Abstract
Polyphenolics and 1,3,4-oxadiazoles are two of the most potent bioactive classes of compounds in medicinal chemistry, since both are known for their diverse pharmacological activities in humans. One of their prominent activities is the antimicrobial/antiviral activities, which are much apparent when the key functional structural moieties of both of them meet into the same compounds. The current COVID-19 pandemic motivated us to computationally screen and evaluate our library of previously-synthesized 2-(3,4,5-trihydroxyphenyl)-1,3,4-oxadiazoles against the major SARS-CoV-2 protein targets. Interestingly, few ligands showed promising low binding free energies (potent inhibitory interactions/affinities) with the active sites of some coronaviral-2 enzymes, specially the RNA-dependent RNA polymerase (nCoV-RdRp). One of them was 5,5'-{5,5'-[(1R,2R)-1,2-dihydroxyethane-1,2-diyl]bis(1,3,4-oxadiazole-5,2-diyl)}dibenzene-1,2,3-triol (Taroxaz-104), which showed significantly low binding energies (−10.60 and −9.10 kcal/mol) with nCoV-RdRp-RNA and nCoV-RdRp alone, respectively. These binding energies are even considerably lower than those of remdesivir potent active metabolite GS-443902 (which showed −9.20 and −7.90 kcal/mol with the same targets, respectively). Further computational molecular investigation revealed that Taroxaz-104 molecule strongly inhibits one of the potential active sites of nCoV-RdRp (the one with which GS-443902 molecule mainly interacts), since it interacts with at least seven major active amino acid residues of its predicted pocket. The successful repurposing of Taroxaz-104 has been achieved after the promising results of the anti-COVID-19 biological assay were obtained, as the data showed that Taroxaz-104 exhibited very significant anti-COVID-19 activities (anti-SARS-CoV-2 EC50 = 0.42 μM) with interesting effectiveness against the new strains/variants of SARS-CoV-2. Further investigations for the development of Taroxaz-104 and its coming polyphenolic 2,5-disubstituted-1,3,4-oxadiazole derivatives as anti-COVID-19 drugs, through in vivo bioevaluations and clinical trials research, are urgently needed.
Keywords: Anti-VOC-202012/01 agent; Anti-COVID-19 drug; SARS-CoV-2 strain/variant/lineage/mutant; Coronavirus RNA-dependent RNA polymerase (RdRp); Polyphenolic 2,5-disubstituted-1,3,4-oxadiazole compound; Taroxaz-104 molecule
Graphical abstract
1. Introduction
More than one year ago, a strange novel coronavirus (2019-nCoV), officially known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), suddenly appeared in Wuhan City of the People's Republic of China [1]. With its ongoing spread, the human respiratory signs/symptoms of the characteristic infection (called coronavirus disease 2019 "COVID-19") were detected and the pandemic was arisen [1,2]. Few weeks ago, at the end of December 2020, more than 85 millions of COVID-19 patients have been confirmed worldwide, with about 1.85 millions of lives sadly lost due to this untreated horrible disease [3]. With expectations of evolution of more virulent/resistant new strains in 2021, the scientific community all over the world is racing time in a challenging mission to find effective potent remedies and therapies capable of combating this virus or, at least, counter almost all the irritating severe/fatal effects of the COVID-19 [2,4].
In the second half of 2020, the first three under-investigation synthetic anti-COVID-19 agents (CoViTris2020, ChloViD2020, and Cyanorona-20) have been successfully discovered and reported [5,6] (Fig. 1 ). CoViTris2020 and ChloViD2020 are the first multitarget anti-SARS-CoV-2 inhibitors in the literature [5]. Both compounds are strong polyphenolic antioxidants with very potent anti-SARS-CoV-2 activities (EC50 = 0.31 and 1.01 μM, respectively) [5]. Specifically, CoViTris2020 has its lowest binding energy with the coronaviral-2 RNA-dependent RNA polymerase (nCoV-RdRp), which is −12.00 kcal/mol [5]. On the other hand, Cyanorona-20 is a very potent monotarget anti-SARS-CoV-2 inhibitor, since it selectively targets the nCoV-RdRp [6]. Cyanorona-20 is the 4-cyanobenzylidene derivative of the antiviral favipiravir at the amino group with extremely-enhanced anti-COVID-19 activities (anti-SARS-CoV-2 EC50 = 0.45 μM), and it was designed to act mainly as a prodrug (like favipiravir and remdesivir) which is metabolized inside the human cells to its active nucleotide triphosphate form Cyanorona-20-ribofuranosyl-5'-triphosphate (Cyanorona-20-RTP) [6]. Medicinal chemists and scientists consider Cyanorona-20 as the first bioactive anti-SARS-CoV-2 derivative based on the favipiravir scaffold.
Fig. 1.
Chemical structures of the under-investigation newly-discovered anti-COVID-19 drugs (CoViTris2020, ChloViD2020, and Cyanorona-20).
Drug repurposing/repositioning is a tactic or trend which uses a known compound or an existing drug (i.e., gives a known molecule a second or new opportunity) to treat a challenging, rare, resistant, or difficult-to-treat disease. This trend is considered as a substitutional drug development plan or strategy that can interestingly be implemented in a reduced/shorter time interval (i.e., less time-consuming) and with minimized expenses (i.e., more cost-effective) as compared to the conventional standard method of developing any new medication [7]. Moreover, as predominant pathogeneses and molecular pathways (e.g., oxidative stress and inflammatory conditions) largely contribute to almost all diseases and disorders, there are great chances in screening and investigating the possibility of known compound repurposing as a therapeutic choice [8,9]. Drug repurposing against SARS-CoV-2 is having a noticeable role in the therapeutic solutions for the current pandemic [10], since some known drugs, e.g., remdesivir, ivermectin, favipiravir, hydroxychloroquine, and arbidol, were suggested to have some potencies and efficacies against this resistant virus [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22]. Making use of this compound repurposing strategy in an effort to help in fighting the COVID-19 pandemic, we have computationally screened and examined the compounds of our small libraries (specially those previously bioevaluated as effective or potent antioxidant, anti-inflammatory, antiviral, and/or antimicrobial agents) [23,24] against almost all the target COVID-19 enzymes, receptors, and proteins. Until mid-2020, more than 181 SARS-CoV-2 protein structures were completely identified and released in the Protein Data Bank (PDB), which are primarily related to about eleven diverse types of the novel coronaviral-2 proteins. Surprisingly, this screening and reevaluation gave rise to the discovery of mainly three potent anti-SARS-CoV-2 agents of the polyphenolic 2,5-disubstituted-1,3,4-oxadiazole type, CoViTris2020/ChloViD2020 (which were previously reported [5]) and Taroxaz-104 (which is reported in this current research paper) (Fig. 2 ), which have three, one, and two 3,4,5-trihydroxyphenyl/1,3,4-oxadiazole group(s), respectively.
Fig. 2.
Chemical structure of the anti-COVID-19 drug candidate Taroxaz-104.
Taroxaz-104 (the word “tar” stands for (2R,3R)-(+)-tartaric acid, the carboxylic acid from which the compound is derived or synthesized; the word “oxaz” stands for the 1,3,4-oxadiazole ring or the oxygen/nitrogen atoms present in the compound; and the number “104” stands for the number of the oxygen and nitrogen atoms present in the compound, which are 10 and 4, respectively) is a polyphenolic molecule (chemically it is 5,5'-{5,5'-[(1R,2R)-1,2-dihydroxyethane-1,2-diyl]bis(1,3,4-oxadiazole-5,2-diyl)}dibenzene-1,2,3-triol) that was previously synthesized and bioevaluated as effective antioxidant compound more than four years ago [23] (Fig. 3 ). It is worth noticing that Taroxaz-104 molecule could be seen as a dimer of the typical 2,5-disubstituted-1,3,4-oxadiazole monomer 5-[5-(hydroxymethyl)-1,3,4-oxadiazol-2-yl]benzene-1,2,3-triol. Polyphenols are one of the most bioactive phytochemical species, since they have potent diverse biological activities, e.g., antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, antiviral, and recently in vitro anticoronaviral-2 activities [23, 24, 25, 26, 27, 28, 29, 30, 31]. Polyphenols potentially inhibit all the steps of the coronaviral-2 life cycle, e.g., prevent binding of SARS-CoV-2 spike (S) protein to human cell membrane enzyme/receptor angiotensin-converting enzyme 2 (ACE2), hinder viral entry into the human cell, block viral RNA replication/transcription, and disturb protein processing [25, 26, 27, 28, 29, 30, 31]. Interestingly, the antioxidant polyphenolic Taroxaz-104 molecule has a high reserve of oxygen atoms (ten atoms, present in the six phenolic hydroxyl groups, two aliphatic hydroxyl groups, and two 1,3,4-oxadiazole nuclei) and nitrogen atoms (four atoms, present in the two 1,3,4-oxadiazole nuclei) in its constructure, rendering it significantly potential inhibitor of the microbial protein (e.g., SARS-CoV-2 proteins). Taroxaz-104 molecule structure is clearly characterized by a high degree of structural flexibility, thus the flexible backbone of this compound is expected to add extra inhibitory binding abilities to its structure when hitting any viral protein [5]. The significant number of hydrogen bond-donating/accepting moieties and aromatic rings (which provide the strong hydrogen bonds and hydrophobic interactions required for the ideal binding with the binding motifs and domains of all active pockets/cavities of the COVID-19 protein targets [5]) is also proposed to synergize and potentiate the inhibitory binding abilities of Taroxaz-104 ligand against the SARS-CoV-2 particles. It is worth mentioning that Taroxaz-104 molecule has considerable analogy with coronaviral RNA nucleosides, giving the compound the potential ability of being successful bioisosteric antimetabolite in COVID-19 treatment. In addition, the oxygenous/nitrogenous constructure of Taroxaz-104 is characterized by an important predicted feature of being a potent multizincophore (i.e., a strong zinc ion/atom carrier), since it has about fourteen zincophoric centers (ten active oxygen atoms and four active nitrogen atoms), rendering it an ideal typical candidate to act as a strong zinc-chelating/transporting agent. Zn2+ ions, in certain concentrations, were found to significantly impair nCoV-RdRp activity and its associated coronaviral-2 replication/transcription in vitro, thus they have pivotal role and function in controlling the performance and activity of the COVID-19 RNA-synthesizing machinery and inhibiting the replication processes of coronaviruses intracellularly [5,15,20,32]. Inhibiting nCoV-RdRp and its accompanying replication process is a newly-established strategy to fight SARS-CoV-2 through searching for zincophoric/multizincophoric molecules that have strong zincophoric properties, thus Taroxaz-104 is expected to act as anti-COVID-19, partly, through acting as a potent anti-SARS-CoV-2 zincophoric compound [5,15,20,32]. The intense resonance of Taroxaz-104 chemical structure may help in reducing the binding free energies and strengthening the inhibitory biochemical stabilities of the complexes of Taroxaz-104 with the active amino acid residues of the SARS-CoV-2 target proteins (e.g., nCoV-RdRp), and, as a result, boosting the total potential anti-COVID-19 activities [5,23]. Logically, the beneficial antioxidant actions of Taroxaz-104 will also support its potential anti-COVID-19 bioactivities through reducing the oxidant parts of almost all target coronaviral-2 enzymes/proteins, leading to inactivation of these active proteins (this antioxidant performance will also have a secondary role in quenching and suppressing the severe inflammatory oxidative stress status of the COVID-19 patients [4,23]). The existence of the two pharmacologically-active antiviral heterocyclic 1,3,4-oxadiazole cores in Taroxaz-104 chemical structure is predicted to significantly support the anticoronaviral-2 bioactions of Taroxaz-104 [5,23]. The desirable moderate and balanced ratios of lipophilic-to-hydrophilic properties of Taroxaz-104 (Taroxaz-104 has a log P value < 5) are substantially required to afford the preferable tolerated pharmacokinetic features [33].
Fig. 3.
Synthetic pathways of the potential anti-COVID-19 drug Taroxaz-104.
The primary computational molecular docking of Taroxaz-104 molecule in almost all the recognized SARS-CoV-2 protein targets (including most known coronaviral-2 functional enzymes) revealed the significantly strong inhibitory binding affinities with the protein structures of SARS-CoV-2 [34], specifically with the nCoV-RdRp. These motivating primary results were further reevaluated through another molecular docking application to confirm the previous results and deeply investigate the nCoV-RdRp-Taroxaz-104 interaction in more details. Interestingly, the computational reevaluation proved the potent inhibitory interactions of the small molecule of Taroxaz-104 with many of the active amino acid residues of the most important binding domains/motifs (i.e., active pockets/cavities of the allosteric and active sites) of the giant molecule of nCoV-RdRp. Based on the previous promising multitarget (i.e., multiple inhibitory actions against the coronaviral-2 particle) and specific-target (i.e., distinctive inhibitory action against the nCoV-RdRp particle) data, the biological assessment of the anti-SARS-CoV-2 activities of Taroxaz-104 was carried out using an in vitro anti-COVID-19 bioassay. Similarly as the simulative computational predictions, the anti-COVID-19 bioassay results were also very interesting, since they even significantly surpassed those of the potent reference drugs. All the preceding promising predictions and results support our mission to prove the potential therapeutic effectiveness of successfully using Taroxaz-104 compound as a highly potent anti-COVID-19 drug. As a conclusion, in this original discovery research paper, the deep theoretical computational investigations and in vitro experimental biological evaluations of the previously-synthesized compound called Taroxaz-104 as efficient and very active anti-COVID-19 agent (i.e., as a strong antidotal anti-SARS-CoV-2 compound) were reported.
2. Methods
2.1. Computational molecular docking studies (speculative anti-COVID-19 activities evaluation) of Taroxaz-104
The primary computational screening and molecular modeling to theoretically predict and evaluate the anti-SARS-CoV-2 properties of Taroxaz-104 were performed using COVID-19 Docking Server [35]. This server is one of the new molecular docking web servers constructed and launched in response to the COVID-19 pandemic in order to examine and predict the binding mechanisms between the known COVID-19 targets and their several potential ligands by implementing AutoDock Vina/CoDockPP docking engines [35]. COVID-19 Docking Server web-based software is a new interactive web server for computational docking of small and complex molecules against known protein targets of COVID-19 (SARS-CoV-2 and human targets) in order to speculatively predict the binding modes between any COVID-19 target and the ligands along with evaluating the anti-SAR-CoV-2 properties of these potential inhibitors. The web server was loaded by the several constructions of all the functional/structural protein targets involved in the coronaviral-2 replication life cycle through using the reliable appropriate methods. 3D coordinate generation/format transformation for the uploaded files were performed using Open Babel. The potent antiviral agent GS-443902 (active metabolite of remdesivir; it is also called GS-441524 triphosphate) was used as the reference or positive control compound for comparison purposes [36]. Taroxaz-104 and GS-443902 structures were preferably uploaded in the form of strict .sdf file format. For virtual docking of each of Taroxaz-104 and GS-443902 molecules, the “Docking” mode box was selected as the implemented computational type. To obtain the highest precise docking results possible, a moderate default exhaustiveness value option of 12 was used. The outputs of the top ten models were visualized by JSmol (3D representations), and all the docking results were easily seen and downloaded from the separate result page.
To confirm the results of the COVID-19 Docking Server methodology and investigate the best binding interactions and modes in much more details, another molecular docking web server, PatchDock, was used [37]. PatchDock web server is a validated rigid molecular docking algorithm which is based mainly on shape complementarity principles [37,38]. The permitted input is two molecules of any type (e.g., proteins, enzymes, DNA, peptides, antibodies, antigens, drugs, and compounds), while the resulted output is a full list of potential complexes arranged by the different criteria of shape complementarity. According to its web site, PatchDock algorithm is principally inspired by object identification/recognition and several image segmentation techniques used in computer vision. Docking in PatchDock methodology is largely analogous to collecting a jigsaw puzzle. When solving the puzzle we try to match two pieces by picking one piece and searching for the complementary one. We concentrate on the patterns that are unique for the puzzle element and look for the matching patterns in the rest of the pieces. As with the jigsaw puzzle, PatchDock technique segments surfaces of the two examined molecules into various distinguishable patches according to the shape of each surface. The patches coincide to patterns that visually differentiate between the puzzle pieces. Once the patches are totally recognized, they could be easily superimposed using the employed shape matching algorithms (each patch passes through the three major stages of the algorithm [37,38]). To begin the docking procedure for each compound, the files of the optimized free structures of the receptor (complex nCoV-RdRp enzyme with the PDB code of 7BV2; the protein with the best inhibitory binding interactions and scores from the previous COVID-19 Docking Server methodology) and ligand (Taroxaz-104 or GS-443902) molecules were firstly uploaded in .pdb format in their respective positions in the docking page of the server. A default clustering RMSD value of 4.0 and an enzyme-inhibitor complex type were selected in our current case (advanced options are also available in this docking tool). Results were seen and easily downloaded in the form of solutions tables (data are sorted in a descending order according to the final score) in the results page. The best solutions from the PatchDock Server were further refined in another linked web server, called FireDock Server, which is used for fast and final refinement of the binding interactions in molecular docking [39].
After performing the previous two major docking procedures, the structure of nCoV-RdRp-Taroxaz-104 complex or nCoV-RdRp-GS-443902 complex was further examined (3D) and precisely analyzed for investigative characterization through utilizing the fully-automated interactive tool of the well-designed Protein-Ligand Interaction Profiler (PLIP) web server [40,41]. Screen shots of the resulted images were taken for investigation and reporting. Data of the interacted active amino acid residues were tabulated for comparison and explanation of the prior docking results to come to final conclusions.
2.2. In vitro anti-COVID-19 bioactivities of Taroxaz-104
This credible and robust in vitro anti-COVID-19 test is based mainly upon the original procedures of Chu and coworkers [11,42] with very slight adjustments. The complete procedures were carried out in a specialized biosafety level 3 (BSL-3) laboratory. The assayed new strain of SARS-CoV-2 virus, the first Variant of Concern from 2020, December (VOC-202012/01), was isolated from the fresh nasopharynx aspirate and throat swab of a confirmed thirty nine-years-aged COVID-19 female patient using Vero E6 cells (ATCC CRL-1586). Stock virus (107.25 TCID50/mL) was prepared after three serial passages in Vero E6 cells in infection media (DMEM supplemented with 4.5 g/L d-glucose, 100 mg/L sodium pyruvate, 2% FBS, 100,000 U/L Penicillin-Streptomycin, and 25 mM HEPES). Following the original procedures in the literature, Taroxaz-104 compound was prepared (starting from gallic acid), purified (> 96% purity, HPLC), fully characterized (color, melting point, IR, 1H NMR, 13C NMR, MS, and elemental analysis), and put in small dark brown glass vials to be ready for the assay [23]. The pure reference compound GS-443902 was purchased from MedChemExpress (MCE®). Preliminary pilot assays were performed mainly to determine and decide the adequate concentration of Taroxaz-104 and GS-443902 to start the in vitro anti-COVID-19 and cytotoxicity tests with. The stocks of the tested compounds were accurately prepared by dissolving each of the two compounds in dimethylsulfoxide (DMSO) (100 mM concentration of each, this was found to be the best stock concentration). Additionally, DMSO was used for the purpose of a negative control comparison to make the study placebo-controlled. To evaluate the in vitro anti-SARS-CoV-2 activity of the target compound, Taroxaz-104, in comparison to that of the positive control drug, GS-443902, along with that of the negative control solvent, DMSO, Vero E6 cells were pretreated with the three compounds diluted in infection media for 1 h prior to infection by the new variant of the SARS-CoV-2 virus at MOI = 0.02. The three tested compounds were maintained with the virus inoculum during the 2-h incubation period. The inoculum was removed after incubation, and the cells were overlaid with infection media containing the diluted test compounds. After 48 h incubation at 37 °C, supernatants were immediately collected to quantify viral loads by TCID50 assay or quantitative real-time RT-PCR "qRT-PCR" (TaqMan™ Fast Virus 1-Step Master Mix) [11,42]. Viral loads in this assay were fitted in logarithm scale (log10 TCID50/mL and log10 viral RNA copies/mL) [11,42], not in linear scale [43], under increasing concentrations of the tested compounds. Four-parameter logistic regression (GraphPad Prism) was used to fit the dose-response curves and determine the EC50 of the tested compounds that inhibit SARS-CoV-2 viral replication (CPEIC100 was also determined for each compound). Cytotoxicity of each of the three tested compounds was evaluated in Vero E6 cells using the CellTiter-Glo® Luminescent Cell Viability Assay (Promega) [11,44]. Final results were represented as the mean (μ) ± the standard deviation (SD) from the triplicate biological experiments. Statistical analysis was performed using SkanIt 4.0 Research Edition software (Thermo Fisher Scientific) and Prism V5 software (GraphPad). All reported data were significant at p < 0.05.
3. Results and discussion
3.1. Computational molecular docking studies (speculative anti-COVID-19 activities evaluation) of Taroxaz-104
The predictive molecular docking investigation of Taroxaz-104 molecule revealed that its maximal effectiveness (i.e., the strongest inhibitory binding interactions) obviously appeared upon hitting the nCoV-RdRp, thus Table 1 specifically focuses on the results for the nCoV-RdRp-Taroxaz-104 complex as compared to the reference nCoV-RdRp-GS-443902 complex. The successful understanding of the nCoV-RdRp-Taroxaz-104 binding interactions will pave the way for the development of this compound as a candidate SARS-CoV-2 inhibitor in the drug discovery journey of COVID-19. This computational prediction helps us to deeply confirm and establish the expected anti-COVID-19 mechanism of action of Taroxaz-104 agent along with its degree of efficacy/potency.
Table 1.
Score values of the computationally-predicted anti-nCoV-RdRp properties of the target 1,3,4-oxadiazole, Taroxaz-104, and the reference drug, GS-443902, respectively, using COVID-19 Docking Server methodology (the table displays the least binding free energy value obtained, in kcal/mol, for each compound with nCoV-RdRp).
| SARS-CoV-2 Target Polymerase |
Top Pose Score Value of Inhibitory Binding Energies for Docking of nCoV-RdRp Protein Target (kcal/mol) |
|
|---|---|---|
| Taroxaz-104 | GS-443902 | |
| RdRp-RNA (RTP site) | −10.60 | −9.20 |
| RdRp (RNA site) | −9.10 | −7.90 |
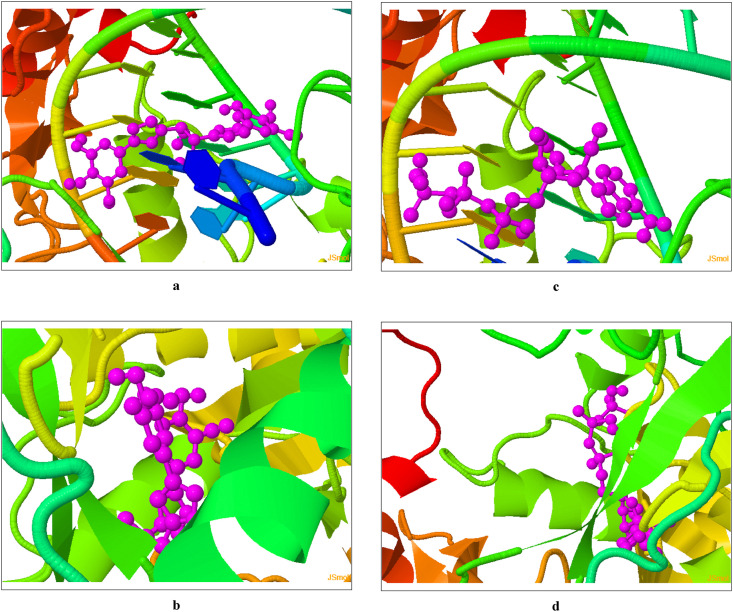
On close observation of the predicted best pose score values (in Table 1) of docking nCoV-RdRp (with and without RNA, respectively) using COVID-19 Docking Server, it is obviously noticed that Taroxaz-104 is superior to GS-443902 in the inhibitory binding affinities/potencies with very good binding energies of −10.60 and −9.10 kcal/mol with RdRp-RNA (RTP site) and RdRp alone (RNA site), respectively. The previous values interestingly reflect the promising expected nCoV-RdRp-inhibiting properties of the potent antioxidant molecule Taroxaz-104, since they significantly exceed those of the positive control agent GS-443902 (it has lower good binding energies of −9.20 and −7.90 kcal/mol with RdRp-RNA and RdRp alone, respectively), through demonstrating the strong interactions and binding to the SARS-CoV-2 polymerase protein with and without RNA forming very stable inhibited complexes. These considerable primary results are very encouraging since they bring to light the significant probability of Taroxaz-104 to be a very potent candidate inhibitor/blocker of nCoV-RdRp. Fig. 4 a–d shows the resulted respective docking images (3D visualized in JSmol application) using COVID-19 Docking Server methodology.
Fig. 4.
Screenshots of COVID-19 Docking Server outputs of the top predicted binding model of docking of Taroxaz-104 molecule (colored pink) in: (a) nCoV-RdRp-RNA “RTP site” (PDB code: 7BV2; colored with other various colors), (b) nCoV-RdRp “RNA site” (PDB code: 7BV2; colored with other various colors); and GS-443902 molecule (colored pink) in: (c) nCoV-RdRp-RNA “RTP site” (PDB code: 7BV2; colored with other various colors), (d) nCoV-RdRp “RNA site” (PDB code: 7BV2; colored with other various colors).
As a complementary confirmation of the COVID-19 Docking Server results, PatchDock Server methodology was performed. The results in Fig. 5 a and b show that Taroxaz-104 interacts with one or more of the predicted active/allosteric pockets (domains and motifs) of the nCoV-RdRp in close mode(s) of action to that of the reference GS-443902. Taroxaz-104 molecule strikes about 50% of the same active amino acid residues that GS-443902 interacts with in chain A of nCoV-RdRp complex structure, namely His256/Tyr265/Ile266/Lys267/Trp268/Pro322/Pro323, respectively, revealing another additional active/allosteric site(s) of the polymerase to the previously-suggested one known for favipiravir [45,46]. Taroxaz-104 binds to the previously-mentioned amino acids with much more stronger interactions than GS-443902, thus predictably exhibits better inhibitory binding interactions than this reference ligand. It was also found that Taroxaz-104 forms higher number of strong hydrogen bonds and hydrophobic interactions with the nCoV-RdRp than what GS-443902 does, as a result of, mainly, the relatively higher number of hydroxyl groups/aromatic rings. These data completely support the previous results obtained upon applying the COVID-19 Docking Server methodology.
Fig. 5.
2D representations of the inhibitory binding interactions, of a) Taroxaz-104; b) GS-443902 (showing opening and rearrangement of the ribofuranosyl moiety into smaller cyclopropane ring during the expected metabolic and docking procedures), with the amino acids residues of one or more of the active sites of nCoV-RdRp (the COVID-19 polymerase).
3D visualization of the best poses of docking of nCoV-RdRp with Taroxaz-104 and GS-443902 molecules in PLIP web server (Fig. 6 a and b) shows the balanced degrees of conformational/orientational flexibility of Taroxaz-104 structure as compared to the less-balanced ones of GS-443902 structure. Taroxaz-104 molecule is expected to be clearly characterized by superflexibility inside the biological systems, since it is a disubstituted or two-wing bulky derivative of tartaric acid with bigger topological polar surface area (TPSA) and more total atoms than GS-443902 molecule. This significant flexibility is much required for any ligand of nCoV-RdRp to provide its structural backbone with the optimal positioning and targeting of the active binding cavities (including active domains and motifs) of the polymerase protein, which leads to more efficient and potent inhibition of the SARS-CoV-2 replication processes performed by the enzyme. Promisingly, all the previous computational predictions support the theoretical expectation for Taroxaz-104 to act as an efficacious potent anti-COVID-19 agent.
Fig. 6.
3D representations of the inhibitory binding interactions, of a) Taroxaz-104; b) GS-443902, with the amino acids residues of one or more of the active sites of nCoV-RdRp.
3.2. In vitro anti-COVID-19 bioactivities of Taroxaz-104
The values resulted from the in vitro anti-COVID-19 and cytotoxicity bioassays (compound concentrations were measured in μM) are shown in Table 2 in details. Recently, more prevalent, infectious, virulent, and resistant strains (variants and lineages) of SARS-CoV-2 have been appeared (e.g., VOC-202012/01, previously identified in the U.K. as the first Variant Under Investigation in December 2020 "VUI-202012/01" and also as the lineage B.1.1.7 or 501Y.V1; and 501.V2, which was mainly detected and traced in South Africa) due to multiple genetic mutations, specially in the spike structure (leading to significant modifications in the spike receptor binding site), and all of them are still under tracing [47, 48, 49, 50]. The data presented in Table 2 interestingly revealed the significantly higher anti-COVID-19 potency of Taroxaz-104 (specially against the new variants or mutants of SARS-CoV-2 appeared until now) as compared to that of the positive control reference drug GS-443902 (on the other hand, the placebo DMSO demonstrated extremely weak and negligible results). Taroxaz-104 compound was found to block SARS-CoV-2 replication/transcription in Vero E6 cells with EC50 much less than 100 μM. Promisingly, Taroxaz-104 (EC50 = 0.42 μM) was found to be about 43 times as potent as the reference GS-443902 (EC50 = 18.05 μM) with respect to the in vitro anti-SARS-CoV-2/anti-VOC-202012/01 bioactivity. According to this assay, CC50 of Taroxaz-104 ligand is much greater than 100 μM, thus this compound is thought to have very considerable clinical selectivity index “SI” (SITaroxaz-104 > 238.10; it is highly wider than that of GS-443902, SIGS-443902 > 5.54), indicating the specific and selective anti-RNA actions of the molecule against the new strains of SARS-CoV-2 rather than the human genome. Taroxaz-104 showed very minute value of the concentration that causes 100% inhibition of the SARS-CoV-2 VOC-202012/01 cytopathic effects in vitro (CPEIC100 = 1.20 μM), which was much lesser than that of GS-443902 (CPEIC100 = 20.50 μM). Moreover, Taroxaz-104 also showed very small value of the concentration that is needed for 50% decrease in the RNA copies number of SARS-CoV-2 (VOC-202012/01 strain) in vitro (0.46 μM), which was much smaller than that of GS-443902 (18.67 μM).
Table 2.
Anti-COVID-19 (anti-SARS-CoV-2/anti-VOC-202012/01) activities (along with human cells toxicities) of Taroxaz-104 (using GS-443902 as the positive control/reference drug, and DMSO as the negative control/placebo drug) against SARS-CoV-2 (VOC-202012/01 strain) in Vero E6 cells.
| Classification | Compound Name |
CC50a (μM) |
Inhibition of SARS-CoV-2in vitro(Anti-VOC-202012/01 Bioactivities) (μM) |
||
|---|---|---|---|---|---|
| 100% CPE Inhibitory Concentration (CPEIC100)b |
50% Reduction in Infectious Virus (EC50)c |
50% Reduction in Viral RNA Copy (EC50)d | |||
| Target Compound | Taroxaz-104 | > 100 | 1.20 ± 0.08 | 0.42 ± 0.02 | 0.46 ± 0.03 |
| Reference Compound | GS-443902 | > 100 | 20.50 ± 0.93 | 18.05 ± 0.88 | 18.67 ± 0.90 |
| Placebo Solvent | DMSO | > 100 | > 100 | > 100 | > 100 |
CC50 or 50% cytotoxic concentration is the concentration of the tested compound that kills half the cells in an uninfected cell culture. CC50 was determined with serially-diluted compounds in Vero E6 cells at 48 h postincubation using CellTiter-Glow Luminescent Cell Viability Assay (Promega).
CPEIC100 or 100% CPE inhibitory concentration is the lowest concentration of the tested compound that causes 100% inhibition of the cytopathic effects (CPE) of SARS-CoV-2 VOC-202012/01 virus in Vero E6 cells under increasing concentrations of the tested compound at 48 h postinfection. Compounds were serially 2-fold or 4-fold diluted from 100 μM concentration.
EC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in infectious SARS-CoV-2 VOC-202012/01 virus particles in vitro. EC50 is determined by infectious virus yield in culture supernatant at 48 h postinfection (log10 TCID50/mL).
EC50 or 50% effective concentration is the concentration of the tested compound that is required for 50% reduction in SARS-CoV-2 VOC-202012/01 viral RNA copies in vitro. EC50 is determined by viral RNA copies number in culture supernatant at 48 h postinfection (log10 RNA copies/mL).
It is worth mentioning that the probability that Taroxaz-104 may undergo some biochemical transformations through biological metabolism inside the human cells into more active forms (e.g., its mono/di/triphosphate forms) is present, which may also significantly differ based on the nature of each tissue type (e.g., hepatic and primary human airway epithelial cells). Based on the metabolism of the analogous natural polyphenolic products in humans, the in vivo metabolic activation is speculatively predicted to attach very minute chemical moieties (which of optimal bioavailability and biocompatibility to the human cells) to Taroxaz-104 molecule scaffold, therefore, increasing the total clinical tolerance and anti-COVID-19 bioactivities of Taroxaz-104 agent. Most experimental biological data observed here in this section are substantially consistent with the previous theoretical/computational data hypothesized in the sections of introduction and molecular docking studies. The questions now, will Taroxaz-104 successfully pass the final preclinical/clinical studies?!, will Taroxaz-104 surpass its brothers CoViTris2020 and ChloViD2020 in the pharmacological activities against the current new variants of SARS-CoV-2?!, and will Taroxaz-104 persist very effective against the other evolved variants of SARS-CoV-2 in the near future?! The coming days will undoubtedly answer all these questions and we will see. Promisingly, the apparent superiority, in almost all anti-COVID-19 parameters, properties, and activities, of Taroxaz-104 over the potent antiviral agent GS-443902 supports Taroxaz-104 candidacy to be a potential unique SARS-CoV-2 killer or antidote.
4. Conclusions
Emergence of the new strains of SARS-CoV-2 represents a critical challenging watershed point in our struggle against the virus and COVID-19 pandemic. The tantalizing mysterious story starts with the appearance and prevalence of SARS-CoV-2 B.1.1.7 strain in London and Southeast England (U.K.) in December of 2020. Most virologists and epidemiologists are pointing to the probability of significant further opportunities for coronaviral-2 evolution, specially in people with considerably-compromised immune systems, since these patients easily tend to suffer from long-lasting chronic infections, during which the SARS-CoV-2 can linger for long periods of weeks and months. Potent inhibition of the most pivotal protein targets of the SARS-CoV-2 particle, e.g., nCoV-RdRp, is considered as the most successful and feasible tactic for efficient development of anti-SARS-CoV-2/anti-COVID-19 drugs. Our continuous efforts led to the effective repositioning/repurposing and discovery of a very promising potent nCoV-RdRp/SARS-CoV-2 inhibitor through the successful biological reevaluation of the previously-synthesized antioxidant ligand Taroxaz-104, which successfully inhibited the most important stage of the coronaviral-2 life cycle, the replication/transcription phase, with interesting EC50 value of 0.42 μM. Taroxaz-104 exhibits about 43-fold anti-SARS-CoV-2 potencies (specially against the newly-evolved SAR-CoV-2 variant, VOC-202012/01) as compared to the standard reference drug GS-443902. Meanwhile, the discovery and arising of the very strong anti-COVID-19 bioactivities of Taroxaz-104 molecule render us welcoming this compound as a third new member (beside the two preceding members, CoViTris2020 and ChloViD2020 compounds) of the first chemical class discovered of anti-COVID-19 agents (the polyphenolic 1,3,4-oxadiazoles or 2,5-disubstituted-1,3,4-oxadiazoles class) [5]. Previous comprehensive computational molecular modeling studies revealed the optimal pharmacokinetic/druglikeness parameters values that Taroxaz-104 has. Molecular docking checking and analysis of the best blocking binding modes of Taroxaz-104 ligand with the different SARS-CoV-2 protein targets showed that the polyhydroxyphenyl/1,3,4-oxadiazole moieties significantly enhance the inhibitory affinities at the active and/or allosteric site(s) of the coronaviral-2 polymerase or nCoV-RdRp (binding energies reach −10.60 kcal/mol) as compared to the reference GS-443902 (which lacks both electron-rich and potent antioxidant moieties). Discovery of Taroxaz-104 presents a major paradigm shift in anti-COVID-19 therapeutic discoveries, since it is the first compound that shows significant inhibiting bioactivities against the more deadly new variants and lineages of the SARS-CoV-2 particles (i.e., Taroxaz-104 agent is specifically characterized by its additional inhibitory anti-COVID-19 activities against the new and resistant strains of SARS-CoV-2, giving this compound a unique featured property as compared to other under-investigation anti-COVID-19 agents). Significantly, Taroxaz-104 is clearly superior to the highly potent antiviral agent GS-443902 in all compared computational and experimental anti-COVID-19 criteria and parameters. Extensive future investigations, including in vivo anti-SARS-CoV-2 studies and randomized controlled anti-COVID-19 clinical trials, are needed for the evolution of Taroxaz-104 and its family of polyphenolic 2,5-disubstituted-1,3,4-oxadiazole analogs/derivatives as anti-COVID-19 drugs. To cut it short, in this novel discovery research article, the antioxidant Taroxaz-104 compound was reported to be effectively repositioned, reevaluated, and repurposed as a very promising anti-SARS-CoV-2 molecule and also as the first reported anti-VOC-202012/01 agent.
Author's contributions
The entire drug discovery, research study, and manuscript were designed, performed, and written, respectively, by a single author (Dr. Amgad M. Rabie).
Declaration of competing interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
I gratefully thank and deeply acknowledge anyone who gave a hand to make this new discovery and work coming out to light.
References
- 1.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J.-Y., You Z., Wang Q., Zhou Z.-J., Qiu Y., Luo R., Ge X.-Y. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020;22:80–85. doi: 10.1016/j.micinf.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.COVID-19 Map (2020) Available from Johns Hopkins Coronavirus Research Center. 2020. https://coronavirus.jhu.edu/map.html Accessed in 30 December, 2020.
- 4.Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerg. Microbes Infect. 2020;9:275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabie A.M. Two antioxidant 2,5-disubstituted-1,3,4-oxadiazoles (CoViTris2020 and ChloViD2020): Successful repurposing against COVID-19 as the first potent multitarget anti-SARS-CoV-2 drugs. New J. Chem. 2021;45:761–771. doi: 10.1039/D0NJ03708G. [DOI] [Google Scholar]
- 6.Rabie A.M. Discovery of (E)-N-(4-cyanobenzylidene)-6-fluoro-3-hydroxypyrazine-2-carboxamide (cyanorona-20): the first potent and specific anti-COVID-19 drug. Chem. Pap. 2021 doi: 10.1007/s11696-021-01640-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 8.Strittmatter S.M. Overcoming drug development bottlenecks with repurposing: Old drugs learn new tricks. Nat. Med. 2014;20:590–591. doi: 10.1038/nm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez J.J., Pryszlak M., Smith L., Yanchus C., Kurji N., Shahani V.M., Molinski S.V. Giving drugs a second chance: overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front. Oncol. 2017;7(273) doi: 10.3389/fonc.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discoveries Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 11.Choy K.-T., Wong A.Y.-L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.-H., Huang X., Peiris M., Yen H.-L. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon A., Le N.T.-T., Selisko B., Eydoux C., Alvarez K., Guillemot J.-C., Decroly E., Peersen O., Ferron F., Canard B. Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 exonuclease active-sites. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin W., Mao C., Luan X., Shen D.-D., Shen Q., Su H., Wang X., Zhou F., Zhao W., Gao M., Chang S., Xie Y.-C., Tian G., Jiang H.-W., Tao S.-C., Shen J., Jiang Y., Jiang H., Xu Y., Zhang S., Zhang Y., Xu H.E. Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science. 2020;368:1499–1504. doi: 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Q., Yang M., Liu D., Chen J., Shu D., Xia J., Liao X., Gu Y., Cai Q., Yang Y., Shen C., Li X., Peng L., Huang D., Zhang J., Zhang S., Wang F., Liu J., Chen L., Chen S., Wang Z., Zhang Z., Cao R., Zhong W., Liu Y., Liu L. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering. 2020;6:1192–1198. doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du Y.-X., Chen X.-P. Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019‐nCoV infection. Clin. Pharmacol. Ther. 2020;108:242–247. doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 19.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med. Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6(16) doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Cao R., Zhang H., Liu J., Xu M., Hu H., Li Y., Zhao L., Li W., Sun X., Yang X., Shi Z., Deng F., Hu Z., Zhong W., Wang M. The anti-influenza virus drug, arbidol is an efficient inhibitor of SARS-CoV-2 in vitro. Cell Discovery. 2020;6(28) doi: 10.1038/s41421-020-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabie A.M., Tantawy A.S., Badr S.M.I. Design, synthesis, and biological evaluation of novel 5-substituted-2-(3,4,5-trihydroxyphenyl)-1,3,4-oxadiazoles as potent antioxidants. Am. J. Org. Chem. 2016;6:54–80. doi: 10.5923/j.ajoc.20160602.02. [DOI] [Google Scholar]
- 24.Rabie A.M., Tantawy A.S., Badr S.M.I. Design, synthesis, and biological evaluation of new 5-substituted-1,3,4-thiadiazole-2-thiols as potent antioxidants. Researcher. 2018;10:21–43. doi: 10.7537/marsrsj100718.04. [DOI] [Google Scholar]
- 25.Paraiso I.L., Revel J.S., Stevens J.F. Potential use of polyphenols in the battle against COVID-19. Curr. Opin. Food Sci. 2020;32:149–155. doi: 10.1016/j.cofs.2020.08.004. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo M., Moccia S., Spagnuolo C., Tedesco I., Russo G.L. Roles of flavonoids against coronavirus infection. Chem. Biol. Interact. 2020;328:109211. doi: 10.1016/j.cbi.2020.109211. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rameshkumar M.R., Indu P., Arunagirinathan N., Venkatadri B., El-Serehy H.A., Ahmad A. Computational selection of flavonoid compounds as inhibitors against SARS-CoV-2 main protease, RNA-dependent RNA polymerase and spike proteins: A molecular docking study. Saudi J. Biol. Sci. 2021;28:448–458. doi: 10.1016/j.sjbs.2020.10.028. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer-Almes F.J. Repurposing approved drugs as potential inhibitors of 3CL-protease of SARS-CoV-2: Virtual screening and structure based drug design. Comput. Biol. Chem. 2020;88:107351. doi: 10.1016/j.compbiolchem.2020.107351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalifa S.A.M., Yosri N., El-Mallah M.F., Ghonaim R., Guo Z., Musharraf S.G., Du M., Khatib A., Xiao J., Saeed A., El-Seedi H.H.R., Zhao C., Efferth T., El-Seedi H.R. Screening for natural and derived bio-active compounds in preclinical and clinical studies: One of the frontlines of fighting the coronaviruses pandemic. Phytomedicine. 2021;85:153311. doi: 10.1016/j.phymed.2020.153311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nebigil C.G., Moog C., Vagner S., Benkirane-Jessel N., Smith D.R., Désaubry L. Flavaglines as natural products targeting eIF4A and prohibitins: From traditional Chinese medicine to antiviral activity against coronaviruses. Eur. J. Med. Chem. 2020;203:112653. doi: 10.1016/j.ejmech.2020.112653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aucoin M., Cooley K., Saunders P.R., Cardozo V., Remy D., Cramer H., Neyre Abad C., Hannan N. The effect of quercetin on the prevention or treatment of COVID-19 and other respiratory tract infections in humans: A rapid review. Adv. Integr. Med. 2020;7:247–251. doi: 10.1016/j.aimed.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.A.J.W. te Velthuis, S.H.E. van den Worm, A.C. Sims, R.S. Baric, E.J. Snijder, M.J van Hemert, Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 34.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COVID-19 Docking Server Web-based Software . Jiangsu University of Technology, Changzhou 213001, China; Available from COVID-19 Docking Server on the Web (homepage:; 2020. COVID-19 Docking Server (Homepage on the Internet), Shan Chang Lab., Institute of Bioinformatics and Medical Engineering, School of Electrical and Information Engineering.http://ncov.schanglab.org.cn ); Results were obtained through using the interactive docking tool in COVID-19 Docking Server (Copyright© 2018–2023, Shan Chang; Version 2020) on this website (accessed and cited in 2020, 12–18 December), and references cited therein. [Google Scholar]
- 36.Eastman R.T., Roth J.S., Brimacombe K.R., Simeonov A., Shen M., Patnaik S., Hall M.D. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 2020;6:672–683. doi: 10.1021/acscentsci.0c00489. doi: 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.PatchDock Server Web-based Software PatchDock Server (Homepage on the Internet); https://bioinfo3d.cs.tau.ac.il/PatchDock Available from PatchDock Server on the Web (homepage: ); Results were obtained through using the docking tool in PatchDock Server (Copyright© 2020-2021; Beta 1.3 Version) on this website (accessed and cited in 2020, 20–30 December), and references cited therein.
- 38.Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33(Web Server issue):W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.http://bioinfo3d.cs.tau.ac.il/FireDock
- 40.https://projects.biotec.tu-dresden.de/plip-web
- 41.Salentin S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015;43:W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 66. 2020:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Lin D., Kusov Y., Nian Y., Ma Q., Wang J., von Brunn A., Leyssen P., Lanko K., Neyts J., de Wilde A., Snijder E.J., Liu H., Hilgenfeld R. α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: Structure-based design, synthesis, and activity assessment. J. Med. Chem. 2020;63:4562–4578. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 45.Sada M., Saraya T., Ishii H., Okayama K., Hayashi Y., Tsugawa T., Nishina A., Murakami K., Kuroda M., Ryo A., Kimura H. Detailed molecular interactions of favipiravir with SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza virus polymerases in silico. Microorganisms. 2020;8 doi: 10.3390/microorganisms8101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picarazzi F., Vicenti I., Saladini F., Zazzi M., Mori M. Targeting the RdRp of emerging RNA viruses: The structure-based drug design challenge. Molecules. 2020;25:5695. doi: 10.3390/molecules25235695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020;371 doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- 48.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M. T.I. de Silva; Sheffield COVID-19 Genomics Group, C. McDanal, L.G. Perez, H. Tang, A. Moon-Walker, S.P. Whelan, C.C. LaBranche, E.O. Saphire, D.C. Montefiori, Tracking changes in SARS-CoV-2 spike: Evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182 doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yi C., Sun X., Ye J., Ding L., Liu M., Yang Z., Lu X., Zhang Y., Ma L., Gu W., Qu A., Xu J., Shi Z., Ling Z., Sun B. Key residues of the receptor binding motif in the spike protein of SARS-CoV-2 that interact with ACE2 and neutralizing antibodies. Cell. Mol. Immunol. 2020;17:621–630. doi: 10.1038/s41423-020-0458-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50."Expert reaction to South African variant of SARS-CoV-2, as mentioned by Matt Hancock at the Downing Street press briefing". Science Media Centre, 23 December 2020. 2020;23 https://www.sciencemediacentre.org/expert-reaction-to-south-african-variant-of-sars-cov-2-as-mentioned-by-matt-hancock-at-the-downing-street-press-briefing Retrieved on 24 December 2020. The South African variant ‘501.V2’ is characterised by N501Y, E484K and K417N mutations in the S protein – so it shares the N501Y mutation with the UK variant, but the other two mutations are not found in the UK variant. Similarly, the South African variant does not contain the 69-70del mutation that is found in the UK variant. [Google Scholar]