Abstract
Water excretion by the kidney is regulated by the neurohypophyseal peptide hormone vasopressin through actions in renal collecting duct cells to regulate the water channel protein aquaporin-2. Vasopressin signaling is initiated by binding to a G-protein–coupled receptor called V2R, which signals through heterotrimeric G-protein subunit Gsα, adenylyl cyclase 6, and activation of the cAMP-regulated protein kinase (PKA). Signaling events coupling PKA activation and aquaporin-2 regulation were largely unknown until the advent of modern protein mass spectrometry techniques that allow proteome-wide quantification of protein phosphorylation changes (phosphoproteomics). This short review documents phosphoproteomic findings in collecting duct cells describing the response to V2R-selective vasopressin agonists and antagonists, the response to CRISPR-mediated deletion of PKA, results from in vitro phosphorylation studies using recombinant PKA, the response to the broad-spectrum kinase inhibitor H89 (N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide), and the responses underlying lithium-induced nephrogenic diabetes insipidus. These phosphoproteomic data sets have been made available online for modeling vasopressin signaling and signaling downstream from other G-protein-coupled receptors.
SIGNIFICANCE STATEMENT
New developments in protein mass spectrometry are facilitating progress in identification of signaling networks. Using mass spectrometry, it is now possible to identify and quantify thousands of phosphorylation sites in a given cell type (phosphoproteomics). The authors describe the use of phosphoproteomics technology to identify signaling mechanisms downstream from a G-protein-coupled receptor, the vasopressin V2 subtype receptor, and its role of the regulation and dysregulation of water excretion in the kidney. Data from multiple phosphoproteomic data sets are provided as web-based resources.
Introduction
Renal water excretion is controlled by the peptide hormone vasopressin largely through regulation of the water channel aquaporin-2 (AQP2) in collecting duct principal cells (Fig. 1). The response is mediated by a G-protein–coupled receptor, the vasopressin V2 receptor (V2R, gene symbol: Avpr2). The V2R signals via the heterotrimeric G-protein stimulatory α subunit, Gsα (Erlenbach et al., 2001), which links to activation of adenylyl cyclase 6 in collecting duct cells (Roos et al., 2012), resulting in increased cAMP production. The V2R can also signal through binding of β arrestins, which act as scaffolds for elements of the ERK mitogen-activated protein kinase cascade, thereby increasing ERK activation in response to ligand binding (Tohgo et al., 2003) (See Effect of Vasopressin on the Phosphoproteome of the Renal Collecting Duct and V2 Receptor Antagonists and Hyponatremic Disorders: How Vaptans Affect the Collecting Duct Phosphoproteome.).
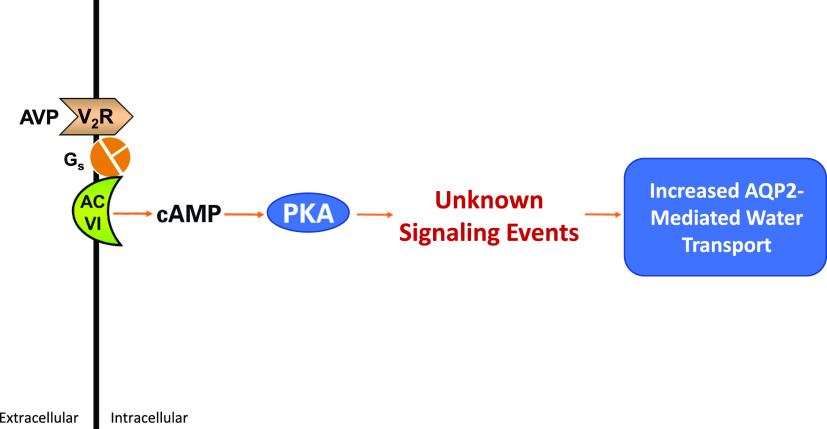
Fig. 1.
Vasopressin signaling in collecting duct cells of the kidney. The physiologic responses at a cellular level are listed in Table 1. AC VI, adenylyl cyclase 6.
Renal collecting ducts can be microdissected from rodent or rabbit kidneys and perfused in vitro to study the water permeability response to vasopressin (Burg et al., 1966). Using this technique, it has been observed that addition of vasopressin results in a rapid increase in transepithelial water permeability (Grantham and Burg, 1966; Wall et al., 1992). The increase begins after an approximately 40-second delay and requires approximately 20 minutes for completion (Wall et al., 1992). If isolated perfused collecting ducts are exposed to cAMP analogs, the transepithelial water permeability increases with a time course similar to that seen with vasopressin (Grantham and Burg, 1966; Star et al., 1988; Wall et al., 1992), implying that the signaling processes that are essential for the water permeability response to vasopressin are cAMP-dependent. The two known effectors of cAMP in collecting duct cells are protein kinase A (PKA) and exchange protein directly activated by cAMP (EPAC) 1 and EPAC2 (coded by Rapgef3 and Rapgef4). Deletion of PKA in collecting duct cells abolishes nearly all of the protein phosphorylation changes associated with vasopressin stimulation, in addition to eliminating Aqp2 gene transcription (Isobe et al., 2017; Datta et al., 2020). On the other hand, EPAC gene deletions did not have a pronounced effect on AQP2 regulation when knocked out in mice (Cherezova et al., 2019), although EPAC has been implicated in vasopressin-induced calcium mobilization (Yip, 2006). Thus, in collecting duct cells, the major aspects of vasopressin signaling are mediated by PKA.
Knowledge of the upstream aspects of vasopressin signaling in the collecting duct through activation of PKA is well established (Fig. 1). Signaling downstream from PKA leading to regulation of AQP2 activity in collecting duct cells is less well understood. Discovery of the relevant signaling pathways in collecting duct cells and how they are changed in disorders of water balance is of interest because of the high prevalence of such disorders. A relatively new tool that is shedding light on vasopressin signaling in health and disease is protein mass spectrometry, especially the use of protein mass spectrometry to identify and quantify phosphorylation events in the cell (phosphoproteomics). The goal of this review is to summarize progress from phosphoproteomic analysis of AQP2-expressing collecting duct cells to identify signaling changes seen in response to vasopressin, signaling changes resulting from deletion of PKA in collecting duct cells, identification of PKA substrates, signaling changes seen in polyuric disorders, and signaling changes associated with water-retaining (hyponatremic) disorders. We also summarize essential features of phosphoproteomic methodology, including bioinformatic analysis and data integration.
Cellular Physiology of AQP2-Expressing Renal Collecting Duct Cells
Detailed studies over several decades have identified a number of cellular-level processes that are regulated in response to vasopressin in collecting duct cells (Table 1) (Ganote et al., 1968; Kirk et al., 1984; Star et al., 1988; Mishler et al., 1990; Champigneulle et al., 1993; Nielsen and Knepper, 1993; Nielsen et al., 1993, 1995; Simon et al., 1993; Naruse et al., 1995; Sabolić et al., 1995; Chou et al., 2000, 2004, 2008; Tamma et al., 2001; Brown, 2003; Yamaguchi et al., 2003; Nunes et al., 2008; Hasler et al., 2009; Nedvetsky et al., 2010; Khositseth et al., 2011; Miller et al., 2013; Sandoval et al., 2013, 2016; Loo et al., 2013). The most thoroughly studied of these processes are the actions of vasopressin to increase Aqp2 gene transcription (Hasler et al., 2002; Sandoval et al., 2016) and the action of vasopressin to regulate membrane trafficking of the AQP2 protein to increase its abundance in the plasma membrane of collecting duct cells (Nielsen et al., 1995). Detailed discussion of the cellular level responses in Table 1 is beyond the scope of this treatise but can be found in prior review articles (Knepper, 1997; Knepper and Inoue, 1997; Brown et al., 1998; Sasaki et al., 1998; Nielsen et al., 1999, 2002; Verkman, 1999; Klussmann and Rosenthal, 2001; Brown, 2003; Noda and Sasaki, 2005; Valenti et al., 2005; Bichet, 2006; Boone and Deen, 2008; Moeller and Fenton, 2012; Fenton et al., 2013; Jung and Kwon, 2016, 2019). One goal of this review is to map recently obtained phosphoproteomic data from both native and cultured collecting duct cells to the processes summarized in Table 1 to identify the signaling events responsible for the physiologic responses. The phosphoproteomic data sets discussed in this review are listed (with their hyperlink addresses) in Table 2. The data sets can also be interrogated at the Kidney Systems Biology Project website (https://hpcwebapps.cit.nih.gov/ESBL/Database/), which allows users to browse and search a large number of data sets acquired from proteomics and next-generation sequencing studies.
TABLE 1.
Cellular processes regulated by vasopressin in collecting duct principal cells
TABLE 2.
List of phosphoproteomic data sets; links to browsing and download sites
| Data Set Name | Publication Year | Cell Type | Experiment | Link |
|---|---|---|---|---|
| Phosphorylation sites in IMCD proteins – response to vasopressin | 2019 | Native rat IMCD cells | Response to V2 agonist dDAVP | https://hpcwebapps.cit.nih.gov/ESBL/Database/IMCD-Phos/ |
| Mouse mpkCCD phosphoprotein database | 2010 | Cultured mpkCCD cells | Response to V2 agonist dDAVP | http://helixweb.nih.gov/ESBL/Database/mpkCCDphos/ |
| Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of Aquaporin-2 phosphorylation at two sites | 2006 | Native rat IMCD cells | Response to V2 agonist dDAVP | https://big.nhlbi.nih.gov/index.jsp |
| TiPD | 2011 | Native rat IMCD cells | Time course of response to V2 agonist dDAVP | http://helixweb.nih.gov/ESBL/Database/TiPD/ |
| Phosphopeptides altered by PKA deletion in mouse mpkCCD cells | 2017 | Cultured mpkCCD cells | Effect of CRISPR-mediated deletion of both PKA catalytic genes | https://hpcwebapps.cit.nih.gov/ESBL/Database/PKAKO/ |
| IMCD phosphoproteome with acute lithium treatment | 2014 | Native rat IMCD cells | Response to short-term treatment of rats with lithium | http://helixweb.nih.gov/ESBL/Database/iPALT/ |
| Effect of satavaptan on phosphoproteome in rat inner medullary collecting duct | 2014 | Native rat IMCD cells | Effect of V2 antagonist satavaptan | https://hpcwebapps.cit.nih.gov/ESBL/Database/Satavaptan/ |
iTRAQ, isobaric tags for relative and absolute quantitation; TiPD, temporal iTRAQ phosphoproteomic database. IMCD, inner medullary collecting duct; mpkCCD, mouse epithelial cell culture line resembling cortical collecting duct.
Phosphoproteomic Methodology and Bioinformatics
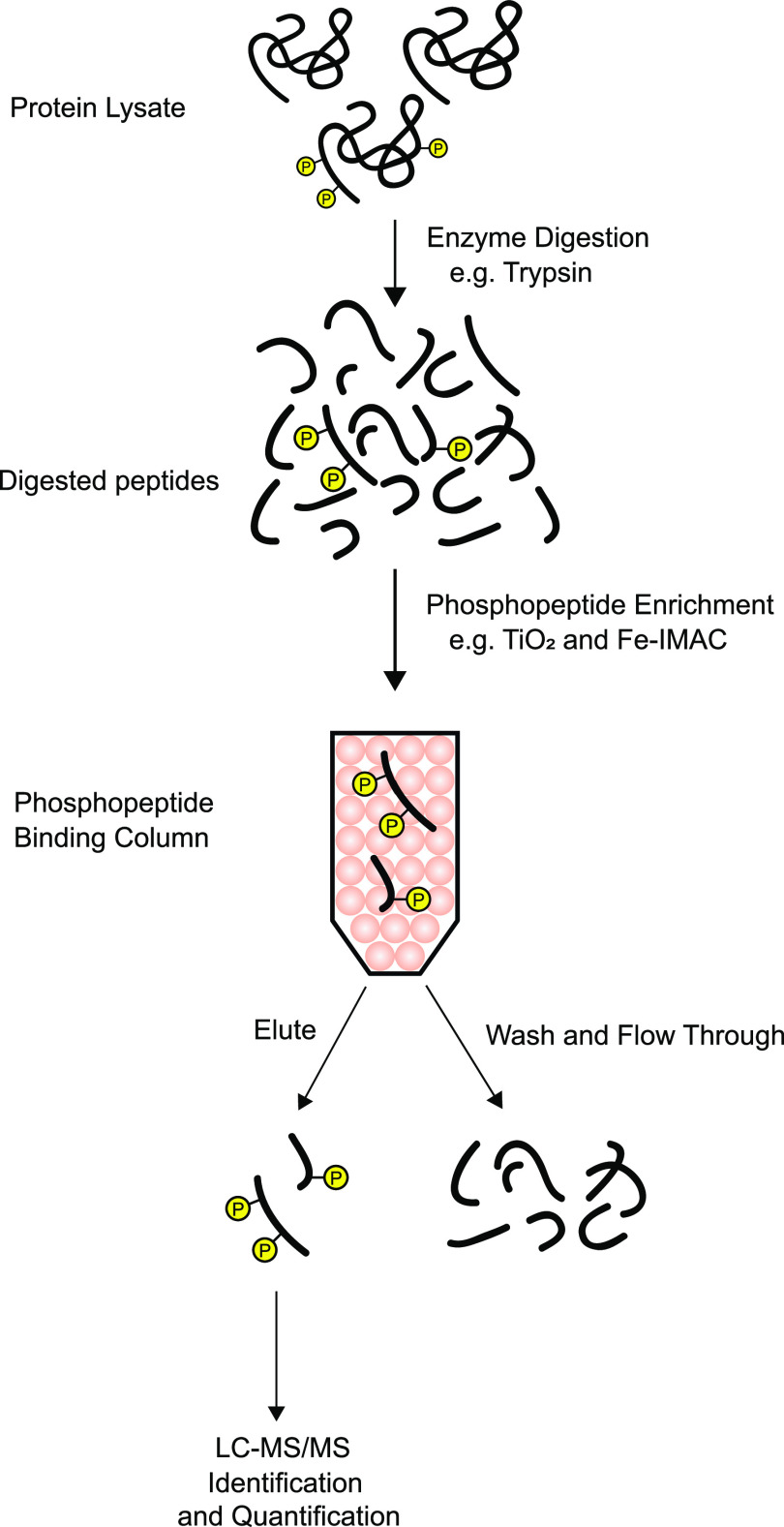
Full details of the methodology used for phosphoproteomic analysis are beyond the scope of this article. The underlying principles of the method are summarized in Fig. 2. The approach is a so-called bottom-up methodology in which proteins are digested with proteases having specific cleavage sites (most commonly trypsin, which cleaves at the carboxyl-terminal side of the amino acids lysine and arginine), and the resulting peptides are sequenced by tandem mass spectrometry coupled to a chromatographic method that stratifies peptide delivery to the mass spectrometer, so-called LC-MS/MS. For phosphoproteomics, additional chromatographic steps are included for phosphopeptide enrichment based on the negative charges of the added phosphate groups (Fig. 2). The mass spectrometer readout is a list of mass-to-charge ratio peaks and their intensities as a function of time. The amino acid sequence for a given peptide is typically determined by fragmentation (e.g., via high-energy collisions of the peptide ions with an inert gas), which produces a series of mass-to-charge ratio peaks that can be used to determine the sequence and phosphorylation site(s) by pattern matching. Many proprietary and open-source programs are available for this task.
Fig. 2.
Simplified basic protocol for phosphoproteomic analysis. A protein sample is subjected to proteolysis with a purified, recombinant protease (typically trypsin). Phosphopeptides are enriched through use of ion chromatography, e.g., with TiO2 or Fe-IMAC columns, which select peptides with negative charges. The column eluate is subjected to LC-MS/MS analysis to identify and quantify phosphopeptides, often after additional fractionation (not shown). IMAC, immobilized metal affinity chromatography. Circled P indicates phosphorylation.
Quantification is usually achieved using labeling techniques. For cells in culture, the most widely used method is SILAC (Ong et al., 2002). With this method, cells are grown in media containing amino acids in which different stable isotopes are incorporated, e.g., C12 and N14 in control cells and C13 and N15 in vasopressin-treated cells. This allows the same peptide to be seen by the mass spectrometer twice (light peptides for control cells, heavy peptides for vasopressin-treated cells in the above example). The relative abundance of a particular peptide in the two samples can be quantified by comparing the peak intensities.
A different quantification strategy, based on isobaric tagging, is usually used in phosphoproteomic analysis of native tissues (Cheng et al., 2016). An example of isobaric tagging methodology is TM (ThermoFisher Inc.), which allows simultaneous quantification of up to 11 multiplexed samples in a single LC-MS/MS run. With this method, chemical tags of equal molecular mass are covalently attached to the processed peptides, a different one for each sample, allowing the samples to be combined. The tagged peptides are fragmented in the mass spectrometer to generate a series of sample-specific reporter ion peaks whose heights give measures of the phosphopeptide abundance in each of the multiplexed input samples. This method is cost-effective because of multiplexing. It allows a high degree of precision and high throughput, allowing tens of thousands of phosphopeptides to be quantified in each LC-MS/MS run.
The tangible product from a quantitative phosphoproteomic analysis is a list of phosphopeptides with the following attributes: 1) parent protein indicated by official gene symbol, 2) the identified phosphorylation site(s), and 3) a quantitation value typically indicated as a ratio between experimental and control values. Added to this are statistical parameters that help to rank the responses with regard to the likelihood that a given response is not a false positive. Typically, three or more replicates are required for reliable statistical analysis.
The bioinformatic challenge is to map the phosphopeptide list to underlying biologic processes to generate hypotheses about molecular mechanisms. For this review, the relevant biologic processes are the ones listed in Table 1. One such process is vasopressin-mediated depolymerization of filamentous actin. The task, then, is to identify the phosphoproteins in a given data set that are involved in regulation of actin polymerization. This can be done with Gene Ontology (GO) biologic function terms. Biologic function terms for a set of regulated phosphoproteins can be identified by inputting the corresponding official gene symbols into computer programs such as the Automated Bioinformatics Extractor (https://helixweb.nih.gov/ESBL/ABE/). This program will provide all GO terms for each regulated phosphoprotein. After transferring this information to a spreadsheet, it is relatively easy to identify which phosphoproteins have GO terms involving actin polymerization. These proteins and their regulated phosphorylation sites can then be studied further, e.g., through use of genome editing techniques (CRISPR-Cas9) (Isobe et al., 2020) to block gene expression or to mutate the phosphorylated amino acid. Usually, however, prior to such reductionist approaches, it is wise to use Bayesian analysis (Bradford et al., 2014; Xue et al., 2017) to assess the strength of prior data pointing to a particular phosphoprotein as a candidate for a mechanistic role in a targeted biologic process.
Another important bioinformatic task is the identification of the protein kinases responsible for observed changes in phosphorylation. It is well known that many protein kinases have sequence preferences. For example, PKA prefers serines or threonines in a motif (R/K)-(R/K)-X-p(S/T)-, where (R/K) indicates either arginine or lysine, X indicates any amino acid, and p(S/T) indicates phosphorylated serine or threonine. Thus, the sequence surrounding a phosphorylation site can be used to narrow down the possible kinases that could phosphorylate that site. An equally important factor that determines kinase/target interactions is colocalization in the cell. Proteomic analysis of subcellular fractions (Bolger et al., 2012; Schenk et al., 2012; Yang et al., 2015; Pickering et al., 2016) can be used to find candidate protein kinases that could target particular phosphoproteins based on colocalization.
Often, regulated phosphoproteins are themselves protein kinases, and these kinases become candidates for mediation of regulated phosphorylation. In a minority of cases, the effect of a given phosphorylation event on kinase activity is known from prior research; these sites can often be identified using data available on two commercial websites, namely PhosphositePlus (https://www.phosphosite.org/) and PhosphoNet (http://www.phosphonet.ca/). When such prior data are available, the change of kinase activity in a particular experiment can be inferred.
To provide a tool for cross-comparing the different data sets described in this paper (Table 2), we used a database program, MySQL, and provided a web-based interrogation interface (https://big.nhlbi.nih.gov/). Users can enter official gene symbols in the submission box, and the output will be all of the quantified sites for that protein in any of the phosphoproteomic studies reported in this paper. Other types of -omics data are included for reference. For example, entering "Ctnnb1" (β catenin) revealed that Ser552 phosphorylation is increased by vasopressin in both native inner medullary collecting duct cells and cultured mouse mpkCCD cells, whereas the V2R antagonist decreases phosphorylation, and lithium treatment had no effect.
Effect of Vasopressin on the Phosphoproteome of the Renal Collecting Duct
The simplest experiment to identify vasopressin signaling pathways in the renal collecting duct is to expose collecting duct cells to vasopressin or its vehicle and carry out mass spectrometry–based quantitative phosphoproteomics. Experiments following this strategy have been done in both cultured collecting duct cells (mouse mpkCCD) (Rinschen et al., 2010; Datta et al., 2020) and suspensions of native inner medullary collecting duct cells from rats (Hoffert et al., 2006, 2012; Deshpande et al., 2019). The studies have used a V2R–selective vasopressin analog desmopressin, D-amino D-arginine vasopressin (dDAVP), which is used clinically for treatment of central diabetes insipidus (Christensen and Rittig, 2006; Oiso et al., 2013). Over the years, protein mass spectrometry has become more and more sensitive, resulting in the ability to quantify more and more phosphorylation sites (Table 3). Despite a relative lack of sensitivity in the earliest phosphoproteomic studies of the vasopressin response in collecting duct cells, they provided critical information that spurred progress, e.g., the identification of a cluster of four vasopressin-regulated phosphorylation sites in the COOH-terminal tail of AQP2 that are critical to regulation of AQP2 trafficking (Hoffert et al., 2006, 2008, 2012; Bansal et al., 2010; Rinschen et al., 2010). However, only in the last 2 or 3 years have we begun to approach comprehensive phosphorylation site quantification in phosphoproteomic analysis.
TABLE 3.
Studies in which phosphoprotemic response to vasopressin was measured. Progress has been marked by a progressive improvement in sensitivity of phosphoproteomic methods used.
| Year | Reference | Tissue | Number of Phosphopeptides Quantified | Quantification Method | Number of Phosphorylation Sites Changed | Number of Phosphorylation Sites Increased |
|---|---|---|---|---|---|---|
| 2006 | Hoffert et al., 2006 | Rat IMCD suspensions | 17 | Label free | 9 | 4 |
| 2010 | Rinschen et al., 2010 | Mouse mpkCCD cells | 338 | SILAC | 45 | 18 |
| 2012 | Hoffert et al., 2012 | Rat IMCD suspensions | 1427 | Isobaric tags (iTRAQ) | 44 | 30 |
| 2019 | Deshpande et al., 2019 | Rat IMCD suspensions | 10,738 | Isobaric tags (TMT) | 219 | 156 |
| 2020 | Datta et al., 2020 | Mouse mpkCCD cells | 19,221 | SILAC | 452 | 205 |
IMCD, inner medullary collecting duct; iTRAQ, isobaric tags for relative and absolute quantitation; mpkCCD, mouse epithelial cell culture line resembling cortical collecting duct; SILAC, stable isotope labeling in cell culture; TMT, tandem mass tag.
A general finding in all phosphoproteomic studies of the vasopressin response in collecting duct cells is that only a small fraction (typically around 2%) of phosphorylation sites among those quantified exhibit changes in response to vasopressin. This finding is compatible with the idea that control of physiologic processes via GPCR-dependent signaling is highly selective with regard to phosphorylation targets. Hence, comprehensive analysis of phosphorylation changes has the potential to uncover specific cellular processes that are selectively regulated. Motif analysis in these studies demonstrates that the phosphorylation sites increased in abundance by vasopressin are dominated by those that fit the motif –(R/K)-(R/K)-X-p(S/T), where (R/K) means either arginine or lysine, X means any amino acid, and p(S/T) means phosphorylation of either serine or threonine. This motif is similar to that associated with the action of PKA but is also consistent with a number of protein kinases, including protein kinase G (Prkg1or Prkg2) (Miller et al., 2008), myotonic dystrophy protein kinase (Dmpk or related kinases) (Miller et al., 2008), and death-associated protein kinase (Dapk1, Dapk2, or Dapk3) (Douglass et al., 2012). In contrast, the phosphorylation sites decreased in abundance in response to vasopressin are dominated by those that fit the motif -X-p(S/T)-P, where P is proline, here in position +1 relative to the phosphorylated residue. Such phosphorylation sites are typically targets of members of two families of protein kinases: the cyclin-dependent kinases (CDKs) and the mitogen-activated kinases (MAPKs). This finding suggests that vasopressin signaling results in inactivation of one or more of these “proline-directed” kinases. Indeed, vasopressin has been found to reduce activation of both ERK1 and ERK2 (Mapk3 and Mapk1, respectively) in collecting duct cells (Pisitkun et al., 2008; Rinschen et al., 2010). The fall in ERK activity in response to vasopressin contrasts with β arrestin–mediated GPCR-dependent activation of the MAPK signaling seen with some GPCRs (Luttrell et al., 2001). Therefore, these results suggest that, with regard to vasopressin signaling in the collecting duct, cAMP-mediated signaling dominates over β arrestin signaling. There is also evidence that vasopressin increases the activity of a proline-directed protein phosphatase called PP2A, through PKA-mediated phosphorylation of a phosphatase inhibitor, cAMP-regulated phosphoprotein 19 (Arpp19) (Deshpande et al., 2019), which could explain some of the downregulated -X-p(S/T)-P sites seen in response to vasopressin.
Vasopressin V2 receptors are also present in two renal cell types that do not express AQP2, namely distal convoluted tubule cells and thick ascending limb cells (Lee et al., 2015), in which vasopressin regulates Na-Cl reabsorption from the tubule lumen to the blood. Large-scale phosphoproteomic studies in cultured distal convoluted tubule cells (Cheng et al., 2015) and suspensions of thick ascending limb cells (Gunaratne et al., 2010) showed that vasopressin elicits a response that is very similar to that seen in collecting duct cells, with increases at basic sites with the motif –(R/K)-(R/K)-X-p(S/T) and decreases in sites with the consensus proline-dependent motif -X-p(S/T)-P. Thus, the general characteristics of the response to vasopressin acting through the V2 receptor are independent of target cell type. Furthermore, parathyroid hormone binding to the parathyroid hormone receptor, a Gsα-coupled receptor, in MC3T3-E1 preosteoblast cells produced the same general pattern of phosphorylation changes (Williams et al., 2016), demonstrating that this response pattern is not unique to the V2 receptor or to the kidney.
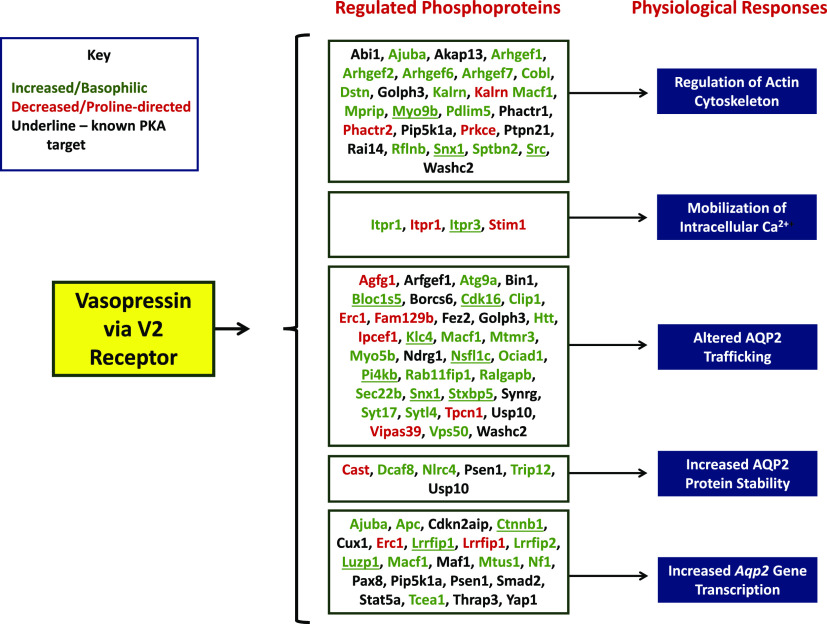
Figure 3 shows some of the results from the most comprehensive vasopressin-response phosphoproteomic study in native collecting duct cells (Deshpande et al., 2019). Several of the phosphoprotein targets of vasopressin signaling mapped to cellular physiologic functions corresponding to those in Table 1. Many of those that had basophilic phosphorylation sites that increased with vasopressin are known targets of PKA (underlined, Fig. 3), cementing the conclusion that vasopressin signaling is largely mediated by PKA activation. Nevertheless, some elements of vasopressin signaling appear to be PKA-independent (Datta et al., 2020). This includes PKA-independent, vasopressin-induced phosphorylation changes associated with activation of one or more kinases of the AMPK-related kinase family (also known as SNF1-family kinases). It also includes increased phosphorylation of the protein kinase coded by Stk39 (commonly called "SPAK") at a site associated with its activation and decreased phosphorylation and decreased activation of atypical protein kinase C iota.
Fig. 3.
Mapping of vasopressin-responsive phosphoproteins to major physiologic effects of vasopressin at a cellular level. Proteins are indicated by their official gene symbols. Those showing increases in phosphorylation in response to vasopressin are indicated in green, whereas those showing decreases are indicated in red. Known PKA targets are underlined.
However, in the study of Deshpande et al. (2019), phosphorylation changes were seen in several other protein kinases at sites that mediate changes in catalytic activity. Specifically, protein kinase D1, PCTAIRE domain kinase 1 (Cdk16), PCTAIRE domain kinase 3 (Cdk18), and Srcundergo phosphorylation changes consistent with increases in activity in response to vasopressin, whereas Araf and Raf1 undergo apparent decreases in activity in response to vasopressin. Protein kinase D1, Cdk16, and Cdk18 are involved in regulation of membrane trafficking, whereas Src, Araf, and Raf1 regulate MAP kinase activities (Deshpande et al., 2019). The full data set describing the phosphoproteomic response to vasopressin in native rat collecting ducts is provided on a publicly accessible website (https://hpcwebapps.cit.nih.gov/ESBL/Database/IMCD-Phos/).
Because protein kinases must come into physical contact with their targets to phosphorylate them, phosphoproteomic analysis can be construed as a form of proximity analysis, potentially identifying subcompartments in the cell that contain the regulated forms of the relevant protein kinases. For example, in the Deshpande et al. (2019) analysis, it was concluded that PKA phosphorylates its targets predominantly at membrane surfaces, particularly targeting plasma membrane proteins.
Effect of PKA Deletion on the Phosphoproteome of the Renal Collecting Duct
It is widely accepted that the downstream effects of activation of the V2 vasopressin receptor are mediated chiefly by PKA (Fig. 1). To identify direct and indirect phosphorylation targets of PKA in collecting duct cells, Isobe et al. (2017) used a combination of genome editing and phosphoproteomics. CRISPR-Cas9 was used to eliminate PKA expression (deletion of both PKA catalytic α and PKA catalytic β) in mouse mpkCCD cells, a cultured cell model of the kidney collecting duct principal cell. The wild-type mpkCCD cells have been previously shown to display both long-term (transcriptional) and short-term (membrane trafficking) regulation of aquaporin-2 in response to vasopressin (Yu et al., 2009). The CRISPR/phosphoproteomics experiments identified 229 direct PKA phosphorylation sites, many of which were novel (Isobe et al., 2017). These phosphoproteins are mapped to specific cellular structures or functions in Fig. 4. Note that the indicated protein groups correspond closely to the known cellular effects of vasopressin in collecting duct cells (Table 1).
Fig. 4.
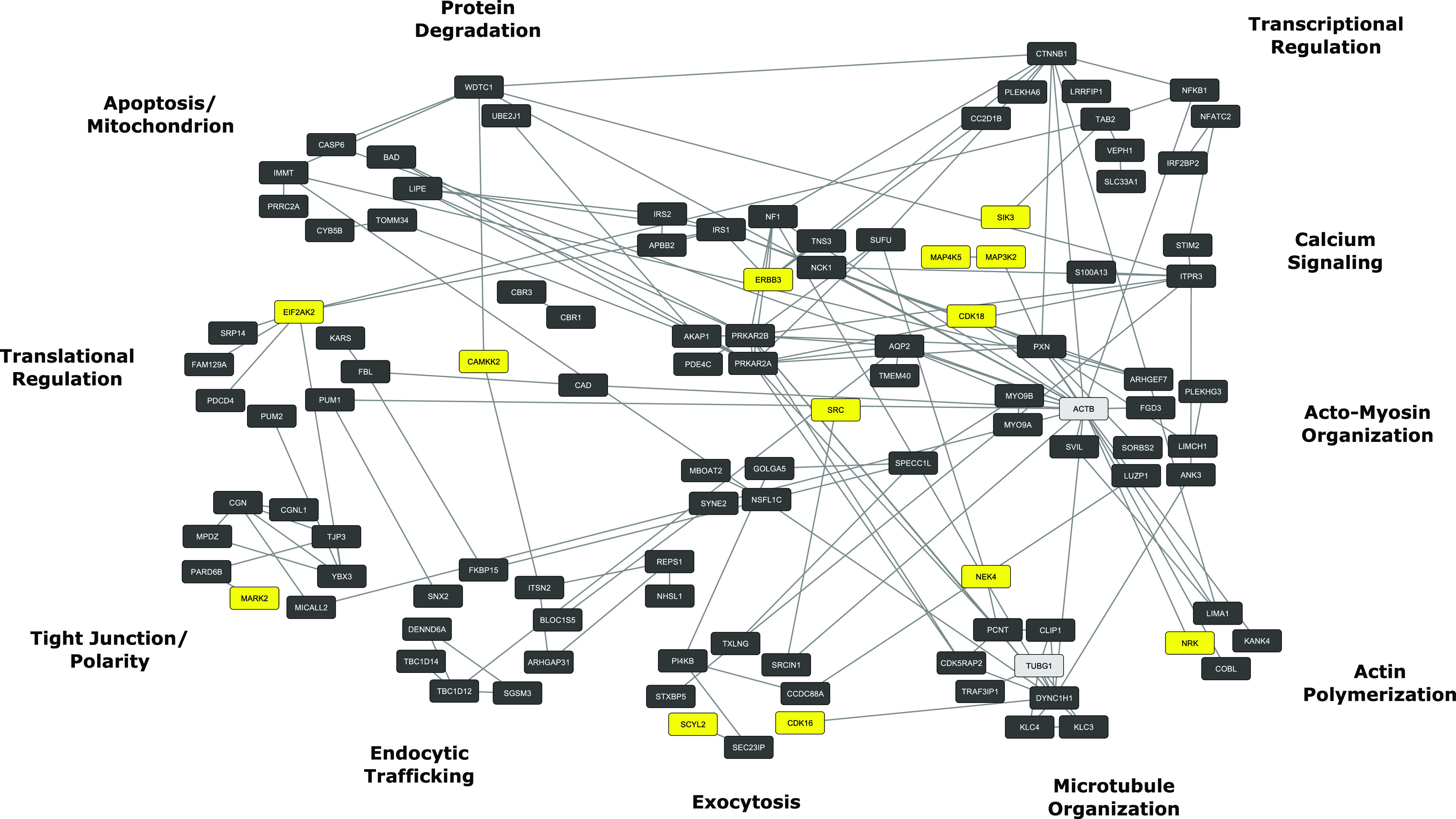
PKA signaling network. PKA target phosphoproteins were identified by deletion of PKA by genome editing techniques followed by SILAC-based phosphoproteomics (Isobe et al., 2017). Functional groups around the periphery correspond to vasopressin-regulated processes in Table 1. Yellow nodes indicate other protein kinases that undergo PKA-mediated phosphorylation. Gray nodes indicate non-PKA targets included to consolidate phosphoprotein clustering. Analysis used a computer application called STRING (https://string-db.org/) for initial mapping followed by additional classification by Gene Ontology biologic process terms. Final visualization used Cytoscape (https://cytoscape.org/).
The group 1 PKA targets (“Transcriptional Regulation,” Fig. 4) include two transcription factors, designated by official gene symbols Nfatc2 and Nfkb1. Members of the NFATc (Li et al., 2007) and NFκB (Hasler et al., 2008) transcription factor families have already been shown to regulate Aqp2 gene transcription. NFATc transcription factors are also regulated by PKA in another way (https://hpcwebapps.cit.nih.gov/ESBL/PKANetwork/Transcription.html), namely by increasing intracellular calcium through phosphorylation of inositol 1,4,5-triphosphate receptor 3 (Gene symbol: Itpr3), a calcium release channel. The vasopressin-mediated calcium mobilization in collecting duct cells is seen as calcium spikes that increase in frequency with addition of vasopressin (Yip, 2002; Pisitkun et al., 2008). The increased calcium activates the phosphatase calcineurin, which dephosphorylates NFATc, causing its translocation into the nucleus. Another group 1 PKA target, β catenin (Ctnnb1), functions as a transcriptional coregulator in the Wnt signaling pathway, which is important to collecting duct maturation and differentiation (Schmidt-Ott and Barasch, 2008).
Identification of Potential PKA Targets by In Vitro Phosphorylation and Mass Spectrometry
Another approach to the identification of potential PKA targets is to carry out in vitro incubation of dephosphorylated proteins with the recombinant active kinase, followed by mass spectrometry–based phosphoproteomics. This has been done in two studies aimed at identifying phosphorylation pathways involved in the regulation of aquaporin-2. One study used a mixture of dephosphorylated proteins from rat whole kidney, liver, brain, and small intestine (Douglass et al., 2012), whereas the other used dephosphorylated proteins isolated from rat inner medullary collecting duct (Bradford et al., 2014), incubating each with recombinant PKA catalytic α subunit. The data are available at https://hpcwebapps.cit.nih.gov/ESBL/Database/PKA-in-vitro/.
In general, these studies confirmed an R-R-X-p(S/T) substrate preference for PKA but also identified many PKA phosphorylation target sites that deviated from this motif, albeit with basic amino acids in at least one upstream position within four amino acids from the phosphorylated serine or threonine. It is recognized that, in the functioning cell, whether a kinase targets a particular substrate is dependent on two factors: kinase specificity and colocalization of the kinase with the substrate (Linding et al., 2008). Thus, in vitro phosphorylation of a particular phosphorylation site by PKA does not necessarily mean that that site will be phosphorylated by PKA in the intact cell.
Effect of Kinase Inhibitor H89 on Protein Phosphorylation in PKA-Null Collecting Duct Cells
N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide (H89) is often used as a PKA specific inhibitor to study the involvement of PKA in signaling pathways. However, evidence from cell-free experiments has suggested that H89 can also inhibit other protein kinases (Davies et al., 2000). Limbutara et al. (2019) used PKA-null and PKA-intact mouse cell lines derived from mpkCCD cells (see Effect of PKA Deletion on the Phosphoproteome of the Renal Collecting Duct) and quantitative phosphoproteomics to investigate the specificity of H89 over the range of concentrations commonly used in the literature. From a total of 14,507 phosphorylation sites quantified, the authors found that 402 phosphorylation sites were significantly changed in abundance in PKA-intact cells, and 217 sites were significantly changed in PKA-null cells. Concentration-response data are available at https://esbl.nhlbi.nih.gov/H89/. Analyses of sequence logos generated from significantly decreased phosphorylation sites in PKA-intact and PKA-null cells both revealed a preference for basic amino acids at position −3 and −2. Thus, H89 appears to inhibit basophilic kinases other than PKA in intact cells. Likely H89 targets include basophilic protein kinases such as protein kinase B, ribosomal S6 inase, AMP-activated protein kinase, and Rho kinase. Thus, the finding that a particular process is altered by H89 cannot be considered sufficient to conclude that PKA is involved in that process.
Polyuric Disorders: Phosphoproteomics of Lithium-Induced Nephrogenic Diabetes Insipidus
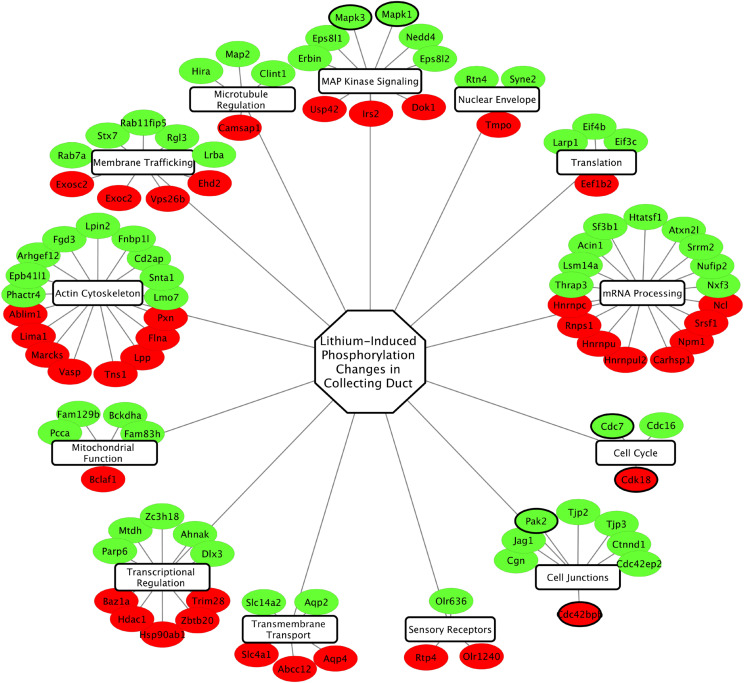
Several disorders of water balance are attributable to loss of vasopressin-mediated regulation of AQP2 in collecting duct cells. Phosphoproteomics can be used in animal models of these disorders to ascertain which signaling pathways are perturbed. In diabetes insipidus, patients excrete excessive amounts of water (polyuria) in an uncontrolled fashion. An example is lithium-induced nephrogenic diabetes insipidus (NDI). Lithium salts provide an effective treatment in many patients with psychiatric manic-depressive disorder (bipolar disorder), but the overall efficacy of lithium salts is limited by concomitant NDI (Knepper et al., 2015). Animal studies have revealed that the polyuria in lithium-induced NDI is due to a nearly complete loss of AQP2 protein from collecting duct cells (Marples et al., 1995) due to loss of Aqp2 gene transcription (Kortenoeven et al., 2012) associated with entry of the cells into the cell cycle with G2 arrest (de Groot et al., 2014). Because the protein kinase glycogen synthase kinase 3-beta (Gsk3β) is directly inhibited by lithium ions (Klein and Melton, 1996), prior studies have focused on the role of Gsk3β in the regulation of Aqp2 gene expression, showing that Gsk3β inhibition or deletion negatively regulates adenylyl cyclase activity (Rao et al., 2010). To carry out an unbiased assessment of signaling changes in collecting duct cells in response to short-term lithium administration in rats, we carried out quantitative phosphoproteomics in native inner medullary collecting ducts (Trepiccione et al., 2014). The study quantified 1093 unique phosphopeptides, including 152 that were increased and 56 that were decreased in response to lithium. The full data set is available online at https://helixweb.nih.gov/ESBL/Database/iPALT/. The phosphoprotein groups with phosphorylation sites that were altered by lithium treatment are shown in Fig. 5. A major finding was that active site phosphorylation in both ERK1 (MAPK3) and ERK2 (MAPK1) was increased in response to lithium, consistent with an increase in ERK activity, i.e., opposite to that seen in response to vasopressin. ERK has many targets, but an important set of targets are transcription factors in the ETS family and AP-1 family whose phosphorylation triggers an immediate early response (Frost et al., 1994; Gille et al., 1995; Kelly and Siebenlist, 1995; Murphy and Blenis, 2006). ERK-mediated phosphorylation of ETS and AP-1 transcription factors can lead to a series of transcriptional changes associated with entry into the cell cycle and dedifferentiation, potentially explaining the loss of AQP2 expression. Subsequent studies using RNA-Seq to identify mRNA changes in microdissected collecting ducts from lithium-treated rats were consistent with this model (Sung et al., 2019). One element of the response was a noncanonical activation of NFκB with selective upregulation of Nfkb2 and Relb transcripts. NFκB is a transcription factor ordinarily associated with inflammation, and its activation had previously been shown to repress Aqp2 gene expression (Hasler et al., 2008).
Fig. 5.
Lithium signaling network. Phosphoproteins altered in rat inner medullary collecting ducts by in vivo lithium administration were clustered according to protein function. Red nodes indicate proteins with phosphosites downregulated by lithium; green nodes indicate proteins with phosphosites that were upregulated by lithium. Nodes with thick borders are protein kinases. Original data were from Trepiccione et al. (2014).
A long-standing hypothesis about the mechanism of lithium inhibition of water transport in the collecting duct is that it inhibits cAMP production (Anger et al., 1990). A detailed transcriptomic analysis using RNA-Seq in collecting ducts microdissected from lithium-treated rats indeed showed a decrease in mRNA coding for the V2 receptor after prolonged exposure, consistent with a role of decreased cAMP production late in the response to lithium (Sung et al., 2019). Thus, based on the combination of phosphoproteomic studies and RNA-Seq studies, the loss of expression of the Aqp2 gene in lithium-induced nephrogenic diabetes insipidus appears to be due to several signaling changes in collecting duct cells. In particular, there is ERK activation triggering an immediate early gene expression pattern followed by an NFκB-dependent inflammatory-like response and, ultimately, decreased V2 receptor expression, all contributing to a decrease in Aqp2 gene transcription.
V2 Receptor Antagonists and Hyponatremic Disorders: How Vaptans Affect the Collecting Duct Phosphoproteome
Water balance abnormalities also include hyponatremic disorders in which patients reabsorb inappropriately large amounts of water from their collecting ducts, resulting in dilution of body fluids and hyponatremia (Knepper et al., 2015). Such disorders are quite common in hospitalized patients, with a prevalence of hyponatremia sometimes greater than 30% in internal medicine services in tertiary care hospitals (Upadhyay et al., 2006). Hyponatremia is sometimes associated with congestive heart failure and hepatic cirrhosis but is most frequently seen with the syndrome of inappropriate antidiuretic hormone hypersecretion. In these syndromes, hyponatremia is the result of nonosmotic release of vasopressin or other antidiuretic substances into the circulation (Knepper et al., 2015). One treatment option is nonpeptide antagonists of vasopressin receptors, the so-called vaptans. Vaptans that antagonize V2 receptors in the kidney have been used clinically to treat chronic hyponatremia (Schrier et al., 2006). In addition, one of the vaptans, namely tolvaptan, has been shown to slow the progression of autosomal dominant polycystic kidney disease (Torres et al., 2012) and has recently been approved by the US Food and Drug Administration for this purpose (Chebib et al., 2018).
Satavaptan was first described as a highly potent and selective V2R antagonist that inhibited vasopressin-stimulated adenylyl cyclase activity and produced a marked aquaretic response in rats (Serradeil-Le Gal et al., 1996). More recently, satavaptan has been demonstrated to act as an inverse agonist of Gsα-mediated vasopressin signaling (i.e., it can decrease adenylyl cyclase activity even in the absence of ligand stimulation) (Azzi et al., 2003) and can act as a partial agonist in the β arrestin pathway (i.e., it can recruit β arrestin and stimulate the MAP kinase pathway) (Azzi et al., 2003).
Hoffert et al. (2014) investigated the effects of satavaptan on vasopressin signaling using quantitative phosphoproteomics in native rat inner medullary collecting ducts. In general, for phosphorylation sites that are altered by vasopressin signaling, satavaptan had opposite effects, consistent with a predominance of antagonism of Gsα-mediated vasopressin signaling (Fig. 6). The authors reported a limited number of phosphorylation sites in which the response to vasopressin was in the same direction as the response to satavaptan and proposed that these sites could be downstream from β arrestin. β Arrestin signaling is generally associated with an activation of the MAP kinase pathway (Daaka et al., 1998). However, active site phosphorylation of ERK1 and ERK2 showed no change in response to satavaptan.
Fig. 6.
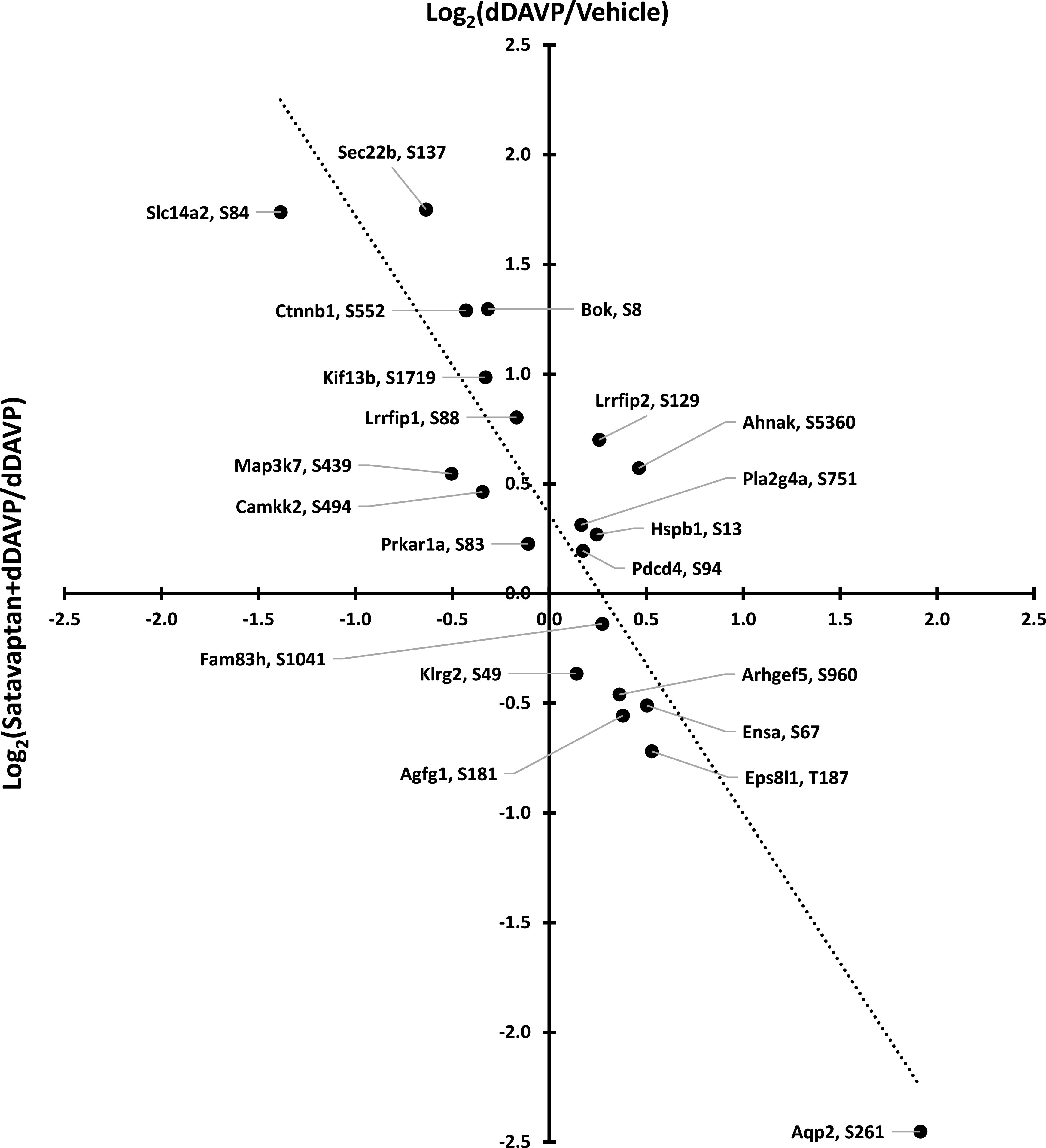
Comparison of phophoproteomic responses to a V2 vasopressin receptor antagonist satavaptan and to a V2 vasopressin receptor agonist dDAVP. Proteins are indicated by official gene symbols, with regulated phosphorylation sites indicated. Dotted line indicates best fit, linear regression.
Integration of Phosphoproteomic Results
An important goal in systems biology is to piece together information from various -omics studies to identify pathways and mechanisms involved in biologic responses. Ultimately, the objective is to derive causal models (directed graphs and associated differential equations) that can be used to organize mechanistic knowledge and make predictions. In our studies investigating vasopressin’s actions in the kidney, we want to create causal models that explain each of the vasopressin responses listed in Table 1. This endeavor can be viewed as consisting of two steps: 1) identifying the essential model elements (proteins) that will be “nodes” in the causal models and 2) identifying how these proteins interact (“edges” in the model). The phosphoproteomic studies described in this review focus mainly on the first step, identification of the elements of the model. It should be emphasized that protein phosphorylation and dephosphorylation are only a part of cell signaling, and other types of -omic data can fill in the information gap, including standard proteomics to detect proteins that change in abundance in response to vasopressin (Khositseth et al., 2011), proteomics of subcellular fractions that can identify translocation of proteins within cells in response to vasopressin (Bolger et al., 2012; Schenk et al., 2012; Yang et al., 2015; Pickering et al., 2016), quantification of other post-translational modifications such as acetylation (Hyndman et al., 2018), and the combination of chromatin precipitation coupled to mass spectrometry (Hwang et al., 2017) and chromatin precipitation coupled to DNA sequencing (Jung et al., 2018) that together can identify proteins that bind to chromatin and their sites of binding. The second step, identification of functional interactions between proteins, is more challenging. Often, functional interactions between two proteins are already documented in the literature and can be identified through fundamental tools like PubMed or more sophisticated tools such as STRING (https://string-db.org/) and Ingenuity Pathway Analysis (http://pages.ingenuity.com/rs/ingenuity/images/IPA_data_sheet.pdf), although the supporting data for these interactions are generally in different cell types than the cell of interest and need experimental confirmation. Ultimately, candidate signaling pathways and networks need to be tested experimentally, e.g., through protein overexpression or siRNA knockdowns/CRISPR deletions, etc., as well as time course studies that can test requirements for causality. Formal computational models can be used to predict the responses within complex networks. Ultimately, more formal tests of candidate models can be achieved by analyzing ensembles of phosphoproteomic data sets via structural equation modeling or path analysis (Stein et al., 2017).
Although the emphasis in this paper is on data sets describing responses to vasopressin in collecting duct cells and how vasopressin signaling may be perturbed in water balance disorders, we propose that the data will be useful for modeling PKA-dependent signaling pathways involving other receptors in other cell types. To facilitate the use of the data, all data sets can be browsed and/or downloaded at the Kidney Systems Biology Project website (https://hpcwebapps.cit.nih.gov/ESBL/Database/).
Abbreviations
- AMPK
AMP-activated protein kinase
- AQP2
aquaporin-2
- CDK
cyclin-dependent kinase
- Dapk
death-associated protein kinase
- dDAVP
D-amino D-arginine vasopressin
- EPAC
exchange protein directly activated by cAMP
- ERK
extracellular signal–regulated kinase
- GO
Gene Ontology
- GPCR
G-protein coupled receptor
- Gsα
heterotrimeric G-protein stimulatory alpha subunit
- H89
N-[2-p-bromocinnamylamino-ethyl]-5-isoquinolinesulphonamide
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
- MAPK
mitogen-activated protein kinase
- mpkCCD
a mouse epithelial cell line with characteristics of cortical collecting duct pricipal cells
- NDI
nephrogenic diabetes insipidus
- PKA
protein kinase A
- RNA-seq
RNA-sequencing
- SILAC
stable isotope labeling of amino acids in cultured cells
- V2R
type 2 vasopressin receptor
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Salhadar, Matthews, Raghuram, Limbutara, Yang, Datta, Chou, Knepper.
Footnotes
This research was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute [Projects ZIA-HL001285 and ZIA-HL006129].
Authors declare that there is no conflict of interest.
References
- Anger MS, Shanley P, Mansour J, Berl T (1990) Effects of lithium on cAMP generation in cultured rat inner medullary collecting tubule cells. Kidney Int 37:1211–1218. [DOI] [PubMed] [Google Scholar]
- Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Piñeyro G (2003) β-arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA 100:11406–11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou C-L, Boja ES, Wang G, Knepper MA (2010) Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol 21:303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet DG (2006) Lithium, cyclic AMP signaling, A-kinase anchoring proteins, and aquaporin-2. J Am Soc Nephrol 17:920–922. [DOI] [PubMed] [Google Scholar]
- Bolger SJ, Hurtado PA, Hoffert JD, Saeed F, Pisitkun T, Knepper MA (2012) Quantitative phosphoproteomics in nuclei of vasopressin-sensitive renal collecting duct cells. Am J Physiol Cell Physiol 303:C1006–C1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone M, Deen PMT (2008) Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch 456:1005–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford D, Raghuram V, Wilson JLL, Chou C-L, Hoffert JD, Knepper MA, Pisitkun T (2014) Use of LC-MS/MS and Bayes’ theorem to identify protein kinases that phosphorylate aquaporin-2 at Ser256. Am J Physiol Cell Physiol 307:C123–C139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D (2003) The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol 284:F893–F901. [DOI] [PubMed] [Google Scholar]
- Brown D, Katsura T, Gustafson CE (1998) Cellular mechanisms of aquaporin trafficking. Am J Physiol 275:F328–F331. [DOI] [PubMed] [Google Scholar]
- Burg M, Grantham J, Abramow M, Orloff J (1966) Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210:1293–1298. [DOI] [PubMed] [Google Scholar]
- Champigneulle A, Siga E, Vassent G, Imbert-Teboul M (1993) V2-like vasopressin receptor mobilizes intracellular Ca2+ in rat medullary collecting tubules. Am J Physiol 265:F35–F45. [DOI] [PubMed] [Google Scholar]
- Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, et al. (2018) A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol 29:2458–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Pisitkun T, Knepper MA, Hoffert JD (2016) Peptide labeling using isobaric tagging reagents for quantitative phosphoproteomics. Methods Mol Biol 1355:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Wu Q, Kortenoeven MLA, Pisitkun T, Fenton RA (2015) A systems level analysis of vasopressin-mediated signaling networks in kidney distal convoluted tubule cells. Sci Rep 5:12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C-L, Christensen BM, Frische S, Vorum H, Desai RA, Hoffert JD, de Lanerolle P, Nielsen S, Knepper MA (2004) Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J Biol Chem 279:49026–49035. [DOI] [PubMed] [Google Scholar]
- Chou CL, Yip KP, Michea L, Kador K, Ferraris JD, Wade JB, Knepper MA (2000) Regulation of aquaporin-2 trafficking by vasopressin in the renal collecting duct. Roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275:36839–36846. [DOI] [PubMed] [Google Scholar]
- Chou C-L, Yu M-J, Kassai EM, Morris RG, Hoffert JD, Wall SM, Knepper MA (2008) Roles of basolateral solute uptake via NKCC1 and of myosin II in vasopressin-induced cell swelling in inner medullary collecting duct. Am J Physiol Renal Physiol 295:F192–F201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezova A, Tomilin V, Buncha V, Zaika O, Ortiz PA, Mei F, Cheng X, Mamenko M, Pochynyuk O (2019) Urinary concentrating defect in mice lacking Epac1 or Epac2. FASEB J 33:2156–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JH, Rittig S (2006) Familial neurohypophyseal diabetes insipidus--an update. Semin Nephrol 26:209–223. [DOI] [PubMed] [Google Scholar]
- Daaka Y, Luttrell LM, Ahn S, Della Rocca GJ, Ferguson SS, Caron MG, Lefkowitz RJ (1998) Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem 273:685–688. [DOI] [PubMed] [Google Scholar]
- Datta A, Yang CR, Limbutara K, Chou CL, Rinschen MM, Raghuram V, Knepper MA (2020) PKA-independent vasopressin signaling in renal collecting duct. FASEB J DOI: 10.1096/fj.201902982R [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot T, Alsady M, Jaklofsky M, Otte-Höller I, Baumgarten R, Giles RH, Deen PMT (2014) Lithium causes G2 arrest of renal principal cells. J Am Soc Nephrol 25:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande V, Kao A, Raghuram V, Datta A, Chou C-L, Knepper MA (2019) Phosphoproteomic identification of vasopressin V2 receptor-dependent signaling in the renal collecting duct. Am J Physiol Renal Physiol 317:F789–F804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J, Gunaratne R, Bradford D, Saeed F, Hoffert JD, Steinbach PJ, Knepper MA, Pisitkun T (2012) Identifying protein kinase target preferences using mass spectrometry. Am J Physiol Cell Physiol 303:C715–C727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenbach I, Kostenis E, Schmidt C, Serradeil-Le Gal C, Raufaste D, Dumont ME, Pausch MH, Wess J (2001) Single amino acid substitutions and deletions that alter the G protein coupling properties of the V2 vasopressin receptor identified in yeast by receptor random mutagenesis. J Biol Chem 276:29382–29392. [DOI] [PubMed] [Google Scholar]
- Fenton RA, Pedersen CN, Moeller HB (2013) New insights into regulated aquaporin-2 function. Curr Opin Nephrol Hypertens 22:551–558. [DOI] [PubMed] [Google Scholar]
- Frost JA, Geppert TD, Cobb MH, Feramisco JR (1994) A requirement for extracellular signal-regulated kinase (ERK) function in the activation of AP-1 by Ha-Ras, phorbol 12-myristate 13-acetate, and serum. Proc Natl Acad Sci USA 91:3844–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganote CE, Grantham JJ, Moses HL, Burg MB, Orloff J (1968) Ultrastructural studies of vasopressin effect on isolated perfused renal collecting tubules of the rabbit. J Cell Biol 36:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE (1995) ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J 14:951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham JJ, Burg MB (1966) Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol 211:255–259. [DOI] [PubMed] [Google Scholar]
- Gunaratne R, Braucht DW, Rinschen MM, Chou CL, Hoffert JD, Pisitkun T, Knepper MA (2010) Quantitative phosphoproteomic analysis reveals cAMP/vasopressin-dependent signaling pathways in native renal thick ascending limb cells. Proc Natl Acad Sci USA 107:15653–15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler U, Leroy V, Jeon US, Bouley R, Dimitrov M, Kim JA, Brown D, Kwon HM, Martin P-Y, Féraille E (2008) NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem 283:28095–28105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler U, Leroy V, Martin PY, Féraille E (2009) Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol 297:F10–F18. [DOI] [PubMed] [Google Scholar]
- Hasler U, Mordasini D, Bens M, Bianchi M, Cluzeaud F, Rousselot M, Vandewalle A, Feraille E, Martin P-Y (2002) Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem 277:10379–10386. [DOI] [PubMed] [Google Scholar]
- Hoffert JD, Fenton RA, Moeller HB, Simons B, Tchapyjnikov D, McDill BW, Yu M-J, Pisitkun T, Chen F, Knepper MA (2008) Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem 283:24617–24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou C-L, Knepper MA (2012) Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics 11:M111.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Pisitkun T, Saeed F, Wilson JL, Knepper MA (2014) Global analysis of the effects of the V2 receptor antagonist satavaptan on protein phosphorylation in collecting duct. Am J Physiol Renal Physiol 306:410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffert JD, Pisitkun T, Wang G, Shen R-F, Knepper MA (2006) Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103:7159–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JR, Chou CL, Medvar B, Knepper MA, Jung HJ (2017) Identification of β-catenin-interacting proteins in nuclear fractions of native rat collecting duct cells. Am J Physiol Renal Physiol 313:F30–F46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyndman KA, Yang CR, Jung HJ, Umejiego EN, Chou CL, Knepper MA (2018) Proteomic determination of the lysine acetylome and phosphoproteome in the rat native inner medullary collecting duct. Physiol Genomics 50:669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe K, Jung HJ, Yang C-R, Claxton J, Sandoval P, Burg MB, Raghuram V, Knepper MA (2017) Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci USA 114:E8875–E8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe K, Raghuram V, Krishnan L, Chou CL, Yang CR, Knepper MA (2020) CRISPR-Cas9/phosphoproteomics identifies multiple noncanonical targets of myosin light chain kinase. Am J Physiol Renal Physiol 318:F600–F616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Kwon TH (2016) Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol 311:F1318–F1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Kwon TH (2019) New insights into the transcriptional regulation of aquaporin-2 and the treatment of X-linked hereditary nephrogenic diabetes insipidus. Kidney Res Clin Pract 38:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Raghuram V, Lee JW, Knepper MA (2018) Genome-wide mapping of DNA accessibility and binding sites for CREB and C/EBPβ in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 29:1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K, Siebenlist U (1995) Immediate-early genes induced by antigen receptor stimulation. Curr Opin Immunol 7:327–332. [DOI] [PubMed] [Google Scholar]
- Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, Yu M-J (2011) Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Mol Cell Proteomics 10:M110.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk KL, DiBona DR, Schafer JA (1984) Morphologic response of the rabbit cortical collecting tubule to peritubular hypotonicity: quantitative examination with differential interference contrast microscopy. J Membr Biol 79:53–64. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA (1996) A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 93:8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klussmann E, Rosenthal W (2001) Role and identification of protein kinase A anchoring proteins in vasopressin-mediated aquaporin-2 translocation. Kidney Int 60:446–449. [DOI] [PubMed] [Google Scholar]
- Knepper MA (1997) Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol 272:F3–F12. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Inoue T (1997) Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr Opin Cell Biol 9:560–564. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Kwon T-H, Nielsen S (2015) Molecular physiology of water balance. N Engl J Med 372:1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenoeven MLA, Schweer H, Cox R, Wetzels JFM, Deen PMT (2012) Lithium reduces aquaporin-2 transcription independent of prostaglandins. Am J Physiol Cell Physiol 302:C131–C140. [DOI] [PubMed] [Google Scholar]
- Lee JW, Chou CL, Knepper MA (2015) Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26:2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-Z, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F (2007) Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292:C1606–C1616. [DOI] [PubMed] [Google Scholar]
- Limbutara K, Kelleher A, Yang C-R, Raghuram V, Knepper MA (2019) Phosphorylation changes in response to kinase inhibitor H89 in PKA-null cells. Sci Rep 9:2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linding R, Jensen LJ, Pasculescu A, Olhovsky M, Colwill K, Bork P, Yaffe MB, Pawson T (2008) NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res 36:D695–D699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo C-S, Chen C-W, Wang P-J, Chen P-Y, Lin S-Y, Khoo K-H, Fenton RA, Knepper MA, Yu M-J (2013) Quantitative apical membrane proteomics reveals vasopressin-induced actin dynamics in collecting duct cells. Proc Natl Acad Sci USA 110:17119–17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ (2001) Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA 98:2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples D, Christensen S, Christensen EI, Ottosen PD, Nielsen S (1995) Lithium-induced downregulation of aquaporin-2 water channel expression in rat kidney medulla. J Clin Invest 95:1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Jensen LJ, Diella F, Jørgensen C, Tinti M, Li L, Hsiung M, Parker SA, Bordeaux J, Sicheritz-Ponten T, et al. (2008) Linear motif atlas for phosphorylation-dependent signaling. Sci Signal 1:ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Sandoval PC, Pisitkun T, Knepper MA, Hoffert JD (2013) Vasopressin inhibits apoptosis in renal collecting duct cells. Am J Physiol Renal Physiol 304:F177–F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishler DR, Kraut JA, Nagami GT (1990) AVP reduces transepithelial resistance across IMCD cell monolayers. Am J Physiol 258:F1561–F1568. [DOI] [PubMed] [Google Scholar]
- Moeller HB, Fenton RA (2012) Cell biology of vasopressin-regulated aquaporin-2 trafficking. Pflugers Arch 464:133–144. [DOI] [PubMed] [Google Scholar]
- Murphy LO, Blenis J (2006) MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31:268–275. [DOI] [PubMed] [Google Scholar]
- Naruse M, Yoshitomi K, Hanaoka K, Imai M, Kurokawa K (1995) Electrophysiological study of luminal and basolateral vasopressin in rabbit cortical collecting duct. Am J Physiol 268:F20–F29. [DOI] [PubMed] [Google Scholar]
- Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, Mutig K, Boltzen M, Petrucci O, Vossenkämper A, et al. (2010) Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21:1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92:1013–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Frøkiaer J, Marples D, Kwon T-H, Agre P, Knepper MA (2002) Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82:205–244. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Knepper MA (1993) Vasopressin activates collecting duct urea transporters and water channels by distinct physical processes. Am J Physiol 265:F204–F213. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Kwon TH, Christensen BM, Promeneur D, Frøkiaer J, Marples D (1999) Physiology and pathophysiology of renal aquaporins. J Am Soc Nephrol 10:647–663. [DOI] [PubMed] [Google Scholar]
- Nielsen S, Muller J, Knepper MA (1993) Vasopressin- and cAMP-induced changes in ultrastructure of isolated perfused inner medullary collecting ducts. Am J Physiol 265:F225–F238. [DOI] [PubMed] [Google Scholar]
- Noda Y, Sasaki S (2005) Trafficking mechanism of water channel aquaporin-2. Biol Cell 97:885–892. [DOI] [PubMed] [Google Scholar]
- Nunes P, Hasler U, McKee M, Lu HAJ, Bouley R, Brown D (2008) A fluorimetry-based ssYFP secretion assay to monitor vasopressin-induced exocytosis in LLC-PK1 cells expressing aquaporin-2. Am J Physiol Cell Physiol 295:C1476–C1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiso Y, Robertson GL, Nørgaard JP, Juul KV (2013) Clinical review: treatment of neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab 98:3958–3967. [DOI] [PubMed] [Google Scholar]
- Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M (2002) Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 1:376–386. [DOI] [PubMed] [Google Scholar]
- Pickering CM, Grady C, Medvar B, Emamian M, Sandoval PC, Zhao Y, Yang CR, Jung HJ, Chou CL, Knepper MA (2016) Proteomic profiling of nuclear fractions from native renal inner medullary collecting duct cells. Physiol Genomics 48:154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisitkun T, Jacob V, Schleicher SM, Chou C-L, Yu M-J, Knepper MA (2008) Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295:F1030–F1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Patel S, Hao C, Woodgett J, Harris R (2010) GSK3β mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol 21:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinschen MM, Yu M-J, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA (2010) Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107:3882–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos KP, Strait KA, Raphael KL, Blount MA, Kohan DE (2012) Collecting duct-specific knockout of adenylyl cyclase type VI causes a urinary concentration defect in mice. Am J Physiol Renal Physiol 302:F78–F84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabolić I, Katsura T, Verbavatz JM, Brown D (1995) The AQP2 water channel: effect of vasopressin treatment, microtubule disruption, and distribution in neonatal rats. J Membr Biol 143:165–175. [DOI] [PubMed] [Google Scholar]
- Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA (2016) Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep 6:34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA (2013) Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24:1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Ishibashi K, Marumo F (1998) Aquaporin-2 and -3: representatives of two subgroups of the aquaporin family colocalized in the kidney collecting duct. Annu Rev Physiol 60:199–220. [DOI] [PubMed] [Google Scholar]
- Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, Hoffert JD, Pisitkun T, Knepper MA (2012) Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23:1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Barasch J (2008) WNT/beta-catenin signaling in nephron progenitors and their epithelial progeny. Kidney Int 74:1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C, SALT Investigators (2006) Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355:2099–2112. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Lacour C, Valette G, Garcia G, Foulon L, Galindo G, Bankir L, Pouzet B, Guillon G, Barberis C, et al. (1996) Characterization of SR 121463A, a highly potent and selective, orally active vasopressin V2 receptor antagonist. J Clin Invest 98:2729–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H, Gao Y, Franki N, Hays RM (1993) Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am J Physiol 265:C757–C762. [DOI] [PubMed] [Google Scholar]
- Star RA, Nonoguchi H, Balaban R, Knepper MA (1988) Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81:1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein CM, Morris NJ, Hall NB, Nock NL (2017) Structural equation modeling. Methods Mol Biol 1666:557–580. [DOI] [PubMed] [Google Scholar]
- Sung C-C, Chen L, Limbutara K, Jung HJ, Gilmer GG, Yang C-R, Lin S-H, Khositseth S, Chou C-L, Knepper MA (2019) RNA-Seq and protein mass spectrometry in microdissected kidney tubules reveal signaling processes initiating lithium-induced nephrogenic diabetes insipidus. Kidney Int 96:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamma G, Klussmann E, Maric K, Aktories K, Svelto M, Rosenthal W, Valenti G (2001) Rho inhibits cAMP-induced translocation of aquaporin-2 into the apical membrane of renal cells. Am J Physiol Renal Physiol 281:F1092–F1101. [DOI] [PubMed] [Google Scholar]
- Tohgo A, Choy EW, Gesty-Palmer D, Pierce KL, Laporte S, Oakley RH, Caron MG, Lefkowitz RJ, Luttrell LM (2003) The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem 278:6258–6267. [DOI] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418.23121377 [Google Scholar]
- Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, Knepper MA, Fenton RA, Christensen BM (2014) Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86:757–767. [DOI] [PubMed] [Google Scholar]
- Upadhyay A, Jaber BL, Madias NE (2006) Incidence and prevalence of hyponatremia. Am J Med 119:S30–S35. [DOI] [PubMed] [Google Scholar]
- Valenti G, Procino G, Tamma G, Carmosino M, Svelto M (2005) Minireview: aquaporin 2 trafficking. Endocrinology 146:5063–5070. [DOI] [PubMed] [Google Scholar]
- Verkman AS (1999) Lessons on renal physiology from transgenic mice lacking aquaporin water channels. J Am Soc Nephrol 10:1126–1135. [DOI] [PubMed] [Google Scholar]
- Wall SM, Han JS, Chou CL, Knepper MA (1992) Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am J Physiol 262:F989–F998. [DOI] [PubMed] [Google Scholar]
- Williams GR, Bethard JR, Berkaw MN, Nagel AK, Luttrell LM, Ball LE (2016) Exploring G protein-coupled receptor signaling networks using SILAC-based phosphoproteomics. Methods 92:36–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z, Chen JX, Zhao Y, Medvar B, Knepper MA (2017) Data integration in physiology using Bayes’ rule and minimum Bayes’ factors: deubiquitylating enzymes in the renal collecting duct. Physiol Genomics 49:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ (2003) Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63:1983–1994. [DOI] [PubMed] [Google Scholar]
- Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA (2015) Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309:C799–C812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KP (2002) Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip KP (2006) Epac-mediated Ca2+ mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol 291:F882–F890. [DOI] [PubMed] [Google Scholar]
- Yu M-J, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DWW, Chou C-L, Pisitkun T, Nelson RD, Knepper MA (2009) Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106:2441–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]