Abstract
The aim of this study was to determine the effect of a 10-week tai chi intervention on psychoemotional state, cognition, and motor learning in older adults during the COVID-19 pandemic. Participants aged 60–78 years were randomized to either a control group (n = 15) or a tai chi group (n = 15) for a 10-week period. The tai chi group received two, 8-form tai chi classes of 60 min duration per week. Changes in psychoemotional state, cognition, and the learning of fast and accurate reaching movements were assessed. In addition, the potential roles of the autonomic nervous system and brain-derived neurotrophic factor (BDNF) were investigated. Tai chi practice decreased (P < 0.05) perceived stress, whereas no change in autonomic nervous system activity was observed. Improvements in mental switching correlated with decreased depressive symptoms and increased BDNF levels (P < 0.05), whereas improvements in inhibitory control tended to correlate with BDNF levels (P = 0.08). Improvements in visuospatial processing tended to correlate with decreased depressive symptoms (P = 0.07) while improved visuospatial processing correlated with improved motor planning during learning tasks (P < 0.05). This study suggests that tai chi is an effective intervention that can be delivered under pandemic conditions to improve mental and physical function in older adults.
Abbreviations: BDNF, brain-derived neurotrophic factor; BMI, body mass index; COVID-19, coronavirus disease 2019; MST, memory search task; PRTT, procedural reaction time task; MGT, matching grids task; GNGT, go/no-go task; HR, heart rate; HF, high-frequency; RMSSD, root mean square of successive differences; BP, blood pressure
Keywords: BDNF, Cognitive function, Depression, Heart rate variability, Motor function, Stress
1. Introduction
Since the beginning of the outbreak in China in December 2019, the novel coronavirus disease (COVID-19) has spread rapidly around the world causing an ongoing pandemic (World Health Organization, 2020). Governments immediately adopted extreme public health measures including shutting down all except essential services and industries, closing borders and restricting travel, promoting adherence to hand hygiene and respiratory etiquette, maintaining physical distance and wearing masks in all settings, and implementing self-isolation and quarantine measures. These changes in individual daily life have had negative effects not only on physical health but also on mental well-being (Holmes et al., 2020; Whitehead, 2020). Increased feelings of anxiety, depression, and perceived stress in response to the COVID-19 pandemic have been well documented (Flesia et al., 2020; Wang et al., 2020; Whitehead, 2020; Yao et al., 2020).
Prolonged stress causes increases in sympathetic nervous system activity and reductions in parasympathetic nervous system activity (Kim et al., 2018) and circulating brain-derived neurotrophic factor (BDNF) levels, which play an important role in the pathophysiology of depression (Duman et al., 2019; Mondal and Fatima, 2019; Phillips, 2017; Won and Kim, 2016) and cognitive impairment (Kim et al., 2006; Shimada et al., 2014). Decreases in both cognitive abilities and BDNF levels may lead to a deterioration in motor performance and learning (Levin and Netz, 2015; Mang et al., 2013; Wu et al., 2016), as well as changes in mood, such as depression and anxiety, which may negatively affect both motor and cognitive performance (Bolmont, 2005; Buyukdura et al., 2011; Perini et al., 2019). Particular attention should be paid to the older population, which already experiences declines in cognitive (e.g., processing speed and certain memory, visuospatial, and executive function abilities) (Harada et al., 2013) and physical (e.g., balance, gait, and the ability to learn and retain a motor skill) (Krishnan et al., 2018; Seidler et al., 2010) functioning. Increased stress levels can accelerate cognitive decline in older adults (Aggarwal et al., 2014; Tschanz et al., 2013) and cognitive decline affect motor learning efficiency and performance (Levin and Netz, 2015; Ren et al., 2013), limiting an individual's ability to cope with daily tasks and decreasing autonomy. Thus, there is an urgent need for research to address how to effectively decrease stress levels, increase BDNF levels, and improve psychoemotional state and cognitive and motor functioning under pandemic conditions.
An increasing amount of scientific evidence suggests that mind–body exercise, such as tai chi, reduces perceived and physiological stress (Audette et al., 2006; Chan et al., 2018; Taylor-Piliae et al., 2006), decreases depressive symptoms (Cho, 2008), and improves mood (Taylor-Piliae et al., 2006) and various aspects of physical functioning (e.g., eye–hand coordination, balance, strength, and endurance) (Audette et al., 2006; Lee et al., 2015; Lu et al., 2016; Riegle van West et al., 2018; Wu et al., 2018; Yan, 1998) and cognition (Lu et al., 2016; Riegle van West et al., 2018; Sungkarat et al., 2018; Tao et al., 2017a, Tao et al., 2017b; Wu et al., 2018; Yang et al., 2020) in healthy older adults. However, no studies have investigated whether tai chi remains effective in older adults who are living in improvement-limiting chronic stress conditions, such as a pandemic. Furthermore, no studies have examined the effects of a tai chi intervention on motor learning. Older adults may need to learn and relearn skills during lockdown to maintain their independence. To date, only one study has suggested that tai chi may lead to cognitive improvements, at least in older adults with mild cognitive impairment, via upregulation of BDNF (Sungkarat et al., 2018). Increase in neuroplasticity mechanisms, such as increased BDNF levels, and improvement in cognition also could be associated with advancement in motor learning (Čekanauskaitė et al., 2020; Levin and Netz, 2015; Mang et al., 2013; Ren et al., 2013). Taken together, these data suggest that tai chi may increase BDNF levels, improve psychoemotional state, autonomic control, and cognition, and result in improved motor learning in older adults during a pandemic.
It is of note that tai chi is a particularly attractive activity in the context of a viral pandemic because it can be practiced not only in groups with an instructor but also individually at home (Riegle van West et al., 2018). Moreover, it is suitable for older people with limitations in their ability to tolerate vigorous exercise (Sungkarat et al., 2018). Thus, the primary aim of this study was to determine the effect of a 10-week tai chi intervention on psychoemotional state, cognition, and learning of fast and accurate reaching movements during the COVID-19 pandemic. Because the specific mechanism underlying the positive behavioral effects of tai chi remains unclear (Sungkarat et al., 2018), the potential role of physiological and subjective stress, as well as BDNF levels in the performance of cognitive and motor tasks during chronic stress conditions (2,5 months) were also explored.
2. Methods
2.1. Participants
Seventy-three participants were assessed for eligibility to participate in this study (Fig. 1 ). Inclusion criteria were adults (1) aged ≥60 years who had never previously practiced tai chi; (2) who were physically inactive (<2 days per week of structured physical activity) for at least the previous 6 months; (3) with a score of ≥24 in the Mini-Mental State Examination to exclude cognitive deficits (Folstein et al., 1975); (4) with absence of alcohol and/or drug addiction, metabolic, cardiovascular, pulmonary, musculoskeletal, oncological diseases, neurological and mental disorders; (5) with normal or corrected-to-normal vision and hearing; and (6) with body mass index (BMI) >18.5 and < 30 kg/m2. Participants were excluded if they smoked; had significant visual impairment or glaucoma; were unable to perform tai chi movements; had yoga, qigong, martial arts or meditation-related experiences; had uncontrolled hypertension (resting systolic blood pressure (BP) >160 or diastolic BP >100 mmHg) and abnormal heart rate (HR) (resting HR >100 bpm; <50 bpm); or used medications and dietary supplements regularly that might affect experimental variables. In total, 30 (4 men) participants (weight: 71.3 ± 8.7 kg; BMI: 26.0 ± 2.7 kg/m2) aged 67.0 ± 5.9 years were enrolled in the study (Fig. 1). All procedures were approved by the University Bioethics Committee (reference number: MNL-KIN(M)-2020–317) and were performed following the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants before the baseline examinations. This study is registered at ClinicalTrials.gov (trial registration number: NCT04690244).
Fig. 1.
CONSORT flow chart of the study.
2.2. Study design
This study was conducted after all participants were familiarized with the cognitive function evaluation procedures as described previously (Solianik and Sujeta, 2018). The participants arrived at the laboratory after overnight fasting (10.7 ± 2.7 h) and were asked to rest in a supine position in a quiet room for 15 min. During the last 10 min, heart rate (HR) and HR variability were measured. Subsequently, blood pressure (BP) and psychoemotional state were assessed, and a venous blood sample was taken for BDNF analysis. Next, assessment of cognitive and motor functions was performed. Participants were then randomly allocated into one of two groups and commenced the 10-week control (n = 15; 2 men) or tai chi (n = 15; 2 men) interventions. All measurements were repeated after 10 weeks in the same order as before the intervention as described above. During the course of the study, no participant in either group was lost to follow-up. Thus, a total of 30 participants were included in the final analysis (Fig. 1).
2.3. Interventions
Interventions began in June 2020 and lasted for 10 weeks. Participants in the tai chi group attended a biweekly 60-min, 8-form Yang-style tai chi practice for 10 weeks. During each session, a physical distance of 4 m between individuals was maintained. Each session included a 15-min warm-up (range of motion and stretching exercises), 40 min of tai chi movements (including [1] repulse monkey, [2] brush knees, [3] part the wild horse's mane, [4] wave hands like clouds, [5] golden rooster stands on one leg, [6] left and right heel kick [7] grasp the peacock's tail, and [8] cross hands) and a 5-min cool-down (breathing exercises). During the performance of tai chi, over the 10 weeks, the participants were led by the same tai chi instructor with 6 years of teaching experience, and they imitated the postures and motions at the same speed as the instructor. The session attendance of participants was recorded by the researcher, and all participants had high adherence rates (98.1 ± 3.0%) to exercise sessions. Participants in the control group did not receive any intervention and were asked to maintain their daily routines and refrain from any new exercise interventions during the 10-week period.
2.4. Assessments
2.4.1. Brain-derived neurotrophic factor level
Venous blood samples for free BDNF analysis from the median antecubital vein were collected into 5 mL vacutainer tubes with a gel separator (Franklin Lakes, NJ, USA). The samples were allowed to clot, and the serum was separated by centrifugation at 1200 ×g for 15 min at 4 °C. Serum samples were stored 4–6 months in 0.5 mL aliquots at −80 °C until analysis. Serum BDNF was measured in duplicates, and the sample from the same participant (pre-post) were run on the same plate with the Quantikine Human BDNF enzyme-linked immunoassay kit (R&D Systems, Inc. Minneapolis, MN) according to the manufacturer's instructions. The intra-assay coefficients were 2.72 ± 2.30%. The trained outcome assessor was blinded to participants' group assignment.
2.4.2. Autonomic nervous system activity
Real-time R–R intervals in supine resting conditions were recorded using a Polar H2 HR sensor (Kempele, Finland) and simultaneously transferred to Polar Pro Trainer 5 software (version 5.41.006; Kempele, Finland). Selected 10-min segments were analyzed using Kubios HR Variability Analysis software (version 2.0; The Biomedical Signal and Medical Imaging Analysis Group, Department of Applied Physics, University of Kuopio, Kuopio, Finland) as described previously (Solianik and Sujeta, 2018). HR and HR variability indices reflecting parasympathetic activity, using the root mean square of successive differences (RMSSD) and high-frequency (HF) power (Shaffer and Ginsberg, 2017), were assessed. Furthermore, RMSSD and HF power were logarithmically transformed (Ln) to correct the skewness of distribution. Resting arterial BP measurements were obtained using an automatic Microlife BP A100 Plus BP monitor (Widnau, Switzerland) in a sitting position.
2.4.3. Psychoemotional state
The 10-item Perceived Stress Scale (PSS-10) was used to measure participants' stress levels (Cohen et al., 1983), and the 14-item Hospital Anxiety and Depression Scale (HADS) was used to measure anxiety and depression symptoms (Snaith, 2003). In the PSS-10, participants were asked to answer 10 questions about feelings and thoughts during the last month on a five-point scale ranging from 0 to 4. Higher scores indicate higher levels of perceived stress. In the HADS, participants were asked to answer 14 questions (i.e., 7 questions each for anxiety and depression subscales) about feelings at that moment on a 4-point scale ranging from 0 to 3. Higher scores indicate higher anxiety and depression symptoms.
2.4.4. Cognitive performance
The computerized Automated Neuropsychological Assessment Metrics version 4 (ANAM-4; Vista Life Sciences, Norman, OK, USA) was used for assessment of multiple cognitive domains (i.e., visuospatial processing [matching grids task (MGT)]; verbal working memory [memory search task (MST)]; response inhibition [go/no-go task (GNGT)]; and choice reaction time and ability to shift mental set [procedural reaction time (PRTT) task]. Detailed descriptions of these tasks can be found elsewhere (Čekanauskaitė et al., 2020; Meyers and Vincent, 2020; Reeves et al., 2007; Solianik et al., 2018; Solianik and Sujeta, 2018). The ANAM-4 test is supported by previous studies that provided evidence for construct validity, reliability, and sensitivity (Reeves et al., 2007).
2.4.5. Motor performance
The previously described fast and accurate reaching task was used to evaluate motor performance using a Dynamic Parameter Analyzer (DPA-1) (Čekanauskaitė et al., 2020). The task was performed with the nondominant hand. The participant was required to position the handle symbol (0.0035 m in diameter) in the start zone on the computer screen, and to react to the target (a red circle, 0.007 m in diameter) that appeared in the same place on the computer screen in front of the start zone at 0.17 m distance by pushing the handle so that the circle of the handle symbol reached the target as fast and accurately as possible. The endpoint of the reaching movement was recorded when the center of the handle symbol stopped in the circle and stayed there for at least 0.03 s. Biofeedback was provided on the computer screen after each trial. A single block of 20 trials was completed before both interventions and five blocks of 20 trials separated by 2-min breaks were completed after interventions.
2.5. Statistical analyses
All statistical analyses were performed with IBM SPSS Statistics version 25.0 (Armonk, NY, USA). Data are reported as means and standard deviations. Changes in fast and accurate reaching movement performance during the learning task are expressed as a percentage of the first averaged 20 movement repetitions (baseline values). The assumption about the homogeneity of variances was confirmed by the Levene's test. A mixed-design analysis of variance (ANOVA) was used to determine the effect of the tai chi intervention on selected variables. If a significant effect was found, the statistical power was calculated. If a significant interaction of time × group was observed, paired t-tests were used to determine the changes evoked within each group. Independent sample t-tests were conducted to examine whether significant differences existed between the two groups. In addition, the effect sizes of evoked significant changes within the control and tai chi groups were estimated by Cohen's d effect size. To explore the mechanisms by which tai chi enhanced function, change scores (e.g., score at follow-up – score at baseline) were evaluated and Pearson correlation coefficients (r) were then calculated. The level of significance was set at 0.05.
3. Results
3.1. Brain-derived neurotrophic factor level
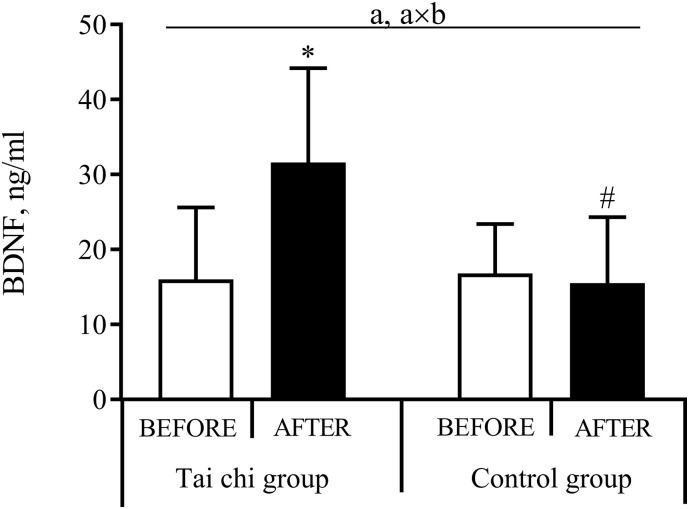
The effect of tai chi on BDNF level is presented in Fig. 2 . A mixed-design ANOVA of the BDNF response revealed a significant effect of time (P < 0.001, OP = 0.98) and a time × group interaction (P < 0.001, OP = 1.00). Subsequent analysis showed that the tai chi intervention significantly increased BDNF levels (P < 0.001, Cohen's d = 1.39), whereas the BDNF level remained unchanged in the control group. A higher BDNF level (P < 0.001) was observed after the tai chi intervention compared with the control group.
Fig. 2.
The effect of tai chi on the brain-derived neurotrophic factor (BDNF) level.
Values are given as mean (standard deviation). aP < 0.05, time effect; axbP < 0.05, time × group interaction effect; *P < 0.05, compared with before; #P < 0.05, compared with tai chi group.
3.2. Autonomic nervous system activity
The effect of tai chi on the physiological stress markers is presented in Table 1 . A mixed-design ANOVA revealed no significant effect of time or any time × group interaction on resting BP and HR variability. Lower HR (P < 0.01) in line with higher RMSSD (P = 0.009) and HF power (P = 0.038) was observed before and after tai chi intervention compared with the control group.
Table 1.
The effect of tai chi on the autonomic nervous system activity.
| Tai chi group |
Control group |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Blood pressure | ||||
| SBP (mm Hg) | 140.0 (17.3) | 141.5 (14.5) | 131.5 (9.4) | 133.8 (10.7) |
| DBP (mm Hg) | 76.6 (4.6) | 78.4 (7.2) | 79.8 (6.9) | 81.5 (5.4) |
| Heart rate variability | ||||
| HR (bpm) | 60.4 (8.7) | 60.6 (7.8) | 69.3 (7.6)# | 68.6 (7.5)# |
| RMSSD (ln (ms)) | 4.0 (0.9) | 3.8 (0.7) | 3.1 (0.5)# | 3.1 (0.5)# |
| HF (ln (ms2)) | 6.1 (1.8) | 6.4 (1.3) | 4.6 (1.0)# | 4.7 (1.2)# |
Values are given as mean (standard deviation). SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; RMSSD, root mean square of the successive differences; HF, high frequency power.
P < 0.05, compared with tai chi group.
3.3. Psychoemotional state
The effect of tai chi on psychoemotional state is presented in Table 2 . A mixed-design ANOVA revealed a significant time × group interaction on perceived stress (P < 0.001, OP = 0.54) and depression symptoms (P = 0.042, OP = 0.54), and significant effects of time on anxiety (P = 0.029, OP = 0.61) and depression (P = 0.031, OP = 0.59) symptoms. Subsequent analysis showed that the tai chi intervention significantly decreased depression (P = 0.034, Cohen's d = 0.62) and perceived stress (P = 0.005, Cohen's d = 0.83), whereas perceived stress increased (P = 0.040, Cohen's d = 0.36) in the control group. Lower perceived stress (P < 0.001) was observed after the tai chi intervention compared with the control group. No significant correlation between psychoemotional state and BDNF level was observed.
Table 2.
The effect of tai chi on the psychoemotional state.
| Tai chi group |
Control group |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Anxietya | 5.9 (3.4) | 3.9 (2.2) | 6.2 (2.4) | 5.5 (2.5) |
| Depressiona, axb | 5.0 (3.9) | 3.0 (2.4)⁎ | 4.5 (4.4) | 4.4 (4.0) |
| Perceived stressaxb | 15.0 (4.9) | 11.5 (3.4)⁎ | 16.5 (6.0) | 18.7 (6.3)⁎, # |
Values are given as mean (standard deviation).
P < 0.05, time effect.
P < 0.05, time × group interaction effect.
P < 0.05, compared with before.
P < 0.05, compared with tai chi group.
3.4. Cognitive performance
The effect of tai chi on the cognitive performance is presented in Table 3 . A mixed-design ANOVA revealed a significant effect of time for reaction time in the MST (P = 0.036, OP = 0.56), and significant time × group interactions for reaction time in the PRTT (P = 0.006, OP = 0.82), and for accuracy in the MGT (P = 0.047, OP = 0.52) and GNGT (P = 0.049, OP = 0.51). Subsequent analysis showed that the tai chi intervention significantly improved reaction time in the PRTT (P = 0.014, Cohen's d = 0.87), and accuracy in the MGT (P = 0.017, Cohen's d = 0.79) and GNGT (P = 0.005, Cohen's d = 0.27). Greater accuracy (P = 0.012) in the MGT was observed after the tai chi intervention compared with the control group.
Table 3.
The effect of tai chi on performance of cognitive and motor tasks.
| Tai chi group |
Control group |
|||
|---|---|---|---|---|
| Before | After | Before | After | |
| Matching grids task | ||||
| Reaction time, s | 2.00 (0.35) | 2.01 (0.27) | 2.06 (0.50) | 2.15 (0.53) |
| Accuracy, %axb | 90.0 (11.2) | 96.7 (4.1)⁎ | 92.0 (7.7) | 91.7 (5.9)# |
| Memory search task | ||||
| Reaction time, sa | 1.12 (0.39) | 0.97 (1.36) | 1.29 (0.36) | 1.13 (0.27) |
| Accuracy, % | 91.8 (9.8) | 95.0 (6.8) | 91.3 (9.1) | 94.6 (7.1) |
| Go/no-go task | ||||
| Reaction time, s | 0.416 (0.05) | 0.40 (0.05) | 0.42 (0.06) | 0.42 (0.43) |
| Accuracy, %axb | 95.6 (3.4) | 96.4 (2.5)⁎ | 96.2 (2.8) | 94.9 (5.0) |
| Procedural reaction time task | ||||
| Reaction time, saxb | 0.75 (0.14) | 0.64 (0.11)⁎ | 0.67 (0.10) | 0.68 (0.11) |
| Accuracy, % | 96.0 (4.2) | 95.0 (4.2) | 97.1 (3.0) | 95.0 (8.6) |
| Reaching task | ||||
| Reaction time, s | 0.38 (0.04) | 0.39 (0.04) | 0.37 (0.05) | 0.40 (0.05) |
| Movement time, s | 1.70 (0.45) | 1.61 (0.23) | 1.66 (0.20) | 1.65 (0.27) |
| Velocity, mm/s | 118.5 (24.9) | 121.7 (18.8) | 118.5 (17.0) | 123.7 (23.9) |
| Distance, mma | 183.5 (6.4) | 179.8 (4.0) | 183.1 (7.9) | 180.7 (6.3) |
Values are given as mean (standard deviation).
P < 0.05, time effect.
P < 0.05, time × group interaction effect.
P < 0.05, compared with before.
P < 0.05, compared with tai chi group.
The changes in depressive symptoms were correlated with changes in the reaction time of the PRTT (r = 0.41, P = 0.023) and tended to correlate with changes in the accuracy of MGT (r = −0.34, P = 0.067), while changes in BDNF level were correlated with changes in the reaction time of PRTT (r = −0.40, P = 0.043) and tended to correlate with changes in accuracy in GNGT (r = 0.36, P = 0.077).
3.5. Motor performance
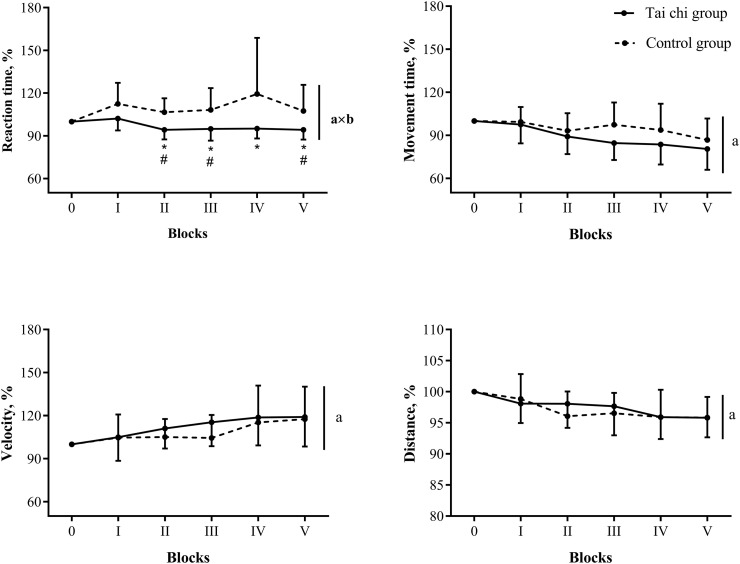
A mixed-design ANOVA revealed a significant effect of time for movement distance to the target (Table 3; P = 0.022, OP = 0.65). However, no significant time × group interactions for parameters of the fast and accurate reaching task were observed. Data on the changes in movement performance indicators during learning of the reaching task are presented in Fig. 3 . A mixed-design ANOVA revealed a significant effect of time for mean values of movement time (P < 0.001, OP = 0.99), velocity (P < 0.001, OP = 1.00), and trajectory (P < 0.001, OP = 1.00), and a significant time × group interaction only for results of mean values of reaction time (P = 0.028, OP = 0.79). Subsequent analysis showed that the tai chi intervention decreased reaction time for blocks 2 to 5 (P < 0.037, Cohen's d > 0.87), whereas in the control group, no changes compared with the baseline block value were observed. A faster reaction time for blocks 2 (P = 0.003), 3 (P = 0.026) and 5 (P = 0.050) was observed in the tai chi group compared with the control group. Nevertheless, no significant correlations between changes in psychoemotional state, BDNF level, and motor learning were found. A change in accuracy in the MGT was negatively correlated with a change in the reaction time in the motor learning task (r = −0.41, P = 0.046).
Fig. 3.
The effect of tai chi on motor learning.
Values are given as mean (standard deviation). aP < 0.05, time effect; axbP < 0.05, time × group interaction effect; *P < 0.05, compared with the baseline (0 block), #P < 0.05, compared with control group.
4. Discussion
To our knowledge, this is the first study to explore the effects of tai chi on mental and physical functioning in older adults during the COVID-19 pandemic. In addition, the potential mechanisms such as autonomic nervous system activity and BDNF levels underlying the functional changes were explored. Tai chi intervention improved cognition, measured in terms of visuospatial processing, inhibitory control and mental switching, and learning of fast and accurate reaching movements in older adults. Because no effect on autonomic nervous system activity was observed, the potential roles of decreased depressive symptoms and improved BDNF levels were investigated. The observed decrement in depressive symptoms and increased BDNF levels significantly correlated with mental switching as well as tendencies of the relationships between depressive symptoms and visuospatial processing, and between BDNF levels and response inhibition were established. Furthermore, improved visuospatial processing correlated with improved motor learning.
In contrast to previous studies in older adults (Audette et al., 2006; Chan et al., 2018; Lee, 2017), we did not observe any changes in BP, HR, or HR variability after tai chi intervention. Notably, baseline values of systolic and diastolic BP were within normal limits (<140/90 mmHg (Aronow, 2015)) in 73% of participants in the tai chi group. Baseline values of HR were within the normal range (Chow et al., 2012) in all participants. Additionally, we observed that parasympathetic activity (i.e., lower HR and greater RMSSD and HF power values (Shaffer and Ginsberg, 2017)) before the intervention was significantly higher in the tai chi group than in the control group, and this difference was maintained after the intervention. In Lee et al. (Lee, 2017) and Chan et al. (Chan et al., 2018), baseline values for BP were reported to be higher than normal before the tai chi intervention. Furthermore, elsewhere (Audette et al., 2006; Chan et al., 2018), longer-duration tai chi programs were implemented (i.e., ≥12 weeks). Thus, in contrast to these reports, changes in our study may not necessarily be expected.
In contrast to the unchanged autonomic nervous system activity, we found that the tai chi intervention decreased both perceived stress and depressive symptoms, and these findings are consistent with previous work (Chan et al., 2018; Cho, 2008; Taylor-Piliae et al., 2006). There is evidence that BDNF levels are associated with clinical changes in depression (Duman et al., 2019; Mondal and Fatima, 2019; Phillips, 2017; Won and Kim, 2016). In our study, baseline scores of the HADS depression subscale were within the normal range (<11 (Snaith, 2003)) in 93.3% of participants. We did not find a significant association between decreased depressive symptoms and increased BDNF levels. Thus, it can be suggested that BDNF does not play a role in mood state in subjects without mood disorders. Nevertheless, programs that reduce perceived stress levels among older adults are likely to have beneficial effects for better mental health (Kwag et al., 2011). Thus, in our study, decreased perceived stress likely improved depressive symptoms. In addition, social support may also have partly contributed to the effect of the tai chi intervention on depressive symptoms (Cho, 2008).
Tai chi practice engages cognitive demands, such as spatiotemporal orientation, memory, and executive control resources as well as attention devoted to multisegmental movements (Li et al., 2014; Sungkarat et al., 2018). Surprisingly, in contrast to our expectations, the present findings revealed that tai chi practice had no effect on verbal working memory. In previous studies, improvements in memory were observed after 8 weeks of 18-form (Riegle van West et al., 2018) and 12 weeks of 24-form Yang-style tai chi intervention (Tao et al., 2017a, Tao et al., 2017b). Such improvements might be the result of learning and memorization of a greater number of movement patterns engaged during tai chi practice resulting in a greater cognitive challenge. A sufficient cognitive challenge seems to play a greater role in obtaining cognitive benefits than high doses of intervention sessions (Gheysen et al., 2018). Although no changes in memory were observed, the tai chi intervention resulted in improved mental switching, inhibitory control, and visuospatial processing in older adults, and again these findings are in line with previous studies (Sungkarat et al., 2017; Wu et al., 2018; Yang et al., 2020). There is evidence that tai chi induces structural and functional changes, which explain the observed behavioral changes. Short-term regular Yang-style tai chi practice increases prefrontal cortex activation and this increase is associated with improvements in mental switching (Wu et al., 2018) and inhibitory control (Yang et al., 2020). Tao et al. (2017b) found that the parahippocampal volume increased after tai chi practice, and it was also shown that the parahippocampal cortex is associated with visuospatial processing (Aminoff et al., 2013).
Sungkarat et al. (2018) suggested that tai chi-induced increases in memory and mental switching are possibly linked to increased circulating BDNF levels in older adults with mild cognitive impairment. It is well established that BDNF facilitates neurogenesis and promotes synaptic plasticity, and exercise-induced increases in BDNF levels benefit cognitive function (Duman et al., 2019; Mang et al., 2013). Our study confirms that changes in BDNF levels were moderately related to changes in improved mental switching and tended to weakly correlate with improved inhibitory control in healthy older adults. Nevertheless, while no relationships between BDNF and visuospatial processing were observed in our study, there is evidence that exercise-induced increases in BDNF levels are associated with changes in hippocampal volume, which in turn, correlate with improved spatial abilities (Erickson et al., 2011). Another suggested mechanism for cognitive benefits is changes in mood state. Chang et al. (2010) suggested that the effects of tai chi on cognition might be influenced by depression. Consistent with this suggestion, our study showed a decrease in depressive symptoms that were moderately related to improved mental switching and tended to weakly correlate with improved visuospatial processing.
In contrast to previous studies (Lee et al., 2015; Yan, 1998), performance on the fast and accurate reaching task was not improved after tai chi, which might be explained by factors such as different duration of tai chi program, practice conditions, and measured variables. In Lee et al. (2015), tai chi practice was performed in a sitting position, where eye–hand coordination was emphasized and there was no need to divert attention to controlling the lower body. Furthermore, in Lee et al. (2015), longer-duration tai chi programs were applied (i.e., 12 weeks). Yan (1998) reported that tai chi practice for 8 weeks improved arm movement smoothness, as measured by jerk but not speed. In our study, movement speed was also unaffected, although we did not measure jerk.
As expected, the tai chi intervention improved the learning of fast and accurate reaching movements. Specifically, it decreased reaction time, which reflects the efficiency of motor planning (Delmas et al., 2018). Benefits in motor planning were not associated with improved BDNF levels or decreased depressive symptoms. However, decreases in reaction time during learning were moderately correlated with improvements in visuospatial processing. While it is not possible to make a causal inference about the direction of this association, the present findings are consistent with previous findings that visuospatial function may be related to motor skill learning in older adults, regardless of other cognitive domains such as attention or working memory (Lingo Van Gilder et al., 2018; Schaefer and Duff, 2017). Moreover, Rosenkranz and Rothwell (2012) showed that the integration of proprioceptive input in the motor cortex provides an important drive for successful motor skill learning. Thus, this finding highlights the need for further investigations to identify whether tai chi practice affects upper-extremity proprioceptive function, and if evoked changes in proprioception modulate motor learning.
The current study has several limitations. First, the sample size was relatively. Second, generalizability of our findings may be limited, as only participants with perceived good health may have volunteered as study participants. No participants reported poor physical health or mood disorders, and our sample may therefore not be representative of the general elderly population. Third, only four men were recruited in this study and it could not be determined if sex played a role. Fourth, the duration of our program was only 10 weeks, and an extended follow-up period was not carried out due to government restrictions related to COVID-19. Thus, the durability of the observed improvements was not investigated. Fifth, tai chi is considered to be a mind–body exercise (Tao et al., 2017a); it is therefore not possible to examine which activity—physical or mental—promotes the functional improvements reported here. Finally, studies show that tai chi affects hemispheric reorganization, such as modified brain activity, functional connectivity, and brain structures, in older adults (Pan et al., 2018). Thus, in future studies, the application of advanced neuroimaging techniques and electroencephalography focused on the underlying mechanisms of cognitive and motor benefits is desirable.
5. Conclusions
Ten weeks of tai chi induced improvements in inhibitory control, mental switching, and visuospatial processing, which were possibly mediated by decreased depressive symptoms and increased BDNF levels. Furthermore, improvement in visuospatial processing was associated with improvements in motor learning. Thus, this study offers an effective intervention that can be delivered under pandemic conditions to improve psychoemotional state, cognition, and motor learning in older adults.
Authors' statement
All authors conceived and designed the experiments. The authors A.Č., L.Ž. and D.M. performed the experiments, and analyzed the data. The author R.S. performed the statistical analysis and drafted the initial manuscript. All authors have read, contributed to, and approved the final version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgments
We would like to thank the Kaunas City Municipality Public Health Bureau, tai chi master Kęstutis Bartusevičius and participants who kindly took the time to participate in the study.
Section Editor: Stephane Baudry
References
- Aggarwal N.T., Wilson R.S., Beck T.L., Rajan K.B., Mendes de Leon C.F., Evans D.A., Everson-Rose S.A. Perceived stress and change in cognitive function among adults aged 65 and older. Psychosom. Med. 2014;76(1):80–85. doi: 10.1097/PSY.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow W.S. Blood pressure goals and targets in the elderly. Curr. Treat. Option Cardiovasc. Med. 2015;17(7):394. doi: 10.1007/s11936-015-0394-x. [DOI] [PubMed] [Google Scholar]
- Audette J.F., Jin Y.S., Newcomer R., Stein L., Duncan G., Frontera W.R. Tai chi versus brisk walking in elderly women. Age Ageing. 2006;35(4):388–393. doi: 10.1093/ageing/afl006. [DOI] [PubMed] [Google Scholar]
- Bolmont B. Causes, Role and Influence of Mood States. Nova Biomedical Books; 2005. Role and influence of moods including anxiety on motor control; pp. 57–73. [Google Scholar]
- Buyukdura J.S., McClintock S.M., Croarkin P.E. Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35(2):395–409. doi: 10.1016/j.pnpbp.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čekanauskaitė A., Skurvydas A., Žlibinaitė L., Mickevičienė D., Kilikevičienė S., Solianik R. A 10-week yoga practice has no effect on cognition, but improves balance and motor learning by attenuating brain-derived neurotrophic factor levels in older adults. Exp. Gerontol. 2020;138 doi: 10.1016/j.exger.2020.110998. [DOI] [PubMed] [Google Scholar]
- Chan A.W.K., Chair S.Y., Lee D.T.F., Leung D.Y.P., Sit J.W.H., Cheng H.Y., Taylor-Piliae R.E. Tai chi exercise is more effective than brisk walking in reducing cardiovascular disease risk factors among adults with hypertension: a randomised controlled trial. Int. J. Nurs. Stud. 2018;88:44–52. doi: 10.1016/j.ijnurstu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- Chang Y.-K., Nien Y.-H., Tsai C.-L., Etnier J.L. Physical activity and cognition in older adults: the potential of Tai Chi Chuan. J. Aging Phys. Act. 2010;18(4):451–472. doi: 10.1123/japa.18.4.451. [DOI] [PubMed] [Google Scholar]
- Cho K.-L. Effect of tai chi on depressive symptoms amongst Chinese older patients with major depression: the role of social support. Med. Sport Sci. 2008;52:146–154. doi: 10.1159/000134295. [DOI] [PubMed] [Google Scholar]
- Chow G.V., Marine J.E., Fleg J.L. Epidemiology of arrhythmias and conduction disorders in older adults. Clin. Geriatr. Med. 2012;28(4):539–553. doi: 10.1016/j.cger.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Delmas S., Casamento-Moran A., Park S.H., Yacoubi B., Christou E.A. Motor planning perturbation: muscle activation and reaction time. J. Neurophysiol. 2018;120(4):2059–2065. doi: 10.1152/jn.00323.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Deyama S., Fogaça M.V. Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur. J. Neurosci. 2019;53(1):126–139. doi: 10.1111/ejn.14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesia L., Monaro M., Mazza C., Fietta V., Colicino E., Segatto B., Roma P. Predicting perceived stress related to the COVID-19 outbreak through stable psychological traits and machine learning models. J. Clin. Med. 2020;9(10):3350. doi: 10.3390/jcm9103350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gheysen F., Poppe L., DeSmet A., Swinnen S., Cardon G., De Bourdeaudhuij I., Chastin S., Fias W. Physical activity to improve cognition in older adults: can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018;15 doi: 10.1186/s12966-018-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada C.N., Natelson Love M.C., Triebel K. Normal cognitive aging. Clin. Geriatr. Med. 2013;29(4):737–752. doi: 10.1016/j.cger.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E.A., O'Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Silver R.C., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A.…Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Lipsitz L.A., Ferrucci L., Varadhan R., Guralnik J.M., Carlson M.C., Fleisher L.A., Fried L.P., Chaves P.H.M. Association between reduced heart rate variability and cognitive impairment in older disabled women in the community: women’s health and aging study I. J. Am. Geriatr. Soc. 2006;54(11):1751–1757. doi: 10.1111/j.1532-5415.2006.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.-G., Cheon E.-J., Bai D.-S., Lee Y.H., Koo B.-H. Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 2018;15(3):235–245. doi: 10.30773/pi.2017.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C., Washabaugh E.P., Reid C.E., Althoen M.M., Ranganathan R. Learning new gait patterns: age-related differences in skill acquisition and interlimb transfer. Exp. Gerontol. 2018;111:45–52. doi: 10.1016/j.exger.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwag K.H., Martin P., Russell D., Franke W., Kohut M. The impact of perceived stress, social support, and home-based physical activity on mental health among older adults. Int. J. Aging Hum. Dev. 2011;72(2):137–154. doi: 10.2190/AG.72.2.c. [DOI] [PubMed] [Google Scholar]
- Lee Y.M. The effects of tai chi on waist circumference and blood pressure in the elderly. J. Phys. Ther. Sci. 2017;29(1):172–175. doi: 10.1589/jpts.29.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.Y.T., Hui-Chan C.W.Y., Tsang W.W.N. The effects of practicing sitting tai chi on balance control and eye-hand coordination in the older adults: a randomized controlled trial. Disabil. Rehabil. 2015;37(9):790–794. doi: 10.3109/09638288.2014.942003. [DOI] [PubMed] [Google Scholar]
- Levin O., Netz Y. Aerobic training as a means to enhance inhibition: what’s yet to be studied? Eur. Rev. Aging Phys. Act. 2015;12 doi: 10.1186/s11556-015-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Harmer P., Liu Y., Chou L.-S. Tai ji quan and global cognitive function in older adults with cognitive impairment: a pilot study. Arch. Gerontol. Geriatr. 2014;58(3):434–439. doi: 10.1016/j.archger.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingo Van Gilder J., Hengge C.R., Duff K., Schaefer S.Y. Visuospatial function predicts one-week motor skill retention in cognitively intact older adults. Neurosci. Lett. 2018;664:139–143. doi: 10.1016/j.neulet.2017.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Siu K.C., Fu S.N., Hui-Chan C.W.Y., Tsang W.W.N. Effects of tai chi training on postural control and cognitive performance while dual tasking—a randomized clinical trial. J. Complement. Integrat. Med. 2016;13(2):181–187. doi: 10.1515/jcim-2015-0084. [DOI] [PubMed] [Google Scholar]
- Mang C.S., Campbell K.L., Ross C.J.D., Boyd L.A. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys. Ther. 2013;93(12):1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J.E., Vincent A.S. Automated neuropsychological assessment metrics (v4) military battery: military normative data. Mil. Med. 2020;185(9–10):e1706–e1721. doi: 10.1093/milmed/usaa066. [DOI] [PubMed] [Google Scholar]
- Mondal A.C., Fatima M. Direct and indirect evidences of BDNF and NGF as key modulators in depression: role of antidepressants treatment. Int. J. Neurosci. 2019;129(3):283–296. doi: 10.1080/00207454.2018.1527328. [DOI] [PubMed] [Google Scholar]
- Pan Z., Su X., Fang Q., Hou L., Lee Y., Chen C.C., Lamberth J., Kim M.-L. The effects of tai chi intervention on healthy elderly by means of neuroimaging and EEG: a systematic review. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini G., Cotta Ramusino M., Sinforiani E., Bernini S., Petrachi R., Costa A. Cognitive impairment in depression: recent advances and novel treatments. Neuropsychiatr. Dis. Treat. 2019;15:1249–1258. doi: 10.2147/NDT.S199746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plasticity. 2017;2017 doi: 10.1155/2017/7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves D.L., Winter K.P., Bleiberg J., Kane R.L. ANAM genogram: historical perspectives, description, and current endeavors. Arch. Clin. Neuropsychol. 2007;22(Suppl. 1):S15–S37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Ren J., Wu Y.D., Chan J.S.Y., Yan J.H. Cognitive aging affects motor performance and learning. Geriatr Gerontol Int. 2013;13(1):19–27. doi: 10.1111/j.1447-0594.2012.00914.x. [DOI] [PubMed] [Google Scholar]
- Riegle van West K., Stinear C., Buck R. The effects of poi on physical and cognitive function in healthy older adults. J. Aging Phys. Act. 2018:1–9. doi: 10.1123/japa.2017-0273. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K., Rothwell J.C. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J. Neurosci. 2012;32(26):9000–9006. doi: 10.1523/JNEUROSCI.0120-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer S.Y., Duff K. Within-session and one-week practice effects on a motor task in amnestic mild cognitive impairment. J. Clin. Exp. Neuropsychol. 2017;39(5):473–484. doi: 10.1080/13803395.2016.1236905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front. Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Makizako H., Doi T., Yoshida D., Tsutsumimoto K., Anan Y., Uemura K., Lee S., Park H., Suzuki T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014;6 doi: 10.3389/fnagi.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith R.P. The hospital anxiety and depression scale. Health Qual. Life Outcomes. 2003;1:29. doi: 10.1186/1477-7525-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solianik R., Sujeta A. Two-day fasting evokes stress, but does not affect mood, brain activity, cognitive, psychomotor, and motor performance in overweight women. Behav. Brain Res. 2018;338:166–172. doi: 10.1016/j.bbr.2017.10.028. [DOI] [PubMed] [Google Scholar]
- Solianik R., Sujeta A., Čekanauskaitė A. Effects of 2-day calorie restriction on cardiovascular autonomic response, mood, and cognitive and motor functions in obese young adult women. Exp. Brain Res. 2018;236(8):2299–2308. doi: 10.1007/s00221-018-5305-4. [DOI] [PubMed] [Google Scholar]
- Sungkarat S., Boripuntakul S., Chattipakorn N., Watcharasaksilp K., Lord S.R. Effects of tai chi on cognition and fall risk in older adults with mild cognitive impairment: a randomized controlled trial. J. Am. Geriatr. Soc. 2017;65(4):721–727. doi: 10.1111/jgs.14594. [DOI] [PubMed] [Google Scholar]
- Sungkarat S., Boripuntakul S., Kumfu S., Lord S.R., Chattipakorn N. Tai chi improves cognition and plasma BDNF in older adults with mild cognitive impairment: a randomized controlled trial. Neurorehabil. Neural Repair. 2018;32(2):142–149. doi: 10.1177/1545968317753682. [DOI] [PubMed] [Google Scholar]
- Tao J., Chen X., Liu J., Egorova N., Xue X., Liu W., Zheng G., Li M., Wu J., Hu K., Wang Z., Chen L., Kong J. Tai chi chuan and baduanjin mind-body training changes resting-state low-frequency fluctuations in the frontal lobe of older adults: a resting-state fMRI study. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Liu J., Liu W., Huang J., Xue X., Chen X., Wu J., Zheng G., Chen B., Li M., Sun S., Jorgenson K., Lang C., Hu K., Chen S., Chen L., Kong J. Tai chi chuan and baduanjin increase grey matter volume in older adults: a brain imaging study. J. Alzheimer's Dis. 2017;60(2):389–400. doi: 10.3233/JAD-170477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Piliae R.E., Haskell W.L., Waters C.M., Froelicher E.S. Change in perceived psychosocial status following a 12-week tai chi exercise programme. J. Adv. Nurs. 2006;54(3):313–329. doi: 10.1111/j.1365-2648.2006.03809.x. [DOI] [PubMed] [Google Scholar]
- Tschanz J.T., Pfister R., Wanzek J., Corcoran C., Smith K., Tschanz B.T., Steffens D.C., Østbye T., Welsh-Bohmer K.A., Norton M.C. Stressful life events and cognitive decline in late life: moderation by education and age. The Cache County study. Int. J. Geriat. Psych. 2013;28(8):821–830. doi: 10.1002/gps.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., Ho R.C. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Public Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead B.R. COVID-19 as a stressor: pandemic expectations, perceived stress, and negative affect in older adults. J. Gerontol. Series B. 2020;76(2):e59–e64. doi: 10.1093/geronb/gbaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won E., Kim Y.-K. Stress, the autonomic nervous system, and the immune-kynurenine pathway in the etiology of depression. Curr. Neuropharmacol. 2016;14(7):665–673. doi: 10.2174/1570159x14666151208113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). Timeline of WHO's response to COVID-19. https://www.who.int/news/item/29-06-2020-covidtimeline.
- Wu Q., Chan J.S.Y., Yan J.H. Mild cognitive impairment affects motor control and skill learning. Rev. Neurosci. 2016;27(2):197–217. doi: 10.1515/revneuro-2015-0020. [DOI] [PubMed] [Google Scholar]
- Wu M.-T., Tang P.-F., Goh J.O.S., Chou T.-L., Chang Y.-K., Hsu Y.-C., Chen Y.-J., Chen N.-C., Tseng W.-Y.I., Gau S.S.-F., Chiu M.-J., Lan C. Task-switching performance improvements after tai chi chuan training are associated with greater prefrontal activation in older adults. Front. Aging Neurosci. 2018;10 doi: 10.3389/fnagi.2018.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J.H. Tai chi practice improves senior citizens’ balance and arm movement control. J. Aging Phys. Act. 1998;6(3):271–284. doi: 10.1123/japa.6.3.271. [DOI] [Google Scholar]
- Yang Y., Chen T., Shao M., Yan S., Yue G.H., Jiang C. Effects of tai chi chuan on inhibitory control in elderly women: an fNIRS study. Front. Hum. Neurosci. 2020;13 doi: 10.3389/fnhum.2019.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Chen J.-H., Xu Y.-F. Patients with mental health disorders in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4) doi: 10.1016/S2215-0366(20)30090-0. [DOI] [PMC free article] [PubMed] [Google Scholar]