Key Points
Question
What are the associations of concurrent LRRK2 G2019S and GBA variations with clinical progression of Parkinson disease (PD)?
Findings
In this cohort study combining data for 1193 participants with PD from multiple studies, individuals with dual LRRK2 G2019S and GBA variation PD had a slower rate of cognitive decline than those with GBA PD alone, and this was not different from individuals with LRRK2 G2019S PD alone, supporting the notion that there is a dominant association of the LRRK2 gene in individuals with both variations. There was also a novel statistical interaction between LRRK2 G2019S and GBA variations in cognitive decline.
Meaning
These findings suggest that there was not a convergent deleterious association of LRRK2 and GBA variations in PD progression, as would be expected based on prior cellular studies.
This cohort study examines the associations of LRRK2 G2019S and GBA variants with longitudinal cognitive and motor decline in Parkinson disease.
Abstract
Importance
Despite a hypothesis that harboring a leucine-rich repeat kinase 2(LRRK2) G2019S variation and a glucocerebrosidase (GBA) variant would have a combined deleterious association with disease pathogenesis, milder clinical phenotypes have been reported in dual LRRK2 and GBA variations Parkinson disease (PD) than in GBA variation PD alone.
Objective
To evaluate the association of LRRK2 G2019S and GBA variants with longitudinal cognitive and motor decline in PD.
Design, Setting, and Participants
This longitudinal cohort study of continuous measures in LRRK2 PD, GBA PD, LRRK2/GBA PD, and wild-type idiopathic PD used pooled annual visit data ranging from 2004 to 2019 from the Mount Sinai Beth Israel, Parkinson Disease Biomarker Program, Harvard Biomarkers Study, Ashkenazi Jewish-LRRK2-Consortium, Parkinson Progression Marker Initiative, and SPOT-PD studies. Patients who were screened for GBA and LRRK2 variations and completed either a motor or cognitive assessment were included. Data were analyzed from May to July 2020.
Main Outcomes and Measures
The associations of LRRK2 G2019S and GBA genotypes on the rate of decline in Montreal Cognitive Assessment (MoCA) and Movement Disorders Society-Unified Parkinson Disease Rating Scale–Part III scores were examined using linear mixed effects models with PD duration as the time scale.
Results
Among 1193 individuals with PD (mean [SD] age, 66.6 [9.9] years; 490 [41.2%] women), 128 (10.7%) had GBA PD, 155 (13.0%) had LRRK2 PD, 21 (1.8%) had LRRK2/GBA PD, and 889 (74.5%) had idiopathic PD. Patients with GBA PD had faster decline in MoCA than those with LRRK2/GBA PD (B [SE], −0.31 [0.09] points/y; P < .001), LRRK2 PD (B [SE], −0.33 [0.09] points/y; P < .001), or idiopathic PD (B [SE], −0.23 [0.08] points/y; P = .005). There was a LRRK2 G2019S × GBA interaction in MoCA decline (B [SE], 0.22 [0.11] points/y; P = .04), but not after excluding severe GBA variations (B [SE], 0.12 [0.11] points/y; P = .28). Patients with GBA PD had significantly worse motor progression compared with those with idiopathic PD (B [SE], 0.49 [0.22] points/y; P = .03) or LRRK2 PD (B [SE], 0.77 [0.26] points/y; P = .004).
Conclusions and Relevance
These findings suggest that longitudinal cognitive decline in patients with GBA PD was more severe than in those with LRRK2/GBA PD, which more closely resembled LRRK2 PD. This further supports the notion of a dominant association of LRRK2 on GBA in individuals who carry both and raises the possibility of an LRRK2 × GBA interaction. However, the biological basis of a dominant association or interaction is not clear and is apparently contrary to basic investigations. Study of a larger cohort of individuals with severe GBA variation is warranted.
Introduction
Variants in the leucine-rich repeat kinase 2 (LRRK2; OMIM 609007) and glucocerebrosidase (GBA; OMIM 606463) genes are frequent and worldwide genetic contributors to Parkinson disease (PD) susceptibility.1,2 LRRK2 variations occur in approximately 2% of PD overall, and up to 40% of PD in certain ethnic groups, particularly individuals of Ashkenazi Jewish or Arabian Berber descent.1 GBA variation and risk variants in GBA may be present in as many of 10% of PD worldwide and in approximately 15% or greater in PD among individuals of Ashkenazi Jewish descent.2,3,4 Wide ranges in conferred disease risk exist based on allelic heterogeneity. The penetrance of LRRK2 variations (specifically G2019S), or the chance that a carrier of this variation will manifest PD by late adulthood, has been estimated at 26% to 43% and is lower in individuals with GBA variations, ranging from 9% to 19%.5,6,7,8 Therefore, additional risk factors are clearly at play.9,10,11 One potential critical interaction is the association of GBA and LRRK2 variations in carriers of both variations (hereafter, LRRKR2/GBA).
Several lines of basic experimental data demonstrate convergent deleterious associations of LRRK2 and GBA variations.12,13 These are consistent with limited clinical reports suggesting earlier age at onset of PD and increased penetrance in carriers of LRRK2/GBA.14,15 However, cross-sectional analyses of patients with established PD with both LRRK2 and GBA variations suggest that carriers of LRRK2/GBA may instead have a less severe clinical course than those harboring GBA variations only,15,16 including less severe motor symptoms, and less dementia, and less severe nonmotor features in LRRK2/GBA PD compared with GBA PD.15,16 Because there are founder variations for LRRK2 G2019S and GBA variations in individuals of Ashkenazi Jewish descent, the likelihood of identifying carriers of LRRK2/GBA is increased in this ethnic group. We expanded on these studies by evaluating longitudinal PD progression, with particular focus on continuous cognitive and motor scores in individuals with LRRK2/GBA PD compared with those with LRRK2 PD, GBA PD, and idiopathic PD (ie, individuals without GBA or LRRK2 variations), and assessing the LRRK2 × GBA gene interaction on cognitive and motor decline. In addition, we extended the sample to include cohorts with individuals not of Ashkenazi Jewish descent as well.
Methods
Ethical approval for the Mount Sinai Beth Israel (MSBI) Biomarker study was received from the Mount Sinai institutional review board, and all other sites and studies had local institutional review board approval. All participants provided written informed consent. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Study Design
To maximize the number of dual LRRK2 G2019S and GBA variant carriers with PD, we combined data from subgroups of prospective longitudinal cohorts of participants with or without Ashkenazi Jewish ancestry and included LRRK2/GBA PD and compared these with LRRK2 PD, GBA PD, and idiopathic PD.
Participants
Data from MSBI Biomarker study, National Institute of Neurological Disorders and Stroke (NINDS) Parkinson Disease Biomarker Program (PDBP),17 the Harvard Biomarkers Study (HBS),18,19,20 the Michael J. Fox Foundation (MJFF), LRRK2 Ashkenazi Jewish Cohort Consortium (AJLCC, including Columbia, Tel Aviv Sourasky Medical Center, and MSBI),21 the SPOT study,22 and Parkinson Progression Marker Initiative (PPMI)23 were included. Study visits were performed between February 25, 2004, and December 31, 2019, depending on the study. The goal was to include the maximum number of individuals with LRRK2/GBA PD. Participants were screened for LRRK2 and GBA variations and were included if they completed either a motor rating (Movement Disorder Society–sponsored revision of the Unified Parkinson Disease Rating Scale–Part III [MDS-UPDRS-III],24 or the original unrevised version [UPDRS-III]25) or a cognitive task (Montreal Cognitive Assessment [MoCA]26 or Mini-Mental State Examination [MMSE]27), with demographic information available. MSBI and AJLCC participants were genotyped for LRRK2 G2019S and the 11 most common GBA variations among individuals of Ashkenazi Jewish descent (ie, N370S, 84GG, IVS2 + 1, V394L, D409G, L444P, A456P, RecNcil, R496H, E326K, and T369M). The PDBP and PPMI studies genotyped participants via NeuroX-derived genotyping or the Immunochip Array, including GBA T369M, E326K, N370S, F298L, C62Y, K13R, F255Y, E150K, E427K, D419N, or A309V, and the HBS study identified variations through targeted next-generation sequencing as previously described.28,29 PPMI genetic data were queried May 2020.
Our comparison groups focused on the subset of LRRK2 PD previously described for progression (AJLCC, including follow-up at MSBI), and the subset of GBA PD not previously reported from the PDBP and MSBI studies (GBA PD and idiopathic PD). To prevent any double representation of participant enrollment, only unique data from a single cohort were included if a participant was enrolled in multiple cohorts. A total of 534 individuals with LRRK2 or idiopathic PD from MSBI and the AJ LCC cohort were also included in prior description of longitudinal progression,30 and 3 participants with LRRK2/GBA PD in the PPMI study were previously reported.16 GBA variants were categorized31 into 4 variation severity categories: severe (including 84GG, L444P, L444R, R120W, RecNcil, V394L, and biallelic), mild (including N370S and R496H), variant (including E326K and T369M), and wild type.
Clinical Measures
Criteria for PD and clinical, demographic, motor, and nonmotor data were collected per each study’s protocol, as previously described17,18,21,22,23 (Table 1). Longitudinal visits occurred a mean (SD) of 12 (3) months apart. Data were harmonized according to published conversions, with discordant scales converted to a single scale for analysis: the UPDRS-III32 subsection score was converted to the MDS-UPDRS III24,25 for the MSBI and AJLCC cohorts, and continuous MMSE27 scores were converted to MoCA26 scores for the HBS cohort as previously described.33
Table 1. Baseline Clinical and Demographic Features.
| Characteristic overall | No. (%) (N = 1193) | P valuea | |||
|---|---|---|---|---|---|
| GBA PD (n = 128) | LRRK2 PD (n = 155) | LRRK2/GBA PD (n = 21) | Idiopathic PD (n = 889) | ||
| Age, mean (SD) y | 64.4 (9.9) | 68.4 (9.2) | 65.7 (9.0) | 66.6 (10.0) | .006 |
| Age at PD onset, yb | 57.5 (10.2) | 60.1 (9.6) | 59.6 (9.9) | 61.1 (10.0) | .003 |
| Duration of PD, y | 6.9 (5.9) | 8.3 (6.5) | 6.0 (6.7) | 5.5 (4.9) | <.001 |
| Sex | |||||
| Women | 58 (45.3) | 78 (50.3) | 12 (57.1) | 342 (38.5) | .015 |
| Men | 70 (54.7) | 77 (49.7) | 9 (42.9) | 547 (61.5) | |
| Cohort | |||||
| MSBI (n = 307) | 55 (43.0) | 56 (36.1) | 6 (28.6) | 190 (20.9) | NA |
| HBS (n = 66) | 26 (20.3) | 0 | 0 | 40 (4.4) | NA |
| PDBP (n = 417) | 27 (21.1) | 0 | 1 (4.8) | 389 (42.8) | NA |
| LCC (n = 394) | 20 (15.6) | 99 (63.9) | 5 (23.8) | 290 (31.9) | NA |
| PPMI (n = 7) | 0 | 0 | 7 (33.3) | 0 | NA |
| SPOT (n = 2) | 0 | 0 | 2 (9.5) | 0 | NA |
| Levodopa equivalent dose | 692.7 (551.1) | 747.4 (545.1) | 283.3 (262.2) | 580.7 (489.7) | <.001f |
| MDS-UPDRS IIIc | 26.4 (13.6) | 24.7 (14.2) | 26.1 (9.1) | 24.5 (13.5) | .680 |
| MoCAc | 24.8 (4.8) | 25.4 (3.1) | 25.7 (2.6) | 25.5 (3.6) | .383 |
Abbreviations: HBS, Harvard Biomarkers Study; LCC, LRRK2 Cohort Consortium; MDS-UPDRS III, Movement Disorder Society–sponsored revision of the Unified Parkinson Disease Rating Scale, part III; MoCA, Montreal Cognitive Assessment; MSBI, Mount Sinai Beth Israel; NA, not applicable; PD, Parkinson disease; PDBP, Parkinson Disease Biomarker Program; PPMI, Parkinson disease Progression Marker Initiative.
P values correspond to test for equality among all groups.
Age at PD determined using age at onset when available and age at diagnoses otherwise.
Participant data was selected for inclusion if there was a complete motor rating (MDS-UPDRS III) or a cognitive task (MoCA) at any visit during their participation and demographic information (sex, age, and age at PD onset) available.
Statistical Analysis
The primary goals were the evaluation of the differences in the longitudinal rate of change in cognition (MoCA) and motor function (MDS-UPDRS III) among idiopathic PD, LRRK2 PD, GBA PD (all variant types included in the main analysis), and LRRK2/GBA PD. Linear mixed effects models with robust variance estimates using PD duration (from age of onset) as the time scale were used to examine the associations of LRRK2 and GBA genotypes on the rate of decline in MoCA and MDS-UPDRS-III scores. The primary models included LRRK2 G2019S and GBA genotypes and their interaction term as the primary independent variables. Cognitive and motor progressions were modeled adjusting for sex, age at baseline, PD duration at baseline, and cohort as fixed effects. Models included a participant-specific random effect to account for the correlation in repeated measurements within the same participant. In addition, motor progression models adjusted for levodopa equivalent dose and for presence of deep brain stimulation implants at time of visit. All analyses were performed using Stata statistical software version 16 (StataCorp). Because there is heterogeneity among GBA variants,31,34 sensitivity analyses excluding severe and biallelic GBA variation carriers were also performed. Individual waves with missing data were excluded. All tests were 2-sided, and P < .05 was considered significant. Data were analyzed from May to July 2020.
Results
Demographic Characteristics and Overall Comparisons
Among 1193 participants with PD (mean [SD] age, 66.6 [9.9] years; 490 [41.2%] women) included, 128 (10.7%) had GBA PD, 155 (13.0%) had LRRK2 PD, 21 (1.8%) had LRRK2/GBA PD, and 889 (74.5%) had idiopathic PD. The mean (SD) duration of study participation was 2.85 (1.43) years.
Site information and baseline clinical and genotype characteristics are presented in Table 1. Participants with LRRK2 PD were older at baseline assessment (mean [SD] age, 68.4 [9.2] years) compared with individuals with idiopathic PD (mean [SD] age, 66.5 [10.0] years; P = .03) or GBA PD (mean [SD] age, 64.8 [9.7] years; P < .001) but not LRRK2/GBA PD (mean [SD] age, 65.7 [9.0] years; P = .18). Similarly, participants with LRRK2 PD had longer mean (SD) duration of motor symptoms at baseline assessment (8.3 [6.5] years) compared with participants with idiopathic PD (5.5 [4.9] years; P < .001) or GBA PD (6.9 [5.9] years; P = .046) but not LRRK2/GBA PD (6.0 [6.7] years; P = .13). Participants with GBA PD were younger at motor symptoms onset (mean [SD] age, 57.5 [10.2] years) than those with LRRK2 PD (mean [SD] age, 60.1 [9.6] years), LRRK2/GBA PD (mean [SD] age, 59.6 [9.9]), or idiopathic PD (mean [SD] age, 61.1 [10.0] years). Idiopathic PD was less common in women (342 women of 889 participants [38.5%]) compared with GBA PD (58 women of 128 participants [45.3%]), LRRK2 PD (78 women of 155 participants [50.3%]), and LRRK2/GBA PD (78 women of 155 participants [57.1%]). There were no baseline differences in MDS-UPDRS III or MoCA scores among groups.
Participants with GBA PD were less likely to have mild variations and more likely to have high-risk variations than participants with LRRK2/GBA PD (biallelic: 8 participants [6.3%] vs 1 participant [4.8%]; severe: 11 participants [8.6%] vs 2 participants [9.5%]; mild: 49 participants [38.3%] vs 16 participants [76.2%]; variant: 60 participants [46.9%] vs 2 participants [9.5%]; overall P = .01) (Table 2); however, when mild or high-risk variant and severe or biallelic variations were combined, the distribution in GBA PD did not differ from LRRK2/GBA PD (109 participants [85.2%] vs 18 participants [85.7%]; P = .62). Information regarding deep brain stimulation was collected in all but 1 cohort, and included 6 participants with idiopathic PD (0.7%), 6 participants with GBA PD (4.7%), 5 participants with LRRK2 PD (3.2%), and 2 participants with LRRK2/GBA PD (9.5%).
Table 2. GBA Gene Variation and Variant Frequencies.
| Variationa | No. (%) | P valueb | ||
|---|---|---|---|---|
| All (n = 149) | GBA only (n = 128) | LRRK2 and GBA (n = 21) | ||
| Severe and biallelic | 22 (14.8) | 19 (14.8) | 3 (14.3) | >.99 |
| Severe | 13 (8.7) | 11 (8.6) | 2 (9.5) | .99 |
| 84GG | 5 (38.5) | 3 (27.3) | 2 (100) | |
| L444P | 3 (24.2) | 3 (27.3) | 0 | |
| L444R | 1 (6.7) | 1 (9.1) | 0 | |
| R120W | 1 (6.7) | 1 (9.1) | 0 | |
| RecNcil | 1 (6.7) | 1 (9.1) | 0 | |
| V394L | 2 (13.3) | 2 (18.2) | 0 | |
| Biallelic | 9 (6.4) | 8 (6.3) | 1 (4.8) | .97 |
| N370S/84GG | 1 (11.1) | 1 (12.5) | 0 | |
| N370S/N370S | 1 (11.1) | 1 (12.5) | 0 | |
| N370S/R496H | 2 (22.2) | 2 (25.0) | 0 | |
| N370S/RecNcil | 5 (55.6) | 4 (50.0) | 1 (100) | |
| Mild and Variant | 127 (85.2) | 109 (85.2) | 18 (85.7) | >.99 |
| Mild | 65 (43.6) | 49 (38.3) | 16 (76.2) | .001 |
| N370S | 60 (92.3) | 45 (91.8) | 15 (93.75) | |
| R496H | 5 (7.7) | 4 (8.2) | 1 (6.25) | |
| Variant | 62 (41.6) | 60 (46.9) | 2 (9.5) | .001 |
| E326K | 46 (74.2) | 44 (73.3) | 2 (100) | |
| E326K/E326K | 1 (1.6) | 1 (1.7) | 0 | |
| T369M | 15 (32.6) | 15 (25.0) | 0 | |
Mount Sinai Beth Israel and LRRK2 Ashkenazi Jewish Cohort Consortium participants were genotyped for both LRRK2 G2019S and the 11 most common GBA variations among individuals of Ashkenazi Jewish descent (ie, N370S, 84GG, IVS2 + 1, V394L, D409G, L444P, A456P, RecNcil, R496H, E326K, or T369M). Parkinson Disease Biomarker Program and Parkinson Progression Marker Initiative genotyped participants via NeuroX-derived genotyping or the Immunochip Array and included GBA variations T369M, E326K, N370S, F298L, C62Y, K13R, F255Y, E150K, E427K, D419N, and A309V. The Harvard Biomarker Study identified variations through targeted next-generation sequencing as previously described.28,29
P values correspond to test for equality among all groups.
Rates of Cognitive Decline
The estimated (SE) rates of decline in total MoCA score were −0.29 (0.05) points/y for idiopathic PD, −0.52 (0.09) points/y for GBA PD, −0.19 (0.06) points/y for LRRK2 PD, and −0.21 (0.06) points/y for LRRK2/GBA PD (Figure 1). There was a slower, although not statistically significant, rate of cognitive decline for carriers with a LRRK2 G2019S variation compared with individuals with idiopathic PD for LRRK2 PD alone (B [SE], 0.10 [0.06] points/y; P = .08) and LRRK2/GBA PD (B [SE], 0.08 [0.05] points/y; P = .12). As anticipated, there was a worse rate of cognitive decline in participants with GBA PD compared with participants with idiopathic PD (B [SE], −0.23 [0.08] points/y; P = .005) and LRRK2 PD (B [SE], −0.33 [0.09] points/y; P < .001), as well as those with LRRK2/GBA PD (B [SE], −0.31 [0.09] points/y; P < .001). However, there was no difference in rate of cognitive change between participants with LRRK2 PD and those with LRRK2/GBA PD (B [SE], 0.01 [0.07] points/y; P = .85).
Figure 1. Longitudinal Trajectories of Mean Montreal Cognitive Assessment (MoCA) and Movement Disorders Society-Unified Parkinson Disease Rating Scale–Part III (MDS-UPDRS III) Rating Across Groups.
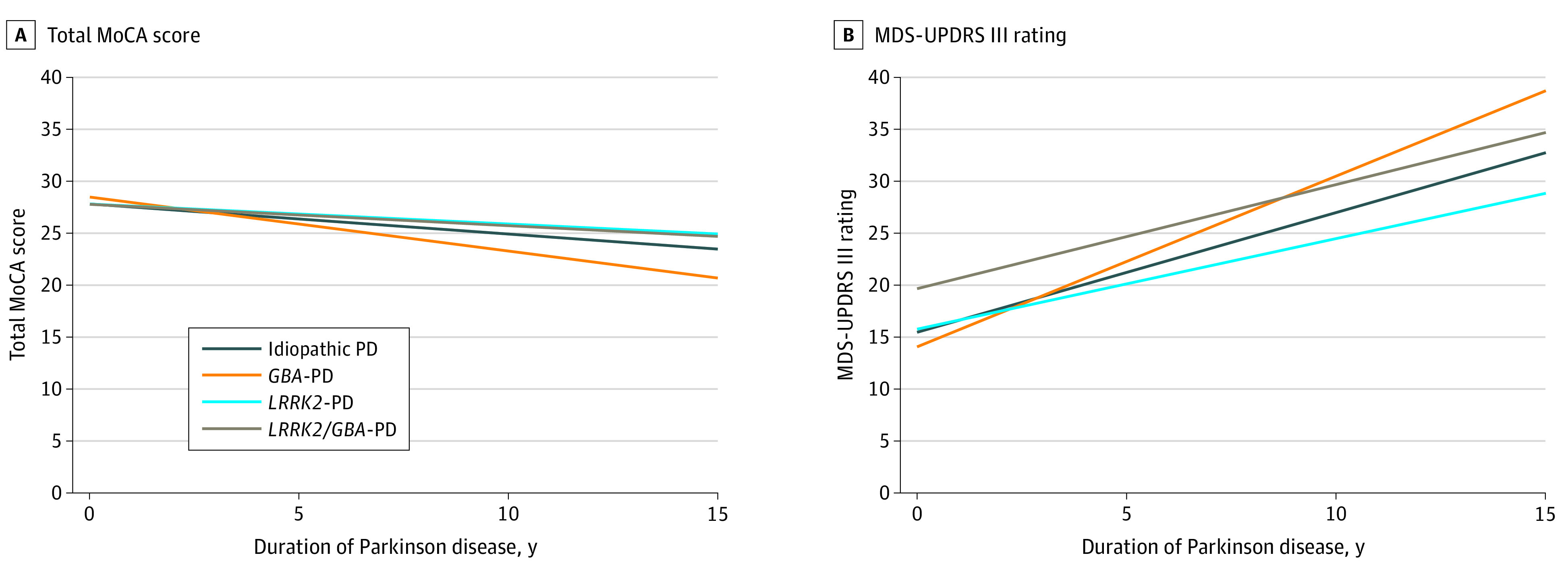
PD indicates Parkinson disease.
There was a significant GBA × LRRK2 G2019S interaction in decline in cognitive scores (B [SE], 0.22 [0.11] points/y; P = .04). This interaction is described in detail in the eTable and eFigure in the Supplement. Specifically, among participants without the LRRK2 G2019S variation, participants with GBA experienced faster decline compared with those without (ie, GBA PD vs idiopathic PD), but among participants with LRRK2 G2019S, those with a GBA variation had no difference compared with participants without a GBA variation (ie, LRRK2/GBA PD vs LRRK2 PD). Similarly, among participants without a GBA variation, carrying a LRRK2 G2019S variation was associated with a slower cognitive decline (LRRK2 PD vs idiopathic PD) as well as among individuals with LRRK2/GBA variations (LRRK2/GBA PD vs GBA PD). In sensitivity analysis excluding participants with severe GBA and dual variations the direction was the same, but the interaction was no longer significant (B [SE], 0.12 [0.11] points/y; P = .28).
Rates of Motor Decline
The estimated (SE) rates of worsening in MDS-UPDRS III scores were 1.15 (0.16) points/y for idiopathic PD, 1.64 (0.26) points/y for GBA PD, 0.87 ± 0.20) points/y for LRRK2 PD, and 1.00 (0.35) points/y for LRRK2/GBA PD. There was no significant difference in rate of motor change between participants with LRRK2 PD and those with idiopathic PD or LRRK2/GBA PD, nor between participants with LRRK2/GBA PD and those with idiopathic PD. Participants with GBA PD had worse motor progression compared with those with idiopathic PD (estimate [SE], 0.49 [0.22] points/y; P = .03) and those with LRRK2 PD (estimate [SE] 0.77 [0.26] points/y; P = .004), and a not statistically significant difference from those with LRRK2/GBA PD (estimate [SE], 0.64 [0.36] points/y; P = .07). There was no significant interaction association between LRRK2 G2019S and GBA variations for motor progression in the main analysis (estimate [SE], −0.36 [0.40] points/y; P = .38) (Table 3).
Table 3. Models Comparing Rate of Change in MoCA Score and MDS-UPDRS III Among PD (Idiopathic PD, GBA PD, LRRK2 PD, and LRRK2/GBA PD).
| Characteristic | MoCA | MDS-UPDRS IIIa | ||
|---|---|---|---|---|
| B (SE) | P value | B (SE) | P value | |
| Age at baseline, per y | −0.13 (0.01) | <.001 | 0.36 (0.04) | <.001 |
| Men (vs women) | −0.66 (0.19) | <.001 | 3.95 (0.78) | <.001 |
| Site (vs PDBP) | ||||
| HBS | 0.46 (0.32) | .15 | 379 (1.46) | .009 |
| MSBI | 0.01 (0.26) | .98 | −3.54 (1.08) | .001 |
| CUIMC LCC | 0.60 (0.32) | .06 | −0.08 (1.44) | .95 |
| Tel Aviv LCC | −1.11 (0.28) | <.001 | 4.78 (1.14) | <.001 |
| PPMI | −0.90 (0.95) | .35 | −0.54 (3.90) | .89 |
| CUIMC SPOT | 0.66 (2.05) | .75 | 5.48 (9.09) | .55 |
| Baseline duration, per y | 0.14 (0.05) | .005 | −0.37 (0.17) | .03 |
| Baseline PD (vs idiopathic PD) | ||||
| GBA | 0.66 (0.55) | .23 | −1.40 (1.92) | .47 |
| LRRK2 | −0.01 (0.51) | .99 | 0.03 (1.90) | .99 |
| LRRK2/GBA | −0.03 (0.67) | .96 | 4.19 (3.78) | .27 |
| PD slope, points/y | ||||
| Idiopathic | −0.29 (0.05) | <.001 | 1.15 (0.16) | <.001 |
| GBA | −0.52 (0.09) | <.001 | 1.64 (0.26) | <.001 |
| LRRK2 | −0.19 (0.06) | .001 | 0.87 (0.20) | <.001 |
| LRRK2/GBA | −0.20 (0.06) | .001 | 1.0 (0.35) | .004 |
| PD slope differences between groups, point/y | ||||
| GBA vs idiopathic | −0.23 (0.08) | .005 | 0.49 (0.22) | .03 |
| LRRK2 vs idiopathic | 0.10 (0.06) | .08b | −0.28 (0.19) | .14 |
| LRRK2/GBA vs idiopathic | 0.08 (0.05) | .12 | −0.15 (0.32) | .64 |
| GBA vs LRRK2 | −0.33 (0.09) | <.001 | 0.77 (0.26) | .004 |
| GBA vs LRRK2/GBA | −0.31 (0.09) | <.001 | 0.64 (0.36) | .07 |
| LRRK2 vs LRRK2/GBA | 0.01 (0.07) | .85 | −0.13 (0.35) | .71 |
| Interaction between GBA and LRRK2 variation | 0.22 (0.11) | .04 | −0.36 (0.40) | .38 |
Abbreviations: CUIMC, Columbia University Irving Medical Center; HBS, Harvard Biomarkers Study; LCC, LRRK2 Cohort Consortium; MDS-UPDRS-III, Movement Disorder Society sponsored revision of the Unified Parkinson Disease Rating Scale, part III; MoCA, Montreal Cognitive Assessment; MSBI, Mount Sinai Beth Israel; PD, Parkinson disease; PDBP, Parkinson Disease Biomarker Program.
For the MDS-UPDRS-III only model, in addition to the covariates listed, levodopa equivalent dose (per 100 mg) and deep brain stimulation therapy received at the time of the visit as fixed-effects covariates were also included. The association of levodopa equivalent dose with progression was estimated in the model as B (SE), −0.13 (0.07) points/y (P = .07) while DBS was estimated as B (SE), −7.75 (3.87) points/y (P = .045).
Tests for the presence of an interaction of variation type (LRRK2 variations and GBA variations) in the rate of cognitive progression (MoCA) and motor progression (MDS-UPDRS III) were performed based on slope estimates from the main models. In secondary models limited to sites of enrollment including participants of Ashkenazi Jewish descent, B (SE) was 0.19 (0.15) points/y (P = .20). In models adjusting for education B (SE) was 0.19 (0.11) points/y (P = .08). In models excluding participants with severe GBA and biallelic variations B (SE) was 0.12 (0.11) points/y (P = .28). In models limited to mild GBA variations, B (SE) was 0.07 (0.16) points/y (P = .64). In these models, the LRRK2 × GBA interactions for decline in cognitive scores were no longer significant. Furthermore, in a final secondary model also adjusting for level of education, the interaction was no longer significant (B [SE], 0.19 [0.11] points/y; P = .08). Of note, while the difference between LRRK2 PD and idiopathic PD did not reach statistical significance here (difference estimate [SE], −0.281 [0.19] points/y; P = .14), the magnitude and direction of the difference was similar to our prior work.30
Discussion
In this cohort study systematically evaluating longitudinal cognitive scores, LRRK2 G2019S and GBA variations did not have a combined deleterious association with disease progression in participants with both mutations. Cognitive decline in participants with LRRK2/GBA PD was less severe than in those with GBA PD and more closely resembled that observed in participants with LRRK2 PD. Our findings are overall consistent with those of 2 major studies evaluating LRRK2/GBA15,16 and are in alignment by finding that nonmotor anomalies were less prominent in individuals with LRRK2/GBA variations than in those with GBA variations. In a 2019 study by Yahalom et al in an Ashkenazi Jewish cohort,15 individuals with LRRK2/GBA PD had significantly less cognitive impairment, more rapid eye movement sleep behavior disorder, and less dementia and psychosis than those with GBA PD. In a larger and separate Israeli study evaluating individuals with LRRK2/GBA PD,16 there were no cross-sectional differences in MoCA performance, but there was less depression in participants with LRRK2/GBA PD than among those with severe variation GBA PD and more preserved olfaction in participants with LRRK2/GBA PD, as well as greater motor function than in those with GBA PD. Omer et al16 suggested a dominant association of the LRRK2 variation in participants with variations in LRRK2 and GBA. Our work extends these prior studies15,16 through longitudinal evaluation of continuous measures of cognition and motor function and with inclusion of participants with and without Ashkenazi ancestry. We specifically demonstrated that the longitudinal cognitive decline in individuals with LRRK2/GBA PD, as measured by MoCA, was more similar to that observed in individuals with LRRK2 PD, consistent with the cross-sectional data from the study by Yahalom et al.15
Our study also expands prior work by identifying a novel statistical interaction between LRRK2 G2019S variations and GBA variations in cognitive decline. However, we cannot be certain that the statistical interaction translates to a biological one. Genetic heterogeneity, including GBA allelic differences, as well as differences in GBA and LRRK2 penetrance, clinical expression, and distribution of types of variations in the GBA and LRRK2/GBA groups may all play roles in explaining the statistical interaction. Sensitivity analyses excluding individuals with severe GBA variations no longer demonstrated significance in the interaction association for cognitive decline. However, it should be noted that the power to detect an interaction was subsequently reduced in this restricted sensitivity sample. Furthermore, although the magnitude of the association was reduced, the direction of the association was maintained, suggesting that the overall association of GBA is still diminished in those with the LRRK2/GBA variation. In a 2020 cross-sectional evaluation of the largest sample of individuals with LRRK2/GBA dual variation,16 the dominant association of LRRK2 G2019S persisted with analysis restricted to individuals with mild GBA variations.
As anticipated from prior studies of single variation data,35,36 we found that harboring a GBA variation was associated with faster motor deterioration. While the slope of motor decline was worse in participants with GBA PD than in those with LRRK2/GBAPD, the difference was not statistically significant. The difference between participants with LRRK2 PD and those with idiopathic PD also did not reach statistical significance, but the magnitude and direction of the difference was similar to our prior work.30
Our overall finding, that cognition was not worse in participants with LRRK2/GBA PD than in those with only the LRRK2 variation, challenges cellular and clinical data suggesting convergent deleterious associations of dual LRRK2 and GBA variations. Studies in GBA1 variant mouse astrocytes13 and in human LRRK2 variation–induced pluripotent stem cell12 derived dopamine neurons support a convergent deleterious association of GBA and LRRK2 variations on some phenotypes; that is, inhibiting the overactivity of LRRK2 kinase improved function in the lysosomal glucocerebrosidase GBA pathway and ameliorated GBA variation–mediated cell loss. However, these findings are in contrast to the elevated peripheral glucocerebrosidase activity observed from dried blood spots from individuals with LRRK2 G2019S PD.22
Furthermore, not all studies that evaluated PD penetrance and age at onset among individuals of Ashkenazi Jewish descent with PD suggested a combined deleterious association in carriers of both LRRK2 G2019S and GBA variations or risk variants. Some have demonstrated a higher odds ratio of developing PD, as well as an earlier age of PD onset, than in dual carriers vs those of only a LRRK2 G2019S or GBA variant.14,15 If these penetrance and age at onset findings are replicated, one explanation that may reconcile these with the finding of less severe nonmotor progression in carriers of dual LRRK2/GBA variations is that LRRK2 and GBA may differentially be associated with the development of PD (penetrance and onset) and the PD disease course (clinical course). We speculate that once PD is established, the joint association might occur in 1 of 2 ways. On the one hand, individuals with dual LRRK2/GBA variations might be more likely to express LRRK2-associated pathologic and clinical expression when LRRK2 G2019S variations and associated gain-of-function renders otherwise deleterious GBA variations inert with respect to GBA progression phenotypes (Figure 2). Alternatively, because most GBA variations have significantly lower penetrance than LRRK2 G2019S, GBA variations in these individuals with LRRK2/GBA variations may be nonpenetrant carriers, and the clinical course may be driven by the LRRK2 variation, with the GBA variation not demonstrating great association, particularly for mild or high-risk variations. However, the biological underpinnings of these possibilities are not clear, and further interrogation of biological mechanisms, including biomarker analysis in a larger sample of individuals with PD harboring dual LRRK2 and severe GBA variations, and an analysis of the consequence of LRRK2 and/or GBA variations on such a biomarker is needed.
Figure 2. Hypothetical Outcomes in LRRK2-Targeted Therapies in Different Genetic Groups.
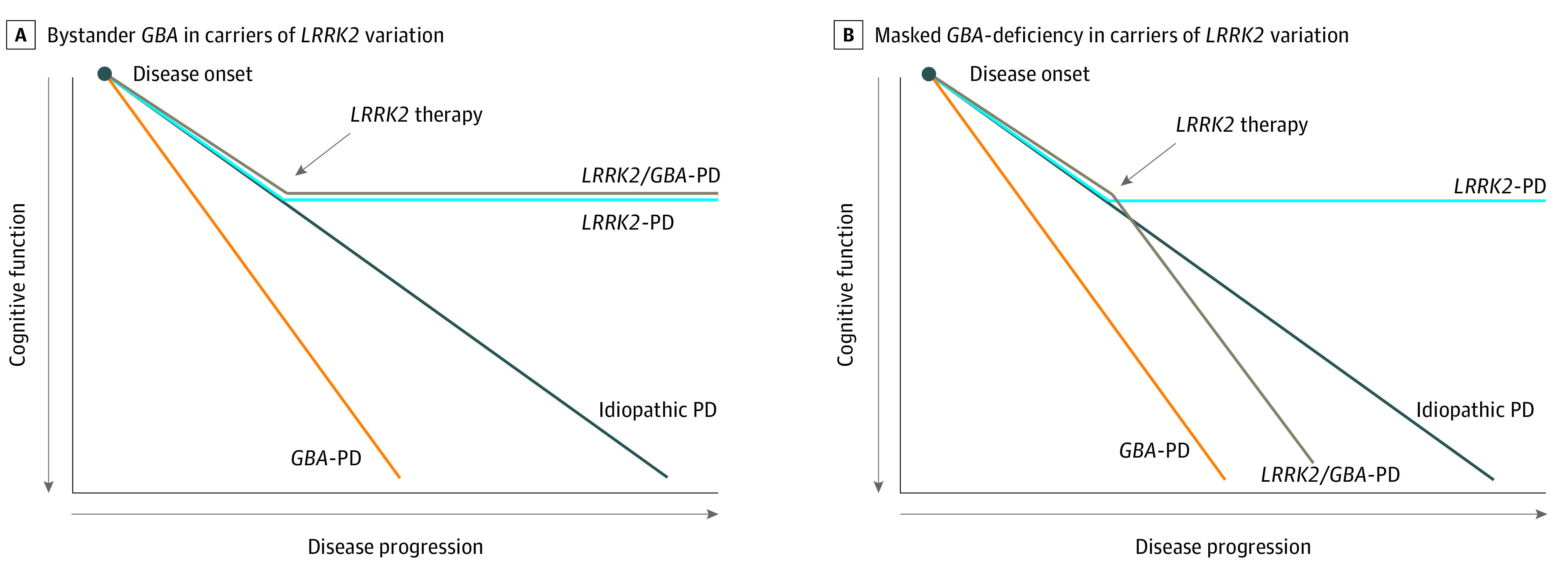
After disease onset, in the case that GBA mutations are benign in LRRK2 Parkinson disease (PD), according to an inert hypothesis for LRRK2/GBA carriers, treatment responses for effective LRRK2-targeting therapies would be identical for carriers of LRRK2 and LRRK2/GBA carriers. Alternatively, in the masked hypothesis for carriers of LRRK2/GBA, LRRK2 variations are biologically masking the effects of GBA variations in disease progression, and LRRK2-targeting therapy would unmask the effects of GBA variations to match disease progression and cognitive decline associated with GBA variations.
A strength of our study is that we have pooled and analyzed standardized, prospective data with up to 7 years of follow-up. Unlike previous studies that have published data on individuals with LRRK2/GBA, our analysis allowed longitudinal assessment to estimate the rate of motor and cognitive progression in LRRK2/GBA PD. Furthermore, to increase the sample size and enrich the genetic cohorts, our study included data collected from multiple cohorts and increased the proportion and variation of GBA variation types represented. However, because Ashkenazi Jewish ethnicity was not queried in 2 sites, we could not stratify the entire study. In sensitivity analysis limited to individuals with known Ashkenazi Jewish ethnicities, the slope of cognitive decline in LRRK2/GBA PD remained less than GBA PD.
Limitations
Our study has some weaknesses, such as the noted genetic heterogeneity across sites and variation groups, including the few individuals with dual LRRK2/GBA variation and severe GBA variations, and the small sample size of individuals with LRRK2/GBA PD, which limits generalizability. As we did not have equivalent GBA sequencing data for all sites, some individuals with GBA variations were likely miscoded as having idiopathic PD. However, this would bias toward the null rather than toward our finding in cognition. Not all data were available or used from all cohorts. For example, HBS data were limited to those associated with the MSBI study, and to avoid any potential overlap, we limited PPMI data to LRRK2/GBA PD and did not include the entire PPMI cohort data. In sensitivity analyses excluding the 7 individuals with dual LRRK2/GBA variation from PPMI, all findings, including group differences in the rate of change in MoCA score and MDS-UPDRS III rating, and an interaction association in MoCA were maintained. Additionally, there was a need to harmonize measures across sites, and furthermore, MoCA scores may be lower in non-English versions.37
Conclusions
The findings of this cohort study support prior, primarily cross-sectional data, suggesting that individuals with dual LRRK2/GBA variations do not have a worse PD clinical course than individuals with GBA PD or LRRK2 PD alone. We also found a statistical interaction between LRRK2 G2019S and GBA that may point to a possible biological association of LRRK2 G2019S canceling the deleterious associations of GBA. However, because the association was no longer significant in sensitivity analyses excluding individuals with severe or dual variations, we cannot exclude that the interaction may be a statistical reflection of genetic heterogeneity in this group, and that the differential associations of GBA variations and the interaction’s biological relevance are not certain. Because it was challenging to identify individuals that harbor both GBA and LRRK2 G2019S variations and we combined diverse groups, further investigation with systematic larger groups, especially including individuals with severe GBA variations, is warranted. This will facilitate better understanding of putative interaction mechanisms and potential therapeutic implications for cognitive progression.
eTable. Interaction Between LRRK2 and GBA for Rate of MoCA Decline
eFigure. Interaction Between LRRK2 and GBA for Rate of MoCA Decline
References
- 1.Healy DG, Falchi M, O’Sullivan SS, et al. ; International LRRK2 Consortium . Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7(7):583-590. doi: 10.1016/S1474-4422(08)70117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651-1661. doi: 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark LN, Nicolai A, Afridi S, et al. Pilot association study of the β-glucocerebrosidase N370S allele and Parkinson’s disease in subjects of Jewish ethnicity. Mov Disord. 2005;20(1):100-103. doi: 10.1002/mds.20320 [DOI] [PubMed] [Google Scholar]
- 4.Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med. 2004;351(19):1972-1977. doi: 10.1056/NEJMoa033277 [DOI] [PubMed] [Google Scholar]
- 5.Rana HQ, Balwani M, Bier L, Alcalay RN. Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med. 2013;15(2):146-149. doi: 10.1038/gim.2012.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AJ, Wang Y, Alcalay RN, et al. ; Michael J. Fox LRRK2 Cohort Consortium . Penetrance estimate of LRRK2 p.G2019S mutation in individuals of non-Ashkenazi Jewish ancestry. Mov Disord. 2017;32(10):1432-1438. doi: 10.1002/mds.27059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marder K, Wang Y, Alcalay RN, et al. ; LRRK2 Ashkenazi Jewish Consortium . Age-specific penetrance of LRRK2 G2019S in the Michael J. Fox Ashkenazi Jewish LRRK2 Consortium. Neurology. 2015;85(1):89-95. doi: 10.1212/WNL.0000000000001708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balestrino R, Tunesi S, Tesei S, Lopiano L, Zecchinelli AL, Goldwurm S. Penetrance of glucocerebrosidase (GBA) mutations in Parkinson’s Disease: a kin cohort study. Mov Disord. 2020;35(11):2111-2114. doi: 10.1002/mds.28200 [DOI] [PubMed] [Google Scholar]
- 9.Blauwendraat C, Reed X, Krohn L, et al. ; 23andMe Research Team . Genetic modifiers of risk and age at onset in GBA associated Parkinson’s disease and Lewy body dementia. Brain. 2020;143(1):234-248. doi: 10.1093/brain/awz350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwaki H, Blauwendraat C, Makarious MB, et al. ; International Parkinson’s Disease Genomics Consortium (IPDGC) . Penetrance of Parkinson’s Disease in LRRK2 p.G2019S carriers is modified by a polygenic risk score. Mov Disord. 2020;35(5):774-780. doi: 10.1002/mds.27974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan-Or Z, Amshalom I, Bar-Shira A, et al. The Alzheimer disease BIN1 locus as a modifier of GBA-associated Parkinson disease. J Neurol. 2015;262(11):2443-2447. doi: 10.1007/s00415-015-7868-3 [DOI] [PubMed] [Google Scholar]
- 12.Ysselstein D, Nguyen M, Young TJ, et al. LRRK2 kinase activity regulates lysosomal glucocerebrosidase in neurons derived from Parkinson’s disease patients. Nat Commun. 2019;10(1):5570. doi: 10.1038/s41467-019-13413-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanyal A, DeAndrade MP, Novis HS, et al. Lysosome and inflammatory defects in GBA1-mutant astrocytes are normalized by LRRK2 inhibition. Mov Disord. 2020;35(5):760-773. doi: 10.1002/mds.27994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein O, Gana-Weisz M, Cohen-Avinoam D, et al. Revisiting the non-Gaucher-GBA-E326K carrier state: is it sufficient to increase Parkinson’s disease risk? Mol Genet Metab. 2019;128(4):470-475. doi: 10.1016/j.ymgme.2019.10.001 [DOI] [PubMed] [Google Scholar]
- 15.Yahalom G, Greenbaum L, Israeli-Korn S, et al. Carriers of both GBA and LRRK2 mutations, compared to carriers of either, in Parkinson’s disease: risk estimates and genotype-phenotype correlations. Parkinsonism Relat Disord. 2019;62:179-184. doi: 10.1016/j.parkreldis.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 16.Omer N, Giladi N, Gurevich T, et al. A possible modifying effect of the G2019S mutation in the LRRK2 gene on GBA Parkinson’s Disease. Mov Disord. 2020;35(7):1249-1253. doi: 10.1002/mds.28066 [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal LS, Drake D, Alcalay RN, et al. ; PDBP consortium . The NINDS Parkinson’s disease biomarkers program. Mov Disord. 2016;31(6):915-923. doi: 10.1002/mds.26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Locascio JJ, Corvol JC, et al. ; HBS; CamPaIGN; PICNICS; PROPARK; PSG; DIGPD; PDBP . Prediction of cognition in Parkinson’s disease with a clinical-genetic score: a longitudinal analysis of nine cohorts. Lancet Neurol. 2017;16(8):620-629. doi: 10.1016/S1474-4422(17)30122-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Boot B, Locascio JJ, et al. ; International Genetics of Parkinson Disease Progression (IGPP) Consortium . Specifically neuropathic Gaucher’s mutations accelerate cognitive decline in Parkinson’s. Ann Neurol. 2016;80(5):674-685. doi: 10.1002/ana.24781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Center for Advanced Parkinson Research . Biobank. Accessed March 17, 2021. http://www.bwhparkinsoncenter.org/biobank
- 21.Alcalay RN, Mirelman A, Saunders-Pullman R, et al. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Mov Disord. 2013;28(14):1966-1971. doi: 10.1002/mds.25647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alcalay RN, Levy OA, Waters CC, et al. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain. 2015;138(Pt 9):2648-2658. doi: 10.1093/brain/awv179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marek K, Chowdhury S, Siderowf A, et al. ; Parkinson’s Progression Markers Initiative . The Parkinson’s Progression Markers Initiative (PPMI)—establishing a PD biomarker cohort. Ann Clin Transl Neurol. 2018;5(12):1460-1477. doi: 10.1002/acn3.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, et al. ; Movement Disorder Society UPDRS Revision Task Force . Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- 25.Goetz CG, Stebbins GT, Tilley BC. Calibration of unified Parkinson’s disease rating scale scores to Movement Disorder Society—Unified Parkinson’s Disease Rating Scale scores. Mov Disord. 2012;27(10):1239-1242. doi: 10.1002/mds.25122 [DOI] [PubMed] [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27.Tombaugh TN, McIntyre NJ. The Mini-Mental State Examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922-935. doi: 10.1111/j.1532-5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- 28.Nalls MA, Bras J, Hernandez DG, et al. ; International Parkinson’s Disease Genomics Consortium (IPDGC); Parkinson’s Disease meta-analysis consortium . NeuroX, a fast and efficient genotyping platform for investigation of neurodegenerative diseases. Neurobiol Aging. 2015;36(3):1605.e7-1605.e12. doi: 10.1016/j.neurobiolaging.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nalls MA, Keller MF, Hernandez DG, Chen L, Stone DJ, Singleton AB; Parkinson’s Progression Marker Initiative (PPMI) investigators . Baseline genetic associations in the Parkinson’s Progression Markers Initiative (PPMI). Mov Disord. 2016;31(1):79-85. doi: 10.1002/mds.26374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders-Pullman R, Mirelman A, Alcalay RN, et al. ; LRRK2 Ashkenazi Jewish Consortium . Progression in the LRRK2-asssociated Parkinson disease population. JAMA Neurol. 2018;75(3):312-319. doi: 10.1001/jamaneurol.2017.4019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaler A, Bregman N, Gurevich T, et al. Parkinson’s disease phenotype is influenced by the severity of the mutations in the GBA gene. Parkinsonism Relat Disord. 2018;55:45-49. doi: 10.1016/j.parkreldis.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 32.Goetz CC; Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease . The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18(7):738-750. doi: 10.1002/mds.10473 [DOI] [PubMed] [Google Scholar]
- 33.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9(5):529-537. doi: 10.1016/j.jalz.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gan-Or Z, Amshalom I, Kilarski LL, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology. 2015;84(9):880-887. doi: 10.1212/WNL.0000000000001315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brockmann K, Srulijes K, Pflederer S, et al. GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord. 2015;30(3):407-411. doi: 10.1002/mds.26071 [DOI] [PubMed] [Google Scholar]
- 36.Winder-Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136(Pt 2):392-399. doi: 10.1093/brain/aws318 [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Mirelman A, Saunders-Pullman R, et al. Differences in performance on English and Hebrew versions of the MoCA in Parkinson’s patients. Clin Park Relat Disord. 2020;3:100042. doi: 10.1016/j.prdoa.2020.100042 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Interaction Between LRRK2 and GBA for Rate of MoCA Decline
eFigure. Interaction Between LRRK2 and GBA for Rate of MoCA Decline


