Abstract
Nutritional ketosis, induced via either the classical ketogenic diet or the use of emulsified medium-chain triglycerides, is an established treatment for pharmaceutical resistant epilepsy in children and more recently in adults. In addition, the use of oral ketogenic compounds, fractionated coconut oil, very low carbohydrate intake, or ketone monoester supplementation has been reported to be potentially helpful in mild cognitive impairment, Parkinson’s disease, schizophrenia, bipolar disorder, and autistic spectrum disorder. In these and other neurodegenerative and neuroprogressive disorders, there are detrimental effects of oxidative stress, mitochondrial dysfunction, and neuroinflammation on neuronal function. However, they also adversely impact on neurone–glia interactions, disrupting the role of microglia and astrocytes in central nervous system (CNS) homeostasis. Astrocytes are the main site of CNS fatty acid oxidation; the resulting ketone bodies constitute an important source of oxidative fuel for neurones in an environment of glucose restriction. Importantly, the lactate shuttle between astrocytes and neurones is dependent on glycogenolysis and glycolysis, resulting from the fact that the astrocytic filopodia responsible for lactate release are too narrow to accommodate mitochondria. The entry into the CNS of ketone bodies and fatty acids, as a result of nutritional ketosis, has effects on the astrocytic glutamate–glutamine cycle, glutamate synthase activity, and on the function of vesicular glutamate transporters, EAAT, Na+, K+-ATPase, Kir4.1, aquaporin-4, Cx34 and KATP channels, as well as on astrogliosis. These mechanisms are detailed and it is suggested that they would tend to mitigate the changes seen in many neurodegenerative and neuroprogressive disorders. Hence, it is hypothesized that nutritional ketosis may have therapeutic applications in such disorders.
Key words: Astrogliosis, ketosis, neurodegeneration, neuroprogression, molecular neurobiology
Introduction
Several nutritional approaches are now available to clinicians wishing to induce ketosis in their patients in the periphery and/or the brain in order to further positive therapeutic outcomes and the details of such approaches are usefully reviewed in [1] and [2] and depicted in Figure 1. A state of induced ketosis via the classical ketogenic diet (KD), the modified KD, or the medium-chain triglyceride (MCT) diet have long been successful therapeutic interventions in the treatment of many children with pharmacologically resistant epilepsy and the efficacy of these diets have been confirmed in large studies [3,4]. More recently, the results of prospective studies and meta-analyses have also confirmed the efficacy of these diets in the treatment of intractable epilepsy in adults [5,6]. There is also some evidence to suggest that the modified Atkins diet may have efficacy irrespective of patient age [7,5].
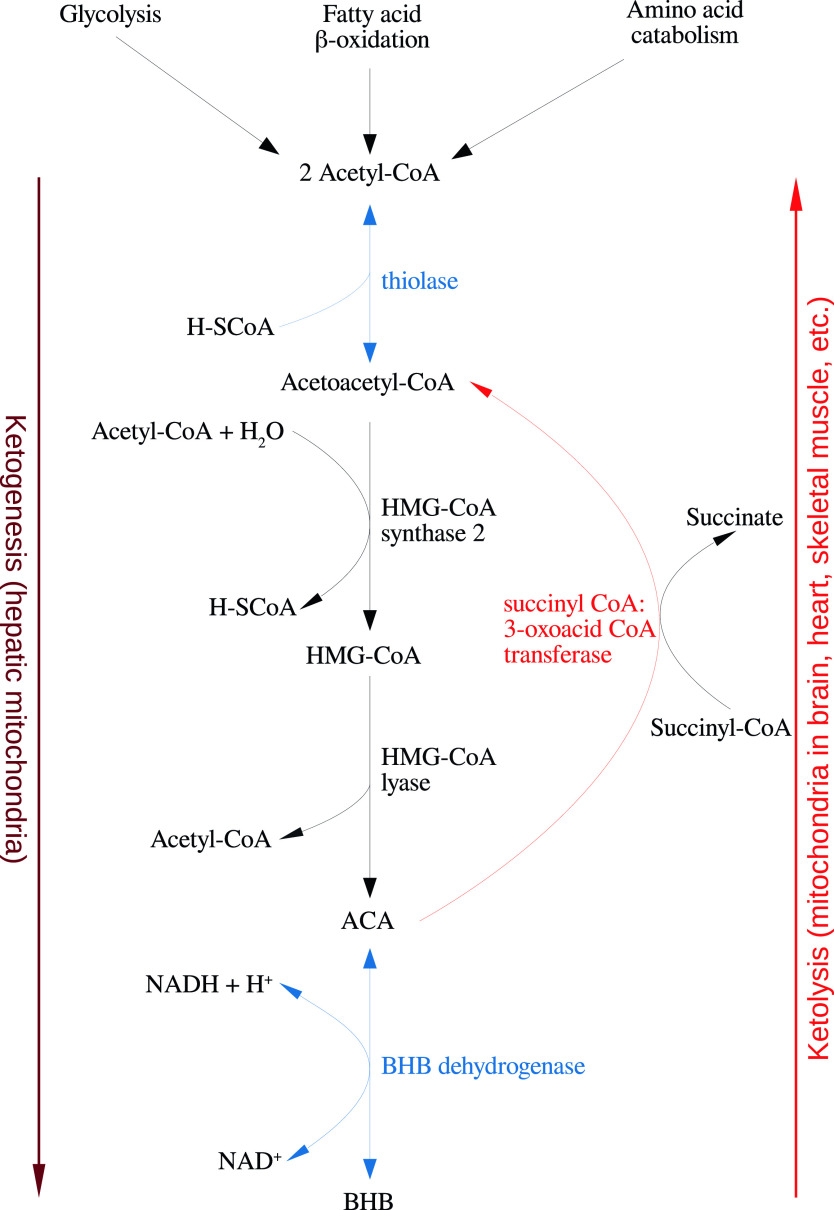
Figure 1.
Summary of the reactions of ketogenesis and ketolysis. Abbreviations: ACA, acetoacetate; BHB, β-hydroxybutyrate.
Unsurprisingly, there has been considerable interest in the putative therapeutic utility of dietary ketosis as a possible treatment approach for neurological and neuropsychiatric (increasingly described as neuroprogressive) illnesses which are very often refractory to current standard pharmaceutical interventions. In this context, it is noteworthy that some research teams investigating this area have reported some success most notably in patients with mild cognitive impairment or early Alzheimer’s disease (AD) [8]. This is also true of interventions based on elevating levels of β-hydroxybutyrate (BHB), which is one of the molecules thought to underpin many of the therapeutic benefits of the classical and modified KD [8]. However, it must be emphasized that this latter approach does not induce ketosis but rather a state described as ketonemia. The different biochemistry involved in these two states is explained in an excellent review by Reger et al. [9].
Importantly, positive results have been reported using a wide range of methods now available for inducing a state of ketosis in animals and humans such as an emulsified MCT diet [10], oral ketogenic compound [11], fractionated coconut oil [12], very low carbohydrate diet [13], and a ketone monoester dietary supplement [14]. There are also encouraging data suggesting that a KD might benefit individuals with Parkinson’s disease (PD) [15]. There is also some evidence to suggest that nutritional ketosis might benefit patients with schizophrenia (SZ) [16], bipolar disorder (BPD) [17], and autistic spectrum disorder (ASD) [17]. However, a literature search by the authors did not to reveal any studies which examined the effect of the KD on patients with major depressive disorder (MDD), which is curious given the existence of data demonstrating a positive effect of the diet, or its variants, on tryptophan metabolism [18], as abnormal tryptophan metabolism is considered to be involved in the pathogenesis of the illness [19,20]. In addition, MDD patients have higher levels of pro-inflammatory cytokines (PICs) [21] and hence a KD can be viewed as a potential treatment for MDD because it has an anti-inflammatory property [18]. In this context, it is noteworthy that commonly used antidepressants have anti-inflammatory activity [22] while anti-cytokine agents can improve anhedonia [23]. Furthermore, KD increases the levels of brain-derived neurotrophic factor (BDNF) [24]. Similarly, novel antidepressants also increase BDNF levels in the hippocampus [25,26]. As a result, the KD has the potential to modulate neurotrophic pathways and inflammatory mechanisms to reduce depressive symptom severity and other dimensions of depressive psychopathology including cognition [18]. The use of a KD in MDD, and indeed other neuroprogressive conditions, is also supported by data gleaned from animal studies, and readers interested in the area are invited to consult an excellent review by Fabrazzo [27]. Unsurprisingly, there has been a plethora of research investigating the mode of action of nutritional ketosis and how it produces its therapeutic effects, and certain themes have emerged.
For example, nutritional ketosis and the influx of ketone bodies (KBs) and medium-chain fatty acids (MCFAs) into the brain provide an alternative source of energy to glucose and exert a glucose sparing effect in the brain in an environment of glucose restriction or relative hypometabolism thereby allowing the preservation or even improvement of brain function and neuronal survival [2, 28–31]. This appears to be potentially of considerable therapeutic potential as far as the treatment of neurodegenerative and neuropsychiatric disorders, henceforth described as neuroprogressive, disorders is concerned, as glucose hypometabolism is observed in AD [32], PD [33], amyloid lateral sclerosis (ALS) [34], Huntington’s disease (HD; [35]), SZ [36], BPD [37] and MDD [38]. The therapeutic importance of addressing the relative glucose hypometabolism and its consequences in patients suffering from neurodegenerative or neuroprogressive disorders are emphasized by data suggesting that this state plays a causative role in the pathogenesis of these illnesses and in some cases may be observed long before symptoms or other recognized drivers of pathology are apparent [39].
In addition, there is accumulating preclinical and clinical evidence that dietary ketosis results in the amelioration of oxidative stress, mitochondrial dysfunction and inflammation in the periphery, and in the brain of animals and humans [40–46]. Such data may also have therapeutic relevance as the weight of evidence strongly suggests that oxidative stress and mitochondrial dysfunction have a causative role in the pathogenesis of neurodegenerative [47,48] and neuroprogressive [49,50] disorders.
Although much of the research in neurodegenerative and neuroprogressive diseases has focused on the detrimental effects of oxidative stress, mitochondrial dysfunction and neuroinflammation on neurone function [51,52], and survival, there is now a growing appreciation that this triad of abnormalities exerts pathology by compromising neurone–glial cell interactions and disrupting the normal roles played by microglia and astrocytes in central nervous system (CNS) homeostasis [53,54]. Mitochondrial dysfunction in astrocytes and microglia is of particular pathological significance from the perspective of compromised homeostasis in the CNS as the regulatory functions of microglia and astrocytes are dependent on optimal mitochondrial function and the maintenance of these glial cells in their physiological state [55–57].
In addition, while disturbed mitochondrial function impairs the ability of microglia and astrocytes to regulate multiple dimensions of CNS homeostasis, the same is true of raised levels of oxidative stress via mechanisms independent of induced mitochondrial dysfunction. In brief, elevated levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS), and/or compromised cellular antioxidant systems sensitize microglia to activation by inflammatory mediators and hence exacerbate levels of inflammation and promote a variable state of reactivity and dysfunction described as astrogliosis [58,59].
The development of astrogliosis leads to impairment or loss of homeostatic functions of these glial cells in regulating brain homeostasis [60]. This is highly problematic from the perspective of brain function and is considered to be a critical event in the pathogenesis and pathophysiology of neurodegenerative and neuroprogressive illnesses as accumulating data strongly suggest that such a reactive and dysfunctional state in astrocytes is a major if not the main driver of neural dysfunction or neurodegeneration seen in these illnesses [61,62].
Unsurprisingly, given the evidence discussed above, the modulation of astroglial activity and function is now considered to be an important therapeutic target in the treatment of neurodegenerative and neuroprogressive diseases [60, 63–65]. From this perspective, it is encouraging that several authors have reported that induced ketosis decreases astrocyte activity, improves astrocyte–neurone interactions [66,67], and exerts positive effects on expression and function of receptors-enabling astrocytes to regulate multiple dimensions of CNS homeostasis [29, 68–71].
Clearly, there is accumulating evidence suggesting that the use of nutritional ketosis may result in a beneficial manipulation of astrocyte activity and function. However, there appear to be few publications relating to dietary ketosis exclusively focusing on this topic. Hence, this article aims to address this apparent gap in the literature by attempting to explain the various biochemical and energetic consequences of dietary ketosis from the perspective of microglia and astrocytes. In order to facilitate this endeavor, we will first outline the processes involved in the generation of a ketotic state before discussing entry of KBs and fatty acids (FAs) into the brain and the consequences of such entry on energy production, cellular antioxidant defences, and levels of neuroinflammation. We will then move on to consider the elements driving astrogliosis and its functional consequences before focusing on the potential remedial effects of nutritional ketosis on the disturbed patterns of astroglial function seen in neurodegenerative and neuroprogressive conditions.
The Biochemistry of Ketogenesis
Under physiological conditions, acetyl CoA produced by FA oxidation enters the tricarboxylic acid (TCA) cycle and subsequently engages in a chemical reaction with oxaloacetate to produce citrate. However, under metabolic conditions induced by the KD, oxaloacetate is exported out of the mitochondria, being utilized for the process of gluconeogenesis [72]. In this scenario, levels of acetyl CoA synthesis greatly exceed the amount of oxaloacetate in the mitochondrial environment and the former engages in a series of condensation reactions, which are the hallmark of ketogenesis [73]. First, two acetyl CoA molecules combine to produce acetoacetyl CoA. This molecule reacts with a further molecule of acetyl CoA to form HMG-CoA in a functionally irreversible and rate limiting reaction enabled by HMG-CoA synthase 2 [74]. Once formed, this compound dissociates to the KB acetoacetate (ACA), which is further reduced to BHB by a reaction enabled by BHB dehydrogenase and involving the NAD+/NADH couple as the hydrogen donor [75]. It should be noted that levels of BHB in the circulation and tissues are much higher than those of ACA, making the former the predominant KB [76,77].
BHB and ACA are exported into the circulation from the liver and ultimately imported by the brain, heart, skeletal muscle, and other tissues with high metabolic demands [73]. Once ensconced in these body compartments, BHB is oxidized to ACA by BHB dehydrogenase, which acts as a prime regulator of the mitochondrial NAD+/NADH ratio status [78]. ACA is then hydrolyzed to form acetoacetyl CoA and succinate in a reaction enabled by the enzyme succinyl CoA:3-oxoacid CoA transferase, and the acetoacetyl CoA is then cleaved to yield acetyl CoA in a reaction catalyzed by thiolase; the acetyl CoA and succinate form substrates for the TCA cycle and complex II of the electron transfer chain (ETC), respectively [79]. This process may also result in increased succinate dehydrogenase activity reported following prolonged administration of the KD in rodents [80]. These pathways are depicted in Figure 1. The effects of the KD may be mimicked by the use of KB supplements and there is at least some evidence to suggest that the production of KBs in the liver, which occurs in physiological conditions may be inhibited in such a scenario although this is not universally accepted [9,81].
KBs are metabolized at a considerably higher rate than glucose and enter the TCA cycle directly as previously discussed, thus bypassing glycolysis [77,82]. Importantly, much evidence suggests that at levels normally induced by ketogenesis, glycolytic ATP generation diminishes and the generation of ATP by oxidative phosphorylation increases [83,84]. Although the β-oxidation of free fatty acids (FFAs) is clearly a factor underpinning such observations, other mechanisms are also involved and we turn to a consideration of these elements in the next section of this article.
Entry of Ketone Bodies and FAs into the Brain
When plasma KB concentration exceeds 4 nM, the uptake of these molecules into the brain increases ([85,86]; reviewed [87]). Several research teams using in vivo PET techniques have reported a magnitude of increase in brain KB concentrations induced by a prolonged KD in humans and rodents of approximately eightfold compared with controls fed a normal diet [31,85,86]. However, the extent of ketosis is of importance as experimental evidence suggests that mild ketosis only produces a doubling of KB levels in the brain [88].
Plasma KB levels are also of importance because such levels are proportional to increased KB levels and metabolism in the brain, which in turn determine the global degree of KB-induced glucose metabolism suppression within the CNS [31]. Evidence suggests that the suppression of glucose metabolism in the CNS induced by KBs increases by approximately 9% for every 1 nM increase in KB levels in the plasma [30,89]. The importance of KBs as an energy source in conditions of ketosis induced by diet or starvation is graphically illustrated by the presence of data demonstrating that these molecules may supply approximately 60–70% of the brain’s energy needs in such conditions [90].
KBs and MCFAs (produced from MCT supplementation in some versions of the KD, as discussed above) transverse the blood–brain barrier (BBB) into the brain via the assistance of monocarboxylate 1 and 2 transporters expressed on brain microvascular endothelial cells [91,92]. The expression of these transporters increases over 10-fold following a protracted period of ketosis [93]. Polyunsaturated fatty acids (PUFAs) can also cross the BBB although the enabling mechanisms are a matter of debate and the assistance of calveolin-1, FA transporters, phospholipid-bound FA translocase, and lipoprotein packaging have all been posited (reviewed by [75]). However, current evidence suggests that long-chain nonesterified FAs (NEFAs) cannot cross the BBB at a sufficient rate to meet energy demands [94].
Consequences of Ketogenesis and Ketolysis in the CNS
Dietary induced ketosis is associated with increased ATP levels in the brain [95–99]. Other in vivo effects include increased phosphocreatine [100,101] and increased ATP synthase [100,102]. These changes are associated with increased numbers of mitochondria [95,100] and improved levels of mitochondrial performance in glia [103] and neurones [104]. It is important to note that this pattern of globally increased metabolism is observed in patients with AD following ingestion of the MCT diet and thus there is good reason to believe that these effects would also occur in patients suffering from other neurological and indeed neuroprogressive disorders [105].
There is also a significant and accumulating body of evidence demonstrating a statistically significant reduction in oxidative and nitrosative stress and upregulation of cellular antioxidant defences in the brains of animals following prolonged dietary ketosis ([106–111]; reviewed by [41]). This decrease would also appear to be clinically significant as several authors have reported a reduction in oxidative damage to neurones and increased neuronal survival as a result of dietary induced ketosis especially in an environment of cerebral glucose deprivation [109, 111–113].
Several mechanisms appear to underpin the reductions in CNS oxidative stress induced by the KD, with ROS scavenging by KBs being the simplest. Another route involves the maintenance of ETC performance, particularly complexes I, II, and III resulting in reduced ROS production by mitochondria [40,109,111,112].
Ingestion of KDs also leads to upregulation of Nrf2 in the brain [43,45,46]. This is of paramount importance as activation of this transcription factor activates a myriad of cellular antioxidant enzymes and nonenzymatic elements of the cellular antioxidant response system. The cellular antioxidant enzymes include superoxide dismutase, catalase, thioredoxin reductase, glutathione peroxidase, glutathione transferase, glutathione reductase, and the peroxidase family [114,115]. The nonenzymatic elements include carbon monoxide, thioredoxin, and reduced glutathione (GSH) [116].
Nutritional ketosis and increased levels of KB can also activate a plethora of other transcription factors and increase levels of several molecules, which can activate many signaling pathways resulting in reduced oxidative stress and metabolic adaptation to energy production via FA oxidation and ketolysis, which also have the effect of reducing mitochondrial ROS generation (reviewed by [117]). For example, the weight of in vivo data associates dietary-induced ketosis with elevated levels and activity of AMP-activated protein kinase (AMPK) in the brain in rodents and humans [118,119]. In vitro data suggest that such upregulation in astrocytes occurs to a much greater degree in these glial cells than neurones [120].
Prolonged ketosis is also associated with upregulation of NAD+ [75,82,121,122]. Increased levels of NAD+ explain the upregulation of the histone deacetylases sirtuin-1 (SIRT-1) and SIRT-3 seen in the brains and peripheral tissues of animals fed a KD, as SIRTs are NAD+-dependent enzymes [123–125].
There exist reports of FOXO3a, PGC-1α, and PPARγ elevation in animals fed a KD [42,126,127]. This is consistent with the work of several other authors who have reported that the upregulation of these transcription factors is driven by the upregulation of NAD+, AMPK, and SIRTs via a number of different routes [1,128]. The upregulation of these molecules also leads to activation of Nrf2 [117], which provides another route for activation of this transcription factor in addition to increases in NO and ROS levels [129].
The activation of the cascade described above results in increased cellular antioxidant systems and a downregulation of oxidative stress together with improved mitochondrial performance generation and a series of long-term metabolic adaptations designed to improve the efficiency of FA oxidation via mechanisms described in [130]. Clearly, the activation of signaling pathways subsequent to KD activation of AMPK, NAD+, and SIRTs explains the beneficial effects of dietary induced ketosis on ATP production, mitochondrial function, and oxidative stress in the brain, which are all therapeutic targets as far as the treatment of neuroprogressive and neurodegenerative diseases is concerned.
However, the data supplied by several research teams describing the activation of PPARs in the CNS of animals following KD ingestion appear worthy of particular focus from the perspective of this article for a number of reasons [127,131,132]. The first stems from the fact that PPARα and PPARγ are the main transcription factor-regulating ketogenesis and ketolysis and both PPAR isoforms are activated by increased levels of FFAs in the periphery and brain, a few days after the advent of ketosis [75,133,134]. The second is that several authors have reported that the upregulation of PPAR isoforms in the brain results in a reduction in neuroinflammation in vivo [125,126,135,136]. The third is that PPAR upregulation has the capacity to rescue mitochondrial dysfunction in the CNS environment typical of neurodegenerative [137] and neuroprogressive disorders [137,138].
Importantly, these effects extend to astrocytes. For example, the in vivo reduction in increased PPAR levels in astrocytes reduces mitochondrial dysfunction, decreases inflammation, and increases cellular antioxidant defences [139–141]. These data are also of importance from the perspective of improving astrocyte function as all these factors are also involved in driving and maintaining a reactive state in these glial cells [142].
Unsurprisingly, given the data discussed above, there are also an accumulating number of in vivo and in vitro studies where the authors report beneficial changes to astrocyte functions including improved glutamate and potassium homeostasis via direct effects on surface receptors [143,144]. There is also a growing awareness that many of these effects ultimately arise from the effects of ketone bodies and FAs translocated from the periphery on astrocyte metabolism and the involvement of these glial cells in mediating de novo ketogenesis in the brain. We will now move on to discuss these elements beginning with the role of astrocytes in FA oxidation and energy production.
Role of Astrocytes in Energy Production and Distribution
Astrocytes are regarded as the main site of FA oxidation in the brain [145,146]. There is also an accumulating body of evidence to support the view that astrocyte-derived KBs produced by FA oxidation in an environment of glucose restriction can be a significant source of oxidative fuel for neurones [147–150]. Indeed, there is a developing consensus that KBs supplied by astrocyte-mediated FA oxidation rather than KBs translocated from the periphery are the dominant source of these molecules in the brain in a state of ketosis [151,152]. This phenomenon is of paramount importance as far as neurone function and survival in an environment of glucose hypometabolism is concerned as the so-called lactate shuttle between astrocytes and neurones, which provides the oxidative substrate for neurones in physiological conditions, becomes compromised in such an environment owing to its dependence on glycogenolysis and glycolysis [153,154].
The reason for the dependence of the lactate shuttle on glycolysis stems from the fact that the astrocyte filopodia responsible for the release of lactate, either via monocarboxylate transporters or by passive diffusion, is too narrow to accommodate mitochondria [153,154]. There are numerous papers discussing the evidence confirming the existence of an astrocyte–neurone lactate shuttle in physiological conditions and detailing the mechanisms underpinning its operation and hence it will not be considered further here. Readers interested in an in-depth treatment of this phenomenon are referred to an excellent review by Zhang et al. [155].
In vitro experiments have produced conflicting results regarding the usage or otherwise of MCFAs as a substrate for FA oxidation most notably with regard to octanoate where authors have either reported that astrocytes do not appear to utilize this FA as a substrate for KB production [156]. Another research team reported a twofold increase in KB production following octanoate addition and a 50% increase in the production of lactate following the addition of decanoate to the culture medium [157]. However, the weight of in vivo evidence is consistent with the latter findings as several authors have reported significantly increased KB production by astrocytes following assimilation of MCFAs translocated across the BBB [158–160].
However, it is worthy of note that the preferred FA substrates of astrocytes in the hippocampus may be different [160] and there is some evidence to suggest that even long-chain FAs may be utilized in some circumstances despite their relative mitotoxicity [160]. Interestingly, data suggest that the relative inhibitory effects of MCFAs on oxidative phosphorylation [161] may increase KB production in astrocytes and improve the efficiency of the astrocyte–neurone shuttle [157], although these observations could also be explained by the inhibitory effect of MCFAs on glycolysis and the resultant improvement in the efficiency of ketogenesis [161]. Finally, it should be noted that MCFAs are not the only substrates enabling KB production in astrocytes as these glial cells may utilize branched-chain amino acids such as leucine for this purpose in certain circumstances [162]. Similarly, not all KB production by astrocytes is destined for neurones as an appreciable amount is used for cholesterol and lipoprotein synthesis [145].
Having discussed the role of astrocytes in ketogenesis and ketolysis in the brain, we now move on to consider how ketogenesis positively modulates the metabolism, signal transduction, and receptor profiles of astrocytes, which may mitigate against the neuropathological consequences of reactive astrogliosis. However, before doing so, it is necessary to explain the origins and consequences of this phenomenon.
Causes and Consequences of Astrogliosis
Causes of astrogliosis
Astrocytes are exquisitely sensitive to very small fluctuations in the CNS intracellular environment and readily attain a reactive phenotype in the face of such changes. One acknowledged cause of increased astrocyte reactivity is increased intracellular levels of PICs, NO, and ROS secreted by activated microglia [163–165]. Other triggers include glucose deprivation, increased levels of ATP and other gliotransmitters, and activation of surface toll-like receptors by commensal lipopolysaccharide (LPS) originally translocated from the intestine. It is important to note that the development of reactive astrogliosis provoked by these stimuli, particularly the inflammatory mediators such as the PICs, ROS, and NO, is accomplished via major changes in astrocyte gene transcription patterns, which drive morphological, physiological, and biochemical changes ([142]; reviewed by [166]).
Although there are a myriad of changes in signaling pathways in activated astrocytes compared with those existing in these glial cells in their physiological state, from the perspective of this article, the most noteworthy change is the chronic activation of the NF-κB, MAP kinase, and Jak/Stat pathways, which result in high intracellular levels of ROS, RNS, and PICs (reviewed by [167]). It is noteworthy that STAT-3 is of paramount importance as the weight of evidence suggests that activity of this transcription factor is an indispensable element in the development and maintenance of reactive astrogliosis [168–170].
Readers interested in the details of mechanisms underpinning the advent and persistence of a reactive or dysfunctional state in astrocytes are invited to consult the work of [171] and [60]. However, the key points to bear in mind during a perusal of the following sections of this article are that many of the factors underpinning the loss of homeostatic functions normally exerted by astrocytes in their physiological state stem from the changes in transcription orchestrated by the chronic activation of the pathways and transcription factors discussed above and/or the ensuing increases in levels of PICs, ROS, and RNS. These adversely affect the transcription and/or function of crucial membrane receptors and impair mitochondrial respiration and dynamics [59,172,173]. The importance of the latter is difficult to overemphasize as the homeostatic roles of astrocytes depend on adequate performance of mitochondria [55,56,174], and on a wider note, a host of neural–glial interactions also depend on optimal mitochondrial function [175]. Hence, a therapeutic approach, such as the KD, capable of reducing oxidative stress and inflammation in the brain in vivo while improving mitochondrial function has the potential to mitigate against the severity of astrogliosis and improve CNS homeostasis.
Consequences of astrogliosis
The normal role of astrocytes in regulating other dimensions of CNS homeostasis such as neurotransmitter levels, water transport, waste product clearance, ion homeostasis, and glucose and oxygen delivery to neurones (reviewed by [176] and summarized in Figure 2) are impaired when astrocytes are in a reactive state [177,178]. These observations are underpinned by the fact that astrogliosis results in adverse changes in astrocyte phenotype, signaling pathways, and surface receptor expression, which normally enable these cells to perform their essential role in the regulation of various dimensions involved in the maintenance of CNS homeostasis as described above. Disruption of the neurovascular unit owing to a loss of astrocyte end-feet and other cellular protrusions is perhaps the most damaging physical change as far as CNS homeostasis is concerned. The physical connection between BBB epithelial cells and neurones is needed to deliver nutrients and oxygen to the latter (reviewed by [179]). Examples of changes in receptor levels and function [180] include oxidative modification of glutamate receptors leading to inhibition of astrocyte-mediated glutamate uptake, nitrosylation of the gap junction channel connexin 43 (Cx43), and several gap junction pannexins (reviewed by [181]) leading to dysregulated calcium signaling and ATP transfer between astrocytes and neurones and other astrocytes [182]. Astrogliosis is also associated with profound disturbances in potassium homeostasis as a result of oxidative modification and downregulation of Na+, K+-ATPase (NKA) [180], and the weak inwardly rectifying Kir family potassium channel Kir4.1 [183,184]. The effects of astrogliosis in disrupting CNS homeostasis are summarized in Figure 3.
Figure 2.
The multiple roles of astrocytes in central nervous system homeostasis.
Figure 3.
Disruption of central nervous system homeostasis resulting from astrogliosis.
Given this information, it probably comes as no surprise to learn that astrocyte dysfunction, astrogliosis, and the accompanying global loss of the physiological functions of astrocytes in the regulation of CNS homeostasis play a major role in the development and acceleration of most if not all neurodegenerative [185,186] and neuroprogressive disorders [187,188]. In addition, treating the causes and/or consequences of astrogliosis is a major therapeutic objective [61,63,166]. How that might be achieved, at least in part, via dietary induced ketosis will be discussed in the next section of this article. In order to facilitate this endeavor, we will divide the following section into effects on the glutamate–glutamine cycle, glutamine synthetase, glutamate secretion, glutamate uptake via EAATs, NKA, Kir4.1 receptors, aquaporin-4 (AQP4), gap junctions, and KATP channels.
Nutritional Ketosis, Astrocyte Functions, and Astrogliosis
Effect of nutritional ketosis on the astrocyte glutamate–glutamine cycle
There is evidence that the entry of KBs into astrocytes stimulates mitochondrial metabolism and accelerates flux through the TCA cycle resulting in a number of consequences including the upregulation of the glutamate–glutamine cycle ([71,189–191]; reviewed by [144]). The mechanisms underpinning these observations are a matter of debate but stimulation of glycolysis, enhanced efficiency of pyruvate utilization, and increased levels of succinate to overcome a shortfall of oxaloacetate in an environment of glucose restriction all appear to be involved ([74,157,192]; reviewed by [193]).
The weight of evidence suggests that this phenomenon results in increased synthesis of glutamine and upregulated levels of GABA coupled with decreased synthesis of glutamate [71, 189–191, 194, 195], although the latter appears to be highly dependent on astrocyte KB concentration [196]. The existence of the glutamate–glutamine cycle is limited to astrocytes as these glial cells uniquely possess the two enzymes needed for its operation, namely pyruvate carboxylase, which enables the synthesis of new TCA cycle intermediates via the replenishment of oxaloacetate, and glutamine synthetase, which enables the synthesis of glutamine [197,198].
These neurotransmitters must be continually re-synthesized as uptake of GABA by astrocytes is limited and glutamate is used as a substrate for oxidation to compensate for the huge energetic costs of glutamate uptake (reviewed by [199]). Readers interested in the biochemistry underpinning the glutamate–glutamine cycle and the re-syntheses of glutamate and glutamine are invited to consult a comprehensive review on these matters by O'Gorman Tuura et al. [198].
However, there are some key points germane to the discussion below. First, the cycle is highly dependent on energy supplied by glucose oxidation and optimal mitochondrial function [200]. Second, KBs are the preferred oxidative substrate for “powering” the glutamate–glutamine cycle in the environment of cerebral glucose hypometabolism seen in neurodegenerative and neuroprogressive disorders [201]. Third, and perhaps expectedly, evidence of dysfunction or dysregulation of this cycle is present in at least some regions of the brain in patients with SZ [202], MDD [203], BPD [204], AD [205], HD [206], ALS [207], and PD [208]. Moreover, impairment of this cycle is a major element in the development of disturbed glutamate homeostasis, with glutamate excitotoxicity, and reduced GABAergic signaling, which play a causative role in the pathogenesis of most if not all neurodegenerative and neuroprogressive conditions [209,210]. Finally, the weight of evidence suggests that the ultimate source of glutamate and GABA dyshomeostasis seen in these illnesses is the presence of chronic astrogliosis [211,212].
Nutritional ketosis, astrogliosis, and glutamate synthetase
Astrogliosis is associated with reduced activity of glutamine synthase and GABA synthesis and relative failure of astrocyte-mediated glutamate reuptake as well as disruption of the glutamate–glutamine cycle [211–213]. Astrogliosis is also associated with increased expression of the cysteine/glutamate antiporter channel (Xc −) resulting in increased glutamate signaling and oxidative downregulation of the astrocyte glutamate uptake receptors EAAT1 and EAAT2, which in turn leads to the development of glutamate-mediated N-methyl-d-aspartate (NMDA) receptor excitotoxicity [166,214,215]. Impaired glutamate synthetase (GS) activity results in the accumulation of glutamate and impaired glutamate uptake thus making a contribution to the development of excitotoxicity [216].
Several research teams have reported that GS activity in humans and animals is downregulated in a cerebral environment of chronic oxidative and nitrosative stress and that such downregulation results in elevated intracellular glutamate and reduced glutamine levels [217–220]. Moreover, there are replicated in vivo data demonstrating that a major cause of GS inhibition in vivo is nitrosylation or nitration of functional residues secondary to excessive NMDA receptor activity and perhaps even more importantly that in vivo inhibition of extrasynaptic NMDA results in the upregulation of GS activity and increased levels of glutamine [221,222].
This is of interest as a mechanism known to downregulate NMDA activity in vivo is NO-mediated S-nitrosylation of functional thiol groups in NMDA receptor subunits and animal studies have reported raised NO levels in the CNS following consumption of a diet aimed at inducing ketosis [223–225].
It is also noteworthy that ex vivo studies have demonstrated a direct inhibitory effect of MCFAs on NMDA receptor excitotoxicity via the inhibition of NMDA AMPA subunits [226–228] which is of interest as MCT supplemented versions of the KD produced significant benefits in the treatment of patients with AD. Ex vivo data also suggest a positive effect of MCFAs on astrocyte mitochondrial biogenesis (reviewed by [229]).
Nutritional ketosis, astrogliosis, glutamate release, and synthesis
There are some ex vivo and in vivo data suggesting that KBs suppress the release of glutamate from astrocytes and neurones by inhibiting the actions of vesicular glutamate transporters (VGLUTs) and via an as yet undelineated mechanism [230]. The reduction of glutamate synthesis by BHB in vitro in a dose-dependent manner has also been reported [196]. An in vivo study involving rodents conducted by Olson et al. also appears to be worthy of consideration in the context of the potential beneficial effects of the KD on glutamate and GABA homeostasis. These authors reported that the reduction in glutamate and increase in GABA levels seen in the hippocampus of their study animals was effected by KD-induced changes to the microbiota [231].
Astrogliosis and EAAT function
However, despite the presence of data suggesting that diet-induced ketosis may have beneficial effects on some drivers of glutamate and GABA dyshomeostasis, it must be noted that there appears to be no direct evidence that a KD exerts positive effects on the activity or levels of astrocyte glutamate transporters (EAAT1 and EAAT2). This is an important point as the weight of evidence suggests that dysfunction and/or downregulated expression of EAAT2, which is responsible for approximately 90% of glutamate reuptake by humans astrocytes, is a major cause, if not the major cause, of glutamate-mediated NMDA receptor excitotoxicity, which appears to be causatively implicated in the pathogenesis and pathophysiology of all neurodegenerative and neuroprogressive disorders [232,233].
The results of human and animal studies point to a major cause of such downregulated expression or dysfunction of these receptors seen in all these illnesses as being the upregulated canonical NF-κB signaling and elevated levels of PICs such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β, ROS and RNS characteristic of the intracellular environment of reactive astrocytes ([234]; reviewed by [233]). TNF-α appears to downregulate the transcription of the EAAT2 gene [209,235] while IL-1β primarily has a negative effect on the membrane density of the receptor by enhancing its endocytosis and sequestration in the cytoplasm [236,237]. There is also evidence suggesting that the function of EAAT2 (and indeed EAAT1) is compromised in neurodegenerative and neuroprogressive illnesses as a result of S-nitrosylation of crucial thiol residues, which play an indispensable role in their function (reviewed by [238]).
There is robust in vivo evidence that increasing ROS scavenging and GSH production, using N-acetylcysteine, can increase EAAT and decrease Xc − expression in reactive astrocytes [239]. Hence, the consumption of a KD, which also results in increased GSH production in the brain via the upregulation of Nrf2 [43,46] would be expected to have a similar beneficial effect. There are also an increasing number of publications reporting reduced levels of NF-κB and PIC levels in astrocytes and indeed other regions of the brain following ingestion of various manifestations of the KD [1,240] and hence the diet seems to have the capacity to exert a corrective influence on several elements driving the EAAT2 downregulation seen in neurodegenerative and neuroprogressive disorders.
In addition, EAAT transcription is a downstream target of PPAR whose upregulation has a range of neuroprotective effects in neuropathological conditions in vivo [241,242]. This is of particular importance as there are several studies reporting upregulation of PPAR activity following the prolonged ingestion of a KD and this provides another mechanism by which diet-induced ketosis could upregulate the transcription of EAATs in reactive astrocytes [127,131]. In addition, there is an accumulating body of data suggesting that the upregulation of PPAR reduces neuroinflammation, which is of interest because the presence of this phenomenon is a major trigger of increased astrocyte reactivity as discussed above [136]. Furthermore, PPAR activation may rescue the compromised mitochondrial bioenergetics and dynamics seen in diseases such as AD and SZ [137,138].
Nutritional ketosis, astrogliosis, and NKA function
This is of paramount importance from the perspective of this article. Glutamate and GABA uptake are energy consuming processes as previously discussed [199,243] due in part to the reliance of EAATs on the option function of NKA receptors which coexist in the same molecular complex [244] (reviewed by [243]). This enzyme, as the name suggests, is in turn dependent on adequate supplies of ATP [245] and hence its function is likely to be compromised in an environment of impaired bioenergetics characteristic of astrocytes in their reactive state [55].
Improving the function of NKA is clearly a desirable therapeutic target and in that context, it is important to note that several research teams have reported that protracted, acute, or intermittent ketosis activates or increases the expression NKA pumps in the brain [68,246,247]. It should be stressed that this finding is not only important from the perspective of glutamate homeostasis as the interplay between NKA and EAATs, but also plays an important role in regulating levels of K+ ions throughout the CNS [248]. From the perspective of K+ homeostasis, however, the weight of evidence suggests NKA is the most important player in this molecular partnership and plays the dominant role in K+ uptake into astrocytes, which in turn regulates neural function and excitability [167]. The dependence of K+ uptake into astrocytes on NKA activity goes some way to explaining evidence supplied by several authors confirming that the maintenance of K+ homeostasis is the most energy intensive role of astrocytes [249] and thus dependent on adequate supplies of ATP [250,251]. The function of NKA and its indispensable role in astrocyte is well documented and hence will not be considered here but any readers interested in a detailed consideration of the biochemistry underpinning its structure and functions are referred to excellent reviews by Roy et al. [245] and Rodrigo et al. [252].
However, from the perspective of this article, it should be emphasized that the activity of NKA is not just dependent on adequate supplies of ATP but is heavily influenced by the redox state of the intracellular and extracellular environment. Unsurprisingly, animal and human studies confirm that oxidative and nitrosative stress inhibits NKA activity in rat hippocampus and prefrontal cortex [253,254] and in patients with SZ and BPD [255,256]. A combination of oxidative stress would also appear to explain the downregulation of NKA activity seen in MDD, AD, and other neurodegenerative diseases (reviewed by [257]). These are important observations as accumulating evidence suggests that downregulation of NKA is partly responsible for the impaired ability of reactive astrocytes to regulate K+ homeostasis [211,258].
The causative role played by oxidative and nitrosative stress in NKA downregulation is further emphasized by studies reporting that prolonged use of antioxidant combinations such as vitamin C and E or N-acetylcysteine, α-tocopherol, and α-lipoic acid can produce clinically significant increases in NKA activity in the brain and periphery [259,260]. Thus, there is a prospect that the well-documented antioxidant properties of the KD may exert a similar effect assuming a similar effect size and may well underpin the observations reporting a positive effect of the KD on improving NKA function discussed above. However, there may be another mechanism by which nutritional ketosis may improve NKA function, which would appear to be under-discussed.
Briefly, several authors have also reported strong negative correlations between the extent of membrane lipid peroxidation and NKA activity in vivo in illnesses as diverse as cardiovascular disease, SZ, and BPD [255,256,261]. The relationship between increased membrane lipid peroxidation and increasing NKA dysfunction appears to be mediated by decreased membrane fluidity and PUFA content [260,262]. This is of importance as several studies have reported a significant increase in NKA activity following dietary supplementation with PUFAs [260,263–265]. These observations may be explained by reference to data confirming that dietary PUFAs can integrate into lipid membranes in the periphery and the brain combatting the drivers of lipid peroxidation and increasing membrane fluidity via mechanism (reviewed by [266]). In this context, it is noteworthy that the KD can elevate levels of PUFAs in the circulation, cerebrospinal fluid, and brain [267,268] and hence this property may afford yet another route by which diet-induced ketosis might upregulate NKA levels and function. There is also some evidence to suggest that reducing lipid membrane peroxidation and improving membrane stability in astrocytes may help to increase the expression of another receptor, which also plays an indispensable role in K+ homeostasis mediated by these glial cells, namely the aforementioned weak inwardly rectifying Kir family containing Kir4.1 [269–271]. This is important as the downregulation of this receptor in reactive astrocytes is the other major cause of impaired K+ homeostasis in the brain [183,184].
Nutritional ketosis, astrogliosis, and Kir4.1 function
These findings are a reflection of the fact that astrocyte-mediated K+ buffering is mainly enabled by the presence of Kir family potassium channels containing Kir4.1 and Kir4.1/5.1 subunits [272,273]. Much evidence suggests that the Kir4.1 is the most important channel in astrocyte-mediated spatial buffering and may be responsible for up to 45% of potassium buffering in the hippocampus [274,275]. Readers interested in regarding the structure and mechanisms underpinning the operation of Kir4.1 and other astrocyte Kir family channels are invited to consult the work of Brill et al. [276].
Kir4.1 activity has been associated with several other elements involved in astrocyte structure and function such as the regulation of astrocyte cell volume, astrocyte K+ conductance, resting membrane potential, and glutamate uptake [277,278]. Importantly, several research teams have reported that inhibition of this channel leads to increased K+ concentrations in the extracellular space and impaired glutamate uptake [279,280]. The resultant increase of glutamate in the synaptic cleft resulting from Kir4.1 inhibition results in abnormal modulation of synaptic transmission and network level communication [277,281] and is associated with the development and maintenance of neuroinflammation [282,283]. The pathological significance of Kir4.1 downregulation in a neuroinflammatory environment is further emphasized by the results of several in vivo studies reporting reduced expression and/or function of this receptor in several neurodegenerative diseases, most notably multiple sclerosis and AD [284–287].
Downregulation of Kir4.1 is also seen in patients with MDD [288] and there is some evidence to suggest that reduced Kir4.1 expression plays a causative role in the development of SZ and ASD [289].
Given the potential therapeutic importance of improving Kir4.1 expression and/or function, it is encouraging to note that there is evidence that some of the elements responsible for the downregulation of astrocytic Kir4.1 receptors in an environment of chronic neuroinflammation are very similar if not identical to the drivers of impaired EAAT expression and activity discussed above with increased IL-1 [270,282,290] and glutathionylation [291] playing important inhibitory roles. Hence, the proven capacity of nutritional ketosis to reduce levels of oxidative stress and inflammation in the brain discussed on several occasions above might be expected to improve the expression and function of this receptor assuming that such reductions are of sufficient magnitude needed to produce such an effect. Other factors known to reduce Kir4.1 expression in vivo seen in neurodegenerative and neuroprogressive conditions include high levels of extracellular glutamate, which stimulate NMDA receptors located on astrocytes leading to Kir4.1 downregulation [292–294].
Hence, improvements in glutamate reuptake by astrocytes coupled with amelioration of glutamate dyshomeostasis, which may stem from nutritional ketosis would be expected to produce concomitant improvements in Kir4.1-mediated astrocyte K+ buffering. Increased levels of ATP fostered by the bioenergetic and metabolic consequences of induced ketosis may also be of therapeutic benefit as far as improving K+ homeostasis mediated by Kir4.1 is concerned as the optimum function of this receptor is also dependent on adequate levels of ATP [285,286,295,296]. Consequently, the documented improvements in oxidative stress, neuroinflammation, glutamate homeostasis, and ATP production in the CNS following prolonged ingestion of various KDs would be expected to result in beneficial effects on Kir4.1 levels and function.
Nutritional ketosis, astrogliosis, AQP4, and gap junction function
There are replicated in vivo data confirming that nutritional ketosis and/or BHB administration may upregulate AQP4 activity [70,297] and normalize Cx43 gap junction function [298–300]. This is also important from the perspective of K+ homeostasis as the activity of Kir4.1 channels in astrocyte-mediated K+ buffering is aided by the activity of AQP4 (reviewed by [301]) and astrocyte gap junctions [275].
The expression, structure, and activity of AQP4 is significantly compromised in the environment of chronic neuroinflammation seen in neurodegenerative and neuroprogressive disorders [302,303]. These ROS- and NO-mediated alterations lead to compromised performance of the receptor in regulating K+ homeostasis and several other dimensions of CNS homeostasis such as water balance, glutamate uptake, adult neurogenesis, and astrocyte migration (reviewed by [304]). Impaired expression of AQP4 perpetuates and exacerbates neuroinflammation and astrogliosis and this phenomenon also underpins the detrimental role played by this receptor in the pathophysiology of AD, PD, MDD, and ASD [305] (reviewed by [303]).
Astrocytic Cx43 gap junctions and pannexin hemichannels are held in an open configuration in a state of chronic neuroinflammation and oxidative stress as a result of the S-nitrosylation and oxidative modification of regulatory cysteine thiol motifs leading to conformational changes and loss of functional plasticity (reviewed by [306,307]). This compromised function not only impairs their role in K+ homeostasis but also negatively impairs astrocyte-mediated glutamate uptake and dispersal [308,309]. There is also evidence that a state of chronically open gap junctions induces and exacerbates neuroinflammation [308,309] (reviewed by [310]).
These consequences would appear to underpin at least in part evidence implicating gap junction and pannexin hemichannel dysfunction as another causative factor in the development of neurodegenerative and neuroprogressive disorders [309,311] (reviewed by [312]).
Nutritional ketogenesis, astrogliosis, and KATP channel function
Several animal studies have reported that prolonged ingestion of the KD or administration of the KD results in the opening of Kir6.1 family KATP channels located in cell plasma membranes (sKATP) and in the outer membranes of mitochondria (mtKATP) [44, 313–315]. It could be argued that this effect results from increasing ATP levels, which are increased following ingestion of various KDs as discussed above. However, given the fact that these channels are opened in an environment of low ATP and high ATP [316,317] and the significant increase in ATP production in the brain induced by the KD, it is possible that KATP opening is mediated by decreasing oxidative stress and neuroinflammation. This argument is strengthened by evidence demonstrating inhibition of KATP channels by glutathionylation in an environment of increased oxidative stress [309–311, 318].
Irrespective of the mechanisms underpinning such upregulation, however, there is evidence to suggest that increased activity of these channels also has positive consequences for astrocyte function. For example, one such consequence is improved sequestration of K+ into mitochondria via a mechanism, which is similar in many respects to the mechanism enabling the sequestration of iron [312,313]. There is also evidence, albeit in vitro, of a positive association between the activation of astrocytic mtKATP channels and upregulation of electrical coupling between astrocytes in the hippocampus which is an effect mediated via increased efficiency of Cx43 gap junction function secondary to upregulated ERK signaling in astrocytic mitochondria (reviewed by [299]). It is also noteworthy that opening of astrocyte mtKATP channels may also be important from the perspective of CNS homeostasis as its upregulation appears to be an important element in maintaining the stability of the wider astrocyte neurovascular unit [314].
There are also replicated data suggesting that opening mtKATP channels may make a significant contribution to astrocyte survival in an environment of chronic inflammation and oxidative stress by inhibiting the translocation of Bax and the release of cytochrome c oxidase from mitochondria into the cytosol thereby inhibiting ROS- or TNF-mediated apoptosis [315–317]. Finally, one team of researchers has produced tantalizing evidence of an association between the opening of KATP channels and the inhibition of astrocyte activation and the prevention of astrogliosis [318].
Caveats and Uncertainty
Although the data reviewed above suggest that BHB entry into the brain is a major driver of the therapeutic benefits of the KD or of KB supplements, it should be emphasized that other factors may be involved and the mechanisms underpinning such therapeutic benefits are not completely understood either in the case of epilepsy or otherwise. For example, several authors have reported profound changes in the composition of the microbiota following the administration of a KD in children with intractable epilepsy, which appear to be important if not essential for seizure control [231,319,320]. In general, increases in Firmicutes and Actinobacteria are seen in KD-responding children. Although, increases in Alistipes and Ruminococcaceae are apparent in nonresponders [319]. Similar patterns have been reported by authors investigating KD effects in animal models of epilepsy, with a positive effect being associated with changes in the composition of the microbiota associated with relative increases in levels of Akkermansia and Parabacteroides [231]. Data suggesting that fecal transplants based on Akkermansia and Parabacteroides also exert an antiseizure effect are also of interest [231]. The association between changes in the microbiota and improved seizure control can be understood in the context of accumulating evidence demonstrating that the composition of the microbiota exerts profound effects on metabolism and inflammatory status via numerous mechanisms such as influencing levels of short-chain FAs and intestinal barrier integrity [321,322]. For example, increased levels of Akkermansia and Parabacteroides increase intestinal barrier integrity via a positive effect on epithelial tight junction proteins and hence reduce the translocation of commensal antigens into the blood, the latter being a powerful driver of peripheral inflammation [323–325]. Elevated levels of BHB also result in the suppression of peripheral inflammation via the inhibition of NF-κB and the NLRP3 inflammasome (reviewed by [1]). This latter point is important because peripheral inflammation is also a driver of pro-inflammatory dysbiosis via mechanisms explained in [326] and [321]. Hence, the reduction in peripheral inflammation seen in individuals following protracted consumption of a KD could potentially explain the positive effects on the composition of the gut population seen above, which in turn could make an independent contribution to the reduction of peripheral inflammation. This is an important point because peripheral inflammation in the guise of elevated PICs is a major driver of microglial and astrocytic activation, proliferation and/or dysfunction [327,328], which are all involved in the development of severe intractable epilepsy [329,330]. Hence, it is tempting to conclude that the reduction in peripheral inflammation explains the positive effects of a KD on seizure control and on the microbiome. However, rodents consuming a KD have an increased GABA/glutamate ratio in their brains, which appears to stem from positive changes to the composition of the microbiota [278]. This observation is supported by other lines of evidence suggesting that manipulation of the gut commensal population can exert positive effects on glutamatergic neurotransmission, which is compromised in patients with intractable epilepsy and neuroprogressive disorders [331–333]. In addition, the reduction of dysbiosis or positive changes to the composition of the microbiota can exert a number of additional neuroprotective effects mediated via the enteric nervous system and the vagus nerve (reviewed by [326]). Thus, the positive effects on seizure control effected by the KD associated with changes in the microbiota are likely underpinned by multifactorial mechanisms.
There is also evidence to suggest that a KD may exert antiseizure and neuroprotective effects by inducing a unique metabolic state of increased serum leptin in combination with reduced serum insulin [334–337]. This state is associated with modification of the PI3k/Akt/mTOR signaling axis and AMPK levels and therefore may be responsible for the reduced levels of mTOR and Akt seen in the hypothalamus following prolonged intake of a KD [338,339]. Increased leptin in the brain may result in improved function of KATP channels, inhibition of AMPA receptors, and improved function of NMDA receptors via a mechanism dependent on increased PI3K signaling [340,341]. Reduced levels of insulin in the periphery in patients with metabolic syndrome or type 2 diabetes mellitus, frequently seen in patients with neuroprogressive disorders [342], can also exert neuroprotective effects by reducing the translocation of ceramide into the CNS which is often described as the liver–brain axis of neurodegeneration (reviewed by [343]). The range of neuroprotective effects potentially resulting from a metabolic state of increased leptin and reduced insulin in the periphery and the mechanisms involved are numerous and readers interested in pursuing this area are invited to consult an excellent review of the subject by [339].
An attempt at explaining the neuroprotective effects of induced ketosis or ketonemia is further complicated by evidence suggesting that a low glycemic index diet may also exert antiseizure activity [344]. This neuroprotective effect could potentially be explained by reduced insulin and triglyceride levels coupled with improved insulin resistance, which both induce anti-inflammatory effects and hence would be expected to reduce levels of glial cell pathology via mechanisms discussed above [345,346]. However, a perusal of the literature suggests that low glycemic carbohydrates have largely been used in the context of a KD and hence the effectiveness of this approach is difficult to assess [347]. A study comparing the effects on seizure control of a low glycemic diet, which does not involve the induction of ketosis compared with the use of low glycemic carbohydrates in the context of a KD may well provide clarity in this area.
Conclusions
This article illustrates how the entry of KBs and FAs into the CNS, as a result of a ketotic state resulting from nutritional ketosis, has effects on the astrocytic glutamate–glutamine cycle, GS activity, and on the function of VGLUTs, EAATs, NKA, Kir4.1, AQP4, Cx34, and KATP, as well as on astrogliosis, which would tend to mitigate the changes seen in a wide range of neurodegenerative and neuroprogressive disorders. These disorders include, but are not limited to, AD, PD, HD, SZ, BPD, MDD, and ASD. It is therefore plausible to hypothesize that nutritional ketosis may have therapeutic applications in the treatment of such disorders. However, it must also be stated that the KD results in a number of effects in the periphery such as changes in the composition of the microbiota, and alterations in levels of leptin and insulin, which combined with a reduction of inflammation, may also contribute to the antiseizure and neuroprotective effects of induced ketosis or ketonemia.
Acknowledgments
M.B. is supported by a National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1059660 and APP1156072).
Glossary
- ACA
acetoacetate
- AD
Alzheimer’s disease
- ALS
amyloid lateral sclerosis
- AQP4
aquaporin-4
- ASD
autistic spectrum disorder
- BBB
blood–brain barrier
- BDNF
brain-derived neurotrophic factor
- BHB
β-hydroxybutyrate
- BPD
bipolar disorder
- CNS
central nervous system
- Cx43
connexin 43
- ETC
electron transfer chain
- FA
fatty acid
- FFA
free fatty acid
- GS
glutamate synthetase
- GSH
reduced glutathione
- HD
Huntington’s disease
- KB
ketone body
- KD
ketogenic diet
- LPS
lipopolysaccharide
- MCFA
medium-chain fatty acid
- MCT
medium-chain triglyceride
- MDD
major depressive disorder
- NEFA
nonesterified fatty acid
- NKA
Na+, K + -ATPase
- PC
pyruvate carboxylase
- PD
Parkinson’s disease
- PIC
pro-inflammatory cytokine
- PUFA
polyunsaturated fatty acid
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SIRT
sirtuin
- SOD
superoxide dismutase
- SZ
schizophrenia
- TCA
tricarboxylic acid
- VGLUT
vesicular glutamate transporter
- Xc-
cysteine/glutamate antiporter channel
Conflict of Interest
The authors have no conflicts of interest to declare.
Authorship Contributions.
All authors contributed to the writing up of the article.
References
- [1]. Pinto A, Bonucci A, Maggi E, Corsi M, Businaro R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: new perspectives for neuroprotection in Alzheimer's disease. Antioxidants. 2018;7(5):63. 10.3390/antiox7050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55(11):2211–2228. 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–506. 10.1016/s1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- [4]. Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50(5):1109–1117. 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- [5]. Klein P, Tyrlikova I, Mathews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology. 2014;83(21):1978–1985. 10.1212/wnl.0000000000001004. [DOI] [PubMed] [Google Scholar]
- [6]. Liu H, Yang Y, Wang Y, Tang H, Zhang F, Zhang Y, et al. Ketogenic diet for treatment of intractable epilepsy in adults: a meta-analysis of observational studies. Epilepsia Open. 2018;3(1):9–17. 10.1002/epi4.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Kim JA, Yoon JR, Lee EJ, Lee JS, Kim JT, Kim HD, et al. Efficacy of the classic ketogenic and the modified Atkins diets in refractory childhood epilepsy. Epilepsia. 2016;57(1):51–58. 10.1111/epi.13256. [DOI] [PubMed] [Google Scholar]
- [8]. Lange KW, Lange KM, Makulska-Gertruda E, Nakamura Y, Reissmann A, Kanaya S, et al. Ketogenic diets and Alzheimer’s disease. Food Sci Hum Wellness. 2017;6(1):1–9. 10.1016/j.fshw.2016.10.003. [DOI] [Google Scholar]
- [9]. Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–314. 10.1016/s0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- [10]. Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer's disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metabol. 2009;6(1):31. 10.1186/1743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Sharma A, Bemis M, Desilets AR. Role of medium chain triglycerides (Axona®) in the treatment of mild to moderate Alzheimer’s disease. Am J Alzheimer's Dis Other Dement. 2014;29(5):409–414. 10.1177/1533317513518650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33(2):425.e419–427.e419. 10.1016/j.neurobiolaging.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer's disease. Alzheimers Dement. 2015;11(1):99–103. 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson's disease: a pilot randomized controlled trial. Mov Disord. 2018;33(8):1306–1314. 10.1002/mds.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Wlodarczyk A, Wiglusz MS, Cubala WJ. Ketogenic diet for schizophrenia: nutritional approach to antipsychotic treatment. Med Hypotheses. 2018;118:74–77. 10.1016/j.mehy.2018.06.022. [DOI] [PubMed] [Google Scholar]
- [16]. Phelps JR, Siemers SV, El-Mallakh RS. The ketogenic diet for type II bipolar disorder. Neurocase. 2013;19(5):423–426. 10.1080/13554794.2012.690421. [DOI] [PubMed] [Google Scholar]
- [17]. Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Kolosowska K, Krzascik P, et al. Is the interaction between fatty acids and tryptophan responsible for the efficacy of a ketogenic diet in epilepsy? The new hypothesis of action. Neuroscience. 2016;313:130–148. 10.1016/j.neuroscience.2015.11.029. [DOI] [PubMed] [Google Scholar]
- [18]. Bostock ECS, Kirkby KC, Taylor BVM. The current status of the ketogenic diet in psychiatry. Front Psychiatry. 2017;8(43). 10.3389/fpsyt.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36(1):32–40. 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. LaManna JC, Salem N, Puchowicz M, Erokwu B, Koppaka S, Flask C, et al. Ketones suppress brain glucose consumption. Adv Exp Med Biol. 2009;645:301–306. 10.1007/978-0-387-85998-9_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Zhang Y, Kuang Y, Xu K, Harris D, Lee Z, LaManna J, et al. Ketosis proportionately spares glucose utilization in brain. J Cereb Blood Flow Metab. 2013;33(8):1307–1311. 10.1038/jcbfm.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Courchesne-Loyer A, Croteau E, Castellano CA, St-Pierre V, Hennebelle M, Cunnane SC. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab. 2017;37(7):2485–2493. 10.1177/0271678x16669366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. de Ceballos ML, Köfalvi A. Boosting brain glucose metabolism to fight neurodegeneration? Oncotarget. 2017;8(9):14273–14274. 10.18632/oncotarget.15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Firbank MJ, Yarnall AJ, Lawson RA, Duncan GW, Khoo TK, Petrides GS, et al. Cerebral glucose metabolism and cognition in newly diagnosed Parkinson's disease: ICICLE-PD study. J Neurol Neurosurg Psychiatry. 2017;88(4):310–316. 10.1136/jnnp-2016-313918. [DOI] [PubMed] [Google Scholar]
- [25]. Endo H, Sekiguchi K, Ueda T, Kowa H, Kanda F, Toda T. Regional glucose hypometabolic spread within the primary motor cortex is associated with amyotrophic lateral sclerosis disease progression: a fluoro-deoxyglucose positron emission tomography study. eNeurologicalSci. 2017;6:74–79. 10.1016/j.ensci.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Seethalakshmi R, Parkar SR, Nair N, Adarkar SA, Pandit AG, Batra SA, et al. Regional brain metabolism in schizophrenia: an FDG-PET study. Indian J Psychiatry. 2006;48(3):149–153. 10.4103/0019-5545.31577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Fabrazzo M. Impaired glucose metabolism in bipolar patients and response to mood stabilizer treatments. J Affect Disord. 2018;245:174–179. 10.1016/j.jad.2018.10.360. [DOI] [PubMed] [Google Scholar]
- [28]. Su L, Cai Y, Xu Y, Dutt A, Shi S, Bramon E. Cerebral metabolism in major depressive disorder: a voxel-based meta-analysis of positron emission tomography studies. BMC Psychiatry. 2014;14:321–321. 10.1186/s12888-014-0321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Zilberter Y, Zilberter M. The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J Neurosci Res. 2017;95(11):2217–2235. 10.1002/jnr.24064. [DOI] [PubMed] [Google Scholar]
- [30]. Nylen K, Velazquez JLP, Sayed V, Gibson KM, Burnham WM, Snead OC IIIrd. The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(−/−) mice. Biochim Biophys Acta. 2009;1790(3):208–212. 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Greco T, Glenn TC, Hovda DA, Prins ML. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J Cereb Blood Flow Metabol. 2016;36(9):1603–1613. 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Hasan-Olive MM, Lauritzen KH, Ali M, Rasmussen LJ, Storm-Mathisen J, Bergersen LH. A ketogenic diet improves mitochondrial biogenesis and bioenergetics via the PGC1alpha-SIRT3-UCP2 axis. Neurochem Res. 2019;44(1):22–37. 10.1007/s11064-018-2588-6. [DOI] [PubMed] [Google Scholar]
- [33]. Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106(3):1044–1051. 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- [34]. Kim DY, Abdelwahab MG, Lee SH, O'Neill D, Thompson RJ, Duff HJ, et al. Ketones prevent oxidative impairment of hippocampal synaptic integrity through KATP channels. PLoS One. 2015;10(4):e0119316. 10.1371/journal.pone.0119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Li X, Valencia A, McClory H, Sapp E, Kegel KB, Difiglia M. Deficient Rab11 activity underlies glucose hypometabolism in primary neurons of Huntington’s disease mice. Biochem Biophys Res Commun. 2012;421(4):727–730. https://doi:10.1016/j.bbrc.2012.04.070. [DOI] [PubMed] [Google Scholar]
- [36]. Milder J, Patel M. Modulation of oxidative stress and mitochondrial function by the ketogenic diet. Epilepsy Res. 2012;100(3):295–303. 10.1016/j.eplepsyres.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis. 2010;40(1):238–244. 10.1016/j.nbd.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, et al. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 2015;74:101–110. 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- [39]. Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8(21):2003–2014. 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13:68. 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M, et al. The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. 2018;84:453–469. 10.1016/j.neubiorev.2017.07.017. [DOI] [PubMed] [Google Scholar]
- [43]. Nakamura T, Lipton SA. SNO'-storms compromise protein activity and mitochondrial metabolism in neurodegenerative disorders. Trends Endocrinol Metab. 2017;28(12):879–892. 10.1016/j.tem.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Sominsky L, De Luca S, Spencer SJ. Microglia: key players in neurodevelopment and neuronal plasticity. Int J Biochem Cell Biol. 2018;94:56–60. 10.1016/j.biocel.2017.11.012. [DOI] [PubMed] [Google Scholar]
- [45]. Du R-H, Sun H-B, Hu Z-L, Lu M, Ding J-H, Hu G. Kir6.1/K-ATP channel modulates microglia phenotypes: implication in Parkinson’s disease. Cell Death Dis. 2018;9(3):404. 10.1038/s41419-018-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Rose J, Brian C, Woods J, Pappa A, Panayiotidis MI, Powers R, et al. Mitochondrial dysfunction in glial cells: implications for neuronal homeostasis and survival. Toxicology. 2017;391:109–115. 10.1016/j.tox.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Kubik LL, Philbert MA. The role of astrocyte mitochondria in differential regional susceptibility to environmental neurotoxicants: tools for understanding neurodegeneration. Toxicol Sci. 2015;144(1):7–16. 10.1093/toxsci/kfu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- [49]. Sarkar S, Malovic E, Harishchandra DS, Ghaisas S, Panicker N, Charli A, et al. Mitochondrial impairment in microglia amplifies NLRP3 inflammasome proinflammatory signaling in cell culture and animal models of Parkinson's disease. NPJ Parkinsons Dis. 2017;3:30. 10.1038/s41531-017-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Ignatenko O, Chilov D, Paetau I, de Miguel E, Jackson CB, Capin G, et al. Loss of mtDNA activates astrocytes and leads to spongiotic encephalopathy. Nat Commun. 2018;9(1):70. 10.1038/s41467-017-01859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Colangelo AM, Alberghina L, Papa M. Astrogliosis as a therapeutic target for neurodegenerative diseases. Neurosci Lett. 2014;565:59–64. 10.1016/j.neulet.2014.01.014. [DOI] [PubMed] [Google Scholar]
- [52]. Pekny M, Pekna M. Reactive gliosis in the pathogenesis of CNS diseases. Biochim Biophys Acta Mol Basis Dis. 2016;1862(3):483–491. 10.1016/j.bbadis.2015.11.014. [DOI] [PubMed] [Google Scholar]
- [53]. Kim R, Healey KL, Sepulveda-Orengo MT, Reissner KJ. Astroglial correlates of neuropsychiatric disease: from astrocytopathy to astrogliosis. Progr Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt A):126–146. 10.1016/j.pnpbp.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V. Pathobiology of neurodegeneration: the role for astroglia. Opera Med Physiol. 2016;1:13–22. [PMC free article] [PubMed] [Google Scholar]
- [55]. Tay TL, Béchade C, D'Andrea I, St-Pierre M-K, Henry MS, Roumier A, et al. Microglia gone rogue: impacts on psychiatric disorders across the lifespan. Front Mol Neurosci. 2018;10:421. 10.3389/fnmol.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Du L, Zhang Y, Chen Y, Zhu J, Yang Y, Zhang HL. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol Neurobiol. 2017;54(10):7567–7584. 10.1007/s12035-016-0245-0. [DOI] [PubMed] [Google Scholar]
- [57]. Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58(5):588–598. 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- [58]. Wu G, Liu Z. Nuclear factor erythroid 2-related factor 2 (Nrf2) mediates neuroprotection in traumatic brain injury at least in part by inactivating microglia. Med Sci Monit. 2016;22:2161–2166. 10.12659/MSM.896568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1):43–58. 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- [60]. Lutas A, Birnbaumer L, Yellen G. Metabolism regulates the spontaneous firing of substantia nigra pars reticulata neurons via KATP and nonselective cation channels. J Neurosci. 2014;34(49):16336–16347. 10.1523/jneurosci.1357-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Woolf EC, Curley KL, Liu Q, Turner GH, Charlton JA, Preul MC, et al. The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model. PLoS One. 2015;10(6):e0130357. 10.1371/journal.pone.0130357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Melo TM, Nehlig A, Sonnewald U. Neuronal–glial interactions in rats fed a ketogenic diet. Neurochem Int. 2006;48(6–7):498–507. 10.1016/j.neuint.2005.12.037. [DOI] [PubMed] [Google Scholar]
- [63]. Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6:27. 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria, and neurological diseases. J Lipid Res. 2014;55(11):2211–2228. 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Dedkova EN, Blatter LA. Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front Physiol. 2014;5:260. 10.3389/fphys.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Grabacka M, Pierzchalska M, Dean M, Reiss K. Regulation of ketone body metabolism and the role of PPARα. Int J Mol Sci. 2016;17(12):2093. 10.3390/ijms17122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Greene AE, Todorova MT, Seyfried TN. Perspectives on the metabolic management of epilepsy through dietary reduction of glucose and elevation of ketone bodies. J Neurochem. 2003;86(3):529–537. [DOI] [PubMed] [Google Scholar]
- [68]. Veech RL, Chance B, Kashiwaya Y, Lardy HA, Cahill GF Jr. Ketone bodies, potential therapeutic uses. IUBMB Life. 2001;51(4):241–247. 10.1080/152165401753311780. [DOI] [PubMed] [Google Scholar]
- [69]. Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304(8):H1060–H1076. 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Sahni PV, Zhang J, Sosunov S, Galkin A, Niatsetskaya Z, Starkov A, et al. Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic-ischemic brain injury in mice. Pediatr Res. 2018;83(2):491–497. 10.1038/pr.2017.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Balietti M, Giorgetti B, Di Stefano G, Casoli T, Platano D, Solazzi M, et al. A ketogenic diet increases succinic dehydrogenase (SDH) activity and recovers age-related decrease in numeric density of SDH-positive mitochondria in cerebellar Purkinje cells of late-adult rats. Micron. 2010;41(2):143–148. 10.1016/j.micron.2009.08.010. [DOI] [PubMed] [Google Scholar]
- [72]. Elamin M, Ruskin DN, Masino SA, Sacchetti P. Ketone-based metabolic therapy: is increased NAD(+) a primary mechanism? Front Mol Neurosci. 2017;10:377. 10.3389/fnmol.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Kim DY, Vallejo J, Rho JM. Ketones prevent synaptic dysfunction induced by mitochondrial respiratory complex inhibitors. J Neurochem. 2010;114(1):130–141. 10.1111/j.1471-4159.2010.06728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, et al. A ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric western diet. Front Physiol. 2016;7:533. 10.3389/fphys.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Bentourkia M, Tremblay S, Pifferi F, Rousseau J, Lecomte R, Cunnane S. PET study of 11C-acetoacetate kinetics in rat brain during dietary treatments affecting ketosis. Am J Physiol Endocrinol Metabol. 2009;296(4):E796–E801. 10.1152/ajpendo.90644.2008. [DOI] [PubMed] [Google Scholar]
- [76]. Pifferi F, Tremblay S, Plourde M, Tremblay-Mercier J, Bentourkia M, Cunnane SC. Ketones and brain function: possible link to polyunsaturated fatty acids and availability of a new brain PET tracer, 11C-acetoacetate. Epilepsia. 2008;49(Suppl 8):76–79. 10.1111/j.1528-1167.2008.01842.x. [DOI] [PubMed] [Google Scholar]
- [77]. Carneiro L, Geller S, Hébert A, Repond C, Fioramonti X, Leloup C, et al. Hypothalamic sensing of ketone bodies after prolonged cerebral exposure leads to metabolic control dysregulation. Sci Rep. 2016;6:34909. 10.1038/srep34909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R, et al. Mild experimental ketosis increases brain uptake of 11C-acetoacetate and 18F-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr Neurosci. 2011;14(2):51–58. 10.1179/1476830510y.0000000001. [DOI] [PubMed] [Google Scholar]
- [79]. Roy M, Nugent S, Tremblay-Mercier J, Tremblay S, Courchesne-Loyer A, Beaudoin JF, et al. The ketogenic diet increases brain glucose and ketone uptake in aged rats: a dual tracer PET and volumetric MRI study. Brain Res. 2012;1488:14–23. 10.1016/j.brainres.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [80]. Hashim SA, VanItallie TB. Ketone body therapy: from the ketogenic diet to the oral administration of ketone ester. J Lipid Res. 2014;55(9):1818–1826. 10.1194/jlr.R046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Hugo SE, Cruz-Garcia L, Karanth S, Anderson RM, Stainier DYR, Schlegel A. A monocarboxylate transporter required for hepatocyte secretion of ketone bodies during fasting. Genes Dev. 2012;26(3):282–293. 10.1101/gad.180968.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82]. Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab. 2013;33(10):1493–1499. 10.1038/jcbfm.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83]. White H, Venkatesh B. Clinical review: ketones and brain injury. Crit Care. 2011;15(2):219. 10.1186/cc10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84]. Januszewicz E, Pająk B, Gajkowska B, Samluk Ł, Djavadian RL, Hinton BT, et al. Organic cation/carnitine transporter OCTN3 is present in astrocytes and is up-regulated by peroxisome proliferators-activator receptor agonist. Int J Biochem Cell Biol. 2009;41(12):2599–2609. 10.1016/j.biocel.2009.08.020. [DOI] [PubMed] [Google Scholar]
- [85]. Nylen K, Velazquez JL, Sayed V, Gibson KM, Burnham WM, Snead OC IIIrd. The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1(−/−) mice. Biochim Biophys Acta. 2009;1790(3):208–212. 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86]. Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5(4):375–380. 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- [87]. DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB Jr. Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3(4):331–337. 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- [88]. Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, Brederode J, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101(5):1316–1326. 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- [89]. Masino S, Kawamura M Jr, Wasser C, Pomeroy L, Ruskin D. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7(3):257–268. 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60(2):223–235. 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- [91]. Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40(6):703–707. 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- [92]. Noh HS, Lee HP, Kim DW, Kang SS, Cho GJ, Rho JM, et al. A cDNA microarray analysis of gene expression profiles in rat hippocampus following a ketogenic diet. Brain Res Mol Brain Res. 2004;129(1–2):80–87. 10.1016/j.molbrainres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- [93]. Achanta LB, Rowlands BD, Thomas DS, Housley GD, Rae CD. beta-hydroxybutyrate boosts mitochondrial and neuronal metabolism but is not preferred over glucose under activated conditions. Neurochem Res. 2017;42(6):1710–1723. 10.1007/s11064-017-2228-6. [DOI] [PubMed] [Google Scholar]
- [94]. Chowdhury GM, Jiang L, Rothman DL, Behar KL. The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J Cereb Blood Flow Metab. 2014;34(7):1233–1242. 10.1038/jcbfm.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Croteau E, Castellano CA, Richard MA, Fortier M, Nugent S, Lepage M, et al. Ketogenic medium chain triglycerides increase brain energy metabolism in Alzheimer's disease. J Alzheimer's Dis. 2018;64(2):551–561. 10.3233/jad-180202. [DOI] [PubMed] [Google Scholar]
- [96]. Ziegler DR, Ribeiro LC, Hagenn M, Siqueira IR, Araujo E, Torres IL, et al. Ketogenic diet increases glutathione peroxidase activity in rat hippocampus. Neurochem Res. 2003;28(12):1793–1797. [DOI] [PubMed] [Google Scholar]
- [97]. Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev. 2009;59(2):293–315. 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145(1):256–264. 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol. 2008;211(1):85–96. 10.1016/j.expneurol.2007.12.029. [DOI] [PubMed] [Google Scholar]
- [100]. Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55(4):576–580. 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- [101]. Kim DY, Davis LM, Sullivan PG, Maalouf M, Simeone TA, van Brederode J, et al. Ketone bodies are protective against oxidative stress in neocortical neurons. J Neurochem. 2007;101(5):1316–1326. 10.1111/j.1471-4159.2007.04483.x. [DOI] [PubMed] [Google Scholar]
- [102]. Julio-Amilpas A, Montiel T, Soto-Tinoco E, Geronimo-Olvera C, Massieu L. Protection of hypoglycemia-induced neuronal death by beta-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab. 2015;35(5):851–860. 10.1038/jcbfm.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103]. Camberos-Luna L, Geronimo-Olvera C, Montiel T, Rincon-Heredia R, Massieu L. The ketone body, beta-hydroxybutyrate stimulates the autophagic flux and prevents neuronal death induced by glucose deprivation in cortical cultured neurons. Neurochem Res. 2016;41(3):600–609. 10.1007/s11064-015-1700-4. [DOI] [PubMed] [Google Scholar]
- [104]. Narasimhan M, Rajasekaran NS. Exercise, Nrf2 and antioxidant signaling in cardiac aging. Front Physiol. 2016;7(241). 10.3389/fphys.2016.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42(12):1838–1850. 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- [106]. Vargas MR, Johnson JA. The Nrf2-ARE cytoprotective pathway in astrocytes. Expert Rev Mol Med. 2009;11:e17. 10.1017/s1462399409001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Miller VJ, Villamena FA, Volek JS. Nutritional ketosis and mitohormesis: potential implications for mitochondrial function and human health. J Nutr Metabol. 2018;2018:27. 10.1155/2018/5157645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52(3):e7–e11. 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Paoli A, Bosco G, Camporesi EM, Mangar D. Ketosis, ketogenic diet and food intake control: a complex relationship. Front Psychol. 2015;6(27). 10.3389/fpsyg.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Blazquez C, Woods A, de Ceballos ML, Carling D, Guzman M. The AMP-activated protein kinase is involved in the regulation of ketone body production by astrocytes. J Neurochem. 1999;73(4):1674–1682. [DOI] [PubMed] [Google Scholar]
- [111]. Elamin M, Ruskin DN, Masino SA, Sacchetti P. Ketogenic diet modulates NAD(+)-dependent enzymes and reduces DNA damage in hippocampus. Front Cell Neurosci. 2018;12:263. 10.3389/fncel.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Xin L, Ipek Ö, Beaumont M, Shevlyakova M, Christinat N, Masoodi M, et al. Nutritional ketosis increases NAD(+)/NADH ratio in healthy human brain: an in vivo study by (31)P-MRS. Front Nutr. 2018;5:62. 10.3389/fnut.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Elamin M, Ruskin DN, Masino SA, Sacchetti P. Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front Cell Neurosci. 2018;12(263). 10.3389/fncel.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20(5):840–855. 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Draznin B, Wang C, Adochio R, Leitner JW, Cornier MA. Effect of dietary macronutrient composition on AMPK and SIRT1 expression and activity in human skeletal muscle. Hormone Metab Res. 2012;44(9):650–655. 10.1055/s-0032-1312656. [DOI] [PubMed] [Google Scholar]
- [116]. Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, et al. A ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric western diet. Front Physiol. 2016;7(533). 10.3389/fphys.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, et al. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. 2011;232(2):195–202. 10.1016/j.expneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- [118]. Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, et al. A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev. 2017;74(Pt A):1–20. 10.1016/j.neubiorev.2017.01.014. [DOI] [PubMed] [Google Scholar]
- [119]. Morris G, Berk M, Carvalho AF, Maes M, Walker AJ, Puri BK. Why should neuroscientists worry about iron? The emerging role of ferroptosis in the pathophysiology of neuroprogressive diseases. Behav Brain Res. 2018;341:154–175. 10.1016/j.bbr.2017.12.036. [DOI] [PubMed] [Google Scholar]
- [120]. Morris G, Maes M, Berk M, Puri BK. Myalgic encephalomyelitis or chronic fatigue syndrome: how could the illness develop? Metabol Brain Dis. 2019;34(2):385–415. 10.1007/s11011-019-0388-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Cullingford T. Peroxisome proliferator-activated receptor alpha and the ketogenic diet. Epilepsia. 2008;49(Suppl 8):70–72. 10.1111/j.1528-1167.2008.01840.x. [DOI] [PubMed] [Google Scholar]
- [122]. Sikder K, Shukla SK, Patel N, Singh H, Rafiq K. High fat diet upregulates fatty acid oxidation and ketogenesis via intervention of PPAR-gamma. Cell Physiol Biochem. 2018;48(3):1317–1331. 10.1159/000492091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Simeone TA, Matthews SA, Samson KK, Simeone KA. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp Neurol. 2017;287(Pt 1):54–64. 10.1016/j.expneurol.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124]. Knowles S, Budney S, Deodhar M, Matthews SA, Simeone KA, Simeone TA. Ketogenic diet regulates the antioxidant catalase via the transcription factor PPARgamma2. Epilepsy Res. 2018;147:71–74. 10.1016/j.eplepsyres.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Esmaeili MA, Yadav S, Gupta RK, Waggoner GR, Deloach A, Calingasan NY, et al. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum Mol Genet. 2016;25(2):317–327. 10.1093/hmg/ddv477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Zolezzi JM, Santos MJ, Bastias-Candia S, Pinto C, Godoy JA, Inestrosa NC. PPARs in the central nervous system: roles in neurodegeneration and neuroinflammation. Biol Rev Camb Philos Soc. 2017;92(4):2046–2069. 10.1111/brv.12320. [DOI] [PubMed] [Google Scholar]
- [127]. Crunkhorn S. Boosting PPARδ blocks neurodegeneration. Nat Rev Drug Discov. 2016;15:83. 10.1038/nrd.2016.1. [DOI] [PubMed] [Google Scholar]
- [128]. Corona JC, Duchen MR. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med. 2016;100:153–163. 10.1016/j.freeradbiomed.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129]. Dello Russo C, Gavrilyuk V, Weinberg G, Almeida A, Bolanos JP, Palmer J, et al. Peroxisome proliferator-activated receptor γ thiazolidinedione agonists increase glucose metabolism in astrocytes. J Biol Chem. 2003;278(8):5828–5836. 10.1074/jbc.M208132200. [DOI] [PubMed] [Google Scholar]
- [130]. Nijland PG, Witte ME, van het Hof B, van der Pol S, Bauer J, Lassmann H, et al. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: implications for multiple sclerosis. Acta Neuropathol Commun. 2014;2(1):170. 10.1186/s40478-014-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131]. Chistyakov DV, Aleshin SE, Astakhova AA, Sergeeva MG, Reiser G. Regulation of peroxisome proliferator-activated receptors (PPAR) α and -γ of rat brain astrocytes in the course of activation by toll-like receptor agonists. J Neurochem. 2015;134(1):113–124. 10.1111/jnc.13101. [DOI] [PubMed] [Google Scholar]
- [132]. Hamby ME, Coppola G, Ao Y, Geschwind DH, Khakh BS, Sofroniew MV. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple G-protein-coupled receptors. J Neurosci. 2012;32(42):14489–14510. 10.1523/jneurosci.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Sada N, Inoue T. Electrical control in neurons by the ketogenic diet. Front Cell Neurosci. 2018;12:208. 10.3389/fncel.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134]. Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;8(Suppl 8):73–75. 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135]. Bruce KD, Zsombok A, Eckel RH. Lipid processing in the brain: a key regulator of systemic metabolism. Front Endocrinol. 2017;8:60. 10.3389/fendo.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136]. Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes. 2014;63(4):1259–1269. 10.2337/db13-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Takahashi S, Iizumi T, Mashima K, Abe T, Suzuki N. Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia. ASN Neuro. 2014;6(5). 10.1177/1759091414550997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, et al. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte–neuron lactate and ketone body shuttle systems. FASEB J. 2016;30(5):1913–1926. 10.1096/fj.201500182. [DOI] [PubMed] [Google Scholar]
- [139]. Guzman M, Blazquez C. Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab. 2001;12(4):169–173. [DOI] [PubMed] [Google Scholar]
- [140]. Guzman M, Blazquez C. Ketone body synthesis in the brain: possible neuroprotective effects. Prostagland Leukot Essent Fatty Acids. 2004;70(3):287–292. 10.1016/j.plefa.2003.05.001. [DOI] [PubMed] [Google Scholar]
- [141]. Freire-Regatillo A, Argente-Arizón P, Argente J, García-Segura LM, Chowen JA. Non-neuronal cells in the hypothalamic adaptation to metabolic signals. Front Endocrinol. 2017;8:51. 10.3389/fendo.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142]. Romano A, Koczwara JB, Gallelli CA, Vergara D, Micioni Di Bonaventura MV, Gaetani S, et al. Fats for thoughts: an update on brain fatty acid metabolism. Int J Biochem Cell Biol. 2017;84:40–45. 10.1016/j.biocel.2016.12.015. [DOI] [PubMed] [Google Scholar]
- [143]. Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–249. 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- [144]. Dienel GA, Cruz NF. Contributions of glycogen to astrocytic energetics during brain activation. Metab Brain Dis. 2015;30(1):281–298. 10.1007/s11011-014-9493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci. 2015;16(11):25959–25981. 10.3390/ijms161125939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146]. Schonfeld P, Reiser G. Inhibition of beta-oxidation is not a valid therapeutic tool for reducing oxidative stress in conditions of neurodegeneration. J Cereb Blood Flow Metab. 2017;37(3):848–854. 10.1177/0271678x16642448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147]. Thevenet J, De Marchi U, Domingo JS, Christinat N, Bultot L, Lefebvre G, et al. Medium-chain fatty acids inhibit mitochondrial metabolism in astrocytes promoting astrocyte-neuron lactate and ketone body shuttle systems. FASEB J. 2016;30(5):1913–1926. 10.1096/fj.201500182. [DOI] [PubMed] [Google Scholar]
- [148]. Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE. Role of VMH ketone bodies in adjusting caloric intake to increased dietary fat content in DIO and DR rats. Am J Physiol Regulat Integr Compar Physiol. 2015;308(10):R872–R878. 10.1152/ajpregu.00015.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149]. Le Foll C, Levin BE. Fatty acid-induced astrocyte ketone production and the control of food intake. Am J Physiol Regul Integr Compar Physiol. 2016;310(11):R1186–R1192. 10.1152/ajpregu.00113.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T. Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem. 2013;288(52):37216–37229. 10.1074/jbc.M113.506238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151]. Schönfeld P, Wojtczak L. Short- and medium-chain fatty acids in energy metabolism: the cellular perspective. J Lipid Res. 2016;57(6):943–954. 10.1194/jlr.R067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, et al. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2016;1367(1):12–20. 10.1111/nyas.12999. [DOI] [PubMed] [Google Scholar]
- [153]. Ishii T, Takanashi Y, Sugita K, Miyazawa M, Yanagihara R, Yasuda K, et al. Endogenous reactive oxygen species cause astrocyte defects and neuronal dysfunctions in the hippocampus: a new model for aging brain. Aging Cell. 2017;16(1):39–51. 10.1111/acel.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Liu FT, Xu SM, Xiang ZH, Li XN, Li J, Yuan HB, et al. Molecular hydrogen suppresses reactive astrogliosis related to oxidative injury during spinal cord injury in rats. CNS Neurosci Therap. 2014;20(8):778–786. 10.1111/cns.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155]. Zhang D, Hu X, Qian L, O'Callaghan JP, Hong J-S. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol. 2010;41(2–3):232–241. 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156]. Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics. 2010;7(4):494–506. 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157]. Ben Haim L, Carrillo-de Sauvage M-A, Ceyzériat K, Escartin C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front Cell Neurosci. 2015;9(278). 10.3389/fncel.2015.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158]. O'Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, Miller DB. Early activation of STAT3 regulates reactive astrogliosis induced by diverse forms of neurotoxicity. PLoS One. 2014;9(7):e102003. 10.1371/journal.pone.0102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Reichenbach N, Delekate A, Plescher M, Schmitt F, Krauss S, Blank N, et al. Inhibition of Stat3‐mediated astrogliosis ameliorates pathology in an Alzheimer's disease model. EMBO Mol Med. 2019;e9665. 10.15252/emmm.201809665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160]. Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med. 2015;21:927. 10.1038/nm.3912. [DOI] [PubMed] [Google Scholar]
- [161]. Sofroniew MV. Astrogliosis. Cold Spring Harbor Perspect Biol. 2014;7(2):a020420. 10.1101/cshperspect.a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162]. Fiskum G, Danilov CA, Mehrabian Z, Bambrick LL, Kristian T, McKenna MC, et al. Postischemic oxidative stress promotes mitochondrial metabolic failure in neurons and astrocytes. Ann N Y Acad Sci. 2008;1147:129–138. 10.1196/annals.1427.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163]. Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, et al. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18(6):844–859. 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- [164]. Voloboueva LA, Suh SW, Swanson RA, Giffard RG. Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem. 2007;102(4):1383–1394. 10.1111/j.1471-4159.2007.4634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165]. Bambrick L, Kristian T, Fiskum G. Astrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection. Neurochem Res. 2004;29(3):601–608. [DOI] [PubMed] [Google Scholar]
- [166]. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168]. Phatnani H, Maniatis T. Astrocytes in neurodegenerative disease. Cold Spring Harbor Perspect Biol. 2015;7(6). 10.1101/cshperspect.a020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169]. Lassmann H, van Horssen J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim Biophys Acta Mol Basis Dis. 2016;1862(3):506–510. 10.1016/j.bbadis.2015.09.018. [DOI] [PubMed] [Google Scholar]
- [170]. Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14(5):2924–2932. 10.1523/jneurosci.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171]. Johnstone SR, Billaud M, Lohman AW, Taddeo EP, Isakson BE. Posttranslational modifications in connexins and pannexins. J Membr Biol. 2012;245(5–6):319–332. 10.1007/s00232-012-9453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172]. Stout CE, Costantin JL, Naus CCG, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277(12):10482–10488. 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- [173]. Pehar M, Harlan BA, Killoy KM, Vargas MR. Role and therapeutic potential of astrocytes in amyotrophic lateral sclerosis. Curr Pharmaceut Des. 2017;23(33):5010–5021. 10.2174/1381612823666170622095802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174]. Bataveljic D, Nikolic L, Milosevic M, Todorovic N, Andjus PR. Changes in the astrocytic aquaporin-4 and inwardly rectifying potassium channel expression in the brain of the amyotrophic lateral sclerosis SOD1(G93A) rat model. Glia. 2012;60(12):1991–2003. 10.1002/glia.22414. [DOI] [PubMed] [Google Scholar]
- [175]. Brambilla L, Martorana F, Rossi D. Astrocyte signaling and neurodegeneration: new insights into CNS disorders. Prion. 2013;7(1):28–36. 10.4161/pri.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176]. Guillamón-Vivancos T, Gómez-Pinedo U, Matías-Guiu J. Astrocytes in neurodegenerative diseases (I): function and molecular description. Neurología. 2015;30(2):119–129. 10.1016/j.nrleng.2014.12.005. [DOI] [PubMed] [Google Scholar]
- [177]. Koyama Y. Functional alterations of astrocytes in mental disorders: pharmacological significance as a drug target. Front Cell Neurosci. 2015;9:261–261. 10.3389/fncel.2015.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178]. Elsayed M, Magistretti PJ. A new outlook on mental illnesses: glial involvement beyond the glue. Front Cell Neurosci. 2015;9:468. 10.3389/fncel.2015.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179]. Yudkoff M, Daikhin Y, Nissim I, Horyn O, Lazarow A, Luhovyy B, et al. Response of brain amino acid metabolism to ketosis. Neurochem Int. 2005;47(1–2):119–128. 10.1016/j.neuint.2005.04.014. [DOI] [PubMed] [Google Scholar]
- [180]. Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostagland Leukotr Essent Fatty Acids. 2004;70(3):277–285. 10.1016/j.plefa.2003.07.005. [DOI] [PubMed] [Google Scholar]
- [181]. McKenna MC. Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res. 2012;37(11):2613–2626. 10.1007/s11064-012-0901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182]. Valdebenito R, Ruminot I, Garrido-Gerter P, Fernandez-Moncada I, Forero-Quintero L, Alegria K, et al. Targeting of astrocytic glucose metabolism by beta-hydroxybutyrate. J Cereb Blood Flow Metab. 2016;36(10):1813–1822. 10.1177/0271678x15613955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183]. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25(2):262–284. 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184]. Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64(3):115–125. 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- [185]. Calderon N, Betancourt L, Hernandez L, Rada P. A ketogenic diet modifies glutamate, gamma-aminobutyric acid and agmatine levels in the hippocampus of rats: a microdialysis study. Neurosci Lett. 2017;642:158–162. 10.1016/j.neulet.2017.02.014. [DOI] [PubMed] [Google Scholar]
- [186]. Hertz L, Chen Y, Waagepetersen HS. Effects of ketone bodies in Alzheimer's disease in relation to neural hypometabolism, beta-amyloid toxicity, and astrocyte function. J Neurochem. 2015;134(1):7–20. 10.1111/jnc.13107. [DOI] [PubMed] [Google Scholar]
- [187]. Anlauf E, Derouiche A. Glutamine synthetase as an astrocytic marker: its cell type and vesicle localization. Front Endocrinol. 2013;4:144. 10.3389/fendo.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188]. Schousboe A, Bak LK, Waagepetersen HS. Astrocytic control of biosynthesis and turnover of the neurotransmitters glutamate and GABA. Front Endocrinol. 2013;4:102. 10.3389/fendo.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189]. McKenna M. Glutamate pays its own way in astrocytes. Front Endocrinol. 2013;4:191. 10.3389/fendo.2013.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190]. Hertz L, Chen Y. Integration between glycolysis and glutamate-glutamine cycle flux may explain preferential glycolytic increase during brain activation, requiring glutamate. Front Integr Neurosci. 2017;11:18. 10.3389/fnint.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191]. Hertz L, Rothman DL. Glucose, lactate, beta-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in the glutamine–glutamate/GABA cycle. Adv Neurobiol. 2016;13:9–42. 10.1007/978-3-319-45096-4_2. [DOI] [PubMed] [Google Scholar]
- [192]. Marsman A, van den Heuvel MP, Klomp DWJ, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull. 2013;39(1):120–129. 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193]. Hashimoto K, Bruno D, Nierenberg J, Marmar CR, Zetterberg H, Blennow K, et al. Abnormality in glutamine-glutamate cycle in the cerebrospinal fluid of cognitively intact elderly individuals with major depressive disorder: a 3-year follow-up study. Translat Psychiatry. 2016;6(3):e744. 10.1038/tp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194]. Chen G, Henter ID, Manji HK. Presynaptic glutamatergic dysfunction in bipolar disorder. Biol Psychiatry. 2010;67(11):1007–1009. 10.1016/j.biopsych.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195]. Madeira C, Vargas-Lopes C, Brandão CO, Reis T, Laks J, Panizzutti R, et al. Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer's disease and depression. Front Psychiatry. 2018;9:561. 10.3389/fpsyt.2018.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196]. Woodman B, Landwehrmeyer GB, Lindenberg KS, Franz P, Behrens PF. Impaired glutamate transport and glutamate–glutamine cycling: downstream effects of the Huntington mutation. Brain. 2002;125(8):1908–1922. 10.1093/brain/awf180. [DOI] [PubMed] [Google Scholar]
- [197]. Pioro EP, Majors AW, Mitsumoto H, Nelson DR, Ng TC. 1H-MRS evidence of neurodegeneration and excess glutamate glutamine in ALS medulla. Neurology. 1999;53(1):71. 10.1212/wnl.53.1.71. [DOI] [PubMed] [Google Scholar]
- [198]. O'Gorman Tuura RL, Baumann CR, Baumann-Vogel H. Beyond dopamine: GABA, glutamate, and the axial symptoms of parkinson disease. Front Neurol. 2018;9:806. 10.3389/fneur.2018.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199]. Clark IA, Vissel B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J Neuroinflam. 2016;13(1):236. 10.1186/s12974-016-0708-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200]. Kim YS, Yoon B-E. Altered GABAergic signaling in brain disease at various stages of life. Exp Neurobiol. 2017;26(3):122–131. 10.5607/en.2017.26.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201]. Robel S, Buckingham SC, Boni JL, Campbell SL, Danbolt NC, Riedemann T, et al. Reactive astrogliosis causes the development of spontaneous seizures. J Neurosci. 2015;35(8):3330–3345. 10.1523/JNEUROSCI.1574-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202]. Robel S, Sontheimer H. Glia as drivers of abnormal neuronal activity. Nat Neurosci. 2016;19(1):28–33. 10.1038/nn.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203]. Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, et al. Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci. 2010;13(5):584–591. 10.1038/nn.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204]. Jackman NA, Uliasz TF, Hewett JA, Hewett SJ. Regulation of system x(c) (−)activity and expression in astrocytes by interleukin-1beta: implications for hypoxic neuronal injury. Glia. 2010;58(15):1806–1815. 10.1002/glia.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205]. Shi J, He Y, Hewett SJ, Hewett JA. Interleukin 1beta regulation of the system xc-substrate-specific subunit, xCT, in primary mouse astrocytes involves the RNA-binding protein HuR. J Biol Chem. 2016;291(4):1643–1651. 10.1074/jbc.M115.697821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206]. Mei Y-Y, Wu DC, Zhou N. Astrocytic regulation of glutamate transmission in schizophrenia. Front Psychiatry. 2018;9:544. 10.3389/fpsyt.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207]. Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, et al. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363(9402):28–37. [DOI] [PubMed] [Google Scholar]
- [208]. van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64(2):326–333. 10.1212/01.wnl.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- [209]. Swamy M, Norlina W, Azman W, Suhaili D, Sirajudeen KNS, Mustapha Z, et al. Restoration of glutamine synthetase activity, nitric oxide levels and amelioration of oxidative stress by propolis in kainic acid mediated excitotoxicity. Afr J Tradition Complement Alt Med. 2014;11(2):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210]. Castegna A, Palmieri L, Spera I, Porcelli V, Palmieri F, Fabis-Pedrini MJ, et al. Oxidative stress and reduced glutamine synthetase activity in the absence of inflammation in the cortex of mice with experimental allergic encephalomyelitis. Neuroscience. 2011;185:97–105. 10.1016/j.neuroscience.2011.04.041. [DOI] [PubMed] [Google Scholar]
- [211]. Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, et al. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43(4–5):493–499. [DOI] [PubMed] [Google Scholar]
- [212]. Rodrigo R, Felipo V. Control of brain glutamine synthesis by NMDA receptors. Front Biosci. 2007;12:883–890. [DOI] [PubMed] [Google Scholar]
- [213]. Noh HS, Kim DW, Cho GJ, Choi WS, Kang SS. Increased nitric oxide caused by the ketogenic diet reduces the onset time of kainic acid-induced seizures in ICR mice. Brain Res. 2006;1075(1):193–200. 10.1016/j.brainres.2005.12.017. [DOI] [PubMed] [Google Scholar]
- [214]. Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, et al. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep. 2018;8(1):6670. 10.1038/s41598-018-25190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215]. Tae-Woo Kim J-MK, Park H-D, Jung K-Y, Kim D-W. Effect of ketogenic diet on the nitric oxide of pilocarpine-induced status epilepticus. Ann Clin Neurophysiol. 2003;5(2):171–176. [Google Scholar]
- [216]. Chang P, Augustin K, Boddum K, Williams S, Sun M, Terschak JA, et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139(Pt 2):431–443. 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217]. Rogawski MA. A fatty acid in the MCT ketogenic diet for epilepsy treatment blocks AMPA receptors. Brain. 2016;139(Pt 2):306–309. 10.1093/brain/awv369. [DOI] [PubMed] [Google Scholar]
- [218]. Augustin K, Williams S, Cunningham M, Devlin AM, Friedrich M, Jayasekera A, et al. Perampanel and decanoic acid show synergistic action against AMPA receptors and seizures. Epilepsia. 2018;59(11):e172–e178. 10.1111/epi.14578. [DOI] [PubMed] [Google Scholar]
- [219]. Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross JH, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17(1):84–93. 10.1016/s1474-4422(17)30408-8. [DOI] [PubMed] [Google Scholar]
- [220]. Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68(1):99–112. 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221]. Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY. The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell. 2018;173(7):1728.e1713–1741.e1713. 10.1016/j.cell.2018.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222]. O'Donovan SM, Sullivan CR, McCullumsmith RE. The role of glutamate transporters in the pathophysiology of neuropsychiatric disorders. NPJ Schizophr. 2017;3(1):32. 10.1038/s41537-017-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223]. Kim K, Lee S-G, Kegelman TP, Su Z-Z, Das SK, Dash R, et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: opportunities for developing novel therapeutics. J Cell Physiol. 2011;226(10):2484–2493. 10.1002/jcp.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224]. Sheng WS, Hu S, Feng A, Rock RB. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem Res. 2013;38(10):2148–2159. 10.1007/s11064-013-1123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225]. Dumont AO, Goursaud S, Desmet N, Hermans E. Differential regulation of glutamate transporter subtypes by pro-inflammatory cytokine TNF-alpha in cortical astrocytes from a rat model of amyotrophic lateral sclerosis. PLoS One. 2014;9(5):e97649. 10.1371/journal.pone.0097649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226]. Yan X, Yadav R, Gao M, Weng HR. Interleukin-1 beta enhances endocytosis of glial glutamate transporters in the spinal dorsal horn through activating protein kinase C. Glia. 2014;62(7):1093–1109. 10.1002/glia.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227]. Prow NA, Irani DN. The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. J Neurochem. 2008;105(4):1276–1286. 10.1111/j.1471-4159.2008.05230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228]. Raju K, Doulias P-T, Evans P, Krizman EN, Jackson JG, Horyn O, et al. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci Signal. 2015;8(384):ra68. 10.1126/scisignal.aaa4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229]. Krzyzanowska W, Pomierny B, Bystrowska B, Pomierny-Chamiolo L, Filip M, Budziszewska B, et al. Ceftriaxone- and N-acetylcysteine-induced brain tolerance to ischemia: Influence on glutamate levels in focal cerebral ischemia. PLoS One. 2017;12(10):e0186243. 10.1371/journal.pone.0186243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230]. Mulrooney TJ, Marsh J, Urits I, Seyfried TN, Mukherjee P. Influence of caloric restriction on constitutive expression of NF-kappaB in an experimental mouse astrocytoma. PLoS One. 2011;6(3):e18085. 10.1371/journal.pone.0018085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231]. Romera C, Hurtado O, Mallolas J, Pereira MP, Morales JR, Romera A, et al. Ischemic preconditioning reveals that GLT1/EAAT2 glutamate transporter is a novel PPARgamma target gene involved in neuroprotection. J Cereb Blood Flow Metab. 2007;27(7):1327–1338. 10.1038/sj.jcbfm.9600438. [DOI] [PubMed] [Google Scholar]
- [232]. Yang Y, Gozen O, Watkins A, Lorenzini I, Lepore A, Gao Y, et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61(6):880–894. 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233]. Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015;40(2):402–409. 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- [234]. Waagepetersen HS, Sonnewald U, Schousboe A. Compartmentation of glutamine, glutamate, and GABA metabolism in neurons and astrocytes: functional implications. Neuroscientist. 2003;9(5):398–403. 10.1177/1073858403254006. [DOI] [PubMed] [Google Scholar]
- [235]. Hertz L, Gerkau NJ, Xu J, Durry S, Song D, Rose CR, et al. Roles of astrocytic Na(+),K(+)-ATPase and glycogenolysis for K(+) homeostasis in mammalian brain. J Neurosci Res. 2015;93(7):1019–1030. 10.1002/jnr.23499. [DOI] [PubMed] [Google Scholar]
- [236]. Kaur G, Kaur K. Effect of acute starvation on monoamine oxidase and Na+,K(+)-ATPase activity in rat brain. Mol Chem Neuropathol. 1990;13(3):175–183. [DOI] [PubMed] [Google Scholar]
- [237]. Vasconcelos AR, Kinoshita PF, Yshii LM, Marques Orellana AM, Bohmer AE, de Sa Lima L, et al. Effects of intermittent fasting on age-related changes on Na,K-ATPase activity and oxidative status induced by lipopolysaccharide in rat hippocampus. Neurobiol Aging. 2015;36(5):1914–1923. 10.1016/j.neurobiolaging.2015.02.020. [DOI] [PubMed] [Google Scholar]
- [238]. Larsen BR, Holm R, Vilsen B, MacAulay N. Glutamate transporter activity promotes enhanced Na(+)/K(+)-ATPase-mediated extracellular K(+) management during neuronal activity. J Physiol. 2016;594(22):6627–6641. 10.1113/jp272531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239]. Dinuzzo M, Mangia S, Maraviglia B, Giove F. The role of astrocytic glycogen in supporting the energetics of neuronal activity. Neurochem Res. 2012;37(11):2432–2438. 10.1007/s11064-012-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240]. Hertz L, Song D, Xu J, Peng L, Gibbs ME. Role of the astrocytic Na(+), K(+)-ATPase in K(+) homeostasis in brain: K(+) uptake, signaling pathways and substrate utilization. Neurochem Res. 2015;40(12):2505–2516. 10.1007/s11064-014-1505-x. [DOI] [PubMed] [Google Scholar]
- [241]. Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of glycogenolysis for uptake of increased extracellular K+ in astrocytes: potential implications for K+ homeostasis and glycogen usage in brain. Neurochem Res. 2013;38(3):472–485. 10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]
- [242]. Larsen BR, Stoica A, MacAulay N. Managing brain extracellular K(+) during neuronal activity: the physiological role of the Na(+)/K(+)-ATPase subunit isoforms. Front Physiol. 2016;7:141. 10.3389/fphys.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243]. Franzon R, Lamers ML, Stefanello FM, Wannmacher CM, Wajner M, Wyse AT. Evidence that oxidative stress is involved in the inhibitory effect of proline on Na(+),K(+)-ATPase activity in synaptic plasma membrane of rat hippocampus. Int J Dev Neurosci. 2003;21(6):303–307. [DOI] [PubMed] [Google Scholar]
- [244]. Novaes LS, Dos Santos NB, Dragunas G, Perfetto JG, Leza JC, Scavone C, et al. Repeated restraint stress decreases Na,K-ATPase activity via oxidative and nitrosative damage in the frontal cortex of rats. Neuroscience. 2018;393:273–283. 10.1016/j.neuroscience.2018.09.037. [DOI] [PubMed] [Google Scholar]
- [245]. Roy S, Dasgupta A, Banerjee U, Chowdhury P, Mukhopadhyay A, Saha G, et al. Role of membrane cholesterol and lipid peroxidation in regulating the Na(+)/K(+)-ATPase activity in schizophrenia. Indian J Psychiatry. 2016;58(3):317–325. 10.4103/0019-5545.192023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246]. Banerjee U, Dasgupta A, Rout JK, Singh OP. Effects of lithium therapy on Na+- K+-ATPase activity and lipid peroxidation in bipolar disorder. Progr Neuropsychopharmacol Biol Psychiatry. 2012;37(1):56–61. 10.1016/j.pnpbp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- [247]. de Lores Arnaiz GR, Ordieres MGL. Brain Na(+), K(+)-ATPase activity in aging and disease. Int J Biomed Sci. 2014;10(2):85–102. [PMC free article] [PubMed] [Google Scholar]
- [248]. Seidel JL, Faideau M, Aiba I, Pannasch U, Escartin C, Rouach N, et al. Ciliary neurotrophic factor (CNTF) activation of astrocytes decreases spreading depolarization susceptibility and increases potassium clearance. Glia. 2015;63(1):91–103. 10.1002/glia.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249]. Thakurta IG, Banerjee P, Bagh MB, Ghosh A, Sahoo A, Chattopadhyay S, et al. Combination of N-acetylcysteine, alpha-lipoic acid and alpha-tocopherol substantially prevents the brain synaptosomal alterations and memory and learning deficits of aged rats. Exp Gerontol. 2014;50:19–25. 10.1016/j.exger.2013.11.008. [DOI] [PubMed] [Google Scholar]
- [250]. Rodrigo R, Miranda-Merchak A, Valenzuela Grau R, Bachler JP, Vergara L. Modulation of (Na,K)-ATPase activity by membrane fatty acid composition: therapeutic implications in human hypertension. Clin Exp Hypertens. 2014;36(1):17–26. 10.3109/10641963.2013.783048. [DOI] [PubMed] [Google Scholar]
- [251]. Namazi G, Jamshidi Rad S, Attar AM, Sarrafzadegan N, Sadeghi M, Naderi G, et al. Increased membrane lipid peroxidation and decreased Na+/K+-ATPase activity in erythrocytes of patients with stable coronary artery disease. Coronary Artery Dis. 2015;26(3):239–244. 10.1097/mca.0000000000000196. [DOI] [PubMed] [Google Scholar]
- [252]. Rodrigo R, Bachler JP, Araya J, Prat H, Passalacqua W. Relationship between (Na+ K)-ATPase activity, lipid peroxidation and fatty acid profile in erythrocytes of hypertensive and normotensive subjects. Mol Cell Biochem. 2007;303(1–2):73–81. 10.1007/s11010-007-9457-y. [DOI] [PubMed] [Google Scholar]
- [253]. Gerbi A, Barbey O, Raccah D, Coste T, Jamme I, Nouvelot A, et al. Alteration of Na,K-ATPase isoenzymes in diabetic cardiomyopathy: effect of dietary supplementation with fish oil (n-3 fatty acids) in rats. Diabetologia. 1997;40(5):496–505. 10.1007/s001250050707. [DOI] [PubMed] [Google Scholar]
- [254]. Gerbi A, Maixent JM. Fatty acid-induced modulation of ouabain responsiveness of rat Na, K-ATPase isoforms. J Membr Biol. 1999;168(1):19–27. [DOI] [PubMed] [Google Scholar]
- [255]. Gerbi A, Maixent JM, Barbey O, Jamme I, Pierlovisi M, Coste T, et al. Alterations of Na,K-ATPase isoenzymes in the rat diabetic neuropathy: protective effect of dietary supplementation with n-3 fatty acids. J Neurochem. 1998;71(2):732–740. [DOI] [PubMed] [Google Scholar]
- [256]. Morris G, Walder K, Puri BK, Berk M, Maes M. The deleterious effects of oxidative and nitrosative stress on palmitoylation, membrane lipid rafts and lipid-based cellular signalling: new drug targets in neuroimmune disorders. Mol Neurobiol. 2016;53(7):4638–4658. 10.1007/s12035-015-9392-y. [DOI] [PubMed] [Google Scholar]
- [257]. Fraser DD, Whiting S, Andrew RD, Macdonald EA, Musa-Veloso K, Cunnane SC. Elevated polyunsaturated fatty acids in blood serum obtained from children on the ketogenic diet. Neurology. 2003;60(6):1026–1029. 10.1212/01.wnl.0000049974.74242.c6. [DOI] [PubMed] [Google Scholar]
- [258]. Taha AY, Ryan MA, Cunnane SC. Despite transient ketosis, the classic high-fat ketogenic diet induces marked changes in fatty acid metabolism in rats. Metabol Clin Exp. 2005;54(9):1127–1132. 10.1016/j.metabol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [259]. Liu X-Q, Kobayashi H, Jin Z-B, Wada A, Nao-I N. Differential expression of Kir4.1 and aquaporin 4 in the retina from endotoxin-induced uveitis rat. Mol Vis. 2007;13:309–317. [PMC free article] [PubMed] [Google Scholar]
- [260]. Hassan I, Luo Q, Majumdar S, Dominguez JM II, Busik JV, Bhatwadekar AD. Tumor necrosis factor alpha (TNF-α) disrupts Kir4.1 channel expression resulting in Müller cell dysfunction in the retinadiurnal rhythm of Kir4.1 in the retina. Investig Ophthalmol Vis Sci. 2017;58(5):2473–2482. 10.1167/iovs.16-20712. [DOI] [PubMed] [Google Scholar]
- [261]. Thompson K, Chen J, Luo Q, Xiao Y, Cummins TR, Bhatwadekar AD. Advanced glycation end (AGE) product modification of laminin downregulates Kir4.1 in retinal Muller cells. PLoS One. 2018;13(2):e0193280. 10.1371/journal.pone.0193280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262]. Sibille J, Dao Duc K, Holcman D, Rouach N. The neuroglial potassium cycle during neurotransmission: role of Kir4.1 channels. PLoS Comput Biol. 2015;11(3):e1004137. 10.1371/journal.pcbi.1004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263]. Ohno Y, Kinboshi M, Shimizu S. Inwardly rectifying potassium channel Kir4.1 as a novel modulator of BDNF expression in astrocytes. Int J Mol Sci. 2018;19(11):3313. 10.3390/ijms19113313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264]. Kinboshi M, Mukai T, Nagao Y, Matsuba Y, Tsuji Y, Tanaka S, et al. Inhibition of inwardly rectifying potassium (Kir) 4.1 channels facilitates brain-derived neurotrophic factor (BDNF) expression in astrocytes. Front Mol Neurosci. 2017;10:408. 10.3389/fnmol.2017.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265]. Bellot-Saez A, Kékesi O, Morley JW, Buskila Y. Astrocytic modulation of neuronal excitability through K+ spatial buffering. Neurosci Biobehav Rev. 2017;77:87–97. 10.1016/j.neubiorev.2017.03.002. [DOI] [PubMed] [Google Scholar]
- [266]. Larsen BR, MacAulay N. Kir4.1-mediated spatial buffering of K(+): experimental challenges in determination of its temporal and quantitative contribution to K(+) clearance in the brain. Channels. 2014;8(6):544–550. 10.4161/19336950.2014.970448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267]. Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, et al. Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci. 2014;111(32):E3343–E3352. 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268]. Olsen ML, Khakh BS, Skatchkov SN, Zhou M, Lee CJ, Rouach N. New insights on astrocyte ion channels: critical for homeostasis and neuron–glia signaling. J Neurosci. 2015;35(41):13827–13835. 10.1523/JNEUROSCI.2603-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269]. Bay V, Butt AM. Relationship between glial potassium regulation and axon excitability: a role for glial Kir4.1 channels. Glia. 2012;60(4):651–660. 10.1002/glia.22299. [DOI] [PubMed] [Google Scholar]
- [270]. Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27(42):11354–11365. 10.1523/jneurosci.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271]. Yaguchi T, Nishizaki T. Extracellular high K+ stimulates vesicular glutamate release from astrocytes by activating voltage-dependent calcium channels. J Cell Physiol. 2010;225(2):512–518. 10.1002/jcp.22231. [DOI] [PubMed] [Google Scholar]
- [272]. Zurolo E, De Groot M, Iyer A, Anink J, Van Vliet E, Heimans J, et al. Regulation of Kir4.1 expression in astrocytes and astrocytic tumors: a role for interleukin-1 β. J Neuroinflammation. 2012;9:280. 10.1186/1742-2094-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273]. Schirmer L, Srivastava R, Kalluri SR, Bottinger S, Herwerth M, Carassiti D, et al. Differential loss of KIR4.1 immunoreactivity in multiple sclerosis lesions. Ann Neurol. 2014;75(6):810–828. 10.1002/ana.24168. [DOI] [PubMed] [Google Scholar]
- [274]. Marnetto F, Valentino P, Caldano M, Bertolotto A. Detection of potassium channel KIR4.1 antibodies in multiple sclerosis patients. J Immunol Methods. 2017;445:53–58. 10.1016/j.jim.2017.03.008. [DOI] [PubMed] [Google Scholar]
- [275]. Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367(2):115–123. 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276]. Brill L, Goldberg L, Karni A, Petrou P, Abramsky O, Ovadia H, et al. Increased anti-KIR4.1 antibodies in multiple sclerosis: could it be a marker of disease relapse? Multiple Sclerosis. 2015;21(5):572–579. 10.1177/1352458514551779. [DOI] [PubMed] [Google Scholar]
- [277]. Wilcock DM, Vitek MP, Colton CA. Vascular amyloid alters astrocytic water and potassium channels in mouse models and humans with Alzheimer's disease. Neuroscience. 2009;159(3):1055–1069. 10.1016/j.neuroscience.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278]. Medina A, Watson SJ, Bunney W Jr, Myers RM, Schatzberg A, Barchas J, et al. Evidence for alterations of the glial syncytial function in major depressive disorder. J Psychiatr Res. 2016;72:15–21. 10.1016/j.jpsychires.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279]. Imbrici P, Camerino DC, Tricarico D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives. Front Genet. 2013;4:76. 10.3389/fgene.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280]. Sun M, Yan H, Zou W, Wang Y, Li H, Wang X. Lipopolysaccharide induces astrocyte activation and downregulates the expression of Kir4.1 channel. Chin J Cell Mol Immunol. 2016;32(2):196–200. [PubMed] [Google Scholar]
- [281]. Jin X, Yu L, Wu Y, Zhang S, Shi Z, Chen X, et al. S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J Physiol. 2012;590(21):5335–5348. 10.1113/jphysiol.2012.236885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282]. Skowronska K, Obara-Michlewska M, Czarnecka A, Dabrowska K, Zielinska M, Albrecht J. Persistent overexposure to N-methyl-D-aspartate (NMDA) calcium-dependently downregulates glutamine synthetase, aquaporin 4, and Kir4.1 channel in mouse cortical astrocytes. Neurotox Res. 2019;35(1):271–280. 10.1007/s12640-018-9958-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283]. Skowronska K, Obara-Michlewska M, Zielinska M, Albrecht J. NMDA receptors in astrocytes: in search for roles in neurotransmission and astrocytic homeostasis. Int J Mol Sciences. 2019;20(2). 10.3390/ijms20020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284]. Obara-Michlewska M, Ruszkiewicz J, Zielinska M, Verkhratsky A, Albrecht J. Astroglial NMDA receptors inhibit expression of Kir4.1 channels in glutamate-overexposed astrocytes in vitro and in the brain of rats with acute liver failure. Neurochem Int. 2015;88:20–25. 10.1016/j.neuint.2014.10.006. [DOI] [PubMed] [Google Scholar]
- [285]. Satoh J-I, Tabunoki H, Ishida T, Saito Y, Konno H, Arima K. Reactive astrocytes express the potassium channel Kir4.1 in active multiple sclerosis lesions. Clin Exp Neuroimmunol. 2013;4(1):19–28. 10.1111/cen3.12011. [DOI] [Google Scholar]
- [286]. Milton M, Smith PD. It's all about timing: the involvement of Kir4.1 channel regulation in acute ischemic stroke pathology. Front Cell Neurosci. 2018;12:36. 10.3389/fncel.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287]. Zhang J, Zhan Z, Li X, Xing A, Jiang C, Chen Y, et al. Intermittent fasting protects against Alzheimer’s disease possible through restoring aquaporin-4 polarity. Front Mol Neurosci. 2017;10(395). 10.3389/fnmol.2017.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288]. Jiang K, Wang J, Zhao C, Feng M, Shen Z, Yu Z, et al. Regulation of gap junctional communication by astrocytic mitochondrial KATP channels following neurotoxin administration in in vitro and in vivo models. Neurosignals. 2011;19(2):63–74. 10.1159/000323575. [DOI] [PubMed] [Google Scholar]
- [289]. Wang J, Li Z, Feng M, Ren K, Shen G, Zhao C, et al. Opening of astrocytic mitochondrial ATP-sensitive potassium channels upregulates electrical coupling between hippocampal astrocytes in rat brain slices. PLoS One. 2013;8(2):e56605. 10.1371/journal.pone.0056605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290]. Ho CF, Chan KW, Yeh HI, Kuo J, Liu HJ, Wang CY. Ketone bodies upregulate endothelial connexin 43 (Cx43) gap junctions. Vet J. 2013;198(3):696–701. 10.1016/j.tvjl.2013.09.069. [DOI] [PubMed] [Google Scholar]
- [291]. Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–1562. 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292]. Lan YL, Fang DY, Zhao J, Ma TH, Li S. A research update on the potential roles of aquaporin 4 in neuroinflammation. Acta Neurol Belg. 2016;116(2):127–134. 10.1007/s13760-015-0520-2. [DOI] [PubMed] [Google Scholar]
- [293]. Xiao M, Hu G. Involvement of aquaporin 4 in astrocyte function and neuropsychiatric disorders. CNS Neurosci Therapeut. 2014;20(5):385–390. 10.1111/cns.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294]. Kong H, Ding J, Gao J, Liu L, Li L, Lu Y, et al. Aquaporin-4 deficiency exacerbates brain oxidative damage and memory deficits induced by long-term ovarian hormone deprivation and D-galactose injection. Int J Neuropsychopharmacol. 2012;15(1):55–68. 10.1017/s1461145711000022. [DOI] [PubMed] [Google Scholar]
- [295]. Foglio E, Rodella LF. Aquaporins and neurodegenerative diseases. Curr Neuropharmacol. 2010;8(2):112–121. 10.2174/157015910791233150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296]. Xu J, Chen L, Li L. Pannexin hemichannels: a novel promising therapy target for oxidative stress related diseases. J Cell Physiol. 2018;233(3):2075–2090. 10.1002/jcp.25906. [DOI] [PubMed] [Google Scholar]
- [297]. Retamal MA. Connexin and Pannexin hemichannels are regulated by redox potential. Front Physiol. 2014;5:80. 10.3389/fphys.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298]. Le HT, Sin WC, Lozinsky S, Bechberger J, Vega JL, Guo XQ, et al. Gap junction intercellular communication mediated by Connexin43 in astrocytes is essential for their resistance to oxidative stress. J Biol Chem. 2014;289(3):1345–1354. 10.1074/jbc.M113.508390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299]. Takeuchi H, Suzumura A. Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci. 2014;8:–189. 10.3389/fncel.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300]. Bennett MV, Garre JM, Orellana JA, Bukauskas FF, Nedergaard M, Saez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15. 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301]. Zhao Y, Xin Y, He Z, Hu W. Function of connexins in the interaction between glial and vascular cells in the central nervous system and related neurological diseases. Neur Plast. 2018;2018:6323901. 10.1155/2018/6323901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302]. Eugenin EA, Basilio D, Saez JC, Orellana JA, Raine CS, Bukauskas F, et al. The role of gap junction channels during physiologic and pathologic conditions of the human central nervous system. J Neuroimmune Pharmacol. 2012;7(3):499–518. 10.1007/s11481-012-9352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303]. Tanner GR, Lutas A, Martinez-Francois JR, Yellen G. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci. 2011;31(23):8689–8696. 10.1523/jneurosci.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304]. Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27(14):3618–3625. 10.1523/jneurosci.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305]. Kawamura M, Ruskin DN, Geiger JD, Boison D, Masino SA. Ketogenic diet sensitizes glucose control of hippocampal excitability. J Lipid Res. 2014;55(11):2254–2260. 10.1194/jlr.M046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306]. Sun H-S, Feng Z-P. Neuroprotective role of ATP-sensitive potassium channels in cerebral ischemia. Acta Pharmacol Sin. 2013;34(1):24–32. 10.1038/aps.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307]. Szeto V, Chen NH, Sun HS, Feng ZP. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin. 2018;39(5):683–694. 10.1038/aps.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308]. Yang Y, Jin X, Jiang C. S-glutathionylation of ion channels: insights into the regulation of channel functions, thiol modification crosstalk, and mechanosensing. Antioxid Redox Signal. 2014;20(6):937–951. 10.1089/ars.2013.5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309]. Yang Y, Shi W, Cui N, Wu Z, Jiang C. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem. 2010;285(49):38641–38648. 10.1074/jbc.M110.162578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310]. Yang Y, Shi W, Chen X, Cui N, Konduru AS, Shi Y, et al. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem. 2011;286(11):9298–9307. 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311]. Yan XS, Ma JH, Zhang PH. Modulation of K(ATP) currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharmacol Sin. 2009;30(10):1399–1414. 10.1038/aps.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312]. Kozoriz MG, Church J, Ozog MA, Naus CC, Krebs C. Temporary sequestration of potassium by mitochondria in astrocytes. J Biol Chem. 2010;285(41):31107–31119. 10.1074/jbc.M109.082073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313]. Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, et al. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane K-ATP channels. Mol Cell Neurosci. 2001;18(6):671–690. 10.1006/mcne.2001.1048. [DOI] [PubMed] [Google Scholar]
- [314]. Sun XL, Hu G. ATP-sensitive potassium channels: a promising target for protecting neurovascular unit function in stroke. Clin Exp Pharmacol Physiol. 2010;37(2):243–252. 10.1111/j.1440-1681.2009.05190.x. [DOI] [PubMed] [Google Scholar]
- [315]. Zhang S, Zhou F, Ding JH, Zhou XQ, Sun XL, Hu G. ATP-sensitive potassium channel opener iptakalim protects against MPP-induced astrocytic apoptosis via mitochondria and mitogen-activated protein kinase signal pathways. J Neurochem. 2007;103(2):569–579. 10.1111/j.1471-4159.2007.04775.x. [DOI] [PubMed] [Google Scholar]
- [316]. Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22(4):431–443. 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- [317]. Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, et al. Targeting mitochondrial ATP-sensitive potassium channels—a novel approach to neuroprotection. Brain Res Rev. 2004;46(3):282–294. 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- [318]. Cao Z, Dai W, Zhang R, Chen L, Yang X, Hu L, et al. Opening of the adenosine triphosphate-sensitive potassium channel attenuates morphine tolerance by inhibiting JNK and astrocyte activation in the spinal cord. Clin J Pain. 2016;32(7):617–623. 10.1097/ajp.0000000000000299. [DOI] [PubMed] [Google Scholar]
- [319]. Spinelli E, Blackford R. Gut microbiota, the ketogenic diet and epilepsy. Pediatr Neurol Briefs. 2018;32:10. 10.15844/pedneurbriefs-32-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320]. Dahlin M, Prast-Nielsen S. The gut microbiome and epilepsy. EBioMedicine. 2019;44:741–746. 10.1016/j.ebiom.2019.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321]. Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M. The role of microbiota and intestinal permeability in the pathophysiology of autoimmune and neuroimmune processes with an emphasis on inflammatory bowel disease type 1 diabetes and chronic fatigue syndrome. Curr Pharm Des. 2016;22(40):6058–6075. 10.2174/1381612822666160914182822. [DOI] [PubMed] [Google Scholar]
- [322]. Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, et al. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. 2017;54(6):4432–4451. 10.1007/s12035-016-0004-2. [DOI] [PubMed] [Google Scholar]
- [323]. Lucas K, Morris G, Anderson G, Maes M. The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS & neurological disorders—drug targets (formerly Curr Drug Targets. 2015;14(7):838–854. [DOI] [PubMed] [Google Scholar]
- [324]. Wang K, Liao M, Zhou N, Bao L, Ma K, Zheng Z, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019;26(1):222.e225–235.e225. 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- [325]. Naito Y, Uchiyama K, Takagi T. A next-generation beneficial microbe: Akkermansia muciniphila. J Clin Biochem Nutr. 2018;63(1):33–35. 10.3164/jcbn.18-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326]. Morris G, Fernandes BS, Puri BK, Walker AJ, Carvalho AF, Berk M. Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Austr New Zealand J Psychiatry. 2018;52(10):924–948. 10.1177/0004867418796955. [DOI] [PubMed] [Google Scholar]
- [327]. Morris G, Berk M, Walder K, Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328]. Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015;13(1):68. 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329]. Zhao X, Liao Y, Morgan S, Mathur R, Feustel P, Mazurkiewicz J, et al. Noninflammatory changes of microglia are sufficient to cause epilepsy. Cell Rep. 2018;22(8):2080–2093. 10.1016/j.celrep.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330]. Hiragi T, Ikegaya Y, Koyama R. Microglia after seizures and in epilepsy. Cells. 2018;7(4):26. 10.3390/cells7040026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331]. Baj A, Moro E, Bistoletti M, Orlandi V, Crema F, Giaroni C. Glutamatergic signaling along the microbiota-gut-brain axis. Int J Mol Sci. 2019;20(6):1482. 10.3390/ijms20061482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332]. Barker-Haliski M, White HS. Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med. 2015;5(8):a022863. 10.1101/cshperspect.a022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333]. Li C-T, Yang K-C, Lin W-C. Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: evidence from clinical neuroimaging studies. Front Psychiatry. 2019;9:767. 10.3389/fpsyt.2018.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334]. Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab. 2009;297(5):E1197–E1204. 10.1152/ajpendo.00357.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335]. Honors MA, Davenport BM, Kinzig KP. Effects of consuming a high carbohydrate diet after eight weeks of exposure to a ketogenic diet. Nutr Metab. 2009;6:46. 10.1186/1743-7075-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336]. Kinzig KP, Honors MA, Hargrave SL. Insulin sensitivity and glucose tolerance are altered by maintenance on a ketogenic diet. Endocrinology. 2010;151(7):3105–3114. 10.1210/en.2010-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337]. Kinzig KP, Honors MA, Hargrave SL, Davenport BM, Strader AD, Wendt D. Sensitivity to the anorectic effects of leptin is retained in rats maintained on a ketogenic diet despite increased adiposity. Neuroendocrinology. 2010;92(2):100–111. 10.1159/000314180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338]. McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52(3):e7–e11. 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [339]. Thio LL. Hypothalamic hormones and metabolism. Epilepsy Res. 2012;100(3):245–251. 10.1016/j.eplepsyres.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340]. Mirshamsi S, Laidlaw HA, Ning K, Anderson E, Burgess LA, Gray A, et al. Leptin and insulin stimulation of signalling pathways in arcuate nucleus neurones: PI3K dependent actin reorganization and KATP channel activation. BMC Neurosci. 2004;5:54. 10.1186/1471-2202-5-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341]. Qiu J, Wagner EJ, Ronnekleiv OK, Kelly MJ. Insulin and leptin excite anorexigenic pro-opiomelanocortin neurones via activation of TRPC5 channels. J Neuroendocrinol. 2018;30:2. 10.1111/jne.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342]. Morris G, Puri BK, Walker AJ, Maes M, Carvalho AF, Walder K, et al. Shared pathways for neuroprogression and somatoprogression in neuropsychiatric disorders. Neurosci Biobehav Rev. 2019;107:862–882. 10.1016/j.neubiorev.2019.09.025. [DOI] [PubMed] [Google Scholar]
- [343]. de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity, type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10(10):1049-1060. [PMC free article] [PubMed] [Google Scholar]
- [344]. Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50(5):1118–1126. 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- [345]. Sun Q, Li J, Gao F. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014;5(2):89–96. 10.4239/wjd.v5.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346]. Radulian G, Rusu E, Dragomir A, Posea M. Metabolic effects of low glycaemic index diets. Nutr J. 2009;8:5. 10.1186/1475-2891-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347]. Karimzadeh P, Sedighi M, Beheshti M, Azargashb E, Ghofrani M, Abdollahe-Gorgi F. Low glycemic index treatment in pediatric refractory epilepsy: the first Middle East report. Seizure. 2014;23(7):570–572. 10.1016/j.seizure.2014.03.012. [DOI] [PubMed] [Google Scholar]