Abstract
Voltage-gated sodium channels (Navs) are tightly regulated by multiple conserved auxiliary proteins, including the four fibroblast growth factor homologous factors (FGFs), which bind the Nav EF-hand like domain (EFL), and calmodulin (CaM), a multifunctional messenger protein that binds the NaV IQ motif. The EFL domain and IQ motif are contiguous regions of NaV cytosolic C-terminal domains (CTD), placing CaM and FGF in close proximity. However, whether the FGFs and CaM act independently, directly associate, or operate through allosteric interactions to regulate channel function is unknown. Titrations monitored by steady-state fluorescence spectroscopy, structural studies with solution NMR, and computational modeling demonstrated for the first time that both domains of (Ca2+)4-CaM (but not apo CaM) directly bind two sites in the N-terminal domain (NTD) of A-type FGF splice variants (FGF11A, FGF12A, FGF13A, and FGF14A) with high affinity. The weaker of the (Ca2+)4-CaM-binding sites was known via electrophysiology to have a role in long-term inactivation of the channel but not known to bind CaM. FGF12A binding to a complex of CaM associated with a fragment of the NaV1.2 CTD increased the Ca2+-binding affinity of both CaM domains, consistent with (Ca2+)4-CaM interacting preferentially with its higher-affinity site in the FGF12A NTD. Thus, A-type FGFs can compete with NaV IQ motifs for (Ca2+)4-CaM. During spikes in the cytosolic Ca2+ concentration that accompany an action potential, CaM may translocate from the NaV IQ motif to the FGF NTD, or the A-type FGF NTD may recruit a second molecule of CaM to the channel.
Keywords: molecular recognition, Ca2+-dependent interaction, voltage-gated sodium channel, protein–protein interaction, NMR, FRET, FHF, biosensor, thermodynamics, allostery, affinity
Abbreviations: CaM, calmodulin; CaMBD, CaM-binding domain; CTD, C-terminal domain; FGF, fibroblast growth factor homologous factor; Nav, voltage-gated sodium channel; NTD, N-terminal domain
The human voltage-gated sodium channels (NaV) are a family of nine proteins (NaV1.1–NaV1.9) that are responsible for the generation and propagation of action potentials in excitable tissues throughout the human body. The functional core of each NaV is a single transmembrane pore-forming α-subunit that interacts with one or more auxiliary β-subunits (Fig. 1, A and B) (1). The physiological function of NaVs requires the α-subunit to rapidly transition among closed, opened, and inactivated states. The importance of rapidly cycling among these functional states is highlighted by the identification of disease-causing mutations throughout the sequences of the NaV isoforms that disrupt this process to cause debilitating conditions including epileptic disorders (2, 3, 4, 5, 6), cardiomyopathies (7, 8, 9, 10), and chronic pain (11, 12, 13).
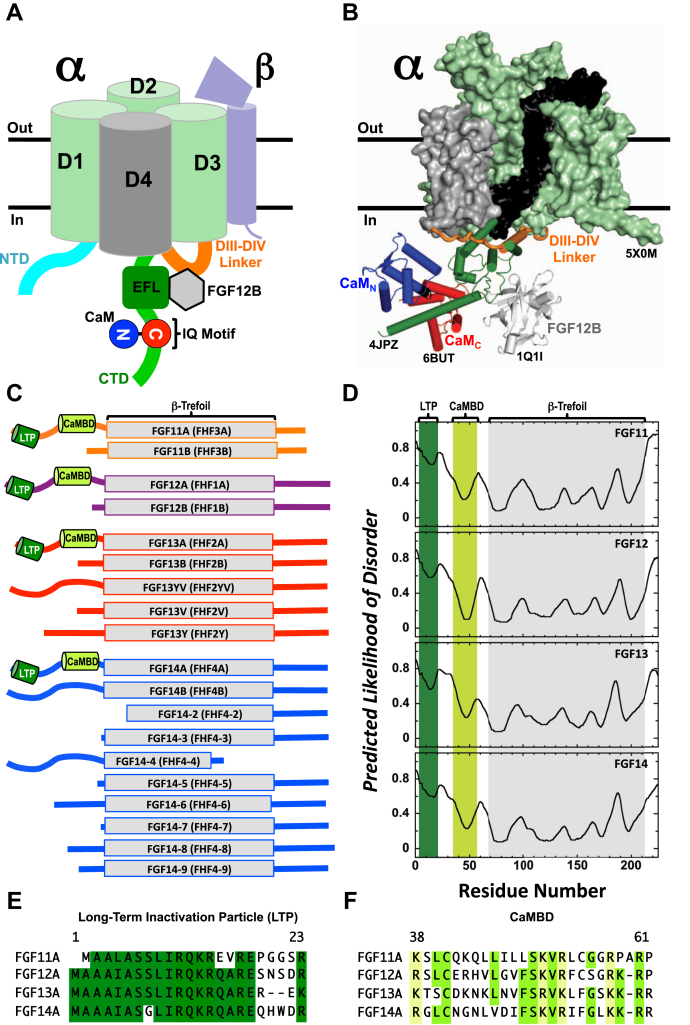
Figure 1.
Architecture of NaV, CaM, and FGFs.A, schematic of human NaV. The α-subunit N-terminal domain (NTD, cyan), transmembrane domains DI-DIII (pale green) and DIV (gray), the linker connecting DIII to DIV (orange), and C-terminal domain (CTD, green) with 4-helix bundle EFL (dark green) that binds FGFs (light gray), and IQ motif that binds CaM (CaMN/blue, CaMC/red) are shown. Auxiliary β-subunit that contains a transmembrane helix and extracellular domain is lavender. B, model of apo CaM and FGF12B bound to NaV. Model is comprised of NaVPAS (5X0M, DI-DIII/pale green surface, DIV voltage-sensing domain/gray surface, DIV pore domain/black surface, DIII-DIV linker/orange cartoon), the NaV1.5CTD (4DCK, forest green), the apo CaM+NaV1.2IQp ensemble (6BUT, CaMN/blue, CaMC/red), and FGF12B (1Q1U, gray). The NaV1.5CTD was aligned to NaVPAS EFL (a.a. 1426–1521), the apo CaM+NaV1.2IQp ensemble was aligned with 4DCK via CaM a.a. 101–112 and 117–128, and FGF12B was aligned with 4DCK using FGF13B a.a. 11–158. For simplicity NaV1.2IQp in 6BUT and CaM FGF13B in 4DCK are not shown. C, schematics of FGF11 (orange), FGF12A (purple), FGF13A (red), and FGF14 (blue) splice variants. The β-trefoil core is shown as a rectangle, and the long-term inactivation particle (LTP, green) and putative CaM-binding domain (CaMBD, limon) are shown as cylinders. D, predicted likelihood of disorder of A-type FGF isoform sequences. The minima shaded green and limon correspond to the LTP and CaMBD sequences, respectively. The folded β-trefoil core is shaded gray. E and F, sequence alignments of the A-type FGF LTP (E) and CaMBD (F). Positions that are conserved in at least three of the four FGF isoforms are shaded (LTP/green, CaMBD/limon). Positions in the CaMBD (F) that contain a basic K or R in all four isoforms are shaded in pale yellow. Alignments were made with COBALT (108).
The transition of an NaV α-subunit among its functional states is tightly regulated by a network of protein–protein interactions. These include intramolecular interactions among cytosolic regions of the α-subunit including the inactivation gate and intermolecular interactions between the cytosolic N-terminal (NTD) and C-terminal domains (CTD) of the channel and several auxiliary proteins (14, 15, 16). The NaV CTD interacts with multiple auxiliary proteins including fibroblast growth factor homologous factors (FGFs or FHFs) that bind to an acidic EF-hand-like domain (EFL) (17, 18, 19, 20, 21) and the ubiquitously expressed and essential Ca2+ sensor calmodulin (CaM) that binds to a highly conserved basic IQ motif (IQxxx[R,K]Gxxx[R,K]) (Fig. 1B) (22, 23, 24, 25, 26, 27, 28).
CaM is composed of two four-helix bundle domains (CaMN and CaMC). They are connected by a flexible linker that allows the domains to move independently in solution. Both CaMN and CaMC contain a pair of EF-hands that bind Ca2+ cooperatively. In free CaM, CaMC has an affinity for Ca2+ that is approximately tenfold higher than that of CaMN (29, 30, 31) resulting in sequential occupancy of the domains. In eukaryotes, CaM regulates many proteins in a Ca2+-dependent manner (32, 33, 34, 35, 36). Binding these targets selectively increases or decreases the Ca2+-binding affinity of one or both domains of CaM, making CaM an effective Ca2+ sensor over a wide range (103) of Ca2+ concentrations (37).
Ca2+-depleted (apo) CaM and (Ca2+)4-CaM bind tightly to many IQ motifs. These basic amphipathic α-helix (BAA) CaM binding domains (CaMBDs) are found in all human NaV isoforms, and CaM–NaV interactions have been especially well studied in NaV1.2 and NaV1.5 (23, 24, 25, 26, 27). Despite both apo and (Ca2+)4-CaM having a high affinity for these IQ motifs, how CaM acts as a Ca2+ sensor to modulate NaV function is poorly understood. Ca2+ binding to CaMC induces a ∼180° rotation of CaMC on the NaV1.2 IQ motif (25). This rotation may require transient release and reassociation of CaMC with the IQ motif, which could also allow CaM to translocate to a different high-affinity CaMBD.
The four FGF isoforms (FGF11–FGF14) that bind the EFL domain of NaVs are a subgroup of the fibroblast growth factor superfamily (38). Crystallographic structures (39, 40) showed that they contain a well-folded β-trefoil core that is nearly identical to that of canonical fibroblast growth factors (41). However, unlike most members of the FGF family, these FGFs are not secreted (38, 42). Rather, they remain in the cytosol and have been implicated in trafficking and modulating NaV channel properties including persistent current (17, 19, 21, 43, 44).
Multiple splice variants have been identified for each of the four FGF isoforms (Fig. 1C). These arise primarily from differential splicing of the first exon and result in sequences that vary in the length and composition of the NTD (42, 45). The effect of FGFs on NaV function depends on the splice variant bound (21, 43, 46, 47). The B-type splice variants typically have a shorter NTD and are associated with changing current density. The A-type splice variants typically have a longer NTD and are specifically associated with an increased rate of inactivation and long-term inactivation of the NaV α-subunit, which has been proposed to result from an interaction between a region in the NTD and the channel (43). The differences suggest important roles for the distinct NTD sequence of each FGF splice variant.
Colocalization experiments have found that CaM and the FGFs interact with multiple NaV isoforms within cells (47, 48, 49, 50). Proteomics studies have shown that CaM and FGF12 interact with NaV1.2 in neurons (16). Crystallographic structures of a B-type FGF (FGF12B and FGF13U) and CaM bound to NaV CTD fragments that contain both the EFL and IQ motif (26, 51) showed that CaM and FGFs are bound near each other. Recently, an indirect allosteric interaction has been proposed to occur between CaM and FGF12B on NaV1.4 (52). However, there has been no evidence supporting CaM directly binding any splice variant of an FGF isoform.
Here we report for the first time that CaM binds the NTD of each A-type FGF (FGF11A, FGF12A, FGF13A, and FGF14A) in a Ca2+-dependent manner. Using steady-state fluorescence spectroscopy, copurification, and solution NMR, we show that CaM binds two sequences in the NTD of each A-type FGF with high affinity and that both domains of (Ca2+)4-CaM mediate this interaction. Focusing on FGF12 because of the cellular and structural studies cited above, we demonstrate that binding of full-length FGF12A to a fragment of the NaV1.2 CTD (NaV1.2CTD, residues 1777–1937) containing the EFL and IQ motif increases the Ca2+ affinity of both CaM domains. Because the IQ motif is known to lower Ca2+ affinity of CaMC, this new finding is consistent with (Ca2+)4-CaM interacting favorably with the NTD of FGF12A in this ternary complex. These results support a model in which the A-type FGFs compete with NaV IQ motifs for (Ca2+)4-CaM and suggest that, during spikes in the local cytosolic Ca2+ concentration, CaM may translocate from an NaV IQ motif to an A-type FGF NTD or that the NTD may recruit an additional CaM molecule to the ternary complex.
Results
Potential CaM-binding sites in NTDs of A-type FGFs
CaM is known to bind tightly to sequences that are intrinsically disordered but that adopt helical geometry when bound by CaM. The NTD sequences of A-type splice variants of intracellular FGFs are thought to be disordered. However, an analysis of the FGF11A, FGF12A, FGF13A, and FGF14A sequences with the Protein Disorder Prediction System (53) showed two minima in the NTD of each FGF isoform (Fig. 1, C and D) suggesting that two segments are capable of adopting ordered secondary structure and might be CaM-binding sites.
One sequence is near the N terminus of the NTD (referred to as the long-term inactivation particle or LTP). It is highly conserved among the four human FGF isoforms (Fig. 1E) and across species (Fig. S1, A–D, Table S1–S4) and was shown to contribute functionally to long-term inactivation of NaVs mediated by A-type FGFs (43). The other sequence is C terminal to the LTP (referred to hereafter as the CaM-binding domain (CaMBD)). Although the CaMBD has a more variable sequence among the four human FGF isoforms (Fig. 1F), the CaMBD sequence of each isoform is highly conserved across species (Fig. S1, E–H, Table S5–S8). Currently it has no known function.
To explore whether the FGF LTP and CaMBD regions might function as CaM-binding sites, α-helical models were made with PyMOL and helical wheels were generated based on the sequence of the FGF12A LTP and CaMBD (Fig. S2, A–F). The sequences each contained an aliphatic patch bracketed by basic residues consistent with other BAA motifs that are known to bind tightly to CaM.
To understand whether these putative sites in the FGF NTD would be accessible to CaM, it would be helpful to have an experimentally determined structure; however, none are available for any of the full-length A-type FGFs. Therefore, structural models of full-length FGF12A were generated with Robetta (54). Each model in the ensemble had a well-folded β-trefoil core that was nearly identical to that of a crystallographically determined structure of FGF12B (Fig. S3, A and B) (39). New insights came from modeling of the FGF12A NTD that included the LTP and CaMBD. Both potential CaM-binding sites were predicted to adopt α-helical secondary structure (Fig. S3B). They were connected by a disordered linker (aa 18–39), and the CaMBD region was connected to the β-trefoil core by another disordered linker (aa 52–69). These linkers would allow the LTP and CaMBD to sample many orientations relative to each other and relative to the β-trefoil core as shown in the set of five lowest-energy (most favorable) conformations (Fig. S3, C–H). The predicted secondary structure and BAA motif sequences of the FGF12A LTP and CaMBD suggested that both were strong candidates for CaM binding.
(Ca2+)4-saturated CaM tightly binds two sites in the A-type FGFs
To determine whether CaM binds the FGF LTP or CaMBD, apo or (Ca2+)4-CaM was added to biosensors in which the sequence of the LTP of FGF12A (FGF12ALTP, residues 1–23) or CaMBD of FGF11A (FGF11ACaMBD, residues 36–62), FGF12A (FGF12ACaMBD, residues 38–64), FGF13A (FGF13ACaMBD, residues 35–60), and FGF14A (FGF14ACaMBD, residues 37–63) was inserted between YFP and CFP (see Experimental procedures).
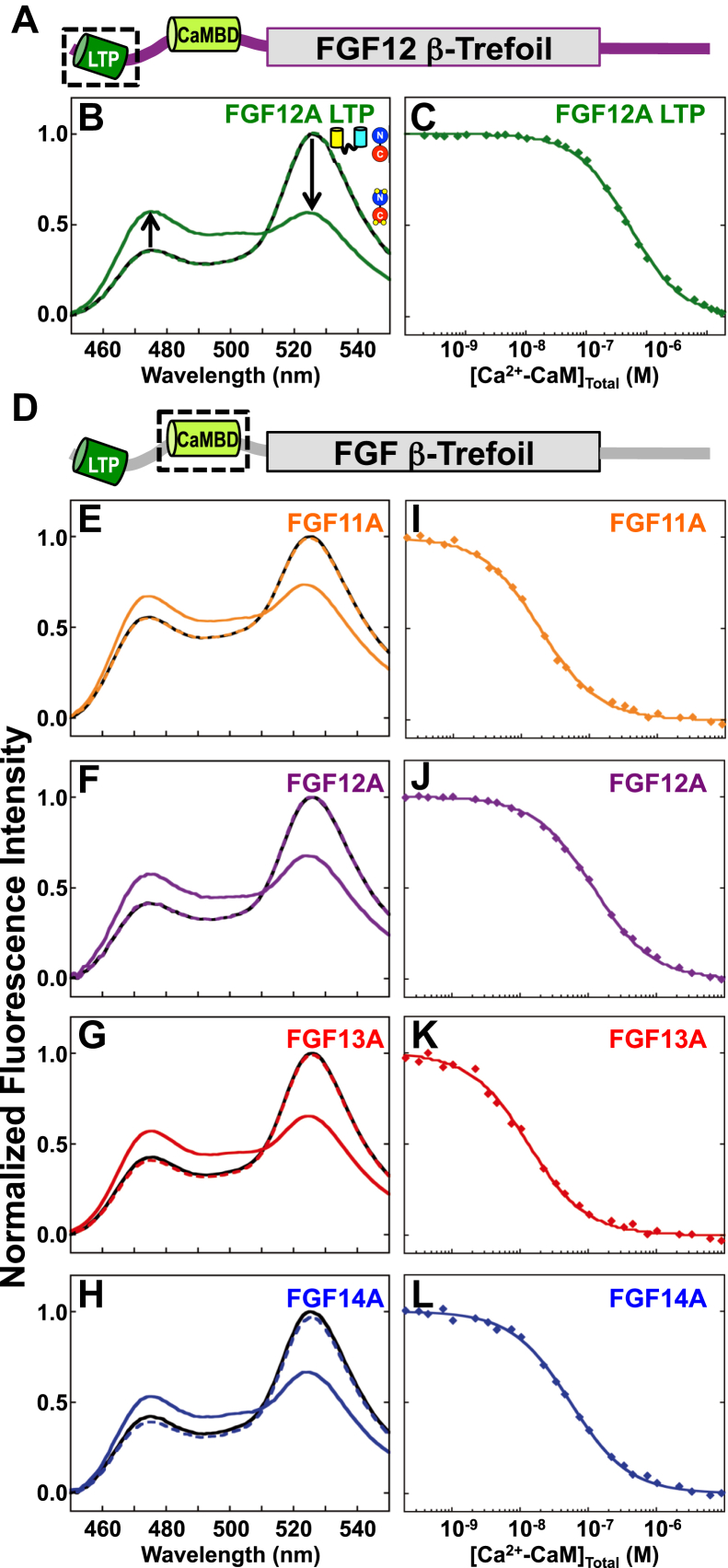
Addition of excess apo CaM to the FGF12ALTP biosensor (200:1 [CaM]:[Biosensor]) caused negligible (∼0%) changes in the emission spectrum (Fig. 2, A and B). However, after addition of saturating Ca2+, reciprocal changes were observed in the intensities of YFP (reduced by ∼50%) and CFP (increased by ∼52%) (Fig. 2B). This indicated that (Ca2+)4-CaM, but not apo CaM, bound to FGF12ALTP. Equilibrium titrations of the FGF12ALTP biosensor with (Ca2+)4-CaM showed that the Kd was 576 nM (Fig. 2C, Table 1).
Figure 2.
(Ca2+)4-CaM binds the FGF12ALTPand FGF CaMBD biosensors.A, schematic of FGF12A. Folded β-trefoil core is shown as a rectangle, and LTP (green) and CaMBD (limon) are shown as cylinders. The black box indicates the position of the FGF12A LTP. B, steady-state emission spectra of the FGF12ALTP biosensor alone (solid black), in the presence of apo (dashed green) and (Ca2+)4-CaM (solid green). C, equilibrium titration of FGF12ALTP biosensor with (Ca2+)4-CaM (green). D, schematic of an A-type FGF. Folded β-trefoil core is shown as a rectangle, and LTP (green) and CaMBD (limon green) are shown as cylinders. The black box indicates the position of the A-type FGF CaMBD. E–H, steady-state fluorescence spectra of FGF11ACaMBD (E, orange), FGF12ACaMBD (F, purple), FGF13ACaMBD (G, red), FGF14ACaMBD (H, blue) biosensors alone (all shown in solid black), and in the presence of apo (dashed/colored) and (Ca2+)4-CaM (solid/colored). I–L, equilibrium titrations of FGF11ACaMBD (I), FGF12ACaMBD (J), FGF13ACaMBD (K), and FGF14ACaMBD (L) biosensors with (Ca2+)4-CaM. FGF isoforms are colored as in panels E–H.
Table 1.
FGF biosensor affinities for WT CaM
| FGFCaMBD | ΔGa | Kdb | |
|---|---|---|---|
| FGF11ACaMBD | −10.43 ± 0.18 | 19 nM | 7 |
| FGF12ACaMBD | −9.40 ± 0.21 | 107 nM | 9 |
| FGF13ACaMBD | −10.63 ± 0.02 | 13 nM | 6 |
| FGF14ACaMBD | −9.87 ± 0.18 | 51 nM | 7 |
ΔG in kcal/mol. Average and standard deviation based on N determinations of biosensors prepared from at least two independent cultures.
Kd, equilibrium dissociation constant, calculated from average value of ΔG reported in this table. Solution Conditions: 50 mM HEPES, 100 mM KCl, 50 μM EGTA, 5 mM NTA, 1 mM MgCl2, 1.5 μM BSA, 500 μM DTT, 1 mM CaCl2; pH 7.4, 22 °C.
As observed for FGF12ALTP, addition of excess apo CaM to the FGF11ACaMBD, FGF12ACaMBD, FGF13ACaMBD, or FGF14ACaMBD biosensor (200:1 [CaM]:[Biosensor]) resulted in negligible spectral changes, while (Ca2+)4-CaM induced robust reciprocal changes in the intensities of YFP (-21%–33%) and CFP (18%–32%) (Fig. 2, D–H). Equilibrium titrations with (Ca2+)4-CaM showed that CaM bound the CaMBD of each FGF isoform with a different affinity (Fig. 2, I–L, Table 1), with FGF13A being most favorable (Kd = 13 nM) and FGF12A least favorable (Kd = 107 nM). The small difference in the affinity of (Ca2+)4-CaM for the CaMBD of FGF11A and FGF13A (0.18 kcal/mol) was statistically significant (p value 0.02358) but would have a very small effect on saturation.
These affinities were 5–44-fold more favorable than the affinity of (Ca2+)4-CaM for the FGF12A LTP (Fig. 2, C and I–L, Table 1). Based on the high degree of conservation in the LTP sequence of all four FGFs, these findings suggest that when a single (Ca2+)4-CaM is bound to a full-length A-type FGF, it would occupy the putative CaMBD rather than the LTP.
Both CaMN and CaMC are required for tight binding to FGF CaMBDs
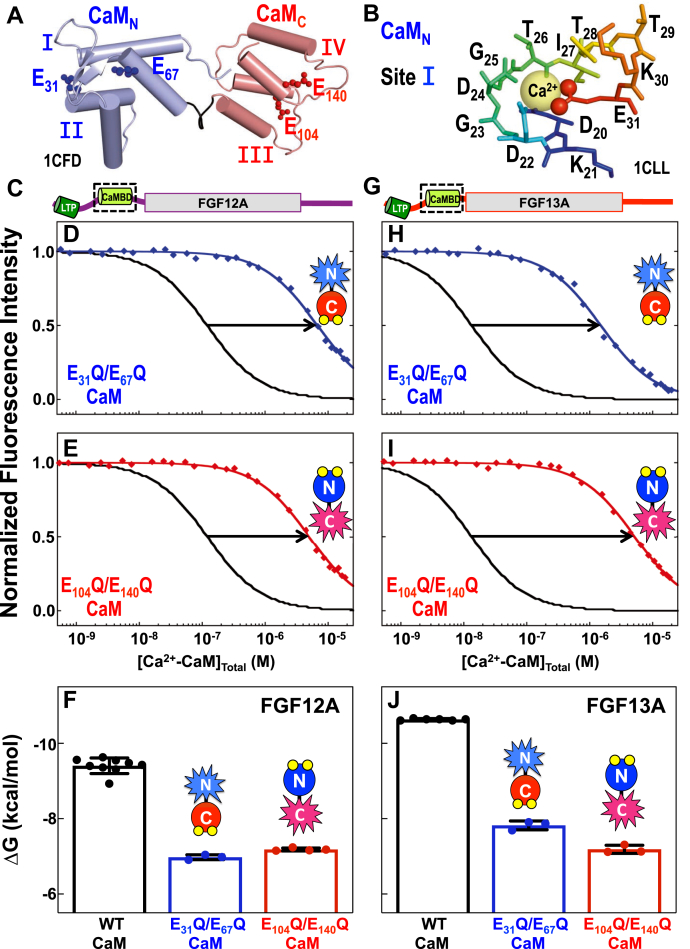
We assessed the energetic contributions of CaMN and CaMC to the binding of FGF12ACaMBD and FGF13ACaMBD. Initial titrations with isolated CaMN (aa 1–80) or CaMC (aa 76–148) showed no evidence of binding at a concentration of 1 μM (data not shown). Because the interaction of CaM with FGF12ACaMBD and FGF13ACaMBD is Ca2+-dependent, the energetic contributions of CaMN and CaMC within full-length CaM were explored by determining their affinity for "knockout mutants" of CaM engineered to significantly reduce Ca2+-binding to one domain. In these mutants the bidentate Glu (position 12) in sites I and II (E31Q/E67Q) or sites III and IV (E104Q/E140Q) was replaced with Gln (Fig. 3, A and B) (31). In these mutants with one domain mutated, the other domain retains a high Ca2+-binding affinity (31).
Figure 3.
Energetic contribution of CaMNand CaMCto FGF12A and FGF13A CaMBD binding by CaM.A, position of E31 (blue), E67 (blue), E104 (red), and E140 (red), shown as ball-and-stick, in apo CaM (1CFD, CaMN/light blue, CaMC/salmon). B, (Ca2+)4-CaM site I (1CLL): Ca2+ (yellow sphere) is surrounded by residues D20 (blue) to E31 (red). The coordinating O atoms of E31 are shown as red spheres. C, schematic of FGF12A. Folded β-trefoil core is shown as a rectangle, and LTP (green) and CaMBD (limon) are shown as cylinders. The black box indicates the position of the FGF12A CaMBD. D and E, equilibrium titrations of the FGF12ACaMBD biosensor with E31Q/E67Q (D, blue) or E104A/E140Q CaM (E, red). A reference titration with WT CaM is shown in black. F, ΔG of WT (black), E31Q/E67Q (blue), and E104Q/E140Q (red) CaM binding the FGF12ACaMBD biosensor. G, schematic of FGF13A. Folded β-trefoil core is shown as a rectangle, and LTP (green) and CaMBD (limon) are shown as cylinders. The black box indicates the position of the FGF13A CaMBD. H and I, equilibrium titrations of the FGF13ACaMBD biosensor with E31Q/E67Q (H, blue) or E104A/E140Q CaM (I, red). A reference titration with WT CaM is shown in black. J, ΔG of WT (black), E31Q/E67Q (blue), and E104Q/E140Q (red) CaM binding the FGF13ACaMBD biosensor.
Equilibrium titrations of FGF12ACaMBD with these mutants showed that E31Q/E67Q CaM bound to the CaMBD with a Kd of 6.74 μM, a 63-fold lower affinity than WT CaM, while E104Q/E140Q CaM bound with a Kd of 4.73 μM, a 44-fold lower affinity (Fig. 3, C–E, Table 2). The affinities of the knockout mutants were very close (Fig. 3F), suggesting that CaMN and CaMC contribute similarly to binding FGF12ACaMBD. Compared with the pattern observed for FGF12ACaMBD, the threefold difference in the affinity of E31Q/E67Q CaM (Kd = 1.59 μM, 122-fold weaker than WT) and E104Q/E140Q CaM (Kd = 4.73 μM, 363-fold weaker than WT) (Fig. 3, G–I, Table 2) for FGF13ACaMBD indicated that CaMC makes an energetic contribution to binding FGF13ACaMBD that is larger than that made by CaMN.
Table 2.
FGF biosensor affinities for mutant CaM
| CaM1-148 | FGF12ACaMBD |
FGF13ACaMBD |
||||
|---|---|---|---|---|---|---|
| ΔGa | Kdb | N | ΔGa | Kdb | N | |
| E31Q/E67Q | −6.98 ± 0.07 | 6.74 μM | 3 | −7.82 ± 0.12 | 1.59 μM | 3 |
| E104Q/E140Q | −7.18 ± 0.04 | 4.73 μM | 4 | −7.18 ± 0.11 | 4.72 μM | 3 |
ΔG in kcal/mol. Average and standard deviation based on N titrations of biosensors prepared from at least two independent cultures.
Kd, equilibrium dissociation constant, calculated from average value of ΔG reported in this table. Solution Conditions: 50 mM HEPES, 100 mM KCl, 50 μM EGTA, 5 mM NTA, 1 mM MgCl2, 1.5 μM BSA, 500 μM DTT, 1 mM CaCl2; pH 7.4, 22 °C.
The mutant E104Q/E140Q CaM contains only a functional CaMN. Given that its affinity for FGF13ACaMBD and FGF12ACaMBD was identical (Fig. 3, F and J, Table 2), this suggests that the separation in the affinity of WT CaM for the CaMBD of FGF12A and FGF13A may result from differences in the interface between CaMC and the CaMBD.
Stoichiometry of copurified (Ca2+)4-CaM+FGF NTD complexes
The finding that (Ca2+)4-CaM can bind to the isolated FGF LTP (Fig. 2, A–C) and CaMBD (Fig. 2, D–L) suggests that the NTD of an A-type FGF may bind two molecules of CaM simultaneously. To test this hypothesis, we utilized revered-phase high-performance liquid chromatography (rpHPLC) to determine the molar ratio of (Ca2+)4-CaM to FGF NTD in copurified complexes of (Ca2+)4-CaM bound to NTD fragments (residues ∼1–70, containing both the LTP and CaMBD), of FGF11A (FGF11ANTD), FGF12A (FGF12ANTD), FGF13A (FGF13ANTD), and FGF14A (FGF14ANTD) (Fig. S4, A and B).
In rpHPLC chromatograms (Fig. S4B) of the copurified complexes, the ratio of the integrated area under the absorbance peaks corresponding to the FGF NTD and (Ca2+)4-CaM showed that each sample contained a 1:1 M ratio of (Ca2+)4-CaM to FGF NTD (Fig. S4C). This is consistent with the isolated NTD fragment of each A-type FGF binding a single molecule of (Ca2+)4-CaM following copurification. However, these results do not indicate the location(s) of CaM and do not discriminate among the possibilities of having a single (Ca2+)4-CaM bound to an LTP or CaMBD site alone, or possibly bridging these two sites with one CaM domain bound to each. Furthermore, these results do not preclude the possibility that a second molecule of (Ca2+)4-CaM may bind the NTD if the local CaM concentration was sufficiently high.
CaMN and CaMC bind identically to FGF12ACaMBDp and FGF12ANTD
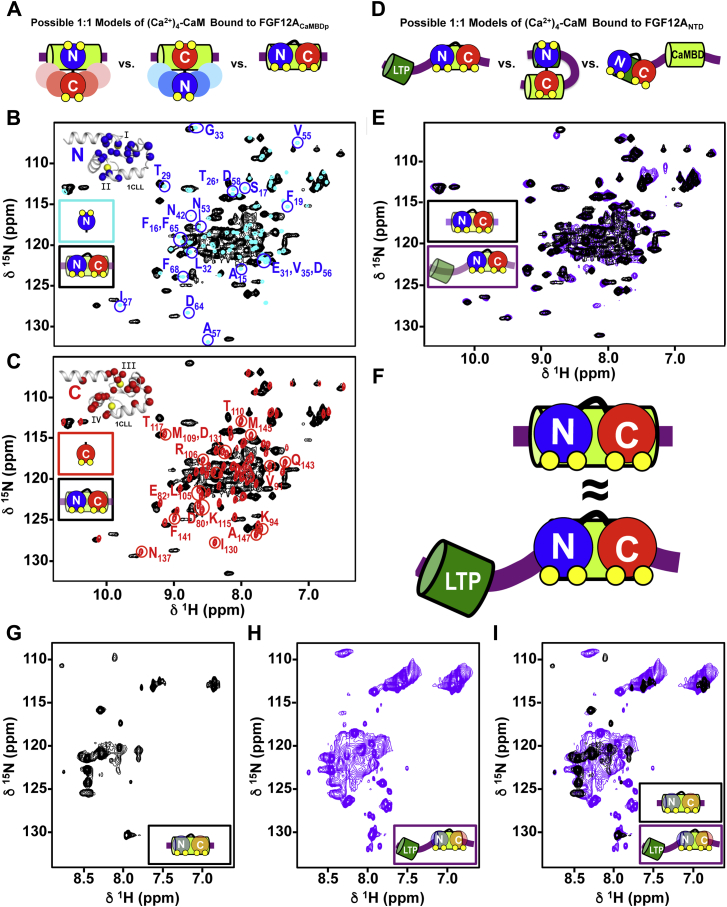
Solution NMR is uniquely capable of monitoring changes in the local environment of individual residues within a protein. To determine how the two domains of (Ca2+)4-CaM rearrange and interact with an FGF NTD at a one-to-one molar ratio, solution NMR was used to monitor FGF12A CaMBD and FGF12ANTD-induced changes in the local environment of residues in labeled (Ca2+)4-CaM.
We first sought to determine how binding of the isolated CaMBD of FGF12A (Fig. 4A) changed CaMN and CaMC within (Ca2+)4-CaM. To do this we compared the 15N-HSQC spectrum of 15N-(Ca2+)4-CaM bound to an unlabeled C-terminal fragment of the FGF12ANTD (14N-FGF12ACaMBDp, residues 41–70), corresponding to roughly half of the FGF12ANTD, to spectra of isolated (Ca2+)2-CaMN (Fig. 4B) and (Ca2+)2-CaMC (Fig. 4C). These showed that FGF12ACaMBDp binding induced changes in the chemical shifts of residues throughout CaMN and CaMC, which is consistent with both domains of (Ca2+)4-CaM interacting directly with FGF12ACaMBDp.
Figure 4.
Interaction of (Ca2+)4-CaM with the FGF12ACaMBDpand FGF12ANTD.A, schematics depicting models of (Ca2+)4-CaM (CaMN/blue, CaMC/red) binding to the FGF12ACaMBDp (limon cylinder) in a 1: 1 M ratio. B and C, overlay of the 15N-HSQC spectra of 15N-(Ca2+)2-CaMN (blue, B) or 15N-(Ca2+)2-CaMC (red, C) and 15N-(Ca2+)4-CaM+14N-FGF12ACaMBDp (black). Peaks labeled in the spectrum of 15N-(Ca2+)2-CaMN (B) or 15N-(Ca2+)2-CaMC (C) are shifted in the 15N-(Ca2+)4-CaM+14N-FGF12ACaMBD spectrum. Insets show the Cα of labeled CaMN (blue spheres, B) or CaMC (red spheres, C) residues on a structure of (Ca2+)4-CaM (1CLL, gray helices). D, schematics depicting models of (Ca2+)4-CaM (CaMN/blue, CaMC/red) binding to the FGF12ANTD in a 1:1 M ratio. FGF12A LTP (green) and CaMBD (limon) are shown as cylinders. E, overlay of the 15N-HSQC spectra of 15N-(Ca2+)4-CaM bound to the 14N-FGF12ACaMBDp (black) or 14N-FGF12ANTD (purple). F, schematic showing that the interface is essentially identical between (Ca2+)4-CaM (CaMN/blue, CaMC/red) and the FGF12ACaMBDp (limon cylinder) or FGF12ANTD (LTP/green cylinder, CaMBD/limon cylinder). G and H, 15N-HSQC spectrum of 14N-(Ca2+)4-CaM bound to 15N-FGF12ACaMBDp (G, black) or 15N-FGF12ANTD (H, purple). I, overlay of the 15N-HSQC spectra of 14N-(Ca2+)4-CaM bound to 15N-FGF12ACaMBDp (black) or 15N-FGF12ANTD (purple).
To determine how (Ca2+)4-CaM interacts with the full NTD that contains both the LTP and CaMBD sequences (Fig. 4D), we compared the 15N-HSQC spectrum of 15N-(Ca2+)4-CaM bound to the complete unlabeled NTD of FGF12A (14N-FGF12ANTD) to that of 15N-(Ca2+)4-CaM bound to the 14N-FGF12ACaMBDp. As observed in the 1:1 complex of CaM bound to the FGF12ACaMBDp, the binding of FGF12ANTD changed the local environment of residues throughout CaMN (Fig. S5, A and B) and CaMC (Fig. S5, C and D). This conclusion alone would be consistent with either CaM bridging the LTP and CaMBD or CaM binding either of these sites exclusively.
Comparison of the 15N-HSQC spectrum of 15N-(Ca2+)4-CaM+14N-FGF12ACaMBDp to that of 15N-(Ca2+)4-CaM+14N-FGF12ANTD revealed that peaks corresponding to (Ca2+)4-CaM residues were in nearly identical positions in both spectra (Fig. 4E). This indicated that residues in (Ca2+)4-CaM have equivalent local environments when bound to the FGF12ACaMBDp or FGF12ANTD, suggesting that the interface between (Ca2+)4-CaM and the FGF12ANTD is identical to that of (Ca2+)4-CaM+FGF12ACaMBDp (Fig. 4F). The simplest explanation is that (Ca2+)4-CaM binds the FGF12ANTD exclusively through the CaMBD, with neither domain of CaM making persistent contacts with the LTP. There was no evidence for more than one conformation though we cannot exclude the possibility that some additional conformations were populated in low abundance.
FGF12ACaMBDp and FGF12ANTD respond similarly to (Ca2+)4-CaM binding
To probe the interface between (Ca2+)4-CaM and the FGF12ANTD from the FGF side, we used solution NMR to examine the effect of unlabeled (Ca2+)4-CaM (14N-(Ca2+)4-CaM) binding on the local chemical environment of residues in the labeled FGF12ACaMBDp (15N-FGF12ACaMBDp) and FGF12ANTD (15N-FGF12ANTD). The isolated FGF12ACaMBDp and FGF12ANTD were not soluble at the concentrations needed for NMR studies. Thus, we made a pairwise comparison of the local chemical environment of FGF residues in 15N-FGF12ACaMBDp (Fig. 4G) to those in 15N-FGF12ANTD (Fig. 4H) when each was bound to 14N-(Ca2+)4-CaM. This was illuminating regarding the preference of CaM for the CaMBD sequence relative to the LTP sequence.
The majority of the peaks in the 15N-HSQC spectrum of 15N-FGF12ACaMBDp or 15N-FGF12ANTD bound to 14N-(Ca2+)4-CaM had a 1H chemical shift between 8.0 and 8.5 ppm (Fig. 4, G and H). Peaks in the15N-HSQC spectrum of 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp were relatively well dispersed (Fig. 4G), which was likely due to the small size of this peptide (30 FGF12A residues with a four-residue tag). In contrast, the spectrum of 14N-(Ca2+)4-CaM+15N-FGF12ANTD was more crowded, as expected for a larger fragment (70 FGF12A residues with a four-residue tag) (Fig. 4H). The higher degree of overlap in that spectrum likely reflects the presence of a disordered linker between the LTP and CaMBD, as predicted in the Robetta models of full-length FGF12A (Fig. S3, B–H).
To assess whether the pattern of peaks in the 15N-HSQC spectra of 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp and 14N-(Ca2+)4-CaM+15N-FGF12ANTD were consistent with the predicted α-helical secondary structure of the FGF12A LTP and CaMBD, the observed peak positions were compared with those predicted with SPARTA+ (55) for residues 41–70 (FGF12ACaMBDp) and 1–70 (FGF12ANTD) (Fig. S6, A–D) from the model of full-length FGF12A. Peak positions in the 15N-HSQC spectra of 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp (Fig. S6C) or +15N-FGF12ANTD (Fig. S6D) agreed well with those predicted from the fragments of the FGF12A model. We inferred that both the FGF12A LTP and CaMBD adopted an α-helical structure in the complexes of (Ca2+)4-CaM bound to the FGF12ACaMBDp or FGF12ANTD.
Comparison of the 15N-HSQC spectrum of 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp (Fig. 4G) to that of 14N-(Ca2+)4-CaM+15N-FGF12ANTD (Fig. 4H) showed that a subset of peaks in the 14N-(Ca2+)4-CaM+15N-FGF12ANTD spectrum were located at positions essentially equivalent to those of peaks in the 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp spectrum (Fig. 4I). This suggests that these peaks correspond to the same residues in the FGF12ACaMBDp and FGF12ANTD and that they have an essentially identical local chemical environment when bound by (Ca2+)4-CaM. That supports a model where (Ca2+)4-CaM is anchored to the FGF12ANTD via the CaMBD sequence when in a one-to-one complex as shown schematically in Figure 4F.
FGF12ACaMBDp and FGF12ANTD increase the Ca2+ affinity of CaMN and CaMC
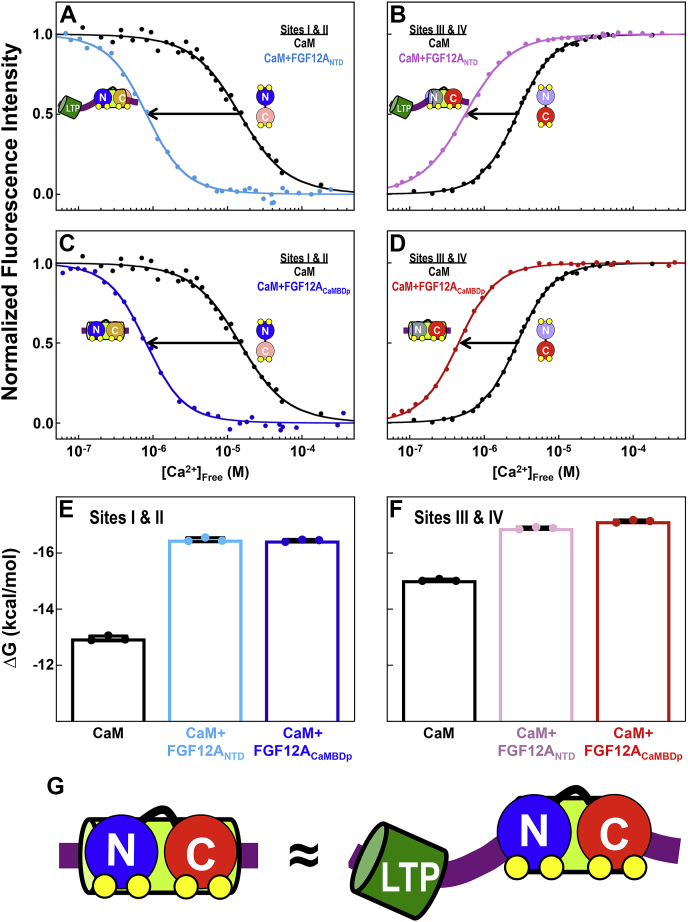
To quantitatively assess the allosteric effect of FGF12ANTD and FGF12ACaMBDp on the Ca2+-binding affinity of CaMN and CaMC, we conducted equilibrium Ca2+ titrations of CaM in the presence of the FGF12ANTD or FGF12ACaMBDp by monitoring changes in the steady-state fluorescence intensity of endogenous Phe and Tyr residues to detect Ca2+ binding by CaMN and CaMC, respectively (31).
Equilibrium Ca2+ titrations of CaM bound to FGF12ANTD showed it increased the Ca2+-binding affinity of sites I and II in CaMN (Kd-app = 0.81 μM) by ∼20-fold (Fig. 5A, Table 3) and sites III and IV in CaMC (Kd-app = 0.45 μM) by approximately fivefold (Fig. 5B, Table 3) relative to free CaM. In a similar pattern, FGF12ACaMBDp increased the affinity of sites I and II (Kd-app = 0.79 μM) by ∼20-fold relative to CaM alone (Fig. 5C, Table 3), while sites III and IV (Kd-app = 0.56 μM) (Fig. 5D, Table 3) bound Ca2+ with an approximately fivefold higher affinity. Thus, FGF12ANTD and FGF12ACaMBDp increased the Ca2+-binding affinity of CaMN (Fig. 5E, no statistically significant difference between the effect of NTD and CaMBD) and CaMC (Fig. 5F, with CaMBD having a larger effect by 0.25 kcal/mol). This is consistent with both domains of (Ca2+)4-CaM binding FGF12ANTD exclusively through the CaMBD region and having an identical interface with both (Fig. 5G). This supports the interpretation of NMR data presented in Figure 4, A–I and the conclusion that the LTP does not participate in the CaM-FGF12A NTD interaction in a 1:1 complex.
Figure 5.
Equilibrium Ca2+titrations of CaM+FGF12ACaMBDpand CaM+FGF12ANTD. A, equilibrium Ca2+ titrations of sites I and II of CaM alone (black) and in the presence of the FGF12ANTD (teal). B, equilibrium Ca2+ titrations of sites III and IV of CaM alone (black) and in the presence of the FGF12ANTD (pink). C, equilibrium Ca2+ titrations of sites I and II of CaM alone (black) and in the presence of the FGF12ACaMBDp (blue). D, equilibrium Ca2+ titrations of sites III and IV of CaM alone (black) and in the presence of the FGF12ACaMBDp (red). E and F, ΔG of Ca2+ binding to sites I and II (E) in CaM alone (black), +FGF12ANTD (teal) or +FGF12ACaMBDp (blue), and sites III and IV (F) in CaM alone (black), +FGF12ANTD (pink) or +FGF12ACaMBDp (red). G, schematic depicting that the interface is essentially identical between (Ca2+)4-CaM (CaMN/blue, CaMC/red) and the FGF12ACaMBDp (lime green cylinder) or FGF12ANTD (LTP/green cylinder, CaMBD/limon cylinder).
Table 3.
Effect of FGF12ACaMBDp and FGF12ANTD on Ca2+ binding of CaM
| CaM1-148 | Sites I and II |
Sites III and IV |
||||
|---|---|---|---|---|---|---|
| ΔG1a (kcal/mol) | ΔG2a (kcal/mol) | Kd-appb (μM) | ΔG1a (kcal/mol) | ΔG2a (kcal/mol) | Kd-appb (μM) | |
| - | −6.27 ± 0.21 | −12.96 ± 0.09 | 15.92 | −6.77 ± 0.09 | −15.03 ± 0.04 | 2.73 |
| + FGF12ACaMBDp | −7.75 ± 0.07 | −16.45 ± 0.04 | 0.81 | −8.08 ± 0.14 | −17.14 ± 0.05 | 0.45 |
| + FGF12ANTD | −7.69 ± 0.04 | −16.48 ± 0.07 | 0.79 | −8.27 ± 0.08 | −16.89 ± 0.04 | 0.56 |
Solution conditions: 50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 1 mM DTT, pH 7.4, 22 °C.
Average ΔG values based on three independent determinations. Titrations fit to Equation 4.
Kd-app (apparent dissociation constant) was calculated from half of the average ΔG2 value reported in this table.
FGF12B and FGF12A differ in effects on Ca2+ binding by CaM+NaV1.2CTD
In isolation, both FGF12ANTD and FGF12ACaMBDp increased the Ca2+-binding affinity of CaMN and CaMC (Fig. 5, A–D). This suggests that the allosteric regulatory roles of FGF12A, which includes the NTD and FGF12B, will not differ because only the A-type splice variant will be capable of binding CaM. Currently there is no evidence of CaM binding to FGF12B.
To understand the complementary roles of CaM and FGF bound to NaV channels, it would be ideal to determine the Ca2+ affinity of CaM in a ternary complex with FGF12A bound to a full-length NaV1.2 channel in a plasma membrane and compare that with the Ca2+ affinity of CaM in a complex with FGF12B bound to an identical full-length NaV1.2. However, no currently available method is capable of measuring this property in these large transmembrane complexes. Therefore, we investigated Ca2+ binding to CaM in soluble ternary complexes containing either full-length FGF12A or FGF12B bound to the NaV1.2CTD that contains both the EFL domain and IQ motif.
Unlike the FGF12A NTD fragments, which had a low abundance of naturally occurring fluorophores (2 Phe, 0 Tyr, 0 Trp), FGF12B (8 Phe, 10 Tyr, 1 Trp), FGF12A (10 Phe, 10 Tyr, 1, Trp), and NaV1.2CTD (9 Phe, 4 Tyr, 1 Trp) all contain multiple aromatic residues (Fig. S7A) that could quench or overwhelm signals coming from CaM. There is also a controversial report that the EFL of NaV1.5 binds Ca2+ (56), and that phenomenon could contribute a Ca2+-dependent change in fluorescence intensity coincident with that of the changes in CaM.
To determine whether the intrinsic fluorescence of FGF12B and NaV1.2CTD was Ca2+-dependent, titrations of the FGF12B+NaV1.2CTD complex were conducted. Its signal was essentially flat (Ca2+-independent) (Fig. S7, B and C), and its excitation (Fig. S7, D and E) and emission (Fig. S7, F and G) spectra were essentially identical under Ca2+-depleted conditions and in excess Ca2+ ([Ca2+]Total = 10 mM). Similarly, Ca2+ titrations of the FGF12A+NaV1.2CTD complex (Fig. S8A) showed that its fluorescence intensity was essentially flat (Fig. S8, B and C), consistent with a lack of intrinsic Ca2+ binding by these FGF-NaV complexes. Thus, in the Ca2+ titrations of CaM bound to FGF12B+NaV1.2CTD or FGF12A+NaV1.2CTD, the change in fluorescence intensity was interpreted as reporting solely on Ca2+ binding to CaM.
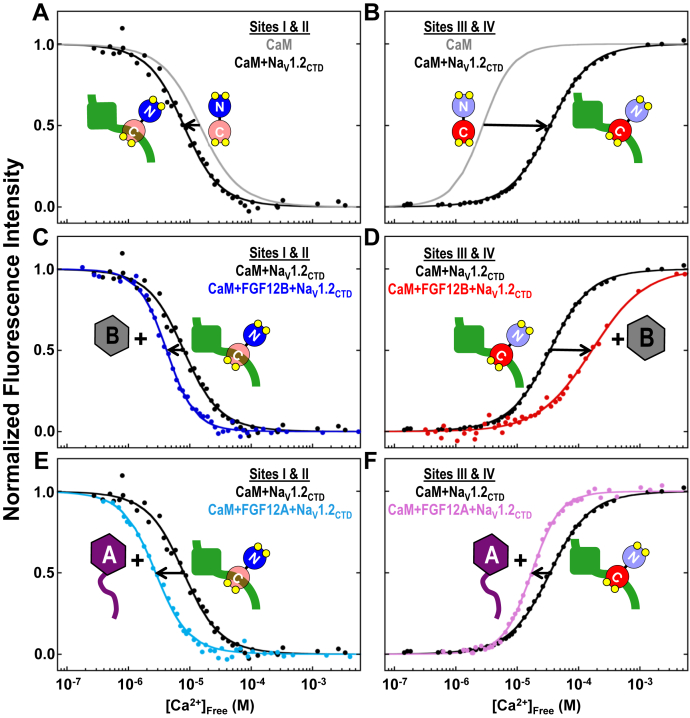
To understand the effect of full-length FGF12B or FGF12A on Ca2+ binding by CaM bound to the NaV1.2CTD, we compared the equilibrium Ca2+ titrations of the ternary complexes to those of the binary CaM+NaV1.2CTD complex (Fig. 6, A and B). In CaM+NaV1.2CTD the Ca2+ affinity of CaM sites I and II (Kd-app = 7.91 μM, Fig. 6A, Table 4) increased approximately twofold compared with free CaM. This change was nearly identical to the difference observed between sites I and II in a CaMN fragment (residues 1–75) and in full-length CaM and was shown to reflect the release of anticooperative interactions between CaMN and residues in the linker between CaM domains (30, 57). This indicates that NaV1.2CTD binding perturbs the thermodynamic linkage between CaMN and CaMC.
Figure 6.
Equilibrium Ca2+titrations of CaM+FGF12B+NaV1.2CTDand CaM+FGF12A+NaV1.2CTD.A and B, equilibrium Ca2+ titrations of sites I and II (A), and sites III and IV (B) of CaM in CaM+NaV1.2CTD. Solid gray lines show reference Ca2+ titrations sites I and II (A) and sites III and IV (B) of CaM alone. C, E, equilibrium Ca2+ titrations of sites I and II of CaM in CaM+NaV1.2CTD (C, E, black), CaM+FGF12B+NaV1.2CTD (C, blue) and CaM+FGF12A+NaV1.2CTD (E, teal). D, F, equilibrium Ca2+ titrations of sites III and IV of CaM in CaM+NaV1.2CTD (D, F, black), CaM+FGF12B+NaV1.2CTD (D, red) and CaM+FGF12A+NaV1.2CTD (F, pink).
Table 4.
Effect of FGFB and FGF12A on Ca2+ binding by CaM bound to NaV1.2CTD
| CaM1-148+ NaV1.2CTD |
Ratioa | Sites I and II |
Sites III and IV |
||||
|---|---|---|---|---|---|---|---|
| ΔG1b (kcal/mol) | ΔG2b (kcal/mol) | Kd-appc (μM) | ΔG1b (kcal/mol) | ΔG2b (kcal/mol) | Kd-appc (μM) | ||
| − | − | −6.27 ± 0.39 | −13.78 ± 0.08 | 7.91 | −6.04 ± 0.19 | −12.09 ± 0.07 | 33.42 |
| + FGF12B | 1:1:1 | −6.00 ± 0.10 | −14.51 ± 0.09 | 4.51 | −5.44 ± 0.11 | −10.27 ± 0.11 | 157.67 |
| + FGF12A | 1:1:1 | −7.18 ± 0.22 | −14.99 ± 0.08 | 2.82 | −5.31 ± 0.07 | −12.91 ± 0.10 | 16.61 |
| CaM1-148+ NaV1.2CTD |
Ratioa | ΔG1-appb (kcal/mol) | ΔG2-appb (kcal/mol) | Kd-appc (μM) | ΔG1-appd (kcal/mol) | ΔG2-appd (kcal/mol) | Kd-appc (μM) |
|---|---|---|---|---|---|---|---|
| + FGF12A | 2:1:1 | −7.59 ± 0.14 | −15.41 ± 0.14 | 1.97 | −7.61 ± 0.24 | −16.05 ± 0.04 | 1.14 |
| + FGF12A | 2:1:1 | – | – | – | −5.03 ± 0.16 | −10.89 ± 0.13 | 92.94 |
Solution Conditions: 50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 1 mM DTT, pH 7.4, 22 °C. Fluorescence controls shown in Figures S7 and S8.
Stoichiometry of CaM:NaV1.2CTD:FGF12B or CaM:NaV1.2CTD:FGF12B.
Titrations fit to Equation 4. Average ΔG values based on three independent determinations monitoring Phe intensity.
Kd-app (apparent dissociation constant) was calculated from half of the average ΔG2 value reported in this table.
Titrations fit to Equation 5. Average ΔG based on three independent determinations monitoring Tyr intensity.
In the CaM+NaV1.2CTD complex, the Ca2+ affinity of sites III and IV decreased by ∼12-fold (Kd-app = 33.42 μM, Fig. 6B, Table 4) relative to free CaM, consistent with preferential binding of the NaV1.2 IQ motif by apo versus (Ca2+)4-CaM (22, 23, 24, 25). Comparing the two domains of CaM to each other in the CaM+NaV1.2CTD complex, CaMN binds Ca2+ with a approximately fourfold higher affinity than CaMC, indicating that NaV1.2CTD binding reverses the sequential occupancy of the CaM domains observed in CaM alone (gray curves in Fig. 6, A and B).
In the ternary CaM+FGF12B+NaV1.2CTD complex, a slight additional increase (approximately twofold) was observed in the Ca2+ affinity of sites I and II (Kd-app = 4.51 μM) relative to CaM bound to the NaV1.2CTD (Fig. 6C, Table 4). Inclusion of FGF12B decreased the Ca2+-binding affinity of sites III and IV (Kd-app = 157.67 μM) by approximately fivefold relative to CaM in the CaM+NaV1.2CTD complex (Fig. 6D, Table 4). Thus, FGF12B binding to the NaV1.2CTD further separated the midpoints of the Ca2+-binding isotherms of CaMN and CaMC. This was an unanticipated result because there are no published reports of direct interactions between CaM and this shorter FGF12 splice variant that consists primarily of the folded β-trefoil core (Fig. 1C).
For CaM in CaM+FGF12A+NaV1.2CTD, the Ca2+ affinity of sites I and II (Kd-app = 2.82 μM) was more favorable than those sites in CaM+NaV1.2CTD (approximately threefold) or CaM+FGF12B+NaV1.2CTD (approximately twofold) (Fig. 6, C and E, Table 4). The binding of FGF12A also increased the Ca2+ affinity of sites III and IV (Kd-app = 16.61 μM) relative to CaM in both the CaM+NaV1.2CTD (approximately twofold) and CaM+FGF12B+NaV1.2CTD (approximately tenfold) complexes. The increased Ca2+-binding affinity of both CaM domains in the CaM+FGF12A+NaV1.2CTD complex is consistent with (Ca2+)4-CaM interacting favorably with the NTD of full-length FGF12A when bound to the NaV1.2CTD.
Binding of multiple CaM molecules to the FGF12A+NaV1.2CTD complex
Ca2+-saturated CaM can bind two sites in the FGF12A NTD (Fig. 2, A–L) and one site in the NaV1.2 IQ motif (22, 23, 24, 25). Thus, at high local concentrations of Ca2+ and CaM, the CTD of NaV1.2 channels with FGF12A bound might bind up to three molecules of (Ca2+)4-CaM. However, (Ca2+)4-CaM binds more weakly to the LTP than the CaMBD (Fig. 2, C and J, Table 1), and the solution NMR data were consistent with (Ca2+)4-CaM binding the FGF12ANTD exclusively through the CaMBD region in a one-to-one complex (Fig. 4, E and I).
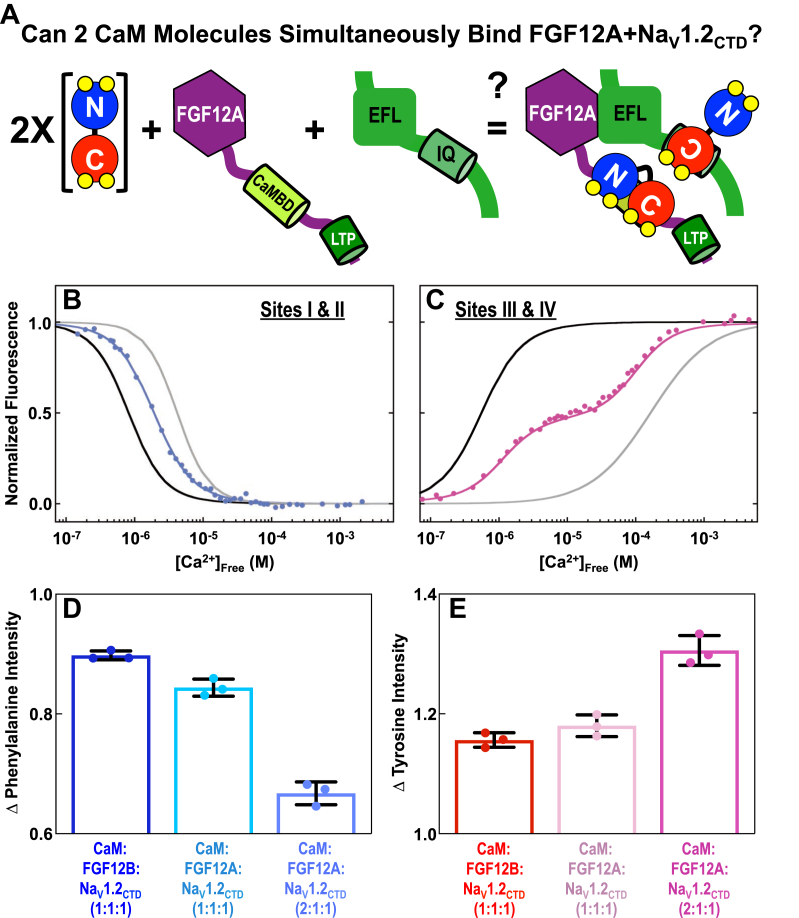
To explore the limits of stoichiometry, we tested whether FGF12A+NaV1.2CTD might recruit a total of two molecules of CaM: one at the NaV1.2 IQ motif and one at the FGF12A CaMBD (Fig. 7A). To do this, Ca2+ titrations were conducted of the CaM+FGF12A+NaV1.2CTD complex at a [CaM]:[FGF12A]:[NaV1.2CTD] ratio of 2:1:1 (CaM+FGF12A+NaV1.2CTD (2:1:1)). In this complex it was anticipated that CaMN would not interact with the IQ motif regardless of the Ca2+ concentration (25) and that it would bind the FGF CaMBD only under Ca2+-saturating conditions. In contrast, CaMC would bind to the NaV1.2 IQ motif constitutively (±Ca2+), but CaMC of a second -(Ca2+)4-CaM molecule might bind at the FGF CaMBD.
Figure 7.
Equilibrium Ca2+titrations of a 2:1:1 complex of CaM+FGF12A+NaV1.2CTD.A, schematic depicting the possible binding of two CaM (CaMN/blue, CaMC/red) molecules the FGF12A (purple)+NaV1.2CTD (green) complex. The FGF12A LTP (green) and CaMBD (limon) are shown as cylinders. B, equilibrium Ca2+ titrations of CaM sites I and II in CaM+FGF12A+NaV1.2CTD (light blue, CaM:FGF12A:NaV1.2CTD = 2:1:1). The solid black and gray lines show reference titrations of CaM sites I and II in CaM+FGF12ANTD and CaM+FGF12B+NaV1.2CTD, respectively. C, Equilibrium Ca2+ titrations of sites III and IV of CaM in CaM+FGF12A+NaV1.2CTD (hot pink, CaM:FGF12A:NaV1.2CTD = 2:1:1). The solid black and gray lines show reference titrations of CaM sites III and IV in CaM+FGF12ANTD and CaM+FGF12B+NaV1.2CTD, respectively. D, maximum decrease in Phe fluorescence intensity in equilibrium Ca2+ titrations of CaM+FGF12B+NaV1.2CTD (blue), CaM+FGF12A+NaV1.2CTD (teal, CaM:FGF12A:NaV1.2 = 1:1:1) and CaM+FGF12A+NaV1.2CTD (light blue, CaM:FGF12A:NaV1.2CTD = 2:1:1). E, maximum increase in Tyr fluorescence intensity in equilibrium Ca2+ titrations of CaM+FGF12B+NaV1.2CTD (red), CaM+FGF12A+NaV1.2CTD (pink, CaM:FGF12A:NaV1.2 = 1:1:1), and CaM+FGF12A+NaV1.2CTD (hot pink, CaM:FGF12A:NaV1.2CTD = 2:1:1).
The Ca2+ titrations of CaMN in CaM+FGF12A+NaV1.2CTD (2:1:1) were monotonic (Fig. 7B, light blue line). Sites I and II bound Ca2+ with an affinity (Kd-app = 1.97 μM) that was between that of CaM in the CaM+FGF12ANTD (Kd-app = 0.81 μM, Fig. 7B, black line) and CaM+FGF12B+NaV1.2CTD (Kd-app = 4.51 μM, Fig. 7B, gray line, Table 4) complexes.
The Ca2+ titrations of CaMC in the CaM+FGF12A+NaV1.2CTD (2:1:1) were biphasic (Fig. 7C, red line), in contrast to the titrations of CaM+FGFNTD (Fig. 5B) and CaM+FGF12A+NaV1.2CTD with a 1:1:1 stoichiometry (Fig. 6F). Fitting these data as a sum of two isotherms (see Experimental procedures), we determined that the dissociation constant of the first phase (Kd-app = 1.14 μM) was similar to that of CaMC in CaM+FGF12ANTD (Kd-app = 0.45 μM, Fig. 7C, black line) while the dissociation constant of the second phase was similar to that of CaMC in CaM+FGF12B+NaV1.2CTD (Kd-app = 157.67 μM, Fig. 7C, gray line, Table 4).
Both the shape of the Ca2+ titration curve of CaM sites III and IV in the 2:1:1 complex and the values of the resolved dissociation constants for each transition suggest that the FGF12A+NaV1.2CTD complex can bind two CaM molecules simultaneously. Consistent with this, the change in intensity of both the Phe (Fig. 7D) and Tyr (Fig. 7E) signals observed in titrations of the CaM+FGF12A+NaV1.2CTD (2:1:1) complex was approximately twofold larger than in Ca2+ titrations of CaM+FGF12B+NaV1.2CTD or CaM+FGF12A+NaV1.2CTD.
The biphasic titration curves of the CaMC sites and the changes in affinity of CaMN and CaMC in the CaM+FGF12A+NaV1.2CTD (2:1:1) complex were consistent with FGF12A+NaV1.2CTD binding two molecules of CaM simultaneously with one molecule of CaM bound via the FGF12A CaMBD and the other bound at the NaV1.2 IQ motif. This suggests that if local concentrations of Ca2+ and CaM were sufficiently high, NaV1.2 channels that have FGF12A associated may recruit a second molecule of CaM through the FGF12A CaMBD independent of CaM binding at the NaV1.2 IQ motif.
Discussion
Under resting conditions, the cytosolic CTD of NaV channels binds one FGF and one CaM (16, 17, 22, 48); however, the mechanism by which these two auxiliary proteins modulate channel function is poorly understood. The thermodynamic and structural studies presented here show a direct, Ca2+-dependent interaction between (Ca2+)4-CaM and the NTD of the four A-type FGF splice variants. We found that (a) (Ca2+)4-CaM preferentially binds a CaMBD in the NTD with a dissociation constant in the low nM range but can bind the LTP with weaker affinity, and (b) both domains of (Ca2+)4-CaM bind the CaMBD. These results suggest that at elevated cytosolic Ca2+ concentrations reached during an action potential, CaM may translocate from the NaV IQ motif to the FGF CaMBD and participate in regulatory functions previously identified as requiring FGF binding to NaVs.
Discovery of novel (Ca2+)4-CaM-binding sites
Members of the intracellular FGF subfamily were reported to directly bind NaV isoforms (17, 18), voltage-gated potassium channels (58), and islet brain protein 2 (59). A recent report proposed an interaction between CaM and FGF12B when bound to NaV1.4 (52); however, there has been no evidence for a direct interaction between CaM and any FGF isoform. Using multiple spectroscopic methods, we have demonstrated that the isolated LTP (Fig. 2, B and C) and CaMBD (Fig. 2, E–L) sequences of A-type FGFs bind (Ca2+)4-CaM but not apo CaM(Fig. 2, B and E–H), mirroring the selectivity of CaMBD sequences in targets such as CaMKII (60), myosin light chain kinase (61), and calcineurin (62).
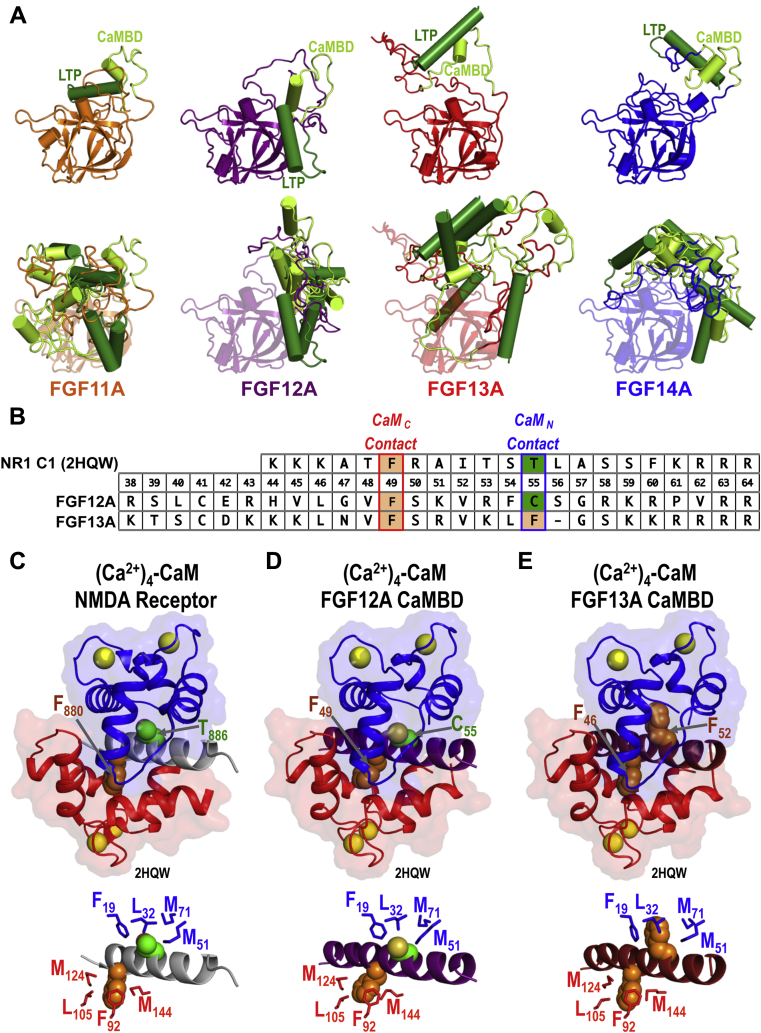
Robetta models of all four full-length A-type FGFs (Fig. 8A) predicted that segments of both LTP and CaMBD sequences would adopt α-helical structure. For FGF12A, this was supported by the 15N-HSQC spectra of 15N-FGF12ACaMBDp and 15N-FGF12ANTD with one bound (Ca2+)4-CaM (Fig. 4, G–I, Fig S6, C and D). The energetically similar models for each FGF show an ensemble of positions for the LTP and CaMBD separated by a disordered linker. The full NTD is tethered to the β-trefoil core with a disordered linker (Fig. 8A). In solution, this would allow the LTP and CaMBD to move independently relative to each other and the β-trefoil core. This flexibility and range of motion may facilitate (Ca2+)4-CaM binding the FGF NTD when the β-trefoil core is bound to a NaV EFL.
Figure 8.
Models of (Ca2+)4-CaM bound to the FGF12A and FGF13A CaMBD. In all structures CaM residues 1–75 are blue, 76–80 are black and 81–148/red, and Ca2+ are yellow spheres. A, top, model 1 in the Robetta (54) generated ensemble of each full-length A-type FGF. Bottom, orientation of the LTP and CaMBD regions in the modeled ensemble of FGF11, FGF12A, FGF13A, and FGF14. The FGF isoforms are colored as follows: FGF11A is orange, FGF12A is purple, FGF13A is red, and FGF14A is blue. The LTP and CaMBD regions of each FGF are shown in green and limon, respectively. Models were aligned with FGF12A a.a. 71–204. B, alignment of the NMDA receptor CaMBD, FGF12A CaMBD, and FGF13A CaMBD sequences. The positions that correspond to F880 and T886in the NMDA receptor are shaded (hydrophobic/tan, polar/green). C: Structure of (Ca2+)4-CaM bound to the NMDA receptor CaMBD (2HQW, gray), F880 (orange), and T886 (green) of the NMDA receptor are shown as spheres (top) and positions of F19, L32, M51, and M71 in CaMN (blue) and F92, L105, M124, and M144 in CaMC (red) are shown as sticks relative to F880 (orange spheres) and T886 (green spheres) in the NMDA receptor CaMBD (lower). D: Model of (Ca2+)4-CaM bound to the FGF12A CaMBD (purple), F49 (orange), and C55 (green) of FGF12A are shown as spheres (top) and positions of F19, L32, M51, and M71 in CaMN (blue) and F92, L105, M124, and M144 in CaMC (red) are shown as sticks relative to F49 (orange spheres) and C55 (green spheres) in the FGF12A CaMBD (bottom). E, model of (Ca2+)4-CaM bound to the FGF13A CaMBD (firebrick), F46 and F52 of FGF13A are shown as orange spheres (top) and positions of F19, L32, M51, and M71 in CaMN (blue) and F92, L105, M124, and M144 in CaMC (red) are shown as sticks relative to F46 (orange spheres) and C52 (green spheres) in the FGF13A CaMBD (bottom).
(Ca2+)4-CaM is known to bridge noncontiguous sites as observed in structures of CaM bound to the STRA6 retinol receptor (63) and the SK channel (64)., However, comparison of NMR spectra (Fig. 4E) and effects of FGF12ACaMBDp and FGF12ANTD on Ca2+ binding by CaM (Fig. 5, A–F, Table 3) were consistent with (Ca2+)4-CaM binding the FGF12A NTD exclusively at the CaMBD site. This suggests that (Ca2+)4-CaM would bind the NTD of the other A-type FGF isoforms (11A, 13A, and 14A) exclusively through the CaMBD in a one-to-one complex. However, the ability of (Ca2+)4-CaM to bind the isolated FGF12A LTP (and its nearly identical sequence in all four A-type FGFs) suggests that if the local CaM concentration is high, the LTP site could recruit a second molecule of CaM to an A-type FGF. Although (Ca2+)4-CaM binds to CaMBD with higher affinity than to LTP, CaM might interact transiently with LTP before binding to CaMBD. The reported thermodynamic studies were conducted under equilibrium conditions and did not address kinetics or explore possible translocation between the sites.
Differences in (Ca2+)4-CaM-FGF CaMBD interface
The two-domain architecture of CaM allows it to recognize target sequences in a variety of ways, with some targets such as NaV and myosin IQ motifs (65), binding a single domain of CaM (CaMC) while others, such as CaMKII (66), bind both. Titrations of the FGF12A and FGF13A CaMBD with E31Q/E67Q CaM (Fig. 3, D and H) and E104Q/E140Q CaM (Fig. 3, E and I), and the 15N-HSQC spectra of labeled (Ca2+)4-CaM bound to the unlabeled FGF12ACaMBDp (Fig. 4, B and C) or FGF12ANTD (Fig. 4E, and Fig. S5, A–D) were consistent with both CaM domains recognizing FGF CaMBD sequences. However, the approximately tenfold difference in the affinity between FGF13A and FGF12A indicates that their CaM–FGF interfaces differ.
To explore possible structural sources for this disparity, we modeled the (Ca2+)4-CaM– FGF12A CaMBD interface on a high-resolution structure (2HQW.pdb) of (Ca2+)4-CaM bound to the NR1C1 site of the N-methyl-D-aspartate (NMDA) receptor (67, 68). In 2HQW, F880 binds the hydrophobic cleft of CaMC while T886 is the primary contact in the cleft of CaMN (Fig. 8, B and C). An alignment of NR1C1 with the CaMBDs of FGF12A and FGF13A (Fig. 8D) suggested that CaMC would bind a Phe in each FGF CaMBD (F49 in FGF12A or F46 in FGF13A) (Fig. 8, B–E). However, CaMN would contact a Cys (C55) in FGF12A (Fig. 8D) and a Phe (F52) in FGF13A (Fig. 8E). In homology models of (Ca2+)4-CaM bound to the FGF12A (Fig. 8D) and FGF13A CaMBD (Fig. 8E), based on 2HQW and minimized using YASARA, the hydrophobic pocket of CaMN would make more favorable close contacts with the bulkier F52 in FGF13A than with the smaller polar C55 of FGF12A. This may explain the approximately tenfold higher affinity of (Ca2+)4-CaM for FGF13A CaMBD.
The structural models shown in Figure 8, D and E were simulated assuming that CaM binds to both FGF12A and FGF13A in an antiparallel arrangement (i.e., CaMN binds the C-terminal half of the CaMBD) that is observed for the majority of CaM–target interactions. However, the binding of knockout mutants having only one functional domain suggests another possible arrangement. We found that Ca2+-saturated E104Q/E140Q CaM (functionally equivalent to apo CaMC tethered to (Ca2+)2-CaMN) bound the FGF12A and FGF13A CaMBDs with nearly identical affinity (Fig. 3, E, F, I, and J). A simple interpretation would be that those titrations represent binding of (Ca2+)2-CaMN to a similar half-site in each FGF CaMBD. Thus, the disparity in the affinity might arise from differences in the interface between CaMC and FGF CaMBDs.
The ability of CaMN and CaMC to recognize a variety of target sequences (68) makes it extremely difficult to predict their orientation on a CaMBD. There are high-resolution structures of some peptides bound to (Ca2+)4-CaM in a parallel orientation. The ability of the hydrophobic clefts of CaMN and CaMC to form more favorable interactions with a Phe versus Cys may cause the functional domain in each CaM knockout mutant to compete for the same sequence in the FGF12A CaMBD, as was previously observed in the binding of (Ca2+)4-CaM to melittin (69). In that complex, the single Trp residue of melittin preferentially binds to CaMC in full-length CaM, and CaMN interacts elsewhere; however, CaMN as an isolated fragment will bind to the Trp residue that is available because CaMC is absent.
In the titrations of the FGF12ACaMBD biosensor with E104Q/E140Q CaM, the functional CaMN may interact with F49 in the FGF12A CaMBD. This would make the interface between E104Q/E140Q CaM with the CaMBD of either FGF12A or FGF13A similar, which could explain the nearly identical affinity of E104Q/E140Q CaM for these sequences.
Although (Ca2+)4-CaM was modeled according to the more common antiparallel orientation, CaM could recognize the FGF12A and FGF13A CaMBD in a parallel orientation as seen in structures of (Ca2+)4-CaM bound to IQ motif peptides from CaV1.2, CaV2.2, and CaV2.3 (70, 71) and a CaMBD from CaM-dependent kinase kinase (72). Alternatively, (Ca2+)4-CaM might bind the CaMBD of FGF12A in an orientation opposite from that adopted when binding to FGF13A. Crystal structures have shown a CaV target peptide bound to CaM in both parallel and antiparallel orientations (71, 73) and TFP, an antipsychotic drug that binds (Ca2+)4-CaM, has also been found in opposing orientations (74, 75, 76).
In the future, high-resolution structures of (Ca2+)4-CaM bound to the FGF12A and FGF13A CaMBD will be required to determine the positions of CaMN and CaMC and how differences in the interface between CaM and these FGF CaMBDs contribute to free energies of binding.
FGF12B lowers Ca2+ affinity of CaMC bound to NaV1.2CTD
In high-resolution structures of apo CaM bound to NaV1.5 CTD fragments in the presence or absence of a B-type FGF, the interface between apo CaMC and the NaV IQ motif is essentially identical (28, 51). Although two residues (Y98 and K144) in the β-trefoil core of the B-type splice variant of FGF13 (FGF13U) are within 5 Å of residues K95 and N111 in CaMC in a crystallographic structure of apo CaM bound to the NaV1.5 CTD with FGF13U (51), no interface is observed between CaMC and FGF13U in this structure. The simplest conclusion is that the interaction of CaMC at the NaV IQ motif is independent of B-type FGF binding to NaV EFL. However, binding of FGF12B to CaM+NaV1.2CTD decreased the Ca2+ affinity of sites III and IV in CaMC (Fig. 6D, Table 4). Thermodynamic linkage requires that FGF12B allosterically alters the energy of CaM binding to NaV1.2CTD.
Because CaM is a highly acidic protein (pI ∼ 4) and FGF12B is basic (pI ∼ 9), we hypothesized that FGF12B may increase the affinity of apo CaM for the NaV1.2CTD through favorable electrostatic interactions. However, in superpositions of apo and (Ca2+)4-CaM bound to the NaV1.2CTD with either FGF12B or FGF12A (Fig. 9C and Fig. S9, A–D), the nearest residues in apo CaMC (K94) or (Ca2+)2-CaMC (I130 and R126) and the large basic patch in the β-trefoil core of FGF12B (K117) or FGF12A (K193) are separated by > 13 Å. Thus, it is unlikely that electrostatic interactions between CaMC and FGF12B are sufficient to explain the FGF12B-induced changes in the energetics of CaM association with the NaV1.2CTD.
Figure 9.
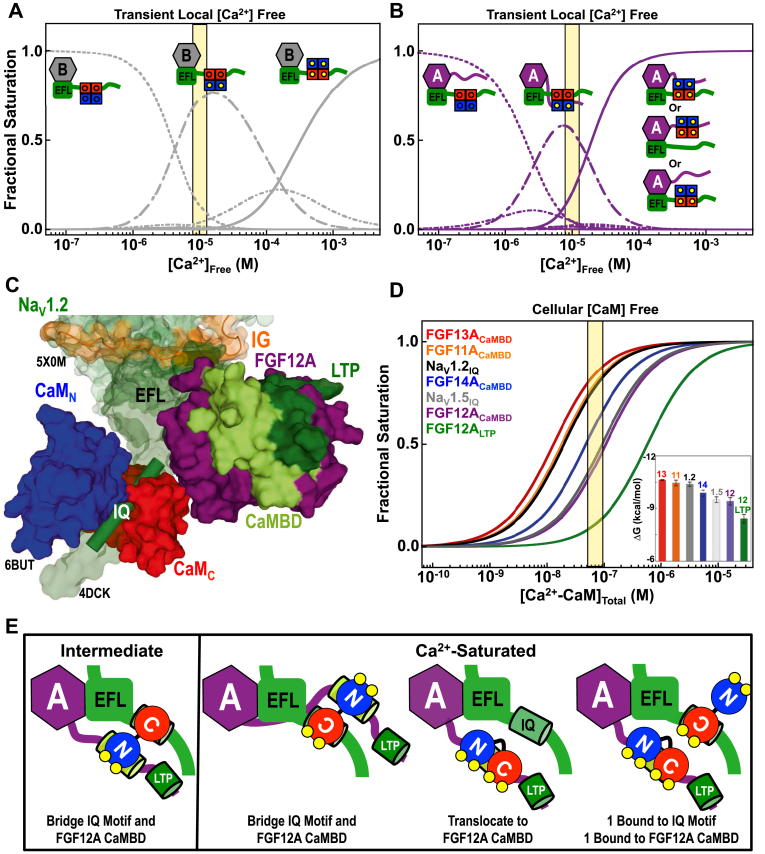
States of CaM bound to FGF12B+NaV1.2CTDor FGF12A+NaV1.2CTD.A and B, abundance of ligated CaM species in the CaM+FGF12B+NaV1.2CTD (A, gray) and CaM+FGF12A+NaV1.2CTD (B, purple) complexes. Simulations are based on the Ca2+-binding affinities reported in Table 4. States of CaM with an abundance >0.25 are shown in the inset schematics that depict the possible orientations of the CaM domains (CaMN/blue, CaMC/red) relative to FGF12B (gray) and NaV1.2CTD (green) (A) or FGF12A (purple) and NaV1.2CTD (green) (B), Ca2+ are shown as yellow circles. The yellow box is centered at physiologically relevant Ca2+ concentration of 10 μM. Representing the occupancy of the four Ca2+-binding sites in CaM (in order I, II, III, IV) with binary, where 0 is emtpy and 1 if filled, the discernible (abundances >0.05) species of CaM in the CaM+FGF12B+NaV1.2CTD complex (A) are 0000 (dotted line), 1100 (dashed and dotted line), 1101 or 1110 (dashed line), and 1111 (solid line). The discernible (abundance >0.5) species of CaM in the CaM+FGF12A+NaV1.2CTD complex (B) are 0000 (dotted line), 1000 or 0100 (dashed line), 1100 (dashed and dotted line), 1111 (solid line). C, model of CaM (CaMN/blue, CaMC/red) bound to an NaV CTD (forest green, surface) relative to the NaV DIII-DIV linker (orange) and FGF12A (purple, LTP/green, CaMBD/limon). The model is a composite of NaV1.5 CTD (4DCK), aligned with NaVPAS (5X0M) EFL (a.a. 1426–1521). NaVPAS a.a. 1122–1160 are shown as the DIII–DIV linker. The apo CaM+NaV1.2IQp ensemble (6BUT) was aligned with 4DCK via CaM a.a. 101–112 and 117–128. The NaV1.2IQp (a.a. 1904–1924) is shown as a forest green cylinder. The model of FGF12A was aligned with 4DCK using FGF13U a.a. 11–158. For clarity CaM and FGF13B in 4DCK are hidden. D, simulation of the saturation of each A-type FGF CaMBD (FGF11A/orange, FGF12A/purple, FGF13A/red, FGF14A/blue), FGF12A LTP (green) or the IQ motifs of NaV1.2 (black) and NaV1.5 (gray) with (Ca2+)4-CaM. Simulations of (Ca2+)4-CaM binding the FGF12A LTP and each FGF CaMBD are based on the affinities reported in Table 1. Simulations of (Ca2+)4-CaM binding to IQ motifs are based on Kd of 19.8 nM for NaV1.2 and 92.3 nM for NaV1.5 (data not shown). The yellow shaded region highlights the estimated concentration range of free CaM (90) within the cell. The inset shows the ΔG of (Ca2+)4-CaM binding to the putative A-type FGF CaMBDs (FGF11A/orange, FGF12A/purple, FGF13A/red, and FGF14/blue), FGF12A LPTD (green) and the NaV1.2 IQ motif (black) and NaV1.5 IQ motif (gray). The affinities are arranged from tightest (left) to weakest (right). E, schematic depiction of different models of partially ((Ca2+)2-CaMN, apo CaMC) and fully Ca2+-saturated CaM (CaMN/blue, CaMC/red) binding to the IQ motif (green cylinder) in NaV1.2CTD (green) and/or FGF12A CaMBD (limon cylinder) in full-length FGF12A (purple). The FGF12A LTP is shown as a green cylinder and Ca2+ is shown as yellow circles.
Ca2+ binding to CaMC induces a ∼180° rotation of CaMC on the NaV1.2 IQ motif (25). This may require transient release and reassociation of CaMC with the NaV1.2 IQ motif, which in turn might be facilitated by conformational flexibility (i.e., a hinge) between the EFL and IQ motif of NaV1.2. In crystallographic structures containing an NaV CTD bound by FGF12B or FGF13U, the FGF contacts both the NaV EFL and residues that precede the IQ motif (26, 51). This suggests that the binding of FGF12B may alter the dynamics between the NaV1.2 EFL and IQ motif, which could perturb the ability of CaMC to reassociate with the NaV1.2 IQ motif following a Ca2+-induced release.
Ca2+ ligation states of CaM bound to NaV1.2
Based on the Ca2+ affinities of the CaM domains when bound to FGF12B+NaV1.2CTD or FGF12A+NaV1.2CTD, we predict that, at high cytosolic Ca2+ concentrations (∼10 μM (77)), NaV1.2 associated with FGF12B will have either apo CaM or half-saturated ((Ca2+)2-CaMN, apo CaMC) CaM bound (Fig. 9A). In contrast, channels bound to FGF12A are expected to be populated by apo, half ((Ca2+)2-CaMN, apo CaMC), and fully Ca2+-saturated CaM (Fig. 9B). Thus, NaV1.2 with either FGF12 splice variant bound could undergo regulatory processes requiring only apo CaM or Ca2+ binding solely to the sites in CaMN. However, only NaV1.2 bound to FGF12A would support modulation requiring Ca2+-induced rotation or release of CaM from the NaV1.2 IQ motif.
The effect of FGF binding on NaV function has been shown to depend upon the FGF splice variant bound (21, 43, 46, 47). Multiple splice variants of a particular FGF isoform have been found to be expressed simultaneously (38, 78), suggesting that within a cell, NaVs would be associated with more than one. Their unique effects on NaV function have been proposed to correlate with variations in their NTD sequences. While it is unclear how CaM and the FGFs modulate NaV function, the FGF splice variant-dependent differences in the available states of CaM during a spike in the local Ca2+ concentration could contribute to their unique effects on the functional states of NaV.
Cellular competition for (Ca2+)4-CaM
Both CaM and FGF colocalize with NaV (19, 47, 50, 79, 80). Because both apo and (Ca2+)4-CaM bind NaV IQ motifs with high affinity (22, 23, 24, 25, 26, 27, 28) and the β-trefoil cores of FGF12 and FGF13 also bind NaV CTD fragments with high affinity (20, 40), it is likely that CaM and an FGF are constitutively associated with an NaV (Fig. 9C). Proteomic studies investigating NaV1.2-associated proteins in rat neurons have found a level of CaM and FGF enrichment in pull-downs of the NaV1.2 α-subunit similar to other constitutively associated proteins such as the NaV β-subunit β2 (16).
Our thermodynamic and structural studies suggest that at elevated cytosolic Ca2+ levels, an FGF CaMBD and NaV IQ motif will compete for (Ca2+)4-CaM in an isoform-dependent manner (Fig. 9D) due to their proximity and similar affinities for (Ca2+)4-CaM. The FGF12A-induced increase in the Ca2+ affinity of both CaM domains when bound to the NaV1.2CTD (Fig. 6, E and F) suggests that CaM translocates from the NaV IQ motif to the FGF CaMBD at elevated cytosolic Ca2+ concentrations.
While the nine NaV and four FGF isoforms have tissue-dependent patterns of expression, multiple isoforms of both are expressed in some tissues (38, 42, 45, 81, 82), implying that multiple NaV-FGF pairings are present across tissues as well as within a particular cell. The different possible forms of FGF CaMBD and NaV IQ motif recognition by (Ca2+)4-CaM may have distinct effects on channel function and could provide a means to elicit unique modulation of NaV by Ca2+, CaM, and FGF in different tissues and potentially across channels within a particular cell. Structural and functional studies investigating (Ca2+)4-CaM bound to complexes composed of CTD fragments of other NaV isoforms and FGF11A, FGF13A or FGF14A will be needed to determine the generality of the allosteric effects of FGFs on Ca2+ binding by CaM observed in this study
Stoichiometry of CaM bound to NaV
The Ca2+ titrations of CaM+FGF12A+NaV1.2CTD (2:1:1) were consistent with the FGF12A+NaV1.2CTD complex binding one molecule of CaM at the FGF12A CaMBD and one at the NaV1.2 IQ motif (Fig. 7, B–D, Table 4). This may allow NaV channels to recruit a second molecule of CaM, as has been reported for CaV1.2 (50, 83). The ability of the FGF CaMBD to anchor a CaM molecule to an NaV may explain the results of a recent report that found FGF13A is sufficient to regulate arrhythmogenic late current in cardiac NaV1.5 channels with an IQ>AA mutation that blocks CaM binding (44). Although those channels would not have CaM bound at the IQ motif, our findings predict that the NTD of FGF13A would bind (Ca2+)4-CaM, which might be sufficient for regulation if the primary role of the IQ motif is to serve as a sink of constitutively bound CaM.
The closely related channel NaV1.4, primarily found in skeletal muscle, bound only a single molecule of CaM in HEK293 cells under resting conditions and during spikes in cytosolic Ca2+ (50). That 1:1 stoichiometry may be related to the fact that HEK293 cells do not express any A-type FGF splice variant (42) and may lack (or have a lower expression level of) other auxiliary proteins as well. When present, other auxiliary proteins could enable multiple CaM molecules to be recruited to an NaV in an excitable cell.
The schematic models in Figure 9E show NaV1.2CTD with both CaM and FGF12A bound to illustrate how partially ((Ca2+)2-CaMN, apo CaMC) and fully Ca2+-saturated CaM may recognize the NaV1.2 IQ motif and FGF12A CaMBD and how two CaM molecules might bind. The stoichiometry between CaM, NaV1.2CTD, and FGF12A and locations of CaM reflect those used in the Ca2+ titrations of the CaM+FGF12A+NaV1.2CTD complex and the results of those experiments (Fig. 6, E and F, and Fig. 7, A–E). However, the finding that (Ca2+)4-CaM binds the isolated FGF12A LTP with a weaker affinity than the CaMBD suggests that at a sufficiently high local CaM concentration a third CaM molecule could also bind to the LTP.
Multiple reports have found that (Ca2+)4-CaM binds the highly conserved linker between domains III and IV (DIII–DIV linker) (56, 84, 85, 86, 87). Recently CaM has also been reported to associate with a site in the NTD of NaV1.5 (88). The direct binding of (Ca2+)4-CaM to the LTP and CaMBD in the NTD of the A-type FGFs suggests that, when bound to an A-type FGF, the CTD of an NaV may associate with three molecules of CaM simultaneously. Thus, in the cell an NaV with a bound A-type FGF could potentially bind multiple CaM molecules.
Within the context of a cell, multiple targets compete for CaM, making free CaM a limiting reagent in the cytosol (89, 90). However, a number of CaM-binding proteins are found near or within the membrane that may serve as sinks. Among these proteins are ion channels including the NaV, CaV, SK channels, and NMDA receptor, which can form clusters in the membrane (91, 92, 93) that would have high local CaM concentrations. Additional CaM-binding targets, such as neurogranin (94, 95) and neuromodulin (96), constitutively bind apo CaM and release it upon Ca2+ binding. This may provide a pool of free CaM on the intracellular side of the plasma membrane that could allow recruitment of additional CaM to an NaV associated with an A-type FGF.
Flux of Na2+ through the pore of the NaV α-subunit is tightly regulated by a network of intramolecular conformational changes and direct interactions with auxiliary proteins that are expressed as different isoforms and splice variants. These may interact with each other as well as with the channel, which could contribute to how they tune NaV function. An understanding of the structural and energetic forces driving direct interactions between individual auxiliary proteins present at an NaV will provide valuable insights into how they work in concert to modulate channel function as well as how mutations within their sequences disrupt regulatory processes.
The thermodynamic and structural studies here are the first to identify a direct Ca2+-dependent interaction between CaM and the NTD of A-type FGFs and suggest that Ca2+-saturated CaM may translocate from the NaV IQ motif to the FGF CaMBD. These findings lay the groundwork for future studies investigating the consequence of the (Ca2+)4-CaM–FGF CaMBD interaction on NaV function.
Experimental procedures
Modeling software and protein structure images
Model structures of human full-length FGF11A (UniProt ID: Q92914), FGF12A (UniProt ID: P61328), FGF13A (UniProt ID: Q92913), and FGF14A (UniProt ID: Q92915) were generated with the Robetta server (https://robetta.bakerlab.org/) (54). Helical wheels of the FGF12A LTP (aa 1–23) and CaMBD (38–64) were made with HeliQuest (https://heliquest.ipmc.cnrs.fr/) (97). PyMOL (Schrödinger LLC) was used to render all protein structures with these color conventions (unless stated otherwise): CaM residue 1–75/blue, 76–80/black, 81–148/red, Ca2+/yellow, NaV/green, FGF12B/light gray, FGF12A/deep purple, FGF12A LTP/forest green, and FGF12A CaMBD/limon, and calculate vacuum electrostatic surfaces.
Protein expression and purification
Full-length (aa 1–148) and domain fragments (CaMN [aa 1–75], CaMC [aa 76–148]) of WT or mutant human CaM sequences were bacterially overexpressed and purified (23, 98). Genes for full-length or domain fragments of WT or mutant CaM sequences [E31Q/E67Q (E32Q/E68Q) and E104Q/E140Q (E105Q/E141Q)] were expressed with a pT7-7 vector (23). The standard protein designation for each mutant is given first. The parenthetical notation corresponds to the UniProt convention in which the initial Met is designated as residue 1. The three human genes for CaM (CALM1, CALM2, and CALM3) all code for the same protein sequence that corresponds to the sequence of WT CaM used in this study.
The FGF12ACaMBDp (aa 41–70, [CERHVLGVFSKVRFCSGRKRPVRRRPEPQL]), FGF11ANTD (aa 1–68) FGF12ANTD (aa 1–70), FGF13ANTD (aa 1–66), FGF14ANTD (aa 1–68), full-length FGF12A (aa 1–243), full-length FGF12B (aa 1–181), and NaV1.2CTD (aa 1777–1937) were expressed with an N-terminal His-GST-tag using a pBG101 vector (99). The FGF12ACaMBDp, FGF11ANTD, FGF12ANTD, FGF13ANTD, FGF14ANTD, FGF12A, FGF12B, and NaV1.2CTD constructs contained four nonnative residues (GPGS) at the N terminus after removal of the His-GST-tag with 3C protease. FGF12B and complexes of full-length CaM bound to human FGF12ACaMBD, FGF11ANTD, FGF12ANTD, FGF13ANTD, FGF14ANTD, or human NaV1.2CTD (SCN2A) were made by bacterial coexpression and purified as previously described (100). Purity was assessed by SDS-PAGE, UV/Vis spectroscopy, and rpHPLC.
Purification and refolding of full-length FGF12A from inclusion bodies
The cell pellet was thawed, resuspended in lysis buffer (50 mM Tris, 500 mM KCl, 0.01% (w/v) NaN3, 1 mM DTT, 1% (v/v) Triton X-100, pH = 7.4), sonicated, and centrifuged (15,000 rpm, 4 °C, 20 min). The supernatant was discarded, and the pellet was stored at –80 °C. Inclusion bodies were solubilized for 2 h at 22–25 °C, with gentle rocking, in 50 mM Tris, 100 mM KCl, 5 mM imidazole, 6 M guanidine HCl, 1 mM DTT, 0.01% (w/v) NaN3, pH 7.4. The sample was then centrifuged (25,000 rpm, 4 °C, 25 min), the supernatant passed through a sterile 0.45 μM PVFD filter and loaded onto 5 ml of nickel sepharose resin (GE Life Sciences) equilibrated in wash buffer (50 mM Tris, 100 mM KCl, 20 mM imidazole, 6 M urea, 1 mM DTT, 0.01% (w/v) NaN3, 1 mM DTT, pH 7.4). The resin was then washed with 25 ml of wash buffer, and the His-GST-tagged FGF12A was eluted with elution buffer (wash buffer with 500 mM imidazole).
Full-length FGF12A was then refolded by rapid dilution into refolding buffer (100 mM Tris, 200 mM KCl, 100 mM L-arginine, 5% (w/v) sucrose, 0.02% NaN3, 2 mM DTT, pH 7.7, 500 ml per L of cell growth) at 4 °C stirred at 700 rpm with a Teflon-coated stir bar. Prior to the addition of the denaturated His-GST-FGF12A, the CaM+NaV1.2CTD complex (final concentration 0.2 μM) was added to the refolding buffer to assist FGF12A refolding. Twenty-four hours after the addition of His-GST-FGF12A, insoluble material was removed by centrifugation (20,000 rpm, 4 °C, 20 min) and the sample was concentrated. The HIS-GST-tag was cleaved with 3C protease and removed by repassing the sample over nickel sepharose resin. Anion exchange chromatography (pH 8.5–7.4, KCl 0–300 mM) separated FGF12A and NaV1.2CTD from CaM to make the FGF12A+NaV1.2CTD complex. Complex purity (>95%) was assessed by SDS-PAGE, rpHPLC, and UV-Vis spectroscopy.
Affinity of CaM for FGF biosensors
YFP-CFP biosensors containing the sequences for WT human FGF11A CaMBD (aa 36–62), FGF12A LTP (aa 1–23), FGF12A CaMBD (aa 38–64), FGF13A CaMBD (aa 35–60), or FGF14A CaMBD (aa 37–63) were expressed from a pET21B vector (25) (parent vector from A. Persechini and D.J. Black (UMKC) (101, 102)). The FGF sequence used in each biosensor is given in Table 5. Biosensors (≥1 nM) were titrated with WT or mutant CaM (50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 1.5 μM BSA, 500 μM DTT, 1 mM CaCl2, pH 7.4, 22 °C) in a stirred, water-jacketed quartz cuvette. The steady-state fluorescence intensity of CFP (λEX 430 nm, λEM 475 nm), YFP (λEX 430 nm, λEM 525 nm), and an experimentally determined isoemissive point (λEX 430 nm, λEM 509–513 nm) were monitored throughout the titration with a PTI QM4 fluorimeter (4 nm excitation, 8 nm emission bandpasses, 4–16 s integration). Emission spectra (λEX 430 nm, 4 nm excitation, 8 nm emission bandpasses, 4 s integration) were collected from 450 to 550 nm.
Table 5.
FGF biosensor sequences
| FGFCaMBD | Start Positiona | Sequenceb | End Positiona | pIc |
|---|---|---|---|---|
| FGF11ACaMBD | 36 | KSLCQKQLLILLSKVRLCGGRPARPDR | 62 | 10.95 |
| FGF12ACaMBD | 38 | RSLCERHVLGVFSKVRFCSGRKRPVRR | 64 | 11.88 |
| FGF13ACaMBD | 35 | KTSCDKNKLNVFSRVKLFGSKKRRRR | 60 | 11.85 |
| FGF14ACaMBD | 37 | KTSCDKNKLNVFSRVKLFGSKKRRRR | 63 | 11.85 |
Start and end positions are numbered according to the UniProt (https://www.uniprot.org/) convention (106).
FGF sequence inserted between the sequences for the YFP and CFP fluorophores in the FGF biosensor constructs (at KpnI and AgeI restriction sites).
Theoretical isoelectric point calculated with ExPASy (https://web.expasy.org/protparam/) (107).
Buffer subtracted and dilution-corrected titrations were fit to Equation 1, as described (25).
| (1) |
where [CaM]free was calculated using Equation 2,
| (2) |
b is (1 + Ka·[biosensor] − Ka·[CaM]total), and the positive value is taken as [CaM]free. The quality of each fit was judged by evaluating the values of the 67% confidence intervals for each parameter, the span and randomness of the residuals, square root of the variance, and the values of the correlation matrix (30). The magnitude of the confidence intervals was typically smaller and within a factor of 2 of the standard deviation of the average determined from the independent replicate titrations. The average ΔG values and standard deviations from three to nine replicate titrations are reported in Tables 1 and 2. Pairwise comparisons were evaluated using the unpaired t-test (GraphPad Prism, StatPlus); all p values were considered to be very (<0.005) or extremely (<0.0001) statistically significant unless noted otherwise.
Molar ratio of CaM to FGF NTD
The molar ratio of CaM to FGF NTD in the copurified complexes of (Ca2+)4-CaM bound to the FGF11ANTD, FGF12ANTD, FGF13ANTD, or FGF14ANTD was analyzed chromatographically with rpHPLC. The complexes were separated with a Supelco C-18 column with a binary solvent system of water (A) and acetonitrile (B), both with 0.1% (v/v) TFA, using the following gradients: 20%–70% B from 1 to 10 min, 70% B from 10 to 14 min, 70%–90% B from 14 to 16 min. The molar ratio between CaM and the FGF11ANTD, FGF12ANTD, FGF13ANTD, or FGF14ANTD was determined by comparing the area of the absorbance peaks at 220 nm for CaM and the FGF NTD construct in rpHPLC chromatograms.
Solution NMR
Spectra were collected at 25 °C on a Bruker Avance II 500 MHz, Varian Unity Inova 600 MHz, or cryoprobe-equipped Bruker Avance NEO 600 MHz spectrometer. Samples were 15N-(Ca2+)2-CaMN (450 μM), 15N-(Ca2+)2-CaMC (300 μM), 15N-(Ca2+)4-CaM+14N-FGF12ACaMBDp (330 μM), 14N-(Ca2+)4-CaM+15N-FGF12ACaMBDp (450 μM), 15N-(Ca2+)4-CaM+14N-FGF12ANTD (290 μM), or 14N-(Ca2+)4-CaM+15N-FGF12ANTD (600 μM) in 50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 0.01% NaN3, 10 mM CaCl2, ±1 mM DTT, pH 7.4. Spectra were processed with NMRPipe (103) and analyzed with CCPN Analysis (104). SPARTA+ (Shifts Predicted from Analogy in Residue-type and Torsion Angle) (55) was used to predict the position of peaks for residues 41–70 (FGF12ACaMBDp) and 1–70 (FGF12ANTD) of model 1 in the ensemble of full-length FGF12A generated with Robetta (54).
Steady-state fluorescence excitation and emission spectra
Excitation (λEM 280 nm, λEX 240–270 nm or λEM 320 nm, λEX 250–310 nm) and emission (λEX 250 nm, λEM 270–370 nm or λEX 277 nm, λEM 290–370 nm) spectra of NaV1.2CTD (4 μM) and FGF12B (5 μM) (50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 500 μM DTT, ± 10 mM CaCl2, pH 7.4, 22 °C) were collected in a stirred, water-jacketed quartz cuvette with a PTI QM4 fluorimeter. Spectra of were collected with 3 (excitation: λEM 320 nm, λEX 250–310 nm, emission: λEX 277 nm, λEM 290–370 nm) or 5 nm (excitation: λEM 280 nm, λEX 240–270 nm, emission: λEX 250 nm, λEM 270–370 nm) bandpasses and a 4 s integration time.
Equilibrium Ca2+ titrations
Equilibrium Ca2+ titrations of full-length WT CaM (4 μM) alone or with the FGF12ACaMBDp (4 μM), FGF12ANTD (4 μM), NaV1.2CTD (4 μM), FGF12B (5 μM) and NaV1.2CTD (4 μM), or FGF12A and NaV1.2CTD (4 μM) in 5 nM Oregon Green or 50 nM XRhod-5F, 50 mM HEPES, 100 mM KCl, 1 mM MgCl2, 50 μM EGTA, 5 mM NTA, 500 μM DTT, pH 7.4, 22 °C were conducted in a water-jacketed quartz cuvette as described (23). Ca2+ titrations of the CaM+FGF12A+NaV1.2CTD complex conducted at a CaM:FGF12A:NaV1.2CTD ratio of 2:1:1 were conducted with 8 μM CaM and 4 μM FGF12A+NaV1.2CTD in the same buffer. Steady-state fluorescence intensity of intrinsic CaM fluorophores, Phe (λEX 250 nm, λEM 280 nm) and Tyr (λEX 277 nm, λEM 320 nm), was monitored with a PTI QM4 fluorimeter. Phe and Tyr-monitored titrations of CaM alone, +FGF12ACaMBDp, or +FGF12ANTD were collected with 3 nm bandpasses and a 4 s integration time. Phe-monitored titrations of the CaM+NaV1.2CTD, +FGF12B+NaV1.2CTD or +FGF12A+NaV1.2CTD were collected using 5 nm bandpasses and a 16 s integration time, and Tyr-monitored Ca2+ titrations of these complexes were collected with 3 nm bandpasses and a 16 s integration time. The free [Ca2+] was calculated from the change in Oregon Green (λEX 494 nm, λEM 521 nm, Kd 34.2 μM) or XRhod-5F (λEX 576 nm, λEM 603 nm, Kd 1.78 μM) emission intensity (31). The average affinity and standard deviation from three to four replicate titrations are reported in Tables 3 and 4.
Analysis of Ca2+-binding affinity
Each domain of CaM can be considered a two-site macromolecule and the affinities of Ca2+ binding to sites I and II or sites III and IV can be fit to a two-site Adair equation (Equation 3) (23)
| (3) |
where K1 (ΔG1 = −RTlnK1) is the sum of the intrinsic constants (k1 + k2) of the two sites in either domain of CaM, K2 (ΔG2 = −RTlnK2) is the product of the intrinsic constants and the cooperativity constant (k1·k2·kc), and [X] is the free [Ca2+].
To account for variations in intensity between experiments, the low and high endpoints of each titration were fit to the function [f(x)] shown in Equation 4 with nonlinear least squares analysis.
| (4) |
The variable Y[X]low corresponds to the intensity of the Ca2+-depleted sample, 2 is the average fractional saturation, and Span accounts for the difference in intensity at the highest and lowest ligand concentrations. Ca2+ titrations of CaM in the presence of the FGF12ACaMBDp, FGF12NTD, NaV1.2CTD, FGF12B and NaV1.2CTD, or FGF12A and NaV1.2CTD were analyzed assuming that each domain of CaM contained two functional Ca2+-binding sites. Pairwise comparisons of ΔG determinations were evaluated using the unpaired t test (GraphPad Prism, StatPlus); all p values were considered to be very (<0.005) or extremely (<0.0001) statistically significant unless noted otherwise.
Analysis of Ca2+-binding affinity of CaM+FGF12A+NaV1.2CTD
Ca2+ titrations of CaM sites I and II in the CaM+FGF12A+NaV1.2CTD complex conducted at a [CaM]:[FGF12A]:[NaV1.2CTD] ratio of 2:1:1 were fit to Equation 5. Ca2+ titrations of CaM sites III and IV in the same complex were fit to the biphasic function [f(x)] shown in Equation 5 as previously described (37).
| (5) |
The variables 2A and 2B are the average fractional saturation of the Ca2+-binding sites that correspond to the first and second transitions, respectively. SpanA and SpanB account for the direction and magnitude of signal change in the first and second transitions, respectively, and Y[X]low is the intensity of the Ca2+-depleted sample.
Fractional population of intermediate states
The fractional populations of ligated species shown in Figure 9, A and B were calculated with a standard Boltzmann distribution, where the probability (ƒs) of a species (s) is given by Equation 6:
| (6) |
where [X] is the free [Ca2+], j is the stoichiometry of Ca2+ bound by species s, and ΔGs represents the free energy of species s, which includes the intrinsic binding affinity for each site (kI and kII for CaMN, and kIII and kIV for CaMC) and cooperative interactions between the sites (kI-II or kIII-IV). The curves in Figure 9, A and B were simulated using the ΔG1 values for Ca2+ binding by CaM in the CaM+FGF12B+NaV1.2CTD or CaM+FGF12A+NaV1.2CTD complexes reported in Table 4 and assumed that the intrinsic Ca2+-binding affinities of both sites in each domain were equal (i.e., kI = kII = kN and kIII = kIV = kC), as described previously (23). For CaM in the CaM+FGF12B+NaV1.2CTD complex kN and kI-II were 1.39 × 104 M−1 and 284.3, respectively, and kC and kIII-IV were 5.36 × 103 M−1 and 1.5, respectively. For CaM in the CaM+FGF12A+NaV1.2CTD complex kN and kI-II were 1.04 × 105 M−1 and 11.9, respectively, and kC and kIII-IV were 4.30 × 103 M−1 and 198.1, respectively.
Modeling of FGF12A and FGF13A CaMBD bound to (Ca2+)4-CaM
Models of (Ca2+)4-CaM bound to the FGF12 (aa 38–64) and FGF13A (aa 35–60) CaMBD were generated by aligning idealized α-helical models of both FGF CaMBDs to the crystallographic structure of (Ca2+)4-CaM bound to the NMDA receptor CaMBD (2HQW.pdb) (68). The α-helical models FGF12A CaMBD and FGF13A CaMBD were aligned to the structure with NMDA receptor CaMBD residues 880–886 in PyMOL (Schrödinger LLC). Coordinates of the initial models were then energy-minimized with YASARA (105) using the default em_runclean macro.
Data availability
Data reported in this publication are shown in the figures and contained within the article or available upon request from the corresponding author (Madeline A. Shea, madeline-shea@uiowa.edu).
Supporting information
This article contains supporting information.
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Brenda R. Sorensen, Holly M. Isbell, Joseph P. Burba, and Imad Isehak for preliminary studies, Krista Bergquist for technical assistance, and Christopher P. Ptak for NMR support.
Funding and additional information
These studies were supported by the Interdisciplinary Training in Pain Research Program National Institutes of Health T32 NS045549 (R. M.); University of Iowa ICRU Fellowship (C. R. R.); University of Iowa Carver College of Medicine Biochemistry Summer Undergraduate Research Fellowship (M. R. H.); the US National Institutes of Health R01 GM57001 (M. A. S.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
R. M. and M. A. S. conceptualization; R. M., C. R. R., S. C. H., M. R. H., and M. A. S. investigation; R. M., S. C. H., and M. A. S. formal analysis; R. M., C. R. R., and M. A. S. visualization; R. M. and M. A. S. writing—original draft; R. M., C. R. R., and M. A. S. writing—review and editing; R. M., C. R. R., M. R. H., and M. A. S. funding acquisition.
Biography

Ryan Mahling conducted this research as a Biochemistry Ph.D. student at the University of Iowa. Focusing on regulation of NaVs by multiple auxiliary proteins, he and undergraduate coworkers determined that calmodulin and intracellular FGFs (11A, 12A, 13A, 14A) interact with high affinity, forming a ternary complex with an NaV1.2 fragment. This suggests a new molecular mechanism of allosteric regulation. He will continue this work as a postdoctoral fellow at Columbia University.
Edited by Karen Fleming
Supporting information
References
- 1.Messner D.J., Catterall W.A. The sodium channel from rat brain. Separation and characterization of subunits. J. Biol. Chem. 1985;260:10597–10604. [PubMed] [Google Scholar]
- 2.Fujiwara T., Sugawara T., Mazaki-Miyazaki E., Takahashi Y., Fukushima K., Watanabe M., Hara K., Morikawa T., Yagi K., Yamakawa K., Inoue Y. Mutations of sodium channel alpha subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic-clonic seizures. Brain. 2003;126(Pt 3):531–546. doi: 10.1093/brain/awg053. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara T., Tsurubuchi Y., Agarwala K.L., Ito M., Fukuma G., Mazaki-Miyazaki E., Nagafuji H., Noda M., Imoto K., Wada K., Mitsudome A., Kaneko S., Montal M., Nagata K., Hirose S. A missense mutation of the Na+ channel αII subunit gene NaV1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6384–6389. doi: 10.1073/pnas.111065098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mantegazza M., Gambardella A., Rusconi R., Schiavon E., Annesi F., Cassulini R.R., Labate A., Carrideo S., Chifari R., Canevini M.P., Canger R., Franceschetti S., Annesi G., Wanke E., Quattrone A. Identification of an NaV1.1 sodium channel (SCN1A) loss-of-function mutation associated with familial simple febrile seizures. Proc. Natl. Acad. Sci. U. S. A. 2005;102:18177–18182. doi: 10.1073/pnas.0506818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogiwara I., Ito K., Sawaishi Y., Osaka H., Mazaki E., Inoue I., Montal M., Hashikawa T., Shike T., Fujiwara T., Inoue Y., Kaneda M., Yamakawa K. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology. 2009;73:1046–1053. doi: 10.1212/WNL.0b013e3181b9cebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi X., Yasumoto S., Kurahashi H., Nakagawa E., Fukasawa T., Uchiya S., Hirose S. Clinical spectrum of SCN2A mutations. Brain Dev. 2012;34:541–545. doi: 10.1016/j.braindev.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Tester D.J., Will M.L., Haglund C.M., Ackerman M.J. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–517. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Kapplinger J.D., Tester D.J., Salisbury B.A., Carr J.L., Harris-Kerr C., Pollevick G.D., Wilde A.A., Ackerman M.J. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–1303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bankston J.R., Yue M., Chung W., Spyres M., Pass R.H., Silver E., Sampson K.J., Kass R.S. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS One. 2007;2:e1258. doi: 10.1371/journal.pone.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Shen J., Splawski I., Atkinson D., Li Z., Robinson J.L., Moss A.J., Towbin J.A., Keating M.T. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–811. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y., Wang Y., Li S., Xu Z., Li H., Ma L., Fan J., Bu D., Liu B., Fan Z., Wu G., Jin J., Ding B., Zhu X., Shen Y. Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J. Med. Genet. 2004;41:171–174. doi: 10.1136/jmg.2003.012153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M.T., Huang P.Y., Yen C.T., Chen C.C., Lee M.J. A novel SCN9A mutation responsible for primary erythromelalgia and is resistant to the treatment of sodium channel blockers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faber C.G., Lauria G., Merkies I.S., Cheng X., Han C., Ahn H.S., Persson A.K., Hoeijmakers J.G., Gerrits M.M., Pierro T., Lombardi R., Kapetis D., Dib-Hajj S.D., Waxman S.G. Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19444–19449. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vassilev P.M., Scheuer T., Catterall W.A. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science. 1988;241:1658–1661. doi: 10.1126/science.241.4873.1658. [DOI] [PubMed] [Google Scholar]
- 15.Mantegazza M., Yu F.H., Catterall W.A., Scheuer T. Role of the C-terminal domain in inactivation of brain and cardiac sodium channel. Proc. Natl. Acad. Sci. U. S. A. 2001;98:15348–15353. doi: 10.1073/pnas.211563298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wildburger N.C., Ali S.R., Hsu W.C., Shavkunov A.S., Nenov M.N., Lichti C.F., LeDuc R.D., Mostovenko E., Panova-Elektronova N.I., Emmett M.R., Nilsson C.L., Laezza F. Quantitative proteomics reveals protein-protein interactions with fibroblast growth factor 12 as a component of the voltage-gated sodium channel 1.2 (Nav1.2) macromolecular complex in Mammalian brain. Mol. Cell Proteomics. 2015;14:1288–1300. doi: 10.1074/mcp.M114.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu C., Dib-Hajj S.D., Waxman S.G. Fibroblast growth factor homologous factor 1B binds to the C terminus of the tetrodotoxin-resistant sodium channel rNaV1.9a (NaN) J. Biol. Chem. 2001;276:18925–18933. doi: 10.1074/jbc.M101606200. [DOI] [PubMed] [Google Scholar]
- 18.Liu C.J., Dib-Hajj S.D., Renganathan M., Cummins T.R., Waxman S.G. Modulation of the cardiac sodium channel NaV1.5 by fibroblast growth factor homologous factor 1B. J. Biol. Chem. 2003;278:1029–1036. doi: 10.1074/jbc.M207074200. [DOI] [PubMed] [Google Scholar]
- 19.Lou J.Y., Laezza F., Gerber B.R., Xiao M., Yamada K.A., Hartmann H., Craig A.M., Nerbonne J.M., Ornitz D.M. Fibroblast growth factor 14 is an intracellular modulator of voltage-gated sodium channels. J. Physiol. 2005;569(Pt 1):179–193. doi: 10.1113/jphysiol.2005.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Wang C., Hoch E.G., Pitt G.S. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage-gated sodium channels. J. Biol. Chem. 2011;286:24253–24263. doi: 10.1074/jbc.M111.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laezza F., Lampert A., Kozel M.A., Gerber B.R., Rush A.M., Nerbonne J.M., Waxman S.G., Dib-Hajj S.D., Ornitz D.M. FGF14 N-terminal splice variants differentially modulate Nav1.2 and Nav1.6-encoded sodium channels. Mol. Cell Neurosciences. 2009;42:90–101. doi: 10.1016/j.mcn.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori M., Konno T., Ozawa T., Murata M., Imoto K., Nagayama K. Novel Interaction of the voltage-dependent sodium channel (VDSC) with calmodulin: Does VDSC acquire calmodulin-mediated Ca2+-sensitivity? Biochemistry. 2000;39:1316–1323. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- 23.Theoharis N.T., Sorensen B.R., Theisen-Toupal J., Shea M.A. The neuronal voltage-dependent sodium channel Type II IQ Motif lowers the calcium affinity of the C-domain of calmodulin. Biochemistry. 2008;47:112–123. doi: 10.1021/bi7013129. [DOI] [PubMed] [Google Scholar]
- 24.Feldkamp M.D., Yu L., Shea M.A. Structural and energetic Determinants of apo calmodulin Binding to the IQ Motif of the Nav1.2 voltage-dependent sodium channel. Structure. 2011;19:733–747. doi: 10.1016/j.str.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hovey L., Fowler C.A., Mahling R., Lin Z., Miller M.S., Marx D.C., Yoder J.B., Kim E.H., Tefft K.M., Waite B.C., Feldkamp M.D., Yu L., Shea M.A. Calcium triggers reversal of calmodulin on nested anti-parallel sites in the IQ motif of the neuronal voltage-dependent sodium channel NaV1.2. Biophys. Chem. 2017;224:1–19. doi: 10.1016/j.bpc.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C., Chung B.C., Yan H., Wang H.G., Lee S.Y., Pitt G.S. Structural analyses of Ca2+/CaM interaction with NaV channel C-termini reveal mechanisms of calcium-dependent regulation. Nat. Commun. 2014;5:4896. doi: 10.1038/ncomms5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoder J.B., Ben-Johny M., Farinelli F., Srinivasan L., Shoemaker S.R., Tomaselli G.F., Gabelli S.B., Amzel L.M. Ca(2+)-dependent regulation of sodium channels NaV1.4 and NaV1.5 is controlled by the post-IQ motif. Nat. Commun. 2019;10:1514. doi: 10.1038/s41467-019-09570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabelli S.B., Boto A., Kuhns V.H., Bianchet M.A., Farinelli F., Aripirala S., Yoder J., Jakoncic J., Tomaselli G.F., Amzel L.M. Regulation of the NaV1.5 cytoplasmic domain by calmodulin. Nat. Commun. 2014;5:5126. doi: 10.1038/ncomms6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klee C.B., Newton D.L., Ni W.C., Haiech J. Regulation of the calcium signal by calmodulin. In: Evered D., Whelan J., editors. Calcium and the Cell. John Wiley & Sons; Chichester, NY: 1986. pp. 162–182. [Google Scholar]
- 30.Sorensen B.R., Shea M.A. Interactions between domains of apo calmodulin alter calcium binding and stability. Biochemistry. 1998;37:4244–4253. doi: 10.1021/bi9718200. [DOI] [PubMed] [Google Scholar]
- 31.Evans T.I., Shea M.A. Energetics of calmodulin domain interactions with the calmodulin binding domain of CaMKII. Proteins. 2009;76:47–61. doi: 10.1002/prot.22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung W.Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem. Biophys. Res. Commun. 1970;38:533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- 33.Schulman H., Greengard P. Stimulation of brain membrane protein phosphorylation by calcium and an endogenous heat-stable protein. Nature. 1978;271:478–479. doi: 10.1038/271478a0. [DOI] [PubMed] [Google Scholar]
- 34.Peterson B.Z., DeMaria C.D., Yue D.T. Calmodulin Is the Ca2+ Sensor for Ca2+-dependent Inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 35.Tzortzopoulos A., Best S.L., Kalamida D., Török K. Ca2+/Calmodulin-dependent activation and inactivation mechanisms of αCaMKII and phospho-Thr286-αCaMKII. Biochemistry. 2004;43:6270–6280. doi: 10.1021/bi035449u. [DOI] [PubMed] [Google Scholar]
- 36.Li Z., Zhang Y., Hedman A.C., Ames J.B., Sacks D.B. Calmodulin lobes facilitate dimerization and activation of estrogen receptor-alpha. J. Biol. Chem. 2017;292:4614–4622. doi: 10.1074/jbc.M116.754804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell S.E., Yu L., Fowler C.A., Shea M.A. Recognition of beta-calcineurin by the domains of calmodulin: Thermodynamic and structural evidence for distinct roles. Proteins. 2011;79:765–786. doi: 10.1002/prot.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smallwood P.M., Munoz-Sanjuan I., Tong P., Macke J.P., Hendry S.H., Gilbert D.J., Copeland N.G., Jenkins N.A., Nathans J. Fibroblast growth factor (FGF) homologous factors: New members of the FGF family implicated in nervous system development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9850–9857. doi: 10.1073/pnas.93.18.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsen S.K., Garbi M., Zampieri N., Eliseenkova A.V., Ornitz D.M., Goldfarb M., Mohammadi M. Fibroblast growth factor (FGF) homologous factors share structural but not functional homology with FGFs. J. Biol. Chem. 2003;278:34226–34236. doi: 10.1074/jbc.M303183200. [DOI] [PubMed] [Google Scholar]
- 40.Goetz R., Dover K., Laezza F., Shtraizent N., Huang X., Tchetchik D., Eliseenkova A.V., Xu C.F., Neubert T.A., Ornitz D.M., Goldfarb M., Mohammadi M. Crystal structure of a fibroblast growth factor homologous factor (FHF) defines a conserved surface on FHFs for binding and modulation of voltage-gated sodium channels. J. Biol. Chem. 2009;284:17883–17896. doi: 10.1074/jbc.M109.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ago H., Kitagawa Y., Fujishima A., Matsuura Y., Katsube Y. Crystal structure of basic fibroblast growth factor at 1.6 A resolution. J. Biochem. 1991;110:360–363. doi: 10.1093/oxfordjournals.jbchem.a123586. [DOI] [PubMed] [Google Scholar]
- 42.Munoz-Sanjuan I., Smallwood P.M., Nathans J. Isoform diversity among fibroblast growth factor homologous factors is generated by alternative promoter usage and differential splicing. J. Biol. Chem. 2000;275:2589–2597. doi: 10.1074/jbc.275.4.2589. [DOI] [PubMed] [Google Scholar]
- 43.Dover K., Solinas S., D'Angelo E., Goldfarb M. Long-term inactivation particle for voltage-gated sodium channels. J. Physiol. 2010;588(Pt 19):3695–3711. doi: 10.1113/jphysiol.2010.192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abrams J., Roybal D., Chakouri N., Katchman A.N., Weinberg R., Yang L., Chen B.X., Zakharov S.I., Hennessey J.A., Avula U.M.R., Diaz J., Wang C., Wan E.Y., Pitt G.S., Ben-Johny M. Fibroblast growth factor homologous factors tune arrhythmogenic late NaV1.5 current in calmodulin binding-deficient channels. JCI Insight. 2020;5 doi: 10.1172/jci.insight.141736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartung H., Feldman B., Lovec H., Coulier F., Birnbaum D., Goldfarb M. Murine FGF-12 and FGF-13: Expression in embryonic nervous system, connective tissue and heart. Mech. Dev. 1997;64:31–39. doi: 10.1016/s0925-4773(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 46.Yang J., Wang Z., Sinden D.S., Wang X., Shan B., Yu X., Zhang H., Pitt G.S., Wang C. FGF13 modulates the gating properties of the cardiac sodium channel Nav1.5 in an isoform-specific manner. Channels (Austin) 2016;10:410–420. doi: 10.1080/19336950.2016.1190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Effraim P.R., Huang J., Lampert A., Stamboulian S., Zhao P., Black J.A., Dib-Hajj S.D., Waxman S.G. Fibroblast growth factor homologous factor 2 (FGF-13) associates with Nav1.7 in DRG neurons and alters its current properties in an isoform-dependent manner. Neurobiol. Pain. 2019;6:100029. doi: 10.1016/j.ynpai.2019.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bosch M.K., Nerbonne J.M., Townsend R.R., Miyazaki H., Nukina N., Ornitz D.M., Marionneau C. Proteomic analysis of native cerebellar iFGF14 complexes. Channels (Austin) 2016;10:297–312. doi: 10.1080/19336950.2016.1153203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao M., Bosch M.K., Nerbonne J.M., Ornitz D.M. FGF14 localization and organization of the axon initial segment. Mol. Cell Neurosciences. 2013;56:393–403. doi: 10.1016/j.mcn.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Johny M., Yue D.N., Yue D.T. Detecting stoichiometry of macromolecular complexes in live cells using FRET. Nat. Commun. 2016;7:13709. doi: 10.1038/ncomms13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C., Chung B.C., Yan H., Lee S.Y., Pitt G.S. Crystal Structure of the ternary Complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure. 2012;20:1167–1176. doi: 10.1016/j.str.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niu J., Dick I.E., Yang W., Bamgboye M.A., Yue D.T., Tomaselli G., Inoue T., Ben-Johny M. Allosteric regulators selectively prevent Ca2+-feedback of CaV and NaV channels. Elife. 2018;7 doi: 10.7554/eLife.35222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishida T., Kinoshita K. Prediction of disordered regions in proteins based on the meta approach. Bioinformatics. 2008;24:1344–1348. doi: 10.1093/bioinformatics/btn195. [DOI] [PubMed] [Google Scholar]
- 54.Kim D.E., Chivian D., Baker D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004;32:W526–W531. doi: 10.1093/nar/gkh468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen Y., Bax A. SPARTA+: A modest improvement in empirical NMR chemical shift prediction by means of an artificial neural network. J. Biomol. NMR. 2010;48:13–22. doi: 10.1007/s10858-010-9433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah V.N., Wingo T.L., Weiss K.L., Williams C.K., Balser J.R., Chazin W.J. Calcium-dependent regulation of the voltage-gated sodium channel hH1: Intrinsic and extrinsic sensors use a common molecular switch. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3592–3597. doi: 10.1073/pnas.0507397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sorensen B.R., Faga L.A., Hultman R., Shea M.A. Interdomain linker increases thermostability and decreases calcium affinity of calmodulin N-domain. Biochemistry. 2002;41:15–20. doi: 10.1021/bi011718+. [DOI] [PubMed] [Google Scholar]
- 58.Pablo J.L., Pitt G.S. FGF14 is a regulator of KCNQ2/3 channels. Proc. Natl. Acad. Sci. U. S. A. 2017;114:154–159. doi: 10.1073/pnas.1610158114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schoorlemmer J., Goldfarb M. Fibroblast growth factor homologous factors are intracellular signaling proteins. Curr. Biol. 2001;11:793–797. doi: 10.1016/s0960-9822(01)00232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schulman H., Hanson P.I., Meyer T. Decoding calcium signals by multifunctional CaM kinase. Cell Calcium. 1992;13:401–411. doi: 10.1016/0143-4160(92)90053-u. [DOI] [PubMed] [Google Scholar]
- 61.Hathaway D.R., Adelstein R.S., Klee C.B. Interaction of calmodulin with myosin light chain kinase and cAMP-dependent protein kinase in bovine brain. J. Biol. Chem. 1981;256:8183–8189. [PubMed] [Google Scholar]
- 62.Hubbard M.J., Klee C.B. Calmodulin binding by calcineurin ligand-induced renaturation of protein immobilized on nitrocellulose. J.Biol.Chem. 1987;262:15062–15070. [PubMed] [Google Scholar]
- 63.Chen Y., Clarke O.B., Kim J., Stowe S., Kim Y.-K., Assur Z., Cavalier M., Godoy-Ruiz R., von Alpen D.C., Manzini C., Blaner W.S., Frank J., Quadro L., Weber D.J., Shapiro L. Structure of the STRA6 receptor for retinol uptake. Science. 2016;353 doi: 10.1126/science.aad8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee C.H., MacKinnon R. Activation mechanism of a human SK-calmodulin channel complex elucidated by cryo-EM structures. Science. 2018;360:508–513. doi: 10.1126/science.aas9466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houdusse A., Gaucher J.F., Krementsova E., Mui S., Trybus K.M., Cohen C. Crystal structure of apo-calmodulin bound to the first two IQ motifs of myosin V reveals essential recognition features. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19326–19331. doi: 10.1073/pnas.0609436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meador W.E., Means A.R., Quiocho F.A. Target enzyme recognition by calmodulin: 2.4 Å Structure of a calmodulin-peptide complex. Science. 1992;257:1251–1255. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]
- 67.Ehlers M.D., Zhang S., Bernhardt J.P., Huganir R.L. Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell. 1996;84:745–755. doi: 10.1016/s0092-8674(00)81052-1. [DOI] [PubMed] [Google Scholar]
- 68.Ataman Z.A., Gakhar L., Sorensen B.R., Hell J.W., Shea M.A. The NMDA receptor NR1 C1 region bound to calmodulin: Structural insights into functional differences between homologous domains. Structure. 2007;15:1603–1617. doi: 10.1016/j.str.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Newman R.A., Van Scyoc W.S., Sorensen B.R., Jaren O.R., Shea M.A. Interdomain cooperativity of calmodulin bound to melittin preferentially increases calcium affinity of sites I and II. Proteins. 2008;71:1792–1812. doi: 10.1002/prot.21861. [DOI] [PubMed] [Google Scholar]
- 70.van Petegem F., Chatelain F.C., Minor D.L., Jr. Insights into voltage-gated calcium channel regulation from the structure of the Ca(V)1.2 IQ domain-Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim E.Y., Rumpf C.H., Fujiwara Y., Cooley E.S., Van Petegem F., Minor D.L., Jr. Structures of CaV2 Ca2+/CaM-IQ domain complexes reveal binding modes that underlie calcium-dependent inactivation and facilitation. Structure. 2008;16:1455–1467. doi: 10.1016/j.str.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osawa M., Tokumitsu H., Swindells M.B., Kurihara H., Orita M., Shibanuma T., Furuya T., Ikura M. A novel target recognition revealed by calmodulin in complex with Ca2+-calmodulin-dependent kinase kinase. Nat. Struct. Biol. 1999;6:819–824. doi: 10.1038/12271. [DOI] [PubMed] [Google Scholar]
- 73.Mori M.X., Vander Kooi C.W., Leahy D.J., Yue D.T. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: High-resolution mechanistic implications for channel regulation by Ca2+ Structure. 2008;16:607–620. doi: 10.1016/j.str.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feldkamp M.D., Gakhar L., Pandey N., Shea M.A. Opposing orientations of the anti-psychotic drug trifluoperazine selected by alternate conformations of M144 in calmodulin. Proteins. 2015;83:989–996. doi: 10.1002/prot.24781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vertessy B.G., Harmat V., Bocskei Z., Naray-Szabo G., Orosz F., Ovadi J. Simultaneous binding of drugs with different chemical structures to Ca2+-calmodulin: Crystallographic and spectroscopic studies. Biochemistry. 1998;37:15300–15310. doi: 10.1021/bi980795a. [DOI] [PubMed] [Google Scholar]
- 76.Cook W.J., Walter L.J., Walter M.R. Drug binding by calmodulin: Crystal structure of a calmodulin-trifluoperazine complex. Biochemistry. 1994;33:15259–15265. doi: 10.1021/bi00255a006. [DOI] [PubMed] [Google Scholar]
- 77.Li R., Leblanc J., He K., Liu X.J. Spindle function in Xenopus oocytes involves possible nanodomain calcium signaling. Mol. Biol. Cell. 2016;27:3273–3283. doi: 10.1091/mbc.E16-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang C., Hennessey J.A., Kirkton R.D., Wang C., Graham V., Puranam R.S., Rosenberg P.B., Bursac N., Pitt G.S. Fibroblast growth factor homologous factor 13 regulates Na+ channels and conduction velocity in murine hearts. Circ. Res. 2011;109:775–782. doi: 10.1161/CIRCRESAHA.111.247957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pablo J.L., Wang C., Presby M.M., Pitt G.S. Polarized localization of voltage-gated Na+ channels is regulated by concerted FGF13 and FGF14 action. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E2665–E2674. doi: 10.1073/pnas.1521194113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Biswas S., Deschenes I., Disilvestre D., Tian Y., Halperin V.L., Tomaselli G.F. Calmodulin regulation of NaV1.4 current: Role of binding to the carboxyl terminus. J. Gen. Physiol. 2008;131:197–209. doi: 10.1085/jgp.200709863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schaller K.L., Caldwell J.H. Expression and distribution of voltage-gated sodium channels in the cerebellum. Cerebellum. 2003;2:2–9. doi: 10.1080/14734220309424. [DOI] [PubMed] [Google Scholar]
- 82.Whitaker W.R., Clare J.J., Powell A.J., Chen Y.H., Faull R.L., Emson P.C. Distribution of voltage-gated sodium channel alpha-subunit and beta-subunit mRNAs in human hippocampal formation, cortex, and cerebellum. J. Comp. Neurol. 2000;422:123–139. doi: 10.1002/(sici)1096-9861(20000619)422:1<123::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 83.Kim E.Y., Rumpf C.H., Van Petegem F., Arant R.J., Findeisen F., Cooley E.S., Isacoff E.Y., Minor D.L., Jr. Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization. EMBO J. 2010;29:3924–3938. doi: 10.1038/emboj.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potet F., Chagot B., Anghelescu M., Viswanathan P.C., Stepanovic S.Z., Kupershmidt S., Chazin W.J., Balser J.R. Functional interactions between distinct sodium channel cytoplasmic domains through the action of calmodulin. J. Biol. Chem. 2009;284:8846–8854. doi: 10.1074/jbc.M806871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarhan M.F., Van Petegem F., Ahern C.A. A double tyrosine motif in the cardiac sodium channel domain III-IV linker couples calcium-dependent calmodulin binding to inactivation gating. J. Biol. Chem. 2009;284:33265–33274. doi: 10.1074/jbc.M109.052910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarhan M.F., Tung C.C., Van Petegem F., Ahern C.A. Crystallographic basis for calcium regulation of sodium channels. Proc. Natl. Acad. Sci. U. S. A. 2012;109:3558–3563. doi: 10.1073/pnas.1114748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson C.N., Potet F., Thompson M.K., Kroncke B.M., Glazer A.M., Voehler M.W., Knollmann B.C., George A.L., Jr., Chazin W.J. A mechanism of calmodulin modulation of the human cardiac sodium channel. Structure. 2018;26:683–694 e3. doi: 10.1016/j.str.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Z., Vermij S.H., Sottas V., Shestak A., Ross-Kaschitza D., Zaklyazminskaya E.V., Hudmon A., Pitt G.S., Rougier J.S., Abriel H. Calmodulin binds to the N-terminal domain of the cardiac sodium channel Nav1.5. Channels (Austin) 2020;14:268–286. doi: 10.1080/19336950.2020.1805999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Persechini A., Cronk B. The Relationship between the free Concentrations of Ca2+ and Ca2+-calmodulin in intact cells. J. Biol. Chem. 1999;274:6827–6830. doi: 10.1074/jbc.274.11.6827. [DOI] [PubMed] [Google Scholar]
- 90.Maier L.S., Ziolo M.T., Bossuyt J., Persechini A., Mestril R., Bers D.M. Dynamic changes in free Ca-calmodulin levels in adult cardiac myocytes. J. Mol. Cell Cardiol. 2006;41:451–458. doi: 10.1016/j.yjmcc.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Westenbroek R.E., Merrick D.K., Catterall W.A. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 92.Clatot J., Hoshi M., Wan X., Liu H., Jain A., Shinlapawittayatorn K., Marionneau C., Ficker E., Ha T., Deschenes I. Voltage-gated sodium channels assemble and gate as dimers. Nat. Commun. 2017;8:2077. doi: 10.1038/s41467-017-02262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou D., Lambert S., Malen P.L., Carpenter S., Boland L.M., Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gerendasy D.D., Herron S.R., Watson J.B., Sutcliffe J.G. Mutational and biophysical studies suggest RC3/neurogranin regulates calmodulin availability. J. Biol. Chem. 1994;269:22420–22426. [PubMed] [Google Scholar]
- 95.Gerendasy D.D., Herron S.R., Jennings P.A., Sutcliffe J.G. Calmodulin stabilizes an amphiphilic a-helix within RC3/neurogranin and GAP-43/neuromodulin only when Ca2+ is absent. J. Biol. Chem. 1995;270:6741–6750. doi: 10.1074/jbc.270.12.6741. [DOI] [PubMed] [Google Scholar]
- 96.Chapman E.R., Au D., Alexander K.A., Nicolson T.A., Storm D.R. Characterization of the calmodulin binding domain of neuromodulin. Functional significance of serine 41 and phenylalanine 42. J. Biol. Chem. 1991;266:207–213. [PubMed] [Google Scholar]
- 97.Gautier R., Douguet D., Antonny B., Drin G. HELIQUEST: A web server to screen sequences with specific alpha-helical properties. Bioinformatics. 2008;24:2101–2102. doi: 10.1093/bioinformatics/btn392. [DOI] [PubMed] [Google Scholar]
- 98.Putkey J.A., Slaughter G.R., Means A.R. Bacterial expression and characterization of proteins derived from the chicken calmodulin cDNA and a calmodulin processed gene. J. Biol. Chem. 1985;260:4704–4712. [PubMed] [Google Scholar]
- 99.Damo S.M., Feldkamp M.D., Chagot B., Chazin W.J. NMR studies of the interaction of calmodulin with IQ motif peptides. Methods Mol. Biol. 2013;963:173–186. doi: 10.1007/978-1-62703-230-8_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahling R., Kilpatrick A.M., Shea M.A. Backbone resonance assignments of complexes of human voltage-dependent sodium channel NaV1.2 IQ motif peptide bound to apo calmodulin and to the C-domain fragment of apo calmodulin. Biomol. NMR Assign. 2017;11:297–303. doi: 10.1007/s12104-017-9767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Romoser V.A., Hinkle P.M., Persechini A. Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators. J. Biol. Chem. 1997;272:13270–13274. doi: 10.1074/jbc.272.20.13270. [DOI] [PubMed] [Google Scholar]
- 102.Black D.J., Leonard J., Persechini A. Biphasic Ca2+-dependent switching in a calmodulin-IQ domain complex. Biochemistry. 2006;45:6987–6995. doi: 10.1021/bi052533w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Delaglio F., Grzesiek S., Vuister G.W., Zhu G., Pfeifer J., Bax A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 104.Vranken W.F., Boucher W., Stevens T.J., Fogh R.H., Pajon A., Llinas M., Ulrich E.L., Markley J.L., Ionides J., Laue E.D. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins. 2005;59:687–696. doi: 10.1002/prot.20449. [DOI] [PubMed] [Google Scholar]
- 105.Krieger E., Vriend G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015;36:996–1007. doi: 10.1002/jcc.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Apweiler R., Bairoch A., Wu C.H., Barker W.C., Boeckmann B., Ferro S., Gasteiger E., Huang H., Lopez R., Magrane M., Martin M.J., Natale D.A., O'Donovan C., Redaschi N., Yeh L.S. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2004;32:D115–D119. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gasteiger E., Gattiker A., Hoogland C., Ivanyi I., Appel R.D., Bairoch A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003;31:3784–3788. doi: 10.1093/nar/gkg563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papadopoulos J.S., Agarwala R. Cobalt: Constraint-based alignment tool for multiple protein sequences. Bioinformatics. 2007;23:1073–1079. doi: 10.1093/bioinformatics/btm076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data reported in this publication are shown in the figures and contained within the article or available upon request from the corresponding author (Madeline A. Shea, madeline-shea@uiowa.edu).