Abstract
Sensorimotor coordination is thought to rely on cerebellar-based internal models for state estimation, but the underlying neural mechanisms and specific contribution of the cerebellar components is unknown. A central aspect of any inferential process is the representation of uncertainty or conversely precision characterizing the ensuing estimates. Here, we discuss the possible contribution of inhibition to the encoding of precision of neural representations in the granular layer of the cerebellar cortex. Within this layer, Golgi cells influence excitatory granule cells, and their action is critical in shaping information transmission downstream to Purkinje cells. In this review, we equate the ensuing excitation–inhibition balance in the granular layer with the outcome of a precision-weighted inferential process, and highlight the physiological characteristics of Golgi cell inhibition that are consistent with such computations.
Keywords: neural computation, neural networks, state estimation, granule cell layer, sensorimotor
1. Introduction
Sensorimotor coordination or control can be regarded as the realization of expected sensation via movement. It involves interactions between an agent and its environment; like when a mouse is actively gathering information with its whiskers. In order to control these interactions, the brain must be able to approximate or predict the consequences of forthcoming action. This relies on accurate estimates of behaviourally relevant states (such as whisker position) generated by an underlying model of how states relate to one another. Estimates are intrinsically uncertain, reflecting stochasticity in sensory channels and dynamics of states. Hence, when considering the neural implementation of an estimation process, it is desirable that neural circuits are capable of representing estimates conditioned on their associated uncertainty; in other words, the underlying models ought to be probabilistic.
The cerebellum has long been posited to instantiate probabilistic internal models for estimation of rapidly varying external states [1], whether somatic, such as limb kinematics [2], or environmental, for example, moving targets [3]. In the cerebellum, these models are deemed to support sensorimotor control [4–6], as well as more abstract mental representations [7], by complementing ongoing neural computations in other brain regions with internally generated, delay-free probabilistic estimates of stochastic external dynamics, built upon past experience and integrating multiple sources of noisy information.
These ideas are long-standing, but it remains unresolved how various components of the cerebellum could specifically contribute to state estimation. In general, only activity and plasticity of Purkinje cells, the output of the cerebellar cortex, have been associated with this computation; however, inferential processes occur all the way through the hierarchy of an internal probabilistic model. Here we consider the first step in cerebellar cortical state estimation, by proposing a role for inhibition in the granular layer. This network, comprising about half of the neurons in the mammalian nervous system, relays all extracerebellar input that is directed via mossy fibres (MFs) to Purkinje cells [8–10] (figure 1). The granular layer is made up of excitatory granule cells and inhibitory Golgi cells, and interactions between these two neuronal populations determine network responses to external (MF) perturbations. Two aspects are key to understanding neural dynamics within this network: firstly, granule cells are numerous but individually receive only a small number of inputs (four excitatory and four inhibitory connections each on average [11,12]); secondly, Golgi cells are sparse relative to granule cells, but each neuron contacts hundreds to thousands of granule cells through an extended axonal arborization. Thus, Golgi cell inhibition is likely to have a big impact on individual granule cell activity and putative inferential processes in the cerebellum.
Figure 1.
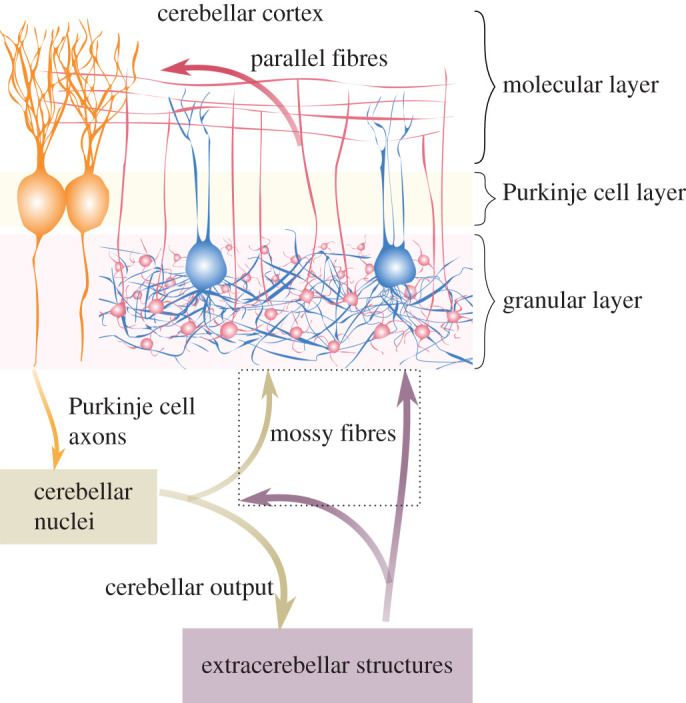
Information flow through the cerebellum. Information from extracerebellar structures enters the cerebellum via MFs (violet arrows) and climbing fibres (not shown). MFs contact both the cerebellar cortex and nuclei; in the former, they synapse in the granular layer, the first stage of information processing along this pathway. Here reside densely packed excitatory granule cells (in red), and sparsely distributed inhibitory Golgi cells (in blue), which inhibit vast and overlapping groups of granule cells. Interaction between these two neuronal populations determine how information is transmitted downstream to Purkinje cells (in orange) through granule cell ascending axons and parallel fibres (red fibres). Purkinje cells generate the sole output of the cerebellar cortex, influencing neural activity in the cerebellar nuclei (brown box). Nuclear neurons depart axons back to extracerebellar structures (brown arrows), also making collaterals that terminate as MFs in the granular layer. The dotted box inscribes all MF terminals in the cerebellum.
We explore this possibility by discussing the role of Golgi cell inhibition in the context of state estimation in the cerebellar cortex, and set out a link between high-level theoretical descriptions of cerebellar computations and their neural substrates. We start from the assumption that neuronal activity encodes estimates or predictions of somatic and environmental states that enable guidance, coordination and refinement of action; then, we argue that Golgi cell inhibition promotes accurate state estimation, by adaptively tuning excitatory responses encoding those estimates. Crucially, such tuning rests upon (neural mechanisms signalling) the precision of the information driving the inferential process, so that the excitation–inhibition balance in granule cell populations becomes the result of a precision-weighting process. This view ultimately affords a new interpretation of observed inhibitory mechanisms in the granular layer.
2. Precision in state estimation
Many aspects of brain functioning can be phrased in terms of probabilistic inference and learning processes [13–15]. In this framework, inference and learning are based on probabilistic models entertained by the brain, representing somatic and environmental variables or states, their dynamical interactions and link to sensory input [16]. Central to this argument is the notion of uncertainty, describing the spread or variance of belief distributions assumed to be implicitly encoded by neural activity. Whatever the exact form of this encoding, one can argue that the variance of the implicit distributions depends in the first place on the quality of data available to the network. In other words, uncertainty represented in neural activity should be a function of input precision, a measure of the reliability of input that determines how much this drives belief updating (box 1).
Box 1.
Input precision changes neural response.
When investigating the functions of a neural network, we usually try to identify which features of the body or world are encoded in the activity of its constituent neurons. Underlying this approach is the assumption that there is a mapping between the activity of the network and the outer states. Because this mapping is indirect—mediated by vicarious input about the system—it licenses an interpretation of neural circuits as internal models inferring causes of their input, like a patch of V1 reflecting the possible presence of a luminous bar projected onto the visual field. Importantly, this mapping is necessarily probabilistic, because the dynamics and interactions between states and sensory signals are noisy. By accounting for this stochasticity, neuronal activity comes to reflect probability distributions over states.
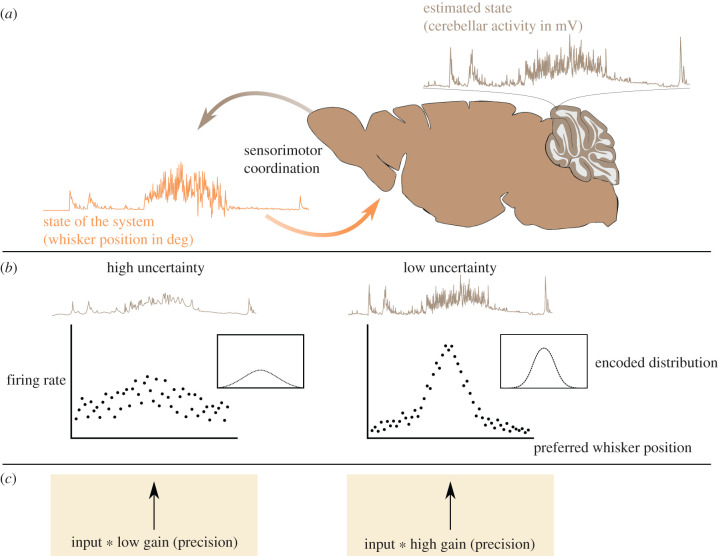
The cerebellum is thought to instantiate internal models for motor and cognitive calibration and adaptation. Neural activity in this region has indeed been observed to accurately encode dynamics of somatic or environmental states, such as whisker position in the mouse cerebellum [17]. These representations in turn contribute to sensorimotor coordination by refining motion [18] and sustaining or altering neural activity in other brain regions, such as the neocortex [19–22] (panel a).
In order for network dynamics in the cerebellum to reflect inferential processes, it is necessary that uncertainty in state estimation influences neural activity. There exist different models of how probability distributions can be encoded by neural populations (see [23] for an example in the cerebellum), some of which highlight the possibility that neural activity scales with the precision of the encoded distribution [24], while becoming sparser as an effect of divisive normalization [25] (panel b). Notably, activity levels in recipient populations result from a combination of input and population gain/responsiveness, which here we associate with input precision. In other words, we argue that (un)certainty in neural representations is determined by the quality of information in the input driving those representations (panel c). Notice that input precision-weighting relies on the capacity of the network to assess this precision. Here we address this possibility and propose that Golgi cells in the cerebellar granular layer mediate the link between neural dynamics and state estimation, by making network excitability sensitive to and reflective of uncertainty in inferential processes.
In biological neural networks, precision naturally translates into population gain [26], which scales or weights presynaptic input and adjusts its capacity to elicit voltage changes in the target population. The underlying idea is that a neural circuit is a system with endogenous or autonomous dynamics, whose activity is not entirely determined by external stimuli; its response to events can contextually vary, conditioned on their precision. Here we assume that inputs reporting more precise representations are associated with higher population gain, that is, a stronger impact on downstream network dynamics—whose output in turn is implicitly linked to more precise distributions.
This brings us to two key points: first, the quality or precision of information is not reducible to its content, meaning that neural mechanisms signalling what is represented can be different from those signalling how it should be represented. For instance, the identity and activity pattern of upstream neurons can be related to the nature of a stimulus encoded, whereas the postsynaptic gain to the amount of information transmitted. Second, the precision of an input, realized as population gain and ultimately translated into patterns of excitation–inhibition balance, is related to the concurrent behavioural context. This can be exemplified through attentional gain modulation in visual and auditory cortex [26–28], where contextualization (weighting) of sensory stimuli by their precision can be accomplished via temporal coincidence of pre and postsynaptic activity, increasing probability of conversion of pre to postsynaptic spikes. In this case, gain-by-synchrony depends on both bottom-up (e.g. intrinsic saliency of the stimulus) and top-down (attentional) effects [29–31], which are a function of behaviour. It follows that the extent to which a stimulus can be relevant for ongoing inference—under a certain behaviour—translates into precision-weighting of that stimulus via gain modulation.
The cerebellar cortex receives input via MFs from virtually every part of the brain. This input is rich, encompassing multiple sensory and motor modalities [32–36] as well as cognitive domains [37]. Moreover, its nature can be both predictive (e.g. anticipatory reward-related signals) and postdictive (e.g. sensory feedback) [38–40], encompassing the entire period of movement execution (e.g. [39]). Consequently, at any given time a huge amount of information can potentially be transmitted to the granular layer via MFs. However, only a fraction of this information is likely to be relevant at any given moment in time; this fact intimates the necessity for the cerebellar cortex to select or prioritize some and not other sources of input, so that only information that is relevant in a particular behavioural context can affect state estimation. For example, while engaged in a visuomotor task, postsynaptic responses to MFs conveying confounding auditory signals might be dampened. With respect to the cerebellar cortex, precision encoded in various extracerebellar regions must be translated and implemented in a common way within the granule cell population, in a manner which is instrumental for state estimation, that is, causing downstream layers to appropriately respond to encoded precision. Accordingly, inhibition in the input layer of the cerebellum appears capable of operating these fundamental operations.
3. Golgi cells underlie precise granular layer computations
The inhibitory network in the cerebellar granular layer is simpler than in cerebral cortical regions, lacking cellular diversity and extremely complicated intra- and inter-areal top-down feedback modulation (cf. [41,42]); nonetheless, it is suited to effectively balance excitation in granule cells. Golgi cells act through both a hyperpolarizing current that lowers granule cell resting potential—efficiently thresholding or gating MF input—and through an increase in membrane conductance or shunting inhibition—associated with faster membrane dynamics and ultimately a biased sensitivity towards synchronous presynaptic activity [43,44]. As a result, Golgi cells can set the excitability or responsiveness of granule cells, approximated by the operative point (position and slope) of their F-I (frequency-current) curve, controlling propagation of MF activity within the cerebellar circuit. From a neural inference perspective, this propagation should be conditional upon the precision of information transmitted, implying that Golgi cell inhibition is sensitive to signals that are most relevant for present belief updating. It is therefore necessary to identify which mechanisms may inform granule cell excitability via inhibition in this context-dependent manner. One distinction mentioned above is between bottom-up and top-down sources of conditioning; beyond this, various biophysical mechanisms might determine how Golgi cells operate. In this section, we highlight those mechanisms that may allow Golgi cell inhibition to perform precision-weighting of the input. First, we see how inhibition sets neural gain to match average levels of activity. Then, we consider time-varying inhibition and its modulation by temporal and spatial properties of the input; in doing so, we characterize the temporal unfolding of MF activity and its spatial organization as a proxy for its intrinsic (bottom-up) precision. Finally, we address mechanisms, such as modulation of Golgi cells by neuromodulators or nucleocortical projections, that do not directly depend or arise from current MF input, yet control how these are transmitted by changing the endogenous state of the granular layer; we refer to these as top-down mechanisms signalling expected precision of the input.
A substantial component of inhibition is tonic, hinging on constantly activated extrasynaptic receptors that are responsive to ambient levels of neurotransmitter concentration [45]. This persistent form of inhibition, arising in part from non-vesicular sources of γ-aminobutyric acid (GABA) [46,47], is favoured by the synaptic organization of the granular layer. Most if not all synaptic connections to granule cells are indeed located in special structures called glomeruli, which form isolated microenvironments where neurotransmitter (both GABA and glutamate) can accumulate and easily diffuse [12,48–50]. In these compartments, ambient concentrations of GABA are sufficient to persistently activate high-affinity α6δ-subunit containing GABAA receptors [51]. In vivo, tonic inhibition minimizes granule cell responsiveness to uncorrelated, temporally scattered inputs [52], while maintaining an exquisite sensitivity to salient (e.g. sensory-evoked) stimuli [53]. Therefore, tonic inhibition appropriately fixes granule cell excitability to match the average levels of MF activity, establishing a slowly changing threshold on neural gain discriminating noise from signals. In mathematical terms, this may be equivalent to a prior over expected precision of the input required for its propagation. At a behavioural level, loss of motor coordination resulting from the disruption of tonic inhibition, for example, owing to alcohol consumption [54], might then reflect global alterations in representational uncertainty.
On top of a persistent inhibitory conductance, feedforward and feedback synaptic loops enable Golgi cells to dynamically modulate granule cells by following rapid variations in network activity [55]—although the exact contribution of these loops is still unknown. Phasic inhibition underlies balanced dynamics of excitation and inhibition in granule cells. Notably, phasic inhibition from Golgi cells can promptly track changes in MF spiking behaviour while, at the same time, accumulate over Golgi cell spike trains to match input firing rates [50,56–58]. The ensuing coordination of excitation and inhibition, on a timescale ranging from few to hundreds of milliseconds, can determine which input patterns elicit responses based on the evoked instantaneous balance. Accordingly, when inhibition is temporally matched to excitation, granule cell firing is reduced but becomes more similar across cells [59]: in vivo, this could favour, for example, selective transmission of the synchronous and invariant component of MF stimuli to Purkinje cells, by virtue of its stronger impact on postsynaptic neurons. Moreover, inhibition can preserve temporal information in granule cell output by rapidly trailing excitation and forcing a sharp integration window of couple of milliseconds for excitatory post-synaptic currents [57]. Overall, balanced dynamics could increase the capacity of granule cells to reliably transmit temporally structured information—here associated with high precision representations. This is in agreement with the general observation that the granular layer faithfully encodes extracerebellar activity [60–64]; and resonates with the idea of a precision-weighting mechanism relying on inhibition and sensitive to bottom-up dynamics, such as synchrony in MF input enhancing temporal coordination across subsets of Golgi cells [65].
Along with temporal features of Golgi cell inhibition, the spatial arrangement of Golgi cell processes may also play a role in the contextualization of incoming information [58]. Notably, there is a mismatch between the narrow granular layer region from which Golgi cells receive excitatory inputs (determined by the dendritic tree), and the region extending hundreds of micrometers over which they exert inhibitory influence (determined by the axonal plexus). In the present discussion, lateral inhibition could be linked to representational precision via its effects over correlations among different streams of MF input. Excitation–inhibition balance at any location in the granular layer could then reflect—via horizontal mixing of Golgi cell signals—the precision of the local information, relative to its surround. In practice, this could lead to an increase of fast correlations among clusters of granule cells that are excited by common MFs, and a simultaneous decrease of slower correlations across competing patches of granular layer—replicating observations in structures that share a similar geometry, like the olfactory bulb [66].
This contextual modulation of granule cell excitability relies on spatial constraints of information driving Golgi and granule cell populations, which in turn depend on different anatomical properties of the network. MFs show substantial anisotropic divergence in the granular layer [34,67], which enables integration of various sources of information at the level of single granule cells, but prevents the emergence of ordered, neocortical-like receptive fields. As a consequence, fast correlations among Golgi cells (and inhibited clusters of granule cells) sharing MF input might be more evident within distributed, scattered groups of cells [68].
Another important anatomical property is the presence of millimetre-long granule-Golgi cell connections mediated by parallel fibres [69]. Parallel fibres have been linked to extended oscillations in the granular layer during rest [70], possibly setting a global pace for network computations and dynamics. Notably, these connections appear to be qualitatively different from local contacts made by ascending granule cell axons onto Golgi cells, which resemble more the faster and stronger MF-Golgi cell synapses [55,71]. It follows that upon localized activation of MF terminals, parallel fibres might preferentially contribute to slow correlation of granule cells across the transverse axis [72], while ascending axons precisely entrain spiking of surrounding Golgi cells. Furthermore, the existence of electrical connections among Golgi cells further increases their sensitivity to temporal coincidence of local excitation, enhancing synchrony or alternatively asynchrony in and between granule cell clusters [65,73,74]. Therefore, different degrees of correlations might coexist in the granular layer, following properties of MF input and connectivity structure within the network, which might result in balanced dynamics of excitation and inhibition reflecting the statistics (precision) of information encoded.
Finally, precision-weighting for state estimation does not depend solely on properties intrinsic to the inputs, but also on selective mechanisms modulating states of the network. Analogously, Golgi cells are both driven by the same MF inputs that elicit activity in granule cells, and are influenced by neural components located within or external to the cerebellum. In vivo, the granular layer is characterized by endogenous activity owing to spontaneous firing of MFs and Golgi cells [62,70,75]; this autonomous state affects the evoked response elicited by a stimulus, and is itself under the control of various mechanisms. In particular, within the cerebellar cortex, climbing fibres, Lugaro cells and Purkinje cells all directly or indirectly modulate Golgi cell activity. [76–78]. From cerebellar nuclei instead, excitatory neurons give rise to MF collaterals innervating glomeruli [79], and inhibitory neurons selectively contact Golgi cells through long-range axons [80]. Moreover, Golgi cells are also sensitive to a variety of neuromodulators including serotonin [81] and noradrenaline [82], which exert opposing actions upon granular layer excitability. Clearly, these sources of input exert very different effects on information processing, and their specific role is still unresolved; nevertheless, this intricate circuit highlights the importance of properly tuning inhibition in the granular layer in order to contextualize incoming information. This is central for putative state estimation in the cerebellar cortex, as it depends not only on current local observations, but also on past inference, systemwise states and coordination with other brain structures.
In conclusion, there appear to be a variety of mechanisms that could inform the granular layer about precision of MF input, irrespective of the extremely diversified nature of those inputs. These mechanisms condition granule cell excitation through Golgi cell inhibition, which constitutes the unique local feedback of the network. In this sense, Golgi cells emerge as a crucial hub for precise state estimation in the cerebellar cortex (figure 2).
Figure 2.
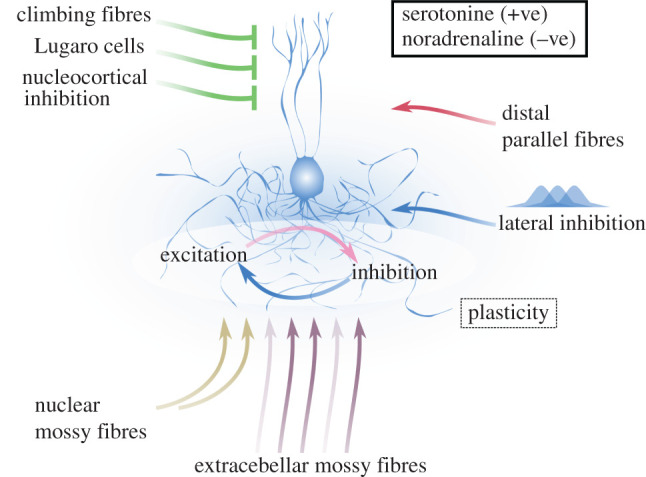
Putative mechanisms for precision-weighting control excitation–inhibition balance in the granular layer. Extracerebellar MFs entering the granular layer are presumed to drive state estimation or belief updating in the cerebellar cortex, whose evoked activity encodes precision-weighted estimates of (somatic and environmental) states of the system. This weighting controls the excitation–inhibition balance in granule cells and is induced by activity of Golgi cells. A variety of factors, which we link to input precision, can condition Golgi cells and therefore granular layer dynamics. These include temporal properties (e.g. synchrony) of MF activity as well as its spatial organization, shaping interactions between neighbouring patches of granular layer via lateral inhibition and distal parallel fibres. At the same time, activity from downstream layers and circuits can dynamically adapt the state of the network by changing Golgi cell activity through climbing fibres, Lugaro cells and nucleocortical inhibition. In addition, the serotoninergic and noradrenergic system too can boost and hinder Golgi cell inhibition respectively, possibly promoting systemwise coordination, for example, between neo- and cerebellar cortex. Finally, present states are also influenced by past inference and experience, through nuclear MF feedbacks and plastic changes in granular layer connectivity [83], determining the impact and spatial organization of extracerebellar MFs input. In this picture, Golgi cells are a crucial hub for state estimation in the cerebellar cortex, contextualizing new MF information and conditioning granule cell response accordingly.
4. Discussion
We have considered how putative state estimation in the cerebellar cortex relies on appropriately tuning of neural dynamics within the granular layer. In this picture, Golgi cell inhibition underlies selective responsiveness or gain of subsets of granule cell population to extracerebellar activity, controlling to what extent the latter drives state estimation. This is a form of input precision-weighting, and depends on information about precision being accessible to the network. Accordingly, several mechanisms related to Golgi cell functioning could provide this information, and therefore adapt granule cell population gain to input precision. In particular, we interpret the optimal signal-to-noise ratio set by tonic inhibition as a prior over expected signal precision, possibly defined by phylogenetic and ontogenetic processes. On the other hand, rapid changes in the excitation–inhibition balance could dynamically accommodate the relative precision of the excitatory drive as a function of its intrinsic properties and top-down modulations, hence controlling precision encoded in the granular layer output (figure 3). The importance of this balance for state estimation is intimated by the remarkable number of mechanisms capable of tuning it. Together, these mechanisms could underpin rapid but accurate changes in neural representations, which would afford to the cerebellar cortex sufficient temporal resolution to encode body and environmental state dynamics [84], in service of well-timed, predictive computations throughout the brain [85–87].
Figure 3.
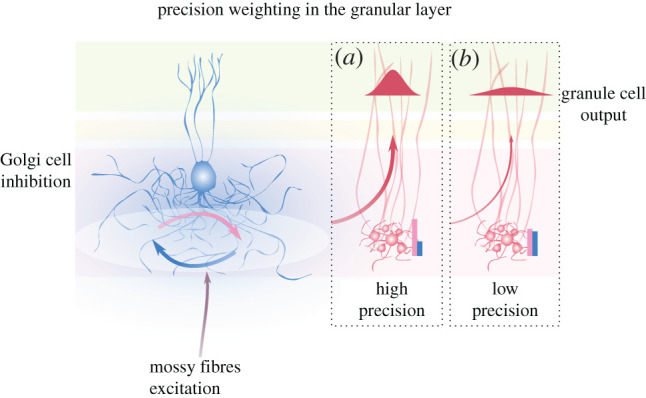
Tuning of the system’s excitability controls precision of representations. Left: the excitation–inhibition balance in the granular layer (circular blue and pink arrows) is a function of both MF input and neural mechanisms signalling its precision by tuning Golgi cell inhibition. Right: within a population, specific neurons might exhibit higher or lower synaptic gain, depending for instance on stimulus overlap with their receptive field, while at a network level, population gain associated with precision of upstream representations dictates the responsiveness of neural ensembles. Golgi cell inhibition sets population gain, such that the balance of excitation and inhibition in granule cells reflects precision-weighted input; and encodes neural representations whose precision determines their transmission and influence on downstream integrative layers via parallel fibres. In (a), MF input is coupled with high population gain and strongly drives granule cells, pushing excitation (pink bar) to overcome inhibition (blue bar). The ensuing population activity then represents states with high precision, exemplified by the red distribution. In (b), MF input convey less reliable information, and the low gain brings inhibition to balance excitation, making the network almost unresponsive. The small network output, in turn, encodes state estimates with low precision, which will not be effective in driving neural inference downstream.
Arguably, neural dynamics underlying state estimation in the cerebellar cortex should necessarily be sensitive to uncertainty associated with inference, as discussed more generally in the context of cortical functioning [26,27,88]. Cortical control of excitation is much more complex than in the granular layer, hinting at a more sophisticated neural inference. Nonetheless, fine-tuning of granule cell activity via Golgi cells also seems to be calibrated by a variety of mechanisms, including top-down signals from cerebellar nuclei, as well as from downstream layers in the cerebellar cortex itself. This in turn should bear on any theory aiming to explain cerebellar computations. In particular, recurrent connectivity within it has usually been neglected or oversimplified; by contrast, a probabilistic inference framework may provide a starting point for explaining this anatomical detail, as shown in this review.
Testing these ideas requires both theoretical and experimental efforts. Here we have assumed a general principle, namely, that precision in neural representations should affect their propagation across the different stages of inference—by tuning population gain. However, future work should aim at investigating the exact nature of this probabilistic encoding throughout the cerebellar circuit. From the experimental side, testing these ideas requires tracking and manipulation of spatiotemporal properties of excitation–inhibition balance in the granular layer, as MF input is transformed into parallel fibre output. The exact shape of ensuing activity patterns depends on many factors, including kinetics variability at the Golgi-granule cell synapses [56], which might result from plastic mechanisms in the granular layer [83]. Previous works have examined the consequences of altering excitation levels in this network, showing a direct link to motor impairment, including tremors, ataxia and reduced reflex adaptation [54,89,90]. Interestingly, Golgi cell ablation alters the spatio-temporal patterns of activity in the granule cell population without necessarily producing overexcitation, as the result of compensatory mechanisms such as reduced N-methyl-D-aspartate activity [91]. This highlights the importance of fine-grained granule cell activity patterns for downstream computations [90]—here argued to be the result of Golgi cell-mediated precision-weighting at the first stage of state estimation in the cerebellar cortex. Ultimately, technical advances in the field [92,93] will make it possible to verify or not verify these ideas.
5. Conclusion
The cerebellum is deemed to support behaviour through predictive processes, adjusting and refining interactions of the organism with its environment. These processes rely on internal models that capture consistent relationships between states of the system and are probabilistic in nature, taking uncertainty into account. Many data confirm this high-level description of the cerebellum, but it remains to be understood how this can emerge from activity of neural networks. In the present work, we link Golgi cell inhibition in the granular layer with mechanisms sensitive to the relative precision of MF input, and the ensuing excitation–inhibition balance in granule cells with a precision-weighted response.
Supplementary Material
Acknowledgements
We thank Laurence Aitchison for feedback on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to the conception of the study. E.R.P. wrote the manuscript and made the figures, all authors contributed to drafting and revision.
Competing interests
We declare we have no competing interests.
Funding
This work was supported from the following sources: Wellcome Trust Neural Dynamics PhD studentship to E.R.P.; Wellcome Trust Investigator Award to P.C.
References
- 1.Paulin MG. 1993. A model of the role of the cerebellum in tracking and controlling movements. Hum. Mov. Sci. 12, 5-16. ( 10.1016/0167-9457(93)90033-L) [DOI] [Google Scholar]
- 2.Liu X, Robertson E, Miall RC. 2003. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J. Neurophysiol. 89, 1223-1237. ( 10.1152/jn.00817.2002) [DOI] [PubMed] [Google Scholar]
- 3.Cerminara NL, Apps R, Marple-Horvat DE. 2009. An internal model of a moving visual target in the lateral cerebellum. J. Physiol. 587, 429-442. ( 10.1113/jphysiol.2008.163337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolpert DM, Miall R, Kawato M. 1998. Internal models in the cerebellum. Trends Cogn. Sci. 2, 338-347. ( 10.1016/S1364-6613(98)01221-2) [DOI] [PubMed] [Google Scholar]
- 5.Ito M. 2006. Cerebellar circuitry as a neuronal machine. Prog. Neurobiol. 78, 272-303. ( 10.1016/j.pneurobio.2006.02.006) [DOI] [PubMed] [Google Scholar]
- 6.Brooks JX, Carriot J, Cullen KE. 2015. Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat. Neurosci. 18, 1310-1317. ( 10.1038/nn.4077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M. 2008. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 9, 304-313. ( 10.1038/nrn2332) [DOI] [PubMed] [Google Scholar]
- 8.Eccles JC, Ito M, János S. 1967. The cerebellum as a neuronal machine. Berlin, Germany: Springer. [Google Scholar]
- 9.Ito M. 1984. The cerebellum and neural control. New York, NY: Raven Press. [Google Scholar]
- 10.Palkovits M, Magyar P, Szentágothai J. 1971. Quantitative histological analysis of the cerebellar cortex in the cat. Brain Res. 32, 15-30. ( 10.1016/0006-8993(71)90152-1) [DOI] [PubMed] [Google Scholar]
- 11.Billings G, Piasini E, Lörincz A, Nusser Z, Silver RA. 2014. Network structure within the cerebellar input layer enables lossless sparse encoding. Neuron 83, 960-974. ( 10.1016/j.neuron.2014.07.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakab RL, Hámori J. 1988. Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat. Embryol. 179, 81-88. ( 10.1007/BF00305102) [DOI] [PubMed] [Google Scholar]
- 13.Knill DC, Pouget A. 2004. The Bayesian brain: the role of uncertainty in neural coding and computation. Trends Neurosci. 27, 712-719. ( 10.1016/j.tins.2004.10.007) [DOI] [PubMed] [Google Scholar]
- 14.Körding KP, Wolpert DM. 2004. Bayesian integration in sensorimotor learning. Nature 427, 244-247. ( 10.1038/nature02169) [DOI] [PubMed] [Google Scholar]
- 15.Fiser J, Berkes P, Orbán G, Lengyel M. 2010. Statistically optimal perception and learning: from behavior to neural representations. Trends Cogn. Sci. 14, 119-130. ( 10.1016/j.tics.2010.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mcnamee D, Wolpert DM. 2019. Internal models in biological control. Annu. Rev. Control, Rob. Auton. Syst. 2, 339-364. ( 10.1146/annurev-control-060117-105206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Augustine GJ, Chadderton P. 2017. Serial processing of kinematic signals by cerebellar circuitry during voluntary whisking. Nat. Commun. 8, 312. ( 10.1038/s41467-017-00312-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker MI, Person AL. 2019. Cerebellar control of reach kinematics for endpoint precision. Neuron 103, 335-348.e5. ( 10.1016/j.neuron.2019.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly RM, Strick PL. 2003. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J. Neurosci. 23, 8432-8444. ( 10.1523/JNEUROSCI.23-23-08432.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proville RD, Spolidoro M, Guyon N, Dugué GP, Selimi F, Isope P, Popa D, Léna C. 2014. Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat. Neurosci. 17, 1233-1239. ( 10.1038/nn.3773) [DOI] [PubMed] [Google Scholar]
- 21.Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N. 2018. A cortico-cerebellar loop for motor planning. Nature 563, 113-116. ( 10.1038/s41586-018-0633-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chabrol FP, Blot A, Mrsic-Flogel TD. 2019. Cerebellar contribution to preparatory activity in motor neocortex. Neuron 103, 506-519.e4. ( 10.1016/j.neuron.2019.05.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paulin MG. 2005. Evolution of the cerebellum as a neuronal machine for Bayesian state estimation. J. Neural Eng. 2, S219-S234. ( 10.1088/1741-2560/2/3/S06) [DOI] [PubMed] [Google Scholar]
- 24.Ma WJ, Beck JM, Latham PE, Pouget A. 2006. Bayesian inference with probabilistic population codes. Nat. Neurosci. 9, 1432-1438. ( 10.1038/nn1790) [DOI] [PubMed] [Google Scholar]
- 25.Orbán G, Berkes P, Fiser J, Lengyel M. 2016. Neural variability and sampling-based probabilistic representations in the visual cortex. Neuron 92, 530-543. ( 10.1016/j.neuron.2016.09.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman H, Friston K. 2010. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 4, 215. ( 10.3389/fnhum.2010.00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao RP. 2005. Bayesian inference and attentional modulation in the visual cortex. NeuroReport 16, 1843-1848. ( 10.1097/01.wnr.0000183900.92901.fc) [DOI] [PubMed] [Google Scholar]
- 28.Heilbron M, Chait M. 2018. Great expectations: is there evidence for predictive coding in auditory cortex? Neuroscience 389, 54-73. ( 10.1016/j.neuroscience.2017.07.061) [DOI] [PubMed] [Google Scholar]
- 29.Fries P, Womelsdorf T, Oostenveld R, Desimone R. 2008. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J. Neurosci. 28, 4823-4835. ( 10.1523/JNEUROSCI.4499-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akam T, Kullmann DM. 2010. Oscillations and filtering networks support flexible routing of information. Neuron 67, 308-320. ( 10.1016/j.neuron.2010.06.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis CM, Ni J, Wunderle T, Jendritza P, Diester I, Fries P. 2020. Gamma-rhythmic input causes spike output. bioRxiv. ( 10.2139/ssrn.3570563) [DOI] [Google Scholar]
- 32.Snider RS, Stowell A. 1944. Receiving areas of the tactile, auditory, and visual systems in the cerebellum. J. Neurophysiol. 7, 331-357. ( 10.1152/jn.1944.7.6.331) [DOI] [Google Scholar]
- 33.Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, Gabrieli JD, Sullivan EV. 1998. Odorant-induced and sniff-induced activation in the cerebellum of the human. J. Neurosci. 18, 8990-9001. ( 10.1523/JNEUROSCI.18-21-08990.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang C-C, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, Hantman AW. 2013. Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. eLife 2, e00400. ( 10.7554/eLife.00400) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chabrol FP, Arenz A, Wiechert MT, Margrie TW, Digregorio DA. 2015. Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat. Neurosci. 18, 718-727. ( 10.1038/nn.3974) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa T, Shimuta M, Häusser M. 2015. Multimodal sensory integration in single cerebellar granule cells in vivo. eLife 4, e12916. ( 10.7554/eLife.12916) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner MJ, Luo L. 2020. Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci. 43, 42-54. ( 10.1016/j.tins.2019.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giovannucci A et al. 2017. Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat. Neurosci. 20, 727. ( 10.1038/nn.4531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. 2017. Cerebellar granule cells encode the expectation of reward. Nature 544, 96-100. ( 10.1038/nature21726) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popa LS, Ebner TJ. 2019. Cerebellum, predictions and errors. Front. Cellular Neurosci. 12, 524. ( 10.3389/fncel.2018.00524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kubota Y. 2014. Untangling GABAergic wiring in the cortical microcircuit. Curr. Opin Neurobiol. 26, 7-14. ( 10.1016/j.conb.2013.10.003) [DOI] [PubMed] [Google Scholar]
- 42.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. 2004. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793-807. ( 10.1038/nrn1519) [DOI] [PubMed] [Google Scholar]
- 43.Hamann M, Rossi DJ, Attwell D. 2002. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33, 625-633. ( 10.1016/S0896-6273(02)00593-7) [DOI] [PubMed] [Google Scholar]
- 44.Mitchell SJ, Silver R. 2003. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433-445. ( 10.1016/S0896-6273(03)00200-9) [DOI] [PubMed] [Google Scholar]
- 45.Farrant M, Nusser Z. 2005. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 6, 215-229. ( 10.1038/nrn1625) [DOI] [PubMed] [Google Scholar]
- 46.Rossi DJ, Hamann M, Attwell D. 2003. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. 548, 97-110. ( 10.1113/jphysiol.2002.036459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Yoon B-E, Berglund K, Oh S-J, Park H, Shin H-S, Augustine GJ, Lee CJ. 2010. Channel-mediated tonic GABA release from glia. Science 330, 790-796. ( 10.1126/science.1184334) [DOI] [PubMed] [Google Scholar]
- 48.Digregorio DA, Nusser Z, Silver R. 2002. Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron 35, 521-533. ( 10.1016/S0896-6273(02)00787-0) [DOI] [PubMed] [Google Scholar]
- 49.Nielsen TA, Digregorio DA, Silver R. 2004. Modulation of glutamate mobility reveals the mechanism underlying slow-rising AMPAR EPSCs and the diffusion coefficient in the synaptic cleft. Neuron 42, 757-771. ( 10.1016/j.neuron.2004.04.003) [DOI] [PubMed] [Google Scholar]
- 50.Rossi DJ, Hamann M. 1998. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron 20, 783-795. ( 10.1016/S0896-6273(00)81016-8) [DOI] [PubMed] [Google Scholar]
- 51.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. 2001. Adaptive regulation of neuronal excitability by a voltage- independent potassium conductance. Nature 409, 88-92. ( 10.1038/35051086) [DOI] [PubMed] [Google Scholar]
- 52.Chadderton P, Margrie TW, Häusser M. 2004. Integration of quanta in cerebellar granule cells during sensory processing. Nature 428, 856-860. ( 10.1038/nature02442) [DOI] [PubMed] [Google Scholar]
- 53.Duguid I, Branco T, London M, Chadderton P, Häusser M. 2012. Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J. Neurosci. 32, 11 132-11 143. ( 10.1523/JNEUROSCI.0460-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. 2005. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat. Neurosci. 8, 339-345. ( 10.1038/nn1398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cesana E, Pietrajtis K, Bidoret C, Isope P, D’Angelo E, Dieudonne S, Forti L. 2013. Granule cell ascending axon excitatory synapses onto Golgi cells implement a potent feedback circuit in the cerebellar granular layer. J. Neurosci. 33, 12 430-12 446. ( 10.1523/JNEUROSCI.4897-11.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crowley JJ, Fioravante D, Regehr WG. 2009. Dynamics of fast and slow inhibition from cerebellar Golgi cells allow flexible control of synaptic integration. Neuron 63, 843-853. ( 10.1016/j.neuron.2009.09.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanichay RT, Silver RA. 2008. Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J. Neurosci. 28, 8955-8967. ( 10.1523/JNEUROSCI.5469-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Angelo E, Solinas S, Mapelli J, Gandolfi D, Mapelli L, Prestori F. 2013. The cerebellar Golgi cell and spatiotemporal organization of granular layer activity. Front. Neural Circuits 7, 93. ( 10.3389/fncir.2013.00093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duguid I, Branco T, Chadderton P, Arlt C, Powell K, Häusser M. 2015. Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proc. Natl Acad. Sci. USA 112, 13 099-13 104. ( 10.1073/pnas.1510249112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jörntell H, Ekerot C-F. 2006. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J. Neurosci. 26, 11 786-11 797. ( 10.1523/JNEUROSCI.2939-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, Häusser M. 2007. High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 450, 1245-1248. ( 10.1038/nature05995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arenz A, Silver RA, Schaefer AT, Margrie TW. 2008. The contribution of single synapses to sensory representation in vivo. Science 321, 977-980. ( 10.1126/science.1158391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bengtsson F, Jörntell H. 2009. Sensory transmission in cerebellar granule cells relies on similarly coded mossy fiber inputs. Proc. Natl Acad. Sci. USA 106, 2389-2394. ( 10.1073/pnas.0808428106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Powell K, Mathy A, Duguid I, Häusser M. 2015. Synaptic representation of locomotion in single cerebellar granule cells. eLife 4, e07290. ( 10.7554/eLife.07290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Welie I, Roth A, Ho SS, Komai S, Häusser M. 2016. Conditional spike transmission mediated by electrical coupling ensures millisecond precision-correlated activity among interneurons in vivo. Neuron 90, 810-823. ( 10.1016/j.neuron.2016.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giridhar S, Doiron B, Urban NN. 2011. Timescale-dependent shaping of correlation by olfactory bulb lateral inhibition. Proc. Natl Acad. Sci. USA 108, 5843-5848. ( 10.1073/pnas.1015165108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sultan F. 2001. Distribution of mossy fibre rosettes in the cerebellum of cat and mice: evidence for a parasagittal organization at the single fibre level. Eur. J. Neurosci. 13, 2123-2130. ( 10.1046/j.0953-816x.2001.01593.x) [DOI] [PubMed] [Google Scholar]
- 68.Gurnani H, Silver RA. 2019. Coordination of inhibitory Golgi cell population activity in the cerebellar cortex. Cosyne Abstracts 2019.
- 69.Harvey RJ, Napper RMA. 1988. Quantitative study of granule and Purkinje cells in the cerebellar cortex of the rat. J. Comp. Neurol. 274, 151-157. ( 10.1002/cne.902740202) [DOI] [PubMed] [Google Scholar]
- 70.Vos BP, Maex R, Volny-Luraghi A, Schutter ED. 1999. Parallel fibers synchronize spontaneous activity in cerebellar Golgi cells. J. Neurosci. 19, RC6. ( 10.1523/JNEUROSCI.19-11-j0003.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watanabe D, Nakanishi S. 2003. mGluR2 postsynaptically senses granule cell inputs at Golgi cell synapses. Neuron 39, 821-829. ( 10.1016/S0896-6273(03)00530-0) [DOI] [PubMed] [Google Scholar]
- 72.Sudhakar SK, Hong S, Raikov I, Publio R, Lang C, Close T, Guo D, Negrello M, De Schutter E. 2017. Spatiotemporal network coding of physiological mossy fiber inputs by the cerebellar granular layer. PLoS Comput. Biol. 13, 1-35. ( 10.1371/journal.pcbi.1005754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dugué GP, Brunel N, Hakim V, Schwartz E, Chat M, Lévesque M, Courtemanche R, Léna C, Dieudonné S. 2009. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron 61, 126-139. ( 10.1016/j.neuron.2008.11.028) [DOI] [PubMed] [Google Scholar]
- 74.Vervaeke K, Lörincz A, Gleeson P, Farinella M, Nusser Z, Silver RA. 2010. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron 67, 435-451. ( 10.1016/j.neuron.2010.06.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Kan PL, Gibson AR, Houk JC. 1993. Movement-related inputs to intermediate cerebellum of the monkey. J. Neurophysiol. 69, 74-94. . ( 10.1152/jn.1993.69.1.74) [DOI] [PubMed] [Google Scholar]
- 76.Nietz AK, Vaden JH, Coddington LT, Overstreet-Wadiche L, Wadiche JI. 2017. Non-synaptic signaling from cerebellar climbing fibers modulates Golgi cell activity. eLife 6, e29215. ( 10.7554/eLife.29215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dieudonné S, Dumoulin A. 2000. Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J. Neurosci. 20, 1837-1848. ( 10.1523/JNEUROSCI.20-05-01837.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Witter L, Rudolph S, Pressler RT, Lahlaf SI, Regehr WG. 2016. Purkinje cell collaterals enable output signals from the cerebellar cortex to feed back to Purkinje cells and interneurons. Neuron 91, 312-319. ( 10.1016/j.neuron.2016.05.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Houck BD, Person AL. 2015. Cerebellar premotor output neurons collateralize to innervate the cerebellar cortex. J. Comp. Neurol. 523, 2254-2271. ( 10.1002/cne.23787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ankri L, Husson Z, Pietrajtis K, Proville R, Léna C, Yarom Y, Dieudonné S, Uusisaari MY. 2015. A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. eLife 4, e06262. ( 10.7554/eLife.06262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fleming E, Hull C. 2019. Serotonin regulates dynamics of cerebellar granule cell activity by modulating tonic inhibition. J. Neurophysiol. 121, 105-114. ( 10.1152/jn.00492.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lanore F, Rothman JS, Coyle D, Silver RA. 2019. Norepinephrine controls the gain of the inhibitory circuit in the cerebellar input layer. bioRxiv. ( 10.1101/567172) [DOI] [Google Scholar]
- 83.Hansel C, Linden DJ, D’Angelo E. 2001. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat. Neurosci. 4, 467-475. ( 10.1038/87419) [DOI] [PubMed] [Google Scholar]
- 84.Therrien AS, Bastian AJ. 2015. Cerebellar damage impairs internal predictions for sensory and motor function. Curr. Opin. Neurobiol. 33, 127-133. ( 10.1016/j.conb.2015.03.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hull C. 2020. Prediction signals in the cerebellum: beyond supervised motor learning. eLife 9, e54073. ( 10.7554/eLife.54073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Narayanan S, Thirumalai V. 2019. Contributions of the cerebellum for predictive and instructional control of movement. Curr. Opin. Physiol. 8, 146-151. ( 10.1016/j.cophys.2019.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li N, Mrsic-Flogel TD. 2020. Cortico-cerebellar interactions during goal-directed behavior. Curr. Opin. Neurobiol. 65, 27-37. ( 10.1016/j.conb.2020.08.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu AJ, Dayan P. 2005. Inference, attention, and decision in a Bayesian neural architecture. In Advances in neural information processing systems 17 (eds LK Saul, Y Weiss, L Bottou), pp. 1577–1584. New York, NY: MIT Press.
- 89.Chiu C-S, Brickley S, Jensen K, Southwell A, Mckinney S, Cull-Candy S, Mody I, Lester HA. 2005. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J. Neurosci. 25, 3234-3245. ( 10.1523/JNEUROSCI.3364-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seja P et al. 2012. Raising cytosolic Cl− in cerebellar granule cells affects their excitability and vestibulo-ocular learning. EMBO J. 31, 1217-1230. ( 10.1038/emboj.2011.488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watanabe D et al. 1998. Ablation of cerebellar Golgi cells disrupts synaptic integration involving GABA inhibition and NMDA receptor activation in motor coordination. Cell 95, 17-27. ( 10.1016/S0092-8674(00)81779-1) [DOI] [PubMed] [Google Scholar]
- 92.Griffiths VA et al. 2020. Real-time 3D movement correction for two-photon imaging in behaving animals. Nat. Methods 17, 741-748. ( 10.1038/s41592-020-0851-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Antonini A et al. 2020. Extended field-of-view ultrathin microendoscopes for high-resolution two-photon imaging with minimal invasiveness. eLife 9, e58882. ( 10.7554/eLife.58882) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.


