Abstract
Purpose:
Accurate preoperative staging of prostate cancer (PCa) is essential for treatment planning. Conventional imaging (CI) is limited in detection of metastases. Our primary aim was to determine if [18F]fluciclovine PET/CT is an early indicator of sub-clinical metastasis among patients with high-risk PCa.
Materials and Methods:
68 patients with unfavorable intermediate to very high-risk PCa without systemic disease on CI were recruited before robotic radical prostatectomy with extended pelvic lymph node dissection (EPLND). Diagnostic performance of [18F]fluciclovine PET/CT and CI for detection of metastatic disease, and correlation of positivity to node and metastatic deposit size were determined.
Results:
57/68 patients completed the protocol, of which 31/57 had nodal metastasis on histology. [18F]fluciclovine PET/CT sensitivity and specificity in detecting nodal metastasis were 55.3% and 84.8% per-patient, 54.8% and 96.4% per-region (right and left pelvis, presacral and non-regional), respectively. Compared with CI, [18F]fluciclovine PET/CT had significantly higher sensitivity on patient-based (55.3% vs 33.3%; p<0.01) and region-based (54.8% vs 19.4%; p<0.01) analysis, detecting metastasis in 7 more patients and 22 more regions; with similar high specificity. Four additional patients had distant disease or other cancer detected on [18F]fluciclovine PET/CT which precluded surgery. Detection of metastasis was related to size of metastatic deposits within LNs, and overall metastatic burden.
Conclusions:
[18F]fluciclovine PET/CT detects occult metastases not identified on CI and may help guide treatment decisions and lymph node dissection due to high specificity for metastatic disease.
Keywords: Prostate cancer, [18F]fluciclovine, staging, robotic radical prostatectomy, extended pelvic lymph node dissection
Introduction
Following initial diagnosis, accurate staging of prostate cancer (PCa) is fundamental to proper treatment planning. Conventional imaging (CI) such as computed tomography (CT), magnetic resonance imaging (MRI) and bone scan are recommended for preoperative evaluation of high-risk disease1, 2. Unfortunately, CI is suboptimal in detection of small volume lymph node (LN) and bone metastasis2, 3. Molecular imaging is increasingly utilized in preoperative evaluation. Positron emission tomography (PET) radiotracers, such as [11C]choline, [18F]choline, [18F]fluciclovine and prostate specific membrane antigen (PSMA) have shown promising results with high specificity for metastasis4–9.
Anti-1-amino-3-[18F]fluorocyclobutyl-1-carboxylic acid (FACBC or [18F]fluciclovine) is a synthetic amino acid analog which has been approved by the United States Food and Drug Administration (Axumin®; Blue Earth Diagnostics, Ltd, Oxford UK) for imaging of suspected recurrent PCa10–14. While its role in detection of recurrent PCa is well established, there is little research evaluating use in staging of primary PCa7, 15. In this prospective trial we set out to evaluate [18F]fluciclovine PET/CT as an early indicator of sub-clinical metastatic disease among patients with high-risk PCa eligible for curative surgery.
Materials and Methods
Prospective clinical trial (NCT03081884; NIHU01-CA113913) conducted between 3/2017 and 12/2019. Ethical approval was obtained from the institutional review board in compliance with Health Insurance Portability and Accountability Act.
Patient Selection
Inclusion criteria included biopsy-proven primary PCa with unfavorable intermediate-risk disease (Grade Group (GG) 2 with either PSA 10–20 ng/ml or clinical stage T2b-c, or GG 3 with PSA <20 ng/ml), high-risk (clinical T3a, or GG 4–5, or PSA >20 ng/ml) or very high-risk (clinical T3b-T4, or GG 5 with primary Gleason pattern 5, or >4 cores with Gleason sum 8–10), without definitive findings of systemic metastasis on CI and eligible for potentially curative radical prostatectomy and extended pelvic LN dissection (RP+EPLND).
Conventional Imaging
Patients had standard-of-care CI including [99mTc]-methylene diphosphonate (MDP) bone scanning and CT or MRI for preoperative staging per institutional protocol.
18[F]fluciclovine PET/CT Imaging Protocol
[18F]fluciclovine was administered under FDA Investigational New Drug Application 72,437 and synthesized via the FastLab Cassette System (GE Healthcare, Marlborough MA).
PET/CT was acquired on a GE Discovery-690 16 slice integrated scanner (GE Healthcare, Waukesha, WI) after intravenous bolus injection of [18F]fluciclovine (366.0±23.6 MBq). Early time-point images were obtained immediately post-injection for pelvis only in 2 consecutive 2.5-minute bed positions. At 5 minutes, 7 consecutive 2.5-minute/frame acquisitions were obtained from the pelvis (below ischium) to the skull base (routine time-point). At 22.5 minutes, delayed time-point pelvis only images were acquired in 2 consecutive 2.5-minute bed positions. Images were reconstructed with iterative technique and reviewed on a MimVista workstation (MIM Software, Cleveland, OH).
Image Analysis
CI was interpreted per institutional protocol; clinical interpretations completed before PET/CT were utilized for this analysis.
[18F]fluciclovine PET/CT was interpreted by a board-certified nuclear radiologist with over 20 years’ experience, blinded to details of clinical history (beyond inclusion criteria) and other imaging. [18F]fluciclovine uptake in prostate and extraprostatic sites were recorded. Routine whole-body time-point images were interpreted for suspicious uptake per established criteria, supplemented by additional information provided by early and delayed pelvic imaging16. Abnormal uptake was assigned a Likert confidence score of 1–5 for malignancy (1-definitely benign; 2-probably benign; 3-equivocal; 4-probably malignant, 5-definitely malignant). A lesion with a score of ≥3 was considered positive for the primary analysis.
Malignant nodes were assigned an anatomical location according to a predefined anatomic surgical template described below. Uptake parameters recorded in the prostate will undergo future analysis. [18F]fluciclovine PET/CT results were reviewed with the surgeon pre- and post-operatively to align LN packet (LNP) nomenclature on imaging with anatomic nomenclature of the resected LNP.
Surgery
Eligible patients underwent robotic RP+EPLND based on a predefined LN template: right and left obturator, external iliac, internal iliac, common iliac and pre-sciatic; presacral, and non-regional. Each group of LNs was removed as a packet and processed separately according to the template during histopathologic examination. Template data were also grouped for region-based analysis (right and left pelvis, presacral, and non-regional LNs).
Histologic Examination and Correlation to Imaging
Histology was the primary reference standard-of-truth. Given that correlation of individual LNs detected on PET/CT with histology is not feasible, correlation was therefore made at the level of the LNP. For example, if ≥1 node were positive in that LNP on imaging, and ≥1 node were positive on histology, the entire packet was considered true-positive. For analysis of detection-rate relative to nodal or metastatic deposit size, the longest diameter was conservatively employed.
Data and Statistical Analysis
Diagnostic performance (sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy) for metastatic disease with the corresponding 95% confidence interval were assessed for [18F]fluciclovine PET/CT and CI per-patient, per-region, and per LN template level. Comparison of diagnostic performance of [18F]fluciclovine PET/CT and CI was done using McNemar test and generalized score statistics methods. Intra-patient correlation was accounted for in the inference of these diagnostic performance measures on region- and surgical template-based levels, by bootstrapping patients. The effect of size of the node and metastatic deposits on PET detection was also evaluated. Data analysis was performed using Statistical Analysis Software (SAS Version 9.4 SAS Institute Inc. Cary, NC). P-value <0.05 was considered statistically significant.
Results
Patients
68 patients were recruited. Three patients were screen failures; 4 patients withdrew or were lost to follow-up. Of the remaining 61 who underwent [18F]fluciclovine PET/CT, 1/61 had prostate cancer treatment deferred after the incidental detection of lung cancer; 3/61 had occult distant metastasis disease detected on PET/CT and managed with systemic therapy. Thus, 57 patients underwent surgery (56 robotic RP+EPLND, 1 EPLND only due to unexpected non-regional metastasis on frozen section which halted prostatectomy). (Supplementary figure 1).
Demographic characteristics are recorded in Table 1.
Table 1:
Demographic, clinical, imaging, and pathology data
| Patient characteristics (n=57) | |
|---|---|
| Age (years) | |
| Mean (SD) | 61.7 (7.0) |
| PSA (ng/ml) | |
| Median (Interquartile range (Q1–Q3)) | 15.0 (7.4–27.6) |
| NCCN Risk Stratification | |
| Intermediate-unfavorable risk | 4 (7.0) |
| High risk | 35 (61.4) |
| Very high risk | 18 (31.6) |
| Post-operative Grade Group* n (%) | |
| 2 (Gleason score 3+4) | 9 (15.0) |
| 3 (Gleason score 4+3) | 11 (18.3) |
| 4 (Gleason score 4+4, 3+5, 5+3) | 1 (1.7) |
| 5 (Gleason scores 4+5, 5+4,5+5) | 35 (58.3) |
| Total number of excised lymph nodes | 2480 |
| Median per patient (Interquartile range (Q1–Q3)) | 40.0 (33.5–51.5) |
| [18F]fluciclovine PET/CT positivity rate (N1 ± M1) n (%) | 21 (36.8) |
| Positivity rate per risk group: | |
| Intermediate-unfavorable risk (n=4) | 2 (50.0) |
| High risk (n=35) | 12 (34.3) |
| Very high risk (n=18) | 7 (38.9) |
| Metastatic disease based on truth (N1 ± M1) n (%) | 31 (54.4) |
| Metastatic disease per risk group: | |
| Intermediate-unfavorable risk (n=4) | 2 (50.0) |
| High risk (n=35) | 17 (48.6) |
| Very high risk (n=18) | 12 (66.7) |
n=56 patients that had radical prostatectomy
Conventional Imaging Analysis
All (57/57) patients who completed the study protocol had negative bone scans. Of these 57 patients, 20 (35.1%) had standard-of-care CT while 37 (72.5%) had MRI. Median interval between CI and PET/CT was 36 days (interquartile range 18–55 days). Metastatic nodal disease was suspected in 14/57 (24.6%) patients on CI. No patient had osseous metastasis on CI.
[18F]fluciclovine PET/CT Analysis
[18F]fluciclovine PET/CT was completed 10.6 ± 7.8 days prior to surgery. All patients had abnormal [18F]fluciclovine uptake in the prostate. Metastatic disease (N1 ± M1) was present in 21/57 (36.8%) patients on PET/CT. Of the 21 patients, 14/21 were classified as N1M0, 1/21 as N0M1b and 6/21 as N1M1a. Seventeen templates in 10 patients were equivocal on PET/CT (Likert 3) and were classified as positive for the primary analysis.
Histologic Analysis
Of the 57 patients who had surgery, multifocal PCa was present in all patients who had prostatectomy. A total of 2480 LNs were excised (median 40 LNs/patient). Nodal metastasis (N1 ± M1) was found in 31/57 (54.4%) patients on histology. Of these patients, 20/31 had regional nodal disease only (N1M0) and 11/31 had non-regional nodal (M1a) disease.
Correlation of CI with Reference Standard
Diagnostic performance:
On the patient level (n=57) sensitivity for metastasis was 33.3% (CI: 21.0%−47.0%), specificity 84.1% (CI: 73.3%−94.4%), PPV 72.2% (CI: 55.6%−88.9%), and NPV 51.2% (CI: 40.0%−63.0%). For region-based analysis (n=57×4 regions), sensitivity was 19.4% (CI: 11.0%−26.0%), specificity 94.6% (CI: 92.3%−97.2%), PPV 57.4% (CI: 40.0%−75.0%) and NPV 75.8% (CI: 71.4%−80.4%). Template level analysis was not feasible.
Correlation of [18F]fluciclovine PET/CT With Reference Standard
Diagnostic performance:
On the patient level (n=57) sensitivity was 55.3% (CI: 43.0%−68.0%), specificity 84.8% (CI: 75.0%−94.7%), PPV 81.5% (CI: 69.2%−93.3%), and NPV 60.8% (CI: 47.8%−73.7%). For region-based analysis (n=57×4 regions), sensitivity was 54.8% (CI: 46.0%−65.0%), specificity 96.4% (CI: 94.2%−99.0%), PPV 85.0% (CI: 76.9%−94.9%), and NPV 85.1% (CI: 81.4%−89.1%). For template-based analysis (n=57×12 templates), sensitivity was 46.2% (CI: 38.0%−54.0%), specificity 98.7% (CI: 98.1%−99.5%), PPV 82.8% (CI: 76.7%−92.6%), and NPV 92.1% (CI: 90.7%−93.7%). (Table 2).
Table 2:
Diagnostic performance of [18F]fluciclovine PET/CT for metastatic disease
| Per patient n=57 |
Per region n=228 |
Per template n=684 |
|
|---|---|---|---|
| True positives | 17 | 34 | 42 |
| True negatives | 22 | 160 | 585 |
| False positives | 4 | 6 | 8 |
| False negatives | 14 | 28 | 49 |
| %Sensitivity (95% CI) | 55.3 (43.0–68.0) | 54.8 (46.0–65.0) | 46.2 (38.0–54.0) |
| %Specificity (95% CI) | 84.8 (75.0–94.7) | 96.4 (94.2–99.0) | 98.7 (98.1–99.5) |
| %PPV (95% CI) | 81.5 (69.2–93.3) | 85.0 (76.9–94.9) | 82.8 (76.7–92.6) |
| %NPV (95% CI) | 60.8 (47.8–73.7) | 85.1 (81.4–89.1) | 92.1 (90.7–93.7) |
| %Accuracy (95% CI) | 68.5 (59.4–78.1) | 85.1 (81.6–88.5) | 91.7 (90.2–93.2) |
Regions (n=57×4=228 regions): Right pelvic, left pelvic, presacral and non-regional groups of lymph nodes.
Templates (n=57×12=684 templates): Right and left common, internal and external iliac, right and left obturator, right and left pre-sciatic, presacral, and non-regional lymph nodes.
Detection-rate and node size:
There was no significant difference between mean long axis diameters of true-positive and false-negatives LNs (16.1 ± 8.0 mm vs 15.6 ± 11.0 mm respectively; p=0.80).The mean long axis diameter of metastatic deposits within true-positive nodes was significantly higher than false-negative nodes (10.6 ± 8.2 mm vs 3.9 ± 3.1 mm, respectively; p<0.01). Detection-rate on PET/CT was related to diameter of nodal metastasis (Table 3). Patients with PET-positive LNs (true-positives) had more metastatic LNPs on histology than those who were PET-negative (false-negatives) (7.5 ± 7.0 LNPs vs 2.6 ± 1.9 LNPs; p=0.02).
Table 3:
Detection rate of nodal metastasis by [18F]fluciclovine PET/CT according to long diameter of metastatic foci.
| Diameter of metastatic foci | Detection rate (%) |
|---|---|
| ≤3 mm | 9/38 (23.7) |
| >3–6 mm | 7/24 (29.2) |
| >6–9 mm | 5/10 (50.0) |
| >9 mm | 20/24 (83.3) |
Correlation to stage:
Of the 36/57 patients staged as N0M0 on PET, there was concordance on pathologic staging in 22/36 patients; 12/36 patients were up-staged to N1M0 and 2/36 patients were up-staged to N1M1a after surgery. Of the 14/57 patients classified as N1M0 on PET, there was concordance with pathologic staging in 8/14 patients; 4/14 patients were down-staged to N0M0 while 2/14 was up-staged to N1M1a after surgery. Of the 7/57 patients classified as M1 disease on PET/CT, there was 100% concordance between PET staging and pathology.
Comparison Between [18F]fluciclovine PET/CT and CI
On patient-based analysis, [18F]fluciclovine PET/CT had significantly higher sensitivity (55.3% vs 33.3%; p<0.01) and NPV (60.8% vs 51.2%; p=0.02) compared to CI for the detection of metastatic disease. There was no statistically significant difference in specificity and PPV. On region-based analysis, [18F]fluciclovine PET/CT had significantly higher sensitivity (54.8% vs 19.4%; p<0.01), PPV (85.0% vs 57.4%; p=0.01), and NPV (85.1% vs 75.8%; p<0.01) compared to CI. Specificity of [18F]fluciclovine PET/CT was comparable to CI (Table 4).
Table 4:
Comparison between [18F]fluciclovine PET/CT and conventional imaging in preoperative lymph node staging of high-risk prostate cancer patients
| Patient-based analysis (n=57) | Region-based analysis (n=228) | |||||
|---|---|---|---|---|---|---|
| [18F]fluciclovine PET/CT | Conventional Imaging | p-value | [18F]fluciclovine PET/CT | Conventional Imaging | p-value | |
| %Sensitivity (95% CI) | 55.3 (43.0–68.0) | 33.3 (21.0–47.0) | <0.01 | 54.8 (46.0–65.0) | 19.4 (11.0–26.0) | <0.01 |
| %Specificity (95% CI) | 84.8 (75.0–94.7) | 84.1 (73.3–94.4) | 1.00 | 96.4 (94.2–99.0) | 94.6 (92.3–97.2) | 0.37 |
| %PPV (95% CI) | 81.5 (69.2–93.3) | 72.2 (55.6–88.9) | 0.35 | 85.0 (76.9–94.9) | 57.4 (40.0–75.0) | 0.01 |
| %NPV (95% CI) | 60.8 (47.8–73.7) | 51.2 (40.0–63.0) | 0.02 | 85.1 (81.4–89.1) | 75.8 (71.4–80.4) | <0.01 |
| %Accuracy (95% CI) | 68.5 (59.4–78.1) | 56.3 (47.2–65.7) | <0.01 | 85.1 (81.6–88.5) | 74.1 (69.8–78.6) | <0.01 |
Representative images are shown in Figures 1 and 2. Supplemental Table 1 is a list of all patients with imaging and histologic findings.
Figure 1:
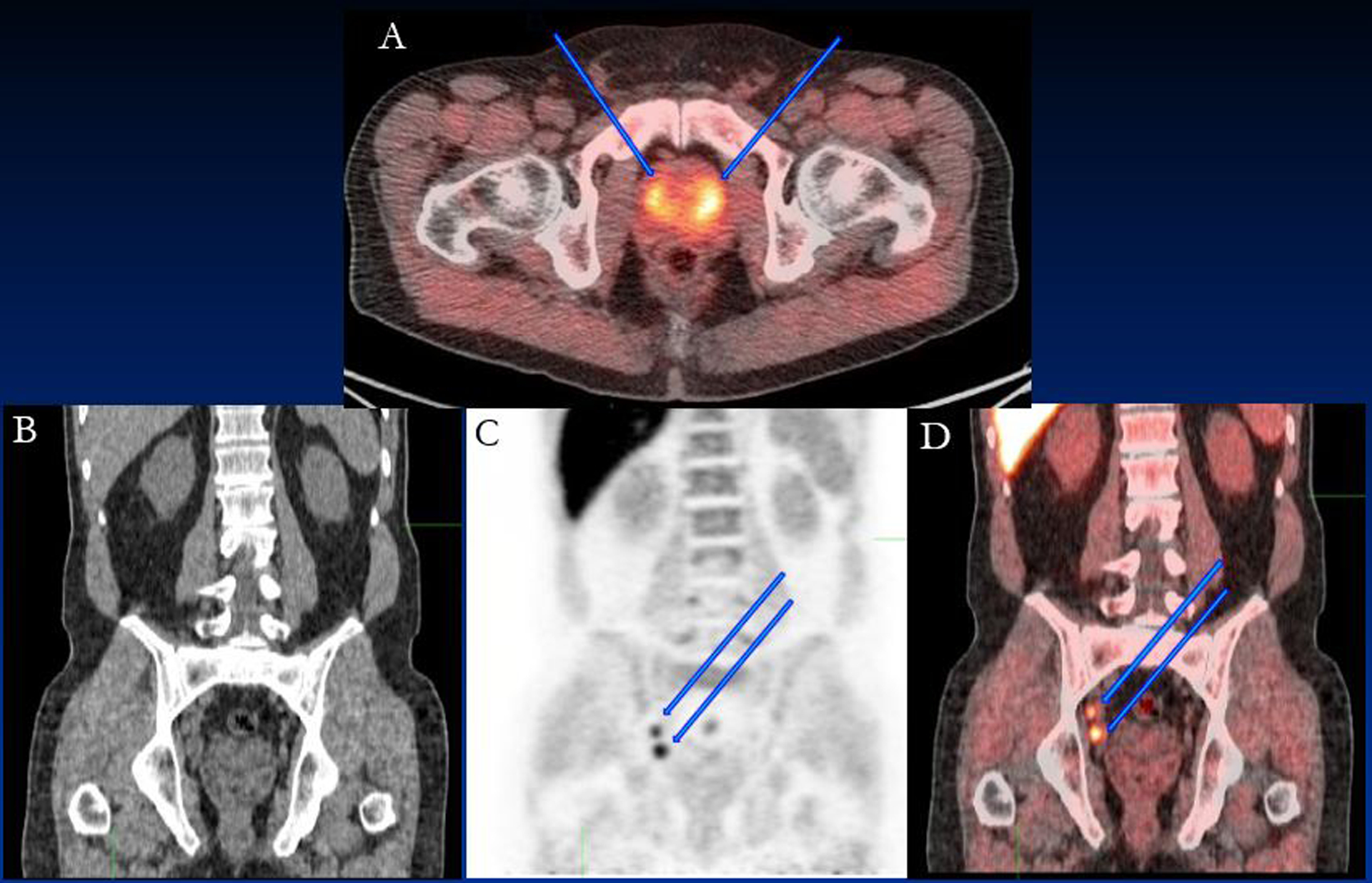
64 year old patient with PSA of 22.22 ng/ml. Bilateral prostate disease was seen on fluciclovine PET; (A) fused PET/CT image showing the prostate lesions (blue arrows). Intense fluciclovine uptake was detected in obturator lymph nodes (arrows) in (B) CT, (C) PET and (D) fused PET/CT images. Histology confirmed metastatic obturator lymph nodes (true-positive).
Figure 2:
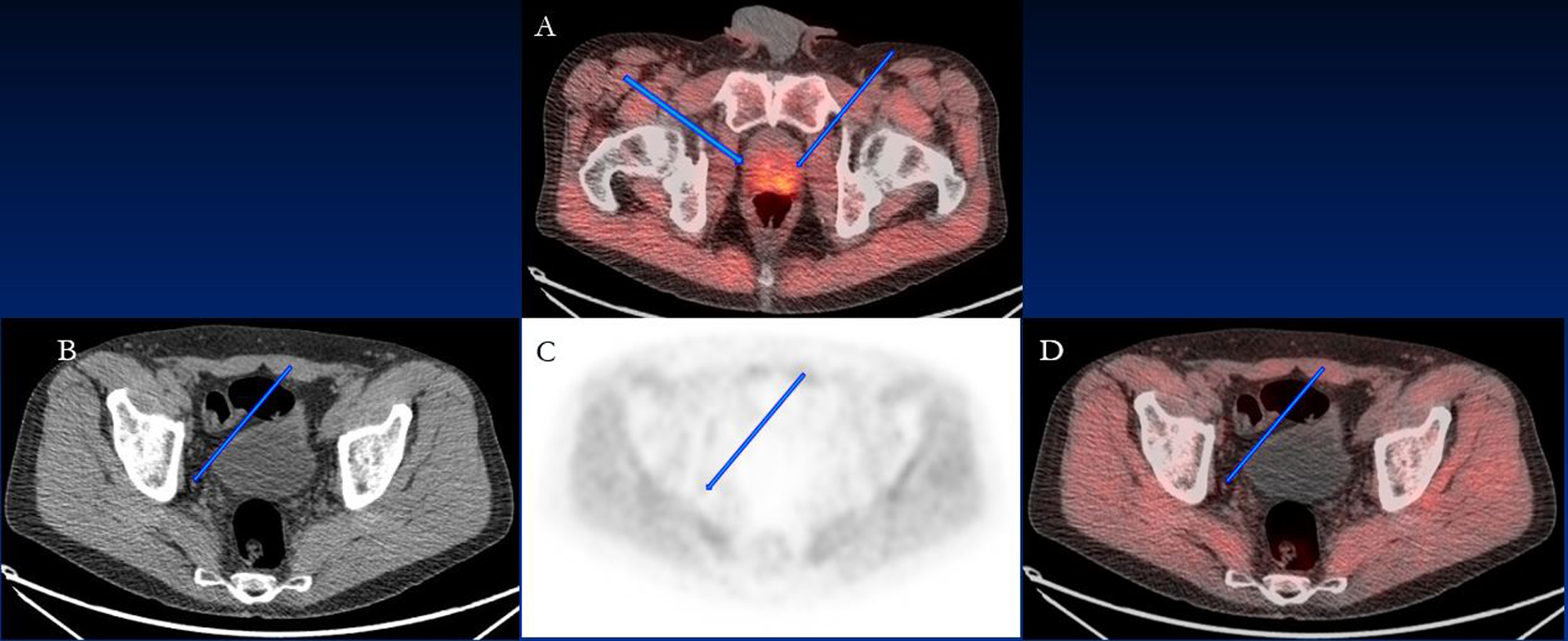
70 year old patient with PSA of 5.3 ng/ml. Bilateral prostate disease was seen on fluciclovine PET; (A) fused PET/CT image showing the prostate lesions (blue arrows). Mild fluciclovine uptake in the right obturator lymph node (arrows) measuring 0.4×0.4 cm was seen on (B) CT, (C) PET and (D) fused PET/CT and considered benign but was malignant on histology (false-negative).
Patients Who Did Not Undergo Surgery
CT suggested pelvic nodal disease in 1 patient, yet PET/CT revealed more disease including mesenteric nodes. Localized prostate cancer was predicted by CI (1 CT; 1 MR) in 2 other patients, but systemic disease was identified on PET/CT. These patients underwent non-surgical therapy and were not utilized for the primary analysis. One other patient had [18F]fluciclovine-avid lung cancer incidentally discovered on PET/CT which obviated prostate cancer surgery.
Discussion
Accurate staging is critical for treatment planning in patients with high-risk primary PCa, as local therapy may be insufficient in patients with metastatic disease. CI is limited in detection of extraprostatic metastases2, 3. Our prospective trial was designed to evaluate [18F]fluciclovine PET/CT as an early indicator of sub-clinical metastatic disease among high-risk PCa patients eligible for curative surgery.
Diagnostic performance of [18F]fluciclovine PET/CT for extraprostatic staging demonstrates sensitivity of 55.3%, 54.8%, and 46.2% and specificity of 84.8%, 96.4%, and 98.7% on per-patient, region and LNP (template) levels, respectively. [18F]fluciclovine PET/CT performs better than CI in detection of metastatic disease, with significantly higher sensitivity and NPV per-patient, and significantly higher sensitivity, PPV and NPV on region-based analysis. Sensitivity and specificity for detection of M1 disease were 63.6% and 100%, respectively. Three additional patients with unsuspected systemic disease, and one patient with lung cancer were detected on [18F]fluciclovine PET/CT which obviated surgery.
PET-detected LN metastatic foci (true-positives) were significantly larger than those that were undetected (false-negatives). Additionally, there was a greater nodal tumor burden in patients who were PET-positive versus PET-negative. Briganti has reported an association between number of metastatic nodes and tumor free survival17. Further studies are required to determine if preoperative [18F]fluciclovine PET/CT can also provide prognostic information.
Selnaes7 evaluated the diagnostic performance of [18F]fluciclovine PET/MR for nodal metastasis in 28 patients with high-risk primary PCa and reported 40% sensitivity per-patient and 30% per-region with 87.5% and 95.7% specificity per-patient and per-region, respectively. While their study and ours had similar high specificity, the higher sensitivity in our study may be related to our higher mean PSA (25.5 ng/ml vs 14.6 ng/ml) and greater percentage of patients with extraprostatic disease. Our findings of detection-rate related to size of the metastatic focus within the LN is also in agreement with Selnaes7 and Suzuki15.
Choline PET in high-risk primary prostate cancer has a reported sensitivity of 10%−80% and specificity of 80–100%8, 18–22. A systematic review and meta-analysis of the diagnostic performance of choline for extraprostatic disease in primary PCa reported a pooled sensitivity of 49.2%6. PSMA PET radiotracers for preoperative staging of intermediate to high-risk prostate cancer have reported a sensitivity of 33%−85% and specificity of 88%−100%5, 23–26. Though the findings from our study are comparable to this range, PSMA radiotracers appear to have higher sensitivity than [18F]fluciclovine for small volume disease27. In a study of 130 patients with intermediate to high-risk PCa who underwent [68Ga]PSMA imaging, retrospective analysis reported a sensitivity of 65.9% per-patient and 73.5% per-template with 98.9%-99.2% specificity23. Gorin reported a sensitivity and specificity of 71.4% and 88.9% per-patient, and a sensitivity and specificity of 66.7% and 92.7% per-template, in a study of [18F]DCFPyL PET/CT for preoperative staging in 25 patients with high-risk PCa27.
Our finding that advanced PET/CT had higher sensitivity than CI agrees with existing literature7, 15,28. [18F]fluciclovine PET/CT had a 22% greater sensitivity per-patient (55.3% vs 33%; p<0·01), and 35% greater sensitivity per-region (54.8% vs 19.4%; p<0.01) compared to CI. This is comparable to findings by Schiavina using [18F]choline who reported a higher sensitivity per-patient for PET/CT of 50.0% versus 21.0% for CI19, and Maurer who reported 65.9% sensitivity for [68Ga] PSMA PET/CT vs 46.2% sensitivity for morphologic CI23.
In our study, [18F]fluciclovine PET/CT identified more patients with regional and non-regional metastasis undetected on standard-of-care imaging resulting in accurate stage migration. All M1 disease detected on [18F]fluciclovine PET/CT was histologically proven. Identification of patients with non-regional disease may facilitate an oligometastatic approach to therapy, though this requires further investigation in well-controlled trials29. Yet, inaccurate stage prediction also occurred in which EPLND identified metastatic disease in patients considered to have localized disease on [18F]fluciclovine PET/CT. This highlights the limitation of [18F]fluciclovine PET/CT, especially in micro-metastatic disease as demonstrated by the relatively low sensitivity in metastatic deposit size <3 mm, as previously reported7, 15. In fact, our use of EPLND increases the possibility of detection of small volume metastasis by histology alone30.
Strengths of our study include prospective design, blinded interpretation, homogenous cohort, and meticulous correlation of imaging results with surgical findings. Limitations include relatively small sample size, single center design and single reader. We classified equivocal interpretation on PET/CT as positive; analysis in which equivocal findings were classified as negative (Supplemental Table 2), resulted in sensitivity of 41.9% and specificity of 100% per-patient. Another limitation is the inability to directly correlate individual LNs on PET with histology. Since this direct correlation is implausible in practice, we employed a conservative approach, using the longest diameter of the largest LN in each packet as the representative sample, which may have overestimated the sizes reported for the true-positive and false-negative LNs. While the preoperative review of images with the surgeon was ethically appropriate, this may have prompted the surgeon to extend the field of otherwise template surgery in selected cases.
Conclusion
[18F]fluciclovine PET/CT can detect extraprostatic metastasis occult to CI with high specificity. While detection rate is related to the size of individual metastasis, the high PPV of [18F]fluciclovine PET/CT suggests utility in clinical staging and identification of patients who may not benefit from surgery alone.
Supplementary Material
Acknowledgements
We acknowledge Bridget Fielder, RN, Almira Catic, Stephanie Giles, Fenton G. Ingram, RT(R), CNMT, PET, Seraphinah Lawal, RT(R), CNMT, PET, Ronald J. Crowe, RPh, BCNP, and the cyclotron/synthesis team from Emory University Center for Systems Imaging.
Financial Disclosure: This study was funded by National Institutes of Health grant U01-CA113913-11.
AAA, OAA: Funding is or has been received from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for other clinical trials using [18F]fluciclovine.
DMS: Funding is or has been received from Blue Earth Diagnostics Ltd. and Nihon Medi-Physics Co., Ltd. through the Emory University Office of Sponsored Projects for other clinical trials using [18F]fluciclovine. Participates through the Emory Office of Sponsored Projects in sponsored grants including those funded or partially funded by Telix Pharmaceuticals (US) Inc., Advanced Accelerator Applications, and FUJIFILM Pharamaceuticals USA, Inc. Consultant, Syncona, Global Medical Solutions (Taiwan) and AIM Specialty Health.
Abbreviations
- CI
Conventional Imaging
- PCa
Prostate cancer
- PET
Positron Emission Tomography
- CT
Computed Tomography
- LN
Lymph node(s)
- LNP
Lymph node packet(s)
- RP+EPLND
Radical prostatectomy and extended lymph node dissection
Footnotes
Emory University: Blue Earth Diagnostics Ltd. provided fluciclovine synthesis cassettes to Emory University. Entitled to royalties derived from the sale of products related to the research described in this manuscript. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
MMG: Entitled to royalties derived from the sale of products related to the research described in this manuscript. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
This study was registered at ClinicalTrials.Gov:
References
- 1.Sanda MG, Cadeddu JA, Kirkby E et al. : Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J Urol, 199: 683, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M et al. : EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol, 71: 618, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Hovels AM, Heesakkers RA, Adang EM et al. : The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol, 63: 387, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Budiharto T, Joniau S, Lerut E et al. : Prospective evaluation of 11C-choline positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur Urol, 60: 125, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Budaus L, Leyh-Bannurah SR, Salomon G et al. : Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol, 69: 393, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Evangelista L, Guttilla A, Zattoni F et al. : Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol, 63: 1040, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Selnaes KM, Kruger-Stokke B, Elschot M et al. : (18)F-Fluciclovine PET/MRI for preoperative lymph node staging in high-risk prostate cancer patients. Eur Radiol, 28: 3151, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Van den Bergh L, Lerut E, Haustermans K et al. : Final analysis of a prospective trial on functional imaging for nodal staging in patients with prostate cancer at high risk for lymph node involvement. Urol Oncol, 33: 109.e23, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Zang S, Zhang C et al. : Comparison of (68)Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med, 15: 230, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster DM, Votaw JR, Nieh PT et al. : Initial experience with the radiotracer anti-1-amino-3–18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med, 48: 56, 2007 [PubMed] [Google Scholar]
- 11.Schuster DM, Nieh PT, Jani AB et al. : Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol, 191: 1446, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach-Gansmo T, Nanni C, Nieh PT et al. : Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol, 197: 676, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DOTATATE P: FDA Approves 18F-Fluciclovine and 68Ga-DOTATATE Products. J Nucl Med, 57: 9N, 2016. 26449838 [Google Scholar]
- 14.Akin-Akintayo O, Tade F, Mittal P et al. : Prospective evaluation of fluciclovine ((18)F) PET-CT and MRI in detection of recurrent prostate cancer in non-prostatectomy patients. Eur J Radiol, 102: 1, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki H, Inoue Y, Fujimoto H et al. : Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate pancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol, 47: 283, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Savir-Baruch B, Banks KP, McConathy JE et al. : ACR-ACNM Practice Parameter for the Performance of Fluorine-18 Fluciclovine-PET/CT for Recurrent Prostate Cancer. Clin Nucl Med, 43: 909, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Briganti A, Karnes JR, Da Pozzo LF et al. : Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol, 55: 261, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hacker A, Jeschke S, Leeb K et al. : Detection of pelvic lymph node metastases in patients with clinically localized prostate cancer: comparison of [18F]fluorocholine positron emission tomography-computerized tomography and laparoscopic radioisotope guided sentinel lymph node dissection. J Urol, 176: 2014, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Schiavina R, Bianchi L, Mineo Bianchi F et al. : Preoperative Staging With (11)C-Choline PET/CT Is Adequately Accurate in Patients With Very High-Risk Prostate Cancer. Clin Genitourin Cancer, 16: 305, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Evangelista L, Cimitan M, Zattoni F et al. : Comparison between conventional imaging (abdominal-pelvic computed tomography and bone scan) and [(18)F]choline positron emission tomography/computed tomography imaging for the initial staging of patients with intermediate- tohigh-risk prostate cancer: A retrospective analysis. Scand J Urol, 49: 345, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Poulsen MH, Bouchelouche K, Hoilund-Carlsen PF et al. : [18F]fluoromethylcholine (FCH) positron emission tomography/computed tomography (PET/CT) for lymph node staging of prostate cancer: a prospective study of 210 patients. BJU Int, 110: 1666, 2012 [DOI] [PubMed] [Google Scholar]
- 22.de Jong IJ, Pruim J, Elsinga PH et al. : Preoperative staging of pelvic lymph nodes in prostate cancer by 11C-choline PET. J Nucl Med, 44: 331, 2003 [PubMed] [Google Scholar]
- 23.Maurer T, Gschwend JE, Rauscher I et al. : Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J Urol, 195: 1436, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Park SY, Zacharias C, Harrison C et al. : Gallium 68 PSMA-11 PET/MR Imaging in Patients with Intermediate- or High-Risk Prostate Cancer. Radiology, 288: 495, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Corfield J, Perera M, Bolton D et al. : (68)Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol, 36: 519, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Gorin MA, Rowe SP, Patel HD et al. : Prostate Specific Membrane Antigen Targeted (18)F-DCFPyL Positron Emission Tomography/Computerized Tomography for the Preoperative Staging of High Risk Prostate Cancer: Results of a Prospective, Phase II, Single Center Study. J Urol, 199: 126, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jilg CA, Drendel V, Rischke HC et al. : Diagnostic Accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics, 7: 1770, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beheshti M, Imamovic L, Broinger G et al. : 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology, 254: 925, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Lecouvet FE, Oprea-Lager DE, Liu Y et al. : Use of modern imaging methods to facilitate trials of metastasis-directed therapy for oligometastatic disease in prostate cancer: a consensus recommendation from the EORTC Imaging Group. Lancet Oncol, 19: e534, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Heidenreich A, Varga Z, Von Knobloch R: Extended pelvic lymphadenectomy in patients undergoing radical prostatectomy: high incidence of lymph node metastasis. J Urol, 167: 1681, 2002 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


