Abstract
Background
The optimal dose and fractionation scheme of stereotactic body radiation therapy (SBRT) for hepatocellular carcinoma (HCC) remains unclear due to different tolerated liver volumes and degrees of cirrhosis. In this study, we aimed to verify the dose-survival relationship to optimize dose selection for treatment of HCC.
Methods
This multicenter retrospective study included 602 patients with HCC, treated with SBRT between January 2011 and March 2017. The SBRT dosage was classified into high dose, moderate dose, and low dose levels: SaRT (BED10 ≥ 100 Gy), SbRT (EQD2 > 74 Gy to BED10 < 100 Gy), and ScRT (EQD2 < 74 Gy). Overall survival (OS), progression-free survival (PFS), local control (LC), and intrahepatic control (IC) were evaluated in univariable and multivariable analyses.
Results
The median tumor size was 5.6 cm (interquartile range [IQR] 1.1–21.0 cm). The median follow-up time was 50.0 months (IQR 6–100 months). High radiotherapy dose correlated with better outcomes. After classifying into the SaRT, SbRT, and ScRT groups, three notably different curves were obtained for long-term post-SBRT survival and intrahepatic control. On multivariate analysis, higher radiation dose was associated with improved OS, PFS, and intrahepatic control.
Conclusions
If tolerated by normal tissue, we recommend SaRT (BED10 ≥ 100 Gy) as a first-line ablative dose or SbRT (EQD2 ≥ 74 Gy) as a second-line radical dose. Otherwise, ScRT (EQD2 < 74 Gy) is recommended as palliative irradiation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13014-021-01778-6.
Keywords: Hepatocellular carcinoma, Radiotherapy dosage, Stereotactic body radiotherapy, Survival rate
Background
Hepatocellular carcinoma (HCC) is highly prevalent in many Asian countries and accounts for nearly 80% of HCC cases worldwide. In China, HCC is the second most common cause of cancer-related deaths and the fourth most commonly diagnosed cancer among men [1]. HCC is resectable in only 10–40% of newly diagnosed patients. Liver resection, transplantation, percutaneous ethanol injection, or radiofrequency ablation (RFA) are the standard treatments for early-stage HCC [2].
The use of external beam radiation therapy (RT) [3], specifically including stereotactic body radiation therapy (SBRT), is increasing in popularity of treatment for HCC [4–11]. It is commonly recommended as an alternative treatment in medically inoperable patients, as a result of its rapid adoption in clinical practice worldwide [12–14]. SBRT for primary HCC provides high rates of durable local control (89–100%) [8, 15–18], but there is no clear evidence of a dose-survival relationship for the commonly used radiation therapy schedules. Increasing radiotherapy dose was associated with improved overall survival in patients treated with SBRT for stage I non-small-cell lung cancer [19–21]. However, the optimal dose and fractionation scheme of SBRT for HCC remains unclear because primary HCCs tend to be associated with different degrees of cirrhosis and tolerated liver volumes. In a previous retrospective study of SBRT for 127 patients with HCCs that were > 5 cm, we preliminarily found that higher biologically effective dose (BED10) and equivalent dose in 2 Gy fractions (EQD2) was associated with better survival [22]. In another prior prospective study, we built normal tissue complication probability models and nomograms for radiation-induced hepatic toxicity to obtain individual liver constraints for HCC patient [23]. In current study, we aimed to verify the dose-survival relationship to optimize dose selection for treatment of HCC.
Methods
Study design and patients
This was a multicenter retrospective study of patients with HCC who underwent SBRT in China between January 2011 and March 2017. HCC diagnosis was established based on histopathology or according to the clinical criteria for diagnosis of HCC [13]. The eligibility criteria were as follows: primary or recurrent/residual HCC patients, who were medically inoperable or refused to undergo surgery and radiofrequency ablative therapy, treated with SBRT. The exclusion criteria were as follows: (a) prior history of abdominal conventional radiotherapy, (b) intrahepatic cholangiocellular carcinoma, (c) gallbladder metastases, and/or (d) liver metastases, (e) patients with incomplete data and lost to follow-up.
Stereotactic body radiation therapy
Briefly, the patients were immobilized with a customized external vacuum-type. All patients were treated using the CyberKnife system (Accuray Incorporated, Sunnyvale, CA, USA), with 6 Mv photons. Three or four gold markers were inserted into the surrounding area of the tumor or into tumor tissue. Gross tumor volume was delineated as the visible tumor. Planning target volume was established as a 0–5 mm expansion of the GTV. No internal target volume was created because tracking was used. A dose of 28–55 Gy was administered in 1–6 fractions on consecutive days at the 50–85% isodose line that covered at least 97% of the planning target volume. Total doses and fractionation schedules were chosen according to size and dose-volume constraints of the organs at risk. The SBRT technique used has been previously described [5, 17, 22–24]
Response evaluation and follow-up
Patients were re-evaluated 1 month after SBRT and every 3–6 months thereafter. In addition, contrast-enhanced CT or/and MRI were performed at each follow-up visit. The Modified RECIST Response Evaluation Criteria in Solid Tumors (mRECIST) guideline was used to evaluate the response of the tumor [25]. The laboratory examinations assessed levels of aspartate transaminase (AST), alanine transaminase (ALT), prothrombin time (PT), levels of albumin, total bilirubin, alpha fetoprotein (AFP).
Calculated values
BED10 and EQD2 were assumed at an α/β ratio of 10, for rapidly proliferating tumor cells. EQD was calculated as: d × n{(α/β + d)/(α/β + dx)}; BED was calculated as: d × n{1 + d/(α/β)}; (d = dose, n = fraction and dx = 2). Based on our previous studies [22, 23], the SBRT dosage was classified into high dose, moderate dose, and low dose levels: SaRT (BED10 ≥ 100 Gy), SbRT (EQD2 > 74 Gy to BED10 < 100 Gy), and ScRT (EQD2 < 74 Gy).
Statistical analysis
Overall survival (OS), progression-free survival (PFS) incidence of local recurrence (LC), and incidence of intrahepatic recurrence (IC) rates were estimated using the Kaplan–Meier method and compared between groups using the log-rank test. Cumulative OS was calculated starting from the date of the first treatment until the date of the final follow-up or death. Cumulative PFS was calculated starting from the date of the first treatment until the date of recurrence or progression or death. LC was calculated starting from the date of the first treatment until the date of local recurrence or progression. IC was calculated starting from the date of the first treatment until the date of intrahepatic recurrence or progression.
Additionally, variables without associations between each other were analyzed by chi-squared/Mann–Whitney-tests. We use univariate with significant value (P < 0.05) to identify non-associated predictive variables that contribute towards the final multivariate. For categorical variables, the Pearson's chi-squared test was used. Kruskal–Wallis test was used to analyze continuous variables.
All statistical analyses were performed using R version 4.0.2 (2020-06-22) software. P < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 602 HCC patients with complete information were included in this study. All patients were classified into three groups according to SBRT dosage: SaRT (n =259), SbRT (n = 163), and ScRT (n = 180). The demographic and clinical characteristics of the patients and their treatment are summarized in Table 1. We observed strong associations between RT dose/fractionation and other prognostic factors, including BCLC class, tumor size, and ALBI grade. In general, patients with small tumors, BCLC stage A, and/or low ALBI score received higher RT doses, whereas those with larger tumors, BCLC B, C, D, and/or higher ALBI score received lower RT doses.
Table 1.
Patient and treatment characteristics for different dose groups
| Factor | Level | SaRT | SbRT | ScRT | P value |
|---|---|---|---|---|---|
| N | 259 | 163 | 180 | ||
| Gender | Female | 38 (14.7%) | 23 (14.1%) | 24 (13.3%) | 0.92 |
| Male | 221 (85.3%) | 140 (85.9%) | 156 (86.7%) | ||
| Age, median (IQR) | 54 (45, 64) | 55 (45, 63) | 51 (44, 58.5) | 0.030 | |
| Age ≥ 60 | No | 164 (63.3%) | 105 (64.4%) | 137 (76.1%) | 0.012 |
| Yes | 95 (36.7%) | 58 (35.6%) | 43 (23.9%) | ||
| HBV | Positive | 188 (72.6%) | 115 (70.6%) | 123 (68.3%) | 0.17 |
| Negative | 33 (12.7%) | 25 (15.3%) | 38 (21.1%) | ||
| Unknown | 38 (14.7%) | 23 (14.1%) | 19 (10.6%) | ||
| AFP status | 0–8 | 80 (30.9%) | 38 (23.3%) | 35 (19.4%) | < 0.001 |
| 8–200 | 95 (36.7%) | 40 (24.5%) | 41 (22.8%) | ||
| 200–400 | 16 (6.2%) | 8 (4.9%) | 11 (6.1%) | ||
| > 400 | 56 (21.6%) | 68 (41.7%) | 86 (47.8%) | ||
| Unknown | 12 (4.6%) | 9 (5.5%) | 7 (3.9%) | ||
| PT, median (IQR) | 13.3 (12.7, 14.3) | 13.4 (12.6, 14.2) | 13.15 (12.5, 14.25) | 0.58 | |
| INR, median (IQR) | 1.11 (1.05, 1.2) | 1.12 (1.05, 1.2) | 1.1 (1.045, 1.215) | 0.86 | |
| Tbil, median (IQR) | 13.7 (9.6, 19.5) | 14 (10, 21.5) | 14.1 (9.85, 20.75) | 0.58 | |
| Dbil, median (IQR) | 5.3 (3.7, 8.7) | 6.3 (4.1, 10.7) | 6.4 (4.5, 10.45) | 0.012 | |
| albumin, median (IQR) | 38 (34.2, 41.7) | 36.9 (33.5, 40.1) | 35.95 (31.8, 39.4) | < 0.001 | |
| AST, median (IQR) | 32 (23, 48) | 37 (25, 55) | 41.5 (26, 59.5) | 0.005 | |
| ALT, median (IQR) | 31 (21, 43) | 31 (20, 46) | 32.5 (23.5, 49.5) | 0.31 | |
| ALP, median (IQR) | 86 (67, 120) | 101 (78, 142) | 111 (87.5, 151.5) | < 0.001 | |
| ALBI score, median (IQR) | − 2.52 (− 2.83, − 2.10) | − 2.38 (− 2.70, − 2.04) | − 2.28 (− 2.62, − 1.93) | < 0.001 | |
| ALBI grade | 1 | 113 (43.6%) | 59 (36.2%) | 47 (26.1%) | 0.002 |
| 2 | 137 (52.9%) | 92 (56.4%) | 123 (68.3%) | ||
| 3 | 9 (3.5%) | 12 (7.4%) | 10 (5.6%) | ||
| CTP class | A | 212 (81.9%) | 131 (80.4%) | 139 (77.2%) | 0.63 |
| B | 45 (17.4%) | 30 (18.4%) | 37 (20.6%) | ||
| C | 2 (0.8%) | 2 (1.2%) | 4 (2.2%) | ||
| TD ≥ 42 Gy | No | 13 (5.0%) | 69 (42.3%) | 73 (40.6%) | < 0.001 |
| Yes | 246 (95.0%) | 94 (57.7%) | 107 (59.4%) | ||
| Fractions | 1 | 6 (2.3%) | 0 (0.0%) | 0 (0.0%) | < 0.001 |
| 2 | 6 (2.3%) | 1 (0.6%) | 0 (0.0%) | ||
| 3 | 230 (88.8%) | 68 (41.7%) | 44 (24.4%) | ||
| 4 | 11 (4.2%) | 86 (52.8%) | 85 (47.2%) | ||
| 5 | 6 (2.3%) | 7 (4.3%) | 47 (26.1%) | ||
| 6 | 0 (0.0%) | 1 (0.6%) | 4 (2.2%) | ||
| Per dose, median (IQR) | 15 (14, 15) | 11.5 (11.125, 13) | 10.5 (9, 10.625) | < 0.001 | |
| Recurrence/residual disease | No | 137 (52.9%) | 83 (50.9%) | 72 (40.0%) | 0.022 |
| Yes | 122 (47.1%) | 80 (49.1%) | 108 (60.0%) | ||
| BCLC stage | A | 139 (53.7%) | 45 (27.6%) | 30 (16.7%) | < 0.001 |
| B | 57 (22.0%) | 42 (25.8%) | 39 (21.7%) | ||
| C | 60 (23.2%) | 74 (45.4%) | 107 (59.4%) | ||
| D | 3 (1.2%) | 2 (1.2%) | 4 (2.2%) | ||
| Tumor size, median (IQR) | 3.7 (2.5, 6) | 6 (4, 9.4) | 8.1 (5.45, 11) | < 0.001 |
AFP, alpha fetal protein; ALBI, albumin-bilirubin score; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; INR, International Normalized Ratio; PT, prothrombin time;
Clinical effectiveness of increasing radiation dose
The median tumor size was 5.6 cm (interquartile range [IQR] 1.1–21.0 cm). The median follow-up time was 50.0 months (IQR 6–100 months). When RT dose was used to classify the patients into the SaRT, SbRT, and ScRT groups, 3 notably different curves were observed for long-term post-SBRT survival.
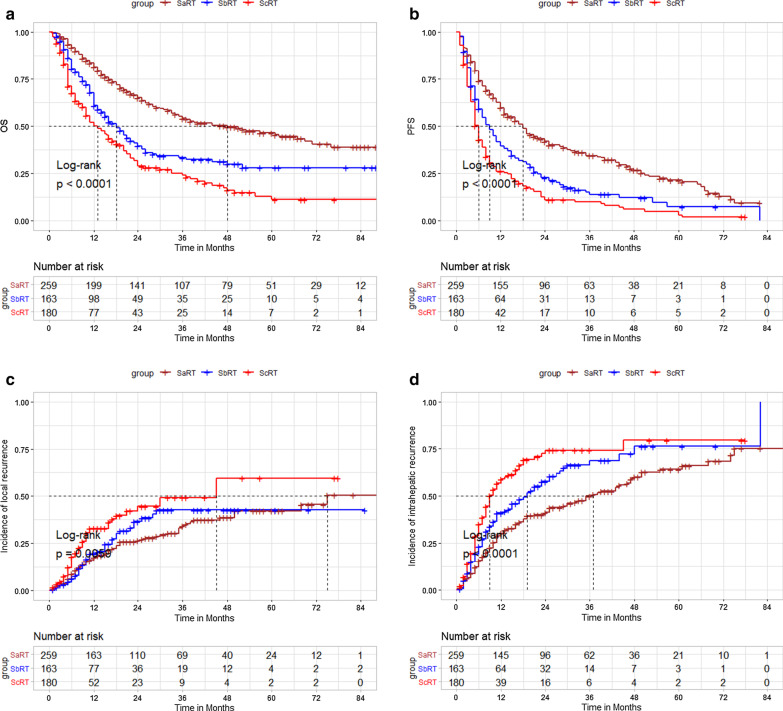
The 1-, 2-, 3-, and 5-year OS rates were 81.4, 64.9, 54.1, and 46.4% in the SaRT group; 67.7, 39.5, 33.3, and 28% in the SbRT group; and 50.0, 28.7, 24.0, and 11.1% in the ScRT group, respectively (log-rank P < 0.0001; Fig. 1a).
Fig. 1.
SaRT versus SbRT or ScRT: a overall survival, b progression-free survival, c local control, d intrahepatic control
The 1-, 2-, 3-, and 5-year PFS rates were 59.6, 41.8, 34.3 and 21.5% in the SaRT group; 39.5, 22.6, 13.8, and 7.2% in the SbRT group; and 22.5, 10.3, 9.3, and 5.2% in the ScRT group, respectively (log-rank P < 0.0001; Fig. 1b).
The 1-, 2-, 3-, and 5-year LC rates were 82.5, 73.7, 65.9 and 57.9% in the SaRT group; 80.6, 63.5, 57.5 and 57.5% in the SbRT group; and 67.2, 55.5, 50.9 and 40.7% in the ScRT group, respectively (log-rank P = 0.00594; Fig. 1c).
The 1-, 2-, 3-, and 5-year IC rates were 69.7, 58.7, 50.1 and 36.0% in the SaRT group; 59.1, 42.5, 31.4 and 23.9% in the SbRT group; and 41.2, 27.6, 25.9 and 20.7% in the ScRT group, respectively (log-rank P < 0.0001; Fig. 1d).
Multivariable Cox analysis
Cox proportional hazards models accounting for clustering were used to compare the SaRT, SbRT, and ScRT groups. The selection of influencing factors without associations between each other, including: age, gender, hepatitis B virus (HBV) status, AFP, PT, AST, ALT, alkaline phosphatase (ALP), albumin-bilirubin (ALBI) score, RT dose, recurrence/residual disease, Barcelona Clinic Liver Cancer (BCLC) stage, and tumor size, were considered for multivariate analysis based on P value < 0.05 in univariable analyses.
Multivariable cox regression analysis of OS (Fig. 2a) showed that 6 independent predictors were RT dosage (SbRT/SaRT: HR = 1.34, 95% CI 1.06–1.7; P = 0.015; ScRT/SaRT: HR = 1.67, 95% CI 1.32–2.1; P < 0.001), ALBI score, BCLC stage, HBV, AST, and AFP level > 400.
Fig. 2.
Multivariable cox analyses of all patients. a overall survival; b progression-free survival; c local control; d intrahepatic control
Multivariable cox regression analysis of PFS (Fig. 2b) showed that 5 independent predictors were RT dosage (SbRT/SaRT: HR = 1.43, 95% CI 1.08–1.9; P = 0.014; ScRT/SaRT: HR = 1.68, 95% CI 1.27–2.2; P < 0.001), ALBI score, BCLC stage, HBV, and tumor size.
Multivariable cox regression analysis of LC (Fig. 2c) showed that BCLC stage was an only independent predictor.
Multivariable cox regression analysis of IC (Fig. 2d) showed that 2 independent predictors were RT dosage (SbRT/SaRT: HR = 1.25, 95% CI 0.92–1.7; P = 0.15; ScRT/SaRT: HR = 1.60, 95% CI 1.12–2.2; P = 0.004) and BCLC stage.
Subgroup analysis of total dose and fractionation scheme for OS and PFS
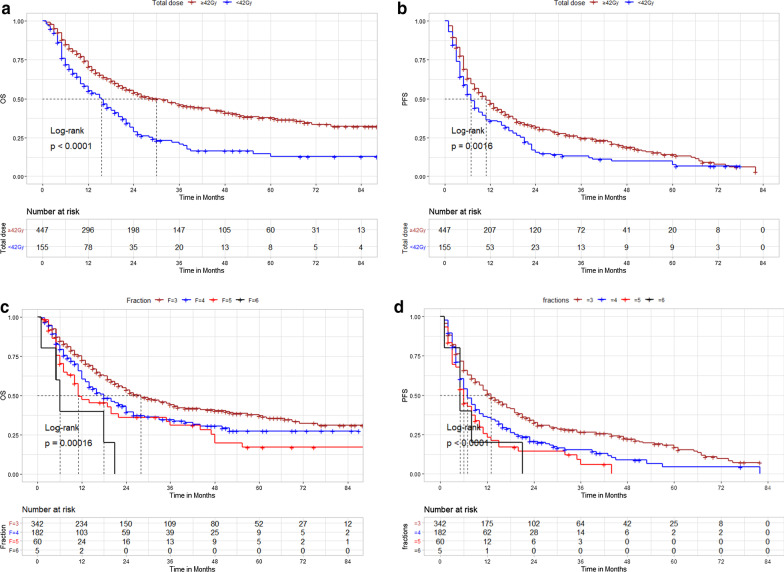
Additionally, we found a significant association between higher total dose (TD) and better OS. The 1-, 3-, and 5-year OS rates were 70.6, 46.0, and 37.8% in the TD ≥ 42 Gy group and 55.1, 28.9, and 12.9% in the TD < 42 Gy group, respectively (log-rank P < 0.001; Fig. 3a). The 1-, 3-, and 5-year PFS rates were 46.8, 31.7, and 14.0% in the TD ≥ 42 Gy group and 35.8, 17.1, and 7.8% in the TD < 42 Gy group, respectively (log-rank P < 0.001; Fig. 3b).Further, patients treated with single fraction (n = 6) and 2 fractions group (n = 7) were excluded (Additional file 1: Supplementary Fig. S1), we found that the use of fewer fractions (= 3 fractions) group was association with significantly better OS (Fig. 3c) and PFS (Fig. 3d) than more fractions (≥ 4 to 6 fractions) group.
Fig. 3.
a Overall survival: total doses ≥ 42 Gy versus < 42 Gy group; b progression-free survival: total doses ≥ 42 Gy versus < 42 Gy group; c overall survival: fractions = 3 versus ≥ 4–6 fractions group; d progression-free survival: fractions = 3 versus ≥ 4–6 fractions group
Additionally, a sensitivity analysis excluding the initial 127 overlapping patients [22], three notably different curves were also obtained for long-term post-SBRT survival for log-rank testing among the high, moderate and low dose groups (Additional file 1: Supplementary material Figure S2–3).
Discussion
Precise SBRT dose is important but uncertain, especially in HCC that can be treated with radical radiotherapy, because primary HCCs tend to be associated with different tolerated liver volumes and degrees of cirrhosis. In the current study, we classified radiotherapy doses into high dose, moderate dose, and low dose levels: SaRT (BED10 ≥ 100 Gy), SbRT (EQD2 > 74 Gy to BED10 < 100 Gy), and ScRT (EQD2 < 74 Gy). Three notably different curves were obtained for long-term post-SBRT survival and intrahepatic control. On multivariate analysis, higher RT dose was associated with improved OS, PFS, and intrahepatic control but not local control. This finding is consistent with a previous study of SBRT for 127 patients with HCCs that were > 5 cm [22].
In the current study, a ≥ 42 Gy total dose and 3 fractions were important indices that were associated with clinical curative effect (e.g. in 42 Gy in 3 fractions, BED10 = 100.8 Gy and EQD2 = 84.0 Gy).Wahl et al. [16] reported that SBRT appears to be a reasonable first-line treatment for inoperable large HCC. They found no significant difference in OS between the SBRT and RFA groups, and also observed that, for tumors sized ≥ 2 cm, SBRT was superior to RFA in terms of freedom from local progression. In contrast, Rajyaguru et al. [26] reported 5-year OS rates of 19.3% in the SBRT group and 29.8% in the RFA group, and 60 of the 235 (26%) received lower radiation doses (< 40 Gy) in SBRT group, a follow-up analysis of patients receiving ablative doses (> 40 Gy) showed no OS difference in comparison with patients receiving RFA [27]. Jang et al. [28] reported SBRT doses escalated from 33 Gy in 3 fractions to 60 Gy in 3 fractions for HCC (longest diameter ≤ 7 cm). The 2-year OS rates for patients treated with doses > 54 Gy, 45–54 Gy, and < 45 Gy were 71%, 64%, and 30%, respectively, while the 2-year local control rates were 100%, 78%, and 64%, respectively. Recently, a fractionated scheme of 45 Gy in 3 fractions (BED10 = 112.5 Gy and EQD2 = 93.8 Gy) was tested in a multi-institutional, single-arm phase II trial of SBRT for the treatment of 74 HCC patients with unifocal liver tumors within ≤ 5 cm in diameter in China. Thirteen patients presented with grade ≥ 2 hepatic adverse reaction and 8 patients presented with decreased CP classification [29]. Another scheme, involving 3–5 fractions of 39–50 Gy (EQD2 = 70.0 Gy to BED10 = 112.5 Gy), has recently been tested in our single-institutional phase II trial of SBRT for the treatment of HCC in patients with a total diameter < 10 cm. A first-line ablative dose of SaRT with a BED10 ≥ 100 Gy or a second-line radical dose of SbRT with an EQD2 ≥ 74 Gy was recommended. Otherwise, palliative irradiation via ScRT with EQD2 < 74 Gy was recommended. In this prior prospective study, 85 patients have been previously reported. None case of classic radiation-induced liver disease was observed. Regarding the Child–Pugh (CP) scores following SBRT, 20 (23.5%) and 12 (14.2%) patients suffered Child–Pugh scores CP + ≥ 1 and ≥ 2, respectively. We further found that pre-CP, V15 (the percentage of normal liver volume receiving more than 15 Gy) and VS10 (the absolute normal liver volume spared from at least 10 Gy) were optimal predictors for radiation-induced hepatic toxicity (RIHT: CP + ≥ 1 and ≥ 2) modelling and nomograms based on normal tissue complication probability (NTCP) models were generated [23]. On the basis of these two studies, optimal selection of SBRT dosage and dose-volume constraints for the liver was recommended to balance the pros and cons (Table 2).
Table 2.
Recommendations for 3–5 fractions SBRT treatment
| Dosimetric constraints for normal liver | Radiation dose for GTV |
|---|---|
| V15 < 21.5%, VS10 ≥ 621.8 mL | SaRT: BED10 ≥ 100 Gy |
| V15 < 33.1%, VS10 ≥ 416.2–621.8 mL | SbRT: EQD2 ≥ 74 Gy |
| Without above conditions or Child–Pugh ≥ B7 class | ScRT: EQD2 < 74 Gy |
GTV, gross tumor volume; V15, percentage of normal liver volume receiving more than 15 Gy; VS10, absolute normal liver volume spared from at least 10 Gy
The present study has some limitations. First, the calculation of BED10 using an α/β ratio of 10 from the linear-quadratic model is controversial, despite being commonly used. BED10 can serve as a simple and straightforward means to perform a comparative and effective analysis among a large variety of dose fractionations prescribed. The clinical efficacy of higher BED10 values has been fully recognized in the use of SBRT for lung cancer [19–21] and live cancer [30, 31]. Conventional radiation dose is difficult to exceed 60–74 Gy in HCC, and we found that EQD2 ≥ 74 Gy was the second-line radical dose in BED10 < 100 Gy. Second, this study was performed in an area in which hepatitis B is endemic; it is unclear whether the dosimetric findings are applicable to cases of HCC associated with other risk factors.
In conclusion, higher radiotherapy doses were associated with better survival in patients undergoing SBRT for the treatment of HCC. If tolerated by normal tissue, we recommend SaRT with BED10 ≥ 100 Gy as the first-line ablative dose or undergoing SbRT with EQD2 ≥ 74 Gy as the second-line radical dose. Otherwise, ScRT with EQD2 < 74 Gy is recommended as palliative irradiation. Future prospective research is warranted to validate the effects of this treatment regimen.
Supplementary Information
Additional file 1. Fig S1: Overall survival based on different fractions. Fig S2: A sensitivity analysis excluding the initial 127 overlapping patients,three notably different curves of long-term post-SBRT survival: S2A) OS, S2A) PFS.
Acknowledgements
None.
Abbreviations
- ALBI
Albumin–bilirubin
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- BCLC
Barcelona Clinic Liver Cancer
- BED
Biologically effective dose
- CT
Computed tomography
- CTP
Child-Turcotte-Pugh
- EQD2
Equivalent dose in 2 Gy fractions
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- IC
Intrahepatic control
- LC
Local control
- MRI
Magnetic resonance imaging
- OS
Overall survival
- PFS
Progression-free survival
- RT
Radiation therapy
- SBRT
Stereotactic body radiotherapy
Authors' contributions
Su made substantial contributions to conception and design of the study and the analysis and interpretation of the data. Su, Liu, Zhu, Liang P, Zhou, Lai, Cheng and Huang made substantial contributions to data acquisition. Su and Zhu participated in drafting the article. All authors participated in revising it critically for important intellectual content. All authors provided final approval of the version to be published.
Funding
This research was supported by the National Natural Science Foundation of China (81903257 and 81960534), and Guangxi Natural Science Foundation (CN) (2020GXNSFAA297171), and National Science and Technology Major Special Project (2017ZX10203207), and China International Medical Foundation-Tumor Precise Radiotherapy Spark Program (2019-N-11-01), and High-level innovation team and outstanding scholar program in Guangxi Colleges and Universities, and Guangxi Medical University Training Program for Distinguished Young Scholars, and Guangxi BaGui Scholars’ Special Fund.
Availability of data and materials
The datasets generated during the current study are not publicly available due to hospital secrets but are available from the corresponding author (Su, sutingshi@163.com) on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Institutional Ethics Committee of Guangxi Medical University Cancer Hospital (LW2019038), and informed consent was waived because of the retrospective nature of this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ting-Shi Su, Email: sutingshi@163.com.
Qiu-Hua Liu, Email: lqhaaa@126.com.
Xiao-Fei Zhu, Email: zhuxiaofei_zxf@163.com.
Ping Liang, Email: 13878111698@163.com.
Shi-Xiong Liang, Email: shixliang@vip.sina.com.
Lin Lai, Email: lailin2856@163.com.
Ying Zhou, Email: zhylsh@163.com.
Yong Huang, Email: 13978623908@163.com.
Tao Cheng, Email: rkct123@163.com.
Le-Qun Li, Email: Li_lequn@263.net.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study of The L, European Organisation For R, Treatment of C: EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012, 56(4):908–943. [DOI] [PubMed]
- 3.Su TS, Li LQ, Meng WW, Wang YD, Chen YT, Li JX, Du YQ, Qu S, Zhao C, Huang DJ, et al. Long-term survival analysis of transarterial chemoembolization plus radiotherapy vs. radiotherapy for hepatocellular carcinoma with macroscopic vascular invasion. Front Oncol. 2020;10:1205. doi: 10.3389/fonc.2020.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durand-Labrunie J, Baumann AS, Ayav A, Laurent V, Boleslawski E, Cattan S, Bogart E, Le Deley MC, Steen V, Lacornerie T, et al. Curative irradiation treatment of hepatocellular carcinoma: a multicenter phase 2 trial. Int J Radiat Oncol Biol Phys. 2020;107(1):116–125. doi: 10.1016/j.ijrobp.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Su TS, Liang P, Liang J, Lu HZ, Jiang HY, Cheng T, Huang Y, Tang Y, Deng X. Long-term survival analysis of stereotactic ablative radiotherapy versus liver resection for small hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2017;98(3):639–646. doi: 10.1016/j.ijrobp.2017.02.095. [DOI] [PubMed] [Google Scholar]
- 6.Sapir E, Tao Y, Schipper MJ, Bazzi L, Novelli PM, Devlin P, Owen D, Cuneo KC, Lawrence TS, Parikh ND, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2018;100(1):122–130. doi: 10.1016/j.ijrobp.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su TS, Liang P, Zhou Y, Huang Y, Cheng T, Qu S, Chen L, Xiang BD, Zhao C, Huang DJ, et al. Stereotactic body radiation therapy vs. transarterial chemoembolization in inoperable barcelona clinic liver cancer stage a hepatocellular carcinoma: a retrospective, propensity-matched analysis. Front Oncol. 2020;10:347. doi: 10.3389/fonc.2020.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, Iwabuchi S, Kunieda E. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53(3):399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Wang Q, Hong ZX, Li WG, He WP, Zhang T, Zhang AM, Fan YZ, Sun YZ, Zheng L, et al. Stereotactic body radiotherapy versus hepatic resection for hepatocellular carcinoma (</= 5 cm): a propensity score analysis. Hepatol Int. 2020;14(5):788–797. doi: 10.1007/s12072-020-10088-0. [DOI] [PubMed] [Google Scholar]
- 10.Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, Imajo K, Aoki Y, Saito H, Kunieda E. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122(13):2041–2049. doi: 10.1002/cncr.30008. [DOI] [PubMed] [Google Scholar]
- 11.Yoon SM, Kim SY, Lim Y-S, Kim KM, Shim JH, Lee D, An J, Jung J, Kim JH, Lee HC: Stereotactic body radiation therapy for small (≤5 cm) hepatocellular carcinoma not amenable to curative treatment: results of a single-arm, phase II clinical trial. Korean J Hepatol 2020, 0(0):0–0. [DOI] [PMC free article] [PubMed]
- 12.Vogel A, Cervantes A, Chau I, Daniele B, Llovet J, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Supplement_4):iv238–iv255. doi: 10.1093/annonc/mdy308. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Sun HC, Wang Z, Cong WM, Wang JH, Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, et al. Guidelines for diagnosis and treatment of primary liver cancer in China liver (2017 Edition) Cancer. 2018;7(3):235–260. doi: 10.1159/000488035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson AB, 3rd, D'Angelica MI, Abbott DE, Abrams TA, Alberts SR, Saenz DA, Are C, Brown DB, Chang DT, Covey AM, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. doi: 10.6004/jnccn.2017.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, Lartigau E, Mirabel X. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS ONE. 2013;8(10):e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su TS, Liang P, Lu HZ, Liang J, Gao YC, Zhou Y, Huang Y, Tang MY, Liang JN. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol. 2016;113(2):181–187. doi: 10.1002/jso.24128. [DOI] [PubMed] [Google Scholar]
- 18.Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croise-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115(2):211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2015;91(2):344–350. doi: 10.1016/j.ijrobp.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Kestin L, Grills I, Guckenberger M, Belderbos J, Hope AJ, Werner-Wasik M, Sonke JJ, Bissonnette JP, Xiao Y, Yan D, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol. 2014;110(3):499–504. doi: 10.1016/j.radonc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, Niibe Y, Karasawa K, Hayakawa K, Takai Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 22.Su TS, Lu HZ, Cheng T, Zhou Y, Huang Y, Gao YC, Tang MY, Jiang HY, Lian ZP, Hou EC, et al. Long-term survival analysis in combined transarterial embolization and stereotactic body radiation therapy versus stereotactic body radiation monotherapy for unresectable hepatocellular carcinoma >5 cm. BMC Cancer. 2016;16(1):834. doi: 10.1186/s12885-016-2894-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su TS, Luo R, Liang P, Cheng T, Zhou Y, Huang Y. A prospective cohort study of hepatic toxicity after stereotactic body radiation therapy for hepatocellular carcinoma. Radiother Oncol. 2018;129(1):136–142. doi: 10.1016/j.radonc.2018.02.031. [DOI] [PubMed] [Google Scholar]
- 24.Su TS, Yang HM, Zhou Y, Huang Y, Liang P, Cheng T, Chen L, Li LQ, Liang SX. Albumin - bilirubin (ALBI) versus Child-Turcotte-Pugh (CTP) in prognosis of HCC after stereotactic body radiation therapy. Radiat Oncol. 2019;14(1):50. doi: 10.1186/s13014-019-1251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 26.Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN, Go RS. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Clin Oncol. 2018;36(6):600–608. doi: 10.1200/JCO.2017.75.3228. [DOI] [PubMed] [Google Scholar]
- 27.Shinde A, Jones BL, Chen YJ, Amini A. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma: does radiation dose make a difference? J Clin Oncol. 2018;36(24):2566–2567. doi: 10.1200/JCO.2018.78.6012. [DOI] [PubMed] [Google Scholar]
- 28.Jang W, Kim M, Bae S, Cho C, Yoo H, Seo Y, Kang J, Kim S, Lee D, Han C, et al. High-dose stereotactic body radiotherapy correlates increased local control and overall survival in patients with inoperable hepatocellular carcinoma. Radiat Oncol. 2013;8:250. doi: 10.1186/1748-717X-8-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Song Y, Liang P, Su T, Zhang H, Zhao X, Yuan Z, Wang P. Analysis of the factors affecting the safety of robotic stereotactic body radiation therapy for hepatocellular carcinoma patients. Onco Targets Ther. 2017;10:5289–5295. doi: 10.2147/OTT.S142025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, Navarria P, Fogliata A, Tomatis S, D'Agostino G, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT) J Cancer Res Clin Oncol. 2015;141(7):1301–1309. doi: 10.1007/s00432-015-1929-y. [DOI] [PubMed] [Google Scholar]
- 31.Sun J, Zhang T, Wang J, Li W, Zhang A, He W, Zhang D, Li D, Ding J, Duan X. Biologically effective dose (BED) of stereotactic body radiation therapy (SBRT) was an important factor of therapeutic efficacy in patients with hepatocellular carcinoma (</=5 cm) BMC Cancer. 2019;19(1):846. doi: 10.1186/s12885-019-6063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig S1: Overall survival based on different fractions. Fig S2: A sensitivity analysis excluding the initial 127 overlapping patients,three notably different curves of long-term post-SBRT survival: S2A) OS, S2A) PFS.
Data Availability Statement
The datasets generated during the current study are not publicly available due to hospital secrets but are available from the corresponding author (Su, sutingshi@163.com) on reasonable request.