Abstract
Serious concerns are expressed on the safe use of red yeast rice (RYR) supplements. The aim of the present study was to analyse cases received by Lareb on RYR‐related adverse health events. These cases were analysed for the number of reports, number of adverse drug reactions (ADRs), causality, seriousness of the reaction, latency‐period, age and sex of the patients, concomitant medication and type of reporter. A total of 94 individual reports were collected by Lareb, corresponding with 187 ADRs. The analysis showed most reported ADRs were labelled musculoskeletal and connective tissue disorders (n = 64), gastrointestinal disorders (n = 33) and general disorders and administration site conditions (n = 23). The use of RYR supplements should be considered as a significant safety concern. Exposure to monacolin K could lead to serious adverse effects. To fully assess the safety profile of RYR supplements, more research is necessary to compose a complete ADR profile of RYR supplements.
Keywords: case series, mechanism, Monascus purpureus, red yeast rice, statins, Stoxicity
What is already known about this subject
Red yeast rice (RYR) supplements are used to lower serum cholesterol levels in patients with statin intolerance.
RYR supplements contain a wide variety of monacolin compounds, of which monacolin K is the primary form being chemically identical to lovastatin.
Serious concerns exist on the safety of RYR supplements, for which general toxicological knowledge on the potential health risks and involved mechanism is lacking.
What this study adds
The present study reveals numerous cases of adverse health effects by RYR and proposes potential toxicological mechanisms involved.
Our study confirms that the use of RYR supplements should be considered as a significant safety concern.
1. INTRODUCTION
Statins belong to the first‐line therapy in the reduction of elevated blood cholesterol levels and have been shown to reduce the rate of cardiovascular events and reduce all‐time mortality. 1 , 2 However, statins have been associated with muscle‐related adverse events that are most commonly reported as a major reason for statin intolerance 3 Several patients with statin intolerance seek alternative products to reduce elevated blood cholesterol levels. Additionally, even healthy consumers use alternative products to lower serum cholesterol. One of the most common alternative products are red yeast rice (RYR) supplements 3 RYR is produced by fermenting rice with Monascus porpureus, forming monacolin compounds 1 The primary monacolin compound present in the RYR supplements is monacolin K, which is chemically identical to the pharmaceutical lovastatin 4 Monacolin K inhibits HMG‐CoA reductase, an enzyme that catalyses the conversion of HMG‐CoA to mevalonate, a precursor of cholesterol. Through the inhibition of HMG‐CoA, monacolin K decreases the production rate of cholesterol 1 , 5 Several clinical trials have shown that RYR supplements are associated with the prevention of primary and secondary cardiovascular events 4 , 6 , 7 , 8 Besides these effects, also anticancer, anti‐inflammatory, antihypertensive and antidiabetic effects have been described 5 , 9
In contrast to these beneficial health effects, serious concerns exist on the safety of RYR supplements. The structural similarity with lovastatin implies that similar adverse drug reactions (ADRs) can be expected. Overall, general toxicological knowledge on the potential health risks of RYR are lacking. Identification of potential risks of RYR products is challenging because food supplements are not usually registered in pharmacovigilance databases where suspected ADRs can be collected. However, The Netherlands Pharmacovigilance Centre Lareb has received numerous spontaneous reports of ADRs by RYR supplements over recent years. The aim of this article is to comprehensively describe the cases received and assess the case series concerning the use of RYR and health complications.
2. METHODS
2.1. Description of cases
In the Netherlands, the Pharmacovigilance Centre Lareb maintains the spontaneous reporting system for drugs, vaccines and natural products with a health claim, such as herbal drugs. By collecting and analysing reported ADRs, knowledge is generated on the occurrence of ADRs in daily practice. Data on reported ADRs was gathered from 1991 (establishment of the centre) until March 2020. The reports can originate from health care professionals or consumers and can be received directly or through pharmaceutical companies.
All directly received reports in the Dutch spontaneous reporting system are individually triaged and assessed by trained assessors at Lareb. For the purpose of this study additional causality assessed was performed by the authors according to the World Health Organization causality model to determine the role of RYR in ADR occurrence 10 ADRs are coded with the Medical Dictionary for Regulatory Activities (MedDRA) 11 In the case of unregistered products, Lareb uses a custom built drug dictionary for coding. 12
The number of reports, number of ADRs, seriousness of the reaction 13 latency‐period, age and sex of the patient, concomitant medication, and type of reporter were described. The reports were grouped based on the MedDRA system organ class (SOC) and preferred term (PT). A line‐listing of all selected cases was made.
3. RESULTS
3.1. Characteristics of reports
From December 2007 to March 2020, a total of 94 individual reports were collected by Lareb, of which only 16 were received before June 2017. In these reports, in total 187 ADRs were reported, coded as PTs according the standardized MedDRA system. Six reports were according the CIOMS criteria classified as serious: 3 times (acute) pancreatitis and 2 times symptoms of rhabdomyolyses such as chromaturia, abnormal urine odor, myalgia, muscle spasms and malaise were reported. One report concerned acute hepatic failure with jaundice where the liver transplantation was required. World Health Organization causality assessment revealed the role of RYR in to be certain (n = 2), probably/likely (n = 24), possible (n = 61) and unlikely (n = 7) in the reported cases.
The majority of reports (n = 75) were submitted by consumers or other non‐health professionals. Nine cases were reported by pharmacists, 8 cases by a physician and 2 reports were reported by both pharmacist and physician. Reported sex was 60 females and 34 males and the mean age was 64 years (range 17–81 years), the median age was 65 years. Concomitant medication use was reported in 55 reports (59.1%).
The most reported comedication was vitamin D (colecalciferol), reported 11 times, followed by clopidogrel (Table S1). It must be noted that 1 report can contain multiple concomitant drugs.
3.2. Action and outcome
In 66 reports, patients discontinued their treatment with RYR supplements; in 47 cases of these the symptoms resolved, were recovering or recovered with sequel. The following actions with RYR supplement occurred in the remaining patients: no adjustment (n = 13), unknown (n = 5), dose reduced (n = 2), not applicable (n = 6) and not reported (n = 2).
3.3. SOC reports
A total of 94 individual reports of ADRs, corresponding to 187 PTs were related to RYR supplement use (Table S3). The most frequently reported PTs were labelled under the SOC musculoskeletal and connective tissue disorders, gastrointestinal disorders, general disorders and administration site conditions and nervous system disorders (Table S2). In total 64 PTs were reported in the SOC musculoskeletal and connective tissue disorders, which included myalgia, muscle spasms, weakness rhabdomyolysis, muscle atrophy, limb discomfort, flank pain, osteoarthritis and tendonitis. Gastrointestinal disorders was reported 33 times, and included inter alia (i.a.) abdominal pain, abdominal discomfort, nausea, diarrhoea and pancreatitis. General disorders and administration site conditions was reported 23 times and included i.a. fatigue, malaise and peripheral oedema. Nervous system disorders, such as headache, dizziness and sleep disorders, were reported 16 times. All SOCs and related no. of ADRs can be found in Table S2.
3.4. Latency
Notable the median latency time for all reported ADRs is 2.1 weeks and also median latency's for the symptoms classified within the 4 most reported SOCs show the same tendency, as it falls between 1.9 weeks for nervous system disorders, 2 weeks for general symptoms, 2.4 weeks for musculoskeletal disorders and 2.9 weeks for gastrointestinal disorders (Figure 1B).
FIGURE 1.
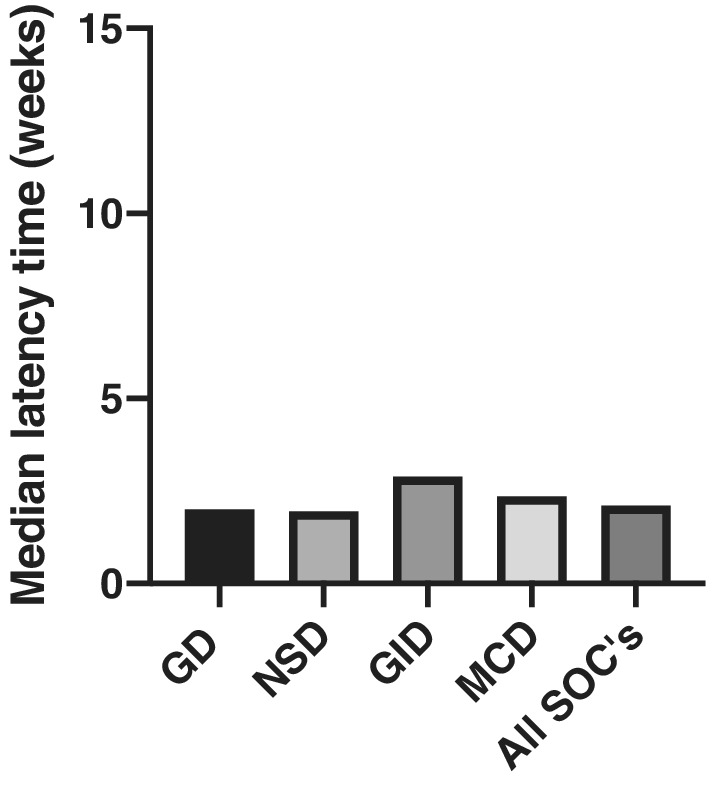
Median latency times for the symptoms in the cases reported to Lareb. GD: general disorders and administration site conditions; NSD: nervous system disorders; GID: gastrointestinal disorders; MCD: musculoskeletal and connective tissue disorders; SOCs: system organ classes
4. DISCUSSION
Although RYR‐containing products have been available on the market as food supplements for several years and their efficacy has been proven, 4 , 6 , 7 , 8 serious concerns about their safety profile remain. The present study reveals the most reported ADRs due to the use of RYR supplements to Lareb. It can be noted that the profile of reported ADRs to RYR of the present study is in line with previous studies, 14 , 15 , 16 ] with musculoskeletal and gastrointestinal, as the most frequently reported adverse effects (AEs). Although similar studies have been published before, these supplements are being used on a large scale and new cases of AEs due to these supplements arise. Our case series adds to the existing case reports and contributes to increasing the awareness for the potential adverse health effects of RYR supplements. Overall, our results demonstrate that the safety profile of RYR supplements is similar to that of the synthetic statins, particularly lovastatin.
Several uncertainties exist that complicate drawing a complete safety profile of RYR supplements. First of all, there is a wide variability in composition of RYR‐containing food supplements. The monacolins in RYR are used in multi‐ingredient botanical preparations containing multiple other components, which have not been fully evaluated individually or in combination. Moreover, it is evident that the ratio of monacolin K and its hydroxyl acid form monacolin KA differs widely in numerous RYR products. 17 Other than monacolin K, there is a lack of data on the bioactivity of the other components in RYR. Therefore, it is difficult to assess the toxicological mechanisms of this mixture of monacolins. Since RYR most abundantly consists of monacolin K, which is a structural homologue to lovastatin, the mechanisms of RYR‐induced adverse effects are expected to be similar to those known for statins.
4.1. Musculoskeletal disorders
Skeletal muscle abnormalities are the most reported and clinically important side effects of RYR, as shown in Table S2. Smith et al. published a case report of a middle‐ aged man presenting with muscle weakness after receiving RYR supplements for 2 months. 18 After examining the patients, they found increased creatine kinase levels of 385 IU/L (normal range is 30–160 IU/L). Additionally, Venhuis et al. reported a similar case report of patients using RYR products. 19 The patients exhibited multiple AEs, including myalgia. Although the exact mechanisms behind the musculoskeletal ADRs are not known, numerous studies have been performed on identification of the mechanism. 20 , 21 Multiple proposed mechanisms include inhibition of coenzyme Q10 synthesis, isoprenoid depletion, decreased or altered sarcolemma membrane cholesterol, disturbed calcium metabolism or autoimmune phenomena 22 , 23 , 24 , 25 The most obvious explanation for RYR‐induced myopathy would be the inhibition of mevalonate, a precursor of coenzyme Q10, due to the HMG‐CoA inhibition. As a consequence of this inhibition, the mitochondrial respiratory chain may be impaired, and this may result in impaired energy production and ultimately cell death 26 This effect is better described for statins, as several studies have shown lower levels of coenzyme Q10 after the use of statins 27 , 28
4.2. Gastrointestinal related disorders
The cases described by the present study show that gastrointestinal reactions are often associated with the use of RYR supplements. These symptoms, varying from dyspepsia to vomiting and abdominal pain, nausea and diarrhoea, are also mostly reported by other studies 6 Mazzanti et al. also found a high percentage of gastrointestinal disorders: 22% of all reported ADRs. In general, the reactions were not serious with the exception of 1 hospitalization 15 Although there is no specific explanation yet for these side effects of RYR supplements, gastrointestinal reactions are not uncommon reactions associated with the use of statins, in which mitochondrial dysfunction is suggested to play an important role 29 , 30
4.3. Hepatic disorders
Besides musculoskeletal and gastrointestinal disorders, adverse effects associated with RYR that are repeatedly reported are elevated levels of hepatic enzymes, indicative for liver injury. This is also shown by a meta‐analysis on the efficacy and safety of RYR supplements that included a total of 13 randomized controlled trials 31 The analysis showed that the serum alanine transaminase and aspartate aminotransferase levels were significantly increased in RYR users. This might explain the 3 cases of liver injury identified by the present study. In addition to the 3 cases reported by our study, multiple other cases of RYR‐induced liver toxicity have been documented 14 , 15 Three individual cases of severe hepatitis due to RYR were published previously, highlighting that, despite the low incidence of liver injury, the use of RYR supplements might have serious health consequences 32 , 33 , 34 The exact mechanism by which RYR causes hepatotoxicity is not well understood yet. However, it is expected that liver injury by RYR supplements may be attributed to the presence of lovastatin, as liver injuries are also among the most reported side effects of statin users 1 , 35 , 36 , 37 Similar to the musculoskeletal adverse effects, mitochondrial dysfunction is proposed to play a major role in the observed liver injury by statins 38 , 39
4.4. Kidney damage
In our case series, kidney damage due to the use of RYR supplements was reported 5 times. The induced kidney damage might be explained by the presence of the mycotoxin citrinin in a significant number of RYR supplement, although this is unfortunately not measured 40 Citrinin is known to have nephrotoxic properties and therefore RYR supplements containing citrinin is a cause for concern, since it can pose a serious health risk: both in vivo and in vitro studies showed that citrinin has potentially mutagenic and mutatoxic properties 41 , 42 , 43 , 44 Another possible explanation for the toxic effects on the kidneys can be found in the muscle damage by RYR supplements. This can result in apoptosis of muscle cells, causing release myoglobin into the blood stream. In the kidneys, myoglobin can be converted to ferrihaem, which is considered a nephrotoxic compound 45
4.5. Possible interactions between comedication and RYR supplements
Comedication influencing the bioavailability of monacolin K could also increase the chance of adverse health events by RYR supplements. The present case series shows that approximately 60% of the reported cases concerned the concomitant use of RYR supplements and medicines (Table S1). The use of comedication can potentially interfere with the kinetics and dynamics of RYR. Statins are metabolized by cytochrome P450 3A4 enzymes, and the administration of RYR with cytochrome enzyme inhibitors can therefore increase the risk of side effects by increasing monacolin K plasma concentrations. Such pharmacokinetic interaction has been reported in a 28‐year‐old kidney transplantation female patient who used a preparation containing RYR and cyclosporine 46 This patient developed rhabdomyolysis several months after starting the use of RYR, after receiving a combination of drugs in her post‐transplant period. Another case report suggests that coadministration of RYR with esomeprazole may increase the plasma concentrations of monacolin K and thus the associated risk of myopathy. The proposed mechanism is competitive inhibition of intestinal P‐glycoprotein, resulting in decreased drug secretion into the intestinal lumen and increased drug bioavailability 47
Also, pharmacodynamic interactions may occur by the combined use of drugs and RYR supplements. This will especially be relevant in case of taking RYR together with other statins. In 2 of our cases, atorvastatin was reported as comedication. This means that a potentiation of the HMCG‐CoA inhibition may occur, potentially leading to an increased risk for the occurrence of the ADRs. Hence, the use of RYR in combination with other drugs needs to be done carefully due to potential interactions that may lead to serious adverse health effects.
5. LIMITATIONS
In the assessment of case series of spontaneously reported cases, several limitations could play a role. Whereas comedications potentially cause interactions with RYR supplements and thereby increase the chance of developing ADRs, they may also have a confounding effect that complicates identifying the role of RYR in the ADRs. There are, for instance, concomitant drugs known to be potentially associated with pancreatitis, such as morphine, perindopril and telmisartan. To exclude this confounding effect of drugs as much as possible, the use of comedications is taken into account in the causality assessment. Another potential limitation of the present case series of spontaneous reports is notoriety bias 48 Although notoriety bias always plays a role when analysing and discussing spontaneous reports, this does not mean that the reported cases to Lareb are no real cases, only that the reporter was triggered to submit their case after the media attention. Seeing that the cases are reported over a period of multiple years, the increase in reports is likely to reflect the growing use of RYR products and is not merely based on more media attention. Finally, the absence of information about content of monacolin K makes it difficult to strongly relate the ADRs to the use of RYR supplements. However, even if such information were available, this would still provide uncertain information for 2 reasons: (i) based on the reports, it cannot be determined how many dosages 1 patient used; (ii) as the supplements were generally not chemically analysed, it remains uncertain what exactly, and how much, is present of monacolins in the supplement. Interestingly, the analysis of 1 RYR sample of 1 of the reports showed that the dose of monacolin K in the supplements varied between pills from 1 jar (7 vs 3 mg). It is therefore impossible to assess the chemicals and doses to which the patients were exposed.
6. CONCLUSION
The intake of monacolins via dietary supplements can lead to serious adverse health effects. Monacolin K is chemically identical to lovastatin and therefore these adverse health effects are similar to those of lovastatin. The use of RYR supplements should therefore be considered as a significant safety concern. So far, exact mechanisms on these toxic effects have not been established. This can be explained by the fact that the RYR supplements contain a wide variety of chemical compounds, most of which have not been toxicologically characterized. To fully assess the safety profile of RYR supplements, more research is needed into all these chemical constituents. Based on remaining uncertainties on the effects of RYR supplements, it remains impossible to identify recommended dietary intake levels of monacolins from RYR that would not give any harmful effect to human health.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All authors substantially contributed to designing the study, acquiring, analyzing and interpreting the data; and drafting and revising the work. All authors approved the submitted final version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
TABLE S1 Top 10 reported comedications
TABLE S2 Number of reported ADRs per SOC
TABLE S3 Overview of all reported cases to Lareb.
Vrolijk MF, van de Koppel S, van Hunsel F. Red yeast rice (Monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br J Clin Pharmacol. 2021;87:2146–2151. 10.1111/bcp.14599
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ong YC, Aziz Z. Systematic review of red yeast rice compared with simvastatin in dyslipidaemia. J Clin Pharm Ther. 2016;41(2):170‐179. [DOI] [PubMed] [Google Scholar]
- 2. Brouwers J, Roeters van Lennep J, Maas A. ‘Rode gist rijst’ als cholesterolverlager? Ned Tijdschr Geneeskd. 2016;160:84. [PubMed] [Google Scholar]
- 3. Gerards MC, Terlou RJ, Yu H, Koks CHW, Gerdes VEA. Traditional Chinese lipid‐lowering agent red yeast rice results in significant LDL reduction but safety is uncertain ‐ a systematic review and meta‐analysis. Atherosclerosis. 2015;240(2):415‐423. [DOI] [PubMed] [Google Scholar]
- 4. Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T. 2009;34(6):313‐327. [PMC free article] [PubMed] [Google Scholar]
- 5. Patel S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons. World J Microbiol Biotechnol. 2016;32(5):87. [DOI] [PubMed] [Google Scholar]
- 6. Moriarty PM, Roth EM, Karns A, et al. Effects of Xuezhikang in patients with dyslipidemia: a multicenter, randomized, placebo‐controlled study. J Clin Lipidol. 2014;8(6):568‐575. [DOI] [PubMed] [Google Scholar]
- 7. Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta‐analysis of randomized controlled trials. Chin Med. 2006;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin‐intolerant patients: a randomized trial. Ann Intern Med. 2009;150(12):830‐839. W147–9 [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Ali Z, Khan SI, Khan IA. Cytotoxic monacolins from red yeast rice, a Chinese medicine and food. Food Chem. 2016;202:262‐268. [DOI] [PubMed] [Google Scholar]
- 10. The use of the WHO‐UMC system for standardised case causality assessment. Available from: https://www.who.int/medicines/areas/quality_safety/safety_efficacy/WHOcausality_assessment.pdf
- 11. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 12. Oosterhuis I, Zweers P, Rümke H, Muller‐Hansma A, Puijenbroek EP. A tailor‐made approach for causality assessment for ADR reports on drugs and vaccines. Pharmacoepidemiol Drug Saf. 2019;28(4):544‐550. [DOI] [PubMed] [Google Scholar]
- 13. IV., C.W.G . Benefit–risk balance for marketed drugs. Geneva: Evaluating safety signals; 1998. [Google Scholar]
- 14. Raschi E, Girardi A, Poluzzi E, et al. Adverse events to food supplements containing red yeast Rice: comparative analysis of FAERS and CAERS reporting systems. Drug Saf. 2018;41(8):745‐752. [DOI] [PubMed] [Google Scholar]
- 15. Mazzanti G, Moro PA, Raschi E, Da Cas R, Menniti‐Ippolito F. Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. Br J Clin Pharmacol. 2017;83(4):894‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Opinion of the French Agency for Food, Environmental andOccupational Health & Safety (ANSES) on the risks associated with the presence of ‘red yeast rice’ in food supplements.. February14, 2014.
- 17. Gordon RY, Cooperman T, Obermeyer W, Becker DJ. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med. 2010;170(19):1722‐1727. [DOI] [PubMed] [Google Scholar]
- 18. Smith DJ, Olive KE. Chinese red rice‐induced myopathy. South Med J. 2003;96(12):1265‐1267. [DOI] [PubMed] [Google Scholar]
- 19. Venhuis BJ, Hunsel F, Koppel S, Keizers PHJ, Jeurissen SMF, De Kaste D. Pharmacologically effective red yeast rice preparations marketed as dietary supplements illustrated by a case report. Drug Test Anal. 2016;8(3–4):315‐318. [DOI] [PubMed] [Google Scholar]
- 20. Bouitbir J, Sanvee GM, Panajatovic MV, Singh F, Krähenbühl S. Mechanisms of statin‐associated skeletal muscle‐associated symptoms. Pharmacol Res. 2020;154:104201. [DOI] [PubMed] [Google Scholar]
- 21. Lotteau S, Ivarsson N, Yang Z, et al. A mechanism for statin‐induced susceptibility to myopathy. JACC Basic Transl Sci. 2019;4(4):509‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abd TT, Jacobson TA. Statin‐induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10(3):373‐387. [DOI] [PubMed] [Google Scholar]
- 23. Toth PP, Harper CR, Jacobson TA. Clinical characterization and molecular mechanisms of statin myopathy. Expert Rev Cardiovasc Ther. 2008;6(7):955‐969. [DOI] [PubMed] [Google Scholar]
- 24. Mohaupt MG, Karas RH, Babiychuk EB, et al. Association between statin‐associated myopathy and skeletal muscle damage. CMAJ. 2009;181(1–2):E11‐E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baker SK. Molecular clues into the pathogenesis of statin‐mediated muscle toxicity. Muscle Nerve. 2005;31(5):572‐580. [DOI] [PubMed] [Google Scholar]
- 26. Marcoff L, Thompson PD. The role of coenzyme Q10 in statin‐associated myopathy: a systematic review. J Am Coll Cardiol. 2007;49(23):2231‐2237. [DOI] [PubMed] [Google Scholar]
- 27. Chatzizisis YS, Vaklavas C, Giannoglou GD. Coenzyme Q10 depletion: etiopathogenic or predisposing factor in statin associated myopathy? Am J Cardiol. 2008;101(7):1071. [DOI] [PubMed] [Google Scholar]
- 28. Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99(10):1409‐1412. [DOI] [PubMed] [Google Scholar]
- 29. Scott RS, Lintott CJ, Wilson MJ. Simvastatin and side effects. N Z Med J. 1991;104(924):493‐495. [PubMed] [Google Scholar]
- 30. Golomb BA, Evans MA. Statin adverse effects: a review of the literature and evidence for a mitochondrial mechanism. Am J Cardiovasc Drugs. 2008;8(6):373‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Jiang L, Jia Z, et al. A meta‐analysis of red yeast rice: an effective and relatively safe alternative approach for dyslipidemia. PLoS One. 2014;9(6):e98611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loubser L, Weider KI, Drake SM. Acute liver injury induced by red yeast rice supplement. BMJ Case Rep. 2019;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grieco A, Miele L, Pompili M, et al. Acute hepatitis caused by a natural lipid‐lowering product: when “alternative” medicine is no “alternative” at all. J Hepatol. 2009;50(6):1273‐1277. [DOI] [PubMed] [Google Scholar]
- 34. Roselle H, Ekatan A, Tzeng J, Sapienza M, Kocher J. Symptomatic hepatitis associated with the use of herbal red yeast rice. Ann Intern Med. 2008;149:516‐517, 7. [DOI] [PubMed] [Google Scholar]
- 35. Ramkumar S, Raghunath A, Raghunath S. Statin therapy: review of safety and potential side effects. Acta Cardiol Sin. 2016;32(6):631‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta‐analysis. Pharmacotherapy. 2004;24(5):584‐591. [DOI] [PubMed] [Google Scholar]
- 37. Bjornsson ES. Hepatotoxicity of statins and other lipid‐lowering agents. Liver Int. 2017;37(2):173‐178. [DOI] [PubMed] [Google Scholar]
- 38. Karahalil B, Hare E, Koç G, Uslu İ, Şentürk K, Özkan Y. Hepatotoxicity associated with statins. Arh Hig Rada Toksikol. 2017;68(4):254‐260. [DOI] [PubMed] [Google Scholar]
- 39. Abdoli N, Azarmi Y, Eghbal MA. Protective effects of N‐acetylcysteine against the statins cytotoxicity in freshly isolated rat hepatocytes. Adv Pharm Bull. 2014;4(3):249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heber D, Lembertas A, Lu QY, Bowerman S, Go VLW. An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents. J Altern Complement Med. 2001;7(2):133‐139. [DOI] [PubMed] [Google Scholar]
- 41. Endo A, Kuroda M. Citrinin, an inhibitor of cholesterol synthesis. J Antibiot (Tokyo). 1976;29(8):841‐843. [DOI] [PubMed] [Google Scholar]
- 42. Sabater‐Vilar M, Maas RF, Fink‐Gremmels J. Mutagenicity of commercial Monascus fermentation products and the role of citrinin contamination. Mutat Res. 1999;444(1):7‐16. [DOI] [PubMed] [Google Scholar]
- 43. Dönmez‐Altuntas H, Dumlupinar G, Imamoglu N, Hamurcu Z, Liman BC. Effects of the mycotoxin citrinin on micronucleus formation in a cytokinesis‐block genotoxicity assay in cultured human lymphocytes. J Appl Toxicol. 2007;27(4):337‐341. [DOI] [PubMed] [Google Scholar]
- 44. Pascual‐Ahuir A, Vanacloig‐Pedros E, Proft M. Toxicity mechanisms of the food contaminant citrinin: application of a quantitative yeast model. Nutrients. 2014;6(5):2077‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nath KA, Belcher JD, Nath MC, et al. Role of TLR4 signaling in the nephrotoxicity of heme and heme proteins. Am J Physiol Renal Physiol. 2018;314(5):F906‐F914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prasad GR, Wong T, Meliton G, Bhaloo S. Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient. Transplantation. 2002;74(8):1200‐1201. [DOI] [PubMed] [Google Scholar]
- 47. Pauli‐Magnus C, Rekersbrink S, Klotz U, Fromm MF. Interaction of omeprazole, lansoprazole and pantoprazole with P‐glycoprotein. Naunyn Schmiedebergs Arch Pharmacol. 2001;364(6):551‐557. [DOI] [PubMed] [Google Scholar]
- 48. Moore N, Hall G, Sturkenboom M, Mann R, Lagnaoui R, Begaud B. Biases affecting the proportional reporting ratio (PPR) in spontaneous reports pharmacovigilance databases: the example of sertindole. Pharmacoepidemiol Drug Saf. 2003;12(4):271‐281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Top 10 reported comedications
TABLE S2 Number of reported ADRs per SOC
TABLE S3 Overview of all reported cases to Lareb.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


