Abstract
Aims
Little is known about the relation between the long-term joint exposure to various ambient air pollutants and the incidence of heart failure (HF). We aimed to assess the joint association of various air pollutants with HF risk and examine the modification effect of the genetic susceptibility.
Methods and results
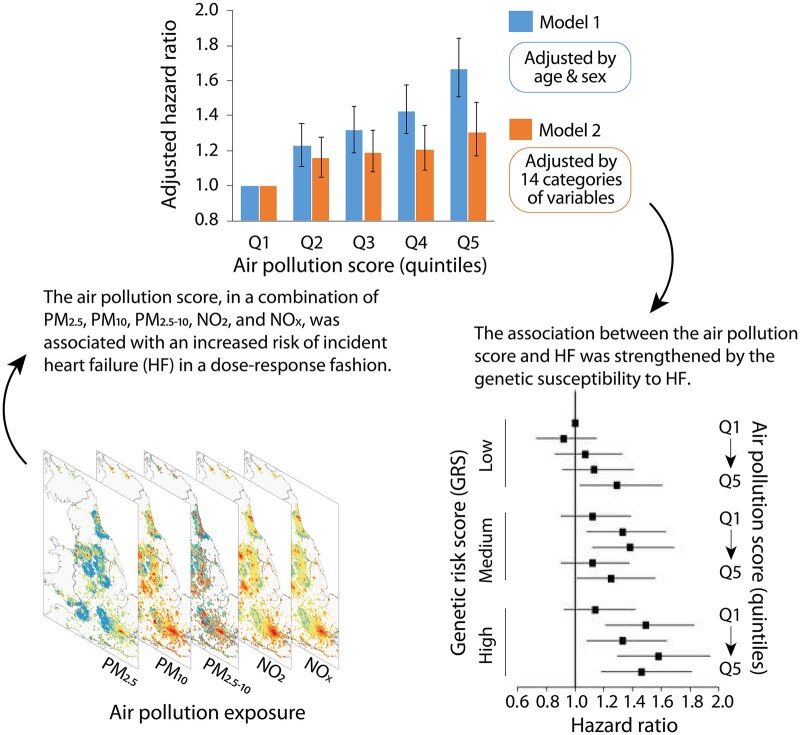
This study included 432 530 participants free of HF, atrial fibrillation, or coronary heart disease in the UK Biobank study. All participants were enrolled from 2006 to 2010 and followed up to 2018. The information on particulate matter (PM) with diameters ≤2.5 µm (PM2.5), ≤10 µm (PM10), and between 2.5 and 10 µm (PM2.5–10) as well as nitrogen oxides (NO2 and NOx) was collected. We newly proposed an air pollution score to assess the joint exposure to the five air pollutants through summing each pollutant concentration weighted by the regression coefficients with HF from single-pollutant models. We also calculated the weighted genetic risk score of HF. During a median of 10.1 years (4 346 642 person-years) of follow-up, we documented 4201 incident HF. The hazard ratios (HRs) [95% confidence interval (CI)] of HF for a 10 µg/m3 increase in PM2.5, PM10, PM2.5–10, NO2, and NOx were 1.85 (1.34–2.55), 1.61 (1.30–2.00), 1.13 (0.80–1.59), 1.10 (1.04–1.15), and 1.04 (1.02–1.06), respectively. We found that the air pollution score was associated with an increased risk of incident HF in a dose–response fashion. The HRs (95% CI) of HF were 1.16 (1.05–1.28), 1.19 (1.08–1.32), 1.21 (1.09–1.35), and 1.31 (1.17–1.48) in higher quintile groups compared with the lowest quintile of the air pollution score (P trend <0.001). In addition, we observed that the elevated risk of HF associated with a higher air pollution score was strengthened by the genetic susceptibility to HF.
Conclusion
Our results indicate that the long-term joint exposure to various air pollutants including PM2.5, PM10, PM2.5–10, NO2, and NOx is associated with an elevated risk of incident HF in an additive manner. Our findings highlight the importance to comprehensively assess various air pollutants in relation to the HF risk.
Keywords: Air pollution, Heart failure, Joint association, Cohort
Graphical Abstract
See page 1592 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa1105)
Introduction
Long-term ambient air pollution exposure remains a major public health threat worldwide.1 Numerous studies have associated air pollution with increased risks of cardiovascular diseases and related mortality.2 , 3 Of note, the hazard of air pollution on patients with chronic coronary syndromes has been included in 2019 European Society of Cardiology (ESC) Guidelines for the first time.4 In particular, heart failure (HF) is a highly prevalent clinical syndrome that contributes to a common cause of mortality from cardiovascular diseases.5 , 6 Several epidemiological studies have shown that short-term exposure to air pollution is associated with an increased risk of mortality and rates of hospitalization due to HF. In addition, patients with congestive HF are at greater risks of pollution-related hospitalizations for ischaemic heart disease7 and chronic obstructive pulmonary disorders.8 However, the evidence on the relation between long-term exposure to air pollution and the incidence of HF remains lacking.9 , 10
Notably, the limited previous studies assessing the association between air pollution and the risk of HF were mainly focused on individual air pollutants, including particulate matter (PM) with diameters ≤2.5 µm (PM2.5), ≤10 µm (PM10), and nitrogen dioxide (NO2).11 , 12 In reality, ambient air pollution consists of a mixture of particles and gaseous pollutants, and their combined health effects may differ from those of the individual air pollutants.13 , 14 However, no prospective cohort study has jointly examined various air pollutants in relation to the risk of incident HF.
In addition, it has been established that both genetic and environmental factors may contribute to HF. In recent years, emerging evidence has revealed that the genetic susceptibility might interact with environmental factors on cardio-metabolic outcomes.15 , 16 Importantly, several studies have indicated that the association between ambient air pollution exposure and cardiovascular diseases could be modified by genetic variations.17 However, whether the genetic predisposition may modify the association between the joint exposure to various air pollutants and HF remains unknown.
Therefore, taking advantage the comprehensive information of air pollution and genetic variations in the UK Biobank study, we newly proposed an air pollution score to assess the joint exposure to a combination of air pollutants including PM2.5, PM10, and PM with diameters between 2.5 and 10 µm (PM2.5–10) as well as nitrogen oxides (NO2 and NOx). We tested the association of the air pollution score with the risk of incident HF and further investigated the joint association of the air pollution score and genetic susceptibility with HF and explored the potential gene-air pollution interaction.
Methods
Study design and participants
The UK Biobank is a population-based prospective cohort study, with the study protocol being described in detail previously.18 Briefly, about 0.5 million residents aged 40–69 years were enrolled from 2006 through 2010 across the UK. The baseline summary characteristics of the cohort are provided on the UK Biobank’s website (www.ukbiobank.ac.uk). The information on lifestyle and health data, physical measurements, and biological samples were collected. The UK Biobank study was approved by the North West Multicenter Research Ethical Committee. All participants provided informed written consent.
Among the 502 506 participants with available data in the current study, we excluded those with HF, atrial fibrillation, or coronary heart disease at baseline. A total of 432 530 participants who had complete data for exposure to air pollution were included in the final analysis. In addition, only participants of European descent were included in the genetic analysis (n = 327 151).
Assessment of outcomes
Prevalent HF was defined based on self-reported information and hospital inpatient records (Supplementary material online, Table S1).19 Date and diagnosis for hospital admissions were determined through record linkage to Health Episode Statistics in England and Wales and the Scottish Morbidity Records in Scotland. Incident HF was ascertained as a hospital admission with International Classification of Diseases, Tenth Revision codes of I11.0, I13.0, I13.2, I50.0, I50.1, and I50.9.
Air pollution estimates
The annual average concentrations of PM2.5, PM10, PM2.5–10, NO2, and NOx were estimated with a Land Use Regression (LUR) model developed from the European Study of Cohorts for Air Pollution Effects project.20 , 21 The spatial variations of annual average air pollutant concentrations were calculated using the LUR model including the geospatial predictor variables generated from the Geographic Information System such as traffic, land use, and topography. Air pollution exposures of all participants in the UK Biobank were linked to the records through residential addresses given at the baseline visit. The exposure data of PM2.5, PM2.5–10, and NOx were collected in 2010, while annual concentration data of NO2 and PM10 were available for several years (2005, 2006, 2007, and 2010 for NO2 and 2007 and 2010 for PM10). The averaged values of NO2 and PM10 were included in the analysis. We made a comparison between air pollution exposures (PM2.5, PM10, NO2, NOx) in the current study and the spatial distributions of air pollutants from the pubic UK Air Information Resources (https://uk-air.defra.gov.uk/data/pcm-data). The results are shown in Supplementary material online, Figure S1. The distribution patterns of air pollution exposures in the current study are similar with the spatial distributions of air pollutants from the public UK Air Information Resources.
Definition of the air pollution score
We created a weighted air pollution score through adding concentrations of the five air pollutants, weighted by the multivariable-adjusted risk estimates (β coefficients) on HF in the present analysis. The equation was: air pollution score = (β [PM2.5] × PM2.5 + β [PM10] × PM10 + β [PM2.5–10] × PM2.5–10 + β [NO2] × NO2 + β [NOx] × NOx) × (5/sum of the β coefficients). The air pollution score ranged from 49.6 to 177.6, as a higher score indicating higher exposure to ambient air pollution. Participants were divided into five groups according to the quintiles of the air pollution score.
In the sensitive analysis, we further constructed a weighted air pollution score based on the four air pollutants without PM2.5–10: air pollution score = (β [PM2.5] × PM2.5 + β [PM10] × PM10 + β [NO2] × NO2 + β [NOx] × NOx) × (4/sum of the β coefficients).
Definition of the genetic risk score
Detailed information about genotyping, imputation, and quality control in the UK Biobank study has been described previously.22 We created a genetic risk score (GRS) for HF using 12 single-nucleotide polymorphisms (SNPs) based on a previous genome-wide association study (Supplementary material online, Table S2).23 A weighted method was used to calculate the HF GRS. Each SNP was recoded as 0, 1, or 2 according to the number of risk alleles; and then multiplied by the risk estimate (β coefficient) on HF obtained from the previous study to calculate the GRS: GRS = (β1 × SNP1 + β2 × SNP2 +…+ β12 × SNP12) × (12/sum of the β coefficients).24 The HF GRS ranged from 1.4 to 18.3, with a higher score indicating a higher genetic predisposition to HF. We classified participants into three groups of low (tertile 1), intermediate (tertile 2), and high (tertile 3) genetic risk of HF.
Measurements of covariates
We included age, sex, race, Townsend Deprivation index, smoking status, alcohol consumption status, physical activity, body mass index (BMI), healthy diet score, blood pressure levels, and prevalent diseases as potential confounders. The Metabolic Equivalent Task (MET) minutes based on items from short International Physical Activity Questionnaire (IPAQ) was adopted to assess physical activity. A healthy diet score was calculated based on the following diet factors: vegetable intake ≥ four tablespoons/day; fruit intake ≥ three pieces/day; fish intake ≥ twice/week; unprocessed red meat intake ≤ twice/week; and processed meat intake ≤ twice/week. Each one point was given for each favourable diet factor, and the healthy diet score ranged from 0 to 5. In addition, height and weight were measured by trained nurses during the baseline assessment centre visit, and BMI was calculated through dividing weight in kilograms by the square of height in meters. The history of hypertension, diabetes, and respiratory diseases (chronic obstructive pulmonary disease [COPD] and emphysema) was based on self-reported information and medical records. Self-reported information on medication use including cholesterol-lowering medication, blood pressure medication, and insulin was also collected. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at baseline using standardized procedures by trained nurses. The mean values of two automated or manual measurements were used.
Statistical analysis
Survival time for each participant was calculated as the duration from the response date of baseline survey through the time of incident HF, death, or date of censoring, whichever came first. The Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI). The proportional hazards assumption was tested using Schoenfeld residuals. Several potential confounders were adjusted in these models, including age (continuous), sex (male, female), race (white European, mixed, South Asian, black, others), UK Biobank assessment centre, Townsend Deprivation index (continuous), alcohol consumption status (current, former, never, missing), smoking status (current, former, never, missing), BMI (kg/m2, continuous), physical activity (MET-minutes/week, continuous), healthy diet score (0, 1, 2, 3, 4, 5), SBP (continuous), DBP (continuous), prevalent diabetes (yes/no), and prevalent hypertension (yes/no). For analyses of genetic data, we further adjusted for the genotyping array and the first ten genetic principal components. In the analysis of individual air pollutants, a single air pollutant was included in the model. The linear trend test was performed by treating the variables continuously. Missing data were coded as a missing indicator category for categorical variables such as smoking and with mean values for continuous variables.
To validate our results, we performed a 10-fold cross-validation analysis.25 , 26 The cohort was randomly divided into 10 batches. In each run, one of the 10 samples was used as the testing data and the remaining nine samples as training data. The Cox regression was applied to the training dataset to obtain the regression coefficient of each air pollutant for the risk of HF and the subsequent air pollution score. Then, the air pollution score was used in the testing dataset to calculate the HRs and 95% CI for HF risk. The process was repeated 10 times, with each of the 10 samples used once as the testing data. A fixed‐effects meta‐analysis was conducted to obtain the comprehensive HR of these 10 groups.
To evaluate whether the genetic susceptibility of HF may modify the association between the air pollution score and HF incidence, we tested the gene–air pollution interaction by setting variable cross-product terms of the air pollution score with the HF GRS in the models.
Several sensitivity analyses were conducted to examine the robustness of our findings. First, since PM2.5–10 was not significantly associated with the risk of incident HF, we excluded PM2.5–10 and only incorporated PM2.5, PM10, NO2, and NOx in the air pollution score. We also additionally adjusted for average total household income (<£18 000, £18 000–£30 999, £31 000–£51 999, £52 000–£100 000, >£100 000, and ‘do not know’ or missing) and education years in the models. In addition, we further adjusted for the history of medication use including anti-hypertensive medication (yes/no), insulin (yes/no), and cholesterol-lowering medication (yes/no) as well as respiratory diseases (yes/no) at baseline. Moreover, we further adjusted for the average 24-h sound level of noise pollution (dB) in the model. Furthermore, we restricted incident HF cases to >2 years from the baseline survey to minimize the reverse causality effect on the observed associations. Finally, we conducted a sensitivity analysis including participants living in the current address for at least five years to assess the long-term effect of air pollution on HF.
All analyses were performed using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) and R software (version 3.5.1). All P-values for the tests were two-sided and P-values <0.05 were considered as statistically significant.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The baseline characteristics of the participants according to incident HF are shown in Table 1. Participants who had incident HF were older, mainly males, and with high BMI and Townsend Deprivation index compared with those without incident HF. In addition, they were more likely to be current smokers but less likely to be current drinkers or have a healthy diet. Moreover, participants with incident HF had a higher prevalence of diabetes and hypertension as well as blood pressure levels at baseline. The mean (standard deviation [SD]) estimates of PM2.5, PM10, PM2.5–10, NO2, and NOx were 10.2 (1.1), 19.4 (1.9), 6.5 (0.9), 30.0 (9.1), and 46.1 (16.8) µg/m3, respectively, among participants with incident HF; and the corresponding concentrations were 10.0 (1.1), 19.3 (2.0), 6.4 (0.9), 29.3 (9.3), 43.9 (15.6) µg/m3 for those without incident HF. The Spearman correlation coefficients among the five air pollutants are shown in Supplementary material online, Table S3.
Table 1.
Baseline characteristics of participants in the UK Biobank study
| Incident heart failure |
||
|---|---|---|
| Yes (N = 4201) | No (N = 428 329) | |
| Characteristics | ||
| Age (years) | 61.6 (6.4) | 56.2 (8.1) |
| Sex, male (%) | 60.2 | 44.0 |
| Race, white (%) | 93.8 | 93.9 |
| Body mass index (kg/m2) | 29.5 (5.9) | 27.3 (4.7) |
| Townsend Deprivation index | −0.8 (3.3) | −1.4 (3.0) |
| Current drinkers (%) | 86.4 | 92.0 |
| Current smokers (%) | 17.0 | 10.2 |
| MET (min/week) | 2478.9 (2283.5) | 2666.8 (2437.3) |
| Healthy diet score (%) | ||
| 0–1 | 13.5 | 11.6 |
| 2–3 | 52.7 | 48.8 |
| 4–5 | 33.8 | 39.6 |
| Prevalent diabetes (%) | 15.2 | 4.5 |
| Prevalent hypertension (%) | 49.7 | 25.5 |
| Systolic blood pressure (mmHg) | 145.6 (20.0) | 137.7 (18.6) |
| Diastolic blood pressure (mmHg) | 83.7 (11.0) | 82.4 (10.1) |
| PM2.5 (µg/m3) | 10.2 (1.1) | 10.0 (1.1) |
| PM10 (µg/m3) | 19.4 (1.9) | 19.3 (2.0) |
| PM2.5–10 (µg/m3) | 6.5 (0.9) | 6.4 (0.9) |
| NO2 (µg/m3) | 30.0 (9.1) | 29.3 (9.3) |
| NOx (µg/m3) | 46.1 (16.8) | 43.9 (15.6) |
Data are mean (SD) unless otherwise indicated. MET, Metabolic Equivalent Task; PM2.5, particular matter with aerodynamic diameter ≤2.5 µm; PM10, particular matter with an aerodynamic diameter ≤10 µm; PM2.5–10, particular matter with an aerodynamic diameter between 2.5 and 10 µm; NO2, nitrogen dioxide; NOx, nitrogen oxides.
During a median of 10.1 years (4 346 642 person-years) of follow-up, we documented 4201 incident HF. The associations between individual air pollutants and HF are shown in Table 2. We observed that PM2.5, PM10, NO2, and NOx each associated with an increased risk of HF in the models adjusted for age, sex, race, UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption, smoking status, BMI, physical activity, healthy diet score, diabetes, hypertension, SBP, and DBP. The HRs (95% CI) of HF for a 10-µg/m3 increase in PM2.5, PM10, NO2, and NOx were 1.85 (1.34–2.55), 1.61 (1.30–2.00), 1.10 (1.04–1.15), and 1.04 (1.02–1.06), respectively. In addition, we observed a non-significant elevated risk of HF associated with a 10-µg/m3 increase in PM2.5–10 (HR = 1.13, 95% CI, 0.80–1.59).
Table 2.
Adjusted hazard ratios a and 95% confidence interval for air pollution concentrations with the risk of incident heart failure in the UK Biobank study
| Air pollution concentrations (quintiles) |
HR (95% CI) for a 10 µg/m3 increase | P for trend | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| PM2.5 | 1.00 | 1.00 (0.90–1.11) | 1.05 (0.94–1.16) | 1.16 (1.05–1.29) | 1.14 (1.02–1.27) | 1.85 (1.34–2.55) | <0.001 |
| PM10 | 1.00 | 1.17 (1.06–1.30) | 1.24 (1.12–1.37) | 1.16 (1.04–1.29) | 1.31 (1.15–1.48) | 1.61 (1.30–2.00) | <0.001 |
| PM2.5–10 | 1.00 | 1.03 (0.93–1.13) | 1.07 (0.97–1.18) | 1.04 (0.94–1.15) | 1.02 (0.93–1.13) | 1.13 (0.80–1.59) | 0.48 |
| NO2 | 1.00 | 1.11 (1.00–1.23) | 1.18 (1.06–1.30) | 1.23 (1.10–1.37) | 1.24 (1.09–1.42) | 1.10 (1.04–1.15) | <0.001 |
| NOx | 1.00 | 1.09 (0.98–1.21) | 1.20 (1.08–1.33) | 1.11 (1.00–1.24) | 1.29 (1.16–1.45) | 1.04 (1.02–1.06) | <0.001 |
CI, confidence interval; HR, hazard ratio; NO2, nitrogen dioxide; NOx, nitrogen oxides; PM2.5, particular matter with aerodynamic diameter ≤2.5 µm; PM10, particular matter with an aerodynamic diameter ≤10 µm; PM2.5–10, particular matter with an aerodynamic diameter between 2.5 and 10 µm.
Adjusted for age, sex, race (white European, mixed, South Asian, black, others), UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption (current, former, never, missing), smoking status (current, former, never, missing), body mass index (kg/m2), physical activity (MET-min/week), healthy diet score (0, 1, 2, 3, 4, 5), diabetes (yes/no), hypertension (yes/no), systolic blood pressure (mmHg), and diastolic blood pressure (mmHg).
Table 3 shows the association between the air pollution score and HF. We found that the air pollution score was significantly associated with a higher risk of incident HF in the age, sex-adjusted, and multivariate-adjusted models. In the age- and sex-adjusted model, a 67% higher risk of incident HF was observed in the highest quintile vs. the lowest quintile of the air pollution score (P trend <0.001). After further adjustment for race, UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption, smoking status, BMI, physical activity, healthy diet score, diabetes, hypertension, SBP, and DBP, the air pollution score was associated with an increased risk of incident HF in a dose–response fashion. The HRs (95% CI) of HF were 1.16 (1.05–1.28), 1.19 (1.08–1.32), 1.21 (1.09–1.35), and 1.31 (1.17–1.48) in higher quintile groups compared with the lowest quintile of the air pollution score (P trend <0.001). The results were largely unchanged after excluding PM2.5–10 in the air pollution score (Supplementary material online, Table S4).
Table 3.
Adjusted hazard ratios and 95% confidence interval for the air pollution score with the risk of incident heart failure in the UK Biobank study
| Air pollution score (quintiles) |
P for trend | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| Cases/N | 677/86506 | 819/86 506 | 850/86 506 | 890/86 506 | 965/86 506 | |
| Model 1a | 1.00 | 1.23 (1.11–1.36) | 1.32 (1.19–1.46) | 1.43 (1.30–1.58) | 1.67 (1.51–1.84) | <0.001 |
| Model 2b | 1.00 | 1.16 (1.05–1.28) | 1.19 (1.08–1.32) | 1.21 (1.09–1.35) | 1.31 (1.17–1.48) | <0.001 |
CI, confidence interval; HR, hazard ratio.
Adjusted for age and sex.
Adjusted for age, sex, race (white European, mixed, South Asian, black, others), UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption (current, former, never, missing), smoking status (current, former, never, missing), body mass index (kg/m2), physical activity (MET-min/week), healthy diet score (0, 1, 2, 3, 4, 5), diabetes (yes/no), hypertension (yes/no), systolic blood pressure (mmHg), and diastolic blood pressure (mmHg).
We further performed cross-validation analysis to evaluate the robustness of our findings. The results of the association between the air pollution score and the risk of HF in the cross-validation analysis are shown in the Supplementary material online, Table S5. The validation tests showed consistently significant results as in the current analysis.
The sensitivity analyses also showed that the association between the air pollution score and the risk of incident HF was robust with further adjustment for average total household income and education years or additionally adjustment for anti-hypertensive medication use, insulin use, cholesterol-lowering medication use, and history of respiratory diseases at baseline (Supplementary material online, Table S6). The results did not change appreciably after further adjustment for the average 24-hour sound level of noise pollution (Supplementary material online, Table S6). In addition, after limiting participants with a follow-up time of more than 2 years, the results did not alter appreciably (Supplementary material online, Table S7). Moreover, the results were robust when only participants living in the current address for at least five years were included (Supplementary material online, Table S8).
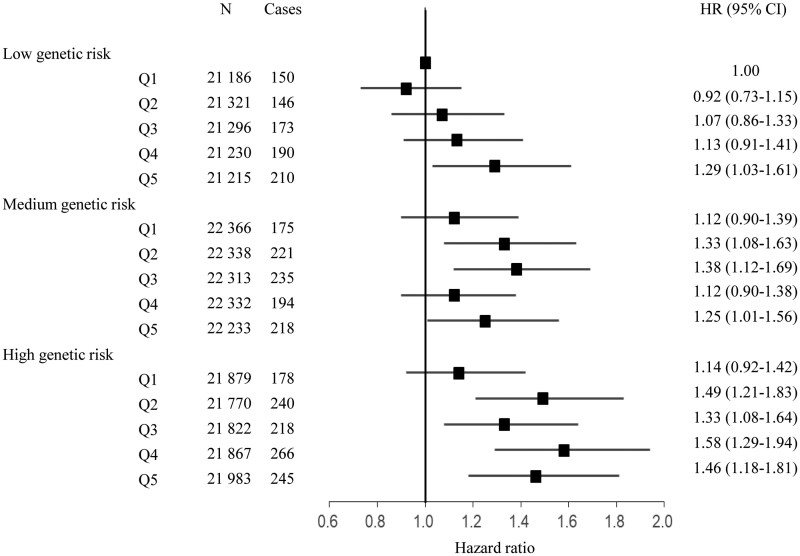
In the models adjusting for sex, age, assessment centre, genotyping batch, and the first ten genetic principal components, we observed a significant association of HF GRS with the risk of incident HF. The HF GRS was associated with a 173% higher risk of incident HF (95% CI, 105–262%). The association remained significant in the multivariable-adjusted model, as the HR (95% CI) of HF was 2.65 (1.99–3.53) for HF GRS (Table 4). We further assessed the joint association of the air pollution score and the HF GRS with the risk of incident HF. We found that participants with high GRS and air pollution score had the highest risk of HF, even though there was no statistically significant interaction between the air pollution score and genetic susceptibility to HF. Participants with high GRS and air pollution score had a 46% greater risk of HF (95% CI, 18%-81%) than participants with low GRS and air pollution score (Figure 1).
Table 4.
Adjusted hazard ratios and 95% confidence interval for heart failure genetic risk score with the risk of incident heart failure in the UK Biobank study
| Heart failure genetic risk score (tertiles) |
|||||
|---|---|---|---|---|---|
| Low genetic risk | Medium genetic risk | High genetic risk | HR (95% CI) for the continuous variable | P for trend | |
| Cases/N | 869/106 248 | 1043/111 582 | 1147/109 321 | ||
| Model 1a | 1.00 | 1.15 (1.05–1.26) | 1.31 (1.20–1.43) | 2.73 (2.05–3.62) | <0.001 |
| Model 2b | 1.00 | 1.15 (1.05–1.25) | 1.30 (1.19–1.42) | 2.65 (1.99–3.53) | <0.001 |
CI, confidence interval; HR, hazard ratio.
Adjusted for age, sex, UK Biobank assessment centre, genotyping batch, and the first 10 genetic principal components.
Adjusted for age, sex, race (white European, mixed, South Asian, black, others), UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption (current, former, never, missing), smoking status (current, former, never, missing), body mass index (kg/m2), physical activity (MET-min/week), healthy diet score (0, 1, 2, 3, 4, 5), diabetes (yes/no), hypertension (yes/no), systolic blood pressure (mmHg), diastolic blood pressure (mmHg), genotyping batch, and the first ten genetic principal components.
Figure 1.
The joint association of the air pollution score (in quintiles) and heart failure genetic risk score with the risk of incident heart failure. The model was adjusted for age, sex, race (white European, mixed, South Asian, black, others), UK Biobank assessment centre, Townsend Deprivation index, alcohol consumption (current, former, never, missing), smoking status (current, former, never, missing), body mass index (kg/m2), physical activity (MET-min/week), healthy diet score (0, 1, 2, 3, 4, 5), diabetes (yes/no), hypertension (yes/no), systolic blood pressure, diastolic blood pressure, genotyping batch, and the first 10 genetic principal components.
Discussion
In this prospective cohort study, we newly created an air pollution score to assess the joint exposure to various air pollutants including PM2.5, PM10, PM2.5–10, NO2, and NOx through summing each pollutant concentration weighted by the regression coefficients with HF from single-pollutant models. We found that the air pollution score was associated with an increased risk of incident HF in a dose–response fashion, independent of traditional risk factors. In addition, we found that the association between the air pollution score and HF risk was strengthened by the genetic susceptibility to HF, even though there was no significant interaction between the air pollution score and HF GRS.
Previous epidemiological studies have associated HF with acute exposures to PM2.5,10 , 27 PM10,10 , 28 , 29 and NO2.10 , 29 The publications with significant associations between short-term PM2.5 exposure and HF are shown in Supplementary material online, Figure S2. An analysis using hospital admissions data of 11.5 million Medicare enrolees showed that PM2.5 was associated with an increased risk for HF hospital admission.30 Furthermore, a time series study in 184 major Chinese cities showed that an increase of 10 μg/m3 in PM2.5 was associated with a 0.27% increase in hospital admissions for HF.31 PM2.5 was also associated with mortality after cardiac transplantation, as the estimated HR per 10 μg/m3 increment in annual exposure to PM2.5 was 1.26 for mortality.32 Moreover, a time-stratified case-crossover analysis in the Vietnamese population showed significant associations of PM2.5 and PM10 with hospital admissions for cardiac failure.33 In addition, PM10 and NO2 were found to be associated with increased risks of hospital cardiac readmissions including HF among myocardial infarction survivors.34 Of note, the associations between NO2 and hospital admissions for HF showed evident heterogeneity across the previous studies.9 Poloniecki et al. 35 did not find a significant association between NO2 and emergency hospital admissions for HF, possibly due to a lack of controlling for confounders such as smoke and blood pressure in the models. However, these studies only assessed the short-term effect of air pollution on HF, the long-term association between air pollution and the incidence of HF is less established. Although several studies have assessed the associations of long-term exposures to PM2.5,36 , 37 PM10,11 , 38 NO2,11 , 39 , 40 and NOx 41 with the incidence of HF, most of the studies only conducted a secondary analysis on HF, while the investigations with HF as the primary outcome are still scarce. Even less studies have comprehensively assessed various air pollutants, and no study has assessed the joint association of different air pollutants with incident HF. In the current study, we comprehensively assessed the associations of long-term exposures to various air pollutants including PM2.5, PM10, PM2.5–10, NO2, and NOx with HF risk and found that the estimated HR risks associated with the individual air pollutants were comparable with several prior investigations showing significant associations of air pollutants with the risk of HF. We have summarized the publications with significant associations between long-term PM2.5 exposure and HF in Supplementary material online, Figure S2. In the present study, PM2.5 showed the strongest association with the risk of HF, followed by PM10, NO2, and NOx. However, we observed an elevated but non-significant risk for incident HF associated with PM2.5–10. Although the reported associations between PM2.5–10 and HF were heterogeneous,37 , 39 , 42 we included PM2.5–10 in the air pollution score to comprehensively assess the joint exposure to various air pollutants.
To our knowledge, this is the first prospective study to assess the association between the joint exposure to various ambient air pollutants and the risk of incident HF. Recently, the importance of evaluating the health effects of multi-pollutant exposures has been increasingly recognized; as the various air pollutants are highly correlated and may be from the same emission sources.43 , 44 We found a relatively stronger association between our newly developed air pollution score and the risk of HF compared with individual air pollutants. Thus, the air pollution score may reflect a more comprehensive measure of the joint exposure to various air pollutants. Previous studies have proposed similar methods by summing air pollutant concentrations to estimate the combined effects of multi-pollutant exposures on human health.45 , 46 For example, Hong et al. 45 examined the combined effects of PM10, NO2, sulphur dioxide, and ozone through summing each air pollutant concentration divided by its mean and found that the combined index had a stronger association with total mortality than individual air pollutants. The use of the statistical approach by adding air pollutant concentrations allows estimating the combined effects of mixtures in the presence of correlation among them. In addition, similar algorithms have been used to assess the joint exposure to other environmental risk factors47 and dietary factors.48 The simple score algorithm makes epidemiological findings easier to be interpreted and may also facilitate public health preventive practice.
Several potential mechanisms might be underlying the observed relations between air pollution and HF. For instance, exposure to air pollution can lead to oxidative stress, systemic inflammation, and autonomic imbalance and then increase blood pressure and decrease cardiac output.12 , 49 , 50 Prolonged or repeated stimulation of these pathways may further result in the progression of endothelial dysfunction, atherosclerosis, diastolic dysfunction, left ventricular hypertrophy, and myocardial fibrosis,50–52 which could eventually increase the risk of HF. In addition, a randomized controlled study of HF (the FILTER-HF Trial) showed that a filter intervention might reduce endothelial dysfunction and B-type natriuretic peptide increases associated with the short-term exposure to diesel exhaust exposure in patients with HF.53 The mechanisms by which the combination of air pollutants might affect the risk of HF remain unclear. Since the association between the air pollution score and HF risk was stronger than those for the individual air pollutants, we assumed that the various air pollutants might have additive effects on the risk of HF through similar biological mechanisms such as oxidative stress and inflammation.49 , 54
We also assessed the joint association of air pollution and genetic susceptibility with the risk of incident HF. We observed that the risk of HF associated with a higher air pollution score was strengthened by high genetic risk, even though the test on the interaction between the air pollution score and genetic susceptibility was not significant. The proportion of HF risk explained by the variants was <10%, which may partially explain the negative interaction. Existing evidence suggests the potential mechanisms for the risk of HF associated with a higher air pollution score strengthened by a high genetic risk. The identified genetic loci for HF in the genome-wide association study were associated with risk factors and traits related to left ventricular structure and function. The genetic loci associated with reduced left ventricular systolic function or atrial fibrillation were also related to the processes of cardiac development, protein homoeostasis, and cellular senescence. In addition, the observed relations between air pollution and HF might be through these aforementioned mechanical changes. Therefore, we assumed that air pollutants and genetic variations for HF risk might have additive effects on the risk of HF through at least certain overlapped biological mechanisms related to cardiac function.
Strengths and limitations
Several strengths of this study include the prospective design and large sample size. Our study is the first prospective study to assess the association between the joint exposure to various ambient air pollutants and the risk of incident HF. The novel findings on the relation between the combined air pollutants and HF may prompt the development of new prevention strategy by considering various air pollutants together. In addition, with HF as the primary disease outcome, the results might provide a global picture about the health effect of air pollution on HF. Moreover, we for the first time examined the joint association of air pollutants and the genetic susceptibility with HF. Furthermore, we conducted a cross-validation analysis and found the results were consistently significant in the validation tests, indicating the robustness of our findings. However, we acknowledge that investigations in other populations are warranted to further validate our findings. The current study also has several potential limitations. First, the results from our observational study were based on a retrospective sub-analysis of the data from the UK Biobank. Thus, the causality of the results should be interpreted with caution. Second, we estimated the weights (regression coefficients) of air pollutants by treating each as a continuous variable. However, air pollutants may not be linearly associated with HF. Although examining non-linearity relations in the single air pollutant models may provide more information, the construction of a simple weighted risk score would not be possible. Third, the air pollution score did not include all air pollutants, such as O3 and ultrafine particles that might be also related to the risk of HF.12 , 28 , 40 Fourth, the air pollution exposure might be over- or underestimated since air pollution exposure in work environment is unavailable in UK Biobank study. Moreover, only a single measurement of air pollution is available in UK Biobank since home addresses of the participants are unavailable during follow-up. Further studies with repeated measurements are needed to confirm the findings. In addition, although we adjusted for the major confounders, residual confounding from unknown or unmeasured factors might be still existing. Finally, the present study was based on UK Biobank and most of the participants were European descent; thus, the generalization of the gene–air pollution score interaction results to other populations should be interpreted with caution.
Conclusions
In summary, for the first time, our results indicate that the long-term joint exposure to various air pollutants including PM2.5, PM10, PM2.5–10, NO2, and NOx, evaluated as an air pollution score, is associated with an elevated risk of incident HF in a dose–response fashion. Our findings suggest potential additive effects of different air pollutants on HF risk and highlight the importance to comprehensively assess various air pollutants in the prevention of cardiovascular diseases.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK115679, DK091718, DK100383, DK078616), and the Fogarty International Center (TW010790). L.Q. is a recipient of the American Heart Association Scientist Development Award (0730094N). L.Q. is also supported by National Institute of General Medical Sciences P20GM109036. M.W. is a recipient of a scholarship under the China Scholarship Council to pursue her study in the United States of America (201906010346).
Contributors
M.W. and L.Q. conceived and designed the study, interpreted the data, and drafted and critically revised the manuscript. M.W., X.L., and Y.S. performed the statistical analysis. All authors contributed to the interpretation of the results and critical revision of the manuscript. All authors approved the final manuscript.
Data sharing
This research has been conducted using the public UK Biobank Resource (www.ukbiobank.ac.uk/).
Conflict of interest: none declared.
Supplementary Material
Contributor Information
Mengying Wang, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA; Department of Epidemiology and Biostatistics, School of Public Health, Peking University, 38 Xueyuan Road, Haidian District, Beijing 100191, China.
Tao Zhou, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA.
Yongze Song, School of Design and the Built Environment, Curtin University, Kent Street, Bentley, Perth, Western Australia 6102, Australia.
Xiang Li, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA.
Hao Ma, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA.
Yonghua Hu, Department of Epidemiology and Biostatistics, School of Public Health, Peking University, 38 Xueyuan Road, Haidian District, Beijing 100191, China.
Yoriko Heianza, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA.
Lu Qi, Department of Epidemiology, School of Public Health and Tropical Medicine, Tulane University, 1440 Canal Street, Suite 1724, New Orleans, LA 70112, USA; Department of Nutrition, Harvard T.H. Chan School of Public Health, 677 Huntington Ave, Boston, MA 02115, USA; Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
References
- 1. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, Feigin V, Freedman G, Hubbell B, Jobling A, Kan H, Knibbs L, Liu Y, Martin R, Morawska L, Pope CA 3rd, Shin H, Straif K, Shaddick G, Thomas M, van Dingenen R, van Donkelaar A, Vos T, Murray CJL, Forouzanfar MH. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017;389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, Brauer M, Kutty VR, Gupta R, Wielgosz A, AlHabib KF, Dans A, Lopez-Jaramillo P, Avezum A, Lanas F, Oguz A, Kruger IM, Diaz R, Yusoff K, Mony P, Chifamba J, Yeates K, Kelishadi R, Yusufali A, Khatib R, Rahman O, Zatonska K, Iqbal R, Wei L, Bo H, Rosengren A, Kaur M, Mohan V, Lear SA, Teo KK, Leong D, O'Donnell M, McKee M, Dagenais G. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study (vol 395, pg 795, 2020). Lancet 2020;395:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pope CA, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 2015;116:108–U258. [DOI] [PubMed] [Google Scholar]
- 4. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ, Neumann F-J, Sechtem U, Banning AP, Bonaros N, Bueno H, Bugiardini R, Chieffo A, Crea F, Czerny M, Delgado V, Dendale P, Flachskampf FA, Gohlke H, Grove EL, James S, Katritsis D, Landmesser U, Lettino M, Matter CM, Nathoe H, Niessner A, Patrono C, Petronio AS, Pettersen SE, Piccolo R, Piepoli MF, Popescu BA, Räber L, Richter DJ, Roffi M, Roithinger FX, Shlyakhto E, Sibbing D, Silber S, Simpson IA, Sousa-Uva M, Vardas P, Witkowski A, Zamorano JL, Achenbach S, Agewall S, Barbato E, Bax JJ, Capodanno D, Cuisset T, Deaton C, Dickstein K, Edvardsen T, Escaned J, Funck-Brentano C, Gersh BJ, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Prescott E, Saraste A, Storey RF, Svitil P, Valgimigli M, Windecker S, Aboyans V, Baigent C, Collet J-P, Dean V, Delgado V, Fitzsimons D, Gale CP, Grobbee D, Halvorsen S, Hindricks G, Iung B, Jüni P, Katus HA, Landmesser U, Leclercq C, Lettino M, Lewis BS, Merkely B, Mueller C, Petersen S, Petronio AS, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa-Uva M, Touyz RM, Benkhedda S, Metzler B, Sujayeva V, Cosyns B, Kusljugic Z, Velchev V, Panayi G, Kala P, Haahr-Pedersen SA, Kabil H, Ainla T, Kaukonen T, Cayla G, Pagava Z, Woehrle J, Kanakakis J, Tóth K, Gudnason T, Peace A, Aronson D, Riccio C, Elezi S, Mirrakhimov E, Hansone S, Sarkis A, Babarskiene R, Beissel J, Maempel AJC, Revenco V, de Grooth GJ, Pejkov H, Juliebø V, Lipiec P, Santos J, Chioncel O, Duplyakov D, Bertelli L, Dikic AD, Studenčan M, Bunc M, Alfonso F, Bäck M, Zellweger M, Addad F, Yildirir A, Sirenko Y, Clapp B; Group ESCSD. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–477. [DOI] [PubMed] [Google Scholar]
- 5. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J; American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011;123:e18–e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savarese G, Lund LH; Division of Cardiology, Department of Medicine, Karolinska Insitutet, Stockholm, Sweden. Global public health burden of heart failure. Card Fail Rev 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mann JK, Tager IB, Lurmann F, Segal M, Quesenberry CP Jr., Lugg MM, Shan J, Van Den Eeden SK. Air pollution and hospital admissions for ischemic heart disease in persons with congestive heart failure or arrhythmia. Environ Health Perspect 2002;110:1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zanobetti A, Schwartz J, Gold D. Are there sensitive subgroups for the effects of airborne particles? Environ Health Perspect 2000;108:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah AS, Langrish JP, Nair H, McAllister DA, Hunter AL, Donaldson K, Newby DE, Mills NL. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet 2013;382:1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu H, Tian YH, Song J, Cao YY, Xiang X, Huang C, Li M, Hu YH. Effect of ambient air pollution on hospitalization for heart failure in 26 of China's largest cities. Am J Cardiol 2018;121:628–633. [DOI] [PubMed] [Google Scholar]
- 11. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long-term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology 2013;24:44–53. [DOI] [PubMed] [Google Scholar]
- 12. Bai L, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Goldberg MS, Lavigne E, Copes R, Martin RV, Kopp A, Chen H. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population-based study of 5.1 million Canadian adults living in Ontario. Environ Int 2019;132:105004. [DOI] [PubMed] [Google Scholar]
- 13. Mauderly JL, Samet JM. Is there evidence for synergy among air pollutants in causing health effects? Environ Health Perspect 2009;117:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearce JL, Neelon B, Bozigar M, Hunt KJ, Commodore A, Vena J. Associations between multipollutant day types and select cardiorespiratory outcomes in Columbia, South Carolina, 2002 to 2013. Environ Epidemiol 2018;2:e030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heianza Y, Qi L. Impact of genes and environment on obesity and cardiovascular disease. Endocrinology 2019;160:81–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, Manson JE, Hu FB, Willett WC, Qi L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ward-Caviness CK. A review of gene-by-air pollution interactions for cardiovascular disease, risk factors, and biomarkers. Hum Genet 2019;138:547–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larsson SC, Back M, Rees JMB, Mason AM, Burgess S. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J 2020;41:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai M-Y, Künzli N, Schikowski T, Marcon A, Eriksen KT, Raaschou-Nielsen O, Stephanou E, Patelarou E, Lanki T, Yli-Tuomi T, Declercq C, Falq G, Stempfelet M, Birk M, Cyrys J, von Klot S, Nádor G, Varró MJ, Dėdelė A, Gražulevičienė R, Mölter A, Lindley S, Madsen C, Cesaroni G, Ranzi A, Badaloni C, Hoffmann B, Nonnemacher M, Krämer U, Kuhlbusch T, Cirach M, de Nazelle A, Nieuwenhuijsen M, Bellander T, Korek M, Olsson D, Strömgren M, Dons E, Jerrett M, Fischer P, Wang M, Brunekreef B, de Hoogh K. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—The ESCAPE project. Atmos Environ 2013;72:10–23. [Google Scholar]
- 21. Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dėdelė A, Dons E, de Nazelle A, Dimakopoulou K, Eriksen K, Falq G, Fischer P, Galassi C, Gražulevičienė R, Heinrich J, Hoffmann B, Jerrett M, Keidel D, Korek M, Lanki T, Lindley S, Madsen C, Mölter A, Nádor G, Nieuwenhuijsen M, Nonnemacher M, Pedeli X, Raaschou-Nielsen O, Patelarou E, Quass U, Ranzi A, Schindler C, Stempfelet M, Stephanou E, Sugiri D, Tsai M-Y, Yli-Tuomi T, Varró MJ, Vienneau D, Klot S. V, Wolf K, Brunekreef B, Hoek G. Development of Land Use Regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ Sci Technol 2012;46:11195–11205. [DOI] [PubMed] [Google Scholar]
- 22. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, Motyer A, Vukcevic D, Delaneau O, O’Connell J, Cortes A, Welsh S, McVean G, Leslie S, Donnelly P, Marchini J. Genome-wide genetic data on ∼500,000 UK Biobank participants. bioRxiv 2017.
- 23. Shah S, Henry A, Roselli C, Lin HH, Sveinbjornsson G, Fatemifar G, Hedman AK, Wilk JB, Morley MP, Chaffin MD, Helgadottir A, Verweij N, Dehghan A, Almgren P, Andersson C, Aragam KG, Arnlov J, Backman JD, Biggs ML, Bloom HL, Brandimarto J, Brown MR, Buckbinder L, Carey DJ, Chasman DI, Chen X, Chen X, Chung J, Chutkow W, Cook JP, Delgado GE, Denaxas S, Doney AS, Dorr M, Dudley SC, Dunn ME, Engstrom G, Esko T, Felix SB, Finan C, Ford I, Ghanbari M, Ghasemi S, Giedraitis V, Giulianini F, Gottdiener JS, Gross S, Gudbjartsson DF, Gutmann R, Haggerty CM, van der Harst P, Hyde CL, Ingelsson E, Jukema JW, Kavousi M, Khaw KT, Kleber ME, Kober L, Koekemoer A, Langenberg C, Lind L, Lindgren CM, London B, Lotta LA, Lovering RC, Luan JA, Magnusson P, Mahajan A, Margulies KB, Marz W, Melander O, Mordi IR, Morgan T, Morris AD, Morris AP, Morrison AC, Nagle MW, Nelson CP, Niessner A, Niiranen T, O'Donoghue ML, Owens AT, Palmer CNA, Parry HM, Perola M, Portilla-Fernandez E, Psaty BM, Rice KM, Ridker PM, Romaine SPR, Rotter JI, Salo P, Salomaa V, van Setten J, Shalaby AA, Smelser DT, Smith NL, Stender S, Stott DJ, Svensson P, Tammesoo ML, Taylor KD, Teder-Laving M, Teumer A, Thorgeirsson G, Thorsteinsdottir U, Torp-Pedersen C, Trompet S, Tyl B, Uitterlinden AG, Veluchamy A, Volker U, Voors AA, Wang XS, Wareham NJ, Waterworth D, Weeke PE, Weiss R, Wiggins KL, Xing HM, Yerges-Armstrong LM, Yu B, Zannad F, Zhao JH, Hemingway H, Samani NJ, McMurray JJV, Yang J, Visscher PM, Newton-Cheh C, Malarstig A, Holm H, Lubitz SA, Sattar N, Holmes MV, Cappola TP, Asselbergs FW, Hingorani AD, Kuchenbaecker K, Ellinor PT, Lang CC, Stefansson K, Smith JG, Vasan RS, Swerdlow DI, Lumbers RT, Abecasis G, Backman J, Bai XD, Balasubramanian S, Banerjee N, Baras A, Barnard L, Beechert C, Blumenfeld A, Cantor M, Chai YT, Chung J, Coppola G, Damask A, Dewey F, Economides A, Eom G, Forsythe C, Fuller ED, Gu ZH, Gurski L, Guzzardo PM, Habegger L, Hahn Y, Hawes A, van Hout C, Jones MB, Khalid S, Lattari M, Li A, Lin N, Liu DR, Lopez A, Manoochehri K, Marchini J, Marcketta A, Maxwell EK, McCarthy S, Mitnaul LJ, O'Dushlaine C, Overton JD, Padilla MS, Paulding C, Penn J, Pradhan M, Reid JG, Schleicher TD, Schurmann C, Shuldiner A, Staples JC, Sun D, Toledo K, Ulloa RH, Widom L, Wolf SE, Yadav A, Ye B, Ctr RG; Regeneron Genetics Center. Genome-wide association and Mendelian randomisation analysis provide insights into the pathogenesis of heart failure. Nat Commun 2020;11: 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ripatti S, Tikkanen E, Orho-Melander M, Havulinna AS, Silander K, Sharma A, Guiducci C, Perola M, Jula A, Sinisalo J, Lokki ML, Nieminen MS, Melander O, Salomaa V, Peltonen L, Kathiresan S. A multilocus genetic risk score for coronary heart disease: case–control and prospective cohort analyses. Lancet 2010;376:1393–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, Tang WHW, Pinto YM, Williams LK, Sabbah HN, Gardell SJ. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail 2017;5:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simon RM, Subramanian J, Li MC, Menezes S. Using cross-validation to evaluate predictive accuracy of survival risk classifiers based on high-dimensional data. Brief Bioinformatics 2011;12:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li M, Wu Y, Tian YH, Cao YY, Song J, Huang Z, Wang XW, Hu YH. Association between PM2.5 and daily hospital admissions for heart failure: a time-series analysis in Beijing. Int J Environ Res Public Health 2018;15:2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Aguiar Pontes Pamplona Y, Arbex MA, Braga ALF, Pereira LAA, Martins LC. Relationship between air pollution and hospitalizations for congestive heart failure in elderly people in the city of Sao Paulo. Environ Sci Pollut Res Int 2020;27:18208–18220. [DOI] [PubMed] [Google Scholar]
- 29. Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol 2005;161:1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006;295:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian Y, Liu H, Wu Y, Si Y, Song J, Cao Y, Li M, Wu Y, Wang X, Chen L, Wei C, Gao P, Hu Y. Association between ambient fine particulate pollution and hospital admissions for cause specific cardiovascular disease: time series study in 184 major Chinese cities. BMJ 2019;367:l6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Kindi SG, Sarode A, Zullo M, Brook J, Burnett R, Oliveira GH, Huang W, Brook R, Rajagopalan S. Ambient air pollution and mortality after cardiac transplantation. J Am Coll Cardiol 2019;74:3026–3035. [DOI] [PubMed] [Google Scholar]
- 33. Nhung NTT, Schindler C, Chau NQ, Hanh PT, Hoang LT, Dien TM, Thanh NTN, Kunzli N. Exposure to air pollution and risk of hospitalization for cardiovascular diseases amongst Vietnamese adults: case-crossover study. Sci Total Environ 2020;703:134637. [DOI] [PubMed] [Google Scholar]
- 34. von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D’Ippoliti D, Elosua R, HöRmann A, Kulmala M, Lanki T, LöWel H, Pekkanen J, Picciotto S, Sunyer J, Forastiere F; Health Effects of Particles on Susceptible Subpopulations Study G. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation 2005;112:3073–3079. [DOI] [PubMed] [Google Scholar]
- 35. Poloniecki JD, Atkinson RW, de Leon AP, Anderson HR. Daily time series for cardiovascular hospital admissions and previous day's air pollution in London, UK. Occup Environ Med 1997;54:535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stockfelt L, Andersson EM, Molnar P, Gidhagen L, Segersson D, Rosengren A, Barregard L, Sallsten G. Long-term effects of total and source-specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ Res 2017;158:61–71. [DOI] [PubMed] [Google Scholar]
- 37. Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, Heo J, Yi SM, Kim K, Youn TJ, Chae IH. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J Am Heart Assoc 2017;6:e007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pranata R, Vania R, Tondas AE, Setianto B, Santoso A. A time-to-event analysis on air pollutants with the risk of cardiovascular disease and mortality: a systematic review and meta-analysis of 84 cohort studies. J Evid Based Med 2020;13:102–115. [DOI] [PubMed] [Google Scholar]
- 39. Downward GS, van Nunen E, Kerckhoffs J, Vineis P, Brunekreef B, Boer JMA, Messier KP, Roy A, Verschuren WMM, van der Schouw YT, Sluijs I, Gulliver J, Hoek G, Vermeulen R. Long-term exposure to ultrafine particles and incidence of cardiovascular and cerebrovascular disease in a prospective study of a Dutch cohort. Environ Health Perspect 2018;126:127007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bai L, Weichenthal S, Kwong JC, Burnett RT, Hatzopoulou M, Jerrett M, van Donkelaar A, Martin RV, Van Ryswyk K, Lu H, Kopp A, Chen H. Associations of long-term exposure to ultrafine particles and nitrogen dioxide with increased incidence of congestive heart failure and acute myocardial infarction. Am J Epidemiol 2019;188:151–159. [DOI] [PubMed] [Google Scholar]
- 41. Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, Gulliver J, Kelly FJ. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med 2016;73:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brunekreef B, Forsberg B. Epidemiological evidence of effects of coarse airborne particles on health. Eur Respir J 2005;26:309–318. [DOI] [PubMed] [Google Scholar]
- 43. Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single-pollutant to a multipollutant approach. Epidemiology 2010;21:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johns DO, Stanek LW, Walker K, Benromdhane S, Hubbell B, Ross M, Devlin RB, Costa DL, Greenbaum DS. Practical advancement of multipollutant scientific and risk assessment approaches for ambient air pollution. Environ Health Perspect 2012;120:1238–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hong YC, Leem JH, Ha EH, Christiani DC. PM(10) exposure, gaseous pollutants, and daily mortality in Inchon, South Korea. Environ Health Perspect 1999;107:873–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stieb DM, Burnett RT, Smith-Doiron M, Brion O, Shin HH, Economou V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J Air Waste Manag Assoc 2008;58:435–450. [DOI] [PubMed] [Google Scholar]
- 47. Park SK, Tao YB, Meeker JD, Harlow SD, Mukherjee B. Environmental risk score as a new tool to examine multi-pollutants in epidemiologic research: an example from the NHANES Study Using Serum Lipid Levels. PLoS One 2014;9:e98632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Arouca A, Moreno LA, Gonzalez-Gil EM, Marcos A, Widhalm K, Molnar D, Manios Y, Gottrand F, Kafatos A, Kersting M, Sjostrom M, Amaro-Gahete FJ, Ferrari M, Huybrechts I, Gonzalez-Gross M, De Henauw S, Michels N. Diet as moderator in the association of adiposity with inflammatory biomarkers among adolescents in the HELENA study. Eur J Nutr 2019;58:1947–1960. [DOI] [PubMed] [Google Scholar]
- 49. Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2054–2070. [DOI] [PubMed] [Google Scholar]
- 50. Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr., Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 51. Franchini M, Mannucci PM. Thrombogenicity and cardiovascular effects of ambient air pollution. Blood 2011;118:2405–2412. [DOI] [PubMed] [Google Scholar]
- 52. Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, Youtz DJ, Wang A, Lucchesi PA, Sun Q, Rajagopalan S. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 2012;5:452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vieira JL, Guimaraes GV, de Andre PA, Cruz FD, Saldiva PH, Bocchi EA. Respiratory filter reduces the cardiovascular effects associated with diesel exhaust exposure: a randomized, prospective, double-blind, controlled study of heart failure: the FILTER-HF Trial. JACC Heart Fail 2016;4:55–64. [DOI] [PubMed] [Google Scholar]
- 54. Brunekreef B, Holgate ST. Air pollution and health. Lancet 2002;360:1233–1242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.