Abstract
Globally fermented foods are in demands due to their functional and nutritional benefits. These foods are sources of probiotic organisms and bioactive peptides, various amino acids, enzymes etc. that provides numerous health benefits. FermFooDb (https://webs.iiitd.edu.in/raghava/fermfoodb/) is a manually curated database of bioactive peptides derived from wide range of foods that maintain comprehensive information about peptides and process of fermentation. This database comprises of 2205 entries with following major fields, peptide sequence, Mass and IC50, food source, functional activity, fermentation conditions, starter culture, testing conditions of sequences in vitro or in vivo, type of model and method of analysis. The bioactive peptides in our database have wide range of therapeutic potentials that includes antihypertensive, ACE-inhibitory, antioxidant, antimicrobial, immunomodulatory and cholesterol lowering peptides. These bioactive peptides were derived from different types of fermented foods that include milk, cheese, yogurt, wheat and rice. Numerous, web-based tools have been integrated to retrieve data, peptide mapping of proteins, similarity search and multiple-sequence alignment. This database will be useful for the food industry and researchers to explore full therapeutic potential of fermented foods from specific cultures.
Keywords: Bioactive peptide, Database, Webserver, Microbial fermentation, Functional food
Bioactive peptide, Database, Webserver, Microbial fermentation, Functional food.
1. Introduction
More food, specifically proteins will be required by the burgeoning world population, which is projected to touch 9.6 billion by 2050. Consumer orientation towards the convenient, healthy nutritious food and need to complement animal protein is driving the demand of protein products that not only taste good but also provide multiple bioactivities in stress relief, mental health, blood pressure and blood sugar control. Proteins are major component of the food as they play many vital roles such as regulation of metabolism, driving biochemical reactions, providing strength to muscles and in boosting of immune system. Different strategies are available for enhancement of solubility of the proteins, however, Industry uses alkaline or salt solutions, as a preferred mode for production of protein hydrolysates and its constituents. The hydrolysis of the protein either with commercially available enzymes or proteolytic enzymes of lactic acid bacteria (LAB) results in the formation of bioactive peptides, which provides health benefits [1, 2].
Lactic acid bacteria (LAB), commonly associated with food fermentations is generally used as starter cultures in dairy, baking and wine industries [3]. LAB is also used in fermentation of different vegetables such as sauerkraut, pickles, olives etc., as they can convert sugars into lactic acid. Lactobacillus species are auxotrophic for amino acids and to accomplish this, these species have established a proteolytic system, that provides the amino acids through protein hydrolysis. This happens more precisely through the cell envelope proteinases (CEP) that cleaves the proteins into peptides that are transported inside the cell. These peptides are finally converted into amino acids with the help of peptidases such as endopeptidases, aminopeptidases, tripeptidases, dipeptidases, and proline-specific peptidases [4].
Lactobacillus species contains six different CEPs viz. PrtB from Lb. delbrueckii subsp. bulgaricus [5], PrtP from Lb. casei and Lb. paracasei [6], PrtR from Lb. plantarum and Lb. rhamnosus [7], PrtH from Lb. helveticus [4], PrtS from Streptococcus thermophilus, and PrtL from Lb. delbrueckii subsp. lactis [8]. Lb. helveticus has several CEP paralogs, such as PrtH1, PrtH2, PrtH3, and PrtH4 displaying different specificities, thereby making it most proficient proteolytic species that generates a great diversity of BAPs [9]. The genetic diversity of CEPs leads to diverse protein hydrolysis patterns [10]. Moreover, Lactobacillus strains have dissimilar specificities for protein hydrolysis, thereby leading to different BAPs [11]. Different types and varying length peptides can be generated through action of lactic acid bacteria on different types of protein substrates [12]. In fermentation of different types of proteins such as milk proteins, cereal, animal and fish-based protein Lactobacillus strains are mostly used [13]. Even the inoculation of the same Lactobacillus strain will produce different peptides on hydrolysing caseins from different milk sources such as cow, goat, and camel.
There is a resurgence of interest in traditional fermented foods, as they contain different kind of bioactive peptides, which can be exploited by the industries for delivering health foods [14]. In addition to several genera of LAB (i.e, Lactobacillus, Streptococcus, and Leuconostoc), other microorganisms such as yeast and fungi also play a significant role in food fermentations. The coculturing of different bacteria and yeast such as Saccharomyces cerevisae or fungi A.oryjae M. purpureus etc.has also been used in various fermentation of food Proteins, which generally results in not only speeding up the process of proteolysis but also generation of different bioactive peptides [15]. In many fermented foods, these microorganisms can function as “microfactories” to produce bioactive molecules with various nutritional and health properties. Fermented foods that are produced commercially across the globe are often used as carriers for probiotic bacteria. These fermented foods contain bioactive peptides, which play an important role in the metabolic functions of living human being as they possess many biological functions such as antihypertensive, antimicrobial, antithrombotic, and antioxidative [16]. The health claims associated with the indigenous fermented foods have been derived from the social community beliefs, and many of these do not yet have a scientific basis to prove or debunk them [17, 18]. However, lot of scientific evidences do exist for many of fermented products such as fermented milk products with hypotensive, hypo-cholesterolemic and antimicrobial effects [19]; fermented legumes with antidiabetic properties [20] and fermented cereal based foods with improved shelf life nutritional value and antioxidant activity [21]. Recently, different studies with in vitro and animal models, have been carried out to corroborate some of these claims associated with the health benefits.
It has been shown in previous studies that bioactive peptides are found in different type of foods like milk, soybean, porcine, egg white and marine byproducts [22, 23, 24, 25, 26]. These peptides can be produced through the meticulous use of peptidases or microorganisms for the formation of functional ingredients in the food [27]. The presence of the proteolytic enzymes in saliva and gastrointestinal tract leads to generation of biologically active peptides, during the digestion of the food in the human body [28]. The fermentation of cheese, wine and meat also produce these peptides [29, 30].
In order to maintain different kind of bioactive peptides, a wide range of databases have been developed in the past. These databases/repositories maintain antimicrobial [31, 32], antiviral [33], cell-penetrating [34], antitumor [35], hemolytic [36] peptides. Most of the information about theses bioactive peptides is available in various databases such as Biopep-UWM, StraPep, FeptideDB, ACEpepDB, BioPD, etc. BioPD is a web based application for evaluation of bioactive peptides, whereas BIpep-UWM, FeptideDB and ACEpepDB describes about the bioactive peptides from the food proteins [35]. Despite the huge potential of bioactive peptides derived from different kinds of fermented foods; till date, there is no consolidated database that maintains biologically active peptides obtained from fermented foods. In order to complement the existing resources on bioactive peptides, we have developed a database of functional peptides obtained from fermented foods. This fermented food peptide database is a sole resource for the food and beverages manufacturers as the different food companies have started not only commercializing the traditional fermented foods but also developing novel products with the starter cultures of the fermented foods across the globe. It will allow one to harness the potential of fermented foods, which are reemerging as a natural food with high nutritional qualities.
2. Material and methods
2.1. Data collection
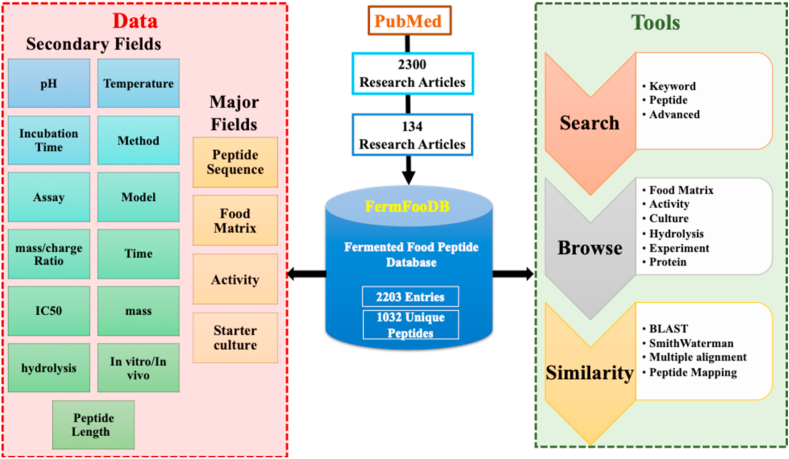
A methodical search was performed in PubMed using different combination of following keywords; fermented/bioactive/biologically active peptides, health benefits, food matrices, lactic acid bacteria, yeast and fungi. These keywords were searched in abstract and title of papers in PUBMED. These searches ended in around 2300 research articles, which were analyzed to extract relevant articles. Next, we manually curated all the research articles to apprehend information present in them. Subsequently, after scrutinizing all the articles, we extracted all relevant papers on peptides derived from the fermented foods for advance processing. For each peptide, we retrieved complete information about each peptide such as mass, IC50, lactic acid bacteria used for the fermentation, and type of food matrices. The structural design of the FermFooDB database is shown in Figure 1.
Figure 1.
The Architecture of the FermFooDB database.
2.2. Architecture and interface of database
A web-based platform has been developed for FermFooDB to access information via web browsers. In order to launch web-based service, we used HTTPD server of Apache on a UNIX-based Ubuntu system. We developed a responsive web interface to make it suitable for wide range of smart devices such as iPhones, iPads, smart phones, and laptops. The web interface has been developed using standard HTML5 and PHP. In order to provide responsive template, we used CSS3 and JavaScript. All the data in this database is managed in form of tables. These tables are stored in MySQL which is an open source software based on relational database management system.
2.3. Database organization
Information in this database is stored in the form of records where each record has several fields related to fermented peptides. Each record maintains different type of peptide properties like sequence, physicochemical properties, length and IC50 value as well as fermentation process. Beside major component of fermentation and by products, we also compile fine detail that includes experimental model, starter culture and PubMed ID of research article. Numerous techniques have been used to evaluate bioactivity of these peptides derived from fermented foods. For example, Spontaneously Hypertensive Rats (SHR) have been used as model to check the antihypertensive effect of peptides. Information was not available for certain fields in few entries hence were marked as ‘NA’, i.e. Not Available. Most of the fermented milk peptides, cheese, goat whey, Kefir and yoghurts are from different types of caseins such as alpha, beta and kappa and various whey peptides from alpha, beta lactoglobulins. Peptides from fermented marine foods such as shrimp have been extracted from the marine proteins.
2.4. Web interface of FermFooDb
All information on bioactive peptides compiled from literature was managed in the form of a database called FermFooDb. In order to provide user-friendly, web-based service to community, a web interface has been developed. It integrates large number of web tools to provide wide range of options to users. The following are major modules in FermFooDb; browsing, searching (e.g., keyword, peptide sequence, advanced search) and similarity search facility.
2.5. Browse tool
Browsing facility was developed to present data in classified form where user can browse data on six major fields like Food Matrix, Activity, Culture, Hydrolysis, Experiment and Protein. Users can extract data of about fifteen different types of food matrices such as milk, cheese, wheat, wine, etc. Under the option of Activity tab, users can browse all different types of functional activities, such as ACE-inhibiting, antioxidant, immunomodulatory, etc. shown by the peptides. Similarly, under the tab of Culture, users can query the peptides by starter cultures from which the peptides collected in this database have been obtained. Further, users can also query this repository based on the enzymes (proteases or peptidases) that are used to generate peptides from proteins. We also added the option to browse using the type of experiment (in-vivo, in-vitro and integrated approaches) to help the users to query the peptides. Integrated approach will use the in-silico method for the prediction of release of bioactive peptide, followed by assessment of bioactive peptide potential either in vitro or in vivo. Users can also extract the peptides based on the proteins from which they are derived.
2.6. Search module
This module allows the user to perform search using keywords, and peptide sequence. It also allows one to perform advanced search. In keyword search, users can search the complete database using specific keywords like Protein name, Food matrix, Pubmed Id and activity etc. User can also select the fields that one wants to display in the results table. The peptide search allows user to search using the peptide sequence. The third type of search called Advance search allows the user to combine the various keywords and search the database. For example, we can use Advance search option to search for “Antihypertensive peptides” in “Milk”. The Advance search is very useful to search for complex user defined queries.
2.7. Similarity module
Similarity search module have been integrated in website that allow users to search similar or identical peptides in the FermFooDB using Smith–Waterman algorithm, and Basic Local Alignment Search Tool (BLAST). This section also allows user to perform alignment with multiple peptides using multiple alignment tool and also perform peptide sub-search using peptide mapping tool.
2.8. Availability
FermFooDb can be accessed freely at https://webs.iiitd.edu.in/raghava/fermfoodb/
3. Results
There is resurging interest in fermented foods due to changes in lifestyle, and growing interest in the health impacts of food [37]. Thus, this database has been developed to enable development of commercial products using novel food ingredients by the food industries. The wide range of bioactive peptide properties maintained in this database will be useful for researchers to evaluate the therapeutic potential of fermented foods. These properties of peptides have been collected and complied from published literature and existing databases like AHTPDB, ACEPepDB, BIOPEP, and MBPDB [38, 39].
3.1. Data statistics
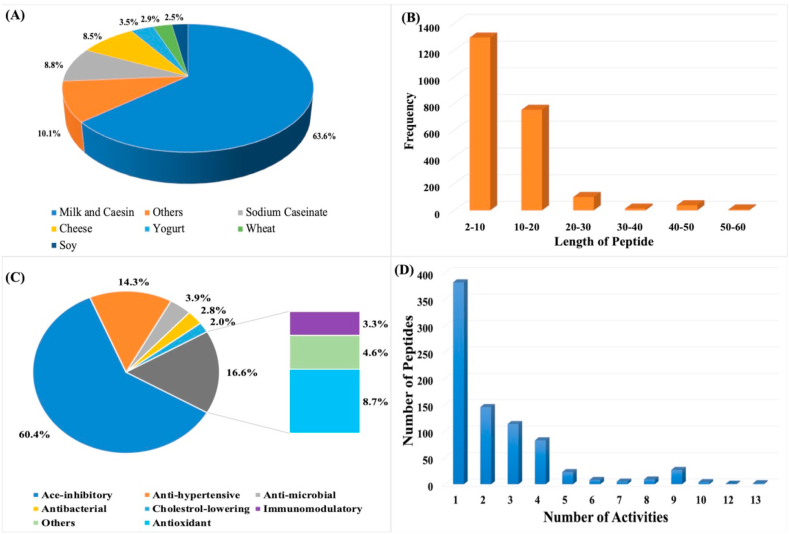
The present release of the database (version 1.0), contain around 2200 entries; most of the peptides were derived from milk and casein which contain around 1530 (63.6%) entries (Figure 2A). It shows the importance of milk as food, as it contains wide range of bioactive peptides with health benefits. The prevalence of milk-derived bioactive peptides shows that milk protein and milk -derived food protein and its peptides are being studied more widely than other food matrices such as fermented plant and animal derived food. The tripeptide from the fermented milk IPP-VPP-LPP by Lactobacillus helveticus NCC 935 (LH53) (CNCM I-3997); Lactobacillus helveticus NCC 1322 (LH111) CNCM I-3998 and Lactobacillus helveticus NCC 1649 (LH158) CNCM I-3999 showed high antihypertensive property. The fermentation by Lactobacillus helveticus NCC 935 (LH53) (CNCM I-3997) at the concentration of 30 mg/L tripeptides and the prescribed limit of daily intake at 5.2 mg per day thus indicated that the daily dose can be completed with the consumption of 150ml of the fermented milk. The ACEI activity of VPP and IPP was very high, 9 μM and 5 μM and they were produced during fermentation only but couldn't be detected in the casein hydrolyzate even after digestion with Lb. helveticus proteinase. As shown in Figure 2A, other major foods are cheese (8.5%), yoghurt (3.5%), wheat (2.9%), and soybeans (2.5%). The percentage of each food represents the proportion of each food compared to all foods in our database. The length distribution of different kinds of peptides in FermFooDB has also been computed and is shown in Figure 2B. The length of most of the peptides is small, around 58% in FermFooDB having less than 10 residues. It is obvious as maintaining lengthy peptides in fermentation are difficult due to presence of enzymes. In addition to small peptides, this database also maintains 34 % peptides having number of residues between 10 to 20 residues. There are 147 peptides having more than 20 amino acids.
Figure 2.
Distribution of peptides according to length (A), Food Matrix (B), Length (C) Activity in FermFooDB database (D) The distribution of Multifunctional Peptides.
3.2. Peptide functions
As shown in Figure 2C, 60.4% of peptide exhibited Ace-inhibitory activity, followed by 14.3% with the antihypertensive properties and 8.7% with antioxidant activities. The rest of the peptides carried the antimicrobial, immunomodulatory and antibacterial properties. It is interesting to note that our database has large number of multifunctional peptides (Figure 2D). A multifunctional peptide is peptide having two or more functions. Even few peptides have more than 10 therapeutic functions (Table1.). Around 400 peptides have single function, the rest of the peptide have multiple functions. In Lactobacillus helveticus, the presence of four different paralogs of CEP PrtH i.e. PrtH1, PrtH2, PrtH3, and PrtH4 and their strain dependent distribution and diverse specificities has made this strain important for generation of great diversity of peptides with multifunction. The process of generating bioactive peptides depends on many components that include substrate selection, microbial strain, proteolytic activity, and hydrolysis [40]. The bioactive properties and structural properties of peptides in hydrolysates of different proteins, is governed by the enzymes specificity, fermentation conditions, degree of hydrolysis [41], therefore, these conditions have been taken as parameters in the preparation of this database. For identification of bioactive peptides, mass spectrometric methods [42], have been used along with the combination of other advanced proteomic technologies such as MALDI-TOF MS, and UPLC-Q/TOF MS. The activities of peptides have also been validated in vivo and/or in vitro in Hypercholestrolemic mice, Humans and spontaneously hypertensive rats (SHR).
Table 1.
Top 10 Multi-functional Peptides in the FermFooDB with starter cultures.
| Peptide Sequence | Activities | Starter Cultures | Food |
|---|---|---|---|
| YP | Ace-inhibitory, Antagonistic, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Opioidantagonist, Cholesterol-lowering, Cytomodulatory, Immunomodulatory, Opioid, Opioid-agonist, Opioid-antagonist | Lactobacillus helveticus CPN4 | Yogurt Like product |
| FP | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, CasoxinC (Opioidantagonist), Cholesterol-lowering, Cytomodulatory, Immunomodulatory, Mitogene, Opioid, Opioid-agonist | Lactococcus lactis subsp. lactis (80%) and Leuconostoc mesenteroides subsp. dextranicum (20%), | Raw Ewe's milk, Manchego cheese |
| PFP | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Cytomodulatory, Immunomodulatory, Mitogene, Opioid, Opioid-agonist | WSE of commercial manchego cheese (ewe's milk) | |
| VP | Ace-inhibitory, Anti-hypertensive, Anti-inflammatory, Anti-microbial, Antioxidant, Attenuate hepatitis, Cholesterol-lowering, Immunomodulatory, Intestinal protection, LOX inhibitor | A. sojae | Fermented Soybean Seasoning |
| QEPV | Ace-inhibitory, Anti-hypertensive, Anti-inflammatory, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Immunomodulatory, Mitogene | Lactobacillus paracasei subsp. paracasei BGHN14 | Beta casein |
| PQ | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Immunomodulatory, LOX inhibitor, Opioid | Water soluble Extract (WSE) of commercial mahon cheese (cow milk) | |
| PK | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cytomodulatory, Immunomodulatory, Intestinal protection, Opioid | WSE of commercial mahon cheese (cow milk) | |
| VRGPFPIIV | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Immunomodulatory, Mitogene | Enterococcus faecalis CECT5727 | Milk |
| VRGPFP | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Immunomodulatory, Mitogene | Lactococcus lactis subsp. lactis (80%) and Leuconostoc mesenteroides subsp. dextranicum (20%), | Raw Ewe's milk, Manchego cheese |
| VRGP | Ace-inhibitory, Anti-hypertensive, Anti-microbial, Antibacterial, Antioxidant, Antithrombotic, Cholesterol-lowering, Immunomodulatory, Mitogene | WSE of commercial goat cheese |
3.3. Case study
In order to describe effective usage of our web server FermFooDb, we demonstrate application by a case study. Ace inhibiting activity of the peptides released during the food processing (gastrointestinal digestion) help in reducing the blood pressure. Thus, fermented foods having this type of bioactive function can be great functional food. Using the advanced search option of our server, we found 859 entries in the FermFooDb database. Of the searched peptides, there is an example peptide “VRGPFPIIV” that is found to have ACE-inhibitor activity and found in E. faecalis hydrolysates along with their IC50 values from three studies. One of these studies shows the activity in In vitro models and other two studies shows the activity in In vivo models. These types of lead peptides which show similar activities in different studies provides high probability candidate peptides for further studies. Many of these lead candidates can be easily searched in the FermFooDb database.
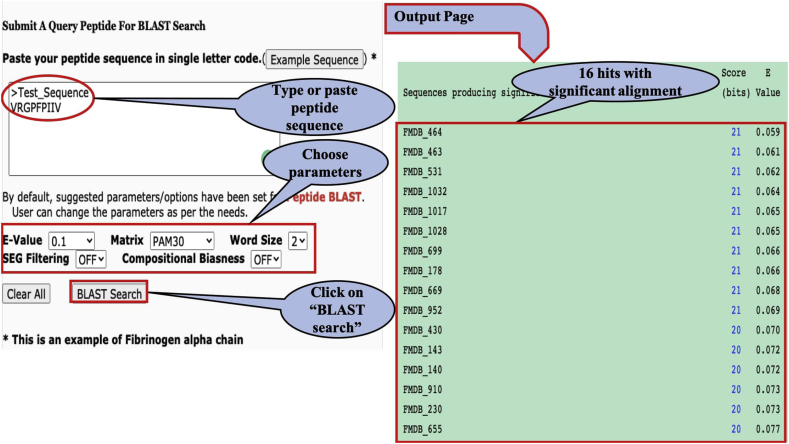
It has been shown in the past that similar proteins sequences exhibit similar protein function. Thus, we integrate many tools in this database, which allow users to perform different types of similarity. In order to demonstrate utility of similarity function, we perform similarity search of a query peptide against peptides in FermFooDb using BLAST. In above, paragraph, we demonstrate application of advance search option. We used same example peptide “VRGPFPIIV” and perform BLAST search using similarity module “BLAST” (with e-value of 0.1, matrix PAM30, word size 2). This results to 16 peptides/entries; these peptides are shows high sequence similarity with query peptide “VRGPFPIIV”. It means there is high probability that these peptides also have Ace inhibiting activity. In order to demonstrate steps involved in similarity, screen shots of submission form and BLAST output are shown in Figure 3.
Figure 3.
Illustration of similarity search module of FermFooDB.
4. Discussion
The fermentation of the food results in the hydrolysis of the protein of different matrices by the proteolytic lactic acid bacteria, which produces novel enzymes that are more stable than the enzymes derived from plants and animals [43]. As comparative to high molecular peptides, low molecular weight peptides (<10 kDa) have been found to be more effective antihypertensive and antioxidants peptides [44, 45, 46]. In fermentation, different microorganisms (e.g., bacteria, yeasts, and fungi) are used to break down the protein substrate into smaller peptides. Various enzymes and the microorganisms are used in exponential growth phase for serving as the starter culture for the protein substrate [13, 47]. Hydrolysis of the protein substrate depends on the protein source, microbial strain, temperature, pH, and incubation time for fermentation. It has been demonstrated that when the whey protein is fermented with Lactobacillus brevis as compared to other Lactobacillus species, the generation of bioactive peptides exhibited stronger ACE-inhibitory activity [48].
The production and characterization of new bioactive peptides depends on the substrate type, microbial strain, proteolytic enzyme, and hydrolysis conditions [40]. After the protein hydrolysis, the hydrolysate is evaluated for biological activity in vitro and bioactive peptide is identified and characterized. However, this method has certain limitations because of its reliance on substrate selection and dearth of standardized protocols for biological activities assessment. This drawback can be overcome by the use of dedicated software tools such as, PeptideCutter software, which can be used to simulate protein hydrolysis and expected sequence of the peptides. With the use of this tool one can select the opposite protein substrate and enzyme for generation of particular bioactive peptides of interest [49].
Across the globe, people have used different fermented milk products, fermented fruit or vegetable proteins, and soybean products (tempeh, tofu, and natto), that are rich sources of food peptides, with bioactive properties [17, 50]. Some individuals have allergy to milk protein, that can be decreased by its hydrolysis during fermentation. The presence of bioactive peptides in milk protein after its hydrolysis, makes it very significant in infant foods throughout the world [51]. Due to ready availability of peptide synthesis, BAPs can be amended for activity improvement, leading to milk protein-derived bioactive peptides analogs [52].
In our database the presence of two broadly studied peptides {b-casein f(74–76) (IPP) and b-casein f (84–86) (VPP)} in fermented milk products obtained through the use of Lactobacillus helveticus LBK16H, have shown antihypertensive effects in vivo. The same peptides have been obtained in the Calpis sour milk through the use of starter culture of Lactobacillus helveticus and Saccharomyces cerevisiae. These two peptides having the blood pressure-reducing properties have also been included in food products as nutraceuticals [53], as IPP and VPP ingestion in human beings, displayed no side effects from their continuous use.
In comparison to commercial ACE-inhibitors, the ACE-inhibitory effect produced by the Lactobacillus helveticus fermentates [54] was relatively low, but the fermented milk reduced the conversion of angiotensin I to angiotensin II [55]. The common blood pressure lowering medicine, captopril, has an IC50 of 0.015 lM [56], and is twenty times more active than the most active ACE-inhibitory peptides of milk proteins. However, this medicine may be accompanied by few side-effects.
The peptide accountable for the antihypertensive effect has also been observed in the Yogurt like product, in which Lactobacillus helveticus CPN4 was used as starter culture. In SHR, YP bioactive peptide showed a decline in systolic blood pressure of -27.4 mmHg with a dosage of 1 mg/kg from 2 to 8h and the effect was maximal at 6 h after oral administration [57]. Similar antihypertensive effect due to YP bioactive peptide was observed in casein hydrolysate produced by Lb. helveticus CP790 proteinase [58]. An IC50 value of 720 μM for YP was observed by Yamamoto and Takano (1999), whereas the presence of proline at the N-terminal end in tripeptide displayed more stronger activity [59]. Bioactive Peptides which exhibits strong ACE-inhibitory activity have also been recognized for their cellular adhesion inhibition activity [60].
“FP” bioactive dipeptide is being formed by the fermentation of Raw Ewe's milk, and Manchego cheese. The potential health promoting ability of bioactive peptide seems to be governed by the suitable peptide length as determined by the structure-activity data [61]. The predominant protein source of all the ACE-inhibitory peptide was from the caseins of different milk of various animals and different cheeses. The proteolysis of caseins of different cheeses by lactic acid bacteria and other bacteria during ripening process produces various peptides of different length.
ACE-inhibitory peptides have become significant because of their function of blood pressure reduction via the inhibition of ACE though binding. Aromatic residues (phenylalanine, tyrosine, tryptophan) and proline enhance binding at the C-terminal end, whilst dicarboxylic residues (glutamic acid, aspartic acid) decrease binding. Whereas, the presence of branched-chain amino acids (leucine, isoleucine, valine) at N-terminus, enhances binding [62]. The positioning of Proline at the third-to-last position improves ACE binding affinity [63]. Moreover, binding to ACE is generally enhanced with the presence of proline as the antepenultimate residue in the functional site of the enzyme [64]. Furthermore, it has been observed that most ACE-inhibitory peptides generally consist of proline residue [59] that enhances the bioactive peptide's resistance to digestion [65].
Most peptides with antioxidant activities were from fermented milk, also from whole wheat flour, spelt, Rye, Kamut, rice wine and red wine. Bioactive peptide with antioxidant properties ranged from 2 to 14 amino acids. In general, at the N-terminal and/or C-terminal position of antioxidative peptides hydrophobic amino acids such as proline, tyrosine or histidine have been found [66], that increases the interaction with fatty acid radicals. In our database, antioxidant peptides sequence either comprised of one histidine/tyrosine/proline. The amino acid Histidine is projected to trap and chelate free radicals and tyrosine helps in reduction of free radicals [66].
Some of the antioxidants peptide also contain “EL” dipeptide at the C- terminal end [67], as we have in our database a bioactive peptide sequence such as “APFPEVFGKEKVNEL” from the fermented lassi, where the Lb.acidophilus NCDC-15, Lactococcus lactis NCDC-167 and S. thermophiles have been used as starter culture. The other sequences (VFGKEKVNEL) and EEL with the EL dipeptide at the C-terminal end and having antioxidant property have been found in the fish oil enriched yoghurt and in water soluble extract of Spanish cheese.
Fermented food database also consists of the peptides which showed antimicrobial properties against the Enterobacter sakazakii ATCC12868, and E.coli DPC5063, Cronobacter sakazakii DPC6440, S. aureus, E.coli ATCC8099 and B. megaterium F6. These antimicrobial peptides were mainly seen in milk, casein (Kefir), casein (raw milk and fermented milk in the form of lassi). Lysine at the different sequences in bioactive peptides and presence of arginine are significant in imparting antimicrobial activity [68] The specific interest in the antimicrobial properties of milk-derived peptides is due to their low toxicity in the people with celiac diseases and good tolerance to bile salts and viability in gastric juices. The mechanism of these antimicrobial peptides is due to the targeted gram positive and gram negative bacterial cell membrane interaction leading to increase in membrane permeability [30]. The antimicrobial peptides exert this effect through the formation of pores in the bacterial cell membrane or by interaction with the macromolecules of microbial cells.
Moreover, due to the growing awareness for health among the youth, and rising interest in vegetarian foods, bioactive peptide rich plant proteins (i.e. from rice and Soy) may find their way in the global market [69]. Food industry generally prepares the bioactive peptides with enzymatic hydrolysis using pepsin, trypsin and papain [27] or microbial fermentation [15] and some uses both methods in combination [70]. For integrated approach, most of the researchers generally combine both in vitro and in vivo studies with the in-silico approaches [71, 72].
4.1. Comparison of FermFoodDb with currently existing databases
Presently, PBDB, is the only database that contains 1730 known probiotics from traditional fermented foods, that provides related biological information and reveals the probiotics mechanisms for the prediction of novel probiotics from unidentified microbial collections [73]. Nevertheless, it doesn't reveal the information about the bioactive peptides which imparts different kinds of multifunction properties to the fermented foods and their human health associated benefits. The database of human milk and dairy derived bioactive peptides, Milk Bioactive Peptide Database, MBPDB [74] delivers the information on multifunctional traits such as antihypertensive, antimicrobial, opioid, antioxidant, and anti-inflammatory properties, described in the literature. This database gives the information on sites of Milk protein with maximum quantity of bioactive peptides and also the various bioactive milk peptides of different species. Nevertheless, this database doesn't reflect about the fermented milk proteins and dairy products from various sources. FeptideDB is a collection of bioactive peptide (BAP) data from both published articles and available bioactive peptide databases which helps in prediction of bioactive peptides from food proteins with the use of peptide cutters and then matching with the various databases [75].
Our manually curated database “FermFooDb” contains mostly the entries from the fermentation of different food-derived protein substrates carried out by different Lactobacilli strains for bioactive peptide production. The present database lacks information about the primary and secondary structures of peptides as well as the lipophilicity and charge of peptides that may govern the peptide transport across the intestinal wall, thereby limiting their bioavailability of food-derived peptides.
The database will be updated regularly through crowd-sourcing of information through the Data submission link on the webpage (https://webs.iiitd.edu.in/raghava/fermfoodb/submit.php). We hope this database will be highly useful to the researchers and industry people.
4.2. Case study
Ace inhibiting activity of peptides released during food processing and gastrointestinal digestion of fermented foods helps in reducing blood pressure. In our database, on using the Browse option, we found that there are 859 entries of ACE-inhibitory peptides. Specifically, Enterococcus faecalis (15 strains) isolated from different food and environmental sources, when grown in bovine skim milk (BSM) produced diverse ACE-inhibitory peptides. Furthermore, two peptides, analogous to β-casein f(133–138) (LHLPLP) and f(58–76) (LVYPFPGPIPNSLPQNIPP), showed ACE inhibitory activity (i.e. IC50 values) as low as 5 μM. Additionally as compared to captopril, these ACE inhibitors derived from food proteins displayed higher in vivo activities than in vitro activities. Hence, these peptides having their origin from food substrates can be explored for the functional foods development. Many of such kinds of peptides can easily be searched in the FermFooDb database.
5. Conclusion
FermFooDB is a web application tool that gives quick identification and analysis of bioactive peptides with a user-friendly interface. This database will be helpful to the food industry in the selection of the bioactive peptides which can be used as a food in the functional foods with different milk, plant and animal protein substrates. Although maximum number of peptides from different food matrices with all possible information has been collected from the available literature but still there are chances of upgradation as the structure–functional properties of such bioactive peptides, their toxicity/bitterness properties, and consistent performance are still missing.
Declarations
Author contribution statement
Anita Chaudhary, Gajendra P. S. Raghava, Girish Sahni: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Sherry Bhalla, Sumeet Patiyal: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by ICAR-IARI, New Delhi.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are thankful for the infrastructure and facilities provided by the Director-IMTECH, Chandigarh for carrying out this activity. Authors also would like to thank Director, ICAR-IARI, New Delhi for sending Dr. Anita Chaudhary on deputation to CSIR-IMTECH, Chandigarh for working on this project.
References
- 1.Li-Chan E.C.Y. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015;1:28–37. [Google Scholar]
- 2.Lassoued I., Mora L., Barkia A., Aristoy M.-C., Nasri M., Toldra F. Bioactive peptides identified in thornback ray skin’s gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. J. Proteomics. 2015;128:8–17. doi: 10.1016/j.jprot.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Carr F.J., Chill D., Maida N. The lactic acid bacteria: a literature survey. Crit. Rev. Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths M.W., Tellez A.M. Lactobacillus helveticus: the proteolytic system. Front. Microbiol. 2013;4:30. doi: 10.3389/fmicb.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hou J., Friedrich A., Gounot J.-S., Schacherer J. Comprehensive survey of condition-specific reproductive isolation reveals genetic incompatibility in yeast. Nat. Commun. 2015;6:7214. doi: 10.1038/ncomms8214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcantara C., Bauerl C., Revilla-Guarinos A., Perez-Martinez G., Monedero V., Zuniga M. Peptide and amino acid metabolism is controlled by an OmpR-family response regulator in Lactobacillus casei. Mol. Microbiol. 2016;100:25–41. doi: 10.1111/mmi.13299. [DOI] [PubMed] [Google Scholar]
- 7.Vukotić G., Strahinić I., Begović J., Lukić J., Kojić M., Fira D. Survey on proteolytic activity and diversity of proteinase genes in mesophilic lactobacilli. Microbiol. (Russian Fed. 2016;85:33–41. [Google Scholar]
- 8.Villegas J.M., Brown L., Savoy de Giori G., Hebert E.M. Characterization of the mature cell surface proteinase of Lactobacillus delbrueckii subsp. lactis CRL 581. Appl. Microbiol. Biotechnol. 2015;99:4277–4286. doi: 10.1007/s00253-014-6258-6. [DOI] [PubMed] [Google Scholar]
- 9.Stefanovic E., Fitzgerald G., McAuliffe O. Advances in the genomics and metabolomics of dairy lactobacilli: a review. Food Microbiol. 2017;61:33–49. doi: 10.1016/j.fm.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Sadat-Mekmene L., Genay M., Atlan D., Lortal S., Gagnaire V. Original features of cell-envelope proteinases of Lactobacillus helveticus. A review. Int. J. Food Microbiol. 2011;146:1–13. doi: 10.1016/j.ijfoodmicro.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z., Harris H.M.B., McCann A., Guo C., Argimon S., Zhang W., Yang X., Jeffery I.B., Cooney J.C., Kagawa T.F., Liu W., Song Y., Salvetti E., Wrobel A., Rasinkangas P., Parkhill J., Rea M.C., O’Sullivan O., Ritari J., Douillard F.P., Paul Ross R., Yang R., Briner A.E., Felis G.E., de Vos W.M., Barrangou R., Klaenhammer T.R., Caufield P.W., Cui Y., Zhang H., O’Toole P.W. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Commun. 2015;6:8322. doi: 10.1038/ncomms9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayyash M., Al-Nuaimi A.K., Al-Mahadin S., Liu S.-Q. In vitro investigation of anticancer and ACE-inhibiting activity, alpha- amylase and alpha-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: a comparative study with fermented bovine milk. Food Chem. 2018;239:588–597. doi: 10.1016/j.foodchem.2017.06.149. [DOI] [PubMed] [Google Scholar]
- 13.Rizzello C.G., Lorusso A., Russo V., Pinto D., Marzani B., Gobbetti M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017;241:252–261. doi: 10.1016/j.ijfoodmicro.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Marco M.L., Heeney D., Binda S., Cifelli C.J., Cotter P.D., Foligne B., Ganzle M., Kort R., Pasin G., Pihlanto A., Smid E.J., Hutkins R. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Chaves-López C., Serio A., Paparella A., Martuscelli M., Corsetti A., Tofalo R., Suzzi G. Impact of microbial cultures on proteolysis and release of bioactive peptides in fermented milk. Food Microbiol. 2014;42:117–121. doi: 10.1016/j.fm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Hayes M., Stanton C., Fitzgerald G.F., Ross R.P. Putting microbes to work: dairy fermentation, cell factories and bioactive peptides. Part II: bioactive peptide functions. Biotechnol. J. 2007;2:435–449. doi: 10.1002/biot.200700045. [DOI] [PubMed] [Google Scholar]
- 17.Leroy F., De Vuyst L. Fermented food in the context of a healthy diet: how to produce novel functional foods? Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:574–581. doi: 10.1097/MCO.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 18.Marsh A.J., Hill C., Ross R.P., Cotter P.D. Fermented beverages with health-promoting potential: past and future perspectives. Trends Food Sci. Technol. 2014;38:113–124. [Google Scholar]
- 19.Ohsawa K., Uchida N., Ohki K., Nakamura Y., Yokogoshi H. Lactobacillus helveticus–fermented milk improves learning and memory in mice. Nutr. Neurosci. 2015;18:232–240. doi: 10.1179/1476830514Y.0000000122. [DOI] [PubMed] [Google Scholar]
- 20.Ademiluyi A.O., Oboh G., Boligon A.A., Athayde M.L. Dietary supplementation with fermented legumes modulate hyperglycemia and acetylcholinesterase activities in Streptozotocin-induced diabetes. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2015;22:195–201. doi: 10.1016/j.pathophys.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Ciesarová Z., Mikušová L., Magala M., Kohajdová Z., Karovičová J. In: Chapter 17 - Nonwheat Cereal-Fermented-Derived Products. Frias J., Martinez-Villaluenga C., in H. E.B.T.-F.F., Peñas D.P., editors. Academic Press; Boston: 2017. pp. 417–432. [Google Scholar]
- 22.Tidona F., Criscione A., Guastella A.M., Zuccaro A., Bordonaro S., Marletta D. I peptidi bioattivi nei prodotti lattiero-caseari. Ital. J. Anim. Sci. 2009;8:315–340. [Google Scholar]
- 23.Fan J., Hu X., Tan S., Zhang Y., Tatsumi E., Li L. Isolation and characterisation of a novel angiotensin I-converting enzyme-inhibitory peptide derived from douchi , a traditional Chinese fermented soybean food. J. Sci. Food Agric. 2009;89:603–608. [Google Scholar]
- 24.O’Keeffe M.B., Norris R., Alashi M.A., Aluko R.E., FitzGerald R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods. 2017;34:77–88. [Google Scholar]
- 25.Rizzetti D.A., Martin A., Corrales P., Fernandez F., Simoes M.R., Pecanha F.M., Vassallo D.V., Miguel M., Wiggers G.A. Egg white-derived peptides prevent cardiovascular disorders induced by mercury in rats: role of angiotensin-converting enzyme (ACE) and NADPH oxidase. Toxicol. Lett. 2017;281:158–174. doi: 10.1016/j.toxlet.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Sable R., Parajuli P., Jois S. Peptides, peptidomimetics, and polypeptides from marine sources: a wealth of natural sources for pharmaceutical applications. Mar. Drugs. 2017;15 doi: 10.3390/md15040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryder K., Bekhit A.E.-D., McConnell M., Carne A. Towards generation of bioactive peptides from meat industry waste proteins: generation of peptides using commercial microbial proteases. Food Chem. 2016;208:42–50. doi: 10.1016/j.foodchem.2016.03.121. [DOI] [PubMed] [Google Scholar]
- 28.Pepe G., Sommella E., Ventre G., Scala M.C., Adesso S., Ostacolo C., Marzocco S., Novellino E., Campiglia P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods. 2016;26:494–505. [Google Scholar]
- 29.Mora L., Escudero E., Arihara K., Toldra F. Antihypertensive effect of peptides naturally generated during Iberian dry-cured ham processing. Food Res. Int. 2015;78:71–78. doi: 10.1016/j.foodres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty D., Jena R., Choudhury P.K., Pattnaik R., Mohapatra S., Saini M.R. Milk derived antimicrobial bioactive peptides: a review. Int. J. Food Prop. 2016;19:837–846. [Google Scholar]
- 31.Théolier J., Fliss I., Jean J., Hammami R. MilkAMP: a comprehensive database of antimicrobial peptides of dairy origin. Dairy Sci. Technol. 2014;94:181–193. [Google Scholar]
- 32.Gogoladze G., Grigolava M., Vishnepolsky B., Chubinidze M., Duroux P., Lefranc M.-P., Pirtskhalava M. DBAASP: database of antimicrobial activity and structure of peptides. FEMS Microbiol. Lett. 2014;357:63–68. doi: 10.1111/1574-6968.12489. [DOI] [PubMed] [Google Scholar]
- 33.Qureshi A., Thakur N., Tandon H., Kumar M. AVPdb: a database of experimentally validated antiviral peptides targeting medically important viruses. Nucleic Acids Res. 2014;42:D1147–D1153. doi: 10.1093/nar/gkt1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gautam A., Singh H., Tyagi A., Chaudhary K., Kumar R., Kapoor P., Raghava G.P.S. CPPsite: a curated database of cell penetrating peptides. Database. 2012;2012 doi: 10.1093/database/bas015. bas015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor P., Singh H., Gautam A., Chaudhary K., Kumar R., Raghava G.P.S. TumorHoPe: a database of tumor homing peptides. PloS One. 2012;7 doi: 10.1371/journal.pone.0035187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gautam A., Chaudhary K., Singh S., Joshi A., Anand P., Tuknait A., Mathur D., Varshney G.C., Raghava G.P.S. Hemolytik: a database of experimentally determined hemolytic and non- hemolytic peptides. Nucleic Acids Res. 2014;42:D444–D449. doi: 10.1093/nar/gkt1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanlier N., Gokcen B.B., Sezgin A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019;59:506–527. doi: 10.1080/10408398.2017.1383355. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R., Chaudhary K., Sharma M., Nagpal G., Chauhan J.S., Singh S., Gautam A., Raghava G.P.S. AHTPDB: a comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015;43:D956–D962. doi: 10.1093/nar/gku1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimsheena V.K., Gowda L.R. Arachin derived peptides as selective angiotensin I-converting enzyme (ACE) inhibitors: structure-activity relationship. Peptides. 2010;31:1165–1176. doi: 10.1016/j.peptides.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Korhonen H. Milk-derived bioactive peptides: from science to applications. J. Funct. Foods. 2009;1:177–187. [Google Scholar]
- 41.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J. Food Sci. 2012;77:R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 42.Valdés A., Cifuentes A., León C. Foodomics evaluation of bioactive compounds in foods. TrAC Trends Anal. Chem. (Reference Ed.) 2017;96:2–13. [Google Scholar]
- 43.Nguyen T.H., Nguyen V.D. Characterization and applications of marine microbial enzymes in biotechnology and probiotics for animal health. Adv. Food Nutr. Res. 2017;80:37–74. doi: 10.1016/bs.afnr.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Musoles R., Manzanares P., Burguete M.C., Alborch E., Salom J.B. In vivo angiotensin I-converting enzyme inhibition by long-term intake of antihypertensive lactoferrin hydrolysate in spontaneously hypertensive rats. Food Res. Int. 2013;54:627–632. [Google Scholar]
- 45.Ruiz-Ruiz J., Dávila-Ortíz G., Chel-Guerrero L., Betancur-Ancona D. Angiotensin i-converting enzyme inhibitory and antioxidant peptide fractions from hard-to-cook bean enzymatic hydrolysates. J. Food Biochem. 2013;37:26–35. [Google Scholar]
- 46.Garcia-Tejedor A., Sanchez-Rivera L., Castello-Ruiz M., Recio I., Salom J.B., Manzanares P. Novel antihypertensive lactoferrin-derived peptides produced by Kluyveromyces marxianus: gastrointestinal stability profile and in vivo angiotensin I-converting enzyme (ACE) inhibition. J. Agric. Food Chem. 2014;62:1609–1616. doi: 10.1021/jf4053868. [DOI] [PubMed] [Google Scholar]
- 47.Aguilar-Toala J.E., Santiago-Lopez L., Peres C.M., Peres C., Garcia H.S., Vallejo-Cordoba B., Gonzalez-Cordova A.F., Hernandez-Mendoza A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017;100:65–75. doi: 10.3168/jds.2016-11846. [DOI] [PubMed] [Google Scholar]
- 48.Ahn J.E., Park S.Y., Atwal A., Gibbs B.F., Lee B.H. Angiotensin I-converting enzyme (ACE) inhibitory peptides from whey fermented BY lactobacillus species. J. Food Biochem. 2009;33:587–602. [Google Scholar]
- 49.Carrasco-Castilla J., Hernández-Álvarez A.J., Jiménez-Martínez C., Gutiérrez-López G.F., Dávila-Ortiz G. Use of proteomics and peptidomics methods in food bioactive peptide science and engineering. Food Eng. Rev. 2012;4:224–243. [Google Scholar]
- 50.Chaudhary A., Sharma D.K., Arora A. 2018. Prospects of Indian Traditional Fermented Food as Functional Foods. [Google Scholar]
- 51.Vandenplas Y. Prevention and management of cow’s milk allergy in non-exclusively breastfed infants. Nutrients. 2017;9 doi: 10.3390/nu9070731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClean S., Beggs L.B., Welch R.W. Antimicrobial activity of antihypertensive food-derived peptides and selected alanine analogues. Food Chem. 2014;146:443–447. doi: 10.1016/j.foodchem.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 53.Cicero A.F.G., Gerocarni B., Laghi L., Borghi C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J. Hum. Hypertens. 2011;25:425–436. doi: 10.1038/jhh.2010.85. [DOI] [PubMed] [Google Scholar]
- 54.Mathur H., Beresford T.P., Cotter P.D. Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients. 2020;12 doi: 10.3390/nu12061679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuglsang A., Rattray F.P., Nilsson D., Nyborg N.C.B. Lactic acid bacteria: inhibition of angiotensin converting enzyme in vitro and in vivo. Antonie Leeuwenhoek. 2003;83:27–34. doi: 10.1023/a:1022993905778. [DOI] [PubMed] [Google Scholar]
- 56.Ben Henda Y., Labidi A., Arnaudin I., Bridiau N., Delatouche R., Maugard T., Piot J.-M., Sannier F., Thiery V., Bordenave-Juchereau S. Measuring angiotensin-I converting enzyme inhibitory activity by micro plate assays: comparison using marine cryptides and tentative threshold determinations with captopril and losartan. J. Agric. Food Chem. 2013;61:10685–10690. doi: 10.1021/jf403004e. [DOI] [PubMed] [Google Scholar]
- 57.Nakamura Y., Yamamoto N., Sakai K., Takano T. Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme. J. Dairy Sci. 1995;78:1253–1257. doi: 10.3168/jds.S0022-0302(95)76745-5. [DOI] [PubMed] [Google Scholar]
- 58.Maeno M., Yamamoto N., Takano T. Identification of an antihypertensive peptide from casein hydrolysate produced by a proteinase from Lactobacillus helveticus CP790. J. Dairy Sci. 1996;79:1316–1321. doi: 10.3168/jds.S0022-0302(96)76487-1. [DOI] [PubMed] [Google Scholar]
- 59.Maruyama S., Mitachi H., Awaya J., Kurono M., Tomizuka N., Suzuki H. Angiotensin I-converting enzyme inhibitory activity of the C-terminal hexapeptide of α s1 -casein. Agric. Biol. Chem. 1987;51:2557–2561. [Google Scholar]
- 60.Aihara K., Ishii H., Yoshida M. Casein-derived tripeptide, Val-Pro-Pro (VPP), modulates monocyte adhesion to vascular endothelium. J. Atherosclerosis Thromb. 2009;16:594–603. doi: 10.5551/jat.729. [DOI] [PubMed] [Google Scholar]
- 61.Clare D.A., Swaisgood H.E. Bioactive milk peptides: a prospectus. J. Dairy Sci. 2000;83:1187–1195. doi: 10.3168/jds.S0022-0302(00)74983-6. [DOI] [PubMed] [Google Scholar]
- 62.Cheung H.S., Wang F.L., Ondetti M.A., Sabo E.F., Cushman D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980;255:401–407. [PubMed] [Google Scholar]
- 63.Rohrbach M.S., Williams E.B.J., Rolstad R.A. Purification and substrate specificity of bovine angiotensin-converting enzyme. J. Biol. Chem. 1981;256:225–230. [PubMed] [Google Scholar]
- 64.Maruyama S., Nakagomi K., Tomizuka N., Suzuki H. Angiotensin i-converting enzyme inhibitor derived from an enzymatic hydrolysate of casein. Ii. isolation and bradykinin-potentiating activity on the uterus and the ileum of rats. Agric. Biol. Chem. 1985;49:1405–1409. [Google Scholar]
- 65.Yang C.M., Russell J.B. Resistance of proline-containing peptides to ruminal degradation in vitro. Appl. Environ. Microbiol. 1992;58:3954–3958. doi: 10.1128/aem.58.12.3954-3958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y.-W., Li B. Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: key sequence positions and their amino acid properties. J. Theor. Biol. 2013;318:29–43. doi: 10.1016/j.jtbi.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 67.Suetsuna K., Ukeda H., Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000;11:128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez-Ordóñez A., Begley M., Clifford T., Deasy T., Considine K., Hill C. Structure-activity relationship of synthetic variants of the milk-derived antimicrobial peptide αs2-casein f(183-207) Appl. Environ. Microbiol. 2013;79:5179–5185. doi: 10.1128/AEM.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salvatore S., Vandenplas Y. Hydrolyzed proteins in allergy. Nestle Nutr. Inst. Workshop Ser. 2016;86:11–27. doi: 10.1159/000442699. [DOI] [PubMed] [Google Scholar]
- 70.Boukil A., Suwal S., Chamberland J., Pouliot Y., Doyen A. Ultrafiltration performance and recovery of bioactive peptides after fractionation of tryptic hydrolysate generated from pressure-treated Β-lactoglobulin. J. Membr. Sci. 2018;556:42–53. [Google Scholar]
- 71.Han J., Tang S., Li Y., Bao W., Wan H., Lu C., Zhou J., Li Y., Cheong L., Su X. In silico analysis and in vivo tests of the tuna dark muscle hydrolysate anti-oxidation effect. RSC Adv. 2018;8:14109–14119. doi: 10.1039/c8ra00889b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agyei D., Bambarandage E., Udenigwe C. 2019. The Role of Bioinformatics in the Discovery of Bioactive Peptides. [Google Scholar]
- 73.Zhao W., Liu Y., Latta M., Ma W., Wu Z., Chen P. Probiotics database: a potential source of fermented foods. Int. J. Food Prop. 2019;22:198–217. [Google Scholar]
- 74.Nielsen S.D., Beverly R.L., Qu Y., Dallas D.C. Milk bioactive peptide database: a comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017;232:673–682. doi: 10.1016/j.foodchem.2017.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panyayai T., Ngamphiw C., Tongsima S., Mhuantong W., Limsripraphan W., Choowongkomon K., Sawatdichaikul O. FeptideDB: a web application for new bioactive peptides from food protein. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.