while counties in Northern Kenya were marginalised across the entire study period.
Northern Kenya is predominately arid and semi-arid (ASAL) with a low amount of rainfall and vegetation (online supplemental file 3) associated with reduced yields from rain-fed agriculture, persistent food insecurity and lack of green pastures for livestock.109 110 They have challenges in accessing clean water and improved sanitation, low education attainment and more poor households, consequently, malnutrition is high in this region.106 110 Healthcare utilisation rates are low due to poor infrastructure and limited geographical access, conflict and insecurity hence low immunisation rates and use of modern contraceptives.71 73 74 101 111 However, in this region, religion and cultural beliefs might be a stronger determinant of contraception use in comparison to poverty and lack of access.112–114 In view of historical and economic similarities between regions in Kenya, six economic blocs were formed. Among them is the Frontier Counties Development Council consisting of ASAL counties mainly in Northern Kenya (Lamu, Tana River, Garissa, Wajir, Mandera, Marsabit, Isiolo, Turkana, Samburu and West Pokot) which aims to enhance socioeconomic development and sustainable development through better cooperation through projects such as livestock strengthening.115 These counties can further harness the assembled data to gain better insights on trends for informed decision making.
Conversely, the counties in Central Kenya with the higher coverage historically have higher agricultural productivity, lower rates of disease, better access to education, clean water and quality sanitation. Spatial access to healthcare is better in most parts of these counties, hence, better healthcare utilisation rates, access to vaccinations and supplementations.71 74 75 116 The infection prevalence of HIV and malaria have declined across much of the country but remain high in Western Kenya and parts of south-east (online supplemental file 3). This has led to targeted interventions, for example, restricting IPTp to high prevalence areas, the pilot introduction of RTSS vaccine, and focused efforts to increase coverage of ITNs, IRS larval source management117 and increased attention to HIV preventative interventions43 118–121 in these two regions.
In this paper, we have not attributed the changing coverage of interventions and infection prevalence variations to disparities in U5M.1 However, preliminary analysis, show that counties with high U5M (western and coastal region) were characterised with high HIV and malaria prevalence. Central region with low infection prevalence and higher coverage of interventions had lower U5M although smaller upsurges. Northern region with low HIV and malaria prevalence but constrained by low access to interventions, poor healthcare utilisation limited access to clean and safe water and sanitation had moderate U5M. The correlation coefficient between these factors and U5M (online supplemental file 5) were statistically significant for all but three factors. Malaria prevalence and early infant breast feeding had moderate correlation.122 This underpins the need for a rigorous examination of the impact of these factors on driving trends in U5M across the 47 counties. Such evidence would be important for the health planners and policy makers and for targeted resource allocation.
bmjgh-2020-004544supp005.pdf (27.4KB, pdf)
The estimates generated in this analysis have important implications on existing efforts to improve child survival across Kenya under the decentralised governance structure while leaving no one behind and reaching the farthest behind, first. County planners can gain insights on coverage and trends to facilitate prioritisation. For example, by 2014, Mandera’s coverage of ANC1, BCG and polio vaccines were ≥70% while improved sanitation and contraceptive use were ≤3% justifying additional funds and prioritisation for the latter factors. These estimates provide opportunities for benchmarking across counties where localised initiatives which have been shown to successfully improve coverage and in reduce disease prevalence.12–15 The success of Afya Uzazi (healthy parenthood) programme in Baringo and Nakuru counties in improving access to quality health services by targeting family planning, pregnancies and deliveries, can be a benchmarking point for neighbouring counties.
At the national level, the government through The Commission on Revenue Allocation can leverage on the generated estimates when distributing national-level resources to 47 counties. The commission uses a weighted average of key factors including health indicators and can leverage on these estimates to better disaggregate the differences between counties. Further, counties in Northern Kenya should be targeted and prioritised during resources allocation and policy formulation to increase intervention coverage while more concerted efforts should be directed to western and coastal parts of the country to lower HIV and malaria infection prevalence. Various divisions such immunisation, national malaria control programme, human nutrition and dietetics unit within the national Ministry of Health can use the estimates as baseline to evaluate the impact of interventions that had been rolled out. This evaluation can be extended to include international development partners such as the GAVI the vaccine alliance to evaluate the impact of their funding or justify support for immunisation outreach programme. The findings should also form a key baseline for monitoring SDGs indicators proposed under the Inter-Agency and Expert Group on SDG Indicators123 and county-specific targets as outlined in each county blue print, the County Integrated Development Plan for the 47 county governments.124 The estimates form a key input in epidemiological studies of child survival across Kenya and these will be made available through a data visualisation web portal. Finally, the modelling framework can be applied to update the subnational estimates and evaluate progress as new data sources becomes available such as the recently concluded population census in Kenya (2019).55 108
We compared our estimates with previous estimates generated from similar and comparable studies that computed the prevalence of wasting, stunting and underweight, access to improved water sources, coverage of DPT3 (for the period 2000–2014)125–127 and skilled birth attendance (SBA) (for the period 2014)128 (online supplemental file 6). The estimates were highly correlated and generally with good concordance, identifying and ranking nearly all similar counties with the highest coverage (or lowest disease prevalence) and those with lowest coverage (or highest disease prevalence) (online supplemental file 6). The slight differences observed with reference to SBA are likely due to more data used in the current study within a spatio-temporal model without covariates relative to fewer data sources within a spatial model with covariates used in the comparator.128
bmjgh-2020-004544supp006.pdf (34.9KB, pdf)
Limitations
There are several caveats to this analysis. Tracking of coverage estimates beyond 2014 was not possible due to lack of data post-2014 limiting the number of the possible applications. Despite interpolating in space and time, household surveys are limited as they are conducted every three to five years powered for precise estimates at provincial level, thus they are not an alternative to quality data from HMIS. Additionally, factors such as human resources for health which affect child survival were not included due to lack of spatio-temporal data. The introduction and expansion of District Health Information System version 2 (DHIS2)129 130 as part of the HMIS in Kenya and the development of approaches to deal with limitations of routine data131–134 will allow incorporation of more variables in future analyses.
Recall and self-report biases were associated with some indicators, especially for longer recall periods, however, this effect was minimised by limiting the recall period to 3 years preceding the survey.62 There was selection bias for some indicators because the survey included only the experiences of mothers with a live birth 3 years preceding a survey leaving out mothers with other birth outcomes or those who might have died during pregnancy or delivery. The coverage estimates are not indicative of the quality of interventions received nor do they measure effective coverage which combines the need for, use and quality of the interventions.12 135
There was bias due to the modifiable areal unit problem, where results obtained might have been different if data had been aggregated into differently sized spatial units. A small proportion of clusters near county boundaries may have been misclassified because the displacement of cluster coordinates (due to confidentiality) was not accounted for. However, the use of CAR models to smooth estimates across adjacent counties potentially reduced this effect. Some small-scale heterogeneities were masked especially in high sized counties of Northern Kenya and disaggregation of the results to units lower than the counties (subcounties) would improve relevance at county level, however, the precision would reduce drastically. This provides an impetus for a strengthening DHIS2 and in the meantime powering household surveys to be representative at county level and conducting them more regularly.
While exploring inequities across other domains such as disparities across wealth quintiles, urban/rural stratifications or education attainment is important for policy making, however, the focus our work was to explore and describe inequities across geographic areas (counties) for 43 factors. Majority of the other domains including wealth quintiles, urban/rural differences have previously been explored through the standard DHS reports and by Keats and colleagues21 in Kenya.
Conclusion
By harnessing and combining multiple data sources including household sample surveys and population censuses within a geospatial framework, levels and trends of 43 factors associated with child survival were generated between 1993 and 2014 in Kenya. The variation between factors over time was wide and estimates were highly heterogeneous between counties and over time. The marginalised counties that have been left behind should be given priority to address health inequities. The subnational estimates are useful to county planners in the current decentralised system of governance for evidence-based priority setting, a key baseline for monitoring and tracking of interventions within the defined local and global targets such as the SDGs.
Footnotes
Handling editor: Seye Abimbola
Twitter: @Pete_M_M
Contributors: PMM undertook the development of the models, analysis and writing of the first draft of the manuscript. NKJ provided support for data assembly, checking, interpretation and data validation. BS, RWS and EO provided scientific and methodological support throughout the project. EO conceived the project, provided overall management and interpretation of results. All authors reviewed the final analysis, have access to the data and approved the final manuscript. All authors read and met ICMJE criteria for authorship.
Funding: PMM is funded under the IDeAL’s Project, DELTAS Africa Initiative (DEL-15-003). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (number 107769/Z/10/Z) and the UK government. RWS is funded by Wellcome Trust Principal Fellowship (numbers 103602 and 212176) that also provided support for PMM. EO is supported as a Wellcome Trust Intermediate Fellow (number 201866) that provided support for NKJ; PMM, NKJ, RWS and EO acknowledge the support of the Wellcome Trust to the Kenya Major Overseas Programme (# 203077). The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Disclaimer: The funder of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The full database of household sample surveys, population censuses, malaria surveys and environmental data that support the findings of this study are available open access from online data repositories available to registered users. Integrated Public Use Microdata Series (IPUMS)—https://international.ipums.org/international/index.shtml; Multiple Indicator Cluster Surveys (MICS)—http://mics.unicef.org/; Demographic and Health Surveys (DHS)—https://dhsprogram.com/; Kenya National Bureau of Statistics (KNBS)—http://statistics.knbs.or.ke/nada/index.php/home; Population Health Harvard Dataverse—https://dataverse.harvard.edu/dataverse/population-health.
References
- 1. Macharia PM, Giorgi E, Thuranira PN, et al. Sub national variation and inequalities in under-five mortality in Kenya since 1965. BMC Public Health 2019;19:146. 10.1186/s12889-019-6474-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landier J, Rebaudet S, Piarroux R, et al. Spatiotemporal analysis of malaria for new sustainable control strategies. BMC Med 2018;16:226. 10.1186/s12916-018-1224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barasa E, Nguhiu P, McIntyre D. Measuring progress towards sustainable development goal 3.8 on universal health coverage in Kenya. BMJ Glob Health 2018;3:e000904. 10.1136/bmjgh-2018-000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marmot M, Bell R. The sustainable development goals and health equity. Epidemiology 2018;29:5–7. 10.1097/EDE.0000000000000773 [DOI] [PubMed] [Google Scholar]
- 5. Stuart E, Woodroffe J. Leaving no-one behind: can the sustainable development goals succeed where the millennium development goals lacked? Gend Dev 2016;24:69–81. 10.1080/13552074.2016.1142206 [DOI] [Google Scholar]
- 6. Ferreira LZ, Blumenberg C, Utazi CE, et al. Geospatial estimation of reproductive, maternal, newborn and child health indicators: a systematic review of methodological aspects of studies based on household surveys. Int J Health Geogr 2020;19:1–15. 10.1186/s12942-020-00239-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corsi DJ, Neuman M, Finlay JE, et al. Demographic and health surveys: a profile. Int J Epidemiol 2012;41:1602–13. 10.1093/ije/dys184 [DOI] [PubMed] [Google Scholar]
- 8. Simpson EK. Spatial modeling with R-INLA : a review. Wiley 2018:1–24. 10.1002/wics.1443 [DOI] [Google Scholar]
- 9. Diggle PJ, Ribeiro PJ. Model-based geostatistics. 1 edn. New York: Springer Science+Business Media, 2007. [Google Scholar]
- 10. Manda S, Haushona N, Bergquist R. A scoping review of spatial analysis approaches using health survey data in sub-Saharan Africa. Int J Environ Res Public Health 2020;17. 10.3390/ijerph17093070. [Epub ahead of print: 28 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. JNK R. Small area estimation. 1 edn. New Jersey: John Wiley & Sons, Ltd, 2003. [Google Scholar]
- 12. Noor AM. Subnational benchmarking of health systems performance in Africa using health outcome and coverage indicators. BMC Med 2015;13:299. 10.1186/s12916-015-0541-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wollum A, Burstein R, Fullman N, et al. Benchmarking health system performance across states in Nigeria: a systematic analysis of levels and trends in key maternal and child health interventions and outcomes, 2000-2013. BMC Med 2015;13:208. 10.1186/s12916-015-0438-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colson KE, Dwyer-Lindgren L, Achoki T, et al. Benchmarking health system performance across districts in Zambia: a systematic analysis of levels and trends in key maternal and child health interventions from 1990 to 2010. BMC Med 2015;13:69. 10.1186/s12916-015-0308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roberts DA, Ng M, Ikilezi G, et al. Benchmarking health system performance across regions in Uganda: a systematic analysis of levels and trends in key maternal and child health interventions, 1990-2011. BMC Med 2015;13:285. 10.1186/s12916-015-0518-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruktanonchai CW, Nilsen K, Alegana VA, et al. Temporal trends in spatial inequalities of maternal and newborn health services among four East African countries, 1999–2015. BMC Public Health 2018;18:1–13. 10.1186/s12889-018-6241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burgert-Brucker CR, Dontamsetti T, Gething PW. The DHS program's modeled surfaces spatial datasets. Stud Fam Plann 2018;49:87–92. 10.1111/sifp.12050 [DOI] [PubMed] [Google Scholar]
- 18. Li Q, Louis TA, Liu L, et al. Subnational estimation of modern contraceptive prevalence in five sub-Saharan African countries: a Bayesian hierarchical approach. BMC Public Health 2019;19:216. 10.1186/s12889-019-6545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. IHME . About the local burden of disease project, 2020. Available: http://www.healthdata.org/lbd/about [Accessed 2 Sep 2020].
- 20. Maina I, Wanjala P, Soti D, et al. Using health-facility data to assess subnational coverage of maternal and child health indicators, Kenya. Bull World Health Organ 2017;95:683–94. 10.2471/BLT.17.194399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keats EC, Akseer N, Bhatti ZNgugi A, Macharia W, et al. Assessment of inequalities in coverage of essential reproductive, maternal, newborn, child, and adolescent health interventions in Kenya. JAMA Netw Open 2018;1:e185152. 10.1001/jamanetworkopen.2018.5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macharia PM, Giorgi E, Noor AM, et al. Spatio-temporal analysis of Plasmodium falciparum prevalence to understand the past and chart the future of malaria control in Kenya. Malar J 2018;17:340. 10.1186/s12936-018-2489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wairoto KG, Joseph NK, Macharia PM, et al. Determinants of subnational disparities in antenatal care utilisation: a spatial analysis of demographic and health survey data in Kenya. BMC Health Serv Res 2020;20:655.. 10.1186/s12913-020-05531-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. GoK . The constitution of Kenya, 2010. Available: http://kenyalaw.org/kl/index.php?id=398 [Accessed 23 May 2016].
- 25. MoH/ GoK. Transforming health: accelerating attainment of universal health coverage: Kenya health sector strategic and investment plan (KHSSP1) July 2013- June 2017, 2014. Available: http://e-cavi.com/wp-content/uploads/2014/11/kenya-health-sector-strategic-investiment-plan-2013-to-2017.pdf [Accessed 22 May 2019].
- 26. Chuma J, Okungu V. Viewing the Kenyan health system through an equity lens: implications for universal coverage. Int J Equity Health 2011;10:22–14. 10.1186/1475-9276-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dennis ML, Abuya T, Campbell OMR, et al. Evaluating the impact of a maternal health voucher programme on service use before and after the introduction of free maternity services in Kenya: a quasi-experimental study. BMJ Glob Health 2018;3:e000726–11. 10.1136/bmjgh-2018-000726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dennis ML, Benova L, Abuya T, et al. Initiation and continuity of maternal healthcare: examining the role of vouchers and user-fee removal on maternal health service use in Kenya. Health Policy Plan 2019;34:120–31. 10.1093/heapol/czz004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gitobu CM, Gichangi PB, Mwanda WO. The effect of Kenya’s free maternal health care policy on the utilization of health facility delivery services and maternal and neonatal mortality in public health facilities. BMC Pregnancy Childbirth 2018;18:1–11. 10.1186/s12884-018-1708-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dennis ML, Benova L, Goodman C, et al. Examining user fee reductions in public primary healthcare facilities in Kenya, 1997–2012: effects on the use and content of antenatal care. Int J Equity Health 2020;19:1–13. 10.1186/s12939-020-1150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janisch CP, Albrecht M, Wolfschuetz A, et al. Vouchers for health: a demand side output-based aid approach to reproductive health services in Kenya. Glob Public Health 2010;5:578–94. 10.1080/17441690903436573 [DOI] [PubMed] [Google Scholar]
- 32. Abuya T, Njuki R, Warren CE, et al. A policy analysis of the implementation of a reproductive health vouchers program in Kenya. BMC Public Health 2012;12:540. 10.1186/1471-2458-12-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MoH/ GoK. National policy guidelines on immunization in Kenya, 2013. Available: https://www.medbox.org/kenya-national-policy-guidelines-on-immunization-2013/download.pdf [Accessed 2 Feb 2018].
- 34. Ahoya B, Kavle JA, Straubinger S, et al. Accelerating progress for complementary feeding in Kenya: key government actions and the way forward. Matern Child Nutr 2019;15:e12723. 10.1111/mcn.12723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clohossey PC, Katcher HI, Mogonchi GO, et al. Coverage of vitamin A supplementation and deworming during Malezi Bora in Kenya. J Epidemiol Glob Health 2014;4:169–76. 10.1016/j.jegh.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maingi M, Kimiywe J, Iron-Segev S. Effectiveness of baby friendly community initiative (BFCI) on complementary feeding in Koibatek, Kenya: a randomized control study. BMC Public Health 2018;18:1–11. 10.1186/s12889-018-5519-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Noor AM, Amin AA, Akhwale WS, et al. Increasing coverage and decreasing inequity in insecticide-treated bed net use among rural Kenyan children. PLoS Med 2007;4:e255. 10.1371/journal.pmed.0040255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MoH/ GoK. The epidemiology and control profile of malaria in Kenya: reviewing the evidence to guide the future vector control, 2016. Available: http://virtual.lshtm.ac.uk/wp-content/uploads/2016/11/Kenya-Epidemiological-Profile.pdf [Accessed 13 Dec 2016].
- 39. Amin AA, Zurovac D, Kangwana BB, et al. The challenges of changing national malaria drug policy to artemisinin-based combinations in Kenya. Malar J 2007;6:72. 10.1186/1475-2875-6-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shretta R, Omumbo J, Rapuoda B, et al. Using evidence to change antimalarial drug policy in Kenya. Trop Med Int Health 2000;5:755–64. 10.1046/j.1365-3156.2000.00643.x [DOI] [PubMed] [Google Scholar]
- 41. Murithi LK, Masho SW, Vanderbilt AA. Factors enhancing utilization of and adherence to prevention of mother-to-child transmission (PMTCT) service in an urban setting in Kenya. AIDS Behav 2015;19:645–54. 10.1007/s10461-014-0939-0 [DOI] [PubMed] [Google Scholar]
- 42. Barnhart DA, Tsikhutsu I, Kirui D, et al. Association of the US president's emergency plan for AIDS relief's funding with prevention of mother-to-child transmission of HIV in Kenya. JAMA Netw Open 2019;2:e1911318. 10.1001/jamanetworkopen.2019.11318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohler PK, Okanda J, Kinuthia J, et al. Community-based evaluation of PMTCT uptake in Nyanza Province, Kenya. PLoS One 2014;9:e110110. 10.1371/journal.pone.0110110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullei K, Wafula CG F. A case study of integrated management of childhood illness (IMCI) implementation in Kenya, 2008. Available: http://www.crehs.lshtm.ac.uk/downloads/publications/IMCI_implementation_in_kenya.pdf [Accessed 16 Nov 2020].
- 45. Keats EC, Ngugi A, Macharia W, et al. Progress and priorities for reproductive, maternal, newborn, and child health in Kenya: a countdown to 2015 country case study. Lancet Glob Health 2017;5:e782–95. 10.1016/S2214-109X(17)30246-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. GoK. A globally competitive and Prosperous Kenya: first medium term plan, 2008. Available: https://www.sida.se/contentassets/855677b831b74ea0b226ce2db4eb93a3/kenya_medium_term_plan_2008-2012.pdf [Accessed 21 May 2019].
- 47. Okaka FO, Odhiambo BDO. Relationship between flooding and out break of infectious Diseasesin Kenya: a review of the literature. J Environ Public Health 2018;2018:1–8. 10.1155/2018/5452938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spencer L. Kenya’s history of political violence: colonialism, vigilantes and militias. Conversat, 2017. Available: https://theconversation.com/kenyas-history-of-political-violence-colonialism-vigilantes-and-militias-83888 [Accessed 1 Dec 2020].
- 49. DCHA, OFDA . Kenya USAID humanitarian assistance in review, 1997 – 2008, 2008. Available: https://reliefweb.int/report/kenya/kenya-usaid-humanitarian-assistance-review-1997-present [Accessed 20 May 2019].
- 50. Venton CC. Economics of resilience to drought - Kenya analysis, 2018. Available: https://www.usaid.gov/sites/default/files/documents/1867/Kenya_Economics_of_Resilience_Final_Jan_4_2018_-_BRANDED.pdf [Accessed 22 Feb 2019].
- 51. UNDP . Kenya natural disaster profile, 2007. Available: http://meteorology.uonbi.ac.ke/sites/default/files/cbps/sps/meteorology/ProjectonDisasters.pdf [Accessed 23 Feb 2018].
- 52. GoK/MoH . Kenya health policy 2014-2030: towards attaining the highest standard of health, 2014. Available: http://www.health.go.ke/images/policydocs/KHPFinalbooklet.pdf [Accessed 25 Mar 2017].
- 53. Barasa EW, Manyara AM, Molyneux S, et al. Recentralization within decentralization: County Hospital autonomy under devolution in Kenya. PLoS One 2017;12:e0182440. 10.1371/journal.pone.0182440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. KPMG . Devolution of healthcare services in Kenya: lessons learnt from other countries, 2013. Available: https://www.kpmg.com/Africa/en/IssuesAndInsights/Articles-Publications/Documents/DevolutionofHCServicesinKenya.pdf[Accessed 22 May 2015].
- 55. Minnesota Population Center . Integrated public use microdata series, International: version 6.5 [Kenya census data], 2016. Available: https://international.ipums.org/international-action/variables/group?id=fert [Accessed 21 Apr 2016].
- 56. KNBS . Kenya national data Archive, 2016. Available: http://statistics.knbs.or.ke/nada/index.php/home [Accessed 21 Apr 2020].
- 57. UNICEF . Unicef MICs surveys, 2016. Available: http://mics.unicef.org/surveys [Accessed 21 Apr 2016].
- 58. ICF . The DHS program : available datasets, 2016. Available: http://dhsprogram.com/data/available-datasets.cfm [Accessed 21 Apr 2016].
- 59. Mosley WH, Chen LC. An analytical framework for the study of child survival in developing countries. Popul Dev Rev 1984;10:25–45. 10.2307/2807954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hill K. Frameworks for studying the determinants of child survival. Bull World Health Organ 2003;81:138–9. [PMC free article] [PubMed] [Google Scholar]
- 61. Corsi DJ, Subramanian SV. Association between coverage of maternal and child health interventions, and under-5 mortality: a repeated cross-sectional analysis of 35 sub-Saharan African countries. Glob Health Action 2014;7:24765. 10.3402/gha.v7.24765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ngandu NK, Manda S, Besada D, et al. Does adjusting for recall in trend analysis affect coverage estimates for maternal and child health indicators? an analysis of DHS and MICs survey data. Glob Health Action 2016;9:32408. 10.3402/gha.v9.32408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. NACC . Kenya HIV prevalence estimates, 2020. Available: https://nacc.or.ke/ [Accessed 22 Oct 2020].
- 64. Funk C, Peterson P, Landsfeld M, et al. The climate hazards infrared precipitation with stations--a new environmental record for monitoring extremes. Sci Data 2015;2:1–21. 10.1038/sdata.2015.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matsushita B, Yang W, Chen J. Sensitivity of the enhanced vegetation index (Evi) and normalized difference vegetation index (NDVI) to topographic effects. Sensors 2007;7:2636–51. 10.3390/s7112636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rajaratnam JK, Marcus JR, Flaxman AD, et al. Neonatal, postneonatal, childhood, and under-5 mortality for 187 countries, 1970-2010: a systematic analysis of progress towards millennium development goal 4. Lancet 2010;375:1988–2008. 10.1016/S0140-6736(10)60703-9 [DOI] [PubMed] [Google Scholar]
- 67. Mehdipour P, Navidi I, Parsaeian M, et al. Application of Gaussian Process Regression (GPR) in estimating under-five mortality levels and trends in Iran 1990 - 2013, study protocol. Arch Iran Med 2014;17:189–92. doi:0141703/AIM.009 [PubMed] [Google Scholar]
- 68. Haji A, Lowther S, Ngan’ga Z, Ngan Z, et al. Reducing routine vaccination dropout rates: evaluating two interventions in three Kenyan districts, 2014. BMC Public Health 2016;16:1–8. 10.1186/s12889-016-2823-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gibson DG, Ochieng B, Kagucia EW, et al. Individual level determinants for not receiving immunization, receiving immunization with delay, and being severely underimmunized among rural Western Kenyan children. Vaccine 2015;33:6778–85. 10.1016/j.vaccine.2015.10.021 [DOI] [PubMed] [Google Scholar]
- 70. Calhoun LM, van Eijk AM, Lindblade KA, et al. Determinants and coverage of vaccination in children in Western Kenya from a 2003 cross-sectional survey. Am J Trop Med Hyg 2014;90:234–41. 10.4269/ajtmh.13-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Joseph NK, Macharia PM, Ouma PO, et al. Spatial access inequities and childhood immunisation uptake in Kenya. BMC Public Health 2020;20:1407. 10.1186/s12889-020-09486-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. MoH/ GoK. Kenya DVI comprehensive Multi-Year plan 2006-2010, 2010. Available: http://e-cavi.com/wp-content/uploads/2014/11/KENYA-IMMUNIZATION-MULTI-YEAR-PLAN-2006_2010.pdf [Accessed 22 May 2020].
- 73. Noor AM, Zurovac D, Hay SI, et al. Defining equity in physical access to clinical services using geographical information systems as part of malaria planning and monitoring in Kenya. Trop Med Int Health 2003;8:917–26. 10.1046/j.1365-3156.2003.01112.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Noor AM, Alegana VA, Gething PW, et al. A spatial National health facility database for public health sector planning in Kenya in 2008. Int J Health Geogr 2009;8:13. 10.1186/1476-072X-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ouma PO, Maina J, Thuranira PN, et al. Access to emergency hospital care provided by the public sector in sub-Saharan Africa in 2015: a geocoded inventory and spatial analysis. Lancet Glob Health 2018;6:e342–50. 10.1016/S2214-109X(17)30488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Snow RW, Kibuchi E, Karuri SW, et al. Changing malaria prevalence on the Kenyan coast since 1974: climate, drugs and vector control. PLoS One 2015;10:e0128792. 10.1371/journal.pone.0128792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lupia R, Chien S-C. Hiv and AIDS epidemic in Kenya: an overview. J Exp Clin Med 2012;4:231–4. 10.1016/j.jecm.2012.06.007 [DOI] [Google Scholar]
- 78. Grabbe KL, Menzies N, Taegtmeyer M, et al. Increasing access to HIV counseling and testing through mobile services in Kenya: strategies, utilization, and cost-effectiveness. J Acquir Immune Defic Syndr 2010;54:317–23. 10.1097/QAI.0b013e3181ced126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Marum E, Taegtmeyer M, Chebet K. Scale-Up of voluntary HIV counseling and testing in Kenya. JAMA 2006;296:859–62. 10.1001/jama.296.7.859 [DOI] [PubMed] [Google Scholar]
- 80. Marum E, Morgan G, Hightower A, et al. Using mass media campaigns to promote voluntary counseling and HIV-testing services in Kenya. AIDS 2008;22:2019–24. 10.1097/QAD.0b013e3283104066 [DOI] [PubMed] [Google Scholar]
- 81. NASCOP . Kenya AIDS indicator survey 2012, final report, 2014. Available: https://nacc.or.ke/kais-2012-final-report/ [Accessed 25 Jun 2016].
- 82. Mwabu G. Health care reform in Kenya: a review of the process. Health Policy 1995;32:245–55. 10.1016/0168-8510(95)00738-E [DOI] [PubMed] [Google Scholar]
- 83. Mwabu G, Mwanzia J, Liambila W. User charges in government health facilities in Kenya: effect on attendance and revenue. Health Policy Plan 1995;10:164–70. 10.1093/heapol/10.2.164 [DOI] [PubMed] [Google Scholar]
- 84. Mwabu GM. Health care decisions at the household level: results of a rural health survey in Kenya. Soc Sci Med 1986;22:315–9. 10.1016/0277-9536(86)90129-2 [DOI] [PubMed] [Google Scholar]
- 85. Collins D, Quick JD, Musau SN, et al. The fall and rise of cost sharing in Kenya: the impact of phased implementation. Health Policy Plan 1996;11:52–63. 10.1093/heapol/11.1.52 [DOI] [PubMed] [Google Scholar]
- 86. Masibo PK, Makoka D. Trends and determinants of undernutrition among young Kenyan children: Kenya demographic and health survey; 1993, 1998, 2003 and 2008-2009. Public Health Nutr 2012;15:1715–27. 10.1017/S1368980012002856 [DOI] [PubMed] [Google Scholar]
- 87. Gewa CA, Yandell N. Undernutrition among Kenyan children: contribution of child, maternal and household factors. Public Health Nutr 2012;15:1029–38. 10.1017/S136898001100245X [DOI] [PubMed] [Google Scholar]
- 88. Matanda DJ, Mittelmark MB, Kigaru DMD. Child undernutrition in Kenya: trend analyses from 1993 to 2008–09. BMC Pediatr 2014;14:1–13. 10.1186/1471-2431-14-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kabubo-Mariara J, Ndenge GK, Mwabu DK. Determinants of children’s nutritional status in Kenya: Evidence from Demographic and Health Surveys. J Afr Econ 2009;18:363–87. 10.1093/jae/ejn024 [DOI] [Google Scholar]
- 90. Matanda DJ, Urke HB, Mittelmark MB. Changes in optimal childcare practices in Kenya: insights from the 2003, 2008-9 and 2014 demographic and health surveys. PLoS One 2016;11:e0161221. 10.1371/journal.pone.0161221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Whaley SE, Sigman M, Neumann C. Animal source foods to improve micronutrient nutrition and human function in developing countries. Proceedings of a conference. June 24-26, 2002. Washington, DC, USA. J Nutr 2003;133:3965–71. 10.1093/jn/133.11.3875S [DOI] [PubMed] [Google Scholar]
- 92. Stephenson K, Amthor R, Mallowa S. Consuming cassava as a staple food places children 2-5 years old at risk for inadequate protein intake, an observational study in Kenya and Nigeria. Plant Foods Hum Nutr 2010;65:64–70. 10.1007/s11130-010-0157-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kamau MW, Makokha AO, Mutai JK, et al. Factors influencing vitamin A supplementation among mothers of children under five years old at Mbagathi district Hospital, Kenya. East Afr Med J 2012;89:134–41. [PubMed] [Google Scholar]
- 94. Kavle JA, Ahoya B, Kiige L, et al. Baby‐Friendly community Initiative—From national guidelines to implementation: a multisectoral platform for improving infant and young child feeding practices and integrated health services. Matern Child Nutr 2019;15:1–19. 10.1111/mcn.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Saha UR, van SA. Does family planning reduce infant mortality? Evidence from surveillance data in Matlab, Bangladesh. Cent Discuss Pap Ser No 2012. 10.2139/ssrn.2009853 [DOI] [Google Scholar]
- 96. Forstet R. The effects of breastfeeding and birth spacing on infant and child mortality in Bolivia. Demography 1994;48:497–511. 10.1080/0032472031000147996 [DOI] [Google Scholar]
- 97. Manda SO. Birth intervals, breastfeeding and determinants of childhood mortality in Malawi. Soc Sci Med 1999;48:301–12. 10.1016/S0277-9536(98)00359-1 [DOI] [PubMed] [Google Scholar]
- 98. Miller RA, Ndhlovu L, Gachara MM, et al. The situation analysis study of the family planning program in Kenya. Stud Fam Plann 1991;22:131–43. 10.2307/1966641 [DOI] [PubMed] [Google Scholar]
- 99. Tumlinson K, Pence BW, Curtis SL, et al. Quality of care and contraceptive use in urban Kenya. Int Perspect Sex Reprod Health 2015;41:69–79. 10.1363/4106915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kimani M. Trends in contraceptive use in Kenya, 1989-1998: the role of socio-economie, cultural and family planning factors. Etude la Popul Africaine 2006;21:1989–98. 10.1016/j.physbeh.2017.03.040 [DOI] [Google Scholar]
- 101. Lunani LL, Abaasa A, Omosa-Manyonyi G. Prevalence and factors associated with contraceptive use among Kenyan women aged 15–49 years. AIDS Behav 2018;22:125–30. 10.1007/s10461-018-2203-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kimani M, Njeru M, Ndirangu G. Regional variations in contraceptive use in Kenya: comparison of Nyanza, Coast and central provinces. Etude la Popul Africaine 2012;27:43–52. 10.11564/27-1-6 [DOI] [Google Scholar]
- 103. GOK/MoH . Family planning 2020 commitment, 2017. Available: http://ec2-54-210-230-186.compute-1.amazonaws.com/wp-content/uploads/2018/02/Kenya_FP2020_Commitment_2017.pdf
- 104. FP2020 . Kenya family planning commitmnet marker since 2012, 2020. Available: http://www.familyplanning2020.org/kenya [Accessed 6 Nov 2020].
- 105. MoH/GoK , 2016. National family planning costed implementation plan. Available: http://www.familyplanning2020.org/sites/default/files/Kenya_National_Family_Planning_Costed_Implementation_Plan_2012-2016.pdf [Accessed 10 Mar 2021].
- 106. Njuguna J. Progress in sanitation among poor households in Kenya: evidence from demographic and health surveys. BMC Public Health 2019;19:1–8. 10.1186/s12889-019-6459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. AMCOW . The Ngor declaration on sanitation and hygiene, 2015. Available: http://www.wsscc.org/resources/resource-news-archive/africasan-2015-ngor-declaration-sanitation-and-hygiene [Accessed 6 Nov 2020].
- 108. KNBS . Kenya population and housing census reports, 2019. Available: https://www.knbs.or.ke/?p=5732 [Accessed 22 Apr 2020].
- 109. Bauer JM, Mburu S. Effects of drought on child health in Marsabit district, Northern Kenya. Econ Hum Biol 2017;24:74–9. 10.1016/j.ehb.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 110. Opiyo F, Wasonga O, Nyangito M, et al. Drought adaptation and coping strategies among the Turkana pastoralists of northern Kenya. Int J Disaster Risk Sci 2015;6:295–309. 10.1007/s13753-015-0063-4 [DOI] [Google Scholar]
- 111. Kumssa A, Jones JF, Herbert Williams J. Conflict and human security in the North Rift and North eastern Kenya. Int J Soc Econ 2009;36:1008–20. 10.1108/03068290910984786 [DOI] [Google Scholar]
- 112. Magadi MA, Curtis SL. Trends and determinants of contraceptive method choice in Kenya. Stud Fam Plann 2003;34:149–59. 10.1111/j.1728-4465.2003.00149.x [DOI] [PubMed] [Google Scholar]
- 113. Bauni E. The low acceptability and use of condoms within marriage : evidence from nakuru District, Kenya. Etude La Popul Africaine 2003;18:51–65. [Google Scholar]
- 114. Anguko AA. Determinants of contraceptive use among women of reproductive age in North eastern Kenya, 2014. Available: http://erepository.uonbi.ac.ke/bitstream/handle/11295/76609/Anguko_DeterminantsofcontraceptiveuseamongwomenofreproductiveageinnortheasternKenya.pdf?sequence=1
- 115. FCDC . Frontier counties development Council, 2018. Available: https://www.fcdc.or.ke/ [Accessed 10 Mar 2021].
- 116. Alegana VA, Maina J, Ouma PO, et al. National and sub-national variation in patterns of febrile case management in sub-Saharan Africa. Nat Commun 2018;9:4994.. 10.1038/s41467-018-07536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Akech S, Chepkirui M, Ogero M, et al. The Clinical Profile of Severe Pediatric Malaria in an Area Targeted for Routine RTS, S/AS01 Malaria Vaccination in Western Kenya. Clin Infect Dis 2020;71:372–80. 10.1093/cid/ciz844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ndege S, Washington S, Kaaria A, et al. Hiv prevalence and antenatal care attendance among pregnant women in a large home-based HIV counseling and testing program in Western Kenya. PLoS One 2016;11:e0144618. 10.1371/journal.pone.0144618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Borgdorff MW, Kwaro D, Obor D, et al. Hiv incidence in Western Kenya during scale-up of antiretroviral therapy and voluntary medical male circumcision: a population-based cohort analysis. Lancet HIV 2018;5:e241–9. 10.1016/S2352-3018(18)30025-0 [DOI] [PubMed] [Google Scholar]
- 120. Reece M, Hollub A, Nangami M, et al. Assessing male spousal engagement with prevention of mother-to-child transmission (pMTCT) programs in Western Kenya. AIDS Care 2010;22:743–50. 10.1080/09540120903431330 [DOI] [PubMed] [Google Scholar]
- 121. Moth IA, Ayayo ABCO, Kaseje DO. Assessment of utilisation of PMTCT services at Nyanza provincial Hospital, Kenya. Sahara J 2005;2:244–50. 10.1080/17290376.2005.9724847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24:69–71. [PMC free article] [PubMed] [Google Scholar]
- 123. IAEG-SDGs . Final list of proposed sustainable development goal indicators, 2016. Available: https://sustainabledevelopment.un.org/content/documents/11803Official-List-of-Proposed-SDG-Indicators.pdf [Accessed 24 Nov 2020].
- 124. CoG . County integrated development plans, 2019. Available: http://cog.go.ke/about-us/20-the-council-of-governors/484-county-integrated-development-plans [Accessed 23 Feb 2019].
- 125. Kinyoki DK, Osgood-Zimmerman AE, Local Burden of Disease Child Growth Failure Collaborators . Mapping child growth failure across low- and middle-income countries. Nature 2020;577:231–4. 10.1038/s41586-019-1878-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mosser JF, Gagne-Maynard W, Rao PC, et al. Mapping diphtheria-pertussis-tetanus vaccine coverage in Africa, 2000-2016: a spatial and temporal modelling study. Lancet 2019;393:1843–55. 10.1016/S0140-6736(19)30226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Deshpande A, Miller-Petrie MK, Lindstedt PA, et al. Mapping geographical inequalities in access to drinking water and sanitation facilities in low-income and middle-income countries, 2000-17. Lancet Glob Health 2020;8:e1162–85. 10.1016/S2214-109X(20)30278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruktanonchai CW, Ruktanonchai NW, Nove A, et al. Equality in maternal and newborn health: modelling geographic disparities in utilisation of care in five East African countries. PLoS One 2016;11:e0162006. 10.1371/journal.pone.0162006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Manya A, Braa J, Øverland L. National roll out of district health information software (DHIS 2) in Kenya, 2011–Central server and cloud based infrastructure. in: IST-Africa 2012 conference proceedings, 2012. Available: http://www.researchgate.net/publication/230559375_National_Roll_out_of_District_Health_Information_Software_(DHIS_2)_in_Kenya_2011__Central_Server_and_Cloud_based_Infrastructure/file/9fcfd501777a933fe0.pdf
- 130. Karuri J, Waiganjo P, Orwa D. DHIS2: the tool to improve health data demand and use in Kenya. J Health Inform Dev Ctries 2014;8:38–60. [Google Scholar]
- 131. Alegana VA, Okiro EA, Snow RW. Routine data for malaria morbidity estimation in Africa: challenges and prospects. BMC Med 2020;18:121. 10.1186/s12916-020-01593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ouma J, Jeffery C, Valadez JJ, et al. Combining national survey with facility-based HIV testing data to obtain more accurate estimate of HIV prevalence in districts in Uganda. BMC Public Health 2020;20:1–14. 10.1186/s12889-020-8436-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Maïga A, Jiwani SS, Mutua MK, et al. Generating statistics from health facility data: the state of routine health information systems in eastern and southern Africa. BMJ Glob Health 2019;4:e001849. 10.1136/bmjgh-2019-001849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dehnavieh R, Haghdoost A, Khosravi A, et al. The district health information system (DHIS2): a literature review and meta-synthesis of its strengths and operational challenges based on the experiences of 11 countries. Health Inf Manag 2019;48:62–75. 10.1177/1833358318777713 [DOI] [PubMed] [Google Scholar]
- 135. Ng M, Fullman N, Dieleman JL, et al. Effective coverage: a metric for monitoring universal health coverage. PLoS Med 2014;11:e1001730. 10.1371/journal.pmed.1001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2020-004544supp001.pdf (209.1KB, pdf)
bmjgh-2020-004544supp002.pdf (43.5KB, pdf)
bmjgh-2020-004544supp003.pdf (4.1MB, pdf)
bmjgh-2020-004544supp004.pdf (36.3KB, pdf)
bmjgh-2020-004544supp005.pdf (27.4KB, pdf)
bmjgh-2020-004544supp006.pdf (34.9KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The full database of household sample surveys, population censuses, malaria surveys and environmental data that support the findings of this study are available open access from online data repositories available to registered users. Integrated Public Use Microdata Series (IPUMS)—https://international.ipums.org/international/index.shtml; Multiple Indicator Cluster Surveys (MICS)—http://mics.unicef.org/; Demographic and Health Surveys (DHS)—https://dhsprogram.com/; Kenya National Bureau of Statistics (KNBS)—http://statistics.knbs.or.ke/nada/index.php/home; Population Health Harvard Dataverse—https://dataverse.harvard.edu/dataverse/population-health.