Introduction
Chilblains are one of the earliest and most commonly described cutaneous manifestations of COVID-19;1 however, more recent data have failed to suggest an association. As widespread vaccinations are underway, reports of delayed-type hypersensitivity reactions in recipients of the mRNA-1273 vaccine have been brought to attention. Here, we present a case of biopsy-confirmed chilblains developing shortly after vaccination in an asymptomatic healthcare worker, who was not tested for SARS-CoV-2.
Case report
A 70-year-old woman presented to our Southern California dermatology clinic with an acute-onset of a pruritic papular rash on the digits of her right hand, which appeared 2 days after she had received her first dose of the mRNA-1273 vaccine. Of note, she was an asymptomatic healthcare worker with no history of COVID-19, and she had never been tested for the disease. A few scattered red edematous papules on an erythematous/violaceous background were noted on the palmar and lateral aspects of the fingers on her right hand (Fig 1). The left hand was unaffected. Associated symptoms included erythema, swelling, and pain with movement of the right proximal interphalangeal joints of the 4th and 5th digits. These joint symptoms resolved within 10 days without treatment; however, the rash persisted. Her medical history was notable for pityriasis lichenoides chronica (PLC), which remained clinically stable with as-needed clobetasol 0.05% ointment. She denied recent exposure to cold or damp environments. A complete physical examination was unremarkable except for a few lesions consistent with PLC located on the extensor surfaces of the extremities. A complete blood count, erythrocyte sedimentation rate, C-reactive protein, rheumatoid factor, Sjögren antibodies (anti-SS-A/anti-SS-B), and antinuclear antibody were either within normal limits or negative.
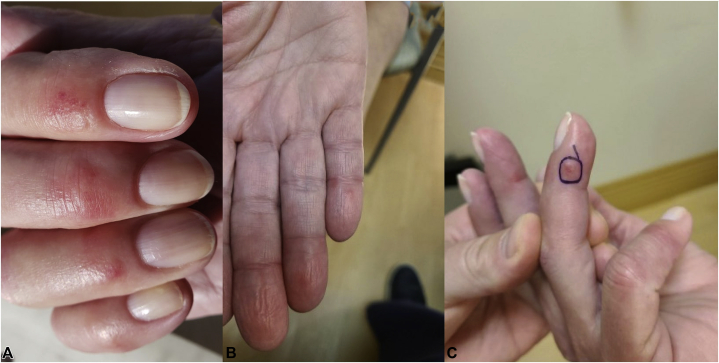
Fig 1.
Chilblain-like lesions of the right hand. Acute-onset pruritic red papules on an erythematous/violaceous base on the distal interphalangeal joints and palmar aspect of the distal phalanxes after administration of the mRNA-1273 vaccine, consistent with chilblains/perniosis. A, Erythematous papules and plaques on the dorsal fingers. B, Red and violaceous erythema of the distal fingers. C, Red papule on the distal finger.
A 3-mm punch biopsy was obtained from the edge of a red papule located on the ulnar side of the 4th distal phalanx on the right hand (Fig 1, C). Histopathology revealed a dense and predominantly perivascular lymphocytic infiltrate within the superficial-to-deep reticular dermis (Fig 2, A). The epidermis appeared normal with no vacuolar changes at the epidermal-dermal junction. There was a notable papillary dermal edema (Fig 2, B). Within the superficial dermis, some vessels exhibited slightly thickened walls with trophism of lymphocytes, although vascular wall hyalinization, neutrocytosis, or intravascular thrombi were not evident (Fig 3). Immunohistochemical analysis demonstrated a majority of CD3+ T cells in the lymphocytic infiltrate. Immunohistochemical results were otherwise negative. A diagnosis of chilblains was established based on histologic findings.
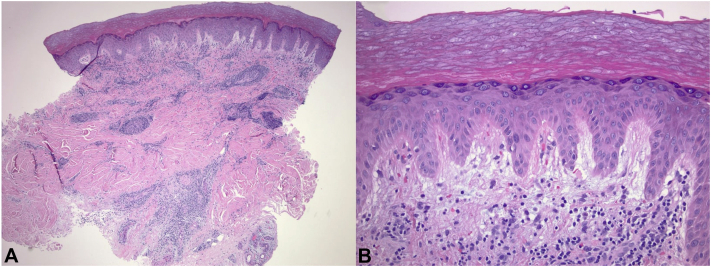
Fig 2.
Chilblains developing shortly following vaccination. A, Histopathology revealed a dense and predominantly perivascular lymphocytic infiltrate within the superficial-to-deep reticular dermis. B, Notable interstitial edema within the papillary dermis. (Hematoxylin-eosin stain).
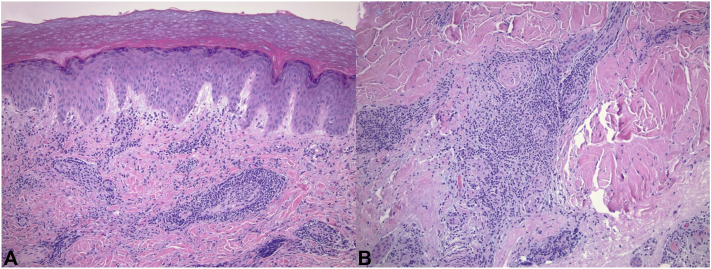
Fig 3.
Chilblains developing shortly following vaccination. A, Within the superficial dermis, some vessels exhibited slightly thickened walls with trophism of lymphocytes, although vascular wall hyalinization, neutrocytosis, or intravascular thrombi were not evident. B, Dense lymphocytic infiltrate with relatively “small” and “mature” appearing lymphoid cells with the majority of lymphocytes being CD3 immunoreactive T-cells in the deep reticular dermis. (Hematoxylin-eosin stain).
The patient was treated with clobetasol 0.05% ointment applied twice daily. At the 2-week follow-up visit, complete resolution of the rash was observed. A similar rash occurred on the same hand 3 days after she received her second dose of the vaccine, which resolved in one week with topical steroid therapy. The injection site was on the left arm in both instances.
Discussion
Due to conflicting evidence, a causal relationship between SARS-CoV-2 infection and chilblains has not been established. Since the beginning of the COVID-19 pandemic, chilblain-like lesions have been observed in adults with severe disease and in asymptomatic or mildly symptomatic younger patients.2,3 The hypothesis of a robust antiviral type I interferon response in younger patients leading to early viremia clearance has been inconclusive, since few patients were tested for SARS-CoV-2 by reverse-transcriptase polymerase chain reaction and serology in the early reports.1, 2, 3 Now with greater availability of resources, researchers are testing larger sample sizes and at different stages of the disease. Recent series have demonstrated a statistically significant number of patients with chilblains who have tested negative for SARS-CoV-2 by reverse-transcriptase polymerase chain reaction and serology, thereby not suggesting any causal relationship between chilblains and COVID-19.4, 5, 6 In a smaller study, SARS-CoV-2 PCR swabs were negative in 6 out of 7 pediatric patients with biopsy-confirmed chilblains. However, immunohistochemical staining was positive for SARS-CoV-2 spike protein in endothelial cells in all patients.7 Interferonopathies, such as classic chilblains and associated variants, are characterized by an abnormal inflammatory and vascular response. Pardi et al8 illustrated the potential of mRNA vaccines to induce potent type I interferon reactions, which are well known to stimulate cytokine-mediated autoinflammatory responses. Interestingly, we observed the development of chilblains shortly after vaccination in an older adult patient, in which such robust immune responses are uncommonly seen.
Chilblains are typically seen following exposure to cold, damp environments, while less commonly seen in warmer climates, such as Southern California; however, chilblains may present during the winter season even in this region. There are 2 cases of chilblains in Southern California reported in the literature,9 and our clinic has seen an increase of chilblains during these past winter months. Our patient was vaccinated during these colder months, which obscures the relationship between her cutaneous eruption and the vaccine. It should be noted that our patient has a history of PLC, which has been described to affect acral surfaces,10 and which can present with lesions resembling those in Fig 1. However, a diagnosis of PLC was ruled out based on histological examination featuring typical characteristics of chilblains. Our case presentation does not prove a causal relationship between the mRNA-1273 vaccine and the development of chilblains. However, the temporal relationship between the rash onset and the date of vaccination and the development of the rash with each subsequent dose are in support of our theory.
Several vaccine surveillance systems are in place to collect safety and adverse reactions data, which can provide further insight, as widespread vaccination continues. Until then, the purpose of this report is to alert clinicians of the possible side effect of the mRNA-1273-induced chilblains and to reassure patients that obtaining the second dose of the vaccine is safe and should not be delayed.
Conflicts of interest
None declared.
Footnotes
Funding sources: None.
References
- 1.Freeman E.E., McMahon D.E., Lipoff J.B. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83(4):1118–1129. doi: 10.1016/j.jaad.2020.06.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccolo V., Neri I., Filippeschi C. Chilblain-like lesions during COVID-19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020;34(7):e291–e293. doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massey P.R., Jones K.M. Going viral: a brief history of Chilblain-like skin lesions ("COVID toes") amidst the COVID-19 pandemic. Semin Oncol. 2020;47(5):330–334. doi: 10.1053/j.seminoncol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeck M., Peeters C., Herman A. Chilblains and COVID-19: further evidence against a causal association. J Eur Acad Dermatol Venereol. 2021;35(1):e2–e3. doi: 10.1111/jdv.16901. [DOI] [PubMed] [Google Scholar]
- 5.Herman A., Peeters C., Verroken A. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020;156(9):998–1003. doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roca-Ginés J., Torres-Navarro I., Sánchez-Arráez J. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. JAMA Dermatol. 2020;156(9):992–997. doi: 10.1001/jamadermatol.2020.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colmenero I., Santonja C., Alonso-Riaño M. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183(4):729–737. doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon R., Arikian A.M., Pakula A.S. Chilblains in Southern California: two case reports and a review of the literature. J Med Case Rep. 2014;8:381. doi: 10.1186/1752-1947-8-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halbesleben J.J., Swick B.L. Localized acral pityriasis lichenoides chronica: report of a case. J Dermatol. 2011;38(8):832–834. doi: 10.1111/j.1346-8138.2010.01089.x. [DOI] [PubMed] [Google Scholar]