Abstract
Objective:
Previous work demonstrated that deployment to sites with open burn pits altered serum microRNA (miRNA) levels. Here, we determined if identified deployment-related exposures alter miRNA expression in primary human lung fibroblasts (HLFs).
Methods:
Benzo(ghi)perylene (BghiP) and 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (HpCDD) were tested for their ability to activate the aryl hydrocarbon receptor (AHR) and induce changes in miRNA. AHR knockdown determined if changes were AHR-dependent.
Results:
HpCDD induced AHR activity in a dose-dependent manner. Four miRNAs linked to occupational open burn pit exposure were altered by HpCDD in HLFs. Knockdown of AHR attenuated changes in miRNA levels.
Conclusions:
These studies confirm that specific miRNAs, previously identified as different in sera from personnel deployed to sites with open burn pits, are altered by HpCDD exposure in human lung cells. HpCDD alters miRNA levels in an AHR dependent manner.
Keywords: microRNA; dioxin; biomarker; deployment; open burn pit; exposure; Benzo(ghi)perylene; 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin; human lung fibroblasts
Introduction
The health consequences of occupational exposure to hazardous environments can be difficult to quantify, especially when environmental monitoring and medical assessments are not readily available or possible. Polycyclic aromatic hydrocarbons (PAHs), polychlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs) are some of the hazardous chemicals that are released into the air when occupational wastes are burned in open burn pits (1,2). Ambient particulate matter containing PAHs from aircraft and diesel fuel combustion are also major occupational hazards on active military bases (3).
The long-term goal of our study is to determine whether post-deployment serum samples contain biomarker signatures of deployment-related exposures to environmental hazards, such as open burn pits (3–6). We previously identified numerous PAHs, PCDDs and PCDFs present in serum samples from both control (not stationed at sites with open burn pits) and case samples (deployed to sites with open burn pits). Serum samples were available for both pre and post deployment times, yielding 4 groups (control pre, control post, case pre and case post) for analysis. Comparisons across the groups yielded some interesting differences and noticed some similarities. Naphthalene, a bicyclic PAH with a relatively short half-life (12 hours), was detected in 83% of all serum samples analyzed (similar number of detections across all 4 groups). Benzo(ghi)perylene (BghiP), a more complex PAH, was detectable in 21% of serum samples. BghiP levels were significantly higher in case post samples compared to control post and case pre samples suggesting BghiP levels provide evidence of deployment-related exposure. With regards to polychlorinated hydrocarbons, 1,2,3,4,6,7,8-Heptachloro-dibenzo-p-dioxin (HpCDD) was found at significantly higher levels in the case post group compared to the control post group (4). No other polychlorinated compounds or PAHs were significantly increased in case post serum samples.
While serum BghiP and HpCDD levels may be markers of occupational exposure, it is unclear if these are truly representative of total exposure to hydrocarbon-based air pollution as PAHs and dioxins are lipophilic compounds that bioaccumulate in lipid rich tissues such as fat (7,8). Occupational exposure to dioxins and PAHs maybe underrepresented by serum values as many of these compounds may have been sequestered in lipid rich tissues where they can reside for years (8). To gain a more complete understanding of the action of these chemicals, it is critical to investigate their effects on human cells to determine what biological pathways they may alter and cause a health burden.
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that binds structurally diverse polyromantic hydrocarbons (9). The AHR is most widely recognized as the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a toxic component of Agent Orange (10). TCDD is an extremely stable molecule that binds the AHR with high affinity to drive constitutive activation of AHR, thus contributing to TCDD-induced toxicological effects (8). Other dioxins and PAHs can also bind the AHR however, the dose and duration of activation can vary significantly (11). AHR-dependent genes include numerous cytochrome P450 enzymes, such as CYP1B1 and CYP1A1, which are involved in metabolism and clearance of xenobiotic PAHs. Recent work has revealed that in addition to traditional messenger RNA (mRNA) target genes, the AHR can alter expression of microRNAs (miRNAs) as well (12). Benzo(a)pyrene, another PAH that serves as an AHR ligand, can influence expression of miRNA in exposed animal lungs (13). Interestingly, liver miRNA levels were unchanged in similar studies by the same group, indicating the lung may function as the key miRNA responsive environmental sensor (14).
The use of miRNA levels for biomarker discovery or diagnostic purposes is a swiftly rising field. Several studies have identified serum miRNAs that correlate with diseases such as Alzheimer’s disease, Type 2 diabetes and COPD (15–17). Previous work by us demonstrated that case post deployment serum samples have altered expression of several miRNAs (5). Additionally, we observed that the serum levels of specific miRNAs correlated with serum PCDD/PCDFs. However, it is unclear if the relationship between deployment, serum miRNA and serum occupational hazard levels are merely correlative or a direct result of occupational exposure. Thus, taken together, the objectives of the current study were the following: 1) to further understand if miRNAs are directly responsive to occupational hazard related chemical exposure, 2) to determine if primary human lung cells are capable of expressing miRNAs in response to occupational chemical exposure, and 3) to determine if these chemicals alter miRNA expression through their ability to activate the AHR. To accomplish these objectives, we determined if putative deployment-related exposures (BghiP and HpCDD) alter miRNA expression in primary human lung fibroblasts (HLFs).
Materials and Methods
Detailed methods are provided in an online supplement. Briefly, primary human lung fibroblasts (HLFs) were derived and grown from human tissue explants as previously described (18,19). Control or “normal” tissues represented surgical waste tissue obtained from patients undergoing diagnostic biopsy and cell lines were established by explant technique from tissue. All patient tissue explants were obtained with written informed consent under the approval of the University of Rochester Medical Center Institutional Review Board (IRB).
To test if compounds activate the AHR, HEK293FT cells were co-transfected with an AHR overexpressing plasmid and an AHR reporter plasmid using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions as reported previously (20). AHR knockdown was accomplished using small interfering RNA (siRNA) as described (21). Western Blotting and quantitative PCR were performed as described previously (21–23) and further details are in the online supplement. All values are presented as mean ± SEM. Experiments were conducted in biological triplicate or quadruplicate wells. Student’s t-test, One-way analysis of variance (ANOVA) and Two-way ANOVA were used for statistical analysis using GraphPad Prism6. P-values of p< 0.05 (*); p< 0.01 (**); and p< 0.001(***); were considered significant.
Results
1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin and benzo(ghi)perylene induce AHR activity in human cells
1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD), benzo(ghi)perylene (BghiP) and naphthalene were previously identified in serum samples from the Department of Defense Serum Repository (DoDSR) . BghiP and naphthalene are polycyclic aromatic hydrocarbons (PAHs) whereas HpCDD is in the class of polychlorinated dibenzo-p-dioxin compounds exemplified by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a component of Agent Orange. One mechanism whereby PAHs and dioxins invoke a physiological response is that they may serve as ligands for the aryl hydrocarbon receptor (AHR). Their structures are shown in Figure 1A.
Figure 1: HpCDD and BghiP induce AHR-driven luciferase activity.
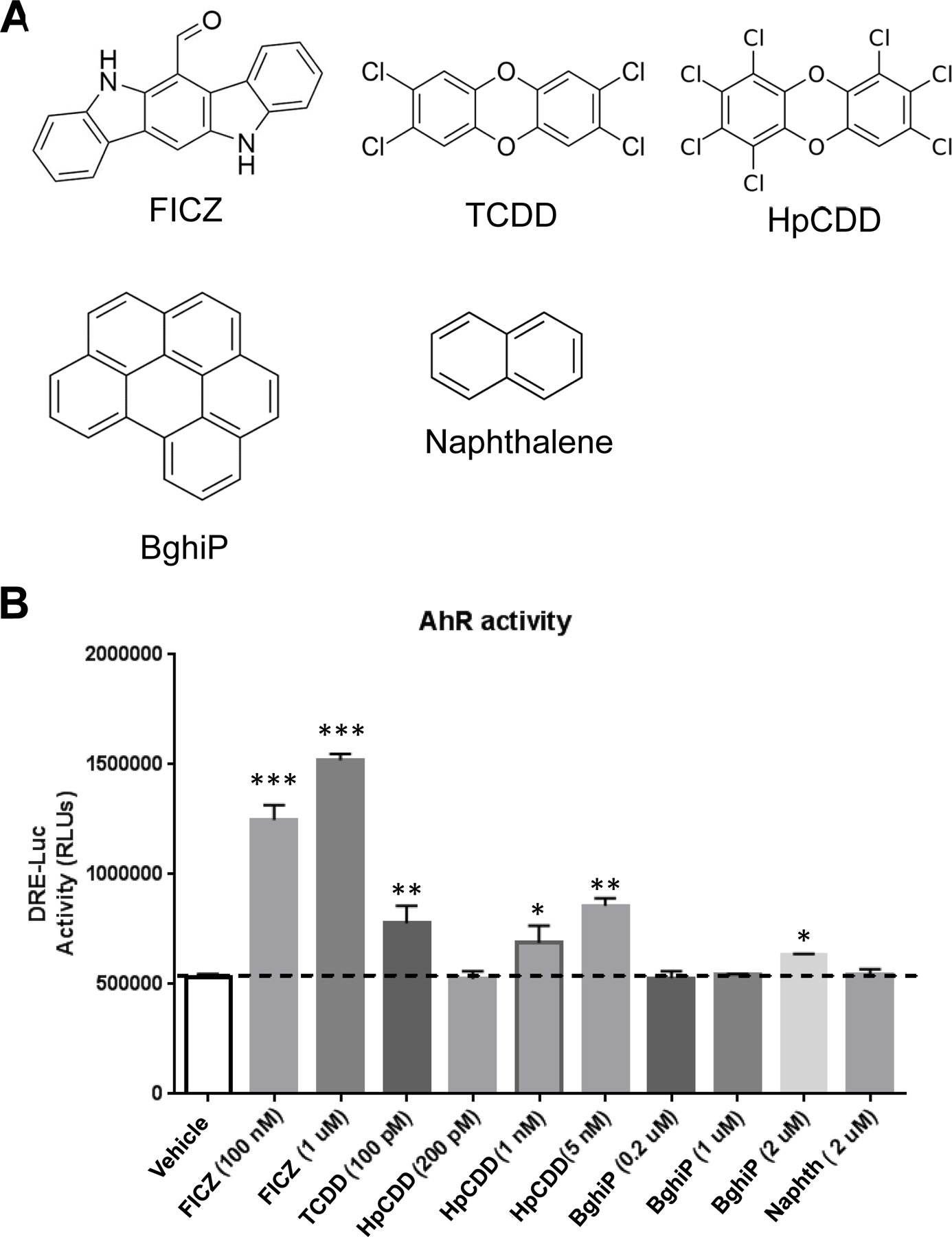
(A) Chemical structures of polycyclic aromatic hydrocarbons (PAHs) and dibenzo-p-dioxins used in this study. The compounds are: 6-formylindolo[3,2-b]carbazole (FICZ), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), 1,2,3,4,6,7,8-Heptachlorodibenzo-p-doxin (HpCDD), Benzo(ghi)perylene (BghiP) and Naphthalene. (B) HEK293FT cells were used to test if the chemicals affect AHR activity. The DRE-luciferase reporter construct (which harbors 2 dioxin response elements (DRE) upstream of the firefly luciferase gene) and the pCMV-AHR plasmid (which constitutively expresses human AHR) were introduced into cells as described in the online supplement. After 20 hours of exposure to the chemicals, cells were collected and luciferase activity measured. FICZ induced a robust increase in luciferase activity at both 100 nM and 1 µM. TCDD induced luciferase activity at 100 pM. HpCDD induced a dose-responsive increase in luciferase activity where 1–5 nM HpCDD elicited a similar response as 100 pM TCDD. BghiP induced a significant induction in DRE-luc at 2 µM. Naphthalene at 2 µM did not induce DRE-luciferase. The experiment was repeated two times in quadruplicate with a representative experiment shown and data presented as means + standard error., * = p < 0.05, ** = p < 0.01, *** = p < 0.001, One-way-ANOVA.
A luciferase reporter gene with two, tandem AHR response elements (termed dioxin response elements or DREs) was used to investigate if these compounds can activate the AHR in human cells (Figure 1B)(24). 6-formylindolo[3,2-b]carbazole (FICZ), an endogenous AHR ligand produced as a byproduct of the tryptophan biosynthetic pathway, was used as a positive control. HEK293FT cells containing the DRE-luciferase reporter and human AHR cDNA were exposed to compounds for 20 hours before luciferase activity was measured. FICZ induced a robust increase in luciferase activity at both 100 nM and 1 μM concentrations. TCDD, the most potent dioxin AHR ligand, significantly induced luciferase activity at 100 pM. HpCDD induced a dose-responsive increase in luciferase activity where 1 and 5 nM HpCDD elicited a comparable response as 100 pM TCDD. Thus, HpCDD can promote activation of the AHR in human cells; however, the dose required appears to be ~10 times higher that of the effective TCDD dose. The PAH, BghiP induced a significant induction in DRE-luc at 2 μM but not at lower doses of 1 or 0.2 μM. Higher concentrations of BghiP resulted in poor cell viability and thus were not used (data not shown). Naphthalene failed to elicit an increase in DRE-luciferase levels even at 2 μM (Figure 1B). Taken together, these data reveal that HpCDD is a robust AHR ligand while BghiP is less responsive in this AHR reporter system.
Induction of CYP1B1 by HpCDD is AHR-dependent
CYP1B1 (cytochrome P450 family 1 subfamily B member 1) is a canonical AHR-dependent gene and acts as the first step in the metabolic breakdown of hydrocarbon pollutants (25,26). To further investigate if HpCDD and BghiP could activate AHR-dependent pathways, we measured induction of CYP1B1. Primary human lung fibroblasts (HLFs) were used for these tests as the likely route of chemical exposure near open burn pits is inhalation of airborne materials. Lung Fibroblasts act as sentinel cells that respond robustly to external stimuli and are the most numerous cell type in the lung (27–30). Short interfering RNAs (siRNAs) that specifically target AHR to reduce its protein expression were used to test whether or not CYP1B1 induction was indeed AHR dependent in HLFs as demonstrated in other fibroblasts by our group previously (21). HLFs were treated with two different siRNAs targeting the AHR or a non-specific control siRNA for 48 hours to allow significant depletion of AHR protein levels. Afterwards, cells were harvested and analyzed for AHR and actin (loading control) protein levels (Figure 2A). HEK293FT cells with or without an AHR overexpressing plasmid were included as a control for the Western blot. In the presence of control siRNA, HLFs robustly express the AHR. HLFs treated with either AHR siRNA # 1 or AHR siRNA # 2 show a dramatic reduction in AHR protein levels (~less than 10% of control siRNA samples) demonstrating effective siRNA mediated depletion.
Figure 2: AHR siRNA reduces AHR protein levels and HpCDD-mediated induction of CYP1B1 mRNA.
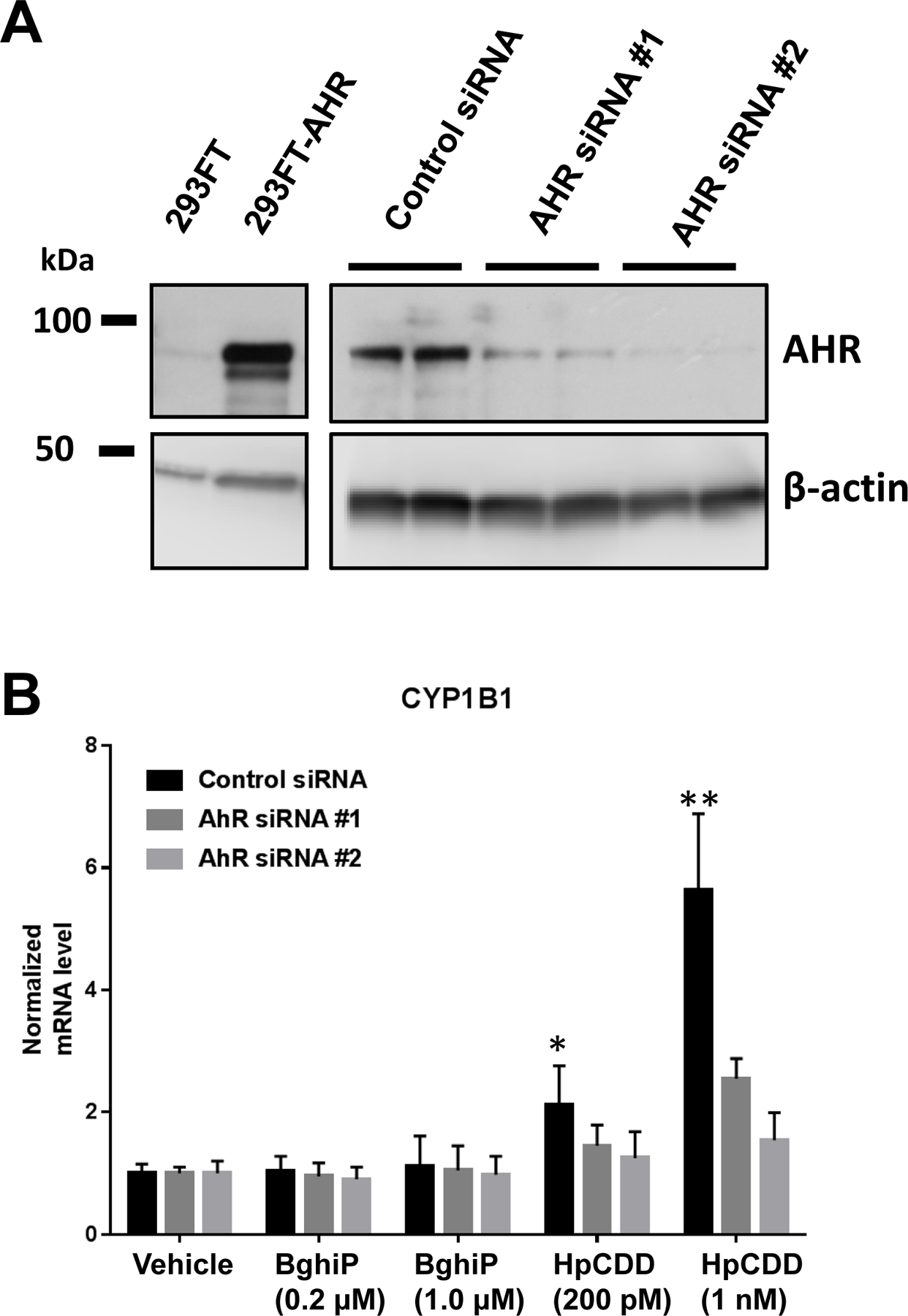
(A) Control or pCMV-AHR plasmid DNA were introduced into HEK293FT cells (left panels) and human lung fibroblasts (right panels) were treated with control or AHR specific siRNA (2 different target sequences) for 72 hours. Then, protein was prepared and analyzed by Western blot for AHR and total actin (loading control). 293FT cells show robust expression of AHR with pCMV-AHR. In HLFs, AHR siRNA #1 reduced AHR protein levels to less than 10% of control siRNA levels and AHR siRNA #2 reduced AHR protein levels to less than 5% of control. (B) HLFs treated with the siRNAs as described in (A) were then exposed to vehicle (DMSO) or BghiP or HpCDD at the doses listed. After 24 hours, total RNA was collected and analyzed by RT-qPCR for the AHR-dependent mRNA, CYP1B1 and reference mRNAs, HPRT1 and TBP. With control siRNA treated cells, CYP1B1 levels were not significantly altered by either 0.2 or 1 µM BghiP, however, both 200 pM and 1 nM HpCDD led to a significant increase in CYP1B1 levels. In samples treated with either AHR siRNA, CYP1B1 induction was dramatically attenuated. The experiment was performed in 2 different HLF strains in triplicate with similar results in both strains. Representative data from 1 strain presented as means ± standard error, ** = p < 0.01, Two-way-ANOVA.
Next, control or AHR siRNA treated HLFs were exposed to vehicle (DMSO), BghiP (0.2 or 1 μM) or HpCDD (200 pM or 1 nM) for 24 hours. After exposure, RNA was collected and analyzed by RT-qPCR for CYP1B1 mRNA levels and the reference mRNAs, HPRT1 and TBP. CYP1B1 levels were normalized to reference mRNA levels and graphed as a fold induction from vehicle exposed cells (Figure 2B). CYP1B1 levels were not significantly upregulated by 0.2 µM BghiP in control siRNA or AHR siRNA treated cells. BghiP at 1 µM slightly elevated CYP1B1 levels (~1.2) in control siRNA treated samples suggesting some AHR-dependent activity. Experiments performed with exposure to higher doses of BghiP (2 µM or more) reduced HLF viability and were excluded. HpCDD exposure at 200 pM and 1 nM significantly increased CYP1B1 mRNA levels, with 200 pM inducing CYP1B1 levels ~2.1 fold and ~5.6 fold for 1 nM. The effects of HpCDD on CYP1B1 mRNA were dependent upon AHR expression as AHR siRNA #1 and #2 significantly attenuated induction of CYP1B1 mRNA.
HpCDD induces expression of miRNA in HLFs
Having established that HpCDD and, to a lesser extent, BghiP activate the AHR in HLFs, we asked whether or not these compounds could alter specific miRNA that were previously identified as potential biomarkers of deployment-related exposures. Let-7d was increased in case-post deployment serum samples compared to control serum samples and was correlated with dioxin/dibenzofuran serum levels (5). miR-103 showed a strong correlation with serum levels of HpCDD and the related dioxin, octachlorodibenzo-p-dioxin (OCDD) (5). Let-7d and miR-103 were also identified as potential biomarkers of exposure in a machine learning approach (Thatcher et al, JOEM this issue). HLFs were treated with vehicle (DMSO), BghiP (0.2 or 1 µM) or HpCDD (200 pM or 1 nM) for 24 hours. Afterwards, total RNA was isolated and miRNA expression evaluated by RT-qPCR. MiRNA levels were normalized to U6 snRNA, HPRT1, TBP and RNU66 (reference RNAs) levels. Both Let-7d and miR-103 were moderately upregulated by 1 µM BghiP (~1.5 and ~1.1 fold, respectively, compared to vehicle) however, the inductions were not statistically significant (Figure 3). Let-7d and miR-103 levels were significantly induced with HpCDD exposure in a dose dependent manner, where at 1 nM HpCDD, Let-7d was upregulated ~3-fold compared to vehicle and miR-103 was upregulated ~1.6-fold (Figure 3).
Figure 3: Exposure to BghiP and HpCDD upregulate Let-7d and miR-103 levels.
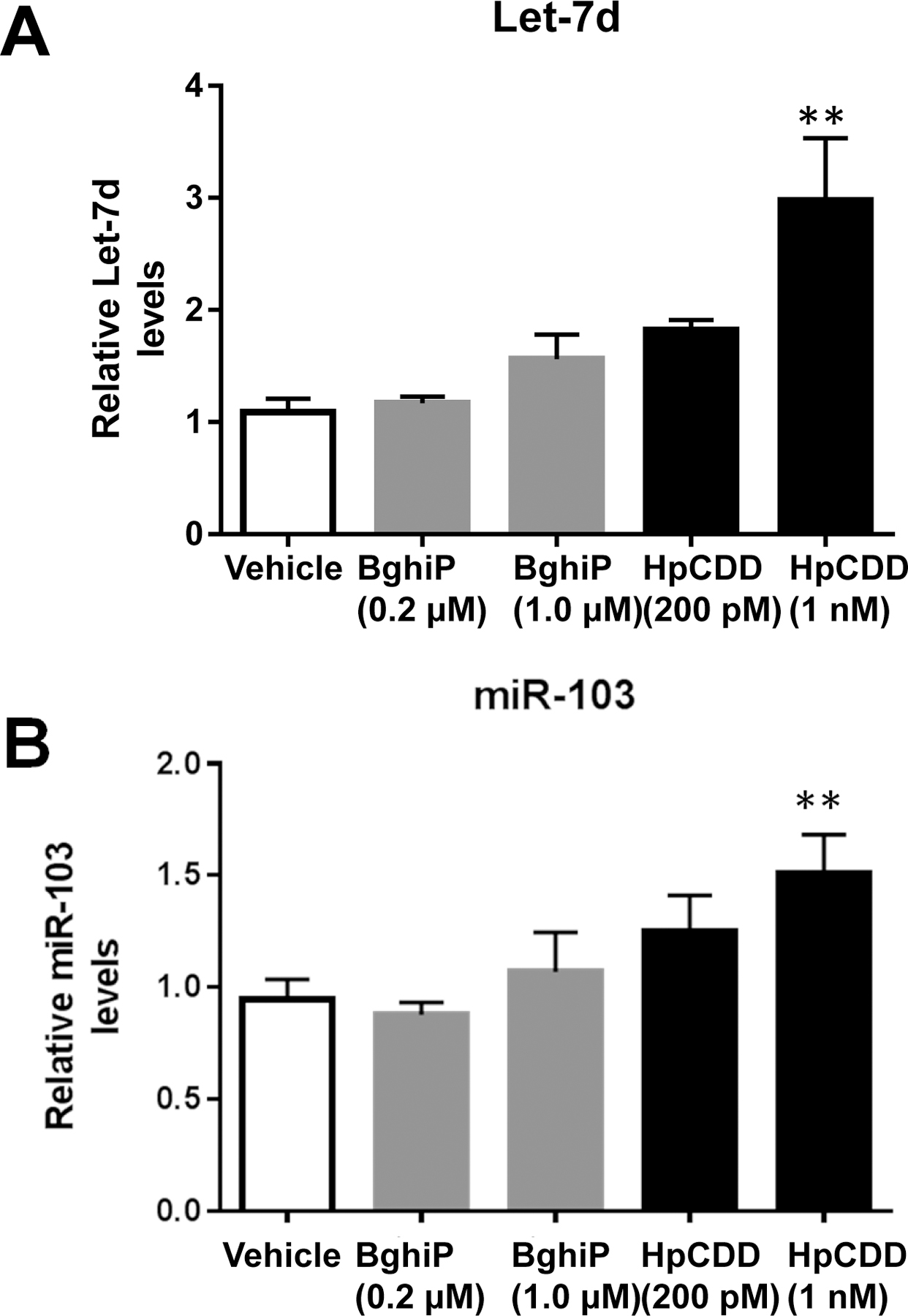
Primary human lung fibroblasts (HLFs) were treated with vehicle (DMSO), BghiP or HpCDD with the doses listed for 24 hours and total RNA was isolated. Let-7d (A), and miR-103 (B) were detected by RT-qPCR. At 1 µM BghiP treatment both Let-7d and miR-103 were upregulated but the induction was not significant. Let-7d and miR-103 levels were significantly induced with HpCDD exposure. The experiment was performed in 4 different HLF strains with similar results in all strains. Representative data from 1 strain presented as means ± standard error, ** = p < 0.01, One-way-ANOVA.
In addition to analyzing Let-7d and miR-103 levels, we analyzed several other miRNAs that were of interest to us based on our previous findings in this study population; miR-144–3p, miR-107–3p, miR-16–5p, miR-34a-5p, miR-17–5p, miR-145–5p, miR-146a-5p and miR-32–5p. These miRNAs are correlated with serum dioxin levels or deployment status or both ((5) and Thatcher et. al., this issue). Here, experiments were performed in HLFs from 3 different donors. MiRNA levels were normalized as described above. Interestingly, miR-107, which is closely related to miR-103, was significantly induced with HpCDD treatment where 1 nM HpCDD led to a ~2.4 fold increase in miR-107(Figure 4A). In contrast, miR-144–3p was decreased by HpCDD in a dose dependent manner where 1 nM HpCDD decrease miR-144–3p by ~80% (Figure 4B). The other miRNAs analyzed (miR-16–5p, miR-34a-5p, miR-17–5p, miR-146a-5p, miR-145–5p and miR-32–5p) were expressed by HLFs but did not change with HpCDD exposure.
Figure 4: HpCDD regulates expression of miR-107 and miR-144–3p in HLFs.
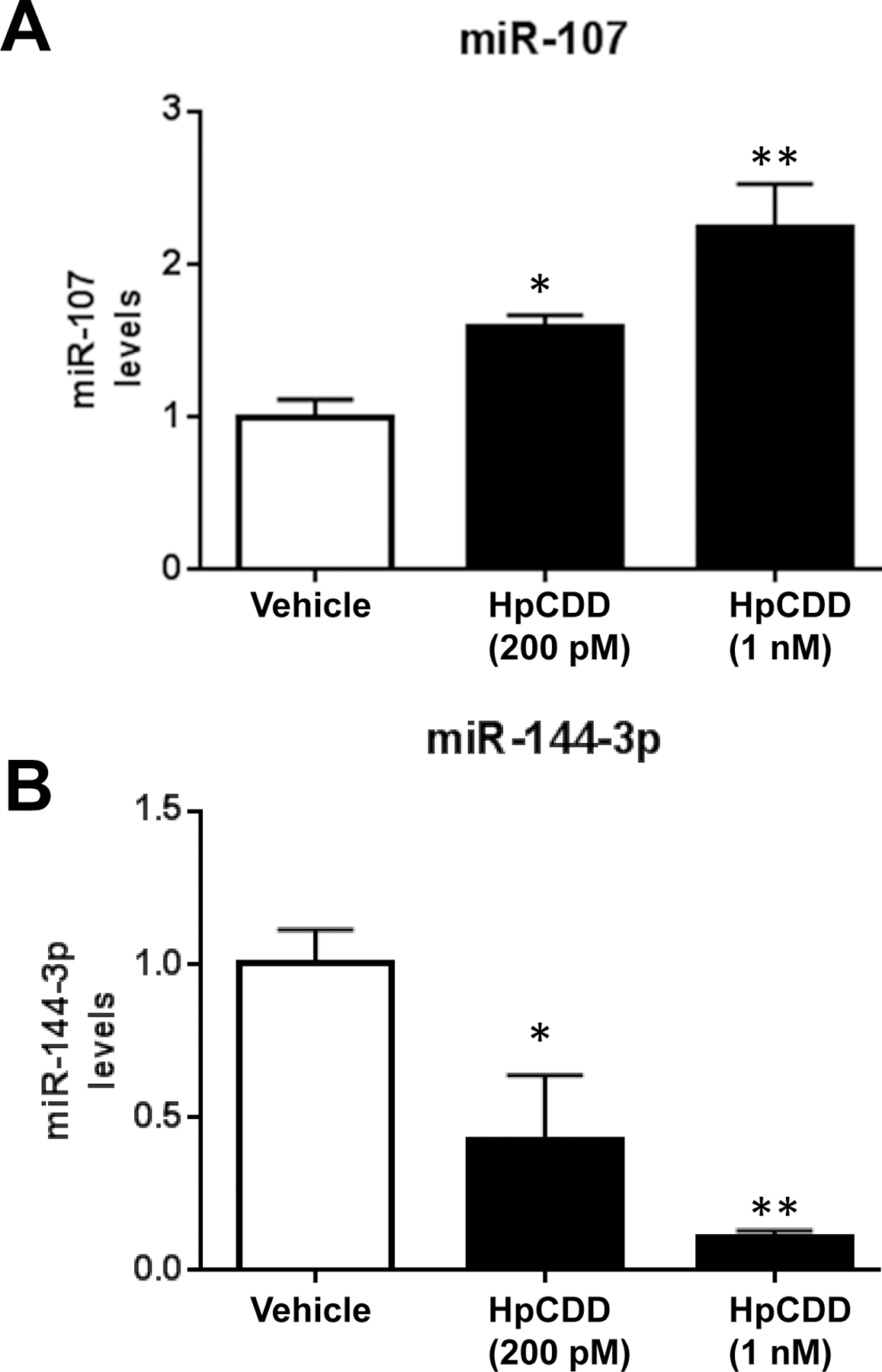
Primary human lung fibroblasts (HLFs) were treated with vehicle (DMSO) or 200 pM or 1 nM HpCDD for 24 hours and total RNA was isolated. MiR-107 (A), miR-144–3p (B) were detected by RT-qPCR. miR-107 was induced with HpCDD treatment whereas miR-144–3p expression was decreased by HpCDD. The experiment was performed in 4 different HLF strains in quadruplicate with similar results in all strains. Representative data from 1 strain presented as means ± standard error, * = p < 0.05, ** = p < 0.01, One-way-ANOVA.
HpCDD-mediated changes in Let-7d, miR-103 and miR-144–3p are AHR-dependent
To determine if changes in the three miRNAs that were most influenced by HpCDD in vitro and associated with case-post deployment samples, HLFs were treated with control or AHR specific siRNA for 48 hours as described above. Next, control and AHR depleted HLFs were exposed to DMSO or HpCDD at 100 pM or 1 nM for 24 hours. After treatments, total RNA was isolated and analyzed by qPCR. In control siRNA treated samples, Let-7d (Figure 5A) and miR-103 (Figure 5B) levels were significantly induced by HpCDD. In AHR siRNA treated samples, however, HpCDD failed to significantly induce expression of these miRNAs. MiR-144–3p, which was decreased by HpCDD exposure was again dose dependently reduced in control siRNA treated samples (Figure 5C). Interestingly, AHR siRNA significantly attenuated the reduction of miR-144–3p in both 200 pM and 1 nM HpCDD exposed HLFs. Taken together, these results demonstrate that these miRNAs are regulated by HpCDD in an AHR-dependent manner.
Figure 5: HpCDD changes in miRNA expression are mediated by the AHR.
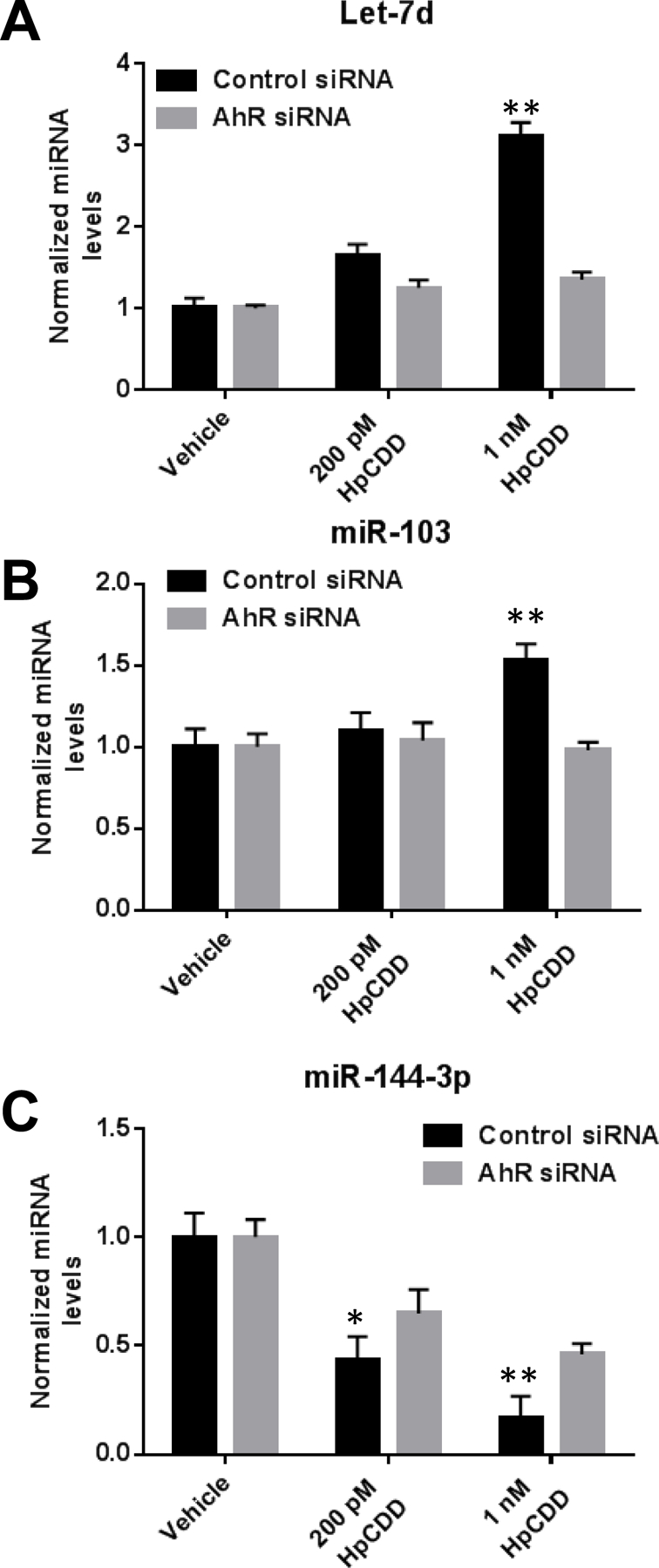
HLFs were treated with control or AHR specific siRNA #2 for 48 hours and then exposed to DMSO or HpCDD at doses indicated for 24 hours. After treatments, Let-7d (A), miR-103 (B), and miR-144–3p (C) were detected by RT-qPCR. AHR siRNA significantly attenuated the effect of 1 nM HpCDD for all 3 miRNAs. At 200 pM HpCDD, AhR siRNA significantly attenuated the reduction in miR-144–3p. The experiment was performed in 2 different HLF strains in quadruplicate with similar results in both strains. Representative data from 1 strain presented as means ± standard error, * = p < 0.05, ** = p < 0.01, Two-way-ANOVA.
Discussion
In previous reports, we have identified several miRNAs as potential biomarkers of deployment or of exposure to hydrocarbons including PAHs and polychlorinated dibenzo-p-dioxins and dibenzo furans (5). Here, we sought to validate these miRNAs as biomarkers of exposure by examining whether their expression was directly affected by dioxin and PAHs in a culture model of primary human lung cells. We used HpCDD and BghiP, two chemicals with elevated levels in post-deployment serum samples. Four miRNAs (Let-7d, miR-103, miR-107 and miR-144–3p) showed a significant difference in expression upon exposure to HpCDD. Additionally, the ability of HpCDD to induce changes in miRNA is dependent upon expression of the AHR, a ligand activated transcription factor capable of mediating genome-wide changes in gene expression. These studies provide in vitro validation for previously documented associations between serum miRNA levels, serum PCDD/PCDF levels, and occupational deployment to sites with open burn pits. Additionally, this work supports the concept that these miRNAs can serve as biomarkers or possibly a sensor panel for establishing occupational exposure to hazardous environmental agents. Indeed, others have suggested plasma miRNA levels as markers for PAH exposure (31,32). Novel biomarkers are urgently needed as real-time exposures are difficult to quantify and evaluate, especially when environmental monitoring and medical assessments are not readily available or possible (6,33). Changes in miRNA expression can persist long after exposure is over and therefore may serve as effective gauges for previous exposure related incidents (34,35). For example, a panel of 12 miRNAs was able to distinguish control and benzene poisoned individuals (36). Another study used genome wide miRNA profiling from the placenta to show that miR-146a was upregulated with previous BPA exposure (37).
HpCDD is closely related to the most well studied dioxin and potent AHR ligand, TCDD. Many studies have revealed that a multitude of toxic effects brought about by TCDD are mediated through its ability to sustain activation of the AHR (38–40). HpCDD most likely drives many of the same toxic effects however, the dose and duration may be different between the two chemicals. In our studies, the concentration of HpCDD required to reach a similar activation of the AHR luciferase reporter as achieved through TCDD was ~10 times that of TCDD (1 nM vs 100 pM). The WHO 2005 toxic equivalency factor (TEF) value for HpCDD is 0.01 compared to the reference value of 1.0 for TCDD (41). However, recent studies show a consensus toxicity factor of HpCDD to be 0.2 in human cells (42). This is more consistent with our results (Figure 1). We noted significant changes in miRNA expression with HLF exposure to 200 pM and 1 nM HpCDD. These doses were chosen as representative of occupational exposure levels and are readily detectable in vivo (4). HpCDD was detected in 291 out of 800 serum samples (36%) and had a mean detection of 100 pM with some serum samples showing up to 1 nM HpCDD (4). Additionally, serum samples did not just contain HpCDD, rather, many other dioxin and dibenzofuran congeners were identified. Thus, the total dioxin burden in some of the samples was likely well over 1 nM HpCDD. Thus, miRNA levels can be altered by biologically detected human serum doses.
AHR is an essential mediator of the biological response to environmental exposures, as it regulates several important genes and pathways involved in detoxifying hydrocarbon pollutants. Here, we are able to map the effect of HpCDD on miRNA expression to an AHR dependent role. In addition to miRNA, many metabolites were also significantly different in case post deployed serum samples (Thatcher et al this Issue, and (43)). Several metabolites in the tryptophan pathway were significantly different including: kynurenine, indoleacrylic acid, and tryptophan itself. Several reports reveal that TCDD disrupts tryptophan homeostasis in animal models (44–46). Increased AHR activation by TCDD or other ligands can lead to perturbations in tryptophan metabolism and it is likely that some of the tryptophan metabolic differences observed in case post serum samples are due to increased activation of the AHR (by HpCDD or other exposure related AHR ligands) (46). For example, indoleamine 2,3-dioxygenase 2 (IDO2), an enzyme that catabolizes tryptophan into kynurenine, is a direct transcriptional target of AHR (47). Interestingly, kynurenine is an endogenous ligand of the AHR. FICZ, the powerful AHR ligand we used as a control for AHR activation, is another tryptophan metabolism product (48,49). Kynurenine and FICZ have been shown to alter many AHR-dependent pathways involved in immune suppression and autoimmune disease (50–52).
We used lung fibroblasts as an effector cell to measure miRNA responses to environmental exposure. Lung fibroblasts are sentinel cells that make up a large fraction of the total cells in the lung and robustly respond to numerous stimuli (18,27,28). An emerging concept is that sentinel cells can produce extracellular vesicles termed exosomes that communicate with other cells, either in close proximity or distally through blood circulation. Lung fibroblasts are capable of producing exosomes (22). Indeed, lung derived miRNAs have recently been shown to be packaged into extracellular exosomes (53) that could be released into the blood. Therefore, the lung is the possible source of these specific serum miRNAs. In support of this, recent studies showed that benzo(a)pyrene exposure led to significant changes in lung miRNA expression but not liver miRNA expression (13). Future studies aimed at purifying serum exosomes and measuring purified miRNA and lung specific markers will answer this central question.
We did not see significant changes in HLF miRNA levels using BghiP exposure. BghiP levels in case post samples were around 100 nM with a max detection of 2.8 µM (4). We used only up to 1 µM BghiP as doses of 2 µM and above showed overt toxic effects (loss of viability and signs of cell death) in HLFs. HLFs may be very sensitive to BghiP and it is possible other cell types could more readily manage higher exposure levels. The half-life of BghiP is approximately24–72 hours (4). In this initial validation study, we decided to analyze miRNA expression after 24 hours of exposure since transcription and miRNA processing based mechanisms could occur in this time frame. However, future studies should include shorter or longer exposure times to determine if a more robust response to BghiP can be obtained. A previous report showed that, like us, BghiP was able to stimulate an AHR dependent reporter (54). Interestingly, BghiP was only a partial agonist and showed preferential activation at 6 hours compared to a 24 hour time point in the rat hepatoma cell line used (54).
Some of the miRNAs we analyzed did not change in response to HpCDD. While these miRNAs were found to be significantly different in case post samples, it is probable that other occupationally relevant stimuli are responsible for mediating these changes. Exposures to different stress environments, microbiomes, flora and fauna, climate differences and weather patterns for example could be responsible for altering these miRNAs (3,6,43). It will be difficult to dissect these pathways without further samples and studies. Alternatively, HLFs may not be the cell type responsible for mediating these effects. Other cell and tissue types also produce miRNA, for example: mesenchymal stem cells, adipocytes, hepatocytes and keratinocytes are all capable of producing miRNA and releasing them into the blood stream via exosome release (55–58). Further studies testing the cell types described above may address this question. Taken together, the miRNAs altered by HpCDD exposure in this report, namely Let-7d, miR-103, miR-107 and miR-144–3p could potentially make or be part of an environmental sensor/biomarker panel. MiRNA levels may persist in serum longer than PAHs (with half-lives of hours to days) and PCDD/PCDFs (longer half-lives but bioaccumulate in lipid rich tissues, thus serum levels may not be representative).
While these miRNAs are interesting as a biomarker panel, they also have the potential to regulate gene expression of a host on key pathways involved in health and disease (Supplemental Figure 1). For example, Let-7d regulates genes involved in mediating cell stress, cell proliferation and differentiation and carcinogenesis (59–61). MiR-103 and miR-107, which share the same seed sequence and many of the same target genes, play a role in insulin signaling, diabetes, lipid accumulation and obesity (23,62–64). Interestingly, miR-144–3p is involved in regulating inflammatory genes and is a putative tumor suppressor miRNA in lung adenocarcinoma (65–67). Given the broad impact that these miRNAs may play in health and disease, future follow-up studies of case post deployed personnel are warranted and indeed may provide additional evidence of miRNA biomarkers and future disease risk.
Supplementary Material
Acknowledgments
This work was supported by The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. grant number HT9404–13–1–0030, and the National Institute of Environmental Health Sciences Grant # P30-ES01247.
Footnotes
Conflict of Interest: none declared.
Literature Cited
- 1.Abraham JH, Eick-Cost A, Clark LL, Hu Z, Baird CP, DeFraites R, Tobler SK, Richards EE, Sharkey JM, Lipnick RJ, and Ludwig SL (2014) A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Military medicine 179, 540–546 [DOI] [PubMed] [Google Scholar]
- 2.Smith B, Wong CA, Boyko EJ, Phillips CJ, Gackstetter GD, Ryan MA, and Smith TC (2012) The effects of exposure to documented open-air burn pits on respiratory health among deployers of the Millennium Cohort Study. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 54, 708–716 [DOI] [PubMed] [Google Scholar]
- 3.Masiol M, Mallon CT, Haines KM Jr., Utell MJ, and Hopke PK (2016) Source Apportionment of Airborne Dioxins, Furans, and Polycyclic Aromatic Hydrocarbons at a United States Forward Operating Air Base During the Iraq War. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia X, Carroll-Haddad A, Brown N, Utell MJ, Mallon CT, and Hopke PK (2016) Polycyclic Aromatic Hydrocarbons and Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans in Microliter Samples of Human Serum as Exposure Indicators. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woeller CF, Thatcher TH, Van Twisk D, Pollock SJ, Croasdell A, Hopke PK, Xia X, Thakar J, Sime PJ, Mallon TM, Utell MJ, and Phipps RP (2016) MicroRNAs as Novel Biomarkers of Deployment Status and Exposure to Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallon CT, Rohrbeck MP, Haines MK, Jones DP, Utell M, Hopke PK, Phipps RP, Walker DI, Thatcher T, Woeller CF, Baird CP, Pollard HB, Dalgard CL, and Gaydos JC (2016) Introduction to Department of Defense Research on Burn Pits, Biomarkers, and Health Outcomes Related to Deployment in Iraq and Afghanistan. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S3–S11 [DOI] [PubMed] [Google Scholar]
- 7.Ronn M, Lind L, van Bavel B, Salihovic S, Michaelsson K, and Lind PM (2011) Circulating levels of persistent organic pollutants associate in divergent ways to fat mass measured by DXA in humans. Chemosphere 85, 335–343 [DOI] [PubMed] [Google Scholar]
- 8.Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, and Jolliet O (2009) Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environmental health perspectives 117, 417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baglole CJ, Maggirwar SB, Gasiewicz TA, Thatcher TH, Phipps RP, and Sime PJ (2008) The aryl hydrocarbon receptor attenuates tobacco smoke-induced cyclooxygenase-2 and prostaglandin production in lung fibroblasts through regulation of the NF-kappaB family member RelB. The Journal of biological chemistry 283, 28944–28957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao N, and Whitelaw ML (2013) The emerging roles of AhR in physiology and immunity. Biochemical pharmacology 86, 561–570 [DOI] [PubMed] [Google Scholar]
- 11.Denison MS, Soshilov AA, He G, DeGroot DE, and Zhao B (2011) Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences : an official journal of the Society of Toxicology 124, 1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecht E, Zago M, Sarill M, Rico de Souza A, Gomez A, Matthews J, Hamid Q, Eidelman DH, and Baglole CJ (2014) Aryl hydrocarbon receptor-dependent regulation of miR-196a expression controls lung fibroblast apoptosis but not proliferation. Toxicology and applied pharmacology 280, 511–525 [DOI] [PubMed] [Google Scholar]
- 13.Halappanavar S, Wu D, Williams A, Kuo B, Godschalk RW, Van Schooten FJ, and Yauk CL (2011) Pulmonary gene and microRNA expression changes in mice exposed to benzo(a)pyrene by oral gavage. Toxicology 285, 133–141 [DOI] [PubMed] [Google Scholar]
- 14.Yauk CL, Jackson K, Malowany M, and Williams A (2011) Lack of change in microRNA expression in adult mouse liver following treatment with benzo(a)pyrene despite robust mRNA transcriptional response. Mutation research 722, 131–139 [DOI] [PubMed] [Google Scholar]
- 15.Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, and Richards CA (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. Journal of Alzheimer’s disease : JAD 14, 27–41 [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Dai X, Zhan J, Zhang Y, Zhang H, Zhang H, Zeng S, and Xi W (2015) Profiling peripheral microRNAs in obesity and type 2 diabetes mellitus. APMIS 123, 580–585 [DOI] [PubMed] [Google Scholar]
- 17.Ovchinnikova ES, Schmitter D, Vegter EL, Ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de Boer RA, Meyer S, Teerlink JR, O’Connor CM, Metra M, Davison BA, Bloomfield DM, Cotter G, Cleland JG, Mebazaa A, Laribi S, Givertz MM, Ponikowski P, van der Meer P, van Veldhuisen DJ, Voors AA, and Berezikov E (2015) Signature of circulating microRNAs in patients with acute heart failure. European journal of heart failure [DOI] [PubMed]
- 18.Baglole CJ, Reddy SY, Pollock SJ, Feldon SE, Sime PJ, Smith TJ, and Phipps RP (2005) Isolation and phenotypic characterization of lung fibroblasts. Methods in molecular medicine 117, 115–127 [DOI] [PubMed] [Google Scholar]
- 19.Ferguson HE, Thatcher TH, Olsen KC, Garcia-Bates TM, Baglole CJ, Kottmann RM, Strong ER, Phipps RP, and Sime PJ (2009) Peroxisome proliferator-activated receptor-gamma ligands induce heme oxygenase-1 in lung fibroblasts by a PPARgamma-independent, glutathione-dependent mechanism. American journal of physiology. Lung cellular and molecular physiology 297, L912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woeller CF, O’Loughlin CW, Pollock SJ, Thatcher TH, Feldon SE, and Phipps RP (2015) Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 29, 920–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woeller CF, Roztocil E, Hammond CL, Feldon SE, and Phipps RP (2016) The Aryl Hydrocarbon Receptor and Its Ligands Inhibit Myofibroblast Formation and Activation: Implications for Thyroid Eye Disease. The American journal of pathology 186, 3189–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacy SH, Woeller CF, Thatcher TH, Pollock SJ, Small EM, Sime PJ, and Phipps RP (2018) Activated Human Lung Fibroblasts Produce Extracellular Vesicles with Anti-Fibrotic Prostaglandins. American journal of respiratory cell and molecular biology [DOI] [PMC free article] [PubMed]
- 23.Woeller CF, Flores E, Pollock SJ, and Phipps RP (2017) THY1 (CD90) expression is reduced by the environmental chemical tetrabromobisphenol-A to promote adipogenesis through induction of microRNA-103. Toxicological sciences : an official journal of the Society of Toxicology [DOI] [PMC free article] [PubMed]
- 24.Gasiewicz TA, Kende AS, Rucci G, Whitney B, and Willey JJ (1996) Analysis of structural requirements for Ah receptor antagonist activity: ellipticines, flavones, and related compounds. Biochemical pharmacology 52, 1787–1803 [DOI] [PubMed] [Google Scholar]
- 25.Jacob A, Potin S, Chapy H, Crete D, Glacial F, Ganeshamoorthy K, Couraud PO, Scherrmann JM, and Decleves X (2015) Aryl hydrocarbon receptor regulates CYP1B1 but not ABCB1 and ABCG2 in hCMEC/D3 human cerebral microvascular endothelial cells after TCDD exposure. Brain research 1613, 27–36 [DOI] [PubMed] [Google Scholar]
- 26.Jacob A, Hartz AM, Potin S, Coumoul X, Yousif S, Scherrmann JM, Bauer B, and Decleves X (2011) Aryl hydrocarbon receptor-dependent upregulation of Cyp1b1 by TCDD and diesel exhaust particles in rat brain microvessels. Fluids Barriers CNS 8, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baglole CJ, Ray DM, Bernstein SH, Feldon SE, Smith TJ, Sime PJ, and Phipps RP (2006) More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunological investigations 35, 297–325 [DOI] [PubMed] [Google Scholar]
- 28.Lacy SH, Woeller CF, Thatcher TH, Maddipati KR, Honn KV, Sime PJ, and Phipps RP (2016) Human lung fibroblasts produce proresolving peroxisome proliferator-activated receptor-gamma ligands in a cyclooxygenase-2-dependent manner. American journal of physiology. Lung cellular and molecular physiology 311, L855–L867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabasa M, Royo D, Molina-Molina M, Roca-Ferrer J, Pujols L, Picado C, Xaubet A, and Pereda J (2013) Lung myofibroblasts are characterized by down-regulated cyclooxygenase-2 and its main metabolite, prostaglandin E2. PloS one 8, e65445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoyles RK, Derrett-Smith EC, Khan K, Shiwen X, Howat SL, Wells AU, Abraham DJ, and Denton CP (2011) An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II transforming growth factor beta receptor. American journal of respiratory and critical care medicine 183, 249–261 [DOI] [PubMed] [Google Scholar]
- 31.Deng Q, Dai X, Guo H, Huang S, Kuang D, Feng J, Wang T, Zhang W, Huang K, Hu D, Deng H, Zhang X, and Wu T (2014) Polycyclic aromatic hydrocarbons-associated microRNAs and their interactions with the environment: influences on oxidative DNA damage and lipid peroxidation in coke oven workers. Environmental science & technology 48, 4120–4128 [DOI] [PubMed] [Google Scholar]
- 32.Deng Q, Huang S, Zhang X, Zhang W, Feng J, Wang T, Hu D, Guan L, Li J, Dai X, Deng H, Zhang X, and Wu T (2014) Plasma microRNA expression and micronuclei frequency in workers exposed to polycyclic aromatic hydrocarbons. Environmental health perspectives 122, 719–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancuso JD, Mallon TM, and Gaydos JC (2015) Maximizing the Capabilities of the DoD Serum Repository to Meet Current and Future Needs: Report of the Needs Panel. Military medicine 180, 13–24 [DOI] [PubMed] [Google Scholar]
- 34.Deiuliis JA (2016) MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond) 40, 88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woeller CF, Thatcher TH, Van Twisk D, Pollock SJ, Croasdell A, Kim N, Hopke PK, Xia X, Thakar J, Mallon CT, Utell MJ, and Phipps RP (2016) Detection of Serum microRNAs From Department of Defense Serum Repository: Correlation With Cotinine, Cytokine, and Polycyclic Aromatic Hydrocarbon Levels. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai W, Chen Y, Yang J, Niu P, Tian L, and Gao A (2014) Aberrant miRNA profiles associated with chronic benzene poisoning. Experimental and molecular pathology 96, 426–430 [DOI] [PubMed] [Google Scholar]
- 37.De Felice B, Manfellotto F, Palumbo A, Troisi J, Zullo F, Di Carlo C, Di Spiezio Sardo A, De Stefano N, Ferbo U, Guida M, and Guida M (2015) Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med Genomics 8, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry EC, Welle SL, and Gasiewicz TA (2010) TCDD and a putative endogenous AhR ligand, ITE, elicit the same immediate changes in gene expression in mouse lung fibroblasts. Toxicological sciences : an official journal of the Society of Toxicology 114, 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S, Dere E, Burgoon LD, Chang CC, and Zacharewski TR (2009) Comparative analysis of AhR-mediated TCDD-elicited gene expression in human liver adult stem cells. Toxicological sciences : an official journal of the Society of Toxicology 112, 229–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters JM, Narotsky MG, Elizondo G, Fernandez-Salguero PM, Gonzalez FJ, and Abbott BD (1999) Amelioration of TCDD-induced teratogenesis in aryl hydrocarbon receptor (AhR)-null mice. Toxicological sciences : an official journal of the Society of Toxicology 47, 86–92 [DOI] [PubMed] [Google Scholar]
- 41.Hong B, Garabrant D, Hedgeman E, Demond A, Gillespie B, Chen Q, Chang CW, Towey T, Knutson K, Franzblau A, Lepkowski J, and Adriaens P (2009) Impact of WHO 2005 revised toxic equivalency factors for dioxins on the TEQs in serum, household dust and soil. Chemosphere 76, 727–733 [DOI] [PubMed] [Google Scholar]
- 42.Larsson M, van den Berg M, Brenerova P, van Duursen MB, van Ede KI, Lohr C, Luecke-Johansson S, Machala M, Neser S, Pencikova K, Poellinger L, Schrenk D, Strapacova S, Vondracek J, and Andersson PL (2015) Consensus toxicity factors for polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls combining in silico models and extensive in vitro screening of AhR-mediated effects in human and rodent cells. Chemical research in toxicology 28, 641–650 [DOI] [PubMed] [Google Scholar]
- 43.Walker DI, Mallon CT, Hopke PK, Uppal K, Go YM, Rohrbeck P, Pennell KD, and Jones DP (2016) Deployment-Associated Exposure Surveillance With High-Resolution Metabolomics. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine 58, S12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unkila M, Pohjanvirta R, and Tuomisto J (1999) Dioxin-induced perturbations in tryptophan homeostasis in laboratory animals. Advances in experimental medicine and biology 467, 433–442 [DOI] [PubMed] [Google Scholar]
- 45.Unkila M, Pohjanvirta R, MacDonald E, Tuomisto JT, and Tuomisto J (1994) Dose response and time course of alterations in tryptophan metabolism by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the most TCDD-susceptible and the most TCDD-resistant rat strain: relationship with TCDD lethality. Toxicology and applied pharmacology 128, 280–292 [DOI] [PubMed] [Google Scholar]
- 46.Unkila M, Ruotsalainen M, Pohjanvirta R, Viluksela M, MacDonald E, Tuomisto JT, Rozman K, and Tuomisto J (1995) Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on tryptophan and glucose homeostasis in the most TCDD-susceptible and the most TCDD-resistant species, guinea pigs and hamsters. Archives of toxicology 69, 677–683 [DOI] [PubMed] [Google Scholar]
- 47.Prendergast GC, Metz R, Muller AJ, Merlo LM, and Mandik-Nayak L (2014) IDO2 in Immunomodulation and Autoimmune Disease. Frontiers in immunology 5, 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei YD, Bergander L, Rannug U, and Rannug A (2000) Regulation of CYP1A1 transcription via the metabolism of the tryptophan-derived 6-formylindolo[3,2-b]carbazole. Archives of biochemistry and biophysics 383, 99–107 [DOI] [PubMed] [Google Scholar]
- 49.Jonsson ME, Franks DG, Woodin BR, Jenny MJ, Garrick RA, Behrendt L, Hahn ME, and Stegeman JJ (2009) The tryptophan photoproduct 6-formylindolo[3,2-b]carbazole (FICZ) binds multiple AHRs and induces multiple CYP1 genes via AHR2 in zebrafish. Chemico-biological interactions 181, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kawasaki H, Chang HW, Tseng HC, Hsu SC, Yang SJ, Hung CH, Zhou Y, and Huang SK (2014) A tryptophan metabolite, kynurenine, promotes mast cell activation through aryl hydrocarbon receptor. Allergy 69, 445–452 [DOI] [PubMed] [Google Scholar]
- 51.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, and Platten M (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478, 197–203 [DOI] [PubMed] [Google Scholar]
- 52.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, and Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185, 3190–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C, Xiao X, Chen M, Aldharee H, Chen Y, and Long W (2018) Liver kinase B1 restoration promotes exosome secretion and motility of lung cancer cells. Oncology reports 39, 376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machala M, Vondracek J, Blaha L, Ciganek M, and Neca JV (2001) Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutation research 497, 49–62 [DOI] [PubMed] [Google Scholar]
- 55.Bail S, Swerdel M, Liu H, Jiao X, Goff LA, Hart RP, and Kiledjian M (2010) Differential regulation of microRNA stability. RNA 16, 1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colombo M, Raposo G, and Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology 30, 255–289 [DOI] [PubMed] [Google Scholar]
- 57.Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, and Deng ZF (2015) Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem cell research & therapy 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, and Wang L (2013) Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC genomics 14, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang LQ, Franck N, Egan B, Sjogren RJ, Katayama M, Duque-Guimaraes D, Arner P, Zierath JR, and Krook A (2013) Autocrine role of interleukin-13 on skeletal muscle glucose metabolism in type 2 diabetic patients involves microRNA let-7. American journal of physiology. Endocrinology and metabolism 305, E1359–1366 [DOI] [PubMed] [Google Scholar]
- 60.Kolenda T, Przybyla W, Teresiak A, Mackiewicz A, and Lamperska KM (2014) The mystery of let-7d - a small RNA with great power. Contemp Oncol (Pozn) 18, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, VandenBoom TG 2nd, Kong D, Wang Z, Ali S, Philip PA, and Sarkar FH (2009) Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer research 69, 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holik AK, Lieder B, Kretschy N, Somoza MM, Held S, and Somoza V (2016) N -Carboxymethyllysine Increases the Expression of miR-103/143 and Enhances Lipid Accumulation in 3T3-L1 Cells. Journal of cellular biochemistry [DOI] [PMC free article] [PubMed]
- 63.Li M, Liu Z, Zhang Z, Liu G, Sun S, and Sun C (2015) miR-103 promotes 3T3-L1 cell adipogenesis through AKT/mTOR signal pathway with its target being MEF2D. Biol Chem 396, 235–244 [DOI] [PubMed] [Google Scholar]
- 64.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, and Stoffel M (2011) MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 474, 649–653 [DOI] [PubMed] [Google Scholar]
- 65.Liu CL, Wang WH, Sun YL, Zhuang HW, Xu M, Chen HF, and Liu JX (2018) MiR-144–3p inhibits the proliferation and metastasis of pediatric Wilms’ tumor cells by regulating Girdin. European review for medical and pharmacological sciences 22, 7671–7678 [DOI] [PubMed] [Google Scholar]
- 66.Wu C, Li X, Zhang D, Xu B, Hu W, Zheng X, Zhu D, Zhou Q, Jiang J, and Wu C (2018) IL-1beta-Mediated Up-Regulation of WT1D via miR-144–3p and Their Synergistic Effect with NF-kappaB/COX-2/HIF-1alpha Pathway on Cell Proliferation in LUAD. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 48, 2493–2502 [DOI] [PubMed] [Google Scholar]
- 67.Wu C, Xu B, Zhou Y, Ji M, Zhang D, Jiang J, and Wu C (2016) Correlation between serum IL-1beta and miR-144–3p as well as their prognostic values in LUAD and LUSC patients. Oncotarget 7, 85876–85887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


