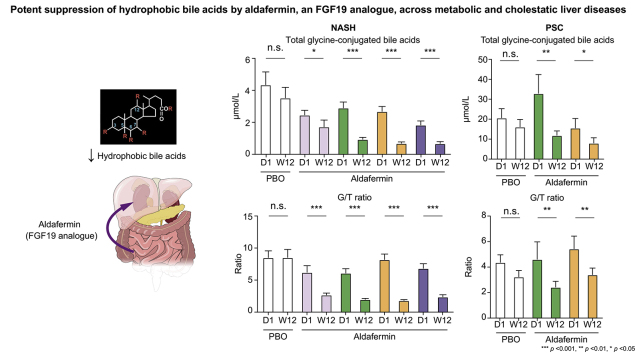
Summary
Background & Aims
Higher serum bile acid levels are associated with an increased risk of cirrhosis and liver-related morbidity and mortality. Herein, we report secondary analyses of aldafermin, an engineered analogue of the gut hormone fibroblast growth factor 19, on the circulating bile acid profile in prospective, phase II studies in patients with metabolic or cholestatic liver disease.
Methods
One hundred and seventy-six patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH) and fibrosis and elevated liver fat content (≥8% by magnetic resonance imaging-proton density fat fraction) received 0.3 mg (n = 23), 1 mg (n = 49), 3 mg (n = 49), 6 mg (n = 28) aldafermin or placebo (n = 27) for 12 weeks. Sixty-two patients with primary sclerosing cholangitis (PSC) and elevated alkaline phosphatase (>1.5× upper limit of normal) received 1 mg (n = 21), 3 mg (n = 21) aldafermin or placebo (n = 20) for 12 weeks. Serum samples were collected on day 1 and week 12 for determination of bile acid profile and neoepitope-specific N-terminal pro-peptide of type III collagen (Pro-C3), a direct measure of fibrogenesis.
Results
Treatment with aldafermin resulted in significant dose-dependent reductions in serum bile acids. In particular, bile acids with higher hydrophobicity indices, such as deoxycholic acid, lithocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, and glycocholic acid, were markedly lowered by aldafermin in both NASH and PSC populations. Moreover, aldafermin predominantly suppressed the glycine-conjugated bile acids, rather than the taurine-conjugated bile acids. Changes in levels of bile acids correlated with changes in the novel fibrogenesis marker Pro-C3, which detects a neo-epitope of the type III collagen during its formation, in the pooled NASH and PSC populations.
Conclusions
Aldafermin markedly reduced major hydrophobic bile acids that have greater detergent activity and cytotoxicity. Our data provide evidence that bile acids may contribute to sustaining a pro-fibrogenic microenvironment in the liver across metabolic and cholestatic liver diseases.
Lay summary
Aldafermin is an analogue of a gut hormone, which is in development as a treatment for patients with chronic liver disease. Herein, we show that aldafermin can potently and robustly suppress the toxic, hydrophobic bile acids irrespective of disease aetiology. The therapeutic strategy utilising aldafermin may be broadly applicable to other chronic gastrointestinal and liver disorders.
Clinical Trials Registration
The study is registered at Clinicaltrials.govNCT02443116 and NCT02704364.
Keywords: Non-alcoholic steatohepatitis, Primary sclerosing cholangitis, Fibrogenesis, Fibroblast growth factor, Bile acid synthesis, Pro-C3
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAAT, bile acid-CoA:amino acid N-acyltransferase; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; ELF test, Enhanced Liver Fibrosis test; FGF19, fibroblast growth factor 19; FXR, farnesoid X receptor; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; G/T ratio, ratio of glycine to taurine conjugates of bile acids; LCA, lithocholic acid; MRI-PDFF, magnetic resonance imaging-proton density fat fraction; NAFLD, non-alcoholic fatty liver disease; NAS, non-alcoholic fatty liver disease activity score; NASH, non-alcoholic steatohepatitis; NASH CRN, NASH Clinical Research Network; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; PSC, primary sclerosing cholangitis; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid; UDCA, ursodeoxycholic acid
Graphical abstract
Highlights
-
•
Higher serum bile acid levels are associated with an increased risk of liver-related morbidity and mortality.
-
•
Aldafermin produces significant dose-dependent reductions in toxic hydrophobic bile acids in NASH and PSC.
-
•
Changes in bile acids correlate with changes in the novel fibrogenesis marker Pro-C3.
-
•
Bile acids may contribute to a pro-fibrogenic microenvironment in the liver.
Introduction
Bile acids are amphipathic metabolites that are synthesised from cholesterol in the liver.1 Once synthesised, they are conjugated with glycine or taurine and then excreted with bile into the duodenum. On reaching the intestine, bile acids facilitate emulsification and absorption of dietary fats, cholesterol, and fat-soluble vitamins. Approximately 95% of bile acids are re-absorbed through the terminal ileum and recirculated to the liver; the remaining ~5% enters the large intestine where the colonic microbiota dehydroxylate the primary bile acids to form secondary bile acids with unique chemical structures.1
Over the past two decades a remarkable body of literature indicates that bile acids have multiple functions which are mediated through both non-specific cytotoxic effects given their amphipathic structure and specific receptor-mediated actions including engagement of their cognate receptor, the farnesoid X receptor (FXR), as well as other receptors such as the G protein-coupled bile acid receptor 1 (GPBAR1, also known as TGR5), the pregnane X receptor (PXR), and the sphingosine-1-phosphate receptor 2 (S1P2).2,3 The bile acids secreted into the bile can further modify the microbiome, alter the intestinal barrier function, modulate the innate immune system, and influence the development and progression of gastrointestinal and liver disease.4
Altered bile acid homeostasis has been clearly linked to multiple chronic liver diseases.5,6 The development of non-alcoholic steatohepatitis (NASH) is associated with elevated circulating total bile acid concentration, with increases in both primary and secondary bile acids.[7], [8], [9] Patients with NASH appear to have an increased rate of hepatic bile acid synthesis.10,11 A similar increase in circulating bile acid level has been described in cholestatic liver disease, where higher serum bile acids are associated with worse clinical outcomes.5 Moreover, serum bile acids correlate with portal hypertension and model for end-stage liver disease score, and can predict decompensation, liver failure, and transplant-free survival.12 Increased serum bile acid has been reported to significantly associate with 28-day mortality in critically ill patients, independently of bilirubin levels and severity of the underlying disease.13
The gut hormone fibroblast growth factor 19 (FGF19) is a core component of the gut–liver axis in maintaining bile acid homeostasis.14,15 FGF19 is induced by the activation of FXR in the intestine, and then travels to the liver where it activates the FGFR4-βKlotho receptor complex on hepatocytes to inhibit bile acid synthesis.14,15 Aldafermin, also known as NGM282 or M70, is an engineered analogue of the human hormone FGF19.11,16,17 Through the FGFR4-KLB receptor, aldafermin potently suppresses cholesterol 7α-hydroxylase (CYP7A1), which encodes the first and rate-limiting enzyme in the biosynthesis of bile acids.16 Through the FGFR1c-KLB receptor, aldafermin improves insulin sensitivity and energy homeostasis.18,19 In a phase II trial in patients with NASH, aldafermin reduced steatosis and improved hepatic inflammation, injury, and fibrosis.20,21 In a phase II trial in patients with primary sclerosing cholangitis (PSC), aldafermin improved serum markers of fibrosis without affecting alkaline phosphatase (ALP).22
There has been increasing interest in understanding the role of bile acids in chronic liver disease, with multiple therapeutics that regulate bile acid metabolism in clinical trials in NASH and cholestatic liver disease.23,24 In the present secondary analyses, we describe detailed bile acid profiles at baseline and end of treatment in patients with NASH or PSC who received 12 weeks of treatment with placebo or varying doses of aldafermin. We use these data to define treatment-induced changes in the individual bile acids and further evaluate how changes in the bile acid profile and fibrogenesis are linked.
Patients and methods
Patients
Both NASH and PSC phase II studies were conducted in compliance with the International Conference on Harmonization, E6 Good Clinical Practice, and Declaration of Helsinki, and all patients provided written informed consent. The study protocols were approved by the local ethics committees before study initiation. A.J.S., L.L., and G.M.H. had access to all of the data and vouch for the integrity of the data analyses.
Patients with NASH
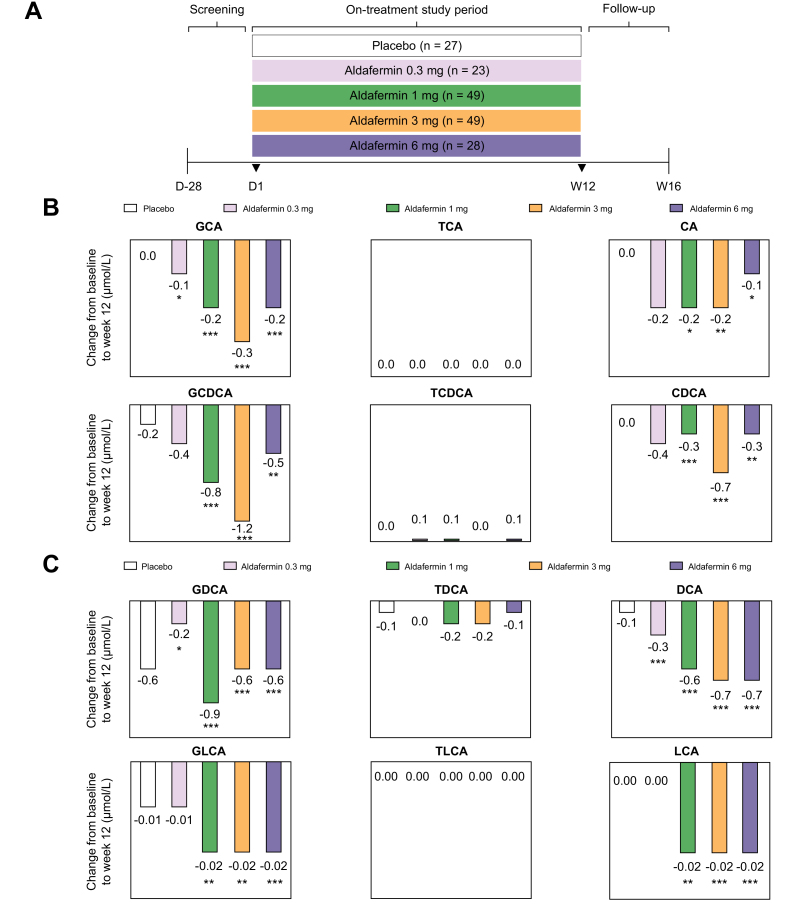
In this phase II study, multiple cohorts of NASH patients were enrolled. Cohort 1 was a placebo-controlled, double-blind study comparing aldafermin 3 mg (n = 27) and 6 mg (n = 28) vs. placebo (n = 27) for 12 weeks20; Cohort 2 was a dose-expansion study evaluating aldafermin 0.3 mg (n = 23), 1 mg (n = 21) and 3 mg (n = 22) for 12 weeks25; Cohort 3 further expanded assessment of aldafermin 1 mg in an additional 28 patients for 12 weeks.21 The primary endpoint was change from baseline to week 12 (end of treatment) in liver fat content as measured by magnetic resonance imaging-proton density fat fraction (MRI-PDFF). Patients were stratified by type 2 diabetes status. Overall, a total of 176 patients with NASH received 0.3 mg (n = 23), 1 mg (n = 49), 3 mg (n = 49), 6 mg (n = 28) aldafermin or placebo (n = 27) daily for 12 weeks, and were pooled in this secondary analysis (Fig. 1A). Details on procedures and treatment in Cohorts 1–3 were previously reported.20,21,25 The clinicaltrials.gov trial number is NCT02443116.
Fig. 1.
Aldafermin reduces the toxic, hydrophobic, glycine-conjugated bile acids in patients with NASH.
(A) A total of 176 patients with biopsy-proven NASH received 0.3 mg (n = 23), 1 mg (n = 49), 3 mg (n = 49), 6 mg (n = 28) aldafermin or placebo (n = 27) for 12 weeks in this phase II trial of aldafermin. Serum samples were collected at baseline (day 1) and week 12 (end of treatment) for bile acid profiling. (B) Change from baseline at week 12 in primary bile acids and conjugates. Note that aldafermin preferentially reduced levels of the more toxic, hydrophobic, glycine-conjugated primary bile acids. (C) Change from baseline at week 12 in secondary bile acids and conjugates. Similarly, aldafermin preferentially reduced levels of the more toxic, hydrophobic, glycine-conjugated secondary bile acids. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001 vs. baseline (Wilcoxon test). CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid.
Patients were eligible if they met the following inclusion criteria: 18–75 years of age at the time of screening; biopsy-confirmed NASH diagnosis as defined by the NASH Clinical Research Network (CRN) histologic scoring system, with a minimum non-alcoholic fatty liver disease (NAFLD) Activity Score (NAS) of 4 (≥1 point in each component of steatosis, lobular inflammation, and hepatocellular ballooning); stage 1, 2, or 3 fibrosis; liver fat content ≥8% as assessed by MRI-PDFF; and elevated alanine aminotransferase (ALT; ≥19 IU/L in females; ≥30 IU/L in males). Exclusion criteria included clinically significant acute or chronic liver disease unrelated to NASH; evidence of drug-induced steatohepatitis; history or presence of compensated or decompensated cirrhosis; liver transplantation; any cardiovascular event or evidence of active cardiovascular disease within 6 months of screening; and type 1 diabetes. Patients taking medications for diabetes were required to be on a stable regimen for at least 3 months before day 1 and maintain a stable regimen during the study period. Initiation of any medications or products for diabetes or weight-loss was prohibited from screening to day 1 until the end of the study.
Patients with PSC
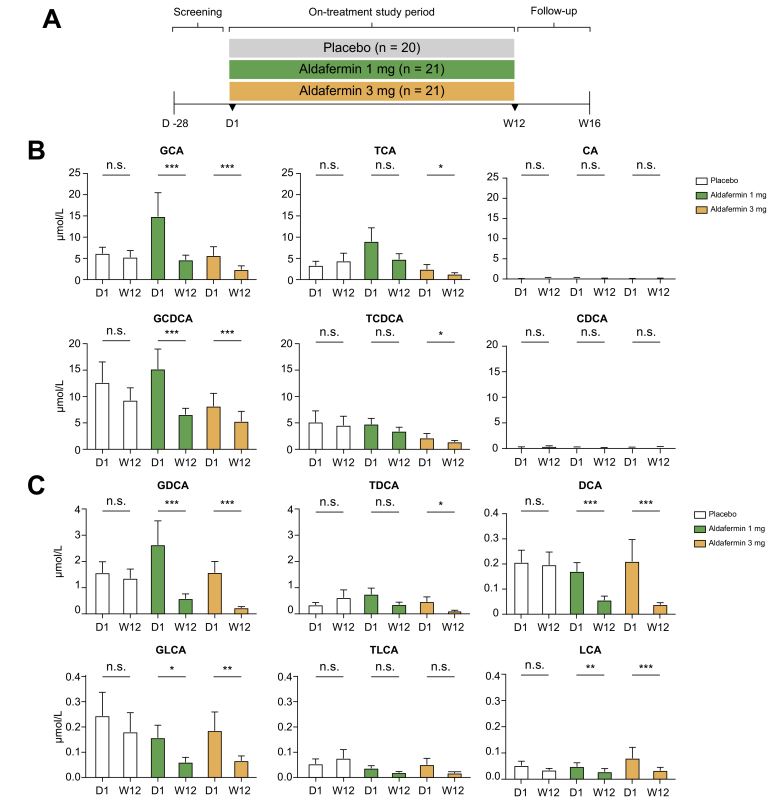
Sixty-two patients were randomised to receive aldafermin 1 mg (n = 21), 3 mg (n = 21) or placebo (n = 20) daily for 12 weeks. The primary endpoint was the change in ALP from baseline to week 12 (end of treatment). Details on procedures and treatment were previously reported.22 The clinicaltrials.gov trial number is NCT02704364.
Patients were eligible if they met the following inclusion criteria: 18–75 years of age at the time of screening; confirmed diagnosis of PSC (based on any 2 of the 3 criteria: abnormal cholangiography consistent with PSC as measured by magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatography, or percutaneous transhepatic cholangiography; liver biopsy consistent with PSC; historical evidence of elevated ALP); ALP >1.5× upper limit of normal; ALT and aspartate aminotransferase (AST) <5× upper limit of normal; bilirubin ≤2.5 mg/dl. Patients taking ursodeoxycholic acid (UDCA; ursodiol) were allowed to enrol if on stable dose (<27 mg/kg per day) within 2 months of screening. Patients were also allowed to enrol if they had dominant strictures with no evidence of clinical concern, inflammatory bowel disease with no episode of flare, autoimmune hepatitis on stable immunosuppressive regimen with no hepatic flare, compensated cirrhosis or pre-sinusoidal oesophageal varices with no history of bleeding and no other evidence of hepatic decompensation. Exclusion criteria included clinically significant acute or chronic liver disease of an aetiology other than PSC; evidence of secondary or immunoglobulin G4-related sclerosing cholangitis; placement of a bile duct stent or percutaneous bile duct drain within 3 months of screening; decompensated cirrhosis; and liver transplantation.
Liver biopsy
During the screening period and before to randomisation, patients with NASH underwent a liver biopsy or provided a liver biopsy tissue specimen obtained within the previous 6 months. Tissue samples were prepared and read by qualified local pathologists to verify NASH according to the NASH CRN histologic scoring system. The NAS (with a score of 8 representing the highest disease severity) is the sum of scores of the 3 components of the histological scoring system (steatosis, ballooning, inflammation). Steatosis in hepatocytes was scored as 0, 1, 2, or 3 if there were less than 5%, 5–33%, 33–66%, or >66% hepatocytes with fat, respectively. Ballooning degeneration in hepatocytes was scored as 0, 1, or 2 if there were none, few ballooned cells, or many ballooned cells, respectively. Lobular inflammation was scored as 0, 1, 2, or 3 if there were no foci, <2 foci, 2–4 foci, or >4 foci per 200× field, respectively. The NASH CRN scoring system of NAFLD fibrosis (0–4 for stages F0–F4) was used to evaluate the stage of fibrosis in each specimen, with higher scores representing more severe fibrosis.
Liver imaging by MRI
NASH patients underwent MRI for assessment of liver fat content by MRI-PDFF at baseline and week 12 (end of treatment). Scans were performed on qualified and standardised instruments at 1.5 Tesla or 3 Tesla field strength. The local MRI facilities completed a qualification process before performing study MRI examinations, and ongoing quality assurance was conducted during the study. MRI-PDFF acquisition protocols included a 6-echo 2D gradient recalled echo sequence, and image data were transferred to the Center for Advanced Magnetic Resonance Development at Duke University for central calculation and measurement of MRI-PDFF using an established technique.
Serum bile acids and 7α-hydroxy-4-cholesten-3-one
Fasting serum samples were collected at day 1 and week 12; concentrations of individual bile acids and 7α-hydroxy-4-cholesten-3-one (a surrogate of bile acid synthesis) were determined by mass spectrometry methods at Mayo Clinic. Given that PSC patients on a stable dose of UDCA were allowed to enrol, UDCA and its derivatives were not included in this secondary analysis to avoid confounding interpretation attributable to UDCA use in a subgroup of patients.
Serum markers of fibrosis
The neoepitope-specific N-terminal pro-peptide of type III collagen (Pro-C3) measures a neo-epitope of type III collagen during collagen formation and reflects true fibrogenic activity.26 Concentrations of serum Pro-C3 were determined by an ELISA method (Nordic Bioscience). The Enhanced Liver Fibrosis (ELF; Siemens) test measures 3 serum parameters (hyaluronic acid, procollagen III amino-terminal peptide, and tissue inhibitor of metalloproteinase 1) in an algorithm which provides a single ELF score, as a non-invasive assessment of liver fibrosis.
Statistical analysis
We used the Kruskal–Wallis test with Dunn’s multiple comparisons correction for comparison of multiple groups. Change from baseline to week 12 (end of treatment) was analysed using the Wilcoxon test. Categorical parameters were compared using χ2 or Fisher’s exact tests. Correlation coefficients were calculated using Spearman’s method. All comparisons were performed at the 5% level of significance. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used to conduct the analyses.
Results
Patient populations
A phase II clinical trial of aldafermin for 12 weeks in patients with NASH was recently conducted to assess the safety and efficacy of aldafermin. Concurrently, a phase II trial of aldafermin for 12 weeks in patients with PSC was conducted and completed. Longitudinal serum samples were taken in the context of respective trials and bile acid profiling was performed to gain insight into the mechanisms of the therapeutic response and bile acid metabolism. In both studies, a novel biomarker of fibrogenesis, Pro-C3, was measured pre-treatment and at week 12 (end of treatment). Overall, a total of 238 patients were included in this secondary analysis.
Table 1 summarises the baseline demographics and characteristics of the 176 patients with biopsy-proven NASH. The mean age of the population was 51.2 (SD 10.6) years; 31% were men; and 49% had type 2 diabetes. Stage 1, 2, or 3 fibrosis was present in 35%, 32%, and 32% of patients, respectively.
Table 1.
Baseline demographics and characteristics of patients with NASH.
| Placebo (n = 27) | 0.3 mg (n = 23) | 1 mg (n = 49) | 3 mg (n = 49) | 6 mg (n = 28) | |
|---|---|---|---|---|---|
| Age (years) | 52.8 (11.1) | 42.9 (11.6) | 50.5 (10.4) | 51.8 (9.2) | 56.4 (7.6) |
| Weight (kg) | 97.6 (19.3) | 104.7 (21.0) | 99.6 (19.7) | 101.8 (25.8) | 98.4 (17.6) |
| BMI (kg/m2) | 35.4 (5.9) | 37.2 (5.3) | 36.7 (6.2) | 37.4 (9.6) | 34.7 (5.5) |
| Sex, n (%) | |||||
| Female | 20 (74) | 13 (56) | 38 (78) | 34 (69) | 16 (57) |
| Male | 7 (26) | 10 (44) | 11 (22) | 15 (31) | 12 (43) |
| Race, n (%) | |||||
| Asian | 0 | 0 | 1 (2) | 1 (2) | 1 (4) |
| Black | 2 (7) | 0 | 0 | 0 | 0 |
| White | 25 (93) | 23 (100) | 47 (96) | 47 (96) | 24 (86) |
| Other | 0 | 0 | 1 (2) | 1 (2) | 3 (11) |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 12 (44) | 11 (48) | 29 (59) | 22 (45) | 8 (28) |
| Non-Hispanic/Latino | 15 (56) | 12 (52) | 20 (41) | 27 (55) | 20 (72) |
| Type 2 diabetes, n (%) | |||||
| Yes | 17 (63) | 10 (43) | 17 (35) | 26 (53) | 17 (61) |
| No | 10 (37) | 13 (57) | 32 (65) | 23 (47) | 11 (39) |
| Histology | |||||
| Fibrosis stage, n (%) | |||||
| 1 | 11 (41) | 12 (52) | 14 (28) | 15 (31) | 10 (36) |
| 2 | 7 (26) | 3 (13) | 21 (43) | 13 (26) | 12 (43) |
| 3 | 9 (33) | 8 (35) | 13 (26) | 20 (41) | 6 (21) |
| 4 | 0 | 0 | 1 (2) | 1 (2) | 0 |
| NAS score, mean (SD) | 5.1 (1.1) | 5.9 (0.8) | 5.3 (1.3) | 5.3 (1.2) | 5.1 (0.9) |
| Fibrogenesis biomarker | |||||
| Pro-C3 (ng/ml) | 19.4 (14.0) | 18.0 (8.4) | 16.8 (9.1) | 22.3 (16.4) | 14.5 (5.7) |
| Primary bile acids | |||||
| Glycine-conjugated primary bile acids | |||||
| GCA (μmol/L) | 0.6 (0.6) | 0.3 (0.3) | 0.5 (0.4) | 0.4 (0.4) | 0.2 (0.2) |
| GCDCA (μmol/L) | 2.2 (1.9) | 1.5 (1.1) | 1.3 (1.1) | 1.6 (1.4) | 0.9 (0.7) |
| Taurine-conjugated primary bile acids | |||||
| TCA (μmol/L) | 0.1 (0.1) | 0.2 (0.2) | 0.1 (0.1) | 0.1 (0.2) | 0.1 (0.1) |
| TCDCA (μmol/L) | 0.2 (0.2) | 0.2 (0.2) | 0.2 (0.3) | 0.2 (0.3) | 0.1 (0.1) |
| Unconjugated primary bile acids | |||||
| CA (μmol/L) | 0.2 (0.5) | 0.3 (0.4) | 0.2 (0.5) | 0.2 (0.4) | 0.1 (0.1) |
| CDCA (μmol/L) | 0.6 (0.9) | 0.6 (0.6) | 0.4 (0.5) | 0.7 (1.1) | 0.3 (0.4) |
| Secondary bile acids | |||||
| Glycine-conjugated secondary bile acids | |||||
| GDCA (μmol/L) | 1.5 (2.0) | 0.6 (0.5) | 1.1 (1.7) | 0.7 (0.8) | 0.6 (0.6) |
| GLCA (μmol/L) | 0.06 (0.06) | 0.03 (0.05) | 0.03 (0.02) | 0.03 (0.03) | 0.03 (0.03) |
| Taurine-conjugated secondary bile acids | |||||
| TDCA (μmol/L) | 0.3 (0.4) | 0.3 (0.2) | 0.3 (0.5) | 0.2 (0.3) | 0.2 (0.2) |
| TLCA (μmol/L) | 0.01 (0.01) | 0.01 (0) | 0.01 (0) | 0.01 (0.01) | 0 (0.01) |
| Unconjugated secondary bile acids | |||||
| DCA (μmol/L) | 0.9 (0.9) | 0.5 (0.4) | 0.7 (0.7) | 0.7 (0.7) | 0.7 (0.6) |
| LCA (μmol/L) | 0.04 (0.04) | 0.02 (0.02) | 0.02 (0.02) | 0.03 (0.02) | 0.03 (0.02) |
Data are presented as mean (SD) or n (%).
CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; LCA, lithocholic acid; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid.
Baseline characteristics of the 62 patients with PSC are shown in Table S1. The mean age of the population was 43.2 (13.7) years; 61% were men; mean duration of PSC was 7.7 years.
Aldafermin reduces the hydrophobic and glycine-conjugated bile acids in patients with NASH
As an FGF19 analogue, aldafermin suppresses CYP7A1, which encodes the key enzyme in the classical bile acid synthetic pathway, through the FGFR4-βKlotho receptor complex located on hepatocytes.16 We measured serum levels of 7α-hydroxy-4-cholesten-3-one, an intermediate of bile acid synthesis and a surrogate marker of hepatic CYP7A1 activity. At week 12, changes from baseline in 7α-hydroxy-4-cholesten-3-one were -28.6 ng/ml (p = 0.004), -27.9 ng/ml (p <0.001), -41.0 ng/ml (p <0.001), and -29.7 ng/ml (p <0.001) in patients receiving aldafermin 0.3 mg, 1 mg, 3 mg, and 6 mg, respectively (Fig. S1). In contrast, a trend of increase of 12.6 ng/ml (p = 0.07) in 7α-hydroxy-4-cholesten-3-one was observed in the placebo group.
The primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesised from cholesterol in hepatocytes, in a complex sequence of enzymatic reactions that lead to oxidation of cholesterol aliphatic side-chain to a carboxylic group and addition of hydroxyl groups to the ring system.27 The carboxyl groups of CA and CDCA are immediately conjugated with glycine or taurine upon synthesis, leading to an increase in their acidity. The conjugation with glycine results in more protonated, hydrophobic bile acids (pKa ~4.5) compared with conjugation with taurine (pKa ~1.5).27
Serum concentrations of glycine-conjugated primary bile acids decreased from baseline with aldafermin, but not placebo treatment (Fig. 1B and Table 2). At week 12, changes in glycocholic acid (GCA) were -0.1 μmol/L (p = 0.025), -0.2 μmol/L (p <0.001), -0.3 μmol/L (p <0.001) and -0.2 μmol/L (p <0.001), and changes in glycochenodeoxycholic acid (GCDCA) were -0.4 μmol/L (p = 0.16), -0.8 μmol/L (p <0.001), -1.2 μmol/L (p <0.001), and -0.5 μmol/L (p = 0.002), in patients receiving aldafermin 0.3 mg, 1 mg, 3 mg, and 6 mg, respectively. Greater reductions in GCA and GCDCA were achieved in the higher dose aldafermin groups (1 mg, 3 mg, and 6 mg) than the lower dose 0.3 mg group. In contrast, no reductions were seen in the more hydrophilic, taurine-conjugated primary bile acids (taurocholic acid [TCA] and taurochenodeoxycholic acid [TCDCA]) in the aldafermin groups. Unconjugated bile acids, being membrane permeable, are highly cytotoxic; aldafermin also lowered unconjugated CA and CDCA in these patients (Fig. 1B and Table 2).
Table 2.
Change in bile acid profile from baseline at week 12 in patients with NASH.
| Change from baseline at week 12 (mean [SD]) |
|||||
|---|---|---|---|---|---|
| Placebo (n = 27) | 0.3 mg (n = 23) | 1 mg (n = 49) | 3 mg (n = 49) | 6 mg (n = 28) | |
| Primary bile acids | |||||
| Glycine-conjugated primary bile acids | |||||
| GCA (μmol/L) | 0 (1.0) p = 0.95 |
-0.1 (0.2) p = 0.025 |
-0.2 (0.2) p <0.001 |
-0.3 (0.4) p <0.001 |
-0.2 (0.2) p <0.001 |
| GCDCA (μmol/L) | -0.2 (2.0) p = 0.68 |
-0.4 (1.2) p = 0.16 |
-0.8 (0.9) p <0.001 |
-1.2 (1.4) p <0.001 |
-0.5 (0.7) p = 0.002 |
| Taurine-conjugated primary bile acids | |||||
| TCA (μmol/L) | 0 (0.2) p = 0.79 |
0 (0.2) p = 0.48 |
0 (0.1) p = 0.48 |
0 (0.2) p = 0.07 |
0 (0.1) p = 0.65 |
| TCDCA (μmol/L) | 0 (0.2) p = 0.83 |
0.1 (0.4) p = 0.12 |
0.1 (0.2) p = 0.009 |
0 (0.3) p = 0.41 |
0.1 (0.3) p = 0.05 |
| Unconjugated primary bile acids | |||||
| CA (μmol/L) | 0 (0.8) p = 0.74 |
-0.2 (0.5) p = 0.12 |
-0.2 (0.3) p = 0.040 |
-0.2 (0.4) p = 0.001 |
-0.1 (0.2) p = 0.017 |
| CDCA (μmol/L) | 0 (0.8) p = 0.96 |
-0.4 (0.6) p = 0.002 |
-0.3 (0.4) p <0.001 |
-0.7 (1.1) p <0.001 |
-0.3 (0.4) p = 0.007 |
| Secondary bile acids | |||||
| Glycine-conjugated secondary bile acids | |||||
| GDCA (μmol/L) | -0.6 (1.6) p = 0.10 |
-0.2 (0.6) p = 0.044 |
-0.9 (1.0) p <0.001 |
-0.6 (0.7) p <0.001 |
-0.6 (0.7) p <0.001 |
| GLCA (μmol/L) | -0.01 (0.06) p = 0.29 |
-0.01 (0.04) p = 0.45 |
-0.02 (0.02) p = 0.008 |
-0.02 (0.04) p = 0.001 |
-0.02 (0.03) p <0.001 |
| Taurine-conjugated secondary bile acids | |||||
| TDCA (μmol/L) | -0.1 (0.3) p = 0.18 |
0 (0.4) p = 0.86 |
-0.2 (0.3) p = 0.003 |
-0.2 (0.3) p = 0.002 |
-0.1 (0.2) p = 0.05 |
| TLCA (μmol/L) | 0 (0.02) p = 0.63 |
0 (0.01) p = 0.08 |
0 (0) p = 0.19 |
0 (0.02) p = 0.43 |
0 (0.01) p = 0.40 |
| Unconjugated secondary bile acids | |||||
| DCA (μmol/L) | -0.1 (1.0) p = 0.77 |
-0.3 (0.3) p <0.001 |
-0.6 (0.5) p <0.001 |
-0.7 (0.8) p <0.001 |
-0.7 (0.7) p <0.001 |
| LCA (μmol/L) | 0 (0.03) p = 1.00 |
0 (0.02) p = 0.17 |
-0.02 (0.03) p = 0.007 |
-0.02 (0.02) p <0.001 |
-0.02 (0.02) p <0.001 |
Data are presented as mean (SD). Values of p by Wilcoxon test.
CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; LCA, lithocholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid.
Gut bacteria transform the primary bile acids CA and CDCA into the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), respectively.1 DCA and LCA have higher hydrophobicity indices,28 higher detergent activity and greater cytotoxicity, causing more cell wall damage than primary bile acids.4 Both DCA and LCA were reported to promote carcinogenesis in the liver and gut in animal models.[29], [30], [31] Aldafermin treatment dose-dependently reduced concentrations of DCA, with changes of -0.3 μmol/L (p <0.001), -0.6 μmol/L (p <0.001), -0.7 μmol/L (p <0.001), and -0.7 μmol/L (p <0.001) at week 12, corresponding to relative changes of -47%, -66%, -84%, and -92%, in patients receiving 0.3 mg, 1 mg, 3 mg, and 6 mg aldafermin, respectively (Fig. 1C and Table 2). In contrast, placebo-treated patients had a 22% increase in DCA. Decreases in LCA were also observed with aldafermin treatment (Fig. 1C and Table 2).
Similar to the observation in primary bile acids, glycine-conjugated secondary bile acids (glycodeoxycholic acid [GDCA] and glycolithocholic acid [GLCA]), but not the more hydrophilic taurine-conjugated secondary bile acids (taurodeoxycholic acid [TDCA] and taurolithocholic acid [TLCA]), were suppressed by aldafermin (Fig. 1C and Table 2).
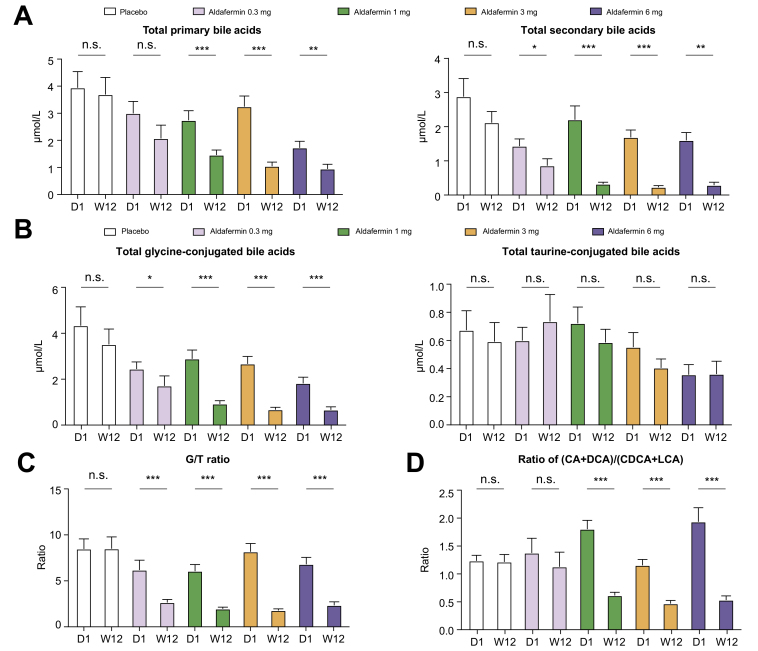
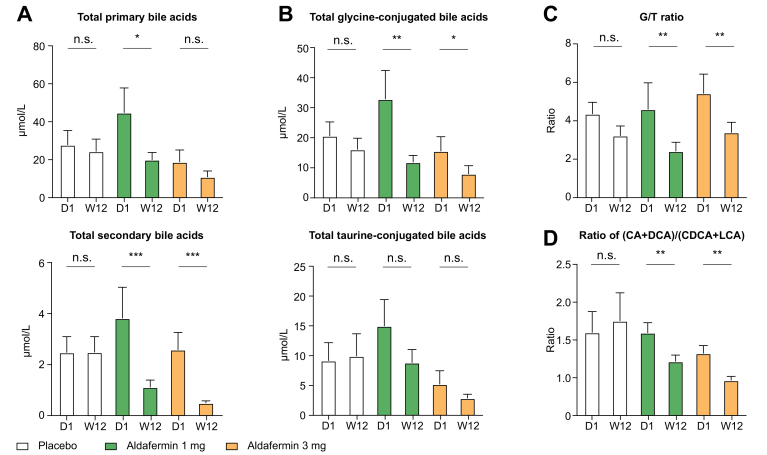
Further analyses revealed that aldafermin reduced total primary bile acids and total secondary bile acids (Fig. 2A), and concentrations of total CA, total CDCA, total DCA and total LCA (Fig. S2). Total glycine-conjugated bile acids, but not total taurine-conjugated bile acids, were decreased with aldafermin treatment (Fig. 2B). Consequently, aldafermin lowered the ratio of glycine to taurine conjugates of bile acids (G/T ratio) (Fig. 2C). The ratio of the sum of (CA + DCA) to the sum of (CDCA + LCA) (Fig. 2D), which reflects the relative contribution of the 12α-hydroxylated bile acids (CA and DCA) vs. non-12α-hydroxylated bile acids (CDCA + LCA) synthetic pathways, was also decreased with aldafermin treatment (Fig. 2D).
Fig. 2.
Aldafermin lowers the G/T ratio and the ratio of 12α-hydroxylated to non-12α-hydroxylated bile acids in patients with NASH.
A total of 176 patients with biopsy-proven NASH received 0.3 mg (n = 23), 1 mg (n = 49), 3 mg (n = 49), 6 mg (n = 28) aldafermin or placebo (n = 27) for 12 weeks. Serum samples were collected at baseline (day 1) and week 12 (end of treatment) for bile acid profiling. (A) Aldafermin reduced total primary and secondary bile acids. (B) Aldafermin reduced total glycine-conjugated, but not total taurine-conjugated bile acids. (C) Aldafermin lowered the G/T ratio in a dose-dependent manner. (D) Aldafermin lowered (CA + DCA)/(CDCA + LCA) ratio in a dose-dependent manner. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001; n.s., not significant (Wilcoxon test). CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; G/T ratio, ratio of glycine to taurine conjugates of bile acids; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis.
In summary, administration of aldafermin produced dose-dependent reductions in circulating bile acids, and the more hydrophobic and the glycine-conjugated bile acids in particular, in patients with NASH. A 12-week treatment with aldafermin produced up to 92% reduction in DCA, 80% reduction in GDCA, 73% reduction in GCA, and 56% reduction in GCDCA, but no reductions in TCA, TCDCA, TDCA, or TLCA.
Correlation between changes in bile acids and 7α-hydroxy-4-cholesten-3-one in patients with NASH
We further evaluated correlation between individual bile acids and 7α-hydroxy-4-cholesten-3-one, a measure of hepatic CYP7A1 activity. We saw robust correlation between levels of 7α-hydroxy-4-cholesten-3-one and individual bile acids. At week 12, changes in 7α-hydroxy-4-cholesten-3-one correlated with changes in the major hydrophobic bile acids (Table S1). The Spearman’s correlation coefficients were: rho = 0.48 (p <0.001) for DCA; rho = 0.48 (p <0.001) for GCA; rho = 0.38 (p <0.001) for GDCA; rho = 0.34 (p <0.001) for GCDCA. No correlations were seen with taurine-conjugated TCA, TCDCA, TDCA, or TLCA (Table S1).
Aldafermin reduces the hydrophobic and glycine-conjugated bile acids in patients with PSC
At baseline, patients with PSC had markedly elevated bile acids compared with patients with NASH (Table S2). Concentrations of both glycine-conjugated primary bile acids (GCA, GCDCA) and taurine-conjugated primary bile acids (TCA, TCDCA) were markedly elevated in patients with PSC compared with those with NASH. PSC patients also had higher levels of ALP, ALT, and AST (Table S2).
Like the observations in the NASH population, there were marked reductions in the levels of individual bile acids – especially the more toxic, hydrophobic bile acids, among them DCA, GDCA, GCA, GCDCA, and GLCA – in patients with PSC who were treated with aldafermin (Fig. 3A–C). In contrast, no significant changes were seen in TDCA, TLCA, TCA, or TCDCA in the aldafermin 1 mg group and the placebo group, and only marginal reductions occurred in the aldafermin 3 mg group.
Fig. 3.
Aldafermin reduces the toxic, hydrophobic, glycine-conjugated bile acids in patients with PSC.
(A) Sixty-two patients with PSC diagnosed according to EASL criteria received 1 mg (n = 21), 3 mg (n = 21) aldafermin or placebo (n = 20) for 12 weeks. Serum samples were collected at baseline (day 1) and week 12 (end of treatment) for bile acid profiling. (B) Concentrations of the primary bile acids and their conjugates. Note that aldafermin preferentially reduced levels of the more toxic, hydrophobic, glycine-conjugated primary bile acids. (C) Concentrations of the secondary bile acids and their conjugates. Similarly, aldafermin preferentially reduced levels of the more toxic, hydrophobic, glycine-conjugated secondary bile acids. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001; n.s., not significant (Wilcoxon test). CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; G/T ratio, ratio of glycine to taurine conjugates of bile acids; LCA, lithocholic acid; PSC, primary sclerosing cholangitis; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid.
In the population with PSC, aldafermin preferentially lowered total secondary bile acids (Fig. 4A) and total glycine-conjugated bile acids (Fig. 4B). The G/T ratio (Fig. 4C), as well as the ratio of (CA + DCA) to (CDCA + LCA) (Fig. 4D), declined in aldafermin-treated but not placebo-treated PSC patients. We further analysed changes in key parameters stratified by UDCA use at baseline. Aldafermin produced similar effects on DCA (Fig. S3A), G/T ratio (Fig. S3B), and the ratio of (CA + DCA) to (CDCA + LCA) (Fig. S3C), in patients who were on UDCA and in those who were not on UDCA. In patients with PSC who had serum bile acids >100 μM at baseline, 12 weeks of aldafermin therapy achieved 70–90% reduction in bile acid levels (Fig. S4).
Fig. 4.
Aldafermin lowers the G/T ratio and the ratio of 12α-hydroxylated to non-12α-hydroxylated bile acids in patients with PSC.
Sixty-two patients with PSC diagnosed according to EASL criteria received 1 mg (n = 21), 3 mg (n = 21) aldafermin or placebo (n = 20) for 12 weeks. Serum samples were collected at baseline (day 1) and week 12 (end of treatment) for bile acid profiling. (A) Aldafermin reduced total secondary bile acids. (B) Aldafermin reduced total glycine-conjugated, but not total taurine-conjugated bile acids. (C) Aldafermin lowered the G/T ratio. (D) Aldafermin lowered (CA + DCA)/(CDCA + LCA) ratio. ∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001; n.s., not significant (Wilcoxon test). CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; G/T ratio, ratio of glycine to taurine conjugates of bile acids; LCA, lithocholic acid; PSC, primary sclerosing cholangitis.
Overall, we saw approximately 50% reduction in DCA, 51% reduction in GDCA, 46% reduction in GCA, and 27% reduction in GCDCA with aldafermin therapy in patients with PSC. Therefore, aldafermin demonstrated robust activity in lowering the major hydrophobic bile acids regardless of disease aetiology.
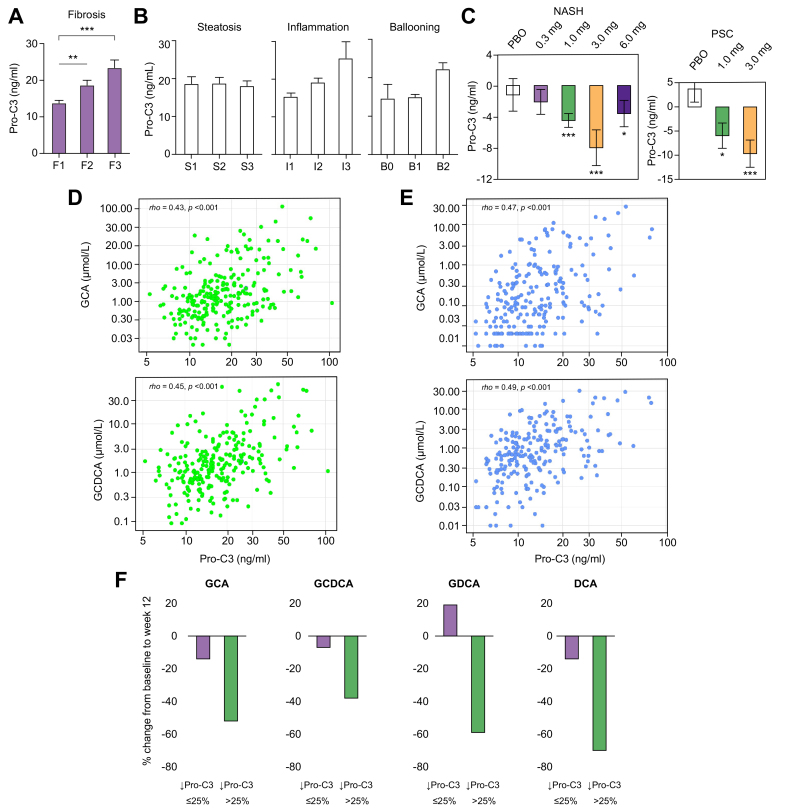
The fibrogenesis marker Pro-C3 correlates with liver fibrosis stage in patients with NASH
The importance of liver fibrosis in predicting clinical outcomes in NASH has been unequivocally established.[32], [33], [34] However, liver biopsy procedure for fibrosis staging is invasive and technically demanding, therefore a second biopsy at the end of treatment is seldom performed in short-term phase II trials. For cholestatic disease such as PSC, biopsy is not routinely performed at all, per society guidelines.35 We sought to identify a serum biomarker that correlates with fibrosis stage for non-invasive monitoring of the antifibrotic response to aldafermin.
Pro-C3 measures a neo-epitope of type III collagen during collagen formation and reflects true fibrogenic activity.26 In these well-characterised, biopsy-proven NASH patients enrolled in the aldafermin phase II trial, serum concentrations of Pro-C3 correlated with fibrosis stage (classified using the NASH-CRN criteria) at baseline (Fig. 5A). Mean Pro-C3 levels were 13.7 ng/ml, 18.6 ng/ml, and 23.3 ng/ml for patients with stage 1, 2, and 3 fibrosis, respectively (F3 vs. F1: p <0.001, F2 vs. F1: p = 0.005; Fig. 5A and Table S3). Pro-C3 levels did not differ significantly among histological grades of steatosis, inflammation or ballooning (Fig. 5B). We did not find a correlation between Pro-C3 concentration and liver fat content (as measured by MRI-PDFF) in patients with NASH (Fig. S5), whereas it did correlate with alkaline phosphatase level in patients with PSC (Fig. S6). No correlation was observed between fibrosis stage and body weight, BMI, or ALT levels, although F3 patients appeared to be older and had more severe ballooning and inflammation on liver biopsy (Table S3).
Fig. 5.
Serum bile acids correlate with the novel fibrogenesis biomarker Pro-C3.
(A) Serum Pro-C3 concentrations increase with fibrosis stage in patients with NASH. A total of 176 patients had liver biopsies evaluated according to the NASH CRN criteria at baseline. Patients with stage 1 fibrosis (F1) had the lowest Pro-C3 levels, whereas those with stage 3 fibrosis (F3, advanced fibrosis) had the highest Pro-C3 levels. ∗∗p <0.01, ∗∗∗p <0.001 vs. F1 (Kruskal–Wallis test with Dunn’s multiple comparison correction). (B) No significant differences in Pro-C3 concentrations by histological grade of steatosis, inflammation, or ballooning. (C) Change from baseline to week 12 in Pro-C3 in NASH and PSC populations.∗p <0.05, ∗∗p <0.01, ∗∗∗p <0.001 (Wilcoxon test). (D) GCA and GCDCA levels correlate with Pro-C3 at baseline in the pooled NASH and PSC populations. A total of 238 patients (176 patients with NASH and 62 patients with PSC) were included in the analysis. The Spearman’s correlation coefficients and p values are labelled on the graphs. (E) GCA and GCDCA levels correlate with Pro-C3 at week 12 (end of treatment) in the pooled NASH and PSC populations. A total of 238 patients were included in the analysis. (F) Percent change in the major, toxic, hydrophobic bile acids in patients who achieved >25% reduction in Pro-C3 vs. those who did not. DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; NASH, non-alcoholic steatohepatitis; NASH CRN, NASH Clinical Research Network; Pro-C3, neoepitope-specific N-terminal pro-peptide of type III collagen; PSC, primary sclerosing cholangitis.
Changes in serum bile acids correlate with changes in the fibrogenesis marker Pro-C3 in NASH and in PSC
Although NASH and PSC have distinct aetiology and clinical manifestations, evidence supports a shared role of bile acid dysregulation in liver inflammation and fibrosis.6 To investigate whether bile acids could be a convergent signal underlying fibrogenesis in both disorders, we assessed the association between Pro-C3 and bile acids.
Circulating Pro-C3, which reflects fibrogenic activity, declined markedly with aldafermin therapy in patients with NASH or PSC (Fig. 5C). In contrast, no change in Pro-C3 was observed in placebo-treated patients. At week 12, changes in Pro-C3 from baseline were -1.2 ng/ml (p = 0.58), -2.1 ng/ml (p = 0.20), -4.5 ng/ml (p <0.001), -8.0 ng/ml (p = 0.001), and -3.6 ng/ml (p = 0.04) in patients with NASH receiving placebo, aldafermin 0.3 mg, 1 mg, 3 mg, and 6 mg, respectively. Changes in Pro-C3 from baseline were +3.7 ng/ml (p = 0.18), -5.9 ng/ml (p = 0.03), and -9.6 ng/ml (p <0.001) in PSC patients receiving placebo, aldafermin 1 mg and 3 mg, respectively.
For correlation analysis, we pooled data from NASH and PSC trials given that both trials evaluated similar doses of aldafermin for the same 12-week duration. As shown in Fig. 5D, levels of bile acids directly correlated with Pro-C3 at baseline. The Spearman’s correlation coefficients were rho = 0.45 (p <0.001) for GCDCA, and rho = 0.43 (p <0.001) for GCA. Similar correlations were observed at week 12, with rho = 0.49 (p <0.001) for GCDCA, and rho = 0.47 (p <0.001) for GCA (Fig. 3D). Importantly, changes from baseline to week 12 in Pro-C3 were associated with reductions in the hydrophobic bile acids (Table 3). The Spearman’s correlation coefficients were: rho = 0.36 (p <0.001) for DCA; rho = 0.34 (p <0.001) for GDCA; rho = 0.33 (p <0.001) for GCA; and no correlations were seen with taurine-conjugated TCA and TCDCA. The strength of the correlations remained unchanged when the NASH or PSC populations were analysed separately (Fig. S7).
Table 3.
Correlation between changes in bile acids and changes in Pro-C3 in the pooled NASH and PSC populations.
| Percent change in bile acids from baseline to week 12 | Percent change in Pro-C3 from baseline to week 12 |
|
|---|---|---|
| rho | p value | |
| Primary bile acids | ||
| Glycine-conjugated primary bile acids | ||
| GCA (μmol/L) | 0.33 | <0.001 |
| GCDCA (μmol/L) | 0.25 | <0.001 |
| Taurine-conjugated primary bile acids | ||
| TCA (μmol/L) | 0.16 | 0.02 |
| TCDCA (μmol/L) | 0.06 | 0.38 |
| Unconjugated primary bile acids | ||
| CA (μmol/L) | 0.23 | 0.001 |
| CDCA (μmol/L) | 0.17 | 0.01 |
| Secondary bile acids | ||
| Glycine-conjugated secondary bile acids | ||
| GDCA (μmol/L) | 0.34 | <0.001 |
| GLCA (μmol/L) | 0.30 | <0.001 |
| Taurine-conjugated secondary bile acids | ||
| TDCA (μmol/L) | 0.31 | <0.001 |
| TLCA (μmol/L) | -0.01 | 0.86 |
| Unconjugated secondary bile acids | ||
| DCA (μmol/L) | 0.36 | <0.001 |
| LCA (μmol/L) | 0.30 | <0.001 |
Data are presented as correlation coefficients. Values of p by Spearman’s method.
CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GDCA, glycodeoxycholic acid; GLCA, glycolithocholic acid; LCA, lithocholic acid; TCA, taurocholic acid; TCDCA, taurochenodeoxycholic acid; TDCA, taurodeoxycholic acid; TLCA, taurolithocholic acid.
Consistent with the observations in Pro-C3, changes from baseline to week 12 in ELF, another serum marker of fibrosis, were associated with reductions in bile acids (Table S4). Similar correlations were seen between changes in the hydrophobic bile acids and ALT, an indicator of liver inflammation and injury (Table S5).
Regardless of disease aetiology, in the pooled analysis, patients who achieved ≥25% reduction in Pro-C3 had greater reduction in the hydrophobic and glycine-conjugated bile acids (DCA, GDCA, GCDCA, and GCA) than those who did not (Fig. 5F). In contrast, no reductions were seen in taurine-conjugated bile acids. These results further support a potential role of the hydrophobic bile acids in extracellular matrix deposition.
In summary, change in circulating levels of bile acids correlated with change in Pro-C3 across metabolic and cholestatic liver diseases, indicating that bile acids may be a molecular driver of hepatic fibrogenesis irrespective of disease aetiology.
Discussion
Preclinical and clinical data have indicated a link between bile acids and the pathophysiology of chronic liver disease. We show here that administration of aldafermin produced dose-dependent reductions in bile acids, and the major hydrophobic bile acids in particular (e.g. DCA, GDCA, GCA, GCDCA), in NASH and in PSC populations. Change in circulating levels of bile acids correlated with change in the fibrogenesis biomarker Pro-C3 independent of disease aetiology. This study is the first to our knowledge to investigate the relationship between individual bile acids and the fibrogenesis biomarker Pro-C3 in a diverse population with various degrees of fibrosis. Our results suggest that the hydrophobic bile acids may contribute to the deposition of extracellular matrix, and that dysregulated bile acid homeostasis may be a shared molecular mechanism, and therapeutic target, underlying fibrosis and disease progression across metabolic and cholestatic liver diseases.
Among all types of bile acids, aldafermin produced the most robust inhibition in the secondary bile acid DCA (up to 92% and 50% reductions in NASH and PSC, respectively). DCA, produced by the 7α-dehydroxylating bacteria in the gut, is highly hydrophobic (with the highest hydrophobicity index among individual bile acids),28 cytotoxic, and known to cause cell and DNA damage.36 DCA is elevated by a high-fat diet and can provoke senescence-associated secretory phenotype in hepatic stellate cells, which in turn secrete various inflammatory and tumour-promoting factors in the liver, thus facilitating the formation of hepatocellular carcinoma.29 DCA has also been implicated as the causative agent in the development of colon cancer, cholangiocarcinomas, and oesophageal cancer.29,30,[37], [38], [39] Given the well-known link between DCA and carcinogenesis, aldafermin may exert antitumour action through its pronounced effect on DCA reduction.
It appears that aldafermin preferentially reduced the glycine-conjugated, rather than the taurine-conjugated, bile acids in NASH and in PSC. Glycine and taurine bile acid derivatives possess different physicochemical properties which contribute to their functional and metabolic differences.1 The different pKa values (~1.5 for taurine-conjugation, ~4.5 for glycine-conjugation) affect the balance between the deprotonated form and the un-ionised form of bile acids under physiological and pathological conditions. Glycine-conjugated bile acids are less water-soluble and more hydrophobic than taurine-conjugated bile acids.40 The protonated glycine-conjugated bile acids can cause uncontrolled damage to hepatocytes and cholangiocytes, especially when the ‘HCO3- umbrella’ is impaired or defective as in chronic liver disease.41 In healthy humans, the relative conjugation of bile acids with G/T ratio approximates a ratio of 3:1.1 It has been known for more than five decades that patients with ileal disorders had a marked elevation in the G/T ratio,42,43 yet the underlying mechanisms remain elusive. The present study, which demonstrates a pronounced effect on the G/T ratio following the administration of an FGF19 analogue, suggests that a hormone of the ileal origin, FGF19, might be the long sought-after explanation. Furthermore, we saw elevated G/T ratios in patients with NASH (~8:1) or PSC (~5:1) in this report, indicating increased bile acid toxicity in both diseases; aldafermin normalised G/T ratios regardless of disease aetiology. Additionally, a reduction in the G/T ratio, as we observed with aldafermin therapy, was previously suggested to be beneficial for cholesterol gallstone dissolution,44 in addition to the reduction of the hepatotoxicity and cholangiotoxicity of the bile acid pool.45 In the human liver, a single enzyme (bile acid-CoA:amino acid N-acyltransferase [BAAT]) is responsible for conjugating bile acids with glycine or taurine.46 Given that BAAT has lower affinity for glycine than taurine,46 suppression of bile acid synthesis and reduced influx of unconjugated bile acids from the blood into the liver may lead to a lower demand for conjugation and hence a relatively greater reduction in the glycine-conjugated bile acids. However, the precise mechanism on the preferential suppression of glycine-conjugated bile acids by aldafermin is not clear and requires further investigation.
Although CYP7A1 catalyses the first and rate-limiting step in the synthesis of primary bile acids, it is CYP8B1 (steroid 12α-hydroxylase) that is at the branch point for CA and CDCA synthesis and determines the ratio of 12α-hydroxylated bile acids (CA and DCA) to non-12α-hydroxylated bile acids (CDCA and LCA).1 Increased ratio of serum 12α-hydroxylated bile acids to non-12α-hydroxylated bile acids (ratio of [CA + DCA] to [CDCA + LCA]) is associated with insulin resistance in humans.47 Aldafermin therapy reduces the ratio of (CA + DCA) to (CDCA + LCA), indicating a crucial role of the FGF19 pathway in regulating steroid 12α-hydroxylase activity and potentially improving insulin sensitivity.
Regardless of the underlying aetiology, complications in chronic liver disease are often driven by liver fibrosis. Liver fibrosis is the strongest determinant of disease progression and the best predictor for liver-related outcomes in patients with NASH.[32], [33], [34] Given that end-of-treatment liver biopsy is not routinely performed because of its invasive and heterogenous nature in short-term trials, we used Pro-C3, which measures type III collagen formation, to allow a non-invasive, more granular analysis of the steady-state and the dynamics of fibrosis. In this report, we show that the fibrogenesis biomarker Pro-C3 correlated with histological stage of liver fibrosis in 176 patients with NASH. In contrast, parameters such as body weight, glucose, or BMI did not correlate with fibrosis on liver biopsy and could not differentiate between mild and advanced fibrosis. We demonstrate that changes in serum levels of glycine-conjugated bile acids, but not taurine-conjugated bile acids, were associated with changes in Pro-C3, highlighting the potential of these hydrophobic bile acids as a molecular link between injury and liver fibrogenesis. Consistent with these findings, a relationship between a decrease in bile acid level and a reduction in ELF score, another serum marker of liver fibrosis, was also observed.
The mechanisms by which bile acids precipitate fibrogenesis may be multifactorial. In addition to cytotoxicity associated with their amphipathic structure, chronic dysregulation of bile acids within the hepatic parenchyma can generate free radical reactive oxygen species, which incites an inflammatory cascade that potentiates hepatocyte cell death.48 Although most experiments on bile acids cytotoxicity were performed in vitro at supra-physiological concentrations,49,50 studies have demonstrated that bile acids, even at low concentrations (10 μmol/L), can impair the function of the complex I and complex III of the electron transport chain in the mitochondria.51 Indeed, the hydrophobic bile acids, but not their taurine conjugates, were shown to inhibit state 3 respiration rates and respiratory control ratios.51 Furthermore, bile acids can directly activate hepatic stellate cells, which differentiate into myofibroblast-like cells, the main collagen-producing cells in the liver.52,53 We noted a correlation between reductions in bile acid and decreases in ALT, a marker of hepatic inflammation and injury. These results suggest that bile acids could be a component of a self-amplifying inflammatory milieu perpetuating liver injury and fibrosis.
Elevated serum bile acids are strongly predictive of progressive decline in liver function, and are associated with clinically significant portal hypertension, hepatic decompensation, HCC, liver failure, and mortality.12,13 Studies in cell culture models and in animals also provided ample evidence on exposure to bile acids as a direct cause of the liver injury seen in patients with chronic liver disease.5 Although such findings make a compelling case for hydrophobic bile acids as the causative agent in liver injury, it must be noted that hydrophilic bile acids such as UDCA can protect against liver injury.54
There are some important limitations to note in this study. First, although the value of histological assessment in liver disease remains unquestioned, most patients with NASH and all patients with PSC in this report did not have paired liver biopsies to allow longitudinal evaluation. Second, although Pro-C3 has the potential to obviate the limitations of liver biopsy by providing important information regarding the state of the liver and extracellular matrix turnover, it only measures the formation of type III collagen. Recent research has demonstrated that not all collagens are created equal, and that an array of collagen-derived molecules has emerged with novel functions still being defined.55 Third, we only evaluated the circulating bile acids in the current study. As concentrations of serum bile acids reflect the dynamics and balance between enterohepatic circulation, hepatic uptake, and synthesis, further research should investigate the bile acid profile in the liver, which is the site of molecular action for aldafermin. Fourth, this study did not enrol patients with cirrhosis, and the total duration of the treatment was only 12 weeks. Finally, this study only evaluated patients with NASH and patients with PSC; to prove the shared, universal mechanism between bile acids and fibrogenesis, further trials are needed to extend these findings to other chronic liver diseases.
The present study suggests that the hydrophobic bile acids may indeed be a trigger of fibrosis in patients with chronic liver disease. In this context, the hydrophobic bile acids may be a direct contributor to the pathophysiology of chronic liver disease rather than merely a marker of a diseased liver. Our findings lay the foundation for selective modulation of the toxic, hydrophobic bile acids by an FGF19 analogue in patients, a therapeutic strategy that may be broadly applicable to other chronic gastrointestinal and liver disorders to slow disease progression and improve outcome.
Financial support
Funding for this study is provided by NGM Biopharmaceuticals.
Authors’ contributions
Participated in study design: L.L., A.M.D., S.A.H., G.M.H. Responsible for data collection: U.B., S.A.H., G.M.H. Participated in data analysis: A.J.S., L.L., G.M.H. Participated in data interpretation: all authors. Responsible for preparation of the tables and figures: A.J.S., L.L. Participated in manuscript review and writing: all authors.
Data availability statement
Due to the confidential nature of the data in the study, the data collected in the study will not be available to access.
Conflict of interest
A.J.S. reports serving as an unpaid consultant to Intercept, Zydus, Echosense, Immuron, Madrigal, Galectin, Blade, Pliant, Albireo, and AMRA; is a consultant to Gilead, Allergan, Bristol-Myers Squibb, Pfizer, Merck, Galmed, Novartis, Novo Nordisk, Lilly, Siemens, Genentech, Boehringer Ingelhiem, Glympse Bio, Genfit, Coherus, Surrozen, Poxel, 89 Bio, Perspectum, AstraZeneca, Medimmune, and Lipocine; owns stock options in Indalo, Durect, Tiziana, Exhalenz, and Northsea; and is president of Sanyal Bio. L.L., A.M.D., and H.D.L. are employees and stockholders of NGM Biopharmaceuticals. U.B. reports consulting for Novartis, Intercept; grant/research support from Norwegian, American, and South African PSC patient foundations and German DCCV; received lecture fees from Abbvie, Falk Foundation, Gilead, Intercept, Novartis, Roche, Shire, and Zambon; and received research support for investigator-initiated studies from Dr. Falk Pharma and Intercept. S.A.H. reports research support from Conatus, Galectin, Galmed, Genfit, Gilead, Intercept, Madrigal, NGM, Akero, Axcella, Metacrine, Sagimet, Enyo, NorthSea, Genentech, Hepion, Cymabay, and Hightide; and is a consulting adviser for the Chronic Liver Disease Foundation, Cirius, Echosens, Akero, Galectin, Galmed, Genfit, Gilead, Intercept, Madrigal, NGM, Novartis, Perspectum, Metacrine, Medpace, Sagimet, Blade, Viking, Poxel, Axcella, Terns, HistoIndex, Hightide, Hepion, Ridgeline Therapeutics, CiVi, and Prometic. G.M.H. reports consulting for Cymabay, Genfit, Falk, Intercept, GSK, Mirum, Roche, and Pliant.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We would like to express our appreciation to all of the patients who participated in this study, along with the study coordinators and staff of the participating clinical centres for their support and assistance.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2021.100254.
Contributor Information
Arun J. Sanyal, Email: arun.sanyal@vcuhealth.org.
Lei Ling, Email: lling@ngmbio.com.
Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Hofmann A.F. Bile acids: trying to understand their chemistry and biology with the hope of helping patients. Hepatology. 2009;49:1403–1418. doi: 10.1002/hep.22789. [DOI] [PubMed] [Google Scholar]
- 2.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 3.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiang J.Y. Recent advances in understanding bile acid homeostasis. F1000Res. 2017;6:2029. doi: 10.12688/f1000research.12449.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trauner M., Meier P.J., Boyer J.L. Molecular pathogenesis of cholestasis. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 6.Arab J.P., Karpen S.J., Dawson P.A., Arrese M., Trauner M. Bile acids and nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Hepatology. 2017;65:350–362. doi: 10.1002/hep.28709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferslew B.C., Xie G., Johnston C.K., Su M., Stewart P.W., Jia W. Altered bile acid metabolome in patients with nonalcoholic steatohepatitis. Dig Dis Sci. 2015;60:3318–3328. doi: 10.1007/s10620-015-3776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri P., Daita K., Joyce A., Mirshahi F., Santhekadur P.K., Cazanave S. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology. 2018;67:534–548. doi: 10.1002/hep.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caussy C., Hsu C., Singh S., Bassirian S., Kolar J., Faulkner C. Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther. 2019;49:183–193. doi: 10.1111/apt.15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouzaki M., Wang A.Y., Bandsma R., Comelli E.M., Arendt B.M., Zhang L. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PloS One. 2016;11 doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePaoli A.M., Zhou M., Kaplan D.D., Hunt S.C., Adams T.D., Learned R.M. FGF19 analog as a surgical factor mimetic that contributes to metabolic effects beyond glucose homeostasis. Diabetes. 2019;68:1315–1328. doi: 10.2337/db18-1305. [DOI] [PubMed] [Google Scholar]
- 12.Horvatits T., Drolz A., Roedl K., Rutter K., Ferlitsch A., Fauler G. Serum bile acids as marker for acute decompensation and acute-on-chronic liver failure in patients with non-cholestatic cirrhosis. Liver Int. 2017;37:224–231. doi: 10.1111/liv.13201. [DOI] [PubMed] [Google Scholar]
- 13.Horvatits T., Drolz A., Rutter K., Roedl K., Langouche L., Van den Berghe G. Circulating bile acids predict outcome in critically ill patients. Ann Intens Care. 2017;7:48. doi: 10.1186/s13613-017-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degirolamo C., Sabba C., Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15:51–69. doi: 10.1038/nrd.2015.9. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer S.A., Mangelsdorf D.J. Bile acids as hormones: the FXR-FGF15/19 pathway. Dig Dis. 2015;33:327–331. doi: 10.1159/000371670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M., Wang X., Phung V., Lindhout D.A., Mondal K., Hsu J.Y. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Canc Res. 2014;74:3306–3316. doi: 10.1158/0008-5472.CAN-14-0208. [DOI] [PubMed] [Google Scholar]
- 17.Zhou M., Learned R.M., Rossi S.J., DePaoli A.M., Tian H., Ling L. Engineered FGF19 eliminates bile acid toxicity and lipotoxicity leading to resolution of steatohepatitis and fibrosis in mice. Hepatol Commun. 2017;1:1024–1042. doi: 10.1002/hep4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DePaoli A., Shankar S., Bashir M.R., Baxter B.A., Phung V., Yan A.Z. NGM313, a novel once-monthly activator of β-Klotho/FGFR1c, significantly reduces hepatic steatosis and key biomarkers of nonalcoholic steatohepatitis: results of a randomized, active-controlled clamp study in obese insulin resistant patients with NAFLD. Hepatology. 2018;69 LB-21. [Google Scholar]
- 19.Baruch A., Wong C., Chinn L.W., Vaze A., Sonoda J., Gelzleichter T. Antibody-mediated activation of the FGFR1/Klothobeta complex corrects metabolic dysfunction and alters food preference in obese humans. Proc Natl Acad Sci U S A. 2020;117:28992–29000. doi: 10.1073/pnas.2012073117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison S.A., Rinella M.E., Abdelmalek M.F., Trotter J.F., Paredes A.H., Arnold H.L. NGM282 for treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2018;391:1174–1185. doi: 10.1016/S0140-6736(18)30474-4. [DOI] [PubMed] [Google Scholar]
- 21.Harrison S.A., Rossi S.J., Paredes A.H., Trotter J.F., Bashir M.R., Guy C.D. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatology. 2020;71:1198–1212. doi: 10.1002/hep.30590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschfield G.M., Chazouilleres O., Drenth J.P., Thorburn D., Harrison S.A., Landis C.S. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 23.Nevens F., Andreone P., Mazzella G., Strasser S.I., Bowlus C., Invernizzi P. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 24.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 25.Rinella M.E., Trotter J.F., Abdelmalek M.F., Paredes A.H., Connelly M.A., Jaros M.J. Rosuvastatin improves the FGF19 analogue NGM282-associated lipid changes in patients with non-alcoholic steatohepatitis. J Hepatol. 2019;70:735–744. doi: 10.1016/j.jhep.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen M.J., Nedergaard A.F., Sun S., Veidal S.S., Larsen L., Zheng Q. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 27.Russell D.W. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res. 2009;50(Suppl):S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heuman D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 29.Yoshimoto S., Loo T.M., Atarashi K., Kanda H., Sato S., Oyadomari S. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 30.Reddy B.S. Role of bile metabolites in colon carcinogenesis. Animal models. Cancer. 1975;36:2401–2406. doi: 10.1002/1097-0142(197512)36:6<2401::aid-cncr2820360619>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360 doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149 doi: 10.1053/j.gastro.2015.04.043. 389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor R.S., Taylor R.J., Bayliss S., Hagstrom H., Nasr P., Schattenberg J.M. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–25. e12. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 35.European Association for the Study of the Liver EASL clinical Practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Ridlon J.M., Harris S.C., Bhowmik S., Kang D.J., Hylemon P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu R., Zhao R., Zhou X., Liang X., Campbell D.J., Zhang X. Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2. Hepatology. 2014;60:908–918. doi: 10.1002/hep.27085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu R., Li X., Hylemon P.B., Zhou H. Conjugated bile acids promote invasive growth of esophageal adenocarcinoma cells and cancer stem cell expansion via sphingosine 1-phosphate receptor 2-mediated yes-associated protein activation. Am J Pathol. 2018;188:2042–2058. doi: 10.1016/j.ajpath.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayerdorffer E., Mannes G.A., Richter W.O., Ochsenkuhn T., Wiebecke B., Kopcke W. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology. 1993;104:145–151. doi: 10.1016/0016-5085(93)90846-5. [DOI] [PubMed] [Google Scholar]
- 40.Hofmann A.F., Roda A. Physicochemical properties of bile acids and their relationship to biological properties: an overview of the problem. J Lipid Res. 1984;25:1477–1489. [PubMed] [Google Scholar]
- 41.Beuers U., Maroni L., Elferink R.O. The biliary HCO(3)(-) umbrella: experimental evidence revisited. Curr Opin Gastroenterol. 2012;28:253–257. doi: 10.1097/MOG.0b013e328352aab2. [DOI] [PubMed] [Google Scholar]
- 42.McLeod G.M., Wiggins H.S. Bile-salts in small intestinal contents after ileal resection and in other malabsorption syndromes. Lancet. 1968;1:873–876. doi: 10.1016/s0140-6736(68)90235-3. [DOI] [PubMed] [Google Scholar]
- 43.Garbutt J.T., Heaton K.W., Lack L., Tyor M.P. Increased ratio of glycine- to taurine-conjugated bile salts in patients with ileal disorders. Gastroenterology. 1969;56:711–720. [PubMed] [Google Scholar]
- 44.Bellentani S., Pecorari M., Cordoma P., Marchegiano P., Manenti F., Bosisio E. Taurine increases bile acid pool size and reduces bile saturation index in the hamster. J Lipid Res. 1987;28:1021–1027. [PubMed] [Google Scholar]
- 45.Dilger K., Hohenester S., Winkler-Budenhofer U., Bastiaansen B.A., Schaap F.G., Rust C. Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health. J Hepatol. 2012;57:133–140. doi: 10.1016/j.jhep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 46.Falany C.N., Johnson M.R., Barnes S., Diasio R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269:19375–19379. [PubMed] [Google Scholar]
- 47.Haeusler R.A., Astiarraga B., Camastra S., Accili D., Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12alpha-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S., Friedman S.L. Hepatic fibrosis: a convergent response to liver injury that is reversible. J Hepatol. 2020;73:210–211. doi: 10.1016/j.jhep.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woolbright B.L., Dorko K., Antoine D.J., Clarke J.I., Gholami P., Li F. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol Appl Pharmacol. 2015;283:168–177. doi: 10.1016/j.taap.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benz C., Angermuller S., Tox U., Kloters-Plachky P., Riedel H.D., Sauer P. Effect of tauroursodeoxycholic acid on bile-acid-induced apoptosis and cytolysis in rat hepatocytes. J Hepatol. 1998;28:99–106. doi: 10.1016/s0168-8278(98)80208-0. [DOI] [PubMed] [Google Scholar]
- 51.Krahenbuhl S., Talos C., Fischer S., Reichen J. Toxicity of bile acids on the electron transport chain of isolated rat liver mitochondria. Hepatology. 1994;19:471–479. doi: 10.1002/hep.1840190228. [DOI] [PubMed] [Google Scholar]
- 52.Svegliati-Baroni G., Ridolfi F., Hannivoort R., Saccomanno S., Homan M., De Minicis S. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042–1055. doi: 10.1053/j.gastro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Saga K., Iwashita Y., Hidano S., Aso Y., Isaka K., Kido Y. Secondary unconjugated bile acids induce hepatic stellate cell activation. Int J Mol Sci. 2018;19:3043. doi: 10.3390/ijms19103043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beuers U., Trauner M., Jansen P., Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond. J Hepatol. 2015;62:S25–S37. doi: 10.1016/j.jhep.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 55.Karsdal M.A., Detlefsen S., Daniels S.J., Nielsen M.J., Krag A., Schuppan D. Is the total amount as important as localization and type of collagen in liver fibrosis attributable to steatohepatitis? Hepatology. 2020;71:346–351. doi: 10.1002/hep.30969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the confidential nature of the data in the study, the data collected in the study will not be available to access.