Summary:
CD19 chimeric antigen receptor (CAR) T cell immunotherapy has demonstrated dramatic results for the treatment of B cell malignancies such as chronic lymphocytic leukemia (CLL). As T cell defects are common in patients with CLL, we compared the T cells from these patients with healthy donors (HDs), and subsequently the CD19 CAR T cells produced from patients and HDs. Despite initial differences when comparing the phenotype of circulating T cells in patients with CLL and HDs, the CD19 CAR T cells manufactured from patients’ or HDs’ cells showed a similar phenotype (effector memory or terminally differentiated), both were specifically activated by and killed CD19+ target cells, and secreted cytokines (ie, IL-2, TNF, and IFN-γ). The frequency of CD19 CAR T cells producing IFN-γ was significantly higher in cells produced from patients as compared with those produced from HDs. Furthermore, our data showed that the polyfunctional profile of CD19 CAR+ T cells was differently modulated by CD19+ K562 cells and autologous B cells. The increased IFN-γ production by CD19 CAR T cells produced from patients with CLL after in vitro stimulation, may if this is also the case in vivo, contribute to a higher risk of a cytokine release syndrome in patients. The different impact by CD19+ target cells on the polyfunctional profile of CD19 CAR T cells in vitro underlines the importance of the choice of CD19+ target cells when assessing CD19 CAR T cells functions.
Keywords: chimeric antigen receptor, CD19, chronic lymphocytic leukemia, T cell
Chimeric antigen receptors (CARs) are synthetic receptors composed of an extracellular recognition domain, usually a single chain variable fragment derived from an antibody, fused to the CD3ζ chain. Second generation CARs that include an intracellular costimulatory domain (eg, CD28) have shown superior effector functions and persistence,1 and demonstrated potent antitumor efficcacy in preclinical and clinical studies. CAR T cells represent one of the most recent promising advances in immunotherapy, best illustrated by the successes of CD19 CAR T cell therapy for the treatment of patients with B cell malignancies such as acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and B-cell lymphoma.2 Complete remission after CD19 CAR treatment in different trials has been reported in 70%–90% of patients with ALL, but the efficacy appears more limited in patients with CLL.3 CLL is the most common leukemia in western countries, characterized by the clonal proliferation and accumulation of mature B cells (usually CD5+) in the blood, lymph node, bone marrow, and spleen.4 Treatments options vary depending on the patient’s age, risk group, but chemotherapy consisting of fludarabine, cyclophosphamide, and rituximab is the standard of care for the initial treatment of eligible patients.5
The efficacy of CAR T cells therapy relies not only on CAR T cells’ antitumor functions, but also on their in vivo expansion and persistence. Therefore, the identification of the optimal cell composition that includes those qualities remains a crucial area of research. In the vast majority of CD19 CAR T cells trials, patients have received autologous derived CAR T cells. However, in patients with cancer such as CLL, T cells have been shown to display impaired functions and a more differentiated phenotype.6 Different studies have shown that CD19 CAR T cells generated from donor-derived allogeneic cells can mediate remission of malignancy without causing graft-versus-host disease.7 In some instances the manufacture of CAR T cells cannot be achieved using patients’ cells (eg, poor cell expansion8), and therefore it may be preferable to use readily available donor-derived or third-party donor-derived cells in order to ensure a reliable production of the most efficient CAR T cells. The advent of gene-editing may further allow the production of “off-the shelf” CAR T cells generated from healthy donors (HDs).9,10
T cells can be delineated into 4 canonical populations, naïve (TN) CD45RA+CCR7+, central memory (TCM) CD45RA− CCR7+, terminally differentiated (TEMRA) CD45RA+CCR7−, and effector memory (TEM) CD45RA−CCR7− T cells, each characterized by different effector functions and proliferative capacities.11 Human T cells with stem cell-like activities (TSCM) were recently discovered. TSCM cells co-express CD45RA and CCR7, display enhanced proliferative potential and are able to generate all memory and effector T cells subsets.12,13 CD19 CAR CD8+ TSCM cells were shown to display enhanced metabolic fitness and provide long-lasting antitumor effect in a preclinical model.14 An other preclinical study showed that the combination (at a 1:1 ratio) of CD8+ CD19 CAR T cells derived from TCM, and CD4+ CD19 CAR T cells derived from TN, had the most efficient antitumor effect in vivo.15 And more recently, 2 clinical trials enrolling patients with ALL have shown the feasibility of administrating CD19 CAR T cells with a defined CD4/CD8 composition.16,17 In this study, we characterized and compared for the first time CD19 CAR T cells produced from either patients with CLL or HDs. We show that despite initial differences in the phenotype of T cells between patients with CLL and HDs, the CD19 CAR T cells generated from patients and HDs displayed a similar phenotype. However, when CD19 CAR T cells were stimulated with CD19+ target cells, patients with CLL, as compared to HDs, showed an increased frequency of cells producing IFN-γ. Lastly, the polyfunctional profile of CD19 CAR T cells was different when stimulated in vitro with CD19+ K562 cells or autologous B cells.
MATERIALS AND METHODS
Donor Samples
Samples of heparinized blood were obtained from HDs and patients with CLL. The median age of the patients was 77 years (52–85 y). Ethical approvals were obtained from the Regional Ethical Review Board (2010/1478–32, 2010/1479–32). Peripheral blood mononuclear cells (PBMCs) were isolated using gradient centrifugation. The patients’ characteristics are shown in Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/JIT/A482).
Generation of CD19 CAR T Cells
The MSGV-FMC63–28Z PG13 packaging cell clone H3 producing the retroviral vector encoding the second generation anti-CD19 CAR with a CD28 costimulatory domain used in this study, was kindly provided by Prof. Steven Rosenberg (from the National Cancer Institute, Bethesda). The production of retroviral supernatant and CAR T-cell manufacturing were performed according to published methods.18 Briefly, On the first day of cell culture PBMCs were activated for 2 days with 50 ng/mL soluble OKT3 (Biolegend) in AIM-V medium (ThermoFisher Scientific, Gibco) supplemented with 5% human AB serum (Karolinska University Hospital) and IL-2 at 300 IU/mL (Proleukine, Novartis). On the third day of cell culture, retroviral transduction was performed in nontreated culture plates coated with 10 μg/mL RetroNectin (Takara). After addition of retroviral supernatants, plates were centrifuged at 2000g for 2 hours at 32°C. The supernatants were aspirated, and OKT3-activated PBMCs, washed and resuspended in fresh AIM-V medium supplemented with 5% human AB serum and IL-2 at 300 IU/mL, were added to the wells. The plates were centrifuged at 2000g for 2 hours at 32° C. Plates were afterwards kept overnight at 37°C and 5% CO2. The next day, PBMCs were removed from the plates, washed, resuspended in fresh AIM-V medium supplemented with 5% heat-inactivated human AB serum and IL-2 at 300 IU/mL at a concentration of 0.5×106 cells/mL and transferred to cell culture flasks. Cells were subsequently cultured at a concentration of 0.5–2×106 cells/mL in AIM-V medium supplemented with 5% heat-inactivated human AB serum and IL-2 at 300 IU/mL at 37°C and 5% CO2.
Cell Lines
K562 cells expressing either CD19 or NGFR were kindly provided by Dr Steven Feldman (from the National Cancer Institute, Bethesda). EBV-transformed B cells were established from HDs’ PBMCs using B95–8 cell supernatant. The cell lines were maintained in culture in RPMI (GE Healthcare Life Sciences, HyClone) supplemented with 10% heat-inactivated fetal bovine serum (ThermoFisher Scientific, HyClone), and 100 U/mL of Penicillin and 100 μg/mL Streptomycin (ThermoFisher Scientific, HyClone).
Phenotypic Analysis by Flow Cytometry
Immunophenotyping was performed using monoclonal antibodies directed against the following surface antigens: CD19 (HIB19 and SJ25-C1 clones), CD5 (UCHT2), CD3 (UCHT1), CD4 (RPA-T4), CD8 (SK1), CD45RA (HI100), CCR7 (150503), PD-1 (MIH4), PDL-1 (MIH1), PDL-2 (MIH18) (all from BD Biosciences), LAG-3 (REA351) and TIM-3 (F38–2E2) (Miltenyi Biotec). CD19 CAR+ T cells were detected using Biotin-SP-conjugated goat anti-mouse IgG, F (ab’)2 fragment specific antibody (#115–065-072), Mouse Gamma Globulin (#015–000-002) (both obtained from Jackson Immunoresearch), and Streptavidin-PE (Biolegend). Cells were analyzed using a FACSCanto flow cytometer (BD Biosciences), data analysis was performed using the FlowJo software (FlowJo, LLC) for the determination of frequencies and median fluorescence intensity (MFI).
Stimulation With CD19+ Target Cells and Intracellular Cytokine Staining (ICS)
CD19 CAR transduced T cells (1×106 cells) were incubated at an effector target ratio of 1:1 with either CD19+ K562, NGFR+ K562 or autologous B cells (CLL cells or EBV-transformed B cells), in AIM-V medium supplemented with 5% heat-inactivated human AB serum in presence of 10 μg/mL brefeldin A (Sigma-Aldrich), GolgiStop (BD Biosciences) and the anti-CD107a (BD Biosciences) antibody for 6 hours at 37°C and 5% CO2. After incubation, monoclonal antibodies directed against CD3 (SK7), CD4 (RPA-T4), and CD8 (SK1) (all from BD Biosciences) were used for cell surface staining, and after fixation and permeabilization with the BD Cytofix/Cytoperm kit (BD Biosciences), antibodies against IL-2 (MQ1–17H12) (Biolegend), TNF (Mab11) (eBioscience), IFN-γ (4S.B3), and IL-17 (N49–653) (BD Biosciences), or in other experiments granzyme B (GB11) (BD Biosciences) were used for intracellular staining. For maximal stimulation controls, PMA and ionomycin (Sigma-Aldrich) were used at 25 ng/mL and 1 μg/mL, respectively, in presence of brefeldin A at 10 μg/mL and GolgiStop for 6 hours at 37°C and 5% CO2. Cells were analyzed by flow cytometry as described above.
Restimulation Assays
One week after transduction, CD19 CAR T cells (1×106 cells) were stimulated (at an effector target ratio of 1:1), and restimulated 7 days later by the addition to the cultures of CD19+ K562 cells (previously irradiated at 55 Gy). After 14 days CD19 CAR T cells were restimulated for 6 hours as described above with CD19+ K562 cells for determination of CD107a expression and cytokine production (ICS).
Flow Cytometric Measurement of Cytotoxic Activity
CD19+ K562 and NGFR+ K562 cells were labelled using the CellTrace Violet dye (Molecular Probes) at a concentration fo 5 μM. K562 cells were then added at a ratio of 1:1 to CD19 CAR transduced T cells in AIM-V medium supplemented with 5% human AB serum and IL-2 at 300 IU/mL. After 24 hours, the cells were analyzed by flow cytometry as described above.
Statistical Analysis
Wilcoxon and Mann-Whitney tests were performed using the GraphPad Prism software (GraphPad Software). P-values <0.05 were considered to be statistically significant.
RESULTS
Increased Frequency of Differentiated T Cells in the Blood of Patients With CLL but No Difference in Expression of Exhaustion Markers
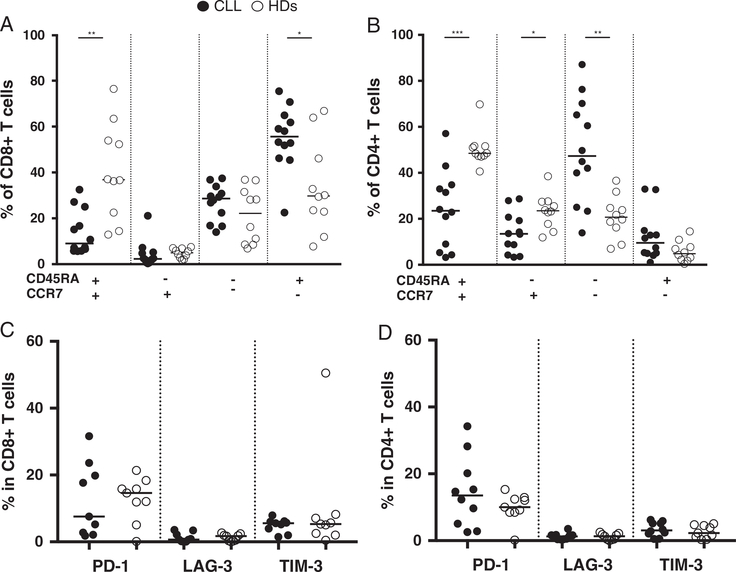
In patients with CLL, the large majority of circulating lymphocytes were CD19+ B cells (median frequency of 89.7%) (Supplementary Fig. 1A, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). CD3+ T cells median frequency was 3.7%. In addition to the lymphocyte gate (based on the side and forward scatter properties of the cells), CD19+ B cells with higher side and forward scatter were identified as blast cells. CD19+ lymphocytes and CD19+ blast cells coexpressed CD5, characteristic of CLL cells (Supplementary Fig. 1B, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). Patients with CLL and HDs showed a comparable CD4/CD8 T cell ratio with a median of 1.8 and 2, respectively. The size of the cohort we studied was small, but we could confirm previous findings by Palma et al6 that circulating T cells in patients with CLL, as compared with HDs, displayed a more diferentiated phenotype, with lower frequencies of TN/TSCM CD8+, TN/TSCM, and TCM CD4+ T cells, while the frequencies of TEMRA CD8+ and TEM CD4+ T cells were significantly higher (Figs. 1A, B).
FIGURE 1.
Comparison of circulating T cells’ phenotype in patients with chronic lymphocytic leukemia (CLL) and healthy donors (HDs). Frequencies of the different T cell subsets defined by CD45RA and CCR7 expression in (A) CD8+ and (B) CD4+ T cells (patients with CLL and HD n=12 and 10, respectively). Expression of exhaustion markers in (C) CD8+ T cells and (D) CD4+ T cells (CLL and HD n=10 and 9, respectively). Black circles represent patients with CLL, and open circles HDs. Mann-Whitney test, *P< 0.04. **P< 0.002. ***P= 0.0004.
The expression of exhaustion markers (ie, PD-1, LAG-3, TIM-3) on T cells was also analyzed. We did not detect significant differences between patients with CLL and HDs in the frequency of CD8+ or CD4+ T cells expressing PD-1+, LAG-3+ or TIM-3+ (Figs. 1C, D), with some T cells expressing either PD-1 or TIM-3 but with <1% (median) of T cells co-expressing 2 or 3 of the markers. Of note, a median of 13% of B cells in patients with CLL expressed PD-1 (data not shown). We did not detect expression of PD-L1 or PD-L2 on B cells neither in patients with CLL nor in HDs (data not shown).
CD19 CAR T Cells From Patients With CLL and HDs Display a Similar Phenotype
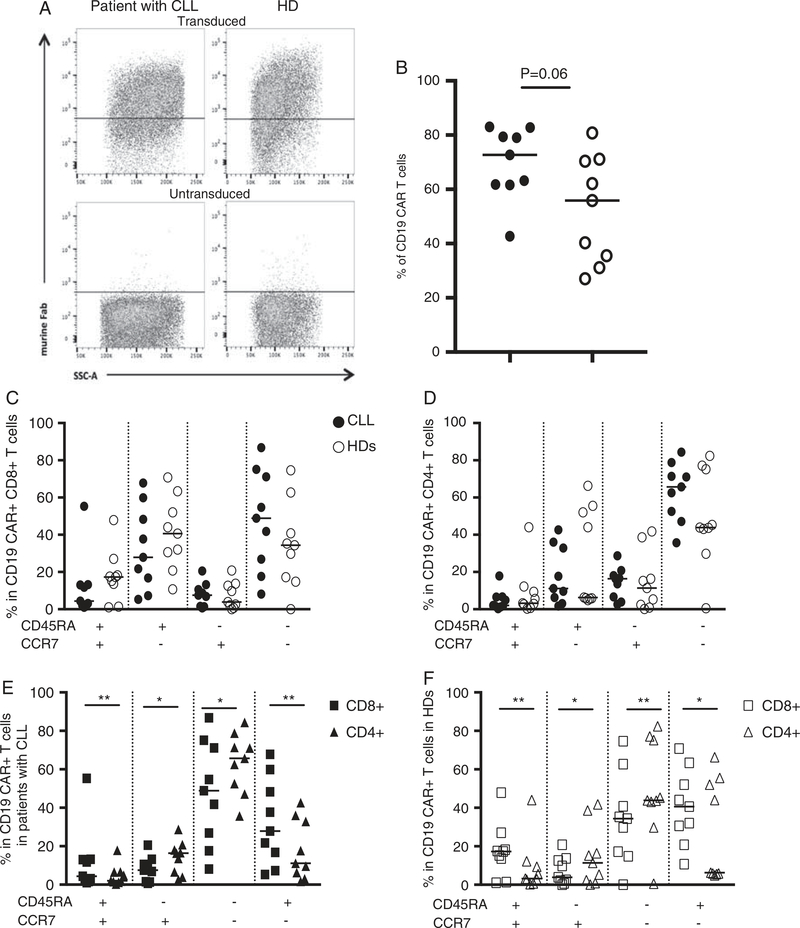
CD19 CAR T cells were produced from patients with CLL and HDs’ PBMCs. For patients with CLL, the median expansion fold of CD3+ T cells (from the start to the end of the cell production) was 35 (ranging from 14 to 108). CD19 CAR cell surface expression was detected by flow cytometric analysis (Fig. 2A). The median transduction efficacy (frequency of T cells expressing CD19 CAR) was for patients with CLL and HDs,73% (43%–83%) and 56% (27%–81%), respectively, with a trend of higher transduction efficiency (P=0.06) in patients with CLL cells (Fig. 2B). Of note, 3 days after transduction, while CD19+ B cells were still present in the nontransduced cell cultures of patients with CLL, we could not detect CD19+ B cells in the cell cultures of CD19 CAR transduced cells (Supplementary Fig. 2A, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). CD19 CAR transduced T cells from patients with CLL and HDs specifically killed CD19+ K562 cells but not NGFR+ K562 cells (Supplementary Fig. 2B, Supplemental Digital Content 1, http://links.lww.com/JIT/A482).
FIGURE 2.
In vitro expanded CD8+ CD19 CAR+ T cells and CD4+ CD19 CAR+ T cells display a different phenotype. Representative flow cytometric analysis showing CD19 CAR T cells detection after retroviral transduction (top panel) in a patient with chronic lymphocytic leukemia (CLL) (left panel) and a healthy donors (HD) (right panel), untransduced samples were used as negative controls (bottom panel) (A). Transduction rate in patients with CLL (n =9) and HDs (n =9) (B). CD45RA and CCR7 expression in patients with CLL (n =9), and in HDs (n=9), comparing the phenotype of CD8+ CD19 CAR+ T cells (C) in patients with CLL versus HDs, the phenotype of CD4+ CD19 CAR+ T cells (D) in patients with CLL versus HDs, and CD8+ versus CD4+ CD19 CAR+ T cells in patients with CLL (E) and in HDs (F). Squares represent CD8+ CD19 CAR+ T cells, and triangles CD4+ CD19 CAR+ T cells. Wilcoxon test, *P< 0.04. **P< 0.004.
The CD4/CD8 ratio of CD19 CAR+ T cells in patients with CLL and HDs was comparable (not statistically significant), with a median of 0.7 and 0.8, respectively (Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). This ratio was also comparable to the CD4/CD8 ratio of CD19 CAR− T cells present in the same cell cultures.
CD19 CAR+ T cells produced from patients with CLL and HDs displayed a similar profile (not significantly different as shown in Figs. 2C, D) after in vitro expansion (5–8 d after transduction). The majority of CD19 CAR+ T cells were TEM or TEMRA cells. However, in both patients with CLL and HDs, CD19 CAR+ CD8+ T cells as compared with CD19 CAR+ CD4+ T cells displayed differences. TN/TSCM and TEMRA CD19 CAR+ CD8+ T cells were more frequent than TN/TSCM and TEMRA CD19 CAR+ CD4+ T cells, while TCM and TEM CD19 CAR+ CD4+ T cells were more frequent than TCM and TEM CD19 CAR+ CD8+ T cells (Figs. 2E, F).
Of note, the majority of CD19 CAR− T cells were also TEM or TEMRA cells, and CD19 CAR− T cells displayed a phenotypic profile similar to CD19 CAR+ T cells (Supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/JIT/A482).
CD19 CAR T Cells’ Degranulation and Cytokine Production Profile After Stimulation With CD19+ Target Cells
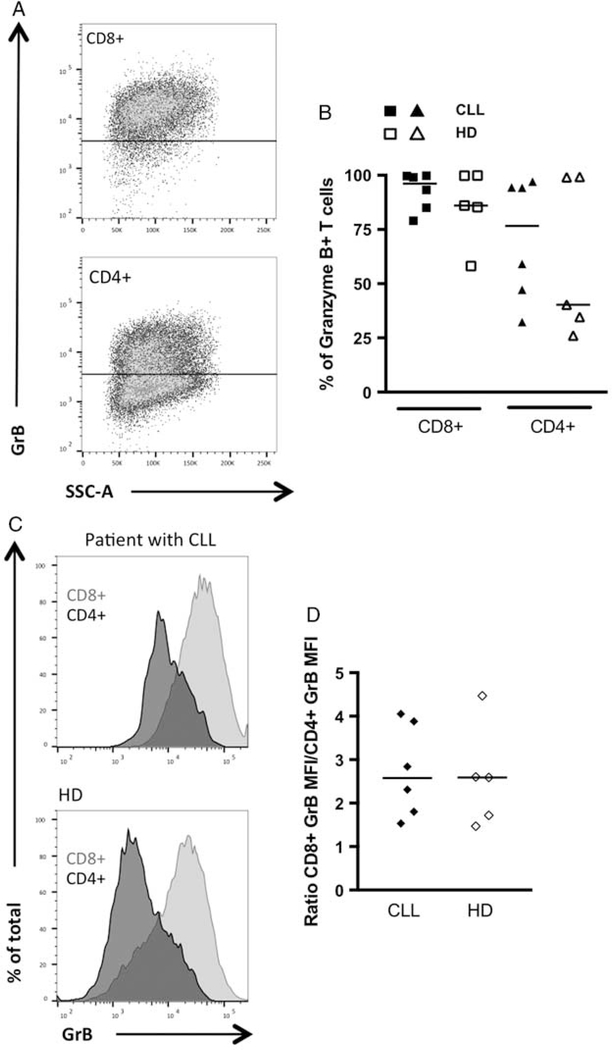
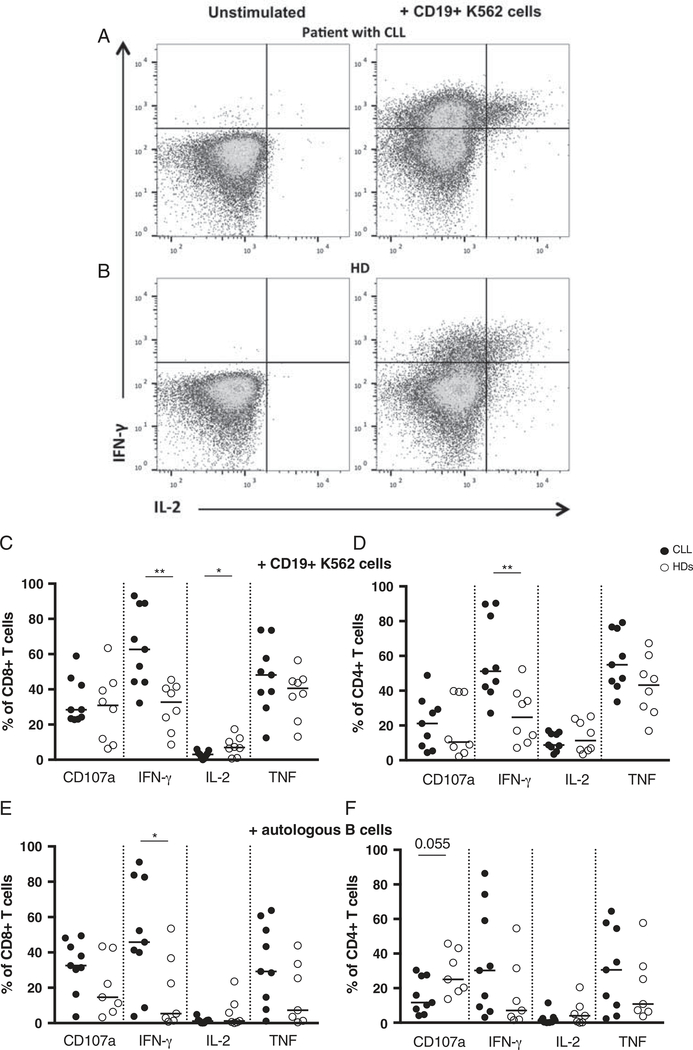
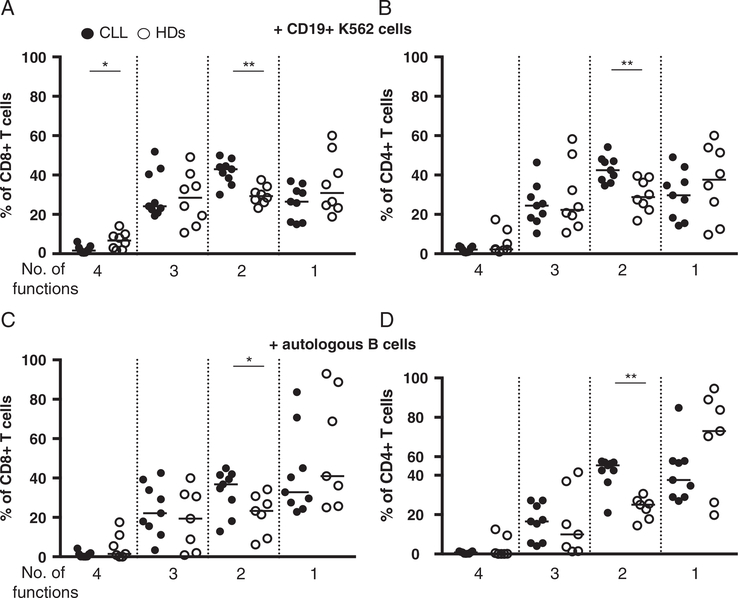
After in vitro expansion (5–8 d after transduction), CD19 CAR transduced T cell cultures were stimulated with either autologous B cells (CLL cells from the patients, or EBV-transformed B cells from the HDs), or CD19+ K562 cells, and the expression of granzyme B, CD107a and production of cytokines were analyzed by flow cytometry. After stimulation with CD19+ K562 cells, in CD19 CAR transduced T cell cultures from patients with CLL and HDs the majority of CD8+ T cells expressed granzyme B (96% and 85%, respectively), while CD4+ cells’ granzyme B expression was more heterogenous (Figs. 3A, B). Granzyme B MFI in CD8+ T cells was higher as compared with granzyme B MFI in CD4+ T cells in both patients and HDs (median ratio of 2.7 and 2.6, respectively) (Figs. 3C, D). In the control experiments (CD19 CAR transduced T cells not stimulated, or stimulated with NGFR+ K562 target cells), there was neither increase of CD107a expression nor production of the cytokines tested (Figs. 4A, B, and Supplementary Figs. 5A, B, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). In response to either autologous B cells or CD19+ K562 cells, CD8+ and CD4+ CD19 CAR transduced T cells from patients with CLL and HDs specifically up-regulated CD107a, produced IFN-γ, TNF, and to a lower extent IL-2 (Figs. 4C–F). A low frequency of CD4+ T cells (with a median comprised between 0.5% and 2.7%) produced IL-17 (data not shown). After stimulation with CD19+ K562 cells, in CD19 CAR transduced T cells from patients with CLL, as compared with HDs cells, a higher frequency of CD8+ and CD4+ T cells produced IFN-γ (P<0.003), and a lower frequency of CD8+ T cells produced IL-2 (P<0.05) (Figs. 4C, D). In response to autologous B cells, in patients with CLL as compared with HDs, a higher frequency of CD8+ T cells produced IFN-γ (P<0.04) (Fig. 4E), and there was a trend toward a lower frequency of CD4+ T cells expressing CD107a (P=0.05) (Fig. 4F). When CD19 CAR transduced T cells were stimulated with PMA/Ionomycin, higher frequencies of cells from patients with CLL produced IFN-γ and TNF as compared with cells from HDs (Supplementary Figs. 5C, D, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). We next analyzed within CD19 CAR transduced T cells that were specifically activated, the level of polyfunctionality (ie, CD107a expression and/or IFN-γ/IL-2/TNF production, IL-17 was not included due to the low levels of production) after stimulation with CD19+ target cells. In patients with CLL and HDs, the majority of activated CD19 CAR transduced T cells displayed more than 1 function, except in the case of CD4+ T cells from HDs stimulated with autologous B cells where a median of 62% of cells displayed only 1 function (Figs. 5A–D). Higher frequencies of CD19 CAR transduced T cells displaying 2 simultaneous functions after stimulation with CD19+ target cells were observed in patients with CLL.
FIGURE 3.
In vitro expanded and stimulated CD8+ and CD4+ CD19 CAR T cells from patients with chronic lymphocytic leukemia (CLL) express granzyme B. CD19 CAR+ T cells from patients with CLL (n=6) or healthy donors (HDs) (n=5) were stimulated with CD19+ K562 cells. A, Representative flow cytometric plot showing granzyme B detection by ICS in CD8+ (top panel) and CD4+ (bottom panel) CD19 CAR T cells from a patient with CLL. B, Frequency of granzyme B+ in CD8+ and CD4+ T cells. C, Overlay of GrB expression in CD4+ and CD8+ T cells in a patient with CLL (top panel) and a HD (bottom panel). D, The median fluorescence intensity (MFI) ratio represents the granzyme B MFI in CD4+ cells/granzyme B MFI in CD8+ cells.
FIGURE 4.
CD107a expression and cytokine production of in vitro expanded CD19 CAR T cells after in vitro stimulation with CD19+ target cells. Representative flow cytometric plot showing IFN-γ and IL-2 production in CD19 CAR transduced CD8+ T cells in a patient with chronic lymphocytic leukemia (CLL) (A) and a healthy donor (HD) (B), either in unstimulated cells (left panel), or after stimulation with CD19+ K562 cells (right panel). Frequencies within (C) CD8+ and (D) CD4+ T cells after stimulation with K562 CD19+ cells in patients with CLL (n=9) and HDs (n=8), and within (E) CD8+ and (F) CD4+ T cells after stimulation with autologous B cells in patients with CLL (n=9) and HDs (n=7). Black circles represent patients with CLL, and open circles HDs. Mann-Whitney test, *P< 0.05. **P< 0.006.
FIGURE 5.
In vitro expanded CD19 CAR T cells polyfunctional profile after stimulation with CD19+ target cells. Number of simultaneous functions (combination of CD107a expression and IFN-γ/IL-2/TNF production) displayed by (A) CD8+ and (B) CD4+ CD19 CAR T cells after stimulation with CD19+ K562 cells in patients with chronic lymphocytic leukemia (CLL) (n=9) and healthy donors (HDs) (n =8). By (C) CD8+ and (D) CD4+ CD19 CAR T cells after stimulation with autologous B cells in patients with CLL (n =9) and HDs (n=7). Black circles represent patients with CLL, and open circles HDs. Mann-Whitney test, *P< 0.05. **P< 0.006.
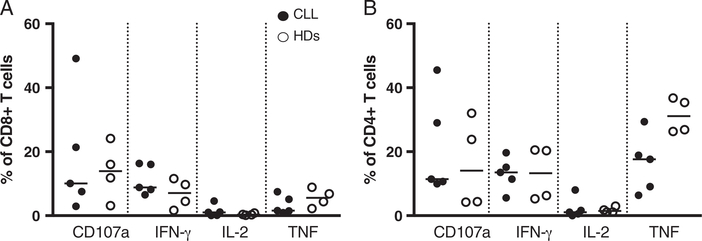
One week after transduction, CD19 CAR T cells from patients with CLL or HDs were kept in culture for 2 additional weeks. The cells were cocultured with irradiated CD19+ K562 cells added on 2 occasions. Two weeks later, the median frequency of CD19 CAR+ T cells was 59% for patients with CLL and 47.5% for HDs, and the cells were then stimulated for 6 hours with CD19+ K562 cells and subsequently analyzed by ICS. Cells from patients with CLL and HDs did not show any difference. CD8+ and CD4+ CD19 CAR transduced T cells, upregulated CD107a, produced IFN-γ and TNF (particularly CD4+ T cells), but almost no cells produced IL-2 (Figs. 6A, B).
FIGURE 6.
CD107a expression and cytokine production of in vitro expanded CD19 CAR T cells after repeated in vitro stimulations with CD19+ K562 cells. CD19 CAR transduced T cells were stimulated during 14 days on 3 occasions with CD19+ K562 cells (on the first 2 stimulations the K562 cells were irradiated) and analyzed after the last stimulation by intracellular cytokine staining, note that the gate included total T cells (not gated on CD19 CAR+ T cells). In (A) CD8+ and (B) CD4+ CD19 CAR T cells in patients with chronic lymphocytic leukemia (CLL) (n=5) and healthy donors (HDs) (n =4). Black circles represent patients with CLL, and open circles HDs.
Note that the different CD19+ target cells used for the stimulation displayed different CD19 expression level (Supplementary Figs. 6A, B, Supplemental Digital Content 1, http://links.lww.com/JIT/A482), with CD19+ K562 cells displaying higher CD19 cell surface expression than CLL cells and HDs’ EBV+ transformed B cells.
Impact of Different CD19+ Target Cells on CD19 CAR T Cells Effector Functions
In order to determine the impact of different CD19+ target cells on the functional profile of CD19 CAR T cells, we compared the different combinations of CD107a expression, IFN-γ, IL-2, and TNF production (Supplementary Fig. 7, Supplemental Digital Content 1, http://links.lww.com/JIT/A482) in activated cells. In patients with CLL, the frequency of CD4+ CD19 CAR transduced T cells being simultaneously IFN-γ+IL-2+TNF+CD107a+/− was significantly higher in response to CD19+ K562 as compared with autologous B cells. The same trend was observed within CD8+ CD19 CAR transduced T cells. In addition, in response to CD19+ K562 as compared with autologous B cells, there was a trend for lower frequency of CD4+ CD19 CAR transduced T cells being CD107a+ only, and higher frequency of CD8+ CD19 CAR transduced T cells being IFN-γ+TNF+. In HDs, the frequency of CD4+ CD19 CAR transduced T cells being CD107a−TNF+ in combination with IFN-γ and/or IL-2, was higher in response to CD19+ K562 as compared with autologous B cells. Inversely, the frequency of CD4+ CD19 CAR transduced T cells being CD107a+TNF+/− was lower in response to CD19+ K562 as compared with autologous B cells. In CD8+ CD19 CAR transduced T cells, in response to CD19+ K562 cells as compared with autologous B cells, the frequency of cells being TNF+IFN-γ+ was higher, but lower for cells expressing only CD107a.
DISCUSSION
Different strategies for CD19 CAR T-cell production have been published,19 and multiple centers have treated patients with CLL8,20,21 but no comparison of CD19 CAR T cells produced from patients and HDs has yet been reported. Therefore, in this study we compared the phenotypic and functional characteristics of CD19 CAR T cells produced from either patients with CLL or HDs.
As reported by others,6,22 circulating T cells of patients with CLL displayed a more differentiated phenotype as compared to HDs. The expression of PD-1 on T cells was heterogenous, particularly in patients, and not statistically different from HDs (Figs. 1C, D). A higher frequency of PD-1+ T cells in patients with CLL not correlated to disease stage or treatment has been reported,22 while others have shown significant differences in CD4+ T cells’ PD-1 expression according to the stage of disease and treatment.23 A recent large cohort study by Palma et al6 showed that in progressive treated patients and in HDs, the median frequencies of PD-1+ CD8+ T cells were 31.3% and 33%, respectively, with the difference lying in the number of PD-1+ CD8+ T cells that was significantly higher in patients due to the higher absolute numbers of T cells in patients. Since immune dysfunction in patients with CLL is a key feature of CLL24 we also investigated the expression of the other exhaustion markers LAG-3 and TIM-3. The frequency of LAG-3+ and TIM-3+ T cells (median <2% and <6%, respectively) was low both in patients and HDs (Figs. 1C, D). Shapiro et al25 have recently shown in patients with CLL that B cells displayed a low extracellular but high intracellular LAG-3 expression. Whether this is also true for T cells regarding the expression of exhaustion markers, and what modulates their surface expression would be of interest. Of note, we could not detect the surface expression of the PD-1 ligands, PD-L1, and PD-L2 on the patients’ CLL cells. Palma et al6 did not detect PD-L1 expression on CLL cells while others previously did, leading the authors to speculate that differences in cell processing may account for this discrepancy.
As CLL typically occurs in elderly patients, the patients’ age in addition to the ongoing disease may impact on T cells’ phenotype and functions. The patients with CLL and HDs included in this study were not aged-matched but CD19 CAR+ T cells could successfully be produced from both patients with CLL and HDs with comparable transduction efficiency, with a trend for higher transduction rate in patients with CLL T cells maybe due to a higher T cell differentiation and activation as compared to HDs’ T cells. Guha et al recently reported lower transduction efficiency (using a retrovirus) when comparing CEA CAR T cells generated from geriatric (older than 65 y) and young (18–45 y) donors.26 Some of the HDs included in our study were anonymous blood donors and we therefore cannot compare the age of the patients (median of 77 y) to the age of the HDs included in our study. However, the median transduction rate of 73% from patients’ derived cells indicates that a relatively high age does not preclude the production of CAR T cells by retroviral transduction.
T cell defects reported in patients with CLL include impairment in T cells’ cytotoxic capacities.27 We produced CD19 CAR T cells without any prior selection (positive selection of T cells, or depletion of B cells), and B cells therefore represented the vast majority of the cells in the cultures at the start of the process. As early as 3 days after transduction we could not detect any CD19+ B cells in transduced cell cultures (containing CD19 CAR+ T cells) from patients with CLL, while in nontransduced cell cultures (devoid of CD19 CAR+ T cells) CD19+ B cells were still present, indicating that the CD19 CAR+ T cells could efficiently kill CD19+ B cells (Supplementary Fig. 2A, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). This was confirmed when incubating patient-derived CD19 CAR transduced T cells with CD19+ but not NGFR+ K562 cells (Supplementary Fig. 2B, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). When comparing patients with HDs, the frequency of granzyme B+ CD19 CAR transduced T cells (Fig. 3), and the CD107a expression of CD19 CAR transduced T cells upon stimulation with CD19+ target cells were comparable (Fig. 4) further showing that no defect in killing capacity was observed in patient-derived CAR T cells. Guha et al26 reported differences between geriatric and young CAR T cells’ killing abilities at 1:20 target/effector ratio (in presence of IL-2 in the cell culture) but not at the 1:1 ratio that we also used in our stimulation assays. The 1:1 ratio we used may reflect better the physiological situation particularly in patients with CLL with lymphocytosis, and also demonstrate the potency of CD19 CAR transduced T cells at this low ratio.
The initial higher frequency of B cells in the cell cultures of patients, implies that the patient-derived CD19 CAR T cells as compared with HDs, encountered higher number of CD19+ target cells and were therefore more activated during the cell expansion. However when we analyzed the phenotype of CD19 CAR+ T cells (in average 1 wk after transduction) we did not find any differences between patients and HDs. Interestingly, both in patients with CLL and HDs, the same differences were observed when compared the phenotype of CD19 CAR+ CD8+ T cells to the phenotype of CD19 CAR+ CD4+ T cells (Figs. 2E, F). In the transduced cell cultures, CD19 CAR− T cells displayed the same phenotype as CD19 CAR+ T cells (Supplementary Fig. 4, Supplemental Digital Content 1, http://links.lww.com/JIT/A482) suggesting that nor the expression of CD19 CAR by T cells, neither an increased activation (increased target cell numbers) drive the differentiation of T cells in our culture conditions (in presence of 300 IU/mL IL-2). Less differentiated T cells such as TSCM endowed with higher proliferative capacities and antitumor effcicay, are better suited for adoptive T-cell therapy.12,28 The use of CD45RA and CCR7 expression did not allow us to distinguish TSCM cells from TN cells. It would have been interesting to compare the frequency of TSCM in CD19 CAR T cells generated from patients with CLL and HDs.
The CD4/CD8 ratio was comparable between the patients and the HDs at the start (median of 1.8 and 2, respectively) and end (in average 1 wk after transduction) (median of 0.7 and 0.8 in CD19 CAR+ T cells, respectively) of the cell manufacturing. Therefore, in our culture conditions, CD8+ T cells outgrew CD4+ T cells, with CD19 CAR expressing and nonexpressing T cells showing a comparable ratio (Supplementary Fig. 3, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). Furthermore, despite initial phenotypical differences, but comparable phenotype upon expansion, the same differences between CD8+ and CD4+ T cells phenotype were observed in patients with CLL and in HDs. Kaartinen et al29 recently demonstrated that a high dose (300 IU/mL) as compared with lower doses of IL-2 induced effector differentiation in CD19 CAR transduced and nontransduced cell cultures. Altogether, these observations underline the impact of the culture conditions and choice of cytokine(s) on CD8+ and CD4+ T cells’ differentiation.
A recent report showed that GD2 CAR T cells derived from the different CD45RO/CCR7 subpopulations (TN, TCM, TEMRA, and TEM) differentiated in culture into a TEM-like phenotype.30 Interestingly however, TN and TEMRA derived cells showed functional differences in response to CAR stimulation as compared with the other subsets. Our data show that CD107a expression, as mentioned above, and TNF production were similar but higher frequencies of CD19 CAR transduced T cells from patients with CLL, as compared with HDs, produced IFN-γ (Fig. 4) after stimulation with CD19+ target cells. PMA/ionomycin stimulation also induced higher frequencies of CD19 CAR transduced T cells from patients with CLL producing IFN-γ and TNF. Higher frequencies of CD8+ T cells producing IFN-γ and TNF upon PMA/ionomycin in early-stage31 and late-stage patients with CLL32 as compared with aged-matched HDs, as well as increased IFN-γ serum levels in patients with CLL33 have previously been reported. This may suggest that the different initial T cell composition between the patients and the HDs, and the T-cell dysregulation in patients with CLL account for the increased IFN-γ production we observed. In addition, we cannot exclude that the presence of a large frequency of B cells in patients with CLL during the production of CD19 CAR T cells and the subsequent activation of CD19 CAR T cells may also play a role. As compared with HDs, patients with CLL showed higher frequencies of CD19 CAR transduced T cells displaying 2 simultaneous functions after stimulation with CD19+ target cells (Fig. 5) indicating that the polyfunctional profile is also altered in patients. Of note, when CD19 CAR transduced CD8+ and CD4+ T cells were kept in culture for an additional 2 weeks and repeatedly stimulated with CD19+ K562 cells, IFN-γ, and TNF production was greatly decreased in both patients and HDs derived cells, while retaining some cytotoxic capacity as denoted by CD107a expression (Fig. 6). In vitro stimulation with relevant antigen expressing cells that can be used to expand CAR T cells may therefore also lead to the loss of CAR T cell function.
Different studies have shown that CD19 CAR T cells generated from donor-derived allogeneic cells can mediate remission of malignancy without causing graft-versus-host disease.7 In 2 reports the same CD19 CAR construct was used in either an allogeneic34 or autologous35 setting, the overall response rate was higher in patients who had received autologous-derived CAR T cells suggesting that autologous CAR T cells may be more efficacious. However, patients who received allogeneic CAR T cells did not undergo chemotherapy while patients who received autologous CAR T cells received low-dose chemotherapy, and therefore the lower response rate may not be only due to the source of CAR T cells.
Cytokine release syndrome (CRS) is the most life-threatening toxicity of this treatment and occurs in many patients after CAR T cell infusion, including patients with CLL.36 IFN-γ has been identified as one of the elevated cytokines (after CAR T cell infusion) associated with severe CRS in patients with ALL.37 Our observation that increased frequencies of CD19 CAR T cells produced IFN-γ in patients with CLL, may indicate that CAR T cells generated from an allogeneic donor instead of patient-derived cells are desirable in order to decrease the risk of CRS after CAR T cell infusion.
CD8+ and CD4+ transduced CD19 CAR T cells, despite a different phenotype, showed very comparable functional capacities (CD107a expression and cytokine production) after stimulation with CD19+ target cells, both in patients and in HDs (Fig. 4). CD4+ CAR T cells have been shown to be important,38 and sufficient39 to mediate antitumor effect in vivo in mice. Our data confirms previous observations40 that CD4+ CD19 CAR T cells are endowed with the capacity to kill CD19+ target cells.
Our results highlight the fact that T cells’ phenotype, particularly when limited to a small set of markers (in this study CD45RA and CCR7), may not be enough to characterize T cells and to infer T cells’ functions. This warrants the use of extended assays, such as extensive panels of markers (eg, activation, exhaustion markers, use of mass cytometry), telomere length measurement, and functional assays in order to better characterize CAR T cells.
T cells polyfunctionality is known to play an important role in mediating efficient antitumor responses.41 In this study, CD19 CAR transduced T cells were stimulated with either autologous CD19+ B cells or CD19+ K562 cells. The choice of CD19+ target cells impacted the CD8+ and CD4+ CD19 CAR T cells polyfunctional profile (Supplementary Fig. 7, Supplemental Digital Content 1, http://links.lww.com/JIT/A482). Upon stimulation with autologous target cells as compared to K562 cells, within cells that were activated, the frequency of CD19 CAR transduced T cells expressing CD107a but no cytokine (IFN-γ/TNF/IL-2) was higher. Target antigen density has been shown to modulate CAR T cells cytokine production.42 Thus, the higher level of CD19 expression by CD19+ K562 cells as compared to CLL and HD’s B cell (Supplementary Fig. 6, Supplemental Digital Content 1, http://links.lww.com/JIT/A482) probably explains some of the differences we observed, but differential expression of inihbitory and/or activatory molecules (not anlayzed here) on the different CD19+ target cells may also play a role. In most studies, cell lines have been used to assess CAR T cells effector functions. Our data underlines the impact of target cells, and importance of using more physiological target cells to identify effector functions that may correlate with clinical responses. In order to expand CAR T cells ex vivo, K562 cells expressing the cognate antigen have also been used as antigen-presenting cells (APCs).43,44 It would be interesting to decipher and compare the impact of stimulation with K562 cells or other artificial APCs for the ex vivo expansion of CAR T cells, on the subsequent cytokines produced by the activated CAR T cells, and on the phenotype and effector functions of the manufactured CAR T cells. The identification of APCs enabling robust CAR T cells ex vivo expansion without loss of multiple antitumor functions and differentiation would be desirable.
Taken together, our data show that while the cytotoxic capacities and phenotype of CD19 CAR T cells from patients with CLL and HDs were similar, the polyfunctional profile and IFN-γ production were different and influenced by the choice of CD19+ target cells used for stimulation.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients, and the nurses who helped with the samples procuration. The authors are grateful to Prof. Steven Rosenberg and Dr Steven Feldman for sharing reagents, protocols, helpful advices and discussions, as well as to Prof. Michael Nishimura and Gina Scurti for helpful advices and discussions.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by grants from the Swedish Children Cancer Foundation (PR2015–0120), Cancer och Allergifonden, and Belding and Rathsman private donation.
Footnotes
All authors have declared that there are no financial conflicts of interest with regard to this work.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.immunotherapy-journal.com.
REFERENCES
- 1.Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geyer MB, Brentjens RJ. Review: current clinical applications of chimeric antigen receptor (CAR) modified T cells. Cytotherapy. 2016;18:1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozman C, Montserrat E. Chronic lymphocytic leukemia. N Engl J Med. 1995;333:1052–1057. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M Chronic lymphocytic leukemia: 2017 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2017; 92:946–965. [DOI] [PubMed] [Google Scholar]
- 6.Palma M, Gentilcore G, Heimersson K, et al. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica. 2017;102:562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anwer F, Shaukat AA, Zahid U, et al. Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy. 2017;9:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9. [DOI] [PubMed] [Google Scholar]
- 11.Kaech SM, Hemby S, Kersh E, et al. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111: 837–851. [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Speiser DE, Lichterfeld M, et al. T memory stem cells in health and disease. Nat Med. 2017;23:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatino M, Hu J, Sommariva M, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016; 128:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126:2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner RA, Finney O, Annesley C, et al. Intent to treat leukemia remission by CD19CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129: 3323–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Feldman SA, Zhao Y, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine BL, Miskin J, Wonnacott K, et al. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2017;4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brentjens RJ, Riviere I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brusa D, Serra S, Coscia M, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98:953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusak M, Eljaszewicz A, Bolkun L, et al. Prognostic significance of PD-1 expression on peripheral blood CD4+ T cells in patients with newly diagnosed chronic lymphocytic leukemia. Pol Arch Med Wewn. 2015;125:553–559. [DOI] [PubMed] [Google Scholar]
- 24.Riches JC, Gribben JG. Immunomodulation and immune reconstitution in chronic lymphocytic leukemia. Semin Hematol. 2014;51:228–234. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro M, Herishanu Y, Katz BZ, et al. Lymphocyte activation gene 3: a novel therapeutic target in chronic lymphocytic leukemia. Haematologica. 2017;102:874–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guha P, Cunetta M, Somasundar P, et al. Frontline science: functionally impaired geriatric CAR-T cells rescued by increased alpha5beta1 integrin expression. J Leukoc Biol. 2017; 102:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorgun G, Holderried TA, Zahrieh D, et al. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. J Clin Invest. 2005;115:1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger C, Jensen MC, Lansdorp PM, et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008; 118:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaartinen T, Luostarinen A, Maliniemi P, et al. Low interleukin-2 concentration favors generation of early memory T cells over effector phenotypes during chimeric antigen receptor T-cell expansion. Cytotherapy. 2017;19:689–702. [DOI] [PubMed] [Google Scholar]
- 30.Schmueck-Henneresse M, Omer B, Shum T, et al. Comprehensive approach for identifying the T cell subset origin of CD3 and CD28 antibody-activated chimeric antigen receptor-modified T Cells. J Immunol. 2017;199:348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallego A, Vargas JA, Castejon R, et al. Production of intracellular IL-2, TNF-alpha, and IFN-gamma by T cells in B-CLL. Cytometry B Clin Cytom. 2003;56:23–29. [DOI] [PubMed] [Google Scholar]
- 33.Buschle M, Campana D, Carding SR, et al. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J Exp Med. 1993;177:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol. 2016;34:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oluwole OO, Davila ML. At the bedside: clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J Leukoc Biol. 2016;100:1265–1272. [DOI] [PubMed] [Google Scholar]
- 37.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adusumilli PS, Cherkassky L, Villena-Vargas J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheadle EJ, Hawkins RE, Batha H, et al. Eradication of established B-cell lymphoma by CD19-specific murine T cells is dependent on host lymphopenic environment and can be mediated by CD4+ and CD8+ T cells. J Immunother. 2009;32:207–218. [DOI] [PubMed] [Google Scholar]
- 40.Liadi I, Singh H, Romain G, et al. Individual motile CD4(+) T cells can participate in efficient multikilling through conjugation to multiple tumor cells. Cancer Immunol Res. 2015;3:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan J, Gnjatic S, Li H, et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci U S A. 2008;105:20410–20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe K, Terakura S, Martens AC, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–920. [DOI] [PubMed] [Google Scholar]
- 43.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maus MV, Thomas AK, Leonard DG, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nat Biotechnol. 2002;20:143–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.