Abstract
As the opioid epidemic continues to grow, opioid use among pregnant women is increasing significantly. This has led to a steady rise in the number of infants born with neonatal opioid withdrawal syndrome (NOWS). Although short-term withdrawal symptoms associated with NOWS are well characterized, there are many gaps in our understanding of the short and long-term effects of prenatal opioid exposure. Current animal models of NOWS are limited by shortened gestational periods, large litter sizes, and primary organogenesis occurring after birth. This often leads to postnatal treatment to mimic drug exposure during third-trimester development. Using the unique rodent species Acomys cahirinus, more commonly known as spiny mice, which have an extended 40-day gestation period, small litter sizes, and increased in utero organogenesis we aim to study the short-term effects of prenatal morphine exposure by assessing withdrawal behavior. To model maternal opioid use, dams were treated daily with morphine (10 and 30 mg/kg S.C.) beginning on gestation day 19 until the day of birth; this resulted in a cumulative exposure of 19–21 days. Withdrawal behaviors for each pup were recorded daily between postnatal days 0–7 (PND 0–7). Our study found that prenatal morphine exposure in spiny mice led to an increase in withdrawal behavior throughout the early postnatal period and validated the use of this species as a novel pre-clinical model of NOWS. We are hopeful this rodent model will further our understanding of the short and long-term consequences of prenatal opioid exposure on neurodevelopment and behavior.
Keywords: Opioids, Morphine, Addiction, Neonatal, Abstinence, Withdrawal
Opioids, Morphine, Addiction, Neonatal, Abstinence, Withdrawal.
1. Introduction
A critical consequence of opioid use disorder during pregnancy is the increase in the diagnosis and treatment of neonatal opioid withdrawal syndrome (NOWS). NOWS is a constellation of withdrawal symptoms experienced by infants shortly after birth due to the abrupt cessation of trans-placental drug exposure from mother to infant. Symptoms associated with NOWS typically affect the central and autonomic nervous systems as well as the gastrointestinal system. Common symptoms include tremors, irritability, excessive crying, poor feeding, sleep disturbances, increased muscle tone, fever, temperature instability, diarrhea, and in extreme cases seizures (Kocherlakota, 2014). Although several drugs have been implicated with withdrawal symptoms in neonates, it is most commonly associated with opioid use (Sutter et al., 2014). In the United States (US) the rate of NOWS increased five-fold between 2004-2014; increasing from 1.6 to 8.8 cases per 1,000 births (Patrick et al., 2012; Winkelman et al., 2018). The rate of NOWS in some US states remains high, for example, in the state of West Virginia where there were 56.2 per 1,000 babies born with NOWS between 2016 – 2017. Similarly, states such as Maine (31.4 per 1,000 births) and Kentucky (23.9 per 1,000 births) also had the highest rates of NOWS. In fact, in 2017 when the national rate of NOWS births was 7.3, a total of nine US states had greater than 10 per 1,000 births that were diagnosed with NOWS, highlighting that maternal opioid abuse disorder and NOWS remains prevalent in the US (Umer et al., 2018). Additionally, the average national hospital costs associated with NOWS are over nine times (~$9,500 per baby) greater than that compared to the cost associated with non-NOWS babies (~$1,100 per baby) (“Agency for Healthcare Research and Quality,” 2020). Although the short-term withdrawal symptoms of NOWS are well characterized and understood, there are still gaps in our understanding of the short and long-term effects on brain development (Boggess and Risher, 2020).
Current rodent models used to study prenatal opioid exposure have limited clinical translatability due to short gestational periods, large litter sizes, and primary organogenesis occurring postnatally. Due to the immature brain development of traditional rodent models at birth, preclinical models of prenatal opioid exposure have been developed using postnatal treatments to mimic third-trimester exposure as seen in humans (Dobbing and Sands, 1979). Unfortunately, these rodent models remove the importance of transplacental exposure of opioids from the mother during gestation limiting there clinical translatability (Ross et al., 2014). Also, large litter sizes observed in traditionally used rodent models fail to mimic human pregnancy. A rodent such as the C57BL/6 mouse species undergo significant postnatal development that limits their use to better our understanding of early, short-term effects on NOWS as seen in humans.
Guinea pigs have been suggested to be a more suitable model for prenatal opioid exposure due to their lengthened gestation, small litter sizes, and precocial pups (Dobbing and Sands, 1970). Additionally, the placental structure and the metabolism of morphine is similar among guinea pigs and humans (Carter et al., 2006; Lawrence et al., 1992; Morrison et al., 2018; Murphey and Olsen, 1993). However, growth and development of the brain occurs much earlier in gestation in guinea pigs compared to humans (Dobbing and Sands, 1979). Therefore, in utero brain development still limits the translatability of this species as a model for prenatal opioid exposure for the purposes of understanding neurological and behavioral consequences. Collectively, these limitations lend to the need for a more translatable preclinical model of NOWS. We believe that spiny mice (Acomys cahirinus) possess several unique biological characteristics that differentiate them from other rodents and thus would better our understanding of NOWS to improve its treatment (early in withdrawal) and the long terms effects NOWS could have on these babies.
Spiny mice are a desert rodent species found across Africa, the Middle East, and Southern Asia that unlike their cousins, possess a menstrual cycle (Bellofiore et al., 2016; Haughton et al., 2016). In recent years, spiny mice have been highlighted as a highly translatable model to investigate neuroprotective interventions against perinatal injury and have been proposed as an ideal rodent model to study in utero development (Ellery et al., 2015; Ireland et al, 2008, 2011). Together, with their ability to menstruate, spiny mice have longer gestational periods (~38–40 days), significant in utero primary organogenesis, small litter sizes (~2–3 pups), and pups that are precocial (Dickinson and Walker, 2007). Due to their lengthened gestation, spiny mice organs undergo substantial development in utero. For example, the liver, kidney, lung, and brain are functionally mature at birth (Brunjes, 1985, 1989; Brunjes et al., 1989; Dickinson et al., 2005; Lamers et al., 1985; Oosterhuis et al., 1984). At 30 days of gestation, the cortical and limbic brain structures are developed to the equivalent of 24–26 weeks’ gestation in the human fetus (Clancy et al., 2001). Additionally, brain development in spiny mice has been shown to more closely resemble human development patterns. Studies have found that spiny mice experience a growth spurt in brain development around the time of birth which is sooner than other rodents like the rat and mouse, but later than the guinea pig, and is more comparable to human brain development (Quinn et al., 2016). The small litter sizes in spiny mice allow us to study the effects of prenatal opioid exposure while minimizing the variables presented by larger litter sizes found in other rodents (Dickinson and Walker, 2007). Spiny mice are a precocial species, at birth, their bodies are covered with hair, open eyes, ears and are capable of locomotion, and self-feeding on the second day of life (Brunjes, 1990; D'Udine et al., 1980). Due to their unique developmental characteristics, spiny mice provide a translational, preclinical model to study the effects of prenatal opioid exposure and spontaneous withdrawal symptoms associated with NOWS. Herein we evaluated the consequences of prenatal morphine exposure on withdrawal behavioral alterations analogous to those of NOWS in spiny mice.
2. Methods
2.1. Breeding
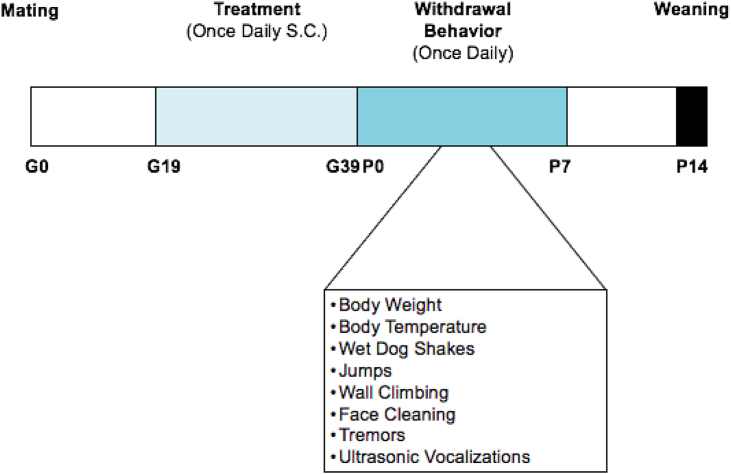
All spiny mice used in this study were obtained from our in-house breeding colony maintained at Purdue University, Fort Wayne, IN. Experiments were conducted per the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and protocols were approved by the Purdue Institutional Animal Care and Use Committee at Purdue University (protocol #1712001654). Spiny mice were bred male: female (1:1) starting between 9 - 12 weeks of age. Spiny mice do not produce a vaginal plug after mating, therefore the date of birth for the first litter was used to determine gestational age (days) of experimental pups. Gestational age was determined from the time of postpartum conception (i.e. mating at 24 h after delivery of a previous litter), as previously described (Dickinson et al., 2005). A timeline of the experiments is shown in Figure 1.
Figure 1.
Experimental timeline. Saline and morphine (10 mg/kg and 30 mg/kg) was administered once-daily (S.C.) beginning on gestational day 19. During daily injections, mice were checked for general health, body weight and body temperature were recorded. Withdrawal behaviors were measured between PND 0 and PND 7 once-daily, and performed in the morning by the same experimenter and data was analyzed by a blinded experimenter. All pups remained in the same cage as their mother's until weaning at PND 14, and were provided food and water ad libitum.
2.2. Morphine treatment
On gestational day 19 (G19), dams were separated from their male partner and remained isolated throughout treatment. Dams were briefly anesthetized using 2% isoflurane and treated (S.C.) once-daily (between 09:00–10:00 h) with either saline (N = 6) or morphine sulfate (Spectrum Chemical, M1167) at two different concentrations, 10 and 30 mg/kg (N = 9/dose). Morphine dosage was based on previous studies of prenatal morphine exposure in rats and mice (Mithbaokar et al., 2016; Slamberova et al, 2003a, 2003b, 2005). Upon birth, all pups (total N's: saline = 17; 10 mg/kg morphine = 24; and 30 mg/kg morphine = 21. See Table 1) remained with the dam until day of weaning at postnatal day (PND) 14. All mice were maintained on a 12h light/dark cycle (lights on at 06:00 h) in a temperature (24–26 °C) and humidity (40–70%) controlled environment. Food and water were available ad libitum.
Table 1.
Maternal and litter characteristics.
| Treatment | Saline | 10 mg/kg | 30 mg/kg | P |
|---|---|---|---|---|
| Number of dams | 6 | 9 | 9 | |
| Number of litters | 6 | 9 | 9 | |
| Number of pups | 17 | 24 | 23 | |
| Number male | 8 | 12 | 12 | |
| Number female | 9 | 12 | 9 | |
| Number unknown | 0 | 0 | 2 | |
| Length of exposure (days) | 20.67 ± 0.49 | 21.33 ± 0.17 | 21.44 ± 0.29 | NS |
| Mean litter size |
2.83 ± 0.17 |
2.67 ± 0.29 |
2.44 ± 0.29 |
NS |
| Deaths | ||||
| Male | 0 | 0 | 0 | |
| Female | 0 | 0 | 4 | |
| Unknown | 0 | 0 | 2 | |
2.3. Maternal measurements
Dams were weighed daily beginning on G19 to determine accurate volume adjustments for daily treatments and to confirm continuous pregnancy. The body temperature of dams was also recorded daily during gestation. Following parturition, body weight and temperature of all dams were also recorded once-daily during the first seven PND's to monitor possible symptoms associated with opioid withdrawal following treatment cessation.
2.4. Litter characteristics
Cages were checked each morning between 08:00–09:00 h for new litters. The day of parturition was designated as PND 0 and pups were examined to determine sex using anogenital distance. On the same day, litter size, weight, and body temperature of the pups were assessed. If any deceased pups were found in the cage it was recorded before being removed; sex was recorded as unknown if the remains of the pup did not allow for an accurate sex classification (Table 1). Beginning on PND 0, each pup was evaluated for NOWS using the following behavioral assays.
2.5. Behavioral procedures
All behavioral testing was conducted daily between 08:00–10:00 h on PND 0–7. Before testing began, mice were placed in the testing room for a minimum of 30 min to acclimate. The testing room temperature was maintained at 24 °C (75 °F) and the humidity was set between 40 - 70%. Spiny mice were tested and recorded to allow for more accurate post-testing observations (blinded) and data collection. The behavioral assays were performed in the order in which they are described below.
2.6. Spontaneous withdrawal
Symptoms of opioid withdrawal were assessed with each pup between PND 0–7. Each day, pups were removed from their home cage and placed in a clear plastic observation chamber. Before each test, the observation chamber was cleaned with 70% ethanol followed by water and dried to remove any olfactory cues. On each day, the body temperature (°C) was measured immediately following removal from the home cage using an infrared thermometer, and the body weight (grams) was measured with a digital scale designed to be used with small animals (Redmon, Peru, IN, USA). Pups were allowed to freely explore the observation chamber for 3 min and behaviors were observed, recorded, and then scored using the open-source Behavioral Observational Research Interactive Software (BORIS; http://www.boris.unito.it) by experimenters blinded to the treatment groups. Withdrawal behaviors that were scored during the 3-min observation period included wet-dog shakes, face cleaning, wall climbing, jumping, and tremors (See Table 2 for definitions of withdrawal behaviors) (Barr et al., 2011; Richardson et al., 2006).
Table 2.
Neonatal opioid withdrawal syndrome (NOWS) behaviors.
| Behavior | Description |
|---|---|
| Wet dog shakes | Rapid shaking of the whole body |
| Jumps | Sudden leaping such that all four paws are off the bottom of the chamber |
| Face cleaning | The continuous movement of paws towards the face |
| Wall climbing | Putting both forepaws on the wall of the observation chamber; typically, with movement |
| Ultrasonic vocalizations | Vocalizations in the ultrasonic range (20–128 kHz) |
| Tremor | Spontaneous shaking or kicking of the hind limbs with full-body movement; Shaking, twitching, curling, or sweeping movement of the tail |
2.7. Ultrasonic vocalizations (USV's)
Immediately following the completion of withdrawal behavior observations, pups were then placed into a glass container housed inside a sound-attenuating box. Before each test, this container was cleaned with 70% ethanol followed by water and dried to remove any olfactory cues. Ultrasonic vocalization (USV) emissions were recorded by the Echo Meter Touch® bat detector (Wild Life Acoustics, Maynard, MA) attached to an iPhone 8 (Apple, Cupertino, CA) that was mounted inside the roof of the sound attenuating box. USV's were recorded daily for each pup for 2 min from PND 0–7. Immediately following completion of recordings, pups were placed back in their home cage. For acoustical analysis, recordings were transferred to RavenPro® software (Cornell Laboratory of Ornithology, Ithaca, NY) and USV's occurring within the range of 20–125 kHz were quantified. All behavioral assays were performed in a quite behavioral suite and completed within 10 min to minimize the stress on pups of being separated from their mother.
2.8. Data analysis
All data are presented as mean ± SEM. Maternal body weight and body temperature were assessed using two-way repeated-measures ANOVA with Tukey's post hoc test, and treatment and PND's as factors. Length of exposure and litter size were assessed using one-way ANOVA with Tukey's post hoc test. The pup death rate was assessed using an ordinary one-way ANOVA with Tukey's post hoc test. Spontaneous withdrawal behaviors were assessed using two-way repeated-measures ANOVA with Tukey's post hoc test, with treatment and PND's as factors. Sex differences within treatment groups were analyzed using two-way repeated-measures ANOVA with Sidak's post hoc test with sex and PND's as factors. All data, including Area under the curve (AUC ±SEM) were analyzed with GraphPad Prism version 8.0 (San Diego, CA, USA) and differences were considered significant for P < 0.05.
3. Results
3.1. General conditions of dams
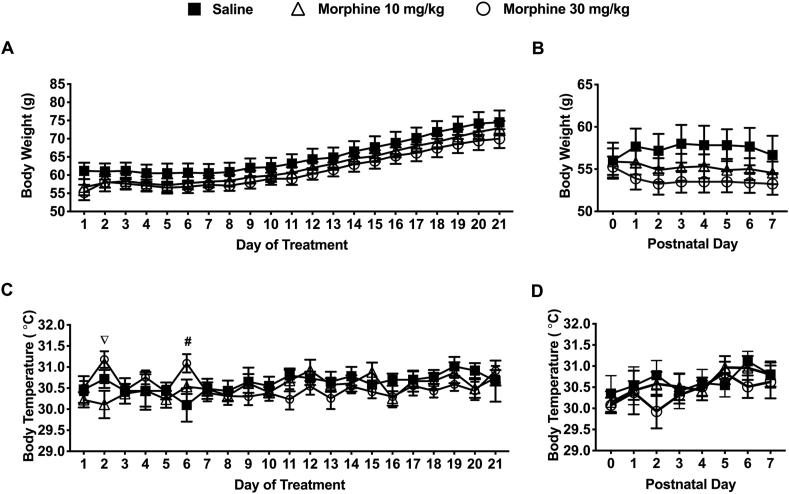
Body weight and body temperatures were measured daily for each dam between G19 and G40 (Figure 2a-c). Morphine treated dams had a slower rate of weight gain throughout this period compared to the saline-treated dams (Figure 2a). Dams from the 30 mg/kg morphine treatment group had significantly higher body temperatures on the 2nd day of treatment compared to the 10 mg/kg morphine group (31.19 ± 0.16 vs. 30.11 ± 0.32). On the 6th day of treatment, dams from the 30 mg/kg morphine group had a significantly higher body temperature compared to the saline-treated group (31.09 ± 0.22 vs. 30.10 ± 0.40) (P < 0.05) (Figure 2c). Bodyweight were also recorded daily on PND's 0–7 (Figure 2b & d). Dams from the morphine treatment groups had lower body weights compared to dams from the saline group (not significant) (Figure 2b). Body temperatures on PND’s were not significantly different from the saline-treated mice (Figure 2d).
Figure 2.
Maternal changes during the gestational treatment period and early post-partum period (PND 0–7). a) There was no significant difference between treatment groups on the body weights of dams during gestation or during the early postpartum period (PND 0–7) parturition (AUC [Weight] – Saline: 1302 21.12; 10 mg/kg: 1251 17.38; 30 mg/kg: 1225 18.66). (b) During the early postpartum period (PND 0–7), 10 mg/kg morphine (N = 9) and 30 mg/kg morphine (N = 9) treated dams had lower (non-significant) body weights compared to saline-treated controls (N = 6) (AUC [Weight] – Saline: 402.5 9.82; 10 mg/kg: 386.3 7.95; 30 mg/kg: 375.3 6.64). (c) On the 2nd day of treatment, dams treated with 30 mg/kg morphine (N = 9) had significantly higher body temperatures compared to 10 mg/kg morphine (N = 9) treated dams. On the 6th day of treatment, spiny mice treated with 30 mg/kg morphine (N = 9) had significantly higher body temperatures than compared to saline-treated controls (N = 6) (AUC [Temperature] – Saline: 612.5 1.99; 10 mg/kg: 610.6 2.11; 30 mg/kg: 610.3 1.85). (d) During the early postpartum period (PND 0–7) there was no significant difference in body temperatures between treatment groups (AUC [Temperature] – Saline: 214.6 1.61; 10 mg/kg: 214.3 1.52; 30 mg/kg: 212.8 1.73). Data are presented as means totals (±SEM) during an approximate 21-day treatment/gestation period and a PND 0–7 period. An pound symbol (# = saline vs. 30 mg/kg morphine) or nabla symbol (∇ = 10 mg/kg morphine vs. 30 mg/kg morphine) are used to indicate a significant difference between groups, P < 0.05.
3.2. Spiny mice pup characteristics
There was no significant difference in the length of exposure (days) between the morphine (10 and 30 mg/kg) and saline-exposed groups (21.33 ± 0.17 and 21.44 ± 0.29 vs. saline: 20.67 ± 0.49 respectively). The average litter size was smaller in the morphine (10 and 30 mg/kg) exposed groups compared to the saline-exposed group (2.67 ± 0.29 and 2.44 ± 0.29 vs. saline: 2.83 ± 0.17 respectively). There was a higher percentage of dead pups (26.09 ± 9.36%) from the 30 mg/kg morphine exposed group compared to the pups from the 10 mg/kg morphine and saline exposed groups (P < 0.05) (See Table 1). In terms of sex, more female pups died from the 30 mg/kg morphine exposed group (44.44 ± 17.57%) compared to male pups from the 30 mg/kg morphine exposed group (P < 0.05) (Table 1).
3.3. The measure of spontaneous opioid withdrawal in pups
3.3.1. Body temperature
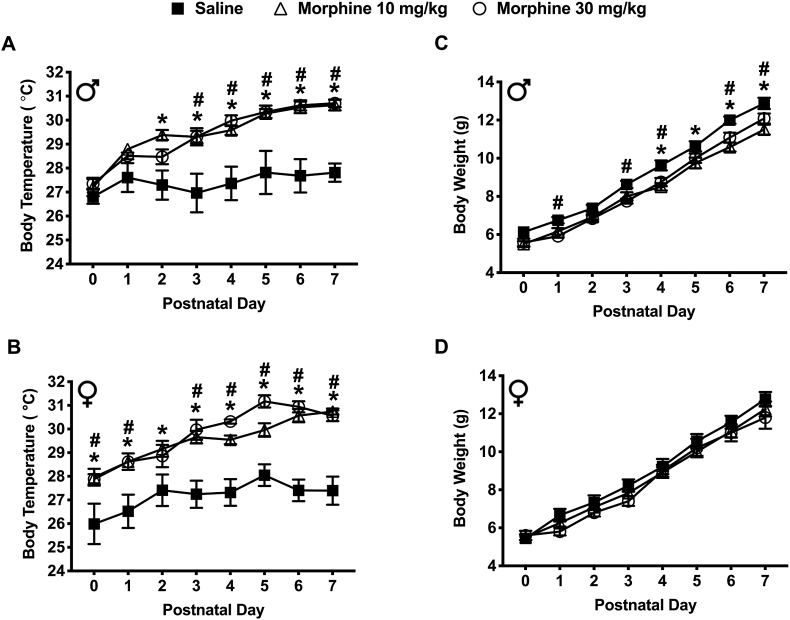
Withdrawal from prenatal morphine exposure increased body temperature in spiny mice pups during the early postnatal period (Figure 3a & b). On PND's 2–7, male mice from both morphine (10 and 30 mg/kg) exposed groups had significantly higher body temperatures compared to saline (P < 0.05) (Figure 3a). Similarly, on PND’s 0–7 female mice from both morphine (10 and 30 mg/kg) exposed groups also had significantly higher average body temperatures compared to saline (P < 0.05) (Figure 3b). No significant difference in body temperatures was found between sexes among all exposure groups and PND's.
Figure 3.
Spontaneous withdrawal following prenatal morphine (10 and 30 mg/kg) exposure affected the body temperature and body weight of spiny mice pups. (a) Male pups exposed to 10 mg/kg morphine (N = 12) and 30 mg/kg morphine (N = 12) had significantly higher body temperatures compared to the saline-exposed controls (N = 8) (AUC [Temperature] – Saline: 192.0 3.63; 10 mg/kg: 206.8 1.52; 30 mg/kg: 206.3 1.63). (b) Similarly, female pups exposed to 10 mg/kg morphine (N = 12) and 30 mg/kg morphine (N = 9) had significantly higher body temperatures compared to the saline-exposed controls (N = 9) (AUC [Temperature] – Saline: 190.6 3.39; 10 mg/kg: 206.9 1.67; 30 mg/kg: 209.1 1.32). (c) Male pups exposed to 10 mg/kg morphine (N = 12) and 30 mg/kg morphine (N = 12) had significantly lower body weights compared to saline-exposed controls (N = 8) (AUC [Weight] – Saline: 64.50 1.13; 10 mg/kg: 58.42 1.36; 30 mg/kg: 59.17 1.30). (d) In contrast, there were no significant differences in body weight in 10 mg/kg morphine (N = 12) or 30 mg/kg morphine (N = 9) exposed female pups compared to the saline-exposed controls (AUC [Weight] – Saline: 62.67 1.96; 10 mg/kg: 60.04 1.57; 30 mg/kg: 58.90 1.48). Data are presented as means total (±SEM) during a PND 0–7 period. An asterisk symbol (∗ = saline vs. 10 mg/kg morphine) or pound symbol (# = saline vs. 30 mg/kg morphine) are used to indicate a significant difference between groups, P < 0.05.
3.3.2. Body weight
Withdrawal from prenatal morphine exposure resulted in a decrease in body weight in spiny mice pups during the early postnatal period (Figure 3c & d). On PND's 4, 5, 6, and 7, male mice exposed to 10 mg/kg morphine had significantly lower body weights compared to the saline exposed males (8.50 ± 0.20; 9.75 ± 0.28; 10.58 ± 0.23 and 11.50 ± 0.26 vs. saline: 9.63 ± 0.26; 10.63 ± 0.26; 12.00 ± 0.19 and 12.88 ± 0.30 respectively) (P < 0.05) (Figure 3c). Similarly, on PND's 1, 3, 4, 6 and 7, male mice exposed to 30 mg/kg morphine group also had significantly lower body weights compared to the saline exposed males (5.92 ± 0.08; 7.75 ± 0.13; 8.75 ± 0.22; 11.08 ± 0.26 and 12.08 ± 0.26 vs. saline: 6.75 ± 0.16; 8.63 ± 0.18; 9.63 ± 0.26; 12.00 ± 0.19 and 12.88 ± 0.30 respectively) (P < 0.05) (Figure 3c). Although a lower body weight was observed in female mice exposed to morphine, no significance was observed when compared to the saline exposed females (Figure 3d). No significant difference in body weight was found between sexes across all exposure groups and PND's.
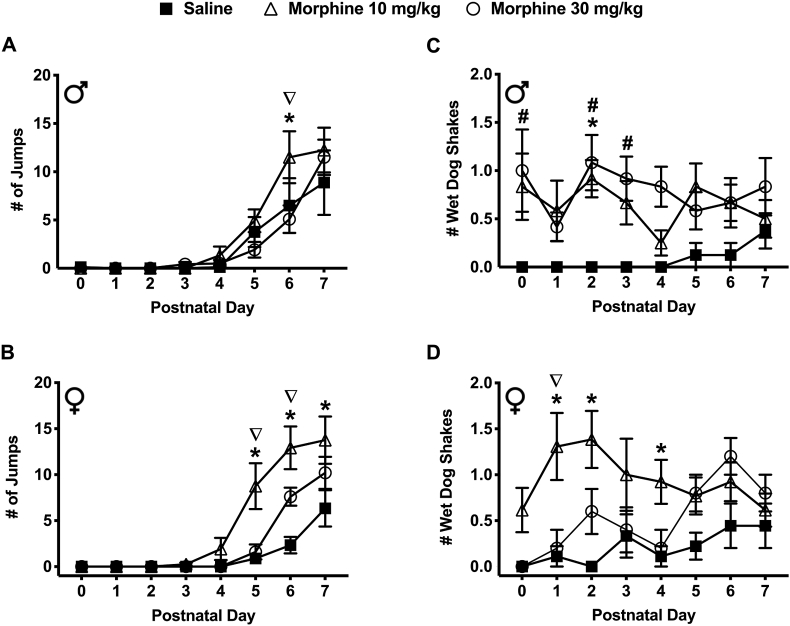
3.3.3. Spontaneous jumps
Withdrawal from prenatal morphine exposure resulted in a significant increase in jumping behavior in spiny mice pups during the early postnatal period (Figure 4a & b). On PND 6, male mice exposed to 10 mg/kg morphine made a significantly greater number of jumps compared to the saline exposed males (11.50 ± 2.69 vs. 6.50 ± 2.84) (P < 0.05) (Figure 4a). Additionally, on PND 6, males exposed to 10 mg/kg morphine made a significantly greater number of jumps compared to the morphine 30 mg/kg exposed males (11.50 ± 2.69 vs. 5.08 ± 1.43). On PND's 5, 6, 7, female mice exposed to 10 mg/kg morphine also made a significantly greater number of jumps compared to the saline exposed females (8.75 ± 2.50; 12.92 ± 2.32 and 13.75 ± 2.57 vs. 0.89 ± 0.56; 2.33 ± 0.90 and 6.33 ± 1.97) (P < 0.05) (Figure 4b). On PND's 5 and 6, female mice exposed to 10 mg/kg morphine had a significantly greater number of jumps compared to the 30 mg/kg morphine exposed females (8.75 ± 2.50; 12.92 ± 2.32 vs. 1.60 ± 0.81; 7.60 ± 0.98) (P < 0.05) (Figure 4b). There was no significant difference in the number of jumps between sexes across all exposure groups and PND's.
Figure 4.
Spontaneous withdrawal following prenatal morphine (10 and 30 mg/kg) exposure affected jumping and wet dog shaking behaviors in spiny mice pups. (a) Male pups on PND-6 exposed to 10 mg/kg morphine (N = 12) made a significantly greater number of jumps compared to the saline-exposed controls (N = 8) and the 30 mg/kg morphine exposed group (N = 12) (AUC [#Jumps] – Saline: 14.88 8.01; 10 mg/kg: 23.88 8.53; 30 mg/kg: 13.67 5.24). (b) Similarly, female pups on PND-5, 6, and 7 from the 10 mg/kg morphine exposed group (N = 12) made a significantly greater number of jumps compared to the saline-exposed controls (N = 9) and 30 mg/kg morphine exposed group (N = 9) on PND-5,6 (AUC [#Jumps] – Saline: 6.39 3.72; 10 mg/kg: 30.71 9.93; 30 mg/kg: 14.30 2.80). (c) Male pups on PND-0, 2, and 3 from the 30 mg/kg morphine exposed group (N = 12) had a significantly greater number of wet dog shakes compared to the saline-exposed controls (N = 8). On PND 2, males from 10 mg/kg morphine exposed group (N = 12) had a significantly greater number of wet dog shakes compared to saline-exposed controls (N = 8) (AUC [#Shakes] – Saline: 0.44 0.44; 10 mg/kg: 4.58 1.56; 30 mg/kg: 5.42 1.56). (d) Female pups on PND-1, 2, and 4 from the 10 mg/kg morphine exposed group (N = 12) had a significantly greater number of wet dog shakes compared to the saline-exposed controls (N = 9) and 30 mg/kg morphine treated mice (N = 9) on PND-2 (AUC [#Shakes] – Saline: 1.44 0.92; 10 mg/kg: 6.92 1.93; 30 mg/kg: 3.80 0.87). Data are presented as means totals (±SEM) during the PND 0–7 period. An asterisk symbol (∗ = saline vs. 10 mg/kg morphine) or pound symbol (# = saline vs. 30 mg/kg morphine) or nabla symbol (∇ = 10 mg/kg morphine vs. 30 mg/kg morphine) are used to indicate a significant difference between groups, P < 0.05.
3.3.4. Wet dog shakes
Withdrawal from prenatal morphine exposure resulted in a significant increase in wet dog shaking behavior in spiny mice pups during the early postnatal period (Figure 4c & d). On PND 2, male mice exposed to 10 mg/kg morphine made a significantly greater number of wet dog shakes compared to the saline exposed males (0.92 ± 0.19 vs. 0.00 ± 0.00) (P < 0.05) (Figure 4c). Similarly, on PND 0, 2, & 3, male mice exposed to 30 mg/kg morphine made a significantly greater number of wet dog shakes compared to males exposed to saline (1.00 ± 0.43; 1.08 ± 0.29 and 0.92 ± 0.23 vs. 0.00 ± 0.00) (P < 0.05) (Figure 4c). On PND 1, 2, & 4, female mice exposed to 10 mg/kg morphine made a significantly greater number of wet dog shakes compared to the saline exposed females (1.31 ± 0.37; 1.39 ± 0.31 and 0.92 ± 0.24 vs. saline: 0.11 ± 0.11; 0.00 ± 0.00 and 0.11 ± 0.11) (P < 0.05) (Figure 4d). On PND 1, female mice exposed to 10 mg/kg morphine made a significantly greater number of wet dog shakes compared to females exposed to 30 mg/kg morphine (1.31 ± 0.37 vs. 0.20 ± 0.20) (P < 0.05) (Figure 4d). There was no significant difference in the number of wet dogs shakes between sexes across all exposure groups and PND's.
3.3.5. Wall climbing
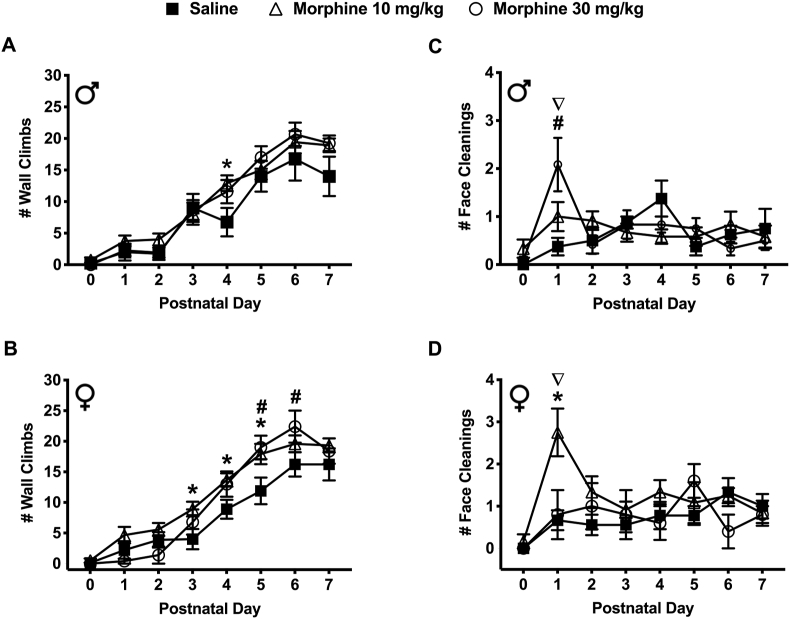
Withdrawal from prenatal morphine exposure increased wall climbing behavior in spiny mice pups during the early postnatal period (Figure 5a & b). On PND 4, male mice exposed to 10 mg/kg morphine made a significantly greater number of wall climbs compared to the saline exposed males (12.92 ± 1.25 vs. 6.75 ± 2.25 respectively) (P < 0.05) (Figure 5a). On PND 3, 4, & 5, female mice exposed to 10 mg/kg morphine also made a significantly greater number of wall climbs compared to the saline exposed females (8.92 ± 1.21; 13.67 ± 1.09 and 17.92 ± 1.66 vs. 4.00 ± 1.66; 8.89 ± 1.55 and 11.89 ± 2.18) (P < 0.05) (Figure 5b). On PND's 5 & 6, female mice exposed to 30 mg/kg morphine made a significantly greater number of wall climbs compared to the saline exposed females (19.00 ± 1.92; 22.40 ± 2.62 vs. 11.89 ± 2.18; 16.22 ± 1.99 respectively) (P < 0.05) (Figure 5b). There was no significant difference in the number of walls climbs between sexes across all exposure groups and PND's.
Figure 5.
Spontaneous withdrawal following prenatal morphine (10 and 30 mg/kg) exposure affected wall climbing and face cleaning behavior in spiny mice pups. (a) Male pups on PND-4 from the 10 mg/kg morphine exposed group (N = 12) had a significantly greater number of wall climbs compared to the saline-exposed controls (N = 8) (AUC [#Climbs] – Saline: 57.38 11.87; 10 mg/kg: 73.00 8.58; 30 mg/kg: 71.71 9.35). (b) Similarly, female pups on PND-3, 4, 5 from the 10 mg/kg morphine exposed group (N = 12) and PND-5, 6 pups from the 30 mg/kg morphine exposed group (N = 9) had a significantly greater number of wall climbs compared to the saline-exposed controls (N = 9) (AUC [#Climbs] – Saline: 55.28 9.69; 10 mg/kg: 80.25 8.11; 30 mg/kg: 72.20 7.64). (c) Male pups on PND-1 from the 30 mg/kg morphine exposed group (N = 12) had a significantly greater number of face cleanings compared to the saline-exposed controls (N = 8) and 10 mg/kg morphine exposed group (N = 12) (AUC [#Cleans] – Saline: 4.50 1.29; 10 mg/kg: 5.04 1.43; 30 mg/kg: 5.54 1.82). (d) Female pups on the PND-1 from the 10 mg/kg morphine exposed group (N = 12) had a significantly greater number of face cleanings compared to saline-exposed controls (N = 9) and the 30 mg/kg morphine exposed group (N = 9) (AUC [#Cleans] – Saline: 5.17 1.43; 10 mg/kg: 9.17 2.33; 30 mg/kg: 5.60 1.92). Data are presented as means totals (±SEM) during the PND 0–7 period. An asterisk symbol (∗ = saline vs. 10 mg/kg morphine) or pound symbol (# = saline vs. 30 mg/kg morphine) or nabla symbol (∇ = 10 mg/kg morphine vs. 30 mg/kg morphine) are used to indicate a significant difference between groups, P < 0.05.
3.3.6. Face cleaning
Withdrawal from prenatal morphine exposure increased face cleaning behavior in spiny mice pups during the early postnatal period (Figure 5c & d). On PND 1, male mice exposed to 30 mg/kg morphine made a significantly greater number of face cleanings compared to the saline exposed males (2.08 ± 0.56 vs. 0.38 ± 0.18 respectively) (P < 0.05) (Figure 5c). Additionally, on PND 1, male mice exposed to 30 mg/kg morphine made a significantly greater number of face cleanings compared to males exposed to 10 mg/kg morphine (2.08 ± 0.56 vs 1.00 ± 0.30 respectively) (P < 0.05) (Figure 5c). On PND 1, female mice exposed to 10 mg/kg morphine also made a significantly greater number of face cleanings compared to the saline exposed (2.75 ± 0.57 vs. 0.67 ± 0.24) and 30 mg/kg morphine exposed females (2.75 ± 0.57 vs. 0.80 ± 0.58) (P < 0.05) (Figure 5d). Additionally, on PND 1, female mice exposed to 10 mg/kg morphine made a significantly greater number of face cleanings compared to male mice exposed to 10 mg/kg morphine (2.75 ± 0.57 vs. 1.00 ± 0.30 respectively) (P < 0.05) (Figure 5d).
3.3.7. Tremors
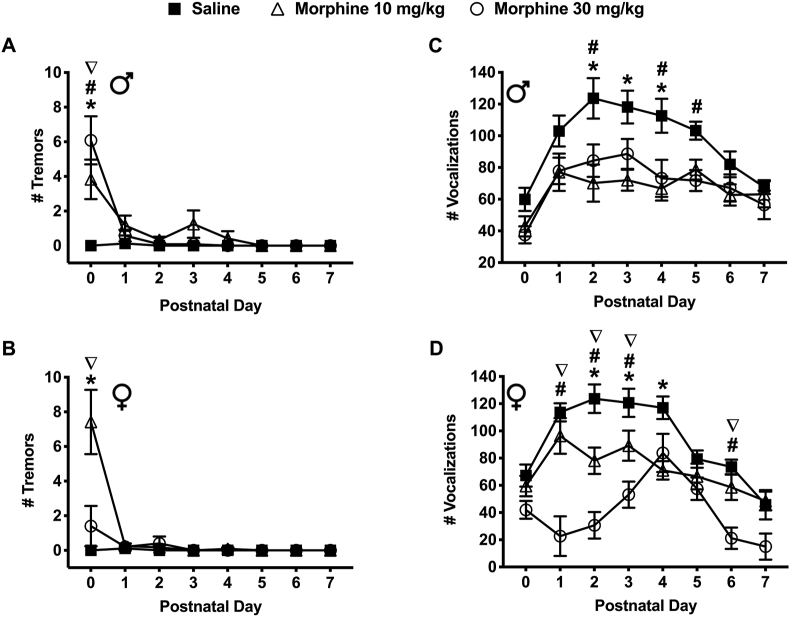
Withdrawal from prenatal morphine exposure increased tremor behavior in spiny mice pups during the early postnatal period (Figure 6a & b). On PND 0, male mice exposed to morphine (10 and 30 mg/kg) experienced a significantly greater number of tremors compared to the saline exposed males (3.83 ± 1.14 and 6.08 ± 1.39 vs. 0.00 ± 0.00, respectively) (P < 0.05) (Figure 6a). In addition, males exposed to 30 mg/kg morphine experienced a significantly greater number of tremors compared to males exposed to 10 mg/kg morphine (6.08 ± 1.39 vs. 3.83 ± 1.14) (P < 0.05) (Figure 6a). On PND 0, female mice exposed to 10 mg/kg morphine also experienced a significantly greater number of tremors compared to the saline exposed mice (7.42 ± 1.86 vs. 0.00 ± 0.00) (P < 0.05) (Figure 6b). Additionally, females exposed to 10 mg/kg morphine experienced a significantly greater number of tremors compared to the 30 mg/kg morphine exposed females (7.42 ± 1.86 vs. 1.40 ± 1.17) (P < 0.05) (Figure 6b). Interestingly, the number of tremors observed in the morphine exposed groups was significantly different between sexes. On PND 0, among pups exposed to 10 mg/kg morphine, females experienced a significantly greater number of tremors compared to males (7.42 ± 1.86 vs. 3.83 ± 1.14) (P < 0.05). In contrast, on PND 0, among pups exposed to 30 mg/kg morphine, male mice experienced a significantly greater number of tremors compared to female mice (6.08 ± 1.39 vs. 1.40 ± 1.17) (P < 0.05) (Figure 6a, b).
Figure 6.
Spontaneous withdrawal following prenatal morphine (10 and 30 mg/kg) exposure affected tremor and vocalization behaviors in spiny mice pups. (a) Male pups on PND-1 from the 10 mg/kg morphine (N = 12) and 30 mg/kg morphine (N = 12) exposed groups experienced a significantly greater number of tremors compared to the saline-exposed controls (N = 8). Pups from the 30 mg/kg morphine exposed group (N = 12) experienced a significantly greater number of tremors compared to the 10 mg/kg morphine exposed group (N = 12) (AUC [#Tremors] – Saline: 0.13 0.25; 10 mg/kg: 5.08 3.30; 30 mg/kg: 3.79 2.56). (b) Similarly, female pups on PND-1 from the 10 mg/kg morphine exposed group (N = 12) experienced a significantly greater number of tremors compared to the saline-exposed controls (N = 9) and 30 mg/kg morphine exposed group (N = 9) (AUC [#Tremors] – Saline: 0.11 0.24; 10 mg/kg: 4.21 3.25; 30 mg/kg: 1.30 1.48). (c) Male pups on PND-2, 3 and 4 from the 10 mg/kg morphine (N = 12) and on PND-2, 4 and 5 from the 30 mg/kg morphine (N = 12) exposed groups emitted a significantly fewer ultrasonic vocalizations compared to the saline-exposed controls (N = 8) (AUC [#Vocalizations] – Saline: 706.4 49.48; 10 mg/kg: 480.2 55.63; 30 mg/kg: 509.7 58.52). (d) Similarly, female pups on PND-1, 2, 3 and 4, from the 10 mg/kg morphine (N = 12) and PND-1, 2, 3 and 6 from the 30 mg/kg morphine exposed group (N = 9) emitted a significantly fewer ultrasonic ultrasonic vocalizations compared to the saline-exposed controls (N = 9). Additionally, on PND-1, 2, 3, 6 female pups from the 30 mg/kg morphine exposed group (N = 9) emitted a significantly fewer ultrasonic vocalizations compared to the 10 mg/kg morphine exposed group (N = 12) and saline-exposed controls (N = 9) (AUC [#Vocalizations] – Saline: 684.4 46.99; 10 mg/kg: 513.7 63.01; 30 mg/kg: 297.5 48.76). Data are presented as means totals (±SEM) during the PND 0–7 period. An asterisk symbol (∗ = saline vs. 10 mg/kg morphine) or pound symbol (# = saline vs. 30 mg/kg morphine) or nabla symbol (∇ = 10 mg/kg morphine vs. 30 mg/kg morphine) are used to indicate a significant difference between groups, P < 0.05.
3.3.8. Ultrasonic vocalizations (USV's)
Withdrawal from prenatal morphine exposure resulted in a significant decrease in the number of ultrasonic vocalizations (USV's) in spiny mice pups during the early postnatal period (Figure 6c & d). On PND's 2, 3 & 4, male mice exposed to 10 mg/kg morphine emitted significantly fewer number of USV's compared to the saline exposed mice (70.17 ± 11.70; 72.00 ± 6.59 and 66.75 ± 7.64 vs. 123.63 ± 12.74; 118.13 ± 10.31 and 112.63 ± 10.72) (P < 0.05) (Figure 6c). Similarly, on PND's 2, 4 & 5, male exposed to 30 mg/kg morphine emitted significantly fewer number of USV's compared to mice from the saline exposed group (84.33 ± 10.25; 73.17 ± 11.66 and 71.75 ± 6.62 vs. 123.63 ± 12.74; 112.63 ± 10.72 and 103.25 ± 5.66) (P < 0.05) (Figure 6c).
Similar to the males, on PND's 2, 3 & 4, female mice exposed to 10 mg/kg morphine emitted a significantly fewer number of USV's compared to mice exposed to saline (78.08 ± 9.70; 89.17 ± 11.06 and 71.00 ± 6.72 vs. 123.67 ± 10.47; 120.67 ± 10.35 and 117.00 ± 8.30) (P < 0.05) (Figure 6d). On PND's 1, 2, 3 & 6, female mice exposed to 30 mg/kg morphine emitted significantly fewer USV's compared to the mice from the saline exposed group (22.67 ± 14.54; 30.67 ± 9.76; 53.17 ± 9.60 and 21.17 ± 7.85 vs. 113.67 ± 6.67; 123.67 ± 10.47; 120.67 ± 10.35 and 73.56 ± 5.38) (P < 0.05) (Figure 6d). On PND's 1, 2, 3 & 6, female mice exposed to 10 mg/kg morphine emitted significantly greater number of USV's compared to female exposed to 30 mg/kg morphine (96.33 ± 13.12; 78.08 ± 9.70; 89.17 ± 11.06 and 58.58 ± 9.31 vs. 30 mg/kg morphine: 22.67 ± 14.54; 30.67 ± 9.76; 53.17 ± 9.60 and 21.17 ± 7.85) (P < 0.05) (Figure 6d). The number of USV's emitted was also significantly different between sexes. On PND's 1, 2, 6 & 7 female mice emitted significantly fewer USV's compared to male mice exposed to 30 mg/kg morphine (22.67 ± 14.54; 30.67 ± 9.76; 21.17 ± 7.85; 15.00 ± 9.62 vs. 77.83 ± 8.34; 84.33 ± 10.25; 67.08 ± 8.53 and 56.42 ± 8.98 respectively) (P < 0.05) (Figure 6c & d).
4. Discussion
Our study is the first to examine the effects of prenatal morphine exposure in spiny mice.
We performed physiological and behavioral tests in spiny mice pups prenatally exposed to morphine to characterize symptoms of withdrawal in this unique rodent species. The lengthened gestational period and increased in utero organogenesis of the spiny mouse allows for improved characterization and understanding of opioid withdrawal in pups following prenatal opioid exposure. Data presented in this study supported our hypothesis that spiny mouse would make an improved preclinical model of NOWS. Our novel model of NOWS relies on trans-placental exposure of morphine instead of postnatal opioid exposure, commonly used in other rodent models to mimic third-trimester exposure. Compared to other rodent species, spiny mice undergo advanced organogenesis and are precocial at birth (Brunjes, 1990). This allowed us to capture withdrawal symptoms that would otherwise not be possible in other rodent models during the early postnatal period.
Dams' body weight and temperature were monitored daily to assess any response to morphine treatment during gestation and during the first 7 days following parturition. Although differences in body weight observed during the gestation and postpartum periods were not significant, a decreased rate of weight gain among dams was measured in the morphine treated groups. Additionally, an increase in body temperature was only observed in dams from the 30 mg/kg morphine treatment group during the first 7 days of treatment. The fluctuations in body temperature during pregnancy may have been due to dams initially adjusting to daily treatments of high dose morphine and also the possible consequence of carrying an unborn litter.
Morphine treatment of pregnant spiny mice also affected litter size. In our study, the average litter size was smaller in the morphine (10 and 30 mg/kg) treated groups compared to saline treated groups. As mentioned above, dams from the morphine treatment groups consistently presented with lower body weights during the periods of morphine treatment and postpartum and it is unclear if the smaller litter sizes from the morphine treated dams could correlate to the reduced weight gain observed in dams. Similarly, we are uncertain if morphine administration could have influenced the spontaneous death of pups and/or fetal absorption in utero as treatment with the higher, 30 mg/kg morphine dose significantly increased the number of deceased pups. More specifically, female pups from the higher, 30 mg/kg morphine group were most affected. The cannibalism of offspring by dams within the 30 mg/kg morphine treatment group was also observed. Endogenous opioids are known to play a key role in the regulation of the neuroendocrine axis and the initiation of maternal caregiving (Bicknell, 1985; Cruz et al., 2010; Farid et al., 2008; Morley, 1981; Stafisso-Sandoz et al., 1998). Previous studies have reported a decrease in maternal care and an increase in cannibalism following gestational opioid exposure (Chen et al., 2015; Wallin et al., 2019). Although not conclusive, it seems as though the deaths associated with morphine treatment were not perhaps directly linked to the lack of maternal care, but instead an effect of morphine on the pup's ability to survive. Careful observations of dams treated with 30 mg/kg morphine showed attempts to feed, groom, and care for their pups. However, pups that were severely affected were typically unable to feed regularly and exhibited signs of failure to thrive.
Prenatal morphine exposure did not result in all pups within a litter dying. Instead, one pup was more effected than their litter mates. Studies have shown that prenatal drug exposure can be complicated by the uterine position of pups; where one pup may have greater drug exposure than another pup in the same litter (Lipton et al., 1998). Fortunately, with the litter size of spiny mice being smaller than other rodents, any intra-pups’ variability of the symptoms associated with withdrawal was minimized. Furthermore, unequal exposure to morphine cannot explain why the female pups from the 30 mg/kg morphine treated group died at a higher rate than their male counterparts. The greater risk posed in the female pups to morphine exposure could be due to sexually dimorphic differences in placental development. A previous study found, the placenta of female spiny mice undergo a greater degree of vascularization compared to males throughout gestation (O'Connell et al., 2013). This difference may lead to a greater degree of drug exposure in female offspring compared to males. Additionally, theses sex differences may be the result of an opioid-induced endocrinopathy in both the pregnant dams, as well as the pups (Seyfried and Hester, 2012). In future studies, we aim to record maternal behaviors during and after morphine treatment to improve our understanding of the role of sex and stress hormones on maternal care. Additionally, we plan to investigate the role the placenta may have on potential mechanisms related to withdrawal severity and survival in both male and female pups.
Prenatal opioid exposure also led to a significant decrease in body weight in male pups, this finding is similar to previous studies (Byrnes and Vassoler, 2017). While changes in body temperature have not been well characterized in rodent models of NOWS, a 2019 study showed that body temperature decreased following prenatal opioid exposure (Wallin et al., 2019). Additionally, studies in adult rodent models of opioid withdrawal have also reported a decrease in body temperature (Belknap, 1989; Lipták et al., 2012). In contrast to these previous reports, the body temperature of spiny mice pups from the morphine exposed groups was significantly higher in both sexes. This difference between body temperatures compared to previous reports may be due to variations in the hormonal profiles between other rodent models and spiny mice. For example, prenatal opioid exposure has been shown to impact both the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system by altering the expression of endogenous opioids which are known to modulate a stress response (Byrnes and Vassoler, 2017; Drolet et al., 2001). Unlike other rodent species, the fetal adrenal gland and brain of spiny mice have been found to produce dehydroepiandrosterone (DHEA) (Lamers et al., 1986). Although the presence of DHEA has been shown to decrease body temperature when administered to rodents, chronic morphine has been shown to decrease the levels of dehydroepiandrosterone sulfate (DHEAS), a precursor to DHEA, in both males and females (Catalina et al., 2002; Daniell, 2006). Also, previous studies in spiny mice have shown that the glucocorticoid cortisol is present when in other rodents, corticosterone is typically found (Lamers et al., 1986; Quinn et al., 2013). These differences in hormonal profiles could account for differences in the dysregulation of the HPA axis among rodent models and may give rise to the increase in body temperature of spiny mice which interestingly, closely resemble fever-like clinical symptoms observed in human infants experiencing NOWS (Kocherlakota, 2014).
Unlike most rodent species, spiny mice are precocial and are capable of walking shortly after birth (Brunjes, 1990). This unique characteristic allowed us to observe and measure withdrawal behaviors that are typically examined in adolescent and adult mice (Jones and Barr, 1995). For example, we measured wet dog shakes and jumping from PND 0–7. We observed an increase in these withdrawal behaviors in pups from the dams treated with morphine. In male pups, wet dog shakes were greater in the 30 mg/kg morphine exposed group, whereas in females wet dog shakes were greater in 10 mg/kg morphine exposed group. Additionally, we observed an increase in jumping behavior in morphine exposed spiny mice. Interestingly, the number of jumps was higher in both male and females exposed to 10 mg/kg morphine and this could be due to the effects on locomotion (motor skills). Previous studies have shown that morphine can have a biphasic effect on locomotion depending on the dose (Patti et al., 2005). Morphine can elicit a initial depression phase followed by a hyperlocomotion phase, however, since our data is from spiny mice pups (0–7 days-old) the increase in jumping may also be related to the development of motor skills during the first seven days of life. Previous studies have demonstrated that morphine at various doses can elicit a hyperlocomotive effect, our data also show a biphasic effect of morphine on locomotor behavior in spiny mice with stimulant effects at 10 mg/kg and depressive effects at 30 mg/kg (Belknap et al., 1998). The exact mechanism for these morphine-mediated effects on motor skills are currently unknown. We also found that spontaneous opioid withdrawal in spiny mice resulted in symptoms such as wall climbing, face cleaning, tremors, and USV's which have previously been characterized in infant rodents (Barr and Wang, 1992; Ceger and Kuhn, 2000; Jones and Barr, 1995; Zhu and Barr, 2004).
In our study, we found sex differences in almost all of these behaviors with the most striking differences observed in tremor behavior and USV's. Tremors are often observed in human infants with NOWS which results from the dysregulation of the autonomic nervous system and the transmission of norepinephrine (Kocherlakota, 2014). Here, we discovered that pups from the morphine exposed groups experienced a significantly greater number of tremors. More specifically, male pups exposed to 30 mg/kg morphine experienced a greater number of tremors. In contrast, females exposed to 10 mg/kg morphine experienced a greater number of tremors. Additionally, we found a marked sex difference between the number of USV's measured. During isolation or in times of distress, pups commonly emit USV's to elicit a dam retrieval response (Ehret, 2005; Elwood, 1979; Smotherman et al., 1974). Previous studies have shown that opioids can reduce USV's (Carden et al., 1991) and when precipitated, opioid withdrawal can result in a greater number of USV's and is thought to be a measure of distress and/or dysphoria (Bell et al., 1971; Covington and Miczek, 2003; Hofer and Shair, 1987). This increase in the number of USV's is now considered a marker of opioid withdrawal in preclinical models (Barr et al., 2011). Here, we report that male and female spiny mice pups from the morphine exposed groups emitted significantly fewer USV's between PND 0–7. Evidence of sex differences in USV's following opioid exposure was recently reported; male rodents emitted a greater number of USV's compared to female rodents (Robinson et al., 2019). Differences observed between male and female spiny mice pups may be attributed to sexually dimorphic transmission of norepinephrine from the locus coeruleus as the dysregulation of norepinephrine has been implicated in opioid addiction and withdrawal as well as in clinical symptoms of NOWS (Aston-Jones and Kalivas, 2008; Kocherlakota, 2014). Following prenatal morphine exposure, an increase in hypothalamic levels of norepinephrine and rate of turnover was observed in male rats. Whereas, females were found to have decreased levels of hypothalamic norepinephrine and turnover rate (Vathy et al., 1994). Additionally, previous studies have found that in untreated mice, males have been shown to emit a greater number of USV's compared to females (Bowers et al., 2013). Thus, our results may be explained by inherent sex differences in USV's (Barr and Wang, 1992; Ceger and Kuhn, 2000; Jones and Barr, 1995; Zhu and Barr, 2004). Studies in rodents have also shown that the emission of USV's changes during early postnatal development with the rate of USV's peaking around PND 7 and subsiding at approximately PND 14 (Elwood and Keeling, 1982). It is important to note that when comparing our findings to other studies, we should carefully consider the use of opioid antagonists used to precipitate opioid withdrawal and/or the postnatal drug regime used to mimic a third-trimester drug exposure. When taken together, it can be difficult to make direct comparisons of our results to those that also measured USV's at later postnatal time-points. Another key difference between other rodent models and spiny mice is that their ears and auditory structures are open and functional at birth which may have an impact on USV's (Brunjes, 1990; Ehret, 1983). Lastly, this is the first time that USV's have been studied in spiny mice, and we hope to gain more insight into this behavior as we learn more about this novel preclincal model of NOWS.
We recognize that our preclinical model of NOWS has limitations. For example, one major limitation of our study was the treatment regime. Our study initiated drug treatment mid-gestation on GD 19. Commonly, women are already engaged in drug abuse before conception and continue throughout gestation and the postpartum periods. However, due to the challenges of confirming pregnancy in spiny mice without an ultrasound machine, we chose to begin treatment mid-gestation following the confirmation of pregnancy measured as a percentage of weight gain from baseline weights; a sustained 1% increase in body weight was indicative of successful pregnancy. Despite this limitation, we believe our study captures a very critical period of gestation that coincides with the peak time of brain development that takes place during the 3rd trimester of pregnancy (Semple et al., 2013). Human studies have shown that infants exposed to opioids during the 3rd trimester have a greater risk for developing NOWS compared to fetus exposed at an earlier period of pregnancy (Desai et al., 2015).
Additionally, the relatively small litter size and longer gestational period of spiny mice limited our ability to scale this study. Cages were checked daily at 08:00 h for new pups and without a 24 h video monitoring system we were unable to record the precise time of births. This may account for any inter- and intra-treatment groups variations in withdrawal behavioral outcomes measured during PND 0–7. Another limitation of our study is the dosing frequency. We injected once-daily to minimize the stress associated with handling and injections in pregnant dams. However, we could have injected twice daily but this could have also introduced more stress that could have impacted the health and behaviors of both dam and pups. Many previously published reports show the successful use of osmotic minipumps to deliver a constant dose of a drug. However, in our study due to the long gestation periods of spiny mice and the 10 and 30 mg/kg morphine doses used it prevented us from utilizing mini-pumps. Additionally, mini pumps would not allow us to adjust the dose of morphine as the body weight of pregnant dams increased.
5. Conclusion
Taken together, our finding validate the use of spiny mice to investigate the effects of prenatal morphine exposure and introduces a novel preclinical model of NOWS. Prenatal morphine exposure affected the development of opioid withdrawal, and perhaps female pups showed more effects than male pups. Our results are consistent with previous research whereby chronic morphine exposure increased jumps, wet dog shakes, wall climbs, face cleaning, and tremors, all well-characterized behaviors associated with opioid withdrawal. Inconsistences between our findings and those in the literature may be due to the variety of doses used, different rodent species, and differences in treatment regimes. Future studies aim to investigate the long-term effects of prenatal opioid exposure on learning and memory in spiny mice and determine cell-specific and molecular changes in the brain induced by prenatal opioid exposure during withdrawal. We are hopeful that our novel mouse model of NOWS will not only provide new insights into the unknown effects of prenatal drug exposure but also highlight the importance of using a more mature and developed rodent species like spiny mice in numerous areas of biomedical research.
Declarations
Author contribution statement
Sarah Stevens PhD: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shekher Mohan PhD: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by research funds from the Dept. of Pharmaceutical Sciences and Pharmacogenomics, Manchester University, School of Pharmacy, Fort Wayne, IN and the Dept. of Integrative Physiology and Pharmacology, Liberty University, College of Osteopathic Medicine, Lynchburg, VA.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to sincerely thank Dr. Ashley Seifert PhD (Univ. Kentucky, Lexington, KY) for providing us spiny mice to help us establish our own colony and to Dr. Ahmed Mustafa PhD (Univ. Purdue, Fort Wayne, IN) for his support, advice and access to space to perform all the animal behavioral experiment's.
References
- Agency for Healthcare Research and Quality [WWW Document] Neonatal abstinence syndrome (NAS) among newborn hospitalizations. 2020. https://www.hcup-us.ahrq.gov/faststats/NASServlet?setting1=IP URL.
- Aston-Jones G., Kalivas P.W. Brain norepinephrine rediscovered in addiction research. Biol. Psychiatr. 2008;63:1005–1006. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr G.A., McPhie-Lalmansingh A., Perez J., Riley M. Changing mechanisms of opiate tolerance and withdrawal during early development: animal models of the human experience. ILAR J. 2011;52:329–341. doi: 10.1093/ilar.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr G.A., Wang S. Tolerance and withdrawal to chronic morphine treatment in the week-old rat pup. Eur. J. Pharmacol. 1992;215:35–42. doi: 10.1016/0014-2999(92)90605-4. [DOI] [PubMed] [Google Scholar]
- Belknap J.K. Components of the opioid withdrawal syndrome in mice are thermoregulatory responses. Pharmacol. Biochem. Behav. 1989;34:241–245. doi: 10.1016/0091-3057(89)90306-7. [DOI] [PubMed] [Google Scholar]
- Belknap J.K., Riggan J., Cross S., Young E.R., Gallaher E.J., Crabbe J.C. Genetic determinants of morphine activity and thermal responses in 15 inbred mouse strains. Pharmacol. Biochem. Behav. 1998;59:353–360. doi: 10.1016/s0091-3057(97)00421-8. [DOI] [PubMed] [Google Scholar]
- Bell R.W., Nitschke W., Gorry T.H., Zachman T.A. Infantile stimulation and ultrasonic signaling: a possible mediator of early handling phenomena. Dev. Psychobiol. 1971;4:181–191. doi: 10.1002/dev.420040209. [DOI] [PubMed] [Google Scholar]
- Bellofiore N., Ellery S.J., Mamrot J., Walker D.W., Temple-Smith P., Dickinson H. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus) Am. J. Obstet. Gynecol. 2016;216:40.e1–40.e11. doi: 10.1016/j.ajog.2016.07.041. [DOI] [PubMed] [Google Scholar]
- Bicknell R.J. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J. Endocrinol. 1985;107:437–446. doi: 10.1677/joe.0.1070437. [DOI] [PubMed] [Google Scholar]
- Boggess T., Risher W.C. Clinical and basic research investigations into the long-term effects of prenatal opioid exposure on brain development. J. Neurosci. Res. 2020 doi: 10.1002/jnr.24642. [DOI] [PubMed] [Google Scholar]
- Bowers J.M., Perez-Pouchoulen M., Edwards N.S., McCarthy M.M. Foxp2 mediates sex differences in ultrasonic vocalization by rat pups and directs order of maternal retrieval. J. Neurosci. 2013;33:3276–3283. doi: 10.1523/JNEUROSCI.0425-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunjes P.C. The precocial mouse, Acomys cahirinus. Psychobiology. 1990;18:339–350. [Google Scholar]
- Brunjes P.C. A comparative study of prenatal development in the olfactory bulb, neocortex and hippocampal region of the precocial mouse Acomys cahirinus and rat. Dev. Brain Res. 1989;49:7–25. doi: 10.1016/0165-3806(89)90055-2. [DOI] [PubMed] [Google Scholar]
- Brunjes P.C. A stereological study of neocortical maturation in the precocial mouse, Acomys cahirinus. Dev. Brain Res. 1985;19:279–287. doi: 10.1016/0165-3806(85)90199-3. [DOI] [PubMed] [Google Scholar]
- Brunjes P.C., Korol D.L., Stern K.G. Prenatal neurogenesis in the telencephalon of the precocial mouse Acomys cahirinus. Neurosci. Lett. 1989;107:114–119. doi: 10.1016/0304-3940(89)90801-x. [DOI] [PubMed] [Google Scholar]
- Byrnes E.M., Vassoler F.M. Modeling prenatal opioid exposure in animals: current findings and future directions. Front. Neuroendocrinol. 2017;51:1–13. doi: 10.1016/j.yfrne.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden S.E., Barr G.A., Hofer M.A. Differential effects of specific opioid receptor agonists on rat pup isolation calls. Dev. Brain Res. 1991;62:17–22. doi: 10.1016/0165-3806(91)90185-l. [DOI] [PubMed] [Google Scholar]
- Carter A.M., Enders A.C., Jones C.J.P., Mess A., Pfarrer C., Pijnenborg R., Soma H. Comparative placentation and animal models: patterns of trophoblast invasion – a workshop report. Placenta. 2006;27:30–33. doi: 10.1016/j.placenta.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Catalina F., Milewich L., Frawley W., Kumar V., Bennett M. Decrease of core body temperature in mice by dehydroepiandrosterone. Exp. Biol. Med. 2002;227:382–388. doi: 10.1177/153537020222700603. [DOI] [PubMed] [Google Scholar]
- Ceger P., Kuhn C.M. Opiate withdrawal in the neonatal rat: relationship to duration of treatment and naloxone dose. Psychopharmacology. 2000;150:253–259. doi: 10.1007/s002130000413. [DOI] [PubMed] [Google Scholar]
- Chen S.-T., Chen H.-H., Chiang Y.-C., Yuan Z.F., Kuo C.-C., Lai M.-D., Hung T.-W., Ho I. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatric Dis. Treat. 2015;11:609–618. doi: 10.2147/NDT.S70585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B., Darlington R.B., Finlay B.L. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Covington H.E., Miczek K.A. Vocalizations during withdrawal from opiates and cocaine: possible expressions of affective distress. Eur. J. Pharmacol. 2003;467:1–13. doi: 10.1016/s0014-2999(03)01558-9. [DOI] [PubMed] [Google Scholar]
- Cruz A. de M., Maiorka P.C., Canteras N.S., Sukikara M.H., Felicio L.F. Morphine treatment during pregnancy modulates behavioral selection in lactating rats. Physiol. Behav. 2010;101:40–44. doi: 10.1016/j.physbeh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Daniell H.W. DHEAS deficiency during consumption of sustained-action prescribed opioids: evidence for opioid-induced inhibition of adrenal androgen production. J. Pain. 2006;7:901–907. doi: 10.1016/j.jpain.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Desai R.J., Huybrechts K.F., Hernandez-Diaz S., Mogun H., Patorno E., Kaltenbach K., Kerzner L.S., Bateman B.T. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ Br. Med. J. (Clin. Res. Ed.) 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson H., Walker D. Managing a colony of spiny mice (Acomys cahirinus) for perinatal research. ANZCCART. 2007:4–11. [Google Scholar]
- Dickinson H., Walker D.W., Cullen-McEwen L., Wintour E.M., Moritz K. The spiny mouse ( Acomys cahirinus ) completes nephrogenesis before birth. Am. J. Physiol. Renal. 2005;289:F273–F279. doi: 10.1152/ajprenal.00400.2004. [DOI] [PubMed] [Google Scholar]
- Dobbing J., Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dobbing J., Sands J. Growth and development of the brain and spinal cord of the Guinea pig. Brain Res. 1970;17:115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Drolet G., Dumont É.C., Gosselin I., Kinkead R., Laforest S., Trottier J.-F. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuro. Psychopharmacol. Biol. Psychiatr. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- D’Udine B., Gerosa E., Drewett R.F. Maternal behavior and the milk ejection reflex in a precocial murid (Acomys cahirinus) Behav. Neural. Biol. 1980;28:378–381. doi: 10.1016/s0163-1047(80)92413-9. [DOI] [PubMed] [Google Scholar]
- Ehret G. Infant rodent ultrasounds – a gate to the understanding of sound communication. Behav. Genet. 2005;35:19–29. doi: 10.1007/s10519-004-0853-8. [DOI] [PubMed] [Google Scholar]
- Ehret G. Auditory processing and perception of ultrasounds in house mice. Adv. Vertebrate Neuroethol. 1983:911–917. undefined. [Google Scholar]
- Ellery S.J., LaRosa D.A., Kett M.M., Gatta P.A.D., Snow R.J., Walker D.W., Dickinson H. Dietary creatine supplementation during pregnancy: a study on the effects of creatine supplementation on creatine homeostasis and renal excretory function in spiny mice. Amino Acids. 2015;48:1819–1830. doi: 10.1007/s00726-015-2150-7. [DOI] [PubMed] [Google Scholar]
- Elwood R.W. Ultrasounds and maternal behavior in the Mongolian gerbil. Dev. Psychobiol. 1979;12:281–284. doi: 10.1002/dev.420120402. [DOI] [PubMed] [Google Scholar]
- Elwood R.W., Keeling F. Temporal organization of ultrasonic vocalizations in infant mice. Dev. Psychobiol. 1982;15:221–227. doi: 10.1002/dev.420150306. [DOI] [PubMed] [Google Scholar]
- Farid W.O., Dunlop S.A., Tait R.J., Hulse G.K. The effects of maternally administered methadone, buprenorphine and naltrexone on offspring: review of human and animal data. Curr. Neuropharmacol. 2008;6:125–150. doi: 10.2174/157015908784533842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughton C.L., Gawriluk T.R., Seifert A.W. The biology and husbandry of the african spiny mouse (Acomys cahirinus) and the research uses of a laboratory colony. J. Am. Assoc. Lab. Anim. Sci. Jaalas. 2016;55:9–17. [PMC free article] [PubMed] [Google Scholar]
- Hofer M.A., Shair H.N. Isolation distress in two-week-old rats: influence of home cage, social companions, and prior experience with littermates. Dev. Psychobiol. 1987;20:465–476. doi: 10.1002/dev.420200410. [DOI] [PubMed] [Google Scholar]
- Ireland Z., Castillo-Melendez M., Dickinson H., Snow R., Walker D.W. A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience. 2011;194:372–379. doi: 10.1016/j.neuroscience.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Ireland Z., Dickinson H., Snow R., Walker D.W. Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am. J. Obstet. Gynecol. 2008;198 doi: 10.1016/j.ajog.2007.10.790. 431.e1–6. [DOI] [PubMed] [Google Scholar]
- Jones K.L., Barr G.A. Ontogeny of morphine withdrawal in the rat. Behav. Neurosci. 1995;109:1189–1198. doi: 10.1037//0735-7044.109.6.1189. [DOI] [PubMed] [Google Scholar]
- Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014;134:e547–e561. doi: 10.1542/peds.2013-3524. [DOI] [PubMed] [Google Scholar]
- Lamers W.H., Mooren P.G., Graaf A., Charles R. Perinatal development of the liver in rat and spiny mouse. Its relation to altricial and precocial timing of birth. Eur. J. Biochem. 1985;146:475–480. doi: 10.1111/j.1432-1033.1985.tb08675.x. [DOI] [PubMed] [Google Scholar]
- Lamers W.H., Mooren P.G., Griep H., Endert E., Degenhart H.J., Charles R. Hormones in perinatal rat and spiny mouse: relation to altricial and precocial timing of birth. Am. J. Physiol. Endoc. M. 1986;251:E78–E85. doi: 10.1152/ajpendo.1986.251.1.E78. [DOI] [PubMed] [Google Scholar]
- Lawrence A.J., Michalkiewicz A., Morley J.S., Mackinnon K., Billington D. Differential inhibition of hepatic morphine UDP-glucuronosyltransferases by metal ions. Biochem. Pharmacol. 1992;43:2335–2340. doi: 10.1016/0006-2952(92)90311-6. [DOI] [PubMed] [Google Scholar]
- Lipták N., Dochnal R., Babits A., Csabafi K., Szakács J., Tóth G., Szabó G. The effect of pituitary adenylate cyclase-activating polypeptide on elevated plus maze behavior and hypothermia induced by morphine withdrawal. Neuropeptides. 2012;46:11–17. doi: 10.1016/j.npep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Lipton J.W., Robie H.C., Ling Z., Weese-Mayer D.E., Carvey P.M. The magnitude of brain dopamine depletion from prenatal cocaine exposure is a function of uterine position. Neurotoxicol. Teratol. 1998;20:373–382. doi: 10.1016/s0892-0362(97)00143-8. [DOI] [PubMed] [Google Scholar]
- Mithbaokar P., Fiorito F., Morte R.D., Maharajan V., Costagliola A. Chronic maternal morphine alters calbindin D-28k expression pattern in postnatal mouse brain. Synapse. 2016;70:15–23. doi: 10.1002/syn.21866. [DOI] [PubMed] [Google Scholar]
- Morley J.E. The endocrinology of the opiates and opioid peptides. Metabolis. 1981;30:195–209. doi: 10.1016/0026-0495(81)90172-4. [DOI] [PubMed] [Google Scholar]
- Morrison J.L., Botting K.J., Darby J.R.T., David A.L., Dyson R.M., Gatford K.L., Gray C., Herrera E.A., Hirst J.J., Kim B., Kind K.L., Krause B.J., Matthews S.G., Palliser H.K., Regnault T.R.H., Richardson B.S., Sasaki A., Thompson L.P., Berry M.J. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J. Physiol. (Lond.) 2018;596:5535–5569. doi: 10.1113/JP274948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey L.J., Olsen G.D. A stereospecific microassay for the determination of morphine-6-β-D-glucuronide and other active morphine metabolites in the neonatal Guinea pig. J. Liq. Chromatogr. 1993;16:2545–2561. [Google Scholar]
- O’Connell B.A., Moritz K.M., Walker D.W., Dickinson H. Sexually dimorphic placental development throughout gestation in the spiny mouse (Acomys cahirinus) Placenta. 2013;34:119–126. doi: 10.1016/j.placenta.2012.11.009. [DOI] [PubMed] [Google Scholar]
- Oosterhuis W.P., Mooren P.G., Charles R., Lamers W.H. Perinatal development of the lung in rat and spiny mouse: its relation to altricial and precocial timing of birth. Neonatology. 1984;45:236–243. doi: 10.1159/000242011. [DOI] [PubMed] [Google Scholar]
- Patrick S.W., Schumacher R.E., Benneyworth B.D., Krans E.E., McAllister J.M., Davis M.M. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307:1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- Patti C.L., Frussa-Filho R., Silva R.H., Carvalho R.C., Kameda S.R., Takatsu-Coleman A.L., Cunha J.L.S., Abílio V.C. Behavioral characterization of morphine effects on motor activity in mice. Pharmacol. Biochem. Behav. 2005;81:923–927. doi: 10.1016/j.pbb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Quinn T.A., Ratnayake U., Dickinson H., Castillo-Melendez M., Walker D.W. The feto-placental unit, and potential roles of dehydroepiandrosterone (DHEA) in prenatal and postnatal brain development: a re-examination using the spiny mouse. J. Steroid Biochem. Mol. Biol. 2016;160:204–213. doi: 10.1016/j.jsbmb.2015.09.044. [DOI] [PubMed] [Google Scholar]
- Quinn T.A., Ratnayake U., Dickinson H., Nguyen T.-H., McIntosh M., Castillo-Melendez M., Conley A.J., Walker D.W. Ontogeny of the adrenal gland in the spiny mouse, with particular reference to production of the steroids cortisol and dehydroepiandrosterone. Endocrinology. 2013;154:1190–1201. doi: 10.1210/en.2012-1953. [DOI] [PubMed] [Google Scholar]
- Richardson K.A., Yohay A.-L.J., Gauda E.B., McLemore G.L. Neonatal animal models of opiate withdrawal. ILAR J. 2006;47:39–48. doi: 10.1093/ilar.47.1.39. [DOI] [PubMed] [Google Scholar]
- Robinson S.A., Jones A.D., Brynildsen J.K., Ehrlich M.E., Blendy J.A. Neurobehavioral effects of neonatal opioid exposure in mice: influence of the OPRM1 SNP. Addiction Biol. 2019 doi: 10.1111/adb.12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.J., Graham D.L., Money K.M., Stanwood G.D. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacol. Offl. Publ. Am. Coll. Neuropsychopharmacol. 2014;40:61–87. doi: 10.1038/npp.2014.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple B.D., Blomgren K., Gimlin K., Ferriero D.M., Noble-Haeusslein L.J. Brain development in rodents and humans: identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013;106(107):1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfried O., Hester J. Opioids and endocrine dysfunction. Br. J. Pain. 2012;6:17–24. doi: 10.1177/2049463712438299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R., Bar N., Vathy I. Long-term effects of prenatal morphine exposure on maternal behaviors differ from the effects of direct chronic morphine treatment. Dev. Psychobiol. 2003;43:281–289. doi: 10.1002/dev.10141. [DOI] [PubMed] [Google Scholar]
- Slamberova R., Rimanoczy A., Bar N., Schindler C.J., Vathy I. Density of μ-opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus. 2003;13:461–471. doi: 10.1002/hipo.10076. [DOI] [PubMed] [Google Scholar]
- Slamberova R., Rimanoczy A., Cao D., Schindler C.J., Vathy I. Alterations of prenatal morphine exposure in μ-opioid receptor density in hypothalamic nuclei associated with sexual behavior. Brain Res. Bull. 2005;65:479–485. doi: 10.1016/j.brainresbull.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Bell R.W., Starzec J., Elias J., Zachman T.A. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav. Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- Stafisso-Sandoz G., Polley D., Holt E., Lambert K.G., Kinsley C.H. Opiate disruption of maternal behavior: morphine reduces, and naloxone restores, c-fos activity in the medial preoptic area of lactating rats. Brain Res. Bull. 1998;45:307–313. doi: 10.1016/s0361-9230(97)00375-4. [DOI] [PubMed] [Google Scholar]
- Sutter M.B., Leeman L., Hsi A. Neonatal opioid withdrawal syndrome. Obstet. Gynecol. Clin. N. Am. 2014;41:317–334. doi: 10.1016/j.ogc.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Umer A., Loudin S., Maxwell S., Lilly C., Stabler M.E., Cottrell L., Hamilton C., Breyel J., Mullins C., John C. Capturing the statewide incidence of neonatal abstinence syndrome in real time: the West Virginia experience. Pediatr. Res. 2018;85:607–611. doi: 10.1038/s41390-018-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathy I., Rimanoczy A., Eaton R.C., Katay L. Modulation of catecholamine turnover rate in brain regions of rats exposed prenatally to morphine. Brain Res. 1994;662:209–215. doi: 10.1016/0006-8993(94)90814-1. [DOI] [PubMed] [Google Scholar]
- Wallin C.M., Bowen S.E., Roberge C.L., Richardson L.M., Brummelte S. Gestational buprenorphine exposure: effects on pregnancy, development, neonatal opioid withdrawal syndrome, and behavior in a translational rodent model. Drug Alcohol Depend. 2019;205:107625. doi: 10.1016/j.drugalcdep.2019.107625. [DOI] [PubMed] [Google Scholar]
- Winkelman T.N.A., Villapiano N., Kozhimannil K.B., Davis M.M., Patrick S.W. Incidence and costs of neonatal abstinence syndrome among infants with medicaid: 2004-2014. Pediatrics. 2018;141 doi: 10.1542/peds.2017-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Barr G.A. The role of AMPA and metabotropic glutamate receptors on morphine withdrawal in infant rats. Int. J. Dev. Neurosci. 2004;22:379–395. doi: 10.1016/j.ijdevneu.2004.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.