Abstract
Background
ECMO is an established supportive adjunct for patients with severe, refractory ARDS from viral pneumonia. However, the exact role and timing of ECMO for COVID-19 patients remains unclear.
Methods
We conducted a retrospective comparison of the first 32 patients with COVID-19-associated ARDS to the last 28 patients with influenza-associated ARDS placed on V-V ECMO. We compared patient factors between the two cohorts and used survival analysis to compare the hazard of mortality over sixty days post-cannulation.
Results
COVID-19 patients were older (mean 47.8 vs. 41.2 years, p = 0.033), had more ventilator days before cannulation (mean 4.5 vs. 1.5 days, p < 0.001). Crude in-hospital mortality was significantly higher in the COVID-19 cohort at 65.6% (n = 21/32) versus 36.3% (n = 11/28, p = 0.041). The adjusted hazard ratio over sixty days for COVID-19 patients was 2.81 (95% CI 1.07, 7.35) after adjusting for age, race, ECMO-associated organ failure, and Charlson Comorbidity Index.
Conclusion
ECMO has a role in severe ARDS associated with COVID-19 but providers should carefully weigh patient factors when utilizing this scarce resource in favor of influenza pneumonia.
Keywords: ECMO, COVID-19, Influenza, Critical care
Introduction
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) is an established supportive adjunct in managing patients with severe acute respiratory distress syndrome (ARDS) unresponsive to conventional and maximal ventilatory strategies.1, 2, 3, 4, 5 Clinical outcomes following V-V ECMO for specific respiratory pathologies (such as viral pneumonia, aspiration pneumonitis, trauma, etc.) and the optimal timing for initiation of ECMO in the critically ill has been well described.6, 7, 8 The best reported clinical outcomes for ECMO are associated with younger age, fewer medical comorbidities, and less than seven ventilator days before cannulation.9
The 2009 H1N1 influenza pandemic was a catalyst for the increasing use of V-V ECMO in ARDS.10 The reported mortality for all critically ill patients with influenza pneumonia is variable and reported to be between 8 and 50%, depending on the patient population, hospital resources, severity of illness, and the viral strain.11, 12, 13 In contrast, several studies have reported an in-hospital mortality as low as 8–21% for patients with H1N1 influenza-associated severe ARDS managed with ECMO, suggesting that ECMO may impart a greater survival benefit in patients with H1N1 influenza infection.6, 7, 8
Unfortunately, ECMO providers are now facing their second pandemic in a decade with COVID-19, where clinical outcomes for critically ill patients are even worse, with early results from China reporting a mortality as high as 60%.14 Initial reports from China on the use of V-V ECMO in COVID-19 demonstrated a mortality as high as 83%, although the studies were small, limiting the ability to draw definitive conclusions.14 , 15 Recent data from the Extracorporeal Life Support Organization (ELSO) registry reports in-hospital mortality for all COVID-19 patients treated with ECMO at less than 40%.16 Unfortunately, the ideal candidate for ECMO and the optimal timing for cannulation in COVID-19 positive patients with severe ARDS is not yet well characterized. This may be attributable, in part, to the older population affected by COVID-19, the associated coagulopathy and other systemic complications observed in COVID-19, and the higher incidence of concomitant severe medical comorbidities in the critically ill COVID-19 patient cohort.17
Currently, there are very little data comparing the clinical characteristics and outcomes of critically ill patients with severe ARDS secondary to COVID-19 to patients with severe ARDS secondary to influenza, with even fewer data comparing the use of V-V ECMO for severe ARDS in these two populations.18, 19, 20, 21 This upcoming winter may compel ECMO providers to triage patients with severe ARDS that will likely benefit from V-V ECMO, given the confluence of increased influenza prevalence amidst the current COVID-19 surge across the United States and Europe. Consequently, we sought to compare our institutional experience between COVID-19 and influenza patients with severe ARDS placed on V-V ECMO, highlighting essential differences in patient characteristics and clinical outcomes.
Materials and methods
We performed a retrospective study comparing patient characteristics and outcomes in patients with severe ARDS secondary to polymerase chain reaction (PCR) confirmed influenza versus reverse-transcriptase polymerase chain reaction (RT-PCR) confirmed COVID-19 infection managed with V-V ECMO at the University of North Carolina Medical Center at Chapel Hill (UNCMC).
UNCMC is a 900-bed quaternary academic medical center with a high-volume ECMO program caring for neonatal, pediatric, and adult patients with cardiac and respiratory failure with an average of 60 adult cannulations each year. The ECMO team consults on patients in the medical intensive care unit (MICU) with severe ARDS and evidence of refractory hypoxia or hypercarbia. If the ECMO provider assesses that the patient is a suitable candidate for ECMO, an ECMO attending (surgical critical care surgeon) cannulates the patient, and the ECMO team subsequently assumes primary care of the patient.
We compared the first 32 adult COVID-19 patients with severe ARDS placed on V-V ECMO with the last 28 adult influenza patients with severe ARDS placed on V-V ECMO. All COVID-19 patients were cannulated between March 2020 and September 2020 while influenza patients were cannulated between November 2013 and March 2020. Per protocol, all cannulated patients were placed on rest ventilatory settings to decrease ventilator-associated lung injury (pressure control of 20 cm H2O, rate of 10 breaths per minute, positive end-expiratory pressure of 10 cm H2O, fraction of inspired oxygen of 40%). The ECMO team then monitored tidal volumes to track improvement in pulmonary compliance over time. All patients in both groups were therapeutically anticoagulated while on ECMO with the same protocol.
Collected variables from the review of the medical records include patient demographics, medical comorbidities, vital signs, and the date of the first positive PCR test or RT-PCR test for influenza and SARS-CoV-2, respectively. We also collected time-dependent variables such as the duration of symptoms, duration of use of non-invasive positive pressure ventilation (NIPPV), ventilator days, and days on ECMO. In addition, we collected data on the presence of barotrauma at the time of ECMO cannulation (defined as pneumothorax, pneumomediastinum, or subcutaneous emphysema on chest x-ray imaging not from another cause), pre-cannulation arterial blood gas values, PaO2/FIO2 (P/F) ratio, and ventilator settings, the Murray score, and the use of prone positioning and paralytics before cannulation.
The primary outcome was survival to hospital discharge. Secondary outcomes included ECMO related complications such as bleeding events, air emergencies, and organ failure (liver, kidney, cardiac). We defined minor hemorrhagic complications as bleeding episodes managed at the bedside from mucosal surfaces, around the cannula or other lines, gastrointestinal bleeding that did not require intervention, or Foley catheter-associated bleeding. We defined major hemorrhagic complications as bleeding episodes requiring interventional radiology, endoscopy, operative exploration, E-aminocaproic acid infusion, or initiation of massive transfusion protocol.
We examined the cohort characteristics by assessing the distribution of variables between COVID-19 patients and influenza patients. We examined categorical and continuous variables by calculating the frequencies of categorical variables and the distribution of continuous variables. We used bivariate analysis to compare variables across the study cohorts and identify potential confounders of the relationship between the viral etiology of ARDS and mortality. We used Pearson’s correlation for the categorical variables and 2-sample t-tests or one-way analysis of variance for continuous variables. Medians of non-normally distributed continuous variables were tested using a Kruskal-Wallis test. We reported means with standard deviations (SD) and medians with interquartile ranges (IQR).
We utilized survival analysis to estimate the hazard ratio for in-hospital mortality through sixty days for ECMO patients with COVID-19 infection compared to influenza infection. We initially plotted an unadjusted Kaplan-Meier curve comparing the two cohorts and utilized a Cox proportional regression model to estimate the unadjusted mortality hazard ratio for patients with COVID-19 infection. We then repeated the Cox proportional regression model adjusting for potential confounders. A potential confounder was included in the model if it substantially affected the adjusted hazard ratio. Among potential confounders, we assessed and corrected for violation of the proportional hazard assumption. The model used 60-days as the time frame to capture all deaths in the cohorts and account for ECMO duration. An adjusted hazard ratio for patients with COVID-19 infection and 95% confidence interval compared to those with influenza infection are reported. In addition, Kaplan-Meier curve adjusting for the same potential confounders is reported.
The University of North Carolina Institutional Review Board approved this study, and the need for consent was waived. We used Stata/SE 16.1 (Stata- Corp LP, College Station, TX) for all statistical analysis.
Results
Overall, we included 32 patients with confirmed COVID-19 and 28 patients with confirmed influenza pneumonia in the analysis. Table 1 summarizes patient characteristics, including demographic data and clinical indicators at the time of cannulation. Notably, COVID-19 patients were significantly older, with a mean age of 47.8 years (SD 10.3) compared to 41.2 years (SD 12.8, p = 0.033) among influenza patients. There was a male preponderance with a similar BMI between the two cohorts. There were significantly more Hispanic patients in the COVID-19 group at 53.1% (n = 17) compared to 7.1% (n = 2, p < 0.001), and fewer Caucasians among COVID-19 patients at 28.1% (n = 9) compared to 64.3% (n = 18). The median Charlson Comorbidity Index (CCI) was similar in both groups (1 in COVID-19 versus 0 in influenza, p = 0.4), but the prevalence of diabetes was much more common among COVID-19 patients, 50.0% (n = 16) versus 10.7% (n = 3, p = 0.001) in the influenza cohort.
Table 1.
A comparison of patient factors between those who were placed on VV ECMO for ARDS secondary to COVID-19 infection versus those with ARDS secondary to confirmed influenza infection.
| Patients with COVID-19 (n = 32) | Patients with Influenza (n = 28) | p value | |
|---|---|---|---|
| Patient Factors | |||
| Age (years) | |||
| Mean (SD) | 47.8 (10.3) | 41.2 (12.8) | 0.033 |
| Sex: n (%) | |||
| Male | 25 (78.1) | 16 (57.1) | 0.08 |
| Female | 7 (21.9) | 12 (42.9) | |
| BMI | |||
| Mean (SD) | 35.1 (7.8) | 37.1 (10.1) | 0.4 |
| Race: n (%) | |||
| Latino | 17 (53.1) | 2 (7.1) | <0.001 |
| African-American | 6 (18.8) | 5 (17.9) | |
| Caucasian | 9 (28.1) | 18 (64.3) | |
| Other | 0 (0.0) | 3 (10.7) | |
| Diabetic? | |||
| Yes: n (%) | 16 (50.0) | 3 (10.7) | 0.001 |
| Charleson Comorbidity Index: | |||
| Median (IQR) | 1 (0–2) | 0 (0–1.5) | 0.4 |
| Clinical Status | |||
| Number of Days from Symptom Onset to Cannulation | |||
| Mean (SD) | 16.2 (8.3) | 8.5 (4.4) | <0.001 |
| Use of Non-invasive Positive-Pressure (NIPPV) Ventilation Prior to Cannulation | |||
| Yes: n (%) | 25 (78.1) | 14 (50.0) | 0.023 |
| Mean Days on Non-Invasive Ventilation: Mean (SD) | 3.3 (4.1) | 0.78 (1.8) | 0.004 |
| Proned Prior to Cannulation? | |||
| Yes: n (%) | 26 (81.3) | 1 (3.6) | <0.001 |
| Number of Days from ICU Admission to Cannulation | |||
| Mean (SD) | 8.7 (7.2) | 1.8 (2.2) | <0.001 |
| Number of Days of Intubation Prior to Cannulation | |||
| Mean Days (SD) | 4.5 (4.3) | 1.5 (1.7) | <0.001 |
| P:F Ratio at Cannulation | |||
| Mean (SD) | 69.3 (19.9) | 58.5 (33.8) | 0.1 |
| Murray Score at Cannulation | |||
| Score 0 | 0 (0.0) | 0 (0.0) | 1.0 |
| Score 0.1–2.5 | 0 (0.0) | 0 (0.0) | |
| Score > 2.5 | 32 (100.0) | 32 (100.0) | |
|
Labs at Cannulation: Mean (SD) |
|||
| Serum pH | 7.31 (0.11) | 7.23 (0.12) | 0.006 |
| Fibrinogen | 695 (249) | 419 (207) | <0.001 |
| D-dimer | 3628 (5277) | 3503 (5287) | 0.9 |
COVID-19 patients had a significantly longer duration of symptoms before ECMO cannulation than influenza patients with a mean of 16.2 days (SD 8.3) compared to 8.5 days (SD 4.4, p < 0.001) (Table 1). Also, COVID-19 patients had a longer ICU admission before cannulation at 8.7 days (SD 7.2) versus 1.8 days (SD 2.2, p < 0.001). The use of non-invasive positive-pressure ventilation (NIPPV) was common in both groups at 78.1% (n = 25) among COVID-19 patients and 50% (n = 14, p = 0.023) among influenza patients. However, the use of prone positioning was much more common in COVID-19 patients at 81.3% (n = 26) versus only 3.6% (n = 1, p < 0.001) of influenza patients. The average number of ventilator-days before cannulation was 4.5 days (SD 4.3) among COVID-19 patients and 1.5 days (SD 1.7, p < 0.001) for influenza patients. The mean Murray Score in both cohorts was 2.5 at the time of cannulation with a mean P:F ratio of 69.3 (SD 19.9) among COVID-19 patients and 58.5 (SD 33.8, p = 0.1) among influenza patients, consistent with severe ARDS in both groups. D-dimer levels at cannulation were equally high in both groups, but fibrinogen levels were higher in the COVID-19 group, and pH was lower in the influenza group (Table 1).
There was a significant difference in V-V ECMO duration between COVID-19 and influenza patients with a mean of 12.4 days (SD 5.7) compared to 7.7 days (SD 5.1, p = 0.002), respectively. (Table 2 ) COVID-19 patients were more likely to require a circuit change due to oxygenator failure, with 46.9% (n = 15) of COVID-19 patients requiring circuit change versus 21.4% (n = 6, p = 0.039) among influenza patients. All oxygenator failures were caused by micro-thrombi in the circuit. There was a statistically significant difference in the incidence of organ failure while on ECMO between the two cohorts. 43.8% (n = 14) of COVID-19 patients developed organ failure while cannulated and 31.3% (n = 10) required continuous renal replacement therapy (CRRT). In contrast, 71.4% (n = 20, p = 0.024) of influenza patients developed organ failure with 67.9% (n = 19, p = 0.005) requiring CRRT. Minor bleeding complications occurred frequently for both cohorts at 68.8% (n = 22) among COVID-19 patients and 67.9% (n = 19, p = 0.9) in influenza patients. Major bleeding episodes were similar between the two groups with three (9.4%) major bleeding episodes among COVID-19 patients and four among influenza patients (14.3%, p = 0.5).
Table 2.
A comparison of circuit outcomes for patients who were placed on VV ECMO for ARDS, stratified by COVID-19 infection versus influenza infection.
| Patients Infected with COVID-19 (n = 32) | Patients Infected with Influenza (n = 28) |
p value | |
|---|---|---|---|
| Number of Days on Circuit | |||
| Mean (SD) | 12.4 (5.7) | 7.7 (5.1) | 0.002 |
| ECMO Circuit Changes Required for Oxygenator Failure | |||
| Required Change: n (%) | 15 (46.9) | 6 (21.4) | 0.039 |
| Required > 1 circuit change: n (%) | 5 (15.6) | 2 (7.1) | 0.1 |
| Developed Organ Failure on Circuit | |||
| Yes: n (%) | 14 (43.8) | 20 (71.4) | 0.031 |
| Placed on CRRT for Renal Failure: n (%) | 10 (31.3) | 19 (67.9) | 0.005 |
| Air Emergency While on Circuit | |||
| Yes: n (%) | 4 (12.5) | 1 (3.6) | 0.2 |
| Bleeding Complications on Circuit | |||
| Minor: n (%) | 22 (68.8) | 19 (67.9) | 0.9 |
| Major: n (%) | 3 (9.4) | 4 (14.3) | 0.5 |
Overall crude 60-day in-hospital mortality was 53.3% (n = 32/60) for all patients. We performed a bivariate analysis to assess for factors associated with mortality across both cohorts, summarized in Table 3 . There was a significant difference in the age of survivors versus non-survivors, with a mean age of 38.6 years (SD 11.2) in survivors and 50.1 years (SD 9.7, p < 0.001) in non-survivors. Sex, BMI, and race were not significantly associated with mortality on bivariate analysis. CCI was slightly higher among those who died but was still low with a median of only 1 (IQR 0–2.5, p = 0.002). There was no significant difference in pre-ECMO ventilator days or days on NIPPV before intubation nor the pre-cannulation P:F ratio between survivors and non-survivors. The mean number of days on ECMO in survivors and non-survivors were 7.3 days (3.2) and 12.8 days (SD 6.6, p < 0.001), respectively.
Table 3.
A comparison of patient factors between those who survived and those died after VV-ECMO cannulation.
| Patients who Survived (n = 28) | Patients who Died (n = 32) | p value | |
|---|---|---|---|
| Patient Factors | |||
| Age (years) | |||
| Mean (SD) | 38.6 (11.2) | 50.1 (9.7) | <0.001 |
| Sex: n (%) | |||
| Male | 17 (60.7) | 24 (75.0) | 0.2 |
| Female | 11 (39.3) | 8 (25.0) | |
| BMI | |||
| Mean (SD) | 37.5 (10.0) | 34.8 (7.8) | 0.2 |
| Race: n (%) | |||
| Latino | 10 (35.7) | 9 (28.1) | 0.1 |
| African-American | 8 (28.6) | 3 (9.4) | |
| Caucasian | 9 (32.1) | 18 (56.3) | |
| Other | 1 (3.6) | 2 (6.3) | |
| Charleson Comorbidity Index: | |||
| Median (IQR) | 0 (0–1) | 1 (0–2.5) | 0.002 |
| Clinical Status | |||
| Number of Days of Intubation Prior to Cannulation | |||
| Mean Days (SD) | 2.2 (3.2) | 3.9 (3.8) | 0.08 |
| Number of Days of Non-Invasive Positive Pressure Ventilation + Intubation Prior to Cannulation | |||
| Mean Days (SD) | 5.4 (9.1) | 9.1 (8.5) | 0.1 |
| Number of Days on ECMO Circuit | |||
| Mean (SD) | 7.3 (3.2) | 12.8 (6.6) | <0.001 |
| P:F Ratio at Cannulation | |||
| Mean (SD) | 67.0 (34.2) | 61.7 (21.5) | 0.5 |
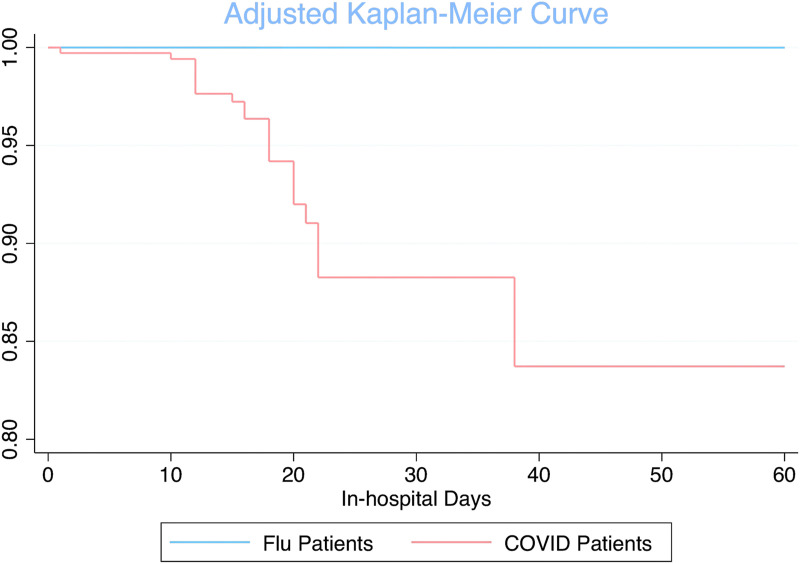
Crude in-hospital mortality was significantly higher in the COVID-19 cohort at 65.6% (n = 21/32) compared to 36.3% (n = 11/28, p = 0.041) for the influenza cohort. We also conducted a survival analysis comparing in-hospital mortality between COVID-19 and influenza patients. Upon Cox regression modeling, the unadjusted hazard ratio over sixty days for COVID-19 patients compared to influenza patients was 1.87 (95% CI 0.90, 3.91). After fitting the model with potential confounders, the adjusted hazard ratio over sixty days for COVID-19 patients compared to influenza patients was 2.81 (95% CI 1.07, 7.35) after controlling for age, race, organ failure while on ECMO, and CCI. Fig. 1 .
Fig. 1.
Adjusted Kaplan-Meier curve comparing patients placed on V-V ECMO with severe COVID-19 infection to patients with severe influenza infection over sixty-days post-cannulation.
Discussion
To our knowledge, this is one of the first comparisons of patients treated with V-V ECMO for severe ARDS secondary to COVID-19 versus influenza pneumonia in the United States. Our study demonstrates that COVID-19 patients were older, had a longer time to cannulation from symptom onset and ICU admission, and required ECMO for a more extended period of time. Ultimately, COVID-19 patients had an almost threefold increased hazard of in-hospital death either during or after ECMO management after adjusting for confounding variables.
The increased mortality for COVID-19 patients raises critical questions about the differences in pathophysiology between severe COVID-19 and severe influenza infection. The descriptions of the contrasts in clinical presentation between the two viruses in the literature are consistent with findings from our study.18 , 22 The older age seen in critically ill COVID-19 patients compared to influenza patients may explain some of the differences in mortality between the two cohorts. Previous data confirm age as a significant risk factor for death after V-V ECMO for respiratory illnesses. Previous studies have also linked associated comorbidities such as obesity and diabetes to critical illness in COVID-19. Our study found that the incidence of diabetes was higher in COVID-19 with a similar BMI between the two groups, however neither factor had a meaningful correlation with mortality.23 This suggests that obesity is an independent risk factor for the development of severe illness in all types of viral pneumonia, not just COVID-19, but its relationship to overall survival needs to be further delineated.24
Despite some of these demographic differences, COVID-19 and influenza patients had similar markers of severe disease. All patients had evidence of severe ARDS based on average PaO2:FiO2 and Murray Scores, demonstrating that the two cohorts were similarly critically ill at the time of ECMO cannulation. However, COVID-19 patients had a significantly longer illness prior to cannulation and a longer ECMO course. While the natural history of severe influenza infection is well established, the clinical course of COVID-19 is not fully understood.
Other comparison studies between these two viral etiologies have found a slower disease progression and a longer duration of critical illness with COVID-19 compared to influenza. These are known factors that could impact the timing of cannulation, duration of ECMO therapy, and the ultimate clinical outcome.19 , 21 Additionally, ECMO may be preferentially delayed for COVID-19 patients as compared to influenza patients, given the frequent use of NIPPV to avoid mechanical ventilation and the extensive use of prone positioning for COVID-19 patients, as demonstrated in our data. Though these treatments may help some patients avoid more invasive therapies, it may also harm a subset of COVID-19 patients who may benefit from early ECMO cannulation. Earlier cannulation may help reduce toxic levels of oxygen or high peak inspiratory pressures from prolonged NIPPV or mechanical ventilation, potentially preventing additional lung damage from barotrauma and ventilator-induced lung injury.25
While factors such as demographic differences and delays to cannulation may contribute to the increased mortality identified among COVID-19 patients, we adjusted for these variables in our survival analysis. Despite this adjustment, COVID-19 still conferred a much higher hazard risk of death over sixty days after cannulation when compared to influenza, suggesting that severe ARDS from COVID-19 is unique. While the exact pathogenesis of COVID-19 remains unclear, speculation has focused on differences in alveolar damage due to inflammation and the higher incidence of microscopic thrombosis in COVID-19 as compared to other viral infections.26 , 27 The number of circuit changes required by our COVID-19 cohort provides some supporting evidence of this phenomenon. This group had a significantly higher number of circuit changes due to oxygenator thrombosis and these oxygenator failures occurred despite therapeutic anticoagulation and even occurred multiple times for some patients. In addition, data have suggested endotheliopathy as a possible etiology of the observed hypercoagulability associated with COVID-19, with evidence that the virus can invade the endothelium leading to widespread vascular damage that is not limited to the lung.28 Goshua et al. correlated the degree of endotheliopathy with an increase in the risk of critical illness and death.29 Another postulated hypotheses is related to the severe inflammatory response detected in COVID-19 compared to other viral pneumonias. The significance of an exaggerated inflammatory milieu is unclear and may be less impactful on mortality than coagulopathy.30 , 31 Further investigations into possible genetic factors associated with severe COVID-19 infection susceptibility may also bring further insights into the pathogenesis.32 In summary, these systemic manifestations of COVID-19 likely play a role in distinguishing it from influenza pneumonia.
Unfortunately, few studies exist that directly compare severe ARDS in COVID-19 to influenza and the available data is equivocal. Cobb et al. published a comparison of critically ill hospitalized COVID-19 and influenza patients managed without ECMO that demonstrated similar demographic differences between the two cohorts and a comparable increase in mortality for COVID-19 patients with an adjusted relative risk of death of 2.13, similar to our study.19 On the other hand, early data of hospitalized patients from China showed a decrease in the adjusted risk of mortality for patients with COVID-19 compared to similarly ill patients with H1N1, although this report came very early in the pandemic.18 Two recently published small institutional studies comparing patients placed on V-V ECMO for COVID-19 and influenza also reported varying results. In contrast to our study, Jäckel et al. reported similar cohorts from Germany in baseline characteristics and did not find a significant mortality difference between those with influenza and COVID-19, although patients in the COVID-19 group were on ECMO significantly longer.20 A similar study from France by Cousin et al. also showed that COVID-19 patients were cannulated later in their clinical course than influenza patients but did not show a higher mortality risk for COVID-19 patients.21
The potential causes of the significant mortality difference between influenza and COVID-19 patients at our center are likely multi-factorial. The relative difference between our extensive experience treating influenza patients compared with managing patients with COVID-19 may be one factor. Recent data from the United Kingdom and the United States has shown a decline in COVID-19 associated mortality over time for hospitalized patients.33 , 34 This may reflect that treatment of COVID-19 has improved as providers have gained more experience treating the novel aspects of the virus along with the development of new COVID-19 therapies. Over time, ECMO-related outcomes may also improve for patients with severe COVID-19. On the other hand, our group’s past experience with influenza also provided valuable lessons when facing the challenges of a new pandemic. Our expertise with ECMO and influenza likely informed some aspects of ECMO management for COVID-19, which may have improved outcomes for some patients. We also acknowledge that our in-hospital mortality for COVID-19 patients on ECMO is higher than that currently reported by ELSO and several other single-center studies.16 , 20 , 21 , 35 , 36 However, unlike many centers, we did not impose strict absolute contraindications based on age or pre-ECMO duration of mechanical ventilation when selecting COVID-19 patients appropriate for ECMO therapy. However, these variables were significantly more common in the COVID-19 group and are associated with worse outcomes.37 , 38 ECMO is an expensive and limited resource even in ECMO centers, necessitating a thoughtful patient selection approach. The patient selection process will only become more difficult as the number of critically ill patients increases. As we approach the winter months, potentially managing both influenza and COVID-19 patients, we need more data to help ECMO providers predict which patients are most likely to benefit from V-V ECMO for severe ARDS associated with COVID-19 in order to optimize the use of this valuable resource.
Our study is limited due to the retrospective and single institution methodology. The timeline for influenza patients stretched over a long period of time but the primary clinician team remained constant throughout the study period. Furthermore, it may be underpowered to identify some significant differences between patients with severe ARDS requiring ECMO for COVID-19 and influenza. However, institutional experiences from high volume centers remain vitally important. In addition, despite relatively small numbers, we were able to identify important risk factors for mortality and make meaningful comparisons between patients with influenza and those with COVID-19.
Conclusion
In this study, we show an increased adjusted hazard for mortality in patients with severe ARDS secondary to COVID-19 virus as compared to influenza following V-V ECMO. Providers should carefully weigh a number of patient factors such as age, medical comorbidities, and the duration of illness when utilizing this scarce resource in favor of influenza pneumonia. Further studies are needed to define best practices of critical care management in COVID-19 patients, including the use of V-V ECMO.
Author contributions
LR, JG, ER, TR, AC, AS, DJ collected data, analyzed data, wrote and edited the manuscript.
Declaration of competing interest
None by any author.
Acknowledgements
No funding was used for this project.
References
- 1.Hardin C., Hibbert K. ECMO for severe ARDS. NEJM. 2018;378(21):2032. doi: 10.1056/NEJMe1802676. [DOI] [PubMed] [Google Scholar]
- 2.Peek G.J., Clemens F., Elbourne D., et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6(1):1–13. doi: 10.1186/1472-6963-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karagiannidis C., Brodie D., Strassmann S., et al. Extracorporeal membrane oxygenation: evolving epidemiology and mortality. Intensive Care Med. 2016;42(5):889–896. doi: 10.1007/s00134-016-4273-z. [DOI] [PubMed] [Google Scholar]
- 4.Combes A., Hajage D., Capellier G., et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. NEJM. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 5.Thiagarajan R.R., Barbaro R.P., Rycus P.T., et al. Extracorporeal life support organization registry international report 2016. Am Soc Artif Intern Organs J. 2017;63(1):60–67. doi: 10.1097/MAT.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 6.Davies A.R., Jones D., Bailey M., et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. J Am Med Assoc. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 7.Buchner J., Mazzeffi M., Kon Z., et al. Single-center experience with venovenous ECMO for influenza-related ARDS. J Cardiothorac Vasc Anesth. 2018;32(3):1154–1159. doi: 10.1053/j.jvca.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Zangrillo A., Biondi-Zoccai G., Landoni G., et al. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit Care. 2013;17(1):1–8. doi: 10.1186/cc12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt M., Bailey M., Sheldrake J., et al. Predicting survival after ECMO for severe acute respiratory failure: the Respiratory ECMO Survival Prediction (RESP) Score. Am J Respir Crit Care Med. 2014;189(11):1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 10.Maurice AMdS., Bridges B.C., Rycus P.T., et al. Global trends in ECMO use and survival of patients with influenza associated illness. Pediatr Crit Care Med. 2016;17(9):876. doi: 10.1097/PCC.0000000000000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan S.J., Jacobson R.M., Dowdle W.R., et al., editors. H1N1 Influenza. Mayo Clinic Proceedings. Elsevier; 2009. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar A., Zarychanski R., Pinto R., et al. Critically ill patients with 2009 influenza A (H1N1) infection in Canada. J Am Med Assoc. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 13.Abaziou T., Delmas C., Vardon Bounes F., et al. Outcome of critically ill patients with influenza infection: a retrospective study. Infect Dis Res Treat. 2020;13 doi: 10.1177/1178633720904081. 1178633720904081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respiratory Med. 2020 May;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. Epub 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Guo Z., Li B., et al. Extracorporeal membrane oxygenation for coronavirus disease 2019 in Shanghai, China. Am Soc Artif Intern Organs J. 2020;66(5):475. doi: 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barbaro R.P., MacLaren G., Boonstra P.S., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covid T.C., Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)-United States, february 12-march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang X., Du R., Wang R., et al. Comparison of hospitalized patients with acute respiratory distress syndrome caused by covid-19 and H1N1. Chest. 2020 Jul;158(1):195–205. doi: 10.1016/j.chest.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cobb N.L., Sathe N.A., Duan K.I., et al. Comparison of clinical features and outcomes in critically ill patients hospitalized with COVID-19 versus influenza. Annals Am Thorac Soc. 2020 Nov 13 doi: 10.1513/AnnalsATS.202007-805OC. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jäckel M., Rilinger J., Lang C.N., et al. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza–a single-center registry study. Artif Organs. 2020 Nov 14 doi: 10.1111/aor.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cousin N., Bourel C., Carpentier D., et al. SARS-CoV-2 versus influenza associated acute respiratory distress syndrome requiring veno-venous extracorporeal membrane oxygenation support. Am Soc Artif Intern Organs J. 2020 Oct 13 doi: 10.1097/MAT.0000000000001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho F.K., Celis-Morales C.A., Gray S.R., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: results from a UK Biobank prospective cohort study. BMJ Open. 2020;10(11) doi: 10.1136/bmjopen-2020-040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Czernichow S., Beeker N., Rives-Lange C., et al. Obesity doubles mortality in patients hospitalized for SARS-CoV-2 in Paris hospitals, France: a cohort study on 5795 patients. Obesity. 2020 Dec;28(12):2282–2289. doi: 10.1002/oby.23014. Epub 2020 Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancuso P. Obesity and respiratory infections: does excess adiposity weigh down host defense? Pulm Pharmacol Therapeut. 2013;26(4):412–419. doi: 10.1016/j.pupt.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. NEJM. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 26.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. NEJM. 2020 Jul 9;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Middleton E.A., He X.-Y., Denorme F., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colmenero I., Santonja C., Alonso-Riaño M., et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7(8):e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha P., Matthay M.A., Calfee C.S. Is a “cytokine storm” relevant to COVID-19? JAMA Int Med. 2020;180(9):1152–1154. doi: 10.1001/jamainternmed.2020.3313. [DOI] [PubMed] [Google Scholar]
- 31.Mangalmurti N., Hunter C.A. Cytokine storms: understanding COVID-19. Immunity. 2020 Jul 14;53(1):19–25. doi: 10.1016/j.immuni.2020.06.017. Epub 2020 Jun 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Group S.C.-G. Genomewide association study of severe Covid-19 with respiratory failure. NEJM. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asch D.A., Sheils N.E., Islam M.N., et al. Variation in US hospital mortality rates for patients admitted with COVID-19 during the first 6 Months of the pandemic. JAMA Intern Med. 2020 Dec 22 doi: 10.1001/jamainternmed.2020.8193. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dennis J.M., McGovern A.P., Vollmer S.J., Mateen B.A. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Critical Care Med. 2021 Feb;49(2):209. doi: 10.1097/CCM.0000000000004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaefi S., Brenner S.K., Gupta S., et al. Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med. 2021 Feb 2:1–4. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biancari F., Mariscalco G., Dalén M., et al. Six-month survival after extracorporeal membrane oxygenation for severe COVID-19. J Cardiothorac Vasc Anesth. 2021 Jan 19;S1053-0770(21):00062–00068. doi: 10.1053/j.jvca.2021.01.027. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopal K., Keller S.P., Akkanti B., et al. Advanced pulmonary and cardiac support of COVID-19 patients: emerging recommendations from ASAIO—a living working document. Circulation: Heart Fail. 2020;13(5) doi: 10.1161/CIRCHEARTFAILURE.120.007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osho A.A., Moonsamy P., Hibbert K.A., et al. Veno-venous extracorporeal membrane oxygenation for respiratory failure in COVID-19 patients: early experience from a major academic medical center in North America. Ann Surg. 2020 Aug;272(2):e75–e78. doi: 10.1097/SLA.0000000000004084. [DOI] [PMC free article] [PubMed] [Google Scholar]