Abstract
Liquid-liquid phase separation is now recognized as a common mechanism for regulating enzyme activity in cells. Insights from studies in cells are complemented by in vitro studies aimed at developing a better understanding of mechanisms underlying such control. These mechanisms are often based on the influence of LLPS on the physicochemical properties of the enzyme’s environment. Biochemical mechanisms underlying such regulation include the potential for concentrating reactants together, tuning reaction rates, and controlling competing metabolic pathways. LLPS is thus a powerful tool with extensive utilities for the cell, e.g., for consolidating cell survival under stress or rerouting metabolic pathways in response to the energy state of the cell. By examining the evidence of how LLPS affects enzyme catalysis, we can begin to understand emerging concepts and expand our understanding of enzyme catalysis in living cells.
Graphical Abstract
Introduction
Enzymatic activity within the cell is delicately balanced through several layers of organization. One example is the compartmentalization of competing reactions into different membrane-bound organelles. Recently, the insight that cells can generate another layer of organization through the process of liquid-liquid phase separation (LLPS) and the resulting formation of membrane-less biomolecular condensates has added a further intriguing layer of complexity to the regulation of enzyme catalysis [1].
LLPS is a dynamic and reversible process through which biomolecules, such as proteins or nucleic acids, associate via multivalent interactions into non-stoichiometric assemblies. The result of this process is the formation of at least two distinct phases, i.e., a dense phase enriched with the biomolecules and often taking the form of large droplets, and the surrounding dilute phase, which is depleted of the biomolecules [2–4]. Phase separation only occurs once a system-specific threshold concentration of participating biomolecules is surpassed. This is often referred to as the saturation concentration (csat) and is dictated by the molecules’ valence, i.e., the number of interactions that can occur between the responsible biomolecules [5].
Evidence is accumulating that LLPS is critical to such cellular processes as cell signaling [6,7], transcription [8–11] and stress response [12–16]. Dysregulation of LLPS has also been causally linked to amyotrophic lateral sclerosis (ALS), inclusion body myopathy (IBM), and other closely related neurodegenerative diseases [17–19], as well as many forms of cancer [20–22]. As the molecular mechanisms of the ties between LLPS and disease are becoming better understood, we are also gaining deeper insights into the functions of biomolecular condensates.
One of the possible functions of condensates is as reaction crucibles, which concentrate enzymes and substrates together and enhance turnover (Figure 1A) or conversely, sequester enzymes and substrate away from each other and reduce turnover [2,3,5,23]. The marrying of enzyme catalysis and LLPS allows for the modulation of many parameters important for enzymatic activity, including local reactant concentrations, presence or absence of reaction influencers, timespans for such localization, and even solvent conditions to be controlled. Several reviews have touched on this topic previously [1,3,24,25], and this review aims to complement these by illuminating recent in vivo discoveries of LLPS functioning to control or alter enzymatic reaction rates. We then discuss the various engineered systems which have recently been implemented to examine the effect of LLPS on enzymatic activity. Finally, we will touch upon the biophysical effects of enzymes in dense phases and discuss under which conditions activities would be expected to be enhanced.
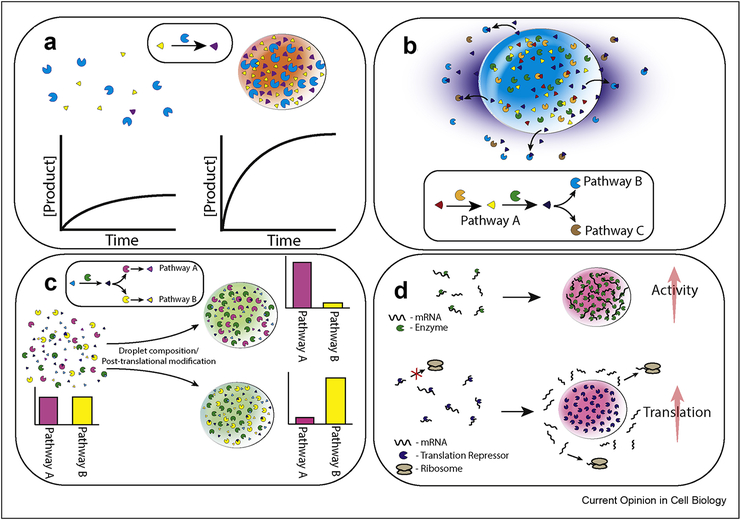
Figure 1: Cells use LLPS to control enzyme activity.
A. LLPS can increase the local concentration of reactants, thus increasing rates of product formation. B. LLPS of enzymes within one particular pathway (pathway A) can lead to increased production of a reagent used in other pathways (pathways B and C). The localized production of the reagent results in localization of these enzymes around the condensate (or even their partitioning into the condensate) [35]. C. Selective co-compartmentalization of certain enzymes in a forked metabolic pathway can divert metabolites toward one pathway. Switching between alternate compartmentalization states may be regulated by post-translational modification of phase-separating proteins, e.g. as a result of the energy state of the cell. D. RNA-binding proteins can phase separate with mRNA and control its translation and degradation, locally and globally.
Phase separation regulates enzyme catalysis in cells
The idea that phase separation can be used to generate protective environments and to control rates of product formation was receiving attention as far back as the beginning of the 20th century. Theories of coacervates functioning as protocells in the origin of life by Alexander Oparin and others were largely respectfully dismissed until recently, when biological condensates began to be linked to an abundance of biological processes [26–28]. The main reason for this belated acknowledgement is largely due to lack of experimental evidence for condensates in cells and in vivo until recently. Currently, we are seeing a wealth of new information arise related to the biological function and mechanism of LLPS, and how it links to enzyme catalysis.
Cellular stresses are frequently seen as a key trigger for LLPS, taking advantage of the reversible nature of condensates to sequester, protect, or coalesce various biological components. Stress granules are the most well-documented example [15,17,29,30], but other unique condensates have been shown to form depending on the source of the stress [12–14,16,31]. Recent discussions have addressed the ability of metabolic enzymes to phase separate under nutrient deprivation [31–33]. Additional works by Fuller, et al. [34] Jin, et al. [35] and Kohnhorst, et al. [36] expanded on our understanding of glycolytic (G) bodies (also called glucosomes), i.e., membraneless condensates which form under hypoxic stress to co-localize glycolytic enzymes and enhance the rate of glycolysis. G bodies alter glycolysis rates as shown by measuring the levels of glycolytic metabolites in wild-type cells and in cells that lacked Snf1p, a conserved AMP-activated protein kinase found to be vital for G body formation [35]. Exclusively hypoxic conditions led to increased levels of upstream metabolites and decreased levels of downstream metabolites in ΔSnf1p cells in comparison to wild-type cells. This is consistent with the idea that G bodies function to enhance glycolytic turnover. This was corroborated with experiments wherein wild-type cells were cultured under hypoxic or normal conditions prior to transfer to new media under exclusively hypoxic conditions. Significantly increased rates of glucose consumption were noted for cells which were initially cultured under hypoxic conditions and had pre-formed G-bodies. Additionally, nearby localization of protein complexes associated with fatty acid synthesis, trehalose-6-phosphate synthesis and protein degradation was noted. The increased rate of glycolysis of G bodies, and subsequent increased adenosine triphosphate (ATP) turnover, appears to provide local energy input for other vital cellular processes to continue, even under hypoxic conditions (Figure 1B). This agreed with demonstrated G body formation near presynaptic sites to meet requirements to sustain synaptic activity [37].
Interestingly, the rate-limiting glycolytic enzymes were found in multienzyme clusters [36]. Parallel to this, a relationship between condensate size and shunting of glycolysis intermediates between energy metabolism and anabolic biosynthetic pathways was demonstrated [36]. Through selective promotion and inhibition of the pentose phosphate pathway or the serine biosynthesis pathway, two anabolic pathways that promote nucleotide and amino acid biosynthesis, respectively, it was determined that cells with medium-sized clusters had a propensity to divert metabolites towards the pentose phosphate pathway. In contrast, cells with larger clusters shunted resources towards the serine biosynthesis pathway. The importance of this is noted by the presence of large-sized clusters in various human cancer cells, and absence in non-cancerous human breast cells. Increased serine biosynthetic rates are considered a hallmark of altered glucose metabolism in cancer [38]. It is likely that the different sizes of clusters reflect the recruitment of different subsets of metabolic enzymes which direct forked metabolic pathways into a specific direction. Switching between these different states may be regulated via post-translational modifications or other inputs from the energy state of the cell (Figure 1C).
G bodies are ribonucleoprotein granules, formed through multivalent protein-protein and protein-RNA interactions [34]. Glycolytic enzymes co-localize with a modest amount of RNA to G bodies in hypoxic conditions, i.e., when oxygen levels are reduced. In fact, RNA serves as a scaffold and is essential for G body formation (Figure 1D). 10 glycolysis enzymes were found to have RNA binding properties even under normal oxygen levels, and they all bind similar RNA sequences, including RNA sequences found in transcripts of glycolysis enzymes. These observations suggest that in the case of G bodies, RNA scaffolding and recruitment has the additional potential for localized translation of the proteins that make up the G body and facilitate its growth. This may be an example in which LLPS does not only influence enzyme activity but also vice versa.
Similar trends have been noted in the purine biosynthesis pathway, in which six enzymes required for de novo purine biosynthesis cluster in ‘purinosomes’ [39–43]. Increased formation of purinosome condensates under hypoxic conditions was demonstrated, similar to G body formation [40]. In fact, treatment with inhibitors of glycolysis and pentose phosphate pathways in cells under hypoxia led to a reduction in purinosome formation. In contrast to G bodies [37], it was determined that there was no up-regulation of enzyme activity within the purinosome under hypoxic conditions. This was attributed to a subsequent down-regulation of mitochondrial one-carbon metabolism under hypoxia and provides a caveat to previous reports stating that purinosomes increased de novo purine biosynthesis [40–42]. The specific reasons as to why purinosome formation is increased under hypoxic conditions therefore remains to be uncovered.
The regulation of enzymatic function by LLPS is not limited to metabolic enzymes, with numerous examples of LLPS functioning to control translation and deadenylation rates. LLPS of Argonaute2 and TNRC6B, two components of the human miRNA-induced silencing complex (miRISC), was shown to sequester target RNA from the surrounding solution and leading to an increased rate of deadenylation [44]. Similarly, FMRP and CAPRIN1, regulator proteins which have been shown to be involved in mRNA stability and translational repression, were found to phase separate with target RNA, but only after phosphorylation of FMRP. Differential Ser/Thr or Tyr phosphorylation tunes rates of deadenylation and translation [45]. This implies that phase separation does not only lead to enhanced activity, but that it can lead to the up- or down-regulation of one reaction pathway over another, simply by tuning the physical characteristics of the condensate via signaling or metabolic inputs (Figure 1C), similar to phenomena observed with G bodies [36]. In contrast, phase separation of cytoplasmic poly(A)-binding protein (PAB1) was found to regulate translation in a very different manner [46]. PAB1 binds to RNA and acts as a translational repressor. Upon phase separation the protein is sequestered away from RNA allowing for its subsequent translation (Figure 1D). These findings give further credence to the diverse but conceptually similar roles LLPS can play in enzyme catalysis.
Taken together, these data demonstrate the multifunctional potential of biomolecular condensates which can be summarized as: 1. Enhancement of activity to negate effects of stress and/or meet local physiological demands (Figure 1A). 2. Selective transfer of substrates between enzymes via the recruitment of enzymes into the vicinity of enzymatically active condensates (Figure 1B). 3. Redirection of metabolites between multiple diverging pathways based on cellular demand by selective compartmentalization (Figure 1C). 4. mRNA binding capabilities to protect cognate mRNA, regulate translation efficiency, and facilitate LLPS (Figure 1D).
Investigations into the effect of LLPS on enzyme activity in de novo designed systems
The pursuit of understanding how phase separation can function in vivo to mediate or regulate enzyme catalysis is complemented by in vitro investigations. Designed systems can push the limits of phase separation and enzyme catalysis and answer questions relating to general mechanisms. These systems often incorporate components from the biological world to tune phase separation abilities by linking so-called ‘scaffold’ molecules, those that control the propensity to phase separate, with an enzyme with a measurable readout. [47]. Adenylate kinase coupled with low complexity domains from Dbp1 and Laf1, a pair of DEAD-box proteins which have been shown to phase separate in vivo, resulted in a significant increase (60- to 100-fold) in enzyme concentration within droplets as compared to the starting concentration and a five-fold increase in initial velocity of reaction when compared to a homogenous solution [48,49]. This increase was attributed to the increased local concentration of reactants but was also notably limited by the increase in viscosity (by 25- to 200-fold), leading to a proposed limitation in turnover. In fact, viscosity has been proposed to limit activity increases in condensates in several cases [2,22] (Figure 2A). This work was built upon by coating magnetic nanoparticles with low complexity domains from Laf1 conjugated to adenylate kinase. This allowed for phase separation in response to changes in pH and ionic strength, and morphology alteration in response to a magnetic field [50].
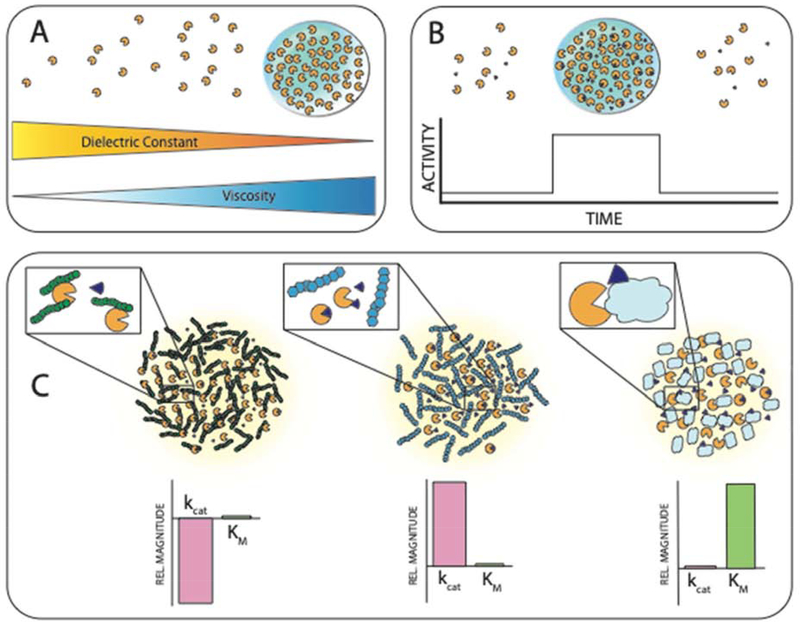
Figure 2: Biophysical and biochemical effects of phase separation can function to regulate enzyme activity.
A. LLPS results in a unique local environment that differs from the surrounding dilute phase. The solvent properties of condensates can resemble organic solvents, allowing tuning of reaction conditions by LLPS. The resulting increase in viscosity has been shown to reduce enzyme activity due to reduced mobility of reagents. This implies a balance between contrasting effects to realize optimal reaction conditions within condensates [2,22,75,76]. B. The high cooperativity of LLPS means that enzymatic reactions can be switched on or off. By balancing the concentration of enzyme at the threshold of phase separation (likely through translational regulation), activity can be rapidly switched on by a small increase in local concentration, and off again by a subsequent small decrease depending on cellular needs [5,14,50,61]. C. Crowding within a condensate can result in a variety of effects on kinetic activity. Interactions between components in a condensate can result in a reduced rate of product turnover by freezing enzyme dynamics critical to turnover. Non-interacting components can reduce the available space through their excluded volume, concentrating enzymes together with their substrates, resulting in increased turnover. Finally, components which form weak, nonspecific interactions with the substrate and reduce accessibility to the enzyme active site can result in an apparent reduction in enzyme-substrate affinity [74].
RNA-scaffolded condensates have been implicated in the development of prebiotic early life [51]. To test the viability of this proposal, condensates of the polycation polydiallyldimethylammonium chloride (PDAC) and a polyA-RNA molecule were formed and shown to alter the rates of ribozymes [52]. The electrostatic interactions that drive formation of RNA condensates were attributed for a 60-fold decrease in activity for the hammerhead ribozyme by disrupting native structure [53]. However, when bulk ribozyme concentrations were kept below the dissociation constant (Kd), activity was increased up to 10-fold in the dense phase when compared to the dilute phase due to a sharp increase in local concentration upon coacervation. This proposes an interesting mechanism of reaction rate control based around the saturation concentration of an enzyme and the Kd or KM of the enzyme with its substrate.
Artificial intrinsically disordered proteins (A-IDPs) with controllable LLPS behavior were shown to recruit small molecules and proteins into condensates and so it was hypothesized that they could be used in the design of enzymatically active condensates [54]. To ensure that the expression of a bulky enzyme attached to the A-IDPs would not affect LLPS, a split version of β-galactosidase was utilized, wherein the active enzyme was only formed when two components of β-galactosidase were recombined. This also had the advantage that the enzyme was only active when the A-IDPs formed complexes. The versatility of the A-IDP system allowed the researchers to investigate the effects of increased A-IDP chain length and aliphatic/aromatic content on kinetic rates. Both alterations enhance the driving force for phase separation [55], which means that a lower concentration of the protein is needed to form dense droplets. The driving force for phase separation increased with chain length and aromatic/aliphatic ratio in vitro and in bacterial cells as expected. Indeed, it was also found that with larger A-IDP chains comes an increased enzymatic efficiency − 2.5 and 7.5 times greater for a 40mer and 80mer, respectively, when compared to a control. Measurable kinetic constants, namely kcat and KM, meant that the increased rates could be attributed to a higher turnover rate (kcat) rather than an increased propensity to form an enzyme-substrate complex (KM). Interestingly, no changes in rates were observed when aromatic content of the A-IDP was altered suggesting that the increase in kcat is decoupled from the driving force for phase separation, probably because the increased aliphatic content accelerated substrate binding kinetics.
Phase separation has the ability to increase enzyme activity more strongly than expected by mass action [56]. An engineered system that used multivalent SH3 domain-containing proteins and proline-rich motif-containing proteins as scaffolds and recruited enzyme and substrate of a SUMOylation pathway, showed 36-fold increased activity of the dense over the dilute phase. Unexpectedly, the total reaction volume had 7-fold higher activity in the presence of phase separation than in the absence, which stemmed from excess activity in the dense phase beyond what was expected by mass action. This effect was only evident for specific scaffold variants and was accompanied by a lower KM value in the dense phase. It was likely caused by the enhanced proximity of enzyme and substrate induced by sterically favorable recruitment to the scaffold. LLPS can thus not only enhance enzyme activity by mass action but also by preorganization of enzyme/substrate complexes [56].
The ability of condensates to facilitate enzymatic catalysis is not limited to single-step reactions. A system was engineered to examine the effect of phase separation on the multienzyme menaquinone biosynthesis and terpene biosynthesis pathways. A trio of synaptic proteins (guanylate kinase-associated protein (GKAP), Shank and Homer) were tagged with a set of high-affinity interacting peptides. This produced a phase-separating system with the ability to selectively recruit ‘guest’ proteins [57]. This system was used to examine the effect of phase separation on the multienzyme menaquinone biosynthesis and terpene biosynthesis pathways. Of the three enzymes in the menaquinone biosynthesis pathway, MenD, MenF, and MenH, only MenH could be tagged and recruited to the condensate. It was found that both tagged and untagged systems had the same reaction rate, regardless of active recruitment, in comparison to a homogenous solution with no phase separation and a lower rate. In contrast, the recruitment of both enzymes in the terpene biosynthesis pathway, isopentenyl diphosphate isomerase and farnesyl diphosphate synthase, had over a 50% increase in activity over the untagged and homogenous controls. The lack of activity enhancement upon recruitment of MenH likely has to do with MenD, not MenH, being the rate-limiting enzyme in the menaquinone biosynthesis pathway [58]. Low levels of enrichment of untagged MenD likely negated any effect of actively recruited MenH. Essentially, an increase in rate of product formation may only be of use to a cell if the recruited enzyme catalyzes a rate-limiting step in a multienzyme pathway.
Utilization of scaffold proteins derived from biological systems offers the ease of genetically encoded constructs and a biologically relevant outlook on the effect of LLPS on enzyme catalysis. Incorporating or substituting scaffold proteins with more artificial components can offer readily tunable phase separation in vitro and the exploration of enzyme activity in synthetic protocells [50,53,59–63]. These are complementary and valuable resources that can provide a robust viewpoint on how enzyme catalysis is influenced by the effects of LLPS. A system composed of three separate liquid phases was used to examine the effect of compartmentalization on a cascade of enzymatic activities [64]. Horseradish peroxidase (HRP) along with a chromogenic substrate was encapsulated within an adenosine triphosphate (ATP)/PDAC coacervate. The surrounding dextran phase contained glucose oxidase. Surrounding this again was a polyethylene glycol (PEG) phase rich in glucose. Oxidation of glucose by glucose oxidase initiated the multi-step reaction, concluding with oxidation of the compartmentalized chromogenic substrate by HRP within the inner ATP/PDAC coacervate. It was found that compartmentalization of HRP with its substrate was necessary, as separate compartmentalization resulted in a significant reduction in activity. Additionally, multiple ATP/PDAC coacervates containing HRP with different substrates demonstrated the potential for multiple separate reactions to occur simultaneously using reactants from one upstream pathway [64]. These results demonstrate the power of compartmentalization to increase functionality.
It has been speculated that LLPS can function as an on/off switch for enzyme activity [5]. This could be the case if enzyme and substrates are too dilute for turnover in the absence of phase separation but sufficiently concentrated in the dense phase. Given the theoretical infinite cooperativity of phase separation, an infinitesimal change in enzyme or substrate concentration would have the potential to suddenly lead to turnover (Figure 2B). The potential of phase separation to regulate enzyme activity in a switch-like manner has now been shown experimentally. Formate dehydrogenase was co-encapsulated within synthetic vesicles containing poly-lysine components and either carboxymethyl-dextran or ATP [61]. By dropping the pH of the vesicle below the pKa of poly-lysine (pH 10.5), phase separation was induced via electrostatic interactions between condensate components. Formate dehydrogenase activity is low at low protein concentrations. When encapsulated, formate dehydrogenase activity was “switched on”, and the rate of conversion of NAD+ to NADH was increased, in agreement with effects expected for an increase in the local concentration of the enzyme. Following return to pH 11, the system returned to a homogenous mixture, and NADH production was switched off.
Phase-separating enzyme systems have also been engineered that reveal the potential of enzymatic reactions to alter the driving force for phase separation. Complex coacervates formed from positively charged peptides and RNA are readily dissolvable by phosphorylation of the peptides and reform upon their dephosphorylation [65]. This system is hence regulatable by opposing kinase and phosphatase activities and could be coupled to another enzyme activity that is only active once the condensate is formed. One could even envision a system that periodically cycles between on- and off-states of enzyme activity (Figure 2B).
The importance of artificial systems for our understanding of the impact of LLPS on enzyme activity lies in the ability to experimentally tune the driving force for phase separation. This allows for intricate balances between enzyme rates, substrate affinity, multi-enzyme reaction bottlenecks and enzyme activation to be studied in the context of condensate saturation concentrations, viscosity, material properties, and stability under varying pH and ionic strengths. The continued investigation into such relationships is of paramount importance to the field, to understand the limits and benefits of regulating enzyme activity via LLPS.
Implications of LLPS on enzyme activity and how a cell can take advantage of this
The dynamic nature of condensates offers a wealth of opportunity to fine-tune reactions, function as selective metabolic crossroads, and act as on/off switches to regulate enzyme activity depending on cellular demand (Figure 2C). The mechanistic basis of these effects likely stem from the unique environment that phase-separated condensates offer. Localizing a particular enzyme within a condensate has the power to increase its concentration; by how much is dependent on the system and the conditions. Some phase-separating molecules differ in dilute and dense phase concentration by a thousand-fold under certain conditions [66]; others experience relatively small increases in concentration, particularly in condensates with several components [29]. Each system can be balanced close to a critical point, where the phase-separating components will be hardly enriched in the dense phase. We expect that the partition coefficient of enzymes into condensates is tightly regulated, but this has not been explored yet.
Not only are enzymes concentrated in condensates, but reactants, cofactors, and products can also be concentrated. Even small molecules can be significantly enriched in dense phases via interactions with macromolecular components [67]. Importantly, condensate components localize dynamically, can diffuse in and out and create a highly active, yet crowded environment. The effect that such crowding has on enzymatic reactions is important, as it is directly tied to how a cell utilizes LLPS to control enzyme activity.
Enzyme crowding has been discussed in detail previously [25,68–71]. However, recent studies from Trylska and coworkers shed new light on the effects of crowding on the activity of enzymes [72–74] (Figure 2C). They showed that the activity of human immunodeficiency virus-1 protease was progressively suppressed by the presence of increasing concentrations of polyethylene glycol (PEG) [72]. This effect was also observed in subsequent experiments involving the hepatitis C virus protease NS3/4A [74]. Molecular dynamics simulations showed PEG-protein interactions predominantly involving side-chain residues, but with no particular obstructions of the active site of NS3/4A. This resulted in a decrease in kcat, consistent with slowing of catalytically important dynamics in the enzyme. Interestingly, Ficoll, a crowder which has been shown to be more inert than PEG, had the opposite effect on activity, with almost an 8-fold increase in rate of activity, likely through excluded volume effects. In addition, Ficoll crowding was found to reduce the activity of Telaprevir, a potent inhibitor of NS3/4A protease. When bovine serum albumin (BSA) was used as the crowder, a different effect was seen again, with a sharp increase in KM observed. This is consistent with crowding affecting the affinity the substrate has for the enzyme rather than affecting turnover of product [74] (Figure 2C).
It is apparent that not only does the biophysical phenomenon of crowding affect enzyme activity, but the biochemical interactions between the molecules being crowded are of significant importance to how the enzyme activity is affected. This points to the importance of the unique solvent properties found within condensates. It has been proposed that the solvent environment in condensates may reflect conditions closer to an organic solvent than water with substantially decreased dielectric constant. [48,75,76]. This was based on the observation that double-stranded DNA was melted upon partitioning into condensates of the DEAD-box helicase 4 low-complexity domain [75]. This implies that cells may be able to control reaction rates by altering the local solvent properties (Figure 2A).
Phase separation also allows for the specific recruitment or exclusion of reactants. Dextranase had different kinetic profiles whether partitioned within a phase separated droplet of dextran, or compartmentalized adjacent to the droplet [64]. Rates were shown to be slower when dextranase was found in the same droplet as dextran whereas when separately compartmentalized, dextranase was more efficient. This was hypothesized to be due to substrate inhibition, but is arguably better characterized as product inhibition, as the product of the hydrolysis of dextran is just a smaller molecule of dextran. When a product has a similar structure to the substrate, it can reversibly bind to the free enzyme, inhibiting activity [77,78]. Nevertheless, the results propose an interesting rationale for compartmentalizing components in a reaction separately rather than partitioning all together.
The mechanism behind such proximity-based activity increase is likely also tied to intrinsic biophysical properties of the condensate. Saleh, et al. investigated circumstances where the phase-separated component consists of the substrate (DNA) whereas the surrounding dilute phase contains the enzyme (a restriction enzyme – Smal) free in solution [79]. Enzymatic activity, and thus degradation of the droplet, was heightened by those enzymes which penetrated within the droplet. Penetration was most efficient for conditions balanced at the phase boundary and led to the formation of dilute phase vacuoles within the condensate. The modulation of enzyme and/or substrate diffusion across phases is likely another way cells can regulate kinetic rates of enzymes through LLPS.
These findings imply that cells can feasibly control reaction rates simply by crowding an enzyme with a component that either encourages enzyme-substrate interaction, offers preferred environmental conditions, controls quench depth or slows formation and release of a product (Figure 2).
Conclusion
Within cells, the delicately balanced parameters that define a biological condensate have been shown to control enzymatic rates, directing reactions towards pathways based on cellular demand, and switching enzyme activity on and off. The emergence of next-generation microscopy techniques, biomolecular engineering capabilities, and advances in enzyme activity quantification offers unparalleled opportunities for the understanding of the extent of this interplay [70,80]. In vitro studies will help characterize the mechanisms underlying cellular phenomena, link them to intrinsic properties of biomolecules and define the potential of the process in cell regulation.
Intrinsic properties of enzymes are seen in a new light. The kinetic constants of KM, Vmax, and kcat depend strongly on local environment. Thus, LLPS can potentially function to increase or decrease affinity between substrate and enzyme, reduce catalytic mobility of an enzyme, increase the encounter probability of substrate and enzyme, modulate product release, or have other effects. Kinetic constants are measurable values, and as such, can allow us to quantify the extent to which LLPS affects enzyme catalysis, understand the mechanism of such an effect, and examine how seemingly unrelated values such as KM and csat could, in fact, be linked. It should be noted, however, that such kinetic measurements are tied to control of not just substrate concentration, but also enzyme concentration. Much of the work discussed herein has characterized ‘enzyme activity’ using initial velocities, which offer limited insight into the true effect of LLPS on an enzyme. Some works that have elucidated kinetic constants are limited in control over local substrate and/or enzyme concentration within condensates. While large leaps have been taken to investigate the link between LLPS and enzyme activity, there is still much to unearth.
Highlights.
LLPS alters enzyme activity in a variety of ways, e.g., via the ability to concentrate enzymes and substrates.
LLPS can be a biomolecular switch that selectively transfers metabolites between pathways or regulates cell survival under stress.
Kinetic properties of enzymes have likely evolved to work in tandem with LLPS.
Unique solvent properties within condensates may regulate enzyme activity.
Acknowledgments
T.M. acknowledges funding by NIH grant R01GM112846, by the St. Jude Children’s Research Hospital Research Collaborative on Membrane-less Organelles in Health and Disease, and by the American Lebanese Syrian Associated Charities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
T.M. is a consultant for Faze Medicines. This affiliation has not influenced the scientific content of this review.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakashima KK, Vibhute MA, Spruijt E: Biomolecular Chemistry in Liquid Phase Separated Compartments. Frontiers in Molecular Biosciences 2019, 6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banani SF, Lee HO, Hyman AA, Rosen MK: Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 2017, 18:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin Y, Brangwynne CP: Liquid phase condensation in cell physiology and disease. Science 2017, 357. [DOI] [PubMed] [Google Scholar]
- 4.Boeynaems S, Alberti S, Fawzi NL, Mittag T, Polymenidou M, Rousseau F, Schymkowitz J, Shorter J, Wolozin B, Van Den Bosch L, et al. : Protein Phase Separation: A New Phase in Cell Biology. Trends Cell Biol 2018, 28:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P, Banjade S, Cheng H-C, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. : Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483:336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, Vale RD: Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Case LB, Zhang X, Ditlev JA, Rosen MK: Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363:1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry J, Weber SC, Vaidya N, Haataja M, Brangwynne CP: RNA transcription modulates phase transition-driven nuclear body assembly. Proc Natl Acad Sci U S A 2015, 112:E5237–5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham BJ, Hannett NM, Zamudio AV, Manteiga JC, et al. : Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li CH, Shrinivas K, Manteiga JC, Hannett NM, et al. : Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175:1842–1855 e1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai D, Feliciano D, Dong P, Flores E, Gruebele M, Porat-Shliom N, Sukenik S, Liu Z, Lippincott-Schwartz J: Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat Cell Biol 2019, 21:1578–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riback JA, Katanski CD, Kear-Scott JL, Pilipenko EV, Rojek AE, Sosnick TR, Drummond DA: Stress-Triggered Phase Separation Is an Adaptive, Evolutionarily Tuned Response. Cell 2017, 168:1028–1040 e1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nuske E, Richter D, Baumeister W, Grill SW, Pappu RV, et al. : Phase separation of a yeast prion protein promotes cellular fitness. Science 2018, 359. [DOI] [PubMed] [Google Scholar]
- 14.Iserman C, Desroches Altamirano C, Jegers C, Friedrich U, Zarin T, Fritsch AW, Mittasch M, Domingues A, Hersemann L, Jahnel M, et al. : Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production. Cell 2020, 181:818–831 e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang P, Mathieu C, Kolaitis RM, Zhang P, Messing J, Yurtsever U, Yang Z, Wu J, Li Y, Pan Q, et al. : G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell 2020, 181:325–345 e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasuda S, Tsuchiya H, Kaiho A, Guo Q, Ikeuchi K, Endo A, Arai N, Ohtake F, Murata S, Inada T, et al. : Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578:296–300. [DOI] [PubMed] [Google Scholar]
- 17.Molliex A, Temirov J, Lee J, Coughlin M, Kanagaraj AP, Kim HJ, Mittag T, Taylor JP: Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 2015, 163:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, Stoynov S, Mahamid J, Saha S, Franzmann TM, et al. : A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162:1066–1077. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie IR, Nicholson AM, Sarkar M, Messing J, Purice MD, Pottier C, Annu K, Baker M, Perkerson RB, Kurti A, et al. : TIA1 Mutations in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Promote Phase Separation and Alter Stress Granule Dynamics. Neuron 2017, 95:808–816 e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Oliveira GAP, Cordeiro Y, Silva JL, Vieira T: Liquid-liquid phase transitions and amyloid aggregation in proteins related to cancer and neurodegenerative diseases. Adv Protein Chem Struct Biol 2019, 118:289–331. [DOI] [PubMed] [Google Scholar]
- 21.Zhang JZ, Lu TW, Stolerman LM, Tenner B, Yang JR, Zhang JF, Falcke M, Rangamani P, Taylor SS, Mehta S, et al. : Phase Separation of a PKA Regulatory Subunit Controls cAMP Compartmentation and Oncogenic Signaling. Cell 2020, 182:1531–1544 e1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouchard JJ, Otero JH, Scott DC, Szulc E, Martin EW, Sabri N, Granata D, Marzahn MR, Lindorff-Larsen K, Salvatella X, et al. : Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments. Molecular cell 2018, 72:19–36.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holehouse AS, Pappu RV: Functional Implications of Intracellular Phase Transitions. Biochemistry 2018, 57:2415–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prouteau M, Loewith R: Regulation of Cellular Metabolism through Phase Separation of Enzymes. Biomolecules 2018, 8:160.** Comprehensive review outlining the current state of phase separation with respect to the control and regulation of metabolic enzymes. The authors categorize observed phase-separating metabolic enzymes based on functions, organism of observation, and trigger, and touch upon the functional relevance of phase separation on metabolism.
- 25.Alberti S: The wisdom of crowds: regulating cell function through condensed states of living matter. Journal of Cell Science 2017, 130:2789–2796. [DOI] [PubMed] [Google Scholar]
- 26.Miller SL, Schopf JW, Lazcano A: Oparin’s “Origin of Life”: sixty years later. Journal of Molecular Evolution 1997, 44:351–353. [PubMed] [Google Scholar]
- 27.Oparin AI, Serebrovskaia KB, Pantskhava SN, Vasileva NV: Enzymatic Synthesis of Polyadenylic Acid in Coacervate Drops. Biokhimiia 1963, 28:671–675. [PubMed] [Google Scholar]
- 28.Oparin AI: The origin of life. New York: The Macmillan Company; 1938. [Google Scholar]
- 29.Guillen-Boixet J, Kopach A, Holehouse AS, Wittmann S, Jahnel M, Schlussler R, Kim K, Trussina I, Wang J, Mateju D, et al. : RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell 2020, 181:346–361 e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. : Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 2020, 181:306–324 e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoo H, Triandafillou C, Drummond DA: Cellular sensing by phase separation: Using the process, not just the products. Journal of Biological Chemistry 2019, 294:7151–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narayanaswamy R, Levy M, Tsechansky M, Stovall GM, O’Connell JD, Mirrielees J, Ellington AD, Marcotte EM: Widespread reorganization of metabolic enzymes into reversible assemblies upon nutrient starvation. Proc Natl Acad Sci U S A 2009, 106:10147–10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Leeuwen W, Rabouille C: Cellular stress leads to the formation of membraneless stress assemblies in eukaryotic cells. Traffic 2019, 20:623–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller GG, Han T, Freeberg MA, Moresco JJ, Ghanbari Niaki A, Roach NP, Yates JR 3rd, Myong S, Kim JK: RNA promotes phase separation of glycolysis enzymes into yeast G bodies in hypoxia. eLife 2020, 9:e48480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin M, Fuller GG, Han T, Yao Y, Alessi AF, Freeberg MA, Roach NP, Moresco JJ, Karnovsky A, Baba M, et al. : Glycolytic Enzymes Coalesce in G Bodies under Hypoxic Stress. Cell Reports 2017, 20:895–908.** This report demonstrates that coalescence of glycolytic enzymes into so called G bodies is a conserved process brought on by hypoxia. By measuring upstream metabolites, it was also shown that G bodies function to enhance the rate of glycolysis. The authors demonstrate the necessity of G body formation for cell survival under hypoxic conditions and identify key factors to their formation.
- 36.Kohnhorst CL, Kyoung M, Jeon M, Schmitt DL, Kennedy EL, Ramirez J, Bracey SM, Luu BT, Russell SJ, An S: Identification of a multienzyme complex for glucose metabolism in living cells. Journal of Biological Chemistry 2017, 292:9191–9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang S, Nelson JC, Bend EG, Rodriguez-Laureano L, Tueros FG, Cartagenova L, Underwood K, Jorgensen EM, Colon-Ramos DA: Glycolytic Enzymes Localize to Synapses under Energy Stress to Support Synaptic Function. Neuron 2016, 90:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, et al. : Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 2012, 491:458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An S, Kumar R, Sheets ED, Benkovic SJ: Reversible compartmentalization of de novo purine biosynthetic complexes in living cells. Science 2008, 320:103–106. [DOI] [PubMed] [Google Scholar]
- 40.Doigneaux C, Pedley AM, Mistry IN, Papayova M, Benkovic SJ, Tavassoli A: Hypoxia drives the assembly of the multienzyme purinosome complex. Journal of Biological Chemistry 2020, 295:9551–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedley AM, Benkovic SJ: A New View into the Regulation of Purine Metabolism: The Purinosome. Trends in Biochemical Sciences 2017, 42:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao H, Chiaro CR, Zhang L, Smith PB, Chan CY, Pedley AM, Pugh RJ, French JB, Patterson AD, Benkovic SJ: Quantitative analysis of purine nucleotides indicates that purinosomes increase de novo purine biosynthesis. Journal of Biological Chemistry 2015, 290:6705–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinzpeter F, Tostevin F, Gerland U: Regulation of reaction fluxes via enzyme sequestration and co-clustering. J R Soc Interface 2019, 16:20190444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheu-Gruttadauria J, MacRae IJ: Phase Transitions in the Assembly and Function of Human miRISC. Cell 2018, 173:946–957.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim TH, Tsang B, Vernon RM, Sonenberg N, Kay LE, Forman-Kay JD: Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 2019, 365:825–829.** FMRP and CAPRIN1 are two interacting translational regulators which phase separate together. Using nuclear magnetic resonance spectroscopy, the authors pinpoint specific interactions that drive phase separation. Additionally, they show that the phosphorylation pattern of the FMRP-CAPRIN1 substituents drives enzymatic activity within the condensate towards either transcription or deadenylation.
- 46.Kini HK, Silverman IM, Ji X, Gregory BD, Liebhaber SA: Cytoplasmic poly(A) binding protein-1 binds to genomically encoded sequences within mammalian mRNAs. RNA 2016, 22:61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banani SF, Rice AM, Peeples WB, Lin Y, Jain S, Parker R, Rosen MK: Compositional Control of Phase-Separated Cellular Bodies. Cell 2016, 166:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Küffner AM, Prodan M, Zuccarini R, Capasso Palmiero U, Faltova L, Arosio P: Acceleration of an Enzymatic Reaction in Liquid Phase Separated Compartments Based on Intrinsically Disordered Protein Domains. ChemSystemsChem 2020, 2.** The authors create an enzymatically active condensate by fusing the phase-separating low-complexity domain from DEAD-box proteins to adenylate kinase. Using this system, they demonstrate an increase in enzyme concentration of up to 140-fold within the phase-separated condensates, leading to an increase in enzyme activity.
- 49.Hondele M, Sachdev R, Heinrich S, Wang J, Vallotton P, Fontoura BMA, Weis K: DEAD-box ATPases are global regulators of phase-separated organelles. Nature 2019, 573:144–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capasso Palmiero U, Kuffner AM, Krumeich F, Faltova L, Arosio P: Adaptive Chemoenzymatic Microreactors Composed of Inorganic Nanoparticles and Bioinspired Intrinsically Disordered Proteins. Angewandte Chemie International Edition 2020, 59:8138–8142. [DOI] [PubMed] [Google Scholar]
- 51.Poudyal RR, Pir Cakmak F, Keating CD, Bevilacqua PC: Physical Principles and Extant Biology Reveal Roles for RNA-Containing Membraneless Compartments in Origins of Life Chemistry. Biochemistry 2018, 57:2509–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poudyal RR, Guth-Metzler RM, Veenis AJ, Frankel EA, Keating CD, Bevilacqua PC: Template-directed RNA polymerization and enhanced ribozyme catalysis inside membraneless compartments formed by coacervates. Nat Commun 2019, 10:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drobot B, Iglesias-Artola JM, Le Vay K, Mayr V, Kar M, Kreysing M, Mutschler H, Tang TD: Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nature Communications 2018, 9:3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dzuricky M, Rogers BA, Shahid A, Cremer PS, Chilkoti A: De novo engineering of intracellular condensates using artificial disordered proteins. Nature Chemistry 2020, 12:814–825.** Artificial intrinsically disordered proteins with varying aromatic content and molecular weights were rationally designed based on the sequences of native IDPs. These A-IDPs were shown to phase separate in vitro and in cells with large variability in propensity – some seven orders of magnitude – depending on molecular weight and composition. The resulting condensates were demonstrated to sequester an enzyme and increase the rate of product formation in proportion to increasing molecular weight of the A-IDP.
- 55.Martin EW, Mittag T: Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry 2018, 57:2478–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peeples W, Rosen MK: Phase Separation Can Increase Enzyme Activity by Concentration and Molecular Organization bioRxiv 2020.** This very careful study demonstrates that LLPS can increase enzyme activity in the dense phase beyond what is expected from the concentration of the enzyme. The effect is likely caused by the enhanced proximity of enzyme and substrate induced by sterically favorable recruitment to the scaffold. LLPS can thus not only enhance enzyme activity by mass action but also by preorganization of enzyme/substrate complexes.
- 57.Liu M, He S, Cheng L, Qu J, Xia J: Phase-Separated Multienzyme Biosynthesis. Biomacromolecules 2020, 21:2391–2399. [DOI] [PubMed] [Google Scholar]
- 58.Jiang M, Cao Y, Guo ZF, Chen M, Chen X, Guo Z: Menaquinone biosynthesis in Escherichia coli: identification of 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate as a novel intermediate and re-evaluation of MenD activity. Biochemistry 2007, 46:10979–10989. [DOI] [PubMed] [Google Scholar]
- 59.Pavlovic M, Plucinski A, Zhang J, Antonietti M, Zeininger L, Schmidt B: Cascade Kinetics in an Enzyme-Loaded Aqueous Two-Phase System. Langmuir 2020, 36:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mountain GA, Keating CD: Formation of Multiphase Complex Coacervates and Partitioning of Biomolecules within them. Biomacromolecules 2020, 21:630–640. [DOI] [PubMed] [Google Scholar]
- 61.Love C, Steinkuhler J, Gonzales DT, Yandrapalli N, Robinson T, Dimova R, Tang TD: Reversible pH-Responsive Coacervate Formation in Lipid Vesicles Activates Dormant Enzymatic Reactions. Angewandte Chemie International Edition 2020, 59:5950–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis BW, Aumiller WM Jr., Hashemian N, An S, Armaou A, Keating CD: Colocalization and Sequential Enzyme Activity in Aqueous Biphasic Systems: Experiments and Modeling. Biophysical Journal 2015, 109:2182–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dora Tang TY, van Swaay D, deMello A, Ross Anderson JL, Mann S: In vitro gene expression within membrane-free coacervate protocells. Chemical Communications 2015, 51:11429–11432. [DOI] [PubMed] [Google Scholar]
- 64.Kojima T, Takayama S: Membraneless Compartmentalization Facilitates Enzymatic Cascade Reactions and Reduces Substrate Inhibition. ACS Applied Materials & Interfaces 2018, 10:32782–32791.** An aqueous multiphase system was used to examine the effect of compartmentalization on enzyme activity. Using an oxidation cascade consisting of glucose oxidase and horseradish peroxidase, they observed the ability of condensates to facilitate multi-step reactions through the interfaces of multiple separate compartments. Additionally, they demonstrated the benefit of proximity-based coacervation, when they observed that dextranase has a higher activity when adjacent to, but sequestered away from its substrate, dextran than in a droplet containing both enzyme and substrate.
- 65.Aumiller WM Jr., Keating CD: Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat Chem 2016, 8:129–137. [DOI] [PubMed] [Google Scholar]
- 66.Martin EW, Holehouse AS, Peran I, Farag M, Incicco JJ, Bremer A, Grace CR, Soranno A, Pappu RV, Mittag T: Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. Science 2020, 367:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klein IA, Boija A, Afeyan LK, Hawken SW, Fan M, Dall’Agnese A, Oksuz O, Henninger JE, Shrinivas K, Sabari BR, et al. : Partitioning of cancer therapeutics in nuclear condensates. Science 2020, 368:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fonin AV, Darling AL, Kuznetsova IM, Turoverov KK, Uversky VN: Intrinsically disordered proteins in crowded milieu: when chaos prevails within the cellular gumbo. Cell Mol Life Sci 2018, 75:3907–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuznetsova IM, Turoverov KK, Uversky VN: What macromolecular crowding can do to a protein. International Journal of Molecular Sciences 2014, 15:23090–23140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zotter A, Bauerle F, Dey D, Kiss V, Schreiber G: Quantifying enzyme activity in living cells. Journal of Biological Chemistry 2017, 292:15838–15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma B, Nussinov R: Structured Crowding and Its Effects on Enzyme Catalysis. In Dynamics in Enzyme Catalysis. Edited by Klinman J, Hammes- Schiffer S: Springer Berlin Heidelberg; 2013:123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maximova K, Wojtczak J, Trylska J: Enzymatic activity of human immunodeficiency virus type 1 protease in crowded solutions. European Biophysics Journal 2019, 48:685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maximova K, Wojtczak J, Trylska J: Enzyme kinetics in crowded solutions from isothermal titration calorimetry. Analytical Biochemistry 2019, 567:96–105. [DOI] [PubMed] [Google Scholar]
- 74.Popielec A, Ostrowska N, Wojciechowska M, Feig M, Trylska J: Crowded environment affects the activity and inhibition of the NS3/4A protease. Biochimie 2020, 176:169–180.** This paper shows the myriad effects crowding can have on an enzyme depending on the chemical nature of the crowder. The authors investigated the activity of the hepatitis C virus protease NS3/4A in the presence of macromolecular crowders. PEG was found to interact with the enzyme resulting in a net loss in kinetic activity. Ficoll was found to not interact with the enzyme, but due to its interlacing capabilities, still limits the available solution space. This resulted in an increase in kinetic activity. Ficoll was additionally found to lower the effectiveness of a potent NS3/4A inhibitor. BSA had a different effect again, with a noted increase in KM in its presence. This was attributed to BSA sequestering the substrate from the enzyme.
- 75.Nott TJ, Craggs TD, Baldwin AJ: Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. Nature Chemistry 2016, 8:569–575. [DOI] [PubMed] [Google Scholar]
- 76.Nott Timothy J, Petsalaki E, Farber P, Jervis D, Fussner E, Plochowietz A, Craggs TD, Bazett-Jones David P, Pawson T, Forman-Kay Julie D, et al. : Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles. Molecular Cell 2015, 57:936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cook PF, Cleland WW: Enzyme Kinetics and Mechanism : CRC Press; 2007. [Google Scholar]
- 78.Huang R, Zhong L, Xie F, Wei L, Gan L, Wang X, Liao A: Purification, Characterization and Degradation Performance of a Novel Dextranase from Penicillium cyclopium CICC-4022. International Journal of Molecular Sciences 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saleh OA, Jeon BJ, Liedl T: Enzymatic degradation of liquid droplets of DNA is modulated near the phase boundary. Proc Natl Acad Sci U S A 2020, 117:16160–16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alberti S, Gladfelter A, Mittag T: Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176:419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]