Abstract
We evaluated the cardiac function recovery following skeletal myoblast cell-sheet transplantation and the long-term outcomes after applying this treatment in 23 patients with ischemic cardiomyopathy. We defined patients as “responders” when their left ventricular ejection fraction remained unchanged or improved at 6 months after treatment. At 6 months, 16 (69.6%) patients were defined as responders, and the average increase in left ventricular ejection fraction was 4.9%. The responders achieved greater improvement degrees in left ventricular and hemodynamic function parameters, and they presented improved exercise capacity. During the follow-up period (56 ± 28 months), there were four deaths and the overall 5-year survival rate was 95%. Although the responders showed higher freedom from mortality and/or heart failure admission (5-year, 81% versus 0%; p = 0.0002), both groups presented an excellent 5-year survival rate (5-year, 93% versus 100%; p = 0.297) that was higher than that predicted using the Seattle Heart Failure Model. The stepwise logistic regression analysis showed that the preoperative estimated glomerular filtration rate and the left ventricular end-systolic volume index were independently associated with the recovery progress. Approximately 70% of patients with “no-option” ischemic cardiomyopathy responded well to the cell-sheet transplantation. Preoperative renal and left ventricular function might predict the patients’ response to this treatment.
Keywords: heart failure, ischemic cardiomyopathy, regenerative therapy, stem cell, sheet transplantation, responder
Graphical abstract
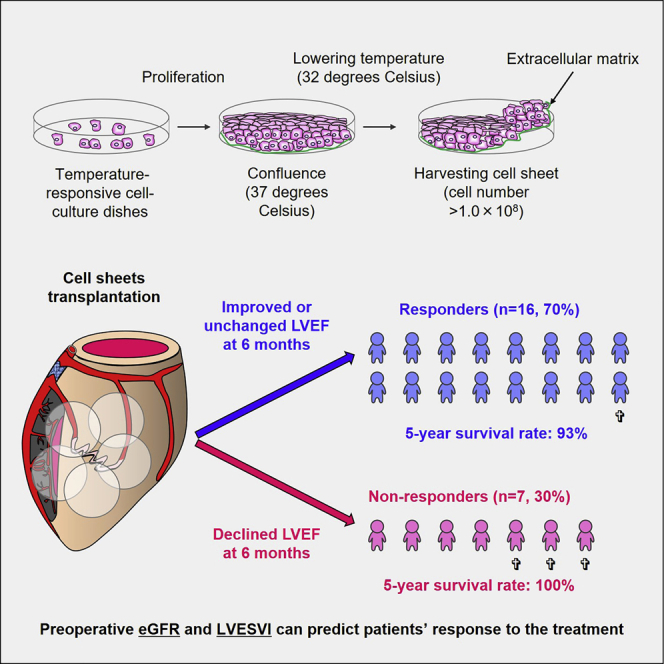
This study demonstrated that approximately 70% of patients with “no-option” ischemic cardiomyopathy responded well to cell-sheet transplantation in terms of LV unloading and improvements in LV systolic and hemodynamic functions and functional capacity. Preoperative renal and LV function might predict the patients’ response to this treatment.
Introduction
Heart failure following myocardial infarction is a major cause of death and disability worldwide.1 Despite the advances in drug and device therapy in recent years, the recovery progress of cardiac function and the degree of prevention of transition to heart failure in patients with myocardial infarction remain unsatisfactory. Cardiac transplantation and/or mechanical circulatory support are the main therapeutic options for patients with severe ventricular dilatation and impaired left ventricular (LV) function, but these choices are limited by the low availability of donor hearts and numerous device-related complications.2 Indeed, in Japan, patients who receive mechanical circulatory support or those who require continuous inotrope administration may wait approximately 900 days for heart transplantation; therefore, many patients might die while waiting.3 In addition, because of the selection criteria, the most common etiology of heart failure in patients who received heart transplantation was dilated cardiomyopathy (average age, 38.1 years); therefore, patients with ischemic cardiomyopathy are less likely to receive this treatment.4 This situation has led clinicians to consider alternative methods for treating heart failure, especially for patients with ischemic cardiomyopathy.
Cellular transplantation represents an important therapeutic option for patients with ischemic cardiomyopathy who are not amenable to percutaneous or surgical treatment options. We previously reported results from a phase I clinical trial demonstrating that autologous skeletal stem cell-sheet transplantation was a safe, feasible, and possibly effective procedure in treating “no-option” ischemic cardiomyopathy patients based on angiogenesis induced by secreted cytokines, which have been approved for clinical use by the Ministry of Health of Japan.5,6 However, there are limited data regarding its long-term therapeutic effects on LV function, functional capacity, and survival. Additionally, patients who would achieve LV recovery following cell-sheet transplantation and thereby benefit from the treatment have not been clearly identified. In the present study, we aimed to clarify the incidence of LV recovery following skeletal stem cell-sheet transplantation, its impact on long-term outcomes, and factors that could identify the possible responders to this treatment among patients with ischemic cardiomyopathy.
Results
Patient characteristics
The baseline characteristics of the patients are summarized in Table 1. All patients had a history of myocardial infarction. In particular, the involved myocardial territories were inferior and posterolateral (without anterior), anterior, and multiple in 1 (4.3%), 6 (26%), and 16 patients (70%), respectively. 4 patients (17%) had a previous history of implantable cardioverter defibrillator (ICD) implantation, while 3 (13%) received cardiac resynchronization-defibrillator therapy. Approximately half of the patients had received cardiac surgery 93 ± 101 months (range, 3.0–296 months) prior to cell-sheet transplantation; 4 (17%), 7 (30%), and 1 patients underwent coronary artery bypass grafting with or without mitral valve surgery or aortic valve replacement, respectively.
Table 1.
Patient demographics
| Variables | All cohort (n = 23) | Responder (n = 16) | Non-responder (n = 7) | p value |
|---|---|---|---|---|
| Clinical variables | ||||
| Age, years | 56 ± 14 | 55 ± 15 | 57 ± 12 | 0.752 |
| Male, n (%) | 21 (91) | 14 (88) | 7 (100) | 0.999 |
| Body surface area, m2 | 1.77 ± 0.18 | 1.73 ± 0.18 | 1.85 ± 0.17 | 0.180 |
| Pre-operation catecholamine use, n (%) | 1 (4.3) | 0 (0) | 1 (14) | 0.233 |
| Territory of previous MI, n (%) | ||||
| Inferior + posterolateral | 1 (4.3) | 1 (6.3) | 0 (0) | 0.111 |
| Anterior only | 6 (26) | 6 (38) | 0 (0) | |
| Multi territory | 16 (70) | 9 (56) | 7 (100) | |
| Previous intervention, n (%) | ||||
| ICD | 4 (17) | 1 (6.3) | 3 (43) | 0.067 |
| CRT-D | 3 (13) | 0 (0) | 3 (43) | 0.020 |
| PCI | 16 (70) | 12 (75) | 4 (57) | 0.626 |
| CABG | 7 (30) | 4 (25) | 3 (43) | 0.626 |
| CABG + MV surgery | 4 (17) | 2 (13) | 2 (29) | 0.557 |
| AVR | 1 (4.3) | 1 (6.3) | 0 (0) | 0.999 |
| Comorbidities, n (%) | ||||
| Hypertension | 16 (70) | 12 (75) | 4 (57) | 0.626 |
| Hyperlipidemia | 19 (83) | 13 (81) | 6 (86) | 0.999 |
| Diabetes | 7 (30) | 4 (25) | 3 (43) | 0.626 |
| Laboratory data | ||||
| eGFR, mL/min/1.73 m2 | 65 ± 24 | 72 ± 25 | 49 ± 11 | 0.029 |
| Medications, n (%) | ||||
| Beta-blockers | 23 (100) | 16 (100) | 7 (100) | 1.000 |
| ACE inhibitors | 15 (63) | 10 (63) | 5 (71) | 0.533 |
| ARB | 5 (22) | 3 (19) | 2 (29) | 0.492 |
| Diuretics | 18 (78) | 11 (69) | 7 (100) | 0.272 |
MI, myocardial infarction; ICD, implantable cardioverter defibrillator; CRT-D, cardiac resynchronization-defibrillator therapy; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; MV, mitral valve; AVR, aortic valve replacement; eGFR, estimated glomerular filtration rate; ACE, angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker.
The baseline functional capacity, LV, and hemodynamic functions are presented in Table 2. The New York Heart Association (NYHA) functional classification at baseline (just 1 week before the treatment) was of class II, III, and IV in 4 (17%), 18 (78%), and 1 (4.3%) patients, respectively. One patient was receiving continuous catecholamine injections and classified as NYHA functional class IV. Before surgery, the functional capacity was impaired, as represented by the shorter 6-min walk distance and higher brain natriuretic peptide (BNP) levels. LV dimensions and volumes were substantially dilated, along with severely impaired LV systolic function.
Table 2.
Baseline and after 6-month functional capacity, LV, and hemodynamic function
| Variables | Preoperative values (baseline) |
Postoperative values (6 months) |
||||||
|---|---|---|---|---|---|---|---|---|
| All cohorts (n = 23) | Responders (n = 16) | Non-responders (n = 7) | p Value | All cohorts (n = 23) | Responders (n = 16) | Non-responders (n = 7) | p value | |
| Functional status | (n = 23) | (n = 16) | (n = 7) | (n = 23) | (n = 16) | (n = 7) | ||
| NYHA class, n (%) | ||||||||
| II | 4 (17) | 4 (25) | 0 (0) | 0.128 | 20 (87) | 15 (94) | 5 (71) | 0.210 |
| III | 18 (78) | 12 (75) | 6 (86) | 3 (13) | 1 (6.3) | 2 (29) | ||
| IV | 1 (4.3) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | ||
| 6-min walk distance, m | 401 ± 107 | 412 ± 106 | 372 ± 116 | 0.447 | 461 ± 121 | 468 ± 124 | 443 ± 120 | 0.682 |
| Plasma BNP, pg/mL | 286 ± 242 | 230 ± 155 | 413 ± 358 | 0.097 | 164 ± 133 | 111 ± 89 | 284 ± 145 | 0.002 |
| Echocardiography | (n = 23) | (n = 16) | (n = 7) | |||||
| LVEDVI, mL/m2 | 110 ± 29 | 102 ± 29 | 127 ± 22 | 0.039 | 106 ± 31 | 94 ± 31 | 128 ± 17 | 0.016 |
| LVESVI, mL/m2 | 81 ± 24 | 75 ± 25 | 96 ± 16 | 0.031 | 77 ± 22 | 65 ± 32 | 98 ± 18 | 0.022 |
| LVEDD, mm | 66 ± 7 | 65 ± 6 | 71 ± 5 | 0.035 | 66 ± 8 | 63 ± 7 | 72 ± 6 | 0.005 |
| LVESD, mm | 58 ± 8 | 56 ± 7 | 64 ± 8 | 0.014 | 58 ± 9 | 55 ± 8 | 66 ± 8 | 0.005 |
| LVEF, % | 26 ± 6 | 27 ± 7 | 26 ± 6 | 0.825 | 29 ± 9 | 32 ± 9 | 22 ± 5 | 0.013 |
| MR grade, n (%) | ||||||||
| None or trivial | 8 (35) | 6 (38) | 2 (29) | 0.693 | 13 (57) | 11 (69) | 2 (29) | 0.046 |
| Mild | 14 (61) | 9 (56) | 5 (71) | 8 (35) | 5 (31) | 3 (43) | ||
| Moderate or severe | 1 (4.3) | 1 (6.3) | 0 (0) | 2 (8.7) | 0 (0) | 2 (29) | ||
| TR grade, n (%) | ||||||||
| None or trivial | 16 (70) | 13 (81) | 3 (43) | 0.111 | 14 (61) | 13 (81) | 1 (14) | 0.003 |
| Mild | 6 (26) | 3 (19) | 3 (43) | 8 (35) | 2 (13) | 6 (86) | ||
| Moderate or severe | 1 (4.3) | 0 (0) | 1 (14) | 1 (4.3) | 1 (6.3) | 0 (0) | ||
| Right heart catheterization | (n = 22) | (n = 15) | (n = 7) | (n = 18) | (n = 12) | (n = 6) | ||
| Heart rate, beat/min | 71 ± 12 | 71 ± 13 | 72 ± 8 | 0.740 | 67 ± 10 | 62 ± 6 | 78 ± 4 | < 0.001 |
| Mean BP, mmHg | 77 ± 12 | 79 ± 10 | 73 ± 15 | 0.307 | 76 ± 10 | 77 ± 11 | 75 ± 9 | 0.624 |
| RAP, mmHg | 6.1 ± 4.4 | 5.4 ± 4.2 | 7.8 ± 5.2 | 0.319 | 4.8 ± 2.3 | 4.8 ± 2.5 | 4.8 ± 2.1 | 0.945 |
| PCWP, mmHg | 15 ± 8 | 14 ± 8 | 17 ± 6 | 0.462 | 13 ± 7.9 | 9.2 ± 5.1 | 20 ± 8 | 0.003 |
| Mean PAP, mmHg | 24 ± 11 | 23 ± 12 | 27 ± 11 | 0.428 | 21 ± 10 | 16 ± 6 | 30 ± 10 | 0.002 |
| PVR, dyne⋅s⋅cm -5 | 188 ± 109 | 179 ± 101 | 207 ± 131 | 0.596 | 148 ± 57 | 128 ± 32 | 184 ± 76 | 0.047 |
| LVSWI, g/m2/beat | 29 ± 12 | 31 ± 14 | 24 ± 6 | 0.169 | 32 ± 11 | 38 ± 10 | 23 ± 6 | 0.004 |
BNP, brain natriuretic peptide; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; TR, tricuspid regurgitation; BP, blood pressure; RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; LVSWI, left ventricular stroke volume index.
At 6 months after cell-sheet transplantation, the LV ejection fraction improved or remained unchanged in 16 (69.6%) patients and declined in 7 (30.4%) patients who were, therefore, considered to be responders and non-responders to this treatment, respectively. There were no intergroup differences in age, sex, body surface area, the territory of previous myocardial infarction, history of percutaneous coronary intervention and cardiac surgeries, LV ejection fraction, and hemodynamic function parameters. However, the non-responder group had a lower estimated glomerular filtration rate (eGFR), higher prevalence of device implantation, and larger LV dimensions and volumes compared to the responder group (Tables 1 and 2).
Early outcomes
The cell-sheet transplantation was performed at 76 ± 50 days after the muscle harvest. Prior to the surgery, intra-aortic balloon pumping was prophylactically introduced for eight (35%) patients. The myoblast cells at a mean number of 3.7 ± 1.7 × 108 (range, 1.1 × 108 to 7.4 × 108) were transplanted over the LV free wall through the left thoracotomy (mainly the fifth intercostal space), without any procedural-related complications. The mean operation time was 133 ± 24 min (range, 83–186 min). There was no 30-day or hospital mortality.
All patients were discharged at 45 ± 52 days (range, 10–222 days) on average. Three patients required prolonged hospitalization (141, 148, and 222 days, respectively) because of the severely deteriorated preoperative cardiac function and the need for meticulous postoperative managements. The remaining 20 patients were discharged at 26 ± 9 days (range, 10–46 days) on average.
During hospitalization, lethal arrhythmias, such as sustained or non-sustained ventricular tachycardia and ventricular fibrillation, were not observed in any patients after performing 24-h electrocardiogram monitoring.
Late outcomes
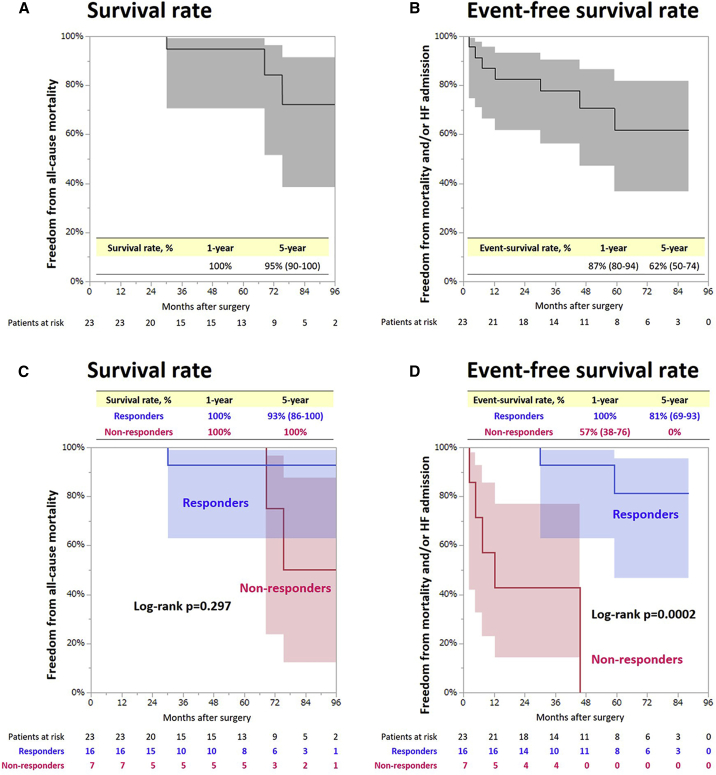
During the follow-up period, there were four cases of cardiac unrelated mortality: one case because of gastrointestinal bleeding in the responder group, and three cases because of gastrointestinal bleeding, pneumonia, and renal failure, respectively, in the non-responder group. The overall 1- and 5-year survival rates were 100% and 95%, respectively (Figure 1A). Seven patients (one and six in the responder and non-responder groups, respectively) experienced heart failure with a mean interval of 24 ± 23 months (range, 2.2–59 months) from the time of surgery to each event. The 1- and 5-year freedom from composite events (mortality and/or heart failure admission) were 87% and 62%, respectively (Figure 1B).
Figure 1.
Freedom from all-cause mortality and composite adverse events in all cases and in each group
(A) Freedom from all-cause mortality in all cases. (B) Composite adverse events in all cases. (C) Freedom from all-cause mortality in responders and non-responders. (D) Composite adverse events in responders and non-responders.
Although there were no intergroup differences in the overall 1-year (100% for responders and non-responders) and 5-year (93% for responders versus 100% for non-responders) survival rates (log-rank p = 0.297), the responders showed higher 1- and 5-year freedom from mortality and heart failure admission (100% and 81%, respectively) compared with non-responders (57% and 0%, respectively) (log-rank p = 0.0002) (Figures 1C and 1D). The data regarding the late outcomes of each patient are also summarized in Table 3.
Table 3.
Summary of changes in LV ejection fraction, outcomes, and predicted survival for each patient
| Case number | Group | LVEF at baseline (%) | LVEF at 6 months (%) | Change in LVEF | Primary endpoint | Cause of death | Follow-up (years) | Predicted 1-year survival (%) | Predicted 3-year survival (%) | Predicted 5-year survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | responder | 28 | 39 | 11 | alive | 5.7 | 86 | 74 | 43 | |
| 2 | responder | 27 | 37 | 10 | alive | 1.3 | 93 | 86 | 66 | |
| 3 | responder | 31 | 41 | 10 | alive | 2.8 | 81 | 65 | 29 | |
| 4 | responder | 33 | 42 | 9 | alive | 7.2 | 94 | 88 | 71 | |
| 5 | responder | 27 | 34 | 7 | alive | 2.7 | 86 | 73 | 42 | |
| 6 | responder | 31 | 37 | 6 | alive | 1.8 | 92 | 84 | 60 | |
| 7 | responder | 21 | 26 | 5 | alive | 6.7 | 96 | 92 | 78 | |
| 8 | responder | 18 | 23 | 5 | alive | 8.5 | 91 | 83 | 59 | |
| 9 | responder | 34 | 38 | 4 | dead | GI bleeding | 2.5 | 94 | 88 | 72 |
| 10 | responder | 27 | 31 | 4 | alive | 4.8 | 80 | 64 | 28 | |
| 11 | responder | 25 | 28 | 3 | alive | 7.4 | 93 | 87 | 68 | |
| 12 | responder | 30 | 32 | 2 | alive | 7.3 | 97 | 95 | 86 | |
| 13 | responder | 16 | 17 | 1 | alive | 2.6 | 86 | 73 | 41 | |
| 14 | responder | 34 | 35 | 1 | alive | 5.1 | 95 | 91 | 79 | |
| 15 | responder | 12 | 12 | 0 | alive | 4.4 | 89 | 80 | 57 | |
| 16 | responder | 32 | 32 | 0 | alive | 5.7 | 89 | 79 | 51 | |
| 17 | non-responder | 20 | 19 | −1 | alive | 1.3 | 85 | 72 | 40 | |
| 18 | non-responder | 33 | 31 | −2 | dead | GI bleeding | 5.7 | 64 | 40 | 7.6 |
| 19 | non-responder | 24 | 21 | −3 | alive | 3.1 | 87 | 76 | 46 | |
| 20 | non-responder | 21 | 17 | −4 | alive | 1.5 | 94 | 88 | 69 | |
| 21 | non-responder | 30 | 24 | −6 | alive | 6.4 | 73 | 53 | 17 | |
| 22 | non-responder | 22 | 16 | −6 | dead | renal failure | 6.3 | 53 | 28 | 2.7 |
| 23 | non-responder | 32 | 26 | −6 | dead | pneumonia | 8.5 | 93 | 86 | 65 |
GI, gastrointestinal.
Serial changes in LV function parameters after the cell-sheet transplantation
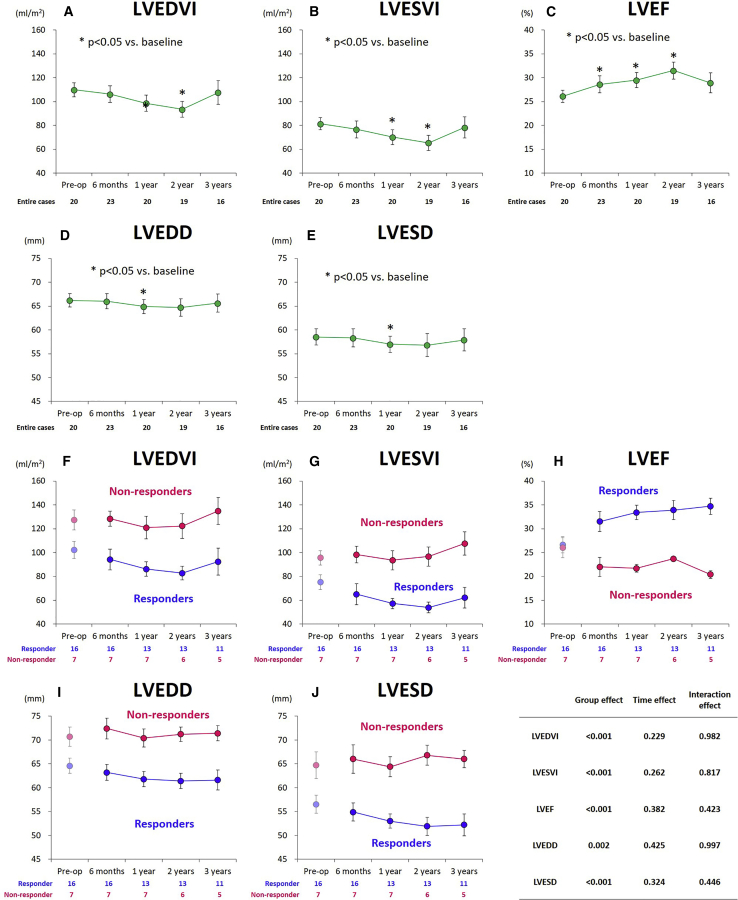
Overall, the LV volumes and dimensions tended to decrease, while the LV ejection fraction improved over time after the treatment (Figures 2A–2E). Moreover, the mitral and tricuspid regurgitation grade did not significantly change during the follow-up period (Figures S1A and S1B).
Figure 2.
Serial echocardiographic assessments in the entire cohorts and according to responders and non-responders
(A–E) Serial echocardiographic assessments in the entire cohorts. (F–J) Serial echocardiographic assessments according to responders and non-responders. (A and F) LVEDVI, (B and G) LVESVI, (C and H) LVEF, (D and I) LVEDD, and (E and J) LVESD. Data are presented as means ± standard error. LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension. (A–E) ∗p < 0.05.
The serial assessments of the echocardiographic parameters according to the study groups are summarized in Table 2 and Figure 2. From baseline (before surgery) to 6 months after the treatment, the LV end-systolic volume index decreased by 13% (from 75 ± 25 to 65 ± 32 mL/m2) in responders, while it increased by 4.4% (from 96 ± 16 to 98 ± 18 mL/m2) in non-responders. Thereafter, the trend was not different, with smaller values for the non-responder group at any follow-up point (interaction effect, p = 0.982; group effect, p < 0.001) (Figures 2F and 2G). The LV ejection fraction increased by 4.9% (from 27% ± 7% to 32% ± 9%) in responders, while it decreased by 4.0% (from 26% ± 6% to 22% ± 5%) in non-responders. Thereafter, the LV ejection fraction gradually but steadily improved over time for up to 3 years in the responder group, whereas it tended to decline in the non-responder group (Figure 2H). Consistently, the values of LV end-diastolic and systolic dimensions were lower for the responder group at any follow-up point (group effect, p < 0.001 for both) (Figures 2I and 2J).
The grades of mitral and tricuspid regurgitation were not different between the groups, but they tended to be less severe in responders than in non-responders (p = 0.046 and 0.003, respectively) (Table 2). The mitral regurgitation grade did not significantly change during the follow-up period, without significant intergroup difference (Figure S1C). However, the tricuspid regurgitation grade was consistently less severe in responders than in non-responders (Figure S1D).
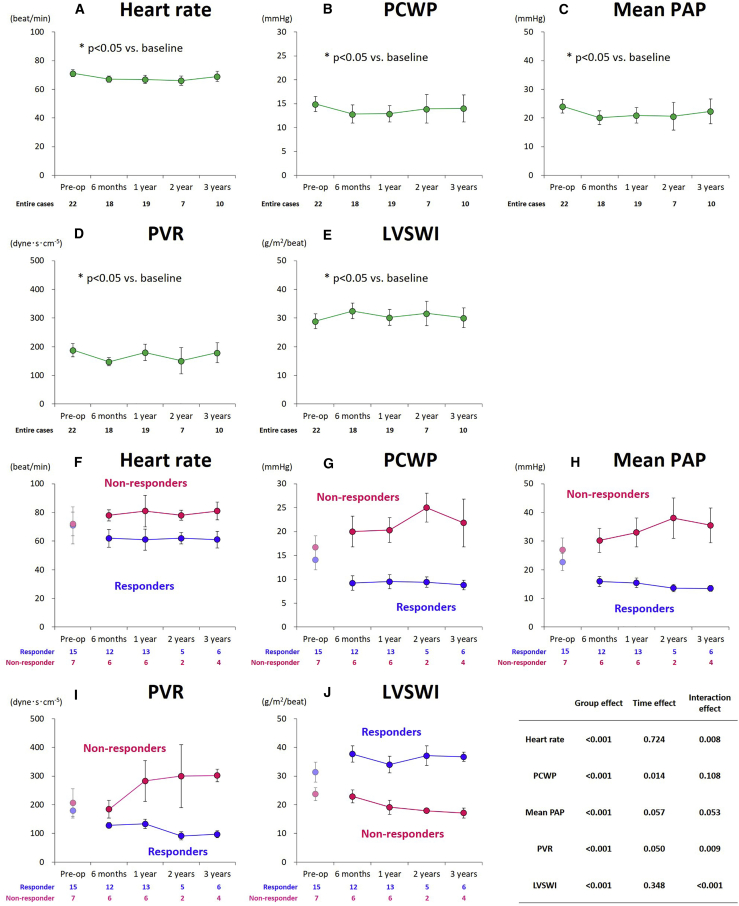
Right heart catheterization
Overall, the hemodynamic variables did not significantly change for up to 3 years after the treatment (Figures 3A–3E). The serial assessments of the hemodynamic parameters according to the groups are summarized in Table 2 and Figure 3. From baseline to 6 months after the treatment, the heart rate tended to decrease (from 71 ± 13 to 62 ± 6 beats/min) in responders and increase (from 72 ± 8 to 78 ± 4 beats/min) in non-responders. Pulmonary capillary wedge pressure (PCWP) substantially decreased (from 14 ± 8 to 9.2 ± 5.1 mmHg) in responders and increased (from 17 ± 6 to 20 ± 8 mmHg) in non-responders. The mean pulmonary artery pressure (PAP) values also decreased (from 23 ± 12 to 16 ± 6 mmHg) in responders, while they increased (from 27 ± 11 to 30 ± 10 mmHg) in non-responders. Likewise, pulmonary vascular resistance (PVR) decreased in responders (from 179 ± 101 to 128 ± 32 dyne⋅s⋅cm−5) and non-responders (from 207 ± 131 to 184 ± 76 dyne⋅s⋅cm-5). In responders, these changes (improvements) in heart rate, PCWP, mean PAP, and PVR observed at 6 months after the treatment were sustained up to 3 years, whereas in non-responders, they tended to increase over time, resulting in significantly larger values at any postoperative follow-up time point (group effect, p < 0.001 for all) (Figures 3F–3I). Furthermore, the LV stroke work index (LVSWI), a good indicator of cardiac performance, was improved after cell-sheet transplantation in responders, while it steadily decreased over time in non-responders, as we obtained lower values at any postoperative follow-up time point (group effect, p < 0.001) (Figure 3J).
Figure 3.
Serial assessments of hemodynamic parameters in the entire cohorts and according to responders and non-responders
(A–E) Serial assessments of hemodynamic parameters in the entire cohorts. (F–J) Serial assessments of hemodynamic parameters according to responders and non-responders. (A and F) Heart rate, (B and G) PCWP, (C and H) mean PAP, (D and I) PVR, and (E and J) LVSWI. Data are presented as means ± standard error. PCWP, pulmonary artery wedge pressure; PAP, pulmonary artery pressure; PVR, pulmonary vascular resistance; LVSWI, left ventricular stroke work index. (A–E) ∗p < 0.05.
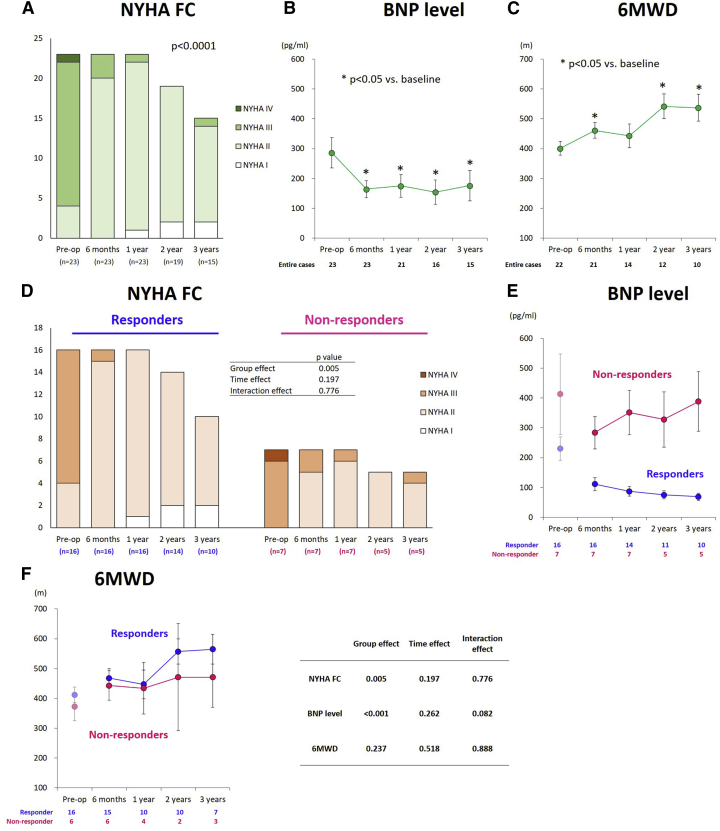
Evaluation of symptoms and exercise capacity
Overall, the NYHA functional class was significantly improved, consistent with the substantial improvements in the serum BNP level and the 6-min walk distance up to 3 years after the treatment (Figures 4A–4C).
Figure 4.
Serial assessments of functional parameters in the entire cohorts according to responders and non-responders
(A–C) Serial assessments of functional parameters in the entire cohorts. (D–F) Serial assessments of functional parameters according to responders and non-responders. (A and D) NYHA FC, (B and E) BNP level, and (C and F) 6-min walk distance. Data are presented as means ± standard error. NYHA FC, New York Heart Association functional class; BNP, brain natriuretic peptide; 6MWD, 6-min walk distance. (B and C) ∗p < 0.05.
The serial assessments of the functional parameters according to the groups are summarized in Figure 4. From baseline to 6 months after the treatment, the NYHA functional class was improved in both groups after the cell-sheet transplantation (responders, 2.8 ± 0.4 [at baseline] to 2.1 ± 0.3 [at 6 months]; non-responders: 3.1 ± 0.4 [at baseline] to 2.3 ± 0.5 [at 6 months]). Thereafter, the values did not substantially change in both groups, although the NYHA class value was consistently lower in responders at any follow-up point (responders, 1.9 ± 0.3, 1.9 ± 0.4, and 1.8 ± 0.4 at 1, 2, and 3 years after surgery, respectively; non-responders, 2.1 ± 0.4, 2.0 ± 0.0, and 2.2 ± 0.4 at 1, 2, and 3 years after surgery, respectively; group effect, p = 0.005), suggesting a more favorable functional capacity compared to non-responders (Figure 4D).
From baseline to 6 months after the treatment, the serum BNP levels decreased from 230 ± 155 to 111 ± 89 pg/mL in responders and from 413 ± 358 to 284 ± 145 pg/mL in non-responders. Thereafter, the BNP levels were well controlled over time in responders, whereas those values fluctuated in non-responders. In particular, larger values were recorded for non-responders at any follow-up time point (group effect, p < 0.001) (Figure 4E).
Likewise, the 6-min walk distance substantially increased from 412 ± 106 to 468 ± 124 m in responders and from 372 ± 116 to 443 ± 120 m in non-responders at 6 months after the treatment. Thereafter, the values tended to improve over time in both groups, without intergroup differences (interaction effect, p = 0.888; group effect, p = 0.237) (Figure 4F).
24-h Holter monitoring analysis
Overall, there were no significant changes in the total number of heart beats and premature ventricular contraction (PVC) values and the percent PVC values throughout the follow-up period, potentially indicating that the treatment did not trigger ventricular arrhythmias (Figures S2A–S2C).
The serial assessments of ventricular arrhythmias according to the groups are summarized in Figures S2D–S2F. From baseline to 6 months after the treatment, the total number of heart beats decreased in the responder group whereas it increased in the non-responder group. Thereafter, the values did not substantially change in both groups, although the value was low in responders at any follow-up point, although it did not reach a statistical significance (Figure S2D). The total number percent values of PVC were lower in the responder group at baseline and these tendencies generally persisted for up to 3 years after the treatment (Figures S2D and S2F). Only one patient in the non-responder group developed amiodarone-induced thyrotoxic thyroiditis and was forced to discontinue amiodarone. The patient subsequently required ICD implantation for non-sustained ventricular tachycardia at 6 months after the treatment.
Clinical associates of becoming responders
Stepwise logistic regression analysis showed that eGFR (adjusted odds ratio [OR], 0.82; 95% confidence interval [CI], 0.62–0.95; p < 0.001) and LV end-systolic volume index (adjusted OR, 1.13; 95% CI, 1.03–1.35; p = 0.003) were independently associated with the possibility to respond to the treatment. The receiver operating characteristic (ROC) curve analysis demonstrated an optimal cutoff value for preoperative eGFR of 63 mL/min/1.73 m2 to determine the possibility to respond to the treatment (100% for ≥63 mL/min/1.73 m2 versus 42% for <63 mL/min/1.73 m2; p = 0.005), which resulted in a sensitivity of 69% and a specificity of 100%, with an area under the curve (AUC) of 0.857 (Figure S3A). An optimal cutoff value of 70 mL/m2 for the preoperative LV end-systolic volume index described responders (56% for ≥70 mL/m2 versus 100% for <70 mL/m2; p = 0.047), which resulted in a sensitivity of 50% and a specificity of 100%, with an AUC of 0.750 (Figure S3B). The model that included the preoperative eGFR and LV end-systolic volume index showed the best accuracy, with an AUC of 0.95 for responders (Figure S3C).
Predictors of composite adverse events after the cell-sheet transplantation
Stepwise Cox regression analysis showed that the LV end-systolic volume index (adjusted hazards ratio [HR], 1.04; 95% CI, 1.00–1.10; p = 0.027) and eGFR (adjusted HR, 0.92; 95% CI, 0.85–0.97; p < 0.001) were independently associated with composite adverse events.
Predicted versus observed survival rate after the cell-sheet transplantation
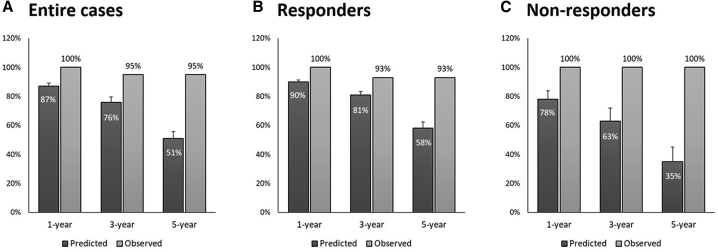
We compared the observed with the predicted survival rates based on the Seattle Heart Failure Model (SHFM) score. In particular, we found that the observed survival rate in the entire cohort was higher than the SHFM-predicted survival rate after a 5-year follow-up period (Figure 5A). Additionally, the observed survival rate was higher than the predicted survival rate in responders and non-responders (Figures 5B and 5C). The outcomes and the predicted 1-, 2-, and 5-year survival rates for each patient are summarized in Table 3.
Figure 5.
Predicted and observed survival rates
(A–C) Survival rates are shown in all cases (A), in responders (B), and in non-responders (C). Data are presented as means ± standard error.
Discussion
The major findings of this study are the following: (1) in patients with refractory heart failure secondary to advanced ischemic cardiomyopathy, autologous skeletal myoblasts cell-sheet transplantation could be safely performed without any procedural-related complication and operative mortality; (2) approximately 70% of patients presented improvement in LV ejection fraction at 6 months after the treatment and were considered as responders; (3) responders achieved substantial LV unloading and improvements in LV systolic and hemodynamic functions and functional capacity over time after the treatment; (4) both groups presented an excellent 5-year survival rate that was higher than that predicted rate using the SHFM; (5) the freedom from composite adverse events was higher in responders; and (6) the preoperative advanced LV remodeling and mild renal dysfunction can predict non-responders and the development of postoperative composite adverse events.
The absolute change in LV ejection fraction from baseline to 6 months after the treatment was 2.2% ± 5.3% (range, −6% to 11%), which was almost consistent with a previous report.7 In this study, we defined responders as patients whose LV ejection fraction improved or remained unchanged at 6 months after the treatment. This definition might be justified by the fact that, in patients with ischemic cardiomyopathy, LV remodeling progressively occurs over time.8, 9, 10 With this definition, we observed that not all, but approximately 70% of patients (considered as responders), presented an improved LV systolic function at 6 months after the treatment, whereas the remaining participants (considered as non-responders) did not, which is one of the most important findings of this study. Most importantly, the responders achieved persistent improvements in LV dimension and systolic function during the follow-up period, which supported the assumption that skeletal myoblast cell-sheet transplantation may overcome the possibly detrimental effects of ventricular remodeling in selected patients. It was interesting, but reasonable, to find that LV systolic function gradually but steadily improved at 2 years after the treatment, as experimental studies have indicated that the skeletal myoblast cell-sheet transplantation could induce robust angiogenic responses (angiogenesis) and establish functionally and structurally mature arterial vascular networks (arteriogenesis) in the ischemic region, thus showing long-term stability and control perfusion.11, 12, 13 Additionally, the sustained improvement in LV function was consistent with the corresponding findings from numerous preclinical studies, demonstrating that the LV ejection fraction improved over time after skeletal myoblasts transplantation, in relationship to attenuation of cardiac hypertrophy and fibrosis and newly formed vasculatures in ischemic and peri-ischemic regions.14, 15, 16, 17, 18 However, we did not observe an improvement in LV systolic function just after the treatment, prompting us to speculate that several months may be needed to improve the contractile function of a hibernating (dysfunctional but viable) myocardium, possibly reflecting a more severe ischemic burden.19, 20, 21 Our speculation may be supported by the finding from the study by Bax et al.,20 in which 31% of patients with ischemic cardiomyopathy undergoing surgical revascularization presented an improved hibernating myocardium contractile function at 3 months, while 61% showed (additional) recovery at 14 months. In responders, besides LV reverse remodeling, PAP and PCWP substantially improved at 6 months after the treatment and, thereafter, remained stable within normal ranges, as evidenced by the serial pressure studies, probably leading to functional improvements. In contrast, in non-responders, global ventricular function gradually, but steadily, deteriorated over time, in association with LV dilation; thus, remodeling presumably was not prevented in these patients. Given the positive relationship between the LV volume and the extent of myocardial infarction, non-responders might not have enough hibernating myocardium to respond to the myoblast sheet transplantation. These data suggested that the skeletal myoblast sheet transplantation, as a sole therapy, can offer sustained improvement in LV and hemodynamic function parameters and heart failure symptoms in selected patients with no-option ischemic cardiomyopathy who have been previously treated with currently available pharmacological, percutaneous, and/or surgical treatments.
Significant differences in long-term clinical outcomes between responders and non-responders and the presence of a tangible proportion of non-responders (i.e., 30%) suggested the importance of predicting responders prior to the treatment. Interestingly, the LV ejection fraction at baseline was similar between the groups and, therefore, it cannot predict responders. However, the LV volume and dimension were significantly larger in the non-responder group. This finding was almost consistent with that obtained from a previous study, which stated that the LV end-systolic volume, but not the LV ejection fraction, is the most powerful predictor of survival in patients with a history of myocardial infarction and impaired LV function.22 The LV end-systolic volume was also a risk factor for poor clinical outcome or death following a variety of surgical interventions, which might support our risk factor analysis.23, 24, 25, 26 These findings can be explained at least partly by the finding that the LV end-systolic volume is determined by the extent of viable myocardium in patients with coronary artery disease and LV dysfunction.27, 28, 29 Therefore, we can speculate that non-responders might have presented a smaller amount of viable myocardium, which made it difficult to adequately respond to the treatment. Notably, a preoperative LV end-systolic volume index of 70 mL/m2 on echocardiography was one of the most important predictors of non-responders and also predicted the development of postoperative adverse events following skeletal myoblast cell-sheet transplantation. This finding indicated that patients whose preoperative LV end-systolic volume index was <70 mL/m2 were more likely to benefit from cell-sheet transplantation. Thus, cell-sheet transplantation should be indicated before LV remodeling severely progresses.
Interestingly, multivariate analysis also identified preoperative renal failure as an independent predictor of the response to skeletal myoblast cell-sheet transplantation. Intriguingly, even mild renal failure, as assessed by an eGFR of approximately 60 (chronic kidney disease stage 3), was associated with the identification of non-responders and the development of postoperative adverse events. The strong association of the mild renal failure with higher incidence of adverse events can be explained by a couple of speculations. First, patients with heart failure complicating with renal impairment might have failed to receive the recommended medical therapy during the postoperative follow-up period.30 Second, it is generally more difficult to control the body fluid volume balance in patients with renal dysfunction than in those with normal renal function, and these patients are more likely to suffer from volume overload in relationship to even a modest increase in body weight.31,32 This finding was supported by the hemodynamic finding that the right atrial pressure was relatively high at any follow-up point in non-responders than in responders (data not shown). Finally, renal function might have deteriorated during the follow-up period in association with heart failure progression, thereby causing a vicious cycle.33 These findings suggested that LV (remodeling) and renal functions should be assessed prior to cell-sheet transplantation to predict who would be at a higher risk for developing postoperative adverse events. In addition, optimizing fluid volume balance is critically important to protect cardiac and renal functions, thereby potentially reducing the postoperative adverse events.
The main limitations of this study were its non-randomized retrospective nature and the limited number of participants. To minimize the potential bias related to patient selection, we excluded those with non-ischemic etiology whose response to regenerative medicine can be affected by the genetic profiles. Therefore, our results would not be applicable to patients with non-ischemic cardiomyopathy. Although we documented the clinical benefits of the cell-sheet treatment, which seemed sustained over time, the magnitude of the changes (improvements) in various parameters (e.g., LV ejection fraction) remained modest. As we did not change the medications or reset the condition of the resynchronization device after performing myoblast cell-sheet transplantation in any of the enrolled patients, there was no significant differences in the preoperative (see Table 1) and postoperative drug regimen. Nevertheless, we cannot deny the possibility that a modest (not significant) difference in drug regimen contributed to differential responses. Finally, the lack of an untreated control group did not allow us to evaluate the impact of cell-sheet transplantation on prognosis of patients enrolled in this study. However, the aforementioned issue encouraged us to calculate the predicted survival using the SHFM for each patient and compare the obtained values with the observed survival rates.34 Importantly, we found that the observed survival rates were higher than the predicted survival rates in responders and non-responders, indicating that skeletal myoblast cell-sheet transplantation could improve survival in both groups and is, therefore, not always contraindicated for patients with advanced LV remodeling and impaired renal function who would become non-responders to the treatment. Nevertheless, our data need to be further confirmed by an additional study with more participants and longer follow-up periods. In addition, whether precision medicine guided by novel technology (e.g., artificial intelligence) could improve the outcomes of regenerative medicine for no-option patients with end-stage cardiomyopathy remained undetermined.
In conclusion, this study’s findings clearly showed that autologous cell-sheet transplantation was safe and potentially effective in improving global LV, hemodynamic functions, and functional capacity in enrolled patients with end-stage ischemic cardiomyopathy. Our data underlined the importance of making an appropriate patient selection, as the preoperative eGFR and the LV end-systolic volume index can predict patients who would benefit from this regeneration therapy.
Materials and methods
This study was approved by the Institutional Review Committee of Osaka University Graduate School of Medicine (Osaka, Japan) and adhered to the principles outlined in the Declaration of Helsinki. Informed consent was obtained from each patient prior to study participation. The procedures followed were in accordance with the institutional guidelines.
Patient characteristics
We enrolled 52 patients with end-stage cardiomyopathy (i.e., LV ejection fraction <35% on echocardiography) who underwent autologous skeletal myoblast cell-sheet transplantation between 2010 and 2018. Before receiving the treatment, all patients had presented with NYHA functional class III or greater heart failure-related symptoms refractory to optimal medical regimens for heart failure, including beta-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers, and diuretics. Of these, those with non-ischemic (n = 27) and congenital etiologies (n = 1) and those who were not followed up for >6 months after the transplantation (n = 1) were excluded. A flow diagram depicting patient selection is shown in Figure S4.
Culture and fabrication of cell sheets
Muscle specimens were harvested from the vastus medialis muscle tissue. The muscle fibers were collected after removing the connective tissues with collagenase and TrypLE select (Invitrogen, Carlsbad, CA, USA), and they were suspended in MCDB131 medium (Invitrogen) with 20% fetal bovine serum. Cell suspensions were cultured and passaged up to P4 for approximately 3 weeks until they expanded to 1.0 × 108 in number. The quality of transplanted cells was controlled by performing endotoxin and mycoplasma tests and ensuring that the cell number was >1.0 × 108. The cultured cells were harvested with TrypLE select, and the cell numbers were assessed using trypan blue. The cell suspensions were placed in temperature-responsive cell-culture dishes (UpCell, Cell Seed, Tokyo, Japan), the surface of which contained a temperature-responsive polymer (poly-N-isopropylacrylamide). Upon reduction of temperature to 32°C, the dish surface rapidly became hydrated, prompting complete detachment of the adherent cells, as a cell sheet.35 After detachment from the temperature-responsive dishes, the top surface of each cell sheet (approximately 4 cm in diameter and 100–150 μm in thickness) was reinforced by fibrin glue.
Cell-sheet transplantation
While the patient was under general anesthesia and single-sided ventilation was provided, the fifth or sixth intercostal space was opened, and the pericardium was opened parallel to the phrenic nerve. The anterior and lateral walls of the left ventricle were dissected where required, and the cell sheet was placed and fixed with a 7-0 Prolene suture. Fibrin glue was added to the surface of the sheet and the LV walls to fix the sheet onto the epicardium.
Outcomes and clinical follow-up examination
Every 6 months to 1 year, the patients were assessed both in our department and by their primary cardiologist. We did not change the medications or reset the condition of the resynchronization device after performing myoblast cell-sheet transplantation in any of the enrolled patients. The primary endpoint was all-cause mortality during the follow-up period. The secondary endpoint was the composite of mortality and re-admission for heart failure. The diagnosis of postoperative heart failure was based on clinical symptoms, physical signs, or radiological evidence of pulmonary congestion. Clinical follow-up examinations were completed in 22 patients (95.7%), with a mean follow-up duration of 56 ± 28 months (range, 15–102 months).
Long-term clinical follow-up examinations and evaluations
Two-dimensional and Doppler echocardiography procedures were performed prior to surgery (baseline), at 6 months, and at 1, 2, and 3 years after cell-sheet transplantation to evaluate the LV end-diastolic and end-systolic volumes and dimensions, and the LV ejection fraction. Regurgitation severity was classified from the color flow Doppler data as none (0), trivial (1+), mild (2+), moderate (3+), or severe (4+). The anatomical and Doppler parameters were measured according to the recommendations of the American Society of Echocardiography.
Right heart catheterization was serially performed with an internal vein approach using a Swan-Ganz catheter to obtain the right atrial pressure, PAP, and PCWP values. We calculated cardiac output based on thermodilution, while PVR was calculated as follows:
Furthermore, the LVSWI was calculated as follows:
The functional status was assessed according to the NYHA criteria for symptoms of heart failure and the serum BNP levels. A 6-min walk test was also serially performed to evaluate exercise capacity. The 6-min walk test measures the distance that a patient could walk during 6 min on a flat surface.
A 24-h Holter monitoring was performed to evaluate the burden of PVC, which was defined as the percent of total PVC number divided by the total beats during monitoring.
Definition of responder
We defined patients as responders to this treatment when their LV ejection fraction remained unchanged or improved at 6 months after the treatment compared with the value at baseline (before the treatment). In contrast, we defined them as non-responders when their LV ejection fraction declined during the corresponding period.
Statistical analysis
The continuous variables are presented as means ± standard deviations and categorical variables as frequencies and proportions. All continuous variables were checked for normality using the Shapiro-Wilk test and normal probability plot. For the continuous variables, comparisons between two study groups (i.e., responders and non-responders) were made using a Student’s t test or Mann-Whitney U test, where appropriate. Likewise, the categorical variables were compared using the chi-square analysis or Fisher’s exact test. The echocardiographic, hemodynamic, and functional variables over time were compared with their baseline values, with the use of a paired t test or Wilcoxon signed rank test. Multiplicity in pairwise comparisons was not corrected. After classifying patients into the two groups at 6 months after the treatment, the above-mentioned variables over time were analyzed using a mixed-effects model for repeated measures, including factors for group, time, and interaction between the groups and time. The variance-covariance matrix in the linear mixed-effects model was assumed to be unstructured. Assessment time points were treated as categorical factors.
Survival analysis was performed using the Kaplan-Meier method for estimation, and a log-rank test was conducted for comparison between the patient groups. As this was a non-comparative, single-arm observational study, we applied the well-validated SHFM to our participants. The SHFM score was calculated for each patient based on the variable values at baseline, and the predicted survival was derived using the original SHFM.34 Stepwise multiple logistic regression analyses were performed to identify the patients that would be responders. As explanatory variables, age, body surface area, history of ICD implantation, eGFR, BNP level, LV end-systolic volume index, mitral and tricuspid regurgitation grades, PCWP, mean PAP, and PVR were introduced into a model based on information from previous studies or clinical knowledge.23,24,36, 37, 38, 39 Likewise, predictors for adverse cardiac time to events were performed using Cox proportional hazards models. Clinically relevant variables were entered appropriately into the multivariate fashion, using stepwise variable selection. The results are summarized as HRs, ORs, and 95% CIs. ROC curves were used to calculate the AUC, while we calculated the sensitivity and specificity rates to determine the optimal cutoff value. Statistical analyses were performed using JMP 7.0 (SAS Institute, Cary, NC, USA) and R (version 3.5.0; R Foundation for Statistical Computing, Vienna, Austria) software.
Acknowledgments
We would like to thank Tomomi Shimamoto for excellent assistance. This research was supported by Health and Labor Sciences Research Grant no. KH24R0020. This study was presented at the meeting of the American Heart Association Scientific Sessions, Philadelphia, Pennsylvania (November 2019).
Author contributions
Conception and design: S.K.; interpretation of data: K.T., Y.Y., H.H., D.Y., T.K., A.K., N.K., T.U., T.K., K.N., F.S., T.O., and Y.S.; drafting of the manuscript or revising it critically for important intellectual content: S.M., Y.I., and H.I.; statistical analysis: T.Y.; final approval of the manuscript submitted: Y.S.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2021.01.004.
Contributor Information
Satoshi Kainuma, Email: s.kainuma19780320@gmail.com.
Yoshiki Sawa, Email: sawasec@surg1.med.osaka-u.ac.jp.
Supplemental information
References
- 1.Shah A.M., Mann D.L. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet. 2011;378:704–712. doi: 10.1016/S0140-6736(11)60894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilic A., Acker M.A., Atluri P. Dealing with surgical left ventricular assist device complications. J. Thorac. Dis. 2015;7:2158–2164. doi: 10.3978/j.issn.2072-1439.2015.10.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakatani T., Fukushima N., Ono M., Saiki Y., Matsuda H., Nunoda S., Sawa Y., Isobe M. The registry report of heart transplantation in Japan (1999–2014) Circ. J. 2016;80:44–50. doi: 10.1253/circj.CJ-15-0975. [DOI] [PubMed] [Google Scholar]
- 4.Fukushima N., Ono M., Saiki Y., Sawa Y., Nunoda S., Isobe M. Registry report on heart transplantation in Japan (June 2016) Circ. J. 2017;81:298–303. doi: 10.1253/circj.CJ-16-0976. [DOI] [PubMed] [Google Scholar]
- 5.Miyagawa S., Domae K., Yoshikawa Y., Fukushima S., Nakamura T., Saito A., Sakata Y., Hamada S., Toda K., Pak K. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. J. Am. Heart Assoc. 2017;6:e003918. doi: 10.1161/JAHA.116.003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawa Y., Yoshikawa Y., Toda K., Fukushima S., Yamazaki K., Ono M., Sakata Y., Hagiwara N., Kinugawa K., Miyagawa S. Safety and efficacy of autologous skeletal myoblast sheets (TCD-51073) for the treatment of severe chronic heart failure due to ischemic heart disease. Circ. J. 2015;79:991–999. doi: 10.1253/circj.CJ-15-0243. [DOI] [PubMed] [Google Scholar]
- 7.Menasché P., Alfieri O., Janssens S., McKenna W., Reichenspurner H., Trinquart L., Vilquin J.T., Marolleau J.P., Seymour B., Larghero J. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 8.Heusch G., Libby P., Gersh B., Yellon D., Böhm M., Lopaschuk G., Opie L. Cardiovascular remodelling in coronary artery disease and heart failure. Lancet. 2014;383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns R.J., Gibbons R.J., Yi Q., Roberts R.S., Miller T.D., Schaer G.L., Anderson J.L., Yusuf S., CORE Study Investigators The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J. Am. Coll. Cardiol. 2002;39:30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 10.Larose E., Rodés-Cabau J., Pibarot P., Rinfret S., Proulx G., Nguyen C.M., Déry J.P., Gleeton O., Roy L., Noël B. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2010;55:2459–2469. doi: 10.1016/j.jacc.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Kainuma S., Miyagawa S., Fukushima S., Pearson J., Chen Y.C., Saito A., Harada A., Shiozaki M., Iseoka H., Watabe T. Cell-sheet therapy with omentopexy promotes arteriogenesis and improves coronary circulation physiology in failing heart. Mol. Ther. 2015;23:374–386. doi: 10.1038/mt.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura M., Miyagawa S., Fukushima S., Saito A., Miki K., Ito E., Sougawa N., Kawamura T., Daimon T., Shimizu T. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation. 2013;128(11, Suppl 1):S87–S94. doi: 10.1161/CIRCULATIONAHA.112.000366. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T., Kawamura T., Kuratani T., Daimon T., Shimizu T. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126(11, Suppl 1):S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 14.Sekiya N., Matsumiya G., Miyagawa S., Saito A., Shimizu T., Okano T., Kawaguchi N., Matsuura N., Sawa Y. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J. Thorac. Cardiovasc. Surg. 2009;138:985–993. doi: 10.1016/j.jtcvs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Narita T., Shintani Y., Ikebe C., Kaneko M., Harada N., Tshuma N., Takahashi K., Campbell N.G., Coppen S.R., Yashiro K. The use of cell-sheet technique eliminates arrhythmogenicity of skeletal myoblast-based therapy to the heart with enhanced therapeutic effects. Int. J. Cardiol. 2013;168:261–269. doi: 10.1016/j.ijcard.2012.09.081. [DOI] [PubMed] [Google Scholar]
- 16.Shudo Y., Miyagawa S., Ohkura H., Fukushima S., Saito A., Shiozaki M., Kawaguchi N., Matsuura N., Shimizu T., Okano T. Addition of mesenchymal stem cells enhances the therapeutic effects of skeletal myoblast cell-sheet transplantation in a rat ischemic cardiomyopathy model. Tissue Eng. Part A. 2014;20:728–739. doi: 10.1089/ten.tea.2012.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito S., Miyagawa S., Sakaguchi T., Imanishi Y., Iseoka H., Nishi H., Yoshikawa Y., Fukushima S., Saito A., Shimizu T. Myoblast sheet can prevent the impairment of cardiac diastolic function and late remodeling after left ventricular restoration in ischemic cardiomyopathy. Transplantation. 2012;93:1108–1115. doi: 10.1097/TP.0b013e31824fd803. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa S., Saito A., Sakaguchi T., Yoshikawa Y., Yamauchi T., Imanishi Y., Kawaguchi N., Teramoto N., Matsuura N., Iida H. Impaired myocardium regeneration with skeletal cell sheets—a preclinical trial for tissue-engineered regeneration therapy. Transplantation. 2010;90:364–372. doi: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 19.Elsässer A., Schlepper M., Klövekorn W.P., Cai W.J., Zimmermann R., Müller K.D., Strasser R., Kostin S., Gagel C., Münkel B. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation. 1997;96:2920–2931. doi: 10.1161/01.cir.96.9.2920. [DOI] [PubMed] [Google Scholar]
- 20.Bax J.J., Visser F.C., Poldermans D., Elhendy A., Cornel J.H., Boersma E., van Lingen A., Fioretti P.M., Visser C.A. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation. 2001;104(12, Suppl 1):I314–I318. doi: 10.1161/hc37t1.094853. [DOI] [PubMed] [Google Scholar]
- 21.Bondarenko O., Beek A.M., Twisk J.W., Visser C.A., van Rossum A.C. Time course of functional recovery after revascularization of hibernating myocardium: a contrast-enhanced cardiovascular magnetic resonance study. Eur. Heart J. 2008;29:2000–2005. doi: 10.1093/eurheartj/ehn266. [DOI] [PubMed] [Google Scholar]
- 22.White H.D., Norris R.M., Brown M.A., Brandt P.W., Whitlock R.M., Wild C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 23.Hamer A.W., Takayama M., Abraham K.A., Roche A.H., Kerr A.R., Williams B.F., Ramage M.C., White H.D. End-systolic volume and long-term survival after coronary artery bypass graft surgery in patients with impaired left ventricular function. Circulation. 1994;90:2899–2904. doi: 10.1161/01.cir.90.6.2899. [DOI] [PubMed] [Google Scholar]
- 24.Athanasuleas C.L., Buckberg G.D., Stanley A.W., Siler W., Dor V., Di Donato M., Menicanti L., Almeida de Oliveira S., Beyersdorf F., Kron I.L., RESTORE group Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J. Am. Coll. Cardiol. 2004;44:1439–1445. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi K., Nakano S., Kawashima Y., Sakai K., Kawamoto T., Sakaki S., Kobayashi J., Morimoto S., Matsuda H. Left ventricular ejection performance, wall stress, and contractile state in aortic regurgitation before and after aortic valve replacement. Circulation. 1990;82:798–807. doi: 10.1161/01.cir.82.3.798. [DOI] [PubMed] [Google Scholar]
- 26.Bonow R.O., Castelvecchio S., Panza J.A., Berman D.S., Velazquez E.J., Michler R.E., She L., Holly T.A., Desvigne-Nickens P., Kosevic D., STICH Trial Investigators Severity of remodeling, myocardial viability, and survival in ischemic LV dysfunction after surgical revascularization. JACC Cardiovasc. Imaging. 2015;8:1121–1129. doi: 10.1016/j.jcmg.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonow R.O., Maurer G., Lee K.L., Holly T.A., Binkley P.F., Desvigne-Nickens P., Drozdz J., Farsky P.S., Feldman A.M., Doenst T., STICH Trial Investigators Myocardial viability and survival in ischemic left ventricular dysfunction. N. Engl. J. Med. 2011;364:1617–1625. doi: 10.1056/NEJMoa1100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzello V., Poldermans D., Biagini E., Schinkel A.F., Boersma E., Boccanelli A., Marwick T., Roelandt J.R.T.C., Bax J.J. Prognosis of patients with ischaemic cardiomyopathy after coronary revascularisation: relation to viability and improvement in left ventricular ejection fraction. Heart. 2009;95:1273–1277. doi: 10.1136/hrt.2008.163972. [DOI] [PubMed] [Google Scholar]
- 29.Bax J.J., Wijns W., Cornel J.H., Visser F.C., Boersma E., Fioretti P.M. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J. Am. Coll. Cardiol. 1997;30:1451–1460. doi: 10.1016/s0735-1097(97)00352-5. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins R., Mandarano L., Gugathas S., Kaski J.C., Anderson L., Banerjee D. Impaired renal function affects clinical outcomes and management of patients with heart failure. ESC Heart Fail. 2017;4:576–584. doi: 10.1002/ehf2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takami Y., Horio T., Iwashima Y., Takiuchi S., Kamide K., Yoshihara F., Nakamura S., Nakahama H., Inenaga T., Kangawa K., Kawano Y. Diagnostic and prognostic value of plasma brain natriuretic peptide in non-dialysis-dependent CRF. Am. J. Kidney Dis. 2004;44:420–428. [PubMed] [Google Scholar]
- 32.Ronco C., McCullough P., Anker S.D., Anand I., Aspromonte N., Bagshaw S.M., Bellomo R., Berl T., Bobek I., Cruz D.N., Acute Dialysis Quality Initiative (ADQI) consensus group Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur. Heart J. 2010;31:703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieger A.C., Myerburg R.J., Florea V., Tompkins B.A., Natsumeda M., Premer C., Khan A., Schulman I.H., Vidro-Casiano M., DiFede D.L. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine. 2019;48:377–385. doi: 10.1016/j.ebiom.2019.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy W.C., Mozaffarian D., Linker D.T., Sutradhar S.C., Anker S.D., Cropp A.B., Anand I., Maggioni A., Burton P., Sullivan M.D. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T., Yamato M., Isoi Y., Akutsu T., Setomaru T., Abe K., Kikuchi A., Umezu M., Okano T. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res. 2002;90:e40. doi: 10.1161/hh0302.105722. [DOI] [PubMed] [Google Scholar]
- 36.Kainuma S., Taniguchi K., Daimon T., Sakaguchi T., Funatsu T., Miyagawa S., Kondoh H., Takeda K., Shudo Y., Masai T. Mitral valve repair for medically refractory functional mitral regurgitation in patients with end-stage renal disease and advanced heart failure. Circulation. 2012;126(11, Suppl 1):S205–S213. doi: 10.1161/CIRCULATIONAHA.111.077768. [DOI] [PubMed] [Google Scholar]
- 37.Kainuma S., Toda K., Miyagawa S., Yoshikawa Y., Hata H., Yoshioka D., Kawamura T., Kawamura A., Ueno T., Kuratani T. Restrictive mitral annuloplasty with or without coronary artery bypass grafting in ischemic mitral regurgitation. ESC Heart Fail. 2020;7:1560–1570. doi: 10.1002/ehf2.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kainuma S., Taniguchi K., Toda K., Funatsu T., Miyagawa S., Kondoh H., Masai T., Otake S., Yoshikawa Y., Nishi H. Restrictive mitral annuloplasty with or without surgical ventricular reconstruction in ischaemic cardiomyopathy: impacts on neurohormonal activation, reverse left ventricular remodelling and survival. Eur. J. Heart Fail. 2014;16:189–200. doi: 10.1002/ejhf.24. [DOI] [PubMed] [Google Scholar]
- 39.Kainuma S., Taniguchi K., Daimon T., Sakaguchi T., Funatsu T., Kondoh H., Miyagawa S., Takeda K., Shudo Y., Masai T., Osaka Cardiovascular Surgery Research (OSCAR) Group Does stringent restrictive annuloplasty for functional mitral regurgitation cause functional mitral stenosis and pulmonary hypertension? Circulation. 2011;124(11, Suppl):S97–S106. doi: 10.1161/CIRCULATIONAHA.110.013037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.