Abstract
Background
Elevated blood pressure is linked to cognitive impairment and Alzheimer’s disease biomarker abnormality. However, blood pressure levels vary over time. Less is known about the role of long-term blood pressure variability in cognitive impairment and Alzheimer’s disease pathophysiology.
Objective
Determine whether long-term blood pressure variability is elevated across the clinical and biomarker spectrum of Alzheimer’s disease.
Methods
Alzheimer’s Disease Neuroimaging Initiative participants (cognitively normal, mild cognitive impairment, Alzheimer’s dementia [n=1421]) underwent baseline exam, including blood pressure measurement at 0, 6, 12 months. A subset (n=318) underwent baseline lumbar puncture to determine cerebral spinal fluid amyloid-β and phosphorylated tau levels. Clinical groups and biomarker-confirmed Alzheimer’s disease groups were compared on blood pressure variability over 12 months.
Results
Systolic blood pressure variability was elevated in clinically diagnosed Alzheimer’s dementia (VIM: F2,1195 = 6.657, p = 0.001, η2 = 0.01) compared to cognitively normal participants (p = .001), and in mild cognitive impairment relative to cognitively normal participants (p = .01). Findings were maintained in biomarker-confirmed Alzheimer’s disease (VIM: F2,850 = 5.216, p = 0.006, η2 = 0.01), such that systolic blood pressure variability was elevated in biomarker-confirmed dementia due to Alzheimer’s disease relative to cognitively normal participants (p = .005) and in biomarker-confirmed mild cognitive impairment due to Alzheimer’s disease compared to cognitively normal participants (p = .04).
Conclusion
Long-term systolic blood pressure variability is elevated in cognitive impairment due to Alzheimer’s disease. Blood pressure variability may represent an understudied aspect of vascular dysfunction in Alzheimer’s disease with potential clinical implications.
Keywords: Blood Pressure, Alzheimer Disease, Amyloid, tau Proteins, Cognitive Dysfunction
INTRODUCTION
A large body of research suggests a link between hypertension and cognitive decline, with deleterious effects noted across cognitive domains that include memory, attention, language, processing speed, and visuospatial perception [1–8]. Moreover, high blood pressure (BP) has been associated with an increased risk for dementia [2,3,8], neuropathological changes in patients with dementia [9–18], and in mouse models of Alzheimer’s disease (AD) [19]. On the other hand, low BP has also been associated with increased dementia risk [2,3,20–24], and BP levels have been shown to decrease with advancing clinical symptoms of AD [25]. Together these studies underscore the potential importance of careful BP assessment and treatment in the prevention of cognitive decline in older adults.
In addition to the importance of average BP levels, blood pressure variability (BPV) over several months and years (e.g., long-term BPV) is thought to be a key index of cardiovascular health [26]. Despite the potential value of BPV, the vast majority of prior observational research and clinical trials have focused on static measures of BP. It is widely appreciated that BP is dynamic and highly variable [26], and BP levels tend to fluctuate over multiple time-scales due to a host of internal and external factors [26]. The inherent variability of BP limits the value and reliability of average BP levels as a biomarker of neurocognitive dysfunction. These fluctuations in BP also have important implications for brain health since variable pressure must be counteracted by homeostatic mechanisms, including baroreflex function and cerebral autoregulation, in order to ensure steady brain perfusion supporting normal neurological function [27–31]. However, these homeostatic processes can be disrupted by a number of pathological processes, including cerebrovascular remodeling due to chronic hypertension, leaving the brain vulnerable to hypoperfusion injury [29,32–39].
Elevated BPV has been reported in AD [40–42], and is recognized as a risk factor for cognitive impairment and dementia in the general older adult population [43–49], even in those with well-controlled average BP [50]. Several of these studies report the prognostic value of BPV in predicting cognitive decline and dementia risk is beyond that of average BP [40,44,46,48,51]. Prior research on BPV in cognitive impairment and AD has a number of limitations, including investigating BPV in a combined group of mild-to-moderate AD patients [40–42], combining cognitively unimpaired and mildly impaired participants into one “non-demented” group [51], lack of characterization of older adult samples [43–47], and reliance on clinical diagnosis without biomarker confirmation [40–44,49,51]. Thus, it remains unclear whether increased BPV occurs in the more mild stages of cognitive impairment and whether it is specific to one etiology [40–51]. Importantly, we are not aware of any studies investigating BPV in mildly impaired participants relative to cognitively unimpaired older adults or to patients with AD dementia. Although one study compared BPV in AD patients versus cognitively normal (CN) controls [41], elevation of mildly impaired participants may be important for early diagnosis and treatment implications. Moreover, examining BPV across the biomarker-confirmed AD clinical spectrum could provide insight into disease-specific profiles. Prior studies have linked elevated BP and cerebrovascular resistance to AD biomarker abnormality [52–56], but to our knowledge no studies to date have examined BPV in patients with AD biomarker abnormality. The aim of the current study was two-fold. First, we compared BPV in older adults with a clinical diagnosis of AD dementia, mild cognitive impairment (MCI) or CN. Second, we compared BPV in older adults with biomarker-confirmed dementia due to AD, MCI due to AD, and CN.
MATERIALS AND METHODS
Participants
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. Volunteer adults aged 55–91 (inclusive) were recruited from more than 50 sites across the United States and Canada, and were enrolled if they had few depressive symptoms (Geriatric Depression Scale score below 6), were free of significant neurological disease (apart from suspected AD), and had low vascular risk (Hachinski Ischemic Score at or below 4). Further information on recruitment and screening can be found on the ADNI website (www.adni-info.org).
Ethics approval was obtained for each institution involved. This study was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki, US 21 CFR Part 50- Protection of Human Subjects, and Part 56- Institutional Review Boards, and pursuant to state and federal HIPAA regulations. Institutional Review Boards were constituted following State and Federal requirements at each participating location. Study protocols were approved by the appropriate Boards and submitted to Regulatory Affairs at the ADNI Coordinating Center prior to the start of the study. All participants and/or authorized representatives and study partners provided written informed consent for the study prior to protocol-specific procedures. For more information, see www.adni-info.org.
For the present study, we included ADNI participants with an initial clinical evaluation and health exam that included BP measurement at baseline, 6 months, and 12 months follow up. A subset of these participants also underwent baseline lumbar puncture for evaluation of cerebral spinal fluid (CSF) AD biomarkers. See Supplementary Table 1 for information on included versus excluded participants in the present study.
Measures
Clinical group assessment
Baseline evaluation determined initial clinical diagnosis. Criteria for ADNI diagnoses of MCI included: subjective memory complaint reported by the participant or informant; Mini-Mental State Examination (MMSE) scores between 24 and 30 (inclusive); global Clinical Dementia Rating (CDR) scale score of 0.5; scores on delayed recall of Story A of the Wechsler Memory Scale Revised (WMS-R) Logical Memory II subtest that are below expected performance based on years of education; general presentation that would disqualify for a diagnosis of AD [57]. A diagnosis of AD dementia was assigned if the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria for probable AD were met, including MMSE scores between 20 and 26 (inclusive), and CDR scores of 0.5 or 1 [58]. Participants were deemed to be CN if neither diagnostic criteria were met.
Alternative diagnostic criteria for MCI were developed in efforts to reduce the known high false-positive rate of MCI classification by the ADNI criteria [59,60]. Given our particular interest in characterizing BPV during milder stages of disease, and the high potential for misclassification, we conducted a cluster analysis of neuropsychological test performance among ADNI-defined MCI participants as previously described [60]. Briefly, neuropsychological test scores (Rey Auditory Verbal Learning Test delayed memory recall, Rey Auditory Verbal Learning Test delayed memory recognition, Animal fluency, Boston Naming Test, Trail Making Test Parts A & B) covering three cognitive domains (memory, language, executive function) were entered into a cluster analysis to derive three previously documented subtypes of MCI (amnestic MCI, dysnomic MCI, and dysexecutive MCI), as well as a cluster-derived CN group [60]. The ADNI-defined CN and the cluster-derived CN were combined into one CN group, and the three cluster-derived MCI subtypes were combined into one MCI group [60]. Thus, our primary analyses included participants with cluster-informed CN and MCI, and ADNI-defined AD.
To contribute to the growing literature using this cluster method, as well as to validate previous study findings of BPV using more conventional criteria [51], identical secondary analyses were conducted in parallel with a sample of participants identified as CN, MCI, and AD dementia using the conventional ADNI criteria (see Supplementary Materials). Potential differences between the diagnostic schemes were examined, as the ADNI database has been used in a previous study of BPV in CN and MCI participants based on ADNI diagnostic criteria [51].
Blood pressure measures
Physiological measures included brachial artery systolic BP and diastolic BP collected during a health exam using a standardized ADNI protocol described elsewhere (www.adni-info.org). Briefly, a calibrated mercury sphygmomanometer recorded BP from the dominant forearm arranged at the horizontal level of the fourth intercostal space at the sternum while the participant was seated and resting. BP assessment was conducted during each exam, including baseline, 6 months, and 12 months follow up. Average BP and BPV (standard deviation [SD], coefficient of variation [CV] [100 × SD / mean], variation independent of mean [VIM]) were calculated for each participant using the three BP measurements collected. VIM is a commonly used index of long-term BPV and has no correlation with average BP levels over visits [42,61]. VIM was calculated using the formula: VIM = SD/meanx, where the power x was derived from non-linear curve fitting (BP SD on y-axis against average BP on x-axis) using the nls package in R Project [61,62].
Other physiological measures
Blood samples were collected by venipuncture and used to determine apolipoprotein E (APOE)-ϵ4 carrier status [63]. Participants were categorized into those with or without at least one copy of the APOE-ϵ4 allele.
Vascular risk factors
Vascular risk factor burden was determined by physical exam and clinical interview as part of the general health evaluation at study entry. For the present study, general health evaluation data were screened and coded for vascular risk factors most relevant to cerebrovascular disease and cognition based on the Framingham Stroke Risk Profile [53,64–66]. Specifically, history of cardiovascular disease (i.e., myocardial infarction, intermittent claudication, angina, heart failure, or other evidence of coronary disease), type 2 diabetes mellitus, atrial fibrillation, evidence of carotid artery disease, and transient ischemic attack or minor stroke were included as vascular risk factors. Each participant was determined to have low vascular risk (i.e., the presence of ≤ 1 vascular risk factor) or high vascular risk (i.e., the presence of ≥ 2 vascular risk factors) based on prior studies linking vascular risk burden to cerebrovascular pathology at autopsy [53,66]. Baseline body mass index (BMI) was calculated as weight (kg) divided by height (meters) squared. Medications taken at baseline evaluation were screened and participants were classified as those taking antihypertensive medication (all major classes of hypertensive medications) versus those who were not taking antihypertensive medication, as well as those who were taking acetylcholinesterase inhibitor (ChEIs) medication versus those who were not taking ChEIs. Hypertensive status was determined based on mean BP.
Biomarker-confirmed Alzheimer’s disease diagnosis
A subset of participants underwent baseline lumbar puncture to obtain CSF samples for assessment of amyloid-β (Aβ) and phosphorylated tau (Ptau) levels using methods detailed elsewhere [67–70]. Briefly, Roche Elecsys Aβ CSF and Elecsys Ptau CSF immunoassays were used to measure Aβ and Ptau levels in CSF aliquots following a Roche Study protocol at the UPENN/ADNI Biomarker Laboratory. Acceptance criteria were met using previously described analyte measuring ranges with lower to upper technical limits [67]. Using established guidelines [69], participants with Aβ values at or above 980 pg/ml were characterized as Aβ negative and participants with values below this cutoff were defined as Aβ positive. Participants were defined as Ptau negative with values of Ptau at or below 21.8, and as Ptau positive with values above this threshold.
To investigate BP in relation to cognitive impairment with biomarker-confirmed AD pathophysiology, participants identified as MCI or AD dementia who had available biomarker data were classified into one of two biomarker groups based on biomarker status of Aβ and Ptau per research recommendations for the diagnosis of AD [71,72]: MCI with two positive biomarkers (MCIAβ+Ptau+), or AD dementia with two positive biomarkers (ADAβ+Ptau+).
To explore the contribution of specific biomarker burden on BPV, CN participants identified using the cluster method who had available biomarker data were further classified into intermediate biomarker groups for exploratory analyses (see Supplementary Methods).
STATISTICAL ANALYSIS
Systolic and diastolic BPV values (SD, CV, VIM) were not normally distributed and were corrected through log transformation. Outliers of each BP measurement (mean, SD, CV, VIM) were removed if they were greater than +/− 3 SD from the mean. One-way analysis of variance (ANOVA) and chi-square tests were used to compare demographic variables (age, sex, BMI, education, APOE-ϵ4 carrier status, antihypertensive medication use, ChEI use, vascular risk level) among clinical and biomarker-confirmed AD groups. Analysis of covariance (ANCOVA) models compared BP measurements across clinical and biomarker-confirmed AD groups after covarying for age, sex, BMI, years of education, APOE-ϵ4 carrier status, vascular risk level, antihypertensive medication use, and ChEI medication use. ANCOVA models also included average BP over the 12 months as a covariate to account for the high degree of correlation between average BP and some measures of BPV [26,61]. Potential interaction effects of group by antihypertensive medication use on BPV, as well as group by average BP on BPV were also examined. Post-hoc Least Significant Difference (LSD) tests and post-hoc chi-squared tests were used in the case of significant main effects to determine specific group differences. All analyses were 2-tailed with significance set at p < .05. Multiple comparison corrections (using the False Discovery Rate [FDR] method) for significant main effects was set at p < .05 [73]. Reported values for ANOVA and ANCOVA models include F-value (F), p-value (p), and partial eta-squared (η2). Reported values for interaction effects include F-value (F) and p-value (p). Reported values for chi-squared tests include x2 values (x2) and p-values (p). See Supplementary Materials for identical secondary and exploratory analyses and results. All analyses were carried out in R Project [62].
RESULTS
Primary analyses of clinical groups included 1421 participants identified through cluster analysis as CN, MCI, and AD who had valid BP measurements taken at a health exam at baseline, 6 months, and 12 months follow up. A subset of 318 participants with valid CSF Aβ and Ptau data from lumbar puncture were included in primary analyses of biomarker-confirmed AD groups (Supplementary Figure 1).
Demographic Findings
Clinical groups
As summarized in Table 1, there were significant differences among clinical groups by sex, BMI, years of education, APOE-ϵ4 carrier status, antihypertensive medication use, and ChEI use.
Table 1.
Clinical and Demographic Data for Clinical Groups
| CN (n=681) | MCI (n=479) | AD (n=261) | F or x2 | p-value | |
|---|---|---|---|---|---|
| Clinical/Demographic | |||||
| Age, yrs | 73.9 (6.8) | 73.6 (7.2) | 75.4 (7.6) | 2.954 | .05 |
| Sex (n,% Male)c | 358 (52.6%) | 295 (61.6%) | 145 (55.6%) | 9.333 | .009 |
| BMI, kg/m2abc | 27.4 (4.9) | 26.6 (4.3) | 25.6 (4.3) | 14.915 | < .001 |
| Education, yrsa | 16.3 (2.7) | 15.9 (2.9) | 15.2 (2.9) | 12.409 | < .001 |
| APOE-ϵ4 carriers (n,%)abc | 232 (34.1%) | 260 (54.3%) | 178 (68.2%) | 102.950 | < .001 |
| Hypertensive (n,%) | 317 (46.6%) | 236 (49.3%) | 134 (51.3%) | 1.936 | .38 |
| Medication Use, (n,%) | |||||
| Antihypertensive agentsab | 128 (18.8%) | 107 (22.3%) | 77 (29.5%) | 12.683 | .002 |
| ACE inhibitors | 38 (29.7%) | 34 (31.8%) | 24 (31.2%) | ||
| Alpha-blockers | 7 (18.3%) | 5 (4.7%) | 7 (9.1%) | ||
| Angiotensin II inhibitors | 29 (22.7%) | 23 (21.5%) | 19 (24.7%) | ||
| Calcium channel-blockers | 37 (28.9%) | 19 (17.8%) | 15 (19.5%) | ||
| Central agonists | 0 (0.0%) | 2 (1.9%) | 0 (0.0%) | ||
| Combined alpha-beta-blockers | 2 (1.6%) | 5 (4.7%) | 0 (0.0%) | ||
| Diuretics | 15 (11.7%) | 19 (17.8%) | 10 (13.0%) | ||
| Vasodilators | 0 (0.0%) | 0 (0.0%) | 2 (2.6%) | ||
| ChEIsabc | 38 (5.6%) | 117 (24.4%) | 112 (42.9%) | 187.38 | < .001 |
| Mean BP, mmHg | |||||
| Systolic | 132.4 (13.0) | 133.2 (13.4) | 133.8 (13.5) | 0.601 | .55 |
| Diastolic | 73.5 (7.9) | 74.0 (7.5) | 74.2 (6.7) | 0.852 | .43 |
| Baseline Vascular Risk Factors, (n,%) | |||||
| Cardiovascular disease | 74 (10.9%) | 60 (12.5%) | 33 (12.6%) | 0.992 | .61 |
| Atrial fibrillation | 28 (4.1%) | 11 (2.3%) | 12 (4.6%) | 3.618 | .16 |
| Type 2 diabetes mellitus | 42 (6.2%) | 44 (9.2%) | 19 (7.3%) | 3.750 | .15 |
| Carotid artery disease | 3 (0.4%) | 6 (1.3%) | 1 (0.4%) | 3.124 | .21 |
| TIA/minor stroke | 16 (2.4%) | 9 (1.9%) | 7 (2.7%) | 0.552 | .76 |
Means and standard deviations shown unless otherwise indicated.
Significant differences (p < .05) among clinical groups are identified in boldface type.
indicates a Least Significant Difference-corrected pairwise difference between AD and CN at p < .05
indicates a Least Significant Difference-corrected pairwise difference between AD and MCI at p < .05
indicates a Least Significant Difference-corrected pairwise difference between MCI and CN at p < .05
Abbreviations: ACE = angiotensin-converting enzyme; APOE = apolipoprotein E; BP = blood pressure; BMI = body mass index; ChEIs = acetylcholinesterase inhibitors; TIA = transient ischemic attack; CN = cognitively normal; MCI = Mild Cognitive Impairment; AD = Alzheimer’s disease
Biomarker-confirmed Alzheimer’s disease groups
As shown in Table 2, biomarker-confirmed AD groups were significantly different on BMI, years of education, APOE-ϵ4 carrier status, and ChEI use.
Table 2.
Clinical and Demographic Data for Biomarker-Confirmed AD Groups
| CN (n=681) | MCIAβ+Ptau+ (n=185) | ADAβ+Ptau+ (n=133) | F or x2 | p-value | |
|---|---|---|---|---|---|
| Clinical/Demographic | |||||
| Age, yrs | 73.9 (6.8) | 73.6 (7.1) | 74.3 (8.0) | 0.557 | .57 |
| Sex (n,% Male) | 358 (52.6%) | 99 (53.5%) | 71 (53.4%) | 0.069 | .97 |
| BMI, kg/m2ac | 27.4 (4.9) | 26.1 (4.1) | 25.3 (4.3) | 13.138 | < .001 |
| Education, yrsa | 16.3 (2.7) | 16.1 (2.8) | 15.4 (2.8) | 5.022 | .007 |
| APOE-ϵ4 carriers (n,%)ac | 232 (34.1%) | 132 (72.4%) | 103 (77.4%) | 143.260 | < .001 |
| Hypertensive (n,%) | 317 (46.6%) | 91 (49.2%) | 68 (51.1%) | 0.991 | .61 |
| Medication Use, (n,%) | |||||
| Antihypertensive agents | 128 (18.8%) | 33 (17.8%) | 30 (22.6%) | 1.259 | .53 |
| ACE inhibitors | 38 (29.7%) | 12 (36.4%) | 11 (36.7%) | ||
| Alpha-blockers | 7 (18.3%) | 1 (3.0%) | 0 (0.0%) | ||
| Angiotensin II inhibitors | 29 (22.7%) | 6 (18.2%) | 7 (23.3%) | ||
| Calcium channel-blockers | 37 (28.9%) | 5 (15.2%) | 9 (30.0%) | ||
| Central agonists | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Combined alpha-beta-blockers | 2 (1.6%) | 0 (0.0%) | 0 (0.0%) | ||
| Diuretics | 15 (11.7%) | 9 (27.3%) | 3 (10.0%) | ||
| Vasodilators | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| ChEIsabc | 38 (5.6%) | 49 (26.5%) | 47 (35.3%) | 118.25 | < .001 |
| Mean BP, mmHg | |||||
| Systolic | 132.4 (13.0) | 133.3 (13.6) | 133.1 (12.4) | 0.319 | .73 |
| Diastolic | 73.5 (7.9) | 73.4 (6.9) | 74.4 (6.4) | 1.407 | .35 |
| Baseline Vascular Risk Factors, (n,%) | |||||
| Cardiovascular disease | 74 (10.9%) | 26 (14.1%) | 19 (14.3%) | 2.233 | .33 |
| Atrial fibrillation | 28 (4.1%) | 4 (0.5%) | 1 (3.0%) | 5.851 | .05 |
| Type 2 diabetes mellitus | 42 (6.2%) | 14 (7.6%) | 8 (6.0%) | 0.515 | .77 |
| Carotid artery disease | 3 (0.4%) | 3 (1.6%) | 1 (0.8%) | 2.923 | .23 |
| TIA/minor stroke | 16 (2.4%) | 4 (2.2%) | 4 (3.0%) | 0.261 | .88 |
Means and standard deviations shown unless otherwise indicated.
Significant differences (p < .05) among biomarker-confirmed AD groups are identified in boldface type.
indicates a Least Significant Difference-corrected pairwise difference between ADAβ+Ptau+ and CN at p < .05
indicates a Least Significant Difference-corrected pairwise difference between ADAβ+Ptau+ and MCIAβ+Ptau+ at p < .05
indicates a Least Significant Difference-corrected pairwise difference between MCIAβ+Ptau+ and CN at p < .05
Abbreviations: ACE = angiotensin-converting enzyme; Aβ = amyloid-β; APOE = apolipoprotein E; BP = blood pressure; BMI = body mass index; ChEIs = acetylcholinesterase inhibitors; Ptau = phosphorylated tau; TIA = transient ischemic attack; CN = cognitively normal; MCI = Mild Cognitive Impairment; AD = Alzheimer’s disease
Demographic findings for secondary and exploratory groups showed a similar pattern (see Supplementary Results and Supplementary Tables 2–4).
Blood Pressure Variability Findings
Clinical groups
After controlling for age, sex, BMI, years of education, APOE-ϵ4 carrier status, vascular risk level, antihypertensive medication use, ChEI use, and average BP, there were significant differences among the clinical groups on systolic BPV (SD: F2,1195 = 5.829, p = 0.003, η2 = 0.01; CV: F2,1194 = 4.447, p = 0.01, η2 = 0.007; VIM: F2,1195 = 6.657, p = 0.001, η2 = 0.01). Post-hoc comparisons indicated participants with a clinical diagnosis of AD showed significantly higher systolic BPV than CN for all measures of variability (SD: p = .002; CV: p = .004; VIM: p = .001). MCI exhibited greater systolic BPV relative to CN on SD (p = .03) and VIM (p = .01) but not CV (p = .08) measures of variability. Clinically diagnosed AD did not significantly differ from MCI on systolic BPV (SD: p = .21; CV: p = .20; VIM: p = .28) (Figure 1a).
Figure 1. Systolic BPV by Clinical Group.
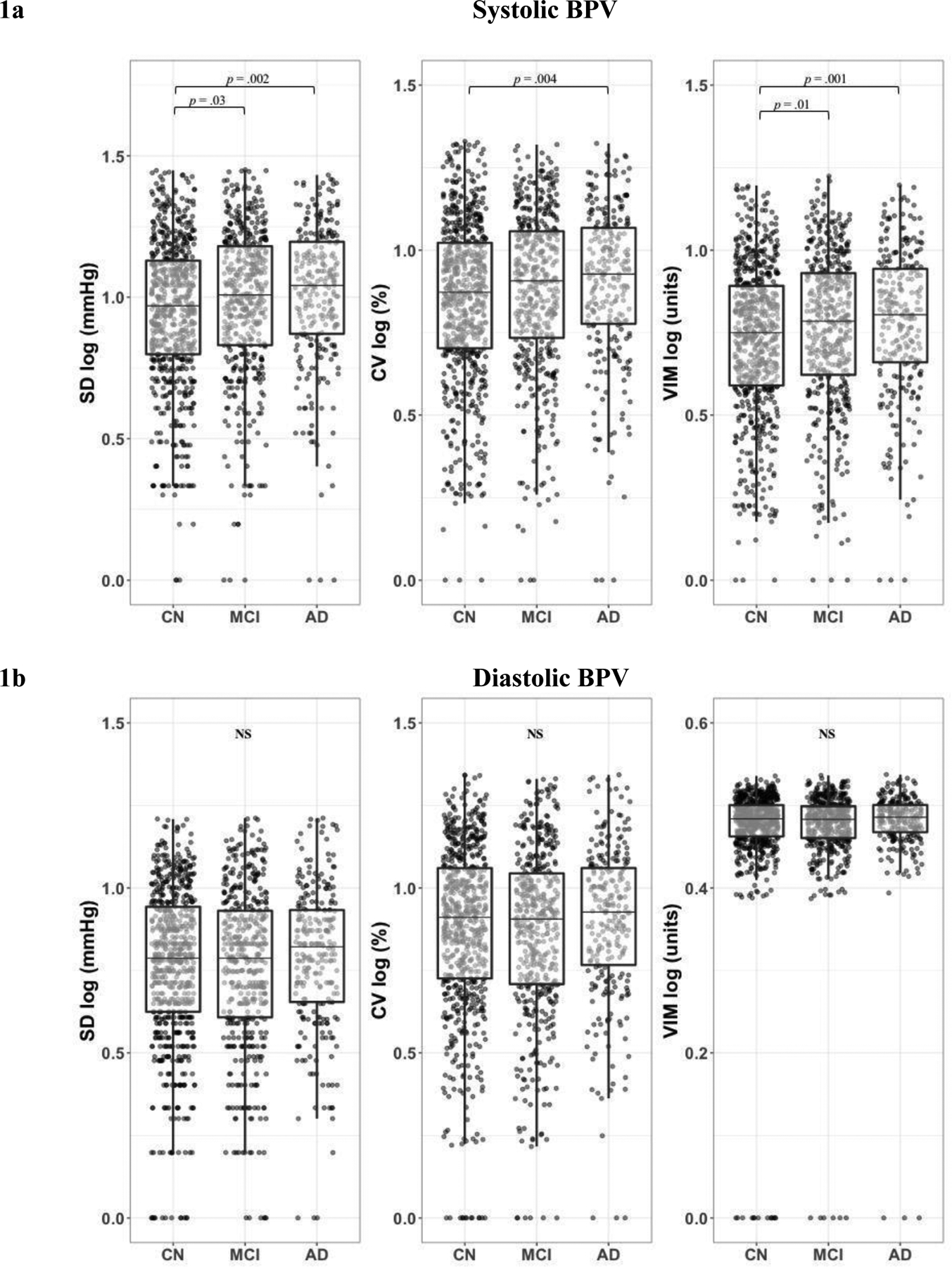
1a. Systolic BPV was greatest in clinically diagnosed AD overall and compared to CN for all measures of BPV. Clinically diagnosed MCI exhibited greater systolic BPV relative to CN on SD and VIM measures of BPV. 1b. Clinical groups did not significantly differ by diastolic BPV. Boxplot lines display minimum, 1st quartile, median, 3rd quartile, and maximum. Abbreviations: BPV = blood pressure variability; CN = cognitively normal; MCI = Mild Cognitive Impairment; AD = Alzheimer’s disease; SD = standard deviation; CV = coefficient of variation; VIM = variation independent of mean
There were no statistically significant differences in diastolic BPV among the clinical groups (SD: F2,1197 = 2.281, p = .10; CV: F2,1197 = 1.904, p = .15; VIM: F2,1197 = 1.992, p = .14) (Figure 1b). There were also no significant interaction effects of clinical group by antihypertensive medication use on BPV (systolic: SD: F2,1193 = 2.203, p = .11; CV: F2,1192 = 2.040, p = .13; VIM: F2,1193 = 2.241, p = .11; diastolic: SD: F2,1195 = 0.014, p = .99; CV: F2,1195 = 0.069, p = .93; VIM: F2,1195 = 1.465, p = .23), or of clinical group by average BP on BPV (systolic: SD: F2,1193 = 1.222, p = .30; CV: F2,1192 = 1.264, p = .29; VIM: F2,1193 = 1.855, p = .16; diastolic: SD: F2,1195 = 0.459, p = .63; CV: F2,1195 = 0.145, p = .87; VIM: F2,1195 = 0.717, p = .49).
Secondary analyses of clinical groups showed a similar pattern of BPV findings (see Supplementary Results and Supplementary Figure 2).
Biomarker-confirmed Alzheimer’s disease groups
Biomarker-confirmed AD groups differed significantly on systolic BPV (SD: F2,850 = 3.955, p = 0.02, η2 = 0.009; VIM: F2,850 = 5.216, p = 0.006, η2 = 0.01; trending CV: F2,850 = 2.928, p = 0.05, η2 = 0.007), such that ADAβ+Ptau+ participants showed significantly higher systolic BPV than CN participants for all measures of variability (SD: p = .007; CV: p = .02; VIM: p = .005). MCIAβ+Ptau+ participants exhibited significantly higher systolic BPV relative to CN on VIM (p = .04) but not SD (p = .21) or CV (p = .38) measures of variability. There were no statistically significant differences in systolic BPV between ADAβ+Ptau+ and MCIAβ+Ptau+ (SD: p = .19; CV: p = .19; VIM: p = .43) (Figure 2a).
Figure 2. Systolic BPV by Biomarker-Confirmed AD Group.
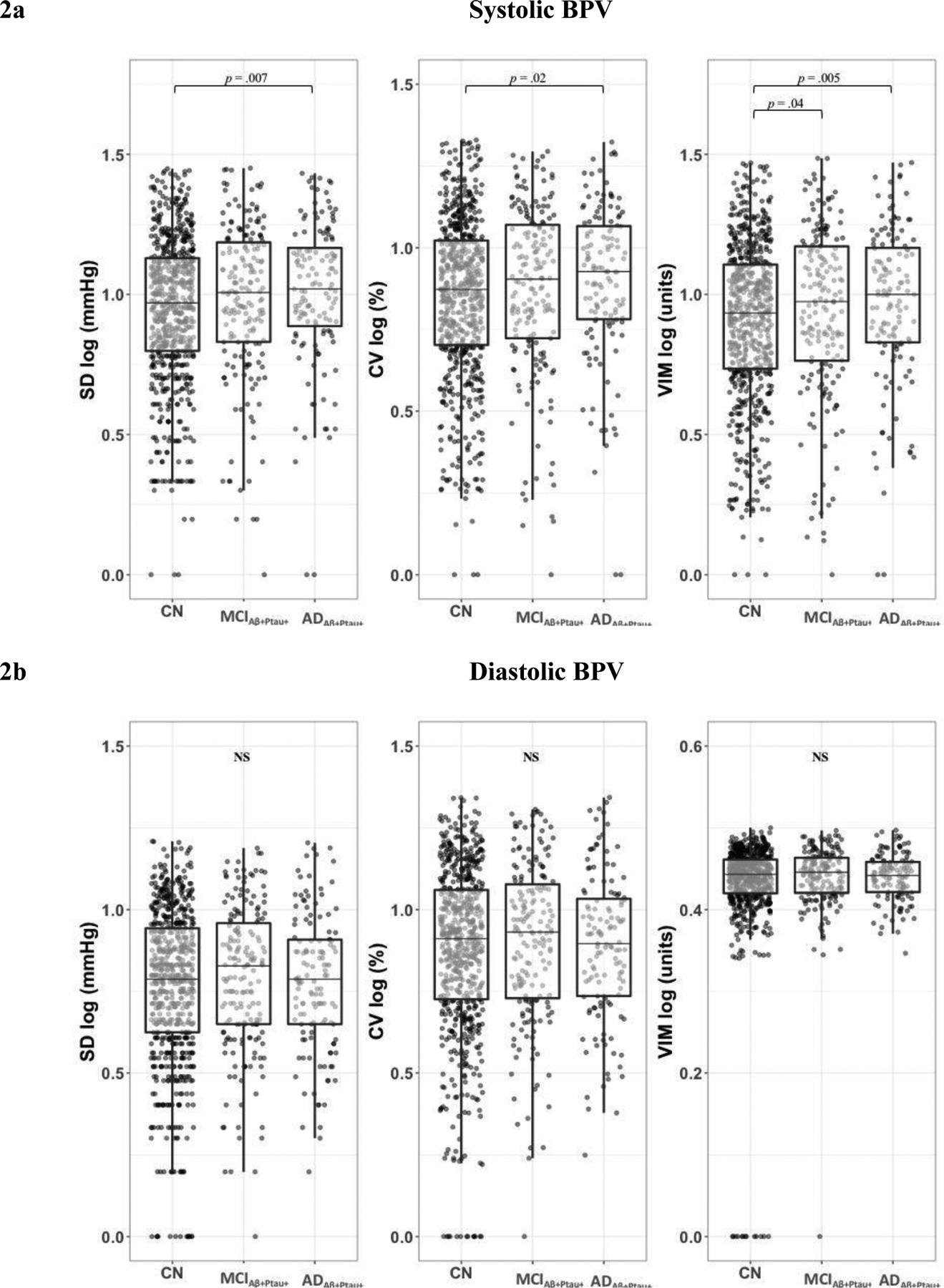
2a. Systolic BPV was greatest in biomarker-confirmed dementia due to AD overall and compared to CN for all measures of BPV. Biomarker-confirmed MCI due to AD exhibited greater systolic BPV relative to CN as measured by VIM. 2b. Biomarker-confirmed AD groups did not significantly differ by diastolic BPV. Boxplot lines display minimum, 1st quartile, median, 3rd quartile, and maximum. Aβ+Ptau+ indicates positive biomarkers for both Aβ and Ptau. Abbreviations: BPV = blood pressure variability; Aβ = amyloid-β; Ptau = phosphorylated tau CN = cognitively normal; MCI = Mild Cognitive Impairment; AD = Alzheimer’s disease; SD = standard deviation; CV = coefficient of variation; VIM = variation independent of mean
Biomarker-confirmed AD groups did not differ significantly on diastolic BPV (SD: F2,851 = 0.236, p = .79; CV: F2,853 = 0.175, p = .84; VIM: F2,851 = 1.331, p = .27) (Figure 2b). There were also no significant interaction effects of biomarker-confirmed AD group by antihypertensive medication use on BPV (systolic: SD: F2,848 = 0.452, p = .64; CV: F2,848 = 0.293, p = .75; VIM: F2,848 = 0.184, p = .83; diastolic: SD: F2,849 = 0.446, p = .64; CV: F2,851 = 0.448, p = .64; VIM: F2,849 = 0.505, p = .60) , or of biomarker-confirmed AD group by average BP on BPV (systolic: SD: F2,848 = 0.738, p = .48; CV: F2,848 = 0.815, p = .44; VIM: F2,848 = 1.366, p = .26; diastolic: SD: F2,849 = 0.972, p = .38; CV: F2,851 = 0.253, p = .78; VIM: F2,849 = 0.084, p = .92).
Secondary analyses of biomarker-confirmed AD groups showed a similar pattern of BPV findings (see Supplementary Results and Supplementary Figure 3).
Exploratory analyses of CN intermediate biomarker groups revealed no significant differences in systolic or diastolic BPV by level of AD biomarker burden (see Supplementary Results and Supplementary Figure 4).
All primary analyses of systolic BPV findings survived FDR correction.
DISCUSSION
Study findings suggest that long-term systolic BPV over one year is elevated in older adults with cognitive impairment due to AD. The present investigation replicated the previously published elevation of systolic BPV in clinically diagnosed AD compared to age-matched controls [41], and extended prior work by using multiple CSF biomarkers to confirm systolic BPV elevation in older adults with a pathological diagnosis of dementia due to AD [71,72]. With regard to more mild levels of symptoms, prior studies of BPV have combined clinical groups [40,42,51], obscuring whether BPV is elevated in MCI. The present study is the first to demonstrate increased systolic BPV at the MCI stage and to further demonstrate that increased systolic BPV specifically applies to MCI in the presence of AD biomarker abnormality. Participant groups did not significantly differ by diastolic BPV in any analyses. Exploratory analyses also revealed no significant differences in systolic or diastolic BPV among cognitively unimpaired participants with varying levels of AD biomarker burden. This suggests that systolic BPV is linked to AD-related cognitive impairment rather than AD in the absence of cognitive dysfunction, a finding that is consistent with recent studies implicating vascular factors in AD-related cognitive decline specifically [74]. Importantly, there were also no significant interactions between BPV and antihypertensive use, or between BPV and average BP levels. Thus, results indicate increased systolic BPV, but not diastolic BPV, may occur in the early stages of cognitive decline in AD. Findings were similar using conventional criteria but were more consistent using cluster-derived groups, a pattern previously observed when using refined MCI classifications to investigate biomarker associations in the ADNI cohort [75]. Study findings provide novel insights into the timeline of BPV elevation with respect to MCI diagnosis and AD pathology, which may have important diagnostic and treatment implications.
The present study does not investigate possible mechanisms responsible for the observed increase in systolic BPV in MCI and AD, but it has been hypothesized that variable BP may induce variability of cerebral perfusion and impact brain health and cognition. Over time, chronic high fluctuations in BP may outreach the homeostatic mechanisms that work to steady changing BP levels, making the brain more vulnerable to waxing and waning levels of cerebral blood flow [76]. Erratic levels of cerebral perfusion threaten the brain’s need for continuous circulation of oxygen and glucose, and may lead to cerebrovascular injury and disrupted functioning [28,30,37,39,76–79].
Another possible explanation for the study findings is that long-term arterial stiffening may be responsible for both inflated BPV [32,80–87] and AD-related cognitive decline [88]. Arterial stiffness may increase BPV through distinct mechanisms involving changes in the timing and buffering of the pulse wave as it is propagated throughout the arterial tree and reflected to the heart [89]. Additionally, arterial stiffness may cause biophysical injury to the brain by passing pulsatile forces into the vulnerable cerebrovasculature, but also by interfering with clearance of toxic proteins along perivascular and/or lymphatic spaces [90,91]. Therefore, arterial stiffness may be responsible for the observed correlation between BPV and brain health. Future studies that directly investigate mechanisms are needed to disentangle these relationships.
Alternatively, neurodegenerative effects on cortical control of autonomic nervous system regulation may cause amplification of BPV [92]. Specifically, the insular cortex, anterior cingulate gyrus, and amygdala regulate autonomic nervous system activity [92], and neurodegeneration in these areas is related to autonomic disruption [93–99]. In addition, the locus coeruleus plays a major role in regulating autonomic activity, and is an early site of AD pathology [100]. Together these studies suggest that AD pathology impacting central nervous system control of autonomic activity may influence BP and BPV.
While increased BPV is a cardiovascular risk factor in the general population, it may be particularly detrimental in AD since these patients already show decreased cerebral perfusion [101], increased cerebrovascular resistance [56], and autonomic abnormalities [2]. Individuals with AD pathology and elevated BPV may be especially promising candidates for therapeutic intervention. Despite the known dynamic nature of BP, most BP therapies focus on modifying average levels [102,103]. Thus, the potential role of BPV with regard to informing diagnosis, treatment and/or prevention of neuropathological processes warrants further investigation.
Dysregulated BP has garnered enormous attention from both the clinical and scientific communities, in part because BP is a highly modifiable risk factor for cardiovascular [104] and cognitive outcomes [103,105]. It is also increasingly recognized that early intervention offers the highest likelihood to alter disease trajectories to prevent dementia [105]. It has been estimated that BP control, particularly in midlife, could significantly reduce the world-wide prevalence of AD [106]. Given the importance of BP as an early modifiable risk factor, understanding the potential role of BPV during the early stages of cognitive impairment may be of great value for early diagnostic and treatment studies.
The present investigation has a number of strengths. First, the study used both CSF Aβ and Ptau biomarkers to confirm AD diagnosis. In doing so, we were able to characterize long-term BPV in cognitive impairment specifically due to AD. Second, the study compared distinct groups of rigorously defined CN, MCI and AD on BPV, providing insights into the characterization of BPV elevation across the spectrum of AD. Third, the study utilized multiple diagnostic methods for distinguishing between CN and MCI participants. Fourth, while some studies on long-term BPV in cognitive impairment and dementia measured BPV over more than 12 months, consistent with other studies [40,45], the present study revealed elevation across groups over just one year, which may suggest the immediate influence variable BP may have in aging adults. Fifth, the study accounted for medication use known to affect BP levels (e.g., antihypertensive agents), and autonomic nervous system activity perhaps especially in patients with cognitive impairment (e.g., ChEIs) [107]. The present study cannot address the role of specific antihypertensive medication classes in the observed clinical and pathological associations with BPV; however, it should be noted that some antihypertensive medications may influence BPV more than others. Specifically, some studies have reported different class effects on risk of stroke [102,108]. The literature is mixed in terms of which specific antihypertensive medication class may have the greatest impact on cognition [109–111], and may vary based on BP dependent and independent effects since some medications may cross the blood-brain barrier to directly influence the central nervous system [112]. A final study strength is that the study had a large sample size and drew from a well-characterized participant pool, which included a detailed panel of vascular health.
Limitations of the present study include the fact that some details of BP measurement were not explicitly standardized across sites and the study utilized three measurements of BP to calculate BPV. Although it has been recommended to use more than three BP measurements to estimate BPV for predicting cardiovascular risk (i.e., stroke) [113], one longitudinal study predicted cognitive impairment and dementia using just three BP measurements [44]. Another study limitation is the demographic differences between included and excluded participants. As summarized in Supplementary Table 1, the included participants were older, less educated, contain a smaller percent of males, and differ by distribution of baseline clinical diagnosis, when compared to participants excluded from the study. It should be noted that ADNI protocols changed mid-study from collecting BP measurement every six months to collecting it every 12 months, which may further influence these differences. Participants excluded from the present study due to missing BP data were largely excluded because they were newly enrolled and only had baseline BP recorded at this point in their study involvement. Other limitations of the present investigation include the cross-sectional nature of the study design and the lack of direct measures of arterial stiffening or other vascular mediators linking BPV to the AD spectrum, such as cerebral perfusion or autoregulation. Future studies will investigate the role these processes may have in the progression to dementia. Future studies will also investigate how cerebrovascular autoregulation and neuropathological factors may moderate the relationship between BPV and cognitive impairment. While the present study included a substantial number of participants with MCI, an even larger sample size would allow evaluation of MCI subtypes (i.e., amnestic, dysnomic, dysexecutive) and intermediate biomarker categories to further determine the timing and etiology of BPV elevation.
CONCLUSION
Study findings indicate that systolic BPV is elevated in cognitive impairment due to AD. Importantly, these findings are independent of average BP levels, which have historically been the main focus of BP research [2] and clinical trials [103]. Given the high overlap of vascular pathology and neurodegeneration [9], the interest to study BPV in the context of cognitive aging is growing. Beyond understanding how BPV is characterized in the general aging population, studying the role of BPV in at-risk populations with AD pathology and neurodegeneration may reveal understudied targets for vascular contributions to cognitive impairment and dementia.
Supplementary Material
ACKNOWLEDGEMENTS
Funding
The study data analysis was supported by NIH/NIA grants (R01AG064228, R01AG060049, R21AG055034, P50 AG005142, and P01 AG052350) and Alzheimer’s Association grant AARG-17-532905.
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE STATEMENT
The authors have no conflict of interest to report.
References
- [1].Wilkie F, Eisdorfer C (1971) Intelligence and blood pressure in the aged. Science (80-. ) 172, 959–962. [DOI] [PubMed] [Google Scholar]
- [2].Qiu C, Winblad B, Fratiglioni L (2005) The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 4, 487–499. [DOI] [PubMed] [Google Scholar]
- [3].Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ (1995) The association between midlife blood pressure levels and late-life cognitive function: The Honolulu-Asia Aging study. JAMA J. Am. Med. Assoc 274, 1846–1851. [PubMed] [Google Scholar]
- [4].Kilander L, Nyman H, Boberg M, Hansson L, Lithell H (1998) Hypertension is related to cognitive impairment: A 20-year follow-up of 999 men. Hypertension 31, 780–787. [DOI] [PubMed] [Google Scholar]
- [5].Kilander L (2000) The association between low diastolic blood pressure in middle age and cognitive function in old age. A population-based study. Age Ageing 29, 243–248. [DOI] [PubMed] [Google Scholar]
- [6].Muela HCS, Costa-Hong VA, Yassuda MS, Moraes NC, Memória CM, Machado MF, Macedo TA, Shu EBS, Massaro AR, Nitrini R, Mansur AJ, Bortolotto LA (2017) Hypertension severity is associated with impaired cognitive performance. J. Am. Heart Assoc 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gifford KA, Badaracco M, Liu D, Tripodis Y, Gentile A, Lu Z, Palmisano J, Jefferson AL (2013) Blood pressure and cognition among older adults: A meta-analysis. Arch. Clin. Neuropsychol 28, 649–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Iadecola C (2014) Hypertension in dementia. Hypertension 64, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. (2016) Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta - Mol. Basis Dis 1862, 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Viswanathan A, Rocca WA, Tzourio C (2009) Vascular risk factors and dementia. Neurology 72, 368 LP – 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S (2011) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42, 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hughes TM, Lockhart SN, Smagula SF (2018) Blood pressure’s role in Alzheimer disease pathology. Am. J. Geriatr. Psychiatry 26, 23–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Arvanitakis Z, Capuano AW, Lamar M, Shah RC, Barnes LL, Bennett DA, Schneider JA (2018) Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 91, e517–e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carnevale D, Perrotta M, Lembo G, Trimarco B (2016) Pathophysiological links among hypertension and Alzheimer’s disease. High Blood Press. Cardiovasc. Prev 23, 3–7. [DOI] [PubMed] [Google Scholar]
- [15].Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C (2013) Blood pressure levels and brain volume reduction: A systematic review and meta-analysis. J. Hypertens 31, 1502–1516. [DOI] [PubMed] [Google Scholar]
- [16].Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, Carmelli D (1998) Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology 51, 986–993. [DOI] [PubMed] [Google Scholar]
- [17].Swan GE, Carmelli D, Larue A (1998) Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke 29, 2334–2340. [DOI] [PubMed] [Google Scholar]
- [18].Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R (2010) Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch. Neurol 67, 564–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cifuentes D, Poittevin M, Dere E, Broquères-You D, Bonnin P, Benessiano J, Pocard M, Mariani J, Kubis N, Merkulova-Rainon T, Lévy BI (2015) Hypertension accelerates the progression of Alzheimer-like pathology in a mouse model of the disease. Hypertension 65, 218–224. [DOI] [PubMed] [Google Scholar]
- [20].Guo Z, Fratiglioni L, Winblad B, Viitanen M (1997) Blood pressure and performance on the Mini-Mental State Examination in the very old: Cross-sectional and longitudinal data from the Kungsholmen project. Am. J. Epidemiol 145, 1106–1113. [DOI] [PubMed] [Google Scholar]
- [21].Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA (2001) Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch. Neurol 58, 1640–1646. [DOI] [PubMed] [Google Scholar]
- [22].Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JCM, Lernfelt B, Van Harskamp F, Hofman A, Breteler MMB (2001) Blood pressure and risk of dementia: Results from the Rotterdam study and the Gothenburg H-70 study. Dement. Geriatr. Cogn. Disord 12, 33–39. [DOI] [PubMed] [Google Scholar]
- [23].Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ (2003) Low blood pressure and the risk of dementia in very old individuals. Neurology 61, 1667–1672. [DOI] [PubMed] [Google Scholar]
- [24].Qiu C, Von Strauss E, Fastbom J, Winblad B, Fratiglioni L (2003) Low blood pressure and risk of dementia in the Kungsholmen project: A 6-year follow-up study. Arch. Neurol 60, 223–228. [DOI] [PubMed] [Google Scholar]
- [25].Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Odén A, Svanborg A (1996) 15-year longitudinal study of blood pressure and dementia. Lancet 347, 1141–1145. [DOI] [PubMed] [Google Scholar]
- [26].Parati G, Ochoa JE, Lombardi C, Bilo G (2013) Assessment and management of blood-pressure variability. Nat. Rev. Cardiol 10, 143–155. [DOI] [PubMed] [Google Scholar]
- [27].Lassen NA (1959) Cerebral blood flow and oxygen consumption in man. Physiol. Rev 39, 183–238. [DOI] [PubMed] [Google Scholar]
- [28].Paulson OB, Strandgaard S, Edvinsson L (1990) Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev 2, 161–192. [PubMed] [Google Scholar]
- [29].La Rovere MT, Pinna GD, Raczak G (2008) Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol 13, 191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cipolla MJ (2009) The Cerebral Circulation., , San Rafael (CA). [PubMed] [Google Scholar]
- [31].Nagai M, Dote K, Kato M, Sasaki S, Oda N, Kagawa E, Nakano Y, Yamane A, Higashihara T, Miyauchi S, Tsuchiya A (2017) Visit-to-visit blood pressure variability and Alzheimer’s disease: Links and risks. J. Alzheimer’s Dis 59, 515–526. [DOI] [PubMed] [Google Scholar]
- [32].Conway J, Boon N, Jones JV, Sleight P (1985) Mechanisms concerned with blood pressure variability throughout the day. Clin. Exp. Hypertens A7, 153–157. [DOI] [PubMed] [Google Scholar]
- [33].Ward MR, Pasterkamp G, Yeung A, Borst C (2000) Arterial remodeling: Mechanisms and clinical implications. Circulation 102, 1186–1191. [DOI] [PubMed] [Google Scholar]
- [34].Faraco G, Iadecola C (2013) Hypertension: A harbinger of stroke and dementia. Hypertension 62, 810–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Capone C, Faraco G, Peterson JR, Coleman C, Anrather J, Milner TA, Pickel VM, Davisson RL, Iadecola C (2012) Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J. Neurosci 32, 4878–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pires PW, Jackson WF, Dorrance AM (2015) Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am. J. Physiol. - Hear. Circ. Physiol 309, H127–H136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Muller M, Van Der Graaf Y, Visseren FL, Mali WPTM, Geerlings MI (2012) Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann. Neurol 71, 825–833. [DOI] [PubMed] [Google Scholar]
- [38].Sykora M, Diedler J, Turcani P, Hacke W, Steiner T (2009) Baroreflex: A new therapeutic target in human stroke? Stroke 40, 678–682. [DOI] [PubMed] [Google Scholar]
- [39].Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lattanzi S, Luzzi S, Provinciali L, Silvestrini M (2014) Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol. Aging 35, 2282–2287. [DOI] [PubMed] [Google Scholar]
- [41].Lattanzi S, Viticchi G, Falsetti L, Buratti L, Luzzi S, Provinciali L, Silvestrini M (2014) Visit-to-visit blood pressure variability in Alzheimer disease. Alzheimer Dis. Assoc. Disord 28, 347–351. [DOI] [PubMed] [Google Scholar]
- [42].de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen JAHR (2019) Blood pressure variability and progression of clinical Alzheimer disease. Hypertension 74, 1172–1180. [DOI] [PubMed] [Google Scholar]
- [43].Oishi E, Ohara T, Sakata S, Fukuhara M, Hata J, Yoshida D, Shibata M, Ohtsubo T, Kitazono T, Kiyohara Y, Ninomiya T (2017) Day-to-day blood pressure variability and risk of dementia in a general Japanese elderly population: The Hisayama study. Circulation 136, 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, Tzourio C (2014) Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimer’s Dement. 10, S330–S337. [DOI] [PubMed] [Google Scholar]
- [45].Nagai M, Hoshide S, Nishikawa M, Masahisa S, Kario K (2014) Visit-to-visit blood pressure variability in the elderly: Associations with cognitive impairment and carotid artery remodeling. Atherosclerosis 233, 19–26. [DOI] [PubMed] [Google Scholar]
- [46].Qin B, Viera AJ, Muntner P, Plassman BL, Edwards LJ, Adair LS, Popkin BM, Mendez MA (2016) Visit-to-visit variability in blood pressure is related to late-life cognitive decline. Hypertension 68, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kanemaru A, Kanemaru K, Kuwajima I (2001) The effects of short-term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens. Res 24, 19–24. [DOI] [PubMed] [Google Scholar]
- [48].Lattanzi S, Vernieri F, Silvestrini M (2018) Blood pressure variability and neurocognitive functioning. J. Clin. Hypertens 20, 645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ma Y, Wolters FJ, Chibnik LB, Licher S, Ikram MA, Hofman A, Ikram MK (2019) Variation in blood pressure and long-term risk of dementia : A population-based cohort study. PLoS Med. 16, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cho N, Hoshide S, Nishizawa M, Fujiwara T, Kario K (2018) Relationship Between Blood Pressure Variability and Cognitive Function in Elderly Patients With Good Blood Pressure Control. Am. J. Hypertens 31, 293–298. [DOI] [PubMed] [Google Scholar]
- [51].Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S (2013) Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J. Am. Geriatr. Soc 61, 2168–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR (2013) Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology 81, 2024–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW (2015) Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 72, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Langbaum JBS, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, Protas HD, Reeder SA, Bandy D, Yu M, Caselli RJ, Reiman EM (2012) Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol. Aging 33, 827.e11–827.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Diaz-Arrastia R, Park DC (2013) Risk factors for β-amyloid deposition in healthy aging: Vascular and genetic effects. JAMA Neurol. 70, 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yew B, Nation DA (2017) Cerebrovascular resistance: Effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 140, 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 74, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939. [DOI] [PubMed] [Google Scholar]
- [59].Bondi M, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko DR, Salmon DP (2014) Neuropsychological criteria for Mild Cognitive Impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimer’s Assoc 42, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Libon DJ, Au R, Galasko D, Salmon DP, Bondi MW (2015) Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s Dement. 11, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR (2010) Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 375, 895–905. [DOI] [PubMed] [Google Scholar]
- [62].R Core Team (2018) R: A language and environment for statistical computing.
- [63].Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, Risacher SL, Nho K, Huentelman MJ, Craig DW, Thompson PM, Stein JL, Moore JH, Farrer LA, Green RC, Bertram L, Jack CR, Weiner MW (2010) Alzheimer’s Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: Genetics core aims, progress, and plans. Alzheimer’s Dement. 6, 265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB (1994) Stroke risk profile: Adjustment for antihypertensive medication: The Framingham Study. Stroke 25, 40–43. [DOI] [PubMed] [Google Scholar]
- [65].Ho JK, Nation DA (2018) Neuropsychological profiles and trajectories in preclinical Alzheimer’s disease. J. Int. Neuropsychol. Soc 24, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bangen KJ, Nation DA, Delano-Wood L, Weissberger GH, Hansen LA, Galasko DR, Salmon DP, Bondi MW (2017) Aggregate effects of vascular risk factors on cerebrovascular changes in autopsy-confirmed Alzheimer’s disease. Physiol. Behav 176, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Shaw LM, Fields L, Korecka M, Waligórska T, Trojanowski JQ, Allegranza D, Bittner T, He Y, Morgan K, Rabe C (2016) Method comparison of AB(1–42) measured in human cerebrospinal fluid samples by liquid chromatography-tandem mass spectrometry, the INNO-BIA AlzBio3 assay, and the Elecsys® B-Amyloid(1–42) assay. Alzheimer’s Dement. 12, P668. [Google Scholar]
- [68].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, Hubeek I, Gibson D, Chu DC, Eichenlaub U, Heiss P, Kobold U, Leinenbach A, Madin K, Manuilova E, Rabe C, Blennow K (2016) Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimer’s Dement. 12, 517–526. [DOI] [PubMed] [Google Scholar]
- [69].Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM (2018) CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s Dement. 14, 1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Seibyl J, Shaw LM, Blennow K, Widmann M, Corradini V, Wahl S, Zink K, Buck K, Eichenlaub U, Hansson O (2017) Amyloid-PET concordance of Elecsys® CSF biomarker immunoassays for Alzheimer’s disease. Alzheimer’s Dement. 13, P199–P200. [Google Scholar]
- [71].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 7, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. R. Stat. Soc [Google Scholar]
- [74].Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, Klein H, Buckley RF, Yang HS, Properzi M, Rao V, Kirn DR, Papp KV., Rentz DM, Johnson KA, Sperling RA, Chhatwal JP (2018) Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: Findings from the Harvard Aging Brain Study. JAMA Neurol. 75, 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Eppig JS, Edmonds EC, Campbell L, Sanderson M, Delano-Wood L, Bondi MW, Alzheimer’s Disease Neuroimaging Initiative (2017) Statistically-derived subtypes and associations with cerebrospinal fluid and genetic biomarkers in mild cognitive impairment: a latent profile analysis. J. Int. Neuropsychol. Soc 23, 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jennings JR, Muldoon MF, Ryan C, Price JC, Greer P, Sutton-Tyrrell K, Van Der Veen FM, Meltzer CC (2005) Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 64, 1358–1365. [DOI] [PubMed] [Google Scholar]
- [77].Power MC, Schneider ALC, Wruck L, Griswold M, Coker LH, Alonso A, Jack CR, Knopman D, Mosley TH, Gottesman RF (2016) Life-course blood pressure in relation to brain volumes. Alzheimer’s Dement. 12, 890–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Glodzik L, Rusinek H, Pirraglia E, McHugh P, Tsui W, Williams S, Cummings M, Li Y, Rich K, Randall C, Mosconi L, Osorio R, Murray J, Zetterberg H, Blennow K, de Leon M (2014) Blood pressure decrease correlates with tau pathology and memory decline in hypertensive elderly. Neurobiol. Aging 35, 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Novak V, Hajjar I (2010) The relationship between blood pressure and cognitive function. Nat. Rev. Cardiol 7, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, Senmaru T, Sakabe K, Ushigome E, Asano M, Yamazaki M, Hasegawa G, Nakamura N (2012) Visit-to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis 220, 155–159. [DOI] [PubMed] [Google Scholar]
- [81].Eto M, Toba K, Akishita M, Kozaki K, Watanabe T, Kim S, Hashimoto M, Sudoh N, Yoshizumi M, Ouchi Y (2003) Reduced endothelial vasomotor function and enhanced neointimal formation after vascular injury in a rat model of blood pressure lability. Hypertens. Res 26, 991–998. [DOI] [PubMed] [Google Scholar]
- [82].Kikuya M, Hozawa A, Ohokubo T, Tsuji I, Michimata M, Matsubara M, Ota M, Nagai K, Araki T, Satoh H, Ito S, Hisamichi S, Imai Y (2000) Prognostic significance of blood pressure and heart rate variabilities: The Ohasama study. Hypertension 36, 901–906. [DOI] [PubMed] [Google Scholar]
- [83].Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K (2011) Visit-to-visit blood pressure variations: New independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J. Am. Soc. Hypertens 5, 184–192. [DOI] [PubMed] [Google Scholar]
- [84].Thrasher TN (2005) Baroreceptors, baroreceptor unloading, and the long-term control of blood pressure. Am. J. Physiol. - Regul. Integr. Comp. Physiol 288, 819–827. [DOI] [PubMed] [Google Scholar]
- [85].Kato T, Kikuya M, Ohkubo T, Satoh M, Hara A, Obara T, Metoki H, Asayama K, Hirose T, Inoue R, Kanno A, Totsune K, Hoshi H, Satoh H, Imai Y (2010) Factors associated with day-by-day variability of self-measured blood pressure at home: The Ohasama study. Am. J. Hypertens 23, 980–986. [DOI] [PubMed] [Google Scholar]
- [86].Imai Y, Aihara A, Ohkubo T, Nagai K, Tsuji I, Minami N, Satoh H, Hisamichi S (1997) Factors that affect blood pressure variability: A community-based study in Ohasama, Japan. Am. J. Hypertens 10, 1281–1289. [DOI] [PubMed] [Google Scholar]
- [87].Nagai M, Hoshide S, Dote K, Kario K (2015) Visit-to-visit blood pressure variability and dementia. Geriatr. Gerontol. Int 15, 26–33. [DOI] [PubMed] [Google Scholar]
- [88].Hughes TM, Wagenknecht LE, Craft S, Mintz A, Heiss G, Palta P, Wong D, Zhou Y, Knopman D, Mosley TH, Gottesman RF (2018) Arterial stiffness and dementia pathology: Atherosclerosis risk in communities (ARIC)-PET study. Neurology 90, e1248–e1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Nichols WW, O’Rourke MF, Vlachopoulos C (2011) McDonald’s Blood Flow in Arteries: Theoretical, Experimental, and Clinical Principles, CRC Press. [Google Scholar]
- [90].Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G (2019) Cerebrovascular smooth muscle cells as the drivers of intramural periarterial drainage of the brain. Front. Aging Neurosci 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 117, 1–14. [DOI] [PubMed] [Google Scholar]
- [92].Nagai M, Hoshide S, Kario K (2010) The insular cortex and cardiovascular system: a new insight into the brain-heart axis. J. Am. Soc. Hypertens 4, 174–182. [DOI] [PubMed] [Google Scholar]
- [93].Sturm VE, Brown JA, Hua AY, Lwi SJ, Zhou J, Kurth F, Eickhoff SB, Rosen HJ, Kramer JH, Miller BL, Levenson RW, Seeley WW (2018) Network architecture underlying basal autonomic outflow: Evidence from frontotemporal dementia. J. Neurosci 38, 8943–8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Guo CC, Sturm VE, Zhou J, Gennatas ED, Trujillo AJ, Hua AY, Crawford R, Stables L, Kramer JH, Rankin K, Levenson RW, Rosen HJ, Miller BL, Seeley WW (2016) Dominant hemisphere lateralization of cortical parasympathetic control as revealed by frontotemporal dementia. Proc. Natl. Acad. Sci. U. S. A 113, E2430–E2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Craig ADB (2009) How do you feel - now? The anterior insula and human awareness. Nat. Rev. Neurosci 10, 59–70. [DOI] [PubMed] [Google Scholar]
- [96].Critchley HD (2005) Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol 493, 154–166. [DOI] [PubMed] [Google Scholar]
- [97].Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD (2012) A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev 36, 747–756. [DOI] [PubMed] [Google Scholar]
- [98].Seeley WW, Zhou J, Kim EJ (2012) Frontotemporal dementia: What can the behavioral variant teach us about human brain organization? Neuroscientist 18, 373–385. [DOI] [PubMed] [Google Scholar]
- [99].Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, Lambert C, Cardenas-Blanco A, Pine K, Passamonti L, Loane C, Keuken MC, Trujillo P, Lüsebrink F, Mattern H, Liu KY, Priovoulos N, Fliessbach K, Dahl MJ, Maaß A, Madelung CF, Meder D, Ehrenberg AJ, Speck O, Weiskopf N, Dolan R, Inglis B, Tosun D, Morawski M, Zucca FA, Siebner HR, Mather M, Uludag K, Heinsen H, Poser BA, Howard R, Zecca L, Rowe JB, Grinberg LT, Jacobs HIL, Düzel E, Hämmerer D (2019) Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Alsop DC, Dai W, Grossman M, Detre JA (2010) Arterial spin labeling blood flow MRI: Its role in the early characterization of Alzheimer’s disease. J. Alzheimer’s Dis 20, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Webb AJ, Fischer U, Mehta Z, Rothwell PM (2010) Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: A systematic review and meta-analysis. Lancet 375, 906–915. [DOI] [PubMed] [Google Scholar]
- [103].Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV., Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT (2015) A randomized trial of intensive versus standard blood-pressure control. N. Engl. J. Med 373, 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K (2016) Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 387, 957–967. [DOI] [PubMed] [Google Scholar]
- [105].Yaffe K (2019) Prevention of cognitive impairment with intensive systolic blood pressure control. JAMA 321, 548–549. [DOI] [PubMed] [Google Scholar]
- [106].Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Umegaki H, Khookhor O (2013) The response of the autonomic nervous system to the cholinesterase inhibitor, donepezil. Neuroendocrinol. Lett 34, 383–387. [PubMed] [Google Scholar]
- [108].Rothwell PM, Howard SC, Dolan E, Brien EO, Dobson JE, Dahlöf B, Poulter NR, Sever PS (2010) Effects of β blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 9, 469–480. [DOI] [PubMed] [Google Scholar]
- [109].Levi Marpillat N, Macquin-Mavier I, Tropeano A-I, Levi-Bachoud A-C, Maison P (2013) Antihypertensive classes, cognitive decline and incidence of dementia: a network meta-analysis. J. Hypertens 31, 1073–1082. [DOI] [PubMed] [Google Scholar]
- [110].Gelber RP, Ross GW, Petrovitch H, Launer LJ, White LR (2013) Antihypertensive medication use and risk of cognitive impairment. Neurology 81, 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yasar S, Yao W, Furberg CD, Xue Q, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, Dekosky ST, Carlson MC (2013) Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo evaluation of memory study. Neurology 81, 896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ho JK, Nation DA, Neuroimaging D (2017) Memory is preserved in older adults taking AT1 receptor blockers. Alzheimer’s Res. Ther 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lim HM, Chia YC, Ching SM, Chinna K (2019) Number of blood pressure measurements needed to estimate long-term visit-to-visit systolic blood pressure variability for predicting cardiovascular risk: A 10-year retrospective cohort study in a primary care clinic in Malaysia. BMJ Open 9, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


