Abstract
There is increasing evidence for graphene associated plant growth promotion, however, the chronic effects of soil-applied graphene remain largely unexplored. The present study investigated the morphological, physiological and biochemical responses of graphene oxide (GO) on Aloe vera L. over the concentration range of 0–100 mg/L for four months. Our results demonstrated that GO, with the best efficiency at 50 mg/L, could enhance the photosynthetic capacity of leaves, increase the yield and morphological characters of root and leaf, improve the nutrient (protein and amino acid) contents of leaf, without reducing the content of the main bioactive compound aloin. Compared with leaves, the effect of GO on root growth was more obvious. Although the electrolyte leakage and MDA content were raised at high concentrations, GO treatment did not increase the root antioxidant enzymes activity or decrease the root vigor, which excluding typical stress response. Furthermore, injection experiments showed that the GO in vivo did not change the plant growth state obviously. Taken together, our study revealed the role of GO in promoting Aloe vera growth by stimulating root growth and photosynthesis, which would provide theory basis for GO application in agriculture and forestry.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12298-021-00979-3.
Keywords: Graphene oxide, Aloe vera L., Growth, Positive effects
Introduction
Graphene, the two dimensional carbon nanoparticle, has attracted increasing attention in the last few years. It is regarded as one of the most promising engineered nanomaterials for its huge surface area, unparalleled mechanical property, electrical and thermal conductivity (Avouris 2010). Graphene oxide (GO) is a graphene derivative that carries many oxygen-containing functional groups, such as carboxyl, epoxy, hydroxyl, and carbonyl groups (De Jesus et al. 2010). These groups endow GO with higher water dispersity and better biocompatibility. GO has been applied to printing electronics, catalysis, separation membranes, energy storage, biomedicine and composites, however, just begun to show its potential for agriculture and forestry (Chakravarty et al. 2015; Kabiri et al. 2017; Li et al. 2015; Tonelli et al. 2015; Zhao et al. 2020). In recent years, researches indicated that GO could be used as the fertilizer carrier to reduce the release rate and improve the utilization efficiency of nutrients, which makes these materials suitable for developing new slow-release fertilizers (Andelkovic et al. 2018; Kabiri et al. 2017; Zhang et al. 2014). As the essential primary component of all ecosystems, plants will be in contact with the graphene released to soil constantly (Spielman-Sun et al. 2017). Therefore, the long-term effects of graphene on plants should be understood before applying large amounts of graphene for agroforestry.
A lot of experiments show that functional graphene could stimulate the physiological processes of certain plants at low concentrations. For instance, hydrated graphene ribbon could promote aged seed germination and root differentiation of wheat at 200 mg/L (Hu and Zhou 2014). The growth of coriander and garlic plants was enhanced by graphene quantum dots at 200 mg/L (Chakravarty et al. 2015). Sulfonated graphene at 50 mg/L motivated maize growth by enhancing ROS scavenging, alleviating oxidative stress, improving the soluble protein content, and decreasing intracellular Ca2+ and cell death in the roots (Ren et al. 2016). GO could act as a water transporter in accelerating seed germination in spinach and chive at 50 mg/L (He et al. 2018). Gossypium hirsutum and Catharanthus roseus exposed to 50 and 200 mg/L of GO exhibited higher germination rate and yield and shorter growing period (Pandey et al. 2019).
In addition, adverse effects were reported by researchers concerning about the environmental risk of graphene (Wang et al. 2019). For example, graphene induces growth inhibition, pH alteration, metabolic disturbance, cell death, oxidative stress, decrease in photosynthetic capacity and nutritional level, morphological defects, and accumulation of heavy metals in cabbage, tomato, red spinach, lettuce, arabidopsis, faba bean, wheat, maize and rice (Anjum et al. 2014; Begum and Fugetsu 2013; Begum et al. 2011; Gao et al. 2019; Guo et al. 2020; Ren et al. 2016; Wang et al. 2019; Zhang et al. 2016). However, almost all these studies were conducted at high concentrations by hydroponic experiment under acute toxicity to plant. In the natural environment, and even for practical production, the GO released to environment may be in the range of ng/L or μg/L (Zhao et al. 2015). Giving the complex compositions of the soil, the effect of GO in soil might differ from that in a liquid medium. Remarkably, the effect of graphene on the terrestrial environment has been assessed using Enchytraeus crypticus, which shows chronic toxicity at high concentrations (Mendonça et al. 2019). The tremendous controversy about the influence of graphene prompted us to screen for suitable graphene for a specific kinds of plants.
Aloe vera is considered an economically important plant, which has been widely employed in the medical, cosmetic, and food industries (Salehi et al. 2018). The production of Aloe vera has reached high-degree industrialization and exhibited huge market potentials (Hazrati et al. 2017). According to the reports from the market research company IMARC, the global Aloe vera market is expected to reach US$ 915 million by 2025. Aloe vera contains nine categories of active ingredients, such as anthraquinones, inorganic compounds, amino acids, fatty acids, alkaloids, carbohydrates, enzymes, vitamins and other miscellaneous compounds (Kumar 2014). As a basic component of anthraquinone, aloin was considered one of the most important active components of Aloe vera. And the aloin content was affected by zeolite treatment and water stress (Hazrati et al. 2017).
In addition to the economic importance, many traits give a vast advantage for Aloe vera as the research material. Aloe vera is mainly used as a cosmetic material, alleviating concerns about food safety to a great extent (Sun et al. 2016). Actually, graphene has been popularly used in facial masks recently. Otherwise, the hypertrophic leaves of Aloe vera are suitable for the injection experiment. No matter as a research material, or future application, Aloe vera is the best choice. To our knowledge, there is no report on the influence of nanomaterials on the growth of Aloe vera. Hence, we conducted a phenotypical, physiological and biochemical analysis to investigate the role of GO in soil with Aloe vera. Our research could help us to learn more about the biological effects of GO and prepare for the application of GO in agroforestry.
Materials and Methods
Materials and characterization
GO suspension was produced by our laboratory and diluted to final concentrations with deionized water. GO solution of different concentrations was sonicated in an ultrasonic bath for 30 min before use. GO was characterized by three techniques. GO suspension was vacuum freeze-dried and observed with a scanning electron microscope (SEM), Fourier transform infrared (FT-IR) and Raman spectra. A scanning electron microscope (TESCAN, MAIA 3 LMH) was used to obtain the morphology of GO. Dry GO powder was suspended in KBr, and FT-IR spectroscopy was performed using a Fourier transform infrared spectrometer (Bruker TENSOR 27, Germany) within the range of 500–4,000 cm−1. Raman spectra were obtained using Renishaw inVia™ Qontor with a 532 nm excitation laser.
Treatments and plant growth status analysis
Four-leaf old Aloe vera seedlings of uniform size and shape were weighed (labeled as m1) and planted in pots containing a mixture of turfy soil and vermiculite (2/1, w/w) after removal of the fibrous root. Plants grown in a culture room at 23 °C and 60–70% relative humidity under a 16-h-light/8-h-dark photoperiod were treated with pure water and GO solution. The plants were harvested after four months for subsequent analysis. For soil treatment, 350 mL GO solution with varying concentrations (0, 10, 20, 50, 100 mg/L) was supplied to every plant twice a month. For injection experiment, Aloe vera seedlings were irrigated with water twice a month throughout the experiment. The injection experiment began the second week after the plant was transplanted into the soil. Leaf injection was conducted by depressing a 1 mL disposable syringe to four leaves of every plant with a modified method twice a month throughout the experiment (Liu et al. 2009).
The longest leaf of every plant was selected for the measurement of leaf length and width. Fresh weight was measured (labeled as m2) after the plant was washed and dryed with filter paper. The root was separated and weighed immediately for root fresh weight (labeled as mroot). Because all the fibrous root was removed before transplanting, the root fresh weight is the net fresh root weight. The net leaf fresh weight was calculated as: mleaf = m2 − m1 − mroot. A fresh leaf sample was weighed before (labeled as mfresh) and after dried (labeled as mdry) following the method of (Xu et al. 2006). Leaf water content (WCleaf) was calculated as WCleaf = (mfresh – mdry) / mfresh. Electrolyte leakage was conducted with a modified method as described by (Chen et al. 2018). Different fresh root sample (0.1 g) was placed in 10 ml water with a centrifuge tube and incubated at room temperature under 100 rpm for 24 h. The electrical conductivity was detected using a conductivity meter (FiveEasy Plus, FE38, Shanghai, China) and labeled as E1. Then, the solution was boiled for 20 min, and measured for the second time after cooling to room temperature (labeled as E2). Electrolyte leakage was calculated as: Electrolyte leakage = E1/E2.
Root morphology analysis
Aloe vera seedlings were rinsed with running water to remove the soil from the roots. The underground part of seedling was cut and placed in a root scanner (EPSON Expression; China). The scanning image was analyzed by WinRHIZO software (Regent Instrument Inc., Montreal, Canada). Total root length, total surface area and total volume were measured in this experiment.
Chlorophyll fluorescence parameters assay
The maximum photochemical efficiency of PSII (Fv/Fm) and the actual photochemical efficiency of PSII (YII) were assessed with Portable Chlorophyll Fluorometer (PAM2500; Heinz Walz, Effeltrich, Germany) after the plants were pre-adapted in the dark for at least 30 min. The measurement was conducted at room temperature with the longest leaf of every plant according to a previously described method (Hazrati et al. 2016).
Analytical Methods
The materials used for total soluble sugars, total protein, amino acid and photosynthetic pigment contents analysis were taken from the middle part of the longest leaf in every plant. The materials used for root vigor, POD, CAT, SOD, MDA analysis were taken from the root of plants. Total soluble sugars were analyzed using a colorimetric method with anthrone (A800666, MACKLIN) and sulphuric acid as described (Jin et al. 2007). Chlorophyll a and chlorophyll b content assays were performed following Lichtenthaler’s method (Lichtenthaler 1987). Total protein was analyzed using Coomassie brilliant blue method, with the kit (A045-2-2) from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The amino acid was analyzed by ninhydrin colorimetry method, with the kit (TC2153) from Beijing Leigen Biotechnology Co. Ltd. (Beijing China). Root vigor was measured using triphenyltetrazolium chloride reduction method, with the assay kit (TP1025) from Beijing Leigen Biotechnology Co. Ltd. (Beijing China). Peroxidase (POD), superoxide dimutase (SOD), catalase (CAT) activities, and malondialdehyde (MDA) content were investigated with the kit (POD-2-Y, SOD-2-Y, CAT-2-Y, MDA-2-Y) from Suzhou Keming Biotechnology Co. Ltd. (Suzhou, China). The content of aloin was measured by Qingdao Sci-tech Innovation Quality Testing Co., Ltd. using a high-performance liquid chromatograph (Agilent, 1260) with chromatographic column (Agilent C18 4.6 mm*150 mm*5 μm). Aloin standard was purchased from Shanghai Yuanye Biotechnology Co., Ltd.
Statistical analysis
Plants were taken from different pots as different biological replicates. The whole experiment was repeated at least twice. For weight, morphological indexes, leaf water content, chlorophyll fluorescence, and electrolyte leakage, each data is the average of at least 5 individual measurements. For biochemical parameters, each data is the average of at least 3 individual measurements. Thus, vertical bars indicate mean ± SD. Data were analyzed mainly by one-way analysis of variance using the programs of SPSS 21. The significance of the difference between means was determined by the least significant difference (LSD) test at the 0.05 probability level.
Results
Characterization of GO
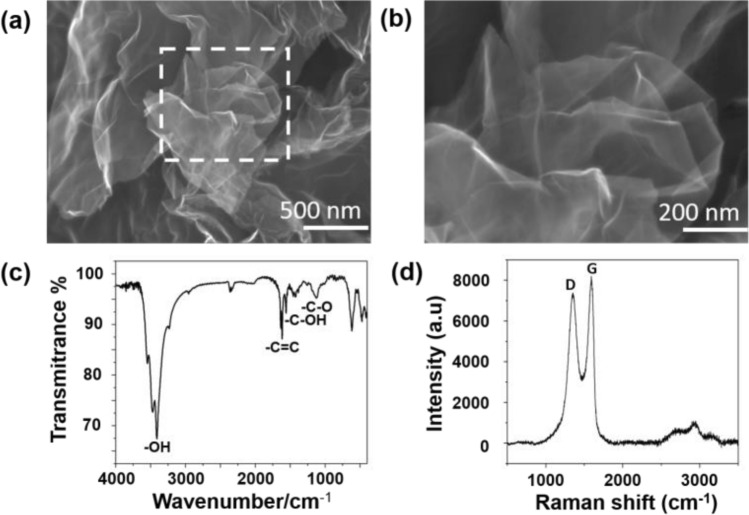
SEM was used to evaluate the morphology of GO. Figure 1a–b showed the typical SEM images. The high magnification image revealed GO with silky, folded and undulating shape as a whole (Fig. 1a). In the partially enlarged image, the GO layer was transparent and light silken, indicating that the number of GO layers was very few (Fig. 1b). The chemical composition of GO was analyzed by Fourier transform infrared spectrometer (FT-IR). FT-IR spectrum (Fig. 1c) showed that GO was significantly oxidized with different functional groups, including the C–O (1093 cm−1), C–OH (1394 cm−1), C=C (1630 cm−1), –OH (3437 cm−1). The Raman spectrum of GO was presented in Fig. 1d. The two main representatives Raman peaks of GO, which called the D band (~ 1350 cm−1) and G band (~ 1581 cm−1), were detected in the Raman spectrum. And the ratio of D band to G band intensity (ID/IG) was about 0.9 in GO sheets.
Fig. 1.
Characterization of graphene oxide (GO). a, b SEM images of GO; c FT-IR spectra of GO; d Raman spectra of GO showing the charactreistic D and G band
Effects of GO on plant growth
Many literatures revealed that nanomaterials in low doses have positive effects on plant growth and stress tolerance (Mukherjee et al. 2016; Zhao et al. 2020). In order to investigate the effects on plant growth in soil, GO solution was applied to different plants over a concentration range of 0–100 mg/L. And Aloe vera showed positive responses to GO treatment after four month (Fig. S1a and 2a).
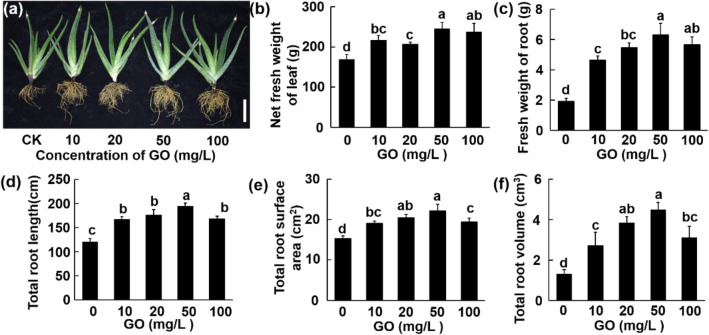
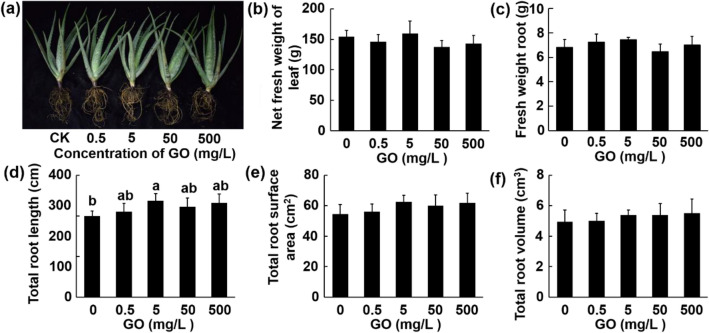
Compared with the control, with GO concentrations ranging from 10 to 100 mg/L, the net fresh weight of leaf was increased by 28.59, 22.69, 45.44, and 40.53%, respectively (Fig. 2b). GO treatments increased the average width significantly at all concentrations, while improved the average length significantly at 50 and 100 mg/L for the longest leaf in every plant (Fig. S1b–c). In comparison with the aerial part, GO showed a greater influence on root growth. Root fresh weight, total root length, total root surface area, and total root volume were all significantly elevated by different concentrations of GO treatments, and reached the peak at 50 mg/L. These four parameters of 50 mg/L group were increased by 229.76%, 61.1%, 44.73%, 238.67% than the control group, respectively (Fig. 2b–f).
Fig. 2.
Phenotype of Aloe vera seedlings after incubation with different concentrations (0–100 mg/L) of GO for 4 months. a Phenotypical image of Aloe vera grown in GO-amended soil (scale bar: 10 cm); b net fresh weight of leaf; c fresh weight of root; d total root length; e total root surface area; f total root volume. Values are means ± SD (n = 5). Means with different letters are significantly different by LSD test (p ≤ 0.05)
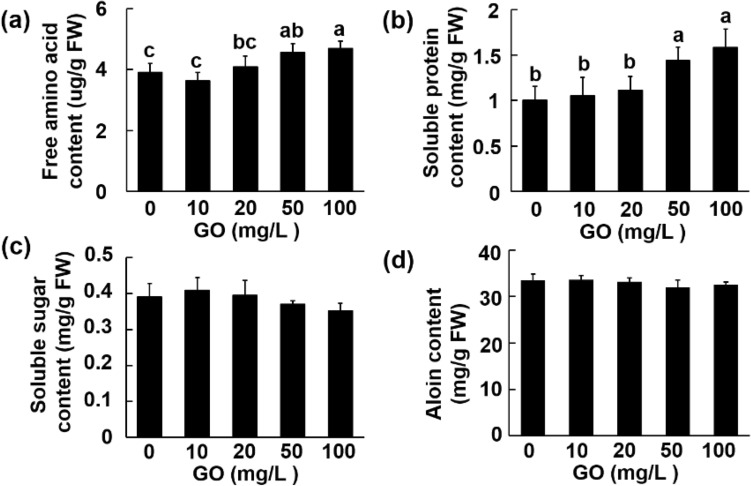
To further analyze the physiological status of the plants after GO treatment, the leaf water content and nutrients content were measured after morphological observation. As is shown in Fig. S2 there is no statistical difference in the leaf water content among different groups. The changes of nutrient contents of Aloe vera are shown in Fig. 3. The free amino acid and soluble protein contents were not altered by treatment at low concentration, however, significantly increased at 50 and 100 mg/L (Fig. 3a–b). The contents of soluble sugars and the bioactive component aloin were not significantly affected compared with the control group (Fig. 3c–d). These results demonstrated that the raised yield of Aloe vera after GO treatment is neither due to increased moisture nor at the expense of reduced nutrients.
Fig. 3.
Effect of GO on free amino acid content (a); soluble protein content (b); soluble sugar content (c); aloin content (d). Values are means ± SD (n = 3). Means with different letters are significantly different by LSD test (p ≤ 0.05)
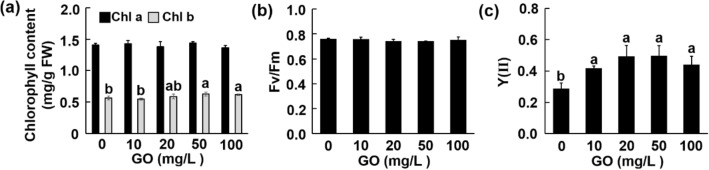
Photosynthesis was significantly inhibited by exogenous stresses (Zhang et al. 2016; Zhao et al. 2020). Pigments and chlorophyll fluorescence parameters were measured as the indicators for assessing the photosynthetic competence. GO treatment did not change the content of chlorophyll a at all concentrations, but significantly increased the content of chlorophyll b at 50 and 100 mg/L compared with control by 11% and 9.25%, respectively (Fig. 4a). There was not much change in the maximal quantum efficiency of PSII (Fv/Fm) (Fig. 4b), which is an important parameter of the physiological state (Krause and Weis 2003). Unlike Fv/Fm, another photosynthetic fluorescence parameter Y(II), which reflects the photosynthetic activity of the leaves (Dong et al. 2020), was much higher after GO treatments. Compared with control, GO enhanced the Y(II) by 45.7, 72.2, 73.7, and 53.7% from 0 to 100 mg/L, respectively (Fig. 4c). According to the data related to photosynthesis, GO treatment did not cause a stress phenotype but improved the photosynthetic capacity of Aloe vera to some extent.
Fig. 4.
Effect of GO on chlorophyll content (a), Fv/Fm level (b), and Y(II) (c). Values are means ± SD (n = 5). Means with different letters are significantly different by LSD test (p ≤ 0.05)
Effects of GO on plant root physiological and biochemical indexes
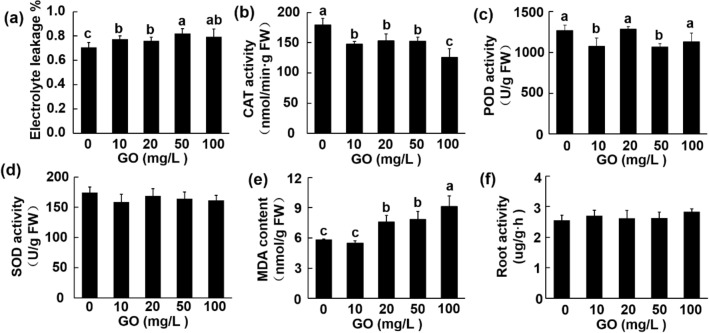
Previous reports identified membrane damage and oxide stress induced by graphene treatment, which often manifested as an elevation in electrolyte leakage, MDA content, and antioxidant enzyme activity (Chen et al. 2018; Guo et al. 2020). Consistent with the previous researches, the electrolyte leakage of root tissue displayed an approximately linear change with GO concentrations (Fig. 5a). To further analyze the characters of the oxidative stress on Aloe vera root, the activity of the antioxidant enzyme (POD, SOD, CAT) and MDA content were measured. To our surprise, none of the three antioxidant enzymes showed elevating enzyme activities after GO treatment. GO significantly inhibited the CAT enzyme activity at all concentrations, whereas the enzyme activity of POD was significantly lower at the concentration of 10 and 50 mg/L (Fig. 5b–c). No obvious difference could be detected in the SOD enzyme activity among different concentrations (Fig. 5d). Unlike the antioxidant enzyme, the content of MDA showed a dose-depended increase, which was significantly raised by 30.7%, 35.5%, and 57.4% at 20, 50 and 100 mg/L, respectively (Fig. 5e). MDA content, an important indicator for stress damage, was abundantly produced after lipid peroxidation (Jiao et al. 2016). To test whether the increase in MDA content means the reduction of physiological activity, root vigor was assessed and no significant difference could be detected after GO treatment (Fig. 5f). These results suggested that GO treatment did not induce a typical stress response.
Fig. 5.
Effects of GO on the electrolyte leakage (a); CAT enzyme activity (b); POD enzyme activity (c); SOD enzyme activity (d); MDA content (e); and root activity of Aloe vera seedlings. Values are means ± SD (n = 3). Means with different letters are significantly different at LSD test (p ≤ 0.05)
Functional test of GO inside the plant body
It is reported that graphene released to soil could be absorbed by root, and transported to shoot (Huang et al. 2018). We asked the question that whether GO plays its part in vivo or outside the root. In order to answer this question, GO solution was injected into the leaves of Aloe vera grown in soil over a concentration range of 0–500 mg/L twice a month. After four months of treatment, no visible variation could be detected among different groups (Fig. 6a). The leaf net fresh weight, root fresh weight and root morphological indexes were further measured and identified no significant difference in all these parameters except that the total root length was increased by 19% after 5 mg/L GO injection (Fig. 6b–f). Based on the results of this experiment, it could be suggested that the Aloe vera leaf could not be promoted by GO in vivo directly.
Fig. 6.
Phenotype of Aloe vera seedlings after injection treatment with different concentrations (0–500 mg/L) of GO for 4 months. a Phenotypical image of Aloe vera grown in soil after injection treatment (scale bar: 10 cm); b net fresh weight of leaf; c fresh weight of root; d total root length; e total root surface area; f total root volume. Values are means ± SD (n = 5). Means with different letters are significantly different at LSD test (p ≤ 0.05)
Discussion
Since the discovery of graphene in 2004, a huge amount of research has been devoted to the biological effects of graphene (Wang et al. 2019; Yao et al. 2019; Zuverza-Mena et al. 2017). However, the purpose of most studies is to analyze the environmental risk of graphene at high concentrations by hydroponic experiment, rather than to analyze the effect of graphene on plants at actual concentrations in soil, thus to promote its application in agriculture and forestry (Wang et al. 2019). In this research, we investigated the long-term impact of GO on Aloe vera growth, and found that GO exhibited positive effect on Aloe vera growth in soil (Fig. 2 and Fig. S1). In contrast to hydroponics, where all nutrients are dissolved in water and can be absorbed directly by the plant, the soil is a complex of minerals, soil organic matter, water, and air (Rutherford and Chiou 1992). Considering the big difference between soil and hydroponics (Di Salvatore et al. 2012; Manzocco et al. 2011), it is not surprising that graphene works differently under different conditions. Therefore, it is necessary to figure out the effects of graphene before future application.
Soil application of GO promoted the growth of leaves and roots, which is manifested in significantly increased fresh weight and various morphological indicators, including average longest leaf length, average longest leaf width, total root length, total root surface area and total root volume (Fig. 2b–f and Fig. S1b–c). The GO treatment did not alter the water content of leaves, but increased the total amino acid and protein content (Fig. 4 and Fig. S2), indicating that the influence of GO on plant growth is not only the enhancement of water absorption, but also the overall improvement in physical activity, which is consistent with the increase in photosynthetic fluorescence parameter Y(II) (Fig. 4c). It has been reported that high concentration of GO treatment could cause cell membrane damage and oxidative stress (Chen et al. 2018; Zhang et al. 2016). In our experiment, although the electrolyte leakage and MDA content was increased after GO treatment, the activities of antioxidant enzymes were not altered significantly (Fig. 5a–e). In particular, the root vigor was not inhibited, indicating that instead of the typical stress effect, soil-applied graphene showed a growth promoting effect (Fig. 5f).
Exogenous substances not only affect plant yield but also often affect nutrients content (Heeb et al. 2006). In some cases, the changes in biomass and nutrients content are not consistent (Lu et al. 2020). Besides the content of soluble sugars was not significantly affected, the content of free amino acid and soluble protein was significantly increased after 50 and 100 mg/L GO treatment (Fig. 3a–c). In addition, we took aloin as an example to analyze the effect of GO treatment on the bioactive compounds of aloe vera (Fig. 3d). The content of aloin was not affected by the GO treatment, which further showed that GO could be used as a new fertilizer additive in the production of aloe vera.
GO could be taken up by the root and transported to the aerial organs (Huang et al. 2018). Although there has been some in vivo research using the nematode Caenorhabditis elegans, no research on the function of GO has been conducted in plants (Chatterjee et al. 2015). By the injection experiment, we found that GO inside the leaves did not display an obvious effect on plant growth (Fig. 6a–f). In other words, the promotion effect of GO is a result of the interaction of root, soil and GO. Of course, we did not rule out the possibilities that GO inside the root may have a direct effect on root growth, or the changes of leaf physiological and biochemical parameters may be outside of our testing. Our research at least raised the inevitable question and provided clues for further research on the mechanism of GO. In addition, this simple method could be used to study the effects of other materials.
The possible long term residual effects of GO accumulated in vivo is an unavoidable problem for future application. Although there is no direct evidence either from the literature or from the results of own experiments, we tend to believe that, if GO does not show significant inhibitory effects on plants in the short term, the long-term residual effects will not be significantly increased. First of all, the GO concentrations used in practice are generally lower than those used experimentally, and the amount of GO entering the body is so small that it would have to be detected by isotope labeling or observed by transmission electron microscopy (Huang et al. 2018; Zhao et al. 2015). Secondly, the GO absorbed into cells could be brokendown into carbon dioxide, further reducing its content (Huang et al. 2018). Afterward, from the chemical point of view, the modified groups of GO are easy to react with various ions in the cytoplasm, resulting in charge neutralization and aggregation. Eventually, our injection experiment showed that GO in vivo did not trigger any visible physiological toxic effects on plant growth (Fig. 6a–f).
Although the mechanism of GO was not clear yet, the application of GO to the soil did enhance root growth. The effects of GO are of great importance to the growth and stress adaption of plants in agriculture and forestry. Firstly, the longer total root length and augmented total root surface area ensured more water and mineral nutrient for plant growth, which in combination with the elevated pigment content and photosynthetic competence, lead to an increase in biomass. Secondly, stronger roots and higher total amino acid and protein content endows plants better osmotic regulation ability to resist various adverse environments (Yu et al. 2020).
In contrast to the wide application in many fields, the application of graphene in agriculture and forestry is still limited. Besides the concerns about the cost and food safety, the unclear effect of graphene on plant growth is the main reason for this dilemma. Along with the diversification of raw materials and the continuous progress of preparation methods of graphene, the cost of graphene production has become gradually lower (Ma et al. 2013; Pei et al. 2018; Raghavan et al. 2017; Zhao et al. 2012). Graphene could be taken up by plants, but was not detected in the grains, indicating that graphene is safe for most grain targeting food crops (Huang et al. 2018). As the biological effects are better understood, especially for ornamental flowers and trees, graphene will play an even greater role in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions:
Jianguo Zhao and Haiyan Wang conceived the project; Xiao Zhang and Huifen Cao designed the experiments, performed the experiment and wrote the manuscript; Zhiwen Chen supervised the project; Jin Zhang provided the GO material and performed the FT-IR analysis; Baoyan Xing performed the SEM analysis; Xinyu Li performed Raman spectra analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Doctoral Scientific Research Foundation of Shanxi Datong University (2018-B-20), The Program for Scientific and Technological Innovation of Higher Education Institutions in Shanxi (2019L0767), Shanxi Datong University Students Innovation and Entrepreneurship Project (XDC2019232), Science and Technology Achievements Transformation Guide project of Shanxi province (201804D131041), The Program for Scientific and Technological Innovation of Higher Education Institutions in Shanxi (2020L0467), Natural Science Foundation of Shanxi Province (201901D211437) and The National Natural Science Foundation of China (52071192).
Data availability
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical standards
Conflicts of Interest
The authors declare no conflict of interest.
Consent to participate
All authors participate to finish the work.
Consent for publication
All authors agreed for publication.
Ethics approval
Compliance with ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiao Zhang and Huifen Cao contributed equally to the work.
Contributor Information
Jianguo Zhao, Email: jgzhaoshi@163.com.
Haiyan Wang, Email: why7135280@126.com.
References
- Andelkovic IB, Kabiri S, Tavakkoli E, Kirby JK, McLaughlin MJ, Losic D. Graphene oxide-Fe(III) composite containing phosphate—a novel slow release fertilizer for improved agriculture management. J Clean Prod. 2018;185:97–104. doi: 10.1016/j.jclepro.2018.03.050. [DOI] [Google Scholar]
- Anjum NA, Singh N, Singh MK, Sayeed I, Duarte AC, Pereira E, Ahmad I. Single-bilayer graphene oxide sheet impacts and underlying potential mechanism assessment in germinating faba bean (Vicia faba L.) Sci Total Environ. 2014;472:834–841. doi: 10.1016/j.scitotenv.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Avouris P. Graphene: electronic and photonic properties and devices. Nano Lett. 2010;10:4285–4294. doi: 10.1021/nl102824h. [DOI] [PubMed] [Google Scholar]
- Begum P, Fugetsu B. Induction of cell death by graphene in Arabidopsis thaliana (Columbia ecotype) T87 cell suspensions. J Hazard Mater. 2013;260:1032–1041. doi: 10.1016/j.jhazmat.2013.06.063. [DOI] [PubMed] [Google Scholar]
- Begum P, Ikhtiari R, Fugetsu B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon. 2011;49:3907–3919. doi: 10.1016/j.carbon.2011.05.029. [DOI] [Google Scholar]
- Chakravarty D, Erande MB, Late DJ. Graphene quantum dots as enhanced plant growth regulators: effects on coriander and garlic plants. J Agric Food Chem. 2015;95:2772–2778. doi: 10.1002/jsfa.7106. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Yang JS, Park K, Oh SM, Park J, Choi J. Screening of toxic potential of graphene family nanomaterials using in vitro and alternative in vivo toxicity testing systems. Environ Anal Health Toxicol. 2015;30:e2015007–e2015000. doi: 10.5620/eht.e2015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Yang L, Li S, Ding W. Various physiological response to graphene oxide and amine-functionalized graphene oxide in wheat (Triticum aestivum) Molecules. 2018;23:1104. doi: 10.3390/molecules23051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jesus LR, Dennis RV, Depner SW, Jaye C, Fischer DA, Banerjee S. Inside and outside: X-ray absorption spectroscopy mapping of chemical domains in graphene oxide. J Phys Chem Lett. 2010;4:3144–3151. doi: 10.1021/jz401717j. [DOI] [PubMed] [Google Scholar]
- Di Salvatore M, Carafa A, Mingo A, Carratù G. Evaluation of heavy metal toxicity on radish: comparison between soil and floating hydroponics systems. Am J Exp Agr. 2012;2:174–185. doi: 10.9734/AJEA/2012/862. [DOI] [Google Scholar]
- Dong Z, Men Y, Liu Z, Li J, Ji J. Application of chlorophyll fluorescence imaging technique in analysis and detection of chilling injury of tomato seedlings. Comput Electron Agr. 2020;168:105109. doi: 10.1016/j.compag.2019.105109. [DOI] [Google Scholar]
- Gao M, Yang Y, Song Z. Effects of graphene oxide on cadmium uptake and photosynthesis performance in wheat seedlings. Ecotox Environ Safe. 2019;173:165–173. doi: 10.1016/j.ecoenv.2019.01.093. [DOI] [PubMed] [Google Scholar]
- Guo Z, Luo W, Xie C, Valsami-Jones E, Lynch I, Abdolahpur Monikh F. Graphene oxide induced pH alteration, iron overload and subsequent oxidative damage in rice (Oryza. sativa L.): A new mechanism of nanomaterial phytotoxicity. Environ Sci Technol. 2020;54:3181–3190. doi: 10.1021/acs.est.9b05794. [DOI] [PubMed] [Google Scholar]
- Hazrati S, Sarvestani T, Modarres-Sanavy S, Mokhtassi-Bidgoli A, Nicola S. Effects of water stress and light intensity on chlorophyll fluorescence parameters and pigments of Aloe vera L Plant. Physiol Bioch. 2016;106:141–148. doi: 10.1016/j.plaphy.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Hazrati S, Sarvestani T, Mokhtassi-Bidgoli A, Modarres-Sanavy SAM, Mohammadi H, Nicola S. Effects of zeolite and water stress on growth, yield and chemical compositions of Aloe vera L. Agr Water Manage. 2017;181:66–72. doi: 10.1016/j.agwat.2016.11.026. [DOI] [Google Scholar]
- He Y, Hu R, Zhong Y, Zhao X, Chen Q, Zhu H. Graphene oxide as a water transporter promoting germination of plants in soil. Nano Res. 2018;11:1928–1937. doi: 10.1007/s12274-017-1810-1. [DOI] [Google Scholar]
- Heeb A, Lundegårdh B, Savage G, Ericsson T. Impact of organic and inorganic fertilizers on yield, taste, and nutritional quality of tomatoes. J Plant Nutr Soil Sci. 2006;169:535–541. doi: 10.1002/jpln.200520553. [DOI] [Google Scholar]
- Hu X, Zhou Q. Novel hydrated graphene ribbon unexpectedly promotes aged seed germination and root differentiation. Sci Rep. 2014;4:3782. doi: 10.1038/srep03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, et al. Transformation of (14) C-labeled graphene to (14) CO2 in the shoots of a rice plant. Angew Chem Int Edit. 2018;57:9759–9763. doi: 10.1002/anie.201805099. [DOI] [PubMed] [Google Scholar]
- Jiao J, et al. The role of graphene oxide on tobacco root growth and its preliminary mechanism. J Nanosci Nanotechno. 2016;16:12449–12454. doi: 10.1166/jnn.2016.12987. [DOI] [Google Scholar]
- Jin Z, Wang C, Liu Z, Gong W. Physiological and ecological characters studies on Aloe vera under soil salinity and seawater irrigation. Process Biochem. 2007;42:710–714. doi: 10.1016/j.procbio.2006.11.002. [DOI] [Google Scholar]
- Kabiri S, Degryse F, Tran DNH, da Silva RC, McLaughlin MJ, Losic D. Graphene oxide: a new carrier for slow release of plant micronutrients ACS. Appl Mater Inter. 2017;9:43325–43335. doi: 10.1021/acsami.7b07890. [DOI] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Biol. 2003;42:313–349. doi: 10.1146/annurev.pp.42.060191.001525. [DOI] [Google Scholar]
- Kumar S. Ethnobotanical and pharmacological properties of Aloe vera: a review. J Med Plants Res. 2014;8(48):1387–1398. [Google Scholar]
- Lichtenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148C:350–382. 10.1016/0076-6879(87)48036-1
- Li F, Xue J, Zhao J, Zhang S. Graphene oxide: a promising nanomaterial for energy and environmental applications. Nano Energy. 2015;16:488–515. doi: 10.1016/j.nanoen.2015.07.014. [DOI] [Google Scholar]
- Liu L, et al. An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J. 2009;61:893–903. doi: 10.1111/j.1365-313X.2009.04109.x. [DOI] [PubMed] [Google Scholar]
- Lu M, et al. Nutritional quality and health risk of pepper fruit as affected by magnesium fertilization. J sci food agric. 2020 doi: 10.1002/jsfa.10670. [DOI] [PubMed] [Google Scholar]
- Ma Q, et al. A rapid and easy approach for the reduction of graphene oxide by formamidinesulfinic acid. Carbon. 2013;54:36–41. doi: 10.1016/j.carbon.2012.10.067. [DOI] [Google Scholar]
- Manzocco L, et al. Influence of hydroponic and soil cultivation on quality and shelf life of ready-to-eat lamb's lettuce (Valerianella locusta L. Laterr) J Sci Food Agr. 2011;91:1373–1380. doi: 10.1002/jsfa.4313. [DOI] [PubMed] [Google Scholar]
- Mendonça M, Rodrigues N, De Jesus M, Amorim M. Graphene-based nanomaterials in soil: ecotoxicity assessment using Enchytraeus crypticus reduced full life cycle. Nanomaterials. 2019 doi: 10.3390/nano9060858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Majumdar S, Servin AD, Pagano L, Dhankher OP, White JC. Carbon nanomaterials in agriculture: a critical review. Front Plant Sci. 2016 doi: 10.3389/fpls.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K, Anas M, Hicks V, Green M, Khodakovskaya M. Improvement of commercially valuable traits of industrial crops by application of carbon-based nanomaterials. Sci Rep. 2019;9:19358. doi: 10.1038/s41598-019-55903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei S, Wei Q, Huang K, Cheng H-M, Ren W. Green synthesis of graphene oxide by seconds timescale water electrolytic oxidation. Nat Commun. 2018 doi: 10.1038/s41467-017-02479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan N, Sakthivel T, Venugopal G. A short review on preparation of graphene from waste and bioprecursors. Appl Mater Today. 2017;7:246–254. doi: 10.1016/j.apmt.2017.04.005. [DOI] [Google Scholar]
- Ren W, Chang H, Teng Y. Sulfonated graphene-induced hormesis is mediated through oxidative stress in the roots of maize seedlings. Sci Total Environ. 2016;572:926–934. doi: 10.1016/j.scitotenv.2016.07.214. [DOI] [PubMed] [Google Scholar]
- Rutherford DW, Chiou CT. Effect of water saturation in soil organic matter on the partition of organic compounds. Environ Sci Technol. 1992;26:965–970. doi: 10.1021/es00029a015. [DOI] [Google Scholar]
- Salehi B, et al. Aloe genus plants: from farm to food applications and phytopharmacotherapy. Int J mol Sci. 2018;19:2843. doi: 10.3390/ijms19092843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman-Sun E, Lombi E, Donner E, Howard D, Unrine JM, Lowry GV. Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum) Environ Sci Technol. 2017;51:7361–7368. doi: 10.1021/acs.est.7b00813. [DOI] [PubMed] [Google Scholar]
- Sun YN, Jo AR, Kim JH, Kang JS, Kim YH. Soluble epoxide hydrolase inhibitory activity of anthraquinone components from Aloe. Planta Med. 2016;81:S1–S381. doi: 10.1055/s-0036-1596599. [DOI] [PubMed] [Google Scholar]
- Tonelli FM, et al. Graphene-based nanomaterials: biological and medical applications and toxicity. Nanomedicine. 2015;10:2423–2450. doi: 10.2217/nnm.15.65. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li C, Wang Y, Que X. Phytotoxicity of graphene family nanomaterials and its mechanisms: a review. Front Chem. 2019;7:00292. doi: 10.3389/fchem.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C-X, Liu Y-L, Zheng Q-S, Liu Z-P. Silicate improves growth and ion absorption and distribution in Aloe vera under salt stress. J Plant Physiol Mol Biol. 2006;32:73–78. [PubMed] [Google Scholar]
- Yao J, Wang H, Chen M, Yang M. Recent advances in graphene-based nanomaterials: properties, toxicity and applications in chemistry, biology and medicine. Microchim Acta. 2019 doi: 10.1007/s00604-019-3458-x. [DOI] [PubMed] [Google Scholar]
- Yu S, Sheng L, Mao H, Huang X, Luo L, Li Y. Physiological response of Conyza Canadensis to cadmium stress monitored by Fourier transform infrared spectroscopy and cadmium accumulation. Spectrochim Acta A. 2020;229:118007. doi: 10.1016/j.saa.2019.118007. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gao B, Chen J, Li Y, Creamer AE, Chen H. Slow-release fertilizer encapsulated by graphene oxide films. Chem Eng J. 2014;255:107–113. doi: 10.1016/j.cej.2014.06.023. [DOI] [Google Scholar]
- Zhang P, Zhang R, Fang X, Song T, Cai X, Liu H, Du S. Toxic effects of graphene on the growth and nutritional levels of wheat (Triticum aestivum L.): short- and long-term exposure studies. J Hazard Mater. 2016;317:543–551. doi: 10.1016/j.jhazmat.2016.06.019. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guo Y, Li Z, Guo Q, Shi J, Wang L, Fan J. An approach for synthesizing graphene with calcium carbonate and magnesium. Carbon. 2012;50:4939–4944. doi: 10.1016/j.carbon.2012.06.024. [DOI] [Google Scholar]
- Zhao S, Wang Q, Zhao Y, Rui Q, Wang D. Toxicity and translocation of graphene oxide in Arabidopsis thaliana. Environ Toxicol Pharmacol. 2015;39:145–156. doi: 10.1016/j.etap.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Zhao L, et al. Nano-biotechnology in agriculture: use of nanomaterials to promote plant growth and stress tolerance. J Agric Food Chem. 2020;68:1935–1947. doi: 10.1021/acs.jafc.9b06615. [DOI] [PubMed] [Google Scholar]
- Zuverza-Mena N, et al. Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses-a review. Plant Physiol Biochem. 2017;110:236–264. doi: 10.1016/j.plaphy.2016.05.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analyzed during the current study are available from the corresponding author on reasonable request.