Key Points
Question
Is epidural labor analgesia (ELA) associated with increased offspring risk of autism spectrum disorder (ASD)?
Findings
In this cohort study of 123 175 offspring born in Manitoba, Canada, from 2005 to 2016, after accounting for maternal sociodemographic, preexisting, pregnancy-related, and birth-specific factors, no association was found between ELA exposure and offspring risk of ASD.
Meaning
Results of this study suggest that ELA is not associated with an increased risk of ASD in offspring.
Abstract
Importance
Epidural labor analgesia (ELA) has been associated with an increased offspring risk of autism spectrum disorder (ASD). Whether this finding may be explained by residual confounding remains unclear.
Objective
To assess the association between ELA and offspring risk of ASD.
Design, Setting, and Participants
Longitudinal cohort study of vaginal deliveries of singleton live infants born from 2005 to 2016 from a population-based data set linking information from health care databases in Manitoba, Canada; offspring were followed from birth until 2019 or censored by death or emigration. Data were analyzed from October 19, 2020, to January 22, 2021.
Exposures
Epidural labor analgesia.
Main Outcomes and Measures
At least 1 inpatient or outpatient diagnosis of ASD in offspring aged at least 18 months. For the full population and a sibling cohort, inverse probability of treatment-weighted Cox proportional hazards regression analyses were used to control for potential confounders.
Results
Of the 123 175 offspring included in this study (62 647 boys [50.9%]; mean [SD] age of mothers, 28.2 [5.8] years), 47 011 (38.2%) were exposed to ELA; 2.1% (985 of 47 011) of exposed vs 1.7% (1272 of 76 164) of unexposed offspring were diagnosed with ASD in the follow-up period (hazard ratio [HR], 1.25; 95% CI, 1.15-1.36). After adjusting for maternal sociodemographic, prepregnancy, pregnancy, and perinatal covariates, ELA was not associated with an offspring risk of ASD (inverse probability of treatment–weighted HR, 1.08; 95% CI, 0.97-1.20). In the within-siblings design adjusting for baseline covariates, ELA was not associated with ASD (inverse probability of treatment–weighted HR, 0.97; 95% CI, 0.78-1.22). Results from sensitivity analyses restricted to women without missing data who delivered at or after 37 weeks of gestation, firstborn infants only, and offspring with ASD classified with at least 2 diagnostic codes were consistent with findings from the main analyses.
Conclusions and Relevance
In a Canadian population-based birth cohort study, no association between ELA exposure and an increased offspring risk of ASD was found.
This population-based cohort study examines the association between epidural labor analgesia and offspring risk of autism spectrum disorder, adjusting for a large set of potential confounders, in the Canadian province of Manitoba.
Introduction
Autism spectrum disorder (ASD) is a group of complex developmental conditions that result in persistent impairments in social interaction, speech, nonverbal communication, and restricted or repetitive behaviors.1 Because the US incidence of ASD increased from 0.66% in 2002 to 1.85% in 2016,2,3 there has been substantial interest in identifying potential genetic, maternal, and neurological risk factors for ASD.4,5,6 Perinatal morbidities and interventions, including birth injury, low birth weight, and cesarean delivery, may lead to neonatal neurological vulnerability and, secondarily, increase the offspring risk of ASD.7,8 More recent research has focused on associations between intrapartum interventions, including epidural labor analgesia (ELA), and the risk of ASD.
Epidural labor analgesia, a highly effective method of providing pain relief,9 is used by 73% of US pregnant women during labor.10 However, findings from a recent population-based cohort study of 147 895 live births in Kaiser Permanente Southern California hospitals reported that ELA was associated with a 37% increased risk of ASD in offspring.11 After publication, 5 medical societies that represent more than 100 000 physicians questioned the biologic plausibility of the reported association and expressed concern that the risk estimates were biased due to residual confounding.12 These findings may have created apprehension among pregnant women and maternal care professionals about the risk to the offspring from ELA exposure.
We performed a population-based study to examine the association between ELA and offspring risk of ASD, adjusting for a large set of potential confounders. Our study used population-based data from the Canadian province of Manitoba, which includes woman-child linkages to identify ELA and ASD diagnoses in offspring. Linkages to multiple health and social data sets allowed for detailed confounder adjustment. We also performed a cosibling analysis to assess stable unmeasured factors shared within families.
Methods
Data Sources and Study Cohort
We obtained a population-based data set by linking information from 4 data providers in Manitoba, Canada: (1) Manitoba Health, Seniors and Active Living (provider of all health services in Manitoba), including the Manitoba Health Insurance Registry, Medical Services, Hospital Abstracts, and Drug Program Information Network; (2) Manitoba Families, including the Social Allowances Management Information Network and the Families First Screen; (3) Manitoba Education, including Enrollment, Marks, and Assessments; and (4) Statistics Canada, including the Canadian Census. The Medical Services data set includes diagnoses from outpatient physician visits.13 Most physicians providing services in Manitoba are reimbursed on a fee-for-service basis; those who are paid on alternate payment plans submit shadow billing claims to Manitoba Health, Seniors and Active Living for administrative purposes.14 The Hospital Abstracts data set contains information on diagnoses and procedures from an inpatient hospitalization, including birth hospitalization information.15 The Drug Program Information Network includes all pharmaceuticals dispensed in outpatient pharmacies.16 Monthly Employment and Income Assistance (analogous to US welfare) use is contained in the Social Allowance Management Information Network.17 The Families First Screen is completed by a public health nurse and used to assess families with newborns within a week after hospital discharge; the screen includes measures on biologic, social, and demographic risk factors and is available for approximately 80 percent of the population.18 The Enrollment, Marks, and Assessments data set includes pupils’ grades in all courses completed in high school; this information is used to ascertain whether an individual completed the required number of high school courses to graduate from high school.19 The Canadian Census contains neighborhood-level information at the dissemination area level; dissemination areas typically consist of 400 to 700 individuals.20 An encrypted personal health number for each woman allowed linkage across all of these deidentified data sets,21,22 with offspring linked to women using hospital birth record information.23 All data are housed at and maintained by the Manitoba Centre for Health Policy.
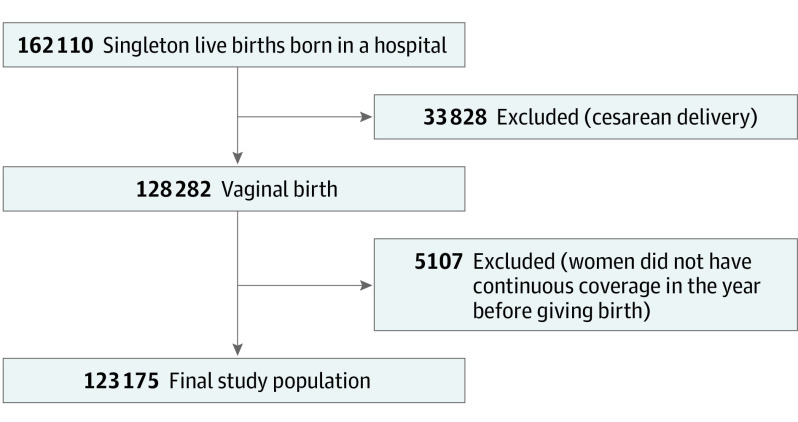
This study includes all singleton live births delivered in a hospital from April 1, 2005, to March 31, 2016, in Manitoba. Data were analyzed from October 19, 2020, to January 22, 2021. All cesarean deliveries were excluded because data were not available to differentiate women who underwent scheduled (prelabor) from unscheduled (intrapartum) cesarean deliveries. To account for women who immigrated into Manitoba within 12 months of delivery, we excluded births in women who did not have 1 year of continuous coverage before giving birth. The final study population was 123 175 offspring (Figure). Among these, 80 459 had at least 1 sibling in the cohort.
Figure. Cohort Selection Process.
This study was approved by the Health Research Ethics Board at the University of Manitoba and the Health Information Privacy Commission at Manitoba Health, Seniors and Active Living. Approval by the Health Research Ethics Board and the Health Information Privacy Commission for use of deidentified administrative data files includes a waiver of informed consent from participants.
Exposure
Using the Hospital Abstracts data set, ELA use was classified using the Canadian Classification of Interventions code (5.MD.xx). Epidural labor analgesia was also classified using an associated Canadian Institute for Healthcare Innovation anesthesia technique code 3 (epidural).24
Outcome
The primary outcome was a diagnosis of ASD in the offspring, classified by the presence of at least 1 diagnosis of ASD after the offspring reached at least 18 months of age.25 The ASD diagnoses were identified using the Medical Services and Hospital Abstracts data sets. Outpatient diagnoses claims were identified using 3-digit International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and inpatient diagnoses were identified using 5-digit International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canadian Modification (ICD-10-CA) codes. Outpatient diagnoses were identified from ICD-9-CM code 299.x and inpatient diagnoses from ICD-10-CA codes F84.x,25 which include autism disorders, Asperger syndrome, or pervasive developmental disorders not otherwise specified. All children were followed up through April 1, 2019, or were censored because of death or emigration.
Covariates
Sociodemographic covariates included maternal age, high school completion, marital status, Employment and Income Assistance receipt during pregnancy, and neighborhood socioeconomic status, defined using the Socioeconomic Factor Index, version 2 (SEFI-2; derived from neighborhood-level rates of high school completion rates, employment rates, single-parent household, and income).26 Prepregnancy covariates included diabetes, hypertension, anxiety, and depression (identified in the year before the birth). Pregnancy-related covariates included parity, gestational diabetes, gestational hypertension or preeclampsia, self-reported and diagnosed drug use, smoking, alcohol use, premature rupture of membranes, antepartum hemorrhage, infection of the amniotic sac and membrane, urogenital infection, antenatal mental health hospitalization, hypothyroidism, benzodiazepine use, antidepressant use, and antiepileptic use. Birth and perinatal covariates included birth year, induction of labor, augmentation of labor, labor dystocia, fetal distress, fetal macrosomia, gestational age at birth, sex of offspring, and hospital type. eTable 1 in the Supplement presents details for the classification of each covariate.
Statistical Analysis
We first examined differences in prepregnancy, pregnancy-related, birth, and perinatal covariates among births with and without ELA using standardized differences. We considered a covariate to be balanced if the standardized difference was less than 0.1.27 The cumulative incidence of ASD diagnoses before April 1, 2019, were compared among offspring exposed and not exposed to ELA.
The association between ELA and ASD was modeled with Cox proportional hazards regression models to account for censored observations in the data. Additionally, we used a robust SE to account for clustering owing to the inclusion of more than 1 birth per woman. To evaluate the covariates and the association between ELA and offspring ASD, we performed a series of regression analyses, sequentially increasing the degree of control for potential confounders. Each analysis used inverse probability of treatment weighting to balance baseline characteristics. Our a priori analysis–defined modeling strategy assessed populationwide associations, adjusting for potential confounders in the following sequence: model 2 consisted of maternal sociodemographic covariates; model 3, all covariates in model 2 plus prepregnancy and pregnancy-related covariates; and model 4, all covariates in models 1 and 2 plus perinatal covariates. Unweighted and weighted standardized differences of baseline characteristics by exposure are presented for each model in eTables 2-19 in the Supplement. After fitting each model, we assessed the proportional hazards assumption by evaluating the scaled Schoenfeld residuals for nonzero slope, finding no evidence that this assumption was violated.
Family context variables, such as family history of ASD, were also associated with offspring risk of ASD but not accounted for in the population-based models.6 To evaluate unmeasured stable familial variables, we conducted a second analysis restricted to siblings. The sibling cohort included an additional model with a fixed effect to allow the underlying hazard to vary between women.
Sensitivity Analyses
To evaluate potential bias, we conducted 3 sensitivity analyses. First, we examined missing sociodemographic information by excluding women with missing data on high school completion or marital status. Second, we reclassified ASD as a child having at least 2 ASD diagnoses on 2 separate days after 18 months of age. Third, we evaluated having multiple births per woman and differences in ASD by parity by including only firstborn offspring. In addition, we restricted our cohort to births at or after term (≥37 weeks of gestation). All data management and analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The final analytic cohort comprised 123 175 births (62 647 boys [50.9%]; mean [SD] age of mothers, 28.2 [5.8] years) that resulted in 1 live offspring per birth. Offspring were followed up for a total of 972 747 years (mean follow-up per offspring, 7.9 [3.4] years). Among the distinct births in the cohort, 47 011 (38.2%) were exposed to ELA. There were substantial differences in maternal sociodemographic, preexisting, pregnancy-related, and birth-specific covariates between births who were exposed vs nonexposed to ELA (Table 1). For example, births exposed to ELA were more likely to be nulliparous, have premature rupture of membranes, antepartum hemorrhage, induction of labor, augmentation of labor, and fetal distress.
Table 1. Maternal and Pregnancy Characteristics of Births Exposed and Not Exposed to ELA From 2005 to 2016.
| Variable | No ELA exposure (n = 76 164) | ELA exposure (n = 47 011) | Standardized difference |
|---|---|---|---|
| Maternal sociodemographic characteristic | |||
| Age range, y | |||
| <20 | 6033 (7.92) | 4960 (10.55) | 0.09 |
| 20-29 | 39 574 (51.96) | 24 539 (52.2) | 0.01 |
| 30-39 | 28 965 (38.03) | 16 756 (35.64) | 0.05 |
| ≥40 | 1592 (2.09) | 756 (1.61) | 0.04 |
| High school completion | |||
| Yes | 44 679 (58.66) | 32 680 (69.52) | 0.23 |
| No | 15 370 (20.18) | 7982 (16.98) | 0.08 |
| Missing | 16 115 (21.16) | 6349 (13.51) | 0.20 |
| Single parent | |||
| Yes | 23 132 (30.37) | 12 653 (26.91) | 0.08 |
| No | 53 032 (69.63) | 34 358 (73.09) | 0.08 |
| Missing | 17 124 (22.48) | 7774 (16.54) | 0.15 |
| Received income assistance in pregnancy | 11 589 (15.22) | 6577 (13.99) | 0.04 |
| Neighborhood SES, mean (SD)a | 0.42 (1.21) | 0.15 (1.06) | 0.23 |
| Maternal preexisting condition | |||
| Diabetes | 766 (1.01) | 500 (1.06) | 0.01 |
| Hypertension | 717 (0.94) | 554 (1.18) | 0.02 |
| Anxiety | 7185 (9.43) | 5530 (11.76) | 0.08 |
| Depression | 3510 (4.61) | 2998 (6.38) | 0.08 |
| Maternal pregnancy-related conditions | |||
| Nulliparous | 20 063 (26.34) | 25 361 (53.95) | 0.59 |
| Drug use in pregnancy | 2491 (3.27) | 1992 (4.24) | 0.05 |
| Alcohol use in pregnancy | 8842 (11.61) | 6814 (14.49) | 0.09 |
| Smoking in pregnancy | 19 481 (25.58) | 11 244 (23.92) | 0.04 |
| Gestational diabetes | 1457 (1.91) | 1629 (3.47) | 0.10 |
| Gestational hypertension/preeclampsia | 2291 (3.01) | 2138 (4.55) | 0.08 |
| Premature rupture of membranes | 3735 (4.9) | 6278 (13.35) | 0.30 |
| Antepartum hemorrhage | 5734 (7.53) | 7856 (16.71) | 0.28 |
| Infection of amniotic sac and membrane | 88 (0.12) | 236 (0.5) | 0.07 |
| Urogenital infection | 1092 (1.43) | 696 (1.48) | 0.01 |
| Mental health hospitalization in pregnancy | 338 (0.44) | 346 (0.74) | 0.04 |
| Hypothyroidism in pregnancy | 1187 (1.56) | 811 (1.73) | 0.01 |
| Antidepressant use in pregnancy | 849 (1.11) | 489 (1.04) | 0.01 |
| Benzodiazepine use in pregnancy | 879 (1.15) | 400 (0.85) | 0.03 |
| Antiepileptic use in pregnancy | 297 (0.39) | 159 (0.34) | 0.01 |
| Birth-specific condition and characteristic | |||
| Year | |||
| 2005-2008 | 24 921 (32.72) | 15 530 (33.03) | 0.01 |
| 2009-2012 | 28 037 (36.81) | 17 120 (36.42) | 0.01 |
| 2013-2016 | 23 206 (30.47) | 14 361 (30.55) | 0.01 |
| Induction of labor | 13 549 (17.79) | 15 253 (32.45) | 0.34 |
| Augmentation of labor | 14 522 (19.07) | 16 262 (34.59) | 0.36 |
| Labor dystocia | 47 (0.06) | 83 (0.18) | 0.03 |
| Fetal distress | 8595 (11.28) | 9465 (20.13) | 0.25 |
| Fetal macrosomia | 10 675 (14.02) | 7185 (15.28) | 0.04 |
| Gestational age, mean (SD), wk | 39.33 (1.96) | 39.25 (1.58) | 0.04 |
| Male offspring | 38 527 (50.58) | 24 120 (51.31) | 0.01 |
| Hospital type | |||
| Tertiary | 45 397 (59.6) | 36 897 (78.49) | 0.42 |
| Urban community | 4949 (6.5) | 5556 (11.82) | 0.19 |
| Rural | |||
| Major | 23 638 (31.04) | 4495 (9.56) | 0.55 |
| Intermediate | 1812 (2.38) | 63 (0.13) | 0.20 |
| Small | 368 (0.48) | 0 | 0.10 |
Abbreviations: ELA, epidural labor analgesia; SES, socioeconomic status.
Defined using the Socioeconomic Factor Index, version 2 (ranging from −5.2 to 4.6; higher values correspond to lower neighborhood SES), which includes neighborhood-level information on unemployment rate, household income, high school graduation, and single-parent household.
In the population cohort, by April 1, 2019, the cumulative risk of ASD was 2.1% for offspring exposed to ELA (985 of 47 011) and 1.7% for offspring unexposed to ELA (1272 of 76 164) (Table 2). Most children (96.1% [2237 of 2326]) received their first ASD diagnosis in an outpatient setting; overall, 74.5% of children (1671 of 2237) received their first ASD diagnosis from a pediatrician (eTable 20 in the Supplement). The median age at first diagnosis was 4.1 years (interquartile range, 3.0-5.8 years) (eTable 21 in the Supplement). The cumulative risk of ASD was 2.0% for exposed siblings and 1.6% for unexposed siblings.
Table 2. Frequency of ASD Diagnoses Among Offspring Exposed and Not Exposed to ELA.
| Cohort | Not exposed to ELA | Exposed to ELA | ||
|---|---|---|---|---|
| No. of offspring | No. of offspring with ASD (%) | No. of offspring | No. of offspring with ASD (%) | |
| Population | 76 164 | 1272 (1.7) | 47 011 | 985 (2.1) |
| Siblings | 53 285 | 825 (1.6) | 27 174 | 550 (2.0) |
Abbreviations: ASD, autism spectrum disorder; ELA, epidural labor analgesia.
ELA exposure was associated with ASD in model 1 (hazard ratio [HR], 1.25; 95% CI, 1.15-1.36) (Table 3). In the inverse probability of treatment-weighting analyses, ELA was also associated with ASD after adjusting for maternal sociodemographic covariates (model 2: inverse probability of treatment-weighted HR, 1.28; 95% CI, 1.17-1.40). After additional adjustment for prepregnancy and pregnancy-related covariates, the HR further attenuated (model 3: inverse probability of treatment-weighted HR, 1.15; 95% CI, 1.04-1.26). After additional adjustment for perinatal covariates, ELA was not associated with ASD (model 4: inverse probability of treatment-weighted HR, 1.08; 95% CI, 0.97-1.20).
Table 3. Association Between ELA and Offspring ASD.
| Cohort | No. of offspring | Hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Model 1: unadjusted model | Model 2: sociodemographic factorsa | Model 3: sociodemographic, preexisting, and pregnancy-related factorsb,c | Model 4: sociodemographic, preexisting, pregnancy-related, and perinatal factorsd | Model 5: sociodemographic, preexisting, pregnancy-related, and perinatal factors, with family fixed effect | ||
| Population | 123 175 | 1.25 (1.15-1.36) | 1.28 (1.17-1.40) | 1.15 (1.04-1.26) | 1.08 (0.97-1.20) | NA |
| Siblings | 80 459 | 1.25 (1.12-1.39) | 1.30 (1.16-1.45) | 1.24 (1.10-1.40) | 1.14 (0.99-1.30) | 0.97 (0.78-1.22) |
Abbreviations: ASD, autism spectrum disorder; ELA, epidural labor analgesia; NA, not available.
Sociodemographic factors: maternal age, high school completion, marital status, receipt of Employment and Income Assistance in pregnancy, and neighborhood socioeconomic status.
Preexisting factors: diabetes, hypertension, anxiety, and depression.
Pregnancy-related factors: parity, drug use in pregnancy, alcohol use in pregnancy, smoking in pregnancy, gestational diabetes, gestational hypertension/preeclampsia, premature rupture of membranes, antepartum hemorrhage, infection of amniotic sac and membrane, urogenital infection, mental health hospitalization in pregnancy, hypothyroidism in pregnancy, antidepressant use in pregnancy, benzodiazepine use in pregnancy, and antiepileptic use in pregnancy.
Perinatal factors: birth year, induction of labor, augmentation of labor, labor dystocia, fetal distress, fetal macrosomia, gestational age, offspring sex, and hospital type.
In the sibling cohort, the ASD risk in ELA-exposed offspring was slightly higher than in unexposed offspring (model 1 HR, 1.25; 95% CI, 1.12-1.39). However, in models adjusting for sociodemographic, prepregnancy, and perinatal covariates (model 4: HR, 1.14; 95% CI, 0.99-1.30) and after further addition of family fixed effects (model 5: inverse probability of treatment-weighted HR, 0.97; 95% CI, 0.78-1.22), ELA exposure was not associated with ASD.
Sensitivity Analyses
Results of the sensitivity analyses revealed similar results to those of our main findings. Results from the population-based models (model 4) restricted to women without missing information on maternal education or marital status, defining ASD as having at least 2 ASD diagnoses on separate days, first births only, and births at or after 37 weeks gestation were similar to the main results (Table 4).
Table 4. Sensitivity Analyses Association Between ELA and Offspring ASD.
| Population | No. of offspring | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|
| Model 1: unadjusted model | Model 2: sociodemographic factorsa | Model 3: sociodemographic, preexisting, and pregnancy-related factorsb,c | Model 4: sociodemographic, preexisting, pregnancy-related, and perinatal factorsd | ||
| Not missing maternal education or marital status information | 90 583 | 1.31 (1.19-1.45) | 1.35 (1.22-1.49) | 1.19 (1.06-1.32) | 1.10 (0.98-1.25) |
| More restrictive definition of ASDe | 123 175 | 1.28 (1.15-1.43) | 1.30 (1.16-1.46) | 1.14 (1.01-1.28) | 1.04 (0.91-1.20) |
| First birth only | 45 424 | 1.15 (1.01-1.31) | 1.13 (0.99-1.28) | 1.07 (0.93-1.23) | 1.03 (0.88-1.20) |
| Term births | 115 668 | 1.27 (1.16-1.39) | 1.29 (1.18-1.42) | 1.16 (1.05-1.28) | 1.09 (0.98-1.22) |
Abbreviations: ASD, autism spectrum disorder; ELA, epidural labor analgesia.
Sociodemographic factors: maternal age, high school completion, marital status, receipt of Employment and Income Assistance in pregnancy, and neighborhood socioeconomic status.
Preexisting factors: diabetes, hypertension, anxiety, and depression.
Pregnancy-related factors: parity, drug use in pregnancy, alcohol use in pregnancy, smoking in pregnancy, gestational diabetes, gestational hypertension/preeclampsia, premature rupture of membranes, antepartum hemorrhage, infection of amniotic sac and membrane, urogenital infection, mental health hospitalization in pregnancy, hypothyroidism in pregnancy, antidepressant use in pregnancy, benzodiazepine use in pregnancy, and antiepileptic use in pregnancy.
Perinatal factors: birth year, induction of labor, augmentation of labor, labor dystocia, fetal distress, fetal macrosomia, gestational age, offspring sex, and hospital type.
A child is defined as having ASD if they have at least 2 ASD diagnoses at or after 18 months of age, diagnosed on separate days.
Discussion
This population-based study of multiple databases from Canada found no association between ELA exposure and offspring risk of ASD. Our findings suggest that ELA use is not associated with an increased offspring risk of ASD.
The unadjusted analysis showed a significant association between ELA and an offspring risk of ASD. However, a null association was observed after adjusting for a large set of sociodemographic, prepregnancy, and perinatal confounders that were identified a priori. To substantiate our main findings, a null association between ELA and ASD was also observed in all sensitivity analyses. Further, findings from the sibling cohort analysis, which accounts for unmeasured stable familial variables (such as potential genetic factors and parental ASD diagnoses), were consistent with those in our main models.
Our findings contrast with those of a cohort study examining 147 895 live births in California in which ELA was associated with a 37% increased risk of ASD in offspring.11 It is possible that residual confounding explains this positive association because key perinatal variables, including induction of labor, labor dystocia, and fetal distress, were not included as confounders in that study. To limit potential bias from unmeasured confounders, we included the aforementioned variables within a wide set of potential confounders.
Epidural labor analgesia offers a number of important benefits to pregnant women during labor. It is recognized as the most effective method of providing labor analgesia.9 In contemporary obstetric anesthesia practice, low concentration local anesthetic infusions combined with ultra-low dose opioid are used to provide high-quality analgesia to women in labor.28 Another notable benefit of ELA is that the presence of an indwelling epidural catheter allows epidural anesthesia to be administered for an unplanned (intrapartum) cesarean delivery, thus secondarily avoiding any maternal complications or fetal exposure from general anesthesia.28 Although these benefits are well established, prior study findings linking ELA to offspring ASD risk may have altered pregnant women’s opinions and health care professionals’ recommendations about ELA use during labor. Future qualitative research is vital for determining the extent to which current and prior study findings may affect the opinions of women and health care professionals about the perceived risk of offspring ASD.
Strengths and Limitations
This cohort study had several strengths. Our analytic sample encompassed a contemporary cohort of vaginal births in a large geographical region (Manitoba, Canada). The large size of our sample allowed for precise risk estimates of the association between ELA and ASD. Data linkages across multiple databases, including medical services, hospital abstracts, pharmaceutical dispensations, educational level, income assistance, and postpartum screens, allowed us to capture a wide set of sociodemographic, prepregnancy, pregnancy-related, and perinatal covariates for inclusion in our regression models. Further, using these linkages, we identified all offspring born to the same woman during the study period. This approach allowed us to adjust for unmeasured factors shared within families in our sibling analysis.
Our analysis also has several limitations. To our knowledge, the accuracy of inpatient and outpatient ICD diagnostic codes for ASD has not been previously examined in Manitoba. However, the accuracy of our approach for classifying ASD using ICD-9-CM and ICD-10 codes has been reported in a large administrative database in British Columbia,25 a Canadian province with a health care system similar to that in Manitoba. In that study,25 ICD-9-CM and ICD-10 codes for ASD had a high positive predictive value (83%). Given this high positive predictive value and assuming nondifferential misclassification of the outcome, HR estimates were expected to be minimally biased. Further, our prevalence estimate for ASD in our study cohort (1.8%) is consistent with the ASD prevalence reported in 7 US states in 2014 (1.4%) and the prevalence seen across 7 provinces and territories in Canada in 2014 (1.5%).29,30 To our knowledge, no accuracy data of ELA coding in Manitoba are available. However, reporting of anesthesia technique (the variable we used to classify ELA exposure) is mandatory in Manitoba and thus likely to be coded with high specificity. Residual confounding is also a potential concern. However, after accounting for a large set of patient-level covariates as well as familial factors in our sibling analysis, we observed complete attenuation, with the point estimate approaching the null.
Information was not available on ELA drug dosing regimens; thus we cannot determine whether drug effects were factors in the observed association between ELA and ASD risk. Further, we did not have access to data on the duration of ELA exposure to women in labor.
Conclusions
Results of this population-based cohort study, including an analysis of exposure-discordant siblings, found no association between ELA and offspring risk of ASD. This finding is of clinical importance in the context of pregnant women and their obstetric and anesthesia care professionals who are considering ELA during labor.
eTable 1. Definitions and Datasets Used to Define Each Covariate
eTable 2. Weighted and Unweighted Standardized Differences, Full Population, Model 2
eTable 3. Weighted and Unweighted Standardized Differences, Full Population, Model 3
eTable 4. Weighted and Unweighted Standardized Differences, Full Population, Model 4
eTable 5. Weighted and Unweighted Standardized Differences, Siblings Population, Model 2
eTable 6. Weighted and Unweighted Standardized Differences, Siblings Population, Model 3
eTable 7. Weighted and Unweighted Standardized Differences, Siblings Population, Model 4
eTable 8. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 2
eTable 9. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 3
eTable 10. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 4
eTable 11. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 2
eTable 12. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 3
eTable 13. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 4
eTable 14. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 2
eTable 15. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 3
eTable 16. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 4
eTable 17. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 2
eTable 18. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 3
eTable 19. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 4
eTable 20. First Inpatient or Outpatient ASD Diagnosis by Provider Type
eTable 21. Age at First Autism Spectrum Disorder Diagnosis
References
- 1.Frith U, Happé F. Autism spectrum disorder. Curr Biol. 2005;15(19):R786-R790. doi: 10.1016/j.cub.2005.09.033 [DOI] [PubMed] [Google Scholar]
- 2.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators; Centers for Disease Control and Prevention . Prevalence of autism spectrum disorders–Autism and Developmental Disabilities Monitoring Network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56(1):12-28. [PubMed] [Google Scholar]
- 3.Maenner MJ, Shaw KA, Baio J, et al. ; EdS1; PhD-7 . Prevalence of autism spectrum disorder among children aged 8 years–Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill Summ. 2020;69(4):1-12. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nevison C, Parker W. California autism prevalence by county and race/ethnicity: declining trends among wealthy whites. J Autism Dev Disord. 2020;50(11):4011-4021. doi: 10.1007/s10803-020-04460-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers SM, Voigt RG, Colligan RC, et al. Autism spectrum disorder: incidence and time trends over two decades in a population-based birth cohort. J Autism Dev Disord. 2019;49(4):1455-1474. doi: 10.1007/s10803-018-3834-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsabbagh M. Linking risk factors and outcomes in autism spectrum disorder: is there evidence for resilience? BMJ. 2020;368:l6880. doi: 10.1136/bmj.l6880 [DOI] [PubMed] [Google Scholar]
- 7.Curran EA, O’Neill SM, Cryan JF, et al. Research review: birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. J Child Psychol Psychiatry. 2015;56(5):500-508. doi: 10.1111/jcpp.12351 [DOI] [PubMed] [Google Scholar]
- 8.Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. 2011;128(2):344-355. doi: 10.1542/peds.2010-1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anim-Somuah M, Smyth RMD, Cyna AM, Cuthbert A. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev. 2018;5(5):CD000331. doi: 10.1002/14651858.CD000331.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butwick AJ, Bentley J, Wong CA, Snowden JM, Sun E, Guo N. United States state-level variation in the use of neuraxial analgesia during labor for pregnant women. JAMA Netw Open. 2018;1(8):e186567. doi: 10.1001/jamanetworkopen.2018.6567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu C, Lin JC, Shi JM, et al. Association between epidural analgesia during labor and risk of autism spectrum disorders in offspring. JAMA Pediatr. 2020;174(12):1168-1175. doi: 10.1001/jamapediatrics.2020.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Society of Anesthesiologists . Labor epidurals do not cause autism; safe for mothers and infants, say anesthesiology, obstetrics, and pediatric medical societies. Published October 11, 2020. Accessed November 8, 2020. https://www.asahq.org/about-asa/newsroom/news-releases/2020/10/labor-epidurals-and-autism-joint-statement
- 13.Manitoba Centre for Health Policy . Manitoba Population Research Data Repository data descriptions. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=MedicalClaims
- 14.Manitoba Centre for Health Policy . Concept: physician/hospital claims. Published 2012. Accessed January 12, 2021. http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?conceptID=1093
- 15.Manitoba Centre for Health Policy . Hospital Abstracts. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=Hospital
- 16.Manitoba Centre for Health Policy . Drug Program Information Network. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=DPIN
- 17.Manitoba Centre for Health Policy . Employment Income Assistance (EIA)/Social Allowance Management Information Network (SAMIN) data. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=Samin
- 18.Brownell M, Chartier M, Santos R, et al. How Are Manitoba's Children Doing? 2nd ed. Manitoba Centre for Health Policy; October 2012. Accessed December 7, 2020. http://mchp-appserv.cpe.umanitoba.ca/reference//mb_kids_report_WEB.pdf
- 19.Manitoba Centre for Health Policy . Enrollment, Marks, and Assessments. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=EMA
- 20.Manitoba Centre for Health Policy . Canada Census. Published 2020. Accessed December 7, 2020. https://umanitoba.ca/faculties/health_sciences/medicine/units/chs/departmental_units/mchp/resources/repository/descriptions.html?ds=Census
- 21.Roos LL, Gupta S, Soodeen RA, Jebamani L. Data quality in an information-rich environment: Canada as an example. Can J Aging. 2005;24(suppl 1):153-170. doi: 10.1353/cja.2005.0055 [DOI] [PubMed] [Google Scholar]
- 22.Roos LL, Nicol JP. A research registry: uses, development, and accuracy. J Clin Epidemiol. 1999;52(1):39-47. doi: 10.1016/S0895-4356(98)00126-7 [DOI] [PubMed] [Google Scholar]
- 23.Currie J, Stabile M, Manivong P, Roos L. Child health and young adult outcomes. J Hum Resour. 2010;45(3):517-548. doi: 10.3368/jhr.45.3.517 [DOI] [Google Scholar]
- 24.Heaman M, Kingston D, Helewa M, et al. Perinatal services and outcomes in Manitoba. Published 2012. Accessed December 7, 2020. http://mchp-appserv.cpe.umanitoba.ca/reference/perinatal_report_WEB.pdf
- 25.Bickford CD, Oberlander TF, Lanphear NE, et al. Identification of pediatric autism spectrum disorder cases using health administrative data. Autism Res. 2020;13(3):456-463. doi: 10.1002/aur.2252 [DOI] [PubMed] [Google Scholar]
- 26.Manitoba Centre for Health Policy . Term: Socioeconomic Factor Index (SEFI): Version 2 (SEFI-2). Published 2016. Accessed October 29, 2020. http://mchp-appserv.cpe.umanitoba.ca/viewDefinition.php?definitionID=103983
- 27.Armstrong K, Lee BK, Leacy FP. Methods in comparative effectiveness research. J Clin Oncol. 2012;30(34):4208-4214. doi: 10.1200/JCO.2012.42.2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim G, Facco FL, Nathan N, Waters JH, Wong CA, Eltzschig HK. A review of the impact of obstetric anesthesia on maternal and neonatal outcomes. Anesthesiology. 2018;129(1):192-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christensen DL, Maenner MJ, Bilder D, et al. Prevalence and characteristics of autism spectrum disorder among children aged 4 years—Early Autism and Developmental Disabilities Monitoring Network, seven sites, United States, 2010, 2012, and 2014. MMWR Surveill Summ. 2019;68(2):1-19. doi: 10.15585/mmwr.ss6802a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofner M, Coles A, Decou ML, et al. Autism Spectrum Disorder Among Children and Youth in Canada 2018: A Report of the National Autism Spectrum Disorder Surveillance System. Public Health Agency of Canada; March 2018. Accessed January 7, 2021. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/diseases-conditions/autism-spectrum-disorder-children-youth-canada-2018/autism-spectrum-disorder-children-youth-canada-2018.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions and Datasets Used to Define Each Covariate
eTable 2. Weighted and Unweighted Standardized Differences, Full Population, Model 2
eTable 3. Weighted and Unweighted Standardized Differences, Full Population, Model 3
eTable 4. Weighted and Unweighted Standardized Differences, Full Population, Model 4
eTable 5. Weighted and Unweighted Standardized Differences, Siblings Population, Model 2
eTable 6. Weighted and Unweighted Standardized Differences, Siblings Population, Model 3
eTable 7. Weighted and Unweighted Standardized Differences, Siblings Population, Model 4
eTable 8. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 2
eTable 9. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 3
eTable 10. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 1, Model 4
eTable 11. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 2
eTable 12. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 3
eTable 13. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 2, Model 4
eTable 14. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 2
eTable 15. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 3
eTable 16. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 3, Model 4
eTable 17. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 2
eTable 18. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 3
eTable 19. Weighted and Unweighted Standardized Differences, Sensitivity Analysis 4, Model 4
eTable 20. First Inpatient or Outpatient ASD Diagnosis by Provider Type
eTable 21. Age at First Autism Spectrum Disorder Diagnosis