Abstract
Although reduced ambient lighting (“dim” light) can cause myopia in emmetropizing chicks, it does not necessarily lead to myopic changes in emmetropizing rhesus monkeys. Because myopia is rarely spontaneous, a question remained whether dim light would hasten the progression of visually induced myopia. To determine the effects of dim light on the development of and recovery from form-deprivation myopia (FDM), seven 3-week-old infant rhesus monkeys were reared under dim light (mean ± SD = 55 ± 9 lux) with monocular diffuser spectacles until ~154 days of age, then maintained in dim light with unrestricted vision until ~337 days of age to allow for recovery. Refractive errors, corneal powers, ocular axial dimensions and sub-foveal choroidal thicknesses were measured longitudinally and compared to those obtained from form-deprived monkeys reared under typical laboratory lighting (504 ± 168 lux). Five of the seven subjects developed FDMs that were similar to those observed among their normal-light-reared counterparts. The average degree of form-deprivation-induced myopic anisometropia did not differ significantly between dim-light subjects (−3.88 ± 3.26D) and normal-light subjects (−4.45 ± 3.75D). However, three of the five dim-light subjects that developed obvious FDM failed to exhibit any signs of recovery and the two monkeys that were isometropic at the end of the treatment period manifest abnormal refractive errors during the recovery period. All refractive changes were associated with alterations in vitreous chamber elongation rates. It appears that dim light is not a strong myopiagenic stimulus by itself, but it can impair the optical regulation of refractive development in primates.
Keywords: form-deprivation, myopia, emmetropization, dim light, ambient light level, non-human primates
1. Introduction
Myopia is a multifactorial refractive disorder (Flitcroft 2013; Wallman & Winawer, 2004), of which the prevalence typically starts to increase during childhood (for a review, see: Holden et al., 2016). Because the underlying ocular changes are irreversible, identifying the environmental factors that associate with the genesis and development of myopia has significant implications for reducing the growing myopia-associated public health burden. Epidemiological studies have shown that children who spend more time outdoors have higher hyperopic refractive errors and a lower chance of developing myopia (Dirani et al., 2009; French, Ashby, Morgan, & Rose, 2013; Guggenheim et al., 2012; Jones et al., 2012; Rose et al., 2008; Wu et al., 2013), speculatively attributed to the higher luminance levels typically associated with outdoor environments. In support of this view, elevated ambient lighting has been shown to slow the development of form-deprivation myopia (FDM) in tree shrews (Siegwart Jr. et al., 2012) and reduce its magnitude in chicks and rhesus monkeys (Ashby et al., 2009; Smith III et al., 2012). These effects on FDM appear to be light-intensity dependent (Karouta and Ashby, 2015). In addition, emmetropization-associated reductions in hyperopia can be slowed or reduced by elevated ambient lighting in chicks and tree shrews (Ashby et al., 2009; Ashby & Schaeffel, 2010; Cohen et al., 2011, 2012; Siegwart Jr. et al., 2012), although not in rhesus monkeys (Smith III et al., 2013). These findings indicate that higher lighting levels may be protective against myopia.
The positive findings with elevated lighting have motivated further investigations as to whether lower lighting levels might contribute to human myopia genesis. However, the limited studies on the effects of reduced ambient lighting on emmetropization showed substantial inter-species discrepancies. Cohen et al. found that, in comparison to animals reared under typical laboratory lighting (500 lux), chickens reared under dim lighting (50 lux) developed absolute myopia (Cohen et al., 2011, 2012). Their findings, along with the early works of Bercovitz et al. (Bercovitz et al., 1972) and Lauber and Kinner (Lauber & Kinner, 1979), suggested that dim light could be myopiagenic. On the other hand, rearing infant rhesus monkeys under reduced ambient lighting interfered with normal emmetropization, increased both inter-individual and interocular variability in refractive errors, but it did not cause myopia (She et al., 2020). These observations suggested that, for primates, dim light does not always cause myopia in otherwise emmetropizing eyes, but could compromise the visual regulation that governs normal refractive development.
For most laboratory animals, emmetropization does not typically end with spontaneous myopia (Cohen et al. 2011; Graham and Judge 1999; Norton and McBrien 1992; Qiao-Grider et al. 2007; Wallman, Adams, and Trachtman 1981; Zhou et al. 2006). However, myopia that is qualitatively similar in nature to common myopic errors in children can be induced by specific visual manipulations in many species (Barathi et al., 2008; Howlett & McFadden, 2006, 2009; Hung et al., 1995; McBrien et al., 2001; Raviola & Wiesel, 1985; Siegwart Jr. & Norton, 1998; Smith III & Hung, 2000; Troilo & Judge, 1993; Troilo & Wallman, 1987; Wallman et al., 1978). In this regard, a related query is whether dim light could alter the development of these visually induced myopias. Direct assessment of this issue was first carried out by Ashby et al. (Ashby et al., 2009) using an avian form-deprivation model of myopia. In their experiment, form-deprived chicks were placed under dim light (50 lux) for 6 of the 12 hours of the daily lights-on cycle from 1 to 4 days of age. In comparison to normal ambient lighting (consistently 500 lux throughout the daily lights-on hours), this intermittent dim-light exposure did not cause more form-deprivation myopia, nor did it produce greater-than-normal reductions in hyperopia in their contralateral, untreated eyes. These observations suggested that, for chickens, dim light might not be a risk factor for vision-induced myopias.
To date, the effects of low ambient lighting levels on visually induced myopias has only been studied in chickens. Given the limited information and the implications for understanding human myopias, we investigated the development of monocular FDM under reduced ambient lighting in non-human primates. In addition, we also examined the recovery from FDM under dim light. Recovery from FDM, i.e. the reduction in the diffuser-induced myopic anisometropias, has been observed in many species (Howlett & McFadden, 2006; Qiao-Grider et al., 2004; Siegwart & Norton, 1998; Wallman & Adams, 1987) and is regarded as the one of the first clear indicators of visual regulation of refractive development (Qiao-Grider et al., 2004; Schaeffel & Howland, 1991; Troilo et al., 2019; Wildsoet & Schmid, 2000). To the best of the authors’ knowledge, there were no prior longitudinal evaluations on the effects of lower ambient lighting levels on the recovery from FDM. The study of this phenomenon could provide information on whether and how the visual regulatory mechanisms for refractive development are influenced by low ambient lighting levels.
2. Method
2.1. Primary subjects and pre-dim-light rearing conditions
Seven infant rhesus monkeys (Macaca mulatta) were acquired at two weeks of age, and then reared in a climate-controlled housing environment until the onset of the experiment. This housing area was illuminated by broadband, white fluorescent lights (GE Ecolux® Starcoat® T8 F32T8/SP35/ECO, General Electric Co., Boston, MA) on a 12 hour-light/12 hour-dark diurnal cycle. The lighting intensity during the daily light phase (7AM – 7PM), as measured at waist-level (approximately the height of the junction between the upper and lower cages), ranged between 312 – 860 lux (“normal” lighting, average illuminance = 504 ± 168 lux, correlated color temperature = 3170K) (She et al., 2020).
2.2. Experimental strategies
Starting at 25 ± 3 days of age, the subjects were reared under reduced ambient illumination with concurrent monocular form deprivation until 154 ± 7 days of age (dim-light-diffuser period). At that time the diffuser lenses were removed, these dim-light-reared, form-deprived monkeys (DL-FD monkeys) then experienced unrestricted vision in both eyes in the dim ambient lighting until 337 ± 10 days of age (dim-light-recovery period).
2.2.1. Experimental “dim” lighting
The reduced ambient lighting (dim light) in the current experiment was maintained throughout the light phase of the diurnal lighting cycle (7AM – 7PM). It was produced by filtering the fluorescent lighting through an aluminum deposited, semi-reflective film (Grafix™ Metalized Dura-Lar®, Silver, 0.05mm-thick; Grafix, Maple Heights, Ohio) that was closely fitted to the ceiling light panels. Ambient illumination was reduced to 55 ± 9 lux at waist level without significant alterations in the spectral composition of the ambient lighting. Light measures obtained with the meter directed out of the front of individual cages, which reflects the visual environment that the animals commonly encountered, varied from 7 to 36 lux (average intensity = 15 ± 8 lux) (She et al. 2020). This average ambient lighting level, which was chosen in part to facilitate between-study comparisons (Cohen et al., 2011 and 2012, Ashby et al., 2009), was identical to that employed in our previous investigation (She et al., 2020). A more detailed rationale for the chosen lighting level can be found in She et al. (2020).
2.2.2. Form-deprivation
Monocular form deprivation was produced using “light perception” (LP) Bangerter occlusion foils (Fresnel Prism and Lens Co., Bloomington MN, USA) that were attached to zero-power (i.e., plano) carrier lenses (diffuser lenses). At the onset of the dim-light-diffuser period, each of the DL-FD monkeys was fitted with a light-weight helmet that held the diffuser lens in front of the treated, form-deprived eye and a plano lens in front of the fellow control eye. The goggle-like helmets were worn continuously except for the brief daily periods when the lenses were cleaned. Throughout the daily light-on cycle, the helmets were inspected frequently and adjusted as needed to ensure proper fit and optics.
The diffuser lenses reduced spatial contrast without significant alterations in the effective lighting levels. Specifically, LP Bangerter diffusers dramatically reduced the modulation transfer at low- and mid-range spatial frequencies and virtually eliminated contrast for higher spatial frequencies (Pérez et al., 2010; Smith III & Hung, 2000). For the specific diffusers employed in the current experiment, viewing through the diffuser lenses was found to reduce human contrast sensitivity by 1.25 log units at 0.1 cycle/degree (cpd) and by 2 log units at 0.5 cpd, with higher spatial frequencies being undetectable (Smith III & Hung, 2000). On the other hand, the decrease in light transmission through the diffusers was very small. As shown in Fig. 1, ambient- and diffuser-attenuated-illumination levels had a linear relationship in log-log coordinates in the dim-to-normal range of ambient illuminations. This relationship predicts an average reduction in ambient illumination on the order of 0.04 log units, which appeared negligible both in comparison to the light-level difference between the two experimental paradigms (approximately 1 log unit) and to the operating range of the primate eye.
Figure 1.
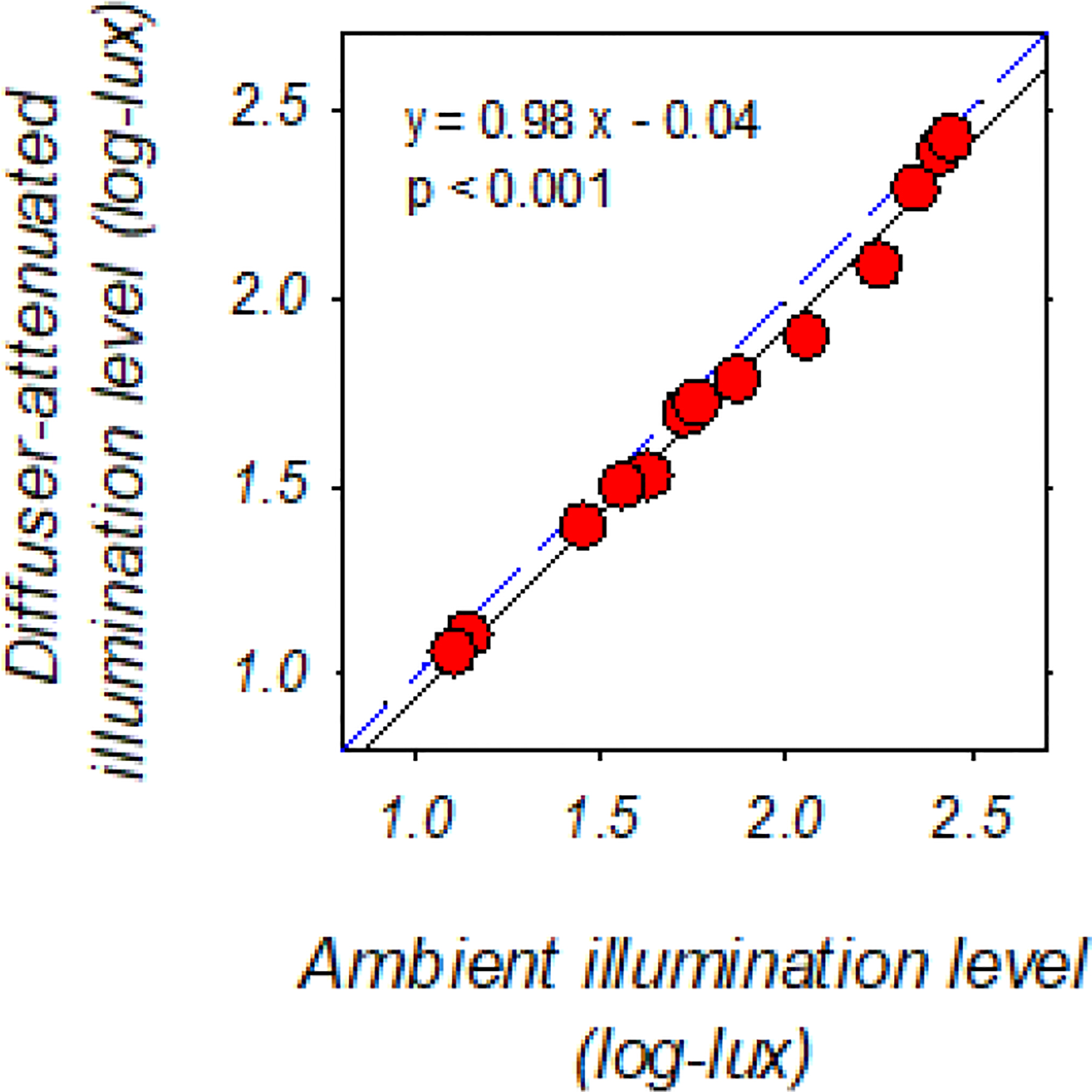
Diffuser-attenuated illumination levels plotted as a function of the corresponding ambient illumination levels on common logarithmic scales. The ambient lighting levels were within the dim-to-normal range employed in the present study. The blue dashed line represents zero diffuser-induced light attenuation, whereas the black regression line represents the linear relationship between diffuser-attenuated and unfiltered ambient illumination levels. To measure the diffuser-attenuated illumination levels, a piece of diffuser foil was attached to a zero-power carrier lens, of which the peripheral area was taped to block stray lights. The center of the diffuser lens was then placed perpendicular to the measurement axis of the spectrophotometer at a 14 mm distance (CL-500A, Konica Minolta Sensing Americas, Inc. NJ, USA). For the measurement of corresponding ambient illumination levels, another taped carrier lens with a clear center of equal size was used in place of the diffuser lens.
2.3. Control data
The primary control subjects were a group of age-matched, monocularly form-deprived monkeys previously reared with the same LP Bangerter diffusers under our “normal” laboratory lighting levels (normal-light-reared, form-deprived, NL-FD monkeys, n = 16). The refractive data for these subjects have been published and discussed (Hung et al., 2018; Smith III & Hung, 2000; Smith III et al., 2012; Smith III et al., 2002). Data from monkeys previously reared with unrestricted vision under “normal” laboratory lighting (normal controls, n = 41) were included as a reference for the vision-induced changes in refractive development (Hung et al., 2018; Hung et al., 2018; Qiao-Grider et al., 2007; Smith III et al., 1999, 2003, 2010, 2015). In addition, refractive error and choroidal thickness data obtained from monkeys that were previously reared with unrestricted vision under identical dim-light conditions (dim-light or DL-controls, n = 7) (She et al., 2020) were included in some analyses (see Section 2.4). Finally, the recovery data from monkeys reared under normal lighting levels with either different strength monocular diffuser lenses or monocular LP diffusers combined with some brief periods of unrestricted vision each day (n = 13) (Smith III et al., 2002; Smith III & Hung, 2000) were included to help characterize the recovery from FDM under typical ambient lighting. The husbandry strategies, diffuser-treatment paradigm, and data collection methodologies for all control subjects were identical to those for the DL-FD monkeys.
2.4. Outcomes and data collection
Refractive errors, ocular axial dimensions, corneal powers, and choroidal thicknesses were measured periodically. Before each measurement session, the animals were cyclopleged (1% tropicamide instilled 25 and 20 minutes before the measurements) and anesthetized (intramuscular injection of 15 – 20 mg/kg of ketamine hydrochloride, combined with 0.15 – 0.20 mg/kg of acepromazine). During the measurement sessions, supplemental topical anesthesia was applied as needed (1% tetracaine ophthalmic solution). Procedures were implemented to ensure that the animals were not exposed to higher ambient lighting during the data collection activities (She et al., 2020).
Refractive error was determined using retinoscopy by two experienced examiners and reported as the mean spherical-equivalent of the spectacle-plane refractive correction for a vertex distance of 14 mm. In previous studies of FDM, interocular differences (IOD, form-deprived eye refraction - control eye refraction) in refractive errors were used as the primary outcome because, with a monocular treatment paradigm, refractive development in the two eyes is largely independent: the lens- or diffuser-treated eyes develop ametropias corresponding to the nature of treatment, whereas their contralateral, untreated eyes typically emmetropize to a relatively normal level (Hung, Arumugam, She, et al., 2018). In this instance, the refractive stability in the untreated eye allows the use of IODs as a sensitive measure of any treatment effects. This metric might not be optimal for the current experiment because our previous study of emmetropization under dim-ambient lighting suggested that control-eye refractive development could be altered by the dim-lighting paradigm (She et al., 2020). Therefore, in this study, we used the absolute refractive error in addition to the IODs in refractive error to determine the main effects of dim-ambient lighting.
Corneal powers and ocular axial dimensions were measured to examine the optical nature of refractive errors. Corneal power was determined using either a hand-held keratometer (Alcon Auto-keratometer: Alcon, Inc., St. Louis, MO, USA) or, in the case when the corneas were too steep (> 62 D, about 5% occurrence rate among 3-week-old monkeys), a corneal topographer (EyeSys 2000; EyeSys Vision, Inc. Houston, TX, USA) (95% limits of inter-instrument agreement = +0.49 to −0.37 D) (Kee et al., 2002). The spherical-equivalent of three independent measurements were averaged to represent the corneal power along the pupillary axis in the 3-mm central region of the cornea. Anterior chamber depth, lens thickness, vitreous chamber depth, and total axial length were assessed using A-scan ultrasonography (OTI-Scan 1000, Ophthalmic Technologies Inc., Downsview, Ontario, Canada). This ultrasonography system used the acoustic velocities for humans eyes to compute the separations between acoustic interfaces (cornea and lens: 1641 m/s, aqueous and vitreous: 1532 m/s) (Byrne & Green, 2002). Ten independent measurements were made along the normal to the corneal apex using a 13 MHz transducer, and the readings were averaged.
Sub-foveal choroidal thickness in the DL-controls and DL-FD monkeys was measured using a spectral-domain, optical coherence tomography system (SD-OCT; Spectralis, Heidelberg, Germany) following the methodology described by Hung et al. (Hung et al. 2018). In brief, images acquired using the OCT “enhanced-depth imaging” mode were manually segmented with a customized Matlab program (2019a, MathWorks, Natick, MA, USA). Choroidal thickness, defined as the distance between Bruch’s membrane and the outer choroidal border, was measured perpendicular to Bruch’s membrane. The average choroidal thickness of a 300-micron region (adjusted for retinal magnification) (Patel et al., 2017) centered at the deepest point of the macula depression (the foveola) was compared interocularly over the course of the experiment. The thickness measures were highly repeatable. The mean (±SD) absolute thickness differences obtained from repeated OCT scans obtained at the same measurement session was 5.07 ± 3.86 μm, about 3% of the mean choroidal thickness in dim-light monkeys.
It has been found that visual manipulations commonly used to induce experimental ametropias consistently and predictably produced choroidal thickness changes. Specifically, in chicks, dramatic choroidal thickening or thinning can occur in response to myopic and hyperopic defocus, respectively, to reduce the presenting optical error (Wallman et al., 1995; Wildsoet & Wallman, 1995). In primates, defocus-induced choroidal thickness changes are qualitatively similar to those observed in chicks, but are much smaller in magnitude and thus have little direct effect on the eye’s refractive state (Hung et al., 2000; Troilo et al., 2000). In this respect, choroidal thickness changes could reflect retinal processing of defocus signals. It is useful particularly for the observations of recovery from FDM under dim light, in which the visual signals that drive the recovery (Qiao-Grider et al., 2004; Schaeffel & Howland, 1991; Wildsoet & Schmid, 2000) might not be sufficiently strong to alter refractive development (Gottlieb et al., IOVS 1991; 32: ARVO abstract; She et al., 2020).
All rearing and measurement procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of Houston’s Institutional Animal Care and Use Committee.
2.5. Statistical methods
Data were analyzed cross-sectionally and longitudinally. Cross-sectional analyses were performed for data obtained at the onset of the dim-light-diffuser period, the onset of the dim-light-recovery period (i.e., the end of dim-light-diffuser period), and the end of dim-light-recovery period. Paired and student’s t-tests were employed for the between-eye and between-group analyses of refractive errors and ocular parameters, respectively.
Multi-level, mixed-effect models were used to compare the longitudinal refractive and vitreous chamber development between DL-FD and NL-FD groups (Rabe-Hesketh & Skrondal, 2012). Because the age-related changes of most ocular parameters are typically curvilinear, the models were constructed as a 2nd order polynomial function of age in a forward selection manner. The statistics for the quadratic effect coefficients were reported only when the effects were statistically significant.
Finally, Pearson correlation and linear regression were used to characterize the relationship between refractive error and ocular parameters. Specifically, we were interested in whether the refractive errors were related to vitreous chamber elongation, the biometrical determinant of most visually induced ametropias in rhesus monkeys. In this respect, the vitreous chamber to corneal radius ratio (VC/CR ratio) was employed for the correlational and regression analyses. In comparison to using absolute vitreous chamber depth, this metric reduces the noise in correlational and regression analyses because it accounts for between-animal differences in the contribution of the cornea to refractive development (She et al., 2020).
All statistical procedures were performed using STATA (MP 14; StataCorp, College Station, TX, USA) at a significance level of 0.05.
3. Results
3.1. Refractive development in the dim-light-diffuser period
The baseline refractive errors and ocular parameters for the two diffuser groups are compared in Table 1. At the onset of the experiment, the DL-FD monkeys were slightly older (2 days) than the NL-FD monkeys (p = 0.02). Despite this difference, the refractive errors and ocular parameters of the DL-FD monkeys were similar to those in NL-FD monkeys. The refractive errors in the two eyes of the DL-FD monkeys were also similar; however, the treated eyes of the NL-FD monkeys were slightly less hyperopic then their control eyes (t (15) = −2.55, p = 0.02). Because no significant between-eye difference in vitreous chamber depth was observed (Table 1), we speculate that this between-eye refractive error difference might reflect measurement variabilities. Finally, the absolute magnitude of the interocular differences in refractive error was similar between the two groups. Considering the degree of anisometropia that can be induced by diffusers, these baseline interocular differences were negligible.
Table 1.
Refractive error and ocular parameters at the onset and end of the dim-light-diffuser period. Asterisks denote significant interocular differences. There were no significant between-group differences in refractive error or any of the ocular parameters at either time point.
| Baseline | End of the dim-light-diffuser period | |||||||
|---|---|---|---|---|---|---|---|---|
| DL-FD (25 ± 3 days)1 | NL-FD (23 ± 2 days) | DL-FD (154 ± 7 days) | NL-FD (149 ± 23 days) | |||||
| Treated eye | Control eye | Treated eye | Control eye | Treated eye | Control eye | Treated eye | Control eye | |
| Refractive error (mean ± SD. D) | 3.73 ± 1.25 | 3.78 ± 1.23 | 4.13 ± 1.29* | 4.29 ± 1.28 | −1.87 ± 4.81* | +2.01 ± 2.78 | −1.30 ± 4.28* | +3.18 ± 1.68 |
| Corneal power (mean ± SD, D) | 62.16 ± 0.97 | 61.93 ± 0.98 | 61.14 ± 1.34 | 60.98 ± 1.22 | 55.73 ± 0.72 | 56.05 ± 0.78 | 55.98 ± 1.52 | 55.45 ± 1.57 |
| Anterior chamber depth (mean ± SD, mm) | 2.38 ± 0.15 | 2.4 ± 0.15 | 2.66 ± 0.16 | 2.61 ± 0.28 | 2.92 ± 0.1 | 2.99 ± 0.11 | 3.12 ± 0.24 | 3.14 ± 0.24 |
| Lens Thickness (mean ± SD, mm) | 3.72 ± 0.08 | 3.72 ± 0.06 | 3.60 ± 0.15 | 3.62 ± 0.2 | 3.72 ± 0.13 | 3.67 ± 0.14 | 3.57 ± 0.15 | 3.59 ± 0.14 |
| Vitreous chamber depth (mean ± SD, mm) | 8.44 ± 0.29 | 8.42 ± 0.3 | 8.62 ± 0.27 | 8.65 ± 0.26 | 10.76 ± 0.75* | 9.93 ± 0.5 | 10.60 ± 0.92* | 9.89 ± 0.58 |
Significant, but negligible, between-group difference in starting age
Significant interocular difference.
After the onset of the dim-light-diffuser period, most of the DL-FD subjects showed obvious alterations in the course of refractive development that reflected the monocular nature of the diffuser treatment (Figure 2, open/closed red symbols). The exceptions were monkeys 672 and 671 (Fig. 2A and B), both of which remained isometropic despite experiencing monocular form deprivation. Specifically, monkey 672 (Fig. 2A) showed a slight increase in hyperopia in the two eyes over time, whereas monkey 671 exhibited moderate reductions in hyperopia that were within the range seen in normally emmetropizing monkeys (Fig. 2B). The interocular differences in refractive error remained small and stable for these two monkeys throughout the dim-light-diffuser period. For the remaining subjects, large reductions in hyperopias took place in their form-deprived eyes (Figs. 2C – 2G). For monkey 674, the early myopic refractive shift appeared to stabilize at approximately 90 days of age (Fig. 2C), but for monkeys 670, 673, 675, and 676 (Figs. 2D – 2G), the myopic alterations continued and eventually resulted in substantial absolute myopias.
Figure 2.
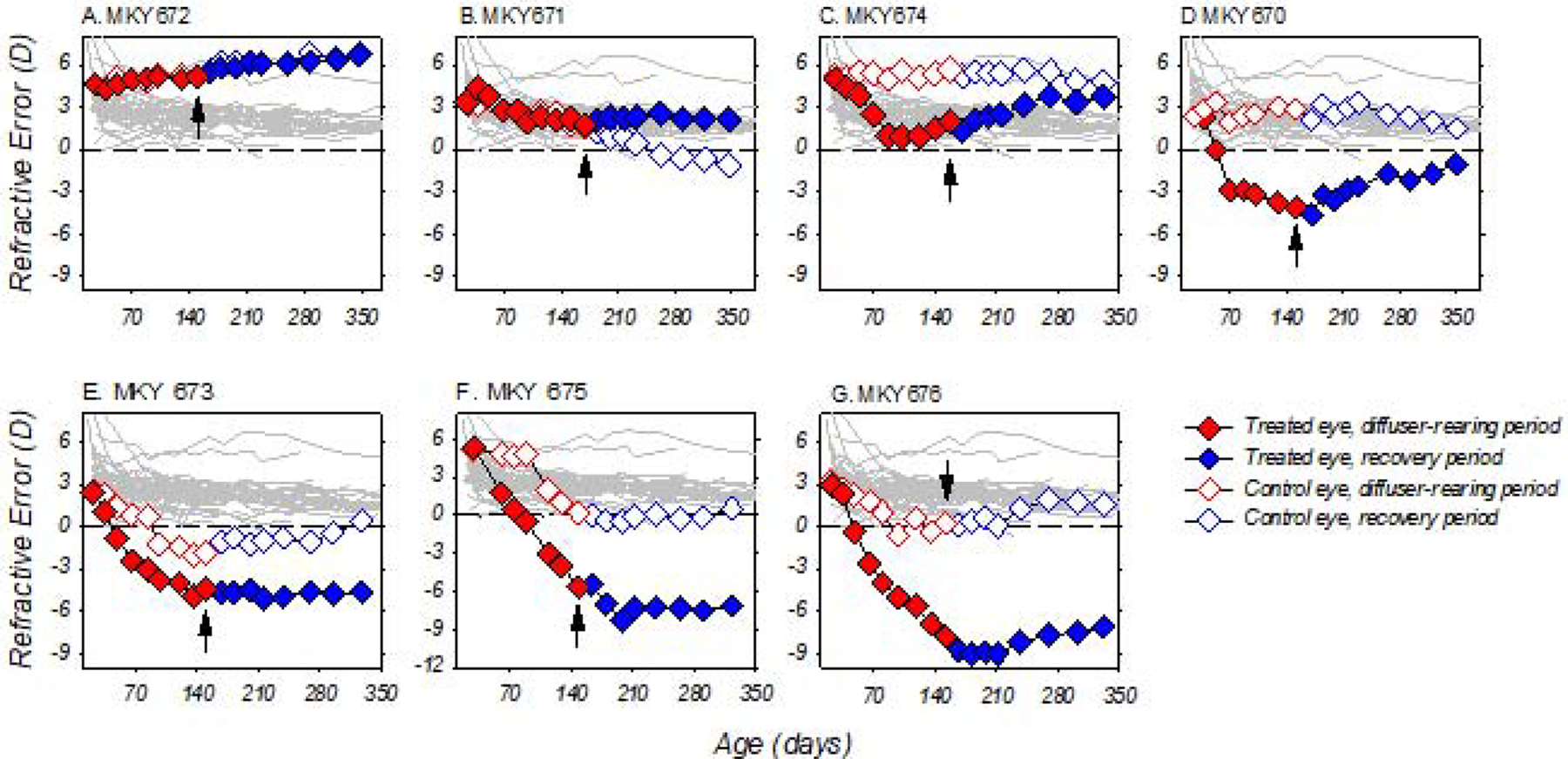
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of age for individual DL-FD subjects. The diffuser-treatment and recovery periods (see section 3.4) are highlighted in red and blue, respectively. Black arrows: the last day of the dim-light-diffuser period and onset of the recovery period; closed symbols: treated eye; open symbols control eyes; thin grey lines: the refractive errors for the right eyes of 41 age-matched, normal-light-reared control monkeys.
With the exception of monkey 671 (Fig. 2B), the treatment regimen also altered the normal refractive development that was anticipated in the fellow control eyes of the DL-FD monkeys. Instead of exhibiting age-related reductions of hyperopia, these eyes either roughly maintained the same amount of hyperopia (monkeys 672, 674 and 670, Figs. 2A, C, and D) or showed greater reductions in hyperopia than what was typically associated with normal emmetropization, and later became more myopic than normal-control monkeys (monkeys 673, 675 and 676, Figs. 2E, F and G). Note that the relative myopic alterations in the fellow eyes were also very different from our previous observations, in which monkeys reared with unrestricted vision under dim light developed age-related increases, rather than decreases, in hyperopia (She et al., 2020).
Dim light appeared to affect the time course for refractive development in the control eyes, but not the diffuser-treated eyes. Figure 3 illustrates the average changes (±SD) in refractive error for the DL-FD and NL-FD monkeys during the diffuser-rearing period. The treated eyes of both diffuser groups showed greater reductions in hyperopia than their respective control eyes and the eyes of normal-control monkeys; however, no significant differences were observed between the treated eyes of the two diffuser groups (Fig. 3A and 3B), indicating that dim-light rearing did not affect the development of FDM. As for the control eyes, the time course for the age-related reductions in hyperopia in the DL-FD monkeys appeared to be initially slower than that of the NL-FD monkeys, but later accelerated towards the end of the dim-light-diffuser period (z = −3.73, p < 0.01 for the quadratic effect coefficients). In addition, the standard deviations of the changes in control-eye refractive error were greater in the DL-FD monkeys than in NL-FD monkeys (Fig. 3A and 3B), a reflection of the individual variability of their refractive development. Despite the variability in the later stages of diffuser rearing, the average fellow-eye refractive changes in the DL-FD monkeys were within the 95% confidence limits for the normal control monkeys (Fig. 3B).
Figure 3.
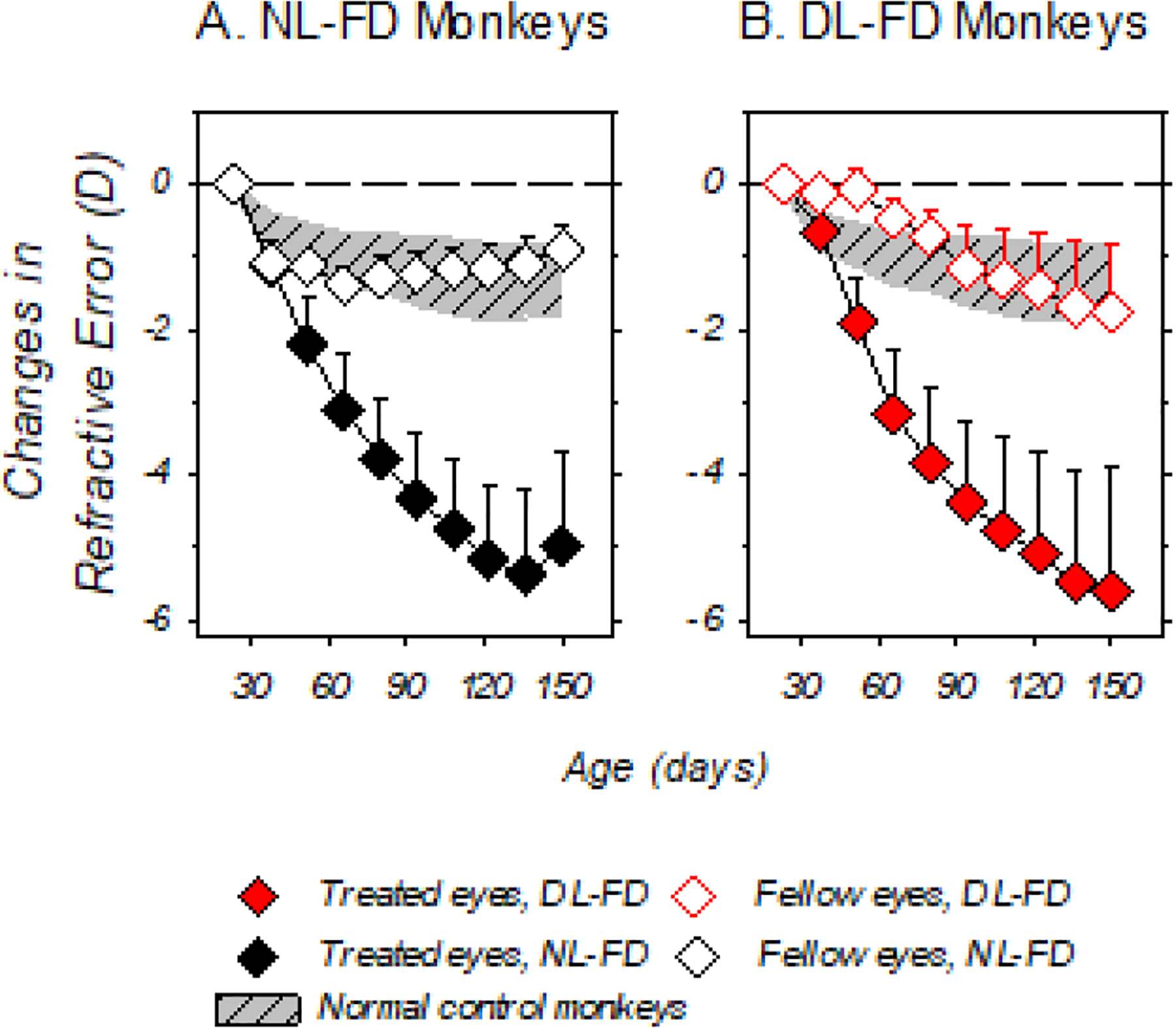
Mean (+SD) refractive-error changes relative to baseline plotted as a function of age. The closed and open symbols represent the treated and control eyes of the NL-FD (Panel A) and DL-FD (Panel B) monkeys, respectively. The shaded areas represent the 95% confidence range for the changes in refractive error in the right eyes of the age-matched, normal-control monkeys.
Dim-light rearing did not appear to affect the magnitude of the refractive changes induced by form deprivation. Figure 4 shows the refractive errors obtained at the end of the diffuser-rearing period for the treated (filled symbols) and control eyes (open symbols) of individual DL-FD and NL-FD monkeys. At the end of the diffuser-rearing period, NL-FD monkeys exhibited various degrees of myopia in their treated eyes in comparison to the NL-controls (NL-FD vs. NL-controls: −1.30 ± 4.28D vs. + 2.34 ± 1.07D, t (55) = −5.1, p < 0.001), whereas their control eyes were relatively more hyperopic (+ 3.18 ± 1.68D vs. +2.34 ± 1.06D, t (55) = 2.24, p = 0.03). Except for the control eye of one DL-FD monkey that fell below the 95% confidence limits for normal-control monkeys, the ranges of treated- and control-eye refractive errors in the DL-FD and NL-FD monkeys were very similar. There were no significant differences between the DL-FD and NL-FD monkeys in the mean refractive errors for either the treated (t (21) = −0.29, p = 0.78) or control eyes (t (21) = 1.26, p = 0.22). In addition, the degree of myopic anisometropias in the NL-FD and DL-FD groups was statistically the same (mean ± SD of IOD, DL- vs. NL-FD: −3.88 ± 3.26 D vs. −4.48 ± 3.73 D, t (21) = −0.37, p = 0.72). On the other hand, due to the excessive reductions in hyperopia in the fellow eyes of DL-FD monkeys 673, 675, and 676, the control eyes of the DL-FD monkeys appeared less hyperopic than those of the DL-control monkeys, although the difference was not statistically significant (+3.70 ± 1.36D vs. +2.01 ± 2.78D, t (6) = 1.52, p = 0.18) (She et al., 2020).
Figure 4.
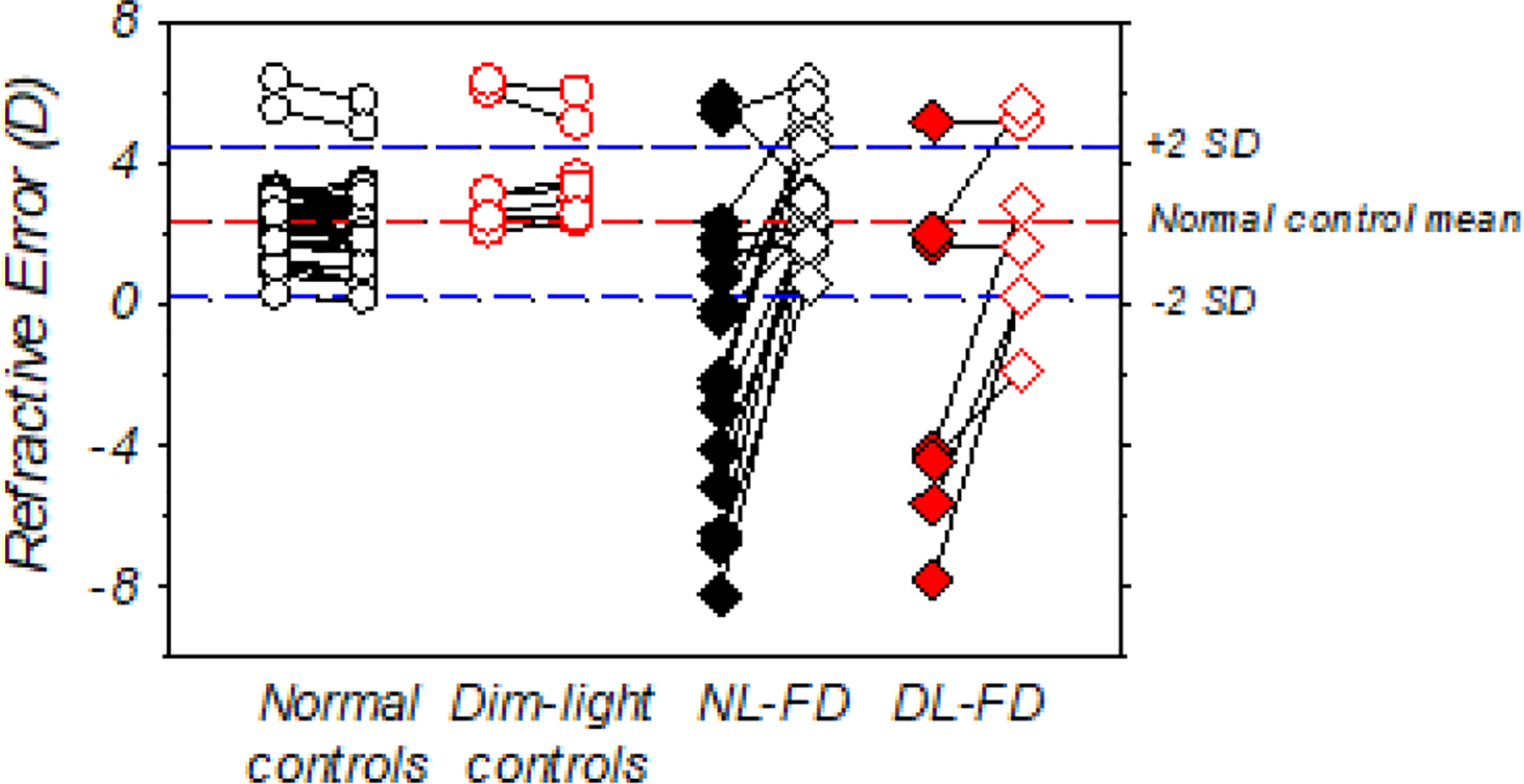
Spherical-equivalent, spectacle-plane refractive corrections for individual animals at the end of the dim-light-diffuser period. The closed and open diamonds represent the treated and control eyes of the DL-FD and NL-FD monkeys, respectively. The circle symbols represent the two eyes of the NL-control (open circles) and DL-control monkeys (open red circles), respectively. The red and blue dashed lines represent the mean and ±2 SD from the mean of the normal-control monkeys.
3.2. Ocular parameters in the dim-light-diffuser period
At the end of diffuser-rearing period, the corneal powers, anterior chamber depths, and lens thicknesses were similar between DL-FD and NL-FD monkeys (Table 1) and were consistently within the ±2 SD range of the normal-control monkeys (Fig. 5A – 5C). In addition, there were no significant between-eye differences in these ocular components.
Figure 5.
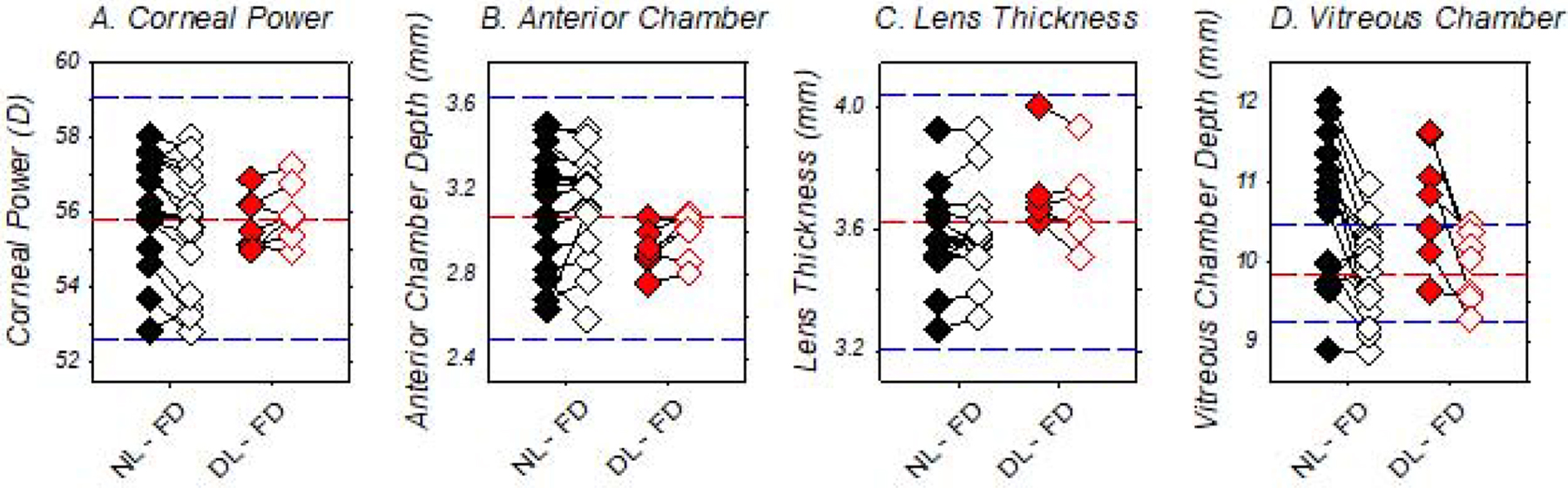
Ocular parameters measured at the end of the diffuser-rearing periods for individual form-deprived monkeys. The closed and open symbols represent the treated and control eyes of the DL-FD (red) and NL-FD monkeys (black), respectively. The red and blue dashed lines represent the mean and ±2 SD from the mean for each respective ocular parameter for the normal-control monkeys.
In agreement with the refractive outcomes noted above, the course of treated-eye vitreous chamber depth development was similar in the DL-FD and NL-FD monkeys (z = 1.45, p = 0.15), indicating that dim-light rearing did not affect the ocular axial elongation that associates with FDM. At the end of the dim-light-diffuser period, vitreous chamber depths of the DL-FD monkeys were greater in their form-deprived eyes than in their control eyes (t (6) = 3.22, p = 0.02), but there were no significant differences between the DL-FD and NL-FD monkeys for either the treated (t (21) = 0.40, p = 0.70) or control eyes (t (21) = −0.15, p = 0.88) (Fig. 5D). As illustrated in Figure 6, at the end of the dim-light-diffuser period, the refractive errors of the DL-FD monkeys were inversely correlated with their vitreous chamber to corneal radius ratios (VC/CR ratio; r = −0.97, p < 0.01), suggesting that vitreous chamber depth remained the primary determinant of the observed refractive errors after accounting, at least in part, for the possible influence of individual differences in corneal power.
Figure 6.
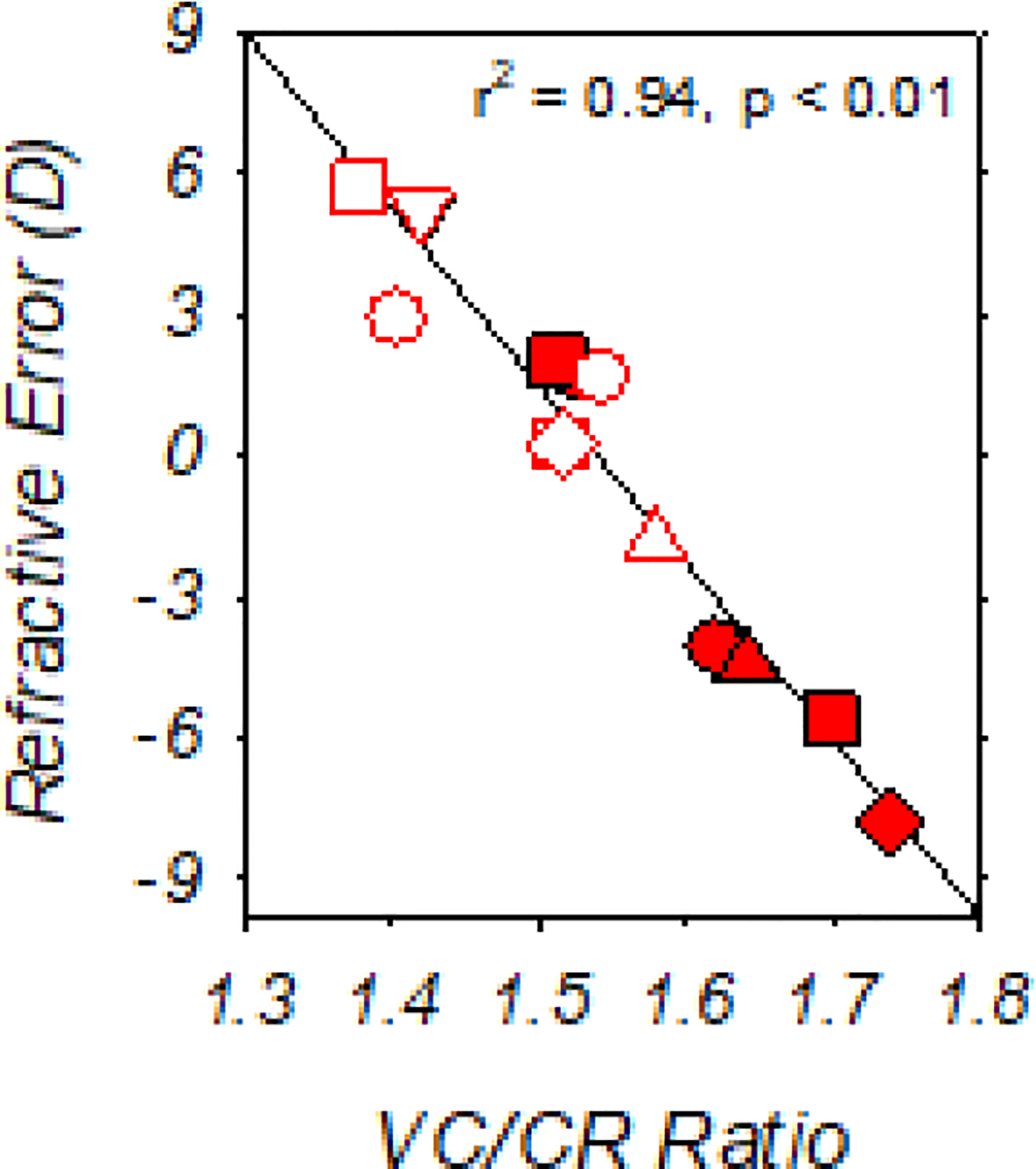
Spherical-equivalent, spectacle-plane refractive corrections plotted as a function of the vitreous chamber to corneal radius ratio (VC/CR ratio) for individual DL-FD monkeys obtained at the end of the dim-light-diffuser period. Filled and open symbols represent the treated and control eyes, respectively. Vitreous chamber depth and the radius of corneal curvature for the calculation of VC/CR ratio were specified in millimeters.
3.3. Sub-foveal choroidal thicknesses in relation to the interocular differences in refractive errors
At the onset of the experiment, the choroidal thicknesses in the treated (average ± SEM thickness: 107.80 ± 6.77 μm) and control eyes of the DL-FD monkeys (108.38 ± 6.88 μm) were similar (t (6) = −0.23, p = 0.83). During the dim-light-diffuser period, the fellow control eyes of all of the DL-FD monkeys exhibited age-associated increases in choroidal thickness. Specifically, sub-foveal choroidal thickness in the control eyes increased in a roughly linear manner after the onset of the dim-light-diffuser period (0.32 micron/day, z = 2.49, p = 0.01) and these longitudinal changes did not differ significantly from those observed in the DL-control monkeys. At the end of the dim-light-diffuser period, the average (±SEM) choroidal thickness in the fellow control eyes of the DL-FD monkeys was similar to the binocularly averaged choroidal thickness observed in the age-matched, DL-control monkeys (DL-FD control eyes vs. DL-controls: 150.76 ± 13.21 μm vs. 167.11 ± 6.24 μm , t (19) = −1.28, p = 0.22) (She et al., 2020) and were not significantly different from the average values in the two eye of the NL-controls (147.18 ± 6.44 μm, t (19) = 0.28, p = 0.79).
The interocular differences in refractive error observed in the DL-FD monkeys were associated with interocular differences in choroidal thickness (Fig. 7). For the two DL-FD monkeys that failed to develop myopic anisometropia during the diffuser-rearing period, choroidal thicknesses were either similar in both eyes or slightly thicker in the deprived eyes (Figs. 7A and B). However, in each of the 5 monkeys that developed obvious myopic anisometropias, the treated-eye choroids were consistently thinner than the choroids in the fellow control eyes, at least during the early stages of diffuser wear (i.e., about the first 30 days of diffuser wear). These initial responses were qualitatively similar to those observed in deprived eyes of chicks, tree shrews, macaque monkeys, and marmosets that were reared under typical laboratory lighting (Hung et al., 2000; Siegwart Jr. & Norton, 1998; Troilo et al., 2000; Wallman et al., 1995). Subsequently, the relative choroidal thinning in the deprived eyes of the DL-FD monkeys quickly slowed; as the diffuser treatment continued, the interocular differences in choroidal thickness were then either roughly maintained (e.g., Figs. 5C, D, E) or decreased (Figs. 5F and G). As a consequence, the average (±SEM) choroidal thickness in the treated and control eyes of the DL-FD monkeys were not significantly different at the end of dim-light-diffuser period (diffuser-treated eyes vs. control eyes: 149.53 ± 48.28 μm vs. 150.76 ± 34.96 μm). The temporal pattern of the relative choroidal thickness changes in the DL-FD monkeys suggested that the relative choroidal thinning in the treated eyes was associated with the ocular responses that eventually led to FDM.
Figure 7.
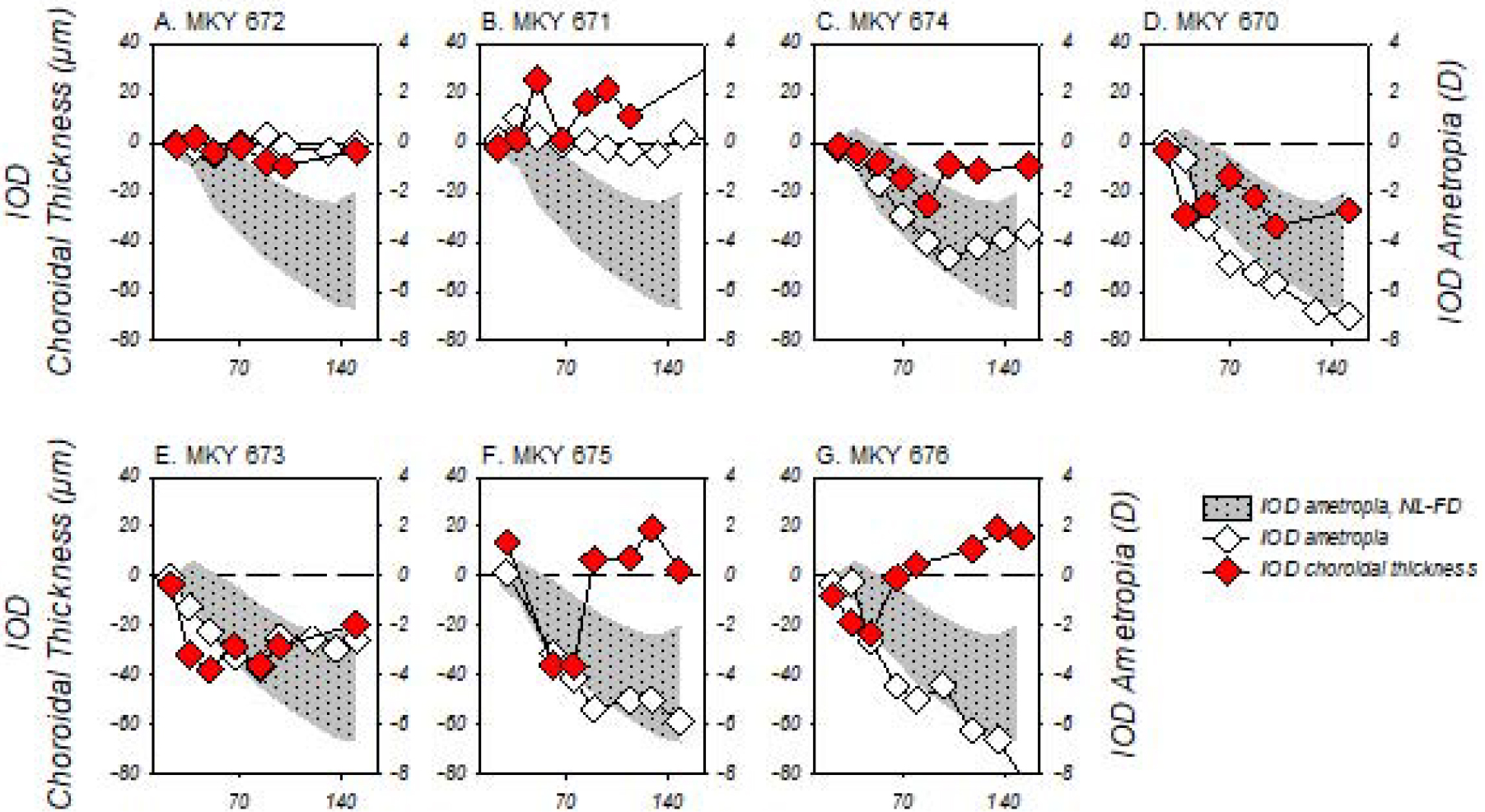
Interocular differences (IOD; treated eye – control eye) in sub-foveal choroidal thickness (red symbols, left ordinates) and refractive error (white symbols, right ordinates) obtained during the dim-light-diffuser periods plotted as a function of age for individual DL-FD subjects. The shaded area represents the 95% confidence range of anisometropias for the NL-FD monkeys during the diffuser-rearing period. The two ordinates are shifted with respect to each other so that the horizontal dashed lines represent zero IODs in refractive error and choroidal thickness.
3.4. Changes in refraction during the recovery period and their relationship with choroidal thickness
Several observations during the recovery period indicated that the dim-light regimen interfered with the normal regulation of the eye’s refractive state. First, the two subjects that did not develop FDM continued to show atypical refractive development after the removal of diffusers. Specifically, monkey 672 (Fig. 2A) remained isometropic, but became progressively more hyperopic in both eyes, i.e. both eyes failed to compensate for the increasing amounts of hyperopic defocus. For monkey 671 (Fig. 2B), the treated eye maintained the low degree of hyperopia that was observed at the end of the diffuser treatment period, but its control eye showed progressive myopic changes that resulted in an obvious hyperopic anisometropia, suggesting that the control eye was not responding appropriately to myopic defocus. Most obviously, for the DL-FD subjects that developed FDM, the anticipated recovery from myopic anisometropias was not consistent. Although the myopic shifts in the treated eyes of monkeys 673, 675, and 676 (Fig. 2E – 3G) stopped after the removal of diffusers, these eyes did not show the rapid hyperopic shift that was observed in monkeys 674 and 670 (Fig. 2C and 2D), thus the degree of their myopic anisometropias did not decrease over the recovery period.
In contrast to the DL-FD monkeys, NL-FD monkeys consistently recovered from FDM when unrestricted vision was restored. Figure 8 compares the recoveries from FDM under normal and dim ambient lightings. In panel A, the recovery data from every NL-FD monkey that had at least 1.5 D of myopic anisometropia at the end of diffuser rearing (filled symbols) are plotted as a function of age. In addition, recovery data from all monkeys previously reared under “normal light” with weaker diffusers (Smith III & Hung, 2000) or with LP diffusers combined with daily brief periods of unrestricted vision (Smith III et al., 2002) and that also exhibited at least 1.5 D of myopic anisometropia are also included (thin solid lines). These data show that the time course of recovery varies with the initial degree of FDM, but given sufficient time even animals with large myopic anisometropias exhibit substantial degrees of recovery (Qiao-Grider et al., 2004; Smith III et al., 2017). Specifically, as illustrated in panel C, which shows the initial degree of myopic anisometropia and that was observed after 156 ± 29 days of recovery for individual animals, all but 1 of the 23 diffuser-reared subjects that developed FDM (a NL-FD subject; yellow symbol in panels A and C) showed systematic reductions in myopic anisometropia following the onset of unrestricted vision. However, for the DL-FD monkeys, only two animals showed similar recovery patterns at rates that were comparable to those of the NL-FD monkeys (Fig. 8B, red symbols); the other three NL-FD monkeys failed to show any systematic reductions in the degree of myopic anisometropia (Fig. 8B, blue symbols), even though the degrees of their myopic anisometropias at the start of the recovery period were similar to those of the two DL-FD subjects that recovered and were within the range of anisometropias where recovery consistently occurred under normal lighting levels (Fig. 8C). A Fisher’s exact test found that dim-light-reared monkeys appeared to be less likely to exhibit a similar recovery pattern (two-sided Fisher’s exact p = 0.08). The average changes in myopic anisometropias in the DL-FD group were smaller than those in the NL-FD group (+0.44 ± 2.99D vs. +2.98 ± 1.53D, p = 0.046), despite the fact that the average length of recovery period was longer in the DL-FD group (181 ± 10 vs. 156 ± 29 days).
Figure 8.
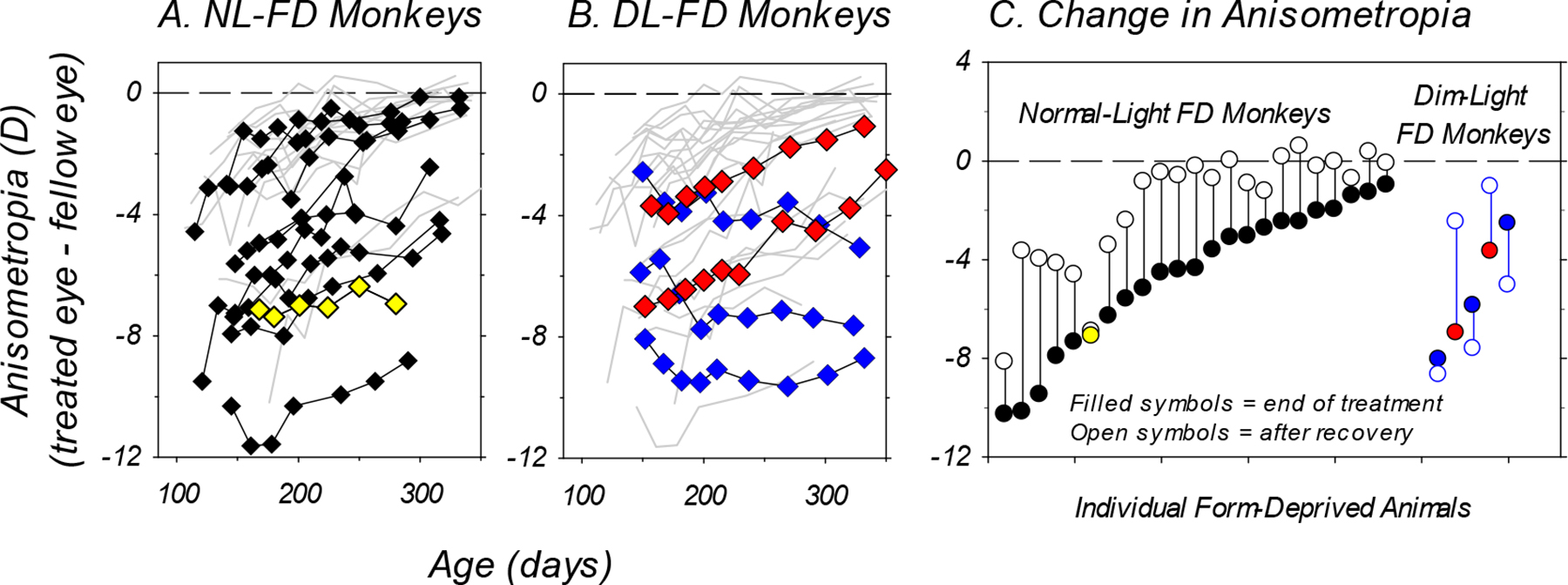
Panels A and B: Anisometropia plotted as a function of age during the recovery period for individual NL-FD (panel A, symbols, n = 10) and DL-FD monkeys (panel B, symbols, n = 5). In panel A, the thin grey lines represent the recovery from FDM for form-deprived monkeys reared with weaker diffusers (Smith III & Hung, 2000) or with intermittent normal vision (Smith III et al., 2002). In panel B, the thin grey lines represent the data from all diffuser-reared monkeys shown in panel A (n = 23). Only monkeys that exhibited at least 1.5 D of myopic anisometropia at the end of the diffuser-treatment period were included in the figure. The one NL-FD monkey that did not recover from FDM is highlighted in yellow (panel A), whereas the three DL-FD monkeys that did not recover are highlighted in blue (panel B). Panel C: Anisometropias obtained at the end of the diffuser treatment period (filled symbols) and at the end of the recovery period (open symbols) plotted for the individual form-deprived animals that are included in panels A and B.
The recovery from monocularly induced FDM in monkeys is normally correlated with interocular differences in vitreous chamber elongation rate (Smith III & Hung, 2000; Troilo & Nickla, 2005). As illustrated in the left column of plots in Figure 9, in which the developmental courses for the IODs in refractive error and vitreous chamber depth (treated eye – fellow control eye values) are plotted for individual DL-FD monkeys, the refractive changes that took place after removing the diffusers were associated with alterations in vitreous chamber elongation rate, and the resulting refractive outcomes were axial in nature. For example, the DL-FD monkey that remained isometropic (672, Fig. 9A) showed no systematic changes in the relative balance of the vitreous chamber depths in its two eyes. Monkey 671 (Fig. 9B), which developed a hyperopic anisometropia during the recovery period, concurrently exhibited a relatively shallower vitreous chamber in its treated eye (i.e., negative IODs). The reductions in the diffuser-induced axial myopic anisometropias in monkeys 674 and 670 were accompanied by synchronized reductions in the interocular differences in vitreous chamber depth (Figs. 9C and 9D). In contrast, the DL-FD monkeys that did not show systematic signs of recovery (monkeys 673, 675, and 676; Figs. 9E, F and G) also failed to show obvious changes or reductions in the interocular differences in vitreous chamber depth.
Figure 9.
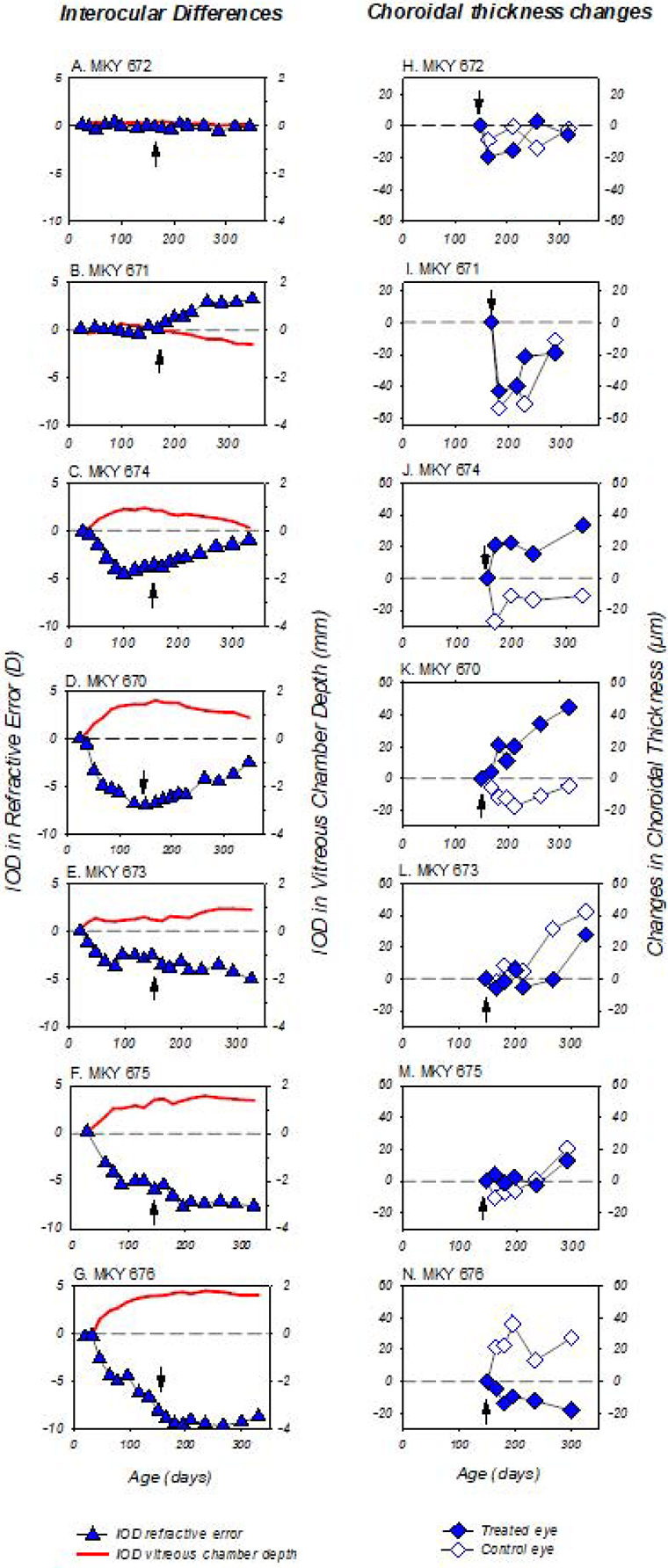
Panels A – G (left column): interocular differences (treated eye – fellow eye) in refractive error (blue symbols) and vitreous chamber depth (solid red lines) obtained during the dim-light-rearing period plotted as a function of age for individual DL-FD subjects. Dashed lines: zero anisometropia/interocular difference in refractive error and vitreous chamber depth. Black arrows: ages that correspond to the end of the diffuser treatment. Note the temporal correlation between the IODs in vitreous chamber and refractive error. Panels H – N (right column): changes in choroidal thickness in the treated (filled symbols) and control eyes (open symbols) of the DL-FD monkeys obtained during the recovery period plotted as a function of age. The first symbol in each plot represents data obtained at the age corresponding to the end of the diffuser-rearing period (i.e. black arrows in panels A – G). The choroidal thicknesses are specified relative to the thicknesses obtained at the end of the dim-light-diffuser period. Dashed lines: zero change in choroidal thickness (right column panels).
The right column of Figure 9 illustrates the changes in choroidal thickness during the recovery period for the treated and control eyes of DL-FD monkeys. For the monkeys that did not develop FDM (monkeys 672 and 671), the choroidal thickness changes were roughly symmetrical in their two eyes (Figs. 9H and 9I). For monkeys that developed FDM, relative choroidal thickening in the treated eye, which was expected in response to myopic defocus, occurred exclusively in the two animals that showed obvious signs of recovery. Specifically, the choroids in the treated eyes of monkeys 674 and 670 thickened (~20 μm) rapidly after the restoration of unrestricted vision, whereas the choroids of their control eyes thinned (Figs. 9J and 9K). These rapid initial relative changes in response to the onset of unrestricted vision, which agree well with the existing knowledge that myopic defocus causes an increase in choroidal thickness (Hung et al., 2000; Troilo et al., 2000; Wallman et al., 1995; Wildsoet & Wallman, 1995), were not observed in monkeys that did not show signs of recovery (673, 675, and 676, Figs. 9L, M and N).
4. Discussion
Our results showed that dim-light rearing increased the variability of control eye refractive development, but it did not change refractive development in the form-deprived eyes, thus had no significant effect on the degree of FDM and the underlying axial elongation. However, dim-light rearing interfered with the anticipated recovery from FDM and promoted the development of abnormal refractive errors during the recovery period in some fellow control eyes. The failure to recover from FDM was accompanied by the absence of defocus-related choroidal thickening in the treated eyes, suggesting that the response to existing optical defocus signals might be impaired under dim light.
4.1. Comparisons to previous studies
In monocularly form-deprived chicks, reduced ambient lighting levels similar to those employed in this study did not alter the course of FDM in the treated eyes or emmetropization in the fellow control eyes (Ashby et al., 2009). In the present study, dim-light rearing also did not prevent nor exacerbate the development of FDM in the treated eyes of DL-FD monkeys, despite the fact that the length of our daily exposure and the total length of our treatment regimen were both much longer than those in the Ashby et al. investigation. Together, these findings indicate that, in the absence of meaningful visual feedback, reduced ambient lighting does not enhance deprivation-induced myopia and thus does not appear to be myopiagenic, per se. Moreover, these results suggest that, in order for ambient lighting levels to influence the phenomenon of FDM, lighting levels must be above our dim lighting levels and above typical indoor lighting levels (Ashby et al., 2009; Chen et al., 2017; Karouta & Ashby, 2015; Siegwart Jr. et al., 2012; Smith III et al., 2012).
On the other hand, dim-light rearing altered the course of refractive development and increased the refractive variability in the fellow control eyes of our DL-FD monkeys. In particular, the fellow-eye myopic changes observed in three DL-FD monkeys that developed myopic anisometropia were very different not only from the relative hyperopic changes found in DL-control monkeys (She et al. 2020), but also from those observed in the fellow eyes of the NL-FD monkeys (Hung, Arumugam, She, et al., 2018; Smith III & Hung, 2000). It is possible that these relative myopic changes came about because dim light influenced the impact of potential interocular factors associated with monocular form deprivation, which have been reported in both chickens and monkeys reared under more typical ambient lighting levels (Bradley et al., 1999; Raviola & Wiesel, 1985; Schmid & Wildsoet, 1996; Smith et al., 1987; Smith III & Hung, 2000; Wildsoet & Wallman, 1995). Considering that none of our DL-control animals developed relative myopias, the myopic tendencies observed in the control eyes suggested that low intensity ambient lighting could be myopiagenic under certain conditions. For the DL-FD monkeys in particular, low ambient lighting was myopiagenic for the control eyes when the treated eye developed vision-induced myopia. Similar interocular effects were not observed in monocularly form-deprived chicks reared in dim ambient lighting, possibly because the length of the observation period was too short (Ashby et al., 2009).
The nature of the observed refractive errors is an important consideration for the discussion of mechanisms related to the refractive effects of ambient lighting levels. Although form-deprivation in typical laboratory lighting (Gottlieb, Fugate-Wentzek, & Wallman, 1987. Also see: Hayes et al., 1986) and in dim lighting (Cohen et al., 2011, 2012) has been reported to induce corneal curvature changes in chickens, neither Ashby et al.’s (Ashby et al., 2009) study of FDM in chickens (again possibly due to the short treatment duration) nor the present study observed systematic alterations in corneal powers. Specifically, in the present study, several observations (such as changes in ocular biometric parameters, correlation between refraction and the VC/CR ratio, and the relative choroidal thinning in the treated eyes) indicate that the FDM observed in the dim-light-reared monkeys maintained its widely conserved axial nature (chicks: Gottlieb, Joshi, and Nickla 1990; Hayes et al. 1986; Troilo et al. 1995; Wallman and Adams 1987; Wallman, Turkel, and Trachtman 1978; Mouse: Schaeffel et al. 2004; tree shrews: Norton and Rada 1995; guinea pigs: Howlett & McFadden, 2006; non-human primates: Smith III et al. 2000; Troilo and Nickla 2005) and was qualitatively similar to that observed under both typical indoor lighting levels (Smith III & Hung, 2000) and elevated ambient lighting levels (Smith III et al., 2012).
4.2. Possible explanations for the absence of effects on FDM
Light-intensity dependency of refractive error has been observed in chicks reared with form-deprivation (Karouta & Ashby, 2015) and unrestricted vision (Cohen et al., 2011, 2012), suggesting that light levels could be quantitatively translated into biological signals that affect eye growth. In this regard, retinal dopamine has been suggested to be a candidate molecule for mediating light intensity effects, although the exact mechanism is not well understood (Feldkaemper & Schaeffel, 2013; Norton & John T. Siegwart Jr., 2013; Zhou et al., 2017). Notably, Norton and Siegwart suggested that lighting levels might act as a continuous factor of influence for refractive error through light-induced changes in retinal dopamine levels (Norton & Siegwart, 2013). In comparison to the phenomena of lens compensation and possibly emmetropization, this hypothesis appears more reasonable for FDM, not only because the form-deprived eyes are unable to use the presenting refractive errors to control refractive development and ocular growth (Park et al., 2003), but also because retinal dopamine levels have been found to be inversely associated with the degree of myopic changes and axial elongation (Stone et al., 1989).
Although the speculation noted above predicts more FDM as dim light potentially reduces retinal dopamine levels, the previous investigation in chicks (Ashby et al., 2009) and our observations in infant monkeys both indicated otherwise. In addition, although Cohen et al., showed that long-term chronic exposure to dim light caused reduced retinal dopamine levels (and more myopia) in emmetropizing chicks (Cohen et al., 2012), there is no evidence that dim light could further reduce the retinal dopamine levels in form-deprived eyes. On the country, the adaptive (Dubocovich et al., 1985; Porceddu et al., 1987) and multifactorial retinal dopaminergic system (Brainard & Morgan, 1987; Cohen, Iuvone, & Neff, 1981; Hadjiconstantinou, Cohen, & Neff, 1983; Iuvone et al., 1978; Iuvone & Rauch, 1983; Marshburn & Iuvone, 1981; Proll, Kamp, & Morgan, 1982; Stone et al., 1989) might be able to compensate for the decreases in dopamine production induced by the relative drop in absolute ambient illuminance (~50 lux vs. ~500 lux), at least for eyes that are permitted unrestricted vision. In this regard, Landis et al. have shown that, whereas the level of 3,4-dihydroxyphenylacetic acid (DOPAC) in the retina showed light-intensity dependency, rearing with chronic exposures to reduced ambient lighting (0.005 lux and 50 lux) did not have significant effects on retinal dopamine levels in mice in comparison to higher ambient lighting (Landis et al., 2019). Based on the available evidences, the refractive effect of reduced ambient lighting levels does not appear to be mediated by quantitative changes in retinal dopamine levels.
It is also possible that dim light did not have a significant refractive effect because the ocular elongation responses in the treated eyes of the DL-FD monkeys were saturated. This rate limitation might arise, for example, from the active biochemical changes that take place in the sclera (scleral remodeling) (Gentle et al., 2003; McBrien et al., 1991, 2001) and/or from the resulting changes in scleral biomechanical properties (Phillips et al., 2000; Siegwart, Jr. & Norton, 1999). In addition, rate limitations could also occur because the visual trigger for FDM had reached the maximum myopiagenic strength when the deprivation paradigm was employed under either reduced or normal lighting levels. The myopiagenic visual trigger associated with form deprivation has been implied to be the degradation in image contrast (Bartmann & Schaeffel, 1994), of which the perception might be worse under dim light. Although some (Bartmann & Schaeffel, 1994; Smith III & Hung, 2000), but not all (Tran et al., 2008), studies suggested that FDM is a graded phenomenon, a limited operating range for such a “dose-response” relationship appears to exist. For example, Tran et al. showed that a quantitatively similar amount of FDM and axial elongation could be induced by diffusers with different nominal strengths (Tran et al., 2008). This possible operating range limitation suggests that dim light might not be able to exacerbate FDM by increasing the myopiagenic strength of certain form-deprivation paradigms. However, it also suggests that the potential myopiagenic effects associated with low lighting levels could become obvious if weaker diffusers are employed. In this respect, our findings cannot exclude the possibility that dim light is a risk factor of myopiagenesis.
Regardless of the underlying events, the results from both chickens and monkeys consistently suggest that ambient lighting levels only affect the course of FDM at lighting levels above those that associate with typical indoor environments.
4.3. Implications of observations made during the recovery period
As observed in other species (Howlett & McFadden, 2006; Siegwart & Norton, 1998; Wallman & Adams, 1987), rhesus monkeys reared under typical laboratory lighting consistently recovered from FDM (Qiao-Grider et al., 2004). On the contrary, only two of the DL-FD subjects that developed FDM exhibited signs of recovery, despite the fact that the progressive myopic changes in these subjects quickly stopped after the diffusers were removed. Considering that the FDMs in the DL-FD monkeys were primarily attributed to diffuser wear rather than dim-light rearing, the cessation of myopia progression after the onset of recovery period was probably not surprising. However, it is less clear whether the cessation of myopia progression was a result of diffuser withdrawal or was related to the presence of myopic defocus (Wildsoet & Schmid, 2000).
In this regard, our choroidal thickness measurement suggested that the absence of obvious signs of recovery might be associated with a failure to detect and/or respond to the presence of the myopic errors. At typical laboratory lighting levels, increases in choroidal thickness have been observed in chicks (Wallman et al., 1995), guinea pigs (Howlett & McFadden, 2006), tree shrews (Siegwart Jr. & Norton, 1998) and non-human primates (Hung et al., 2000; Troilo et al., 2000) recovering from induced myopic ametropias. In the current study, we found that relative increases in choroidal thickness were observed only in the treated eyes of monkeys that showed signs of recovery, whereas monkeys that did not recover showed qualitatively different choroidal thickness change patterns. Because the recovery from FDM under typical ambient lighting has been shown to be driven by the myopic defocus in the treated eyes (Schaeffel & Howland, 1991; Wildsoet & Schmid, 2000), the absence of appropriate choroidal thickness alterations suggests that the treated eyes of these DL-FD monkeys might have failed to detect and/or process the myopic defocus.
The manner in which dim light interfered with the recovery from FDM and the abnormal refractive errors observed in some animals during the recovery period were somewhat analogous to the failure of emmetropization observed in DL-control monkeys (She et al., 2020). In both cases, the probability that alterations in ocular growth would compensate for an existing refractive-error signal was decreased. Although we had previously speculated that some DL-control monkeys were unable to emmetropize because the hyperopic errors were not sufficiently strong to trigger and/or maintain emmetropization (She et al., 2020), the absence of consistent recovery responses in monkeys that experienced large and sustained degrees of FDM suggests that the functional state of the emmetropization process was impaired. In support of this view, the two DL-FD monkeys that did not initially develop FDM also exhibited abnormal refractive development after the removal of the diffuser lenses, suggesting that the emmetropization process did not respond to the presenting defocus signals. Together, these observations indicated that low ambient lighting levels could be a potential risk factor for refractive anomalies in primates (She et al., 2020).
4.4. Conclusions and implications
The observations that dim-light rearing did not affect the time course or degree of FDM in rhesus monkeys and chicks suggests that low intensity ambient lighting, by itself, is not a strong environmental enhancer of visually induced myopia. In view of our previous observations in DL-control monkeys (She et al., 2020), the inability of dim light to alter the unregulated, “intrinsic” ocular growth rate that underlies FDM suggests that dim light primarily affects the mechanisms that operate to eliminate defocus errors. The reduced probability to recover from FDM and the absence of relative choroidal thickening that normally is produced by myopic defocus provide support for this speculation. Due to our small sample size and the variabilities in the final degree of FDM and its recovery, further studies on the compensating responses to imposed defocus are required to confirm the association between dim light and possible defects in the mechanisms that process defocus signals.
Acknowledgement
This work was supported by National Institutes of Health Grants EY-03611 and EY-07551, funds from the Brien Holden Vision Institute, and the University of Houston Foundation.
The authors would like to thank Diana Tran for her work in research data management, and Dr. Nimesh Patel for the development and maintenance of the Matlab program used in choroidal thickness analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: E. L. Smith III, (p) patents on optical and pharmaceutical treatment strategies for myopia, (C) consultant to Nevakar, SightGlass Vision, Treehouse Eyes, Acucela Inc. and Essilor of America; L.-F. Hung, None; B. Arumugam, None; Z. She, None; K. M. Beach, None.
5. Reference
- Ashby RS, Ohlendorf A, & Schaeffel F (2009). The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology and Visual Science, 50(11), 5348–5354. 10.1167/iovs.09-3419 [DOI] [PubMed] [Google Scholar]
- Ashby RS, & Schaeffel F (2010). The effect of bright light on lens compensation in Chicks. Investigative Ophthalmology and Visual Science, 51(10), 5247–5253. 10.1167/iovs.09-4689 [DOI] [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EPH, & Beuerman RW (2008). Two models of experimental myopia in the mouse. Vision Research. 10.1016/j.visres.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Bartmann M, & Schaeffel F (1994). A simple mechanism for emmetropization without cues from accommodation or colour. Vision Research. 10.1016/0042-6989(94)90037-X [DOI] [PubMed] [Google Scholar]
- Bercovitz AB, Harrison PC, & Leary GA (1972). Light induced alterations in growth pattern of the avian eye. Vision Research, 12(7), 1253–1259. 10.1016/0042-6989(72)90196-4 [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, & Boothe RG (1999). Emmetropization in the rhesus monkey (Macaca mulatta): Birth to young adulthood. Investigative Ophthalmology and Visual Science. [PubMed] [Google Scholar]
- Brainard GC, & Morgan WW (1987). Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Research, 424(1), 199–203. 10.1016/0006-8993(87)91211-X [DOI] [PubMed] [Google Scholar]
- Byrne SF, & Green RL (2002). Ultrasound of the Eye and Orbit. 2nd Edition. St.Louis, Mo.; London:Mosby. [Google Scholar]
- Chen S, Zhi Z, Ruan Q, Liu Q, Li F, Wan F, Reinach PS, Chen J, Qu J, & Zhou X (2017). Bright light suppresses form-deprivation myopia development with activation of dopamine d1 receptor signaling in the ON pathway in retina. Investigative Ophthalmology and Visual Science. 10.1167/iovs.16-20402 [DOI] [PubMed] [Google Scholar]
- Cohen J, Iuvone MP, & Neff NH (1981). Neuroleptic drugs activate tyrosine hydroxylase of retinal amacrine cells. Journal of Pharmacology and Experimental Therapeutics. [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, & Polat U (2011). Dependency between light intensity and refractive development under light-dark cycles. Experimental Eye Research, 92(1), 40–46. 10.1016/j.exer.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, & Solomon AS (2012). Ambient illuminance, retinal dopamine release and refractive development in chicks. Experimental Eye Research, 103, 33–40. 10.1016/j.exer.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Dirani M, Tong L, Gazzard G, Zhang X, Chia A, Young TL, Rose KA, Mitchell P, & Saw SM (2009). Outdoor activity and myopia in Singapore teenage children. British Journal of Ophthalmology. 10.1136/bjo.2008.150979 [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Lucas RC, & Takahashi JS (1985). Light-dependent regulation of dopamine receptors in mammalian retina. Brain Research, 335(2), 321–325. 10.1016/0006-8993(85)90485-8 [DOI] [PubMed] [Google Scholar]
- Feldkaemper M, & Schaeffel F (2013). An updated view on the role of dopamine in myopia. Experimental Eye Research, 114, 106–119. 10.1016/j.exer.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Flitcroft DI (2013). Is myopia a failure of homeostasis? Experimental Eye Research, 114, 16–24. 10.1016/j.exer.2013.02.008 [DOI] [PubMed] [Google Scholar]
- French AN, Ashby RS, Morgan IG, & Rose KA (2013). Time outdoors and the prevention of myopia. Experimental Eye Research, 114, 58–68. 10.1016/j.exer.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Gentle A, Liu Y, Martin JE, Conti GL, & McBrien NA (2003). Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. Journal of Biological Chemistry. 10.1074/jbc.M300970200 [DOI] [PubMed] [Google Scholar]
- Gottlieb MD, Marran L, Xu A, Nickla DL, & Wallman J (1991). The emmetropization process in chicks is compromised by dim light. Investigative Ophthalmology and Visual Science, 32, 1203. [Google Scholar]
- Gottlieb Michael D., Fugate-Wentzek LA, & Wallman J (1987). Different visual deprivations produce different ametropias and different eye shapes. Investigative Ophthalmology and Visual Science, 28(8), 1225–1235. [PubMed] [Google Scholar]
- Gottlieb Michael D., Joshi HB, & Nickla DL (1990). Scleral changes in chicks with form-deprivation myopia. Current Eye Research. 10.3109/02713689009003472 [DOI] [PubMed] [Google Scholar]
- Graham B, & Judge SJ (1999). Normal development of refractive state and ocular component dimensions in the marmoset (Callithrix jacchus). Vision Research, 39(2), 177–187. 10.1016/S0042-6989(98)00188-6 [DOI] [PubMed] [Google Scholar]
- Guggenheim JA, Northstone K, McMahon G, Ness AR, Deere K, Mattocks C, St. Pourcain B, & Williams C (2012). Time outdoors and physical activity as predictors of incident myopia in childhood: A prospective cohort study. Investigative Ophthalmology and Visual Science. 10.1167/iovs.11-9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiconstantinou M, Cohen J, & Neff NH (1983). Epinephrine: A Potential Neurotransmitter in Retina. Journal of Neurochemistry. 10.1111/j.1471-4159.1983.tb00843.x [DOI] [PubMed] [Google Scholar]
- Hayes BP, Fitzke FW, Hodos W, & Holden AL (1986). A morphological analysis of experimental myopia in young chickens. Investigative Ophthalmology and Visual Science, 27(6), 981–991. [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, & Resnikoff S (2016). Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology, 123(5), 1036–1042. 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2006). Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Research, 46(1–2), 267–283. 10.1016/j.visres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2009). Spectacle lens compensation in the pigmented guinea pig. Vision Research. 10.1016/j.visres.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, Ostrin L, Patel N, Trier K, Jong M, & Smith III EL (2018). The adenosine receptor antagonist, 7-methylxanthine, alters emmetropizing responses in infant macaques. Investigative Ophthalmology and Visual Science, 59(1), 472–486. 10.1167/iovs.17-22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Arumugam B, She Z, Ostrin L, Smith EL, Smith III EL, & Smith EL (2018). Narrow-band, long-wavelength lighting promotes hyperopia and retards vision-induced myopia in infant rhesus monkeys. Experimental Eye Research, 176(June 2018), 147–160. 10.1016/j.exer.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L-F, Crawford MLJ, & Smith III EL (1995). Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine, 1(8), 765. 10.1038/nm0895-761 [DOI] [PubMed] [Google Scholar]
- Hung L-F, Wallman J, & Smith EL (2000). Vision-dependent changes in the choroidal thickness of macaque monkeys. Investigative Ophthalmology and Visual Science. [PubMed] [Google Scholar]
- Iuvone MP, Galli CL, Garrison-Gund CK, & Neff NH (1978). Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science, 202(4370), 901–902. 10.1126/science.30997 [DOI] [PubMed] [Google Scholar]
- Iuvone MP, & Rauch AL (1983). Alpha2-adrenergic receptors influence tyrosine hydroxylase activity in retinal dopamine neurons. Life Sciences. 10.1016/0024-3205(83)90640-9 [DOI] [PubMed] [Google Scholar]
- Jones LA, Sinnott LT, Cotter SA, Kleinstein RN, Manny RE, Mutti DO, Daniel Twelker J, & Zadnik K (2012). Time outdoors, visual activity, and myopia progression in juvenile-onset myopes. Investigative Ophthalmology and Visual Science. 10.1167/iovs.11-8336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karouta C, & Ashby RS (2015). Correlation between light levels and the development of deprivation myopia. Investigative Ophthalmology and Visual Science, 56(1), 299–309. 10.1167/iovs.14-15499 [DOI] [PubMed] [Google Scholar]
- Kee CS, Hung L-F, Qiao Y, Habib A, & Smith III EL (2002). Prevalence of astigmatism in infant monkeys. Vision Research, 42(11), 1349–1359. 10.1016/S0042-6989(02)00060-3 [DOI] [PubMed] [Google Scholar]
- Landis EG, Park H. na, Chrenek M, Sidhu C, He L, Strickland R, Iuvone MP, & Pardue MT (2019). Light exposure history alters dopamine activity in the retina. Investigative Ophthalmology & Vision Science, 60(9), 3152. [Google Scholar]
- Lauber JK, & Kinner A (1979). Eye enlargement in birds induced by dim light. Canadian Journal of Ophthalmology, 14(4), 265–269. http://europepmc.org/abstract/MED/550921 [PubMed] [Google Scholar]
- Marshburn PB, & Iuvone MP (1981). The role of GABA in the regulation of the dopamine/tyrosine hydroxylase-containing neurons of the rat retina. Brain Research 10.1016/0006-8993(81)91198-7 [DOI] [PubMed] [Google Scholar]
- McBrien NA, Cornell LM, & Gentle A (2001). Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investigative Ophthalmology and Visual Science, 42(10), 2179–2187. [PubMed] [Google Scholar]
- McBrien NA, Moghaddam HO, Reeder AP, & Moules S (1991). Structural and biochemical changes in the sclera of experimentally myopic eyes. Biochem Soc Trans, 19(4), 861–865. 10.1042/bst0190861 [DOI] [PubMed] [Google Scholar]
- Norton TT, & Siegwart John T. Jr. (2013). Light Levels, Refractive Development, and Myopia – a Speculative Review. Experimental Eye Research, 114(205), 48–57. 10.1016/j.exer.2013.05.004.Light [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT, & McBrien NA (1992). Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Research. 10.1016/0042-6989(92)90026-F [DOI] [PubMed] [Google Scholar]
- Norton TT, & Rada JA (1995). Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Research, 35(9), 1271–1281. 10.1016/0042-6989(94)00243-F [DOI] [PubMed] [Google Scholar]
- Park TW, Winawer J, & Wallman J (2003). Further evidence that chick eyes use the sign of blur in spectacle lens compensation. Vision Research, 43(14), 1519–1531. 10.1016/S0042-6989(03)00180-9 [DOI] [PubMed] [Google Scholar]
- Patel NB, Hung L-F, & Harwerth RS (2017). Postnatal maturation of the fovea in Macaca mulatta using optical coherence tomography. Experimental Eye Research, 164, 8–21. 10.1016/j.exer.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez GM, Archer SM, & Artal P (2010). Optical characterization of bangerter foils. Investigative Ophthalmology and Visual Science, 51(1), 609–613. 10.1167/iovs.09-3726 [DOI] [PubMed] [Google Scholar]
- Phillips JR, Khalaj M, & McBrien NA (2000). Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Investigative Ophthalmology and Visual Science. [PubMed] [Google Scholar]
- Porceddu ML, De Montis G, Mele S, Ongini E, Biggio G, De Montis G, Mele S, Ongini E, Biggio G, Porceddu ML, De Montis G, Mele S, Ongini E, & Biggio G (1987). D1 dopamine receptors in the rat retina: effect of dark adaptation and chronic blockade by SCH 23390. Brain Research, 424(2), 264–271. 10.1016/0006-8993(87)91470-3 [DOI] [PubMed] [Google Scholar]
- Proll MA, Kamp CW, & Morgan WW (1982). Use of liquid chromatography with electrochemistry to measure effects of varying intensities of white light on DOPA accumulation in rat retinas. Life Sciences, 30(1), 11–19. 10.1016/0024-3205(82)90630-0 [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C, Ramamirtham R, & Smith III EL (2004). Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology and Visual Science, 45(10), 3361–3372. 10.1167/iovs.04-0080 [DOI] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung L-F, Kee C, Ramamirtham R, & Smith III EL (2007). Normal ocular development in young rhesus monkeys (Macaca mulatta). Vision Research, 47(11), 1424–1444. 10.1016/j.visres.2007.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, & Skrondal A (2012). Multilevel and Longitudinal Modeling Using Stata vol. II: Categorical Responses, Counts, and Survival. In Stata Press. [Google Scholar]
- Raviola E, & Wiesel TN (1985). An Animal Model of Myopia. New England Journal of Medicine, 312(25), 1609–1615. 10.1056/NEJM198506203122505 [DOI] [PubMed] [Google Scholar]
- Rose KA, Morgan IG, Ip JM, Kifley A, Huynh S, Smith W, & Mitchell P (2008). Outdoor Activity Reduces the Prevalence of Myopia in Children. Ophthalmology. 10.1016/j.ophtha.2007.12.019 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, Burkhardt E, Howland HC, & Williams RW (2004). Measurement of Refractive State and Deprivation Myopia in Two Strains of Mice. Optometry and Vision Science. 10.1097/00006324-200402000-00008 [DOI] [PubMed] [Google Scholar]
- Schaeffel F, & Howland HC (1991). Properties of the feedback loops controlling eye growth and refractive state in the chicken. Vision Research, 31(4), 717–734. 10.1016/0042-6989(91)90011-S [DOI] [PubMed] [Google Scholar]
- Schmid KL, & Wildsoet CF (1996). Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research, 36(7), 1023–1036. 10.1016/0042-6989(95)00191-3 [DOI] [PubMed] [Google Scholar]
- She Z, Hung LF, Arumugam B, Beach KM, Smith III EL, & Smith EL (2020). Effects of low intensity ambient lighting on refractive development in infant rhesus monkeys (Macaca mulatta). Vision Research, 176(July), 48–59. 10.1016/j.visres.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (1999). Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Research, 39(2), 387–407. 10.1016/S0042-6989(98)00150-3 [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (1998). The susceptible period for deprivation-induced myopia in tree shrew. Vision Research. 10.1016/S0042-6989(98)00053-4 [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr., Ward AH, & Norton TT (2012). Moderately Elevated Fluorescent Light Levels Slow Form Deprivation and Minus Lens-Induced Myopia Development in Tree Shrews. Investigative Ophthalmology & Visual Science, 53(14), 3457. [Google Scholar]
- Smith EL, Harwerth RS, Crawford MLJ, & Von Noorden GK (1987). Observations on the effects of form deprivation on the refractive status of the monkey. Investigative Ophthalmology and Visual Science, 28(8), 1236–1245. [PubMed] [Google Scholar]
- Smith EL III, & Hung L-F (2000). Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research, 40(4), 371–381. 10.1016/S0042-6989(99)00184-4 [DOI] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Arumugam B, Holden BA, Neitz M, Neitz J, Smith EL, Hung LF, Arumugam B, Holden BA, Neitz M, & Neitz J (2015). Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Investigative Ophthalmology and Visual Science, 56(11), 6490–6500. 10.1167/iovs.15-17025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Arumugam B, & Huang J (2013). Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Investigative Ophthalmology and Visual Science, 54(4), 2959–2969. 10.1167/iovs.13-11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Arumugam B, Wensveen JM, Chino YM, & Harwerth RS (2017). Observations on the relationship between anisometropia, amblyopia and strabismus. Vision Research, 134, 26–42. 10.1016/j.visres.2017.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, & Harwerth RS (1999). Developmental visual system anomalies and the limits of emmetropization. Ophthalmic and Physiological Optics, 19(2), 90–102. 10.1016/S0275-5408(98)00070-2 [DOI] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, & Huang J (2012). Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology and Visual Science, 53(1), 421–428. 10.1167/iovs.11-8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Huang J, Blasdel TL, Humbird TL, & Bockhorst KH (2010). Effects of optical defocus on refractive development in monkeys: Evidence for local, regionally selective mechanisms. Investigative Ophthalmology and Visual Science, 51(8), 3864–3873. 10.1167/iovs.09-4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Kee C-S, Qiao-Grider Y, & Ramamirtham R (2003). Continuous ambient lighting and lens compensation in infant monkeys. Optometry and Vision Science, 80(5), 374–382. 10.1097/00006324-200305000-00012 [DOI] [PubMed] [Google Scholar]
- Smith EL III, Hung L-F, Kee CS, & Qiao Y (2002). Effects of brief periods of unrestricted vision on the development of form-deprivation myopia in monkeys. Investigative Ophthalmology and Visual Science, 43(2), 291–299. [PubMed] [Google Scholar]
- Stone RA, Lin T, Laties AM, & Iuvone MP (1989). Retinal dopamine and form-deprivation myopia. Proceedings of the National Academy of Sciences of the United States of America, 86(2), 704–706. 10.1073/pnas.86.2.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N, Chiu S, Tian Y, & Wildsoet CF (2008). The significance of retinal image contrast and spatial frequency composition for eye growth modulation in young chicks. Vision Research, 48(15), 1655–1662. 10.1016/j.visres.2008.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, & Judge SJ (1993). Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vision Research, 33(10), 1311–1324. 10.1016/0042-6989(93)90039-Y [DOI] [PubMed] [Google Scholar]
- Troilo D, Li T, Glasser A, & Howland HC (1995). Differences in eye growth and the response to visual deprivation in different strains of chicken. Vision Research. 10.1016/0042-6989(94)00230-J [DOI] [PubMed] [Google Scholar]
- Troilo D, & Nickla DL (2005). The response to visual form deprivation differs with age in marmosets. Investigative Ophthalmology and Visual Science, 46(6), 1873–1881. 10.1167/iovs.04-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, & Wildsoet CF (2000). Choroidal thickness changes during altered eye growth and refractive state a primate. Investigative Ophthalmology and Visual Science. [PubMed] [Google Scholar]
- Troilo D, Smith EL, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA, Gawne TJ, Pardue MT, Summers JA, Kee CS, Schroedl F, Wahl S, & Jones L (2019). IMI – Report on experimental models of emmetropization and myopia. Investigative Ophthalmology and Visual Science, 60(3), M31–M88. 10.1167/iovs.18-25967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, & Wallman J (1987). Changes in corneal curvature during accommodation in chicks. Vision Research. 10.1016/0042-6989(87)90186-6 [DOI] [PubMed] [Google Scholar]
- Wallman J, Adams JI, & Trachtman JN (1981). The eyes of young chickens grow toward emmetropia. Investigative Ophthalmology and Visual Science. [PubMed] [Google Scholar]
- Wallman J, & I. Adams J (1987). Developmental aspects of experimental myopia in chicks: Susceptibility, recovery and relation to emmetropization. Vision Research. 10.1016/0042-6989(87)90027-7 [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J, & Trachtman J (1978). Extreme myopia produced by modest change in early visual experience. Science, 201(4362), 1249–1251. 10.1126/science.694514 [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, & Christensen AM (1995). Moving the retina: Choroidal modulation of refractive state. Vision Research, 35(1), 37–50. 10.1016/0042-6989(94)E0049-Q [DOI] [PubMed] [Google Scholar]
- Wallman J, & Winawer J (2004). Homeostasis of eye growth and the question of myopia. Neuron, 43(4), 447–468. 10.1016/j.neuron.2004.08.008 [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, & Schmid KL (2000). Optical correction of form deprivation myopia inhibits refractive recovery in chick eyes with intact or sectioned optic nerves. Vision Research. 10.1016/S0042-6989(00)00138-3 [DOI] [PubMed] [Google Scholar]
- Wildsoet C, & Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research, 35(9), 1175–1194. 10.1016/0042-6989(94)00233-C [DOI] [PubMed] [Google Scholar]
- Wu PC, Tsai CL, Wu HL, Yang YH, & Kuo HK (2013). Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 10.1016/j.ophtha.2012.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone MP, & Qu J (2017). Dopamine signaling and myopia development: What are the key challenges. In Prog Retin Eye Res. 10.1016/j.preteyeres.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Qu J, Xie R, Wang R, Jiang L, Zhao H, Wen J, & Lu F (2006). Normal development of refractive state and ocular dimensions in guinea pigs. Vision Research. 10.1016/j.visres.2006.01.027 [DOI] [PubMed] [Google Scholar]


