Abstract
A significant proportion of individuals with attention-deficit/hyperactivity disorder (ADHD) show persistence into adulthood. The genetic and neural correlates of ADHD in adolescents versus adults remain poorly characterized. We investigated ADHD polygenic risk score (PRS) in relation to previously identified gray matter (GM) patterns, neurocognitive, and symptom findings in the same ADHD sample (462 adolescents & 422 adults from the NeuroIMAGE and IMpACT cohorts). Significant effects of ADHD PRS were found on hyperactivity and impulsivity symptoms in adolescents, hyperactivity symptom in adults, but not GM volume components. A distinct PRS effect between adolescents and adults on individual ADHD symptoms is suggested.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood-onset neuropsychiatric disorder characterized by inattention and/or hyperactivity-impulsivity (American Psychiatric Association, 2013). The disorder is associated with alterations of brain structure and function mostly found in the caudate nucleus, right globus palidus and putamen, fronto-striatal-parietal pathway, and cerebellum (Dickstein, Bannon, Castellanos, & Milham, 2006; Faraone et al., 2005; Frodl & Skokauskas, 2012; Halperin & Schulz, 2006; Hoogman et al., 2017; Nakao, Radua, Rubia, & Mataix-Cols, 2011; Polanczyk & Rohde, 2007; Valera, Faraone, Murray, & Seidman, 2007). ADHD is also often marked by impairments in cognitive functioning; including deficits in working memory, inhibitory control, and cognitive flexibility (Alderson, Kasper, Hudec, & Patros, 2013; Lijffijt, Kenemans, Verbaten, & van Engeland, 2005; Martinussen, Hayden, Hogg-Johnson, & Tannock, 2005; Tarver, Daley, & Sayal, 2014). The persistence rate of ADHD from childhood into adulthood is estimated between 15 to 60%, depending on the definition of persistence (Chandra, Biederman, & Faraone, 2016).
Symptom profiles, neuroanatomical features, and cognitive deficits also appear to differ between children and adults with ADHD. In children, hyperactivity is the more common presentation, whereas inattention, restlessness, and working memory deficits are more common in adulthood (Agnew-Blais et al., 2016). In addition, previous literature has shown different neuroanatomical features between the age groups with adolescents showing more significant alterations in the bilateral Crus I, insula, caudate, thalamus, and middle occipital gyrus, adults showing more significant alterations in the middle frontal gyrus (Duan et al., 2018; Jiang et al., 2019), and children (age 4–9 years) having the greatest reduction in cortical surface area among all the age groups (Hoogman et al., 2019).
ADHD is considered among the most heritable psychiatric disorders with a heritability percentage estimate of 76% (Biederman, Faraone, Keenan, Knee, & Tsuang, 1990; Wolfers et al., 2016). Twelve independent loci on 11 different chromosomes were identified as surpassing genome-wide significance to carry the risk to ADHD (Demontis et al., 2019). However, only a small percentage of heritability was accounted for, indicating a need for further investigation into the common variants of ADHD (Demontis et al., 2019).
Given the differing symptom profiles, neuroanatomical features, and cognitive deficits, examination of the genetic underpinnings of adult ADHD is needed. We aimed to investigate the differences in genetic effects between adolescents and adults with ADHD. Specifically, we investigated how ADHD polygenic risks scores (PRS) based on a genome wide association children and adult study from the Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH; https://ipsych.au.dk/downloads/) may influence brain structures and symptoms in ADHD that has persisted into adulthood, and how these genetic effects differ from those in adolescence (Duan et al., 2018; Jiang et al., 2019).
Methods
2.1. Participants
This study included adolescents and adults with ADHD, siblings of individuals with ADHD, and unrelated healthy controls (462 adolescent participants from the NeuroIMAGE cohort, 278 adult participants from the NeuroIMAGE cohort, and 144 adult participants from the Dutch IMpACT consortium). The NeuroIMAGE projects included relatives of both the adolescent participants and the adult participants, while the IMpACT cohort were unrelated. Participant breakdown and demographics are further explained in Supplemental Appendix 1. Participant recruitment, consent process, and enrollment are detailed in the original studies (Mostert et al., 2015; Onnink et al., 2016; von Rhein et al., 2015).
2.2. Clinical and Neurocognitive measures
In brief, individuals with ADHD were included if they met the DSM-IV (NeuroIMAGE project) (American Psychiatric Association, 1994) or DSM-IV-TR (IMpACT consortium) (American Psychiatric Association, 2000) criteria for ADHD. Two symptom domains, inattention and hyperactivity/impulsivity, were evaluated between the two cohorts based on the 18 DSM-IV symptom questions (American Psychiatric Association, 1994). The symptom scores for both domains ranged from 0 to 9, with larger scores indicating more severe symptoms (Duan et al., 2018; Noordermeer et al., 2017). To examine working memory capacity, the WAIS Digit Span test (Wechsler, 2000) with maximum forward and backward scores was assessed in both NeuroIMAGE and IMpACT participants. Further assessment information is detailed in Supplemental Appendix 1.
2.3. Neuroimaging
T1-weighted images were acquired from three 1.5T scanners (Amsterdam using Siemens SONATA and Siemens AVANTO, and Nijmegen using Siemens SONATA). The imaging preprocessing procedure was the same as in previous studies and is further detailed in Supplemental Appendix 2. In brief, the Jacobian-scaled modulated images were regressed for age, sex, and site prior to analyses.
2.4. Structural brain decomposition
The preprocessed images went through component estimation using the minimum description length algorithm (Rissanen, 1978). Twenty distinct gray matter (GM) components were computed by the infomax algorithm (Bell & Sejnowski, 1995) ICA (Xu, Groth, Pearlson, Schretlen, & Calhoun, 2009) within the GIFT toolbox (http://mialab.mrn.org/software/gift). ICASSO (Himberg, Hyvarinen, & Esposito, 2004) with 10 ICA runs was used to ensure the stability of components. Detailed information about the GM brain components identified in the previous studies is described in Supplemental Appendix 3.
2.5. Genetic data and PRS Construction
We used PRSice-2 (https://www.prsice.info/) for PRS calculations (Choi & O’Reilly, 2019). Detailed information of genetic data and preprocessing is further described in Supplemental Appendix 4. The Lundbeck Foundation Initiative for Integrative Psychiatric Research (iPSYCH; https://ipsych.au.dk/downloads/) child and adult ADHD summary statistics were used as the base file, and the preprocessed genetic data were used as the target file for adolescent and adult samples.
2.6. Association analyses of PRS, structural brain components, and behavior data
Our previous research identified GM components, which were greater in controls than individuals with ADHD (Duan et al., 2018; Jiang et al., 2019). The association between PRS and those GM components that showed differences between cases and controls, symptom score, or neurocognitive differences were analyzed in separate linear mixed models (LMM). In the LMMs, the GM component was the dependent variable. Age, diagnosis, medication use (yes/no), and PRS were included as fixed effect with family as a random effect on the intercept. The quadratic effect of age2 (testing possible non-linear age effects) was added into the fixed effect for adolescents only.
The associations between PRS and symptom score and neurocognitive data were also tested with similar LMMs. The individual ADHD symptoms of hyperactivity and inattention, and the working memory assessments of WAIS digital span forward and backward, were included in four separate LMMs as dependent variables. Again, age, sex, medication, and PRS were included as fixed effect with family as a random effect on the intercept. Significance corrections for multiple comparisons were done using false discovery rate (FDR) correction (p < 0.05) (Genovese, Lazar, & Nichols, 2002).
Results
Detailed demographic information can be found in Supplementary Tables 1 and 2 for adolescents and adults, respectively. In the adolescent sample, the p-value threshold to compute PRS was 0.0025 with 3% of the case vs. control variance explained (p = 1.29E–04) (Figure 1a). Using this threshold, a total of 1,789 SNPs were included in the PRS model. In the adult sample, the p-value threshold to compute PRS was 0.065 with 5.6% of the case vs. control variance explained (p = 2.94E–04). A total of 15,908 SNPs were included in this model (Figure 1b).
Figure 1. Polygenic Risk Model Estimation.
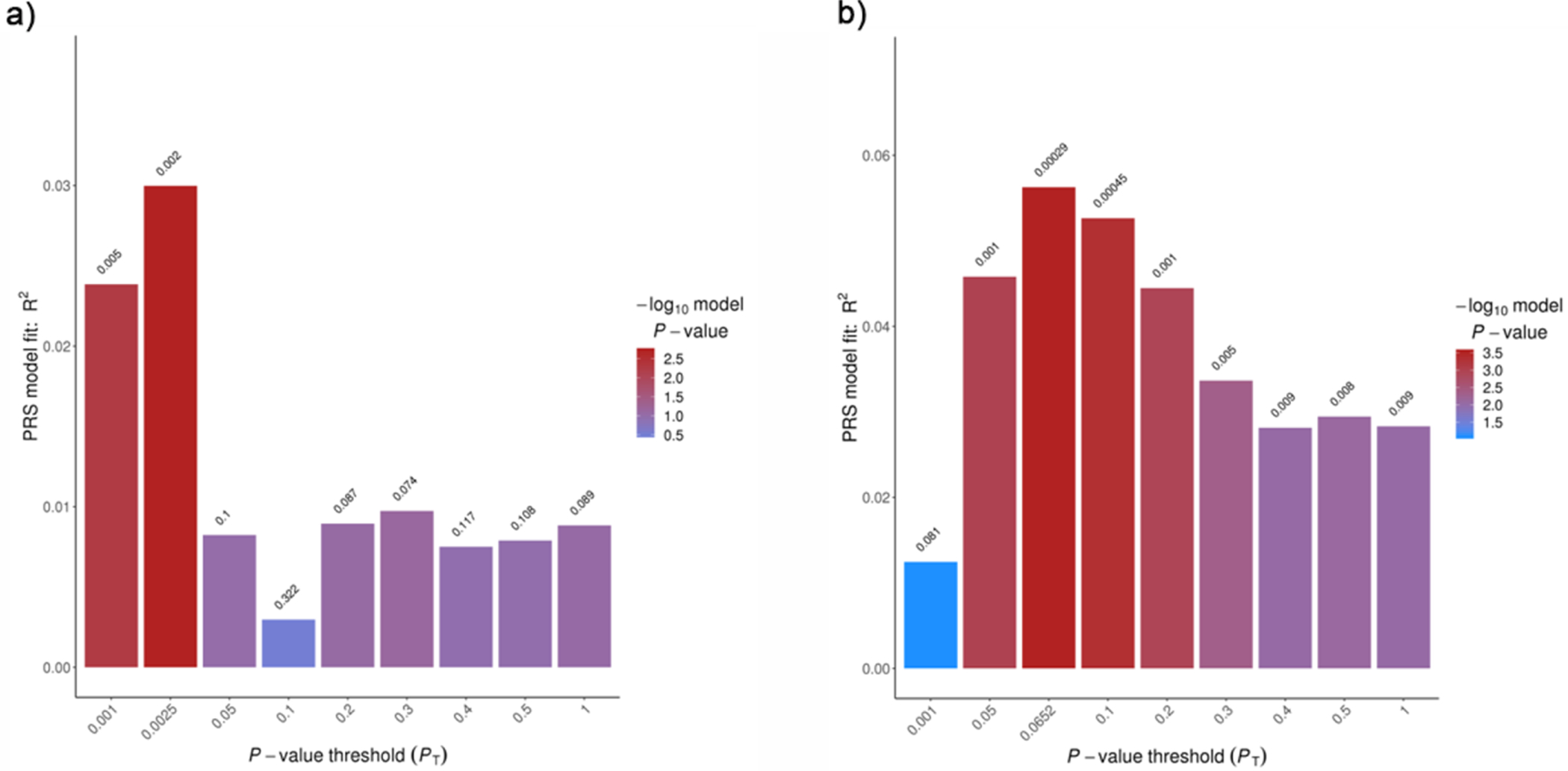
a) The polygenic risk model estimation based on iPSYCH data and case/control phenotypes in adolescent sample. The risk scores set at P value threshold of 0.0025 were included in the following analyses. b) The polygenic risk model estimation based on iPSYCH data and case/control phenotypes in adult sample. The risk scores set at P value threshold of 0.0652 were included in the following analyses.
In adolescents, there were no significant associations between PRS and any of the GM components previously reported (Supplemental Appendix 5). In adolescents, PRS were positively related to hyperactivity scores (β = 0.10, p = 7.52E4 (FDR corrected)) and inattention scores (β = 0.09, p = 0.02 (FDR corrected)) after controlling for age, sex, and medication. In adults, PRS were positively related to hyperactivity scores (β = 0.19, p = 3.58E–03 (FDR corrected)) while controlling for age, sex, and medication, but not inattention scores (β = 0.06, p = 0.15 (FDR corrected)). There were no significant associations with the previously reported GM components for adults (Supplemental Appendix 6).
Discussion
In this study, we assessed PRS effects on ADHD diagnosis, symptoms, and brain networks implicated in ADHD separately in two age cohorts: adolescents and adults. Our findings did not show a PRS effect on any of the previously identified GM components (see Supplemental Appendices 5 and 6) related to ADHD in either adolescents or adults (Duan et al., 2018; Jiang et al., 2019). However, our results did show a PRS effect on individual symptom domains of ADHD; in adolescents this held for both hyperactivity and inattention scores, while in adulthood this was only found for hyperactivity scores.
Inattention is the prominent symptom profile of adults with ADHD, not hyperactivity (Spencer, Biederman, & Mick, 2007). Previous PRS literature has shown associations between PRS and individual externalizing symptoms, hyperactivity among others, but not internalizing symptoms including inattention (Brikell et al., 2018). Our findings are in line with this previous literature. This may partially explain why our results showed no association between PRS and inattention in adults. In adolescents, symptoms of hyperactivity and inattention were highly correlated, and therefore, the dual results could be capturing the same behavioral presentation. These results may offer new insights into the genetic effects of the different behavioral phenotypes of ADHD through the lifespan.
The variable persistence rate of ADHD from childhood to adulthood has previous lead to the speculation that adults with ADHD may present a more homogeneous phenotype of ADHD. Therefore, children with ADHD could be a more muddled representation of ADHD; perhaps representing varied phenotypes, environmental factors, or eventually simply “grow out” of their clinical diagnosis. Adults who have had the diagnosis of ADHD persist through adolescence into their adulthood, may be a more severe and consistent representation of the disorder. A recent study by Rovira and colleagues also found that the PRS for persistent ADHD (or adulthood ADHD) relates to a more severe and consistent clinical phenotype when compared to the PRS for childhood ADHD (Rovira et al., 2020). Our previous and current results support this notion that adulthood ADHD differs from childhood ADHD in phenotypic presentation, in the affected brain structures, and now, genetically.
Limitations in our study include a relatively broad age range for the adolescent data (7 to 18 years old; mean = 14.65, SD = 2.24) that we counteracted by completing a voxelwise correction with the quadratic effect of age (age^2) in the analyses. Our sample sizes are also relatively small and should be replicated with larger samples as these results are meant to serve as preliminary findings for ADHD in adolescents and adults.
In conclusion, the finding of different age groups with ADHD presenting with distinct symptom profiles partially explained by PRS is an important addition to the ADHD literature. We demonstrated a difference between adolescents and adults in the effects of PRS on individual symptom domains. These results may be explained by differences in the genetic effects of the symptom domains of ADHD and should serve as a starting point for future genetic studies of adults with ADHD.
Supplementary Material
Highlights.
We investigated ADHD polygenic risk score (PRS) related to previously identified gray matter (GM) patterns, neurocognitive functions, and symptoms in ADHD
Different age cohorts revealed different symptom profiles related to PRS
Distinct PRS effects between adolescents and adults with ADHD is suggested
Acknowledgments
This study was supported by the National Institutes of Health and The National Institute of Mental Health through the grant 1R01MH106655. This NeuroIMAGE study was supported by NIH Grant R01MH62873, NWO Large Investment Grant 1750102007010 and grants from Radboud University Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. This work was also supported by grants from NWO Brain & Cognition (433-09-242 and 056-13-015) and from ZonMW (60-60600-97-193). Further support was received from the European Union’s FP7 program under grant agreement no. 278948 (TACTICS), no. 602450 (IMAGEMEND), no. 602805 (Aggressotype), and from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 667302 (CoCA) and no. 728018 (Eat2beNICE). Barbara Franke receives funding from a personal Vici grant (to Barbara Franke) of the Netherlands Organization for Scientific Research (NWO, grant numbers 433-09-229 and 016-130-669) and a pilot grant of the Dutch National Research Agenda for the NeuroLabNL project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Barbara Franke has received educational speaking fees from Shire and Medice. Other authors report no conflict of interest.
References
- Agnew-Blais JC, Polanczyk GV, Danese A, Wertz J, Moffitt TE, & Arseneault L (2016). Evaluation of the Persistence, Remission, and Emergence of Attention-Deficit/Hyperactivity Disorder in Young Adulthood. JAMA Psychiatry, 73(7), 713–720. doi: 10.1001/jamapsychiatry.2016.0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson RM, Kasper LJ, Hudec KL, & Patros CH (2013). Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: a meta-analytic review. Neuropsychology, 27(3), 287–302. doi: 10.1037/a0032371 [DOI] [PubMed] [Google Scholar]
- Bell AJ, & Sejnowski TJ (1995). An information-maximization approach to blind separation and blind deconvolution. Neural Comput, 7(6), 1129–1159. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Keenan K, Knee D, & Tsuang MT (1990). Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry, 29(4), 526–533. doi: 10.1097/00004583-199007000-00004 [DOI] [PubMed] [Google Scholar]
- Brikell I, Larsson H, Lu Y, Pettersson E, Chen Q, Kuja-Halkola R, … Martin J (2018). The contribution of common genetic risk variants for ADHD to a general factor of childhood psychopathology. Mol Psychiatry. doi: 10.1038/s41380-018-0109-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Biederman J, & Faraone SV (2016). Assessing the Validity of the Age at Onset Criterion for Diagnosing ADHD in DSM-5. J Atten Disord. doi: 10.1177/1087054716629717 [DOI] [PubMed] [Google Scholar]
- Choi SW, & O’Reilly PF (2019). PRSice-2: Polygenic Risk Score software for biobank-scale data. Gigascience, 8(7). doi: 10.1093/gigascience/giz082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis D, Walters RK, Martin J, Mattheisen M, Als TD, Agerbo E, … Neale BM (2019). Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet, 51(1), 63–75. doi: 10.1038/s41588-018-0269-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, & Milham MP (2006). The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry, 47(10), 1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x [DOI] [PubMed] [Google Scholar]
- Duan K, Chen J, Calhoun VD, Lin D, Jiang W, Franke B, … Liu J (2018). Neural correlates of cognitive function and symptoms in attention-deficit/hyperactivity disorder in adults. Neuroimage Clin, 19, 374–383. doi: 10.1016/j.nicl.2018.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, & Sklar P (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry, 57(11), 1313–1323. doi: 10.1016/j.biopsych.2004.11.024 [DOI] [PubMed] [Google Scholar]
- Frodl T, & Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand, 125(2), 114–126. doi: 10.1111/j.1600-0447.2011.01786.x [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, & Nichols T (2002). Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage, 15(4), 870–878. doi: 10.1006/nimg.2001.1037 [DOI] [PubMed] [Google Scholar]
- Halperin JM, & Schulz KP (2006). Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychol Bull, 132(4), 560–581. doi: 10.1037/0033-2909.132.4.560 [DOI] [PubMed] [Google Scholar]
- Himberg J, Hyvarinen A, & Esposito F (2004). Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage, 22(3), 1214–1222. doi: 10.1016/j.neuroimage.2004.03.027 [DOI] [PubMed] [Google Scholar]
- Hoogman M, Bralten J, Hibar DP, Mennes M, Zwiers MP, Schweren LSJ, … Franke B (2017). Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry, 4(4), 310–319. doi: 10.1016/S2215-0366(17)30049-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, … Franke B (2019). Brain Imaging of the Cortex in ADHD: A Coordinated Analysis of Large-Scale Clinical and Population-Based Samples. Am J Psychiatry, 176(7), 531–542. doi: 10.1176/appi.ajp.2019.18091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Duan K, Rootes-Murdy K, Hoekstra PJ, Hartman C, Oosterlaan J, … Turner J (2019). Structural Brain Alterations and Their Association with Cognitive Function and Symptoms in Attention-Deficit/Hyperactivity Disorder Families. bioRxiv, 863605. doi: 10.1101/863605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, & van Engeland H (2005). A meta-analytic review of stopping performance in attention-deficit/hyperactivity disorder: deficient inhibitory motor control? J Abnorm Psychol, 114(2), 216–222. doi: 10.1037/0021-843X.114.2.216 [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, & Tannock R (2005). A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 44(4), 377–384. doi: 10.1097/01.chi.0000153228.72591.73 [DOI] [PubMed] [Google Scholar]
- Mostert JC, Onnink AMH, Klein M, Dammers J, Harneit A, Schulten T, … Hoogman M (2015). Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: A systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol, 25(11), 2062–2074. doi: 10.1016/j.euroneuro.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, & Mataix-Cols D (2011). Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry, 168(11), 1154–1163. doi: 10.1176/appi.ajp.2011.11020281 [DOI] [PubMed] [Google Scholar]
- Noordermeer SDS, Luman M, Weeda WD, Buitelaar JK, Richards JS, Hartman CA, … Oosterlaan J (2017). Risk factors for comorbid oppositional defiant disorder in attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry, 26(10), 1155–1164. doi: 10.1007/s00787-017-0972-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onnink AM, Franke B, van Hulzen K, Zwiers MP, Mostert JC, Schene AH, … Hoogman M (2016). Enlarged striatal volume in adults with ADHD carrying the 9–6 haplotype of the dopamine transporter gene DAT1. J Neural Transm (Vienna), 123(8), 905–915. doi: 10.1007/s00702-016-1521-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, & Rohde LA (2007). Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry, 20(4), 386–392. doi: 10.1097/YCO.0b013e3281568d7a [DOI] [PubMed] [Google Scholar]
- Rissanen J (1978). Modeling by Shortest Data Description. Automatica, 14, 465–471. [Google Scholar]
- Rovira P, Demontis D, Sanchez-Mora C, Zayats T, Klein M, Mota NR, … Ribases M (2020). Shared genetic background between children and adults with attention deficit/hyperactivity disorder. Neuropsychopharmacology, 45(10), 1617–1626. doi: 10.1038/s41386-020-0664-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, & Mick E (2007). Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol, 32(6), 631–642. doi: 10.1093/jpepsy/jsm005 [DOI] [PubMed] [Google Scholar]
- Tarver J, Daley D, & Sayal K (2014). Attention-deficit hyperactivity disorder (ADHD): an updated review of the essential facts. Child Care Health Dev, 40(6), 762–774. doi: 10.1111/cch.12139 [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, & Seidman LJ (2007). Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry, 61(12), 1361–1369. doi: 10.1016/j.biopsych.2006.06.011 [DOI] [PubMed] [Google Scholar]
- von Rhein D, Mennes M, van Ewijk H, Groenman AP, Zwiers MP, Oosterlaan J, … Buitelaar J (2015). The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. Eur Child Adolesc Psychiatry, 24(3), 265–281. doi: 10.1007/s00787-014-0573-4 [DOI] [PubMed] [Google Scholar]
- Wechsler D, Van der Steene G, Vertommen H, Bleichrodt N, Uiterwijk J,. (2000). WAIS-III: Nederlandstalige bewerking.: Swets Test Publishers; Harcourt Test Publishers. [Google Scholar]
- Wolfers T, van Rooij D, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, … Marquand AF (2016). Quantifying patterns of brain activity: Distinguishing unaffected siblings from participants with ADHD and healthy individuals. Neuroimage Clin, 12, 227–233. doi: 10.1016/j.nicl.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Groth KM, Pearlson G, Schretlen DJ, & Calhoun VD (2009). Source-based morphometry: the use of independent component analysis to identify gray matter differences with application to schizophrenia. Hum Brain Mapp, 30(3), 711–724. doi: 10.1002/hbm.20540 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


