INTRODUCTION
Ticks are obligate hematophagous ectoparasites distributed worldwide and serve as vectors of human and animal diseases 1. The focus of this review is on Ixodid or hard ticks, one of three families within the suborder Ixodida that includes about 700 species 2. Ixodid ticks are vectors of numerous human and livestock diseases 3. Since the bite of a tick is the only route of natural transmission of tick-borne pathogens, several strategies have been explored over the last few decades to prevent getting bitten by ticks. Acaricides, a first-line strategy used to control tick populations in endemic areas, may be detrimental to the environment, and ticks rapidly develop resistance to acaricides making them ineffective 4, 5. Biocontrol measures using entomopathogenic fungi have also shown promise in controlling diverse Ixodid tick populations 6. However, the logistical difficulties in the implementation of biocontrol strategies are hampered by environmental variables including temperature and humidity. Vaccines that can target and impair tick feeding or fecundity have been shown to be effective at controlling tick populations 7. Tick infestations of livestock animals result not just in disease transmission, but also in weight loss and anemia and this impacts milk and meat production resulting in significant economic losses to the animal industry 8. From the veterinary disease perspective, controlling tick densities at specific localities is, therefore, highly valuable. This is exemplified by the success of the Bm86 protein subunit vaccine, the only commercially available anti-tick vaccine, based on a gut protein of Rhipicephalus microplus that impairs tick feeding and significantly reduces R. microplus infestations on cattle 9.
While reducing tick populations in endemic areas also impacts human disease prevalence by reducing the probability of tick encounters, vaccines that can efficiently prevent disease transmission are also possible 10. Traditionally, transmission-blocking vaccines have largely relied on targeting the pathogen, and not the vector. A vaccine to prevent tick-transmitted tick-borne encephalitis virus (TBEV) infection of humans is available for human use in Europe 11. The vaccine is an antigen extract derived from in vitro grown TBE virus that is filtered and inactivated in formaldehyde 12. In the USA, a vaccine to prevent Lyme disease based on OspA, a surface antigen of the Lyme disease agent, was approved for human use in 1998 13, 14, but was withdrawn from the market in 2002 by the manufacturer 15. Global warming is affecting the spread of diverse vectors of human and livestock pathogens, including ticks and tick-borne pathogens 16-18. In the past two decades alone, several newly recognized tick-borne pathogens have been recognized in the Western hemisphere 19. Therefore, there is an urgent need to develop vaccine strategies that would simultaneously reduce tick populations and also prevent transmission of tick-borne pathogens to mammalian hosts. Towards this goal, several research efforts are focused on identifying tick antigens that may serve as effective vaccine targets to thwart tick feeding and consequently also prevent pathogen transmission. The sequencing of transcriptomes and genomes of multiple tick species in the last decade 20-24 coupled with technological advancements have added critical molecular tools 25-27 and collectively accelerated our efforts to gain functional insights into the tick genome.
While, we have overcome the paucity of genomic data that plagued tick research a decade ago, the task of sifting through these meta datasets to identify critical antigens that may serve as vaccine targets remains a daunting challenge. Providing a functional paradigm to address this challenge is the phenomenon of acquired tick resistance or ATR 28. Seminal observations by William Trager in 1939 showed that non-permissive hosts mount a robust immune response to critical tick salivary antigens, and thwart tick feeding. Research efforts to identify salivary proteins targeted by ATR have yielded a diverse list of salivary antigens with functions relevant to tick feeding 29-32. However, immunity elicited against these antigens individually or as subset of cocktails have only partially recapitulated ATR. It is clear that the molecular basis of this phenomenon that has remained a puzzle 33, 34 poses a bottleneck in our ability to fully exploit this robust paradigm towards defining tick salivary vaccine targets. This review will examine our current and expanding understanding of ATR, and highlight how this understanding might reveal key events at the tick-host interface that enable or disable tick feeding. This understanding will educate the prioritization of salivary antigens that may be vaccine targeted and guide the development of an anti-tick vaccine.
TICK SALIVARY PROTEOME: PARAMOUNT FOR TICK FEEDING
Hard ticks (Ixodidae) are obligate hematophagous arthropods and obtain their blood meal by attaching to the skin of vertebrate hosts, tearing the skin and feeding from the pool of blood formed at the bite site. Successful hematophagy is central to the completion of the life cycle of the hard tick. Since the life cycles of the pathogens transmitted by hard ticks are entwined with that of the tick, there is significant impetus to gain a molecular understanding of how ticks acquire a bloodmeal. Tick attachment to the host involves tearing the skin, which is accomplished by a pair of barbed, articulated chelicerae at the tip of the mouthparts. The chelicerae flex and retract, inserting the hypostome and pushing the tick further into the host until the mouthparts are completely embedded into the dermis of the skin 35. Slicing mouthparts create a feeding lesion into which saliva is secreted at continuous intervals over the course of the 4-10 day feeding period typical for Ixodes complex ticks. In order to remain stably tethered to the host over the prolonged feeding period the tick secretes an adhesive cement within hours of attachment to the host 36. Tick cement is shown to be composed of a mixture of proteins, lipids, amino acids such as glycine, serine and tyrosine, and carbohydrates 37 that together form a viscous gel-like cone composed of a core cement that fits snugly around the hypostome and a cortical cement secreted outside the core cement 37. Providing an impenetrable physical barrier to the tick mouth parts is thought to be one of the prime functions of tick cement 38. Until a decade or so ago, only a descriptive understanding of tick cement was available 39, 40 . Advances in molecular techniques, and the availability of an artificial feeding system for ticks have helped circumvent qualitative and quantitative limitations in cement research 37 Cement-specific proteins have been identified predominantly from Rhipicephalus species 41-44 and from Amblyomma species 45, 46 with potential antimicrobial and antihemostatic activities. A cement antigen from Rhipicephalus appendiculatus named 64P 41 was shown to be represented in multiple Ixodid tick species 47 with the potential to serve as a broad-spectrum anti-tick vaccine. Further progress in the molecular understanding of tick cement may reveal novel strategies to thwart tick feeding.
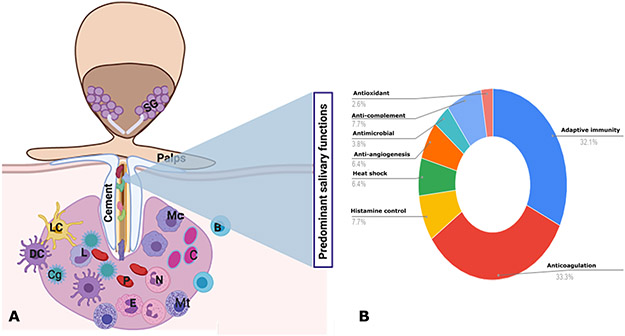
Tick saliva contains an array of immunomodulators, anticoagulants, and hemostatic compounds, which allow the tick to manipulate and maintain the feeding site and subvert host defenses 48-50 and is pivotal for the tick to feed to repletion. An overview of the predominant functions elaborated in the saliva of hard ticks is provided in Figure 1. The first step in hemostasis is the development of a platelet plug and salivary proteins that interfere with this process are secreted by multiple hard tick species. Prostacyclin 51 and the serine protease inhibitor (serpin) IxscS-1E1 52 from I. scapularis, IRS-2 from I. ricinus 53, R. microplus serpins, RmS-3 and RmS-17 54, and Variabilin from D. variabilis 55 disrupt platelet aggregation. In addition to platelet aggregation, multiple pro-coagulation factors are activated by the host to prevent blood loss. Several I. scapularis salivary proteins that inhibit various steps of coagulation have been identified including, Salp14, 56, Innonexin 57, TIX-5 58, Ixolaris 59, and Penthalaris 60. Other anticoagulants from Ixodes ticks include metalloproteases 61-64, various serpins 52, 65, 66, Ir-CPI 67, Iris 68 and Rhippilin-1 and −2 from R. hemaphysaloides 69. Haemaphysalis ticks have a particularly large number of demonstrated and putative thrombin inhibitors, including: madanin-1 and −2 70, 71, chimadanin 72, and the serpin HLS1 73 from H. longicornis; haemathrin from H. bispinosa 74; and serpins HDS1 and HDS2 from H. doenitzi 75. Iris from I. ricinus 68 has also demonstrated anti-thrombin activity, in addition to BmGTI 76, the serpin RmS-15 77, BmAP 78, and microphilin 79 from R. microplus. Haemaphysalis proteins longistatin 80 and enolase 81 stimulate this pathway by activating plasminogen, that negatively regulates the coagulation cascade.
Figure 1. Hard tick attached to host skin and secreting pharmacologically active salivary components into the feeding lesion.
A. Ixodid ticks attach to the host skin by inserting their hypostome into the dermis of the host skin and adhere firmly with the help of salivary cement that is deposited at the bite-site and around the hypostome. Saliva secreted into the feeding lesion thwarts defense responses of the host. SG, salivary glands; Mc, macrophage; N, neutrophils; Lc, Langerhans cells; Dc, dendritic cells; E, eosinophils; Mt, mast cells; B, B-cells; L, lymphocytes; Cg, procoagulants; C, complement factors. B. Brief overview of predominant salivary functions characterized in tick saliva. Percent calculations based on the literature surveyed in this review.
Histamine released at the bite site by platelets, basophils and mast cells can be detrimental to tick attachment by inducing pain and itch responses at the bite site that eventually result in the host grooming the tick off 33, 82. Inhibiting histamine-related inflammation is therefore essential for the tick to remain attached to the host 83. Multiple histamine binding proteins have been identified in the saliva of R. appendiculatus 83, D. reticulatis 84, I. persulcatus 85 I. ricinus 86, and I. scapularis 61. Ticks also maintain their feeding lesion by interfering with host immune responses. Pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α are down-regulated by salivary proteins such as sialostatins (L, L2, Ip-sL1, and Ip-sL2) 87-89, Salp15 90, Iris 91, 92, Isac 93, Ir-SPI 94, IRS-2 95, and Iristatin 96. Other anti-inflammatory salivary proteins include: serpins 97, Hl-p36 98, and Longistatin (99 from H. longicornis; PGE2 from D. variabilis 100, and evasins from R. sanguineas 101, 102. Interestingly, Japanin from R. appendiculatus and PGE2 from D. variabilis and I. scapularis 100, 103, 104 upregulate the anti-inflammatory cytokine IL-10. A sphingomylenase enzyme activity in I. scapularis saliva was shown to promote IL-4 production by CD4-T cells 105. These functions of the tick sialome are suggested to skew the host immune response towards a Th2 profile that may be advantageous to the survival of tick-borne pathogens during transmission and is a key element of saliva-assisted pathogen transmission 106. Tick sialome also encodes functions that prevents immune cell activation and proliferation 91, 94, 96-98, 104, 107-112
Salivary proteins also impair the host complement system that represents a major innate immune response triggered by microbes, cell damage, antigen-antibody complex, and glycans and lead to increased inflammation detrimental to tick feeding. I. scapularis proteins Salp20 and Isac 113-116; I. ricinus proteins IRAC I and II 117, 118 and R. pulchellus protein Cirp-T 119 interrupt the alternative pathway. Tick Salivary Lectin Pathway Inhibitors (TSLPI) in I. scapularis and I. ricinus interfere with the lectin pathway 120, 121. Recent reports suggest that Amblyomma species may encode functions that also impair the classical pathway 50. Additionally, antioxidant salivary proteins have been described in Ixodid ticks and function to quench reactive oxygen and nitrogen species generated by immune cells such as neutrophils and macrophages that migrate to the tick bite-site 29, 122-124.
It is important to note that not all salivary proteins are expressed and secreted throughout the course of feeding. The tick sialome is dynamic and the composition changes over time 125, 126, potentially orchestrated by the different phases of tick feeding 127. Tick salivary protein functions are also exploited by pathogens as they transit to and from the host skin 106, 128, 129. Therefore, it is logical to envision that salivary molecules may serve as viable vaccine targets to abrogate tick feeding and prevent pathogen transmission. Temporal changes in the salivary composition in conjunction with the functional complexity, and redundancy of the tick sialome have rendered the search for salivary vaccine targets a task akin to searching for the proverbial “needle in the haystack”. An opportunity to circumvent this challenge has come from the phenomenon of acquired tick resistance (ATR), originally described by William Trager in 1939 28. Since ATR results in thwarting tick feeding and pathogen transmission, expanding a molecular and mechanistic understanding of ATR may offer a robust paradigm to define the critical subset of salivary proteins that may be vaccine targeted.
ANIMAL MODELS OF ACQUIRED TICK RESISTANCE
Trager, in 1939 28, showed that upon repeated tick infestations of guinea pigs with Dermacentor variabilis nymphs, animals developed an immune response that was potent enough to derail subsequent tick challenges. Tick-immune animals rapidly rejected ticks within the first 24 hours of tick attachment. This phenomenon of acquired tick resistance (ATR) is characterized by visible erythema at the tick bite-site due to the rapid recruitment of immune cells, predominantly composed of basophils and eosinophils and hence termed cutaneous basophilic hypersensitivity in guinea pigs 130, also known as Jones-Mote hypersensitivity in humans 131. The immune responses recruited to the tick bite site on tick-resistant animals are thought to be detrimental to tick feeding resulting in early tick detachment and decreased engorgement weights. Similar observations with additional tick species, as well as the generation of acquired tick resistance in rabbits, mice and cattle 132-135 have demonstrated that the phenomenon of ATR is widespread in tick-host interactions.
Ticks deposit a diverse set of salivary proteins into the host skin in order to facilitate acquisition of a blood meal, as outlined above. It is generally believed that when ticks feed on non-natural hosts, the host mounts a robust immune response towards the deposited salivary proteins 33. Attesting to this hypothesis, experiments wherein animals were immunized with salivary gland extract or saliva have shown that immunity against salivary components is indeed capable of eliciting several parameters of tick resistance including erythema at the bite-site, and impaired feeding 136. It is however, important to note that that none of the effects were as robust as naturally acquired tick resistance. These immune responses potentially neutralize salivary functions essential for feeding, and salivary functions essential for keeping detrimental cells at bay. Activation and degranulation of basophils is thought to result in the release of basophil components including histamine that is noxious to the tick during the early stages of feeding 82, 137. Whether basophilic components released upon degranulation impairs tick feeding due to host responses such as vasodilation, pain and itching or whether it enters the tick gut and causes damage to the organ is not clear.
Guinea pigs have been shown to acquire tick immunity following repeated tick infestations with Amblyomma americanum 138, 139, Rhipicephalus species 140, I. scapularis 125, 141 and Dermacentor andersoni 142. Resistance has been observed at the larval, nymphal and adult stages. Guinea pigs and rabbits support I. scapularis feeding, but they are not the natural host species. Peromyscus leucopus, are the natural host for I. scapularis larvae and nymphs and do not develop tick immunity following repeated tick infestations 143. Similarly, laboratory mouse models also do not acquire tick resistance after repeated I. scapularis tick infestations by mechanisms that are not understood 144, 145.
ATR has been observed in C57BL/6 mice repeatedly infested with Haemaphysalis longicornis larvae 137. Larger mammals such as deer, horse, and cattle, and birds are the natural hosts for H. longicornis, whereas mice are not natural hosts. Cattle have been shown to develop tick resistance following repeated exposures to Rhipicephalus microplus. Importantly, ATR impaired Babesia transmission to cattle 146. Unlike guinea pigs, mice and rabbits, generation of tick immunity in cattle is dependent on the breed of cattle 147. Bos taurus indicus cattle develop immunity to R. microplus, whereas Bos taurus taurus do not develop immunity 147. Several studies have attempted to identify the genetic markers that could account for the differences, but, no clear markers have emerged 148. Histological analysis of the bite site revealed robust infiltration of basophils, mast cells and eosinophils in the skin of the resistant Bos Taurus indicus cattle. In comparison, the bite site of permissive Bos taurus taurus cattle was primarily infiltrated by neutrophils 39, 149, 150.
Evidence for tick immunity in humans is primarily anecdotal. There have been reported cases of individuals developing hypersensitivity reactions at the tick bite site, similar to the observation in tick immune animals 151, 152. Additionally, people with frequent exposures to tick bites have been shown to develop antibodies to tick proteins 153, 154. Importantly, individuals that report itching at the tick bite site may have a decreased chance of acquiring B. burgdorferi 155. Collectively, these results suggest that prior exposure to ticks and the development of immunity towards tick proteins could potentially trigger itching at the bite site and grooming to remove ticks early. Since B. burgdorferi is transmitted only after 24-36 hours of tick attachment, 156, 157, provoking the immunological parameters of acquired tick resistance upon tick attachment may offer an effective strategy for preventing tick transmission of Lyme disease 125, 141. Pathogens such as Babesia microti, Anaplasma phagocytophilum and Powassan virus that reside primarily in the salivary glands may be transmitted within 24 hours or earlier 158, 159. Whether, ATR may impact the transmission of these tick-borne pathogens remains to be seen.
MOLECULAR BASIS OF ACQUIRED TICK RESISTANCE
The mechanisms mediating tick rejection are complex and involve multiple components of the host’s adaptive immune system. As described above, cattle and guinea pigs readily acquire tick immunity following multiple tick infestations and have been used extensively to examine the tick bite site 143, 160, 161. Although the reagents required to characterize the immune response in guinea pigs and cattle are not fully available, several studies have identified a response dependent on both cellular and humoral responses. Repeated tick infestation results in robust immune cell infiltration to the bite site. The bite site of I. scapularis immune guinea pigs is heavily infiltrated by heterophils and macrophages by day 2, followed by an intense infiltration of leukocytes on days 3 and 4 143. Infiltrating basophils have also been observed at the tick bite site in guinea pigs repeatedly infested with Dermacentor andersoni and Amblyomma americanum 160, 162, 163, and at the bite site of tick immune cattle and mice 82, 161.
Basophils are a major source for histamine upon activation and have an important role during tick rejection. Additionally, degranulation of basophils plays an important role in the recruitment of eosinophils to the bite site, although the role of eosinophils requires further investigation 139. In a study to define the role of basophils, the authors demonstrated that tick rejection was abolished when immune guinea pigs were administered anti-basophil sera prior to challenge with Amblyomma americanum 139. However, anti-histamines administered at the time of challenge with A. americanum demonstrated that the critical role of basophils occurs through a histamine-independent mechanism 130. This is in contrast to tick rejection with other tick species that demonstrated that anti-histamines delivered to tick immune guinea pigs, mice and cattle ablate tick immunity towards R. microplus, D. andersoni and H. longicornis 82, 164, 165. Overall, these results suggest that basophils are important for immunity against multiple tick species; however, the mechanism of restriction can be species-specific.
Although degranulation of basophils has been demonstrated to be critical for tick rejection, the mechanism of degranulation is not understood. Salivary proteins deposited into the skin during feeding generate homocytotropic antibodies, i.e., antibodies that only engage with cells of the same or closely related species, bind predominantly to mast cells and basophils, resulting in degranulation 166. Cutaneous basophil hypersensitivity responses have been proposed to be mediated by IgG1 antibodies in guinea pigs resistant to Amblyoma americanum 166, although Wada et al 137 show that IgE antibodies play a role in rejection of H. longicornis ticks. Clearly, the role of antibodies in mediating tick rejection is complex and varies depending on the life stage of the ticks, host species, as well as the tick species 142. Passive transfer of lymph node cells, but not serum, from guinea pigs resistant to Dermacentor andersoni larvae conferred resistance to naïve guinea pigs 167. Similar results have also been observed with passive transfer of lymph node cells from guinea pigs resistant to A. americanum larvae 162. However, passive transfer with sera from guinea pigs infested with adult D. andersoni was able to confer protection 142. These results suggest that the life stage of the tick may also be important for the induction of protective antibody titers 142.
The role of complement has also been demonstrated to play an important role during tick rejection. Resistance to D. andersoni larvae in guinea pigs was ablated when the animals were administered cobra venom factor, a protein which depletes serum complement, prior to tick challenge 168, 169. This was further evaluated using C4-deficient guinea pigs, which are deficient in the classical complement activation pathway 170. In this study, C4-deficient guinea pigs were able to acquire resistance to D. andersoni larvae, suggesting that the alternate pathway of complement activation is important for tick rejection 170. This is also consistent with the observations that tick salivary proteomes, in general, do not contain proteins to inhibit the classical pathway of complement.
The immune reaction at the tick bite-site of immune animals can induce significant changes in the skin, which may play a role to limit tick feeding. In guinea pigs, repeat tick infestation with I. scapularis results in epicutaneous erythema, severe epidermal hyperplasia, edema and hyperkeratosis at the tick bite site 143. Similar results to dermal tissue have been observed in guinea pigs exposed to multiple infestations with Rhipicephalus sanguineus and A. americanum 160, 171. Unlike guinea pigs, Peromyscus leucopus fail to acquire tick immunity following repeat exposures to I. scapularis nymphs 143. Examination of the bite site in P. leucopus revealed minimal disruption to the dermal tissue following repeat tick infestation 143. The observed changes to dermal tissue are speculated to contribute towards tick rejection in guinea pigs, whereas the lack of dermal changes in P. leucopus could support repeat tick feeding.
Basophils were mistakenly thought to be absent in mice 172, until mouse basophils were described by Dvorak et al 173. Over the last two decades, an understanding of the enigmatic role of basophils in Th2-immunity has started to unravel 174-176 and these studies have also provided insights into the possible mechanisms of ATR. While the lack of immunological reagents has hampered our ability to dissect the molecular basis of ATR using the guinea pig and cattle model, this has been circumvented using the mouse model of ATR that is elicited by repeated infestations with H. longicornis 137. Using mice expressing the diphtheria toxin receptor under the control of basophil-specific mast cell protease 8 (Mcpt8) promoter, Wada et al 137 selectively ablated basophils by administering diphtheria toxin to the animals after the first tick infestation and showed that basophils, play a non-redundant role in eliciting ATR to H. longicornis nymphs. Ohta et al 177 then demonstrated that basophil recruitment is promoted by IL-3 secreted by CD4+ memory T cells that arrive at the skin after a tick-challenge. Dissecting the mechanism of ATR, Tabakawa et al 82 showed that histamine released by skin-infiltrating basophils, but not by skin-resident mast cells, promotes H. longicornis rejection. The exact mechanism/s by which histamine promotes tick rejection remains to be deciphered. While the molecular understanding of H. longicornis-provoked ATR in mice is beginning to clarify, given the heterogeneity in the immunological responses of host species to tick species and stages, a single unifying mechanism may not emerge.
DICHOTOMOUS IMMUNE RESPONSES TO TICK BITES
Although, ticks can feed on diverse hosts, each tick species appears to have a host preference in nature that is likely determined by a combination of host, tick, and ecological factors. In general, preferred hosts serve as reservoir hosts for the specific tick and are able to host multiple infestations of the tick species without developing any immune responses detrimental to tick feeding. This characteristic is essential in order to ensure the successful completion of the tick’s life cycle. For example, I. scapularis nymphs and larvae predominantly feed on P. leucopus, the reservoir host 178. Expectedly, in summer and fall when nymphs and larvae are active, P. leucopus are fed upon multiple times by these ticks without any evidence of ATR. Mus musculus has served as a laboratory model of reservoir host for I. scapularis studies despite the fact that P. leucopus and M. musculus belong to different genera and M. musculus is not the natural reservoir host 178. The genetic traits that allow both P. leucopus and M. musculus species to host I. scapularis ticks without developing ATR are not known. Guinea pigs, and rabbits are non-natural hosts for I. scapularis that readily develop ATR. This dichotomy in immune response to tick feeding is thought to be multifactorial as summarized in Figure 2. Proposing a lock and key hypothesis to explain this dichotomy, Ribeiro suggested that tick salivary molecules have co-evolved with reservoir hosts like P. leucopus and geared to efficiently engage with and diffuse adaptive immune responses of P. leucopus 179. Conversely, I. scapularis salivary components engage poorly with immune components of non-natural hosts, hence unable to thwart host adaptive immune responses. In essence, natural/permissive hosts do not have an immunological memory of tick bites, while non-natural/non-permissive hosts do.
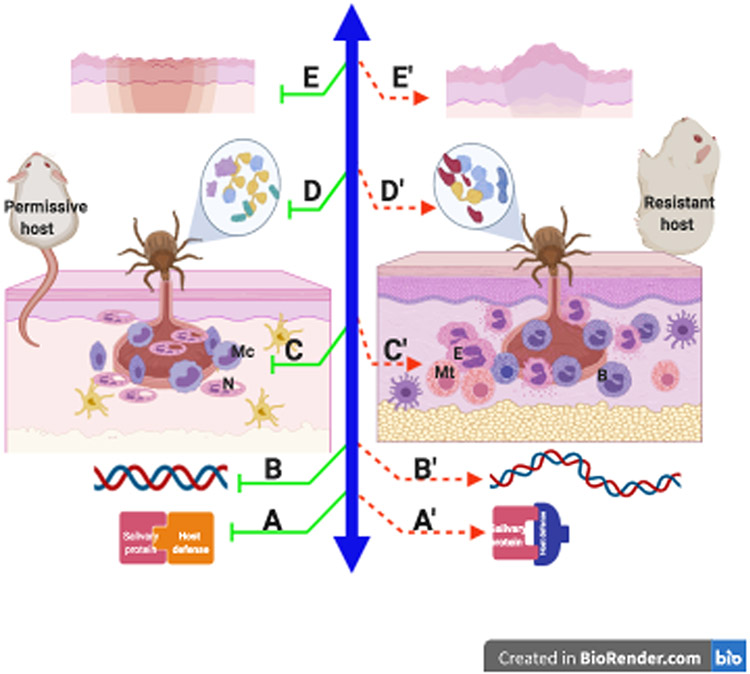
Figure 2. Potential mechanisms of acquired tick resistance.
Factors that may drive the dichotomous immune responses to tick bites on permissive or resistant host species include: Optimal (A) or suboptimal (A’) engagement of salivary proteins with host defense responses; genetic predisposition to decreased (B) or increased (B’) inflammatory responses to salivary proteins; structural and immunological differences in the skin (C, C’); Host-specific salivary proteome that is sufficient (D) or deficient (D’) in modulating host defense responses; and differences in wound healing without scar (E) or with scar (E’) formation.
Another explanation that does not exclude the lock and key hypothesis, but adds another facet to the dichotomous immune response on natural and non-natural host is that the tick sialome is different when it feeds on different hosts. Narasimhan et al 145 compared the salivary composition of I. scapularis nymphs fed on M. musculus, the laboratory model of natural host, and on guinea pigs, the model non-natural host and observed that the sialome composition is host-specific. Tirloni et al 180 also observed that the protein composition of adult I. scapularis and A. americanum stimulated by exposing them to specific host skin semiochemicals of rabbit, dog or human was indeed different. This iterated that the tick salivary composition is not just temporally modulated, but also modulated by host skin components. Narasimhan et al’s study 145 showed that mouse splenocytes incubated with mouse-fed salivary gland extracts elicited significantly less IL-4, a Th2-defining cytokine, when compared to the amounts elicited by guinea pig-fed salivary gland extracts. Consistent with this, Franzin et al 181 showed that R. microplus fed on tick susceptible Bos taurus taurus expressed significantly increased amounts of salivary transcripts for genes encoding immune modulators such evasins, and metalloproteases, when compared to that in ticks fed on tick-resistant Bos taurus indicus. This differential expression of secreted salivary immunomodulators could account for differential evasion of host defense responses. Therefore, host-modulated differences in the tick sialome composition could inadvertently account, in part, for functional differences critical to dampen host immune responses. A detailed characterization of the qualitative differences between tick-susceptible host-fed and tick-resistant host-fed tick sialomes would offer molecular insights into ATR.
The dichotomous immune response to tick feeding could also be driven by the inherent structural and immunological differences between the guinea pig and mouse skin. While the epidermis, dermis and hypodermis are well defined and thick in the guinea pig, these layers are thin in mice 182, 183. Since the mouse skin is covered by fur, the fat-laden hypodermis that normally provides thermal homeostasis in guinea pig and human skin 184, is significantly reduced in the murine skin 183. Mice have an additional subcutaneous layer called panniculus carnosus, an extra layer of muscle that is thought to allow wound healing via contraction resulting in little or no scarring 185. While guinea pigs also have the panniculus carnosus layer, wound healing may occur via re-epithelization leading to scar formation 186 and could account for the epidermal hyperplasia and hyperkeratosis observed at the I. scapularis bite-site on guinea pigs, but not on mice 143 . While, the immunology of the guinea pig skin is not fully understood, presumably it behaves more like the human skin than the murine skin. The Langerhans cells, the dendritic cell subset of the epidermis, in mice are not critical for priming CD8+ T cells, unlike in humans 187, 188. Lymphocytes in the murine epidermis are predominantly populated by γδT cells, while in humans it is predominantly αβT cells 189.
Although, paucity of immunological tools to examine traditional animal models of ATR such as the guinea pig and cattle has stymied progress in this research, Franzin et al 181, More et al 190, and Kurokawa et al 191 have harnessed the power of rapidly evolving next generation RNA sequencing tools to examine host components at the tick bite-site that may serve as molecular drivers of ATR. Franzin et al 181 underscore the genetic predisposition to resistance or susceptibility and show that R. microplus resistant B. taurus indicus have a higher baseline expression of genes encoding proinflammatory cytokines. Consistent with histological examination of tick bite sites of resistant animals, the tick-resistant breed of Bos taurus demonstrated increased expression of basophil and T lymphocyte recruiting cytokine CCL2 when compared to tick-sensitive animals. More interestingly, their study showed that the skin of the susceptible breed expresses higher levels of enzymes involved in detoxification and this also generates volatile metabolites that serve as semiochemicals that are attractive to ticks. More et al 190 showed that upon repeated infestations of tick-resistant breed of B. taurus with R. microplus expression of genes involved in skin remodeling and in basophil activation were increased at the bite-site when compared to that in tick-susceptible breed. Further, transcripts encoding for CCL13, a cytokine invoked in eosinophil recruitment was upregulated at the tick bite-site. This was consistent with the findings of Kurokawa et al 191 that eosinophils are increased at the tick bite-sites on tick-resistant guinea pigs. Further, Robbertse et al 192 addressed lymphocyte populations the skin and draining lymph nodes of resistant and susceptible B. taurus after challenge with R. microplus and showed that the numbers of B lymphocytes and T-helper lymphocytes were decreased in the lymph nodes of tick-susceptible animals. Increased variability in WC1+γδ T lymphocytes populations was also associated with increased susceptibility to R. microplus ticks. Kurokawa et al 191 demonstrated that FCεRI-signaling, complement pathways and procoagulation pathways are activated in the guinea pig skin, but not in mice skin upon repeated infestations with I. scapularis nymphs.
These genetic, transcriptomic and immunologic studies of host responses to tick bites have begun to reveal new insights into the differential responses to tick bites on susceptible and resistant hosts. However, an understanding of what components of the tick saliva drive these differential responses on different host species will be essential to develop strategies to prevent tick feeding and tick-transmission of pathogens. An interesting facet of ATR that may additionally help to shed light on the molecular mechanisms of ATR is the observation that ATR against one tick genus may be cross-protective against another related tick genus.
ATR- MEDIATED CROSS PROTECTION
Cross protective immunity is the phenomenon wherein ATR against a tick species of primary exposure confers host resistance against a secondary tick species for which the host has no prior exposure 28, 193, 194. Numerous tick antigens are conserved among hard tick species including many salivary proteins 195 and could, in part, explain the acquisition of cross-protective immunity. This is also likely the reason why immunity against truncated protein constructs (64TRP) of the 64P cement antigen from Rhipicephalus appendiculatus was shown to induce immunity in rabbits and hamsters against Ixodes ricinus, and Rhipicephalus sanguineus that impacted tick feeding and mortality 47. Cross species protection is important for several reasons related to vaccine development against ticks. Closely related tick species are frequently competent vectors of similar pathogens. For example, the western blacklegged tick, Ixodes pacificus transmits Lyme disease spirochetes (Borrelia burgdorferi) in the West Coast region of the United States, while Lyme spirochetes are transmitted by I. scapularis in other geographic parts of the US. In Europe and Eurasia, the castor bean tick, I. ricinus is the primary vector for Lyme spirochetes. Cross protective immunity could also potentially be useful in areas where multiple medically important tick species occur in close proximity and parasitize the same hosts. This could include the eastern United States, where I. scapularis, Dermacentor variabilis, and A. americanum frequently co-exist and utilize some of the same host species, including white-tail deer (Odocoileus virginianus). Therefore, a molecular understanding of salivary proteins that are involved in cross-protective immunity would help define and prioritize vaccine targets that may serve to simultaneously impair the feeding and fecundity of multiple tick species in endemic regions. It is conceivable that a broad-spectrum anti-tick vaccine applied to wild deer could limit the density of tick populations, potentially reducing enzootic transmission of Borrelia, Anaplasma, Ehrlichia, Rickettsia, and Babesia species transmitted by these ticks. Such an approach would also be operationally cost-effective.
CONCLUSIONS
The phenomenon of acquired tick resistance to ticks remains a puzzling facet of tick-host interactions, since its first description almost 80 years ago 28. Technological advancements in molecular tools to address tick and host functional genomes, transcriptomes and immunomes have provided the much-needed momentum to unravel a new understanding of this phenomenon. A molecular understanding of this phenomenon will enhance approaches to define the subset of antigens that may serve as potential vaccine targets. Translating insights from ATR to tick vaccine development will also be accelerated by recent advancements in vaccine delivery platforms. Although, protein-based vaccines have been the mainstay of vaccinology for the last century 196, the rapidly evolving science of vaccinomics has highlighted the exciting possibility of using DNA 197-199 and mRNA 200-based vaccines to deliver nucleotide sequences encoding target antigens into the host. This enables the in-vivo generation of recombinant antigens by the host translational machinery and immunological presentation of these antigens elicit B and T cell-mediated responses critical for effective and long-lasting immunity 200-202, These approaches may circumvent the cumbersome process of optimization of protein production and formulation and spur progress in tick vaccine development. Importantly, deciphering a mechanistic understanding of ATR will reveal interesting facets of mammalian immunology, and of tick biology that will transcend the field of tick research.
ACKNOWLEDGEMENTS
We are grateful to Ms Aine Kaminski for excellent technical assistance during literature collation. This research was supported by grants from the Steven and Alexandra Cohen Foundation, and the NIH (AI138949, AI126033 and AI128182).
DATA AVAILABILITY
Data cited in this review are published and available on-line or upon request from the authors of the respective publications. No unpublished data is included in this manuscript.
Literature Cited
- 1.Goodman JL, Dennis DT and Sonenshine DE, Tick-Borne Diseases of Humans, ed. Goodman JL, Dennis DT and Sonenshine DE 2005: ASM Press, Washington, DC. 401. [Google Scholar]
- 2.Anderson JM, Ammerman NC, and Norris DE, Molecular differentiation of metastriate tick immatures. Vector Borne Zoonotic Dis, 2004. 4(4): p. 334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jongejan F and Uilenberg G, The global importance of ticks. Parasitology, 2004. 129 Suppl: p. S3–14. [DOI] [PubMed] [Google Scholar]
- 4.George JE, Pound JM, and Davey RB, Chemical control of ticks on cattle and the resistance of these parasites to acaricides. Parasitology, 2004. 129 Suppl: p. S353–66. [DOI] [PubMed] [Google Scholar]
- 5.Graf JF, et al. , Tick control: an industry point of view. Parasitology, 2004. 129 Suppl: p. S427–42. [DOI] [PubMed] [Google Scholar]
- 6.Perinotto WM, et al. , Susceptibility of different populations of ticks to entomopathogenic fungi. Exp Parasitol, 2012. 130(3): p. 257–60. [DOI] [PubMed] [Google Scholar]
- 7.de la Fuente J and Kocan KM, Strategies for development of vaccines for control of ixodid tick species. Parasite Immunol, 2006. 28(7): p. 275–83. [DOI] [PubMed] [Google Scholar]
- 8.Jonsson NN, Davis R, and De Witt M, An estimate of the economic effects of cattle tick (Boophilus microplus) infestation on Queensland dairy farms. Aust Vet J, 2001. 79(12): p. 826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de la Fuente J, et al. , A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev, 2007. 8(1): p. 23–8. [DOI] [PubMed] [Google Scholar]
- 10.Willadsen P, Anti-tick vaccines. Parasitology, 2004. 129 Suppl: p. S367–87. [DOI] [PubMed] [Google Scholar]
- 11.Heinz FX, et al. , Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis, 2013. 19(1): p. 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogovic P and Strle F, Tick-borne encephalitis: A review of epidemiology, clinical characteristics, and management. World J Clin Cases, 2015. 3(5): p. 430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Embers ME and Narasimhan S, Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol, 2013. 3: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotkin SA, Need for a New Lyme Disease Vaccine. N Engl J Med, 2016. 375(10): p. 911–3. [DOI] [PubMed] [Google Scholar]
- 15.Nigrovic LE and Thompson KM, The Lyme vaccine: a cautionary tale. Epidemiol Infect, 2007. 135(1): p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell-Lendrum D, et al. , Climate change and vector-borne diseases: what are the implications for public health research and policy? Philos Trans R Soc Lond B Biol Sci, 2015. 370(1665). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrada-Pena A, et al. , Effects of environmental change on zoonotic disease risk: an ecological primer. Trends Parasitol, 2014. 30(4): p. 205–14. [DOI] [PubMed] [Google Scholar]
- 18.Estrada-Pena A, Tick-borne pathogens, transmission rates and climate change. Front Biosci (Landmark Ed), 2009. 14: p. 2674–87. [DOI] [PubMed] [Google Scholar]
- 19.Rochlin I and Toledo A, Emerging tick-borne pathogens of public health importance: a mini-review. J Med Microbiol, 2020. 69(6): p. 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chmelar J, et al. , Sialomes and Mialomes: A Systems-Biology View of Tick Tissues and Tick-Host Interactions. Trends Parasitol, 2016. 32(3): p. 242–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artigas-Jeronimo S, De La Fuente J, and Villar M, Interactomics and tick vaccine development: new directions for the control of tick-borne diseases. Expert Rev Proteomics, 2018. 15(8): p. 627–635. [DOI] [PubMed] [Google Scholar]
- 22.Nene V, Tick genomics--coming of age. Front Biosci (Landmark Ed), 2009. 14: p. 2666–73. [DOI] [PubMed] [Google Scholar]
- 23.Kotsyfakis M, et al. , Deep Sequencing Analysis of the Ixodes ricinus Haemocytome. PLoS Negl Trop Dis, 2015. 9(5): p. e0003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia N, et al. , Large-Scale Comparative Analyses of Tick Genomes Elucidate Their Genetic Diversity and Vector Capacities. Cell, 2020. 182(5): p. 1328–1340 e13. [DOI] [PubMed] [Google Scholar]
- 25.Ramakrishnan VG, et al. , Application of RNA interference in tick salivary gland research. J Biomol Tech, 2005. 16(4): p. 297–305. [PMC free article] [PubMed] [Google Scholar]
- 26.Kurtti TJ, et al. , Transgene expression and silencing in a tick cell line: A model system for functional tick genomics. Insect Biochem Mol Biol, 2008. 38(10): p. 963–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliver JD, et al. , An Ixodes scapularis cell line with a predominantly neuron-like phenotype. Exp Appl Acarol, 2015. 66(3): p. 427–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trager W, Accquired immunity to ticks. J. Parasitology, 1939. 25: p. 57–81. [Google Scholar]
- 29.Das S, et al. , Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis, 2001. 184(8): p. 1056–64. [DOI] [PubMed] [Google Scholar]
- 30.Schuijt TJ, et al. , Identification and characterization of Ixodes scapularis antigens that elicit tick immunity using yeast surface display. PLoS One, 2011. 6(1): p. e15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro SZ, Voigt WP, and Fujisaki K, Tick antigens recognized by serum from a guinea pig resistant to infestation with the tick Rhipicephalus appendiculatus. J Parasitol, 1986. 72(3): p. 454–63. [PubMed] [Google Scholar]
- 32.Rego ROM, et al. , Counterattacking the tick bite: towards a rational design of anti-tick vaccines targeting pathogen transmission. Parasit Vectors, 2019. 12(1): p. 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wikel SK, Host immunity to ticks. Annu Rev Entomol, 1996. 41: p. 1–22. [DOI] [PubMed] [Google Scholar]
- 34.Willadsen P and Jongejan F, Immunology of the tick-host interaction and the control of ticks and tick-borne diseases. Parasitol Today, 1999. 15(7): p. 258–62. [DOI] [PubMed] [Google Scholar]
- 35.Richter D, et al. , How ticks get under your skin: insertion mechanics of the feeding apparatus of Ixodes ricinus ticks. Proc Biol Sci, 2013. 280(1773): p. 20131758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonenshine DE, Biology of ticks. 1991, New York: Oxford University Press. v. [Google Scholar]
- 37.Suppan J, et al. , Tick attachment cement - reviewing the mysteries of a biological skin plug system. Biol Rev Camb Philos Soc, 2018. 93(2): p. 1056–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moorhouse DE and Tatchell RJ, The feeding processes of the cattle-tick Boophilus microplus (Canestrini): a study in host-parasite relations. I. Attachment to the host. Parasitology, 1966. 56(4): p. 623–32. [DOI] [PubMed] [Google Scholar]
- 39.Tatchell RJ and Moorhouse DE, The feeding processes of the cattle tick Boophilus microplus (Canestrini). II. The sequence of host-tissue changes. Parasitology, 1968. 58(2): p. 441–59. [DOI] [PubMed] [Google Scholar]
- 40.Cowdry EV a.D.W.B.C., Studies on East Coast Fever. II. Behaviour of the Parasite and the Development of Distinctive Lesions in susceptible Animals1. Parasitology, 1933. 25(1): p. 1–63. [Google Scholar]
- 41.Havlikova S, et al. , Functional role of 64P, the candidate transmission-blocking vaccine antigen from the tick, Rhipicephalus appendiculatus. Int J Parasitol, 2009. 39(13): p. 1485–94. [DOI] [PubMed] [Google Scholar]
- 42.Mulenga A, et al. , Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect Immun, 1999. 67(4): p. 1652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bishop R, et al. , A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int J Parasitol, 2002. 32(7): p. 833–42. [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, et al. , Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol Res, 2006. 100(1): p. 77–84. [DOI] [PubMed] [Google Scholar]
- 45.Karim S and Ribeiro JM, An Insight into the Sialome of the Lone Star Tick, Amblyomma americanum, with a Glimpse on Its Time Dependent Gene Expression. PLoS One, 2015. 10(7): p. e0131292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bullard R, et al. , Structural characterization of tick cement cones collected from in vivo and artificial membrane blood-fed Lone Star ticks (Amblyomma americanum). Ticks Tick Borne Dis, 2016. 7(5): p. 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trimnell AR, et al. , A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine, 2005. 23(34): p. 4329–41. [DOI] [PubMed] [Google Scholar]
- 48.Brossard M and Wikel SK, Tick immunobiology. Parasitology, 2004. 129 Suppl: p. S161–76. [DOI] [PubMed] [Google Scholar]
- 49.Francischetti IM, et al. , The role of saliva in tick feeding. Front Biosci, 2009. 14: p. 2051–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotal J, et al. , Modulation of host immunity by tick saliva. J Proteomics, 2015. 128: p. 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribeiro JM, Makoul GT, and Robinson DR, Ixodes dammini: evidence for salivary prostacyclin secretion. J Parasitol, 1988. 74(6): p. 1068–9. [PubMed] [Google Scholar]
- 52.Ibelli AM, et al. , A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting. Int J Parasitol, 2014. 44(6): p. 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chmelar J, et al. , A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood, 2011. 117(2): p. 736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tirloni L, et al. , The putative role of Rhipicephalus microplus salivary serpins in the tick-host relationship. Insect Biochem Mol Biol, 2016. 71: p. 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, et al. , Variabilin, a novel RGD-containing antagonist of glycoprotein IIb-IIIa and platelet aggregation inhibitor from the hard tick Dermacentor variabilis. J Biol Chem, 1996. 271(30): p. 17785–90. [DOI] [PubMed] [Google Scholar]
- 56.Narasimhan S, et al. , Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A, 2004. 101(5): p. 1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assumpcao TC, et al. , Ixonnexin from Tick Saliva Promotes Fibrinolysis by Interacting with Plasminogen and Tissue-Type Plasminogen Activator, and Prevents Arterial Thrombosis. Sci Rep, 2018. 8(1): p. 4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuijt TJ, et al. , Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation, 2013. 128(3): p. 254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francischetti IM, et al. , Ixolaris, a novel recombinant tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick, Ixodes scapularis: identification of factor X and factor Xa as scaffolds for the inhibition of factor VIIa/tissue factor complex. Blood, 2002. 99(10): p. 3602–12. [DOI] [PubMed] [Google Scholar]
- 60.Francischetti IM, Mather TN, and Ribeiro JM, Penthalaris, a novel recombinant five-Kunitz tissue factor pathway inhibitor (TFPI) from the salivary gland of the tick vector of Lyme disease, Ixodes scapularis. Thromb Haemost, 2004. 91(5): p. 886–98. [DOI] [PubMed] [Google Scholar]
- 61.Valenzuela JG, et al. , Exploring the sialome of the tick Ixodes scapularis. J Exp Biol, 2002. 205(Pt 18): p. 2843–64. [DOI] [PubMed] [Google Scholar]
- 62.Decrem Y, et al. , A family of putative metalloproteases in the salivary glands of the tick Ixodes ricinus. FEBS J, 2008. 275(7): p. 1485–99. [DOI] [PubMed] [Google Scholar]
- 63.Decrem Y, et al. , The impact of gene knock-down and vaccination against salivary metalloproteases on blood feeding and egg laying by Ixodes ricinus. Int J Parasitol, 2008. 38(5): p. 549–60. [DOI] [PubMed] [Google Scholar]
- 64.Ali A, et al. , Reprolysin metalloproteases from Ixodes persulcatus, Rhipicephalus sanguineus and Rhipicephalus microplus ticks. Exp Appl Acarol, 2014. 63(4): p. 559–78. [DOI] [PubMed] [Google Scholar]
- 65.Mulenga A, Khumthong R, and Chalaire KC, Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC Genomics, 2009. 10: p. 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pichu S, et al. , Purification of a serine protease and evidence for a protein C activator from the saliva of the tick, Ixodes scapularis. Toxicon, 2014. 77: p. 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Decrem Y, et al. , Ir-CPI, a coagulation contact phase inhibitor from the tick Ixodes ricinus, inhibits thrombus formation without impairing hemostasis. J Exp Med, 2009. 206(11): p. 2381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevot PP, et al. , Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J Biol Chem, 2006. 281(36): p. 26361–9. [DOI] [PubMed] [Google Scholar]
- 69.Cao J, et al. , Characterization of a new Kunitz-type serine protease inhibitor from the hard tick Rhipicephalus hemaphysaloides. Arch Insect Biochem Physiol, 2013. 84(2): p. 104–13. [DOI] [PubMed] [Google Scholar]
- 70.Figueiredo AC, de Sanctis D, and Pereira PJ, The tick-derived anticoagulant madanin is processed by thrombin and factor Xa. PLoS One, 2013. 8(8): p. e71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iwanaga S, et al. , Identification and characterization of novel salivary thrombin inhibitors from the ixodidae tick, Haemaphysalis longicornis. Eur J Biochem, 2003. 270(9): p. 1926–34. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima C, et al. , A novel gene encoding a thrombin inhibitory protein in a cDNA library from Haemaphysalis longicornis salivary gland. J Vet Med Sci, 2006. 68(5): p. 447–52. [DOI] [PubMed] [Google Scholar]
- 73.Imamura S, et al. , A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine, 2005. 23(10): p. 1301–11. [DOI] [PubMed] [Google Scholar]
- 74.Brahma RK, et al. , Expression and characterization of haemathrins, madanin-like thrombin inhibitors, isolated from the salivary gland of tick Haemaphysalis bispinosa (Acari: Ixodidae). Thromb Res, 2017. 152: p. 20–29. [DOI] [PubMed] [Google Scholar]
- 75.Du W, et al. , Expression and function assessment of two serpin-type serine protease inhibitors from Haemaphysalis doenitzi. Res Vet Sci, 2020. 132: p. 1–9. [DOI] [PubMed] [Google Scholar]
- 76.Ricci CG, et al. , A thrombin inhibitor from the gut of Boophilus microplus ticks. Exp Appl Acarol, 2007. 42(4): p. 291–300. [DOI] [PubMed] [Google Scholar]
- 77.Xu T, Lew-Tabor A, and Rodriguez-Valle M, Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick Borne Dis, 2016. 7(1): p. 180–187. [DOI] [PubMed] [Google Scholar]
- 78.Horn F, Coutinho dos Santos P.c., and Termignoni C, Boophilus microplus Anticoagulant Protein: An Antithrombin Inhibitor Isolated from the Cattle Tick Saliva. Archives of Biochemistry and Biophysics, 2000. 384(1): p. 68–73. [DOI] [PubMed] [Google Scholar]
- 79.Ciprandi A, et al. , Boophilus microplus: its saliva contains microphilin, a small thrombin inhibitor. Exp Parasitol, 2006. 114(1): p. 40–6. [DOI] [PubMed] [Google Scholar]
- 80.Anisuzzaman, et al. , Longistatin, a plasminogen activator, is key to the availability of blood-meals for ixodid ticks. PLoS Pathog, 2011. 7(3): p. e1001312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu XL, Cheng TY, and Yang H, Enolase, a plasminogen receptor isolated from salivary gland transcriptome of the ixodid tick Haemaphysalis flava. Parasitol Res, 2016. 115(5): p. 1955–64. [DOI] [PubMed] [Google Scholar]
- 82.Tabakawa Y, et al. , Histamine Released From Skin-Infiltrating Basophils but Not Mast Cells Is Crucial for Acquired Tick Resistance in Mice. Front Immunol, 2018. 9: p. 1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paesen GC, et al. , Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol Cell, 1999. 3(5): p. 661–71. [DOI] [PubMed] [Google Scholar]
- 84.Sangamnatdej S, et al. , A high affinity serotonin- and histamine-binding lipocalin from tick saliva. Insect Mol Biol, 2002. 11(1): p. 79–86. [DOI] [PubMed] [Google Scholar]
- 85.Konnai S, et al. , Molecular identification and expression analysis of lipocalins from blood feeding taiga tick, Ixodes persulcatus Schulze. Exp Parasitol, 2011. 127(2): p. 467–74. [DOI] [PubMed] [Google Scholar]
- 86.Valdes JJ, et al. , Substrate prediction of Ixodes ricinus salivary lipocalins differentially expressed during Borrelia afzelii infection. Sci Rep, 2016. 6: p. 32372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sajiki Y, et al. , Immunosuppressive effects of sialostatin L1 and L2 isolated from the taiga tick Ixodes persulcatus Schulze. Ticks Tick Borne Dis, 2020. 11(2): p. 101332. [DOI] [PubMed] [Google Scholar]
- 88.Lieskovska J, et al. , Tick sialostatins L and L2 differentially influence dendritic cell responses to Borrelia spirochetes. Parasit Vectors, 2015. 8: p. 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kotsyfakis M, et al. , Selective cysteine protease inhibition contributes to blood-feeding success of the tick Ixodes scapularis. J Biol Chem, 2007. 282(40): p. 29256–63. [DOI] [PubMed] [Google Scholar]
- 90.Hovius JW, et al. , Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog, 2008. 4(2): p. e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leboulle G, et al. , Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem, 2002. 277(12): p. 10083–9. [DOI] [PubMed] [Google Scholar]
- 92.Prevot PP, et al. , Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J, 2009. 276(12): p. 3235–46. [DOI] [PubMed] [Google Scholar]
- 93.Ullmann AJ, et al. , Immunization with adenoviral-vectored tick salivary gland proteins (SALPs) in a murine model of Lyme borreliosis. Ticks Tick Borne Dis, 2013. 4(1-2): p. 160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Blisnick AA, et al. , The Immunomodulatory Effect of IrSPI, a Tick Salivary Gland Serine Protease Inhibitor Involved in Ixodes ricinus Tick Feeding. Vaccines (Basel), 2019. 7(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palenikova J, et al. , Ixodes ricinus salivary serpin IRS-2 affects Th17 differentiation via inhibition of the interleukin-6/STAT-3 signaling pathway. Infect Immun, 2015. 83(5): p. 1949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kotal J, et al. , The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin. Cell Mol Life Sci, 2019. 76(10): p. 2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang F, et al. , The immunosuppressive functions of two novel tick serpins, HlSerpin-a and HlSerpin-b, from Haemaphysalis longicornis. Immunology, 2020. 159(1): p. 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konnai S, et al. , Suppression of cell proliferation and cytokine expression by HL-p36, a tick salivary gland-derived protein of Haemaphysalis longicornis. Immunology, 2009. 126(2): p. 209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anisuzzaman, Alim MA, and Tsuji N, Longistatin in tick-saliva targets RAGE. Oncotarget, 2015. 6(34): p. 35133–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poole NM, et al. , Prostaglandin E(2) in tick saliva regulates macrophage cell migration and cytokine profile. Parasit Vectors, 2013. 6(1): p. 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Deruaz M, et al. , Ticks produce highly selective chemokine binding proteins with antiinflammatory activity. J Exp Med, 2008. 205(9): p. 2019–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denisov SS, et al. , Tick saliva protein Evasin-3 modulates chemotaxis by disrupting CXCL8 interactions with glycosaminoglycans and CXCR2. J Biol Chem, 2019. 294(33): p. 12370–12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Preston SG, et al. , Novel immunomodulators from hard ticks selectively reprogramme human dendritic cell responses. PLoS Pathog, 2013. 9(6): p. e1003450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sa-Nunes A, et al. , Prostaglandin E2 is a major inhibitor of dendritic cell maturation and function in Ixodes scapularis saliva. J Immunol, 2007. 179(3): p. 1497–505. [DOI] [PubMed] [Google Scholar]
- 105.Alarcon-Chaidez FJ, et al. , A novel sphingomyelinase-like enzyme in Ixodes scapularis tick saliva drives host CD4 T cells to express IL-4. Parasite Immunol, 2009. 31(4): p. 210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nuttall PA and Labuda M, Tick-host interactions: saliva-activated transmission. Parasitology, 2004. 129 Suppl: p. S177–89. [DOI] [PubMed] [Google Scholar]
- 107.Kotsyfakis M, et al. , The crystal structures of two salivary cystatins from the tick Ixodes scapularis and the effect of these inhibitors on the establishment of Borrelia burgdorferi infection in a murine model. Mol Microbiol, 2010. 77(2): p. 456–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anguita J, et al. , Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity, 2002. 16(6): p. 849–59. [DOI] [PubMed] [Google Scholar]
- 109.Gillespie RD, et al. , Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis. J Immunol, 2001. 166(7): p. 4319–26. [DOI] [PubMed] [Google Scholar]
- 110.Beaufays J, et al. , Ir-LBP, an ixodes ricinus tick salivary LTB4-binding lipocalin, interferes with host neutrophil function. PLoS One, 2008. 3(12): p. e3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toyomane K, et al. , Identification and the preliminary in vitro characterization of IRIS homologue from salivary glands of Ixodes persulcatus Schulze. Ticks Tick Borne Dis, 2016. 7(1): p. 119–125. [DOI] [PubMed] [Google Scholar]
- 112.Coutinho ML, et al. , Rhipicephalus microplus serpins interfere with host immune responses by specifically modulating mast cells and lymphocytes. Ticks Tick Borne Dis, 2020. 11(4): p. 101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tyson K, et al. , Biochemical and functional characterization of Salp20, an Ixodes scapularis tick salivary protein that inhibits the complement pathway. Insect Mol Biol, 2007. 16(4): p. 469–79. [DOI] [PubMed] [Google Scholar]
- 114.Hourcade DE, et al. , Anti-complement activity of the Ixodes scapularis salivary protein Salp20. Mol Immunol, 2016. 69: p. 62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tyson KR, Elkins C, and de Silva AM, A novel mechanism of complement inhibition unmasked by a tick salivary protein that binds to properdin. J Immunol, 2008. 180(6): p. 3964–8. [DOI] [PubMed] [Google Scholar]
- 116.Valenzuela JG, et al. , Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. J Biol Chem, 2000. 275(25): p. 18717–23. [DOI] [PubMed] [Google Scholar]
- 117.Daix V, et al. , Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol Biol, 2007. 16(2): p. 155–66. [DOI] [PubMed] [Google Scholar]
- 118.Schroeder H, et al. , The paralogous salivary anti-complement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect, 2007. 9(2): p. 247–50. [DOI] [PubMed] [Google Scholar]
- 119.Reichhardt MP, et al. , An inhibitor of complement C5 provides structural insights into activation. Proc Natl Acad Sci U S A, 2020. 117(1): p. 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schuijt TJ, et al. , A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe, 2011. 10(2): p. 136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wagemakers A, et al. , An Ixodes ricinus Tick Salivary Lectin Pathway Inhibitor Protects Borrelia burgdorferi sensu lato from Human Complement. Vector Borne Zoonotic Dis, 2016. 16(4): p. 223–8. [DOI] [PubMed] [Google Scholar]
- 122.Tsuji N, et al. , Molecular characterization of a peroxiredoxin from the hard tick Haemaphysalis longicornis. Insect Mol Biol, 2001. 10(2): p. 121–9. [DOI] [PubMed] [Google Scholar]
- 123.Kusakisako K, et al. , 2-Cys peroxiredoxin is required in successful blood-feeding, reproduction, and antioxidant response in the hard tick Haemaphysalis longicornis. Parasit Vectors, 2016. 9: p. 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hernandez EP, et al. , Characterization and expression analysis of a newly identified glutathione S-transferase of the hard tick Haemaphysalis longicornis during blood-feeding. Parasit Vectors, 2018. 11(1): p. 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Narasimhan S, et al. , Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS ONE, 2007. 2(5): p. e451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim TK, et al. , Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl Trop Dis, 2016. 10(1): p. e0004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson JF and Magnarelli LA, Biology of ticks. Infect Dis Clin North Am, 2008. 22(2): p. 195–215, v. [DOI] [PubMed] [Google Scholar]
- 128.Wikel SK, Tick-host-pathogen systems immunobiology: an interactive trio. Front Biosci (Landmark Ed), 2018. 23: p. 265–283. [DOI] [PubMed] [Google Scholar]
- 129.Kurokawa C, et al. , Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brown SJ and Askenase PW, Rejection of ticks from guinea pigs by anti-hapten-antibody-mediated degranulation of basophils at cutaneous basophil hypersensitivity sites: role of mediators other than histamine. J Immunol, 1985. 134(2): p. 1160–5. [PubMed] [Google Scholar]
- 131.Askenase PW and Atwood JE, Basophils in tuberculin and "Jones-Mote" delayed reactions of humans. J Clin Invest, 1976. 58(5): p. 1145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hewetson RW, The inheritance of resistance by cattle to cattle tick. Aust Vet J, 1972. 48(5): p. 299–303. [DOI] [PubMed] [Google Scholar]
- 133.Allen JR and Kemp DH, Observations on the behaviour of Dermacentor andersoni larvae infesting normal and tick resistant guinea-pigs. Parasitology, 1982. 84(Pt 2): p. 195–204. [DOI] [PubMed] [Google Scholar]
- 134.Fivaz BH, Tucker S, and Petney T, Cross-resistance between instars of the brown ear-tick Rhipicephalus appendiculatus (Acarina: Ixodidae). Exp Appl Acarol, 1991. 11(4): p. 323–6. [DOI] [PubMed] [Google Scholar]
- 135.Gebbia JA, et al. , Acquired resistance in dogs to repeated infestation with Ixodes scapularis (Acari: Ixodidae) reduces tick viability and reproductive success. Exp Appl Acarol, 1995. 19(10): p. 593–605. [DOI] [PubMed] [Google Scholar]
- 136.Narasimhan S, et al. , Ixodes scapularis saliva components that elicit responses associated with acquired tick-resistance. Ticks Tick Borne Dis, 2020. 11(3): p. 101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wada T, et al. , Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest, 2010. 120(8): p. 2867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brown SJ and Askenase PW, Amblyomma americanum: physiochemical isolation of a protein derived from the tick salivary gland that is capable of inducing immune resistance in guinea pigs. Exp Parasitol, 1986. 62(1): p. 40–50. [DOI] [PubMed] [Google Scholar]
- 139.Brown SJ, et al. , Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol, 1982. 129(2): p. 790–6. [PubMed] [Google Scholar]
- 140.Brown SJ, Worms MJ, and Askenase PW, Rhipicephalus appendiculatus: larval feeding sites in guinea pigs actively sensitized and receiving immune serum. Exp Parasitol, 1983. 55(1): p. 111–20. [DOI] [PubMed] [Google Scholar]
- 141.Nazario S, et al. , Prevention of Borrelia burgdorferi transmission in guinea pigs by tick immunity. Am J Trop Med Hyg, 1998. 58(6): p. 780–5. [DOI] [PubMed] [Google Scholar]
- 142.Whelen AC and Wikel SK, Acquired resistance of guinea pigs to Dermacentor andersoni mediated by humoral factors. J Parasitol, 1993. 79(6): p. 908–12. [PubMed] [Google Scholar]
- 143.Anderson JM, et al. , Ticks, Ixodes scapularis, Feed Repeatedly on White-Footed Mice despite Strong Inflammatory Response: An Expanding Paradigm for Understanding Tick-Host Interactions. Front Immunol, 2017. 8: p. 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wikel SK, et al. , Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun, 1997. 65(1): p. 335–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Narasimhan S, et al. , Host-specific expression of Ixodes scapularis salivary genes. Ticks Tick Borne Dis, 2019. 10(2): p. 386–397. [DOI] [PubMed] [Google Scholar]
- 146.FRANCIS JL, D. G. , Resistance of droughtmaster cattle to tick infestation and babesiosis. Australian Veterinary Journal, 1964. 40: p. 247–253. [Google Scholar]
- 147.Wambura PN, et al. , Breed-associated resistance to tick infestation in Bos indicus and their crosses with Bos taurus. Vet Parasitol, 1998. 77(1): p. 63–70. [DOI] [PubMed] [Google Scholar]
- 148.Porto Neto LR, et al. , Molecular genetic approaches for identifying the basis of variation in resistance to tick infestation in cattle. Vet Parasitol, 2011. 180(3-4): p. 165–72. [DOI] [PubMed] [Google Scholar]
- 149.Schleger AV, et al. , Boophilus microplus: cellular responses to larval attachment and their relationship to host resistance. Aust J Biol Sci, 1976. 29(5-6): p. 499–512. [DOI] [PubMed] [Google Scholar]
- 150.Tabor AE, et al. , Cattle Tick Rhipicephalus microplus-Host Interface: A Review of Resistant and Susceptible Host Responses. Front Cell Infect Microbiol, 2017. 7: p. 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beaudouin E, et al. , Unusual manifestations of hypersensitivity after a tick bite: report of two cases. Ann Allergy Asthma Immunol, 1997. 79(1): p. 43–6. [DOI] [PubMed] [Google Scholar]
- 152.Garcia MV, et al. , Successful Feeding of Amblyomma coelebs (Acari: Ixodidae) Nymphs on Humans in Brazil: Skin Reactions to Parasitism. J Med Entomol, 2015. 52(2): p. 117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schwartz BS, Ribeiro JM, and Goldstein MD, Anti-tick antibodies: an epidemiologic tool in Lyme disease research. Am J Epidemiol, 1990. 132(1): p. 58–66. [DOI] [PubMed] [Google Scholar]
- 154.Schwartz BS, Goldstein MD, and Childs JE, Antibodies to Borrelia burgdorferi and tick salivary gland proteins in New Jersey outdoor workers. Am J Public Health, 1993. 83(12): p. 1746–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burke G, et al. , Hypersensitivity to ticks and Lyme disease risk. Emerg Infect Dis, 2005. 11(1): p. 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Piesman J, et al. , Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol, 1987. 25(3): p. 557–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Crippa M, Rais O, and Gern L, Investigations on the mode and dynamics of transmission and infectivity of Borrelia burgdorferi sensu stricto and Borrelia afzelii in Ixodes ricinus ticks. Vector Borne Zoonotic Dis, 2002. 2(1): p. 3–9. [DOI] [PubMed] [Google Scholar]
- 158.Hodzic E, et al. , Acquisition and transmission of the agent of human granulocytic ehrlichiosis by Ixodes scapularis ticks. J Clin Microbiol, 1998. 36(12): p. 3574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hermance ME and Thangamani S, Tick Saliva Enhances Powassan Virus Transmission to the Host, Influencing Its Dissemination and the Course of Disease. J Virol, 2015. 89(15): p. 7852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allen JR, Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol, 1973. 3(2): p. 195–200. [DOI] [PubMed] [Google Scholar]
- 161.Allen JR, Doube BM, and Kemp DH, Histology of bovine skin reactions to Ixodes holocyclus Neumann. Can J Comp Med, 1977. 41(1): p. 26–35. [PMC free article] [PubMed] [Google Scholar]
- 162.Brown SJ and Askenase PW, Cutaneous basophil responses and immune resistance of guinea pigs to ticks: passive transfer with peritoneal exudate cells or serum. J Immunol, 1981. 127(5): p. 2163–7. [PubMed] [Google Scholar]
- 163.Brown SJ and Knapp FW, Response of hypersensitized guinea pigs to the feeding of Amblyomma americanum ticks. Parasitology, 1981. 83(Pt 1): p. 213–23. [DOI] [PubMed] [Google Scholar]
- 164.Tatchell RJ and Bennett GF, Boophilus microplus: antihistaminic and tranquilizing drugs and cattle resistance. Exp Parasitol, 1969. 26(3): p. 369–77. [PubMed] [Google Scholar]
- 165.Wikel SK, Histamine content of tick attachment sites and the effects of H1 and H2 histamine antagonists on the expression of resistance. Ann Trop Med Parasitol, 1982. 76(2): p. 179–85. [DOI] [PubMed] [Google Scholar]
- 166.Brown SJ, Graziano FM, and Askenase PW, Immune serum transfer of cutaneous basophil-associated resistance to ticks: mediation by 7SIgG1 antibodies. J Immunol, 1982. 129(6): p. 2407–12. [PubMed] [Google Scholar]
- 167.Wikel SK and Allen JR, Acquired resistance to ticks. I. Passive transfer of resistance. Immunology, 1976. 30(3): p. 311–6. [PMC free article] [PubMed] [Google Scholar]
- 168.Wikel SK and Allen JR, Acquired resistance to ticks. III. Cobra venom factor and the resistance response. Immunology, 1978. 32(4): p. 457–65. [PMC free article] [PubMed] [Google Scholar]
- 169.Kock MA, et al. , Structure and function of recombinant cobra venom factor. J Biol Chem, 2004. 279(29): p. 30836–43. [DOI] [PubMed] [Google Scholar]
- 170.Wikel SK, Acquired resistance to ticks: expression of resistance by C4-deficient guinea pigs. Am J Trop Med Hyg, 1979. 28(3): p. 586–90. [PubMed] [Google Scholar]
- 171.Szabo MP and Bechara GH, Sequential histopathology at the Rhipicephalus sanguineus tick feeding site on dogs and guinea pigs. Exp Appl Acarol, 1999. 23(11): p. 915–28. [DOI] [PubMed] [Google Scholar]
- 172.Urbina C, Ortiz C, and Hurtado I, A new look at basophils in mice. Int Arch Allergy Appl Immunol, 1981. 66(2): p. 158–60. [DOI] [PubMed] [Google Scholar]
- 173.Dvorak AM, et al. , Anaphylactic degranulation of guinea pig basophilic leukocytes. II. Evidence for regranulation of mature basophils during recovery from degranulation in vitro. Lab Invest, 1982. 46(5): p. 461–75. [PubMed] [Google Scholar]
- 174.Cromheecke JL, Nguyen KT, and Huston DP, Emerging role of human basophil biology in health and disease. Curr Allergy Asthma Rep, 2014. 14(1): p. 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sokol CL and Medzhitov R, Role of basophils in the initiation of Th2 responses. Curr Opin Immunol, 2010. 22(1): p. 73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Min B, Basophils induce Th2 immunity: is this final answer? Virulence, 2010. 1(5): p. 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ohta T, et al. , Skin CD4(+) Memory T Cells Play an Essential Role in Acquired Anti-Tick Immunity through Interleukin-3-Mediated Basophil Recruitment to Tick-Feeding Sites. Front Immunol, 2017. 8: p. 1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barbour AG and Fish D, The biological and social phenomenon of Lyme disease. Science, 1993. 260(5114): p. 1610–6. [DOI] [PubMed] [Google Scholar]
- 179.Ribeiro JM, Role of saliva in tick/host interactions. Exp Appl Acarol, 1989. 7(1): p. 15–20. [DOI] [PubMed] [Google Scholar]
- 180.Tirloni L, et al. , Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts. Front Cell Infect Microbiol, 2017. 7: p. 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Franzin AM, et al. , Immune and biochemical responses in skin differ between bovine hosts genetically susceptible and resistant to the cattle tick Rhipicephalus microplus. Parasit Vectors, 2017. 10(1): p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sueki H, et al. , Hairless guinea pig skin: anatomical basis for studies of cutaneous biology. Eur J Dermatol, 2000. 10(5): p. 357–64. [PubMed] [Google Scholar]
- 183.Gudjonsson JE, et al. , Mouse models of psoriasis. J Invest Dermatol, 2007. 127(6): p. 1292–308. [DOI] [PubMed] [Google Scholar]
- 184.Savoji H, et al. , Skin Tissue Substitutes and Biomaterial Risk Assessment and Testing. Front Bioeng Biotechnol, 2018. 6: p. 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Naldaiz-Gastesi N, et al. , The panniculus carnosus muscle: an evolutionary enigma at the intersection of distinct research fields. J Anat, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kassem R, et al. , Harnessing the skin-thyroid connection for wound healing: a prospective controlled trial in guinea pigs. Clin Exp Dermatol, 2012. 37(8): p. 850–6. [DOI] [PubMed] [Google Scholar]
- 187.Flacher V, et al. , Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med, 2014. 6(9): p. 1191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mutyambizi K, Berger CL, and Edelson RL, The balance between immunity and tolerance: the role of Langerhans cells. Cell Mol Life Sci, 2009. 66(5): p. 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Summerfield A, Meurens F, and Ricklin ME, The immunology of the porcine skin and its value as a model for human skin. Mol Immunol, 2015. 66(1): p. 14–21. [DOI] [PubMed] [Google Scholar]
- 190.More DD, et al. , Network analysis uncovers putative genes affecting resistance to tick infestation in Braford cattle skin. BMC Genomics, 2019. 20(1): p. 998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kurokawa C N S; Vidyarthi A,.; Booth CJ.; Mehta S, Meistere L, Diktas H, Strank N, Lynn G, DePonte K, Craft J, Fikrig, , Repeat tick exposure elicits distinct immune responses in guinea pigs and mice. Ticks Tick Borne Dis, 2020. 11(6): p. 101529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Robbertse L, Richards SA, and Maritz-Olivier C, Bovine Immune Factors Underlying Tick Resistance: Integration and Future Directions. Front Cell Infect Microbiol, 2017. 7: p. 522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heller-Haupt A, Kagaruki LK, and Varma MG, Resistance and cross-resistance in rabbits to adults of three species of African ticks (Acari: Ixodidae). Exp Appl Acarol, 1996. 20(3): p. 155–65. [DOI] [PubMed] [Google Scholar]
- 194.Parizi LF, et al. , The quest for a universal vaccine against ticks: cross-immunity insights. Vet J, 2012. 194(2): p. 158–65. [DOI] [PubMed] [Google Scholar]
- 195.Kim TK, et al. , Time-resolved proteomic profile of Amblyomma americanum tick saliva during feeding. PLoS Negl Trop Dis, 2020. 14(2): p. e0007758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Plotkin SA, Vaccines: the fourth century. Clin Vaccine Immunol, 2009. 16(12): p. 1709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Suschak JJ, Williams JA, and Schmaljohn CS, Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother, 2017. 13(12): p. 2837–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ruiz LM, et al. , Immune response in mice and cattle after immunization with a Boophilus microplus DNA vaccine containing bm86 gene. Vet Parasitol, 2007. 144(1-2): p. 138–45. [DOI] [PubMed] [Google Scholar]
- 199.Hassan IA, et al. , Cross protection induced by combined Subolesin-based DNA and protein immunizations against adult Haemaphysalis longicornis. Vaccine, 2020. 38(4): p. 907–915. [DOI] [PubMed] [Google Scholar]
- 200.Pardi N, et al. , mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov, 2018. 17(4): p. 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.VanBlargan LA, et al. , An mRNA Vaccine Protects Mice against Multiple Tick-Transmitted Flavivirus Infections. Cell Rep, 2018. 25(12): p. 3382–3392 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Aberle JH, et al. , Humoral and cellular immune response to RNA immunization with flavivirus replicons derived from tick-borne encephalitis virus. J Virol, 2005. 79(24): p. 15107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data cited in this review are published and available on-line or upon request from the authors of the respective publications. No unpublished data is included in this manuscript.