Abstract
The SARS-CoV-2 virus has spread globally and has severely impacted public health and the economy. Hand hygiene, social distancing, and the usage of personal protective equipment are considered the most vital tools in controlling the primary transmission of the virus. Converging evidence indicated the presence of SARS-CoV-2 in wastewater and its persistence over several days, which may create secondary transmission of the virus via waterborne and wastewater pathways. Although, researchers have started focusing on this mode of virus transmission, limited knowledge and societal unawareness of the transmission through wastewater may lead to significant increases in the number of positive cases. To emphasize the severe issue of virus transmission through wastewater and create societal awareness, we present a state of the art critical review on transmission of SARS-CoV-2 in wastewater and the potential remedial strategies to effectively control the viral spread and safeguard society. For low-income countries with high population densities, it is suggested to identify the virus in large scale municipal wastewater plants before following up with one-to-one testing for effective control of the secondary transmission. Ultrafiltration is an effective method for wastewater treatment and usually more than 4 logs of virus removal are achieved while safeguarding good protein permeability. Decentralized wastewater treatment facilities using solar-assisted disinfestation methods are most economical and can be effectively used in hospitals, isolation wards, and medical centers for reducing the risk of transmission from high local concentration sites, especially in tropical countries with abundant solar energy. Disinfection with chlorine, sodium hypochlorite, benzalkonium chloride, and peracetic acid have shown potential in terms of virucidal properties. Biological wastewater treatment using micro-algae will be highly effective in removal of virus and can be incorporated into membrane bio-reaction to achieve excellent virus removal rate. Though promising results have been shown by initial research for inactivation of SARS-CoV-2 in wastewater using physical, chemical and biological based treatment methods, there is a pressing need for extensive investigation of COVID-19 specific disinfectants with appropriate concentrations, their environmental implications, and regular monitoring of transmission. Effective wastewater treatment methods with high virus removal capacity and low treatment costs should be selected to control the virus spread and safeguard society from this deadly virus.
Keywords: COVID-19, Wastewater, Disinfectants, Secondary transmission, Wastewater treatment
Graphical abstract
Abbreviations
- C/N
Carbon to nitrogen loading
- COVID-19
Coronavirus Disease 2019
- DNA
Deoxyribonucleic acid
- LRV
Log Reduction Value
- MERS
Middle East respiratory syndrome
- MHV
Mouse hepatitis virus
- MID
Minimal infectious dose
- ORF
Open reading frame
- PFU
Plaque-forming units
- RNA
Ribonucleic acid
- RT-PCR
Real-time reverse transcription polymerase chain reaction
- RT-qPCR
Reverse transcription-quantitative polymerase chain reaction
- SARS-CoV
Severe Acute Respiratory Syndrome Coronavirus
- SARS-CoV-1
Severe Acute Respiratory Syndrome Coronavirus 1
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- UF
Ultrafiltration
- UV
Ultraviolet
- WHO
World Health Organization
1. Introduction
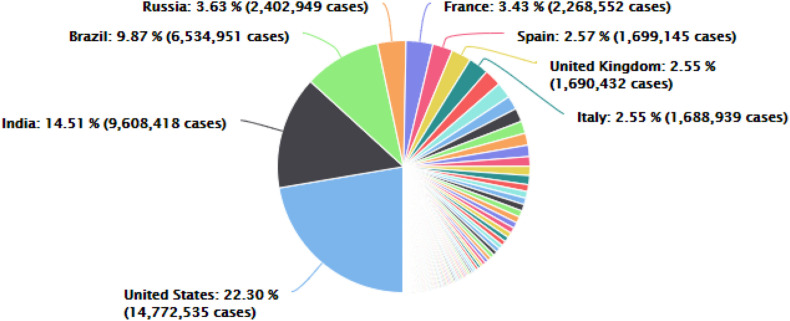
The newly identified coronavirus disease ‘COVID-19’ was first identified as a pneumonia virus causing respiratory illness which is thought to have originated from a local seafood market in Huanan, Wuhan, China and was named ‘SARS-CoV-2’ by the World Health Organization (WHO) on January 12, 2020. The WHO declared COVID-19 as a worldwide health emergency on 30th January, and later, it was declared a pandemic on March 11, 2020. This SARS-CoV-2 has spread across 210 countries and 66, 231, 472 confirmed cases and 1,524,473 deaths were reported by December 5, 2020 as shown in Fig. 1 ( Worldometer, 2020). The SARS-CoV-2 virus is a pleomorphic ribonucleic acid (RNA) virus, belonging to the coronavirus family having crown-shape peplomers (size - 80 to 160 nm) with positive polarity (27–32 kb) (Sahin et al., 2020). COVID-19 virus genome sequence is 96.2%, similar to the ‘BatCoV RaTG13’ bat coronavirus (Yan et al., 2020) and a low mortality rate of ~2%.
Fig. 1.
COVID-19 confirmed cases and their distribution country wise, as of 5th December 2020.
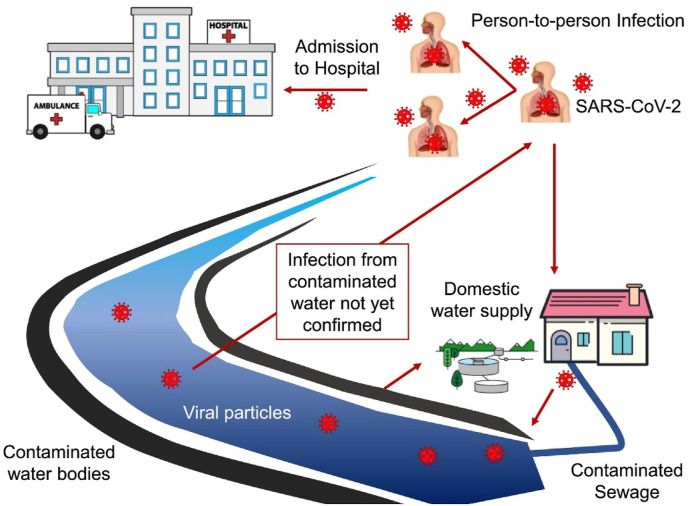
However, the spreading rate of COVID-19 amongst humans is higher than SARs and MERS, with an incubation time of 24 days (Yan et al., 2020). The major path of transmission of the COVID-19 virus among humans occurs through inhalation of saliva and sputum droplets along with person-to-person physical contacts (WHO, 2020; Kitajima et al., 2018). Recently, Doremalen et al. (2020) compared the surface stability and aerosol transmission behavior of SARS-CoV-2 and SARS-CoV-1 and illustrated that SARS-CoV-2 can stay suspended for 3 h in the air (Suthar et al., 2021), with an identical drop in its rate of infections compared to SARS-CoV-1. It was also revealed that the properties of SARS-CoV-2 and SARS-CoV-1 are identical in terms of the formation and air particles stability. Nevertheless, human receptors affinity for the initial variant of SARS-CoV-2 is 10 times greater than SARS-CoV-1. Several studies have shown that the COVID-19 virus can also be shed in feces from infected patients displaying acute symptoms, from asymptomatic individuals, and from patients cured without any further symptoms (Dhama et al., 2021; Pan et al., 2020; Tang et al., 2020; Xiao et al., 2020). In addition, COVID-19 viral RNA was detected in urine samples of infected patients (Ling et al., 2020). COVID-19 RNA was also reported in the community wastewater and hospital sewage (Lodder and de Roda Husman, 2020). Although the risk of spread of COVID-19 virus to people through water including wastewater is still not clear, the identification of COVID-19 virus RNA in both treated and untreated wastewater (Venugopal et al., 2020) raises the alarming situation of the potential for virus transmission through this medium, and consequent occupational exposure concerns for wastewater treatment plant workers. The potential for transmission of viruses through water bodies is gaining attention recently among the research community, following the immediate response to the current pandemic which predominantly focused on prevention of transmission from person-to-person. The increasing number of testing facilities, hospitals, isolation wards and research centers developed worldwide was essential to expedite the detection of infected patients and accommodate them for further testing and to carry out advanced research about this new deadly virus. It is quite obvious that these facilities have increased the generation of wastewater contaminated by the virus, and that if incorrectly handled this will certainly pose a threat to society. Virus transmission through wastewater might be a major worry in regions where there is a lack of water treatment facilities and inadequate sanitation. In countries with lower income, domestic wastewater is often released directly into the environment and may over time find its way towards groundwater (Omosa et al., 2012). As the majority of people fulfill their water needs using groundwater sources in rural and peri-urban areas (Kookana et al., 2020), the potential community transmission of the SARS-COV-2 virus through infected and untreated groundwater is thus possible.
Apart from direct contact with wastewater, breathing of droplets/aerosols which are contaminated with infectious viral particles is considered as the major source of virus transmission in wastewater treatment plants. However, given that this is the first pandemic on such a global scale, very few studies have taken into consideration the risks posed to wastewater treatment plant workers and hence, there is a tremendous need to investigate and highlight the potential of this exposure route as a route of infection. Even though it is stated that the existing disinfection methods can deactivate viruses in water bodies, the fate of SARS-COV-2 virus in water/wastewater bodies is yet to be elucidated (Nghiem et al., 2020). In addition, there is still a substantial knowledge gap regarding to what extent the early detection of virus is possible, i.e., before the occurrence of widespread symptomatic cases, owing to the limitation in identification techniques which mostly depend on the viral load in the patient's fecal matter. Furthermore, very few authors have reported quantitative assessments to predict the loading of virus in wastewater and its correlation to the official case statistics, although these of course are also hugely variable depending on testing rates and approach.
With a second wave of the pandemic occurring across most parts of the world, our focus here is to highlight and draw attention towards the potential transmission of the SARS-COV-2 virus through wastewater. In this regard, to help society fill the knowledge gap, the major objective of this critical review is to synthesize current knowledge on approaches for treating wastewater contaminated with the virus so as to decrease COVID-19's transmission chances, and to support prioritization of the further research needs and the current barriers to implementation of the various treatment methodologies in developing countries. The methodology used for selecting the appropriate recent manuscripts, based on the objective of this work, are discussed in detail. Further, the potential transmission and detection of SARS-COV-2 virus in wastewater, along with the risks of infection through droplets/aerosols contaminated with infectious viral particles, and the quantitative detection methods are discussed in detail to present a clear picture of the current state of knowledge to the readers. Finally, the various remedial approaches for wastewater treatment such as decentralized wastewater treatment and different potential disinfectants for wastewater treatment, are presented and their advantages and disadvantages discussed. In tropical countries with abundantly available solar energy, a sustainable low-cost approach for wastewater treatment is also highlighted. With increasing COVID-19 cases, uncertainty in transmission paths and less-societal knowledge and awareness, our review aims to create awareness and draw the attention of researchers and society towards the potential severity of virus transmission through wastewater and its potential remedies.
2. Methodology
The articles for the present state of the art critical review were carefully selected by considering the impact of the reported research and the quality of the journals, respectively. Identification of published work assessing the potential spread of SARS-CoV-2 virus through wastewater and the various strategies for wastewater treatment to effectively control viral spread was carried out through systematic searches in the Google Scholar, Science Direct (Elsevier), Web of Science, Pub Med and Scopus databases using appropriate keywords such as "SARS/SARS-CoV-2 virus in wastewater", "secondary SARS-CoV-2 virus transmission", "advanced wastewater treatment for virus spread control". Further searches were made using keywords such as "wastewater treatment" and "SARS-CoV-2 virus" for identification of the most relevant literature (up to March 2021) on SARS-CoV-2 virus transmission through wastewater and various methods to control the spread. To select the suitable literature from the so-collected manuscripts in the context to wastewater treatment process exclusively to control the spread of SARS-CoV-2 virus, the following key points were considered:
-
➢
Inclusion of all studies that describe SARS/SARS-CoV-2 virus transmission by any kind of water sources;
-
➢
Inclusion of manuscripts that reported a mechanism of virus transmission in water/wastewater;
-
➢
Inclusion of manuscripts that report detection methods for SARS-CoV-2 virus in water/wastewater;
-
➢
Inclusion of work that reports on impact and severity of virus spread at the social-community level;
-
➢
Inclusion of articles that focus on potential treatment of water/wastewater for deactivating viruses;
-
➢
Inclusion of articles on sustainable treatment strategies for viral deactivation
-
➢
Exclusion of manuscripts that are entirely based on primary transmission of SARS-CoV-2 virus;
-
➢
Exclusion of studies which don't report quantitative outcomes or merely repeat existing results (i.e., review articles).
The mapping of literature content and bifurcation of the selected studies were executed based on the following criteria:
-
➢
What specific virus identification approach in water/wastewater was adopted?
-
➢
What is the transmission mode of the virus through the water/wastewater system?
-
➢
Is there any specific technique implemented to monitor the growth and spread of virus in the water/wastewater system? If so, what are the methods to deactivate the viruses in water bodies?
-
➢
What treatment parameters were adopted to deactivate the virus?
-
➢
What are the sustainable approaches for treatment of wastewater?
-
➢
What hinders implementation of the wastewater treatment method in low-income countries?
-
➢
What specific conclusions are made regarding the effectiveness of the water treatment tactic in controlling the spread of SARS-CoV-2 virus?
All the collected manuscripts were broadly classified based on the sources of virus secondary transmission in water/wastewater and the individual wastewater treatment technologies were further segregated depending upon the nature of treatment (decentralized, physical methods including membrane technology and sedimentation approaches, solar assisted wastewater disinfection, ozonation, chemical based disinfectants and biological based treatment including algae) and its effectiveness for virus deactivation. Under each category, the different virus deactivation approaches and the advanced techniques implemented for deactivating the virus spread are organized and discussed in detail.
3. Potential transmission and detection of COVID-19 virus in wastewater
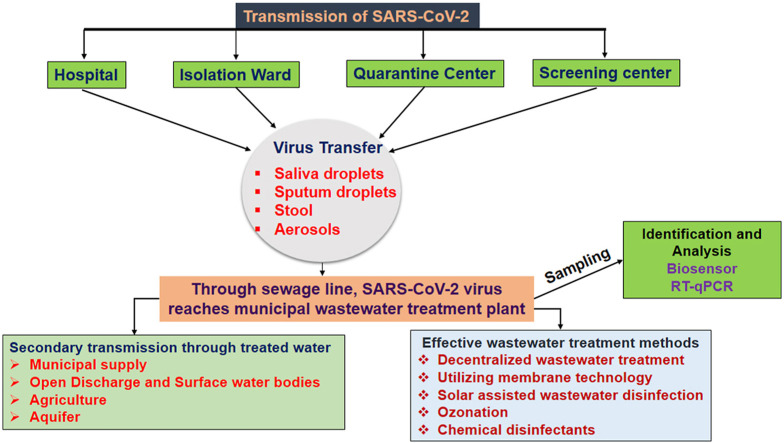
SARS-COV-2 RNA can enter wastewater systems via various pathways, as shown in Fig. 2 which highlights the virus's potential transmission pathways. These include discharged wastewater from isolation or quarantine centers, and hospitals. Urine, stool, and feces related contamination are the most common means of spreading contamination through wastewater systems. It was reported that nearly 67% of the stool samples of infected people tested positive for COVID-19 RNA, with counts reaching almost as high as those in sputum (109 copies/mL in sputum versus 108 copies/mL in stool) ( Chen et al., 2020). It is also interesting that SARS-COV-2 RNA is commonly found in stool even after the respiratory infection has resolved and, in some cases, even after the respiratory samples are found negative ( Xiao et al., 2020). Recently, a compartmental epidemic logical model using the data from Wuhan, China, showed that the fecal-oral path is significant in spreading the virus (Danchin et al., 2020), which is indicative of poor hand-washing, often associated with water scarcity and/or lack of access to clean water (Hannah et al., 2020). The Danchin study revealed that virus replication in the gastrointestinal tract is highly possible (Danchin et al., 2020). Therefore, contaminated wastewater can be supposed to carry a substantial amount of infective virus. Moreover, surface waters such as lakes and streams, where contaminated wastewater is often directly released without appropriate treatment in low-income countries, can also be a possible carrier for the SARS-COV-2 through the water-channel into different parts of society. Likewise, groundwater resources are also not safe, as there might be viral contamination through groundwater recharge. Fig. 2 shows the different pathways for SARS-CoV-2 transmission through water systems. Furthermore, if the wastes from hospitals and isolation wards are disposed without suitable treatment into the water bodies, this may lead to disease transmission. Hence, safeguarding the water systems is highly essential to inhibit unpredictable yet preventable contamination of available water resources from SARS-COV-2 and other microorganisms.
Fig. 2.
Sources and pathways of SARS-CoV-2 in water systems (Adelodun et al., 2020). Copyright 2020 Elsevier.
Detection of SARS-COV-2 in wastewater is highly challenging and presently different approaches such as quantitative molecular methods and in vitro counts by the number of plaque-forming units (PFU) are used to detect and monitor viruses. The PFU method provides a measurable assessment of the infectious viral-particle load; however, it is difficult and slow owing to the requirement for a suitable host for in vitro cultivation ( Wigginton et al., 2015; Madigan et al., 2012). Molecular methods show the ability to estimate (the COVID-19) viral RNA in wastewater samples, but this method doesn't measure viral infectivity ( Wigginton et al., 2015). It is also important to understand that the viral identification sensitivity could be limited further by the cytotoxicity of co-contaminants usually seen in wastewater samples. Moreover, virus concentrations in wastewater samples need to be high compared to the RNA detection limit (>106 copies/mL) in order to distinguish infectious viral particles. Generally, real-time reverse transcription polymerase chain reaction (RT-PCR) is considered the gold standard for determining SARS-CoV-2 using a direct assay of human extraction, where the samples are collected from the upper respiratory system using swabs. In real-time RT-PCR, the limit of detection is ~100 copies of viral RNA/mL of the transport medium; however, the RNA detection limit is > 106 copies/mL in the case of wastewater. Therefore, the wastewater measurement method needs to be more accurate with higher sensitivity for detecting the virus than that needed for clinical samples detection. In order to achieve this, intact virions are concentrated on a cell-free substrate coated with the analogous receptors after the enzyme treatment to eliminate the broken virions. Later, the bound virions are amplified and measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). This method was used in recent studies to identify the SARS-COV-2 in water samples ( Medema et al., 2020 ).
Table 1 presents the recently conducted studies on detection of SARS-CoV-2 in different wastewater samples globally through different targeted genes like ORF1ab, N1, N2, and N3 using RT-qPCR. Very few studies reported on whether the genetic material was present in free nucleic acids or in intact virus particles. It was seen that the majority of the samples tested at multiple different locations globally (per 100,000 people) had demonstrated the presence of SARS-CoV-2 in untreated wastewater through RT-qPCR. Although RT-qPCR shows good outcomes in detecting SARS-CoV-2 in wastewater, other methods must be developed in order to accurately determine the infection in wastewater samples even under low virus load concentration. Presently, the minimal infectious dose (MID) of the COVID-19 virus is unknown for humans ( Kitajima et al., 2020). However, this novel virus's rapid transmission shows that its MID is lower than or identical to those of other enveloped viruses ( Watanabe et al., 2020; Lindsley et al., 2020).
Table 1.
Recent studies assessing the presence of SARS-CoV-2 in wastewater samples.
| Reference | Region of study | Genes analyzed | Outcomes |
|---|---|---|---|
| Kumar et al. (2020) | Ahmedabad, India | ORF1ab, N and S | 2/2 influent water samples - Positive 2/2 effluent water samples - Negative |
| Sherchan et al. (2020) | Louisiana, USA | N1 and N2 | 2/15 raw wastewater samples - Positive All effluent water samples - Negative |
| Randazzo et al. (2020) | Valencia, Spain | N1, N2 and N3 | 35/42 influent water samples - Positive 2/18 secondary treated water samples - Positive 0/12 tertiary effluent water samples - Positive |
| Nemudryi et al. (2020) | Bozeman, Montana, USA | N1 and N2 | 7/7 samples - Positive in March/April 2020 |
| Wu et al. (2020) | Massachusetts, USA | N1, N2 and N3 | 10/10 raw wastewater samples - Positive |
| Istanbul, Turkey | RdRp | 9/9 sludge samples – Positive | |
| Haramoto et al. (2020) | Yamanashi Prefecture, Japan | N1 and N2 | 0/5 influent samples – Positive 1/5 secondary effluent samples – Positive 0/3 river water samples – Positive |
It is interesting to note that a range of factors affects the virions of SARS-COV-2 in water bodies such as organic content, water temperature, and water pH. The survival time of the SARS-COV-2 is estimated from the time needed for 90% inactivation (T90) (Bogler et al., 2020). Under different environmental conditions, the virus can remain infective for many days. However, the method through which the virus translates into severe infection risk is still unknown, especially as human activities on, and exposure to, water varies across seasons and regions. Lower temperatures support longer persistence of SARS-CoVs infectivity; at 4 °C it has been shown to remain infective for 14 days in wastewater whereas it remained viable for only 2 day at 25 °C (Wang et al., 2020). Therefore, lower ambient temperatures under cold climatic conditions support a higher survival of the SARS-COV-2. In view of the possible transmission of the SARS-COV-2 through water and wastewater, precautionary measures must be taken to manage wastewater effectively. During winter or cold climatic conditions, hospitals located in the middle/high latitudes can increase the wastewater treatment temperature by between 20 °C and 25 °C to reliably and rapidly inactivate the SARS-COV-2.
4. Risk of infection through droplets/aerosols contaminated with infectious particles and its quantitative analysis
With increasing threat of secondary transmission, the major exposure risk is associated with the wastewater treatment plant worker, who can be directly exposed to the sewage through faults or leaks in plumbing or sewer networks. In addition, water treatment workers could also be prone to inhaling aerosols/droplets which are contaminated with the infectious viral-particles and there is very high chance of such cases. Gholipour et al. (2021) examined and reported the detection of Covid-19 virus RNA in about 40% of the air samples (6/15) of wastewater treatment plants, when the prevalence of SARS-CoV-2 virus was very high in the region. Covid-19 virus RNA was identified in the range of 5–188 genomic copies/liter of air and the maximum concentration was investigated at the wastewater pumping station. However, very few occupationally exposed cases of this indirect transmission risk through the wastewater aerosols/droplet have been studied or reported as yet, and thus there is a lack of knowledge and reported literature regarding this kind of possible infection. In view of societal equality and generalized safety, it is very important to safeguard wastewater plants workers, who played a pivotal role for society during the lockdown. Various factors affect the probability of infections arising in wastewater plant workers through the inhalation of aerosols which are contaminated with corona virus, as follows:
-
❖
Climatic conditions: Wind velocity and its direction along with turbulence and deposition are the major factors which determine the transmission of virus through aerosols. These factors can critically impact the generated aerosols height and the distance covered before they settle. It is also expected that high wind velocity may lead to enhanced exposure of aerosols to the populations living downwind of wastewater plants.
-
❖
Volume of the infectious viral-particles inhaled: Volume of lung, inhalation rate and viral particle size and density are crucial factor in view of infection likelihood (Wilkinson et al., 2012). Generally, males have bigger nasal-cavities and higher, longer and narrower nasal floors than females with similar body size (García-Martínez et al., 2016). This could result in males breathing a higher volume of infectious viral-particles than female workers, which could result in greater risk of contracting the SARS-COV-2 virus infection.
-
❖
Health response of workers: The most critical factor for assessing the potential for infection of the workers are the host response to the inhaled particles. With the available data, critical infections of SARS-COV-2 virus are predominantly seen in patients with underlying health conditions such as chronic-lung disease, diabetics, and cardiovascular disease (Bonow et al., 2020). Previous studies of health effects in wastewater treatment workers have shown enhanced prevalence of cardiovascular and breathing related conditions compared to control populations (Albatanony et al., 2011). Nevertheless, healthy persons are also infected by particle exposure and therefore, all wastewater plants workers regardless of their health conditions are at risk of infection, especially during severe outbreaks where viral loading may be very elevated.
4.1. Quantitative analysis
Aerosols emitted from wastewater plants possess higher risk and therefore, must be assessed. A human-fecal shedding technique was used for determination of the concentration of SARS-CoV-2 in wastewater (Barker, 2014; Zaneti et al., 2021).
Exposure assessment – The aerosols generated in aeration tanks and pumping stations are predominantly of diameter ≤10 μm, which are considered to be respirable and could deposit in the respiratory tract and reach the alveolar region of the lungs (USEPA, 2011).
The daily dose (dd, TCID50/day) of SARS-COV-2 aerosols inhaled by the wastewater treatment workers can be calculated using an equation developed by (Barker, 2014) for other airborne microbes:
| (1) |
where, C c is the SARS-COV-2 concentration in wastewater (TCID50/L), PC w-ar is the microbial ‘water to air’ partitioning coefficient (L/m3), AIR is the average rate of inhalation (m3/h), t exp is everyday exposure time (8 h for professional exposure), and ARR is the retention rate of aerosol in lungs, determined using the following equation (Schoen et al., 2011):
| (2) |
where, FFi 1 is the fraction of aerosols with size range of ‘i’ and FFi 2 is the fraction of size range i which were deposited onto the lower respiratory tract.
The virus concentration in wastewater is determined using the following equation (Barker, 2014).
| (3) |
where, C i is the cumulative number of COVID-19 cases, PRfs is the % of people with fecal-shedding of SARS-COV-2. Sr and Sd are the shedding rate (copy/gram) and shedding duration (d) of SARS-COV-2 in the feces of patients, respectively, FP is the everyday production of fecal matter by patients (gram feces/person/day), dt is the time period of study, Qf is the flow-rate of wastewater in the plant and CF is the conversion factor of genomic copy number to TCID50. When determining viral concentration through quantitative analysis, care must be taken to account for uncertainty and variability inherent in the biological systems as suitable for the investigated conditions.
5. Potential wastewater treatment options for COVID-19 inactivation
Different precautionary measures have been suggested by the WHO to effectively control the spread of COVID-19, such as face masking at indoor and outdoor gatherings, social distancing, and frequent hand washing with alcohol-based sanitizer or soap. Although the precautionary measures are effective in controlling the transmission, the fear of potential community transmission is very high, particularly in the countries with lower income where several families share (often limited) water systems and sanitation faciliites. Hence, extensive measures are needed to effectively control the spread from wastewater through effective treatment techniques. These treatment approaches include physical, chemical and biological treatment methods, which are focused on removal of suspended solids and bio-degradable organics Crini et al. (2019), (Fu et al., 2010). The efficiency of pathogen removal from water-treatment processes is characterized by the Log Reduction Value (LRV), expressed as the relative number of live-microbes removed from the system through any removal procedure and is represented as:
| (4) |
where, Cmb and Cma signify the viable microbe numbers before and after the treatment. Various wastewater treatment processes and potential remedial approaches such as decentralized wastewater treatment, sedimentation and membrane technology (physical treatment processes), chemical and biological processes including microalgae based treatment techniques are discussed in the following section.
5.1. Decentralized wastewater treatment for preventing virus spread
The COVID-19 pandemic has created huge stress on the availability of clean water for maintaining hygiene and contaminated wastewater treatment in order to safeguard communities and reduce exposure. In general, the dedicated COVID-19 isolation wards and health centers to monitor and treat the patients share the same sewerage systems with nearby societies. People living in the same society are likely to use common water resources, especially in low-income countries, and they may potentially be exposed to the SARS-COV-2 virus through shared water resources. Furthermore, the inappropriate dumping of wastewater from hospitals without any treatment or disinfection approach may cause community health risks and may spread the infection. As there is a potential for the SARS-COV-2 virus spread in a centralized wastewater treatment systems, the decentralized wastewater treatment strategies with affordable and low maintenance cost could play a significant role (Matto and Singhal, 2020) during COVID-19. Design and development of small-scale wastewater treatment plants can be viable alternatives to the centralized treatment plants in COVID hotspots, such as isolation wards and quarantine centers, which are a high potential source for spreading the virus through wastewater. In a decentralized treatment system, utilization of UV radiation and some promising ecofriendly virucidal alternatives, such as performic acid, peracetic acid and sodium dichloro isocyanurate, appear to be effective in disinfecting the Covid-19 virus and thus combatting any potential contamination through wastewater transmission. Decentralized wastewater treatment facilities that consist of light emitting diode (LED) based UV could be highly useful (Naddeo and Liu, 2020). In low-income countries, where the infrastructure is not good, and construction of a complete wastewater treatment plant is not possible in a short time span, the usage of mobile wastewater treatment services with disinfection devices could be a better and more feasible option. Rural solar toilets can also be a viable alternative as they can easily achieve water temperatures up to 44 °C, which helps in the removal of pathogens (Moe and Izurieta, 2003). Additionally, sanitary landfills/wetlands or ponds are also an effective method of discharging the wastewater, and they can be treated with economic disinfectants like sodium hypochlorite. It has been inferred from the above discussion that a cost effective method for controlling the viral spread can be achieved through decentralized treatment of wastewater, and various dimensions should be taken into consideration while designing this system, particularly the local issues related to the suitability and availability while selecting this technology.
5.2. Prevention of virus spread through wastewater by utilizing membrane technology
Membrane filtration technology is considered to be a robust, non-invasive and non-toxic technique. It is highly preferred for removing virus and considered as the most advanced method for wastewater treatment. In this technology, ultrafiltration (UF) is an effective method for removing viruses, macromolecules, pyrogens, and bacteria. UF utilizes membranes with 1000 kilo-Dalton molecular weight cut-off that are explicitly designed to retain viruses, and usually more than 4 logs of virus removal are attained while safeguarding good protein permeability. In filtration, viruses of size less than the pore-size are carried with the fluid and pass through the pores of membranes, while if the size of the pore is less than that of the virus, the virus get retained. Size exclusion is the key mechanism of clearance by filtration. UF has potential to provide a complete barrier to COVID-19 virus spread, as it can easily remove the virus whose diameter is 100 nm.
Filtration capability can be further enhanced using different surface characteristics of the filtration membrane such as hydrophobic and charged regions which attract groups on the viral envelope, leading to the removal of sizes beyond exclusion owing to the electrostatic and hydrophobic interactions (Chaudhry et al., 2015; Bodzek et al., 2019). The usage of UF membranes in bioreactors has further improved the virus removal capability through the combination of three distinct mechanisms: steric removal, adsorption, and inactivation during treatment (Bodzek et al., 2019). Owing to the advanced features of UF membrane in bioreactors, they removed bacteriophage MS2 (virus) with high efficiency (4–7 log). Moreover, nano-filtration (pore size < 2 nm) with high pressure, using a tight and dense membrane system along with forward and reverse osmosis membranes, can completely remove SARS-CoVs (Pendergast et al., 2011). These filtration technologies are most efficient when used in tangential flow or cross flow mode, and are being exploited in various virus removal and wastewater purification processes as they are cheaper than other methods such as chromatography and also easier to implement. Nano-filtration possesses features for separating the COVID-19 virus from wastewater, however extensive experimental studies are needed prior to design and execution especially for wastewater applications. Various effective experimental methods for predicting flux exist, but still there is a lack of theoretical studies, predominantly those targeting the calculation of filtration efficiency related to log reduction value, and hence, filtration efficiency should be considered for advanced design and efficient operation of UF in terms of virus separation.
In addition to filtration, sedimentation is also reported to remove viruses (Verbyla et al., 2105; Shin et al., 2015). Viral adsorption onto large-size settleable solids followed by sedimentation is considered to be the main removal mechanism in many treatment plants (Verbyla et al., 2105). The terminal velocity (V) of the dispersed solid settling due to gravity is represented by the following equation:
| (5) |
where D is diameter of the solid particle (m), g is acceleration due to gravity (m/s), ρp is the density of the solid particle (kg/m3), ρw is density of water (kg/m3), and CD is the drag coefficient. Drag coefficient is calculated using the Reynolds's number (Re), which is expressed as:
| (6) |
where μ denotes the viscosity of water and u denotes the relative velocity, respectively.
It can be seen from these equations that increased settling velocity will be achieved by increasing the diameter or volume of the particles (Mohammed et al., 2013) and similar concepts have been used for removal of viruses in wastewater. As the virus attaches to the suspended-solids, the combined agglomerated particles exhibit a larger size diameter with greater density, which in turn leads to enhanced sedimentation and consequently to removal of the virus. LRV of 0.65–2.85 were achieved for eleven different viruses during conventional activated sludge processes and similarly, LRVs of 1.4–1.7 were achieved for noroviruses, rotaviruses and enteroviruses (Kitajima et al., 2014; Zhou et al., 2015). Therefore, sedimentation is the main mechanism to reduce the viral concentration in wastewater. However, only selected strains of rotavirus and norovirus were removed by this process (Da Silva et al., 2008) and hence, further studies are required to make this technology more effective and to assess its suitability for removal of the COVID-19 virus from wastewater.
5.3. Potential disinfectant strategies for prevention of virus spread
5.3.1. Solar assisted wastewater disinfection
Solar assisted wastewater disinfection is a highly feasible and applicable option in several types of aquatic environments (Nelson et al., 2018). Solar based drinking water disinfection is a sustainable approach for disinfecting water, and it is widely promoted (Thakur et al., 2018a, 2018b, 2020b, 2021a, 2021b, 2021c, 2021d; Thakur et al., 2020a; Kumar et al., 2017). It mainly depends on the intensity of solar radiation, the optical, physical, and chemical properties of the wastewater, and the type of virus (Verbyla et al., 2015). Solar energy has abundant availability with yearly solar irradiance higher than 2000 kWh/m2/y in most places on earth except Russia, Canada, Japan, and South Korea. Tropical countries like India have plenty of sunshine, and average daily solar radiation varies between 4 and 7 kWh/m2 for different parts of the country. With an average of 250–300 clear sunny days in a year, India receives about 5000 TWh of solar insolation per year, and it shows excellent potential for solar assisted wastewater disinfection facilities. Various mechanisms are used for the disinfection of wastewater by solar radiation such as the direct mechanism, which needs photon absorption directly by the virus or an endogenous component such as proteins, nucleic acids and other biomolecules. They absorb the UV-B fraction of solar radiation that leads to structural change and thus, inactivates the virus. Recently, Sagripanti et al. (2020) examined and explored the role of virus inactivation by the UV-B in sunlight in various populated cities across the world. The results showed comparatively faster inactivation of COVID-19 virus (than influenza A) during the summer time, demonstrating the important role of solar radiation on its occurrence and spread. The authors concluded that more than 90% of COVID-19 virus was inactivated by exposure to mid-day solar radiation after 11–34 min in the majority of US cities and world cities during their respective summer. Ratnesar-Shumate et al. (2020) explored the role of simulated solar radiation on the survival of COVID-19 virus dispersed in simulated saliva or culture medium (Vero cells ‘ATCC CCL-81’ cultured at 37 °C and under 5% CO2 in complete growth medium ‘gMEM’). A solar simulator was designed to produce natural sunlight, specifically in the range of ultraviolet. It was observed that solar radiation had a direct effect on survival of the virus with 90% of the infectious virus being inactivated in 6.8 min in simulated saliva under the simulated conditions; however, for culture medium, the time taken was around 14.3 min. Under all simulated conditions, the virus's inactivation rate was greater when dispersed in simulated saliva than in the culture medium. Fisher et al. (2011) examined the role of simulated solar radiation on the inactivation of a single-stranded RNA bacteriophage ‘MS2’ and a double-stranded DNA bacteriophage ‘PRD1’ in clear water (no exogenous sensitizers). It was observed that UVA (320–400 nm) and UV-B (280–320 nm) could inactivate ‘PRD1’; however ‘MS2’ was inactivated only by the UVB light. It is inferred from the above discussion that solar based disinfection is the most sustainable way of wastewater treatment as well as being a cost-effective approach, which has potential for treating contaminated water and deactivating the COVID-19 coronavirus.
5.3.2. Ozonation for wastewater disinfection
Ozone (O3) is an oxidizing agent that can effectively inactivate viruses by oxidative damage due to free radicals. As the viruses multiply only within their host cell, they transform host cell protein into their own protein. O3 inactivates the virus by diffusing through its protein coat into the nucleic acid core, leading to the viral RNA damage. Once O3 interacts with a virus, protein is converted into protein hydroxides and protein hydroperoxides, resulting in the creation of oxidative stress, against which viruses have no self-protection mechanisms. Recently, Tizaoui (2020) proposed that usage of O3 can be effective for SARS-CoV-2 virus as O3 can disorder the lipids and proteins of the virus's spikes. O3 acts on the cytoplasmic membrane through breaking the lipid molecules, thereby inactivating the virus (Kataki et al., 2020). In general, an initial dose of O3 (3–10 mg/L) with 10 min contact time demonstrates Ct values (the product of the concentration of the disinfectant and the contact time with the water being disinfected) of 30–100 mg/min, which has been suggested is the requirement for successful ozonation (Paraskeva and Graham 2002). Ozone is also considered as a significantly stronger disinfectant (10 times) than chlorine in wastewater treatment (Hajiali et al., 2018). Even after dissolving in water, it did not irritate skin, nor did it form a chemical film. It is a stronger disinfectant where the oxidation reaction takes place several time faster than chlorine to inactivate viruses, bacterial and water-borne pathogens. However, for wastewater treatment, ozonation's major issue is the increasing acidity level in the treated water (Zaied et al., 2020) and it needs further investigation.
5.3.3. Chemical based disinfectants for wastewater disinfection
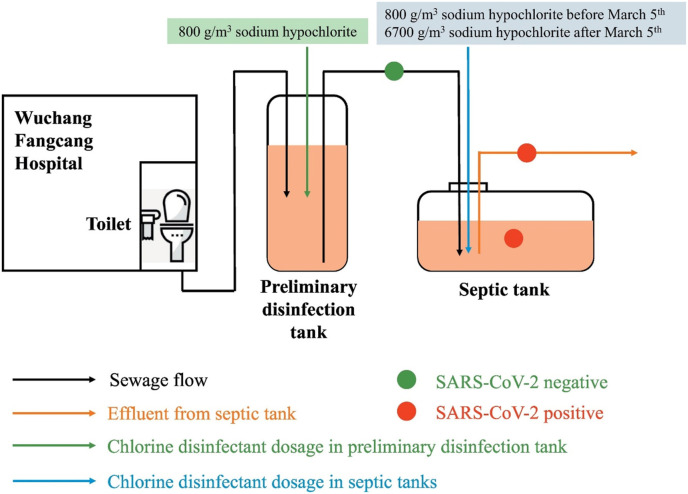
Chlorine-based disinfectants are widely used for water disinfection. Inactivation of microorganisms by chlorine is mainly governed by various factors such as the oxidation of sulfhydryl enzymes and amino acids, reduced nutrient uptake, loss of intracellular contents, reduced oxygen uptake, inhibited protein synthesis, and decreased ATP production. Various literature has shown the efficiency of chlorine towards the virus, but greater tolerance of the virus can be seen for chlorine disinfectants (compared to ozonation or solar assisted disinfection) owing to the absence of a metabolic enzyme system as compared to bacteria, which means that in viruses there are less targets upon which the chlorine can act. Previous research revealed that 0.2–0.5 mg/L of free chlorine residual is sufficient to disinfect the SARS virus in municipal wastewater (Wang et al., 2005). Engelbrecht et al. (1980) investigated the chlorine (0.1% available chlorine) effectiveness against six enteric viruses and revealed a broader range of susceptibility of viruses towards chlorine disinfection. pH is considered as the most important factor for achieving inactivation of viruses in wastewater; the deactivation rate is greater at lower pH (6) than at higher pH (10), yet also with a deviation in the relative sensitivity in respect of different viruses. pH is the regulating factor which controls the dissociation of hypochlorous acid to the less microbicidal form OCl−. With increasing pH, transformation of undissociated hypochlorous acid to OCl− takes place and the disinfecting ability of Cl− reduces. Therefore, at pH higher than 7, the time needed to achieve the same degree of inactivation increases, requiring from 1.5 to 6 fold longer (Clarke et al., 1956; Weidenkopf, 1958). Recently, Zhang et al. (2020) evaluated the existence of SARS-CoV-2 RNA in septic tanks of Wuchang Cabin Hospital, and Fig. 3 shows the schematic arrangement for the disinfection process of the septic tanks of hospital.
Fig. 3.
Schematic illustration of the disinfection process occurring in septic tanks of the Wuchang Cabin Hospital. Copyright 2020 Elsevier.
It was found that utilization of sodium hypochlorite for a contact period of 1.5 h at a dose of 800–6700 g/m3 effectively deactivated the SARS-CoV-2 in the hospital's septic tanks. It was also suggested to revise the present WHO recommended disinfection scheme (freely available chlorine ≥ 0.5 mg/L for at least 30 min) and the China Center for Disease Control and Prevention's current guidance (freely available chlorine above 6.5 mg/L for 1.5 h) in order to completely remove the COVID-19 viral RNA using a decentralized disinfection system. Kampf (2020) reported that usage of sodium hypochlorite (0.21%) solution could be highly efficient for 4 log reduction of COVID-19 in 1 min. Wang et al. (2005) illustrated that a free chlorine residual ‘0.2–0.5 mg/L’ in the municipal wastewater is enough to sterilize the SARS virus. Dellanno et al. (2009) showed a reduction of 3 log in surrogate of the coronavirus mouse hepatitis virus (MHV) using 0.21% sodium hypochlorite as a common disinfectant for a contact period of 30 s. Similarly, Ansaldi et al. (2004) found that utilization of 0.05% hypochlorite solution can completely inactivate the SARS-CoV with a contact time of ˂ 1 min.
Quaternary ammonium-based compounds, being eco-friendly disinfectants, are also recommended for wastewater treatment. For example, benzalkonium chloride (BKC), a quaternary ammonium compound, can be an effective disinfectant for water treatment. The hydrophilic cationic section of benzalkonium chloride generates electrostatic interfaces with a pathogen's surface (negatively charged components), leading to the destabilization of the germs (McDonnell and Russel, 1999). 1% benzalkonium chloride ‘1000 ppm’ was used by Ansaldi et al. (2004) for SARS-CoV, and outcomes showed that the virus lost viability after 30 min exposure. Rabenau et al. (2005) revealed that BKC inactivated SARS-CoV under the limit of detection with a reduction factor >4. However, owing to the restricted action of ammonium compounds with the viruses, it is required to use it in combination with other disinfectants to achieve optimal results. WHO also recommends peracetic acid (PAA) for virucide of SARS-CoV-2. It is reliable and possesses excellent disinfectant characteristics with extensive anti-microbicidal activity (Antonelli et al., 2013). Ansaldi et al. (2004) revealed that SARS-CoV-1 was disrupted using 35 ppm PAA with a contact period of <2 min, while there was no effect after 30 min with the same concentration and further investigation is thus needed. Although chemical-based disinfectants are preferred for wastewater treatment, their role in the deactivation of SARS-CoV-2 is less explored. In addition, optimization of the disinfectant concentration and their reduced efficiency in high organic loaded wastewater needs to be further explored.
5.4. Biological wastewater treatment
Biological wastewater treatment techniques rely on microorganisms’ (such as bacteria, algae or fungi) cellular activity under aerobic/anaerobic conditions in order to achieve the oxidation of the organic matter present in wastewater (Samer, 2015). Biological wastewater treatment methods include membrane bio-reactors, activated sludge, bio-chemical systems, biological contactors and anaerobic digesters. Since the majority of studies assessing the removal of viruses have concentrated on membrane bio-reactors and granular reactors, these are described in greater detail.
5.4.1. Membrane bio-reactors
These consist of a combined arrangement of membrane based filtration with a suspended-growth biological reactor. This approach is a suitable alternative method for achieving virus removal from wastewater owing to the excellent features like a reduced ecological footprint and high effluent quality (Marti et al., 2011). The principal mechanism of pathogenic bacteria removal is the process of size exclusion, whereas the mechanism of virus removal is less studied and not fully understood. Sepehri et al. (2018) highlighted that membrane fouling in membrane bio-reactors mainly depends on microbial cell density and their population structure. The authors concluded that suitable organic carbon to nitrogen (C/N) loading ratio could control the microbial population and benefit the nitrifiers, considerably mitigating the fouling. Various studies have highlighted the role of mixed liquor suspended solids and backwashed membranes in the inactivation of viruses (Xagoraraki et al., 2014; Miura et al., 2015). Da Silva et al. (2007) determined an LRV for norovirus of 5.2–5.5 in a membrane bio-reactor. Similarly, LRVs of 4.8, 6.3, and 6.8 were achieved in a membrane bio-reactor for noroviruses, adenoviruses, and enteroviruses, respectively (Simmons et al., 2011). In contrast, Zhou et al. (2015) concluded that complete removal of several viruses, including rotaviruses, noroviruses and enteroviruses could not be achieved by this method. This discrepancy could be attributed to the fact that the membrane bio-reactor method mainly focusses on physical removal of viruses, whereas the removal is greatly governed by the virus structure, mixed liquor suspended solids, solids and hydraulic retention time along with frequent cleaning of the membrane in order to achieve effective removal. In addition, this method is energy intensive, has a high operational cost, and requires proper disposal of the virus-contaminated sludge produced. The aforementioned drawbacks of this technology can be overcome by utilizing microalgae-based process either alone or coupled with the membrane technology to generate safe biologically treated water.
5.4.2. Microalgae-based wastewater treatment
The usage of macro/micro algae is gaining enormous attention in removal of pollutants including viruses from wastewater in recent years (Prajapati et al., 2014). Several researchers have studied the cultivation of microalgae in membrane bio-reactors, oxidation ponds and biofilm reactors to estimate their efficacy for wastewater disinfection. Recently, Delanka-Pedige et al. (2020) demonstrated that utilization of microalgae in wastewater treatment through employment of extremophile Galdieria sulphuraria leads to high removal rates of noroviruses (1.49 ± 0.16) and enteroviruses (1.05 ± 0.32). Scalable and sustainable filter paper made from Pithophora cellulose were studied for drinking water purification purposes. Results showed that all types of bacterial and infectious viruses were successfully removed from sample water by this filter paper. Sepehri et al. (2020) demonstrated that the aeration system in conventional nitrification processes can be substituted by a microalgae based cleaning process which will result in less metabolite generation, improved carbon capture, augmented nutrient removal, and decreased sludge production. Similarly, Sepehri et al. (2019) found that a nitratation intensification strategy and nitrite-oxidizing bacterial enrichment using a zero C/N ratio reduced microbial metabolites by 50% as compared to the conventional process and improved the nitrification efficacy in the activated sludge involved process. This improved efficiency should also lead to increased effectiveness of viral deactivation.
5.5. Large scale community wise monitoring and testing of COVID-19 RNA in wastewater
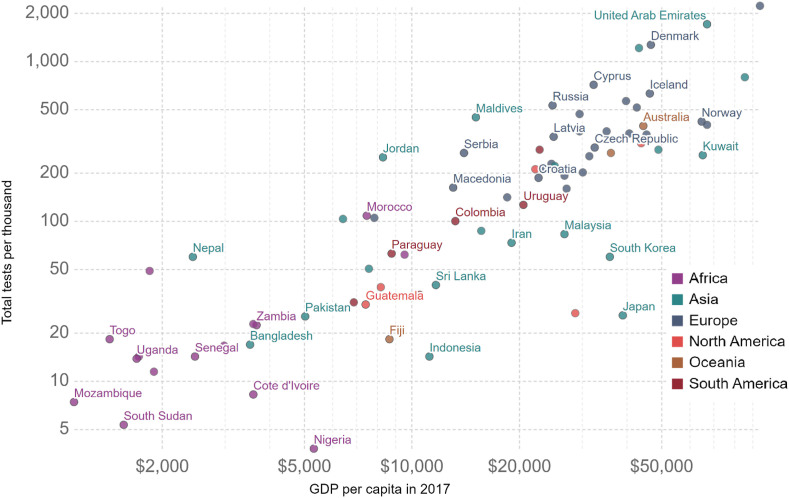
The countries with lower income, determined according to GDP per capita, have in general conducted lower testing for COVID-19 compared to the developed nations, as shown in Fig. 4 (till December 2nd, 2020). There is presently a substantial gap in COVID-19 testing in various low-income nations, with only 779,708 persons tested so far in Nigeria as of December 4, 2020, out of about 200 million population, which is Africa's most populated country (NCDC, 2020). Unfortunately, the transmission of the Covid-19 virus in these nations has been ascribed to the incompetence of quickly detecting the infected people before the virus transmits to others and thus spreads the COVID-19 virus (Mehtar et al., 2020). As the initial identification of the virus could be made through feces (Orive et al., 2020) rapid testing and monitoring of the virus in the municipal/societal wastewater might be an effective technique to control the spread. This method will be more suitable for low-income countries where the virus testing in communities is still limited. Initial surveillance should be done for the pervasiveness of the COVID-19 infection in the populace by observing the abundance of COVID-19 virus in wastewater, and then, the currently applied inspection of symptomatic and/or likely exposed individuals should be carried out for episodic analysis. Recently, Daughton (2020) emphasized the significance of large-scale community wide testing as an economical method for monitoring the status and development of COVID-19 infections. Moreover, improved water quality and adequate sanitation are also essential to effectively prevent the unexpected spread of the COVID-19 and other potential human enteric related viruses that might originate from an infected person's feces.
Fig. 4.
Total COVID-19 tests per 1000 population vs. GDP per capita (Our world in data, 2020).
6. Viewpoint and conclusion
The present effort of public health experts and medical professionals dealing with the COVID-19 pandemic is understandably focused on controlling its direct human-to-human spread and the care of infected individuals. Nevertheless, the potential spread of the virus through secondary transmission must not be underrated. Evidence for the existence of COVID-19 viral RNA in wastewater systems is seen globally, and the risks associated with waterborne transmission should be considered as severe. This needs to be quickly evaluated, especially in low-income countries where higher population density, poor sanitation infrastructure, lack of appropriate wastewater treatment facilities and direct exposure to aerosolized wastewater are major concerns, and may damage the hard-won achievements of the present control measures to reduce individual contacts, leading to a huge spike in COVID-19 cases. Various studies have confirmed the existence of viruses at sewage plants, however, there is no data related to the effectiveness of current disinfection approaches as utilized on real wastewater in the treatment facilities against Covid-19. Therefore, extensive research should be carried out urgently to identify the prevalence of SARS-CoV-2 viral particles in wastewater in order to gain the crucial information related to the virus's abundance in raw and treated wastewater, in order to (1) evaluate the effectiveness of existing disinfection methods for inactivation of SARS-COV-2 and where additional disinfection regimes are needed temporarily to deal with this new challenge; (2) ensure the reduction of potential secondary exposures by appropriately treating wastewater, and ensuring effluents are virus-free; and (3) facilitate monitoring and early-warning of potential hot-spots of infection, enabling local preventative responses to be implemented in a timely manner. Further, the requirement for disinfectants and application regimes should be evaluated according to the loading of the virus. Thus, surveillance should be a core aspect of policymaking and wasterwater treatment modalities in order to effectively monitor and control the spread of SARS-CoV-2 infections in society.
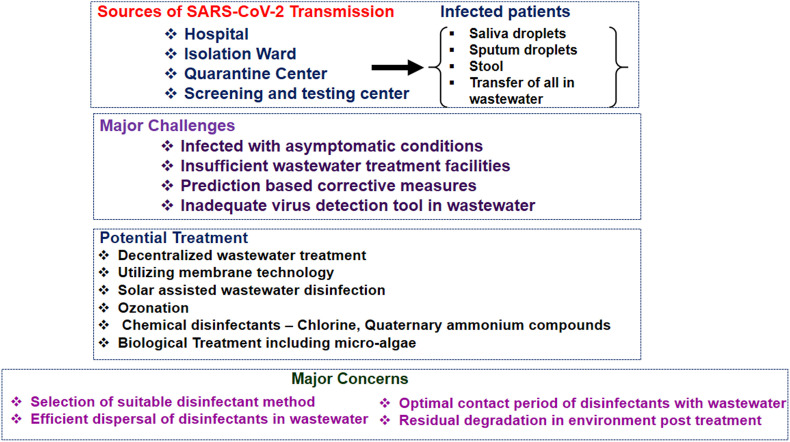
To minimize human exposure to the Covid-19 virus via waterborne transmission, contaminated wastewater from isolation wards, hospitals, testing centers, and quarantine centers should be disinfected and treated correctly before being discharged into the main sewerage systems. In low-income countries with inadequate centralized wastewater plants, decentralized wastewater treatment with solar energy utilization can be incorporated to efficiently inactivate the virus locally. Fig. 5 presents a summary of the major considerations for determination of the optimal treatment to implement locally depending on local conditions (e.g., UV availability, volume or wastewater to be treated, current waste infrastructure capabilities etc.).
Fig. 5.
Various potential wastewater treatment strategies and concerns during Covid-19 pandemic.
In tropical countries like India, where there is abundant solar energy availability, a solar-based disinfectant solution can be a viable option for wastewater treatment. Simple wastewater treatments such as wetlands, ponds, or lagoons could be a superior choice for viral inactivation under the joint effect of solar radiation, comparatively long retention time, high pH, and microbial action. The usage of chemical disinfectants such as the widely available chlorine, sodium hypochlorite, benzalkonium chloride, peracetic acid etc. have shown potential in terms of virucidal properties. Biological treatments including microalgae can be a viable solution for viral removal from wastewater. Fig. 5 presents the different strategies towards the treatment of wastewater and some major concerns that must be considered during the selection of the most appropriate treatment in the view of the SARS-CoV-2 virus.
Nevertheless, further technical evidence is needed to confirm the effectiveness of viral disinfection policies in wastewater, and the impact of different viral loadings on the treatment efficiency. In conclusion, there is a pressing need for improved monitoring and risk assessment along with the implementation of management policies for controlling the spread of COVID-19 via wastewater. To effectively control the spread of the novel coronavirus, policymakers should emphasize systematic testing of the disinfectants' efficiency and concentration ranges under different environmental conditions (e.g., different organic loadings, different water quality scenarios, etc.). Beyond SARS-CoV-2 infections, these methods will also be helpful in improving the identification, response, and inactivation of future viral disease outbreaks, and indeed in controlling other enteric viruses responsible for diarrhea and other intestinal conditions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Adelodun Bashir, Odedishemi Ajibade Fidelis, Gbemisola Ibrahim Rahmat, Olalekan Bakare Hashim, Choi Kyung-Sook. Snowballing transmission of COVID-19 (SARS-CoV-2) through wastewater: any sustainable preventive measures to curtail the scourge in low-income countries? Sci. Total Environ. 2020:140680. doi: 10.1016/j.scitotenv.2020.140680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albatanony M.A., El-Shafie M.K. Work-related health effects among wastewater treatment plants workers. Int. J. Occup. Environ. Med. 2011;2(4):237–244. [PubMed] [Google Scholar]
- Ansaldi F., Banfi F., Morelli P., Valle L., Durando P., Sticchi L., Contos S., Gasparin R., Crovari P. SARS CoV, influenza A and syncitial respiratory virus resistance against common disinfectants and ultraviolet irradiation. J. Prev. Med. Hyg. 2004;45:5–8. [Google Scholar]
- Antonelli M., Turolla A., Mezzanotte V., Nurizzo C. Peracetic acid for secondary effluent disinfection: a comprehensive performance assessment. Water Sci. Technol. 2013;68:2638–2644. doi: 10.2166/wst.2013.542. [DOI] [PubMed] [Google Scholar]
- Barker S.F. Risk of norovirus gastroenteritis from consumption of vegetables irrigated with highly treated municipal wastewater-evaluation of methods to estimate sewage quality. Risk Anal. 2014;34:803–817. doi: 10.1111/risa.12138. [DOI] [PubMed] [Google Scholar]
- Bodzek M., Konieczny K., Rajca M. Membranes in water and wastewater disinfection – review. Arch. Environ. Protect. 2019;45:3–18. [Google Scholar]
- Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (covid-19) with myocardial injury and mortality. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- Chaudhry R.M., Nelson K.L., Drewes J.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49:2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen L., Deng Q., Zhang G., Wu K., Ni L., Yang Y., Liu B., Wang W., Wei C., Yang J., Ye G., Cheng Z. The presence of SARS-CoV-2 RNA in feces of COVID-19 patients. J. Med. Virol. 2020;92:833–840. doi: 10.1002/jmv.25825. [DOI] [PubMed] [Google Scholar]
- Clarke N.A., Stevenson R.E., Kabler P.W. The inactivation of purified type 3 adenovirus in water by chlorine. Am. J. Hyg. 1956;64:314–319. doi: 10.1093/oxfordjournals.aje.a119844. [DOI] [PubMed] [Google Scholar]
- Crini G., Lichtfouse E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019;17:145–155. [Google Scholar]
- Da Silva A.K., Le Saux J.C., Parnaudeau S., Pommepuy M., Elimelech M., Le Guyader F.S. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl. Environ. Microbiol. 2007;73 doi: 10.1128/AEM.01428-07. 7891–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva A.K., Le Guyader F.S., Le Saux J.C., Pommepuy M., Montgomery M.A., Elimelech M. Norovirus removal and particle association in a waste stabilization pond. Environ. Sci. Technol. 2008;42:9151–9157. doi: 10.1021/es802787v. [DOI] [PubMed] [Google Scholar]
- Danchin A., Wai Ng P.T., Turinic G. A new transmission route for the propagation of the SARS-CoV-2 coronavirus. Biology. 2021;1:10. doi: 10.3390/biology10010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. The international imperative to rapidly and inexpensively monitor community-wide Covid-19 infection status and trends. Sci. Total Environ. 2020;726:138149. doi: 10.1016/j.scitotenv.2020.138149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanka-Pedige H.M.K., Cheng X., Munasinghe-Arachchige S.P., Abeysiriwardana-Arachchige I.S.A., Xu J., Nirmalakhandan N., Zhang Y. Metagenomic insights into virus removal performance of an algal-based wastewater treatment system utilizing Galdieria sulphuraria. Algal Res. 2020;47:101865. doi: 10.1016/j.algal.2020.101865. [DOI] [Google Scholar]
- Dellanno C., Vega Q., Boesenberg D. The antiviral action of common household disinfectants and antiseptics against murine hepatitis virus, a potential surrogate for SARS coronavirus. Americ. J. infec. Con. 2009;37:649–652. doi: 10.1016/j.ajic.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhama K., Patel S.K., Yatoo M.I., Tiwari R., Khan S., Dhama J., Natesan S., Malik Y.S., Singh K.P., Harapan H. SARS-CoV-2 existence in sewage and wastewater: a global public health concern. J. Environ. Manag. 2021;280:111825. doi: 10.1016/j.jenvman.2020.111825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht R.S., Weber M.J., Salter B.L., Schmidt C.A. Comparative inactivation of viruses by chlorine. Appl. Environ. Microbiol. 1980;40(2):249–256. doi: 10.1128/aem.40.2.249-256.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M.B., Love D.C., Schuech R., Nelson K.L. Simulated sunlight action spectra for inactivation of MS2 and PRD1 bacteriophages in clear water. Environ. Sci. Technol. 2011;45:9249–9255. doi: 10.1021/es201875x. [DOI] [PubMed] [Google Scholar]
- Fu C.Y., Xie X., Huang J.J., Zhang T., Wu Q.Y., Chen J.N., Hu H.Y. Monitoring and evaluation of removal of pathogens at municipal wastewater treatment plants. Water Sci. Technol. 2010;61:1589–1599. doi: 10.2166/wst.2010.757. [DOI] [PubMed] [Google Scholar]
- García-Martínez D., Torres-Tamayo N., Torres-Sanchez I., García-Río F., Bastir M. Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am. J. Phys. Anthropol. 2016;161:467–477. doi: 10.1002/ajpa.23051. [DOI] [PubMed] [Google Scholar]
- Gholipour S., Mohammadi F., Nikaeen M., Shamsizadeh Z., Khazeni A., Sahbaei Z., Mousavi S.M., Ghobadian M., Mirhendi H. COVID-19 infection risk from exposure to aerosols of wastewater treatment plants. Chemosphere. 2021;273:129701. doi: 10.1016/j.chemosphere.2021.129701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G. Potential role of inanimate surfaces for the spread of coronaviruses and their inactivation with disinfectant agents. Infect. Prev. Pract. 2020;2:100044. doi: 10.1016/j.infpip.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Iker B.C., Pepper I.L., Gerba C.P. Relative abundance and treatment reduction of viruses during wastewater treatment processes - identification of potential viral indicators. Sci. Total Environ. 2014;488–489:290–296. doi: 10.1016/j.scitotenv.2014.04.087. [DOI] [PubMed] [Google Scholar]
- Kitajima M., Sassi H.P., Torrey J.R. Pepper mild mottle virus as a water quality indicator. npj Clean Water. 2018;1:19. [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Kumar Patel A., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Thakur A., Kumar Pathak S. Single basin solar still with varying depth of water: optimization by computational method. Iran. J. Energy Environ. 2017;8(3):216–223. [Google Scholar]
- Lindsley W.G., Pearce T.A., Hudnall J.B., Davis K.A., Davis S.M., Fisher M.A., Khakoo R., Palmer J.E., Clark K.E., Celik I., Coffey C.C., Blachere F.M., Beezhold D.H. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J. Occup. Environ. Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H., Wu F., Song Z.H., Huang W., Chen J., Hu BiJ., Wang S., Mao E.Q., Zhu L., Zhang W.H., Lu H.Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. 2020;9:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. lancet. Gastroenterol. Hepatol. 2020;1253:30087. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M.T., Martinko J.M., Parker J., Brock T.D. thirteenth ed. Pearson; London: 2012. Brock Biology of Microorganisms. [Google Scholar]
- Marti E., Monclús H., Jofre J., Rodriguez-Roda I., Comas J., Balc'azar J.L. Removal of microbial indicators from municipal wastewater by a membrane bioreactor (MBR) Bioresour. Technol. 2011;102:5004–5009. doi: 10.1016/j.biortech.2011.01.068. [DOI] [PubMed] [Google Scholar]
- Matto M., Singhal S. 2020. COVID-19: the Need Is to Decentralise How We Manage Wastewater.https://www.downtoearth.org.in/blog/water/covid-19-the-need-is-to-decentralise-how-we-manage-wastewater-70991 Available at: [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS Coronavirus-2 in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Mehtar S., Preiser W., Lakhe N.A., Bousso A., TamFum J.-J.M., Kallay O., Seydi M., Zumla A., Nachega J.B. Limiting the spread of COVID-19 in Africa: one size mitigation strategies do not fit all countries. Lancet Glob. Heal. 2020:2019–2021. doi: 10.1016/S2214-109X(20)30212-6. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T., Okabe S., Nakahara Y., Sano D. Removal properties of human enteric viruses in a pilot-scale membrane bioreactor (MBR) process. Water Res. 2015;75:282–291. doi: 10.1016/j.watres.2015.02.046. [DOI] [PubMed] [Google Scholar]
- Moe C.L., Izurieta R. Proceedings of the Second International Symposium on Ecological Sanitation, Lubeck, Germany. 2003. Longitudinal study of double vault urine diverting toilets and solar toilets in El Salvador; pp. 295–302. [Google Scholar]
- Mohammed M.A.R., Halagy D.A.E. Studying the factors affecting the settling velocity of solid particles in non-Newtonian fluids. Al-Nahrain J. Eng. Sci. 2013;16:41–50. [Google Scholar]
- Naddeo V., Liu H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Water Res. Technol. Environ. Sci. 2020;6:1213–1216. [Google Scholar]
- NCDC . 2020. Nigeria Centre for Disease Control on Coronavirus COVID-19 Update 2020.https://covid19.ncdc.gov.ng/ [Google Scholar]
- Nelson K.L., Boehm A.B., Davies-Colley R.J., Dodd M.C., Kohn T., Linden K.G., Nguyen T.H., Parker K.M., Rodriguez R.A., Sassoubre L.M., Silverman A.I., Wigginton K.R., Zepp R.G. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Environ. Sci. Processes Impacts. 2018;20:1089–1122. doi: 10.1039/c8em00047f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemudryi A., Nemudraiam A., Surya K., Wiegand T., Buyukyoruk M., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARSCoV- 2 in municipal wastewater. Cell Rep Med. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nghiem L.D., Morgan B., Donner E., Short M.D. The COVID-19 pandemic: considerations for the waste and wastewater services sector. Case Studies in Chem. Environ. Eng. 2020:100006. doi: 10.1016/j.cscee.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omosa I.B., Wang H., Cheng S., Li F. Sustainable tertiary wastewater treatment is required for water resources pollution control in Africa. Environ. Sci. Technol. 2012;46:7065–7066. doi: 10.1021/es3022254. [DOI] [PubMed] [Google Scholar]
- Orive G., Lertxundi U., Barcelo D. Early SARS-CoV-2 outbreak detection by sewage-based epidemiology. Sci. Total Environ. 2020:183135. doi: 10.1016/j.scitotenv.2020.139298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our world in data 2020. https://ourworldindata.org/grapher/tests-of-covid-19-per-thousand-people-vs-gdp-per capita?.Tab=chart&stackMode=absolute&time=latest&country=®ion=World
- Pan X., Chen D., Xia Y. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskeva P., Graham N.J. Ozonation of municipal wastewater effluents. Water Environ. Res. 2002;74:569–581. doi: 10.2175/106143002x140387. [DOI] [PubMed] [Google Scholar]
- Pendergast M.M., Hoek E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011;4:1946–1971. [Google Scholar]
- Prajapati S.K., Choudhary P., Malik A., Vijay V.K. Algae mediated treatment and bioenergy generation process for handling liquid and solid waste from dairy cattle farm. Bioresour. Technol. 2014;167:260–268. doi: 10.1016/j.biortech.2014.06.038. [DOI] [PubMed] [Google Scholar]
- Rabenau H., Kampf G., Cinatl J., Doerr H. Efficacy of various disinfectants against SARS coronavirus. J. Hosp. Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas Ferrando E., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnesar-Shumate S., Williams G., Green B., Krause M., Holland B., Wood S., Bohannon J., Boydston J., Freeburger D., Hooper I., Beck K. Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J. Infect. Dis. 2020;222:214–222. doi: 10.1093/infdis/jiaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti J.L., Lytle C.D. Estimated inactivation of coronaviruses by solar radiation with special reference to COVID‐19. Photochem. Photobiol. 2020;96:731–737. doi: 10.1111/php.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin A.H., Aysegul E., Pelin M.A., Yeliz D., Ahmet Y.C., Mahmut E.S., Ramazan A.O., Ali Muhittin T. 2019 novel coronavirus (COVID-19) outbreak: a review of the current of the current literature. EJMO. 2020;4(1):1–7. [Google Scholar]
- Samer M. Wastewater Treat. Eng., InTech; 2015. Biological and Chemical Wastewater Treatment Processes. [DOI] [Google Scholar]
- Schoen M.E., Ashbolt N.J. An in-premise model for Legionella exposure during showering events. Water Res. 2011;45:5826–5836. doi: 10.1016/j.watres.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Sepehri A., Sarrafzadeh M.-H., Avateffazeli Effect of nitrifiers community on fouling mitigation and nitrification efficiency in a membrane bioreactor. Chem Eng Process CHEM ENG PROCESS. 2018;128:10–18. [Google Scholar]
- Sepehri A., Sarrafzadeh M.-H., Avateffazeli Activity enhancement of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria in activated sludge process: metabolite reduction and CO2 mitigation intensification process. Appl. Water Sci. 2019;9:131. [Google Scholar]
- Sepehri A., Sarrafzadeh M.-H., Avateffazeli M. Interaction between Chlorella vulgaris and nitrifying-enriched activated sludge in the treatment of wastewater with low C/N. J. Clean. Prod. 2020;247:119164. [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin G.A., Sobsey M.D. Removal of norovirus from water by coagulation, flocculation and sedimentation processes. Water Sci. Technol. Water Supply. 2015;15:158–163. [Google Scholar]
- Simmons F., Kuo D., Xagoraraki I. Removal of human enteric viruses by a fullscale membrane bioreactor during municipal wastewater processing. Water Res. 2011;45(9):2739–2750. doi: 10.1016/j.watres.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Suthar S., Das S., Nagpure A., Madhurantakam C., Tiwari S.B., Gahlot P., Tyagi V.K. Epidemiology and diagnosis, environmental resources quality and socio-economic perspectives for COVID-19 pandemic. J. Environ. Manag. 2021;280:111700. doi: 10.1016/j.jenvman.2020.111700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A., Tong Z., Wang H., Dai Y., Li K., Liu J., Wu W., Yuan C., Yu M., Li P., Yan J. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg. Inf. Disp. J. 2020;26:1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A.K., Chandramohan V.P. Advances in Energy Research. second ed. Springer; Singapore: 2020. Productivity enhancement of passive type solar still using copper and aluminum based absorber plate with Al2O3 nanofluid in water basin; pp. 273–281. [Google Scholar]
- Thakur A.K., Agarwal D., Khandelwal P., Dev S. Springer; 2018. Comparative Study and Yield Productivity of Nano-Paint and Nano-Fluid Used in a Passive-type Single Basin Solar Still, Advances in Smart Grid and Renewable Energy; pp. 709–716. [Google Scholar]
- Thakur A.K., Khandelwal P., Sharma B. In: Nanotechnology for Energy and Water. ICNEW 2017. Springer. Proceedings in Energy. Anand G., Pandey J., Rana S., editors. Springer; Cham: 2018. Productivity comparison of solar still with nano fluid and phase changing material with same depth of water. [DOI] [Google Scholar]
- Thakur A.K., Vikrama M.P., Christopher S. In: Advances in Greener Energy Technologies. Green Energy and Technology. Bhoi A., Sherpa K., Kalam A., Chae G.S., editors. Springer; Singapore: 2020. Augmented yield productivity of solar still using energy storage materials: experimental investigation under the climatic conditions of Rajasthan. [DOI] [Google Scholar]
- Thakur A.K., Sathyamurthy R., Sharshir W.S., Ahmed M.S., Hwang J.Y. A novel reduced graphene oxide based absorber for augmenting the water yield and thermal performance of solar desalination unit. Mater. Lett. 2021;286:128867. [Google Scholar]
- Thakur A.K., Sharshir S.W., Ma Z., Thirugnanasambantham A., Christopher S.S., Vikram M.P., Li S., Wang P., Zhao W., Kabeel A.E. Performance amelioration of single basin solar still integrated with V- type concentrator: energy, exergy, and economic analysis. Environ. Sci. Pollut. Res. 2021;28:3406–3420. doi: 10.1007/s11356-020-10625-2. [DOI] [PubMed] [Google Scholar]
- Thakur A.K., Sathyamurthy R., Sharshir W.S., Kabeel A.E., Elkadeem Ma Z., Manokar A.M., Arıcı M., Pandey A.K., Saidur R. Performance analysis of a modified solar still using reduced graphene oxide coated absorber plate with activated carbon pellet. Sustain. Energy Technol. Assess. 2021;45:101046. [Google Scholar]
- Thakur A.K., Sathyamurthy R., Sharshir S.W., Kabeel A.E., Manokar A.M., Zhao W. An experimental investigation of a water desalination unit using different microparticle-coated absorber plate: yield, thermal, economic, and environmental assessments. Environ. Sci. Pollut. Res. Int. 2021 doi: 10.1007/s11356-021-12837-6. [DOI] [PubMed] [Google Scholar]
- Tizaoui C. Ozone: a potential oxidant for COVID-19 virus (SARS-CoV-2) Ozone Sci. Eng. 2020;42:378–385. [Google Scholar]
- USEPA . Final Report; Washington, DC: 2011. Exposure Factors Handbook 2011 Edition. [Google Scholar]
- Venugopal A., Ganesan H., Sudalaimuthu Raja S.S., Govindasamy V., Arunachalam M., Narayanasamy A., Sivaprakash P., Rahman P.K.S.M., Gopalakrishnan A.V., Siama Z., Vellingiri B. Novel wastewater surveillance strategy for early detection of coronavirus disease 2019 hotspots. Curr. Opin. Environ. Sci. Heal. 2020;17:8–13. doi: 10.1016/j.coesh.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbyla M.E., Mihelcic J.R. A review of virus removal in wastewater treatment pond systems. Water Res. 2015;71:107–124. doi: 10.1016/j.watres.2014.12.031. [DOI] [PubMed] [Google Scholar]
- Wang X.W., Li J.S., Jin M., Zhen B., Kong Q.X., Song N., Xiao W.J., Yin J., Wei W., Wang G.J., Si B.Y., Guo B.Z., Liu C., Ou G.R., Wang M.N., Fang T.Y., Chao F.H., Li J.W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Bartrand T.A., Weir M.H., Omura T., Haas C.N. Development of a dose-response model for SARS coronavirus. Risk Anal. 2010;30:1129–1138. doi: 10.1111/j.1539-6924.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenkopf S.J. Inactivation of type I poliomyelites virus with chlorine. Virol. 1958;5:56–67. doi: 10.1016/0042-6822(58)90005-9. [DOI] [PubMed] [Google Scholar]
- Wigginton K.R., Ye Y., Ellenberg R.M. Emerging investigators series: the source and fate of pandemic viruses in the urban water cycle. Environ. Sci. Water Res. Technol. 2015;1:735–746. [Google Scholar]
- Wilkinson D.M., Koumoutsaris S., Mitchell E.A., Bey I. Modelling the effect of size on the aerial dispersal of microorganisms. J. Biogeogr. 2012;39:89–97. [Google Scholar]
- World Health Organization Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipcprecautionrecommendations
- Worldometer graph, https://www.worldometers.info/coronavirus/worldwide-graphs/(accessed as on 5 December 2020).
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao Q., Tang Y., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shan H., Jiang G., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5:434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xagoraraki I., Yin Z., Svambayev Z. Fate of viruses in water systems. J. Environ. Eng. 2014;140 [Google Scholar]
- Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Shin W.I., Pang Y.X., Meng Y., Lai J., You C., Zhao H., Lester E., Wu T., Pang C.H. The first 75 Days of novel coronavirus (SARS-CoV-2) outbreak: recent advances, prevention, and treatment. Inter. Environ. Res. Public Health. 2020;17:2323. doi: 10.3390/ijerph17072323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaied B.K., Rashid M., Nasrullah M., Zularisam A.W., Pant D., Singh L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020:138095. doi: 10.1016/j.scitotenv.2020.138095. [DOI] [PubMed] [Google Scholar]
- Zaneti R.N., Girardi V., Spilki F.R., Mena K., Westphalen A.P.C., da Costa Colares E.R., Pozzebon A.G., Etchepare R.G. Quantitative microbial risk assessment of SARS-CoV-2 for workers in wastewater treatment plants. Sci. Total Environ. 2021;754:142163. doi: 10.1016/j.scitotenv.2020.142163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of fangcang hospital. Sci. Total Environ. 2020:140445. doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Wang X.C., Ji Z., Xu L., Yu Z. Source identification of bacterial and viral pathogens and their survival/fading in the process of wastewater treatment, reclamation, and environmental reuse. World J. Microbiol. Biotechnol. 2015;31:109–120. doi: 10.1007/s11274-014-1770-5. [DOI] [PubMed] [Google Scholar]