Abstract
Study Objectives
We compared the basic cognitive functions of adolescents undergoing split (nocturnal sleep + daytime nap) and continuous nocturnal sleep schedules when total sleep opportunity was either below or within the recommended range (i.e. 6.5 or 8 h).
Methods
Adolescent participants (age: 15–19 year) in the 8-h split (n = 24) and continuous (n = 29) sleep groups were compared with 6.5-h split and continuous sleep groups from a previous study (n = 58). These protocols involved two baseline nights (9-h time-in-bed [TIB]), 5 nights of sleep manipulation, 2 recovery nights (9-h TIB), followed by a second cycle of sleep manipulation (3 nights) and recovery (2 nights). Cognitive performance, subjective sleepiness, and mood were evaluated daily; sleep was assessed using polysomnography.
Results
Splitting 6.5 h of sleep with a mid-afternoon nap offered a boost to cognitive function compared to continuous nocturnal sleep. However, when total TIB across 24 h increased to 8 h, the split and continuous sleep groups performed comparably in tests evaluating vigilance, working memory, executive function, processing speed, subjective sleepiness, and mood.
Conclusions
In adolescents, the effects of split sleep on basic cognitive functions vary by the amount of total sleep obtained. As long as the total sleep opportunity across 24 h is within the recommended range, students may fulfill sleep requirements by adopting a split sleep schedule consisting of a shorter period of nocturnal sleep combined with a mid-afternoon nap, without significant impact on basic cognitive functions.
Clinical trial registration
Keywords: adolescents, cognition, naps, continuous sleep, split sleep, vigilance
Statement of Significance.
Splitting sleep into main nocturnal period and a daytime nap has been shown to mitigate deterioration in adolescents’ basic cognitive functions when total sleep opportunity over 24 h is less than recommended (i.e. 6.5 h). With total sleep opportunity at 8 h—the minimum recommended, we found that a split sleep schedule with 6.5-h nocturnal sleep and a 1.5-h nap achieved similar performance in basic cognitive tasks, subjective alertness, and mood as the nocturnal sleep only schedule. Our findings suggest that adopting a split sleep schedule in this manner may be a plausible option for adolescents unable to obtain sufficient sleep at night.
Introduction
Sleep curtailment in adolescents is a problem for many societies, but is particularly challenging in societies with highly competitive school environments [1–3]. In Singapore, 85% of students [4] report sleeping less than the recommended 8–10 h a night [5, 6]. In such societies, later bedtimes arise not only from the maturational delay in circadian rhythms [7, 8] and slower build-up of homeostatic sleep pressure [9], but also a perceived need to study longer and later into the night [10]. More than 60% of teens in such societies go to bed after midnight on school nights [4]. Late bedtimes, together with resistance to altering early school start times, significantly curtail the amount of nocturnal sleep a student can obtain [11, 12]. While sleeping the recommended nocturnal duration may still be best, a pragmatic remedy to afford satisfactory total sleep duration in societies with entrenched cultural beliefs is to adopt a split sleep schedule where an acceptable total duration of sleep is split into a shorter nocturnal sleep period combined with a mid-afternoon nap.
In our previous study examining the neurocognitive impact of split sleep schedules across two successive weeks, we found that the group that split a restricted total sleep opportunity of 6.5 h in 24 h into a 5-h nocturnal period combined with a 1.5-h mid-afternoon nap were markedly more vigilant compared to the group who had all of their sleep allocated to night time [13]. This benefit on vigilance may be attributed to having two opportunities to dissipate homeostatic sleep pressure over 24 h [13, 14]. Prior work on adults suggests that when provision for total sleep time (TST) is adequate, for example, 9–10 h of time-in-bed (TIB), apportioning this into two equal periods across 24–28 h [15, 16] does not influence daytime cognitive performance. It is however, an open question whether the cognitive benefits of splitting sleep with a daytime nap would still be observed when total sleep opportunity across 24 h lies within the recommended nocturnal sleep duration for adolescents.
To address this, the present study sought to characterize basic neurocognitive functions of split versus continuous sleep schedules in adolescents wherein total sleep opportunity is 8 h, that is, the minimum amount of sleep recommended for this age group. We compared these 8 h sleep opportunity groups (8-h TIB at night vs. 6.5-h TIB at night plus 1.5-h nap opportunity) to the 6.5 h sleep opportunity groups from our previous study (6.5-h TIB at night vs. 5-h TIB at night plus 1.5-h nap opportunity [13]). If the neurocognitive outcomes of a split sleep schedule partitioning 8 h into nocturnal sleep and a daytime nap were found to be comparable to sleeping the full 8 h at night, such a split schedule could be an alternative to sleeping the 8 h allocation solely at night.
Methods
Participants
A total of 59 adolescents (29 males) were recruited into the fifth Need for Sleep study (NFS5)—a series of experimental studies that aim at characterizing adolescents’ cognitive functions under different sleep manipulations. Participants were selected from a pool of 121 volunteers, following inclusion criteria used in four previous NFS studies [13, 17–19]. Adolescents were eligible if they were between 15 and 19 years of age, reported no known health conditions or sleep disorders, had a body mass index (BMI) of ≤30, consumed ≤5 cups of caffeinated beverages per day, were not habitual short sleepers (actigraphically estimated average TIB of <6 h with weekend sleep extension of ≤1 h), and did not travel across >2 time zones in the month prior to the experiment.
Participants were randomized into either a split sleep group (6.5-h TIB at night plus 1.5-h nap opportunity) or a continuous sleep group (8-h TIB at night). Due to personal reasons, two participants withdrew before the experiment, and four withdrew within 3 days after the experiment had begun. There were 53 participants in the final analyses (the continuous sleep group: n = 29; the split sleep group: n = 24). Their data were compared with our prior study that provided for a 6.5-h total sleep opportunity over each 24 h. The participants in the earlier study were either on a continuous 6.5-h TIB schedule (n = 29) or a split schedule consisting of a 5-h nocturnal TIB with a 1.5-h afternoon nap (n = 29; NFS4) [13].
The four groups did not differ significantly in the following measures assessed during screening: age, sex, BMI, daily caffeine intake, morningness-eveningness preference (Morningness Eveningness Questionnaire [20]), symptoms of excessive daytime sleepiness (Epworth Sleepiness Scale [21]), symptoms of chronic sleep reduction (Chronic Sleep Reduction Questionnaire [22]), self-reported sleep (Pittsburgh Sleep Quality Index [23]), and actigraphically assessed sleep parameters (p > 0.22; Table 1).
Table 1.
Characteristics of all split and continuous sleep groups
| Total TIB of 6.5 h | Total TIB of 8 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Split | Continuous | Split | Continuous | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | P | |
| N | 29 | – | 29 | – | 24 | – | 29 | – | – |
| Age (years) | 16.55 | 0.74 | 16.58 | 1.12 | 16.72 | 1.16 | 16.18 | 0.88 | 0.22 |
| Gender (% male) | 51.70 | – | 51.70 | – | 45.83 | – | 48.28 | – | 0.97 |
| Body mass index | 20.7 | 2.80 | 21.3 | 3.5 | 20.2 | 3.13 | 20.5 | 3.08 | 0.64 |
| Daily caffeine intake (cups) | 0.55 | 0.69 | 0.58 | 0.80 | 0.63 | 0.82 | 0.72 | 1.00 | 0.87 |
| Morningness-Eveningness Questionnaire | 50.7 | 7.07 | 49.0 | 7.54 | 48.8 | 6.80 | 49.5 | 6.18 | 0.73 |
| Epworth Sleepiness Scale | 7.86 | 3.78 | 8.21 | 3.43 | 7.42 | 3.09 | 7.66 | 3.04 | 0.85 |
| Chronic Sleep Reduction Questionnaire | 36.10 | 4.66 | 35.24 | 5.96 | 37.00 | 5.23 | 36.59 | 4.04 | 0.61 |
| Pittsburgh Sleep Quality Index | |||||||||
| Weekday TIB (h) | 6.78 | 0.89 | 6.85 | 1.35 | 7.36 | 1.16 | 7.59 | 1.38 | 0.33 |
| Weekend TIB (h) | 8.76 | 1.23 | 8.93 | 1.18 | 9.10 | 1.32 | 8.52 | 1.32 | 0.74 |
| Weekday TST (h) | 6.47 | 0.86 | 6.46 | 1.19 | 6.87 | 1.22 | 6.62 | 1.00 | 0.75 |
| Weekend TST (h) | 8.41 | 1.18 | 8.56 | 1.20 | 8.78 | 2.19 | 8.43 | 1.25 | 0.97 |
| Nap duration (min) | 62.9 | 65.7 | 68.5 | 64.8 | 58.5 | 60.0 | 42.6 | 53.7 | 0.42 |
| Global score | 4.17 | 1.77 | 4.48 | 1.50 | 4.22 | 1.24 | 4.48 | 1.70 | 0.82 |
| Actigraphy | |||||||||
| Weekday TIB (h) | 6.84 | 1.13 | 7.00 | 0.77 | 7.22 | 0.85 | 7.21 | 0.74 | 0.32 |
| Weekend TIB (h) | 8.15 | 1.05 | 8.45 | 1.13 | 8.40 | 1.17 | 8.36 | 1.04 | 0.74 |
| Weekday TST (h) | 5.50 | 0.89 | 5.51 | 0.75 | 5.74 | 0.84 | 5.73 | 0.65 | 0.50 |
| Weekend TST (h) | 6.64 | 1.00 | 6.76 | 1.14 | 6.74 | 1.26 | 6.65 | 0.94 | 0.97 |
| Average TST (h) | 5.83 | 0.73 | 5.86 | 0.68 | 5.98 | 0.78 | 5.97 | 0.58 | 0.80 |
| Sleep efficiency (%) | 81.0 | 6.64 | 79.0 | 5.57 | 79.9 | 6.87 | 79.5 | 6.1 | 0.65 |
| Nap at least once a week (%) | 66.7 | – | 47.4 | – | 44.0 | – | 53.6 | – | 0.45 |
P-values from the ANOVA and chi-squared tests contrasting the four groups are listed. Characteristics of the 6.5-h total TIB groups were obtained from a previous study [13].
During the week prior to the experiment, participants refrained from napping and adhered to a 9-h nocturnal sleep schedule (11:00 pm–08:00 am) in order to minimize the effects of prior sleep loss and to facilitate circadian entrainment. Compliance was verified with actigraphy.
Study protocol
NFS5 was a 15-day experiment that was held during school vacation time at a student dormitory (Figure 1). Details of living arrangements have been reported in previously published work [18]. On the first two nights of the protocol (B1 and B2), all participants had a 9-h nocturnal sleep opportunity (11:00 pm–08:00 am) for adaptation and baseline characterization. This was followed by two cycles of sleep manipulation (continuous or split sleep) and recovery nights. The first cycle began with five nights of manipulation (M11–M15) and ended with two nights of 9-h recovery sleep opportunity (R11–R12), simulating a typical school week. This was followed by the second cycle which included three manipulation nights (M21–M23) and two recovery nights (R21–R22). This mimicked the recurrent pattern of restricted sleep on school nights and recovery sleep on weekends in a school term. During the manipulation periods, the continuous sleep group had 8-h TIB at night (11:30 pm–07:30 am), while the split sleep group had 6.5-h TIB at night (00:15 am–06:45 am) followed by a 1.5-h nap opportunity in the mid-afternoon the next day (2:00 pm–3:30 pm). The 6.5-h manipulation groups from NFS4 [13] followed the same cycles of manipulation and recovery with the continuous sleep group having 6.5-h TIB at night (00:15 am–06:45 am) and the split-sleep group having 5-h TIB at night (01:00 am–06:00 am) and a 1.5-h daytime nap opportunity (2:00 pm–3:30 pm).
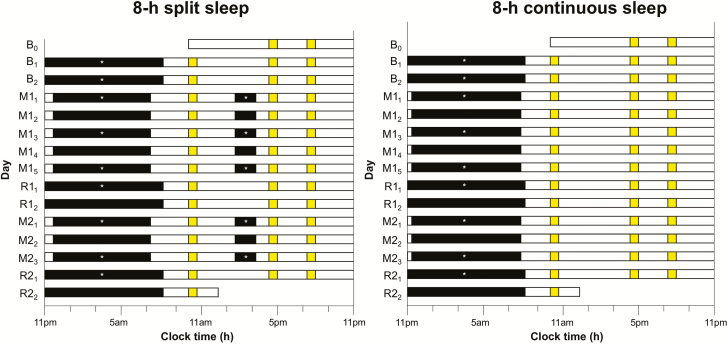
Figure 1.
Protocol. In this 15-day protocol, both the 8-h split sleep group and the 8-h split continuous group had two adaptation and baseline nights (B1 and B2; TIB indicated by black bars = 9 h from 11:00 pm to 08:00 am). The first cycle of sleep opportunity manipulation lasted 5 nights (M11–M15) followed by two nights of recovery sleep (R11 and R12; TIB = 9 h). The second cycle consisted of three manipulation nights (M21–M23) and two recovery nights (R21 and R22). During the two sleep opportunity manipulation periods, the split sleep group had a nocturnal TIB of 6.5 h (00:15 am–06:45 am) and a 1.5-h nap opportunity between 2:00 pm and 3:30 pm, while the continuous sleep group had a nocturnal TIB of 8 h (11:30 pm–07:30 am). Asterisks indicate nocturnal sleep and daytime nap episodes with polysomnographic recordings. A cognitive test battery (yellow bars) was administered at 10:00 am, 4:15 pm, and 8:00 pm daily, except during the first and last days of the protocol.
Cognitive performance was assessed with a computerized test battery administered three times daily (except on the first and final days): at 10:00 am, 4:15 pm, and 8:00 pm. Outside of scheduled activities during the day, participants were kept under constant supervision by research staff and prohibited from napping, consuming caffeinated food and beverages, and engaging in strenuous physical activity. Sleep–wake patterns were monitored throughout the experiment using wrist-worn actigraphy. Polysomnography (PSG) was recorded on nine nights (B1, B2, M11, M13, M15, R11, M21, M23, and R21) for adaptation and baseline characterization, as well as for characterization of the sleep architecture associated with different TIBs. Daytime naps were also monitored with PSG on five days (M11, M13, M15, M21, and M23).
This study was approved by the Institutional Review Board of the National University of Singapore, and conducted according to the principles in the Declaration of Helsinki. Participants and their legal guardians were briefed about the study aims and procedures, and provided written informed consent.
Actigraphy
Participants wore an Actiwatch (Actiwatch 2, Philips Respironics Inc., Pittsburgh, PA) on the wrist of their non-dominant hand (1) for 1 week for screening purposes, (2) during the week prior to the experiment for verifying compliance with the 9-h TIB sleep schedule, and (3) throughout the 15-day protocol. Data were collected in 2-min epochs and scored using the Actiware software (version 6.0.7). TST was calculated using the software algorithm set to medium sensitivity (wake events defined as having an activity count of ≥40). For (1) and (2), bed and wake times were determined based on the participant’s self-reported timings recorded in a sleep diary, as well as event markers on the actogram. If necessary, changes in light and activity levels were referred to for defining the sleep period.
Polysomnography
Electroencephalography (EEG) was performed using a SOMNOtouch recorder (SOMNOmedics GmbH, Randersacker, Germany) on two channels (C3 and C4) in the international 10–20 system), referenced to contralateral mastoids. Cz and Fpz were used as common reference and ground electrodes, respectively. All EEG electrodes were kept to an impedance of below 5 kΩ. Electrooculography (EOG) and submental electromyography were also performed with impedances kept below 10 kΩ. Pulse oximetry was measured on the first night (B1) to screen for undiagnosed sleep apnea.
Signal was sampled at 256 Hz and band-pass filtered between 0.2 and 35 Hz for EEG, and between 0.2 and 10 Hz for EOG. Automated scoring of sleep stages and artifacts was performed using an updated version of the Z3Score algorithm (https://z3score.com) [24] in conjunction with the FASST EEG toolbox, and visually checked by trained research staff following standards set by the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events [25].
The following parameters were computed for each recording: TST, N2 latency (time from lights off to N2 onset), durations of N1, N2, N3, and rapid eye movement (REM) sleep, as well as wake after sleep onset (WASO). Slow wave activity (SWA) was computed in the first hour of sleep from N2 onset as a measure of homeostatic sleep pressure.
Cognitive performance test battery
The test battery was conducted in a classroom setting and consisted of five cognitive tasks and two questionnaires administered in the following order: Karolinska Sleepiness Scale (KSS [26]), Symbol Digit Modalities Test (SDMT [27]), verbal 1- and 3-back tasks [28], Mental Arithmetic Test (MAT [29]), Positive and Negative Affect Scale (PANAS [30]), and a 10-min Psychomotor Vigilance Task (PVT [31]), taking approximately 25 min to complete. Details of each task or questionnaire have been published in previous work [13, 18, 19]. Participants wore earphones during testing sessions for tone presentations and to minimize noise distractions. All tasks were programmed in E-Prime 3.0 (Psychology Software Tools, Inc., Sharpsburg, PA).
Statistical analysis
Differences in screening variables between the 8-h manipulation groups and 6.5-h manipulation groups (NFS4 [13]) were tested using ANOVA and chi-squared tests.
For each outcome measure from the test battery, group differences in the changes across the protocol were tested using a mixed model in SAS 9.4 (SAS Institute, Cary, NC) with group (8-h continuous, 8-h split, 6.5-h continuous, and 6.5-h split sleep groups) as a between-subject factor and day (B2 to R21) as a within-subject factor. We examined the effects of group, day, and their interaction on the number of PVT lapses (response times >500 ms) averaged across the three sessions each day. To control for group differences in baseline performance, we included as a covariate the performance of the last PVT on B1. This statistical model was also applied to the number of PVT lapses in the morning, afternoon, and evening separately. The effects of group, day, and their interaction were examined for the other cognitive functions and mood using the same statistical models. In this report, for pairwise comparisons, we focused on the group contrasts between the two groups with a total TIB of 6.5 h per 24 h (6.5-h continuous and 6.5-h split sleep groups), and those between the two groups with a total TIB of 8 h per 24 h (8-h continuous and 8-h split sleep groups).
For TST and the duration of each sleep stage, a mixed model involving group (8-h continuous and split sleep groups) and day (all nights/days with PSG recordings, except B1 which was for acclimatization) was applied. We applied this model to the sum across each 24-h period, as well as for nocturnal sleep and daytime nap separately. WASO was analyzed separately for nocturnal sleep and daytime nap. To assess the effects of our manipulation on homeostatic sleep pressure, we applied this model to N2 latency and SWA from the first hour of nocturnal sleep. In addition, we used ANOVAs to determine whether all four groups differed in any of the PSG parameters during the baseline night.
Results
Polysomnographically assessed sleep duration
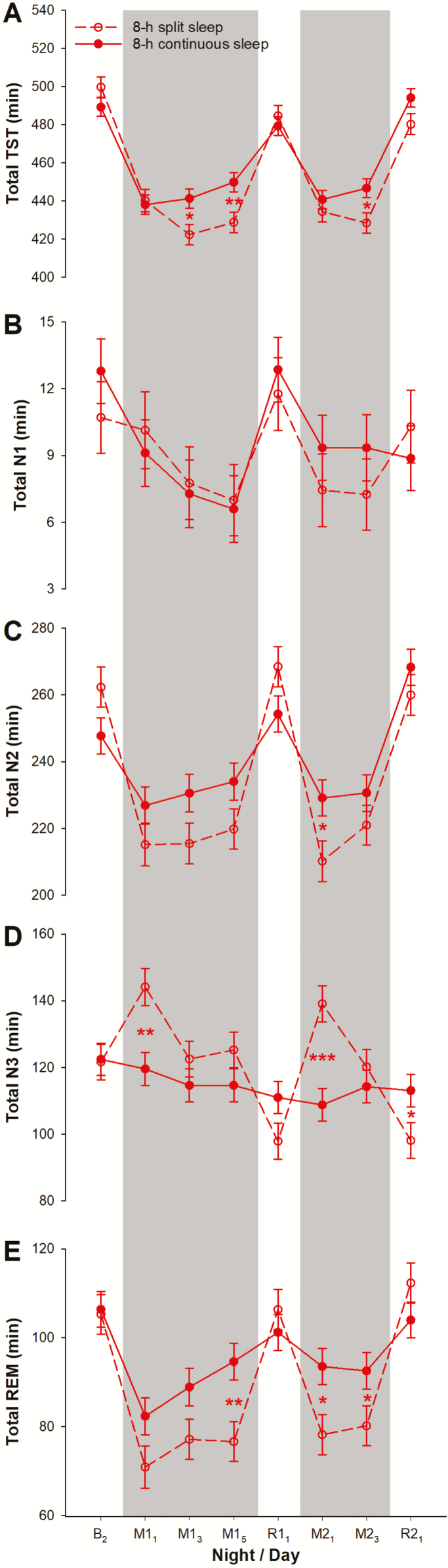
There was no significant difference in any of the PSG parameters during the baseline night among all four groups of participants (p > 0.10; Supplementary Table S1). Furthermore, even when we restricted our analyses to the 8-h continuous and the 8-h split sleep groups, no significant group difference was observed in TST, the durations of N1, N2, N3, and REM sleep (p > 0.07; Figure 2), and N2 sleep latency (p = 0.15; Figure 3, A). At baseline, no significant difference was found between the 8-h continuous and the 8-h split sleep groups in any of the PSG-assessed sleep parameters, including TST, the durations of N1, N2, N3, and REM sleep (p > 0.07; Figure 2), and N2 sleep latency (p = 0.15; Figure 3, A).
Figure 2.
Sleep duration and macrostructure per 24-h period. The least square means and standard errors estimated with general linear mixed models are plotted for polysomnographically assessed (A) TST and duration of (B) N1, (C) N2, (D), N3, and (E) rapid-eye-movement (REM) sleep across each 24-h period separately for the 8-h split sleep group (red open circles and dotted line) and the 8-h continuous sleep group (red filled circles and solid line) during the second baseline night (B2), the sleep opportunity manipulation nights (M; gray shaded areas), and the recovery nights (R). ***p < 0.001, **p < 0.01, and *p < 0.05 for significant group contrasts.
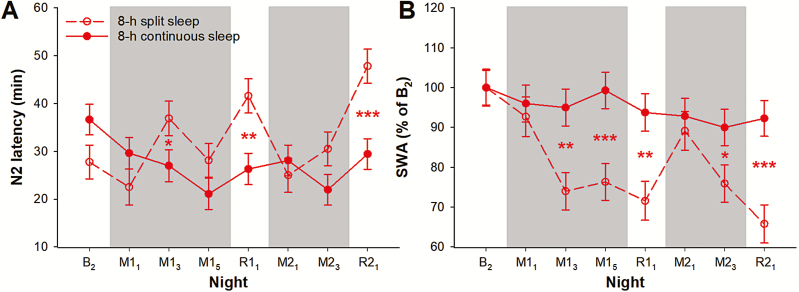
Figure 3.
Markers of homeostatic sleep pressure. The least square means and standard errors of (A) N2 sleep latency and (B) SWA in the first hour of nocturnal sleep from N2 sleep onset are plotted for the 8-h split sleep group (red open circles and dotted line) and the 8-h continuous sleep group (red filled circles and solid line) from the second baseline night (B2) to the first and second cycles of sleep opportunity manipulation (M; gray shaded areas) and recovery (R). ***p < 0.001, **p < 0.01, and *p < 0.05 for significant group contrasts.
In the first 24 h of both sleep manipulation periods, out of their total TIB of 8 h, both groups had a similar total daily TST of 7 h 14 min to 7 h 20 min (M11: p = 0.78; M21: p = 0.38; Figure 2, A). Although the 8-h split sleep group had considerably shorter (73–82 min; p < 0.001) TST at night relative to the 8-h continuous sleep group, they slept an average of about 76 min during their 90-min nap opportunity (Supplementary Figure S1, A). Despite the similar total daily TST of both groups, the two groups differed in sleep macrostructure. During both M11 and M21, the 8-h split sleep group had 25–30 min more N3 sleep in total compared to the 8-h continuous sleep group (Figure 2, D) because of the 30–31 min of N3 sleep afforded by the nap opportunities, when nocturnal N3 durations did not differ between groups (p = 0.47 and 0.87; Supplementary Figure S1, D). In contrast, relative to the 8-h continuous sleep group, the 8-h split sleep group appeared to have shorter total daily durations of N2 and REM sleep at the beginning of each sleep manipulation period (Figure 2, C and E), since the first nap episodes consisted of only 31–35 min of N2 sleep and 9–12 min of REM sleep. This would not make up for the reduced nocturnal N2 (47–50 min) and REM (20–27 min) sleep (Supplementary Figure S1, C and E).
For the rest of the sleep manipulation periods, total daily TST was 18–21 min shorter among the 8-h split sleep than the 8-h continuous sleep participants (p < 0.01; Figure 2, A). This was primarily driven by the shorter total REM sleep duration of 12–18 min (p < 0.06; Figure 2, E). The 7–12 min of REM sleep during the daytime naps in the 8-h split sleep group did not offset the 20–25 min reduction in REM sleep at night compared with the 8-h continuous sleep group (p < 0.001; Supplementary Figure S1, E). Total N2 duration and total N3 duration did not differ between the two 8-h sleep groups (p > 0.07; Figure 2, C and D) because curtailment of these sleep stages during the 6.5-h nocturnal TIB (p < 0.004) was remedied by the daytime naps that consisted of mainly of N2 and N3 sleep (Supplementary Figure S1, C and D). SWA in the first hour of the nocturnal sleep episodes was significantly and consistently lower in the 8-h split sleep group (p < 0.03; Figure 3, B), indicating the effectiveness of daytime napping in dissipating some of the homeostatic sleep pressure built up during the preceding wake period. Longer N2 sleep latency at night might also be observed as a result (e.g. night M13: p = 0.04; Figure 3, A).
During the recovery periods, minimal differences in TST and sleep macrostructure were found between the two 8-h groups (Figure 2 and Supplementary Figure S1). However, on nights R11 and R21, compared with the 8-h continuous sleep group, the 8-h split sleep group had longer N2 sleep latency (p < 0.002; Figure 3, A) and lower SWA in the first hour of their sleep (p < 0.002; Figure 3, B). This could firstly be attributed to the nap opportunity these participants had on the immediately preceding days. Also, bedtime during the recovery nights was 75 min earlier than the manipulation nights (11:00 pm vs. 00:15 am). Consequently, the shorter duration of wakefulness prior to the first recovery sleep episodes might have reduced sleep pressure further.
Vigilance
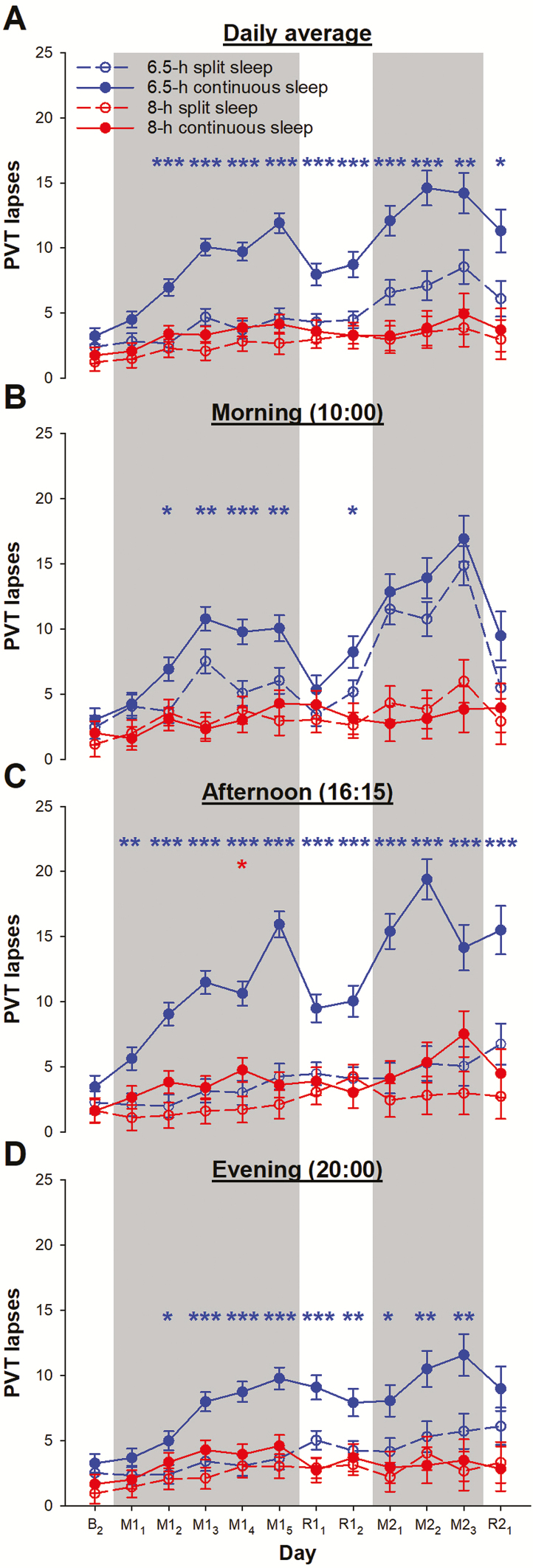
With a total TIB of 8 h provisioned, vigilance performance of the split and the continuous sleep groups did not differ significantly throughout the protocol (8-h split vs. 8-h continuous sleep: p > 0.19). A group × day interaction in the daily average PVT lapse count was statistically significant (F = 2.62, p < 0.001; Figure 4, A). This was attributed to the greater number of PVT lapses in the continuous sleep group relative to the split sleep group when total TIB was restricted to 6.5 h (6.5-h split vs. 6.5-h continuous sleep: p < 0.02 from day M12). Also, PVT performance remained relatively stable for the two groups with a total TIB of 8 h per 24 h. In contrast, compounded effects of two sleep restriction periods on vigilance was observed in both the 6.5-h split and the 6.5-h continuous sleep groups.
Figure 4.
Vigilance performance during a split or continuous sleep schedule when total TIB was below or within the recommended range. The numbers of lapses in the PVT are shown (A) averaged across the three tests each day, and separately for tests taken in the (B) morning, (C) afternoon, and (D) evening. PVT results are plotted after the last baseline night (day B2), during the first cycle of sleep opportunity manipulation (days M11–M15; gray shading) and after recovery nights (R11 and R12), to the second cycle of sleep manipulation (days M21–M23 in gray shading) and recovery sleep (R21). Observations for the 8-h split sleep group are shown in red open circles and dotted lines, while those for the 8-h continuous sleep group are illustrated in red filled circles and solid lines. For comparison, performance in a 6.5-h split sleep group (blue open circles and dotted lines) and a 6.5-h continuous sleep group (blue filled circles and solid lines) from a previous study [13] are also presented. The least square means and standard errors estimated with general linear mixed models are plotted. ***p < 0.001, **p < 0.01, and *p < 0.05 for significant contrasts between the split and the continuous sleep groups (red for the two 8-h sleep groups and blue for the two 6.5-h sleep groups).
Similar patterns were observed when PVT performance was considered for each time of day. In the morning (group × day interaction: F = 3.56, p < 0.001; Figure 4, B), the number of PVT lapses was reduced on most days in the first sleep restriction and recovery cycle for the 6.5-h split sleep group relative to the 6.5-h continuous sleep group. In contrast, no significant contrast was found for the two 8-h groups (p > 0.37). Prior to their nap on M23, the 8-h split sleep group was found to have a small increase in the number of PVT lapses from baseline (4.83 ± 1.92, p = 0.01), whereas the 8-h continuous sleep group’s morning vigilance was at baseline level during the entire study (p > 0.09).
In the afternoon (group × day interaction: F = 3.58, p < 0.001; Figure 4, C) and evening (F = 1.41, p = 0.06; Figure 4, D), compared to sleeping the 6.5 h continuously, splitting sleep with a mid-afternoon nap led to reduction in the number of PVT lapses (from M11 in the afternoon: p < 0.004; from M12 in the evening: p < 0.01). However, when total TIB was 8 h, the split and the continuous sleep schedules resulted in similar levels of vigilance performance at these two times of day (p > 0.05, except for the afternoon on M14, p = 0.03). In the afternoon, PVT performance of the 8-h split sleep group was at the baseline level throughout the protocol (p > 0.06), and the number of PVT lapses was only slightly elevated from baseline on some days in the 8-h continuous sleep group (M14, M22, and M23: mean increase = 3.14–5.90, p < 0.04). The number of PVT lapses in the evening was minimally elevated from baseline for the 8-h split sleep group on R12 and M22 (mean increase = 2.21–3.08, p < 0.05) and the 8-h continuous sleep group on M13–M15 (mean increase = 2.62–2.93, p < 0.03).
Other neurobehavioural functions
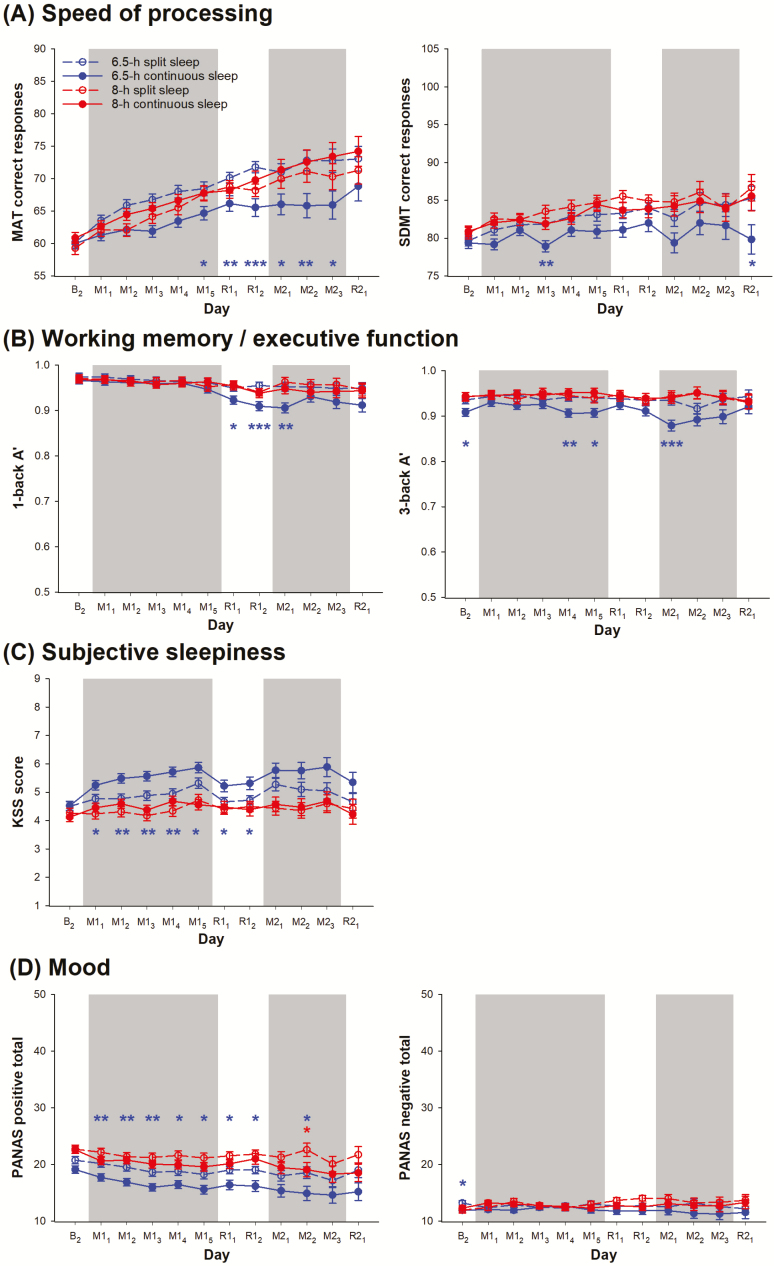
Despite the statistically non-significant group × day interactions on speed of processing, and working memory/executive function (F < 0.86, p > 0.70), a split sleep schedule appeared to confer more prominent benefits on these cognitive domains over a continuous sleep schedule when total TIB per 24 h was 6.5 h than 8 h. Specifically, no significant difference in the number of correct responses in the MAT was found between the 8-h split and continuous sleep groups (p > 0.07), while significant contrasts between the two 6.5-h groups were found on multiple protocol days (Figure 5, A, left panel). Albeit less prominently than in the MAT, a similar pattern was observed for the number of correct responses in the SDMT (Figure 5, A, right panel) and A’ in both the 1- and 3-back tasks (Figure 5, B) without systematic group differences in response bias in the two working memory/executive function tasks (Supplementary Figure S2).
Figure 5.
Other neurobehavioral functions during a split or continuous sleep schedule when total TIB was below or within the recommended range. The least square means and standard errors estimated with general linear mixed models are plotted for the daily average in (A) the number of correct responses in the MAT and the SDMT as measures of speed of processing, (B) A’ in the 1- and 3-back tasks as measures of working memory/executive function, (C) the score on the KSS as a measure of subjective sleepiness, and (D) the positive and negative affect scores on the PANAS. Data on the last baseline day (B2), as well as during the sleep opportunity manipulation periods (M; gray shading) and the recovery periods (R) are plotted in red open circles and dotted lines for the 8-h split sleep group and red filled circles and solid lines for the 8-h continuous sleep group. For comparison, performance in a 6.5-h split sleep group (blue open circles and dotted lines) and a 6.5-h continuous sleep group (blue filled circles and solid lines) from a previous study [13] also illustrated. ***p < 0.001, **p < 0.01, and *p < 0.05 for significant contrasts between the split and the consolidated sleep groups (red for the two 8-h sleep groups and blue for the two 6.5-h sleep groups).
Data from the KSS also showed no protective effect of split sleep for participants with a total TIB of 8 h (group × day interaction: F = 0.90, p = 0.64) because no significant difference was found between the 8-h split and 8-h continuous sleep groups (p > 0.18; Figure 5, C). In contrast, relative to the 6.5-h continuous sleep group, the 6.5-h split sleep group reported significantly lower levels of subjective sleepiness consistently during the first cycle of sleep opportunity manipulation and recovery (p < 0.04).
Similarly, although the group × day interaction on positive mood was not statistically significant (F = 0.37, p > 0.99), the effects of split sleep appeared to depend on the total TIB during the manipulation periods. Positive mood did not seem to benefit from a split sleep schedule when total TIB was 8 h, since no significant difference was found in the PANAS positive score between the 8-h split and 8-h continuous groups during the entire protocol (p > 0.11), except for M22 (p = 0.04; Figure 5, D left panel). However, when total TIB was restricted to 6.5 h per 24 h, the split sleep group reported higher levels of positive mood consistently throughout the protocol relative to the continuous sleep group (Figure 5, D, left panel). Our sleep opportunity manipulations did not have any statistically significant impact on PANAS negative scores (Figure 5, D, right panel; group × day interaction: F = 0.88, p = 0.66).
Discussion
We split an 8-h total sleep opportunity over 24 h to allow for a 1.5-h nap in the afternoon to supplement a shortened nocturnal sleep opportunity of 6.5 h, and found that this split sleep schedule yielded comparable cognitive performance, subjective sleepiness, and mood as when adolescents slept the entire allocated sleep opportunity at night. The findings held up over multiple nights on this schedule and suggest that when the total sleep opportunity across 24 h is within the recommended range, scheduling part of the total sleep opportunity for an afternoon nap may have little impact on neurobehavioral functions. This offers a practical solution to students who are unable to obtain the full sleep recommendation at night wherein a mid-afternoon nap can be utilized to fulfill sleep requirements without significant costs to basic cognitive functions, alertness, and mood.
The present work, the first examining split sleep schedules in adolescents with total sleep duration in the recommended range, converges with findings from studies performed in adults in laboratory settings showing that splitting at least 8-h TIB into two opportunities yielded similar vigilance outcomes compared to continuous sleep [15, 16, 32, 33]. This pattern was consistent across variations in how much sleep was allocated between the two opportunities—while our present study examined a longer nocturnal period with a 1.5-h day time nap, other studies have split the recommended amount of sleep into two opportunities of equal duration [15, 16, 33], and one examined several combinations of a longer anchor nocturnal period plus a nap ranging from 0.4-h to 2.4-h [32]. These findings suggest that when adolescents and adults obtain at least the minimum recommended TIB, how sleep is apportioned across 24 h may not impact basic neurobehavioral functions relative to sleeping the equivalent amount continuously at night. Nevertheless, future work in adolescents should aim to contrast different allocations of sleep within the two opportunities to examine if splitting adolescents’ sleep in different ways would continue to be comparable to a continuous sleep schedule.
Notably, the pattern found in adolescents diverges from that of adults when total sleep opportunity falls below the recommended range. While our adolescent findings suggest that splitting sleep into a nocturnal period and mid-day nap preferentially boosts cognitive function for sleep restricted adolescents, in adults, a study reported that splitting any amount of sleep up, including durations as short as 6 h, does not affect daytime vigilance differently compared to a consolidated sleep period of the same total amount [32, 34]. Whereas in adults it appears that vigilance is a function of the total time in bed per 24 h independent of how sleep is distributed [32, 35], our studies suggest that in adolescents, there are differential effects of splitting sleep that vary by the amount of total sleep obtained. This difference under conditions of sleep restriction may reflect ongoing maturation of brain systems governing arousal and cognitive processes in adolescence [36]. Compared to adults, adolescents’ are potentially more vulnerable to sleep loss [18, 37]. As such, for adolescents, having two opportunities to dissipate sleep pressure over 24 h may be more important when sleep is restricted in order to maintain performance across the day.
It is important to note that for both adults and adolescents the findings relating to split sleep apply to basic cognitive functions, measured by tasks that involve repeated administration of single shot tests. Fewer studies have examined the impact of split sleep on higher order functions like learning and memory that utilize a network of knowledge structures, and of which performance is less time sensitive but rather dependent on stable integration of information. In contrast with effects on vigilance, memory performance does not always show consistent deficits under adolescent sleep restriction protocols [38, 39]. For example, Voderholzer et al. [38] found that restricting nocturnal sleep to 5 h for four consecutive nights had no impact on adolescents’ declarative and procedural memory consolidation. In addition, in our previous study, we found that even though the split sleep group (5 h nocturnal + 1.5 h nap) experienced a relative dip in vigilance in the morning [13], they were still able to effectively learn biology facts at that time [40], suggesting a potential dissociation in the effects of split sleep on tests measuring basic cognitive processes compared to those involving higher order cognitive functions that may be more interesting and cognitively engaging, and hence, less sensitive to momentary drop outs in performance. Interestingly, studies in undergraduates have also found that even after a normal night of 7–9 h TIB [41, 42], a midday nap can still confer benefits on long-term memory. It thus appears that split sleep schedules, even without sleep restriction could still have a facilitative effect on memory.
The present study sheds light on the implications of splitting sleep on sleep architecture. Notably, split sleep schedules did not shorten the total amount of N2 and N3 obtained, as the naps that comprised mainly N2 and N3 sleep filled in for the shortened duration of these stages at night. However, these daytime naps consisted of a small amount of REM sleep and which did not offset the loss of nocturnal REM sleep due to earlier wake times in the split sleep group (06:45 am vs. 07:30 am). The 8-h split sleep group obtained 12–18 min less REM compared to the 8-h continuous sleep group. This said, we did not find group differences in basic cognitive performance, sleepiness, or mood, suggesting that the decreased REM duration may not be severe enough to affect these functions. Lastly, we found that a 1.5-h afternoon nap reduced SWA in the first hour of the subsequent nocturnal sleep episode, indicating that the daytime nap was effective in dissipating the homeostatic sleep pressure that built up with 6.5 h TIB at night. This could have contributed to the relatively stable vigilance performance of the split sleep group throughout the protocol.
Although it has been suggested that individuals who report napping regularly and those who do not may differ in their ability to nap [43], we found that all our adolescent participants were able to nap (minimum TST across all nap episodes = 32 min) regardless of whether or not they did so on a regular basis. Based on sleep diaries collected during the screening week, only 44% of those in the 8-h split sleep group reported taking a daytime nap at least once within the week. Yet, all were able to effectively utilize the 90-min nap opportunity regularly provided across a 15-day period, falling asleep within an average of 12.3 min and achieving an average sleep efficiency of 83.9%. While napping appeared to increase nocturnal sleep latency on some nights likely as a result of reduced sleep pressure on those nights [44], this effect was inconsistent across the manipulation period (significant only on M13), and was most pronounced on the first recovery nights following manipulation (R11 and R21) in which the split sleep group had an earlier bedtime (11:00 pm vs. 00:15 am) in addition to a daytime nap. Overall, these findings suggest that the prevalence of habitual napping in adolescents may be related to opportunity as opposed to ability, and support the feasibility of implementing midday naps in schools as a strategy to boost sleep health and neurobehavioral functions.
Limitations
The present study only examined splitting sleep between a long nocturnal period and a 1.5-h daytime nap. As such, the present conclusions cannot be generalized to other forms of splitting sleep. It is possible that patterns of cognitive functions, subjective sleepiness, and mood measured across the day will differ when nocturnal sleep is reduced to <5 h TIB, for example, when sleep is split into two equal periods (4 h + 4 h). Additionally, the nap opportunity used in our study might have been relatively long to be practicable (Table 1). Future studies may seek to evaluate additional combinations of split sleep schedules.
Adolescents are recommended to sleep 8–10 h each night. Although we used the minimum recommended sleep duration in this study, we cannot preclude the possibility that this TIB was not sufficient for some of our participants. Future studies should examine if our findings will replicate with a 10-h TIB per 24 h.
The present work did not include measures of glucose metabolism. While our previous study found a greater increase in blood glucose during an oral glucose tolerance test in the split sleep group (5-h nocturnal TIB + 1.5-h nap) compared to the continuous sleep group (6.5-h TIB at night) [13], the metabolic outcomes of splitting sleep durations within the recommended range remain unknown.
In order to compare the present study to previous protocols and not to over-test participants, we sampled performance on the test battery three times across the day. This limited the ability to assess performance on a finer time scale, and in particular immediately before the nap when homeostatic sleep pressure would be higher in the split sleep group.
Lastly, as the present work contrasted groups of students in the same age range selected one year apart, there may be potential cohort effects. Nonetheless, participants in the NFS4 and NFS5 studies were matched on all relevant demographic variables as well as sleep habits assessed during screening.
Conclusions
In adolescents, we found that there are differential effects of splitting sleep on basic cognitive functions, alertness, and mood that depend the total sleep opportunity. These findings suggest that as long as the total duration of sleep obtained across 24 h is satisfactory, many students may be able to adopt a split sleep schedule incorporating a mid-afternoon nap combined with a shorter period of nocturnal sleep. This work adds to the growing literature on practical strategies that may be employed to boost sleep health in modern societies.
Supplementary Material
Acknowledgments
The authors thank Elaine van Rijn, James Cousins, Stijn Massar, Soon Chun Siong, Andrew Dicom, Christina Chen, Xin Yu Chua, Hosein Golkashani, Ksenia Vinogradova, Zhenghao Pu, Teck Boon Teo, Brian Teo, Jesisca Tandi, Tiffany Koa, Jessica Lee, James Teng, Kian Wong, Zaven Leow, Litali Mohapatra, Caryn Yuen, Yuvan C, Aleksi Rantanen, and Karthika Muthiah for their assistance in data collection.
Disclosure statement Funding
This research was supported by the STaR Investigator Award from the National Medical Research Council, Singapore (STaR/0015/2013) awarded to Dr Chee, grant NRF2016-SOL002-001 from the National Research Foundation, Singapore, awarded to Drs Chee, Gooley, and Lo, as well as the Far East Organization. Manpower was also supported by research contract PA/9016104043 from the Defence Science & Technology Agency, Singapore, awarded to Dr Gooley, and grant MOE2015-T2-077 from the Ministry of Education, Singapore, awarded to Dr Gooley.
Conflict of interest statement. M.W.L.C. and J.L.O. have a patent for the Z3-score framework. There are no other conflicts of interest.
References
- 1. Jiang X, et al. Sleep duration, schedule and quality among urban Chinese children and adolescents: associations with routine after-school activities. PLoS One. 2015;10(1):e0115326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patte KA, et al. Modifiable predictors of insufficient sleep durations: a longitudinal analysis of youth in the COMPASS study. Prev Med. 2018;106:164–170. [DOI] [PubMed] [Google Scholar]
- 3. Twenge JM, et al. Decreases in self-reported sleep duration among U.S. adolescents 2009–2015 and association with new media screen time. Sleep Med 2017;39:47–53. [DOI] [PubMed] [Google Scholar]
- 4. Yeo SC, et al. Associations of sleep duration on school nights with self-rated health, overweight, and depression symptoms in adolescents: problems and possible solutions. Sleep Med. 2019;60:96–108. [DOI] [PubMed] [Google Scholar]
- 5. Hirshkowitz M, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1(1):40–43. [DOI] [PubMed] [Google Scholar]
- 6. Paruthi S, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J Clin Sleep Med. 2016;12(6):785–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Crowley SJ, et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PLoS One. 2014;9(11):e112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenni OG, et al. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446–1454. [DOI] [PubMed] [Google Scholar]
- 10. Yeo SC, et al. Associations of time spent on homework or studying with nocturnal sleep behaviour and depression symptoms in adolescents from Singapore. Sleep Health 2020; (in press). 10.1016/j.sleh.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 11. Adolescent Sleep Working G, et al. School start times for adolescents. Pediatrics 2014;134:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson NF, et al. Delaying middle school and high school start times promotes student health and performance: an American academy of sleep medicine position statement. J Clin Sleep Med. 2017;13(4):623–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lo JC, et al. Differential effects of split and continuous sleep on neurobehavioral function and glucose tolerance in sleep-restricted adolescents. Sleep 2019;42(5). doi: 10.1093/sleep/zsz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ong JL, et al. EEG changes accompanying successive cycles of sleep restriction with and without naps in adolescents. Sleep 2017;40(4). doi: 10.1093/sleep/zsx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson ML, et al. Investigation of the effectiveness of a split sleep schedule in sustaining sleep and maintaining performance. Chronobiol Int. 2014;31(10):1218–1230. [DOI] [PubMed] [Google Scholar]
- 16. Kosmadopoulos A, et al. The effects of a split sleep-wake schedule on neurobehavioural performance and predictions of performance under conditions of forced desynchrony. Chronobiol Int. 2014;31(10):1209–1217. [DOI] [PubMed] [Google Scholar]
- 17. Cousins JN, et al. Memory encoding is impaired after multiple nights of partial sleep restriction. J Sleep Res. 2018;27(1):138–145. [DOI] [PubMed] [Google Scholar]
- 18. Lo JC, et al. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lo JC, et al. Neurobehavioral impact of successive cycles of sleep restriction with and without naps in adolescents. Sleep 2017;40(2). doi: 10.1093/sleep/zsw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horne JA, et al. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 21. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 22. Meijer AM. Chronic sleep reduction, functioning at school and school achievement in preadolescents. J Sleep Res. 2008;17(4):395–405. [DOI] [PubMed] [Google Scholar]
- 23. Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 24. Patanaik A, et al. An end-to-end framework for real-time automatic sleep stage classification. Sleep 2018;41(5). doi: 10.1093/sleep/zsy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iber C, et al. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine, 2007. [Google Scholar]
- 26. Akerstedt T, et al. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 27. Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services, 1991. [Google Scholar]
- 28. Lo JC, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7(9):e45987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Klein KE, et al. Air operations and circadian performance rhythms. Aviat Space Environ Med. 1976;47(3):221–230. [PubMed] [Google Scholar]
- 30. Watson D, et al. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. [DOI] [PubMed] [Google Scholar]
- 31. Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Meth Instr Comp. 1985;17:652–655. [Google Scholar]
- 32. Mollicone DJ, et al. Response Surface mapping of neurobehavioral performance: testing the feasibility of split sleep schedules for space operations. Acta Astronaut. 2008;63(7–10):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Short MA, et al. The effect of split sleep schedules (6h-on/6h-off) on neurobehavioural performance, sleep and sleepiness. Appl Ergon. 2016;54:72–82. [DOI] [PubMed] [Google Scholar]
- 34. Belenky G, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 35. Belenky G, et al. Split sleeper berth use and driver performance: a review of the literature and application of a mathematical model predicting performance from sleep/wake history and circadian phase. Spokane, WA: Washington State University, 2008. [Google Scholar]
- 36. Campbell IG, et al. Differential and interacting effects of age and sleep restriction on daytime sleepiness and vigilance in adolescence: a longitudinal study. Sleep 2018;41(12). doi: 10.1093/sleep/zsy177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Talbot LS, et al. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10(6):831–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leong RLF, et al. Multiple nights of partial sleep deprivation do not affect prospective remembering at long delays. Sleep Med. 2018;44:19–23. [DOI] [PubMed] [Google Scholar]
- 39. Voderholzer U, et al. Sleep restriction over several days does not affect long-term recall of declarative and procedural memories in adolescents. Sleep Med. 2011;12(2):170–178. [DOI] [PubMed] [Google Scholar]
- 40. Cousins JN, et al. Does splitting sleep improve long-term memory in chronically sleep deprived adolescents? NPJ Sci Learn. 2019;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alger SE, et al. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem. 2012;98(2):188–196. [DOI] [PubMed] [Google Scholar]
- 42. Mander BA, et al. Wake deterioration and sleep restoration of human learning. Curr Biol. 2011;21(5):R183–R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Milner CE, et al. Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J Sleep Res. 2009;18(2):272–281. [DOI] [PubMed] [Google Scholar]
- 44. Werth E, et al. Dynamics of the sleep EEG after an early evening nap: experimental data and simulations. Am J Physiol. 1996;271(3 Pt 2):R501–R510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.